Biogas Production from Microalgal Biomass Produced in the Tertiary Treatment of Urban Wastewater: Assessment of Seasonal Variations
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Harvest from GreenDune Wastewater Treatment System
2.2. Anaerobic Digestion of Microalgal Biomass
2.2.1. Biogas Production, Monitoring, and Energetic Capacity Evaluation
2.2.2. Thermoacid Hydrolysis Pretreatment
2.2.3. Reducing Sugars Quantification
2.2.4. Methane Quantification
2.2.5. Chemical Oxygen Demand (COD)
2.2.6. Statistical Analysis
3. Results and Discussion
3.1. Biomass Composition
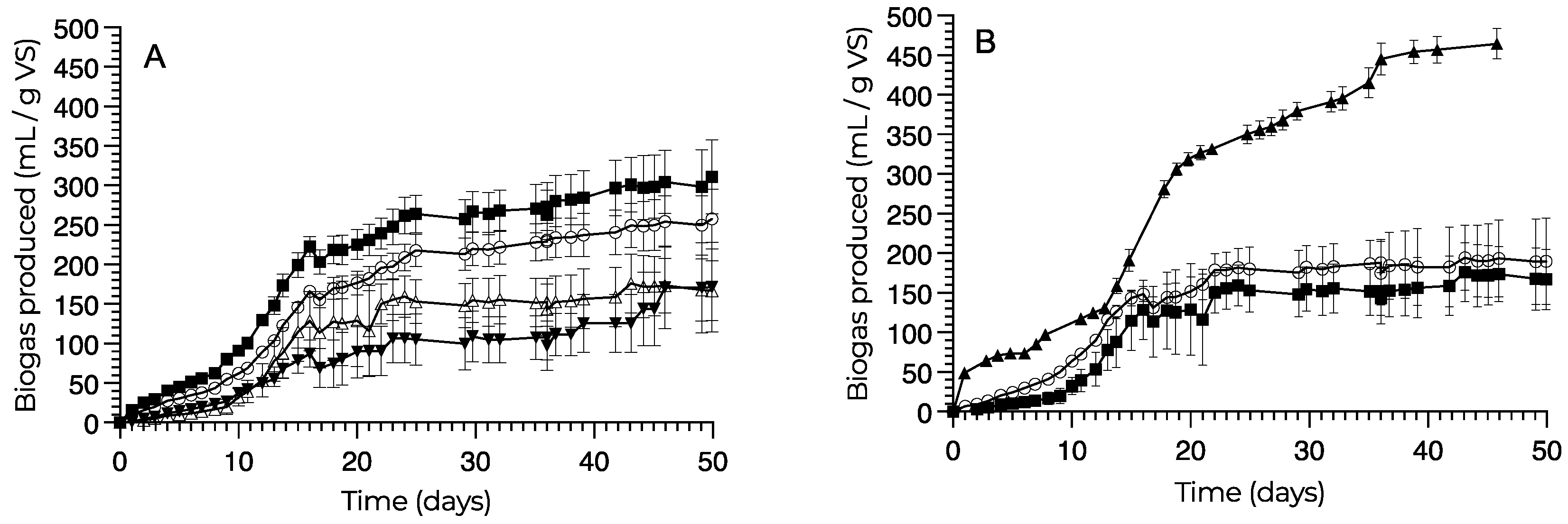
3.2. Biogas Production
3.3. Energy Potential of the Generated Biomass
4. Conclusions
- The biogas production potential is only marginally dependent on seasonality: the biomass produced in the novel pilot GreenDune photobioreactor system applied to tertiary wastewater treatment had a maximum biogas production of 311 mL/g VS with a methane yield of 252 mL/g VS with the spring samples. No significant difference was observed from the production with the summer samples (258 mL/g VS).
- The biogas production potential is not dependent of the hydraulic retention time under which the system is operated as the composition of the biomass samples did not show any significant differences.
- This biomass has a biogas production potential lower than that of purposefully cultivated microalgal species.
- This low biogas production potential is probably not related to difficulties in mobilizing biodegradable organic matter by the anaerobic digestion inoculum, but rather reflects a low intrinsic biodegradability of the volatile solids contained in the biomass, given the type of microalgae consortia formed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magro, F.G.; Freitag, J.F.; Bergoli, A.; Cavanhi, V.A.F.; Colla, L.M. Microalgae Consortia Cultivation Using Efflu-ents for Bioproduct Manufacture. Rev. Environ. Sci. Bio/Technol. 2021, 20, 865–886. [Google Scholar] [CrossRef]
- Molfetta, M.; Morais, E.G.; Barreira, L.; Bruno, G.L.; Porcelli, F.; Dugat-Bony, E.; Bonnarme, P.; Minervini, F. Protein Sources Alternative to Meat: State of the Art and Involvement of Fermentation. Foods 2022, 11, 2065. [Google Scholar] [CrossRef] [PubMed]
- Zewdie, D.T.; Ali, A.Y. Cultivation of microalgae for biofuel production: Coupling with sugarcane-processing factories. Energy Sustain. Soc. 2020, 10, 27. [Google Scholar] [CrossRef]
- Rafa, N.; Ahmed, S.F.; Badruddin, I.A.; Mofijur, M.; Kamangar, S. Strategies to Produce Cost-Effective Third-Generation Biofuel From Microalgae. Front. Energy Res. 2021, 9, 517. [Google Scholar] [CrossRef]
- Morais, E.G.; Cristofoli, N.L.; Maia, I.B.; Magina, T.; Cerqueira, P.R.; Teixeira, M.R.; Varela, J.; Barreira, L.; Gouveia, L. Microalgal Systems for Wastewater Treatment: Technological Trends and Challenges towards Waste Recovery. Energies 2021, 14, 8112. [Google Scholar] [CrossRef]
- Veerabadhran, M.; Natesan, S.; MubarakAli, D.; Xu, S.; Yang, F. Using Different Cultivation Strategies and Methods for the Production of Microalgal Biomass as a Raw Material for the Generation of Bioproducts. Chemosphere 2021, 285, 131436. [Google Scholar] [CrossRef]
- Xia, A.; Murphy, J.D. Microalgal Cultivation in Treating Liquid Digestate from Biogas Systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J.; Shilton, A.N. Wastewater Treatment High Rate Algal Ponds for Biofuel Production. Bioresour. Technol. 2011, 102, 35–42. [Google Scholar] [CrossRef]
- Passos, F.; Hom-Diaz, A.; Blanquez, P.; Vicent, T.; Ferrer, I. Improving Biogas Production from Microalgae by Enzymatic Pretreatment. Bioresour. Technol. 2016, 199, 347–351. [Google Scholar] [CrossRef]
- Adinurani, P.G.; Hendroko, S.R.; Wahono, S.K.; Nindita, A.; Mairziwan, M.; Sasmito, A.; Nugroho, Y.A.; Liwang, T. The Performance of Jatropha Curcas Linn. Capsule Husk as Feedstocks Biogas in One Phase Anaerobic Digestion. Procedia Chem. 2015, 14, 316–325. [Google Scholar] [CrossRef][Green Version]
- Yang, Z.; Guo, R.; Xu, X.; Fan, X.; Luo, S. Hydrogen and Methane Production from Lipid-Extracted Microalgal Biomass Residues. Int. J. Hydrogen Energy 2011, 36, 3465–3470. [Google Scholar] [CrossRef]
- Klassen, V.; Blifernez-Klassen, O.; Wobbe, L.; Schlüter, A.; Kruse, O.; Mussgnug, J.H. Efficiency and Biotechnological Aspects of Biogas Production from Microalgal Substrates. J. Biotechnol. 2016, 234, 7–26. [Google Scholar] [CrossRef]
- Passos, F.; Ferrer, I. Influence of Hydrothermal Pretreatment on Microalgal Biomass Anaerobic Digestion and Bioenergy Production. Water Research 2015, 68, 364–373. [Google Scholar] [CrossRef]
- Marcilhac, C.; Sialve, B.; Pourcher, A.M.; Ziebal, C.; Bernet, N.; Béline, F. Control of Nitrogen Behaviour by Phosphate Concentration during Microalgal-Bacterial Cultivation Using Digestate. Bioresour. Technol. 2015, 175, 224–230. [Google Scholar] [CrossRef]
- Santarelli, M.; Briesemeister, L.; Gandiglio, M.; Herrmann, S.; Kuczynski, P.; Kupecki, J.; Lanzini, A.; Llovell, F.; Papurello, D.; Spliethoff, H.; et al. Carbon Recovery and Re-Utilization (CRR) from the Exhaust of a Solid Oxide Fuel Cell (SOFC): Analysis through a Proof-of-Concept. J. CO2 Util. 2017, 18, 206–221. [Google Scholar] [CrossRef]
- Bona, D.; Papurello, D.; Flaim, G.; Cerasino, L.; Biasioli, F.; Silvestri, S. Management of Digestate and Exhausts from Solid Oxide Fuel Cells Produced in the Dry Anaerobic Digestion Pilot Plant: Microalgae Cultivation Approach. Waste Biomass Valoriz. 2020, 11, 6499–6514. [Google Scholar] [CrossRef]
- Franchino, M.; Tigini, V.; Varese, G.C.; Mussat Sartor, R.; Bona, F. Microalgae Treatment Removes Nutrients and Reduces Ecotoxicity of Diluted Piggery Digestate. Sci. Total Environ. 2016, 569–570, 40–45. [Google Scholar] [CrossRef]
- Hernández, D.; Riaño, B.; Coca, M.; García-González, M.C. Saccharification of Carbohydrates in Microalgal Biomass by Physical, Chemical and Enzymatic Pre-Treatments as a Previous Step for Bioethanol Production. Chem. Eng. J. 2015, 262, 939–945. [Google Scholar] [CrossRef]
- Sanchez Rizza, L.; Sanz Smachetti, M.E.; do Nascimento, M.; Salerno, G.L.; Curatti, L. Bioprospecting for Native Microalgae as an Alternative Source of Sugars for the Production of Bioethanol. Algal Res. 2017, 22, 140–147. [Google Scholar] [CrossRef]
- Bader, A.N.; Sanchez Rizza, L.; Consolo, V.F.; Curatti, L. Efficient Saccharification of Microalgal Biomass by Trichoderma Harzianum Enzymes for the Production of Ethanol. Algal Res. 2020, 48, 101926. [Google Scholar] [CrossRef]
- de Farias Silva, C.E.; Meneghello, D.; de Souza Abud, A.K.; Bertucco, A. Pretreatment of Microalgal Biomass to Improve the Enzymatic Hydrolysis of Carbohydrates by Ultrasonication: Yield vs Energy Consumption. J. King Saud Univ. Sci. 2020, 32, 606–613. [Google Scholar] [CrossRef]
- Constantino, A.; Rodrigues, B.; Raposo, S. Chemo-Enzymatic Saccharification Strategy of Microalgae. In Proceedings of the International Congress on Engineering and Sustainability, Faro, Portugal, 9–11 October 2019; pp. 409–420. [Google Scholar] [CrossRef]
- Baeyens, J.; Kang, Q.; Appels, L.; Dewil, R.; Lv, Y.; Tan, T. Challenges and Opportunities in Improving the Production of Bio-Ethanol. Prog. Energy Combust. Sci. 2015, 47, 60–88. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; Lavín, P.L.; Lanfer Marquez, U.M.; Aidar, E. Distribution of Intracellular Nitrogen in Marine Microalgae: Calculation of New Nitrogen-to-Protein Conversion Factors. Eur. J. Phycol. 2007, 39, 17–32. [Google Scholar] [CrossRef]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet Food with Nutritional Health Benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Maia, I.B.; Carneiro, M.; Magina, T.; Malcata, F.X.; Otero, A.; Navalho, J.; Varela, J.; Pereira, H. Diel Biochemical and Photosynthetic Monitorization of Skeletonema Costatum and Phaeodactylum Tricornutum Grown in Outdoor Pilot-Scale Flat Panel Photobioreactors. J. Biotechnol. 2022, 343, 110–119. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 27 June 2022).
- Vargas-Estrada, L.; Longoria, A.; Arenas, E.; Moreira, J.; Okoye, P.U.; Bustos-Terrones, Y.; Sebastian, P.J. A Review on Current Trends in Biogas Production from Microalgae Biomass and Microalgae Waste by Anaerobic Digestion and Co-Digestion. BioEnergy Res. 2021, 15, 77–92. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic Digestion of Microalgae as a Necessary Step to Make Microalgal Biodiesel Sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Ferrer, I.; Pániker, C.C.; Gómez-Pinchetti, J.L.; Rousseau, D.P.L.; van Hulle, S.W.H.; Garfí, M. Natural Pigments and Biogas Recovery from Microalgae Grown in Wastewater. ACS Sustain. Chem. Eng. 2020, 8, 10691–10701. [Google Scholar] [CrossRef]
- Constantino, A.; Rodrigues, B.; Leon, R.; Barros, R.; Raposo, S. Alternative Chemo-Enzymatic Hydrolysis Strategy Applied to Different Microalgae Species for Bioethanol Production. Algal Res. 2021, 56, 102329. [Google Scholar] [CrossRef]
- Alzate, M.E.; Muñoz, R.; Rogalla, F.; Fdz-Polanco, F.; Pérez-Elvira, S.I. Biochemical Methane Potential of Microalgae Biomass after Lipid Extraction. Chem. Eng. J. 2014, 243, 405–410. [Google Scholar] [CrossRef]
- Industrial Scale Demonstration of Sustainable Algae Cultures for Biofuel Production | ALL-GAS Project | Fact Sheet | FP7 | CORDIS | European Commission. Available online: https://cordis.europa.eu/project/id/268208 (accessed on 27 June 2022).

| Summer | Autumn | Spring | Winter24 | Winter48 | Skeletonema * | |
|---|---|---|---|---|---|---|
| Proteins | 39.5 ± 0.8 | 24.9 ± 0.4 | 36.7 ± 1.9 | 26.1 ± 0.9 | 25.2 ± 0.3 | 22.2 ± 3.7 |
| Carbohydrates | 29.3 ± 0.5 | 35.5 ± 2.0 | 25.3 ± 0.9 | 41.8 ± 0.9 | 39.4 ± 2.1 | 29.6 ± 2.8 |
| Lipids | 11.5 ± 0.3 | 6.7 ± 1.3 | 7.7 ± 0.5 | 5.4 ± 0.2 | 6.2 ± 1.6 | 14.1 ± 1.9 |
| Ash | 19.8 ± 0.4 | 32.9 ± 1.4 | 30.3 ± 0.2 | 26.7 ± 0.9 | 29.2 ± 0.9 | 32.1 ± 2.4 |
| Product | Summer | Autumn | Spring | Winter24 | Winter48 | Skeletonema |
|---|---|---|---|---|---|---|
| Biogas NH | 258 ± 38 b,c | 172 ± 57 c | 311 ± 47 b | 167 ± 37 c | 190 ± 55 b,c | 464 ± 19 a |
| Methane NH | 211 ± 97 *,# | 155 ± 32 *,# | 252 ± 36 *,# | 149 ± 24 *,# | 135 ± 63 *,# | - |
| Methane H | 189 ± 8 # | 81 ± 11 # | 239 ± 12 # | 191 ± 29 # | 137 ± 21 # | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, R.; Raposo, S.; Morais, E.G.; Rodrigues, B.; Afonso, V.; Gonçalves, P.; Marques, J.; Cerqueira, P.R.; Varela, J.; Teixeira, M.R.; et al. Biogas Production from Microalgal Biomass Produced in the Tertiary Treatment of Urban Wastewater: Assessment of Seasonal Variations. Energies 2022, 15, 5713. https://doi.org/10.3390/en15155713
Barros R, Raposo S, Morais EG, Rodrigues B, Afonso V, Gonçalves P, Marques J, Cerqueira PR, Varela J, Teixeira MR, et al. Biogas Production from Microalgal Biomass Produced in the Tertiary Treatment of Urban Wastewater: Assessment of Seasonal Variations. Energies. 2022; 15(15):5713. https://doi.org/10.3390/en15155713
Chicago/Turabian StyleBarros, Raúl, Sara Raposo, Etiele G. Morais, Brígida Rodrigues, Valdemira Afonso, Pedro Gonçalves, José Marques, Paulo Ricardo Cerqueira, João Varela, Margarida Ribau Teixeira, and et al. 2022. "Biogas Production from Microalgal Biomass Produced in the Tertiary Treatment of Urban Wastewater: Assessment of Seasonal Variations" Energies 15, no. 15: 5713. https://doi.org/10.3390/en15155713
APA StyleBarros, R., Raposo, S., Morais, E. G., Rodrigues, B., Afonso, V., Gonçalves, P., Marques, J., Cerqueira, P. R., Varela, J., Teixeira, M. R., & Barreira, L. (2022). Biogas Production from Microalgal Biomass Produced in the Tertiary Treatment of Urban Wastewater: Assessment of Seasonal Variations. Energies, 15(15), 5713. https://doi.org/10.3390/en15155713






