Metal–Organic Frameworks (MOFs) Containing Adsorbents for Carbon Capture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
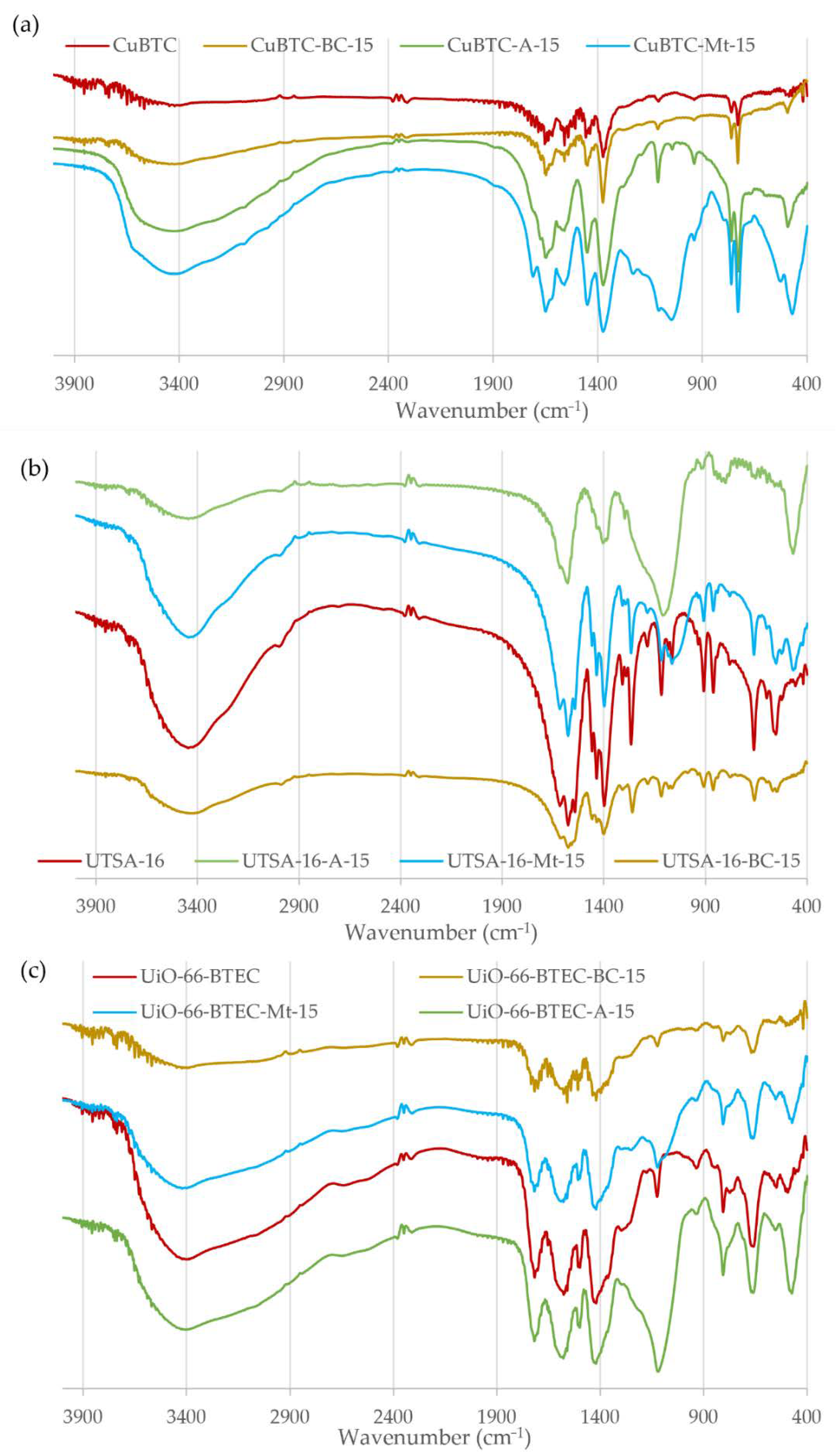
2.2. Synthesis of CuBTC and CuBTC-BC, CuBTC-Mt, CuBTC-A
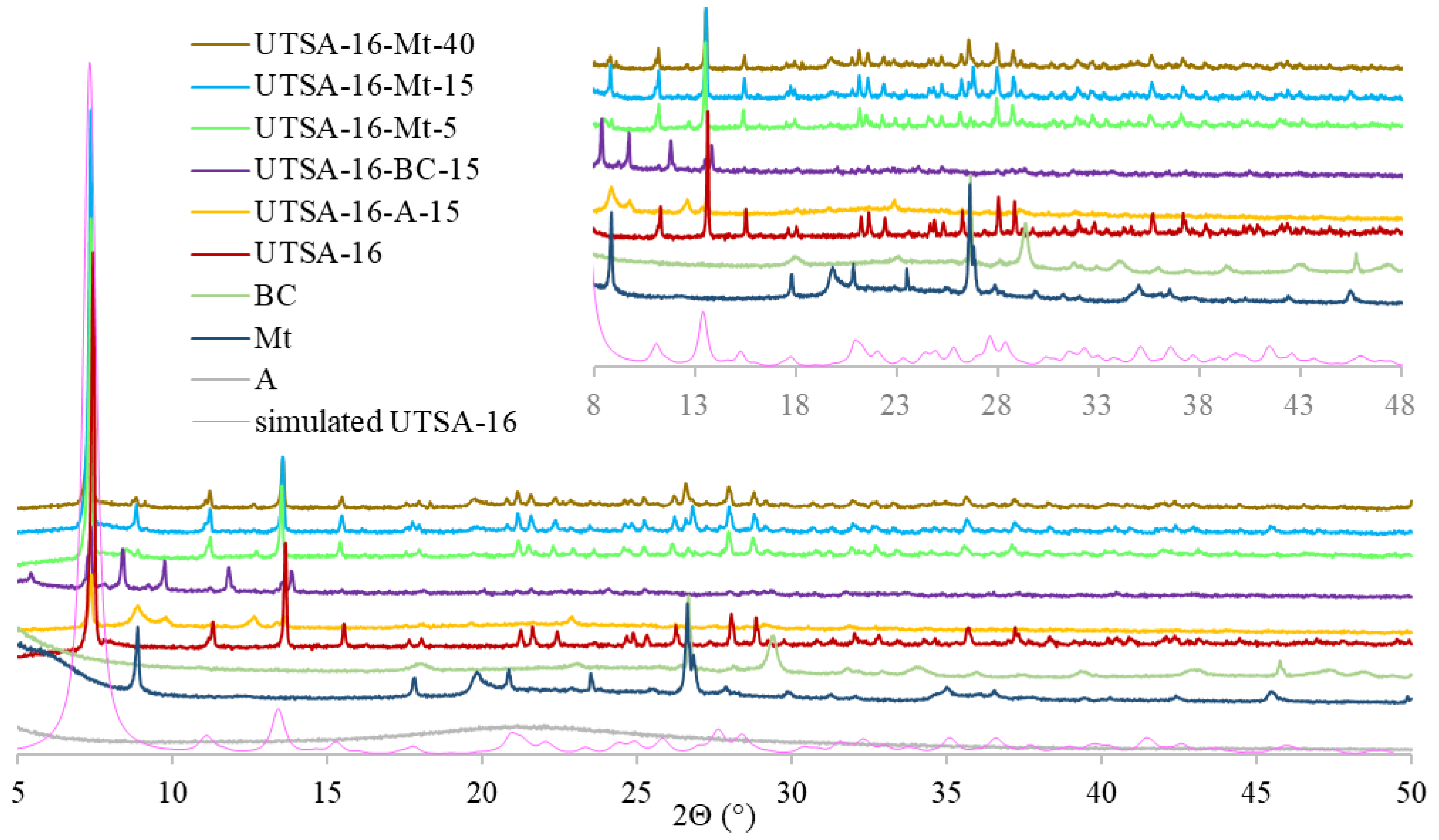
2.3. Synthesis of UTSA-16 and UTSA-16-BC, UTSA-16-Mt, and UTSA-16-A
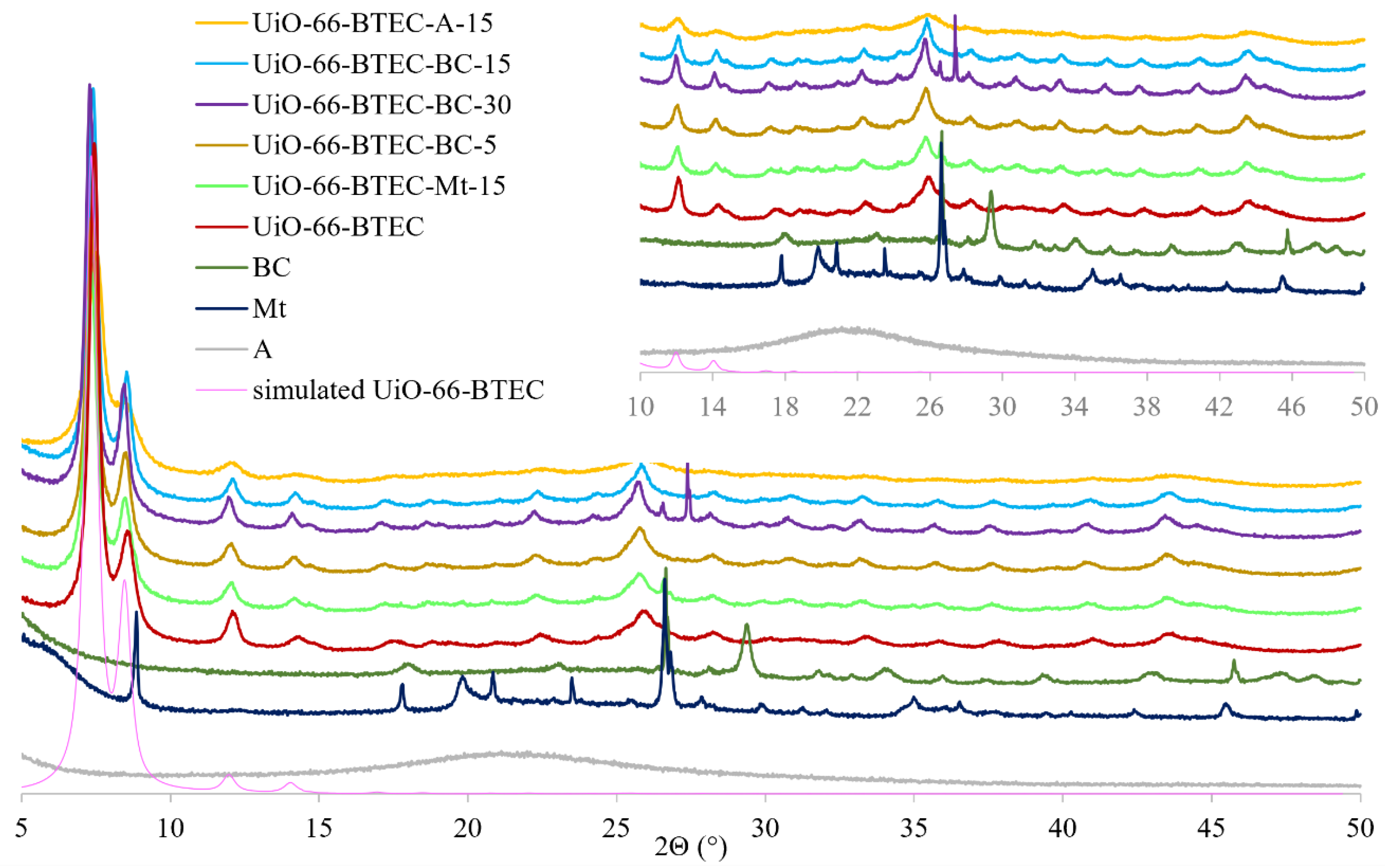
2.4. Synthesis of UiO-66-BTEC and UiO-66-BTEC-BC, UiO-66-BTEC-Mt, and UiO-66-BTEC-A
2.5. Characterization of MOF-Composites
2.6. Carbon Dioxide Adsorption Experiments
3. Results and Discussion
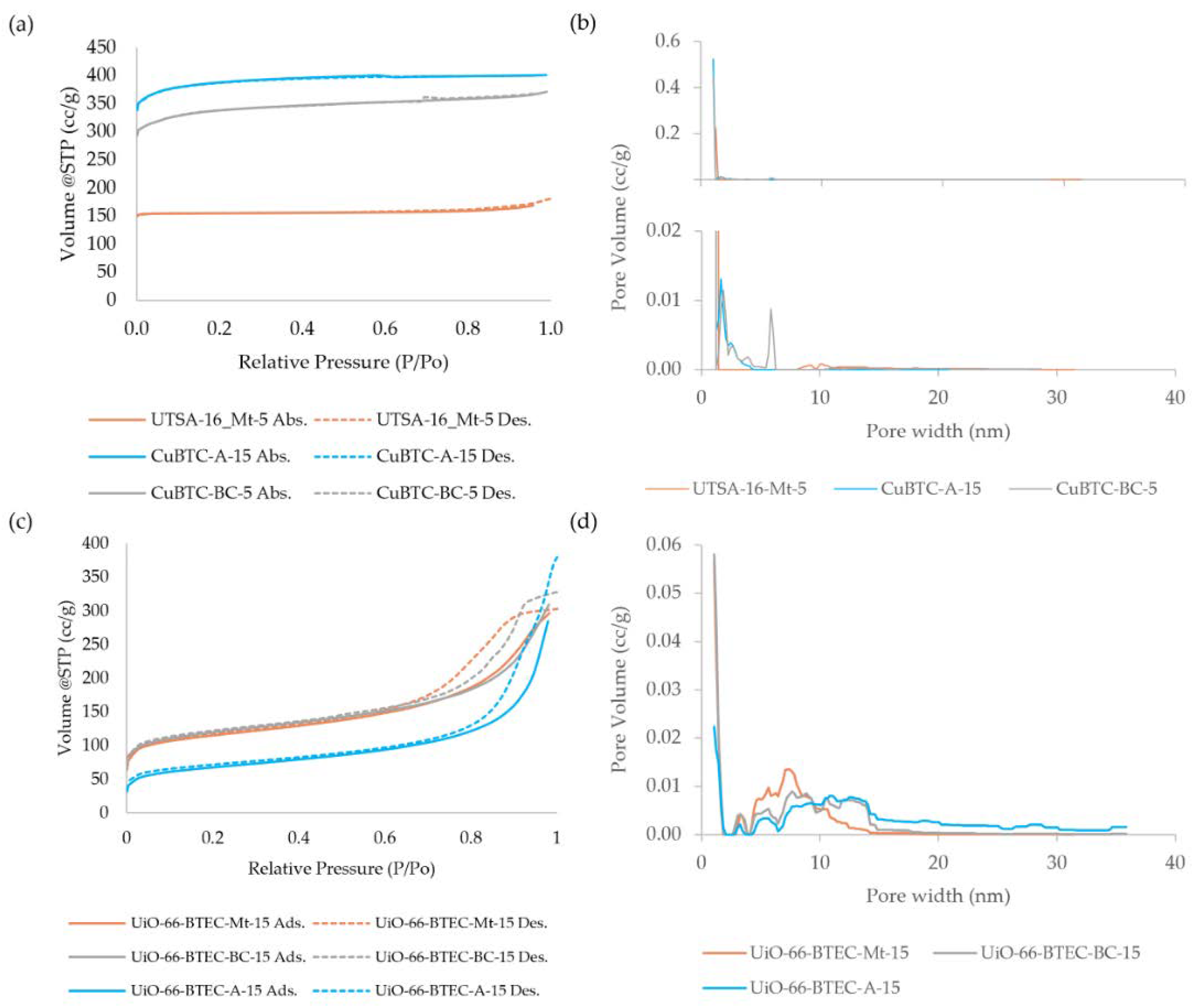
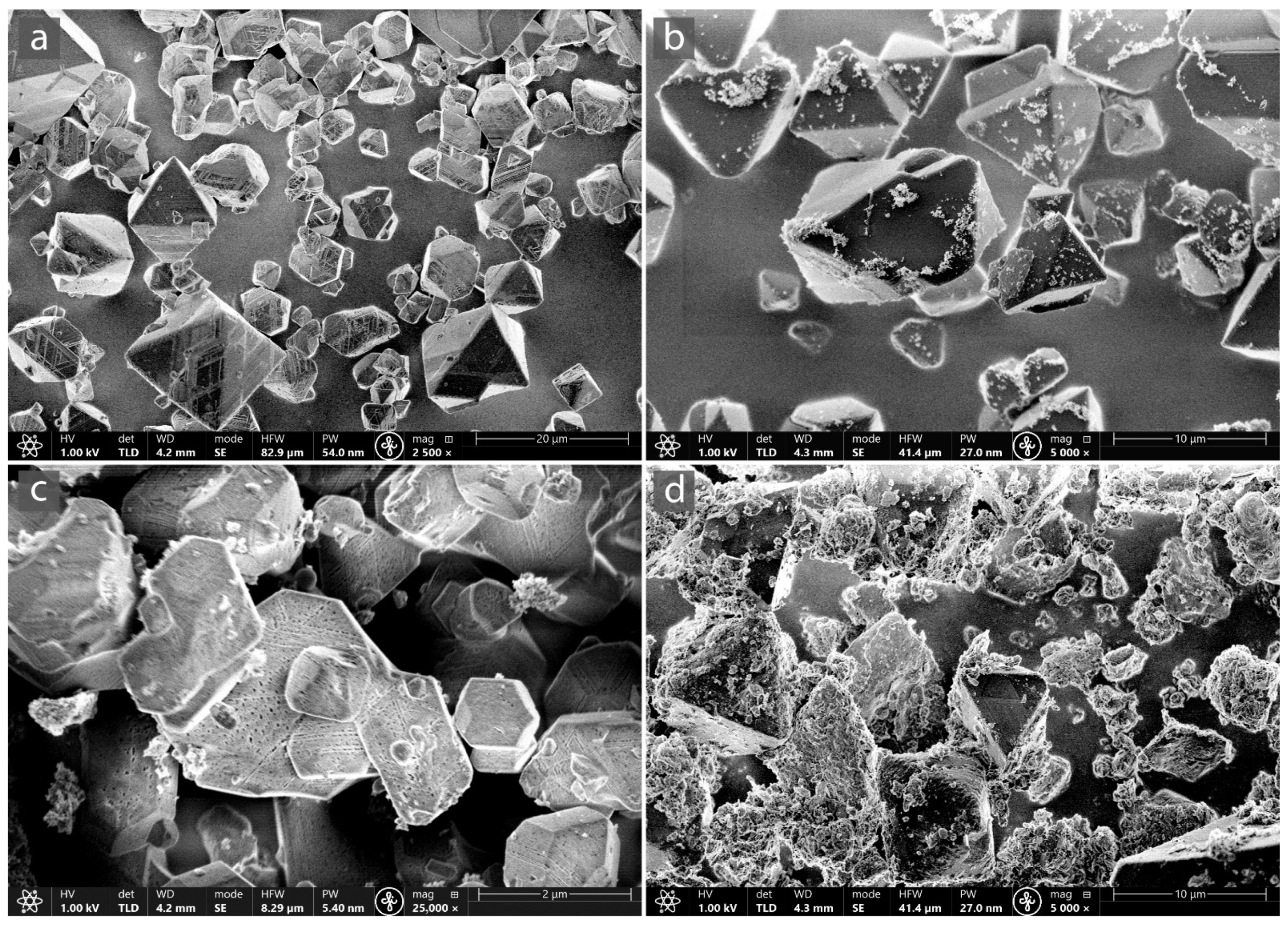
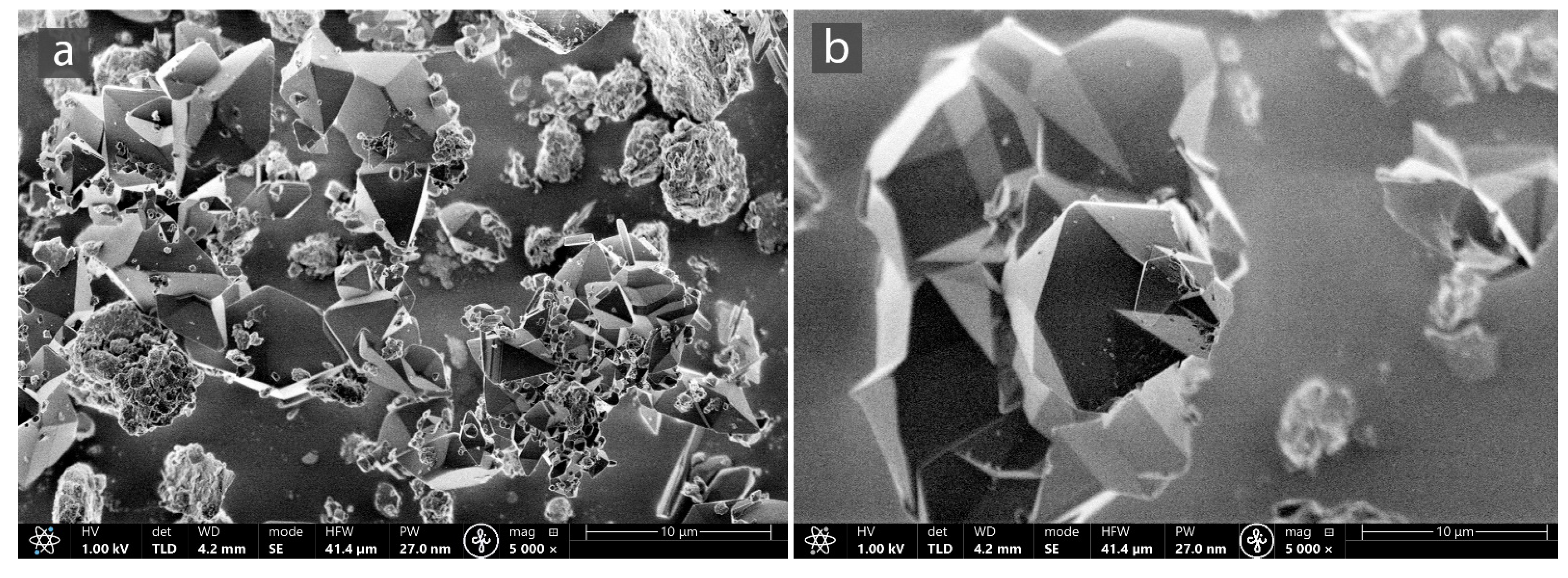
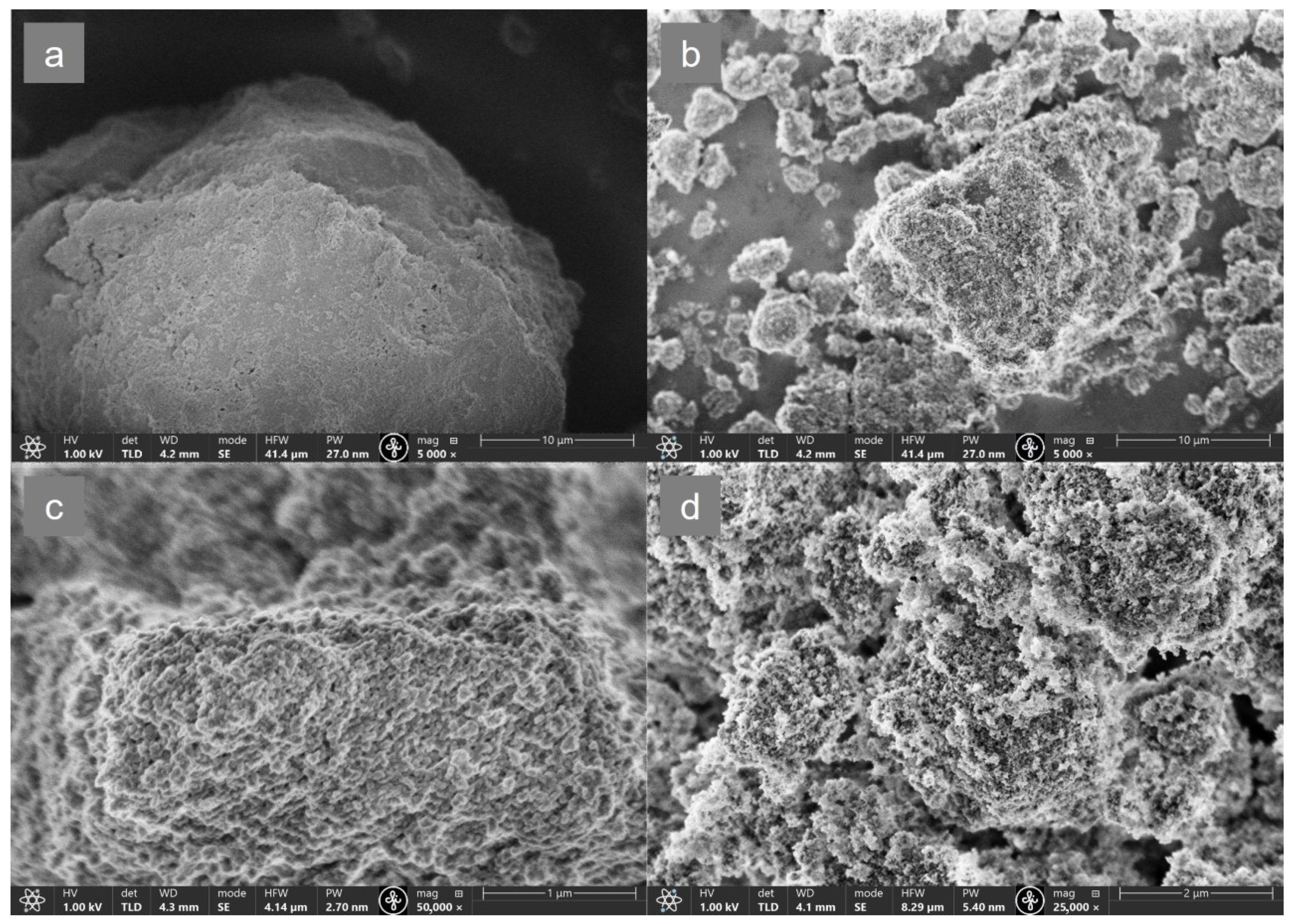
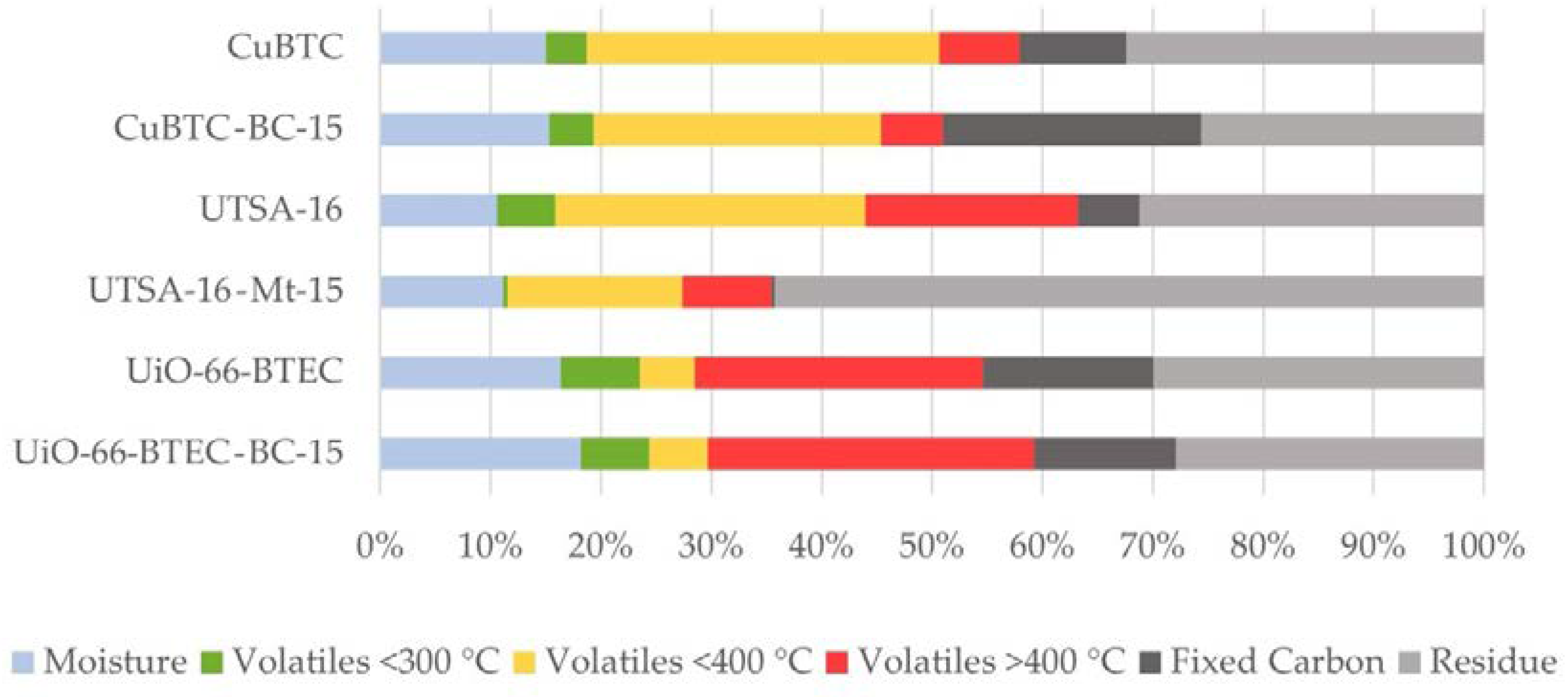
3.1. Characterization of MOF-Composites
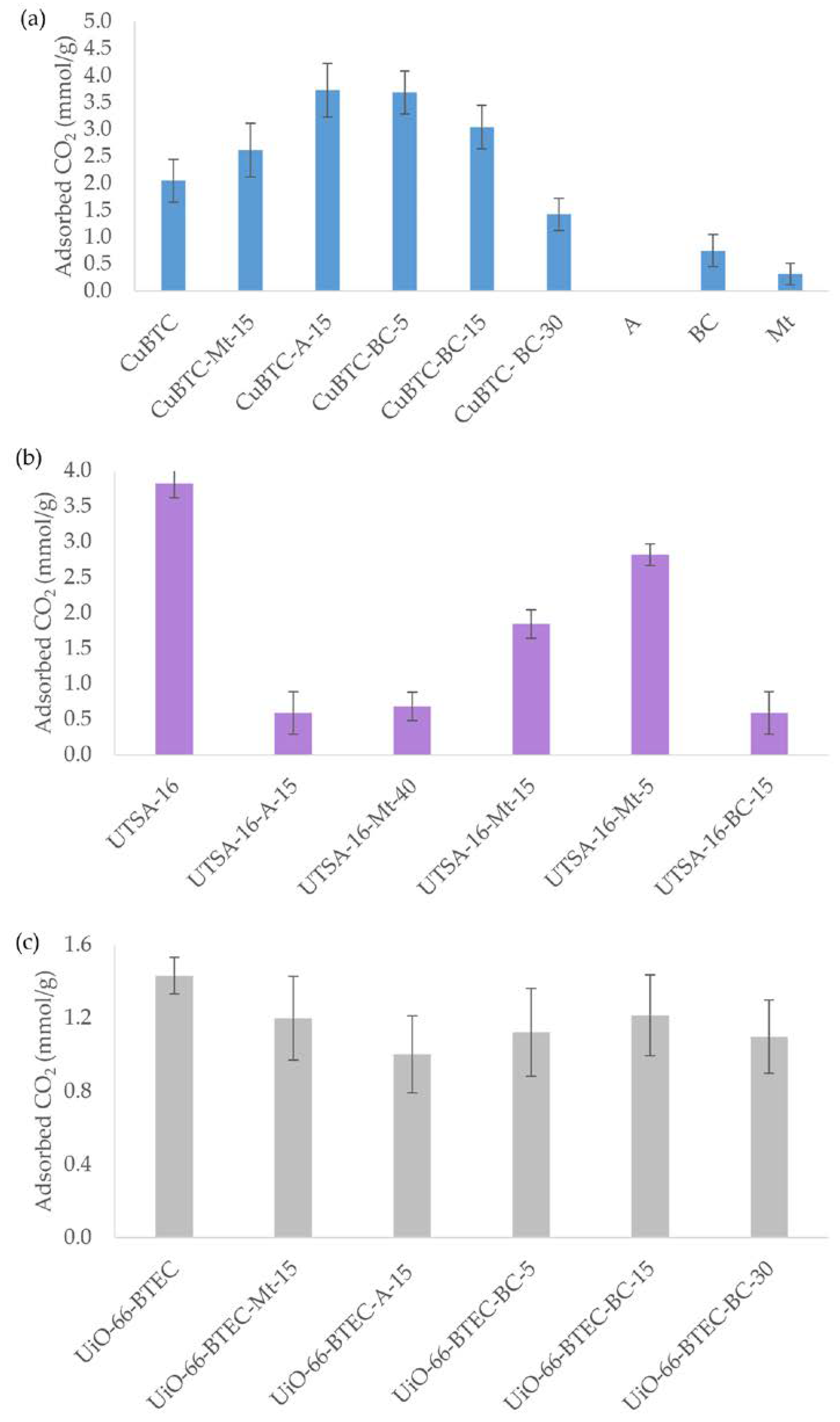
3.2. Carbon Dioxide Adsorption onto MOF-Composite Adsorbents
| MOF-Composite | Surface Area (BET), m2/g | CO2 Adsorption | CO2 Adsorption Conditions | Reference |
|---|---|---|---|---|
| HCM-Cu3(BTC)2-1 | 270 | 2.36 mmol/g | 25 °C | [95] |
| CuBTC-OMC | 1288 | 4.35 mmol/g | 25 °C, 1 bar | [22] |
| CuBTC-AC | 1368 | 4.49 mmol/g | 25 °C, 1 bar | [22] |
| CuBTC-NC | 1364 | 4.51 mmol/g | 25 °C, 1 bar | [22] |
| CuBTC/GA | 1048 | 3.26 mmol/g | 298 K, 1 bar | [23] |
| MWCNTs@CuBTC | 727 | 3.26 mmol/g | ambient conditions | [88] |
| HS-1 | 1745 | 108.0 cm3/g | 25 °C, 1 bar | [24] |
| CuBTC | 1760 | 5.33 mmol/g | 25 °C, 1 bar | [54] |
| CuBTC/GO2 | 1820 | 5.12 mmol/g | 25 °C, 1 bar | [54] |
| CuBTC/GO5 | 1520 | 4.79 mmol/g | 25 °C, 1 bar | [54] |
| CuBTC/GO10 | 1380 | 4.11 mmol/g | 25 °C, 1 bar | [54] |
| CuBTC | 892 | 2.46 mmol/g | 295 K, 0.12 MPa | [45] |
| CuBTC/GO | 1010 | 3.09 mmol/g | 295 K, 0.12 MPa | [45] |
| CuBTC/GO-U3 | 1367 | 4.78 mmol/g | 295 K, 0.12 MPa | [45] |
| CuBTC | 1594 | 3.06 mmol/g | 25 °C, 100 kPa | [55] |
| CuBTC@MWCNT | 1150 | 3.4 mmol/g | 25 °C, 100 kPa | [55] |
| HKUST-1 | 1322 | 114.92 cm3/g | 298 K | [80] |
| HKUST-1/ATP | 1158 | 127.88 cm3/g | 298 K | [80] |
| CuBTC | 1580 | 8.02 mmol/g | 273 K, 1 bar | [58] |
| CuBTC@1%GO | 1772 | 8.90 mmol/g | 273 K, 1 bar | [58] |
| CuBTC-AC-2 | 1381 | 5.35 mmol/g | 298 K, 1 bar | [25] |
| CuBTC-AC-2 | 1381 | 8.1 mmol/g | 273 K, 1 bar | [25] |
| Zeolite 13X | 570 | 210 mg/g | ambient temperatures, 1 bar | [109] |
| Zeolite 13X | 616 | 1.77 mmol/g | 293 K, 1 bar | [102] |
| UTSA-16 | 628 | 189 mg/g | ambient temperatures, 1 bar | [109] |
| UTSA-16@Co-kaolin | 620 | 3.1 mmol/g | 25 °C, 1 bar | [82] |
| UTSA-16/carbon composites | 211 | 2.0 mmol/g | room temp., 1 bar | [110] |
| UTSA-16(Co)-cordierite monolith | 223 | 1.1 mmol/g | 298 K, 1 bar | [99] |
| UTSA-16 monolith containing bentonite | 568 | 3.0 mmol/g | 25 °C, 1.1 bar | [92] |
| UTSA-16 | 727 | 3.5 mmol/g | 25 °C, 1.1 bar | [92] |
| UiO-66-BTEC | 568 (Langmuir surf. area) | 1.05 mmol/g | 303 K, 0.99 bar | [34] |
| HKUST-1@GO-2 | 1550 | 8.5 mmol/g | 0 °C, atmospheric pressure | [56] |
| CuBTC | 1305 | 6.39 mmol/g | 273 K, 1 atm | [44] |
| CG-3 (CuBTC-GO-3) | 1470 | 7.94 mmol/g | 273 K, 1 atm | [44] |
| CG-9 (CuBTC-GO-9) | 1532 | 8.26 mmol/g | 273 K, 1 atm | [44] |
| CG-15 (CuBTC-GO-15) | 500 | 2.97 mmol/g | 273 K, 1 atm | [44] |
| Cu3(BTC)2 | 933 | 2.77 mmol/g | 298 K, 1 bar | [46] |
| Cu3(BTC)2/GO-l | 898 | 3.13 mmol/g | 298 K, 1 bar | [46] |
| Cu3(BTC)2/GO-m | 837 | 3.37 mmol/g | 298 K, 1 bar | [46] |
| Cu3(BTC)2/GO-h | 743 | 2.66 mmol/g | 298 K, 1 bar | [46] |
| Cu3(BTC)2 | 1587 | 295 mg/g | 298 K, 18 bar | [47] |
| CNT@Cu3(BTC)2 | 1458 | 595 mg/g | 298 K, 18 bar | [47] |
| HKUST-1 | 434 | 1.59 mmol/g | 25 °C, 1 bar | [111] |
| HKUST-1/GO | 369 | 0.98 mmol/g | 25 °C, 1 bar | [111] |
| HKUST-1 | 1410 | 7.92 mmol/g | 196 K, 1 bar | [112] |
| 5wt% SWCNT@HKUST-1 | 1714 | 8.75 mmol/g | 196 K, 1 bar | [112] |
| HKUST-1 | 1379.87 | 3.55 mmol/g | 25 °C, 1 bar | [113] |
| HKUST-1@GO | 1096.46 | 2.53 mmol/g | 25 °C, 1 bar | [113] |
3.3. Future Work and Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Ghussain, L. Global warming: Review on driving forces and mitigation. Environ. Prog. Sustain. Energy 2019, 38, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for mitigation of climate change: A review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Green Deal. Striving to the First Climate-Neutral Continent. 2021. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 24 February 2022.).
- Alami, A.H.; Hawili, A.A.; Tawalbeh, M.; Hasan, R.; Al Mahmoud, L.; Chibib, S.; Aokal, K.; Mahmood, A.; Rattanapanya, P. Materials and logistics for carbon dioxide capture, storage and utilization. Sci. Total Environ. 2020, 717, 137221. [Google Scholar] [CrossRef] [PubMed]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernandez, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Sabri, M.A.; Al Jitan, S.; Bahamon, D.; Vega, L.F.; Palmisano, G. Current and future perspectives on catalytic-based integrated carbon capture and utilization. Sci. Total Environ. 2021, 790, 148081. [Google Scholar] [CrossRef]
- Pant, D.; Nadda, A.K.; Pant, K.K.; Agarwal, A.K. Advances in Carbon Capture and Utilization. In Advances in Carbon Capture and Utilization; Springer: Singapore, 2021; pp. 3–7. [Google Scholar]
- Pellegrini, L.A.; De Guido, G.; Ingrosso, S. Thermodynamic Framework for Cryogenic Carbon Capture. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2020; Volume 48, pp. 475–480. [Google Scholar] [CrossRef]
- Shah, S.; Shah, M.; Shah, A.; Shah, M. Evolution in the membrane-based materials and comprehensive review on carbon capture and storage in industries. Emerg. Mater. 2020, 3, 33–44. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Z.; Gani, R.; Zhou, T. Comparative economic analysis of physical, chemical, and hybrid absorption processes for carbon capture. Ind. Eng. Chem. Res. 2020, 59, 2005–2012. [Google Scholar] [CrossRef]
- Ghanbari, T.; Abnisa, F.; Daud, W.M.A.W. A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef]
- Sharma, H.; Dhir, A. Capture of carbon dioxide using solid carbonaceous and non-carbonaceous adsorbents: A review. Environ. Chem. Lett. 2021, 19, 851–873. [Google Scholar] [CrossRef]
- Siegelman, R.L.; Kim, E.J.; Long, J.R. Porous materials for carbon dioxide separations. Nat. Mater. 2021, 20, 1060–1072. [Google Scholar] [CrossRef]
- Osman, A.I.; Hefny, M.; Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: A review. Environ. Chem. Lett. 2021, 19, 797–849. [Google Scholar] [CrossRef]
- Piscopo, C.G.; Loebbecke, S. Strategies to enhance carbon dioxide capture in metal-organic frameworks. ChemPlusChem 2020, 85, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Luedtke, Z.; Aro, M.; Sun, Z.; Toan, S. Carbon Capture Materials and Technologies: A Review. Curr. Res. Mater. Chem. 2020, 3, 108. [Google Scholar] [CrossRef]
- Aniruddha, R.; Sreedhar, I.; Reddy, B.M. MOFs in carbon capture-past, present and future. J. CO2 Util. 2020, 42, 101297. [Google Scholar] [CrossRef]
- Younas, M.; Rezakazemi, M.; Daud, M.; Wazir, M.B.; Ahmad, S.; Ullah, N.; Ramakrishna, S. Recent progress and remaining challenges in post-combustion CO2 capture using metal-organic frameworks (MOFs). Prog. Energy Combust. 2020, 80, 100849. [Google Scholar] [CrossRef]
- Peedikakkal, A.M.; Aljundi, I.H. Mixed-metal Cu-BTC metal−organic frameworks as a strong adsorbent for molecular hydrogen at low temperatures. ACS Omega 2020, 5, 28493–28499. [Google Scholar] [CrossRef]
- Al-Janabi, N.; Deng, H.; Borges, J.; Liu, X.; Garforth, A.; Siperstein, F.; Fan, X. A facile post-synthetic modification method to improve hydrothermal stability and CO2 selectivity of CuBTC metal-organic framework. Ind. Eng. Chem. Res. 2016, 55, 7941–7949. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Cao, L.; Wei, S.; Zhang, X.; Chen, L. A first principles study of gas adsorption on charged Cu-BTC. Comput. Theor. Chem. 2011, 976, 153–160. [Google Scholar] [CrossRef]
- Liu, Y.; Ghimire, P.; Jaroniec, M. Copper benzene-1,3,5-tricarboxylate (Cu-BTC) metal-organic framework (MOF) and porous carbon composites as efficient carbon dioxide adsorbents. J. Colloid Interface Sci. 2019, 535, 122–132. [Google Scholar] [CrossRef]
- Ren, W.; Wei, Z.; Xia, X.; Hong, Z.; Li, S. CO2 adsorption performance of CuBTC/graphene aerogel composites. J. Nanopart. Res. 2020, 22, 191. [Google Scholar] [CrossRef]
- Chen, C.; Li, B.; Zhou, L.; Xia, Z.; Feng, N.; Ding, J.; Wang, L.; Wan, H.; Guan, G. Synthesis of hierarchically structured hybrid materials by controlled self-assembly of Metal−Organic Framework with mesoporous silica for CO2 adsorption. ACS Appl. Mater. Interfaces 2017, 9, 23060–23071. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Achari, A.; Eswaramoorthy, M.; Maji, T.K. MOF-aminoclay composites for superior CO2 capture, separation and enhanced catalytic activity in chemical fixation of CO2. Chem. Commun. 2016, 52, 11378–11381. [Google Scholar] [CrossRef] [PubMed]
- Abdoli, Y.; Razavian, M.; Fatemi, S. Bimetallic Ni–Co-based metal–organic framework: An open metal site adsorbent for enhancing CO2 capture. Appl. Organometal. Chem. 2019, 33, e5004. [Google Scholar] [CrossRef]
- Peh, S.B.; Xi, S.; Karmakar, A.; Yeo, J.Y.; Wang, Y.; Zhao, D. Accelerated formation kinetics of a multicomponent metal−organic framework derived from preferential site occupancy. Inorg. Chem. 2020, 59, 9350–9355. [Google Scholar] [CrossRef] [PubMed]
- Masasla, A.; Grifasi, F.; Atzori, C.; Vitillo, J.G.; Mino, L.; Bonino, F.; Chierotti, R.; Bordiga, S. CO2 adsorption sites in UTSA-16: Multitechnique approach. J. Phys. Chem. C 2016, 120, 12068–12074. [Google Scholar] [CrossRef] [Green Version]
- Masala, A.; Vitillo, J.G.; Bonino, F.; Manzoli, M.; Grabde, C.A.; Bordiga, S. New insights into UTSA-16. Phys. Chem. Chem. Phys. 2016, 18, 220. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Li, Z.; Wang, L.; Ye, Y.; Liu, Q.; Ma, X.; Chen, Q.; Zhang, Z.; Xiang, S. Cobalt–citrate framework armored with graphene oxide exhibiting improved thermal stability and selectivity for biogas decarburization. J. Mater. Chem. A 2015, 3, 593–599. [Google Scholar] [CrossRef]
- Usman, M.; Helal, A.; Abdelnaby, M.M.; Alloush, A.M.; Zeama, M.; Yamani, Z.H. Trends and prospects in UiO-66 metal-organic framework for CO2 capture, separation, and conversion. Chem. Rec. 2021, 21, 1771–1791. [Google Scholar] [CrossRef]
- Khabzina, Y.; Dhainaut, J.; Ahlhelm, M.; Richter, H.-J.; Reinsch, H.; Stock, N.; Farruseng, D. Synthesis and shaping scale-up study of functionalized UiO-66 MOF for ammonia air purification filters. Ind. Eng. Chem. Res. 2018, 57, 8200–8208. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Ahmed, I.; Lee, H.J.; Jhung, S.H. Metal-organic frameworks bearing free carboxylic acids: Preparation, modification, and applications. Coord. Chem. Rev. 2022, 450, 214237. [Google Scholar] [CrossRef]
- Moreira, M.A.; Dias, R.O.M.; Lee, U.H.; Chang, J.S.; Ribeiro, A.M.; Ferreira, A.F.P.; Rodrigues, A.E. Adsorption equilibrium of carbon dioxide, methane, nitrogen, carbon monoxide, and hydrogen on UiO-66(Zr)_(COOH)2. J. Chem. Eng. Data 2019, 64, 4724–4732. [Google Scholar] [CrossRef]
- Wang, G.; Graham, E.; Zheng, S.; Zhu, J.; Zhu, R.; Hongping, H.; Zhiming, S.; Mackinnon, I.D.R.; Xi, Y. Diatomite-Metal-Organic framework composite with hierarchical pore structures for adsorption/desorption of hydrogen, carbon dioxide and water vapor. Materials 2020, 13, 4700. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, H.; Song, F.; Huang, T.; Ji, J.; Zhong, Q.; Chu, W.; Xu, Q. UiO-66-NH2/GO composite: Synthesis, characterization and CO2 adsorption performance. Materials 2018, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Vaghi, O.M. Carbon capture and conversion using metal-organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef]
- Kinik, F.P.; Altintas, C.; Balci, V.; Koyuturk, B.; Uzun, A.; Keskin, S. [BMIM][PF6] Incorporation doubles CO2 selectivity of ZIF-8: Elucidation of interactions and their consequences on performance. ACS Appl. Mater. Interfaces 2016, 8, 30992–31005. [Google Scholar] [CrossRef]
- Cota, I.; Martinez, F.F. Recent advances in the synthesis and applications of metal organic frameworks doped with ionic liquids for CO2 adsorption. Coord. Chem. Rev. 2017, 351, 189–204. [Google Scholar] [CrossRef]
- Sezginel, K.B.; Keskin, S.; Uzun, A. Tuning the gas separation performance of CuBTC by ionic liquid incorporation. Langmuir 2016, 32, 1139–1147. [Google Scholar] [CrossRef]
- Ding, N.; Li, H.; Feng, X.; Wang, Q.; Wang, S.; Ma, L.; Zhou, J.; Wang, B. Partitioning MOF-5 into confined and hydrophobic compartments for carbon capture under humid conditions. J. Am. Chem. Soc. 2016, 138, 10100–10103. [Google Scholar] [CrossRef]
- Darunte, L.A.; Oetomo, A.D.; Walton, K.S.; Sholl, D.S.; Jones, C.W. Direct air capture of CO2 using amine functionalized MIL-101(Cr). ACS Sustain. Chem. Eng. 2016, 4, 5761–5768. [Google Scholar] [CrossRef]
- Kumar, R.; Raut, D.; Ramamurty, U.; Rao, C.N.R. Remarkable improvement in the mechanical properties and CO2 uptake of MOFs brought about by covalent linking to graphene. Angew. Chem. Int. Ed. 2016, 55, 7857–7861. [Google Scholar] [CrossRef]
- Liu, S.; Sun, L.; Xu, F.; Zhang, J.; Jiao, C.; Li, F.; Li, Z.; Wang, S.; Wang, Z.; Jiang, X.; et al. Nanosized Cu-MOFs induced by graphene oxide and enhanced gas storage capacity. Energy Environ. Sci. 2013, 6, 818–823. [Google Scholar] [CrossRef]
- Zhao, Y.; Seredcyh, M.; Jagiello, J.; Zhong, Q.; Bandosz, T.J. Insight into the mechanism of CO2 adsorption on Cu–BTC and its composites with graphite oxide or aminated graphite oxide. Chem. Eng. J. 2014, 239, 399–407. [Google Scholar] [CrossRef]
- Bian, Z.; Xu, J.; Zhang, S.; Zhu, X.; Liu, H.; Hu, J. Interfacial growth of metal organic framework/graphite oxide composites through pickering emulsion and their CO2 capture performance in the presence of humidity. Langmuir 2015, 31, 7410–7417. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Hu, Z.; Cao, D.; Yang, W.; Lu, J.; Han, B.; Wang, W. Metal–organic frameworks with incorporated carbon nanotubes: Improving carbon dioxide and methane storage capacities by lithium doping. Angew. Chem. Int. Ed. 2011, 50, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, S.; Huang, X.; Pang, H. Recent advances and challenges of metal–organic framework/graphene-based composites. Compos. B Eng. 2022, 230, 109532. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Vedenyapina, M.D.; Kurmysheva, A.Y.; Weichgrebe, D.; Nair, R.R.; Nguyen, N.P.T.; Kustov, L.M. Modern carbon–based materials for adsorptive removal of organic and inorganic pollutants from water and wastewater. Molecules 2021, 26, 6628. [Google Scholar] [CrossRef]
- Liu, X.W.; Sun, T.Y.; Hu, J.L.; Wang, S.D. Composites of metal-organic frameworks and carbon-based materials: Preparations, functionalities and applications. J. Mater. Chem. A. 2016, 4, 3584–3616. [Google Scholar] [CrossRef]
- Ahmed, I.; Jhung, S.H. Composites of metal–organic frameworks: Preparation and application in adsorption. Mater. Today 2014, 17, 136–146. [Google Scholar] [CrossRef]
- Azhari, K.M.K. Development of Metal-Organic Framework Carbon Composites for Carbon Dioxide and Methane Separation. Ph.D. Thesis, Ecole Nationale Supérieure Mines-Télécom Atlantique, Universiti Teknologi PETRONAS, Seri Iskandar, Perak, Malaisie, 2020. [Google Scholar]
- Szczesniak, B.; Choma, J.; Jaroniec, M. Gas adsorption properties of hybrid graphene-MOF materials. J. Colloid Interface Sci. 2018, 514, 801–813. [Google Scholar] [CrossRef]
- Szczesniak, B.; Choma, J. Graphene-containing microporous composites for selective CO2 adsorption. Microporous Mesoporous Mater. 2020, 292, 109761. [Google Scholar] [CrossRef]
- Zhang, Y.; Wibowo, H.; Zhong, L.; Horttanainen, M.; Wang, Z.; Yu, C.; Yan, M. Cu-BTC-based composite adsorbents for selective adsorption of CO2 from syngas. Sep. Purif. Technol. 2021, 279, 119644. [Google Scholar] [CrossRef]
- Doman, A.; Klebert, S.; Madarasz, J.; Safran, G.; Wang, Y.; Laszlo, K. Graphene oxide protected copper benzene-1,3,5-tricarboxylate for clean energy gas adsorption. Nanomaterials 2020, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, Z.; Yang, B. Selective gas adsorption and separation of carbon dioxide in metal-organic frameworks and composites. J. Phys. Conf. Ser. 2021, 2021, 012004. [Google Scholar] [CrossRef]
- Shang, S.; Tao, Z.; Yang, C.; Hanif, A.; Li, L.; Tsang, D.C.; Gu, Q.; Shang, J. Facile synthesis of CuBTC and its graphene oxide composites as efficient adsorbents for CO2 capture. Chem. Eng. J. 2020, 393, 124666. [Google Scholar] [CrossRef]
- Chen, Y.; Lv, D.; Wu, J.; Xiao, J.; Xi, H.; Xia, Q.; Li, Z. A new MOF-505@GO composite with high selectivity for CO2/CH4 and CO2/N2 separation. Chem. Eng. J. 2017, 308, 1065–1072. [Google Scholar] [CrossRef]
- International Biochar Initiative. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil; International Biochar Initiative: Toronto, ON, Canada, 2015. [Google Scholar]
- Wang, L.; Ok, Y.S.; Tsang, D.C.W.; Alessi, D.S.; Rinklebe, J.; Mašek, O.; Bolan, N.S.; Hou, D. Biochar composites: Emerging trends, field successes and sustainability implications. Soil Use Manag. 2022, 38, 14–38. [Google Scholar] [CrossRef]
- Yuan, N.; Zhang, X.; Wang, L. The marriage of metal–organic frameworks and silica materials for advanced applications. Coord. Chem. Rev. 2020, 421, 213442. [Google Scholar] [CrossRef]
- Tari, N.E.; Tadjarodi, A.; Tamanloo, J.; Fatemi, S. One pot microwave synthesis of MCM-41/Cu based MOF composite with improved CO2 adsorption and selectivity. Microporous Mesoporous Mater. 2016, 231, 154–162. [Google Scholar] [CrossRef]
- Ma, M.; Lu, L.; Li, H.; Xiong, Y.; Dong, F. Functional metal organic framework/SiO2 nanocomposites: From versatile synthesis to advanced applications. Polymers 2019, 11, 1823. [Google Scholar] [CrossRef] [Green Version]
- Lestari, W.W.; Saraswati, T.E.; Krisnandi, Y.K.; Arrozi, U.S.F.; Heraldy, E.; Kadja, G.T.M. Composite material consisting of HKUST-1 and Indonesian activated natural zeolite and its application in CO2 Capture. Open Chem. 2020, 17, 1279–1287. [Google Scholar] [CrossRef]
- Krūmiņš, J.; Kļaviņš, M.; Ozola-Davidāne, R.; Ansone-Bērtiņa, L. The Prospects of Clay Minerals from the Baltic States for Industrial-Scale Carbon Capture: A Review. Minerals 2022, 12, 349. [Google Scholar] [CrossRef]
- Xie, X.-Y.; Qian, X.-Y.; Qi, S.-C.; Wu, J.-K.; Liu, X.-Q.; Sun, L.-B. Endowing Cu-BTC with improved hydrothermal stability and catalytic activity: Hybridization with natural clay attapulgite via vapor-induced crystallization. ACS Sustain. Chem. Eng. 2018, 6, 10. [Google Scholar] [CrossRef]
- Shen, J.; Wang, N.; Guang Wang, Y.; Yu, D.; Ouyang, X. Efficient adsorption of Pb(II) from aqueous solutions by metal organic framework (Zn-BDC) coated magnetic montmorillonite. Polymers 2018, 10, 1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennabi, S.; Belbachir, M. Synthesis and Characterization of a new hybrid material (MOF-5/Mag-H+) based on a Metal-Organic Framework and a Proton Exchanged Montmorillonite Clay (Maghnite-H+) as catalytic support. J. Mater. Environ. Sci. 2017, 12, 4391–4398. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Zhou, Q. Strong Foam-like Composites from Highly Mesoporous Wood and Metal-Organic Frameworks for Efficient CO2 Capture. ACS Appl. Mater. Interfaces 2021, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Israr, F.; Kim, D.K.; Kim, Y.; Oh, S.J.; Ng, K.C.; Chun, W. Synthesis of porous Cu-BTC with ultrasonic treatment: Effects of ultrasonic power and solvent condition. Ultrason. Sonochem. 2016, 29, 186–193. [Google Scholar] [CrossRef]
- Li, M.; Huang, W.; Tang, B.; Song, F.; Lv, A.; Ling, X. Preparation of a composite material AC/Cu-BTC with improved water stability and n-hexane vapor adsorption. J. Nanomater. 2019, 2019, 5429264. [Google Scholar] [CrossRef]
- Yang, Q.; Vaesen, S.; Ragon, F.; Wiersum, A.D.; Wu, D.; Lago, A.; Devic, T.; Martineau, C.; Taulelle, F.; Llewellyn, P.L.; et al. A water stable Metal–Organic Framework with optimal features for CO2 capture. Angew. Chem. Int. Ed. 2013, 52, 10316–10320. [Google Scholar] [CrossRef]
- Song, J.; Srivastava, V.; Kohout, T.; Sillanpää, M.; Sainio, T. Montmorillonite-anchored magnetite nanocomposite for recovery of ammonium from stormwater and its reuse in adsorption of Sc3−. Nanotechnol. Environ. Eng. 2021, 6, 55. [Google Scholar] [CrossRef]
- Waqas, M.; Aburiazaiza, A.S.; Minadad, R.; Rehan, M.; Barakat, M.A.; Nizami, A.S. Development of Biochar as Fuel and Catalyst in Energy Recovery Technologies. J. Clean. Prod. 2018, 188, 477–488. [Google Scholar] [CrossRef]
- Kamal, K.; Bustam, M.A.; Shariff, A.M.; Pre, P.; Hamon, L. Indexing PXRD structural parameters of graphene oxide-doped metal-organic frameworks. Int. J. Recent Technol. 2019, 8, 550–553. [Google Scholar] [CrossRef]
- Graham, A.J.; Tan, J.-C.; Allan, D.R.; Moggach, S.A. The effect of pressure on Cu-btc: Framework compression vs. guest inclusion. ChemComm 2012, 48, 1535–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binns, J.; Kamenev, K.V.; Marriott, K.E.R.; McIntyre, G.J.; Moggach, S.A.; Murrie, M.; Parsons, S. A non-topological mechanism for negative linear compressibility. ChemComm 2016, 52, 7486–7489. [Google Scholar] [CrossRef] [Green Version]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. JACS 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Miao, J.; Sun, X.; Xiao, J.; Li, Y.; Wang, H.; Xia, Q.; Li, Z. Mechanochemical synthesis of Cu-BTC@GO with enhanced water stability and toluene adsorption capacity. Chem. Eng. J. 2016, 298, 191–197. [Google Scholar] [CrossRef]
- Elnour, A.Y.; Alghyamah, A.A.; Shaikh, H.M.; Poulose, A.M.; Al-Zahrani, S.M.; Anis, A.; Al-Wabel, M.I. Effect of pyrolysis temperature on biochar microstructural evolution, physicochemical characteristics, and its influence on biochar/polypropylene composites. Appl. Sci. 2019, 9, 1149. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Zhan, Y.; Hu, J.; Zhang, G.; Zhao, S.; Feng, Q.; Yang, W. Chemically stable two-dimensional MXene@UIO-66-(COOH)2 composite lamellar membrane for multi-component pollutant-oil-water emulsion separation. Compos. B Eng. 2020, 197, 108188. [Google Scholar] [CrossRef]
- Grajciar, L.; Wiersum, A.D.; Llewellyn, P.L.; Chang, J.-S.; Nachtigall, P. Understanding CO2 adsorption in CuBTC MOF: Comparing combined DFT-ab initio calculations with microcalorimetry experiments. J. Phys. Chem. C 2011, 115, 17925–17933. [Google Scholar] [CrossRef]
- Ragon, F.; Campo, B.; Yang, Q.; Martineau, C.; Wiersum, A.D.; Lago, A.; Guillerm, V.; Hemsley, C.; Eubank, J.F.; Vishnuvarthan, M.; et al. Acid-functionalized UiO-66(Zr) MOFs and their evolution after intra-framework cross-linking: Structural features and sorption properties. J. Mater. Chem. A 2015, 3, 3294–3309. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, Y.; Zhong, Q. CO2 capture on metal-organic framework and graphene oxide composite using a high-pressure static adsorption apparatus. J. Clean Energy Technol. 2014, 2, 34–37. [Google Scholar] [CrossRef] [Green Version]
- Baumann, A.E.; Aversa, G.E.; Roy, A.; Falk, M.L.; Bedford, N.M.; Thoi, V.S. Promoting sulfur adsorption using surface Cu sites in metal–organic frameworks for lithium sulfur batteries. J. Mater. Chem. A 2018, 6, 4811. [Google Scholar] [CrossRef]
- Vishnyakov, A.; Ravikovitch, P.I.; Neimark, A.V.; Bulow, M.; Wang, Q.M. Nanopore structure and sorption properties of Cu−BTC metal−organic framework. Nano Lett. 2003, 3, 713–718. [Google Scholar] [CrossRef]
- Ullah, S.; Sharif, A.M.; Bustam, M.A.; Elkhalifah, A.E.I.; Gonfa, G.; Kareem, F.A.A. The role of multiwall carbon nanotubes in Cu-BTC metal-organic frameworks for CO2 adsorption. J. Chin. Chem. Soc. 2016, 63, 1022–1032. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Yang, X.; Li, M.; Chen, C.; Zhang, N. The fixation of carbon dioxide with epoxides catalyzed by cation-exchanged metal-organic framework. Microporous Microporous Mater. 2018, 258, 55–61. [Google Scholar] [CrossRef]
- Foldvari, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Occasional Papers of the Geological Institute of Hungary; Geological Institute of Hungary: Budapest, Hungary, 2011. [Google Scholar]
- Wu, H.; Simmons, M.; Srinivas, G.; Zhou, W.; Yildirim, T. Adsorption sites and binding nature of CO2 in prototypical metal-organic frameworks: A combined neutron diffraction and first-principles study. J. Phys. Chem. Lett. 2010, 1, 1946–1951. [Google Scholar] [CrossRef]
- Thakkar, H.; Eastman, S.; Al-Naddaf, Q.; Rownaghi, A.A.; Rezaei, F. 3D-Printed metal−organic framework monoliths for gas adsorption processes. ACS Appl. Mater. Interfaces 2017, 9, 35908–35916. [Google Scholar] [CrossRef]
- Kitao, T.; Zhang, Y.; Kitagawa, S.; Wang, B.; Uemura, T. Hybridization of MOFs and polymers. Chem. Soc. Rev. 2017, 46, 3108–3133. [Google Scholar] [CrossRef]
- Alfe, M.; Policicchio, A.; Lisi, L.; Gargiulo, V. Solid sorbents for CO2 and CH4 adsorption: The effect of metal organic framework hybridization with graphene-like layers on the gas sorption capacities at high pressure. Renew. Sustain. Energy Rev. 2021, 141, 110816. [Google Scholar] [CrossRef]
- Qian, D.; Lei, C.; Hao, G.-P.; Li, W.-C.; Lu, A.-H. Synthesis of hierarchical porous carbon monoliths with incorporated metal−organic frameworks for enhancing volumetric based CO2 capture capability. ACS Appl. Mater. Interfaces 2012, 4, 6125–6132. [Google Scholar] [CrossRef]
- Wan, H.; Que, Y.; Chen, C.; Wu, Z.; Gu, Z.; Meng, J.; Wang, L.; Guan, G. Preparation of metal-organic framework/attapulgite hybrid material for CO2 capture. Mater. Lett. 2017, 194, 107–109. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Lawson, S.; Al-Naddaf, Q.; Krishnamurthy, A.; Amor, M.S.; Griffin, C.; Rownaghi, A.A.; Knox, J.C.; Rezaei, F. UTSA-16 growth within 3D-printed Co-Kaolin monoliths with high selectivity for CO2/CH4, CO2/N2, and CO2/H2 separation. ACS Appl. Mater. Interfaces 2018, 10, 19076–19086. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Lawson, S.; Hosseini, H.; Thakkar, H.; Hajari, A.; Monjezi, S.; Rownaghi, A.A. MOF-74 and UTSA-16 film growth on monolithic structures and their CO2 adsorption performance. Chem. Eng. J. 2017, 313, 1346–1353. [Google Scholar] [CrossRef]
- Gaikwad, S.; Han, S. A microwave method for the rapid crystallization of UTSA-16 with improved performance for CO2 capture. Chem. Eng. J. 2019, 371, 813–820. [Google Scholar] [CrossRef]
- Hu, Z.; Peng, Y.; Kang, Z.; Qian, Y.; Zhao, D. A modulated hydrothermal (MHT) approach for the facile synthesis of UiO-66-type MOFs. Inorg. Chem. 2015, 54, 4862–4868. [Google Scholar] [CrossRef]
- Liang, Z.; Marshall, M.; Chaffee, A.L. CO2 Adsorption-based separation by metal organic framework (Cu-BTC) versus Zeolite (13X). Energy Fuels 2009, 23, 2785–2789. [Google Scholar] [CrossRef]
- Zagho, M.M.; Hassan, M.K.; Khraisheh, M.; Al-Maadeed, M.A.A.; Nazarenko, S. A review on recent advances in CO2 separation using zeolite and zeolite-like materials as adsorbents and fillers in mixed matrix membranes (MMMs). Chem. Eng. J. Adv. 2021, 6, 100091. [Google Scholar] [CrossRef]
- Karka, S.; Kodukula, S.; Nandury, S.V.; Pal, U. Polyethylenimine-modified zeolite 13X for CO2 capture: Adsorption and kinetic studies. ACS Omega 2019, 4, 16441–16449. [Google Scholar] [CrossRef] [Green Version]
- Moura, P.A.S.; Bezerra, D.P.; Vilarrasa-Garcia, E.; Bastos-Neto, M.; Azevedo, D.C.S. Adsorption equilibria of CO2 and CH4 in cation-exchanged zeolites 13X. Adsorption 2016, 22, 71–80. [Google Scholar] [CrossRef]
- Pardakhti, M.; Jafari, T.; Tobin, Z.; Dutta, B.; Moharreri, E.; Shemshaki, N.S.; Suib, S.; Srivastava, R. Trends in solid adsorbent materials development for CO2 capture. ACS Appl. Mater. Interfaces 2019, 11, 34533–34559. [Google Scholar] [CrossRef]
- Querejeta, N.; Gil, M.V.; Pevida, C.; Centeno, T.A. Standing out the key role of ultramicroporosity to tailor biomass-derived carbons for CO2 capture. J. CO2 Util. 2018, 26, 1–7. [Google Scholar] [CrossRef]
- Politakos, N.; Cordero-Lanzac, T.; Tomovska, R. Understanding the adsorption capacity for CO2 in reduced graphene oxide (rGO) and modified ones with different heteroatoms in relation to surface and textural characteristics. Appl. Sci. 2021, 11, 9631. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Shah, B.B.; Zhao, D. CO2 capture in metal–organic framework adsorbents: An engineering perspective. Adv. Sustain. Syst. 2019, 3, 1800080. [Google Scholar] [CrossRef] [Green Version]
- Lawson, S.; Rownaghi, A.A.; Rezaei, F. Development of carbon hollow fiber-supported metal-organic framework composites for gas adsorption. Energy Technol. 2018, 6, 694–701. [Google Scholar] [CrossRef]
- Pokhrel, J.; Bhoria, N.; Wu, C.; Reddy, K.S.K.; Margetis, H.; Anastasiou, S.; George, G.; Mittal, V.; Romanos, G.; Karonis, D.; et al. Cu- and Zr-based metal organic frameworks and their composites with graphene oxide for capture of acid gases at ambient temperature. J. Solid State Chem. 2018, 266, 233–243. [Google Scholar] [CrossRef]
- Cortés-Súarez, J.; Celis-Arias, V.; Beltrán, H.I.; Tejeda-Cruz, A.; Ibarra, I.A.; Romero-Ibarra, J.E.; Sánchez-González, E.; Loera-Serna, S. Synthesis and characterization of an SWCNT@HKUST-1 composite: Enhancing the CO2 adsorption properties of HKUST-1. ACS Omega 2019, 4, 5275–5282. [Google Scholar] [CrossRef] [Green Version]
- Varghese, A.M.; Reddy, K.S.K.; Bhoria, N.; Singh, S.; Pokhrel, J.; Karanikolos, G.N. Enhancing effect of UV activation of graphene oxide on carbon capture performance of metal-organic framework/graphene oxide hybrid adsorbents. Chem. Eng. J. 2021, 420, 129677. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansone-Bertina, L.; Ozols, V.; Arbidans, L.; Dobkevica, L.; Sarsuns, K.; Vanags, E.; Klavins, M. Metal–Organic Frameworks (MOFs) Containing Adsorbents for Carbon Capture. Energies 2022, 15, 3473. https://doi.org/10.3390/en15093473
Ansone-Bertina L, Ozols V, Arbidans L, Dobkevica L, Sarsuns K, Vanags E, Klavins M. Metal–Organic Frameworks (MOFs) Containing Adsorbents for Carbon Capture. Energies. 2022; 15(9):3473. https://doi.org/10.3390/en15093473
Chicago/Turabian StyleAnsone-Bertina, Linda, Viesturs Ozols, Lauris Arbidans, Linda Dobkevica, Kristaps Sarsuns, Edgars Vanags, and Maris Klavins. 2022. "Metal–Organic Frameworks (MOFs) Containing Adsorbents for Carbon Capture" Energies 15, no. 9: 3473. https://doi.org/10.3390/en15093473
APA StyleAnsone-Bertina, L., Ozols, V., Arbidans, L., Dobkevica, L., Sarsuns, K., Vanags, E., & Klavins, M. (2022). Metal–Organic Frameworks (MOFs) Containing Adsorbents for Carbon Capture. Energies, 15(9), 3473. https://doi.org/10.3390/en15093473









