Abstract
In this study, renewable and sustainable biofuel production from waste cooking oil and its blends with diesel fuel are investigated in terms of specific fuel properties. The fuel blends are named “Renewable Biofuel (RBF) 20” (20% biofuel–80% diesel), “Renewable Biofuel 50” (50% biofuel–50% diesel), and “Renewable Biofuel 100” (100% biofuel). The acid number, flash point, viscosity, cloud point, density, and pour point fuel properties of the new Renewable Biofuels are experimentally obtained and compared with diesel fuel. The viscosities of the biofuels are found to be 2.774 mm2/s for Renewable Biofuel 20, 3.091 mm2/s for Renewable Biofuel 50, and 4.540 mm2/s for Renewable Biofuel 100. Renewable Biofuel 20 has the minimum density value among biofuels. The density of Renewable Biofuel 20, Renewable Biofuel 50, and Renewable Biofuel 100 are obtained as 835 kg/m3, 846 kg/m3, and 884 kg/m3, respectively. More energy can be released with the use of Renewable Biofuel 100 in terms of heating value. The new fuel specification of biofuels can contribute to the fuel industry and help the studies on fuels for diesel engines.
1. Introduction
Energy has been used for industrialization, transportation, heating, etc. It is consumed every year due to the increase in excessive energy demand. In line with the increasing demand for energy supply, power demand is increasing day by day for developed and developing countries. Therefore, considering the insufficiency of energy resources, it is undoubtedly known that the efficient use of energy directly affects the future of those countries. In this context, the tendency towards alternative energy sources is increasing as a contribution to existing energy resources [1,2,3]. Renewable energy plays an important role in understanding the relationship between increased energy use and CO2. Increasing demand for renewable energy contributes to energy access and a secure energy supply. In addition, its effects on human health are too important to be ignored [4].
As the demand for energy supply increases with each passing year, the trend towards alternative raw materials for energy also increases. Rapid industrialization and population growth trigger an increase in energy demand. Considering these developments, energy production from plants is among the most important alternatives. However, the increase in waste generation is one of the major threats to be concerned about, as it has negative environmental impacts [5,6]. Although diesel fuels are widely used, their emission values still pose a threat to the environment. The activities of people, such as transportation related to the use of fossil fuels, cause an increase in the concentration of greenhouse gases and global warming. In this regard, considering the disadvantages of fossil fuels, it is said that the need for alternative and renewable fuels has gained more importance. Biofuel, an alternative to fossil fuel and one of the renewable fuels, is one of those alternatives. One of the most important factors in the use of biofuels as an alternative to fossil fuels is that they are produced from sustainable sources with appropriate chemical properties [7,8,9].
The use of biodiesel plays an important role in the energy sector. Biofuels accounted for 4.5% of the total energy supply, together with the use of road transport and non-road machinery in 2017. Biodiesel fuels are biofuels with the highest percentage of the total biofuel energy demand, making up about 62.3%. Percentages of biofuels other than biodiesel in the total energy supply are as follows: 17.5% bioethanol, 1.7% upgraded biogas, and 1.1% bio-Ethyl-Tertiary-Butyl Ether [10].
Among the reasons why biofuels are preferred is that carbon monoxide, particulate matter, and unburned hydrocarbon emissions, which are the most harmful in fossil fuels, are reduced. Fuels in which animal fats and vegetable oils are synthesized by transesterification with monohydric alcohols (methanol-CH3OH, ethanol-C2H5OH) are biofuels. Chemically, catalysts such as potassium hydroxide (KOH) and sodium hydroxide (NOH) are generally used during biofuel production in the laboratory [11].
One of the main sources from which biofuel is produced is waste edible oils. Cooking oil produced from plants is the main source of edible oils, and it can be used in biofuel production in order to benefit from the waste of cooking oils used in kitchens [12]. The focus is on the use of waste cooking oil for biofuel production [13]. In some countries and regions, waste oil production is carried out by the state administration with legal regulations. Waste cooking oil collection and recycling studies facilitate the use of waste cooking oil as a fuel source. Between 0.1 and 0.5 million tons of waste cooking oil is produced annually in Japan. The recycling model of waste cooking oil in Japan strengthens price competition thanks to the convenience provided to biofuel enterprises. Therefore, this model helps to prevent illegal producers, reduce the production costs of biofuel enterprises, and increase the recovery rate of waste cooking oil even more [14].
Biodiesel production from waste cooking oil is a very important energy loss control mechanism that can strengthen energy security, as well as minimize environmental pollution and waste, and promote a circular economy. Many developed countries have legalized the production of biofuels from waste cooking oil and encouraged the shift to alternative energy sources [15].
Chai et al. [16] studied the importance of wastewater treatment. In this context, it was concluded that the role of algae was important for its environmental contributions. According to the results of the study, information was given about the purification of heavy metals thanks to microalgae and, thus, the cleaning of wastewater. With the use of microalgae, it was concluded that no extra energy was required, as it prevents secondary pollution during the conversion of water and carbon dioxide into organic compounds. Cong et al. [17] studied the synthesis of biofuels from waste oils. Waste oils were alkalized with sodium hydroxide in a one-step process, without pre-treatment of the used bleaching clay ash at low temperatures alone. According to study results, a solid base was produced by alkalization of bleaching clay ash with a high acid value of 9.71 mg KOH/g without pre-treatment, with a biofuel yield of 95.8% at just 65 °C.
In the study by Janbarari and Ahmadian Behrooz [18], waste cooking oil was used as a raw material for biofuel. As a result of a transesterification reaction with sodium hydroxide, the waste oil was converted into biofuel. The optimization problems with more than one uncertain parameter were seen. Arcigni et al. [19] studied the assessment of bio-energy potential. In addition, energy consumption was also taken into account. All stages of biodiesel production, including plant cultivation required for biodiesel production and biodiesel production, were discussed. Results were analyzed in terms of energy consumption. Khounani et al. [20] studied the potential of walnut shell extract for the biofuel production process. Waste cooking oil biofuel augmented by walnut husk methanolic extract was more environmentally advantageous than waste cooking oil biofuel augmented by propyl gallate in all the investigated damage categories. The results revealed that the use of the resulting biofuel had fewer negative impacts on human health by 12.13%, on ecosystem quality by 0.32%, and on climate change by 8.37%.
O’Connell et al. [21] conducted a study on the effects of aviation biofuel alternatives planned for future use to attain better greenhouse gas emissions and energy balances. In addition to examining the greenhouse gas emissions, energy efficiency results were also discussed. It was concluded that second-generation biofuels showed promising results for better greenhouse gas emission reduction, although their overall energy efficiency performances were lower. Sharma et al. [22] studied biodiesel production from microalgae for energy security. Biodiesel fuel was used as a non-toxic and biodegradable alternative fuel. Microalgae biofuels, which were distinguished by the presence of carbohydrates, some nutrients, large fat content, and proteins, were identified as resources that did not damage other crops. Noorollahi et al. [23] studied energy self-sufficiency and considered biofuels in the agricultural sector. The investigation was conducted on energy potential and demand on the local scale. By comparing the energy consumption in the agricultural sector with bioenergy production potential, it was determined that the potentials in different provinces were different.
In the current study, the biofuel source obtained from waste cooking oils was converted by the authors into biofuel using chemical processes in the laboratory. The specifications of the new biofuel samples obtained by the authors using chemical processes are also determined in the laboratory for each fuel blend. In terms of the methods used and the production of specific fuels, the present study is an original research study compared to other studies in the literature. All processes were applied by the authors, from the collection of waste cooking oil to the ascertainment of the properties of the fuels. The renewable and sustainable biofuels were produced from waste cooking oil, and their blends with diesel fuel were examined. The main aim of this study was to obtain renewable and sustainable biofuel in different specifications, as well as to evaluate the fuel properties (such as density of fuels, the viscosity of fuels, etc.) of the biofuel and its blends as new energy sources for a sustainable energy supply. In addition, it aimed to contribute to energy recovery. This study is also original due to its new biofuel production with a new acid number, kinematic viscosity, density, flash point, cloud point, and pour point that add new data to the fuel literature. It can be said that the production and use of new alternative biofuels will play a significant role in meeting energy demand and in the recovery of waste energy.
2. Methodology
The new waste cooking-oil-based biofuels (fatty acid methyl esters) and their blends with diesel fuel are studied in this present study. New Renewable Biofuels (RBF) are named according to their biofuel percent by mass content. The biofuel blend contents are given in Table 1.

Table 1.
Biofuel blend contents [24].
The specifications of fuels are determined by experimental methods in the Pollars Laboratory (Japan). The general view of the sustainable biofuel production process is shown in Figure 1. The specifications and characteristics of the fuels are determined as follows:
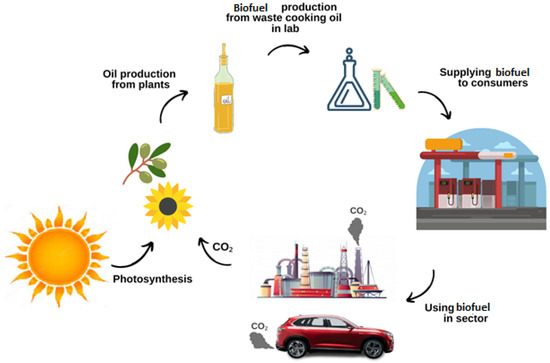
Figure 1.
General view of the sustainable biofuel production process.
2.1. Acid Number
The mass of potassium hydroxide (KOH) in milligrams that is required to neutralize one gram of chemical substance is called the “acid number”. The acid number is expressed as a measurement of the number of carboxylic acid groups, such as fatty acids [25].
2.2. Viscosity
The resistance to deformation caused by shear or tensile stress can be expressed as the viscosity of a fluid. The stress required for the model that occurs in the deformation is directly related to the viscosity of the fluid. Viscosity control in fuels is possible by controlling the temperature and viscosity of the fluid to control combustion in some vehicles (such as diesel engines and generators). Temperature and viscosity are inversely proportional to each other. As the temperature value increases, the viscosity value decreases [26].
2.3. Flash Point
The flash point method is used to separate flammable liquids, such as petrol, from each other. The flash point method is also used to determine the fire potential of flammable liquids. Although it varies according to the standard used, liquids with a flash point above 37.8 °C or 60.5 °C are called combustible, while liquids below this temperature are known as flammable. Determination of the percentage content of the flammable liquid in the air is provided using temperature. Therefore, the content of flammable liquid vapor in the air is different for each flammable liquid. The lowest temperature at which there will be enough flammable vapor to ignite when an ignition source is applied is called the flash point of a flammable liquid [27].
2.4. Cloud Point and Pour Point
If a liquid is cooled, the temperature at which the wax crystal cloud first forms is the cloud point of the liquid. That is why the cloud point plays an important role in determining the temperature up to which a liquid can be used in cold weather conditions. Since solidified wax clogs fuel filters and injectors and thickens the fuel, cloud point detection is an important method to obtain information to prevent such harmful situations for engines. It is a feature that is of great importance in order to avoid this negative situation in cold weather. In addition to the cloud point, the pour point is the lowest temperature at which fuel can be easily pumped and oil movement can be observed. Therefore, the pour point can be expressed as the exact opposite of the cloud point [28].
2.5. Density
The ratio of the unit mass of a substance to its unit volume defines its density. The high density of the fuel also increases the pumping and storage of the fuel. Increasing the molecular weight of the component atoms of the fuel molecules causes the fuel density to increase [29].
3. Results and Discussion
In the present study, waste cooking oil-based Renewable Biofuel 20 (RBF20), Renewable Biofuel 50 (RBF50), and Renewable Biofuel 100 (RBF100) fuels and diesel were examined in terms of fuel properties. RBF20 fuel contains 20% biodiesel and 80% diesel fuel. Further, Renewable Biofuel 50 fuel is called “RBF50” because it contains 50% biodiesel and 50% diesel fuel. However, RBF100 fuel contains 100% biodiesel [30]. In addition, the properties of the fuels were determined using an experimental study method. These results are new to the fuel literature as they contain specific properties. The obtained specifications of the new fuels (RBF20, RBF50, and RBF100) and diesel fuel are given in Table 2.

Table 2.
Obtained specifications of the new biofuels and diesel fuel [24].
The acid numbers of the RBF20, RBF50, and RBF100 biofuels were obtained as 0.146 mgKOH/g, 0.181 mgKOH/g, and 0.241 mgKOH/g, respectively. The acid number of diesel fuel was determined to be 0.048 mgKOH/g. Diesel fuel had the minimum value, while RBF100 fuel had the maximum rate in terms of the acid number of the fuels. The order of acid numbers of fuels was: RBF100 > RBF50 > RBF20 > diesel fuel.
RBF100 was found to have the maximum flash point, while RBF20 fuel was found to have the minimum value. The flash points of the biofuels were found to be 70 °C for RBF20 fuel, 75 °C for RBF50 fuel, and 166 °C for RBF100 fuel. However, the flash point was determined to be 72 °C for diesel fuel. The order of flash point of fuels is RBF100 > RBF50 > diesel fuel > RBF20.
The kinematic viscosities of the biofuels were found to be 2.774 mm2/s for RBF20, 3.091 mm2/s for RBF50, and 4.54 mm2/s for RBF100. However, the kinematic viscosity was 2.537 mm2/s for diesel fuel. When the kinematic viscosities of fuels were examined, the order of kinematic viscosities of fuels was: RBF100 > RBF50 > RBF20 > diesel fuel. According to this result, the maximum kinematic viscosity was found in RBF100. Additionally, the minimum rate was found in the diesel fuel.
The cloud points of the RBF20, RBF50, and RBF100 fuels were obtained as 3 °C, 3 °C, and 8.5 °C, respectively. However, the cloud point was −1 °C for diesel fuel. The order of cloud point of fuels was: RBF100 > RBF50 ≈ RBF20 > diesel fuel.
The density of the RBF20, RBF50, and RBF100 fuels were obtained as 835 kg/m3, 846 kg/m3, and 884 kg/m3, respectively. Considering the densities of biofuels, RBF20 had the lowest density value. However, RBF100 had the highest value. In addition, considering all of the fuels examined, the diesel fuel had the lowest density value in this study. The density of diesel fuel was found to be 823 kg/m3. The order of density of fuels was: RBF100 > RBF50 > RBF20 > diesel fuel.
RBF100 obtained the maximum pour point. However, diesel fuel obtained the minimum pour point. The pour points of the biofuels were found to be −7.5 °C for RBF100, −15.1 °C for RBF50, and −17 °C for RBF100. However, the pour point of diesel fuel was −18.2 °C. The order of pour point of fuels was: RBF100 > RBF50 > RBF20 > diesel fuel.
As the mass percentage of the cooking-oil-based biofuel increased in the fuel blends, higher acid number values were obtained. In addition, diesel fuel had the lowest value when the acid number of diesel fuel was compared to the acid number of all the biofuels. On the other hand, when the kinematic viscosities of the fuels were examined, it was determined that the viscosity of diesel fuel was the minimum value. The kinematic viscosity reduction of the fuel mixture was due to the increase in the percentage content of diesel in the fuel blend. When the densities of fuels were examined, all the Renewable Biofuels had higher densities than diesel fuel. RBF20 had the lowest density value among the fuel blends, while RBF100 had the highest density rate. In this context, these results may help the researchers to determine a more accurate working method for diesel engines and fuels.
The evaluation of fuels can be investigated in detail by using biofuels in various energy systems (e.g., internal combustion engines). The amount of energy potential can be obtained by realizing the combustion of fuels in a system. Since the fuel gives its energy content, heating value is a very important feature for fuels. The heating value is directly related to the energy potential of the fuel and can give an idea of the analysis results [31]. The current study is compared with other studies in the literature in Table 3. Ong et al. [32] studied fuel containing 20% Cerbera Manghas Biodiesel and fuel containing 50% Cerbera Manghas Biodiesel, in contrast to the current research study, which used RBF20 fuel containing 20% biofuel and RBF50 fuel containing 50% biofuel. If an evaluation is made among these fuels, the acid number value of Cerbera Manghas Biodiesel 20 fuel was 0.05 mgKOH/g. However, the acid number value of the RBF20 fuel in the current study was obtained as 0.146 mgKOH/g. The flash point, cloud point, and pour point values of Cerbera Manghas Biodiesel 20 fuel were determined as 82.5 °C, −1 °C, and 1.5 °C, respectively. Moreover, the flash point, cloud point, and pour point values of RBF20 fuel were determined in the present study to be 70 °C, 3 °C, and −17 °C, respectively. Further, the flash point, cloud point, and pour point values of Cerbera Manghas Biodiesel 50 fuel were determined to be 87.5 °C, 3.8 °C, and 5.2 °C, respectively. However, the flash point, cloud point, and pour point values of RBF50 fuel were 75 °C, 3 °C, and −15.1 °C, respectively, in the present study. On the other hand, Cerbera Manghas Biodiesel 20 fuel had a viscosity of 3.57 mm2/s and a density of 823.1 kg/m3. However, the viscosity of RBF20 was found to be 2.77 mm2/s, and the density of RBF20 was determined to be 835 kg/m3. The acid number of the diesel fuel of Ong et al. [32] had a minimum rate of 0.01 mgKOH/g compared to the current study (Japanese Diesel No.2: 0.048 mgKOH/g). The acid numbers of biofuels were found to be higher than diesel fuels in the studies.

Table 3.
Comparison of the present study with other studies in the literature.
As in the current study, Mori et al. [33] examined 20% biodiesel, 50% biodiesel, 100% biodiesel, and diesel fuels. The acid number values of 20% biodiesel, 50% biodiesel, 100% biodiesel, and diesel fuel were 0.115 mgKOH/g, 0.127 mgKOH/g, 0.255 mgKOH/g, and 0.082 mgKOH/g, respectively. In the current study, the acid number of RBF20 was 0.146 mgKOH/g, the acid number of RBF50 was 0.181 mgKOH/g, the acid number of RBF100 was 0.241 mgKOH/g, and the acid number of diesel fuel was 0.048 mgKOH/g. On the other hand, the flash point, cloud point, and pour point values of 20% biodiesel fuel were 74 °C, 1 °C, and −15 °C, respectively. However, the flash point, cloud point, and pour point values of RBF20 were calculated to be 70 °C, 3 °C, and −17 °C, respectively, in the current study. Similarly, the flash point, cloud point, and pour point values of 50% biodiesel fuel were found to be 80 °C, 1 °C, and −12.5 °C, respectively. In contrast, the flash point, cloud point, and pour point values of RBF50 were defined as 75 °C, 3 °C, and −15.1 °C, respectively. While the flash point was 178 °C, the cloud point was 6 °C, and the pour point was −5 °C for 100% biodiesel fuel, the flash point was 166 °C, the cloud point was 8.5 °C, and the pour point was −7.5 °C for RBF100. In addition, the flash point, cloud point, and pour point values of diesel fuel were found to be 70 °C, −5 °C, and −20 °C, respectively, in the study of Mori et al. [33]. The flash point, cloud point, and pour point values of diesel fuel were calculated to be 72 °C, −1 °C, and −18.2 °C, respectively, in this study. Moreover, the viscosity and density values were 2.43 mm2/s and 826 kg/m3 for 20% biodiesel, respectively. The viscosity of 50% biodiesel and 100% biodiesel were 2.99 mm2/s and 4.25 mm2/s, respectively. However, the density of 50% biodiesel and 100% biodiesel were 885 kg/m3 and 849 kg/m3, respectively. Further, the viscosity was 2.23 mm2/s, and the density was 817 kg/m3 for diesel fuel. In the present study, the viscosity and density values were found to be 2.77 mm2/s and 835 kg/m3, respectively, for RBF20. Moreover, the viscosity was defined as 3.09 mm2/s, and the density was found to be 846 kg/m3 for RBF50. However, the viscosity and density values were 4.54 mm2/s and 884 kg/m3, respectively, for RBF100. Also, the viscosity was obtained as 2.54 mm2/s, and the density was calculated as 823 kg/m3 for diesel fuel. The maximum acid number of biofuel was found in 100% biodiesel fuel as 0.255 mgKOH/g in Mori et al. [33]. Since biofuels are produced from vegetable and animal oils, they have fatty acids, and therefore, their acid numbers are higher than diesel fuels. In addition, the maximum flash point was determined to be 178 °C for 100% biodiesel fuel by Mori et al. [33], while the maximum flash point in the present study was found to be 166 °C for RBF100. When the flash points of the studies in the literature were evaluated, the maximum flash point was determined in the 100% biodiesel fuel of Mori et al. [33] to be 178 °C. It is seen that the flash point of diesel fuel is lower than that of the biofuels. In the present study, the flash point of RBF100 was calculated as 166 °C. As the percentage of biofuel in the fuel blend increased, the flash point also increased. This result indicates that, in the present study, as the percentage of biofuel, which is a denser fuel, increased in the fuel mixture, the flash point reached higher rates.
Baldwin et al. [34] evaluated the B100 fuel (which is a biofuel with 100% content) and Diesel #2 fuel. These fuels are comparable to the fuels in the current study. While the flash point of B100 fuel was determined to be about 93 °C, the flash point of Diesel #2 fuel was found to be about 52 °C. However, in the present study, the flash point of RBF100 fuel was 166 °C, and the flash point of diesel fuel was 72 °C. Baldwin et al. [34] obtained the flash point of Difusel Carbonate Biodiesel as 176.9 °C, and this result is relatively high among the studies assessed here.
Brock et al. [35] studied the properties of diesel fuel. The cloud point of diesel fuel was found to be −10 °C. Moreover, the viscosity and density values were 3.80 mm2/s and 840 kg/m3, respectively. However, the cloud point of diesel fuel was determined to be −1 °C in the current study. Further, the viscosity was 2.54 mm2/s, and the density was 823 kg/m3 for diesel fuel. When the kinematic viscosities of fuels were examined, the maximum kinematic viscosity of fuel was found to be 7.02 mm2/s for the Tributyrin biofuel of Brock et al. [35], while the minimum kinematic viscosity was obtained as 0.91 mm2/s for the 2-methylbutyl ether biodiesel fuel of Baldwin et al. [35]. However, the viscosity of RBF100 was obtained as 4.540 mm2/s in the present study. In addition, when the viscosity values of the current study were examined, it was found that the viscosity increased as the percentage of biofuel in the fuel mixture increased. In the present study, the diesel fuel was more advantages than derived biofuels in terms of kinematic viscosity because the fuel viscosity directly affected the heating value (energy) of the fuel that is used for diesel engines.
Devarajan et al. [36] examined diesel fuel and 50% biodiesel in their research study. While the flash point of diesel fuel was determined to be 75 °C, the flash point of 50% biodiesel fuel was calculated to be 94 °C. However, the flash point of diesel fuel was 72 °C, and the flash point of RBF50 was 166 °C in the present study. Moreover, the viscosity and the density values were found to be 3.40 mm2/s and 840 kg/m3, respectively, for diesel fuel by Devarajan et al. [36]. However, the viscosity and the density values were determined as 2.54 mm2/s and 823 kg/m3, respectively, for diesel fuel in the current study. In addition, the viscosity of 50% biodiesel was 3.90 mm2/s, and the density of 50% biodiesel was 855 kg/m3 in the study of Devarajan et al. [36]. However, the viscosity and the density values were calculated to be 4.54 mm2/s and 884 kg/m3, respectively, for RBF50 in this study.
Considering the current study and the reviewed literature studies, the fuel with the highest density is Difusel Carbonate Biodiesel fuel with a density value of 910 kg/m3 in the study of Baldwin et al. [30,34]. When the general density values of the fuels are examined, the density value of Diesel Carbonate Biodiesel fuel is significantly higher than other fuels. If the fuel densities in the current study are examined, the highest fuel density was obtained for RBF100, while the minimum fuel density was found for the diesel fuel. Fuel density is directly related to the energy of the fuel in terms of heating value. Further, emissions are affected since there is a rich mixture in blends [37].
4. Conclusions
In this study, the specific fuel properties of diesel fuel, waste cooking-oil-based Renewable Biofuel (100% biofuel), and their blends of RBF20 and RBF50 were examined. The specifications of fuels changed according to the different mass percentages in the blends. The production of the Renewable Biofuels and their blends were explained experimentally, and Renewable Biofuel/blends were compared with diesel fuel in terms of fuel specifications that are new in the literature. Among the fuels, considering the acid number data, the diesel fuel had the minimum rate. However, the RBF100 had the maximum value. In addition, the minimum kinematic viscosity was found in diesel fuel, while the maximum kinematic viscosity was obtained in RBF100. The viscosity of RBF100 was higher than the values of other obtained fuels. When all the fuels were examined, diesel fuel had the lowest density value. However, RBF20 had the minimum density rate among biofuels. The minimum flash point value was obtained for RBF20 among all fuels tested here. The cloud point of diesel fuel was calculated as the minimum value, while the cloud point of RBF100 was found to be the maximum value in terms of all fuels used here. The pour point of RBF100 is obtained as the maximum, while the minimum pour point is found in the diesel fuel. In fuel blends obtained as a mixture of biofuel and diesel, the values obtained were generally lower as the mass percentage of diesel fuel increased because as the mass percentage of the diesel fuel decreased, the mass percentage of the biofuel increased, and the fuel blend turned into a denser fuel.
Waste cooking oils emerge as a degraded raw material as a result of chemical and physical reactions. Therefore, considering the exhaust emissions and engine performance of biofuels produced from waste cooking oils, the quality of the produced biodiesel is of great importance. In this context, by examining the specific fuel properties of the obtained biofuel blend in detail, significant findings can be determined about the quality of the produced biodiesel. As a simple example, it is inevitable that high-density biofuel will reduce the operating efficiency of the engine, cause damage to the engine, and worsen the exhaust emission values. Since this will negatively affect the viscosity, the quality of the fuel is related to the fuel properties. Because the quality of the obtained biofuel is one of the most important factors in terms of its practical use, it is desirable that the obtained biofuel does not harm the engine system and is environmentally preferable. The characteristic results of the biofuels that were obtained from this study can contribute to the fuel industry and help the studies on fuels for diesel engines.
Author Contributions
Conceptualization, H.C. and I.Y.; Methodology, H.C., I.Y. and K.M.; Validation, H.C., I.Y. and K.M.; Formal analysis, H.C., I.Y. and K.M.; Investigation, H.C., I.Y. and K.M.; Resources, H.C., I.Y. and K.M.; Data curation, H.C., I.Y. and K.M.; Writing—original draft, H.C. and I.Y.; Writing—review & editing, H.C. and I.Y.; Visualization, H.C. and I.Y.; Supervision, H.C. and K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
Ibrahim Yildiz, one of the authors of the present paper, is supported under the 100/2000 Council of Higher Education (YÖK) Ph.D. Scholarship Program by YÖK and The Scientific and Technological Research Council of Turkey (TÜBİTAK) 2211/C National Ph.D. Scholarship Program in the Priority Fields in Science and Technology by TÜBİTAK.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| °C | Degrees Centigrade |
| KOH | Potassium hydroxide |
| m3 | Cubic meter |
| mm2 | Square millimeter |
| NaOH | Sodium hydroxide |
Abbreviations
| kg | Kilogram |
| MJ | Megajoule |
| RBF | Renewable Biofuel |
| s | Second |
References
- Yildiz, I.; Caliskan, H.; Mori, K. Effects of Cordierite Particulate Filters on Diesel Engine Exhaust Emissions in Terms of Pollution Prevention Approaches for Better Environmental Management. J. Environ. Manag. 2021, 293, 112873. [Google Scholar] [CrossRef]
- Kurańska, M.; Malewska, E. Waste cooking oil as starting resource to produce bio-polyol—Analysis of transesteryfication process using gel permeation chromatography. Ind. Crops Prod. 2021, 162, 113294. [Google Scholar] [CrossRef]
- Balli, O.; Caliskan, N.; Caliskan, H. Aviation, energy, exergy, sustainability, exergoenvironmental and thermoeconomic analyses of a turbojet engine fueled with jet fuel and biofuel used on a pilot trainer aircraft. Energy 2023, 263, 126022. [Google Scholar] [CrossRef]
- Agostini, A.; Serra, P.; Giuntoli, J.; Martani, E.; Ferrarini, A.; Amaducci, S. Biofuels from perennial energy crops on buffer strips: A win-win strategy. J. Clean. Prod. 2021, 297, 126703. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef]
- Yildiz, I.; Caliskan, H. Energetic and Exergetic Carbon Dioxide Equivalents and Prices of the Energy Sources for Buildings in Turkey. Environ. Prog. Sustain. Energy 2018, 37, 912–925. [Google Scholar] [CrossRef]
- Watts, N.; Amann, M.; Arnell, N.; Ayeb-Karlsson, S.; Belesova, K.; Berry, H.; Bouley, T.; Boykoff, M.; Byass, P.; Cai, W.; et al. The 2018 report of the Lancet Countdown on health and climate change: Shaping the health of nations for centuries to come. Lancet 2018, 392, 2479–2514. [Google Scholar] [CrossRef]
- Yildiz, I.; Caliskan, H.; Mori, K. Thermodynamic Analysis and Nanoparticle Assessment of Japanese Diesel No 2 Fueled Truck Engine. Energy Procedia 2018, 144, 104–110. [Google Scholar] [CrossRef]
- Balli, O.; Caliskan, H. Energy, Exergy, Environmental and Sustainability Assessments of Jet and Hydrogen Fueled Military Turbojet Engine. Int. J. Hydrog. Energy 2022, 47, 26728–26745. [Google Scholar] [CrossRef]
- Puricelli, S.; Cardellini, G.; Casadei, S.; Faedo, D.; van den Oever, A.E.M.; Grosso, M. A review on biofuels for light-duty vehicles in Europe, Renew. Sustain. Energy Rev. 2021, 137, 110398. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Tabatabaei, M.; Khalife, E.; Shojaei, T.R.; Dadak, A. Exergoeconomic analysis of a DI diesel engine fueled with diesel/biodiesel (B5) emulsions containing aqueous nano cerium oxide. Energy 2018, 149, 967–978. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Aghbashlo, M.; Dehhaghi, M.; Panahi, H.K.S.; Mollahosseini, A.; Hosseini, M.; Soufiyan, M.M. Reactor technologies for biodiesel production and processing: A review. Prog. Energy Combust. Sci. 2019, 74, 239–303. [Google Scholar] [CrossRef]
- Cvengroš, J.; Cvengrošová, Z. Used frying oils and fats and their utilization in the production of methyl esters of higher fatty acids. Biomass Bioenergy 2004, 27, 173–181. [Google Scholar] [CrossRef]
- Teixeira, M.R.; Nogueira, R.; Nunes, L.M. Quantitative assessment of the valorisation of used cooking oils in 23 countries. Waste Manag. 2018, 78, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Tsoutsos, T.; Tournaki, S.; Paraíba, O.; Kaminaris, S. The Used Cooking Oil-to-biodiesel chain in Europe assessment of best practices and environmental performance. Renew. Sustain. Energy Rev. 2016, 54, 74–83. [Google Scholar] [CrossRef]
- Chai, W.S.; Tan, W.G.; Halimatul Munawaroh, H.S.; Gupta, V.K.; Ho, S.-H.; Show, P.L. Multifaceted roles of microalgae in the application of wastewater biotreatment: A review. Environ. Pollut. 2021, 269, 116236. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.-J.; Wang, Y.-T.; Li, H.; Fang, Z.; Sun, J.; Liu, H.-T.; Liu, J.-T.; Tang, S.; Xu, L. Direct production of biodiesel from waste oils with a strong solid base from alkalized industrial clay ash. Appl. Energy 2020, 264, 114735. [Google Scholar] [CrossRef]
- SJanbarari, S.R.; Behrooz, H.A. Optimal and robust synthesis of the biodiesel production process using waste cooking oil from different feedstocks. Energy 2020, 198, 117251. [Google Scholar] [CrossRef]
- Arcigni, F.; Friso, R.; Collu, M.; Venturini, M. Venturini, Harmonized and systematic assessment of microalgae energy potential for biodiesel production. Renew. Sustain. Energy Rev. 2019, 101, 614–624. [Google Scholar] [CrossRef]
- Khounani, Z.; Hosseinzadeh-Bandbafha, H.; Nizami, A.-S.; Sulaiman, A.; Goli, S.A.H.; Tavassoli-Kafrani, E.; Ghaffari, A.; Rajaeifar, M.A.; Kim, K.-H.; Talebi, A.F.; et al. Unlocking the potential of walnut husk extract in the production of waste cooking oil-based biodiesel, Renew. Sustain. Energy Rev. 2020, 119, 109588. [Google Scholar] [CrossRef]
- O’Connell, A.; Kousoulidou, M.; Lonza, L.; Weindorf, W. Considerations on GHG emissions and energy balances of promising aviation biofuel pathways, Renew. Sustain. Energy Rev. 2019, 101, 504–515. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, V. Microalgal biodiesel: A possible solution for India’s energy security. Renew. Sustain. Energy Rev. 2017, 67, 72–88. [Google Scholar] [CrossRef]
- Noorollahi, Y.; Janalizadeh, H.; Yousefi, H.; Jahangir, M.H. Biofuel for energy self-sufficiency in agricultural sector of Iran. Sustain. Energy Technol. Assess. 2021, 44, 101069. [Google Scholar] [CrossRef]
- Caliskan, H.; Mori, K. Thermodynamic, environmental and economic effects of diesel and biodiesel fuels on exhaust emissions and nano-particles of a diesel engine. Transp. Res. Part D Transp. Environ. 2017, 56, 203–221. [Google Scholar] [CrossRef]
- Nwaokobia, K.; Idibie, C.A.; Okolie, P.L. Effects of Extraction Solvents on the Yield and Physicochemical Properties of Mangifera indica L. Seed Oil, Dev Sanskriti Interdiscip. Int. J. 2018, 12, 43–51. [Google Scholar] [CrossRef][Green Version]
- Çengel, Y.A.; Cimbala, J.M. Chapter Two: Properties of Fluids. In Fluid Mechanics: Fundamentals and Applications; McGraw-Hill Education, The McGraw-Hill Companies, Inc., 1221 Avenue of the Americas: New York, NY, USA, 2006; pp. 46–51. ISBN 9780071257640. [Google Scholar]
- Arunachaleswara, P.R.; Kanna, R.; Nair, S.G.; Francis, C.; Bhaktan, N.; Kuruvila, A. Effect of Free Water and Rust on Flash Point of Diesel. Int. J. Eng. Sci. 2017, 7, 21–24. [Google Scholar]
- Ghobadian, B.; Rahimi, H.; Tavakouli, H.T.; Khatami, F.M. Production of Bioethanol and Sunflower Methyl Ester and Investigation of Fuel Blend Properties. J. Agric. Sci. Technol. 2008, 10, 1–8. [Google Scholar]
- Axion, Space, Fuel, Density. 2016. Available online: http://members.axion.net/~enrique/space_fueldensity.html (accessed on 25 July 2017).
- Yildiz, I.; Caliskan, H.; Mori, K. Assessment of biofuels from waste cooking oils for diesel engines in terms of waste-to-energy perspectives. Sustain. Energy Technol. Assess. 2022, 50, 101839. [Google Scholar] [CrossRef]
- Lois, E.; Keating, E.; Gupta, A. Fuels. In Encyclopedia of Physical Science and Technology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 275–314. [Google Scholar] [CrossRef]
- Ong, H.C.; Silitonga, A.; Mahlia, T.; Masjuki, H.; Chong, W. Investigation of Biodiesel Production from Cerbera Manghas Biofuel Sources. Energy Procedia 2014, 61, 436–439. [Google Scholar] [CrossRef]
- Mori, K.; Sorimachi, K.; Eguchi, K.; Kawase, J.; Suzuki, R. Study for Effects of Bio-Diesel Fuel and Engine Oil on Exhaust Emission and PN of Diesel Engine; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2015. [Google Scholar] [CrossRef]
- Baldwin, L.C.; Davis, M.C.; Woodroffe, J. Potential oxygenated biofuels synthesized from fusel pentanols. Fuel 2020, 270, 117505. [Google Scholar] [CrossRef]
- Brock, D.; Koder, A.; Rabl, H.-P.; Touraud, D.; Kunz, W. Optimising the biodiesel production process: Implementation of glycerol derivatives into biofuel formulations and their potential to form hydrofuels. Fuel 2020, 264, 116695. [Google Scholar] [CrossRef]
- Devarajan, Y.; Beemkumar, N.; Ganesan, S.; Arunkumar, T. An experimental study on the influence of an oxygenated additive in diesel engine fuelled with neat papaya seed biodiesel/diesel blends. Fuel 2020, 268, 117254. [Google Scholar] [CrossRef]
- Çukurova Üniversitesi, YAKIT ÖZELLİKLERİ TESTİ 2, Adana, TURKEY, 2019. Available online: https://autoeng.cu.edu.tr/storage/LabFöyler/Türkçe/E-YakıtÖzellikleriDeneyi(2019-2020).pdf (accessed on 27 December 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).