Ignition and Emission Characteristics of Waste Tires Pyrolysis Char Co-Combustion with Peat and Sawdust
Abstract
:1. Introduction
2. Materials and Methods
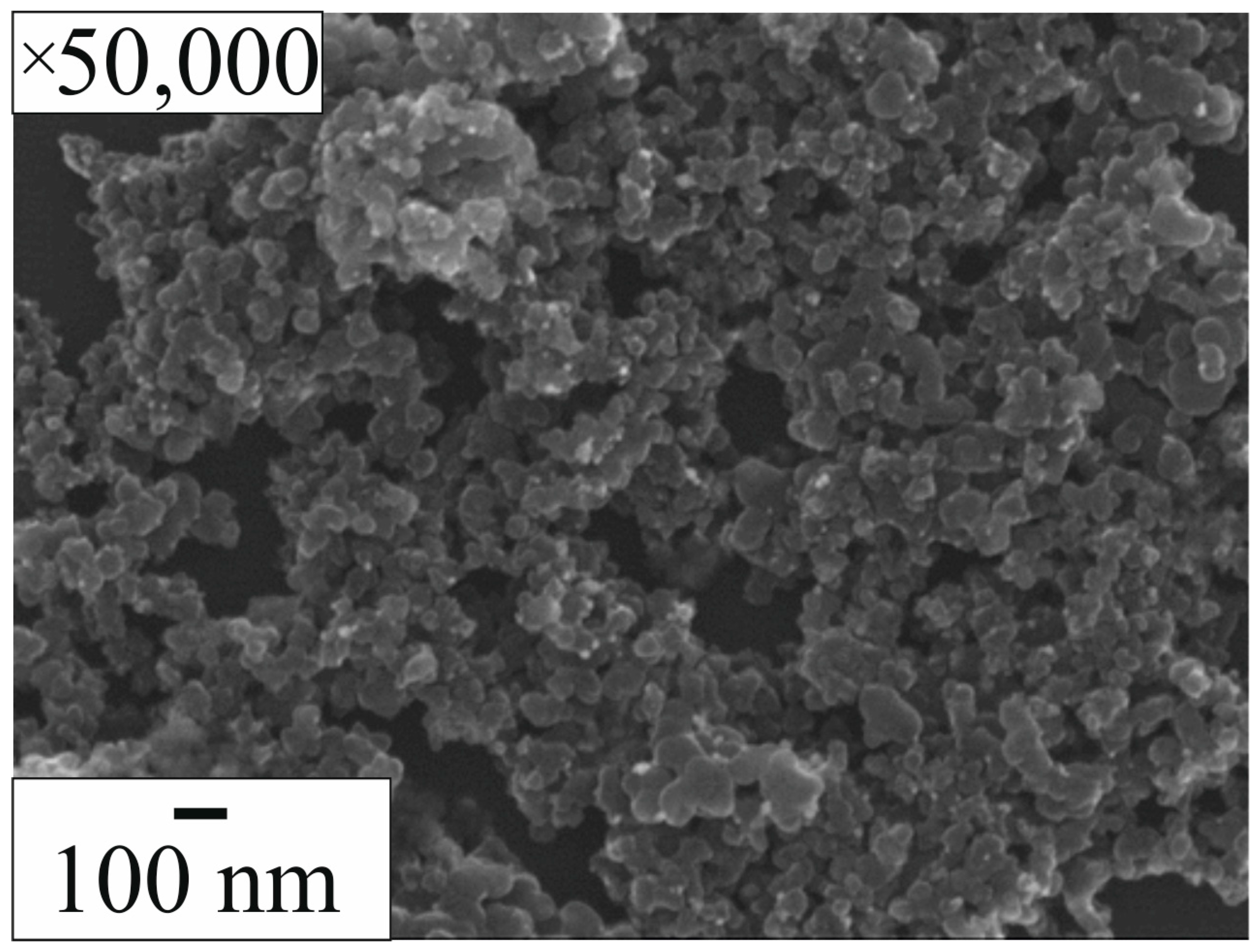
2.1. Initial Sample
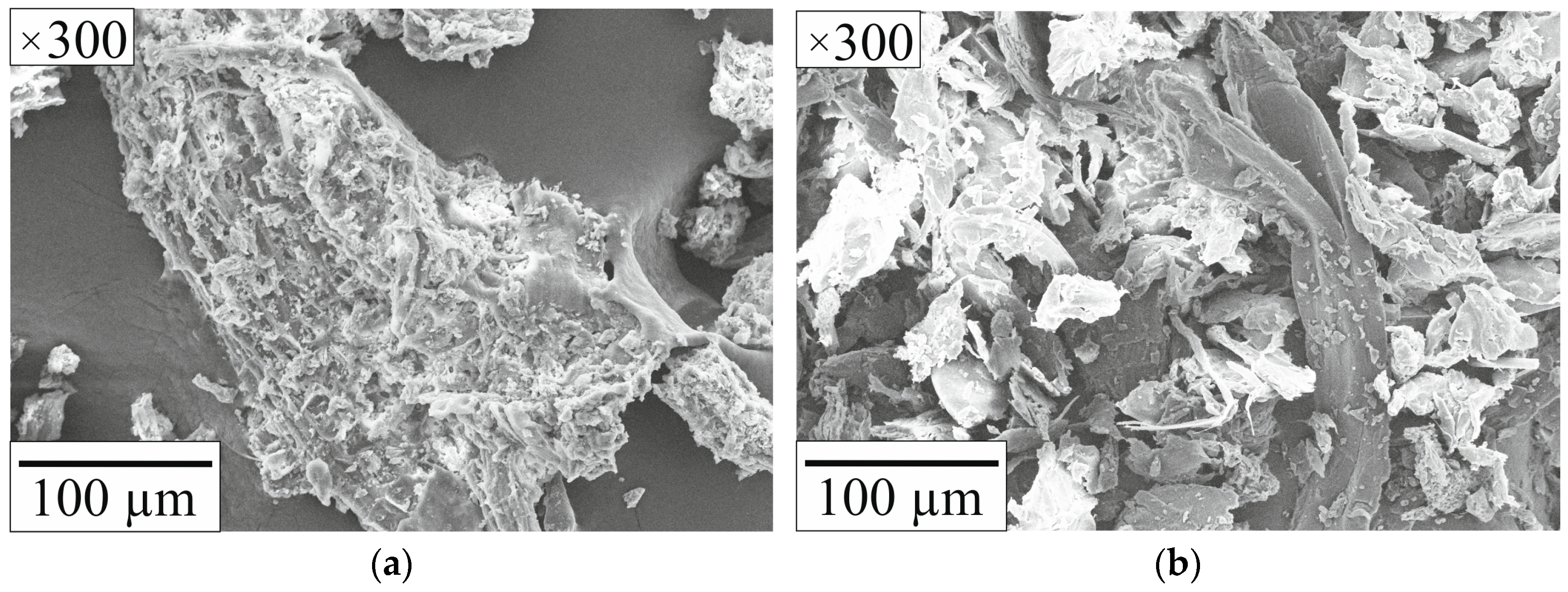
2.2. Fuel Mixtures Characteristics
2.3. Thermal Analysis
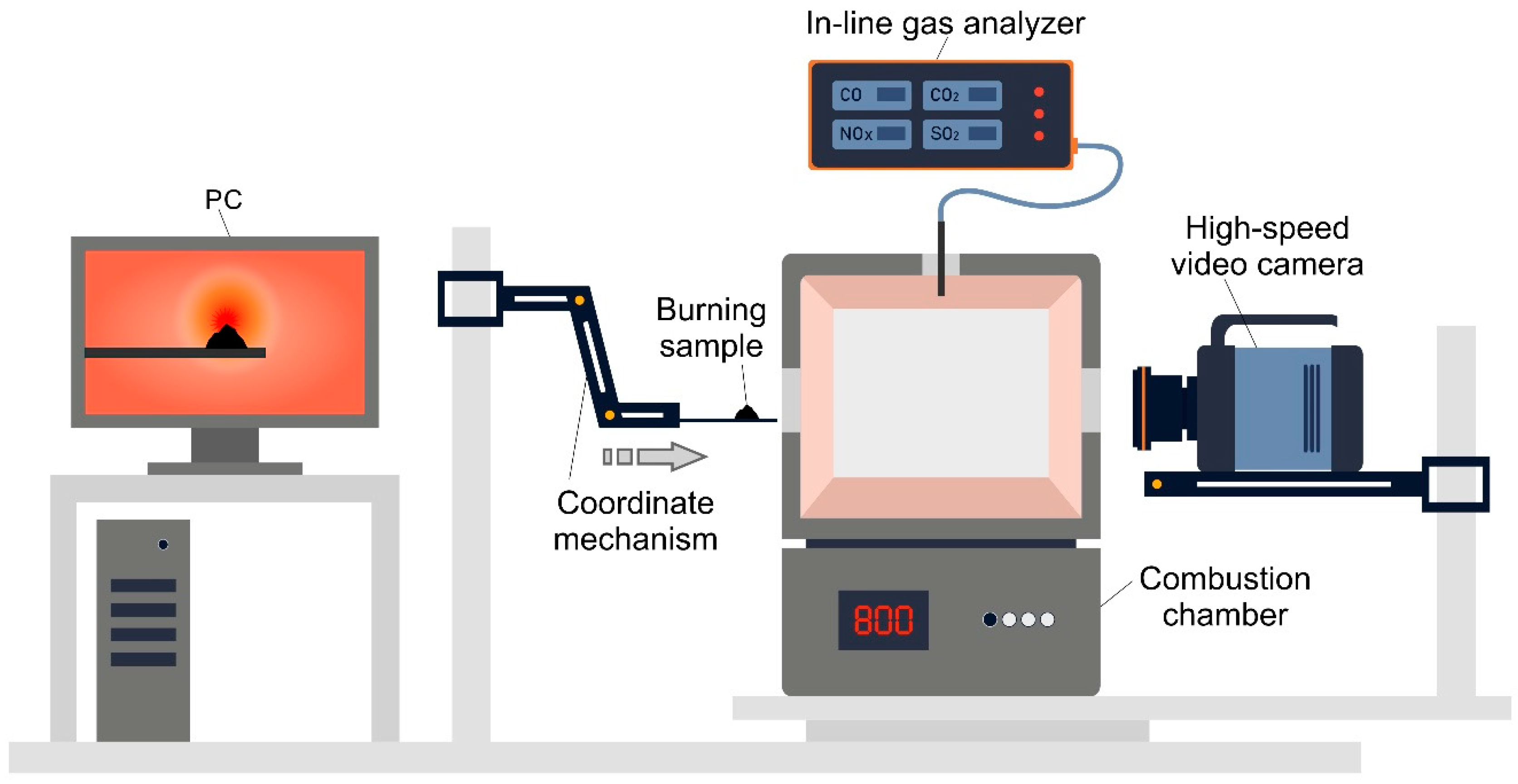
2.4. Research on Ignition and Combustion
2.5. XRD Analysis of Ash Residue
3. Results
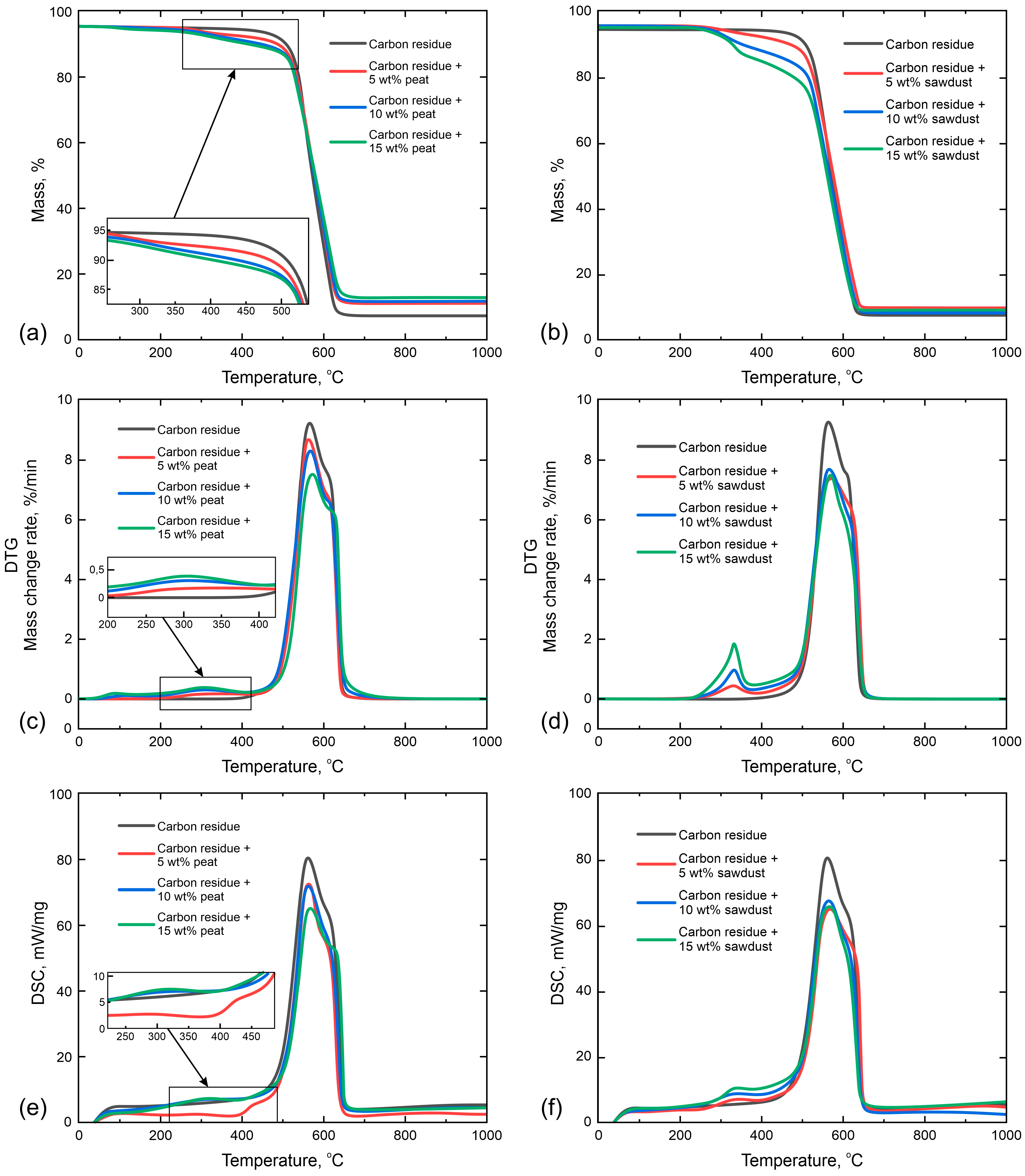
3.1. Thermal Analysis
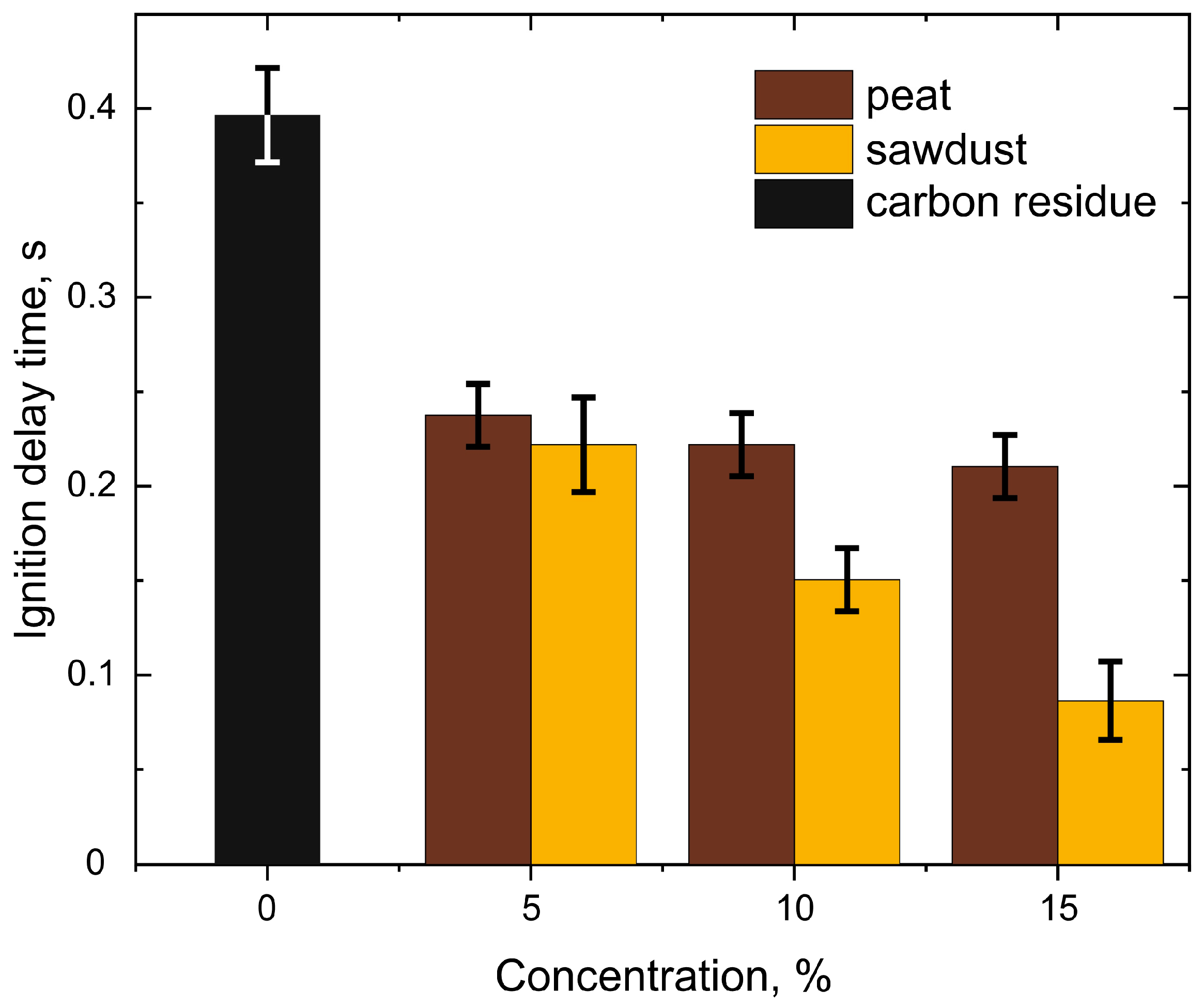
3.2. Ignition and Combustion
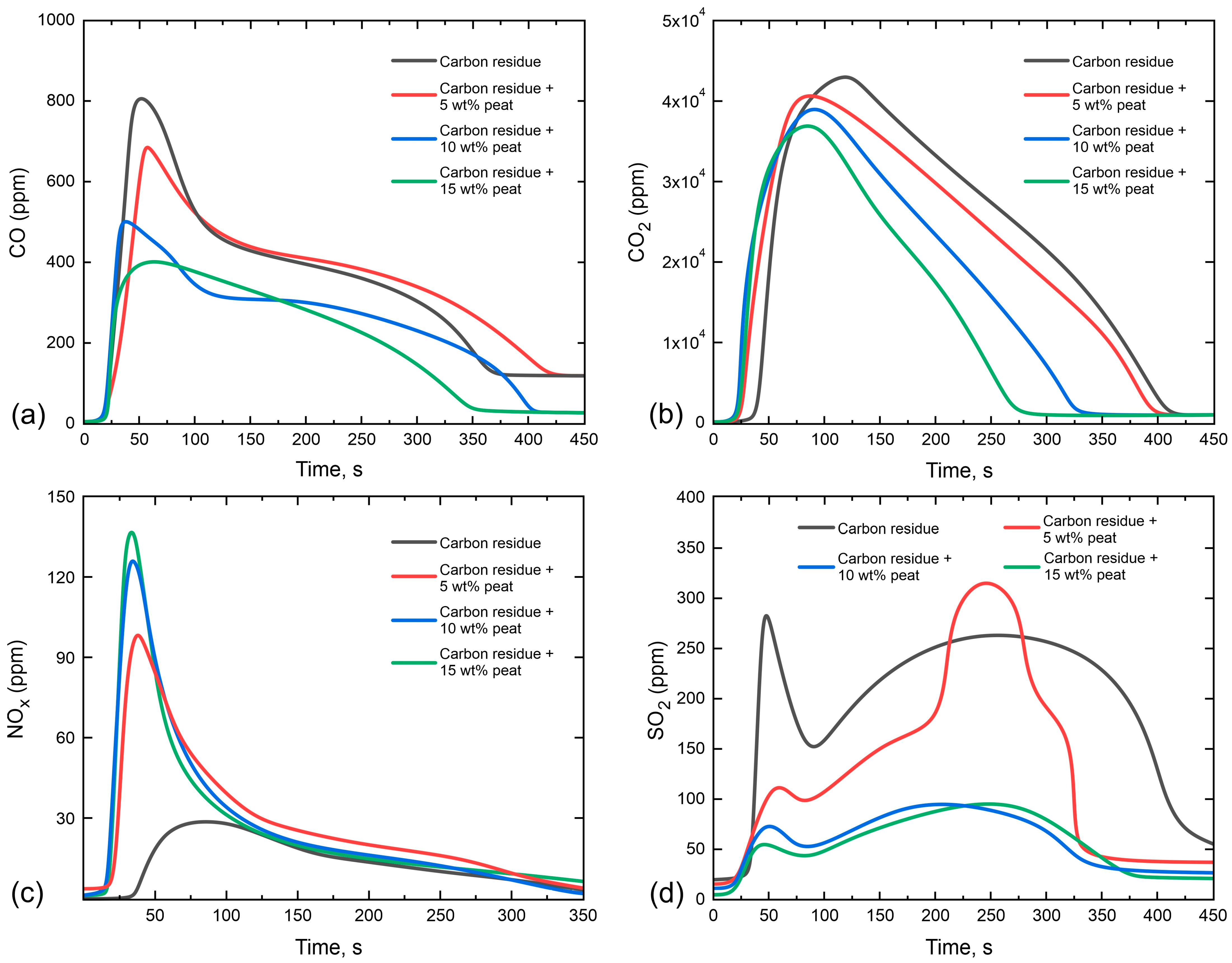
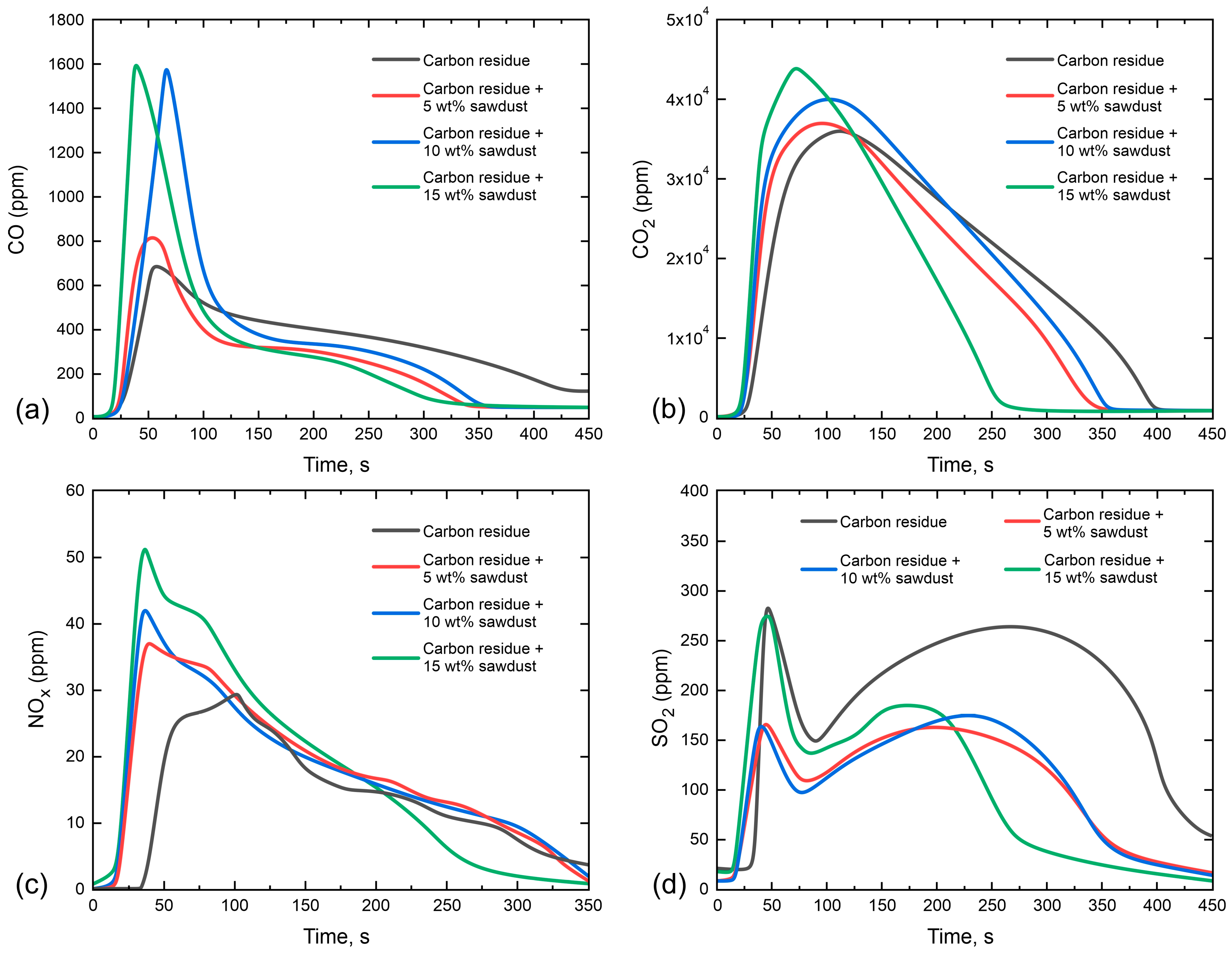
3.3. Gas-Phase Combustion Products
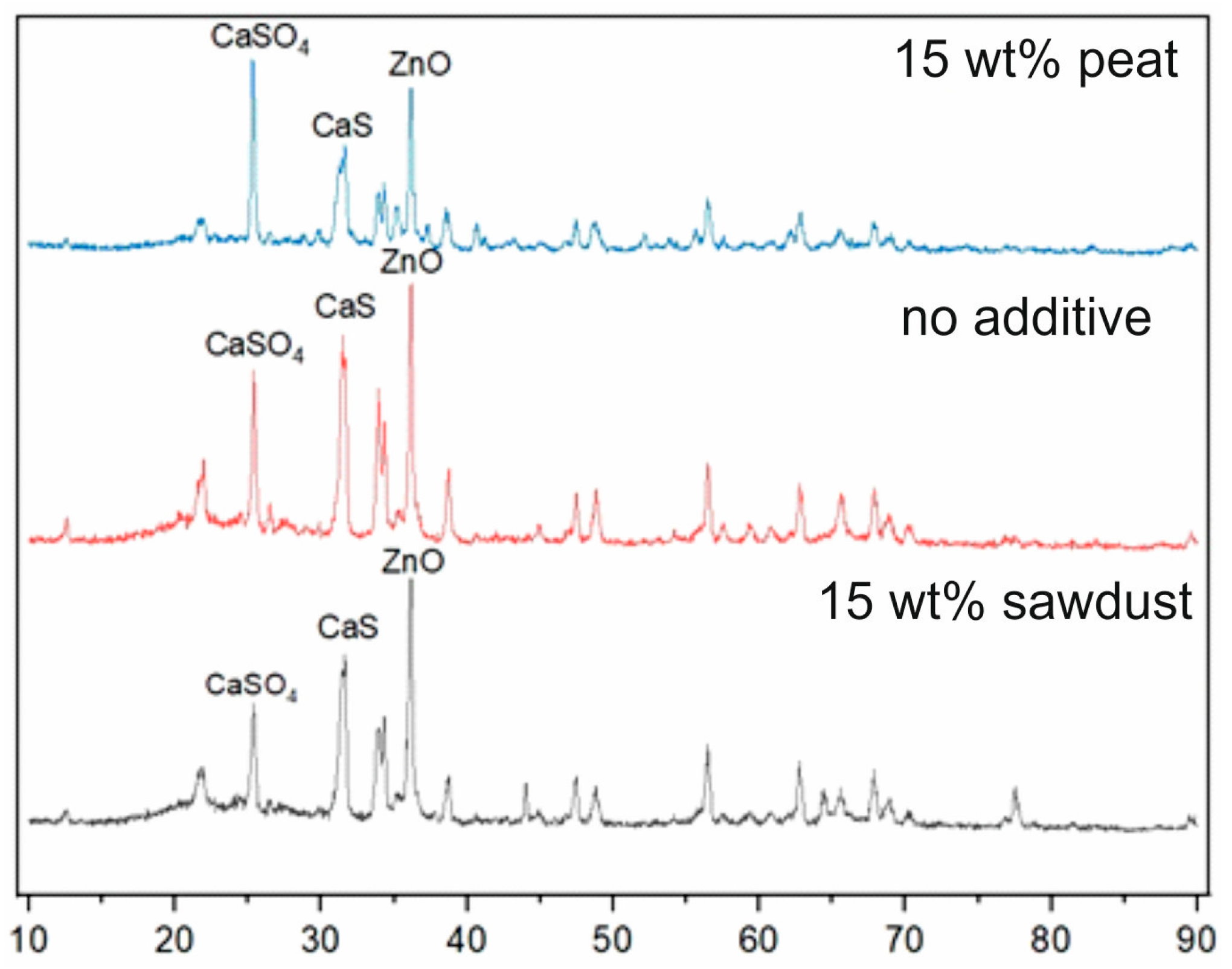
3.4. XRD Analysis of Ash Residue
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, N.; Wang, F.; Quan, C.; Santamaria, L.; Lopez, G.; Williams, P.T. Tire pyrolysis char: Processes, properties, upgrading and applications. Prog. Energy Combust. Sci. 2022, 93, 101022. [Google Scholar] [CrossRef]
- Qiao, Y.; Chen, Z.; Wu, X.; Zheng, Y.; Guan, S.; Li, J.; Yuan, Z.; Li, Z. Analysis of comprehensive utilization of waste tire pyrolysis char by combustion method. Fuel 2022, 312, 122996. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, X.; Zhao, N.; Wang, P.; Che, D. Experimental investigation on combustion and NO formation characteristics of semi-coke and bituminous coal blends. Fuel 2019, 247, 87–96. [Google Scholar] [CrossRef]
- Huan, X.; Tang, Y.G.; Xu, J.J.; Lan, C.Y.; Wang, S.Q. Structural characterization of graphenic material prepared from anthracites of different characteristics: A comparative analysis. Fuel Process. Technol. 2019, 183, 8–18. [Google Scholar] [CrossRef]
- He, J.Y.; Zou, C.; Zhao, J.X.; Ma, C.; Zhang, X.R. Effects of microstructural evolutions of pyrolysis char and pulverized coal on kinetic parameters during combustion. J. Iron Steel Res. Int. 2019, 26, 1273–1284. [Google Scholar] [CrossRef]
- Larionov, K.B.; Slyusarskiy, K.V.; Tsibulskiy, S.A.; Kaltaev, A.Z.; Berezikov, N.I.; Gorshkov, A.S.; Lavrinenko, S.V.; Gubin, V.E. Activation of Anthracite Combustion Using Pyrolysis Oil from Thermal Conversion of Waste Car Tires. ACS Omega 2021, 6, 19731–19739. [Google Scholar] [CrossRef]
- Berezikov, N.I.; Gorshkov, A.S.; Zenkov, A.V.; Larionov, K.B. Intensification of ignition and combustion processes of low-reaction solid fuels by liquid hydrocarbons. AIP Conf. Proc. 2021, 2422, 030001. [Google Scholar]
- Dorokhov, V.V.; Kuznetsov, G.V.; Nyashina, G.S.; Strizhak, P.A. Composition of a gas and ash mixture formed during the pyrolysis and combustion of coal-water slurries containing petrochemicals. Environ. Pollut. 2021, 285, 117390. [Google Scholar] [CrossRef]
- Vershinina, K.Y.; Dorokhov, V.V.; Romanov, D.S.; Strizhak, P.A. Combustion dynamics of droplets of aqueous slurries based on coal slime and waste oil. J. Energy Inst. 2022, 104, 98–111. [Google Scholar] [CrossRef]
- Valiullin, T.R.; Vershinina, K.Y.; Glushkov, D.O.; Shevyrev, S.A. Droplet Ignition of Coal-Water Slurries Prepared from Typical Coal-and Oil-Processing Wastes. Coke Chem. 2017, 60, 40–48. [Google Scholar] [CrossRef]
- Glushkov, D.O.; Kuznetsov, G.V.; Strizhak, P.A.; Syrodoy, S.V. Mathematical model simulating the ignition of a droplet of coal water slurry containing petrochemicals. Energy 2018, 150, 262–275. [Google Scholar] [CrossRef]
- Feoktistov, D.V.; Glushkov, D.O.; Nurpeiis, A.E.; Orlova, E.G.; Samoilo, A.S.; Zhizhaev, A.M.; Zhuikov, A.V. Impregnation of different coals and biomass with rapeseed oil for intensifying their ignition in a heated air stream during oil-free boiler start-up. Fuel Process. Technol. 2022, 236, 107422. [Google Scholar] [CrossRef]
- Chen, L.; Wen, C.; Wang, W.; Liu, T.; Liu, E.; Liu, H.; Li, Z. Combustion behaviour of biochars thermally pretreated via torrefaction, slow pyrolysis, or hydrothermal carbonisation and co-fired with pulverised coal. Renew. Energy 2020, 161, 867–877. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Lang, B.; Xia, L.; Lin, R.; Wang, X. ReaxFF molecular dynamics study of co-combustion of waste tires with petroleum coke gasification residue: Combustion characteristics, gas evolution and micro mechanism. Fuel 2023, 335, 127034. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, W.; Liang, S.; Bi, X.; Sun, R.; Pan, X. Comparative study on the co-combustion behavior of torrefied biomass blended with different rank coals. Biomass Convers. Biorefin. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Rago, Y.P.; Collard, F.X.; Görgens, J.F.; Surroop, D.; Mohee, R. Co-combustion of torrefied biomass-plastic waste blends with coal through TGA: Influence of synergistic behaviour. Energy 2022, 239, 121859. [Google Scholar] [CrossRef]
- Yi, Q.; Qi, F.; Cheng, G.; Zhang, Y.; Xiao, B.; Hu, Z.; Liu, S.; Cai, H.; Xu, S. Thermogravimetric analysis of co-combustion of biomass and biochar. J. Therm. Anal. Calorim. 2013, 112, 1475–1479. [Google Scholar] [CrossRef]
- Dang, H.; Xu, R.; Zhang, J.; Wang, M.; Jia, G.; Wang, Y.; Duan, W. Investigation on co-combustion behavior and kinetic analysis of bamboo char/waste plastics (PVC) hydrochar. Thermochim. Acta 2023, 722, 179466. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, T.Y.; Yu, J.; Jeon, C.H. Influence of biomass pretreatment on co-combustion characteristics with coal and biomass blends. J. Mech. Sci. Technol. 2019, 33, 2493–2501. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Q.C.; Jiang, L.J.; Liu, G.Q.; Ren, S.; Yang, J.; Yao, L.; Meng, F. Thermal Behavior and Kinetics of Raw/Pyrolytic Wood and Coal Blends during Co-combustion Process. J. Iron Steel Res. Int. 2016, 23, 917–923. [Google Scholar] [CrossRef]
- Kazagic, A.; Smajevic, I. Synergy effects of co-firing wooden biomass with Bosnian coal. Energy 2009, 34, 699–707. [Google Scholar] [CrossRef]
- Yankovskii, S.A.; Kuznetsov, G.V.; Galaktionova, A.A. Experimental Substantiation of the Sulfur Oxide Concentration Reduction Mechanism in Coal and Biomass Particle Mixture Pyrolysis Products. Therm. Eng. 2022, 69, 608–614. [Google Scholar] [CrossRef]
- Larionov, K.B.; Slyusarskiy, K.V.; Ivanov, A.A.; Mishakov, I.V.; Pak, A.Y.; Jankovsky, S.A.; Stoyanovskii, V.O.; Vedyagin, A.A.; Gubin, V.E. Comparative analysis of the characteristics of carbonaceous material obtained via single-staged steam pyrolysis of waste tires. J. Air Waste Manag. Assoc. 2022, 72, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Miller, B. Introduction. In Fossil Fuel Emissions Control Technologies; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–45. [Google Scholar]
- Speight, J.G. Handbook of Coal Analysis; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Rostam-Abadi, M.; Hsi, H.C.; Chen, S.; Rood, M.; Chang, R.; Carey, T.R.; Richardson, C.F.; Rosenhoover, B. Development of carbon-based adsorbents for removal of mercury emissions from coal combustion flue gas. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1999; pp. 459–483. [Google Scholar]
- Wu, G.; Asai, S.; Sumita, M.; Yui, H. Entropy Penalty-Induced Self-Assembly in Carbon Black or Carbon Fiber Filled Polymer Blends. Macromolecules 2002, 35, 945–951. [Google Scholar] [CrossRef]
- Miguel, G.S.; Fowler, G.D.; Dall’Orso, M.; Sollars, C.J. Porosity and surface characteristics of activated carbons produced from waste tyre rubber. J. Chem. Technol. Biotechnol. 2002, 77, 1–8. [Google Scholar] [CrossRef]
- Guangwei, W.; Zhang, J.L.; Tao, X.; Jiu-Gang, S.; Huzheng-wen, Q.I.U. Investigation on Co-Combustion Kinetics of Anthracite Coal and Biomass Char by Thermogravimetric Analysis. In Proceedings of the 8th Pacific Rim International Congress on Advanced Materials and Processing, Waikoloa, Hawaii, 4–9 August 2013; Springer International Publishing: Cham, Switzerland, 2013; pp. 155–165. [Google Scholar]
- Larionov, K.B.; Slyusarskiy, K.V.; Mishakov, I.V.; Tsibulskiy, S.A.; Tabakaev, R.B.; Vedyagin, A.A.; Gromov, A.A. Combustion of bituminous coal loaded with copper salts. Fuel 2021, 286, 119366. [Google Scholar] [CrossRef]
- Pronobis, M. Evaluation of the influence of biomass co-combustion on boiler furnace slagging by means of fusibility correlations. Biomass Bioenergy 2005, 28, 375–383. [Google Scholar] [CrossRef]


| Parameter | Value |
|---|---|
| Moisture content a, wt% | 0.5 |
| Ash content d, wt% | 15.6 |
| Volatile matter content daf, wt% | 8.8 |
| Calorific value (lower), MJ/kg | 27.2 |
| Elemental composition daf, wt% | |
| C | 94.7 |
| H | 1.0 |
| N | 0.3 |
| S | 4.0 |
| № | Element | Concentration, mg/kg | Relative Composition, wt% |
|---|---|---|---|
| 1 | Al | 8833.18 | 1.68 |
| 2 | Ba | 50.14 | <0.1 |
| 3 | Ca | 12,184.33 | 2.32 |
| 4 | Cd | 3251.61 | 0.62 |
| 5 | Co | 63.13 | <0.1 |
| 6 | Cr | 17.14 | <0.1 |
| 7 | Cu | 2985.25 | 0.57 |
| 8 | Fe | 4642.40 | 0.88 |
| 9 | K | 5903.23 | 1.12 |
| 10 | Li | 252.53 | <0.1 |
| 11 | Mg | 10,119.82 | 1.93 |
| 12 | Mn | 69.68 | <0.1 |
| 13 | Mo | 20.28 | <0.1 |
| 14 | Na | 6802.76 | 1.30 |
| 15 | Ni | 232.26 | <0.1 |
| 16 | Pb | 221.29 | <0.1 |
| 17 | Si | 201,659.00 | 38.41 |
| 18 | Sr | 450.69 | <0.1 |
| 19 | V | 24.24 | <0.1 |
| 20 | W | 59.91 | <0.1 |
| 21 | Zn | 267,096.80 | 50.88 |
| 22 | Zr | 24.70 | <0.1 |
| Sample | Humidity, , wt% | Ash Content , wt% | Volatile Matter Content , wt% | Calorific Value , MJ/kg | Elemental Composition d, wt% | ||||
|---|---|---|---|---|---|---|---|---|---|
| C d | H d | N d | S d | O d | |||||
| Peat | 9.9 | 22.8 | 74.8 | 11.8 | 40.2 | 4.9 | 2.8 | 0.2 | 29.2 |
| Sawdust | 7.0 | 1.6 | 83.4 | 18.1 | 51.7 | 6.5 | 0.2 | – | 40.1 |
| Sample | The Composition of the Main Components of Ash, % | ||||||
|---|---|---|---|---|---|---|---|
| SiO2 | AL2O3 + TiO2 | Fe2O3 | SO3 | CaO | MgO | Other (K2O, Na2O, P2O5 et al.) | |
| Peat | 3.8 | 7.6 | 16.2 | 0.2 | 54.3 | 1.6 | 16.3 |
| Sawdust | 6.7 | 2.1 | 1.5 | 2.8 | 31.1 | 4.3 | 51.5 |
| Sample | Moisture Content , % | Ash Content , % | Volatile Matter Content , % | Calorific Value , MJ/kg | Elemental Composition, wt% | ||||
|---|---|---|---|---|---|---|---|---|---|
| C d | H d | N d | S d | O d | |||||
| 5% peat | 1.0 | 15.9 | 12.1 | 26.4 | 73.8 | 1.0 | 0.3 | 3.1 | 5.9 |
| 10% peat | 1.4 | 16.3 | 15.4 | 25.7 | 72.0 | 1.2 | 0.5 | 2.9 | 7.1 |
| 15% peat | 1.9 | 16.7 | 18.7 | 24.9 | 70.3 | 1.4 | 0.6 | 2.8 | 8.3 |
| 5% pine sawdust | 0.8 | 14.9 | 12.5 | 26.7 | 74.4 | 1.1 | 0.2 | 3.1 | 6.4 |
| 10% pine sawdust | 1.2 | 14.2 | 16.3 | 26.3 | 73.2 | 1.4 | 0.2 | 2.9 | 8.2 |
| 15% pine sawdust | 1.5 | 13.5 | 20.0 | 25.8 | 72.0 | 1.7 | 0.2 | 2.7 | 9.9 |
| Component | Mass, wt% | Ti, °C | Tf, °C | wmax, %/min | Tmax, °C | ∂m, % |
|---|---|---|---|---|---|---|
| Char | 100 | -/510.3 | -/635.5 | 9.26 | 565.1 | 91.52 |
| Peat | 5 | 302.5/515.1 | 471.1/632.5 | 8.69 | 562.5 | 88.02 |
| 10 | 277.5/505.1 | 460.6/630.4 | 8.31 | 567.5 | 87.4 | |
| 15 | 272.5/502.5 | 452.5/635.1 | 7.55 | 570.1 | 86.11 | |
| Pine sawdust | 5 | 325/507.5 | 472.5/637.5 | 0.6/7.4 | 335/575 | 89.66 |
| 10 | 320.1/500.1 | 447.5/632 | 1.22/7.7 | 335/567.5 | 91.45 | |
| 15 | 317.5/502.5 | 452.5/627.5 | 1.87/7.5 | 332/570.1 | 90.52 |
| Fraction of Additive, % | Biomass Additive | |||||||
|---|---|---|---|---|---|---|---|---|
| Peat | Pine Sawdust | |||||||
| Gas-Phase Combustion Product, Smod/Sref | ||||||||
| CO | CO2 | NOx | SO2 | CO | CO2 | NOx | SO2 | |
| 5 | 0.97 | 0.95 | 1.86 | 0.58 | 1.02 | 1.01 | 1.24 | 0.52 |
| 10 | 0.69 | 0.73 | 1.82 | 0.27 | 1.03 | 1.04 | 1.26 | 0.52 |
| 15 | 0.53 | 0.58 | 1.85 | 0.27 | 1.07 | 1.06 | 1.27 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slyusarsky, K.; Tolokolnikov, A.; Gubin, A.; Kaltaev, A.; Gorshkov, A.; Asilbekov, A.; Larionov, K. Ignition and Emission Characteristics of Waste Tires Pyrolysis Char Co-Combustion with Peat and Sawdust. Energies 2023, 16, 4038. https://doi.org/10.3390/en16104038
Slyusarsky K, Tolokolnikov A, Gubin A, Kaltaev A, Gorshkov A, Asilbekov A, Larionov K. Ignition and Emission Characteristics of Waste Tires Pyrolysis Char Co-Combustion with Peat and Sawdust. Energies. 2023; 16(10):4038. https://doi.org/10.3390/en16104038
Chicago/Turabian StyleSlyusarsky, Konstantin, Anton Tolokolnikov, Artur Gubin, Albert Kaltaev, Alexander Gorshkov, Askar Asilbekov, and Kirill Larionov. 2023. "Ignition and Emission Characteristics of Waste Tires Pyrolysis Char Co-Combustion with Peat and Sawdust" Energies 16, no. 10: 4038. https://doi.org/10.3390/en16104038
APA StyleSlyusarsky, K., Tolokolnikov, A., Gubin, A., Kaltaev, A., Gorshkov, A., Asilbekov, A., & Larionov, K. (2023). Ignition and Emission Characteristics of Waste Tires Pyrolysis Char Co-Combustion with Peat and Sawdust. Energies, 16(10), 4038. https://doi.org/10.3390/en16104038







