Innovative Pathways for the Valorization of Biomass Gasification Char: A Systematic Review
Abstract
1. Introduction
| Precursor | Technology | Gasifying Agent | Scale | C | Ash | SBET * | Activation | Reference |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (m2 g−1) | ||||||
| Spruce Woodchips | Floating fixed-bed | Air | Commercial | 91.4 | 3.7 | 308 | [21] | |
| Woodchips | Downdraft | Air | Lab | 76.0 | 3.3 | 379 | [22] | |
| 82.1 | 2.2 | 385 | ||||||
| 81.3 | 2.4 | 517 | ||||||
| Spruce Woodchips | Dual-stage | Air | Pilot | 87.6 | 1253 | [23] | ||
| Spruce, Pine, and Fir Sawdust | Dual fluidized bed | Steam | Pilot | 91.1 | 13.2 | 458 | [24] | |
| Woodchips | Dual-stage | Air | Commercial | 81.3 | 14.6 | 603 | [25] | |
| Wood Pellets | Rising co-current | 81.2 | 16.1 | 403 | ||||
| Woodchips | Downdraft | 80.6 | 15.8 | 427 | ||||
| Woodchips | Dual-stage | 75.9 | 15.1 | 774 | KOH | |||
| Woodchips | Dual-stage | 78.1 | 15.0 | 739 | ZnCl2 | |||
| Woodchips | Dual-stage | Air | Commercial | 79.0 | 22.2 | 587 | [26] | |
| Woodchips | Downdraft | Air | Commercial | 68.6 | 27.8 | 352 | [27] | |
| Wood Pellets | Rising co-current | 83.4 | 13.5 | 128 | ||||
| Woodchips | Downdraft | 48.0 | 49.5 | 78 | ||||
| Woodchips | Downdraft | 87.6 | 8.7 | 281 | ||||
| Woodchips | Dual-stage | 91.4 | 4.2 | 272 | ||||
| Mesquite Woodchips | Downdraft | Air | Pilot | 84.5 | 9.8 | 777 | CO2 | [28] |
| 84.5 | 9.8 | 737 | H2O | |||||
| Gliricidia Wood | Air | Commercial | 50.0 | 714 | [29] | |||
| Woodchips | Downdraft | Air | Pilot | 52.1 | 590 | CO2 | [30] | |
| Pine Woodchips | Fluidized bed | Air | Pilot | 72.0 | 23.0 | 1509 | K2CO3 | [31] |
| Poplar Wood | Fluidized bed | CO2 | Lab | 435 | [32] | |||
| Lab | 687 | |||||||
| Rubber Tree Roots | Commercial | 68.0 | 5.5 | 478 | KOH | [33] | ||
| Almond Shells | Downdraft | Air | Commercial | 63 | [34] | |||
| Corncob Char | Downdraft | Air | Commercial | 78.5 | 8.6 | 162 | [35] | |
| Switchgrass | Downdraft | Air | Pilot | 73.1 | 944 | KOH | [36] | |
| Sunflower Husks | Fluidized bed | Pilot | 56.8 | 21.7 | 5 | [37] | ||
| Poultry Litter | 12.6 | 74.9 | 12 | |||||
| Wood Pellets | 29.1 | 53.6 | 5 | |||||
| Wood Waste 1 | 39.6 | 45.2 | 2 | |||||
| Wood Waste 2 | 39.4 | 48.9 | 2 | |||||
| Paper and Plastic Waste 1 | 34.4 | 45.1 | 65 | |||||
| Paper and Plastic Waste 2 | 26.2 | 55.1 | 42 | |||||
| Paper and Plastic Waste 3 | 15.8 | 75.4 | 20 | |||||
| MSW | Fixed-bed downdraft | Air/Steam | Commercial | 48.3 | 50.4 | 3 | [38] | |
| 29.7 | 54.5 | 11 | ||||||
| MSW | Fluidized bed | Commercial | 56.2 | 39.4 | 13 | [39] |
2. Methods and Data Analysis
2.1. Literature Review Strategy
2.2. Study Selection and Data Extraction
2.2.1. Pyrolysis and Gasification
2.2.2. Subsequent Gasification of Pyrolysis Char
3. Char Applications
3.1. Adsorption
3.1.1. Water Treatment
Organic Micropollutants
Dyes
Heavy Metals
Other Pollutants
3.1.2. Gas Adsorption and Soil Remediation
3.1.3. Removal Mechanisms
3.1.4. Challenges
3.2. Catalysis
3.2.1. Tar Reforming
3.2.2. Catalyst Support
3.3. Other Applications
3.3.1. Agriculture
3.3.2. Polymers
3.3.3. Anaerobic Digestion and Composting
3.3.4. Electrochemistry
3.3.5. Construction
4. Outlook
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cazzaniga, N.E.; Jasinevičius, G.; Mubareka, S. Sankey Diagrams of Woody Biomass Flows in the European Union-2021 Release; European Commission: Brussels, Belgium, 2022.
- Molino, A.; Chianese, S.; Musmarra, D. Biomass Gasification Technology: The State of the Art Overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.L.; Zhao, X.Y.; Cao, J.P. Methanation of Syngas from Biomass Gasification: An Overview. Int. J. Hydrogen Energy 2020, 45, 4223–4243. [Google Scholar] [CrossRef]
- Thomson, R.; Kwong, P.; Ahmad, E.; Nigam, K.D.P. Clean Syngas from Small Commercial Biomass Gasifiers; a Review of Gasifier Development, Recent Advances and Performance Evaluation. Int. J. Hydrogen Energy 2020, 45, 21087–21111. [Google Scholar] [CrossRef]
- Hrbek, J. Status Report on Thermal Gasification of Biomass and Waste; IEA Bioenergy: Paris, France, 2021. [Google Scholar]
- Patuzzi, F.; Basso, D.; Vakalis, S.; Antolini, D.; Piazzi, S.; Benedetti, V.; Cordioli, E.; Baratieri, M. State-of-the-Art of Small-Scale Biomass Gasification Systems: An Extensive and Unique Monitoring Review. Energy 2021, 223, 120039. [Google Scholar] [CrossRef]
- Bain, R.L.; Broer, K. Chapter 3: Gasification. In Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and Power; Brown, R.C., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 47–77. ISBN 9781119990840. [Google Scholar]
- Hansen, V.; Müller-Stöver, D.; Ahrenfeldt, J.; Holm, J.K.; Henriksen, U.B.; Hauggaard-Nielsen, H. Gasification Biochar as a Valuable By-Product for Carbon Sequestration and Soil Amendment. Biomass Bioenergy 2015, 72, 300–308. [Google Scholar] [CrossRef]
- Benedetti, V.; Patuzzi, F.; Baratieri, M. Gasification Char as a Potential Substitute of Activated Carbon in Adsorption Applications. Energy Procedia 2017, 105, 712–717. [Google Scholar] [CrossRef]
- Shackley, S.; Carter, S.; Knowles, T.; Middelink, E.; Haefele, S.; Sohi, S.; Cross, A.; Haszeldine, S. Sustainable Gasification–Biochar Systems? A Case-Study of Rice-Husk Gasification in Cambodia, Part I: Context, Chemical Properties, Environmental and Health and Safety Issues. Energy Policy 2012, 42, 49–58. [Google Scholar] [CrossRef]
- Brewer, C.E.; Unger, R.; Schmidt-Rohr, K.; Brown, R.C. Criteria to Select Biochars for Field Studies Based on Biochar Chemical Properties. BioEnergy Res. 2011, 4, 312–323. [Google Scholar] [CrossRef]
- Wiedner, K.; Rumpel, C.; Steiner, C.; Pozzi, A.; Maas, R.; Glaser, B. Chemical Evaluation of Chars Produced by Thermochemical Conversion (Gasification, Pyrolysis and Hydrothermal Carbonization) of Agro-Industrial Biomass on a Commercial Scale. Biomass Bioenergy 2013, 59, 264–278. [Google Scholar] [CrossRef]
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.W.; Kwon, E.E.; Lee, J.; Wang, C.-H. A Critical Review on Sustainable Biochar System through Gasification: Energy and Environmental Applications. Bioresour. Technol. 2017, 246, 242–253. [Google Scholar] [CrossRef]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the Circular Economy: An Analysis of 114 Definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- de Coninck, H.; Revi, A.; Babiker, M.; Bertoldi, P.; Buckeridge, M.; Cartwright, A.; Dong, W.; Ford, J.; Fuss, S.; Hourcade, J.-C.; et al. Strengthening and Implementing the Global Response. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018. [Google Scholar]
- Shahbaz, M.; AlNouss, A.; Ghiat, I.; Mckay, G.; Mackey, H.; Elkhalifa, S.; Al-Ansari, T. A Comprehensive Review of Biomass Based Thermochemical Conversion Technologies Integrated with CO2 Capture and Utilisation within BECCS Networks. Resour. Conserv. Recycl. 2021, 173, 105734. [Google Scholar] [CrossRef]
- Huang, Y.; Wan, Y.; Liu, S.; Zhang, Y.; Ma, H.; Zhang, S.; Zhou, J. A Downdraft Fixed-Bed Biomass Gasification System with Integrated Products of Electricity, Heat, and Biochar: The Key Features and Initial Commercial Performance. Energies 2019, 12, 2979. [Google Scholar] [CrossRef]
- Pio, D.T.; Tarelho, L.A.C. Industrial Gasification Systems (>3 MWth) for Bioenergy in Europe: Current Status and Future Perspectives. Renew. Sustain. Energy Rev. 2021, 145, 111108. [Google Scholar] [CrossRef]
- European Biogas Association. Gasification—A Sustainable Technology for Circular Economies; European Biogas Association: Brussels, Belgium, 2021. [Google Scholar]
- Arora, S.; Jung, J.; Liu, M.; Li, X.; Goel, A.; Chen, J.; Song, S.; Anderson, C.; Chen, D.; Leong, K.; et al. Gasification Biochar from Horticultural Waste: An Exemplar of the Circular Economy in Singapore. Sci. Total Environ. 2021, 781, 146573. [Google Scholar] [CrossRef]
- Back, J.O.; Hupfauf, B.; Rößler, A.; Penner, S.; Rupprich, M. Adsorptive Removal of Micropollutants from Wastewater with Floating-Fixed-Bed Gasification Char. J. Environ. Chem. Eng. 2020, 8, 103757. [Google Scholar] [CrossRef]
- Pelaez-Samaniego, M.R.; Perez, J.F.; Ayiania, M.; Garcia-Perez, T. Chars from Wood Gasification for Removing H2S from Biogas. Biomass Bioenergy 2020, 142, 105754. [Google Scholar] [CrossRef]
- Ravenni, G.; Sárossy, Z.; Sanna, S.; Ahrenfeldt, J.; Henriksen, U.B. Residual Gasification Char Applied to Tar Reforming in a Pilot-Scale Gasifier: Performance and Evolution of Char Properties for Perspective Cascade Uses. Fuel Process. Technol. 2020, 210, 106546. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, Z.; Zhang, Z.; Guo, C.; Ellis, N.; Bi, X.; Paul Watkinson, A.; Grace, J.R. Tar Elimination from Biomass Gasification Syngas with Bauxite Residue Derived Catalysts and Gasification Char. Appl. Energy 2020, 258, 114088. [Google Scholar] [CrossRef]
- Benedetti, V.; Cordioli, E.; Patuzzi, F.; Baratieri, M. CO2 Adsorption Study on Pure and Chemically Activated Chars Derived from Commercial Biomass Gasifiers. J. CO2 Util. 2019, 33, 46–54. [Google Scholar] [CrossRef]
- Cordioli, E.; Patuzzi, F.; Baratieri, M. Thermal and Catalytic Cracking of Toluene Using Char from Commercial Gasification Systems. Energies 2019, 12, 3764. [Google Scholar] [CrossRef]
- Benedetti, V.; Patuzzi, F.; Baratieri, M. Characterization of Char from Biomass Gasification and Its Similarities with Activated Carbon in Adsorption Applications. Appl. Energy 2018, 227, 92–99. [Google Scholar] [CrossRef]
- Maneerung, T.; Liew, J.; Dai, Y.; Kawi, S.; Chong, C.; Wang, C.H. Activated Carbon Derived from Carbon Residue from Biomass Gasification and Its Application for Dye Adsorption: Kinetics, Isotherms and Thermodynamic Studies. Bioresour. Technol. 2016, 200, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Mayakaduwa, S.S.; Kumarathilaka, P.; Herath, I.; Ahmad, M.; Al-Wabel, M.; Ok, Y.S.; Usman, A.; Abduljabbar, A.; Vithanage, M. Equilibrium and Kinetic Mechanisms of Woody Biochar on Aqueous Glyphosate Removal. Chemosphere 2016, 144, 2516–2521. [Google Scholar] [CrossRef] [PubMed]
- Kilpimaa, S.; Runtti, H.; Kangas, T.; Lassi, U.; Kuokkanen, T. Physical Activation of Carbon Residue from Biomass Gasification: Novel Sorbent for the Removal of Phosphates and Nitrates from Aqueous Solution. J. Ind. Eng. Chem. 2015, 21, 1354–1364. [Google Scholar] [CrossRef]
- Galhetas, M.; Mestre, A.S.; Pinto, M.L.; Gulyurtlu, I.; Lopes, H.; Carvalho, A.P. Chars from Gasification of Coal and Pine Activated with K2CO3: Acetaminophen and Caffeine Adsorption from Aqueous Solutions. J. Colloid Interface Sci. 2014, 433, 94–103. [Google Scholar] [CrossRef]
- Klinghoffer, N.B.; Castaldi, M.J.; Nzihou, A. Catalyst Properties and Catalytic Performance of Char from Biomass Gasification. Ind. Eng. Chem. Res. 2012, 51, 13113–13122. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Ahmad, M.A.; Yahaya, N.K.E.M.; Karim, J. Adsorption of Malachite Green by Activated Carbon Derived from Gasified Hevea Brasiliensis Root. Arab. J. Chem. 2021, 14, 103104. [Google Scholar] [CrossRef]
- Catizzone, E.; Sposato, C.; Romanelli, A.; Barisano, D.; Cornacchia, G.; Marsico, L.; Cozza, D.; Migliori, M. Purification of Wastewater from Biomass-Derived Syngas Scrubber Using Biochar and Activated Carbons. Int. J. Environ. Res. Public Health 2021, 18, 4247. [Google Scholar] [CrossRef]
- Yao, X.; Xu, K.; Li, Y. Physicochemical Properties and Possible Applications of Waste Corncob Fly Ash from Biomass Gasification Industries of China. BioResources 2016, 11, 3783–3798. [Google Scholar] [CrossRef]
- Bhandari, P.N.; Kumar, A.; Bellmer, D.D.; Huhnke, R.L. Synthesis and Evaluation of Biochar-Derived Catalysts for Removal of Toluene (Model Tar) from Biomass-Generated Producer Gas. Renew. Energy 2014, 66, 346–353. [Google Scholar] [CrossRef]
- Fuente-Cuesta, A.; Diaz-Somoano, M.; Lopez-Anton, M.A.; Cieplik, M.; Fierro, J.L.G.; Martínez-Tarazona, M.R. Biomass Gasification Chars for Mercury Capture from a Simulated Flue Gas of Coal Combustion. J. Environ. Manag. 2012, 98, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Damertey, D.; Ohemeng-boahen, G.; Sung, D.; Han, S. Characterization and Adsorption Performance Evaluation of Waste Char By-Product from Industrial Gasification of Solid Refuse Fuel from Municipal Solid Waste. Waste Manag. 2019, 91, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Assima, G.P.; Marie-Rose, S.; Lavoie, J.M. Role of Fixed Carbon and Metal Oxides in Char during the Catalytic Conversion of Tar from RDF Gasification. Fuel 2018, 218, 406–416. [Google Scholar] [CrossRef]
- Meyer, S.; Glaser, B.; Quicker, P. Technical, Economical, and Climate-Related Aspects of Biochar Production Technologies: A Literature Review. Environ. Sci. Technol. 2011, 45, 9473–9483. [Google Scholar] [CrossRef]
- Demirbaş, A. Gaseous Products from Biomass by Pyrolysis and Gasification: Effects of Catalyst on Hydrogen Yield. Energy Convers. Manag. 2002, 43, 897–909. [Google Scholar] [CrossRef]
- Lee, J.; Sarmah, A.K.; Kwon, E.E. Chapter 1—Production and Formation of Biochar. In Biochar from Biomass and Waste; Ok, Y.S., Tsang, D.C.W., Bolan, N., Novak, J.M.B.T.-B., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–18. ISBN 978-0-12-811729-3. [Google Scholar]
- Ighalo, J.O.; Rangabhashiyam, S.; Dulta, K.; Umeh, C.T.; Iwuozor, K.O.; Aniagor, C.O.; Eshiemogie, S.O.; Iwuchukwu, F.U.; Igwegbe, C.A. Recent Advances in Hydrochar Application for the Adsorptive Removal of Wastewater Pollutants. Chem. Eng. Res. Des. 2022, 184, 419–456. [Google Scholar] [CrossRef]
- Lu, J.; Watson, J.; Liu, Z.; Wu, Y. Elemental Migration and Transformation during Hydrothermal Liquefaction of Biomass. J. Hazard. Mater. 2022, 423, 126961. [Google Scholar] [CrossRef]
- Kota, K.B.; Shenbagaraj, S.; Sharma, P.K.; Sharma, A.K.; Ghodke, P.K.; Chen, W.H. Biomass Torrefaction: An Overview of Process and Technology Assessment Based on Global Readiness Level. Fuel 2022, 324, 124663. [Google Scholar] [CrossRef]
- Amalina, F.; Syukor Abd Razak, A.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Advanced Techniques in the Production of Biochar from Lignocellulosic Biomass and Environmental Applications. Clean. Mater. 2022, 6, 100137. [Google Scholar] [CrossRef]
- Basu, P. Chapter 5: Gasification Theory and Modeling of Gasifiers. In Biomass Gasification and Pyrolysis; Academic Press: Cambridge, MA, USA, 2010; pp. 117–165. ISBN 978-0-12-374988-8. [Google Scholar]
- Fryda, L.; Visser, R. Biochar for Soil Improvement: Evaluation of Biochar from Gasification and Slow Pyrolysis. Agriculture 2015, 5, 1076–1115. [Google Scholar] [CrossRef]
- Pena, J.; Villot, A.; Gerente, C. Pyrolysis Chars and Physically Activated Carbons Prepared from Buckwheat Husks for Catalytic Purification of Syngas. Biomass Bioenergy 2020, 132, 105435. [Google Scholar] [CrossRef]
- Brewer, C.E.; Schmidt-rohr, K.; Satrio, J.A.; Brown, R.C. Characterization of Biochar from Fast Pyrolysis and Gasification Systems. Environ. Prog. Sustain. Energy 2009, 28, 386–396. [Google Scholar] [CrossRef]
- EBC European Biochar Certificate. Guidelines for a Sustainable Production of Biochar; Version 9.2E. 2 December 2020; European Biochar Foundation (EBC): Arbaz, Switzerland, 2012; Available online: https://European-biochar.org (accessed on 4 March 2023).
- Huygens, D.; Saveyn, H.; Tonini, D.; Eder, P.; Delgado Sancho, L. Technical Proposals for Selected New Fertilising Materials under the Fertilising Products Regulation (Regulation (EU) 2019/1009); Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Sircar, I.; Sane, A.; Wang, W.; Gore, J.P. Experimental and Modeling Study of Pinewood Char Gasification with CO2. Fuel 2014, 119, 38–46. [Google Scholar] [CrossRef]
- Zhai, M.; Zhang, Y.; Dong, P.; Liu, P. Characteristics of Rice Husk Char Gasification with Steam. Fuel 2015, 158, 42–49. [Google Scholar] [CrossRef]
- Yan, F.; Luo, S.Y.; Hu, Z.Q.; Xiao, B.; Cheng, G. Hydrogen-Rich Gas Production by Steam Gasification of Char from Biomass Fast Pyrolysis in a Fixed-Bed Reactor: Influence of Temperature and Steam on Hydrogen Yield and Syngas Composition. Bioresour. Technol. 2010, 101, 5633–5637. [Google Scholar] [CrossRef]
- Ferhan, C.; Ozgur, A. Activated Carbon for Water and Wastewater Treatment: Integration of Adsorption and Biological Treatment; Wiley-VCH: Weinheim, Germany, 2011; ISBN 9783527319626. [Google Scholar]
- Qian, K.; Kumar, A.; Patil, K.; Bellmer, D.; Wang, D.; Yuan, W.; Huhnke, R.L. Effects of Biomass Feedstocks and Gasification Conditions on the Physiochemical Properties of Char. Energies 2013, 6, 3972–3986. [Google Scholar] [CrossRef]
- To, M.-H.; Hadi, P.; Hui, C.-W.; Ki Lin, C.S.; Al Ansari, T.; Saleem, J.; Parasarathy, P.; McKay, G. Waste Biomass Gasification Char Derived Activated Carbon for Pharmaceutical Carbamazepine Removal from Water. Resour. Environ. Inf. Eng. 2019, 1, 36–44. [Google Scholar] [CrossRef]
- Ramanayaka, S.; Tsang, D.C.W.; Hou, D.; Ok, Y.S.; Vithanage, M. Green Synthesis of Graphitic Nanobiochar for the Removal of Emerging Contaminants in Aqueous Media. Sci. Total Environ. 2020, 706, 135725. [Google Scholar] [CrossRef]
- do Nascimento, B.F.; de Araujo, C.M.B.; do Nascimento, A.C.; da Costa, G.R.B.; Gomes, B.F.M.L.; da Silva, M.P.; da Silva Santos, R.K.; da Motta Sobrinho, M.A. Adsorption of Reactive Black 5 and Basic Blue 12 Using Biochar from Gasification Residues: Batch Tests and Fixed-Bed Breakthrough Predictions for Wastewater Treatment. Bioresour. Technol. Rep. 2021, 15, 100767. [Google Scholar] [CrossRef]
- Kelm, M.A.P.; da Silva Junior, M.J.; de Barros Holanda, S.H.; de Araujo, C.M.B.; de Assis Filho, R.B.; Freitas, E.J.; dos Santos, D.R.; da Motta Sobrinh, M.A. Removal of Azo Dye from Water via Adsorption on Biochar Produced by the Gasification of Wood Wastes. Environ. Sci. Pollut. Res. 2019, 26, 28558–28573. [Google Scholar] [CrossRef]
- Dias, D.; Lapa, N.; Bernardo, M.; Ribeiro, W.; Matos, I.; Fonseca, I.; Pinto, F. Cr(III) Removal from Synthetic and Industrial Wastewaters by Using Co-Gasification Chars of Rice Waste Streams. Bioresour. Technol. 2018, 266, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Runtti, H.; Tuomikoski, S.; Kangas, T.; Lassi, U.; Kuokkanen, T.; Rämö, J. Chemically Activated Carbon Residue from Biomass Gasification as a Sorbent for Iron(II), Copper(II) and Nickel(II) Ions. J. Water Process Eng. 2014, 4, 12–24. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.; Wei, X.; Zhang, S.; Chen, J.; Ren, Z.J. CO2 Activation Promotes Available Carbonate and Phosphorus of Antibiotic Mycelial Fermentation Residue-Derived Biochar Support for Increased Lead Immobilization. Chem. Eng. J. 2018, 334, 1101–1107. [Google Scholar] [CrossRef]
- Carnimeo, C.; Colatorti, N.; D’Orazio, V.; Trotti, P.; Loffredo, E. Potential of Biochar from Wood Gasification to Retain Endocrine Disrupting Chemicals. Materials 2023, 16, 569. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, P.; Sajjad, S.; Saleem, J.; Alherbawi, M.; Mckay, G. A Review of the Removal of Dyestuffs from Effluents onto Biochar. Separations 2022, 9, 139. [Google Scholar] [CrossRef]
- Rubio-Clemente, A.; Gutiérrez, J.; Henao, H.; Melo, A.M.; Pérez, J.F.; Chica, E. Adsorption Capacity of the Biochar Obtained from Pinus Patula Wood Micro-Gasification for the Treatment of Polluted Water Containing Malachite Green Dye. J. King Saud Univ.-Eng. Sci. 2021; in press. [Google Scholar] [CrossRef]
- Dias, D.; Bernardo, M.; Pinto, F.; Fonseca, I.; Lapa, N. Cr(III) Dynamic Removal in a Fixed-Bed Column by Using a Co-Gasification Char. Int. J. Environ. Sci. Technol. 2022, 19, 8145–8158. [Google Scholar] [CrossRef]
- do Nascimento, B.F.; de Araujo, C.M.B.; do Nascimento, A.C.; da Silva, F.L.H.; de Melo, D.J.N.; Jaguaribe, E.F.; Lima Cavalcanti, J.V.F.; da Motta Sobrinho, M.A. Detoxification of Sisal Bagasse Hydrolysate Using Activated Carbon Produced from the Gasification of Açaí Waste. J. Hazard. Mater. 2021, 409, 124494. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Choi, S.W.; Igalavithana, A.D.; Yang, X.; Tsang, D.C.W.; Wang, C.H.; Kua, H.W.; Lee, K.B.; Ok, Y.S. Sustainable Gasification Biochar as a High Efficiency Adsorbent for CO2 Capture: A Facile Method to Designer Biochar Fabrication. Renew. Sustain. Energy Rev. 2020, 124, 109785. [Google Scholar] [CrossRef]
- Marchelli, F.; Cordioli, E.; Patuzzi, F.; Sisani, E.; Barelli, L.; Baratieri, M.; Arato, E.; Bosio, B. Experimental Study on H2S Adsorption on Gasification Char under Different Operative Conditions. Biomass Bioenergy 2019, 126, 106–116. [Google Scholar] [CrossRef]
- Kumarathilaka, P.; Ahmad, M.; Herath, I.; Mahatantila, K.; Athapattu, B.C.L.; Rinklebe, J.; Ok, Y.S.; Usman, A.; Al-Wabel, M.I.; Abduljabbar, A.; et al. Influence of Bioenergy Waste Biochar on Proton- and Ligand-Promoted Release of Pb and Cu in a Shooting Range Soil. Sci. Total Environ. 2018, 625, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Kumarathilaka, P.; Vithanage, M. Influence of Gliricidia Sepium Biochar on Attenuate Perchlorate-Induced Heavy Metal Release in Serpentine Soil. J. Chem. 2017, 2017, 6180636. [Google Scholar] [CrossRef]
- Oba, S.N.; Ighalo, J.O.; Aniagor, C.O.; Igwegbe, C.A. Removal of Ibuprofen from Aqueous Media by Adsorption: A Comprehensive Review. Sci. Total Environ. 2021, 780, 146608. [Google Scholar] [CrossRef] [PubMed]
- Iovino, P.; Canzano, S.; Capasso, S.; Erto, A.; Musmarra, D. A Modeling Analysis for the Assessment of Ibuprofen Adsorption Mechanism onto Activated Carbons. Chem. Eng. J. 2015, 277, 360–367. [Google Scholar] [CrossRef]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface Water Pollution by Pharmaceuticals and an Alternative of Removal by Low-Cost Adsorbents: A Review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the Removal of Contaminants from Soil and Water: A Review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Lin, S. Advances in the Study of Heavy Metal Adsorption from Water and Soil by Modified Biochar. Water 2022, 14, 3894. [Google Scholar] [CrossRef]
- Hong, N.; Cheng, Q.; Goonetilleke, A.; Bandala, E.R.; Liu, A. Assessing the Effect of Surface Hydrophobicity/Hydrophilicity on Pollutant Leaching Potential of Biochar in Water Treatment. J. Ind. Eng. Chem. 2020, 89, 222–232. [Google Scholar] [CrossRef]
- Castiglioni, M.; Rivoira, L.; Ingrando, I.; Meucci, L.; Binetti, R.; Fungi, M.; El-Ghadraoui, A.; Bakari, Z.; Del Bubba, M.; Bruzzoniti, M.C. Biochars Intended for Water Filtration: A Comparative Study with Activated Carbons of Their Physicochemical Properties and Removal Efficiency towards Neutral and Anionic Organic Pollutants. Chemosphere 2022, 288, 132538. [Google Scholar] [CrossRef]
- Speight, J.G. Handbook of Gasification Technology: Science, Technology, and Processes; Wiley-Scrivener: Austin, TX, USA, 2020; ISBN 9781118773536. [Google Scholar]
- Ren, J.; Cao, J.P.; Zhao, X.Y.; Liu, Y.L. Fundamentals and Applications of Char in Biomass Tar Reforming. Fuel Process. Technol. 2021, 216, 106782. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and Utilization of Biochar: A Review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Buentello-Montoya, D.A.; Zhang, X.; Li, J. The Use of Gasification Solid Products as Catalysts for Tar Reforming. Renew. Sustain. Energy Rev. 2019, 107, 399–412. [Google Scholar] [CrossRef]
- Singh, S.; Kumar Bhaumik, S.; Dong, L.; Li, C.Z.; Vuthaluru, H. An Integrated Two-Step Process of Reforming and Adsorption Using Biochar for Enhanced Tar Removal in Syngas Cleaning. Fuel 2022, 307, 121935. [Google Scholar] [CrossRef]
- Assima, G.P.; Paquet, A.; Lavoie, J. Utilization of MSW-Derived Char for Catalytic Reforming of Tars and Light Hydrocarbons in the Primary Syngas Produced During Wood Chips and MSW-RDF Air Gasification. Waste Biomass Valorization 2019, 10, 1203–1222. [Google Scholar] [CrossRef]
- Korus, A.; Ravenni, G.; Loska, K.; Korus, I.; Samson, A.; Szlęk, A. The Importance of Inherent Inorganics and the Surface Area of Wood Char for Its Gasification Reactivity and Catalytic Activity towards Toluene Conversion. Renew. Energy 2021, 173, 479–497. [Google Scholar] [CrossRef]
- Ravenni, G.; Elhami, O.H.; Ahrenfeldt, J.; Henriksen, U.B.; Neubauer, Y. Adsorption and Decomposition of Tar Model Compounds over the Surface of Gasification Char and Active Carbon within the Temperature Range 250–800 °C. Appl. Energy 2019, 241, 139–151. [Google Scholar] [CrossRef]
- Hervy, M.; Weiss-Hortala, E.; Pham Minh, D.; Dib, H.; Villot, A.; Gérente, C.; Berhanu, S.; Chesnaud, A.; Thorel, A.; Le Coq, L.; et al. Reactivity and Deactivation Mechanisms of Pyrolysis Chars from Bio-Waste during Catalytic Cracking of Tar. Appl. Energy 2019, 237, 487–499. [Google Scholar] [CrossRef]
- Frazier, R.S.; Jin, E.; Kumar, A. Life Cycle Assessment of Biochar versus Metal Catalysts Used in Syngas Cleaning. Energies 2015, 8, 621–644. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A. Reforming of Lignin-Derived Tars over Char-Based Catalyst Using Py-GC/MS. Fuel 2015, 162, 47–54. [Google Scholar] [CrossRef]
- Parrillo, F.; Ruoppolo, G.; Arena, U. The Role of Activated Carbon Size in the Catalytic Cracking of Naphthalene. Energy 2020, 190, 116385. [Google Scholar] [CrossRef]
- Benedetti, V.; Ail, S.S.; Patuzzi, F.; Baratieri, M. Valorization of Char from Biomass Gasification as Catalyst Support in Dry Reforming of Methane. Front. Chem. 2019, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, V.; Ail, S.S.; Patuzzi, F.; Cristofori, D.; Rauch, R.; Baratieri, M. Investigating the Feasibility of Valorizing Residual Char from Biomass Gasification as Catalyst Support in Fischer-Tropsch Synthesis. Renew. Energy 2020, 147, 884–894. [Google Scholar] [CrossRef]
- Bazargan, A.; Kostić, M.D.; Stamenković, O.S.; Veljković, V.B.; McKay, G. A Calcium Oxide-Based Catalyst Derived from Palm Kernel Shell Gasification Residues for Biodiesel Production. Fuel 2015, 150, 519–525. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Bellmer, D.; Yuan, W.; Wang, D.; Eastman, M.A. Physical Properties and Reactivity of Char Obtained from Downdraft Gasification of Sorghum and Eastern Red Cedar. Fuel 2015, 143, 383–389. [Google Scholar] [CrossRef]
- Pedrazzi, S.; Santunione, G.; Minarelli, A.; Allesina, G. Energy and Biochar Co-Production from Municipal Green Waste Gasification: A Model Applied to a Landfill in the North of Italy. Energy Convers. Manag. 2019, 187, 274–282. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.; Tai, M.H.; Lin, A.; Owyong, S.; Li, X.; Leong, K.; Yusof, M.L.M.; Ghosh, S.; Wang, C.H. Integrated Applications of Water Hyacinth Biochar: A Circular Economy Case Study. J. Clean. Prod. 2022, 378, 134621. [Google Scholar] [CrossRef]
- Bruun, E.W.; Petersen, C.T.; Hansen, E.; Holm, J.K.; Hauggaard-Nielsen, H. Biochar Amendment to Coarse Sandy Subsoil Improves Root Growth and Increases Water Retention. Soil Use Manag. 2014, 30, 109–118. [Google Scholar] [CrossRef]
- Bruun, E.W.; Müller-Stöver, D.; Pedersen, B.N.; Hansen, L.V.; Petersen, C.T. Ash and Biochar Amendment of Coarse Sandy Soil for Growing Crops under Drought Conditions. Soil Use Manag. 2022, 38, 1280–1292. [Google Scholar] [CrossRef]
- Martos, S.; Mattana, S.; Ribas, A.; Albanell, E.; Domene, X. Biochar Application as a Win-Win Strategy to Mitigate Soil Nitrate Pollution without Compromising Crop Yields: A Case Study in a Mediterranean Calcareous Soil. J. Soils Sediments 2020, 20, 220–233. [Google Scholar] [CrossRef]
- Yang, X.; Tsibart, A.; Nam, H.; Hur, J.; El-Naggar, A.; Tack, F.M.G.; Wang, C.H.; Lee, Y.H.; Tsang, D.C.W.; Ok, Y.S. Effect of Gasification Biochar Application on Soil Quality: Trace Metal Behavior, Microbial Community, and Soil Dissolved Organic Matter. J. Hazard. Mater. 2019, 365, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Hien, T.T.T.; Tsubota, T.; Taniguchi, T.; Shinogi, Y. Comparison of Consecutive Impacts of Wood and Rice Husk Gasification Biochars with Nitrogen Fertilizer on Soybean Yield. Paddy Water Environ. 2022, 20, 303–313. [Google Scholar] [CrossRef]
- Tonon, G. WOOD-UP—Valorizzazione Della Filiera di Gassificazione di Biomasse Legnose per l’energia, la Fertilità del Suolo e la Mitigazione dei Cambiamenti Climatici; Bozen-Bolzano University Press: Bolzano, Italy, 2020; ISBN 9788860461773. [Google Scholar]
- Schirra, A.; Ali, A.B.; Renz, F.; Sindelar, R.; Pedrazzi, S.; Allesina, G. Preliminary Investigation of Possible Biochar Use as Carbon Source in Polyacrylonitrile Electrospun Fiber Production. Appl. Sci. 2022, 12, 4441. [Google Scholar] [CrossRef]
- Benedetti, V.; Scatto, M.; Baratieri, M.; Riello, P. Valorization of Biomass Gasification Char as Filler in Polymers and Comparison with Carbon Black. Waste Biomass Valorization 2020, 12, 3485–3496. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Ignacio-de Leon, P.A.A.; Schoene, R.P.; Urgun-Demirtas, M. Towards a Sustainable Paradigm of Waste-to-Energy Process: Enhanced Anaerobic Digestion of Sludge with Woody Biochar. J. Clean. Prod. 2016, 135, 1054–1064. [Google Scholar] [CrossRef]
- Salehiyoun, A.R.; Zilouei, H.; Safari, M.; Di Maria, F.; Samadi, S.H.; Norouzi, O. An Investigation for Improving Dry Anaerobic Digestion of Municipal Solid Wastes by Adding Biochar Derived from Gasification of Wood Pellets. Renew. Energy 2022, 186, 1–9. [Google Scholar] [CrossRef]
- Bona, D.; Beggio, G.; Weil, T.; Scholz, M.; Bertolini, S.; Grandi, L.; Baratieri, M.; Schievano, A.; Silvestri, S.; Pivato, A. Effects of Woody Biochar on Dry Thermophilic Anaerobic Digestion of Organic Fraction of Municipal Solid Waste. J. Environ. Manag. 2020, 267, 110633. [Google Scholar] [CrossRef] [PubMed]
- Ottani, F.; Parenti, M.; Pedrazzi, S.; Moscatelli, G.; Allesina, G. Impacts of Gasification Biochar and Its Particle Size on the Thermal Behavior of Organic Waste Co-Composting Process. Sci. Total Environ. 2022, 817, 153022. [Google Scholar] [CrossRef]
- Huggins, T.; Wang, H.; Kearns, J.; Jenkins, P.; Ren, Z.J. Biochar as a Sustainable Electrode Material for Electricity Production in Microbial Fuel Cells. Bioresour. Technol. 2014, 157, 114–119. [Google Scholar] [CrossRef]
- Huggins, T.M.; Pietron, J.J.; Wang, H.; Ren, Z.J.; Biffinger, J.C. Graphitic Biochar as a Cathode Electrocatalyst Support for Microbial Fuel Cells. Bioresour. Technol. 2015, 195, 147–153. [Google Scholar] [CrossRef]
- Huggins, T.M.; Latorre, A.; Biffinger, J.C.; Ren, Z.J. Biochar Based Microbial Fuel Cell for Enhanced Wastewater Treatment and Nutrient Recovery. Sustainability 2016, 8, 169. [Google Scholar] [CrossRef]
- Esfahani, R.A.M.; Osmieri, L.; Specchia, S.; Yusup, S.; Tavasoli, A.; Zamaniyan, A. H2-Rich Syngas Production through Mixed Residual Biomass and HDPE Waste via Integrated Catalytic Gasification and Tar Cracking plus Bio-Char Upgrading. Chem. Eng. J. 2017, 308, 578–587. [Google Scholar] [CrossRef]
- Restuccia, L.; Ferro, G.A.; Suarez-Riera, D.; Sirico, A.; Bernardi, P.; Belletti, B.; Malcevschi, A. Mechanical Characterization of Different Biochar-Based Cement Composites. Procedia Struct. Integr. 2020, 25, 226–233. [Google Scholar] [CrossRef]
- Sirico, A.; Bernardi, P.; Sciancalepore, C.; Vecchi, F.; Malcevschi, A.; Belletti, B.; Milanese, D. Biochar from Wood Waste as Additive for Structural Concrete. Constr. Build. Mater. 2021, 303, 124500. [Google Scholar] [CrossRef]
- Sirico, A.; Bernardi, P.; Belletti, B.; Malcevschi, A.; Dalcanale, E.; Domenichelli, I.; Fornoni, P.; Moretti, E. Mechanical Characterization of Cement-Based Materials Containing Biochar from Gasification. Constr. Build. Mater. 2020, 246, 118490. [Google Scholar] [CrossRef]
- Mobili, A.; Cosoli, G.; Bellezze, T.; Revel, G.M.; Tittarelli, F. Use of Gasification Char and Recycled Carbon Fibres for Sustainable and Durable Low-Resistivity Cement-Based Composites. J. Build. Eng. 2022, 50, 104237. [Google Scholar] [CrossRef]
- Malchiodi, B.; Barbieri, L.; Lancellotti, I.; Pozzi, P. Char Valorization into Sustainable and Performant Polyurethane Insulating Panels. Macromol. Symp. 2022, 404, 2100333. [Google Scholar] [CrossRef]
- Fernández-Pereira, C.; De La Casa, J.A.; Gómez-Barea, A.; Arroyo, F.; Leiva, C.; Luna, Y. Application of Biomass Gasification Fly Ash for Brick Manufacturing. Fuel 2011, 90, 220–232. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Yun, B.Y.; Yang, S.; Kim, S. Encapsulation of Dodecane in Gasification Biochar for Its Prolonged Thermal/Shape Stability, Reliability, and Ambient Enthalpy Storage. Chem. Eng. J. 2022, 437, 135407. [Google Scholar] [CrossRef]
- Engineering GmbH SynCraft. SYNCRAFT®—Das Holzkraftwerk. Available online: https://en.syncraft.at/references/details/holzkraftwerk-cw1800-400x4 (accessed on 9 May 2023).
- Wurzer, C.; Oesterle, P.; Jansson, S.; Mašek, O. Hydrothermal Recycling of Carbon Absorbents Loaded with Emerging Wastewater Contaminants. Environ. Pollut. 2023, 316, 120532. [Google Scholar] [CrossRef]
- Wurzer, C.; Jayakumar, A.; Mašek, O. Sequential Biochar Systems in a Circular Economy. In Biochar in Agriculture for Achieving Sustainable Development Goals 2022; Academic Press: Cambridge, MA, USA, 2022; pp. 305–319. [Google Scholar] [CrossRef]
- Wurzer, C.; Sohi, S.; Masek, O. Synergies in Sequential Biochar Systems. In Advanced Carbon Materials from Biomass—An Overview; Zenodo: Geneva, Switzerland, 2019; pp. 147–159. [Google Scholar]

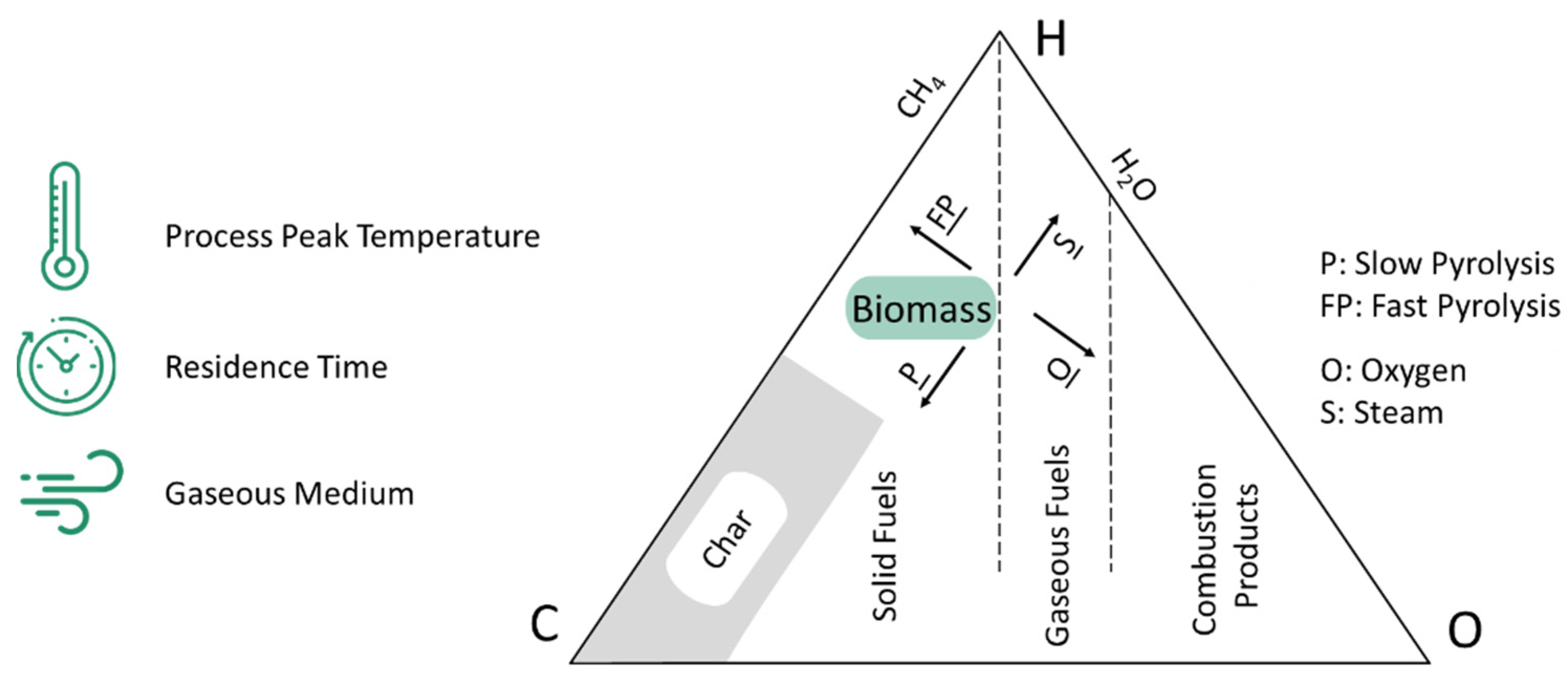
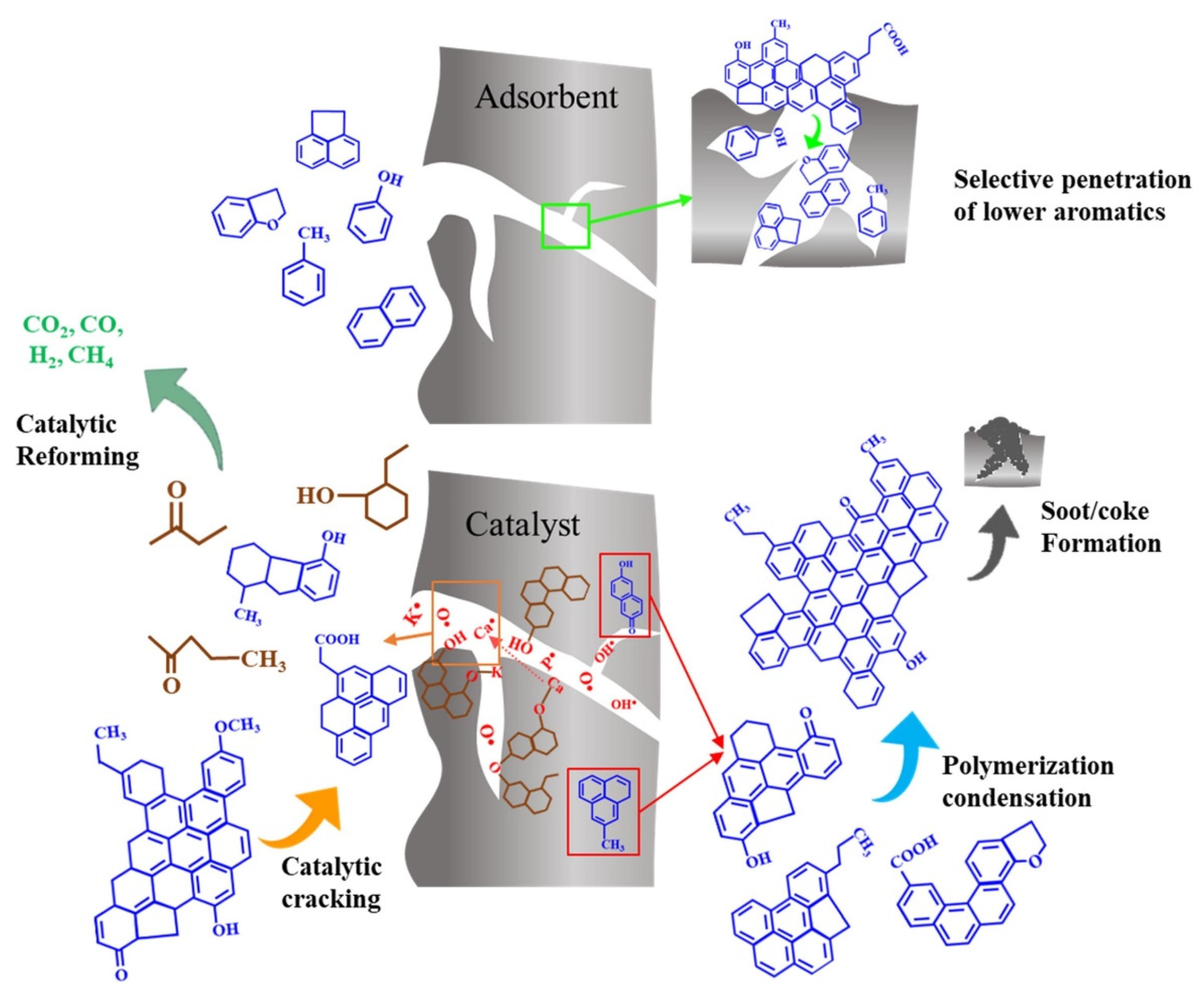
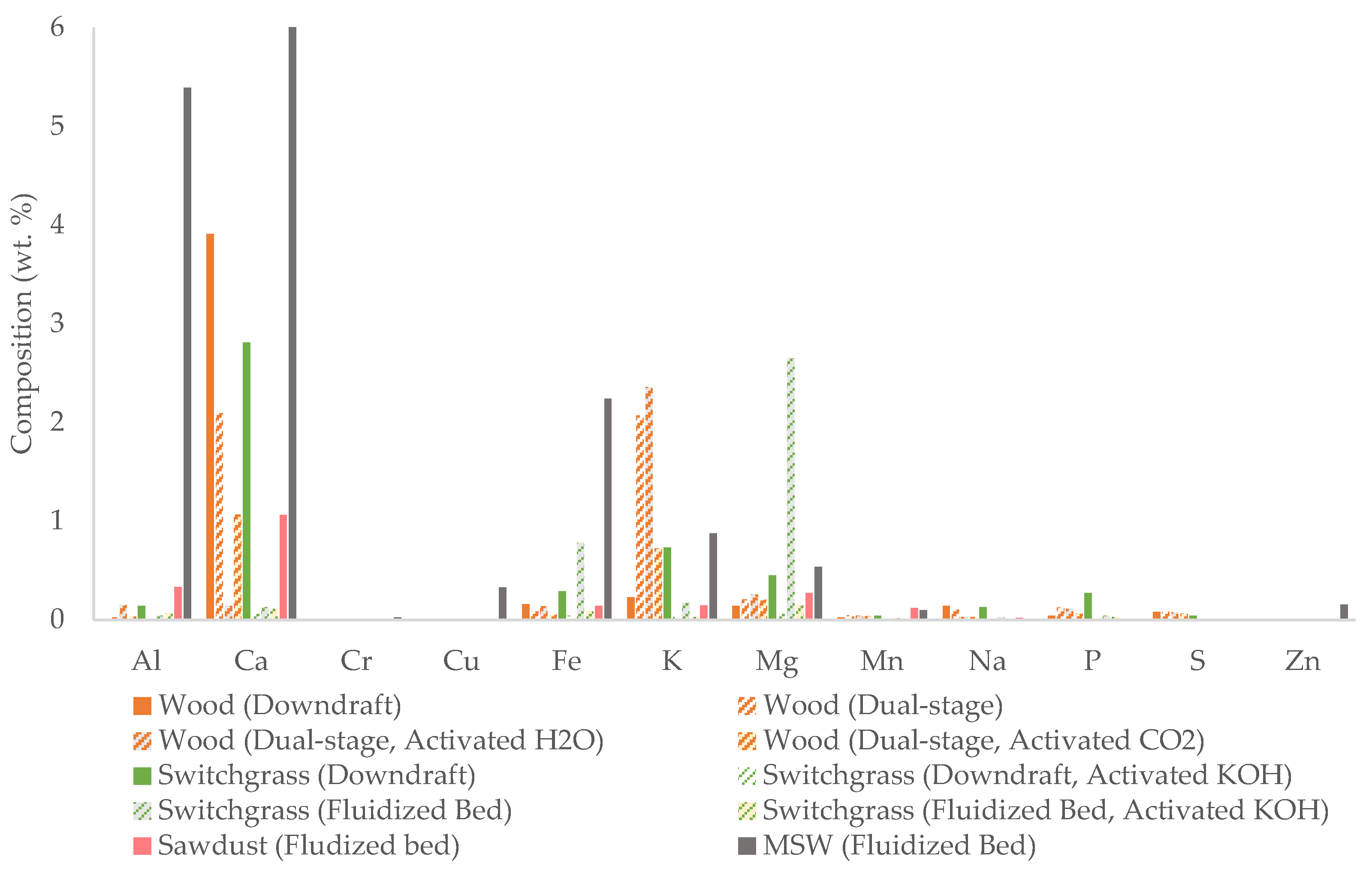
| Gasification | Char | Application | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biomass | Scale | C | Ash | SBET | Pollutant | Matrix | Initial Concentration | Adsorption Capacity | Adsorption Capacity (AC or Similar) | |
| % | % | m2 g−1 | mg L−1 | mg g−1 | mg g−1 | |||||
| Organic Micropollutants | ||||||||||
| Spruce Woodchips | Commercial | 91.4 | 3.7 | 308 | Benzotriazole | Wastewater Treatment Plant Effluent | 5.60 × 10−3 | 166.9 a | 635.8 | [21] |
| Carbamazepine | 0.28 × 10−3 | 9.3 a | 46.5 | |||||||
| Diclofenac | 1.6 × 10−3 | 20.9 a | 126.0 | |||||||
| Metoprolol | 0.76 × 10−3 | 58.7 a | 172.7 | |||||||
| Gliricidia Woodchips | Commercial | 28 | Oxytetracycline | Deionized | 500 | 520.0 c | [59] | |||
| Palm Kernel Shell | Commercial | 712 * | Carbamazepine | Ultrapure | 268.7 b | [58] | ||||
| Pine Woodchips | Pilot | 72.0 | 23.0 | 1509 ** | Acetaminophen | Ultrapure | 434.8 a | 267.7 | [31] | |
| Caffeine | 500.0 a | 296.3 | ||||||||
| Dyes | ||||||||||
| Rubber Tree Roots | Commercial | 68.0 | 5.5 | 478 ** | Malachite Green | Deionized | 300 | 259.5 d | [33] | |
| Biomass Residues | Pilot | 404 | Reactive Black 5 | 35.7 a | 128.2 | [60] | ||||
| Basic Blue 12 | 80.4 a | 86.2 | ||||||||
| Wood Residue | 350 | Black NF1200 | 400 | 805.0 a | [61] | |||||
| Mesquite Woodchips | 776 * | Rhodamine B | 30 | 189.8 a | [28] | |||||
| Heavy Metals | ||||||||||
| Gliricidia Woodchips | Commercial | 28 | Cr (VI) | Deionized | 7.5 c | [59] | ||||
| Cd (II) | 922.0 c | |||||||||
| Rice Husk and Polyethylene | Pilot | 25.9 | 68.3 | 5 | Cr (III) | Industrial Wastewater | 100 | 14.9 | 14.0 | [62] |
| Pine and Spruce Woodchips | Pilot | 61.8 | 259 ** | Fe (II) | Milli-Q | 25–125 | 21 | 13.9 | [63] | |
| Cu (II) | 23 | 5.1 | ||||||||
| Ni (II) | 18 | 2.9 | ||||||||
| Other Pollutants | ||||||||||
| Almond Shells | Commercial | 63 | Phenol | Deionized | 5 × 103 | 65.0 a | 270.0 | [34] | ||
| Gliricidia Woodchips | Commercial | 28 | Glyphosate | 250 | 83.0 c | [59] | ||||
| Gliricidia Woodchips | Commercial | 50.0 | 19.7 | 714 | Glyphosate | Distilled | 100 | 44.0 a | 48.0 | [29] |
| Woodchips | Pilot | 52.1 | 590 * | Phosphates | 140 | 30.2 a | 8.7 | [30] | ||
| Nitrates | 11.2 a | 14.6 | ||||||||
| Gasification | Char | Application | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biomass | Scale | C | Ash | SBET | Hazard | Matrix (Flow) | Temperature | Uptake | Uptake (AC or Similar) | |
| % | % | m2 g−1 | mL min−1 | °C | mg g−1 | mg g−1 | ||||
| Woodchips | Commercial | 76 | 15 | 774 | CO2 | CO2:N2 (40) | 50 | 3.7 (%) | 3.0 (%) | [25] |
| Woodchips and Chicken Manure | Pilot | 72 | 1409 | CO2 (100) | 25 | 128.5 | 95.8 | [70] | ||
| Woodchips | Commercial | 78 | 15 | 587 | H2S | H2S:N2 (100) | 25 | 6.9 | 2.6 | [71] |
| Pinus Patula | Lab | 76 | 3 | 379 | Synthetic Syngas * (20) | 25 | 18.0 | 20.3 | [22] | |
| Eucalyptus Grandis | 82 | 2 | 385 | 15.5 | ||||||
| Paper and Plastic Waste | Pilot | 34 | 45 | 65 | Hg | Synthetic Gas ** (500) | 150 | 0.17 | 0.23 | [37] |
| Gasification | Char | Application | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomass | Scale | C | Ash | SBET | Hazard | Matrix | Reduced Dissolution Rates | Immobilized Bioavailability | |
| Gliricidia Sepium | Commercial | 49 | 21 | 714 | Pb | 17 g Pb/kg soil and 10 wt.% char | Pb: 10.0 to 99.5% | [72] | |
| Cu | 1.1 g Cu/kg soil and 10 wt.% char | Cu: 15.6 to 99.5% | |||||||
| Ni | 6.5 g Ni/kg soil and 5 wt.% char | Ni: 68–92% | [73] | ||||||
| Mn | 2.6 g Mn/kg soil and 5 wt.% char | Mn: 76–93% | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelaal, A.; Benedetti, V.; Villot, A.; Patuzzi, F.; Gerente, C.; Baratieri, M. Innovative Pathways for the Valorization of Biomass Gasification Char: A Systematic Review. Energies 2023, 16, 4175. https://doi.org/10.3390/en16104175
Abdelaal A, Benedetti V, Villot A, Patuzzi F, Gerente C, Baratieri M. Innovative Pathways for the Valorization of Biomass Gasification Char: A Systematic Review. Energies. 2023; 16(10):4175. https://doi.org/10.3390/en16104175
Chicago/Turabian StyleAbdelaal, Ali, Vittoria Benedetti, Audrey Villot, Francesco Patuzzi, Claire Gerente, and Marco Baratieri. 2023. "Innovative Pathways for the Valorization of Biomass Gasification Char: A Systematic Review" Energies 16, no. 10: 4175. https://doi.org/10.3390/en16104175
APA StyleAbdelaal, A., Benedetti, V., Villot, A., Patuzzi, F., Gerente, C., & Baratieri, M. (2023). Innovative Pathways for the Valorization of Biomass Gasification Char: A Systematic Review. Energies, 16(10), 4175. https://doi.org/10.3390/en16104175







