Valve Regulated Lead Acid Battery Evaluation under Peak Shaving and Frequency Regulation Duty Cycles
Abstract
:1. Introduction
2. Failure Modes for Lead Acid Batteries
- Positive grid corrosion
- Positive active material softening and shedding
- Electrolyte stratification (VRLA)
- Electrolyte dry-out (VRLA)
- Negative active material sulfation
2.1. Flooded Batteries
2.2. VRLA Batteries
3. Experimental Setup
3.1. Module Specification
3.2. Test Protocols
3.2.1. Reference Performance Capacity Test
3.2.2. DC Current Pulse Test to Measure Internal Resistance
3.2.3. Peak Shaving Duty Cycle Test
3.2.4. Frequency Regulation Duty Cycle Test
4. Results and Discussion
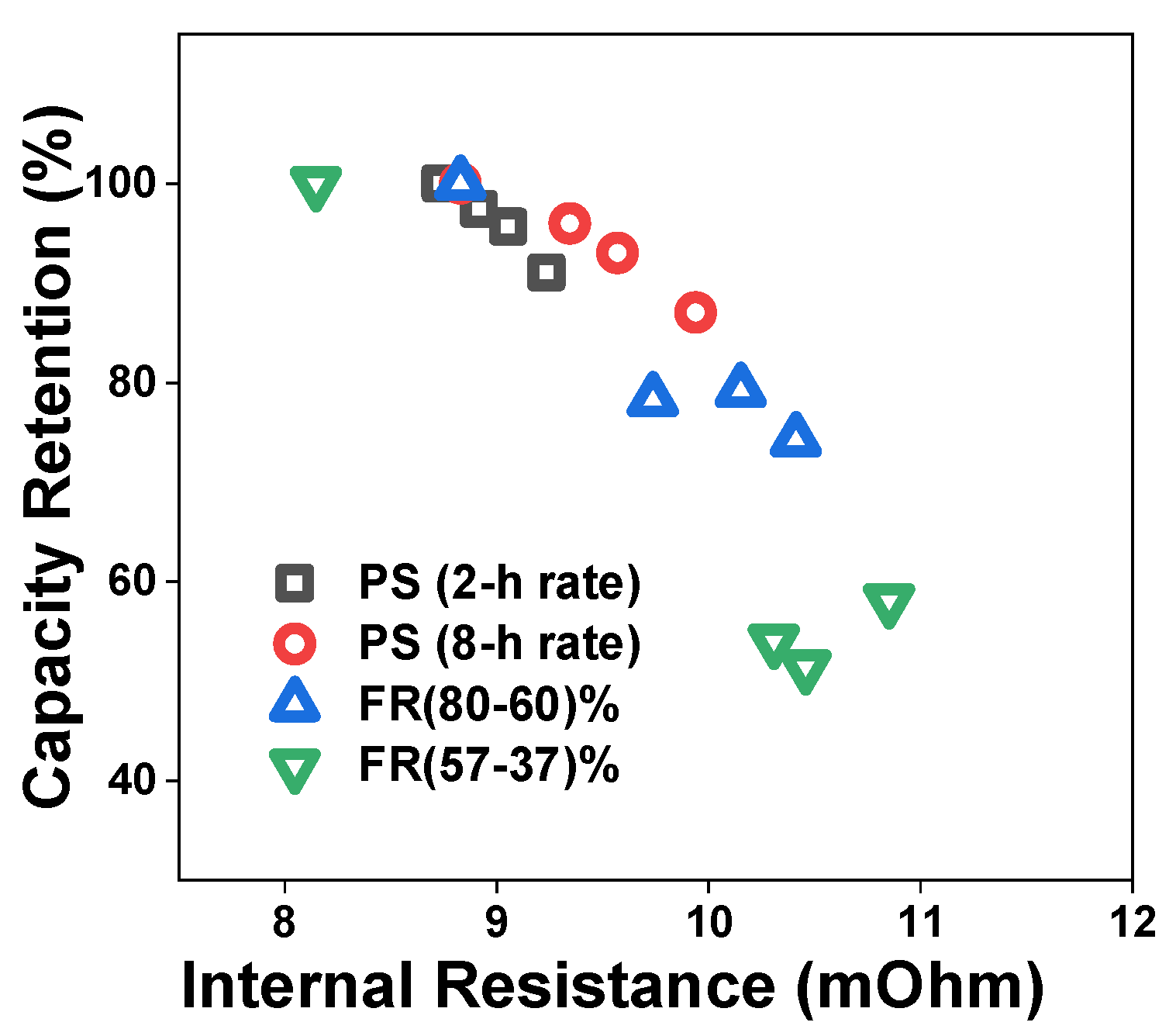
4.1. Reference Performance Test Results
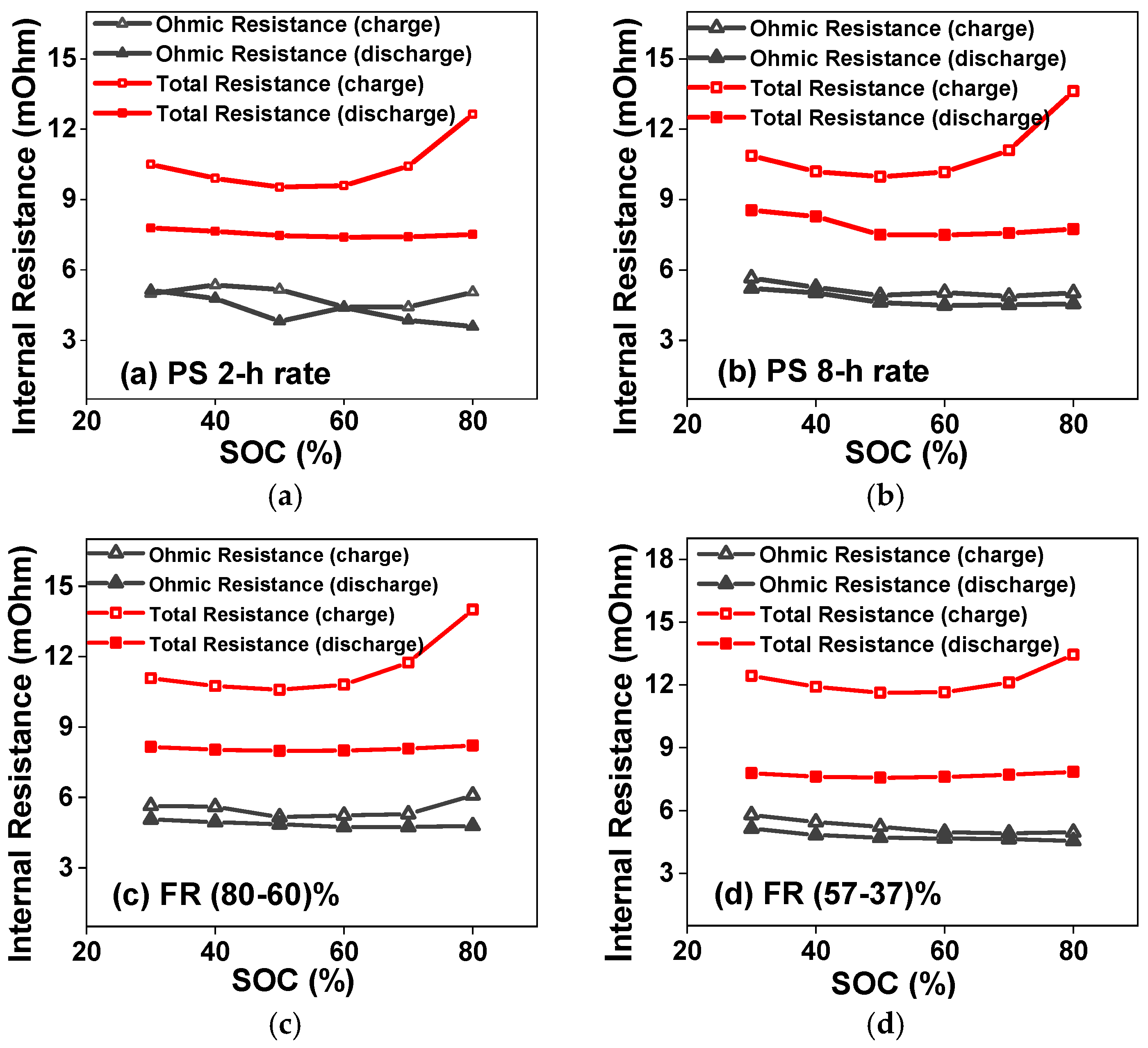
4.2. Pulse Resistance Test Results
4.3. Degradation Analysis
4.4. Recommended Tests to Validate the Proposed Failure Mechanism
- (1)
- Determine mass of negative, positive and separator. The negative electrode needs to be transferred immediately in deionized water to avoid oxidation in air.
- (2)
- Determine electrolyte content in positive, negative and separator by measuring wet and dry weight. The negative needs to be dried in a vacuum oven.
- (3)
- Determine electrolyte specific gravity by titrating a known electrolyte volume obtained by squeezing electrolyte from separator.
- (4)
- Determine electrolyte distribution at various heights of electrodes and separator (to estimate electrolyte stratification).
- a.
- Cut the electrode into three parts, top, middle, and bottom, measuring wet weight, followed by titration to determine acid content for each part, followed by measurement of dry weight.
- (5)
- Determine acid content at the top, middle and bottom parts of the separator, followed by filtration and drying of the retained separator.
- a.
- Immerse each separator piece in deionized water after weighing and titrate vs. 1 M NaOH.
- (6)
- Determine porosity and pore size distribution by BET and mercury porosimetry to correlate results with mass transport resistance.
- (7)
- Determine elemental composition using energy dispersive X-ray analysis (EDX) for the top, middle and bottom part of positive and negative electrodes.
4.5. Internal Resistance vs. SOC Trends for the Duty Cycles
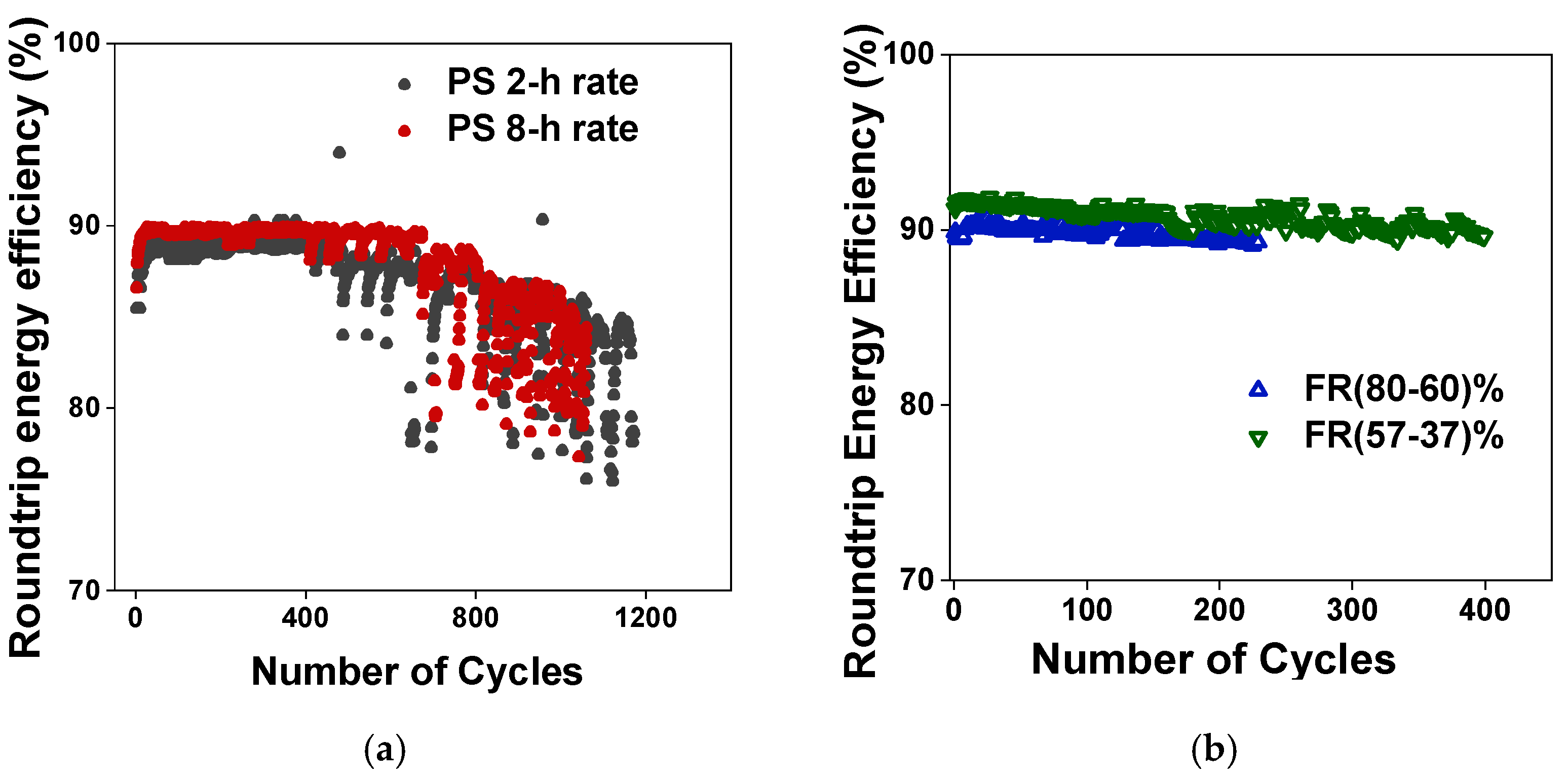
4.6. Round Trip Efficiency Results for the Duty Cycles
4.7. Mapping Hybrid Electric Vehicle (HEV) Degradation with Grid Services
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malhotra, A.; Battke, B.; Beuse, M.; Stephan, A.; Schmidt, T. Use cases for stationary battery technologies: A review of the literature and existing projects. Renew. Sustain. Energy Rev. 2016, 56, 705–721. [Google Scholar] [CrossRef]
- May, G.J.; Davidson, A.; Monahov, B. Lead batteries for utility energy storage: A review. J. Energy Storage 2018, 15, 145–157. [Google Scholar] [CrossRef]
- Choi, D.; Shamim, N.; Crawford, A.; Huang, Q.; Vartanian, C.K.; Viswanathan, V.V.; Paiss, M.D.; Alam, M.J.E.; Reed, D.M.; Sprenkle, V.L. Li-ion battery technology for grid application. J. Power Sources 2021, 511, 230419. [Google Scholar] [CrossRef]
- Cook, G.; Spindler, W. Low-maintenance, valve-regulated, lead/acid batteries in utility applications. J. Power Sources 1991, 33, 145–161. [Google Scholar] [CrossRef]
- Da Silva, V.C.; Gimenes, A.L.V.; Udaeta, M.E.M. Energy Storage and Multi-Source System for Reduction Energy Costs in the Consumer-Side. J. Power Energy Eng. 2021, 9, 80–105. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of current development in electrical energy storage technologies and the application potential in power system operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in electrical energy storage system: A critical review. Prog. Nat. Sci. 2009, 19, 291–312. [Google Scholar] [CrossRef]
- Ibrahim, H.; Ilinca, A.; Perron, J. Energy storage systems—Characteristics and comparisons. Renew. Sustain. Energy Rev. 2008, 12, 1221–1250. [Google Scholar] [CrossRef]
- Gandhi, K.S. Modeling of Sulfation in a Flooded Lead-Acid Battery and Prediction of its Cycle Life. J. Electrochem. Soc. 2020, 167, 13538. [Google Scholar] [CrossRef]
- David, G.; Enos, S.N.L. Lead-Acid Batteries and Advanced Lead-Carbon Batteries; Sandia National Lab: Albuquerque, NM, USA, 2014. [Google Scholar]
- Nelson, R. The basic chemistry of gas recombination in lead-acid batteries. JOM 2001, 53, 28–33. [Google Scholar] [CrossRef]
- Culpin, B.; Rand, D. Failure modes of lead/acid batteries. J. Power Sources 1991, 36, 415–438. [Google Scholar] [CrossRef]
- Moseley, P. Improving the valve-regulated lead–acid battery. J. Power Sources 2000, 88, 71–77. [Google Scholar] [CrossRef]
- Cooper, A.; Moseley, P. Progress in overcoming the failure modes peculiar to VRLA batteries. J. Power Sources 2003, 113, 200–208. [Google Scholar] [CrossRef]
- Moseley, P.T.; Rand, D.A.; Peters, K. Enhancing the performance of lead–acid batteries with carbon – In pursuit of an understanding. J. Power Sources 2015, 295, 268–274. [Google Scholar] [CrossRef]
- Bullock, K.R. Carbon reactions and effects on valve-regulated lead-acid (VRLA) battery cycle life in high-rate, partial state-of-charge cycling. J. Power Sources 2010, 195, 4513–4519. [Google Scholar] [CrossRef]
- Culpin, B. Separator design for valve-regulated lead/acid batteries. J. Power Sources 1995, 53, 127–135. [Google Scholar] [CrossRef]
- Pavlov, D. Chapter 1—Invention and Development of the Lead–Acid Battery. In Lead-Acid Batteries: Science and Technology; Pavlov, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 3–28. [Google Scholar]
- Rand, D.A.J.; Moseley, P.T. 3—Lead–Acid Battery Fundamentals. In Lead-Acid Batteries for Future Automobiles; Garche, J., Karden, E., Moseley, P.T., Rand, D.A.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 97–132. [Google Scholar]
- Pavlov, D. Chapter 4—Lead Alloys and Grids. Grid Design Principles. In Lead-Acid Batteries: Science and Technology; Pavlov, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 149–221. [Google Scholar]
- Lam, L.; Ceylan, H.; Haigh, N.; Lwin, T.; Rand, D. Influence of residual elements in lead on oxygen- and hydrogen-gassing rates of lead-acid batteries. J. Power Sources 2010, 195, 4494–4512. [Google Scholar] [CrossRef]
- Pavlov, D. Chapter 14—Valve-Regulated Lead–Acid (VRLA) Batteries. In Lead-Acid Batteries: Science and Technology, 2nd ed.; Pavlov, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 593–620. [Google Scholar]
- Wagner, R. 8—Positive Active-Materials for Lead–Acid Battery Plates. In Lead-Acid Batteries for Future Automobiles; Garche, J., Karden, E., Moseley, P.T., Rand, D.A.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 235–267. [Google Scholar]
- Pavlov, D. Chapter 7—Additives to the Pastes for Positive and Negative Battery Plates. In Lead-Acid Batteries: Science and Technology; Pavlov, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 311–361. [Google Scholar]
- Fernández, M.; Valenciano, J.; Trinidad, F.; Muñoz, N. The use of activated carbon and graphite for the development of lead-acid batteries for hybrid vehicle applications. J. Power Sources 2010, 195, 4458–4469. [Google Scholar] [CrossRef]
- Pavlov, D.; Nikolov, P.; Rogachev, T. Influence of expander components on the processes at the negative plates of lead-acid cells on high-rate partial-state-of-charge cycling. Part I: Effect of lignosulfonates and BaSO4 on the processes of charge and discharge of negative plates. J. Power Sources 2010, 195, 4435–4443. [Google Scholar] [CrossRef]
- Boden, D.; Loosemore, D.; Spence, M.; Wojcinski, T. Optimization studies of carbon additives to negative active material for the purpose of extending the life of VRLA batteries in high-rate partial-state-of-charge operation. J. Power Sources 2010, 195, 4470–4493. [Google Scholar] [CrossRef]
- C&D Technologies, Carbon Battery. Available online: http://www.energyinsight.co.za/wp-content/uploads/2015/06/Lead-Carbon-gen-brochure-en-1411-Final.pdf (accessed on 28 April 2022).
- Conover, D.R.; Crawford, A.J.; Viswanathan, V.V.; Ferreira, S.; Schoenwald, D. Protocol for Uniformly Measuring and Expressing the Performance of Energy Storage Systems; PNNL-22010 Rev 2; Pacific Northwest National Lab: Richland, WA, USA, 2016. [Google Scholar]
- NH Research Inc. 9200 Series Battery Module/Pack Test System; NH Research Inc.: Irvine, CA, USA; Available online: https://nhresearch.com/power-electronics-test-systems-and-instruments/battery-module-pack-test-systems/high-power-battery-charge-discharge-test-systems-9200-series/ (accessed on 9 February 2022).
- Research, P.A. Basics of Electrochemical Impedance Spectroscopy. In Instruments, Complex Impedance in Corrosion; In Application Note AC-1; Gamry Instruments Inc.: Warminster, PA, USA, 2006. [Google Scholar]
- Alavi, S.M.M.; Birkl, C.R.; Howey, D.A. Time-domain fitting of battery electrochemical impedance models. J. Power Sources 2015, 288, 345–352. [Google Scholar] [CrossRef]
- Schweiger, H.-G.; Obeidi, O.; Komesker, O.; Raschke, A.; Schiemann, M.; Zehner, C.; Gehnen, M.; Keller, M.; Birke, P. Comparison of Several Methods for Determining the Internal Resistance of Lithium Ion Cells. Sensors 2010, 10, 5604–5625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barai, A.; Uddin, K.; Widanage, W.D.; McGordon, A.; Jennings, P. A study of the influence of measurement timescale on internal resistance characterisation methodologies for lithium-ion cells. Sci. Rep. 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.; Rand, D.A.J.; Moseley, P.T. 7—Performance-Enhancing Materials for Lead–Acid Battery Negative Plates. In Lead-Acid Batteries for Future Automobiles; Garche, J., Karden, E., Moseley, P.T., Rand, D.A.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 213–234. [Google Scholar]
- Williford, R.E.; Viswanathan, V.V.; Zhang, J.-G. Effects of entropy changes in anodes and cathodes on the thermal behavior of lithium ion batteries. J. Power Sources 2009, 189, 101–107. [Google Scholar] [CrossRef]
- Salkind, A.J.; Kelley, J.J.; Cannone, A.G. Handbook of Batteries, 2nd ed.; McGraw-Hill, Inc.: New York, NY, USA, 1995. [Google Scholar]
- Electropaedia, Thermal Management. Available online: https://www.mpoweruk.com/thermal.htm#thermochemical (accessed on 1 February 2022).
- Kohya, Y.; Takeda, T.; Takano, K.; Kohno, M.; Yotsumoto, K.; Ogata, T. A deterioration estimating system for 200-Ah sealed lead-acid batteries. In Proceedings of the Intelec 94, Vancouver, BC, Canada, 30 October–3 November 1994. [Google Scholar]
- Hollenkamp, A.; Constanti, K.; Huey, A.; Koop, M.; Aputeanu, L. Premature capacity-loss mechanisms in lead/acid batteries. J. Power Sources 1992, 40, 125–136. [Google Scholar] [CrossRef]
- Battery University. BU-804b: Sulfation and How to Prevent It. Last Updated 2 November 2021. Available online: https://batteryuniversity.com/article/bu-804b-sulfation-and-how-to-prevent-it (accessed on 9 February 2022).
- Badeda, J.; Huck, M.; Sauer, D.U.; Kabzinski, J.; Wirth, J. 16—Basics of Lead–Acid Battery Modelling and Simulation. In Lead-Acid Batteries for Future Automobiles; Garche, J., Karden, E., Moseley, P.T., Rand, D.A.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 463–507. [Google Scholar]
- Kurisawa, I.; Iwata, M. Internal resistance and deterioration of VRLA battery-analysis of internal resistance obtained by direct current measurement and its application to VRLA battery monitoring technique. In Proceedings of the Power and Energy Systems in Converging Markets, Melbourne, Australia, 23–23 October 1997. [Google Scholar]
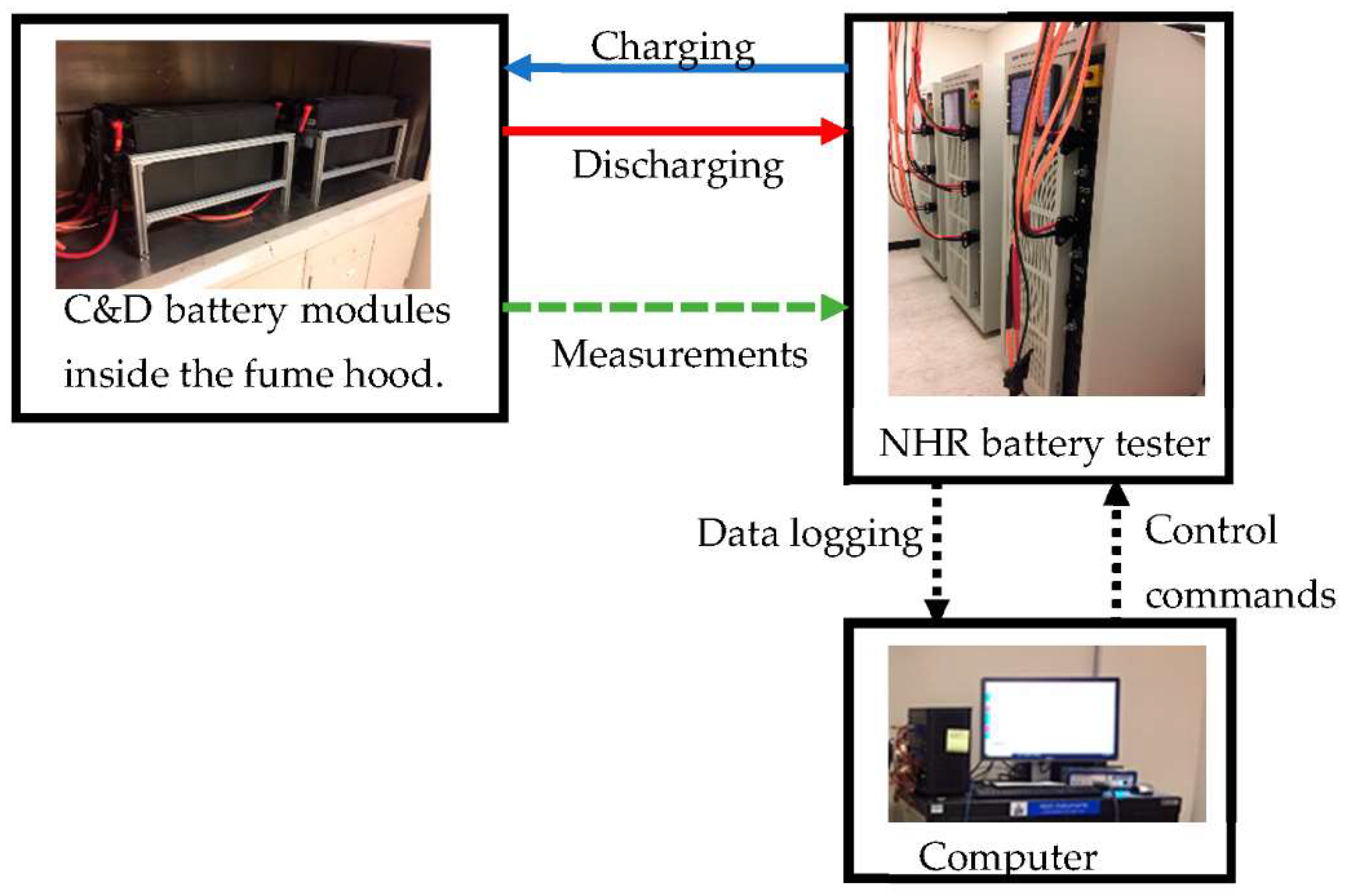
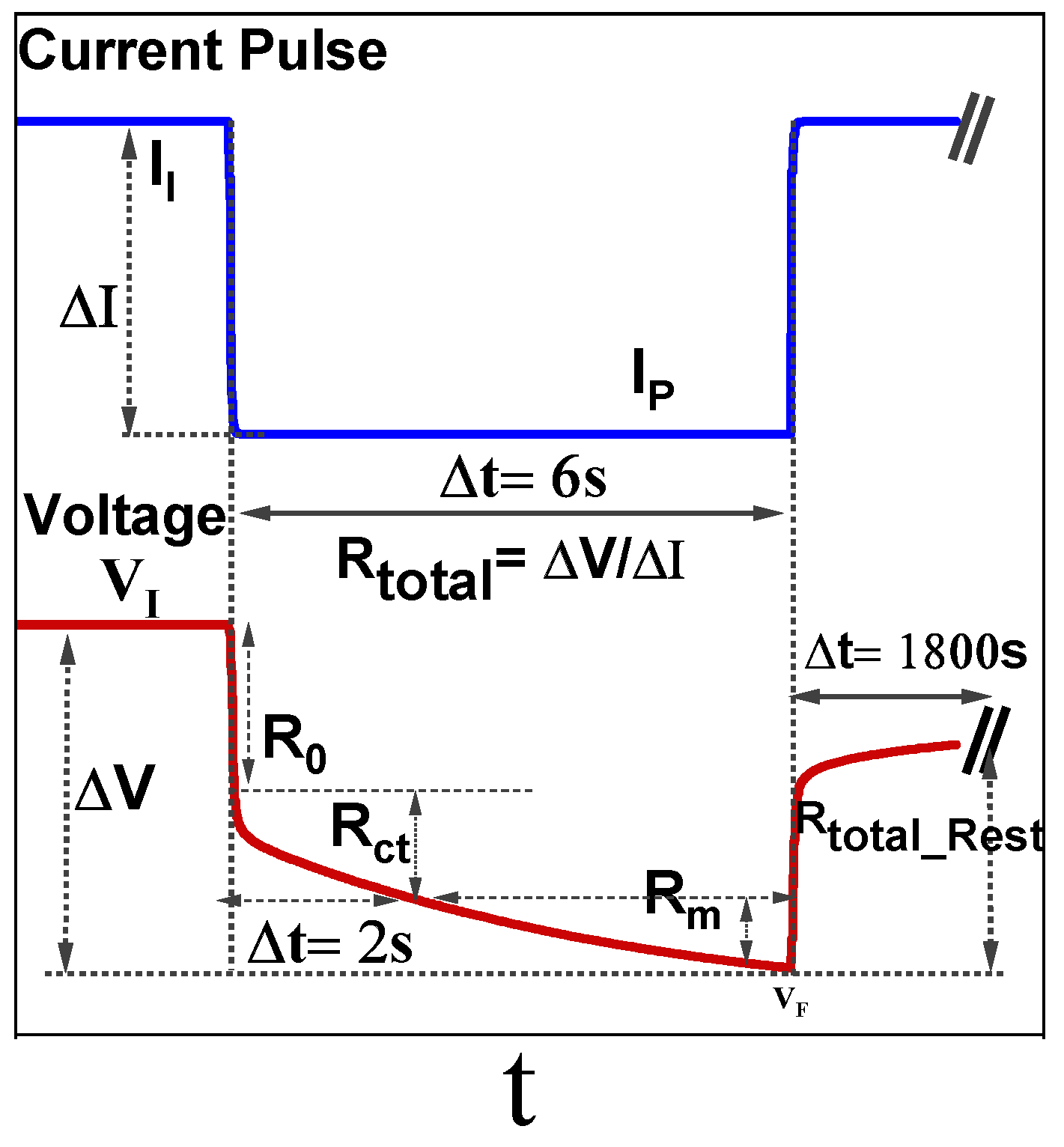
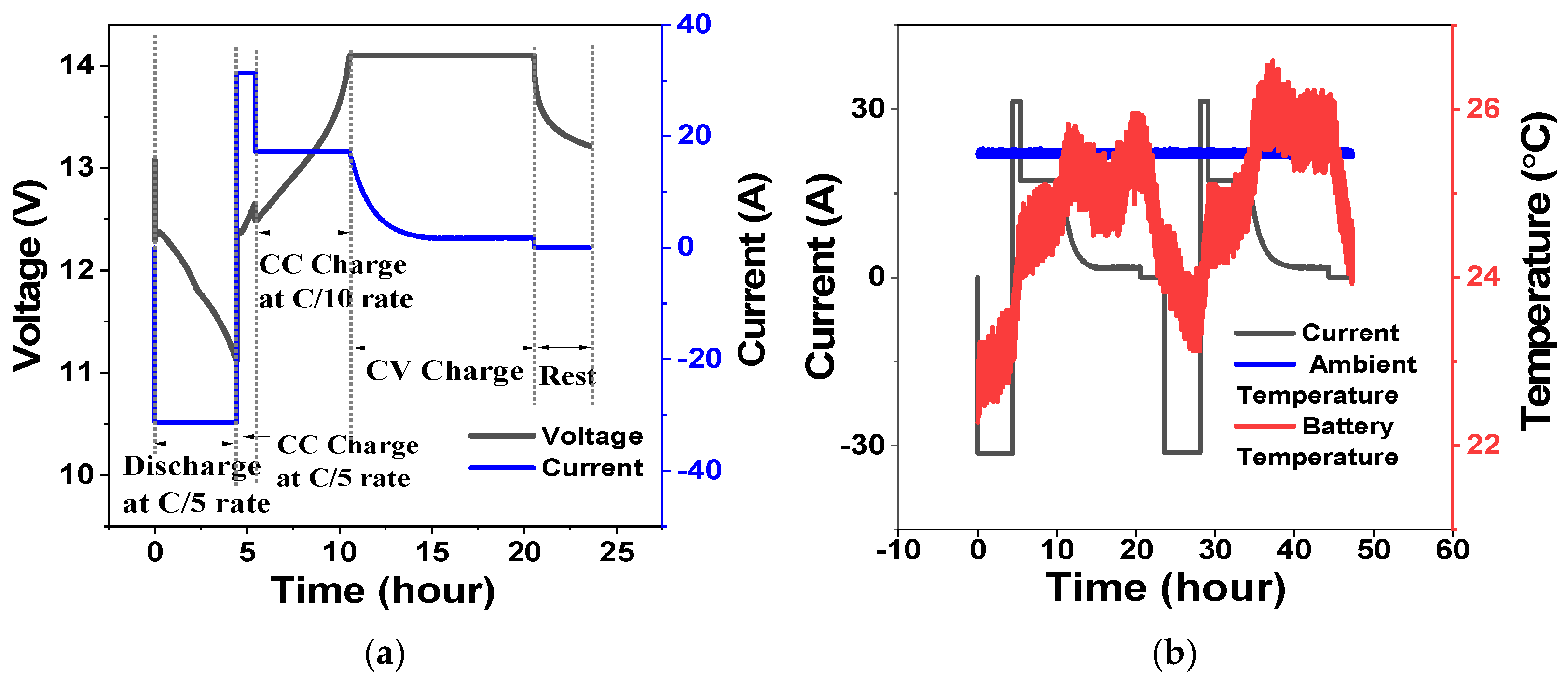

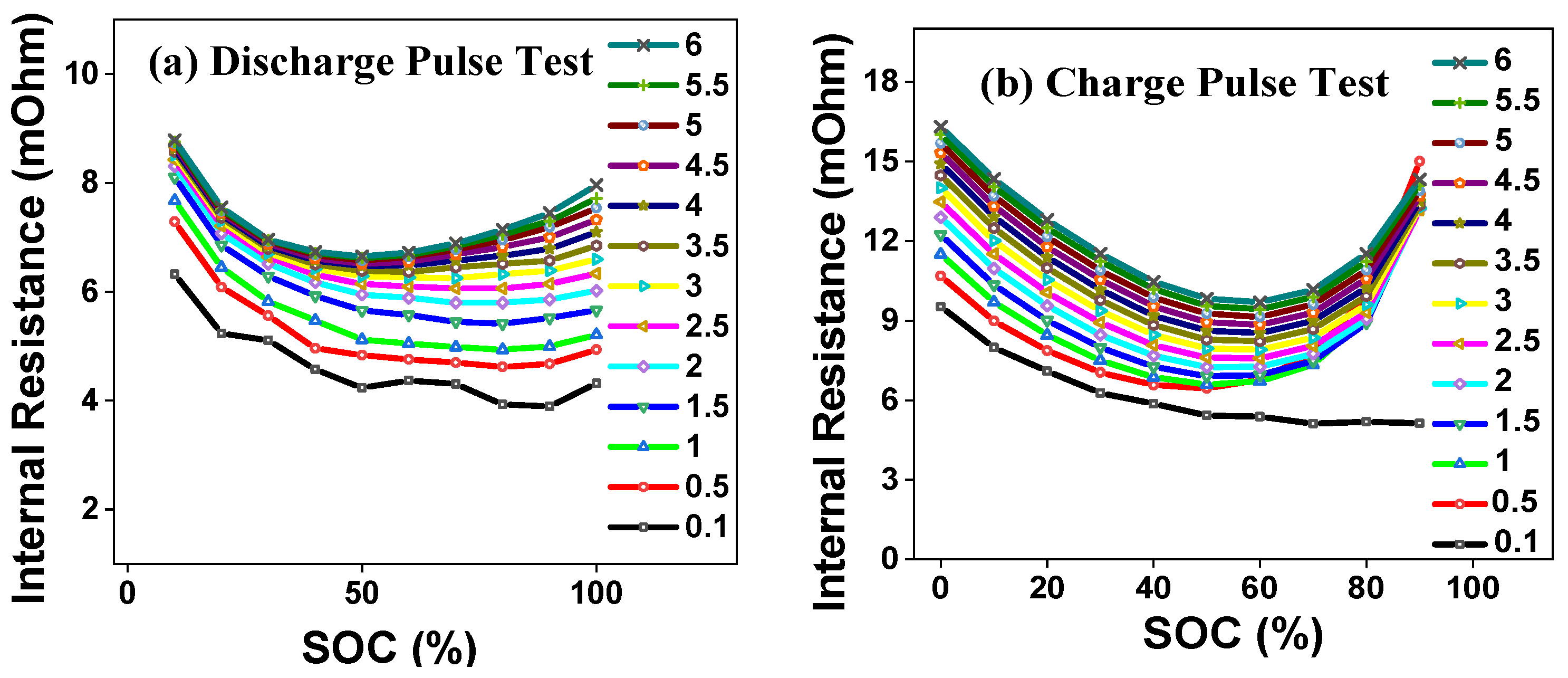

| Failure Mode | Cause | Mitigation |
|---|---|---|
| Positive grid corrosion | Negative electrode depolarization during recombination for CV charge Water loss resulting in higher electrolyte concentration Electrolyte stratification, resulting in greater corrosion at electrode bottom | Mitigate water loss Reduce stratification by appropriate choice of separator fiber diameter Use of Pb-Ca-Sn alloy to increase creep strength and reduce grid corrosion |
| Water loss | Charge current exceeds recombination rate Charge voltage not temperature compensated Positive grid corrosion | Proper charge control Mitigate grid corrosion Adjust initial electrolyte amount, negative excess and optimize separator porosity to minimize hydrogen evolution through the design life |
| Positive active material shedding | Grid growth due to corrosion Active material expansion by 2× during charge | Mitigate grid corrosion Optimize separator fiber diameter distribution to balance mechanical strength (large diameter) versus wicking characteristics (small diameter) |
| Electrolyte stratification | Poor separator wicking | Optimize separator diameter distribution |
| α-PbO2 formation | Electrolyte stratification Water loss | Mitigate stratification and water loss |
| Negative electrode sulfation | Low surface area leads to poor charge acceptance Partial state of charge cycling | Expanders that increase nucleation rate of lead sulfate formation and inhibit crystal growth High surface area carbon to increase surface area and electronic conductivity of partially charged electrode |
| Parameter | Value | |
|---|---|---|
| Module | 1 | 2 |
| DOD | 50% | 50% |
| Initial SOC | 100% | 100% |
| Highest discharge current | 2-h rate | 8-h rate |
| Discharge power (W) | 730 | 246 |
| Discharge duration (h) | ~1.2 | ~3.5 |
| Rest after discharge (h) | 1 | 1 |
| Charge back power (W) | 265 | 265 |
| Charge time (h) | ~8.5 | ~7.5 |
| Rest after charge (h) | 2 | 2 |
| Total duration for each PS cycle (h) | ~13 | ~14 |
| Parameter | Value | |
|---|---|---|
| Module | 1 | 2 |
| DOD | 20% | 20% |
| Initial SOC | 80%, | 57% |
| Ending SOC | 60%, | 37% |
| Discharge duration (h) | 24 | 24 |
| Maximum power (W) | 1090 | 1090 |
| Charge back current | 10-h rate | 10-h rate |
| Total duration for each FR cycle (h) | ~29 | ~29 |
| Drive Cycle | Micro HEV | Mild HEV | Full HEV |
|---|---|---|---|
| SOC range | 90–85% | 90–70% | 80–30% |
| Sulfation of negative | Negligible | Mild | Highest |
| Grid Corrosion | Medium | Lower | Lowest |
| Water loss | Medium | Lower | Lowest |
| Inactive α-PbO2 | Medium | Lower | Lowest |
| Grid services that map to drive cycles | PS 2-h lower sulfation, PS 8-h higher sulfation. Both expected to have similar grid corrosion, similar water loss. FR (80–60)% similar grid corrosion and water loss, greater sulfation | FR (80–60)% lower sulfation, FR (57–37)% higher sulfation, lower grid corrosion and water loss. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamim, N.; Viswanathan, V.V.; Thomsen, E.C.; Li, G.; Reed, D.M.; Sprenkle, V.L. Valve Regulated Lead Acid Battery Evaluation under Peak Shaving and Frequency Regulation Duty Cycles. Energies 2022, 15, 3389. https://doi.org/10.3390/en15093389
Shamim N, Viswanathan VV, Thomsen EC, Li G, Reed DM, Sprenkle VL. Valve Regulated Lead Acid Battery Evaluation under Peak Shaving and Frequency Regulation Duty Cycles. Energies. 2022; 15(9):3389. https://doi.org/10.3390/en15093389
Chicago/Turabian StyleShamim, Nimat, Vilayanur V. Viswanathan, Edwin C. Thomsen, Guosheng Li, David M. Reed, and Vincent L. Sprenkle. 2022. "Valve Regulated Lead Acid Battery Evaluation under Peak Shaving and Frequency Regulation Duty Cycles" Energies 15, no. 9: 3389. https://doi.org/10.3390/en15093389
APA StyleShamim, N., Viswanathan, V. V., Thomsen, E. C., Li, G., Reed, D. M., & Sprenkle, V. L. (2022). Valve Regulated Lead Acid Battery Evaluation under Peak Shaving and Frequency Regulation Duty Cycles. Energies, 15(9), 3389. https://doi.org/10.3390/en15093389








