Analyses of Interpolant Ion Effects on Smart Water Core Flooding in Carbonate
Abstract
1. Introduction
2. Methodology
2.1. Core Flooding Modeling
2.2. Simulated Scenarios
2.3. Smart Water Mechanisms
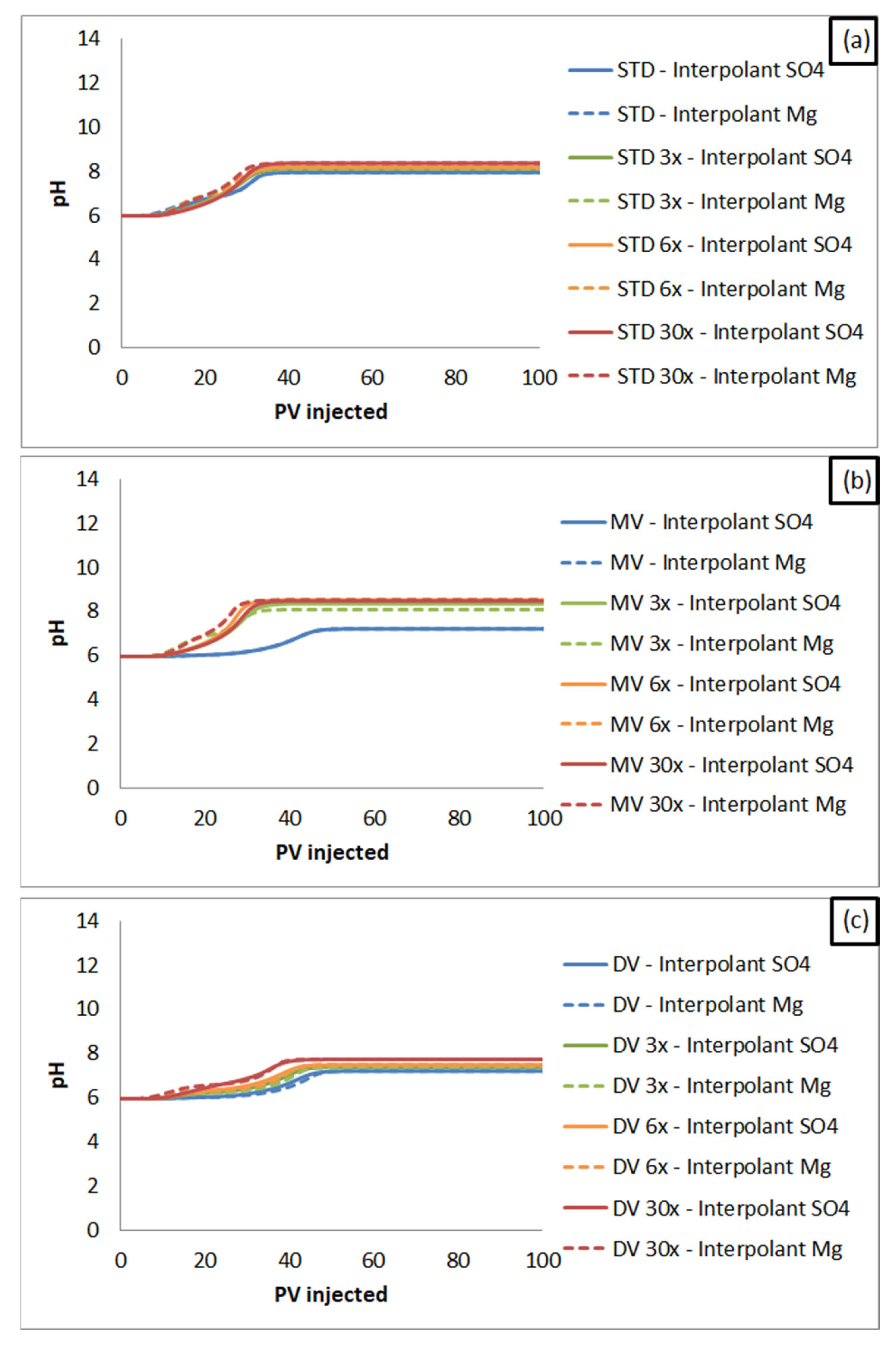
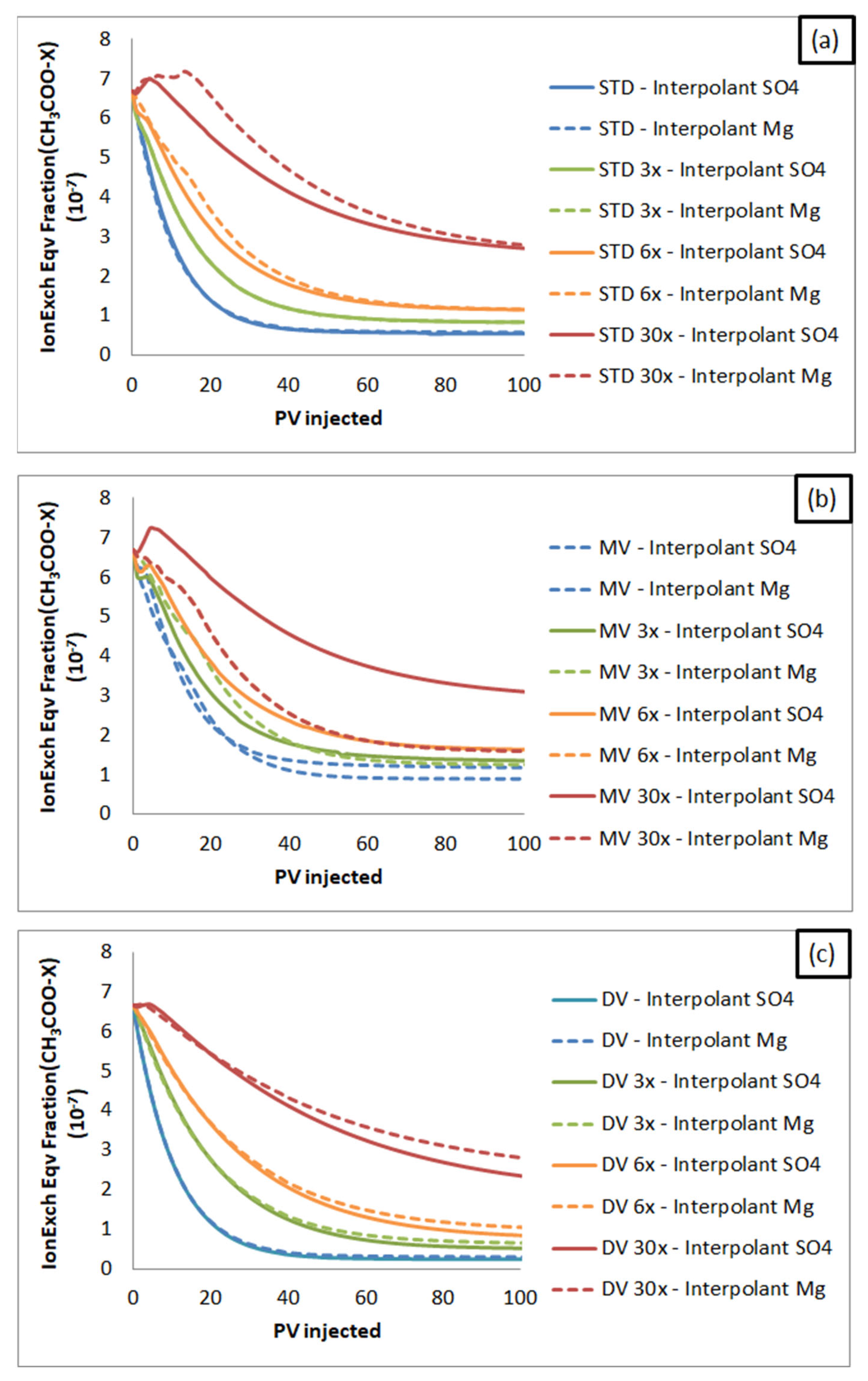
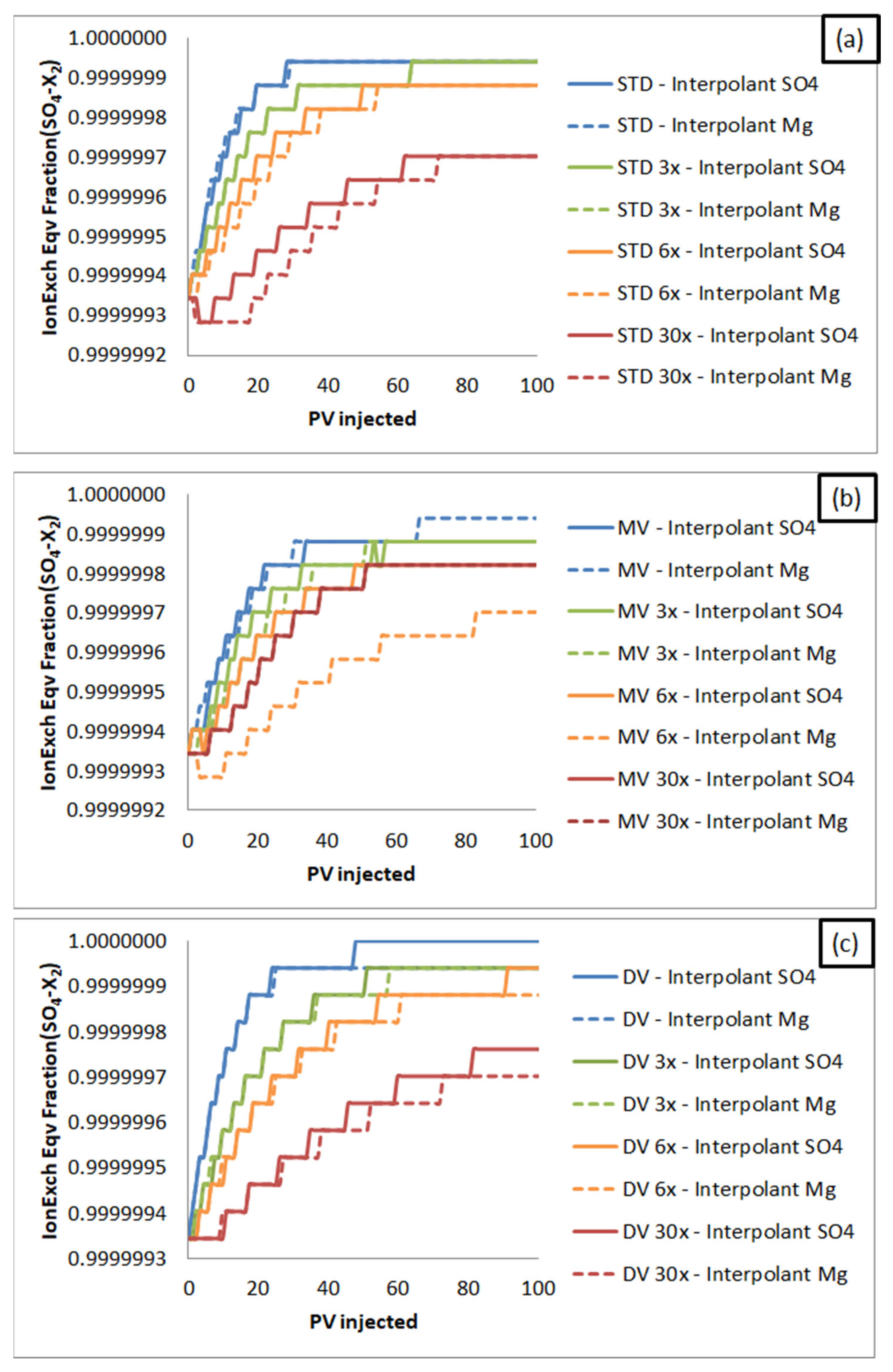
3. Results and Discussion
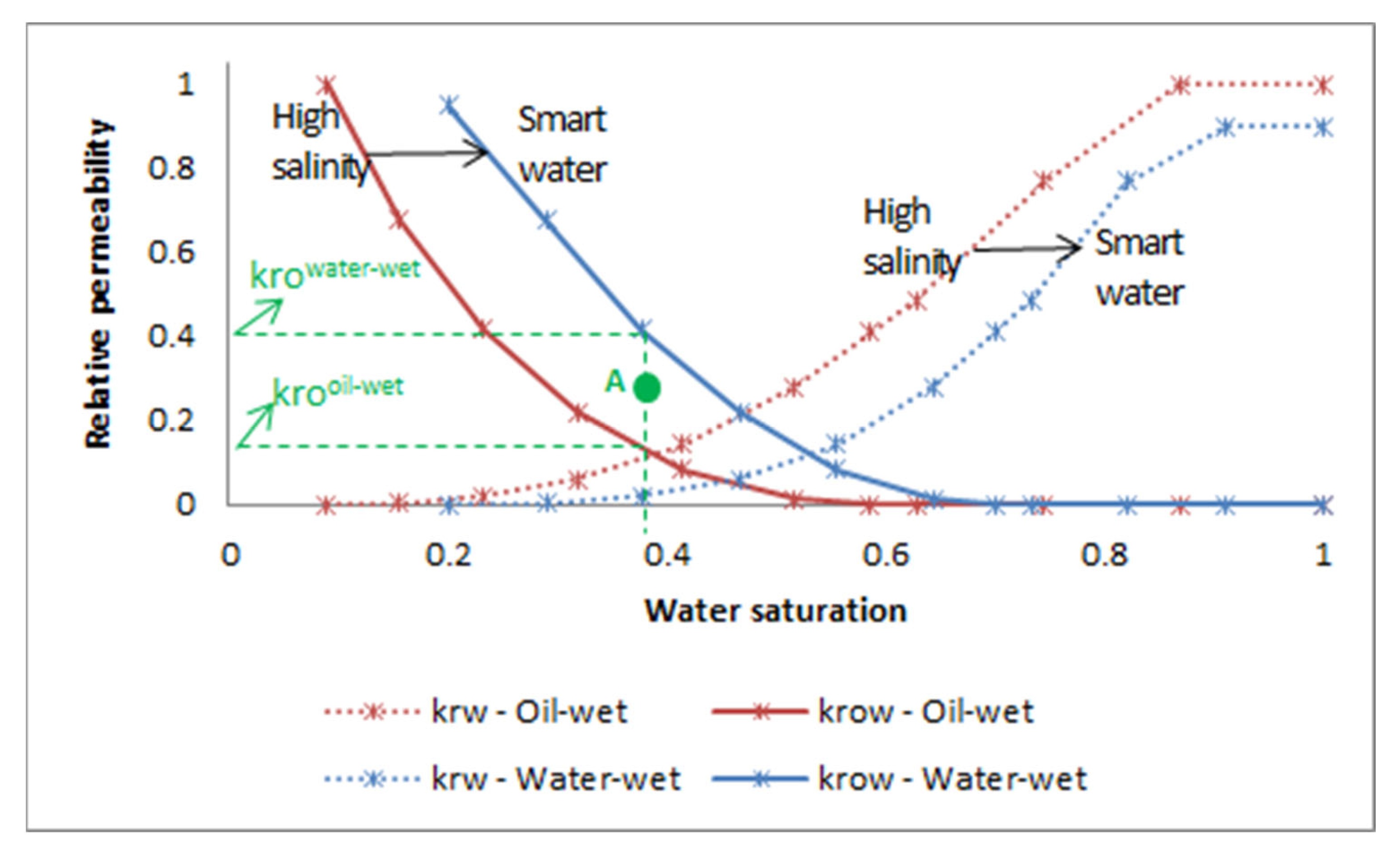
3.1. Oil Behavior Model
3.2. Core Flooding Simulations
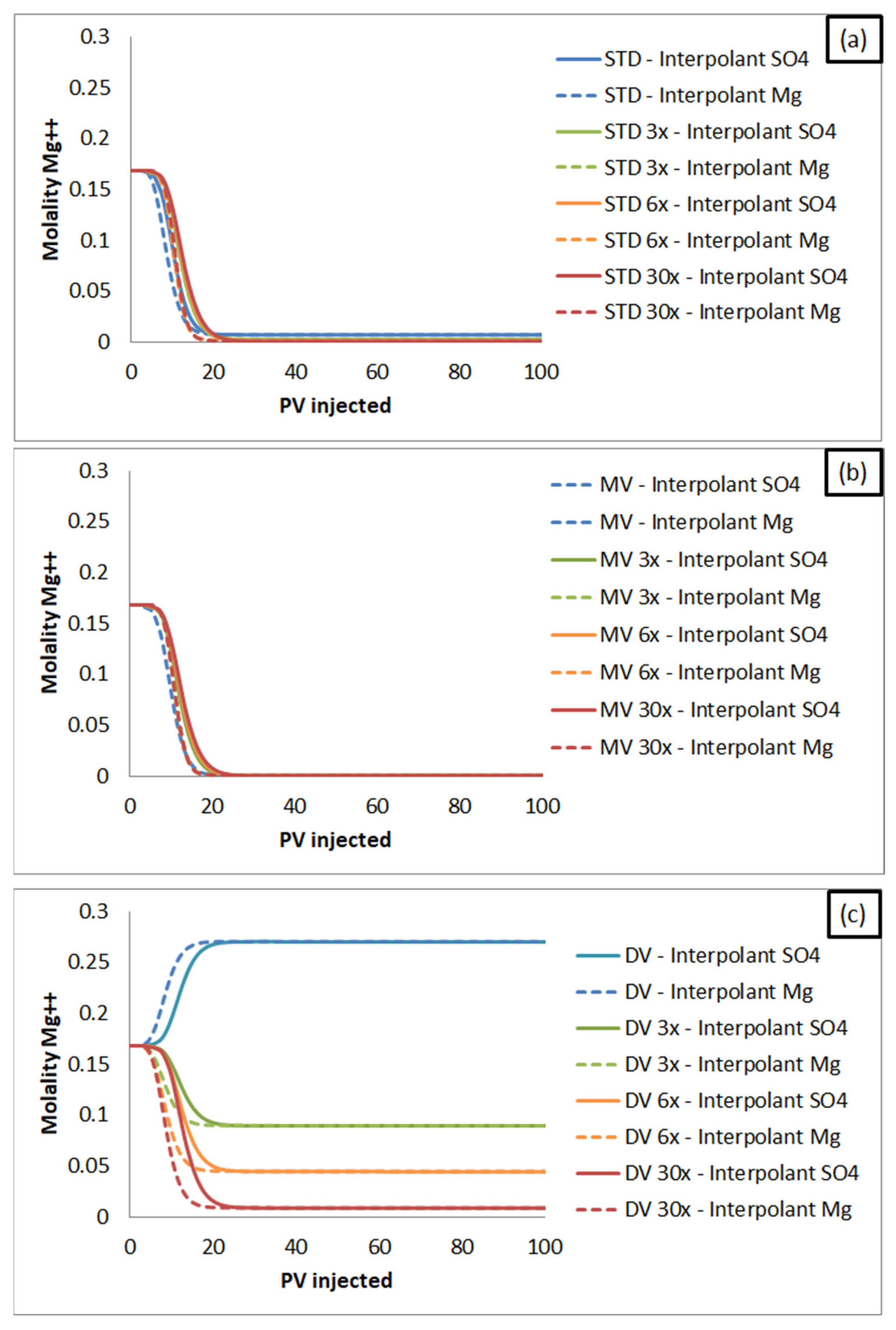
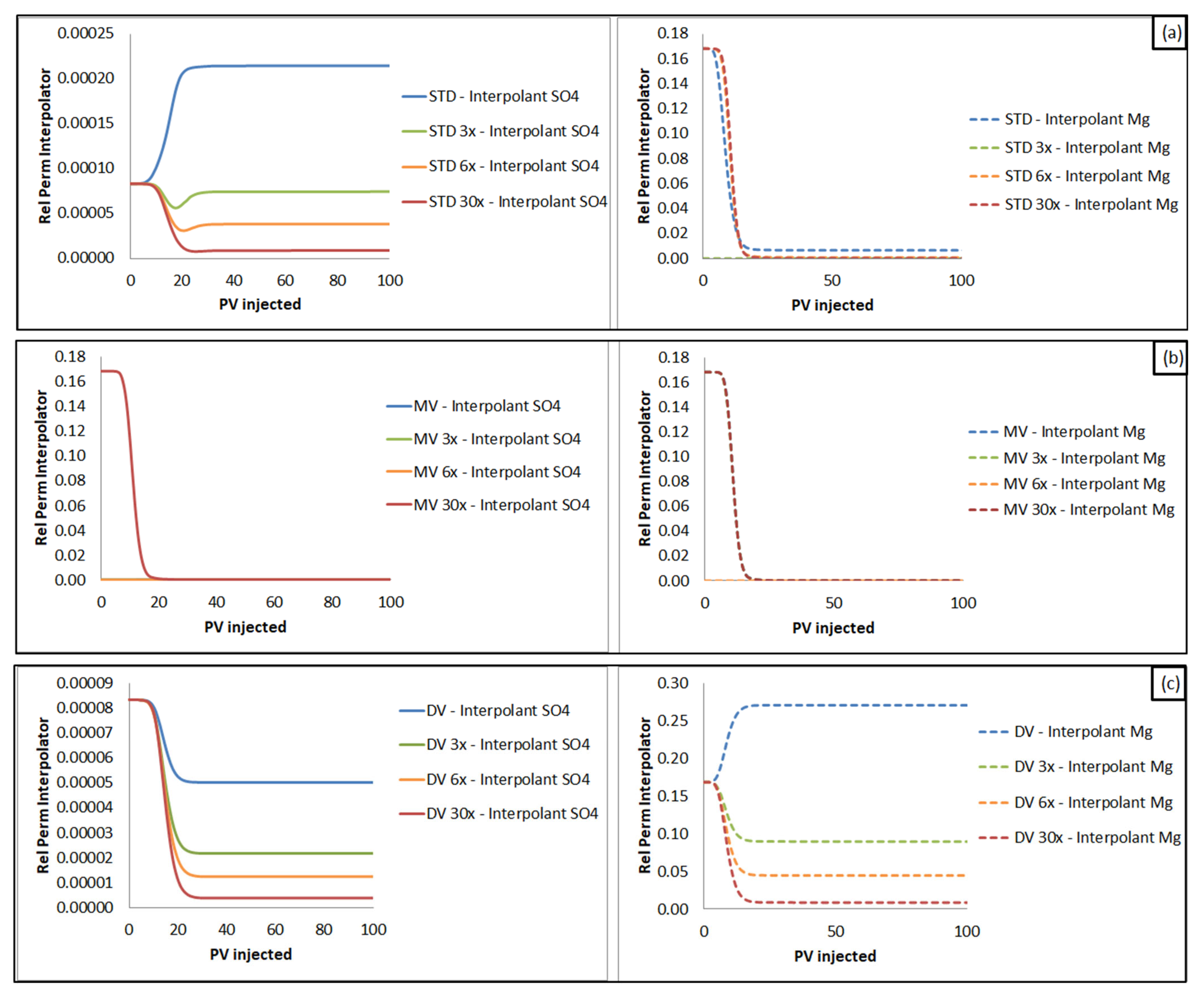
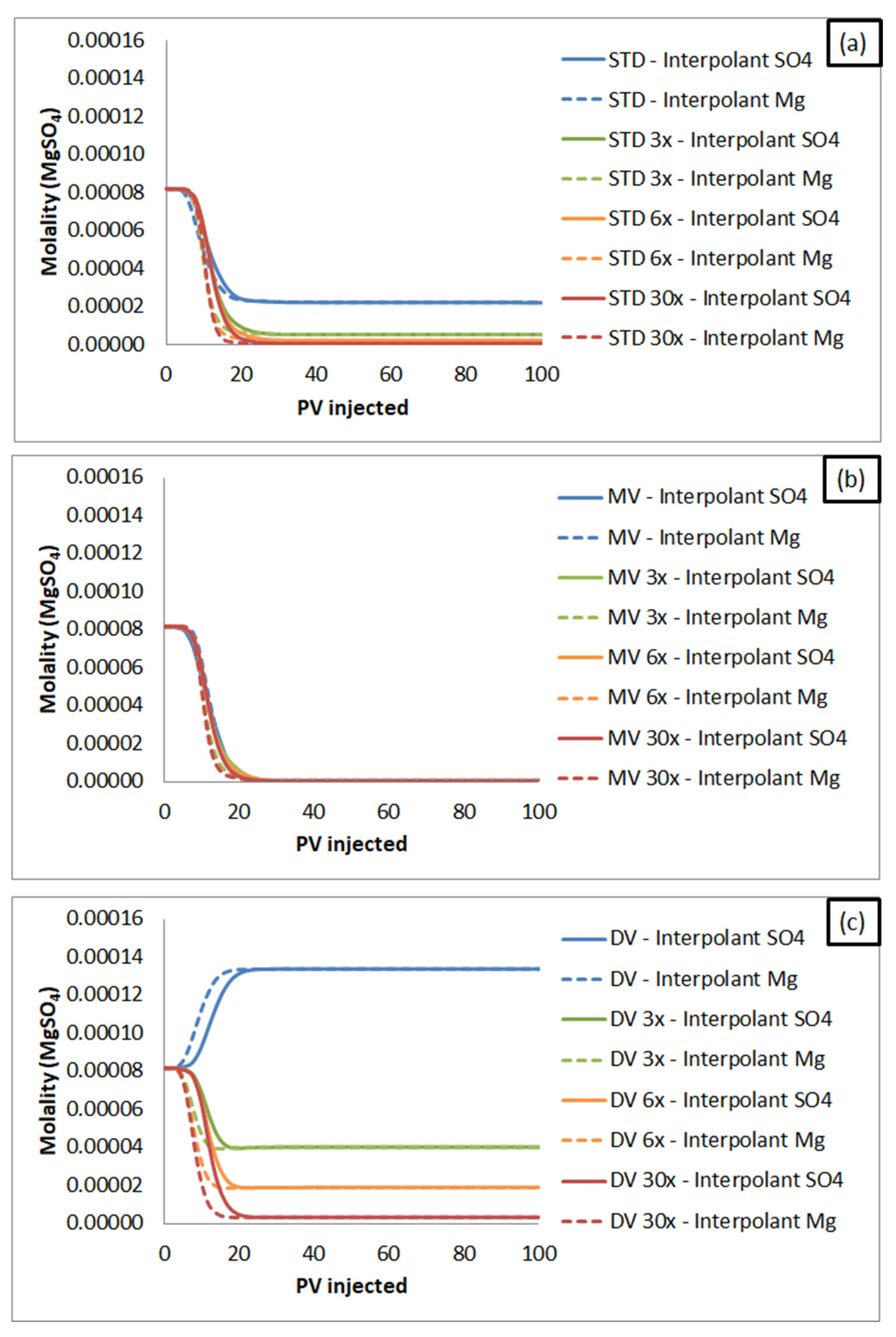
3.2.1. Monovalent Versus Divalent Cations Effluent Concentration: Influence on Oil Recovery Factor, According to SWF Mechanisms
- When sulfate is the interpolant ion, the estimated oil recovery follows a decreasing order: brines rich in divalent cations (DV) > synthetic seawater (STD) > brines rich in monovalent cations (MV). This trend is reversed as long as brine dilutions are evaluated. The recovery is the same for the injection of brine rich in monovalent ions and seawater brine, as seen in the condition of maximum dilution investigated (30x).
- When magnesium is the interpolant ion, the estimated oil recovery occurs in the following order: brines rich in monovalent cations (MV) > synthetic seawater (STD) > brines rich in divalent cations (DV). The recovery factor estimate for injections of synthetic seawater (STD) becomes closer to the results for brines rich in monovalent ions (MV) as the dilution factor increases. For all cases, the recoveries with brines rich in divalent cations (DV) are lower than in the other scenarios.
3.2.2. Dilution Effects: Influence on Oil Recovery Factor, According to SWF Mechanisms
3.2.3. Smart Water Flooding Mechanisms Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahimi, A.; Honarvar, B.; Safari, M. The Role of Salinity and Aging Time on Carbonate Reservoir in Low Salinity Seawater and Smart Seawater Flooding. J. Pet. Sci. Eng. 2020, 187, 106739. [Google Scholar] [CrossRef]
- Sohal, M.A.; Thyne, G.; Søgaard, E.G. Review of Recovery Mechanisms of Ionically Modified Waterflood in Carbonate Reservoirs. Energy Fuels 2016, 30, 1904–1914. [Google Scholar] [CrossRef]
- Egbe, D.I.O.; Jahanbani Ghahfarokhi, A.; Nait Amar, M.; Torsæter, O. Application of Low-Salinity Waterflooding in Carbonate Cores: A Geochemical Modeling Study. Nat. Resour. Res. 2021, 30, 519–542. [Google Scholar] [CrossRef]
- Xu, Z.X.; Li, S.Y.; Li, B.F.; Chen, D.Q.; Liu, Z.Y.; Li, Z.M. A Review of Development Methods and EOR Technologies for Carbonate Reservoirs. Pet. Sci. 2020, 17, 990–1013. [Google Scholar] [CrossRef]
- Al-Shalabi, E.W.; Sepehrnoori, K.; Pope, G. Geochemical Interpretation of Low-Salinity-Water Injection in Carbonate Oil Reservoirs. SPE J. 2015, 20, 1212–1226. [Google Scholar] [CrossRef]
- Zallaghi, M.; Khaz’ali, A.R. Experimental and Modeling Study of Enhanced Oil Recovery from Carbonate Reservoirs with Smart Water and Surfactant Injection. Fuel 2021, 304, 121516. [Google Scholar] [CrossRef]
- Lashkarbolooki, M.; Ayatollahi, S.; Riazi, M. Mechanistical Study of Effect of Ions in Smart Water Injection into Carbonate Oil Reservoir. Process. Saf. Environ. Prot. 2017, 105, 361–372. [Google Scholar] [CrossRef]
- Yousef, A.A.; Al-Saleh, S.; Al-Jawfi, M. New Recovery Method for Carbonate Reservoirs through Tuning the Injection Water Salinity: Smart WaterFlooding. In Proceedings of the SPE EUROPEC/EAGE Annual Conference and Exhibition, Vienna, Austria, 23–26 May 2011. [Google Scholar]
- Yousef, A.A.; Al-Saleh, S.; Al-Jawfi, M. Smart Waterflooding for Carbonate Reservoirs: Salinity and Role of Ions. SPE Middle East Oil Gas Show Conf. MEOS Proc. 2011, 1, 632–642. [Google Scholar] [CrossRef]
- Yousef, A.A.; Liu, J.; Blanchard, G.; Al-Saleh, S.; Al-Zahrani, T.; Al-Zahrani, R.; Al-Tammar, H.; Al-Mulhim, N. SmartWater Flooding: Industry’s First Field Test in Carbonate Reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, Texas, USA, 8–10 October 2012. [Google Scholar]
- Rassenfoss, S. Scaling Up Smart Water. J. Pet. Technol. 2016, 68, 39–41. [Google Scholar] [CrossRef]
- Hao, J.; Mohammadkhani, S.; Shahverdi, H.; Esfahany, M.N.; Shapiro, A. Mechanisms of Smart Waterflooding in Carbonate Oil Reservoirs—A Review. J. Pet. Sci. Eng. 2019, 179, 276–291. [Google Scholar] [CrossRef]
- Elgendy, A.M.S.; Porta, G.M. Impact of Reservoir Geochemistry on Low Salinity Waterflooding: Global Sensitivity Analysis. J. Pet. Sci. Eng. 2021, 207, 109056. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Li, C.; Sun, S. Effect of Salinity on Oil Production: Review on Low Salinity Waterflooding Mechanisms and Exploratory Study on Pipeline Scaling. Oil Gas Sci. Technol. 2020, 75, 50. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Pourafshary, P. Investigation of the Effect of Engineered Water/Nanofluid Hybrid Injection on Enhanced Oil Recovery Mechanisms in Carbonate Reservoirs. J. Pet. Sci. Eng. 2021, 196, 107662. [Google Scholar] [CrossRef]
- Al Shalabi, E.W.; Sepehrnoori, K.; Delshad, M. Mechanisms behind Low Salinity Water Injection in Carbonate Reservoirs. Fuel 2014, 121, 11–19. [Google Scholar] [CrossRef]
- Awolayo, A.N.; Sarma, H.K.; Nghiem, L.X. A Comprehensive Geochemical-Based Approach at Modeling and Interpreting Brine Dilution in Carbonate Reservoirs. In Proceedings of the SPE Reservoir Simulation Conference 2017, Montgomery, TX, USA, 20–22 February 2017; pp. 648–674. [Google Scholar] [CrossRef]
- Strand, S.; Puntervold, T. Recent Updates on Smart Water EOR in Limestone. In Proceedings of the Offshore Technology Conference Brasil, Rio de Janeiro, Brazil, 29–31 October 2019; pp. 29–31. [Google Scholar] [CrossRef]
- Shapoval, A.; Haryono, D.; Cao, J.L.; Rahman, S.S. Pore-Scale Investigation of the Ion-Tuned Water Effect on the Surface Properties of Limestone Reservoirs. Energy Fuels 2021, 35, 10475–10487. [Google Scholar] [CrossRef]
- Rezaeidoust, A.; Puntervold, T.; Strand, S.; Austad, T. Smart Water as Wettability Modifier in Carbonate and Sandstone: A Discussion of Similarities/Differences in the Chemical Mechanisms. Energy Fuels 2009, 23, 4479–4485. [Google Scholar] [CrossRef]
- Al-Shalabi, E.W.; Sepehrnoori, K.; Delshad, M.; Pope, G. A Novel Method to Model Low-Salinity-Water Injection in Carbonate Oil Reservoirs. SPE J. 2015, 20, 1154–1166. [Google Scholar] [CrossRef]
- Nande, S.B.; Patwardhan, S.D. A Review on Low Salinity Waterflooding in Carbonates: Challenges and Future Perspective. J. Pet. Explor. Prod. Technol. 2022, 12, 1037–1055. [Google Scholar] [CrossRef]
- Fathi, S.J.; Austad, T.; Strand, S. Water-Based Enhanced Oil Recovery (EOR) by “Smart Water” in Carbonate Reservoirs. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012; Volume 1, pp. 349–362. [Google Scholar] [CrossRef]
- Moradpour, N.; Pourafshary, P.; Zivar, D. Experimental Analysis of Hybrid Low Salinity Water Alternating Gas Injection and the Underlying Mechanisms in Carbonates. J. Pet. Sci. Eng. 2021, 202, 108562. [Google Scholar] [CrossRef]
- Dang, C.T.Q. Mechanistic Modeling of Low Salinity Waterflooding: Flow and Transport Model. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, 2015; p. 324. [Google Scholar] [CrossRef]
- Alshakhs, M.J.; BuKhamseen, N.Y.; AlGarni, S.A. Paradigm Shift in Smartwater Simulation Methods. In Proceedings of the International Petroleum Technology Conference 2020, Dhahran, Saudi Arabia, 13–15 January 2020; pp. 13–15. [Google Scholar] [CrossRef]
- Dang, C.; Nghiem, L.; Nguyen, N.; Chen, Z.; Nguyen, Q. Modeling and Optimization of Low Salinity Waterflood. In Proceedings of the SPE Reservoir Simulation Symposium, Houston, TX, USA, 23–25 February 2015; pp. 1–19. [Google Scholar] [CrossRef]
- Al-Shalabi, E.W.; Sepehrnoori, K.; Pope, G. Mysteries behind the Low Salinity Water Injection Technique. J. Pet. Eng. 2014, 2014, 304312. [Google Scholar] [CrossRef]
- Nguyen, N.; Dang, C.; Gorucu, S.E.; Nghiem, L.; Chen, Z. The Role of Fines Transport in Low Salinity Waterflooding and Hybrid Recovery Processes. Fuel 2020, 263, 116542. [Google Scholar] [CrossRef]
- Gorucu, S.E.; Dang, C.; Nghiem, L.; Shrivastava, V. Modeling and Optimization of Low Salinity Waterflood with Fines Transport. In Proceedings of the SPE Annual Technical Conference and Exhibition, Calgary, AB, Canada, 30 September–2 October 2019. [Google Scholar] [CrossRef]
- Esene, C.; Onalo, D.; Zendehboudi, S.; James, L.; Aborig, A.; Butt, S. Modeling Investigation of Low Salinity Water Injection in Sandstones and Carbonates: Effect of Na+ and SO42−. Fuel 2018, 232, 362–373. [Google Scholar] [CrossRef]
- Adegbite, J.O.; Al-Shalabi, E.W.; Ghosh, B. Geochemical Modeling of Engineered Water Injection Effect on Oil Recovery from Carbonate Cores. J. Pet. Sci. Eng. 2018, 170, 696–711. [Google Scholar] [CrossRef]
- Kadeethum, T.; Sarma, H.K.; Maini, B.B.; Jaruwattanasakul, C. Uncertainties–Extension of Smart Waterflooding from Core to Field Scale. In Proceedings of the IOR 2017—19th European Symposium on Improved Oil Recovery, Stavanger, Norway, 24–27 April 2017. [Google Scholar] [CrossRef]
- Moradpour, N.; Karimova, M.; Pourafshary, P.; Zivar, D. Effects of Slug Size, Soaking, and Injection Schemes on the Performance of Controlled Ions Water Flooding in Carbonates. ACS Omega 2020, 5, 18155–18167. [Google Scholar] [CrossRef]
- Sequeira, D.S. Compositional Effects on Gas-Oil Interfacial Tension and Miscibility at Reservoir Conditions. Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2006. [Google Scholar]
- Purswani, P.; Karpyn, Z.T. Laboratory Investigation of Chemical Mechanisms Driving Oil Recovery from Oil-Wet Carbonate Rocks. Fuel 2019, 235, 406–415. [Google Scholar] [CrossRef]
- Sarvestani, A.D.; Ayatollahi, S.; Bahari Moghaddam, M. Smart Water Flooding Performance in Carbonate Reservoirs: An Experimental Approach for Tertiary Oil Recovery. J. Pet. Explor. Prod. Technol. 2019, 9, 2643–2657. [Google Scholar] [CrossRef]
- Dang, C.T.Q.; Nghiem, L.X.; Chen, Z.; Nguyen, N.T.B.; Nguyen, Q.P. CO2 Low Salinity Water Alternating Gas: A New Promising Approach for Enhanced Oil Recovery. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014; Volume 1, pp. 578–596. [Google Scholar]
- Sagbana, I.P.; Sarkodie, K.; Nkrumah, W.A. A Critical Review of Carbonate Reservoir Wettability Modi Fi Cation during Low Salinity Water Flooding. Petroleum 2022, in press. [Google Scholar] [CrossRef]
- Mohammadkhani, S.; Shahverdi, H.; Esfahany, M.N. Impact of Salinity and Connate Water on Low Salinity Water Injection in Secondary and Tertiary Stages for Enhanced Oil Recovery in Carbonate Oil Reservoirs. J. Geophys. Eng. 2018, 15, 1242–1254. [Google Scholar] [CrossRef]
- Peng, D.Y.; Robinson, D.B. A New Two-Constant Equation of State. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Jossi, J.A.; Stiel, L.I.; Thodos, G. The Viscosity of Pure Substances in the Dense Gaseous and Liquid Phases. AIChE J. 1962, 8, 59–63. [Google Scholar] [CrossRef]
- Yoonm, P.; Thodos, G. Viscosity of Nonpolar Gaseous Mixtures at Normal Pressures. AIChE J. 1970, 16, 300–304. [Google Scholar] [CrossRef]
- Kestin, J.; Khalifa, H.E.; Sookiazian, H.; Wakeham, W.A. Experimental Investigation of Pressure Effect on the Viscosity of Water in the Temperature Range 10–15 °C. Ber. Bunsenges./Phys. Chem. Chem. Phys. 1978, 82, 180–188. [Google Scholar] [CrossRef]




| Ion | Na+ | K+ | Ca2+ | Mg2+ | H+ | Cl− | SO42− | TDS |
|---|---|---|---|---|---|---|---|---|
| Concentration (ppm) | 54,370 | 2850 | 19,740 | 4070 | 0.0001 | 139,800 | 8 | 220,838 |
| Component | Molar Composition (%) | Component | Molar Composition (%) | ||
|---|---|---|---|---|---|
| CO2 | 0.036 | N2 | 0.023 | ||
| CH4 | 23.736 | C2H6 | 0.009 | ||
| C3H8 | 0.064 | NC4 | 0.342 | ||
| iC4 | 0.117 | NC5 | 0.786 | ||
| iC5 | 0.822 | C6 | 2.644 | ||
| C7+ | 71.423 | ||||
| Molecular weight (g/mol) | 164 | Gas-oil ratio (ft3/bbl) | 167 | Specific Gravity @15 °C | 0.8359 |
| STD | STD 3x | STD 6x | STD 30x | MV | MV 3x | MV 6x | MV 30x | DV | DV 3x | DV 6x | DV 30x | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | 11,075 | 3691.7 | 1845.8 | 369.2 | 11,376 | 3792.0 | 1896.0 | 379.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| K+ | 393 | 131.0 | 65.5 | 13.1 | 404 | 134.7 | 67.3 | 13.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ca2+ | 142 | 47.3 | 23.7 | 4.7 | 0.0 | 0.0 | 0.0 | 0.0 | 5239 | 1746.3 | 873.2 | 174.6 |
| Mg2+ | 170 | 56.7 | 28.3 | 5.7 | 0.0 | 0.0 | 0.0 | 0.0 | 6541 | 2180.3 | 1090.2 | 218.0 |
| Cl− | 18,847 | 6282.3 | 3141.2 | 628.2 | 18,847 | 6282.3 | 3141.2 | 628.2 | 18,847 | 6282.3 | 3141.2 | 628.2 |
| SO42− | 24 | 8.0 | 4.0 | 0.8 | 24 | 8.0 | 4.0 | 0.8 | 24 | 8.0 | 4.0 | 0.8 |
| TDS | 30,651 | 10,217 | 5108.5 | 1021.7 | 30,651 | 10,217 | 5108.5 | 1021.7 | 30,651 | 10,217 | 5108.5 | 1021.7 |
| Scenario | Injection Brine | Scenario | Injection Brine | Scenario | Injection Brine | Interpolant Ion |
|---|---|---|---|---|---|---|
| 1 | STD | 9 | MV | 17 | DV | Mg2+ |
| 2 | 10 | 18 | SO42− | |||
| 3 | STD 3x | 11 | MV 3x | 19 | DV 3x | Mg2+ |
| 4 | 12 | 20 | SO42− | |||
| 5 | STD 6x | 13 | MV 6x | 21 | DV 6x | Mg2+ |
| 6 | 14 | 22 | SO42− | |||
| 7 | STD 30x | 15 | MV 30x | 23 | DV 30x | Mg2+ |
| 8 | 16 | 24 | SO42− |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastos, L.d.S.; Lins, I.E.d.S.; Costa, G.M.N.; Vieira de Melo, S.A.B. Analyses of Interpolant Ion Effects on Smart Water Core Flooding in Carbonate. Energies 2023, 16, 446. https://doi.org/10.3390/en16010446
Bastos LdS, Lins IEdS, Costa GMN, Vieira de Melo SAB. Analyses of Interpolant Ion Effects on Smart Water Core Flooding in Carbonate. Energies. 2023; 16(1):446. https://doi.org/10.3390/en16010446
Chicago/Turabian StyleBastos, Ladislane dos Santos, Igor Emanuel da Silva Lins, Gloria Meyberg Nunes Costa, and Silvio Alexandre Beisl Vieira de Melo. 2023. "Analyses of Interpolant Ion Effects on Smart Water Core Flooding in Carbonate" Energies 16, no. 1: 446. https://doi.org/10.3390/en16010446
APA StyleBastos, L. d. S., Lins, I. E. d. S., Costa, G. M. N., & Vieira de Melo, S. A. B. (2023). Analyses of Interpolant Ion Effects on Smart Water Core Flooding in Carbonate. Energies, 16(1), 446. https://doi.org/10.3390/en16010446









