Bioprocesses for the Biodiesel Production from Waste Oils and Valorization of Glycerol
Abstract
:1. Introduction
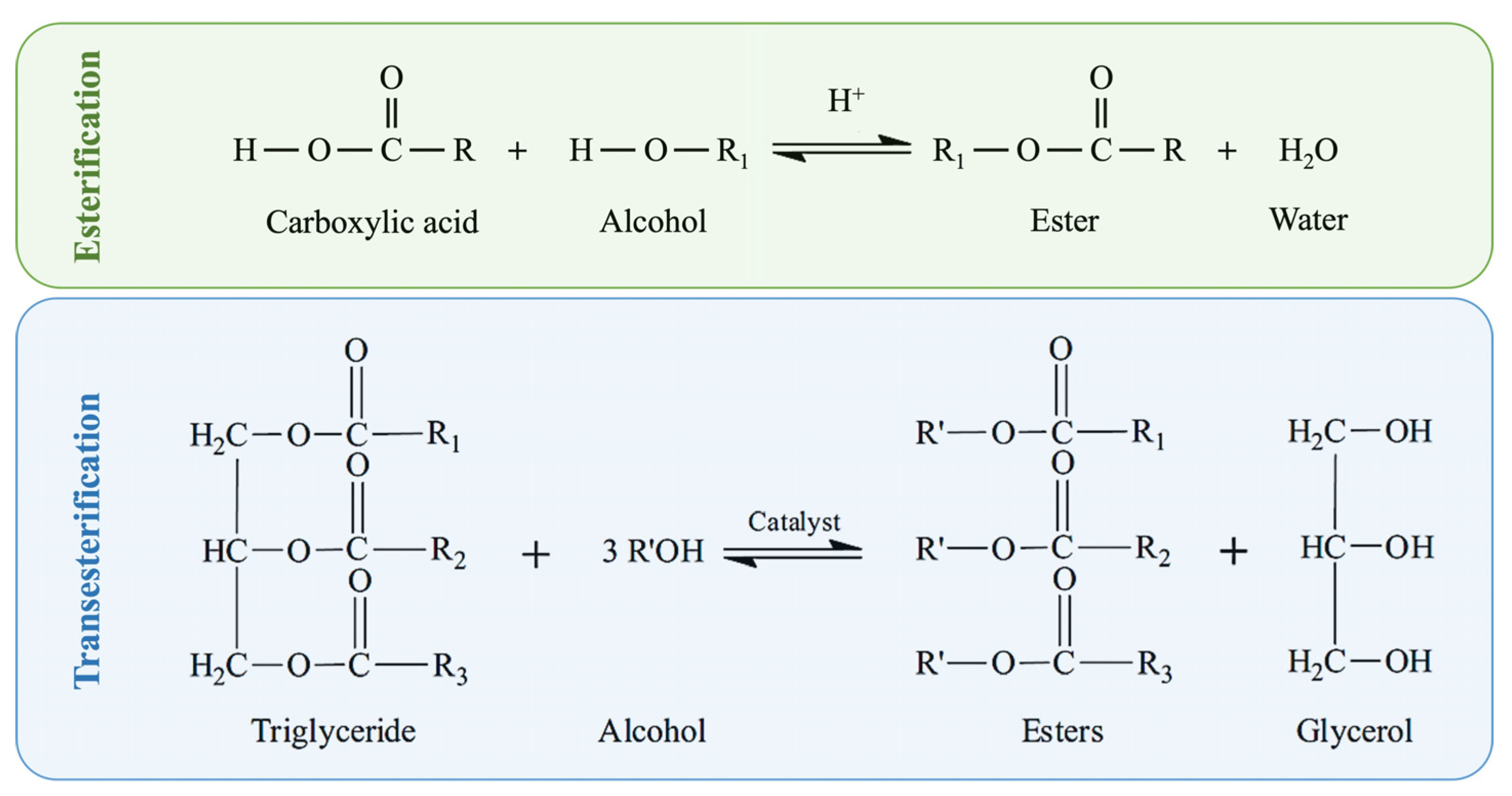
1.1. Biodiesel Production Routes
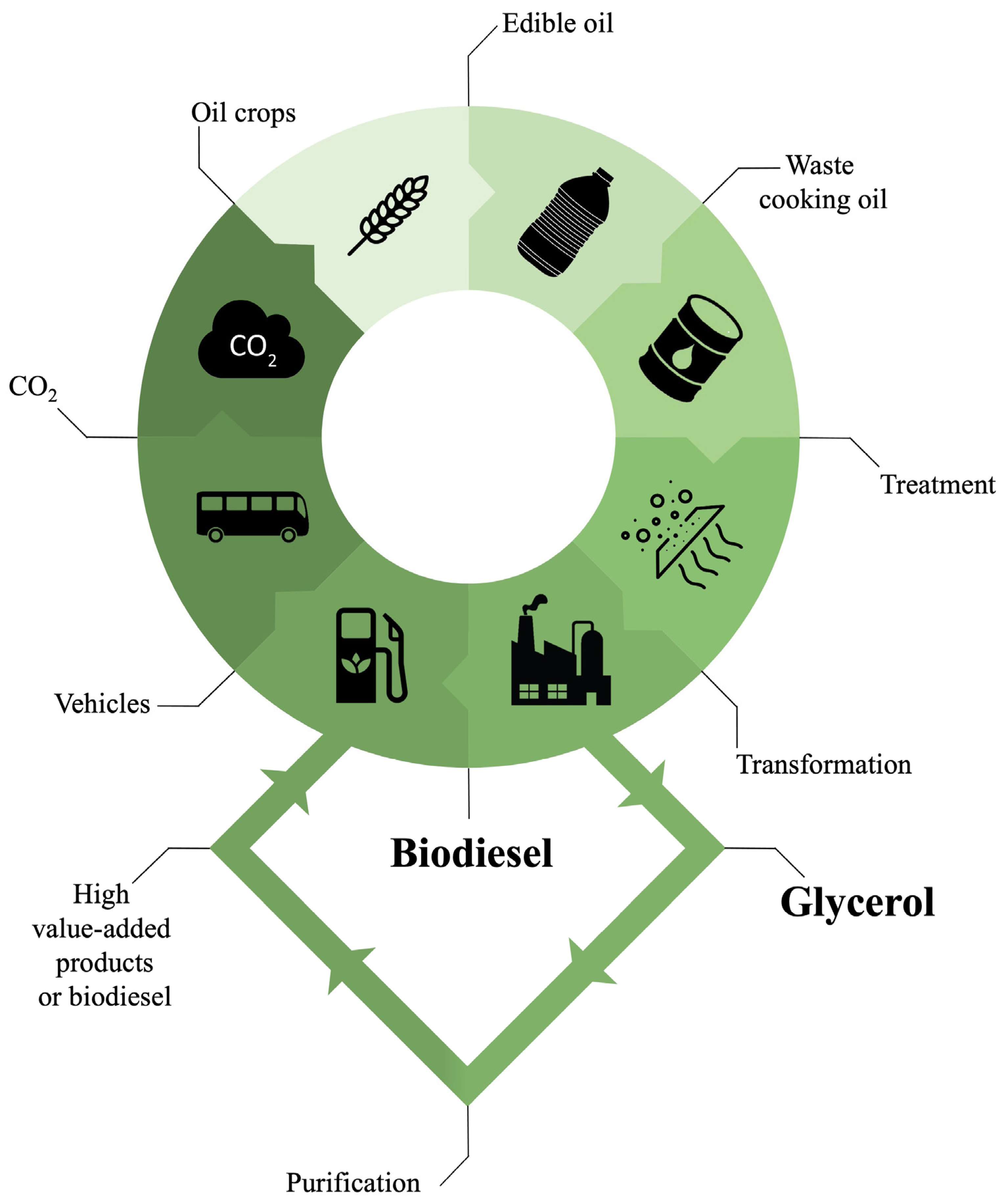
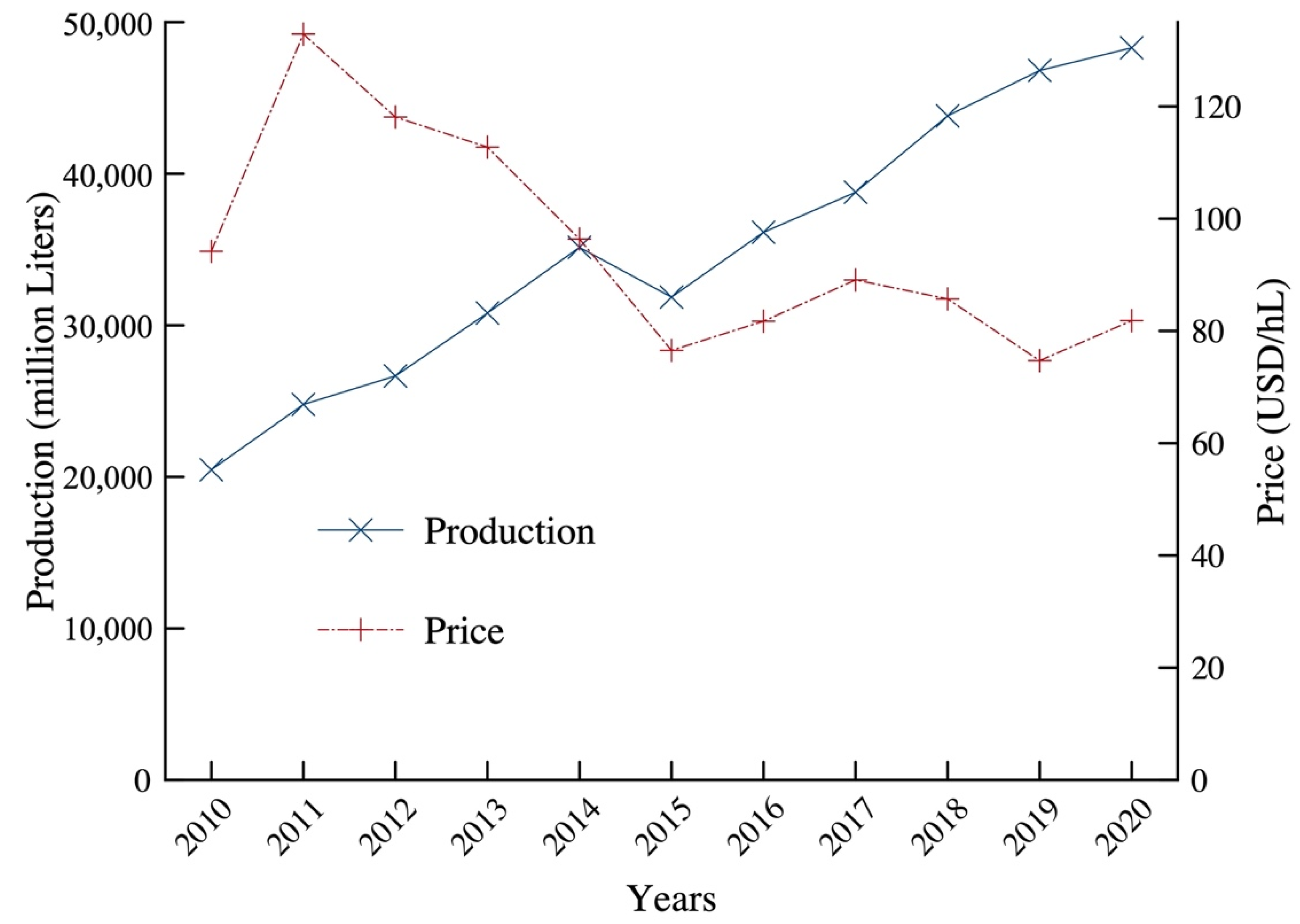
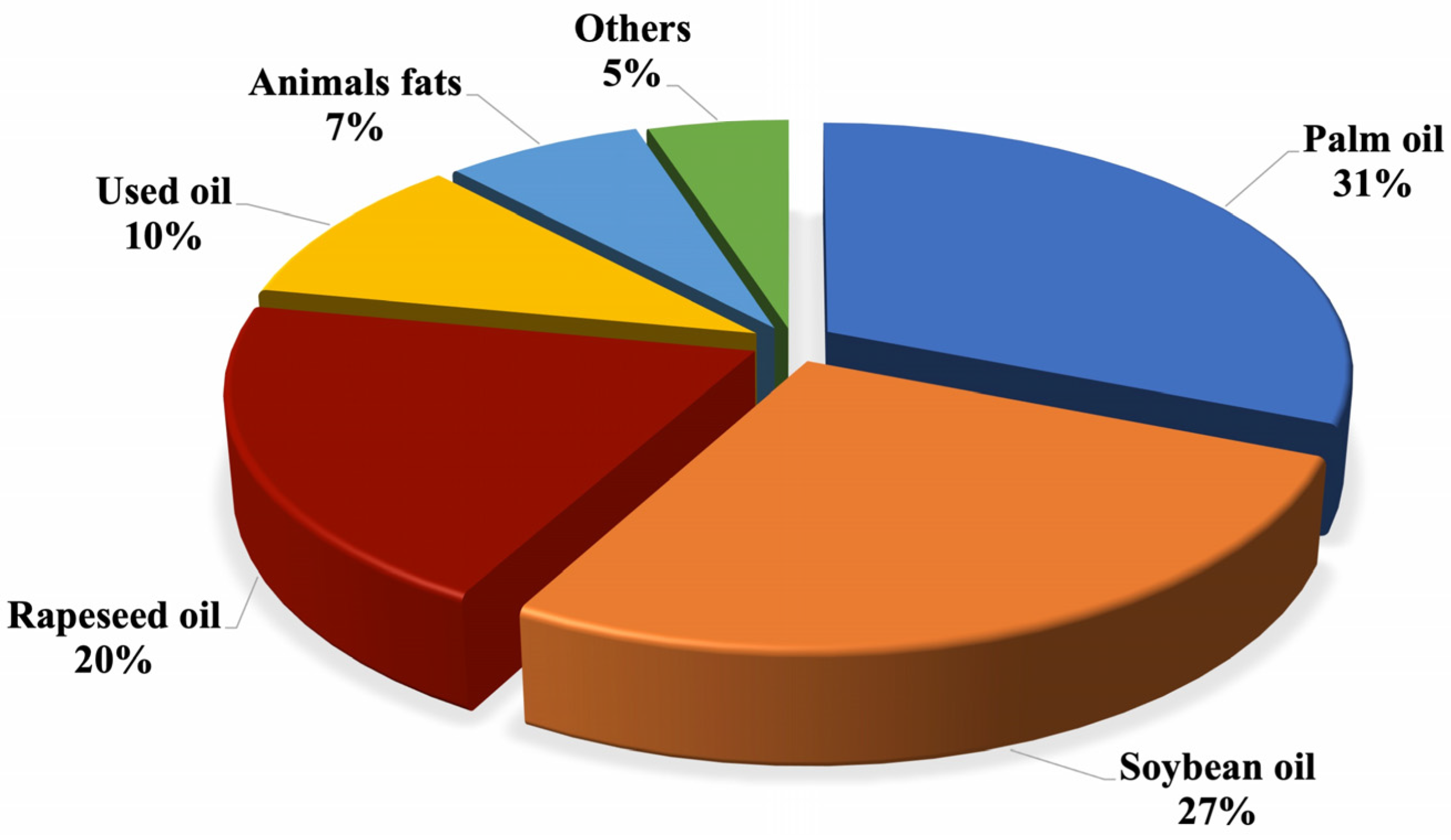
1.2. Current Market for Biodiesel from Waste Oils
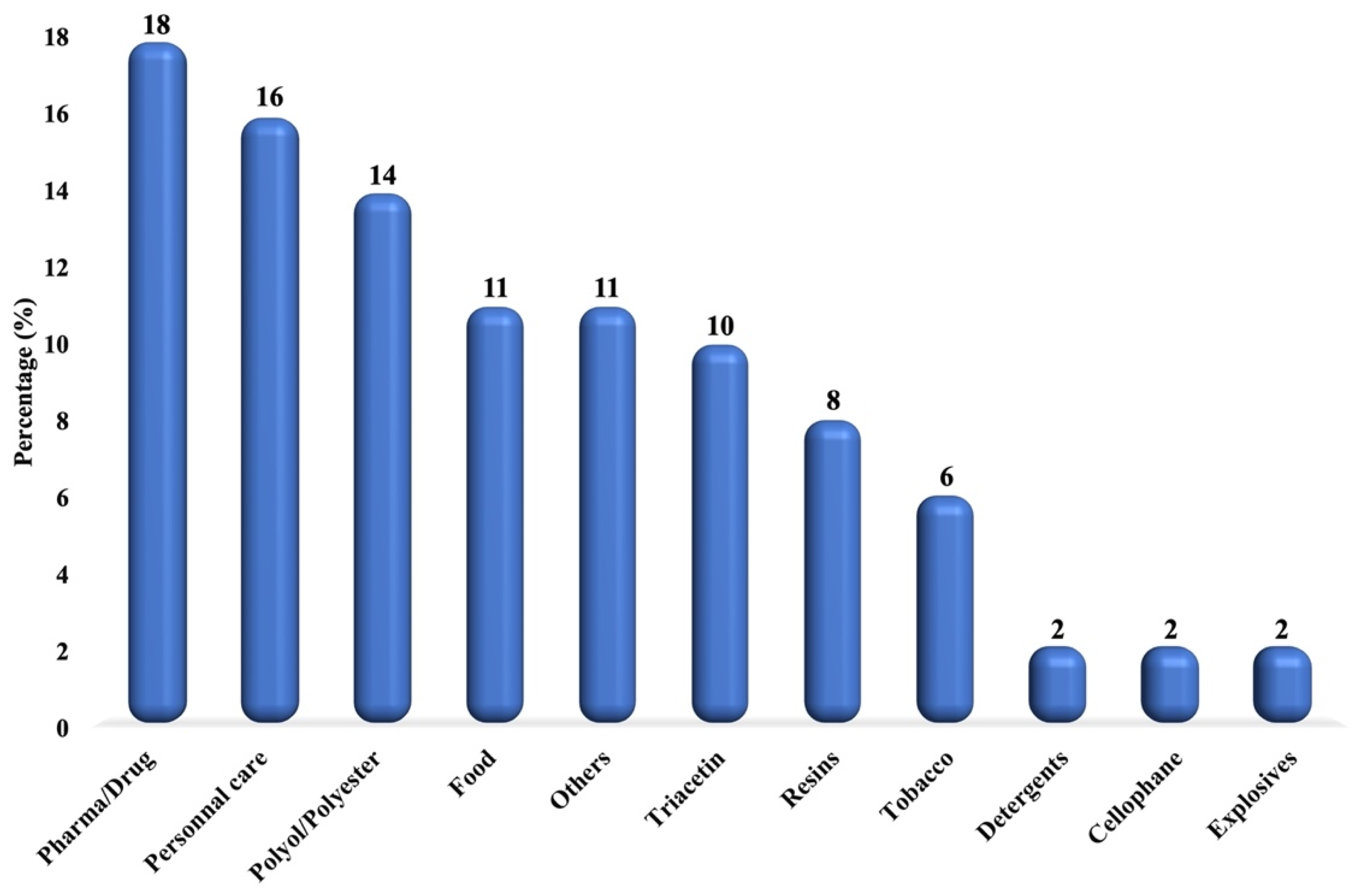
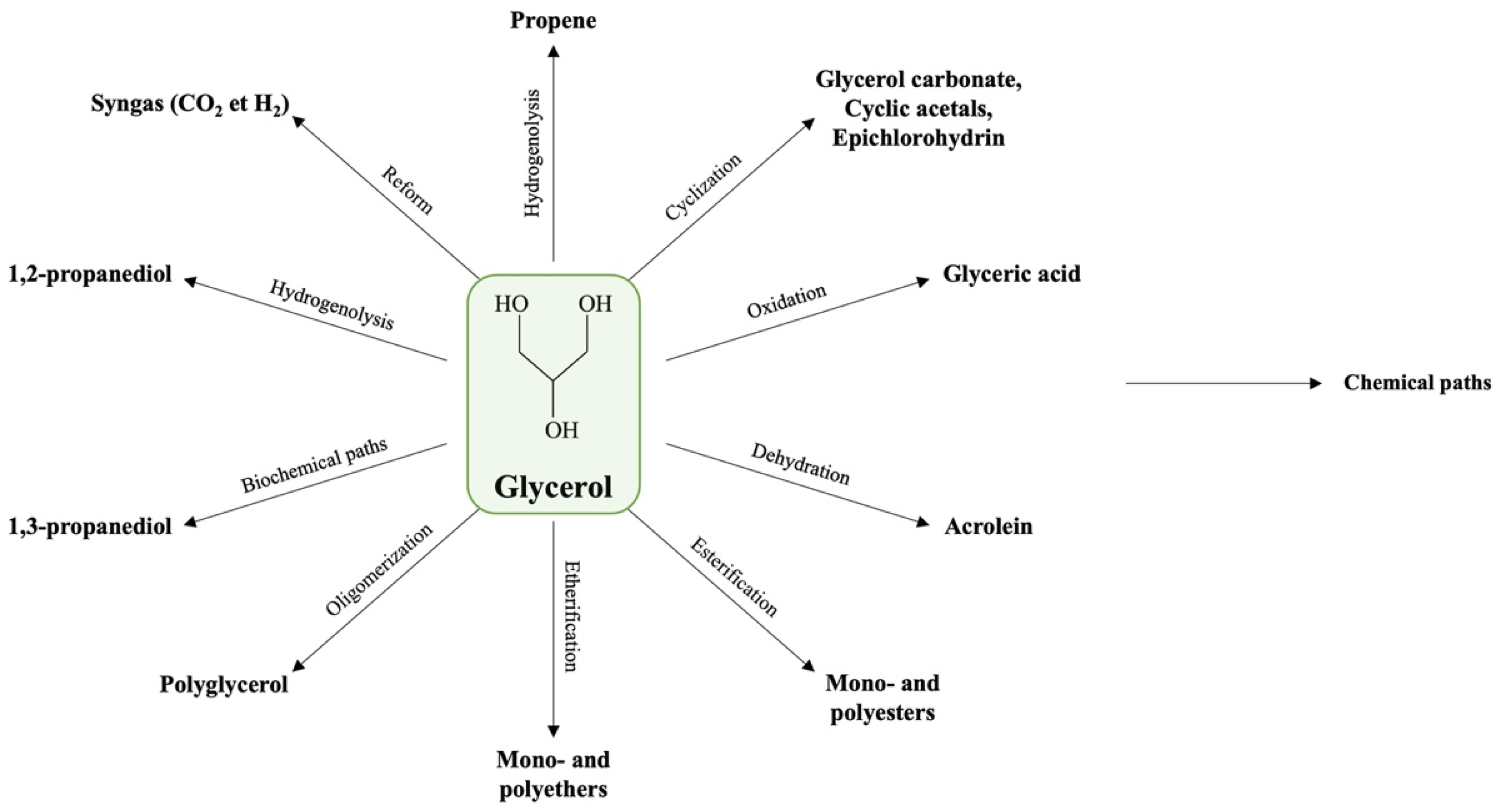
1.3. Current Glycerol Market and Applications
1.4. Advantages and Disadvantages
2. Enzymatic Production of Biodiesel from Waste Oils
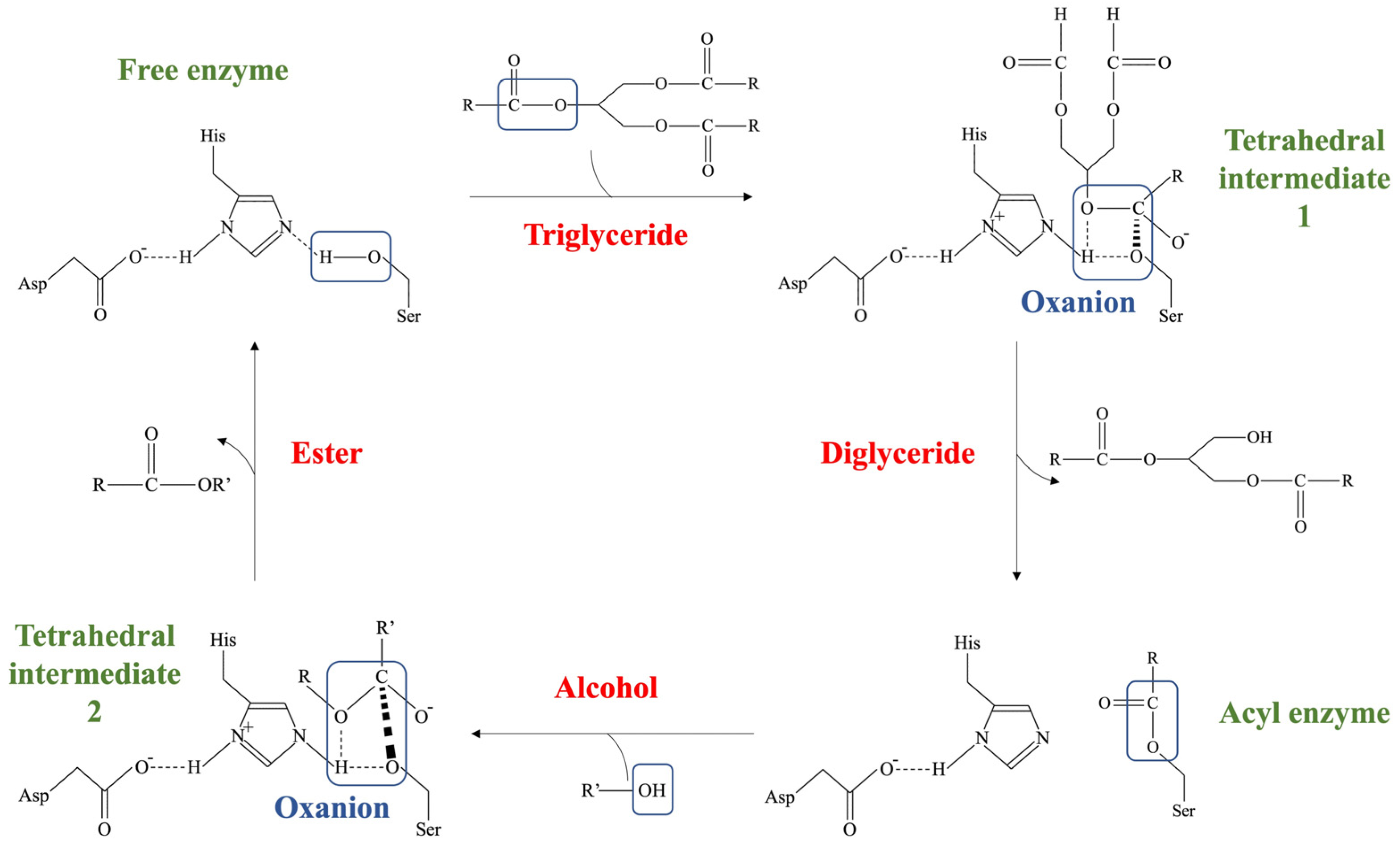
2.1. Enzymatic Transformation
| Pretreatment | Lipase | Acyl Acceptor | Support | Time (h) | Temp. (°C) | Enzyme Content | Water Content | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Free enzymes | |||||||||
| None | Eversa Transform 2.0 | MeOH (4:1 MeOH:oil) | - | 24 | 40 | 0.2 wt% | 2 wt% | 97 | [37] |
| Filtration | Eversa Transform 2.0 | MeOH (6.3:1 MeOH:oil) | - | 8 | 40 | 0.7 wt% | 4 wt% | 96.2 | [38] |
| None | Kocuria flava | MeOH (2:1 MeOH:oil) | - | 5 | 60 | 1 mL | None | 83.08 | [39] |
| None | Thermomyces lanuginosus | MeOH (2eq.; 6 shots) | - | 12 | 37 | 0.2 wt% | 3 wt% | 96 | [40] |
| None | Thermomyces lanuginosus | MeOH (3:1 MeOH:oil) | - | 24 | 30 | 0.3 wt% | 2.5:1 H2O:oil | 89.04 | [41] |
| None | Rhizopus stolonifer Aspergillus tamarii | MeOH (3:1 MeOH:oil) | - | 48 | 30 | 10 wt% | 10 wt% | 92.3 | [42] |
| None | Eversa Transform | MeOH (1.5 eq.) | - | 24 | 45 | 0.3 wt% | 1.5 wt% | 94 | [43] |
| None | Candida antarctica Rhizomucor miehi | EtOH (5:1 EtOH:oil) | - | 3 | 30 | 15 wt% | None | 89.95 | [44] |
| Degumming | Callera Trans L | MeOH (1.5 eq.) | - | 24 | 35 | 1 wt% | 3.5 wt% | >95 | [45] |
| None | NS81006 | MeOH (4.4:1 MeOH:oil) | - | 8 | 55 | 1.5 mL | 10 wt% | ≈80 | [46] |
| None | Eversa | MeOH (1.5 eq.) | - | 16 | 35 | 1 wt% | 2.5 wt% | 94.89 | [47] |
| None | Thermomyces lanuginosus Pseudozyma antarctica | EtOH (2 eq.) | - | 48 | 30 | 20 g | 1.5 wt% | 97.6 | [48] |
| Heating | Pseudomonas fluorescens | MeOH (3:1 MeOH:oil) | - | 24 | 45 | 5 wt% | None | 55.6 | [49] |
| None | Streptomyces sp. | MeOH (1:1 MeOH:oil) | - | 48 | 40 | 0.2 mL | None | >80 | [50] |
| None | Oreochromis niloticus | MeOH (4:1 MeOH:oil) | - | 28 | 45 | 3 kU | 3 wt% | 96.5 | [51] |
| Immobilized enzymes | |||||||||
| None | Rhizopus oryzae | MeOH (2:1 MeOH:oil) | Purolite D6308 | 16 | 30 | 0.2 wt% | None | 100 | [55] |
| None | Thermomyces lanuginose | MeOH (3:1 MeOH:oil) | N.I. | 3 | 35 | 0.5 wt% | None | 69.3 | [56] |
| None | Eversa Transform 2.0 | Isoamyl alcohol (6:1 Alcohol:oil) | CLEA | 72 | 40 | 2.13 U/g oil | None | 90 | [57] |
| None | Thermomyces lanuginosus | MeOH (4:1 MeOH:oil) | Hydrotalcite | 105 | 45 | 4 wt% | None | 92.8 | [58] |
| None | Candida antarctica | MeOH (3:1 MeOH:oil) | Resin | 12 | 40 | 4 wt% | None | 88 | [59] |
| None | Candida antarctica | MeOH (25:1 MeOH:oil) | Resin | 4 | 50 | 10 wt% | None | 89.1 | [60] |
| None | Thermomyces lanuginosus | MeOH (6:1 MeOH:oil) | MPPM | 24 | 65 | 1 g | 15 wt% | 90.2 | [61] |
| Discoloration | Penicillium expansum | MeOH (1 eq.) | Resin | 7 | 35 | 168 U | None | 92.8 | [62] |
| Filtration | Aspergillus niger | MeOH (1 eq.) | Whole-cell | 72 | 30 | 40 pcs | 10 wt% | 91.8 | [63] |
| Filtration/ Acidification Activated charcoal | Rhizopus oryzae | MeOH (3:1 MeOH:oil) | Whole-cell | 24 | 30 | 10 wt% | None | 94 | [64] |
| None | Escherichia coli (Candida antarctica/Thermomyces lanuginosus) | MeOH (1:1 MeOH:oil) | Whole-cell | 48 | 30 | 4 wt% | None | 95 | [65] |
| None | Pichia pastoris (Thermomyces lanuginosus) | MeOH (4:1 MeOH:oil) | Whole-cell | 84 | 40 | 12 wt% | None | 82 | [66] |
| None | Candida antarctica | MeOH (0.5 eq.) | Resin | 10 | 30 | N.I. | None | 93.4 | [67] |
| None | Talaromyces thermophilus | MeOH (6:1 MeOH:oil) | Chitosan | 5 | 50 | 25 wt% | None | 98 | [68] |
| Heating | Candida antarctica | Dimethyl carbonate (6:1 DMC:oil) | Resin | 4 | 60 | 10 wt% | None | 86.61 | [69] |
| None | Candida antarctica | MeOH (6.2:1 MeOH:oil) | Resin | 8 | 50 | 1 wt% | None | 90 | [70] |
| Extraction | Rhizomucor miehei | MeOH (6:1 MeOH:oil) | Resin | N.I. | 40 | 16 wt% | None | 96.7 | [71] |
| None | Burkholderia cepacia | MeOH (6:1 MeOH:oil) | SPION-silica | 35 | 35 | 25 wt% | 10 wt% | 91 | [72] |
| None | Rhizomucor miehei | MeOH (4:1 MeOH:oil) | m-MWCNTs-PAMAM | 30 | 50 | 6 wt% | 8 wt% | 94 | [73] |
| None | Candida antarctica | MeOH (14%) | Lewatit resin | 6 | 40 | N.I. | None | 78 | [74] |
| None | Thermomyces lanuginosus | MeOH (3:1 MeOH:oil) | styrene/ methacrylate | 6 | 30 | 10 wt% | 1 wt% | 79 | [75] |
| None | Candida antarctica | MeOH (3:1 MeOH:oil) | Fe3O4@SiO2 | 96 | 50 | 4.5 wt% | None | ≈100 | [76] |
| None | Bacillus licheniformis | MeOH (3:1 MeOH:oil) | mCLEAs-lip | 36 | 35 | 0.3 wt% | None | 71 | [77] |
| None | Candida antarctica | EtOH (36:1 EtOH:oil) | Resin | 8 | 35 | 50 wt% | None | 82.91 | [78] |
| Filtration | Aspergillus terreus | MeOH (6:1 MeOH:oil) | Fe3O4_PDA | 30 | 37 | 10 wt% | 0.6 wt% | 92 | [79] |
| Filtration | Pseudomonas fluorescens | EtOH (4:1 MeOH:oil) | Na-SBA-15 | 48 | 37 | N.I. | None | 91.4 | [80] |
| Extraction/Filtration/Drying | Thermomyces lanuginosus | MeOH (3:1 MeOH:oil) | octadecyl/ methacrylate | 24 | 35 | 10 wt% | 1 wt% | 75.3 | [81] |
| None | Pseudomonas fluorescens | EtOH (1.5:1 EtOH:oil) | styrene-divinylbenzene | 1.3 | 40 | 15 wt% | None | 94.1 | [13] |
2.2. Nature of Biodiesel by-Products
2.3. Characterization of Biodiesel
| Physical Properties | Biodiesel | Diesel |
|---|---|---|
| Kinematic viscosity (40 °C, mm2/s) | 4.06 | 2.42 |
| Cloud point (°C) | 6 | −5 |
| Pour point (°C) | 5 | −6 |
| Density (g/cm−3) | 0.889 | 0.857 |
| Flash point (°C) | 153 | 78 |
| Cetane number | 59 | 53 |
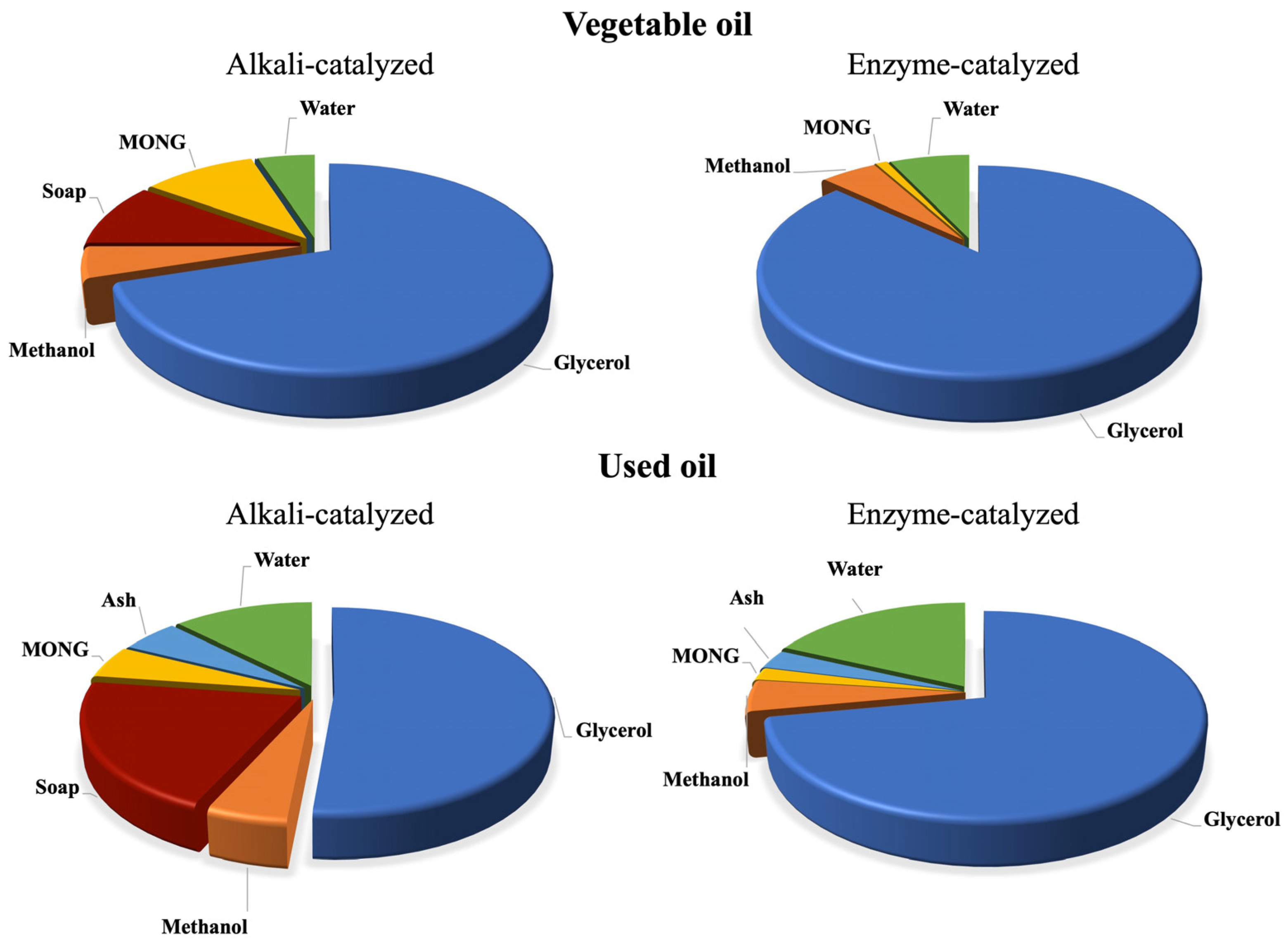
2.4. Separation and Purification of Biodiesel from Its By-Products
3. Purification of Biodiesel Glycerol from Waste Oils
3.1. Purity of Glycerol and Influence of Its Impurities
3.2. Glycerol Characterization Techniques
3.2.1. Properties of Glycerol
3.2.2. Purity of Glycerol
3.2.3. Impurities Measurement
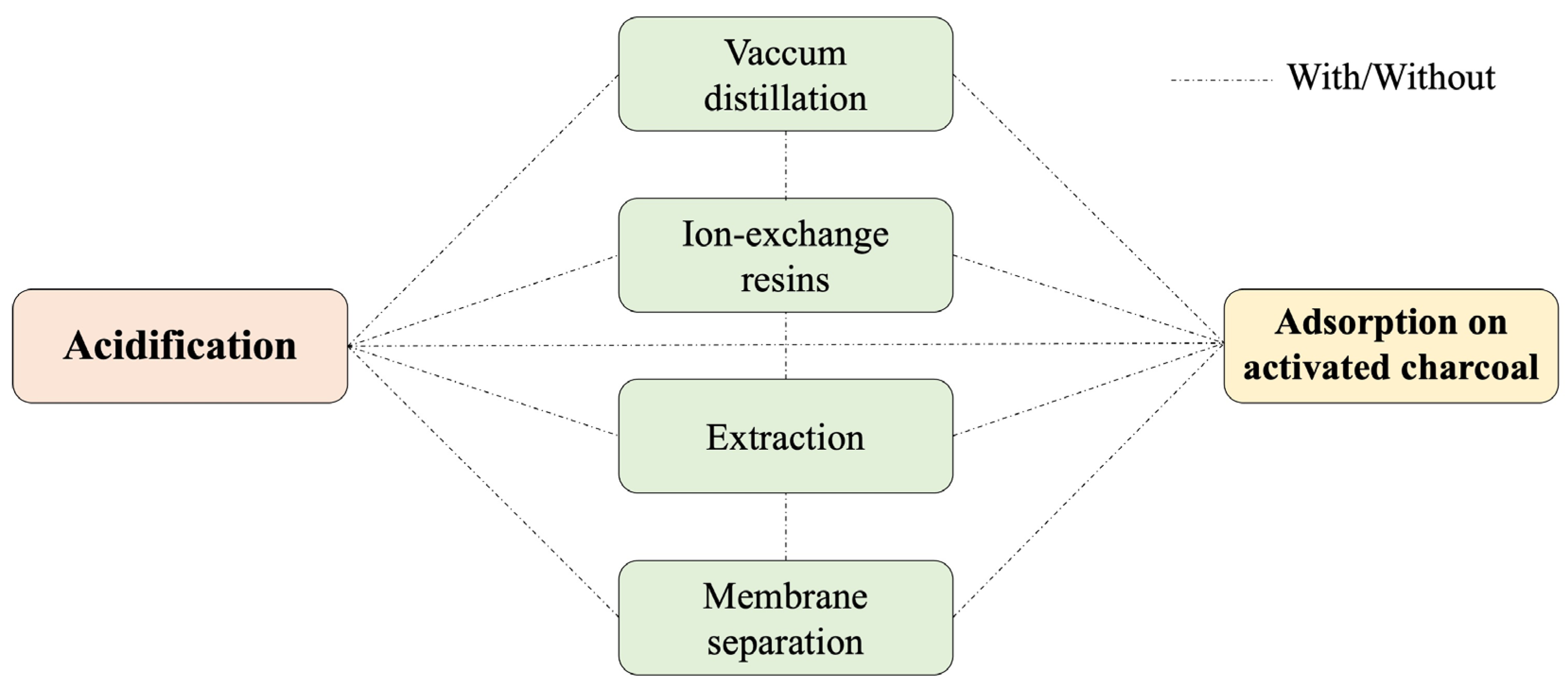
3.3. Glycerol Purification Methods
3.3.1. Acidification/Neutralization
3.3.2. Vacuum Distillation
3.3.3. Ion-Exchange Resins
3.3.4. Adsorption on Activated Charcoal
3.3.5. Extraction
3.3.6. Membrane Separation
3.3.7. Multi-Step Methods
3.4. Possible Applications of Glycerol Purification Methods from Biodiesel Produced by Enzymatic Processes on Waste Oils
3.5. Bioconversion of Glycerol from Waste Oils to High Value-Added Products
| Glycerol (%) | Glycerol Treatment | Final Product | Process or Microorganism | Temperature (°C) | Time (h) | Yield | Ref. |
|---|---|---|---|---|---|---|---|
| N.I. |
| Methane | Anerobic digestion | 25 | 2 | 54% | [167] |
| N.I. |
| Methane | Anerobic digestion | 2 | 0.8 | 0.306 m3 CH4/kg | [168] |
| 83.41 |
| Glycolipids | Ustilago maydis | 30 | 196.8 | 32.1 g/L | [169] |
| N.I. |
| Ethanol | Klebsiella variicola | 25 | 24 | 9.8 g/L | [170] |
| 47.5 |
| Hydrogen | E. coli/Enterobacter spH1 | 37 | 120 | 69.1 mM | [171] |
| 10.41 |
| Hydrogen | Anerobic digestion | 37 | 19.1 | 2.2 mol H2 L−1 | [172,173] |
| N.I. |
| Lactic acid | Rhizopus microsporus | 37 | 1.3 | 1.33 g/L | [174] |
| 49.30 |
| 1,3-propanediol / lactate | E. coli/Enterobacter spH1 | 37 | 1.3 | 27.77 g/L 1,3-PDO 14.68 g/L LA | [175] |
| 69 |
| 1,3-propanediol | Klebsiella pneumoniae | 37 | 12 | 0.64 mol1,3-PDO/mol glycerol | [176] |
| 31.8 |
| Biodiesel | Trichosporon oleaginosus | 28 | 72 | 5.24 g/L | [177] |
| 32.97 |
| Triacylglycerols | Rhodosporidium toruloides | 30 | 160 | 13.4 g/L | [178] |
| 64.5 |
| Biodiesel | Candida viwanathii Y-E4 | 30 | 166 | 13.6 g/L | [179] |
| 82 |
| 2-phenylethanol | Yarrowia lipolytica CH1/5 | 27 | 200 | 2.2 g/L | [180] |
| N.I. |
| Serinol | Gluconobacter oxydans transaminase | 30 | 44 | 36 mM | [181] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meher, L.C.; Vidya Sagar, D.; Naik, S.N. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Moazeni, J.; Chen, Y.C.; Zhang, G. Enzymatic transesterification for biodiesel production from used cooking oil, a review. J. Clean. Prod. 2019, 216, 117–128. [Google Scholar] [CrossRef]
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Sher, F.; Farooq, M.U.; Jamil, M.A.; Kausar, Z.; Sabah, N.U.; Shah, M.F.; Rehman, H.Z.U.; Rehman, A.U. Potential of waste cooking oil biodiesel as renewable fuel in combustion engines: A review. Energies 2021, 14, 2565. [Google Scholar] [CrossRef]
- Gecco. Available online: https://www.gecco.fr/projet-biohec-life/ (accessed on 15 February 2022).
- Kong, P.S.; Aroua, M.K.; Daud, W.M.A.W. Conversion of crude glycerol and pure glycerol into derivates: A feasibility evaluation. Renew. Sustain. Energy Rev. 2016, 63, 533–555. [Google Scholar] [CrossRef]
- Zulqarnain; Ayoub, M.; Yusoff, M.H.M.; Nazir, M.H.; Zahid, I.; Ameen, M.; Sher, F.; Floresyona, D.; Budi Nursanto, E. A comprehensive review on oil extraction and biodiesel production technologies. Sustainability 2021, 13, 788. [Google Scholar] [CrossRef]
- Mahmood Khan, H.; Iqbal, T.; Haider Ali, C.; Javaid, A.; Iqbal Cheema, I. Sustainable biodiesel production from waste cooking oil utilizing waste ostrich (Struthio camelus) bones derived heterogeneous catalyst. Fuel 2020, 277, 118091. [Google Scholar] [CrossRef]
- Analyse de Cycle de vie Appliquée aux Biocarburants de 1ère Génération, ADEME. Available online: https://librairie.ademe.fr/consommer-autrement/1394-analyses-de-cycle-de-vie-appliquees-aux-biocarburants-de-premiere-generation-consommes-en-france.html (accessed on 13 February 2020).
- Shahid, E.M.; Jamal, Y. Production of biodiesel: A technical review. Renew. Sustain. Energy Rev. 2011, 15, 4732–4745. [Google Scholar] [CrossRef]
- Pereira, C.O.; Portilho, M.F.; Henriques, C.A.; Zotin, F.M.Z. SnSO4 as catalyst for simultaneous transesterification and esterification of acid soybean oil. J. Braz. Chem. Soc. 2014, 25, 2409–2416. [Google Scholar]
- Dash, S.K.; Lingfa, P. A review on production of biodiesel using catalyzed transesterification. AIP Conf. Proc. 2017, 1859, 020100. [Google Scholar]
- Costa, M.J.; Silva, M.R.L.; Ferreira, E.E.A.; Carvalho, A.K.F.; Basso, R.C.; Pereira, E.B.; de Castro, H.F.; Mendes, A.A.; Hirata, D.B. Enzymatic biodiesel production by hydroesterification using waste cooking oil as feedstock. Chem. Eng. Process.–Process Intensif. 2020, 157, 108131. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.C.; Guarieiro, L.L.N.; Rezende, M.J.C.; Ribeiro, N.M.; Torres, E.A.; Lopes, W.A.; de P. Pereira, P.A.; de Andrade, J.B. Biodiesel: An overview. J. Braz. Chem. Soc. 2005, 16, 1313–1330. [Google Scholar] [CrossRef] [Green Version]
- Akoh, C.C.; Chang, S.W.; Lee, G.C.; Shaw, J.F. Enzymatic approach to biodiesel production. J. Agric. Food Chem. 2007, 55, 8995–9005. [Google Scholar] [CrossRef] [PubMed]
- OECD/FAO. Available online: https://www.oecd-ilibrary.org/agriculture-and-food/oecd-fao-agricultural-outlook-2020-2029_1112c23b-en (accessed on 16 July 2020).
- Abomohra, A.E.F.; Elsayed, M.; Esakkimuthu, S.; El-Sheekh, M.; Hanelt, D. Potential of fat, oil and grease (FOG) for biodiesel production: A critical review on the recent progress and future perspectives. Prog. Energy Combust. Sci. 2020, 81, 100868. [Google Scholar] [CrossRef]
- Lv, L.; Dai, L.; Du, W.; Liu, D. Progress in enzymatic biodiesel production and commercialization. Processes 2021, 9, 355. [Google Scholar] [CrossRef]
- Rouhany, M.; Montgomery, H. Global biodiesel production: The state of the art and impact on climate change. Biofuel Biorefin. Technol. 2019, 8, 1–14. [Google Scholar]
- Yaqoob, H.; Teoh, Y.H.; Sher, F.; Ashraf, M.U.; Amjad, S.; Jamil, M.A.; Jamil, M.M.; Mujtaba, M.A. Jatropha curcas biodiesel: A lucrative recipe for Pakistan’s energy sector. Processes 2021, 9, 1129. [Google Scholar] [CrossRef]
- Ayoub, M.; Abdullah, A.Z. Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Hejna, A.; Kosmela, P.; Formela, K.; Piszczyk, L.; Haponiuk, J.T. Potential applications of crude glycerol in polymer technology-Current state and perspectives. Renew. Sustain. Energy Rev. 2016, 66, 449–475. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Bellière-Baca, V.; Rey, P.; Dumeignil, F. Glycerol dehydration to acrolein in the context of new uses of glycerol. Green Chem. 2010, 12, 2079–2098. [Google Scholar] [CrossRef]
- UFOP Report on Global Market Supply 2018/2019, European and World Demand for Biomass for the Purpose of Biofuel Production in Relation to Supply in the Food and Feedstuff Markets; Union zur Förderung von oel-Und Proteinpflanzen E.V. (UFOP): Berlin, Germany, 2019.
- Degfie, T.A.; Mamo, T.T.; Mekonnen, Y.S. Optimized biodiesel production from waste cooking oil (WCO) using calcium oxide (CaO) nano-catalyst. Sci. Rep. 2019, 9, 18982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anuar, M.R.; Abdullah, A.Z. Challenges in biodiesel industry with regards to feedstock, environmental, social and sustainability issues: A critical review. Renew. Sustain. Energy Rev. 2016, 58, 208–223. [Google Scholar] [CrossRef]
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Hussein, R.; Ong, M.Y. Sustainability of biodiesel production in Malaysia by production of bio-oil from crude glycerol using microwave pyrolysis: A review. Green Chem. Lett. Rev. 2018, 11, 135–157. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (OECD). OECD-FAO Agricultural Outlook; OECD: Paris, France, 2015. [Google Scholar]
- Chozhavendhan, S.; Praveen Kumar, R.; Elavazhagan, S.; Barathiraja, B.; Jayakumar, M.; Varjani, S.J. Utilization of Crude Glycerol from Biodiesel Industry for the Production of Value-Added Bioproducts. In Waste to Wealth; Singhania, R., Agarwal, R., Kumar, R., Sukumaran, R., Eds.; Energy, Environment, and Sustainability; Springer: Singapore, 2018. [Google Scholar]
- Mishra, V.K.; Goswami, R. A review of production, properties and advantages of biodiesel. Biofuels 2017, 9, 273–289. [Google Scholar] [CrossRef]
- Gog, A.; Roman, M.; Toşa, M.; Paizs, C.; Irimie, F.D. Biodiesel production using enzymatic transesterification-Current state and perspectives. Renew. Energy 2012, 39, 10–16. [Google Scholar] [CrossRef]
- Jegannathan, K.R.; Abang, S. Production of biodiesel using immobilized lipase-A critical review. Rev. Biotechnol. 2008, 28, 253–264. [Google Scholar] [CrossRef]
- Yaakob, Z.; Mohammad, M.; Alherbawi, M.; Alam, Z.; Sopian, K. Overview of the production of biodiesel from waste cooking oil. Renew. Sustain. Energy Rev. 2013, 18, 184–193. [Google Scholar] [CrossRef]
- Chang, M.Y.; Chan, E.-S.; Song, C.P. Biodiesel production catalysed by low-cost liquid enzyme Eversa Transform 2.0: Effect of free fatty acid content on lipase methanol tolerance and kinetic model. Fuel 2021, 283, 119266. [Google Scholar] [CrossRef]
- Wancura, J.H.C.; Fantinel, A.L.; Ugalde, G.A.; Donato, F.F.; de Oliviera, J.V.; Tres, M.V.; Jahn, S.L. Semi-continuous production of biodiesel on pilot scale via enzymatic hydroesterification of waste material: Process and economics considerations. J. Clean. Prod. 2021, 285, 124838. [Google Scholar] [CrossRef]
- Najjar, A.; Hassan, E.A.; Zabermawi, N.; Saber, S.H.; Bajrai, L.H.; Almuhayawi, M.S.; Abujamel, T.S.; Almasaudi, S.B.; Azhar, L.E.; Moulay, M.; et al. Optimizing the catalytic activities of methanol and thermotolerant Kocuria flava lipases for biodiesel production from cooking oil wastes. Sci. Rep. 2021, 11, 13659. [Google Scholar] [CrossRef] [PubMed]
- Mibielli, G.M.; Fagundes, A.P.; Bohn, L.R.; Cavali, M.; Bueno, A.; Bender, J.P.; Oliveira, J.V. Enzymatic production of methyl esters from low-cost feedstocks. Biocatal. Agric. Biotechnol. 2020, 24, 101558. [Google Scholar] [CrossRef]
- da Silva, J.R.P.; da Costa, F.P.; Lerin, L.A.; Ninow, J.L.; Oliviera, J.V.; de Oliviera, D. Application of different methodologies to produce fatty acid esters using waste chicken fat catalyzed by free NS 40116 lipase. Ind. Biotechnol. 2019, 15, 293–302. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Mohamed, S.S.; Amin, H.A.; Moharam, M.E.; El-bendary, M.A.; Hawash, S.I. Semi-pilot scale production of biodiesel from waste frying oil by genetically improved fungal lipases. Prep. Biochem. Biotechnol. 2020, 50, 915–924. [Google Scholar] [CrossRef]
- Coppini, M.; Magro, J.D.; Martello, R.; Valério, A.; Zenevicz, M.C.; de Oliviera, D.; Oliveira, L.V. Production of methyl esters by enzymatic hydroesterification of chicken fat industrial residue. Braz. J. Chem. Eng. 2019, 36, 923–928. [Google Scholar] [CrossRef] [Green Version]
- Rocha, T.G.; Gomes, P.H.d.L.; de Souza, M.C.M.; Monteiro, R.R.C.; dos Santos, J.C.S. Lipase cocktail for optimized biodiesel production of free fatty acids from residual chicken oil. Catal. Lett. 2020, 151, 1155–1166. [Google Scholar] [CrossRef]
- Cesarini, S.; Haller, R.F.; Diaz, P.; Nielsen, P.M. Combining phospholipases and a liquid lipase for one-step biodiesel production using crude oils. Biotechnol. Biofuels 2014, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Du, W.; Liu, D. Exploration on the effect of phospholipids on free lipase-mediated biodiesel production. J. Mol. Catal. B Enzym. 2014, 102, 88–93. [Google Scholar] [CrossRef]
- Remonatto, D.; Santin, C.M.T.; de Oliviera, D.; Di Luccio, M.; de Oliviera, V. FAME production from waste oils through commercial soluble lipase Eversa Catalysis. Ind. Biotechnol. 2016, 12, 254–262. [Google Scholar] [CrossRef]
- Kleiner, B.; Fleischer, P.; Schörken, U. Biocatalytic synthesis of biodiesel utilizing deep eutectic solvents: A two-step-on-pot approach with free lipases suitable for acidic and used oil processing. Process Biochem. 2016, 51, 1808–1816. [Google Scholar] [CrossRef]
- Charpe, T.W.; Rathod, V.K. Biodiesel production using waste frying oil. Waste Manag. 2011, 31, 85–90. [Google Scholar] [CrossRef]
- Mander, P.; Yoo, H.Y.; Kim, S.W.; Choi, Y.H.; Cho, S.S.; Yoo, J.C. Transesterification of waste cooking oil by an organic solvent-tolerant alkaline lipase from Streptomyces sp. CS273. Appl. Biochem. Biotechnol. 2014, 172, 1377–1389. [Google Scholar] [CrossRef]
- Patchimpet, J.; Simpson, B.K.; Sangkharak, K.; Klomklao, S. Optimization of process variables for the production of biodiesel by transesterification of used cooking oil using lipase from Nile tilapia. Renew. Energy 2020, 153, 861–869. [Google Scholar] [CrossRef]
- Christopher, L.P.; Kumar, H.; Zambare, V.P. Enzymatic biodiesel: Challenges and opportunities. Appl. Energy 2014, 119, 497–520. [Google Scholar] [CrossRef]
- Hartmann, M.; Jung, D. Biocatalysis with enzymes immobilized on mesoporous hosts: The status quo and future trends. J. Mater. Chem. 2010, 20, 844–857. [Google Scholar] [CrossRef]
- Decarpigny, C.; Bleta, R.; Ponchel, A.; Monflier, E. Confinement of Candida antarctica lipase B in a multifunctional cyclodextrin-derived silicified hydrogel and its application as enzymatic nanoreactor. ACS Appl. Bio Mater. 2019, 2, 5569–5581. [Google Scholar] [CrossRef]
- López-Fernández, J.; Benaiges, M.D.; Valero, F. Second- and third-generation biodiesel production with immobilised recombinant Rhizopus oryzae lipase: Influence of the support, substrate acidity and bioprocess scale-up. Bioresour. Technol. 2021, 334, 125233. [Google Scholar] [CrossRef]
- Abdul Manab, N.S.; Veny, H.; Gusniah, A.; Sulaiman, S.; Aziz, N. Stepwise methanolysis of waste cooking oil using immobilized Thermomyces lanuginose lipase within ultrasonic-assisted condition. ASEAN J. Chem. Eng. 2021, 21, 19–26. [Google Scholar] [CrossRef]
- Guimarães, J.R.; Miranda, L.P.; Fernandez-Lafuente, R.; Tardioli, P.W. Immobilization of Eversa Transform via CLEA technology converts it in a suitable biocatalyst for biolubricant production using waste cooking oil. Molecules 2021, 26, 193. [Google Scholar] [CrossRef] [PubMed]
- Yagiz, F.; Kazan, D.; Akin, A.N. Biodiesel production from waste oils by using lipase immobilized on hydrotalcite and zeolites. Chem. Eng. J. 2007, 134, 262–267. [Google Scholar] [CrossRef]
- Halim, S.F.A.; Kamaruddin, A.H. Catalytic studies on lipase on FAME production from waste cooking palm oil in a tert-butanol system. Process Biochem. 2008, 43, 1436–1439. [Google Scholar] [CrossRef]
- Macreiras, R.; Vega, M.; Costa, C.; Ramos, P.; Márquez, M.C. Effect of methanol content on enzymatic production of biodiesel from waste frying oil. Fuel 2009, 88, 2130–2134. [Google Scholar] [CrossRef]
- Dizge, N.; Aydiner, C.; Imer, D.Y.; Bayramoglu, M.; Tanriseven, A.; Keskinle, B. Biodiesel production from sunflower, soybean, and waste cooking oils by transesterification using lipase immobilized onto a novel microporous polymer. Bioresour. Technol. 2009, 100, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Li, N.W.; Zong, M.H.; Wu, H. Highly efficient transformation of waste oil to biodiesel by immobilized lipase from Penicillium expansum. Process Biochem. 2009, 44, 685–688. [Google Scholar] [CrossRef]
- Xiao, M.; Obbard, J.P. Whole cell-catalysed transesterification of waste vegetable oil. Glob. Chang. Biol. Bioenergy 2010, 2, 346–352. [Google Scholar] [CrossRef]
- Balasubramaniam, B.; Perumal, A.S.; Jayaraman, J.; Mani, J.; Ramanujam, P. Comparative analysis for the production of fatty acid alkyl esterase using whole cell biocatalyst and purified enzyme from Rhizopus oryzae on waste cooking oil (sunflower oil). Waste Manag. 2012, 32, 1539–1547. [Google Scholar] [CrossRef]
- Yan, J.; Li, A.; Xu, Y.; Ngo, T.P.N.; Phua, S.; Li, Z. Efficient production of biodiesel from waste grease: One-pot esterification and transesterification with tandem lipases. Bioresour. Technol. 2012, 123, 332–337. [Google Scholar] [CrossRef]
- Yan, J.; Zheng, X.; Li, S. A novel and robust recombinant Pichia pastoris yeast whole cell biocatalyst with intracellular overexpression of a Thermomyces lanuginosus lipase: Preparation, characterization and application in biodiesel production. Bioresour. Technol. 2014, 151, 43–48. [Google Scholar] [CrossRef]
- Hama, S.; Yoshida, A.; Tamadani, N.; Noda, H.; Kondo, A. Enzymatic production of biodiesel from waste cooking oil in a packed-bed reactor: An engineering approach to separation of hydrophilic impurities. Bioresour. Technol. 2013, 135, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, I.B.B.; Romdhane, Z.B.; Bouzid, M.; Gargouri, A.; Belghith, H. Application of a chitosan-immobilized Talaromyces thermophilus lipase to a batch biodiesel production from waste frying oils. Appl. Biochem. Biotechnol. 2013, 171, 1986–2002. [Google Scholar] [CrossRef] [PubMed]
- Gharat, N.; Rathod, V.K. Ultrasound assisted enzyme catalyzed transesterification of waste cooking oil with dimethyl carbonate. Ultrason. Sonochem. 2013, 20, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Haigh, K.F.; Abidin, S.Z.; Vladisavljević, G.T.; Saha, B. Comparison of Novozyme 435 and Purolite D5081 as heterogeneous catalysts for the pretreatment of used cooking oil for biodiesel production. Fuel 2013, 111, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Gameiro, M.; Lisboa, P.; Paiva, A.; Barreiros, S.; Simões, P. Supercritical carbon dioxide-based integrated continuous extraction of oil from chicken feather meal, and its conversion to biodiesel in a packed-bed enzymatic reactor, at pilot scale. Fuel 2015, 153, 135–142. [Google Scholar] [CrossRef]
- Karimi, M. Immobilization of lipase onto mesoporous magnetic nanoparticles for enzymatic synthesis of biodiesel. Biocatal. Agric. Biotechnol. 2016, 8, 182–188. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, G.; Su, F.; Li, K.; Xu, L.; Han, X.; Yan, Y. Lipase oriented-immobilized on dendrimer-coated magnetic multi-walled carbon nanotubes toward catalyzing biodiesel production from waste vegetable oil. Fuel 2016, 178, 172–178. [Google Scholar] [CrossRef]
- Pollardo, A.A.; Lee, H.S.; Lee, D.; Kim, S.; Kim, J. Effect of supercritical carbon dioxide on the enzymatic production of biodiesel from waste animal fat using immobilized Candida antarctica lipase B variant. BMC Biotechnol. 2017, 17, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Tacias-Pascacio, V.G.; Virgen-Ortíz, J.J.; Jiménez-Pérez, M.; Yates, M.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Evaluation of different lipase biocatalysts in the production of biodiesel from used cooking oil: Critical role of the immobilization support. Fuel 2017, 200, 1–10. [Google Scholar] [CrossRef]
- Mehrasbi, M.R.; Mohammadi, J.; Peyda, M.; Mohammadi, M. Covalent immobilization of Candida antarctica lipase on core-shell magnetic nanoparticles for the production of biodiesel from waste cooking oil. Renew. Energy 2017, 101, 593–602. [Google Scholar] [CrossRef]
- Badoei-dalfard, A.; Malekabadi, S.; Karami, Z.; Sargazi, G. Magnetic cross-linked enzyme aggregates of Km12 lipase: A stable nanobiocatalyst for biodiesel synthesis from waste cooking oil. Renew. Energy 2019, 141, 874–882. [Google Scholar] [CrossRef]
- Marín-Suárez, M.; Méndez-Mateos, D.; Guadix, A.; Guadix, E.M. Reuse of immobilized lipases in the transesterification of waste fish oil for the production of biodiesel. Renew. Energy 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Touqeer, T.; Mumtaz, M.W.; Mukhtar, H.; Irfan, A.; Akram, S.; Shabbir, A.; Rashid, U.; Nehdi, I.A.; Choong, T.S.Y. Fe3O4-PDA-lipase as substrate functionalized nano biocatalyst for the production of biodiesel using waste cooking oil as feedstock: Characterization and process optimization. Energies 2020, 13, 177. [Google Scholar] [CrossRef] [Green Version]
- Ferrero, G.O.; Faba, E.M.S.; Rickert, A.A.; Eimer, G.A. Alternatives to rethink tomorrow: Biodiesel production from residual and non-edible oils using biocatalyst technology. Renew. Energy 2020, 150, 128–135. [Google Scholar] [CrossRef]
- Ching-Velasquez, J.; Fernández-Lafuente, R.; Rodriguez, R.C.; Plata, V.; Rosales-Quintero, A.; Torrestiana-Sánchez, B.; Tacias-Pascacio, V.G. Production and characterization of biodiesel from oil of fish waste by enzymatic catalysis. Renew. Energy 2020, 153, 1346–1354. [Google Scholar] [CrossRef]
- Osorio-González, C.S.; Gómez-Falcon, N.; Sandoval-Salas, F.; Saini, R.; Brar, S.K.; Ramírez, A.A. Production of biodiesel from castor oil: A review. Energies 2020, 13, 2467. [Google Scholar] [CrossRef]
- Eze, V.C.; Harvey, A.P. Continuous reactive coupling of glycerol and acetone—A strategy for triglyceride transesterification and in-situ valorisation of glycerol by-product. Chem. Eng. J. 2018, 347, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2019, 3, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Biodiesel production from high FFA rubber seed oil. Fuel 2005, 84, 335–340. [Google Scholar] [CrossRef]
- Juan, J.C.; Kartika, D.A.; Wu, T.W.; Hin, T.Y.Y. Biodiesel production from jatropha oil by catalytic and non-catalytic approaches: An overview. Bioresour. Technol. 2011, 102, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhao, W.; Ma, P.; Zhao, Z.; Xu, G.; Yang, C.; Chen, H.; Lin, H.; Han, S. Ternary blends of biodiesel with petro-diesel and diesel from direct coal liquefaction for improving the cold flow properties of waste cooking oil biodiesel. Fuel 2016, 177, 46–52. [Google Scholar] [CrossRef]
- ASTM D445-19a; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2019. Available online: www.astm.org (accessed on 16 June 2020).
- ASTM D2500-17a; Standard Test Method for Cloud Point of Petroleum Products and Liquid Fuels. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D97-17b; Standard Test Method for Pour Point of Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2017.
- Saeedi Dehaghani, A.H.; Rahimi, R. An experimental study of diesel fuel cloud and pour point reduction using different additives. Petroleum 2019, 5, 413–416. [Google Scholar] [CrossRef]
- ASTM D4052-18a; Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D7215-16; Standard Test Method for Calculated Flash Point from Simulated Distillation Analysis of Distillate Fuels. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D6890-18; Standard Test Method for Determination of Ignition Delay and Derived Cetane Number (DCN) of Diesel Fuel Oils by Combustion in a Constant Volume Chamber. ASTM International: West Conshohocken, PA, USA, 2018.
- Giakoumis, E.G.; Sarakatsanis, C.K. A comparative assessment of biodiesel cetane number predictive correlations based on fatty acid composition. Energies 2019, 12, 422. [Google Scholar] [CrossRef] [Green Version]
- Jason, Y.J.J.; How, H.G.; Teoh, Y.H.; Sher, F.; Chuah, H.G.; The, J.S. Tribological behaviour of graphene nanoplatelets as additive in pongamia oil. Coatings 2021, 11, 732. [Google Scholar] [CrossRef]
- István Barabás and Ioan-Adrian Todoruț. Biodiesel Quality, Standards and Properties, Biodiesel-Quality, Emissions and By-Products, Gisela Montero and Margarita Stoytcheva, IntechOpen. 16 November 2011. Available online: https://www.intechopen.com/books/biodiesel-quality-emissions-and-by-products/biodiesel-quality-standards-and-properties (accessed on 18 June 2020). [CrossRef] [Green Version]
- Sharma, Y.C.; Singh, B.; Upadhyay, S.N. Advancements in development and characterization of biodiesel: A review. Fuel 2008, 87, 2355–2373. [Google Scholar] [CrossRef]
- Enweremadu, C.C.; Mbarawa, M.M. Technical aspects of production and analysis of biodiesel from used oil-A review. Renew. Sustain. Energy Rev. 2009, 13, 2205–2224. [Google Scholar] [CrossRef]
- Robles-Medina, A.; González-Moreno, P.A.; Esteban-Cerdán, L.; Molina-Grima, E. Biocatalysis: Towards ever greener biodiesel production. Biotechnol. Adv. 2009, 27, 398–408. [Google Scholar] [CrossRef]
- Gao, Z.; Ma, Y.; Wang, Q.; Zhang, M.; Wang, J.; Liu, Y. Effect of crude glycerol impurities on lipid preparation by Rhodosporidium toruloides yeast 32489. Bioresour. Technol. 2016, 218, 373–379. [Google Scholar] [CrossRef]
- Sarma, S.J.; Dhillon, G.S.; Brar, S.K.; Bihan, Y.L.; Buelna, G.; Verna, M. Investigation of the effect of different crude glycerol components on hydrogen production by Enterobacter aerogenes NRRL B-407. Renew. Energy 2013, 60, 566–571. [Google Scholar] [CrossRef]
- Yang, X.; Jin, G.; Gong, Z.; Shen, H.; Bai, F.; Zhao, Z.K. Recycling biodiesel-derived glycerol by the oleaginous yeast Rhodosporidium toruloides Y4 through the two-stage lipid production process. Biochem. Eng. J. 2014, 91, 86–91. [Google Scholar] [CrossRef]
- Uprety, B.K.; Samavi, M.; Rakshit, S.K. Contribution of specific impurities in crude glycerol towards improved lipid production by Rhodosporidium toruloides ATCC 10788. Bioresour. Technol. Rep. 2018, 3, 27–34. [Google Scholar] [CrossRef]
- Isahak, W.N.R.W.; Ramli, Z.A.C.; Ismail, M.; Jahim, J.M.; Yarmo, M.A. Recovery and purification of crude glycerol from vegetable oil transesterification. Sep. Purif. Rev. 2015, 44, 250–267. [Google Scholar] [CrossRef]
- ASTM D5002-19; Standard Test Method for Density, Relative Density, and API Gravity of Crude Oils by Digital Density Analyzer. ASTM International: West Conshohocken, PA, USA, 2019.
- Harabi, M.; Bouguerra, S.N.; Marrakchi, F.; Chrysikou, L.P.; Bezergianni, S.; Bouaziz, M. Biodiesel and crude glycerol from waste frying oil: Production, characterization and evaluation of biodiesel oxidative stability with diesel blends. Sustainability 2019, 11, 1937. [Google Scholar] [CrossRef] [Green Version]
- Hunsom, M.; Autthanit, C. Adsorptive purification of crude glycerol by sewage sludge-derived activated carbon prepared by chemical activation with H3PO4, K2CO3 and KOH. Chem. Eng. J. 2013, 229, 334–343. [Google Scholar] [CrossRef]
- ASTM D1093-98; Standard Test Method for Acidity of Hydrocarbon Liquids and Their Distillation Residues. ASTM International: West Conshohocken, PA, USA, 1998.
- ASTM D240-19; Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D482-03; Standard Test Method for Ash from Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2003.
- ASTM D4377-00e1; Standard Test Method for Water in Crude Oils by Potentiometric Karl Fischer Titration. ASTM International: West Conshohocken, PA, USA, 2000.
- ASTM D4017-02(2015); Standard Test Method for Water in Paints and Paint Materials by Karl Fischer Method. ASTM International: West Conshohocken, PA, USA, 2015.
- Cocks, L.V.; Devine, J.; Gibbs, F.B. Determination of MONG in place of organic residue for glycerine analysis. Analyst 1970, 95, 278–283. [Google Scholar] [CrossRef]
- Ardi, M.S.; Aroua, M.K.; Hashim, N.A. Progress, prospect and challenges in glycerol purification process: A review. Renew. Sustain. Energy Rev. 2015, 42, 1164–1173. [Google Scholar] [CrossRef] [Green Version]
- Kumar, L.R.; Yellapu, S.K.; Tyagi, R.D.; Zhang, X. A review on variation in crude glycerol composition, bio-valorization of crude and purified glycerol as carbon source for lipid production. Bioresour. Technol. 2019, 293, 1221555. [Google Scholar] [CrossRef]
- Hájek, M.; Skopal, F. Treatment of glycerol phase formed by biodiesel. Bioresour. Technol. 2010, 101, 3242–3245. [Google Scholar] [CrossRef] [Green Version]
- Gil, S.; Marchena, M.; Fernández, C.M.; Sánchez-Silva, L.; Romero, A.; Valverde, J.L. Catalytic oxidation of crude glycerol using catalysts based on Au supported on carbonaceous materials. Appl. Catal. A Gen. 2013, 450, 189–203. [Google Scholar] [CrossRef]
- Velázquez-Hernández, I.; Zamudio, E.; Rodríguez-Valadez, F.J.; García-Gómez, N.A.; Álvarez-Contreras, L.; Guerra-Balcázar, M.; Arjona, N. Electrochemical valorization of crude glycerol in alkaline medium energy conversion using Pd, Au and PdAu nanomaterials. Fuel 2020, 262, 116556. [Google Scholar] [CrossRef]
- Posada, J.A.; Naranjo, J.M.; López, J.A.; Higuita, J.C.; Cardona, C.A. Design and analysis of poly-3-hydroxybutyrate processes from crude glycerol. Process Biochem. 2011, 46, 310–317. [Google Scholar] [CrossRef]
- Skrzyńska, E.; Wondolowska-Grabowska, A.; Capron, M.; Dumeignil, F. Crude glycerol as a raw material for the liquid phase oxidation reaction. Appl. Catal. A Gen. 2014, 482, 245–257. [Google Scholar] [CrossRef]
- Remón, J.; Jarauta-Córdoba, C.; García, L.; Arauzo, J. Analysis and optimization of H2 production from crude glycerol by steam reforming using a novel two step process. Fuel Process. Technol. 2016, 145, 130–147. [Google Scholar] [CrossRef] [Green Version]
- Valerio, O.; Horvath, T.; Pond, C.; Misra, M.; Mohanty, A. Improved utilization of crude glycerol from biodiesel industries: Synthesis and characterization of sustainable biobased polyesters. Ind. Crops Prod. 2015, 78, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Ge, X.; Cui, S.; Li, Y. Value-added processing of crude glycerol into chemicals and polymers. Bioresour. Technol. 2016, 215, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Nasir, N.F.; Mirus, M.F.; Ismail, M. Purification of crude glycerol from transesterification reaction of palm oil using direct method and multistep method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 243, 012015. [Google Scholar] [CrossRef] [Green Version]
- Isahak, W.N.R.W.; Jahim, J.M.; Ismail, M.; Nasir, N.F.; Ba-Abbad, M.M.; Yarmo, M.A. Purification of crude glycerol from industrial waste: Experimental and simulation studies. J. Eng. Sci. Technol. 2016, 11, 1056–1072. [Google Scholar]
- Abdul Raman, A.A.; Tan, H.W.; Buthiyappan, A. Two-step purification of glycerol as a value added by product from the biodiesel production process. Front. Chem. 2019, 7, 774–782. [Google Scholar] [CrossRef] [Green Version]
- Lopes, A.P.; Souza, P.R.; Bonafé, E.G.; Visentainer, J.V.; Martins, A.F.; Canesin, E.A. Purified glycerol is produced from the frying oil transesterification by combining a pre-purification strategy performed with condensed tannin polymer derivative followed by ionic exchange. Fuel Process. Technol. 2019, 187, 73–83. [Google Scholar] [CrossRef]
- Rodrigues, A.; Bordado, J.C.; dos Santos, R.G. Upgrading the glycerol from biodiesel production as source of energy carriers and chemicals-A technological review for three chemical pathways. Energies 2017, 10, 1817. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.W.; Abdul Aziz, A.R.; Aroua, M.K. Glycerol production and its application as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Kongjao, S.; Damronglerd, S.; Hunsom, M. Purification of crude glycerol derived from waste used-oil methyl ester plant. Korean J. Chem. Eng. 2010, 27, 944–949. [Google Scholar] [CrossRef]
- Demaman Oro, C.E.; Bonato, M.; Oliveira, J.V.; Tres, M.V.; Mignoni, M.L.; Dallago, R.M. A new approach for salts removal from crude glycerin coming from industrial biodiesel production unit. J. Environ. Chem. Eng. 2019, 7, 102883. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiao, G.; Varma, A. A universal procedure for crude glycerol purification from different feedstocks in biodiesel production: Experimental and simulation study. Ind. Eng. Res. 2013, 52, 14291–14296. [Google Scholar] [CrossRef]
- Strathmann, H. Membrane separation processes: Current relevance and future opportunities. AIChE J. 2001, 47, 1077–1087. [Google Scholar] [CrossRef]
- Gomes, M.C.S.; Pereirra, N.C.; de Barros, S.T.D. Separation of biodiesel and glycerol using ceramic membranes. J. Membr. Sci. 2010, 352, 271–276. [Google Scholar] [CrossRef]
- Sdrula, N. A study using classical or membrane separation in the biodiesel process. Desalination 2010, 250, 1070–1072. [Google Scholar] [CrossRef]
- Liang, B.; He, X.; Hou, J.; Li, L.; Tang, Z. Membrane separation in organic liquid: Technologies, achievements, and opportunities. Adv. Mater. 2018, 1806090. [Google Scholar] [CrossRef] [PubMed]
- 2007 EET Corp., Web Information; HEEPMTM Technology: Harriman, TN, USA, 2007.
- Indok Nurul Hasyimah, M.A.; Mohammad, A.W.; Markom, M. Influence of triglycerides on fouling of glycerol-Water with ultrafiltration membranes. Ind. Eng. Chem. Res. 2011, 50, 7520–7526. [Google Scholar] [CrossRef]
- Mah, S.K.; Leo, C.P.; Wu, T.Y.; Chai, S.P. A feasibility investigation on ultrafiltration of palm oil and oleic acid removal from glycerin solutions: Flux decline, fouling pattern, rejection and membrane characterizations. J. Membr. Sci. 2012, 389, 245–256. [Google Scholar] [CrossRef]
- Jeromin, L.; Johannisbauer, W.; Blum, S.; Sedelies, R.; Moormann, H.; Holforth, B.; Plachenka, J. Process for the Purification of Crude Glycerol Water. U.S. Patent 5,527,974 A, 18 June 1996. [Google Scholar]
- Vadthya, P.; Kumari, A.; Sumana, C.; Sridhar, S. Electrodialysis aided desalination of crude glycerol in the production of biodiesel from oil feed stock. Desalination 2015, 362, 133–140. [Google Scholar] [CrossRef]
- Kalafatakis, S.; Braekevelt, S.; Carlsen, V.; Lange, L.; Skiadas, I.V.; Gavala, H.N. On a novel strategy for water recovery and recirculation in biorefineries through application of forward osmosis membranes. Chem. Eng. J. 2017, 311, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Zulfikar, M.A.; Mohammad, A.W.; Hilal, N. Preparation and characterization of novel porous PMMA-SiO2 hybrid membranes. Desalination 2006, 192, 262–270. [Google Scholar] [CrossRef]
- Xie, K.; Yu, Y.; Shi, Y. Synthesis and characterization of cellulose/silica hybrid materials with chemical crosslinking. Carbohydr. Polym. 2009, 78, 799–805. [Google Scholar] [CrossRef]
- Shaari, N.Z.K.; Rahman, N.A. Performance of Thin Film Composite in the Purification of Crude Glycerol; IEEE Business Engineering and Industrial Applications Colloquium (BEIAC): Langkawi, Malaysia, 2013. [Google Scholar]
- Siyal, M.I.; Lee, C.K.; Park, C.; Khan, A.A.; Kim, J.O. A review of membrane development in membrane distillation for emulsified industrial or shale gas wastewater treatments with feed containing hybrid impurities. J. Environ. Manag. 2019, 243, 45–66. [Google Scholar] [CrossRef]
- Shirazi, M.M.A.; Kargari, A.; Tabatabaei, M.; Ismail, A.F.; Matsuura, T. Concentration of glycerol wastewater using sweeping gas membrane distillation. Chem. Eng. Process. Process Intensif. 2014, 78, 58–66. [Google Scholar] [CrossRef]
- Pal, P.; Chaurasia, S.P.; Upadhyaya, S.; Agarwal, M.; Sridhar, S. Glycerol purification using membrane technology. In Membrane Processes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 431–463. [Google Scholar]
- Yalcinkaya, F.; Boyraz, E.; Maryska, J.; Kucerova, K. A review on membrane technology and chemical surface modification for the oily wastewater treatment. Materials 2020, 13, 493. [Google Scholar] [CrossRef] [Green Version]
- Manosak, R.; Limpattayanate, S.; Hunsom, M. Sequential-refining of crude glycerol derived from waste used-oil methyl ester plant via a combined process of chemical and adsorption. Fuel Process. Technol. 2011, 92, 92–99. [Google Scholar] [CrossRef]
- Hunsom, M.; Saila, P.; Chaiyakam, P.; Kositnan, W. Comparison and combination of solvent extraction and adsorption for crude glycerol enrichment. Int. J. Renew. Energy Res. 2013, 3, 364–371. [Google Scholar]
- Pitt, F.D.; Domingos, A.M.; Chivanga Barros, A.A. Purification of residual glycerol recovered from biodiesel production. S. Afr. J. Chem. Eng. 2019, 29, 42–51. [Google Scholar] [CrossRef]
- Javani, A.; Hasheminejad, M.; Tahvildari, K.; Tabatabaei, M. High quality potassium phosphate production through step-by-step glycerol purification: A strategy to economize biodiesel production. Bioresour. Technol. 2012, 104, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Poirier, M.A.; Chunbao, X. Purification of crude glycerol using acidification: Effects of acid types and product characterization. Austin J. Chem. Eng. 2014, 1, 7–13. [Google Scholar]
- Contreras-Andrade, I.; Avella-Moreno, E.; Sierra-Cantor, J.F.; Guerrero-Fajardo, C.A.; Sodré, J.R. Purification of glycerol from biodiesel production by sequential extraction monitored by 1H NMR. Fuel Process. Technol. 2015, 132, 99–104. [Google Scholar] [CrossRef]
- Muniru, O.S.; Ezeanyanaso, C.S.; Fagbemigun, T.K.; Akubueze, E.U.; Oyewole, A.O.; Okunola, O.J.; Asieba, G.; Shifatu, A.O.; Igwe, C.C.; Elemo, G.N. Valorization of biodiesel production: Focus on crude glycerine refining/purification. J. Sci. Res. Rep. 2016, 11, 1–8. [Google Scholar] [CrossRef]
- Dhabhai, R.; Ahmadifeijani, E.; Dalai, A.K.; Reaney, M. Purification of crude glycerol using a sequential physico-chemical treatment, membrane filtration, and activated charcoal adsorption. Sep. Purif. Technol. 2016, 168, 101–106. [Google Scholar] [CrossRef]
- Sadhukhan, S.; Sarkar, U. Production of purified glycerol using sequential desalination and extraction of crude glycerol obtained during trans-esterification of Crotalaria juncea oil. Energy Convers. Manag. 2016, 118, 450–458. [Google Scholar] [CrossRef]
- Chol, C.G.; Dhabhai, R.; Dalai, A.K.; Reaney, M. Purification of crude glycerol derived from biodiesel production process: Experimental studies and techno-economic analyses. Fuel Process. Technol. 2018, 178, 78–87. [Google Scholar] [CrossRef]
- Sidhu, M.S.; Roy, M.M.; Wang, W. Glycerine emulsions of diesel-biodiesel blends and their performance and emissions in a diesel engine. Appl. Energy 2018, 230, 148–159. [Google Scholar] [CrossRef]
- de Farias, B.S.; Vidal, É.M.; Ribeiro, N.T.; da Silveiro, N., Jr.; da Silva Vaz, B.; Kuntzler, S.G.; de Morais, M.G.; Cadaval, T.R.S., Jr.; de Almeida Pinto, L.A. Electrospun chitosan/poly(ethylene oxide) nanofibers applied for the removal of impurities from biodiesel production by biosorption. J. Mol. Liq. 2018, 268, 365–370. [Google Scholar] [CrossRef]
- Hobden, R. Commercializing enzymatic biodiesel production. Biodiesel Mag. 2014, 11, 34–37. [Google Scholar]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Vivek, N.; Sindhu, R.; Madhavan, A.; Anju, A.J.; Castro, E.; Faraco, V.; Pandey, A.; Binod, P. Recent advances in the production of value added chemicals and lipids utilizing biodiesel industry generated crude glycerol as a substrate–Metabolic aspects, challenges and possibilities: A overview. Bioresour. Technol. 2017, 239, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Nuchdang, S.; Phalakornkule, C. Anaerobic digestion of glycerol and co-digestion of glycerol and pig manure. J. Environ. Manag. 2012, 101, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Siles López, J.Á.; de los Ángeles Martín Santos, M.; Chica Pérez, A.F.; Martín, A.M. Anaerobic digestion of glycerol derived from biodiesel manufacturing. Bioresour. Technol. 2009, 100, 5609–5615. [Google Scholar] [CrossRef]
- Liu, Y.; Koh, C.M.J.; Ji, L. Bioconversion of crude glycerol to glycolipids in Ustilago maydis. Bioresour. Technol. 2011, 102, 3927–3933. [Google Scholar] [CrossRef]
- Suzuki, T.; Nishikawa, C.; Seta, K.; Shigeno, T.; Nakajima-Kambe, T. Ethanol production from glycerol-containing biodiesel waste by Klebsiella variicola shows maximum productivity under alkaline conditions. New Biotechnol. 2014, 31, 246–253. [Google Scholar] [CrossRef]
- Maru, B.T.; López, F.; Kengen, S.W.M.; Constantí, M.; Medina, F. Dark fermentative hydrogen and ethanol production from biodiesel waste glycerol using a co-culture of Escherichia coli and Enterobacter sp. Fuel 2016, 186, 375–384. [Google Scholar] [CrossRef]
- Rodrigues, C.V.; Santana, K.O.; Nespeca, M.G.; de Oliveira, J.E.; Maintinguer, S.I. Crude glycerol by transesterification process from used cooking oils: Characterization and potentialities on hydrogen bioproduction. Int. J. Hydrogen Energy 2016, 41, 14641–14651. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.V.; Nespeca, M.G.; Sakamoto, I.K.; de Oliveira, J.E.; Amâncio Varesche, M.B.; Maintinguer, S.I. Bioconversion of crude glycerol from waste cooking oils into hydrogen by sub-tropical mixed and pure cultures. Int. J. Hydrogen Energy 2019, 44, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Yuwa-amornpitak, T.; Chookietwatana, K. Bioconversion of waste cooking oil glycerol from cabbage extract to lactic acid by Rhizopus microsporus. Braz. J. Microbiol. 2018, 49, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Zhou, J.J.; Sun, Y.Q.; Xiu, Z.L. Bioconversion of raw glycerol from waste cooking-oil-based biodiesel production to 1,3-propanediol and lactate by a microbial consortium. Front. Bioeng. Biotechnol. 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Jiang, H.; Hector, S.B.; Xiao, Z.; Li, J.; Liu, R.; Li, C.; Zeng, B.; Liu, G.-Q.; Zhu, Y. Adaptability of Klebsiella pneumoniae 2e, a newly isolated 1,3-propanediol-producing strain, to crude glycerol as revealed by genomic profiling. Appl. Environ. Microbiol. 2019, 85, e00254-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Yan, S.; Zhang, X.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Chemical and biological conversion of crude glycerol derived from waste cooking oil to biodiesel. Waste Manag. 2018, 71, 164–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Zhao, X.; Wang, W.; Du, W.; Liu, D. Microbial conversion of biodiesel byproduct glycerol to triacylglycerols by oleaginous yeast Rhodosporidium toruloides and the individual effect of some impurities on lipid production. Biochem. Eng. J. 2012, 65, 30–36. [Google Scholar] [CrossRef]
- Guerfali, M.; Ayadi, I.; Sassi, H.E.; Belhassen, A.; Gargouri, A.; Belghith, H. Biodiesel-derived crude glycerol as alternative feedstock for single cell oil production by the oleaginous yeast Candida viwanathii Y-E4. Ind. Crops Prod. 2020, 145, 112103. [Google Scholar] [CrossRef]
- Braga, A.; Freitas, B.; Cordeiro, A.; Belo, I. Valorization of crude glycerol as carbon source for the bioconversion of L-phenylamine to 2-penylethanol by Yarrowia species. J. Chem. Technol. Biotechnol. 2021, 96, 2940–2949. [Google Scholar] [CrossRef]
- Ripoll, M.; Velasco-Lozano, S.; Jackson, E.; Diamanti, E.; Betancor, L.; López-Gallego, F. One-pot biotransformation of glycerol into serinol catalyzed by biocatalytic composites made of whole cells and immobilised enzymes. Green Chem. 2021, 23, 1140–1146. [Google Scholar] [CrossRef]
- Dobson, R.; Gray, V.; Rumbold, K. Microbial utilization of crude glycerol for the production of value-added products. J. Ind. Microbiol. Biotechnol. 2012, 39, 217–226. [Google Scholar] [CrossRef]



| Exhaust Gases Measurements | GECCO B30 vs. Gas Oil | GECCO B100 vs. Gas Oil |
|---|---|---|
| Fine particles emissions (PM10) | −46% | −63% |
| Unburned products (HC) | −37% | −50% |
| Carbon monoxide (CO) | −11% | −18% |
| Nitrogen oxide (NOx) | Similar | +7% |
| Life Cycle Analysis Results | Gas Oil [9] | Rapeseed FAME [9] | Palm FAME [9] | GECCO FAEE | Variation vs. Gas Oil | Variation vs. Rapeseed FAME | Variation vs. Palm FAME |
|---|---|---|---|---|---|---|---|
| Global warming (kg CO2 eq./MJ) | 9.14 × 10−2 | 3.73 × 10−2 | 2.18 × 10−2 | 1.45 × 10−2 | −84% | −61% | −33% |
| Human toxicity (kg 1,4-DB eq./MJ) | 4.12 × 10−1 | −6.48 × 10−1 | −6.46 × 10−1 | −6.72 × 10−1 | −263% | 4% | 4% |
| Eutrophication (kg PO4 eq./MJ) | 3.71 × 10−5 | 3.64 × 10−4 | 1.84 × 10−4 | 2.16 × 10−5 | −42% | −94% | −88% |
| Non-renewable energy (MJ primary/MJ) | 1.25 | 4.31 × 10−1 | 2.71 × 10−1 | 3.45 × 10−1 | −72% | −20% | 27% |
| Properties | Standard | Limits |
|---|---|---|
| Ester content | EN 14105 | 96.5% (min) |
| Monoglyceride levels | EN 14105 | 0.80% (max) |
| Diglyceride levels | EN 14105 | 0.20% (max) |
| Triglyceride levels | EN 14105 | 0.20% (max) |
| Total glycerol content | EN 14105 | 0.25% (max) |
| Type of Oil | Purification Techniques | Glycerol (%) | Purified Glycerol (%) | Ref. |
|---|---|---|---|---|
| Waste oil |
| 36.7 | 96.2 | [152] |
| Waste oil |
| 29.8 | 99.0 | [153] |
| Waste oil (lab. scale) |
| 51.88 | 78.72 | [154] |
| Waste oil (ind. scale) |
| 29.99 | 60.6 | [154] |
| Waste oil |
| 40.6 | 96.08 | [155] |
| N.I. |
| 12.0 | 96 | [156] |
| Waste oil |
| 74.0 | 99.2 | [157] |
| Waste oil |
| 35.66 | 97.37 | [158] |
| N.I. |
| 40.0 | 97.5 | [159] |
| Virgin (hemp) oil |
| 51.38 | 93.89 | [160] |
| N.I. |
| 40.00 | 93.70 | [161] |
| Virgin (canola) oil |
| N.I. | 98.1 | [162] |
| N.I. |
| N.I. | N.I. | [163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decarpigny, C.; Aljawish, A.; His, C.; Fertin, B.; Bigan, M.; Dhulster, P.; Millares, M.; Froidevaux, R. Bioprocesses for the Biodiesel Production from Waste Oils and Valorization of Glycerol. Energies 2022, 15, 3381. https://doi.org/10.3390/en15093381
Decarpigny C, Aljawish A, His C, Fertin B, Bigan M, Dhulster P, Millares M, Froidevaux R. Bioprocesses for the Biodiesel Production from Waste Oils and Valorization of Glycerol. Energies. 2022; 15(9):3381. https://doi.org/10.3390/en15093381
Chicago/Turabian StyleDecarpigny, Cédric, Abdulhadi Aljawish, Cédric His, Bertrand Fertin, Muriel Bigan, Pascal Dhulster, Michel Millares, and Rénato Froidevaux. 2022. "Bioprocesses for the Biodiesel Production from Waste Oils and Valorization of Glycerol" Energies 15, no. 9: 3381. https://doi.org/10.3390/en15093381
APA StyleDecarpigny, C., Aljawish, A., His, C., Fertin, B., Bigan, M., Dhulster, P., Millares, M., & Froidevaux, R. (2022). Bioprocesses for the Biodiesel Production from Waste Oils and Valorization of Glycerol. Energies, 15(9), 3381. https://doi.org/10.3390/en15093381