Abstract
One of the main goals of the shipping industry is to decarbonize the fuels used in maritime transportation. Ammonia is thought to be a potential alternative for hydrogen storage in the future, allowing for CO2-free energy systems. Ammonia’s beneficial characteristics with regard to hydrogen storage include its high volumetric hydrogen density, low storage pressure, and long-term stability. However, ammonia is characterized by toxicity, flammability, and corrosiveness, making safety a challenge compared to other alternative fuels. In specific circumstances, leakage from ammonia bunkering can cause risks, dispersion, and unsafe areas due to its flammability and toxicity. Based on an analysis of 118 research papers and 50 regulations and guidelines, this review report evaluates various aspects of the hazards associated with the ammonia bunkering processes, considering both current and future implications. This report also includes the latest advancements and potential developments related to the safety of ammonia as a marine fuel. Several related regulations and standards for ammonia supply systems are discussed. This paper examines experiments and numerical investigations conducted using different methods of ammonia bunkering, such as terminal-to-ship, ship-to-ship, and truck-to-ship transfers. This review shows that the toxicity of ammonia is more relevant to the topics of vapor cloud dispersion and ammonia bunkering than its flammability. Finally, the main challenges and recommendations for the implementation of ammonia bunkering and further development of ammonia as a marine fuel are proposed. This review suggests new directions to overcome the disadvantages and research gaps associated with the leakage of ammonia during bunkering periods.
1. Introduction
Maritime transportation is a primary contributor to the world economy, accounting for over 80% of global transport by volume [1,2]. Compared to other modes of transportation, marine transportation has the advantages of a large carrying capacity, high safety, and low operating costs. However, with the expansion of shipping and maritime activities, a significant and rising amount of greenhouse gas emissions (GHGs) is predicted to be released by maritime transportation activities in the coming years [3]. Thus, the maritime shipping sector is actively seeking opportunities to lower its global GHG emissions [4]. The International Maritime Organization (IMO) has established goals for decreasing greenhouse gas (GHG) emissions by a minimum of 50% by 2050, as well as reducing carbon dioxide (CO2) emissions per instance of transportation by 40% by 2030 and 70% by 2050, compared to 2008 [5,6]. To improve ship energy efficiency and reduce CO2 emissions, the IMO implemented MARPOL Annex VI [7], the Ship Energy Efficiency Management Plan (SEEMP), the Energy Efficiency Design Index (EEDI), and the Energy Efficiency Operational Indicator (EEOI), which went into effect on 1 January 2013 [8]. It is essential to find alternative fuel sources to meet the demands of marine transportation. Hydrogen is a carbon-free energy source with a high mass energy density [9]. It can be used as fuel in various technologies, such as internal combustion engines (ICEs), fuel cells, or gas turbines, to meet green emission targets [10]. However, hydrogen has low volumetric density, requiring large storage space and shorter ship voyage times when used as a marine fuel. This mainly causes economic and vessel management efficiency disadvantages. Thus, there is a need to find a hydrogen carrier fuel source that meets both emission reduction targets and vessel operation management requirements [11]. Ammonia is a potential marine fuel alternative attracting a great deal of interest due to its carbon-free chemical composition, which is in line with the IMO’s objectives [12,13]. Ammonia is also being considered as a hydrogen storage and transport medium because it allows for liquid-phase hydrogen storage at room temperature [6], and has greater hydrogen volumetric density [5] than liquid hydrogen [14]. Ammonia has a high-octane rating of 110–130 and a shallow flammability range, making it relatively safe in terms of explosion risk. It also has a relatively high power-to-fuel-to-power (PFP) efficiency and a wide distribution network already in place; these factors contribute to its use as a fuel [15]. The use of ammonia as fuel has increased over the years [16] and, subsequently, the demand for ammonia bunkering has also increased [17]. For maritime applications and ammonia-fueled ships, the ammonia bunkering process is necessary and unavoidable. Under ambient conditions, anhydrous ammonia is in the gas phase. Therefore, ammonia must be liquified for storage, transportation, and the bunkering process. Compared to conventional liquid fuel bunkering, ammonia bunkering is associated with possible risks related to cryogenic liquid/high-pressure liquid transfer and vapor return, which calls for particular attention to ensuring safe procedures. Significant risks could result from the unintentional release of such a dangerous material during the ammonia bunkering process [18,19]. This could take place in the following manner: after a series of failures, one or more safety systems that were in place to prevent the release of ammonia deactivate, and ammonia is released into the environment and distributed [19,20]. If the vapor is ignited, fire or explosion could result, disrupting the normal functioning of bunkering. Risk assessment models can be used to calculate the likelihood of an ammonia release and its effects [21,22]. Thus, the safety of ammonia bunkering should be considered [23,24].
In terms of risk assessment, there are two main approaches to defining the safety zone during the ammonia bunkering process: the deterministic approach and the risk-based approach. The appropriate approach should be selected based on the target bunkering scenario [25]. Normally, for standard scenarios, such as port-to-ship, ship-to-ship, truck-to-ship, and a portable ammonia fuel tank, a qualitative approach (deterministic approach) is employed [26]. A quantitative approach (risk-based approach) is preferred for other scenarios. The spirit and theory of these two approaches are described below in Figure 1. Considering that the ammonia facilities under discussion often use basic methods, the possibility of an ammonia leak during bunkering can be examined using a single scenario. The representative scenario is delineated using the worst-case or specified conditions, and the necessary safety distance surrounding the facility is then roughly computed, taking into account the flammability range and dispersion characteristics.
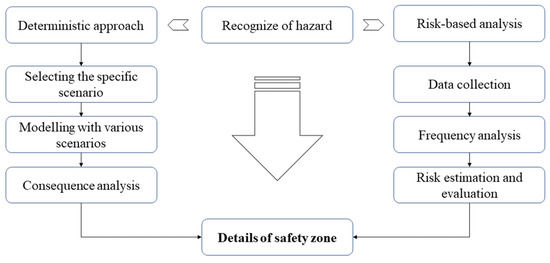
Figure 1.
Main approaches for risk and safety assessment of ammonia bunkering.
Currently, most safety zones associated with the bunkering operations of LNG-fueled ships are determined using qualitative methodology because these operations often run according to standard scenarios, during which additional activities are restricted.
In the case of ammonia-fueled ships, a risk-based approach would be more appropriate, as it allows for a comprehensive assessment of potential risks and their impacts. This approach considers the probability of various scenarios and their potential consequences, leading to a more accurate determination of the safety zone. The safety zone is then established based on the calculated risks and the level of acceptable risk.
For facilities with simultaneous operations (SIMOPs) of ammonia-fueled ships, additional factors, such as the proximity of other vessels or structures, weather conditions, and potential environmental impacts, should also be considered in the risk assessment. This approach ensures that all the potential risks are identified and addressed, reducing the likelihood of accidents and ensuring the safety of personnel, the facility, and the environment. This review aims to answer the following review research questions (RRQs):
RRQ1: What are the main risks associated with using ammonia as fuel?
RRQ2: Do the existing regulations and standards adequately cover all aspects of ammonia bunkering safety? Are there any gaps in the combination of safety regulations?
RRQ3: Are there any gaps in the scientific research and understanding of ammonia bunkering safety?
RRQ4: What are the challenges and recommendations for improving the safety of ammonia bunkering?
RRQ1 is addressed and discussed in the literature review. RRQ2 and RRQ3 are addressed by reviewing the relevant legislation, standards, guidelines, and scientific research publications. Based on these findings, challenges and recommendations are presented.
Overall, this paper not only provides a review of current regulations and guidelines, potential technologies to improve the safety of the ammonia bunkering process, and the important role of determining the safety distance to minimize harm and damage in the event of an ammonia leakage, but also proposes challenges and recommendations for good safety practices during the ammonia bunkering process.
2. Characteristics of Ammonia
2.1. General Information and Physical Properties of Ammonia
Ammonia is composed of one nitrogen and three hydrogen atoms (NH3). The calorific value of ammonia is 22.5 MJ/kg [27]. Ammonia is a colorless gas with a pungent odor [28] that consists of 17.6% hydrogen by weight [29]. The average unit price of ammonia is about USD 250–300 [30]. The contribution of the ammonia production process to the total GHG emissions of the world has been estimated as about 1% [31]. Ammonia is known to have a variety of advantageous properties as fuel, making it appealing as a possible medium for hydrogen storage. The volumetric hydrogen density of ammonia is 45% greater than that of liquid hydrogen. This suggests that there is more hydrogen in liquid ammonia than there is in an equivalent volume of liquid hydrogen [32]. Compared to ethanol, methanol, liquid hydrogen, and gasoline, ammonia is a hydrogen carrier with a higher volumetric hydrogen density [33]. The storage of ammonia is simpler than the storage of hydrogen, the other carbon-free fuel. The storage of ammonia takes place either at room temperature at 10 bars or at 33 °C at 1 bar [28]. The basic properties of ammonia are presented in Table 1.

Table 1.
Basic physical properties of ammonia [34,35,36].
Special safety precautions are necessary for the storage of ammonia given its toxic and corrosive nature. Compared to commonly used fuels such as methanol and diesel, the hazard level of ammonia is over three times higher [37]. The event of an ammonia leak into water can be harmful to aquatic life, but its natural degradation process and the nitrogen cycle can facilitate the regeneration of aquatic life. It should be noted that ammonia has a very low odor threshold (0.037 to 1.0 ppm), making it detectable by most individuals even in small amounts that do not pose a health risk.
Gaseous ammonia has a lower density than air (1.225 kg/m3 compared to 0.769 kg/m3 at STP), and under normal atmospheric conditions, it can quickly dissipate into the atmosphere, lowering the risk of explosion or fire in the event of a leak. In addition, compared to hydrogen, which has an auto-ignition temperature of 520 °C, ammonia’s higher auto-ignition temperature (650 °C) means a lower risk of fire. Liquid ammonia is highly toxic and has a vapor pressure relative to toxicity at atmospheric temperature that is roughly three orders of magnitude higher than those of gasoline and methanol.
2.2. Ammonia Bunkering Methods
Bunkering is a vital operation that supplies fuel to power the machinery of a ship. Ammonia bunkering, similar to that of alternative fuels such as LNG, LPG, and hydrogen, can be categorized into four main types: ship-to-ship (STS), terminal-to-ship (TTS), truck-to-ship (T-TS), and ammonia portable tank (APT). The suitable ammonia bunkering method is selected after considering the amount of ammonia bunkering required, operational circumstances, and time constraints. Figure 2 illustrates the three most common ammonia bunkering methods.
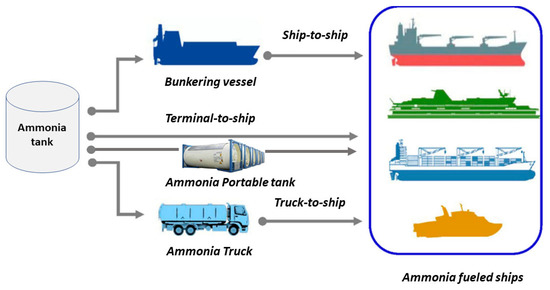
Figure 2.
Ammonia bunkering methods.
A comparison of the advantages and disadvantages of each ammonia bunkering method is shown below in Table 2.

Table 2.
Comparison of ammonia bunkering methods.
2.3. Hazards of the Ammonia Bunkering Process
The dangers of ammonia can be grouped under three characteristics: (i) toxicity, (ii) corrosiveness, and (iii) flammability.
2.3.1. Toxic Effects of Ammonia on Humans
Ammonia is a gas that is colorless and poisonous, and it has a strong pungent smell at concentrations between 5 and 30 ppm. This gas has a lower density than air, and liquid ammonia can cause severe burns and injuries if it comes into contact with skin. There is a high risk of death when large quantities of ammonia are released, as it can form a toxic cloud that can be inhaled far away from the release site. Fatalities usually occur when people are exposed to high concentrations of the gas, or if they are trapped without an escape route, due to the strong odor of ammonia, which is intolerable at concentrations well below those that are harmful. Ammonia rapidly absorbs water and can cause dehydration upon contact with skin, while anhydrous ammonia can cause a loss of water from body tissues and chemical burns via the production of ammonium hydroxide. Additionally, frostbite can occur when liquid ammonia vaporizes, causing the removal of heat from body tissues within seconds. The effects of ammonia on human health are presented in Table 3.

Table 3.
Effects of ammonia on humans [38].
The probability of human death due to the effects of an ammonia vapor cloud formed following a leak can be estimated by [39].
where represents the probability of human death by exposure to toxic ammonia and represents the probability of human death.
Here, t represents exposure time, C represents the cloud dispersion concentration (mg/m3), and erf(x) represents the Gaussian error.
It is important to note that the effects of ammonia toxicity can be cumulative, meaning that repeated exposure even to low concentrations of ammonia over a prolonged period can have significant health consequences. Therefore, it is essential to take appropriate precautions to prevent exposure to ammonia, such as wearing appropriate personal protective equipment (PPE) and working in well-ventilated areas.
2.3.2. Toxic Effects of Ammonia on the Environment
When ammonia leaks occur in seawater during bunkering, the absorbed ammonia can have a severe impact on aquatic life, as lethal levels are easily surpassed, causing death to most species in close proximity. Due to its exothermic reaction with water, ammonia quickly evaporates. As ammonia gas is lighter than air, it will rise to the top of the atmosphere in the form of a cloud. However, this ammonia gas cloud can pose a significant danger to creatures in its immediate vicinity, as it can expose them to deadly amounts of ammonia. This cloud remains a threat until it is completely diluted through the processes of cloud evaporation and continuous air mixing.
Overall, it is important to minimize the release of ammonia into the environment by taking appropriate precautions during storage, handling, and use. This includes proper ventilation, safe disposal methods, and the careful use of fertilizers and other ammonia-based products.
2.3.3. Flammability of Ammonia
The auto-ignition temperature of ammonia under atmospheric conditions is 651 °C, with a flammable range of 15.15–27.35%. Compared to other fuels, the likelihood of ammonia auto-igniting is extremely low due to its high minimum ignition energy of 680 mJ, which is 2000 times greater than that of CH4. However, if ammonia spills from a high-pressure storage container, it can cause severe harm since it is lighter than air and diffuses more quickly. Moreover, due to the increasing use of hydrogen as an alternative for fuel storage, refrigeration, and the post-treatment of combustion exhaust gas in industrial settings, it is important to implement fire protection measures.
Similar to other hydrocarbons, condensed ammonia does not burn continuously. The reason for this is that the heat emitted from the flames is not sufficient to reach the pool. If an external heat source such as the ground or water is present, enough ammonia can vaporize to keep the fire burning.
As presented in Figure 3, the primary dangers related to ammonia bunkering are fires and explosions, which may occur due to leaks and spills around ignition sources. Without ignition, ammonia dissipates by vaporization and forms a vapor cloud that disperses in the air. However, in the event of ignition, there are four potential risk scenarios for ammonia, including vapor cloud flash fire, jet fire, pool fire, and vapor cloud explosion. Additionally, the consequences of ammonia fires and explosions are dependent on factors such as the initial temperature and composition of the ammonia and the diameter of the pool fire. Compared to LNG and LPG, ammonia has a lower risk of fire due to its lower burning rate.
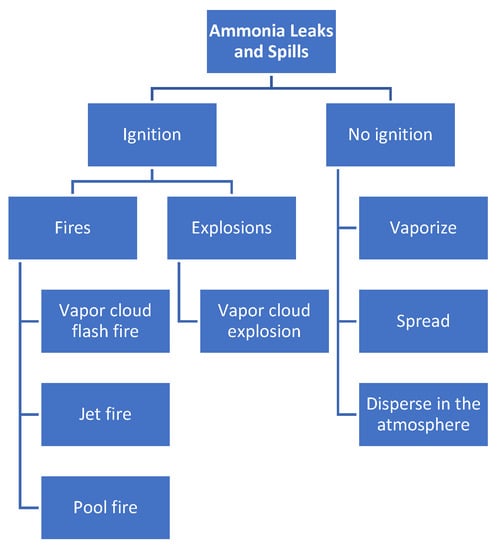
Figure 3.
Main hazards of the ammonia bunkering process.
The use of water spray, fog, or foam can be effective in extinguishing large ammonia flames, while dry chemicals or CO2 are more appropriate for small ammonia fires. However, it is important to avoid directing a water jet directly towards a leak or liquid ammonia source as this may cause a hazardous reaction. Responders must always wear protective equipment with an oxygen supply, even if the ammonia concentration is as low as 25 ppm.
2.3.4. Corrosiveness
Ammonia is a substance that can cause corrosion and harm to various materials, such as metals, plastics, and rubber. The corrosive effect of ammonia is due to its ability to react with water and form ammonium hydroxide, which is strongly alkaline in solution. The following are some of the ways in which ammonia can cause corrosion:
Metals: Ammonia can cause the corrosion of metals, particularly those that are not resistant to alkaline substances. When ammonia comes into contact with metal surfaces, it can cause the metal to become pitted, corroded, or even discolored. This can lead to weakened structural integrity and the potential failure of metal components.
Plastics: Ammonia can also cause damage to certain types of plastics, particularly those that are not resistant to alkaline substances. When ammonia comes into contact with plastic surfaces, it can cause the plastic to become brittle, cracked, or even discolored. This can lead to the potential failure of plastic components, particularly those that are subjected to stress or pressure.
Rubber: Ammonia can cause damage to rubber materials, particularly those that are not resistant to alkaline substances. When ammonia comes into contact with rubber surfaces, it can cause the rubber to become soft, swollen, or even disintegrate. This can lead to the potential failure of rubber components, particularly those that are subjected to pressure or friction.
Overall, it is important to handle ammonia carefully and take appropriate precautions to prevent its exposure to materials that are vulnerable to its corrosive effects. This includes proper storage and handling procedures, as well as the use of protective coatings and materials that are resistant to alkaline substances.
2.3.5. Other Hazards
Ammonia is highly susceptible to hydraulic shock due to its high boiling point and expansion rate. Hydraulic shock is the result of a sudden change in liquid flow velocity, causing a localized pressure surge that may lead to severe damage to equipment, valves, and piping. When a refrigerated system is exposed to a defrosted system, this may cause hydraulic shock.
Additionally, the release of pressurized ammonia into the environment poses another risk. About 8–9% of the ammonia in a container will vaporize and expand rapidly once released, continuing to do so even after the pressure is brought down. Ammonia can expand up to 710 times from its liquid state to a vapor state, and it can continue to evaporate even after it rains out. A catastrophic tank failure at room temperature could result in a significant release of ammonia.
A BLEVE (boiling liquid expanding vapor explosion) is a type of physical explosion that can occur when a pressurized liquid boils rapidly due to a loss in pressure. For a BLEVE to occur, the temperature of the liquid at the time of pressure loss must be higher than its superheating level (TSL). The critical temperature for ammonia is 89.8 °C, which is significantly higher than its recommended ambient and storage temperatures. Therefore, it can be assumed that the risk of a BLEVE is low.
2.3.6. Accidents in Ammonia Bunkering
Ammonia is denser than air and can cause harm to humans when it is released into the environment, especially to the eyes, nose, and respiratory system. At a temperature of −33.33 °C, liquid ammonia is less dense than seawater with a density of 0.696 g/cm3, which makes it float in seawater [40]. It is difficult to ignite ammonia due to its high auto-ignition temperature of approximately 651 °C and narrow flammable range between 15.75% and 27.35%. In the case of a leak, ammonia has the potential to corrode various metals and compounds, including copper and zinc. Hence, it is vital to have a venting mechanism in place that can safely release ammonia during an emergency. Table 1 provides additional information on the properties of ammonia.
The widespread application of ammonia in the industrial sector has led to significant consequences for society, property, the environment, and facilities, particularly during unexpected incidents. According to statistics, from 1985 to 2019, there were approximately 71 accidents involving anhydrous ammonia. The primary causes of deaths and injuries were identified as inhalation of the gas or fires [41]. Chemical-based hazards have a high percentage of injuries, fatalities, and evacuations, which is in line with the alarming number of serious incidents caused by ammonia leaks. Accidents involving ammonia explosions and dispersion can be caused by various factors, such as mechanical failures, operational difficulties, and human error.
There have been numerous incidents where ammonia leaked and negatively impacted the ecosystem. Typical accidents caused by released ammonia are described in Table 4.

Table 4.
Records of ammonia incidents.
Based on the analysis of typical accidents in ammonia plants and operations, we determined that ammonia can be released due to various reasons, such as human error, operational mistakes, and maintenance and inspection failures during storage tank operations, the bunkering process, and pipeline operations. A release of ammonia may pose a threat to nearby areas, and its level of toxicity depends on the extent of diffusion, which can be estimated based on the release grade.
The key method to prevent unforeseen incidents and reduce the negative impact of ammonia release on people and facilities is to conduct a risk and consequence analysis for potential ammonia leakage. Therefore, it is essential to create a safety zone that prohibits unauthorized individuals and vehicles from operating within the ammonia bunkering area.
3. Regulations on Ammonia Bunkering
This section provides a discussion and analysis of the current regulations and safety standards related to the process of ammonia bunkering. Additionally, the guidelines for safe procedures during bunkering are also examined. The typical regulatory framework of ammonia utilization in the maritime sector is summarized and presented in Table 5.

Table 5.
Regulatory frameworks for ammonia in the maritime sector.
Due to the potential toxicity and severe health risks associated with ammonia, ensuring the safety of the bunkering process is crucial. Obtaining approval from classification societies and administration authorities is necessary to ensure compliance with safety standards. Several organizations, such as ISO/TS 18683, ClassNK, the Korean Register, DNVGL, Lloyd’s Register (LR), the Norwegian Maritime Authority, and the Society for Gas as a Marine Fuel (SGMF), have established regulations and guidelines for safe ammonia bunkering. It is recommended that a safety zone be designated to strictly limit access to personnel not involved in the bunkering process.
To this end, significant classification societies and organizations have released safety guidelines for bunkering procedures and equipment. It is crucial to follow these regulations, guidelines, and standards to avoid leakage caused by unexpected incidents and to control ignition sources during the operation of ammonia and bunkering processes. It is important to understand and comply with all domestic and international regulations regarding ammonia safety and apply them to the bunkering process while considering all possible scenarios that may arise. To contribute to the development of safety regulations and guidelines, Luo et al. [58] searched for ammonia leak accidents in China in the period from 2010 to 2020 and analyzed their causes. The results show that human factors and failure of equipment were the two main causes of accidents. It is necessary to develop detailed regulations for safe decision making.
According to the established definitions, risk is the combination of the likelihood that a recognized hazard will occur and the severity of its impact. Risk is determined by the frequency of accidents in a given time period. During the bunkering process, risk factors are classified and evaluated through risk assessment for approval. In the marine industry, various methods are used for risk evaluation, including HAZID/HAZOP, FTA, and FMEA. HAZID/HAZOP is a critical first step for identifying abnormalities and hazards in a risk assessment. Table 6 provides a detailed classification framework for risk assessment [59].

Table 6.
Classification framework for risk analysis with regard to ammonia bunkering.
As for the ammonia bunkering process, the accidents that may occur as a result of ammonia leakage and release are shown below in Figure 4. In the case of ammonia leakage or a spill, the accident outcomes consist of vapor cloud explosion (VCE), flash fire, pool fire, jet fire, and explosion [60]. When released, ammonia that has been kept at a pressure that is significantly higher than the atmospheric pressure will partly flash (rapidly evaporate) [61]. Additional event outcomes, such as fireballs, rocket fires, and BLEVE (boiling liquid expanding vapor explosion) are expected as a result [62]. Further developments include dispersion without fire, fire, and explosion. These three possible scenarios, identified from the literature review and field experiments, are reflected in each of the following trees [63]. The materials cited in this article may contain actual instances of these occurrences.
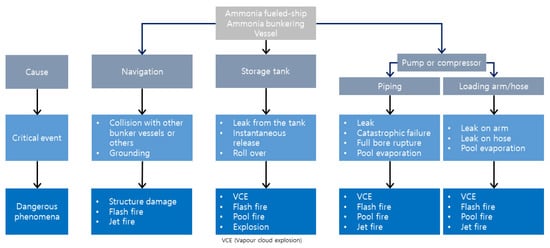
Figure 4.
Event tree of ammonia risk assessment.
In this event tree, the initial event is an ammonia release. Leakage of ammonia can be caused by a failure in navigation or the storage tank, compressor, or pump events. The release can either be small or large. If the release is small, it can either have no impact or have a localized or widespread impact. The localized impact can result in minor injury, major injury, or fatality. The same consequences can occur with a widespread impact. Similarly, if the release is large, it can either have no impact or have a localized or widespread impact. Again, the localized or widespread impact can result in vapor cloud dispersion, flash fire, explosion, or pool fire. This event tree can be used to evaluate the potential consequences of an ammonia release and develop mitigation strategies to prevent or minimize harm.
It was estimated that approximately 71% of the published literature on ammonia risk analysis uses conventional methods of risk assessment, as opposed to the 29% that employ a dynamic risk assessment. The trend is steadily moving towards the development of more efficient integrated risk analysis tools (combining techniques) to evaluate the risk of complex and dynamic assets, such as ammonia plants.
4. Safety Assessment of the Ammonia Bunkering Process
Ammonia bunkering is the process of transferring ammonia fuel from a supply vessel to a receiving vessel for use as fuel. As with any bunkering process, safety is a primary concern, and there are several aspects to consider when assessing the safety of the ammonia bunkering process:
- (i)
- Ammonia storage and handling: Strict safety regulations must be followed for the storage and handling of ammonia due to its hazardous nature. Storage containers for ammonia must be specifically designed and certified for this purpose. Trained personnel who wear suitable protective gear are responsible for handling and transferring ammonia.
- (ii)
- Bunkering procedures: It is crucial to plan and carry out the bunkering process meticulously with trained personnel. The crew of the receiving vessel should be notified about the procedure and any necessary safety precautions. The process should be monitored closely to ensure it is executed safely.
- (iii)
- Ventilation: Proper ventilation is essential during the bunkering process to avoid the accumulation of ammonia vapors. The area where the bunkering is carried out must have adequate ventilation to disperse any leaks or spills swiftly.
- (iv)
- Emergency response: In the event of an accident or spill during bunkering, there must be emergency response plans in place. The crew should be trained to handle an ammonia spill, and necessary equipment, such as personal protective gear and spill containment equipment, should be easily accessible.
- (v)
- Regulatory compliance: The ammonia bunkering process should conform to applicable regulations and guidelines. The International Maritime Organization (IMO) has established safety guidelines for the proper utilization of ammonia as a marine fuel. These guidelines comprise specifications for the design of ammonia bunkering systems and training requirements for the personnel involved in the bunkering process.
Thus, a safety assessment of the ammonia bunkering process involves several critical aspects, such as ammonia storage and handling, bunkering procedures, ventilation, emergency response, and regulatory compliance. All these aspects must be taken into consideration to ensure the safety of the bunkering process.
Data gathering, scenario analysis, frequency analysis, outcome analysis, and risk assessment contribute to the common risk assessment process for finding a vessel’s safety zone. Thus, a comprehensive risk assessment procedure for the ammonia bunkering process includes identifying hazards, determining consequences, evaluating risks, identifying control measures, implementing them, and monitoring and reviewing the entire process regularly. This procedure helps minimize the risk of accidents and ensure that the bunkering process is carried out safely. The flow chart of the general risk assessment procedure for the ammonia bunkering process is summarized and presented in Figure 5 below.
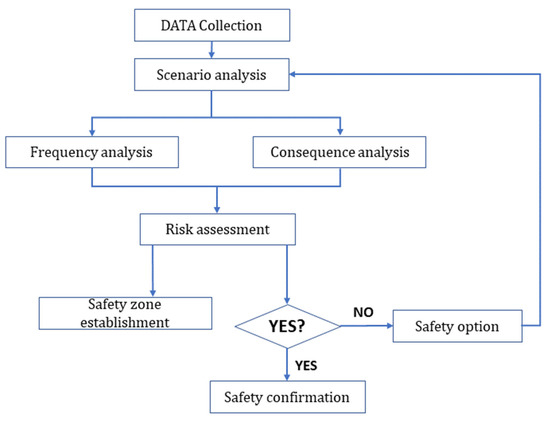
Figure 5.
Risk assessment outline.
4.1. CFD and Theorical Analysis
It is obvious that some level of social cost will be incurred during the ammonia bunkering process. Humans might suffer blindness, lung injury, or even death as a result of exposure to deadly ammonia gas, which also causes burning of the eyes, nostrils, throat, and respiratory system. In addition, it is important to note the dangers posed by ammonia’s flammability. Ammonia is being investigated as a maritime propellant, but not much information is available as of yet. There have been few studies investigating the dangers of using ammonia as fuel for transportation. Figure 6 presents a computational algorithm and procedure of risk assessment.
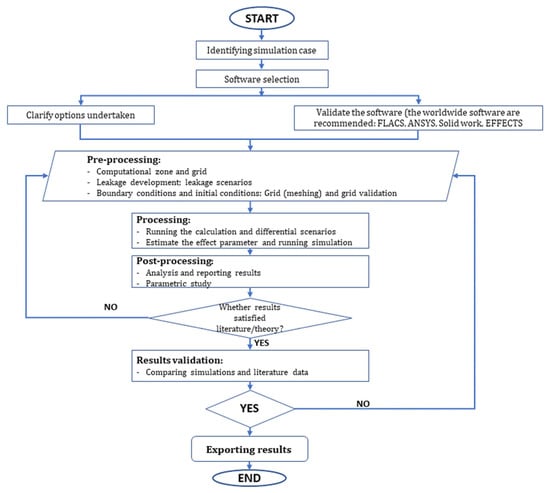
Figure 6.
A typical framework of deterministic approach.
According to typical studies on the modeling of ammonia leakage and release [64,65,66,67,68,69,70,71,72,73,74,75,76,77], the common algorithmic diagram for the computational analysis of ammonia leakage is shown in Figure 5, and the computational algorithm and procedure are presented in Figure 6. The six steps required to use computational fluid dynamics (CFD) for the modeling and consequence analysis of ammonia leakage or accidents include the following:
- (i)
- Identify the simulation case: This step is to clarify the purpose of the simulation and which leakage/release case will be addressed.
- (ii)
- Software selection: Depending on the situation and output requirements of the analyzed case, a suitable simulation tool is selected. Typical 2D simulation software products include EFFECTS (Gexcon) and Safeti (DNV), while 3D simulation software products include FLACS (Gexcon), Phast and KFX (DNV), and ANSYS Fluent (Ansys).
- (iii)
- Software validation and verification.
- (iv)
- Pre-processing: At this stage, the boundary conditions are selected. Grid validation needs to be carried out to estimate the optimal grid size for the simulation. The larger the number of grids, the higher the calculation accuracy, but computational cost is also high. Therefore, the grid number should be selected while keeping the simulation time, computational cost, and simulation accuracy balanced.
- (v)
- Post-processing: Scenarios are established with various influencing factors in ammonia leakage and release. The theories of leakage and dispersion are compared, and the results are considered.
- (vi)
- Result validation: The results are analyzed and compared with theories in the literature.
The algorithm in Figure 7 below describes a procedure for risk analysis using risk-based methods similar to those seen in [78,79,80,81,82,83].
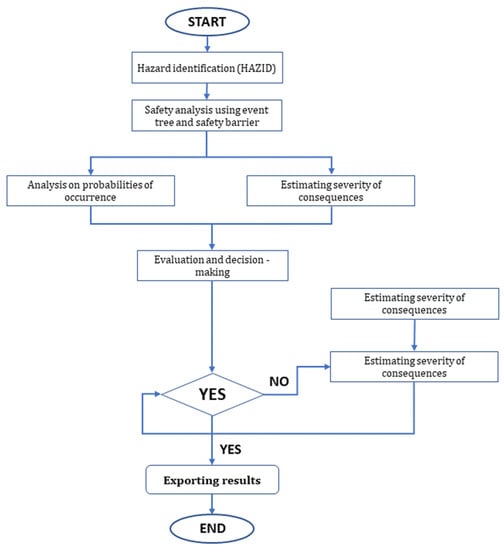
Figure 7.
A typical framework of quantitative risk assessment.
To prevent unexpected damage due to the leakage and release of ammonia during the bunkering process, the establishment of a safety zone where ignition sources are limited and only authorized persons and activities are allowed is essential.
Ammonia leakage and dispersion have heavy gas dispersion characteristics [84,85,86,87]. As presented in Figure 8, the ammonia leakage process can be roughly divided into four stages [88,89,90]. First, leakage occurs from the ammonia storage tank, piping, hoses, etc. At this time, the ammonia is in contact with the air, and since ammonia in a low-temperature state is heavier than air, it forms a low-temperature pool [91]. In the second stage, the ammonia collected in the low-temperature pool on the ground or on a water surface spreads widely [92,93]. Third, since the atmospheric temperature is generally higher than the boiling point of ammonia, the ammonia is evaporated by the surrounding heat. As a result, a wide, low-temperature vapor cloud is formed. Finally, it undergoes a process of diffusion by wind.
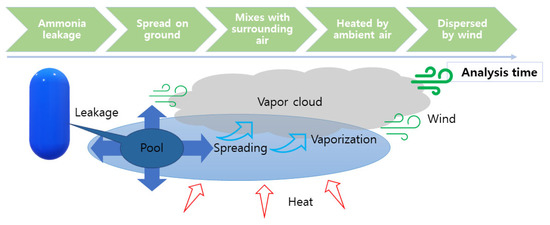
Figure 8.
Ammonia leakage process.
A schematic diagram of the ammonia leakage and diffusion process is shown in Error! Reference source not found.; there are differences in the state of the leaked ammonia, the amount of leakage, and the degree of diffusion in each step of the process. In addition, since it takes time for ammonia to leak, absorb heat, evaporate, and diffuse, the leakage time and CFD analysis time are two important factors for establishing safety zones.
However, in previous studies conducted on the topic of safety zones, the difference between the leakage time and analysis time is not large. Due to this, it may be difficult to include various changes occurring in the ammonia leakage process. When performing an analysis to establish a safety zone, we recommend analyzing the leakage trends and the lower flammable limit range according to the analysis time and reflecting the results in the designation of the safety zone range.
The safety zone procedure established in this study is an improved version of the quantitative risk assessment method recommended by [87,94,95]. Our procedure contains five main steps in accordance with the purpose and scope of this study: (i) review of legal documents and historical cases, (ii) data collection, (iii) scenario and consequence analysis, (iv) risk assessment, and (v) results analysis and conclusion.
- ∗
- Step 1: Review of legal documents and historical cases
In the first step, the regulations and rules from classification societies and management authorities are reviewed. The lessons from each are used as foundations to build and determine safety zones for specific cases.
- ∗
- Step 2: Data collection
A field survey at the bunkering area is carried out to gather and measure the geometry, weather, and other potential influencing factors. The wind speed and wind direction in the designated area are measured. In the following steps, the probability of occurrence and the consequent impact of all accidental scenarios determined during scenario analysis are evaluated.
- ∗
- Step 3: Scenario and consequence analysis
Various bunkering scenarios are designed and considered. The effects of ship size, environmental conditions, loading conditions, and bunkering conditions are estimated and transformed into bunkering scenarios. The frequency of each accidental scenario can be calculated by multiplying the probability of each variable under the given conditions.
- ∗
- Step 4: Risk assessment
The likelihood of each accidental scenario associated with initial gas dispersion behavior is represented by the results of a frequency analysis. Meanwhile, the outcome of the consequence analysis is expressed in critical distances and the number of casualties within critical areas.
- ∗
- Step 5: Simulation results and analysis
A detailed analysis is performed to determine the safety zone for a particular instance of ammonia bunkering.
Table 7 below summarizes some of the theoretical research regarding safety assessments for the ammonia bunkering process.

Table 7.
Ammonia release risk assessment research.
There are several other quantitative analysis studies that we referred to [21,106,107,108,109]. However, the practice of using simulation software to analyze ammonia leakage scenarios, as mentioned above, still has some shortcomings.
CFD models are used to simulate ammonia leakage and dispersion in a designated area, such as an engine room or ammonia storage room. Due to the software limitations of these models, the door, ventilation tube, equipment, and barrier dimensions are modified to suit the simulation. Furthermore, each simulation software uses a specific turbulence model such as , LES Smagorinsky, etc. The results change depending on which turbulence model is used, thus affecting accuracy. Additionally, we present the following considerations:
- Some simulations model congested areas. Therefore, in reality, the dispersion characteristics are affected by walls and barriers.
- However, interior modeling does not take into account barriers or room ventilation. These are only simulated in the case of outdoor gas distribution.
- Leakage characteristics, such as the leak rate, leak duration, and leak direction that affect the critical distance of vapor cloud dispersion are not fully considered.
- CFD modeling is performed on a full-scale model, while wind tunnel testing is performed on a scaled-down model. Although the findings are convincing, CFD modeling should be repeated on a smaller scale to obtain more precise results.
Ultimately, prior theorical research has not yet attempted to create safety zones for ammonia bunkering zones, therefore it is still unclear what regulations should be set for the ammonia-fueled ships currently being developed or planned.
4.2. Experimental Studies
Bouet et al. [110] experimented with releasing ammonia at the Centre of Scientific and Technical Studies of Aquitaine in France. Three 12 m3 units of liquified ammonia were installed in the testing area. In this test, one of these tanks was linked with release equipment to represent an accident in the bunkering process. Sensors and thermocouples were arranged downwind of the leak to record data related to ammonia leakage and dispersion. The experiment’s results show that ammonia dispersion behaves similarly to a heavy gas. Of the more than 2 tons of ammonia released, it was observed that more than 50% of the leakage was in liquid form, with a temperature of around −60 °C. This liquid pool did not evaporate quickly. Thus, the leakage time and leakage diameter (leak amount) are the most important factors to determine the safety zone during the bunkering process. However, this experiment had fixed wind conditions. Therefore, further assessment of safety zones should be performed with changing weather conditions.
Tan et al. [111] tested ammonia leakage and dispersion in a food factory to estimate the ammonia dispersion law. Their sensors were installed at different heights throughout the area to record the ammonia concentrations at different wind speeds and leakage flow rates. The experiments revealed that after increasing the mass flow rate of ammonia leakage, the ammonia concentrations at the measuring sensors increased accordingly. However, with increasing wind speed, the ammonia concentrations first increased and then decreased. In this experiment, the effect of wind speed on the ammonia concentration was bigger than the effect of the mass flow rate.
Witlox et al. [112] used an experimental database and a model built using Phast software version 8.1 to consider more factors affecting dispersion characteristics. The simulation results show good alignment with data from accidental releases.
Additionally, there have been several more experimental studies of ammonia leakage and dispersion, which are summarized below in Table 8.

Table 8.
Experimental studies of ammonia leakage.
As per our review of the literature, comprehensive analyses and criteria to determine the vapor cloud dispersion of ammonia during the ammonia bunkering process are still lacking. Accidents involving dispersion and explosion that may occur during the bunkering process were one of the motivations for this work and will also motivate future studies on the establishment of safety zones. The other motivation for this study came from the limitations of prior studies and the present rules.
4.3. Safety Zone during Ammonia Bunkering Process
The safety zone during the ammonia bunkering process refers to the designated area surrounding the bunkering operation where access is restricted and the necessary safety measures are implemented. The size of the safety zone should be determined by a risk assessment that takes into account factors such as the quantity and properties of the ammonia being bunkered, the configuration of the bunkering operation, and the potential consequences of an accidental release.
In general, the safety zone should be large enough to protect personnel, property, and the environment from harm in the event of an ammonia release. It should also be clearly marked and its boundaries communicated to all personnel involved in the bunkering operation and others working nearby. Depending on the specific circumstances, the safety zone may include physical barriers or other safety measures to prevent unauthorized access. It is important to note that the safety zone is not a static concept and may need to be adjusted based on changing circumstances or the results of ongoing risk assessments. Regular training and drills can also help ensure that all personnel are familiar with the safety measures in place and know what to do in the event of an emergency.
Regarding ammonia-fueled ships, the process of refueling with ammonia is mandatory and unavoidable. However, due to the hazardous nature of ammonia, including its toxicity and flammability, extreme caution must be taken to ensure safe ammonia bunkering operations. Therefore, the risks associated with ammonia bunkering need to be thoroughly considered and analyzed. As previously mentioned, the regulations that provide detailed and quantified guidelines for establishing safety zones during the ammonia bunkering process are currently limited and inadequate. Therefore, it is crucial to provide step-by-step risk assessment guidance for ammonia bunkering aimed at minimizing harm to people and equipment, as well as reducing the possibility of ignition sources.
Establishing a safety zone during the ammonia bunkering process requires a thorough assessment of the associated risks. The safety zone should be determined based on potential hazards, such as the release of ammonia gas or the risk of fire or explosion. The safety zone should be established in consultation with relevant authorities, including the port authority, local emergency responders, and other stakeholders.
Two approaches are available for establishing a safety zone during ammonia bunkering: deterministic and risk-based methods. To prevent unauthorized access, it is necessary to place clear signs and barriers. Equipping personnel with personal protective equipment (PPE) and providing proper training in emergency response procedures is also crucial. It is recommended to conduct regular safety drills and exercises to ensure that everyone involved is familiar with the emergency procedures. These measures, when implemented, can create a secure and safe zone for the ammonia bunkering process.
Kim et al. [120] estimated the safety zone area for the bunkering process of a 30 GT class ammonia-fueled ship. This research describes a new method of assessing the risks involved in ammonia bunkering that utilizes two different types of assessment methods to determine the appropriate safety zones. The findings of the study indicate that, for the ship under consideration, a safety zone of 10 m can be established when the 5.0 × 10−5/year safety criterion is used. However, a zone of 57 m is required for the 1.0 × 10−5/year criterion, and 373 m is required for the 5.0 × 10−6/year criterion. Additionally, this study provides general guidance on establishing proper safety zones for ammonia bunkering and sheds light on the risks associated with using ammonia fuel in the maritime industry, which are not yet fully understood.
Clara et al. [121] simulated the influencing factors affecting ammonia release and dispersion. According to this study, there are significant differences in how ammonia spreads over a sea’s surface compared to land-based dispersion. To investigate this, the researchers analyzed the dispersion of ammonia over both land and sea surfaces in Singapore, taking into account the region’s unique weather conditions. The research evaluated ammonia dispersion following both day and night releases. They found that when ammonia is released from a height exceeding 5 m, the lethality footprint substantially increases. Releasing ammonia in a downward or diagonal direction from the horizontal position has the potential to decrease its lethality footprint. It is important to prevent ammonia from being released upwards, as this can increase its lethality footprint by 3.8 times during the day and 17 times at night compared to a horizontal release.
Previous studies have not furnished clear instructions and measurable techniques for creating safety zones for ammonia bunkering. Therefore, it is uncertain what safety measures should be adopted for ammonia-powered vessels either under development or in their planning stages. When creating safety zones for ammonia bunkering, particular factors and concerns must be considered:
- (i)
- Under the deterministic approach, the extent of flammable gas dispersion and the corresponding safety zone boundary can vary based on the particular leak scenario being examined, resulting in different safety zones. The Society for Gas as a Marine Fuel (SGMF) has established guidelines for industry practice that suggest using a leak size equal to 6% of the transfer line diameter for modeling purposes. Following this guideline could help in applying the deterministic approach to ammonia leak scenarios in a more general manner.
- (ii)
- The method of creating a safety zone layout that considers the probability and consequences of ammonia leaks during bunkering is inspired by quantitative risk assessment (QRA) methods. This process involves evaluating different leak scenarios and their frequencies and integrates their impacts into a single safety zone layout. This approach enables a more comprehensive and accurate assessment of the associated risks.
- (iii)
- The feasibility of a hybrid approach was demonstrated through the creation of a safety zone plan for ammonia bunkering. The relevant study revealed that the hybrid approach yielded a more consistent safety zone design compared to the deterministic approach across various bunkering situations. This highlights the usefulness of combining deterministic and risk-based elements in safety zone planning to create a more adaptable and robust approach.
- (iv)
- Establishing a safety zone between the ammonia-fueled ship and the bunkering vessel is crucial for improving the safety of ship-to-ship ammonia bunkering. However, the industry lacks clear guidelines and detailed instructions for specific cases.
- (v)
- This study focuses on the variables associated with the risks of ammonia bunkering. This review also identifies general trends and relationships among these variables. Our research findings are expected to serve as a starting point for obtaining valuable information, particularly because there are no established industry practices for determining safety zones in ammonia bunkering. However, to practically establish safety zones, a probabilistic analysis should be conducted using a range of plausible scenarios that reflect all potential events and relevant changes in critical factors.
5. Challenges and Recommendations
Although ammonia technology is more advanced in terms of commercialization compared to other hydrogen storage methods, such as complex metal hydrides, its use as a potential hydrogen carrier is still limited due to various concerns. Despite extensive research on carbon-based hydrogen carriers, such as light hydrocarbons and methanol, problems related to CO2 for end-users still exist. To satisfy the key requirements for hydrogen storage material, including fast kinetics, high storage capacity, availability, and low cost, storing ammonia in metal ammines appears to be a promising alternative.
To mitigate the impact of an accidental release of ammonia, strategies to reduce the leakage rate and duration during bunkering should be developed. It is crucial to carefully plan the loading of cargo during bunkering, as the ship’s design and environmental factors can affect the extent of gas spread. Additionally, when establishing the safety zone for ammonia bunkering, the wind direction and speed should be considered to minimize the risks related to leaked gas dispersion.
In summary, establishing a safety zone for ammonia bunkering can be a challenging task due to several factors. Following are some of the challenges that may arise:
- (i)
- Lack of detailed industry guidelines: Due to the fact that ammonia bunkering is a fairly new technology, there are currently no well-defined industry guidelines, regulations, or standards in place regarding safety zones. As a result, this can create ambiguity and discrepancies in the requirements for safety zones.
- (ii)
- The management of dangerous materials is crucial, as ammonia is an extremely hazardous substance that demands specific safety protocols and handling procedures. When establishing a safety zone for ammonia bunkering, it is imperative to prioritize the safety of workers and the surrounding environment.
- (iii)
- Technical limitations: When determining a safety zone, it is important to take into account the vessel’s size, shape, and bunkering infrastructure. The technical constraints of the bunkering equipment and vessel design should be considered to effectively establish a safety zone.
- (iv)
- Local regulations: The creation of a safety zone for ammonia bunkering can be complicated due to the possible influence of local regulations, such as zoning laws, environmental regulations, and safety standards. The inconsistency of these regulations across different regions may pose a challenge in establishing a standardized safety zone for ammonia bunkering.
- (v)
- Public perception: The hazardous nature of ammonia bunkering may lead to concerns from the public about its safety. To establish a safety zone, it may be necessary to address these concerns and effectively communicate the safety measures in place to ensure transparency and promote public confidence.
The following general recommendations can be implemented to improve safety during the ammonia bunkering process:
Firstly, it is essential to adhere to both international and local regulations, guidelines, and standards that pertain to ammonia safety during bunkering.
Secondly, a comprehensive risk assessment should be conducted utilizing established methods, such as HAZID/HAZOP, FTA, FMEA, and ETA, to identify and mitigate any potential hazards or risks during bunkering.
Thirdly, a safety zone should be established that restricts access to personnel not involved in the bunkering process.
Fourthly, it is important to provide thorough training to all personnel involved in the bunkering process, covering topics such as ammonia safety, emergency response protocols, and risk mitigation measures.
Additionally, all equipment, pipelines, and storage tanks used during bunkering should be properly maintained and inspected.
An effective emergency response plan should be implemented that includes procedures for detecting and responding to ammonia leaks or spills, evacuating personnel, and minimizing the impact of a potential ammonia release on the environment.
Furthermore, appropriate sensors and monitoring systems should be used to monitor ammonia levels and conditions during bunkering.
Finally, during bunkering, appropriate personal protective equipment (PPE) and safety equipment, such as gas detectors, respirators, protective clothing, and eye and face protection, should be used.
6. Conclusions
The maritime sector is preparing to use ammonia as an alternative power source to meet its decarbonization objectives. Based on our comprehensive review of safety during the bunkering process, the four main review questions stated in Section 1 are well answered and discussed. The use of ammonia as fuel poses safety issues that are different from those of traditional fuels since ammonia is toxic, corrosive, and flammable. To avoid corrosion, the appropriate materials must be chosen. However, a comprehensive risk evaluation is required because of the lack of clarity about the consequences of toxic gas dispersion and fire. Factors related to the release and dispersion of ammonia, including weather conditions, leak characteristics, outside structure, and traffic conditions, must be discussed and ranked in terms of relevance. The existing ammonia bunkering safety guidelines are insufficient. To avoid dispersion, fire, and explosion hazards on ships, it is crucial to conduct thorough risk analyses. Detailed theorical research and simulations should be carried out to examine the influences of individual factors on ammonia dispersion. Five challenges and eight recommendations to improve the safety of ammonia bunkering are reviewed and discussed in this report.
One of the studies in our review introduced a methodical technique for determining the appropriate size of the safety zone, and the findings indicate that the volume of the ammonia leakage, the duration of the leak, and weather conditions have the most significant impact on the safety zone, as anticipated. However, this study also emphasized the crucial role of external variables, such as the direction of the leak, leak area configuration, wind direction, ship structure, and cargo state, in determining the safety zone. By addressing the constraints of the current approach, which tends to disregard or undervalue these variables, this proposal is expected to increase the safety of ammonia bunkering.
Creating a safety zone and determining the distance between the bunkering vessel and the receiving vessel are crucial to improve the safety of ammonia bunkering. We reviewed case studies that illustrate the extent of flammable gas dispersion that may arise during various methods of ammonia bunkering and provide valuable results. Ship designers, owners, and regulators can utilize this information to develop improved safety zone guidelines for accidental ammonia leaks during bunkering.
According to studies from the literature, factors affecting the flow influence the safety of the ammonia bunkering process:
The dispersion of an ammonia vapor cloud is influenced by its density, leak characteristics, weather conditions, and surroundings. A comprehensive assessment of these variables is essential to comprehend the dispersion of an ammonia vapor cloud.
When ammonia leaks occur, a circular pool of liquid is formed that releases heat into the environment and evaporates into low-temperature steam. The area surrounding the storage tank is the primary target for early warning predictions since it has a high concentration of fuel and a prolonged duration of exposure.
The size of the leakage opening affects the heat transfer between the ammonia and the environment, and methods must be developed to minimize the rate and duration of a leakage to decrease the impact of accidental ammonia discharge.
The dispersion time of an ammonia leak is impacted by the direction of the breach and the location of the grounded leak. When establishing the safety zone for bunkering, it is essential to consider the fuel’s toxicity. This study provides recommendations for comprehending worst-case scenarios, preventing leaks, and creating safety zones for ammonia bunkering. These findings can be utilized to develop guidelines and regulations for the safety zone during ship-to-ship ammonia bunkering.
The proposals put forward for examining the safety of ammonia bunkering have the potential to tackle current knowledge gaps and improve the current research framework.
Author Contributions
Formal analysis, P.A.D.; investigation, B.R.R.; methodology, P.A.D., H.V.N. and M.K.S.; supervision, H.K. and D.N.; writing—original draft, P.A.D.; writing—review and editing, H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was conducted with the support of the Korea Maritime Transportation Safety Authority (KOMSA) internal project “Establishment of safety zone for LNG bunkering of small LNG fueled ships using tank lorry”. This research was supported by the project “Test evaluation for LNG bunkering equipment and development of test technology (Grant No. 20180048)” funded by the Ministry of Oceans and Fisheries (Korea). This research was supported by a Korea Evaluation Institute of Industrial Technology (KEIT) grant funded by the Korea Government (MOTIE) (RS-2022-00144116). This work was also supported by the Research Promotion Program through the Korea Maritime & Ocean University Research Fund in 2022.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ABS | American Bureau of Shipping |
| BLEVE | Boiling liquid expanding vapor explosion |
| CFD | Computational fluid dynamics |
| EEOI | Energy Efficiency Operational Indicator |
| ETA | Event tree analysis |
| EMSA | European Maritime Safety Agency |
| EEDI | Energy Efficiency Design Index |
| FTA | Fault tree analysis |
| FMEA | Failure mode and effect analysis |
| GHG | Greenhouse gas emission |
| IMO | International Maritime Organization |
| ICE | Internal combustion engine |
| IGF Code | The International Code of Safety for Ships Using Gases or Other Low-Flashpoint Fuels |
| IGC Code | International standard for the safe carriage by sea in bulk of liquefied gases |
| IACS | International Association of Classification Society |
| KR | Korean Register |
| LNG | Liquefied natural gas |
| LPG | Liquefied petroleum gas |
| LR | Lloyd’s Register |
| MARPOL | The International Convention for the Prevention of Pollution from Ships |
| PFP | Power-to-fuel-to-power |
| RRQ | Review research questions |
| SOLAS | International Convention for the Safety of Life at Sea |
| SEEMP | Ship energy efficiency management plan |
| SIMOPS | Simultaneous operation |
| STS | Ship-to-ship |
| SGMF | Society for Gas as a Marine Fuel |
| SIGTTO | The Society of International Tanker and Terminal Owners |
| TTS | Terminal-to-ship |
| T-TS | Truck-to-ship |
| VCE | Vapor cloud explosion |
References
- Ni, P.; Wang, X.; Li, H. A review on regulations, current status, effects and reduction strategies of emissions for marine diesel engines. Fuel 2020, 279, 118477. [Google Scholar] [CrossRef]
- Sèbe, M.; Scemama, P.; Choquet, A.; Jung, J.-L.; Chircop, A.; Razouk, P.M.-A.; Michel, S.; Stiger-Pouvreau, V.; Recuero-Virto, L. Maritime transportation: Let’s slow down a bit. Sci. Total Environ. 2021, 811, 152262. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Wang, Q. Emissions in maritime transport: A decomposition analysis from the perspective of production-based and consumption-based emissions. Mar. Policy 2022, 143, 105125. [Google Scholar] [CrossRef]
- Lee, H.; Ryu, B.; Anh, D.P.; Roh, G.; Lee, S.; Kang, H. Thermodynamic analysis and assessment of novel ORC- DEC integrated PEMFC system for liquid hydrogen fueled ship application. Int. J. Hydrogen Energy 2023, 48, 3135–3153. [Google Scholar] [CrossRef]
- Duong, P.A.; Ryu, B.; Jung, J.; Kang, H. Thermal Evaluation of a Novel Integrated System Based on Solid Oxide Fuel Cells and Combined Heat and Power Production Using Ammonia as Fuel. Appl. Sci. 2022, 12, 6287. [Google Scholar] [CrossRef]
- Duong, P.A.; Ryu, B.; Kim, C.; Lee, J.; Kang, H. Energy and Exergy Analysis of an Ammonia Fuel Cell Integrated System for Marine Vessels. Energies 2022, 15, 3331. [Google Scholar] [CrossRef]
- International Maritime Organization. Resolution MEPC.305(73), Amendments to MARPOL Annex VI; International Maritime Organization: London, UK, 2018; Volume 1, pp. 1–15. [Google Scholar]
- Zincir, B. Environmental and economic evaluation of ammonia as a fuel for short-sea shipping: A case study. Int. J. Hydrogen Energy 2022, 47, 18148–18168. [Google Scholar] [CrossRef]
- Ye, M.; Sharp, P.; Brandon, N.; Kucernak, A. System-level comparison of ammonia, compressed and liquid hydrogen as fuels for polymer electrolyte fuel cell powered shipping. Int. J. Hydrogen Energy 2022, 47, 8565–8584. [Google Scholar] [CrossRef]
- Ryu, B.R.; Duong, P.A.; Kang, H. Comparative analysis of the thermodynamic performances of solid oxide fuel cell–gas turbine integrated systems for marine vessels using ammonia and hydrogen as fuels. Int. J. Nav. Arch. Ocean Eng. 2023, 15, 100524. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P.; Mielenz, J.R. Renewable Hydrogen Carrier—Carbohydrate: Constructing the Carbon-Neutral Carbohydrate Economy. Energies 2011, 4, 254–275. [Google Scholar] [CrossRef]
- Al-Aboosi, F.Y.; El-Halwagi, M.M.; Moore, M.; Nielsen, R.B. Renewable ammonia as an alternative fuel for the shipping industry. Curr. Opin. Chem. Eng. 2021, 31, 100670. [Google Scholar] [CrossRef]
- Shi, J.; Zhu, Y.; Feng, Y.; Yang, J.; Xia, C. A Prompt Decarbonization Pathway for Shipping: Green Hydrogen, Ammonia, and Methanol Production and Utilization in Marine Engines. Atmosphere 2023, 14, 584. [Google Scholar] [CrossRef]
- Lamb, K.E.; Dolan, M.D.; Kennedy, D.F. Ammonia for hydrogen storage; A review of catalytic ammonia decomposition and hydrogen separation and purification. Int. J. Hydrogen Energy 2019, 44, 3580–3593. [Google Scholar] [CrossRef]
- Herbinet, O.; Bartocci, P.; Dana, A.G. On the use of ammonia as a fuel—A perspective. Fuel Commun. 2022, 11, 100064. [Google Scholar] [CrossRef]
- Yüzbaşıoğlu, A.E.; Avşar, C.; Gezerman, A.O. The current situation in the use of ammonia as a sustainable energy source and its industrial potential. Curr. Res. Green Sustain. Chem. 2022, 5, 100307. [Google Scholar] [CrossRef]
- Yang, M.; Lam, J.S.L. Operational and economic evaluation of ammonia bunkering—Bunkering supply chain perspective. Transp. Res. Part D Transp. Environ. 2023, 117, 103666. [Google Scholar] [CrossRef]
- Roy, A.; Srivastava, P.; Sinha, S. Dynamic failure assessment of an ammonia storage unit: A case study. Process. Saf. Environ. Prot. 2015, 94, 385–401. [Google Scholar] [CrossRef]
- Kukkonen, J.; Savolainen, A.; Valkama, I.; Juntto, S.; Vesala, T. Long-range transport of ammonia released in a major chemical accident at Ionava, Lithuania. J. Hazard. Mater. 1993, 35, 1–16. [Google Scholar] [CrossRef]
- Gaskin, S.; Pisaniello, D.; Edwards, J.W.; Bromwich, D.; Reed, S.; Logan, M.; Baxter, C. Application of skin contamination studies of ammonia gas for management of hazardous material incidents. J. Hazard. Mater. 2013, 252–253, 338–346. [Google Scholar] [CrossRef]
- Anjana, N.; Amarnath, A.; Nair, M.H. Toxic hazards of ammonia release and population vulnerability assessment using geographical information system. J. Environ. Manag. 2018, 210, 201–209. [Google Scholar] [CrossRef]
- Moura, R.; Beer, M.; Patelli, E.; Lewis, J.; Knoll, F. Learning from major accidents to improve system design. Saf. Sci. 2016, 84, 37–45. [Google Scholar] [CrossRef]
- Michaels, R.A. Emergency Planning and the Acute Toxic Potency of Inhaled Ammonia. Environ. Health Perpet. 1999, 107, 617–627. [Google Scholar] [CrossRef]
- Eliopoulou, E.; Papanikolaou, A.; Voulgarellis, M. Statistical analysis of ship accidents and review of safety level. Saf. Sci. 2016, 85, 282–292. [Google Scholar] [CrossRef]
- Guidelines, R. Revised Guidelines for Formal Safety Assessment (Fsa) for Use in the Imo Rule-Making Process; IMO: London, UK, 2018; Volume 44. [Google Scholar]
- Park, S.-I.; Kim, S.-K.; Freng, J.K.P. Safety-zone layout design for a floating LNG-Fueled power plant in bunkering process. Ocean Eng. 2020, 196, 106774. [Google Scholar] [CrossRef]
- Al-Enazi, A.; Okonkwo, E.C.; Bicer, Y.; Al-Ansari, T. A review of cleaner alternative fuels for maritime transportation. Energy Rep. 2021, 7, 1962–1985. [Google Scholar] [CrossRef]
- Ayvali, T.; Edman Tsang, S.C.; Van Vrijaldenhoven, T. The position of ammonia in decarbonising maritime industry: An overview and perspectives: Part I. Johns. Matthey Technol. Rev. 2021, 65, 275–290. [Google Scholar] [CrossRef]
- Yapicioglu, A.; Dincer, I. A review on clean ammonia as a potential fuel for power generators. Renew. Sustain. Energy Rev. 2019, 103, 96–108. [Google Scholar] [CrossRef]
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I.; Zamfirescu, C.; Vezina, G.; Raso, F. Comparative life cycle assessment of various ammonia production methods. J. Clean. Prod. 2016, 135, 1379–1395. [Google Scholar] [CrossRef]
- Le Fevre, C. A Review of Demand Prospects for LNG as a Marine Transport Fuel; Oxford Institute for Energy Studies: Oxford, UK, 2018. [Google Scholar]
- Inal, O.B.; Dere, C.; Deniz, C. Onboard hydrogen storage for ships: An overview. In Proceedings of the Fifth International Hydrogen Technologies Congress, Online, 26–28 May 2021. [Google Scholar]
- National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ammonia (accessed on 1 March 2023).
- Jeong, S.Y.; Jang, D.; Lee, M.C. Property-based quantitative risk assessment of hydrogen, ammonia, methane, and propane considering explosion, combustion, toxicity, and environmental impacts. J. Energy Storage 2022, 54, 105344. [Google Scholar] [CrossRef]
- Li, M.; Zhu, D.; He, X.; Moshammer, K.; Fernandes, R.; Shu, B. Experimental and kinetic modeling study on auto-ignition properties of ammonia/ethanol blends at intermediate temperatures and high pressures. Proc. Combust. Inst. 2022; in press. [Google Scholar] [CrossRef]
- Mckinlay, C.J.; Turnock, S.R.; Hudson, D.A. A Comparison of Hydrogen and Ammonia for Future Long Distance Shipping Fuels. In Proceedings of the LNG/LPG and Alternative Fuels, London, UK, 29–30 January 2020. [Google Scholar]
- Class NK. Part C ‘Guidelines for the Safety of Ships Using Ammonia as Fuel’ of Guidelines for Ships Using Alternative Fuels; Class NK: Tokyo, Japan, 2018; pp. 63–73. [Google Scholar]
- Declerck, L. Quantitative risk assessment. Top. Model. Clust. Data 2002, 6, 157–172. [Google Scholar] [CrossRef]
- Dharmavaram, S.; Tilton, J.; Gardner, R. Fate and transport of ammonia spilled from a barge. J. Hazard. Mater. 1994, 37, 475–487. [Google Scholar] [CrossRef]
- Jain, P. What Has the Industry Experience Been with Ammonia Manufacturing Plants? What Is Their Track Record for Having Serious Process Safety Incidents? What Root Causes Have Typically Led to Them? 2019. Available online: https://engineering.purdue.edu/P2SAC/presentations/documents/Industry-Experience-With-Ammonia-Manufacturing-Plants-Fall-2019.pdf (accessed on 20 March 2023).
- Junior, M.M.; e Santos, M.S.; Vidal, M.; Carvalho, P. Overcoming the blame game to learn from major accidents: A systemic analysis of an Anhydrous Ammonia leakage accident. J. Loss Prev. Process. Ind. 2012, 25, 33–39. [Google Scholar] [CrossRef]
- Ojha, M.; Dhiman, A. Problem, Failure and Safety Analysis of Ammonia Plant: A Review. Int. Rev. Chem. Eng. 2010, 2, 631–646. [Google Scholar]
- U.S. Chemical Safety and Hazard Investigation Board. Key Lessons for Preventing Hydraulic Shock in Industrial Refrigeration Systems Anhydrous Ammonia Release at Millard; U.S. Chemical Safety and Hazard Investigation Board: Washington, DC, USA, 2015; pp. 1–15. [Google Scholar]
- Reuters. 2013. Available online: www.reuters.com (accessed on 20 March 2023).
- International Maritime Organization. Code of safety for ships using gases or other low-flashpoint fuels (IGF CODE). Marit. Saf. Comm. 2015, 391, 1–124. [Google Scholar]
- International Maritime Organization. Resolution MSC.370(93) (Adopted on 22 May 2014) Amendments to the International Code for the Construction and Equipment of Ships Carrying Liquefied Gases in Bulk (IGC Code); International Maritime Organization: London, UK, 2015; Volume 411. [Google Scholar]
- Korean Register (KR). Guidelines for Ships Using Ammonia as Fuel; Korean Register: Busan, Republic of Korea, 2021. [Google Scholar]
- Hammer, L.S.; Leisner, M.; Eide, M.S.; Sverud, T.; Mjøs, N. Ammonia as a Marine Fuel Safety Handbook; Green Shipping Programme: Oslo, Norway, 2021; pp. 1–28. [Google Scholar]
- DNV Energy Systems. External Safety Study-Bunkering of Alternative Marine Fuel for Seagoing Vessels; Port of Amsterdam: Amsterdam, The Netherlands, 2021. [Google Scholar]
- American Bureau of Shipping. Ammonia_as_Marine_Fuel_Whitepaper_20188 ABS; American Bureau of Shipping: Washington, DC, USA, 2020. [Google Scholar]
- International Association of Classification Societies, Container Ships. Guidelines for Surveys, Assessment and Repair of Hull Structures; International Association of Classification Societies: Paris, France, 2018. [Google Scholar]
- Laursen, R.; Barcarolo, D.; Patel, H.; Dowling, M.; Penfold, M.; Faber, J.; Király, J.; van der Ven, R.; Pang, E.; van Grinsven, A. Potential of Ammonia as Fuel in Shipping; The European Maritime Safety Agency: Lisbon, Portugal, 2022. [Google Scholar]
- The Society of International Tanker and Terminal Owners (SIGTTO). Guidelines List of SIGTTO; The Society of International Tanker and Terminal Owners: London, UK, 2023; Volume 22. [Google Scholar]
- Thomson, H.; Corbett, J.J.; Winebrake, J.J. Natural gas as a marine fuel. Energy Policy 2015, 87, 153–167. [Google Scholar] [CrossRef]
- Bond, S. Gas as a Marine Fuel—Recommendation of Controlled Zones during LNG Bunkering; The Society for Gas as a Marine Fuel: London, UK, 2018. [Google Scholar]
- NEA. QRA Technical Guidance Revision 3; NEA: Paris, France, 2016; pp. 1–39. [Google Scholar]
- Luo, C.; Zhao, Y.; Xu, K. Study on the Regularity of Ammonia-Related Refrigeration Accidents in China from 2010 to 2020. Int. J. Environ. Res. Public Health 2022, 19, 8230. [Google Scholar] [CrossRef]
- Animah, I.; Shafiee, M. Application of risk analysis in the liquefied natural gas (LNG) sector: An overview. J. Loss Prev. Process. Ind. 2020, 63, 103980. [Google Scholar] [CrossRef]
- Morosuk, T.; Tesch, S. Concepts for Regasification of LNG in Industrial Parks. In Advances in Natural Gas Emerging Technologies; Intech Open: London, UK, 2017; pp. 27–53. [Google Scholar]
- Vandebroek, L. Risk Assessment Study Supplying Flemish Ports with LNG as a Marine Fuel. 2017. Available online: https://www.researchgate.net/publication/321825437_Risk_assessment_Study_-_Supplying_Flemish_ports_with_LNG_as_a_marine_fuel_Executive_Summary?channel=doi&linkId=5a338ea40f7e9b2a288a2dbd&showFulltext=true (accessed on 20 March 2023).
- Bolado-lavin, R.; Mengolini, A. Best Practices and Methodological Guidelines for Conducting Gas Risk Assessments; Publications Office of the EU: Luxembourg, 2012. [Google Scholar]
- Zwęgliński, T. Conventional Event Tree Analysis on Emergency Release of Liquefied Natural Gas. Int. J. Environ. Res. Public Health 2022, 19, 2961. [Google Scholar] [CrossRef] [PubMed]
- Ni, J. Mechanistic Models of Ammonia Release from Liquid Manure: A Review. J. Agric. Eng. Res. 1999, 72, 1–17. [Google Scholar] [CrossRef]
- Kaiser, G.D. A Review of Models for Predicting the Dispersion of Ammonia. Plant/Oper. Prog. 1989, 8, 58–64. [Google Scholar] [CrossRef]
- Petrou, I.; Psistaki, K.; Dokas, I.M.; Paschalidou, A.K. Modelling the atmospheric dispersion of ammonia in an industrial area in northern Greece. IOP Conf. Ser. Earth Environ. Sci. 2022, 1123, 012075. [Google Scholar] [CrossRef]
- Abbaslou, H.; Karimi, A. Modeling of Ammonia Emission in the Petrochemical Industry. Jundishapur J. Health Sci. 2019, 11, 6. [Google Scholar] [CrossRef]
- Montes, F.; Rotz, C.A.; Chaoui, H. Process Modeling of Ammonia Volatilization from Ammonium Solution and Manure Surfaces: A Review with Recommended Models. Trans. ASABE 2009, 52, 1707–1720. [Google Scholar] [CrossRef]
- Bjerg, B.; Cascone, G.; Lee, I.-B.; Bartzanas, T.; Norton, T.; Hong, S.-W.; Seo, I.-H.; Banhazi, T.; Liberati, P.; Marucci, A.; et al. Modelling of ammonia emissions from naturally ventilated livestock buildings. Part 3: CFD modelling. Biosyst. Eng. 2013, 116, 259–275. [Google Scholar] [CrossRef]
- Ren, C.; Huang, X.; Liu, T.; Song, Y.; Wen, Z.; Liu, X.; Zhu, T. A dynamic ammonia emission model and the online coupling with WRF-Chem (WRF-SoilN-Chem v1.0): Development and evaluation. Geosci. Model Dev. 2023, 16, 1641–1659. [Google Scholar] [CrossRef]
- Gerdroodbary, M.B.; Mokhtari, M.; Bishehsari, S.; Fallah, K. Mitigation of Ammonia Dispersion with Mesh Barrier under Various Atmospheric Stability Conditions. Asian J. Atmos. Environ. 2016, 10, 125–136. [Google Scholar] [CrossRef]
- Jorqueira, D.S.S.; Neto, A.M.B.; Rodrigues, M.T.M. Modeling and Numerical Simulation of Ammonia Synthesis Reactors Using Compositional Approach. Adv. Chem. Eng. Sci. 2018, 8, 124–143. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, K.; Shen, J.; Jia, L.; Niu, R.; Yang, Z. Simulation Analysis of Ammonia Leakage and Dispersion in a Large-Scale Refrigeration System. Fluid Dyn. Mater. Process. 2022, 18, 1049–1066. [Google Scholar] [CrossRef]
- Danasa, A.S.; Soesilo, T.E.B.; Martono, D.N.; Sodri, A.; Hadi, A.S.; Chandrasa, G.T. The ammonia release hazard and risk assessment: A case study of urea fertilizer industry in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 399, 012087. [Google Scholar] [CrossRef]
- Naserzadeh, Z.; Atabi, F.; Moattar, F.; Nejad, N.M. Modeling the impact of consequence of ammonia release from ship loading arm by. Bulg. Chem. Commun. 2017, 49, 42–52. [Google Scholar]
- Che, B.; Che, R.; Puvaneswaran, B.; Aziz, A.; Raman, A. A Case Study of Consequences Analysis of Ammonia Transportation by Rail from Gurun to Port Klang in Malaysia Using Safti Computer Model. J. Saf. Health Environ. Res. 2009, 6, 1–19. [Google Scholar]
- Science Applications International Corporation. Model Risk Management Program and Plan for Ammonia Refrigeration; Science Applications International Corporation: Reston, VA, USA, 1996; p. 112. [Google Scholar]
- De Rademaeker, E.; Fabiano, B.; Buratti, S.S.; Storti, L.; Buccoliero, D.; Paesani, C. A Risk Based Approach on Selection of Refrigerated Ammonia Storage. Chem. Eng. Trans. 2013, 31, 571–576. [Google Scholar]
- Liu, Z.; Tian, W.; Cui, Z.; Wei, H.; Li, C. An intelligent quantitative risk assessment method for ammonia synthesis process. Chem. Eng. J. 2021, 420, 129893. [Google Scholar] [CrossRef]
- Rathinam, P.; Subburaj, S.; Roseline, A.A.; Kalaiselvam, S. Quantitative resilient investigation using RIPSHA approach and ANOVA validation for the ammonia storage unit. Process. Saf. Prog. 2022, 41, 708–720. [Google Scholar] [CrossRef]
- Cheliotis, M.; Boulougouris, E.; Trivyza, N.; Theotokatos, G.; Livanos, G.; Mantalos, G.; Stubos, A.; Stamatakis, E.; Venetsanos, A. Review on the Safe Use of Ammonia Fuel Cells in the Maritime Industry. Energies 2021, 14, 3023. [Google Scholar] [CrossRef]
- Biscotti, P.S.; Reinheimer, M.A.; Scenna, N.J. A risk–based design of ammonia refrigeration systems in food manufacturing plants. Lat. Am. Appl. Res. Int. J. 2019, 49, 55–60. [Google Scholar] [CrossRef]
- Environment Canada. Proposed Risk Management Strategy addressing Ammonia, Inorganic Chloramines and Chlorinated Wastewater Effluents under CEPA 1999; Environment Canada: Ottawa, ON, Canada, 2002. [Google Scholar]
- Wu, X.; Li, C.; He, Y.; Jia, W. Dynamic Modeling of the Two-Phase Leakage Process of Natural Gas Liquid Storage Tanks. Energies 2017, 10, 1399. [Google Scholar] [CrossRef]
- Operations, S.; Lng, W.B.; Vessels, F. Lng Bunkering: Addressing Simops Conducting Simultaneous Operations While Bunkering Lng Fueled Vessels. Available online: https://www.abs-group.com/content/documents/gated-resources/Conducting_Simultaneous_Operations_SIMOPS_while_Bunkering_LNG_Fueled_Vessels.pdf (accessed on 1 March 2023).
- Duijm, N.J.; Markert, F.; Paulsen, J.L. Safety Assessment of Ammonia as a Transport Fuel; National Laboratory for Sustainable Energy: Roskilde, Denmark, 2005; p. 4. [Google Scholar]
- Jeong, B.; Park, S.; Ha, S.; Lee, J.-U. Safety evaluation on LNG bunkering: To enhance practical establishment of safety zone. Ocean Eng. 2020, 216, 107804. [Google Scholar] [CrossRef]
- Dong, S.-E.; He, Y.; Dong, J.; Peng, Z.; Fu, G. A Review of Leakage and Dispersion of LNG on the Ground. Energy Eng. 2020, 118, 103–118. [Google Scholar] [CrossRef]
- Yong Ung, Y.; Sung Ho, P.; Dong Ho, J.; Chang Hee, L. Improving Liquefied Natural Gas Bunkering in Korea Through the Chinese and Japanese Experiences. Sustainability 2020, 12, 9585. [Google Scholar] [CrossRef]
- Sveistrup Jacobsen, D.M.; Krantz, R.; Mouftier, L.; Skov Christiansen, E. Ammonia as a Shipping Fuel. 2022, pp. 1–6. Available online: https://www.globalmaritimeforum.org/content/2022/03/Insight-brief_Ammonia-as-a-shipping-fuel.pdf (accessed on 20 March 2023).
- Raj, P.K. Models for cryogenic liquid spill behavior on land and water. J. Hazard. Mater. 1981, 5, 111–130. [Google Scholar] [CrossRef]
- DNV. Ammonia Bunkering of Passenger Vessel-Concept Quantitative Risk Assessment—Green Coastal Shipping Programme; DNV: Bærum, Norvegia, 2021; pp. 1–114. [Google Scholar]
- Chen, H.; Deal, L. Considerations for Proponents when Conducting QRA for LNG Bunkering SIMOPS; American Petroleum Institute: Washington, DC, USA, 2016. [Google Scholar]
- Park, S.; Jeong, B.; Yoon, J.Y.; Paik, J.K. A study on factors affecting the safety zone in ship-to-ship LNG bunkering. Ships Offshore Struct. 2018, 13, 312–321. [Google Scholar] [CrossRef]
- Jeong, B.; Lee, B.S.; Zhou, P.; Ha, S.-M. Evaluation of safety exclusion zone for LNG bunkering station on LNG-fuelled ships. J. Mar. Eng. Technol. 2017, 16, 121–144. [Google Scholar] [CrossRef]
- Fan, H.; Enshaei, H.; Jayasinghe, S.G.; Tan, S.H.; Zhang, C. Quantitative risk assessment for ammonia ship-to-ship bunkering based on Bayesian network. Process. Saf. Prog. 2022, 41, 395–410. [Google Scholar] [CrossRef]
- Yadav, A.; Jeong, B. Safety evaluation of using ammonia as marine fuel by analysing gas dispersion in a ship engine room using CFD. J. Int. Marit. Safety Environ. Aff. Shipp. 2022, 6, 99–116. [Google Scholar] [CrossRef]
- Rosa, A.C.; de Souza, I.T.; Terra, A.; Hammad, A.W.; Di Gregório, L.T.; Vazquez, E.; Haddad, A. Quantitative risk analysis applied to refrigeration’s industry using computational modeling. Results Eng. 2021, 9, 100202. [Google Scholar] [CrossRef]
- Pomonis, T.; Jeong, B. Engine room fre safety evaluation of ammonia as marine fuel. J. Int. Marit. Saf. Environ. Aff. Shipp. 2022, 6, 67–90. [Google Scholar]
- Orozco, J.; Van Caneghem, J.; Hens, L.; González, L.; Lugo, R.; Díaz, S.; Pedroso, I. Assessment of an ammonia incident in the industrial area of Matanzas. J. Clean. Prod. 2019, 222, 934–941. [Google Scholar] [CrossRef]
- Pandya, N.; Gabas, N.; Marsden, E. Sensitivity analysis of Phast’s atmospheric dispersion model for three toxic materials (nitric oxide, ammonia, chlorine). J. Loss Prev. Process. Ind. 2012, 25, 20–32. [Google Scholar] [CrossRef]
- Mastellone, M.L.; Ponte, M.; Arena, U. Design of mitigation systems for indoor and outdoor ammonia releases. J. Loss Prev. Process. Ind. 2003, 16, 93–101. [Google Scholar] [CrossRef]
- Salamonowicz, Z.; Majder-Lopatka, M.; Dmochowska, A.; Rogula-Kozlowska, W.; Piechota-Polanczyk, A.; Polanczyk, A. Ammonia Dispersion in the Closed Space of an Ammonia Engine Room with Forced Ventilation in an Industrial Plant. Atmosphere 2022, 13, 1062. [Google Scholar] [CrossRef]
- Galeev, A.; Salin, A.; Ponikarov, S. Consequence analysis of aqueous ammonia spill using computational fluid dynamics. J. Loss Prev. Process. Ind. 2013, 26, 628–638. [Google Scholar] [CrossRef]
- Salamonowicz, Z.; Makowski, R. Numerical modelling of dispersion of ammonia and chlorine in urban areas during emergency accident. E3S Web Conf. 2018, 44, 00159. [Google Scholar] [CrossRef]
- Zhang, Z.-C.; Liu, H.; Sun, S.-M. Analysis on Dispersion and Toxic Effect of Liquefied Ammonia Tank Release Based on PHAST. DEStech Trans. Environ. Energy Earth Sci. 2017, 1–7. [Google Scholar] [CrossRef]
- Jabbari, M.; Atabi, F.; Ghorbani, R. Key airborne concentrations of chemicals for emergency response planning in HAZMAT road transportation- margin of safety or survival. J. Loss Prev. Process. Ind. 2020, 65, 104139. [Google Scholar] [CrossRef]
- Bubbico, R.; Mazzarotta, B. Accidental release of toxic chemicals: Influence of the main input parameters on consequence calculation. J. Hazard. Mater. 2008, 151, 394–406. [Google Scholar] [CrossRef]
- Bondžić, J.; Sremački, M.; Popov, S.; Mihajlović, I.; Vujić, B.; Petrović, M. Exposure to hazmat road accidents—Toxic release simulation and GIS-based assessment method. J. Environ. Manag. 2021, 293, 112941. [Google Scholar] [CrossRef]
- Bouet, R.; Duplantier, S.; Salvi, O. Ammonia large scale atmospheric dispersion experiments in industrial configurations. J. Loss Prev. Process. Ind. 2005, 18, 512–519. [Google Scholar] [CrossRef]
- Tan, W.; Du, H.; Liu, L.; Su, T.; Liu, X. Experimental and numerical study of ammonia leakage and dispersion in a food factory. J. Loss Prev. Process. Ind. 2017, 47, 129–139. [Google Scholar] [CrossRef]
- Witlox, H.W.; Fernandez, M.; Harper, M.; Oke, A.; Stene, J.; Xu, Y. Verification and validation of Phast consequence models for accidental releases of toxic or flammable chemicals to the atmosphere. J. Loss Prev. Process. Ind. 2018, 55, 457–470. [Google Scholar] [CrossRef]
- Hanna, S.R.; Strimaitis, D.G.; Chang, J.C. Evaluation of fourteen hazardous gas models with ammonia and hydrogen fluoride field data. J. Hazard. Mater. 1991, 26, 127–158. [Google Scholar] [CrossRef]
- Bauer, T.J. Comparison of chlorine and ammonia concentration field trial data with calculated results from a Gaussian atmospheric transport and dispersion model. J. Hazard. Mater. 2013, 254-255, 325–335. [Google Scholar] [CrossRef]
- INERIS—Accident Risks Division. WORK STUDY Large-Scale Atmospheric Dispersion Tests; Technical Reports, No. INERIS-DRA-DRAG—2005-10072—RBo—Ammonia; INERIS—Accident Risks Division: Verneuil-en-Halatte, France, 2005. [Google Scholar]
- Weber, M. Some Safety Aspects on the Design of Sparger Systems for the oxidation of organic liquids. Process Saf. Prog. 2006, 25, 326–330. [Google Scholar] [CrossRef]
- Soltanzadeh, A.; Yarandi, M.S.; Mahdinia, M.; Barazandeh, J. Evaluation of the toxic effects of ammonia dispersion: Consequence analysis of ammonia leakage in an industrial slaughterhouse. Med Gas Res. 2021, 11, 24–29. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, J.; Hua, M.; Pan, X. Experimental research on decontamination effect of water curtain containing compound organic acids on the leakage of ammonia. Process. Saf. Environ. Prot. 2017, 105, 250–261. [Google Scholar] [CrossRef]
- Nielsen, M.; Ott, S.; Jørgensen, H.E.; Bengtsson, R.; Nyrén, K.; Winter, S.; Ride, D.; Jones, C. Field experiments with dispersion of pressure liquefied ammonia. J. Hazard. Mater. 1997, 56, 59–105. [Google Scholar] [CrossRef]
- Kim, I.-S.; Jeong, B.-U.; Song, M.-K.; Nam, D. Determination of bunkering safety zones for ammonia-fueled ship. J. Korean Soc. Mar. Eng. 2022, 46, 261–269. [Google Scholar] [CrossRef]
- Ng, C.K.L.; Liu, M.; Lam, J.S.L.; Yang, M. Accidental release of ammonia during ammonia bunkering: Dispersion behaviour under the influence of operational and weather conditions in Singapore. J. Hazard. Mater. 2023, 452, 131281. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).