Effect of In-Pore Wettability on Mass Transfer Performance of Fuel Cell Gas Diffusion Layer
Abstract
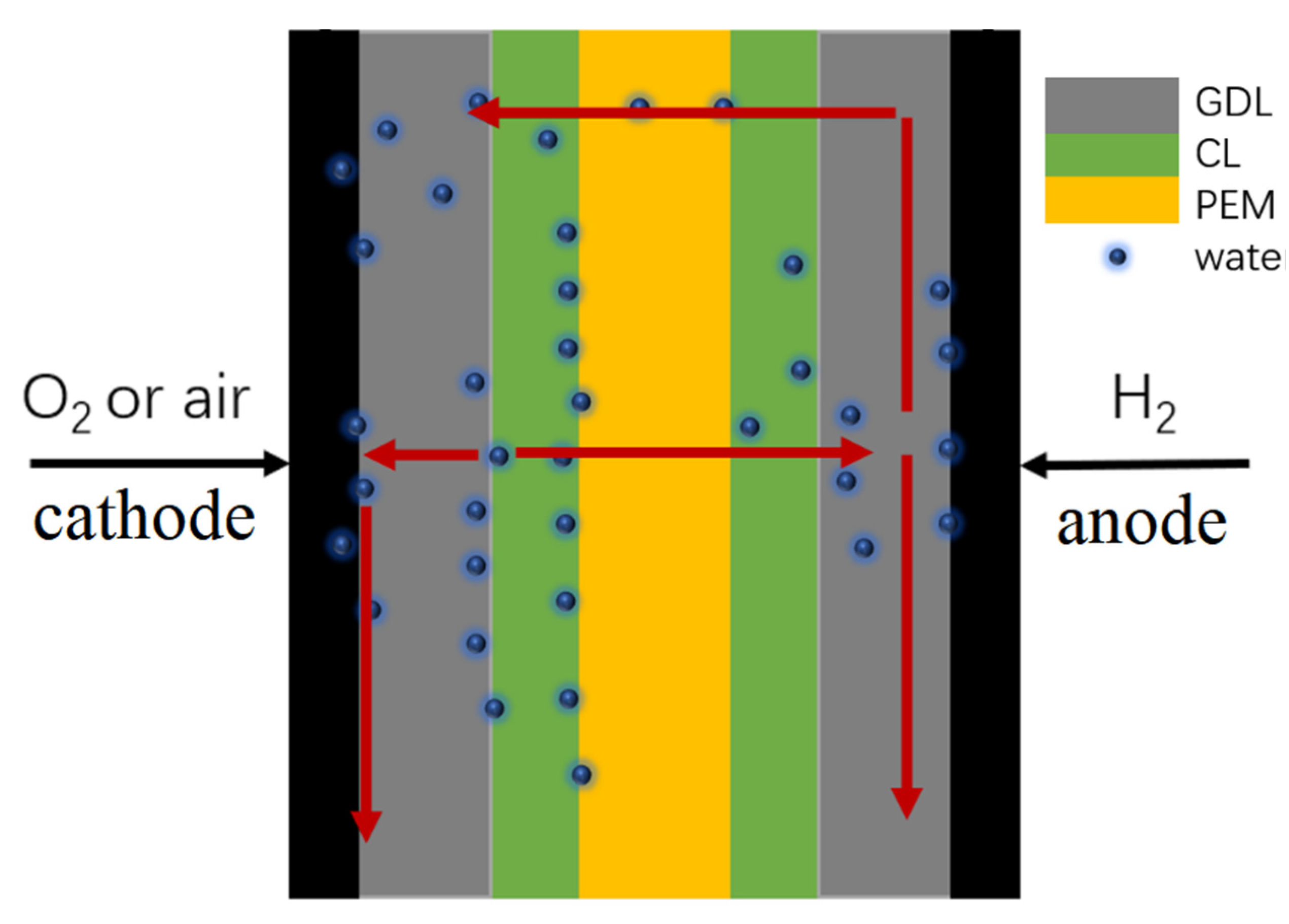
:1. Introduction
2. Experiment and Method
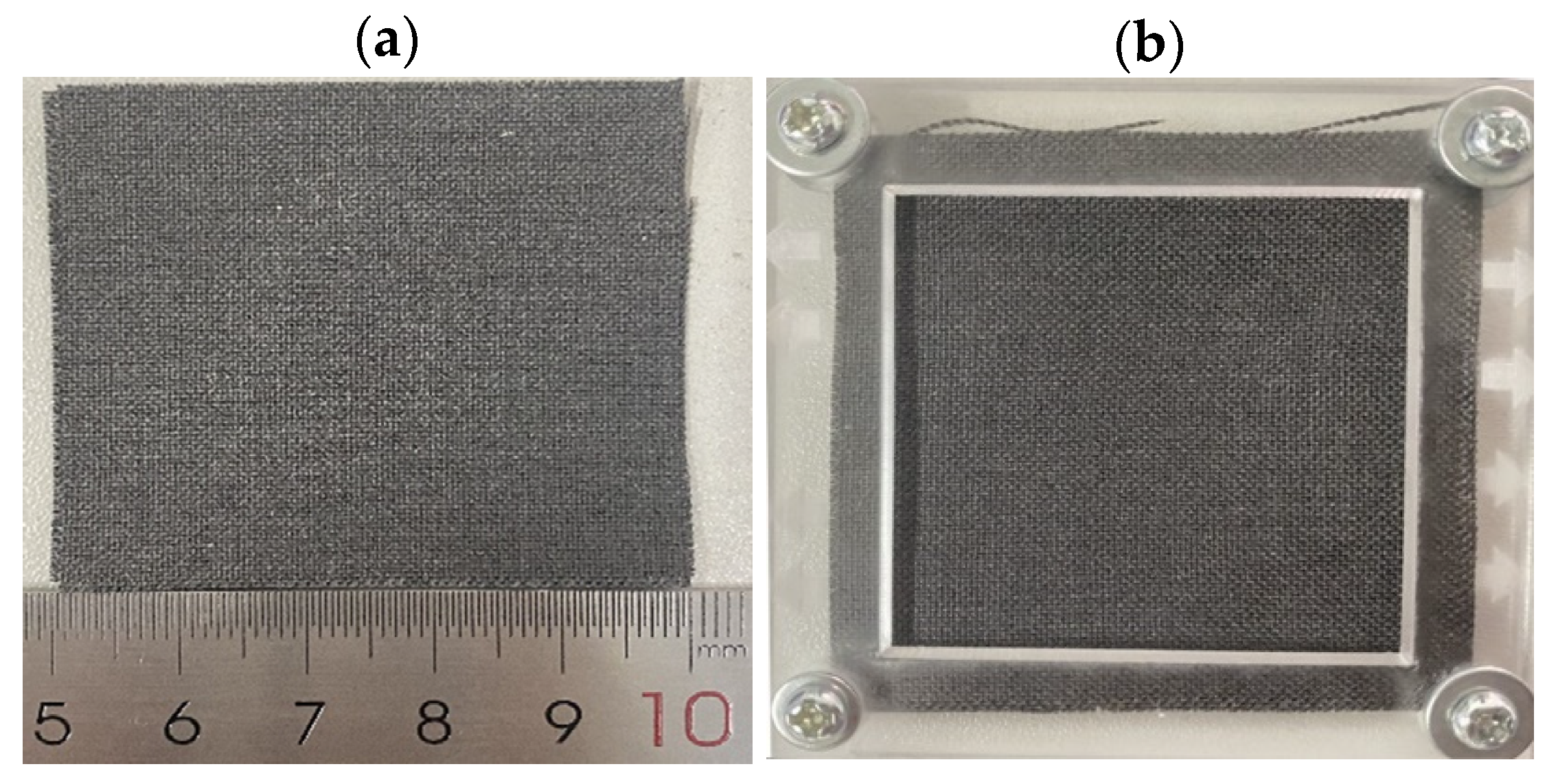
2.1. Preparation of Hydrophilic and Hydrophobic Carbon Cloth
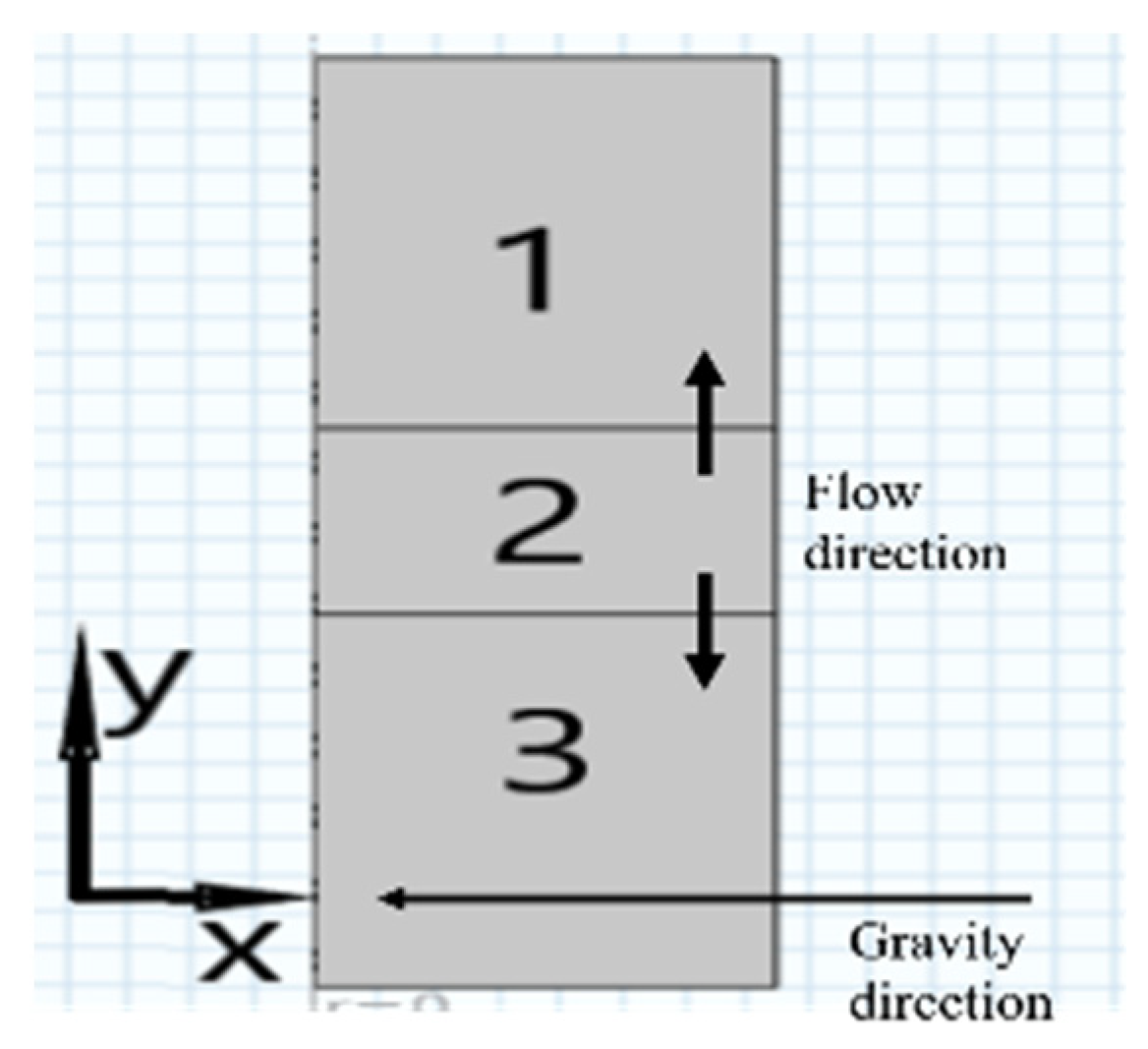
2.2. Numerical Simulation
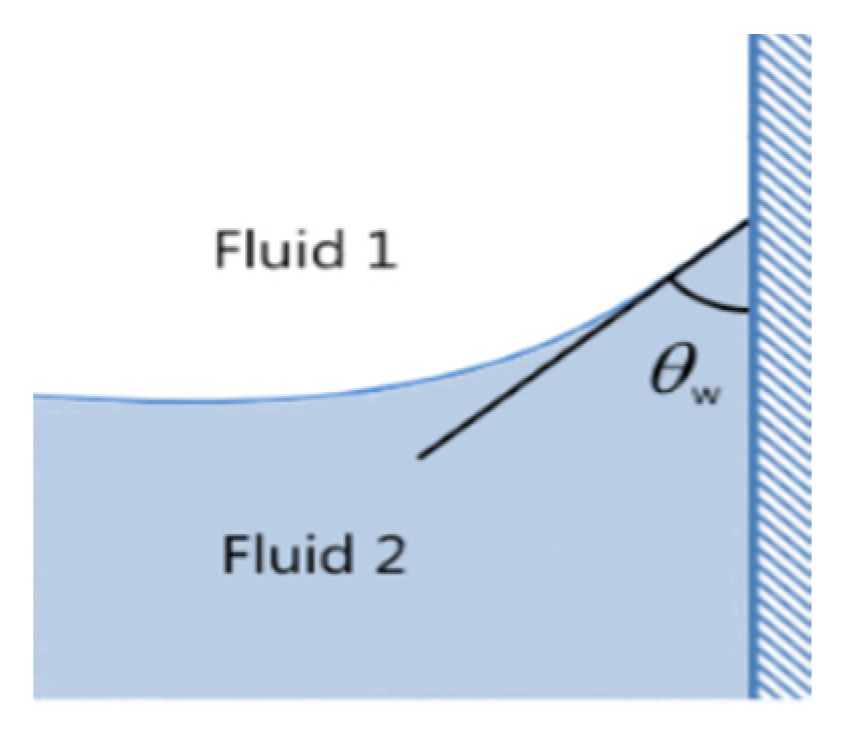
2.2.1. Representation of Fluid Interfaces and Convection
2.2.2. Mass and Momentum Transfer
2.2.3. Boundary Conditions
3. Results and Discussion
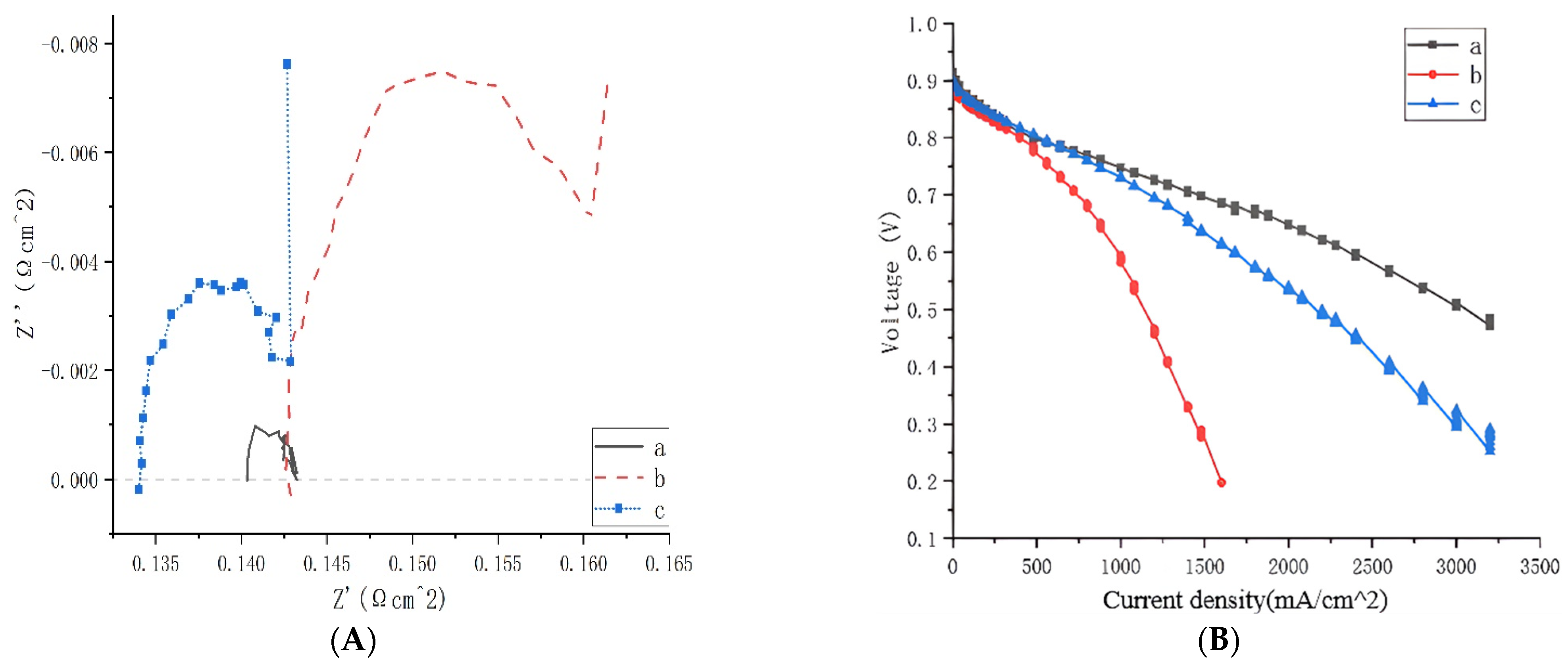
3.1. Electrochemical Test
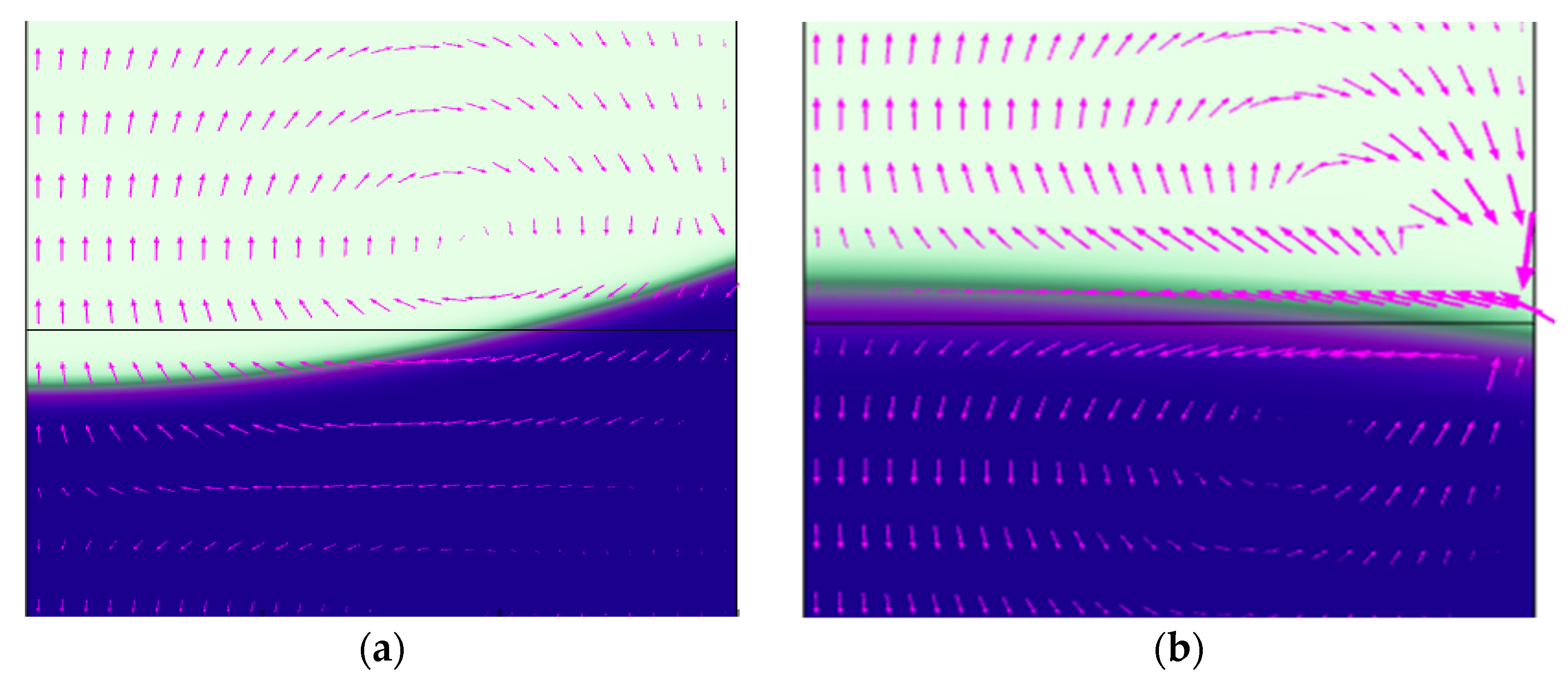
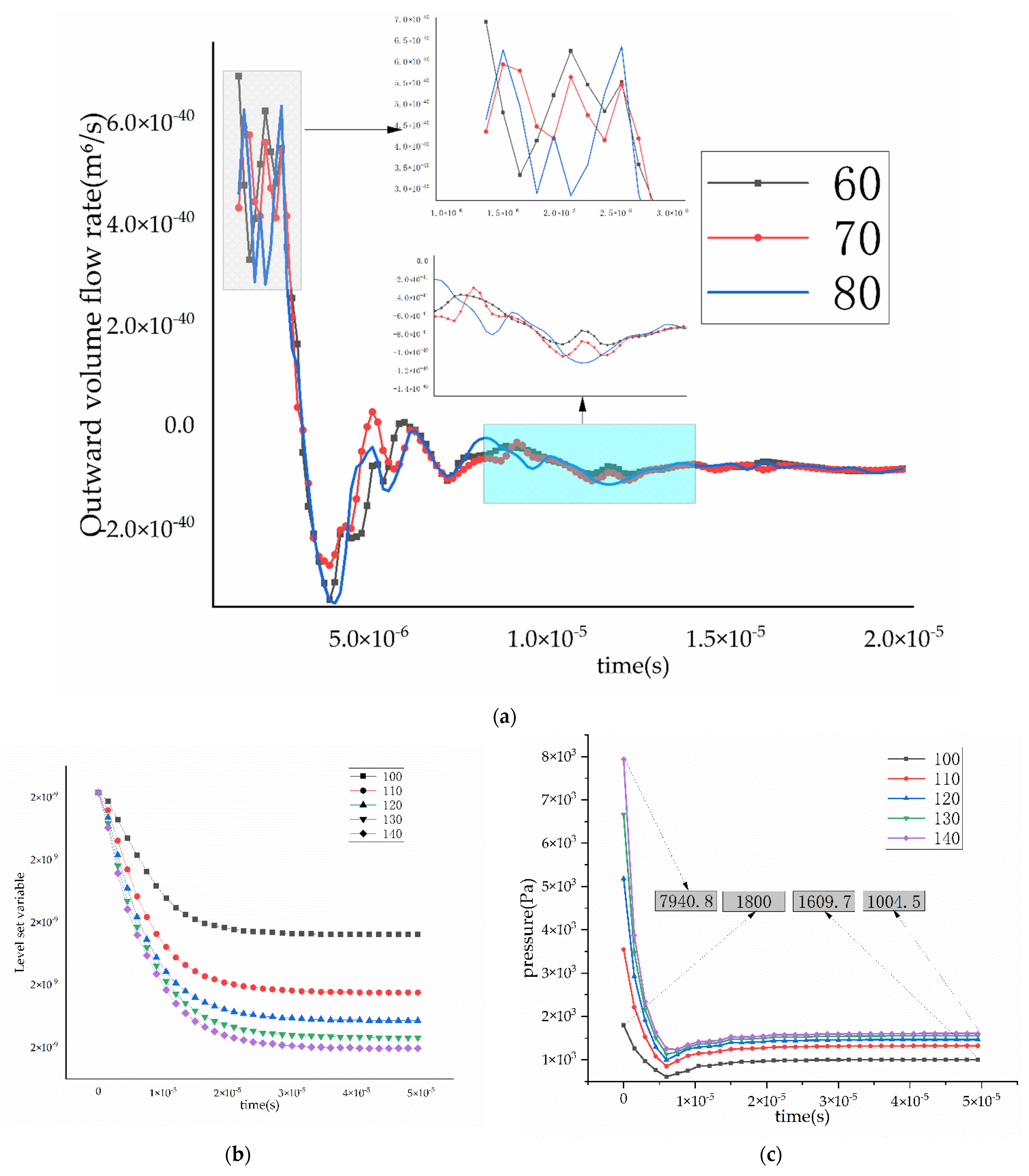
3.2. Influence of Intrapore Wettability on Mass Transfer during Natural Diffusion
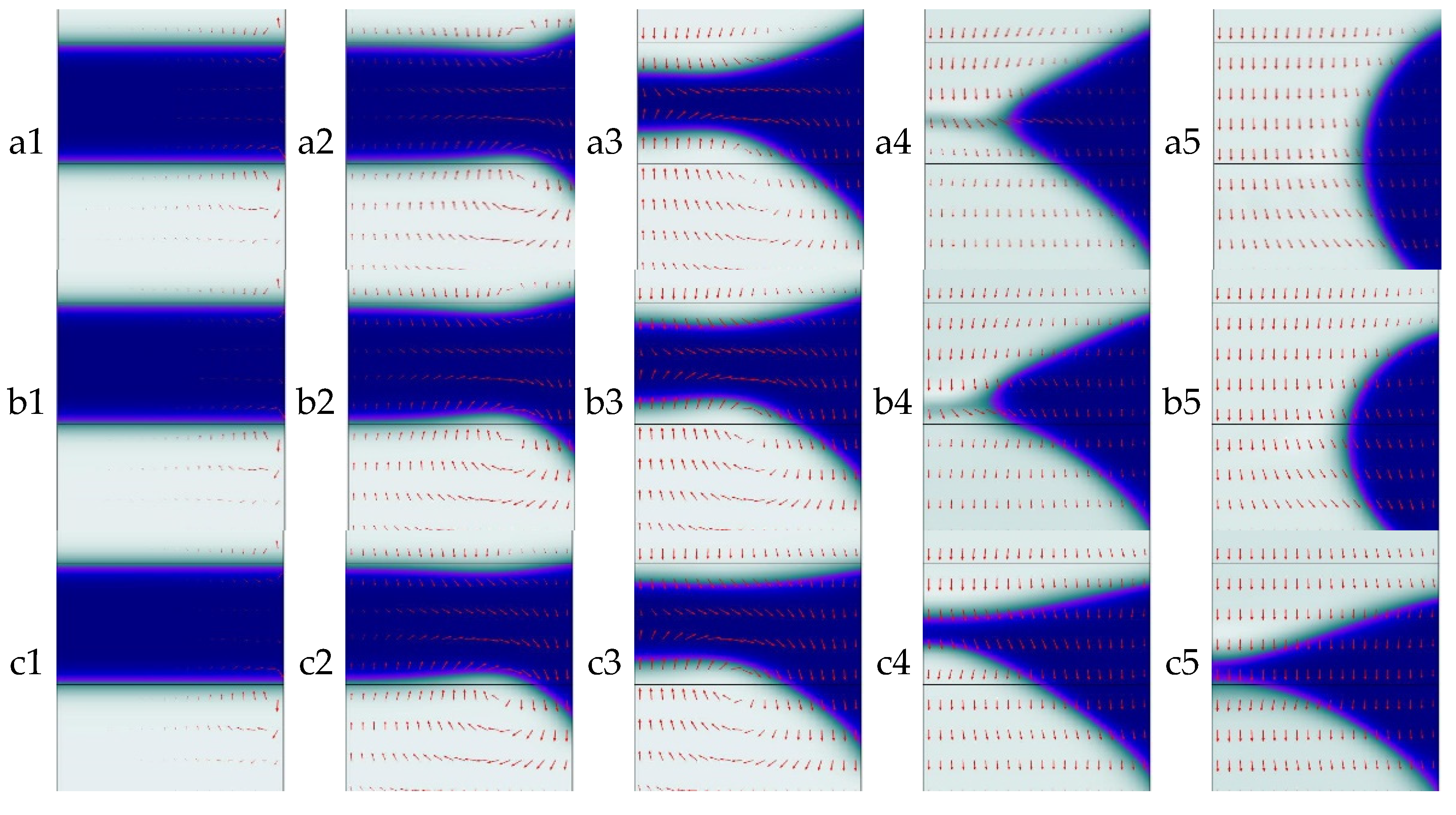
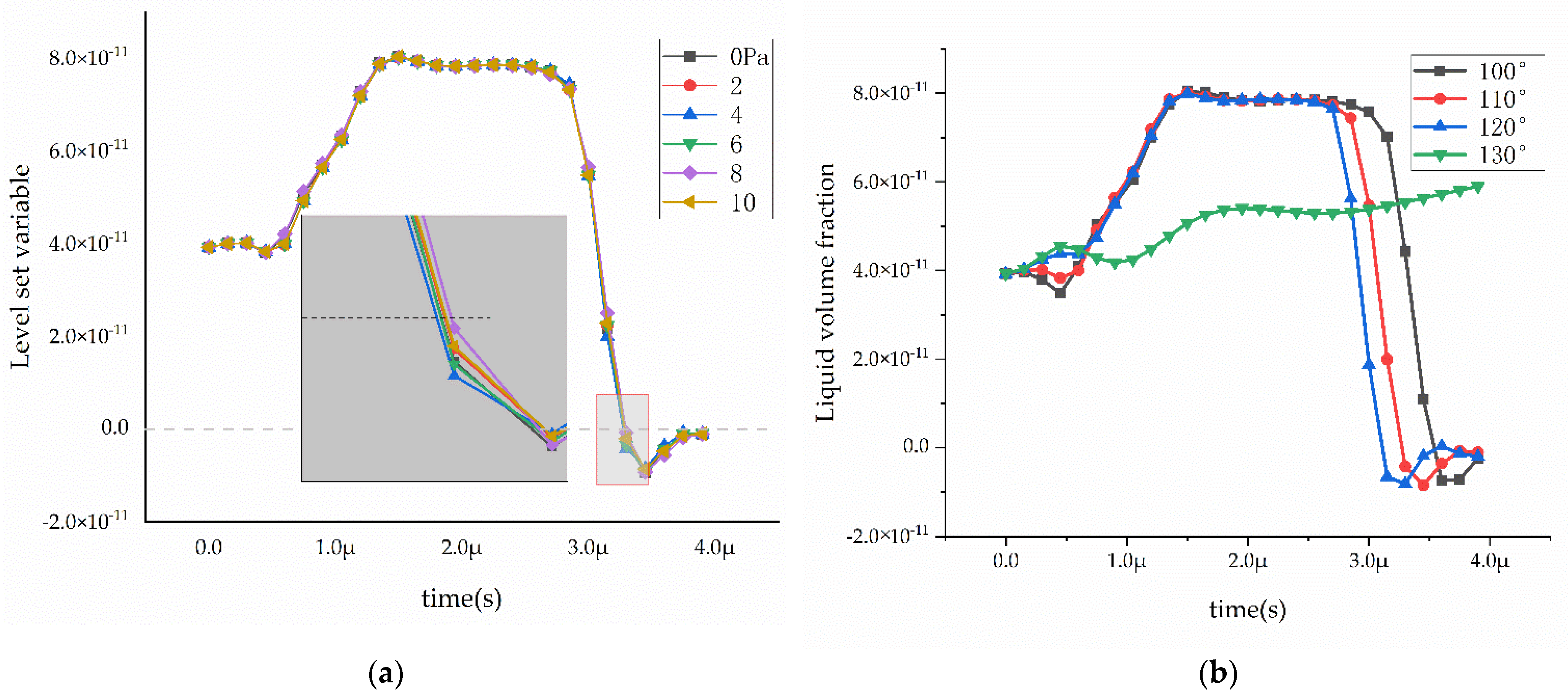
3.3. Effect of Wettability on Mass Transfer at Different Inlet Pressure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daokuan, J.; Kui, J.; Qing, D. Two-Phase Flow in Porous Electrodes of Proton Exchange Membrane Fuel Cell. Trans. Tianjin Univ. 2020, 26, 51–61. [Google Scholar]
- Fan, M.; Duan, F.; Wang, T.; Kang, M.; Zeng, B.; Xu, J.; Anderson, R.; Du, W.; Zhang, L. Effect of Pore Shape and Spacing on Water Droplet Dynamics in Flow Channels of Proton Exchange Membrane Fuel Cells. Energies 2021, 14, 1250. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Otor, C. A review of gas diffusion layer properties and water management in proton exchange membrane fuel cell system. Int. J. Energy Res. 2020, 45, 3780–3800. [Google Scholar] [CrossRef]
- Zhou, K.; Li, T.; Han, Y.; Wang, J.; Chen, J.; Wang, K. Optimizing the hydrophobicity of GDL to improve the fuel cell performance. RSC Adv. 2021, 11, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.; Xiaoyan, S.; Zhou, X.; Fanlin, H. Effect of compression on two-phase flow in gas diffusion layers treated with different hydrophobicity. J. Eng. Thermophys. 2020, 41, 3–8. [Google Scholar]
- Nagy, K.A.; Tóth, I.Y.; Ballai, G.; Varga, Á.T.; Szenti, I.; Sebők, D.; Kopniczky, J.; Hopp, B.; Kukovecz, Á. Wetting and evaporation on a carbon cloth type gas diffusion layer for passive direct alcohol fuel cells. J. Mol. Liq. 2020, 304, 2698. [Google Scholar] [CrossRef]
- Bao, Y.; Gan, Y. Roughness effects of gas diffusion layers on droplet dynamics in PEMFC flow channels. Int. J. Hydrogen Energy 2020, 45, 17869–17881. [Google Scholar] [CrossRef]
- Bazylak, A.; Sinton, D.; Liu, Z.S.; Djilali, N. Effect of compression on liquid water transport and microstructure of PEMFC gas diffusion layers. J. Power Sources 2007, 163, 784–792. [Google Scholar] [CrossRef]
- Alink, R.; Gerteisen, D. Modeling the Liquid Water Transport in the Gas Diffusion Layer for Polymer Electrolyte Membrane Fuel Cells Using a Water Path Network. Energies 2013, 6, 4508–4530. [Google Scholar] [CrossRef] [Green Version]
- Ji, M.; Wei, Z. A Review of Water Management in Polymer Electrolyte Membrane Fuel Cells. Energies 2009, 2, 1057–1106. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.; Nguyen, T.V. Effect of Thickness and Hydrophobic Polymer Content of the Gas Diffusion Layer on Electrode Flooding Level in a PEMFC. J. Electrochem. Soc. 2005, 152, A1942–A1948. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; St-Pierre, J.; Artyushkova, K.; Rocheleau, R.; Atanassov, P.; Bender, G.; Ulsh, M. Multianalytical Study of the PTFE Content Local Variation of the PEMFC Gas Diffusion Layer. J. Electrochem. Soc. 2013, 160, F1305–F1315. [Google Scholar] [CrossRef]
- Chun, J.H.; Park, K.T.; Jo, D.H.; Lee, J.Y.; Kim, S.G.; Park, S.H.; Lee, E.S.; Jyoung, J.-Y.; Kim, S.H. Development of a novel hydrophobic/hydrophilic double micro porous layer for use in a cathode gas diffusion layer in PEMFC. Int. J. Hydrogen Energy 2011, 36, 8422–8428. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, F. Impact of PTFE content and distribution on liquid–gas flow in PEMFC carbon paper gas distribution layer: 3D lattice Boltzmann simulations. Int. J. Hydrogen Energy 2016, 41, 8550–8562. [Google Scholar] [CrossRef]
- Kwon, S.H.; Lee, S.Y.; Kim, H.-J.; Jang, S.S.; Lee, S.G. Distribution characteristics of phosphoric acid and PTFE binder on Pt/C surfaces in high-temperature polymer electrolyte membrane fuel cells: Molecular dynamics simulation approach. Int. J. Hydrogen Energy 2021, 46, 17295–17305. [Google Scholar] [CrossRef]
- Pradecta, M.R.; Prakoso, T.; Indarto; Widyaparaga, A. Contact angle dynamics during the impact of single water droplet onto a hot flat practical stainless steel surface under medium Weber numbers. Heat Mass Transf. 2021, 57, 1097–1106. [Google Scholar]
- Janssen, D.; Palma, R.D.; Verlaak, S.; Heremans, P.; Dehaen, W. Static solvent contact angle measurements, surface free energy and wettability determination of various self-assembled monolayers on silicon dioxide. Thin Solid Film. 2006, 515, 1433–1438. [Google Scholar] [CrossRef]
- Qian, X.; Jie, L.I. Analysis of the Influence Factors of the Steady State Contact Angle Measurement. Sci. Technol. Vis. 2020, 101, 418–426. [Google Scholar]
- Gurtin, M.E. Generalized Ginzburg-Landau and Cahn-Hilliard equations based on a microforce balance. Phys. D Nonlinear Phenom. 1996, 92, 178–192. [Google Scholar] [CrossRef]
| Variable | Value |
|---|---|
| Contact angle θW (°) | 60–140 |
| Aperture size (μm) | 5, 15 |
| Inlet pressure (Pa) | 0–10, 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Q.; Li, M.; Lai, L. Effect of In-Pore Wettability on Mass Transfer Performance of Fuel Cell Gas Diffusion Layer. Energies 2022, 15, 3474. https://doi.org/10.3390/en15103474
Niu Q, Li M, Lai L. Effect of In-Pore Wettability on Mass Transfer Performance of Fuel Cell Gas Diffusion Layer. Energies. 2022; 15(10):3474. https://doi.org/10.3390/en15103474
Chicago/Turabian StyleNiu, Qinchuan, Minglin Li, and Lianfeng Lai. 2022. "Effect of In-Pore Wettability on Mass Transfer Performance of Fuel Cell Gas Diffusion Layer" Energies 15, no. 10: 3474. https://doi.org/10.3390/en15103474
APA StyleNiu, Q., Li, M., & Lai, L. (2022). Effect of In-Pore Wettability on Mass Transfer Performance of Fuel Cell Gas Diffusion Layer. Energies, 15(10), 3474. https://doi.org/10.3390/en15103474






