Numerical Simulation of Oil Shale Pyrolysis under Microwave Irradiation Based on a Three-Dimensional Porous Medium Multiphysics Field Model
Abstract
:1. Introduction
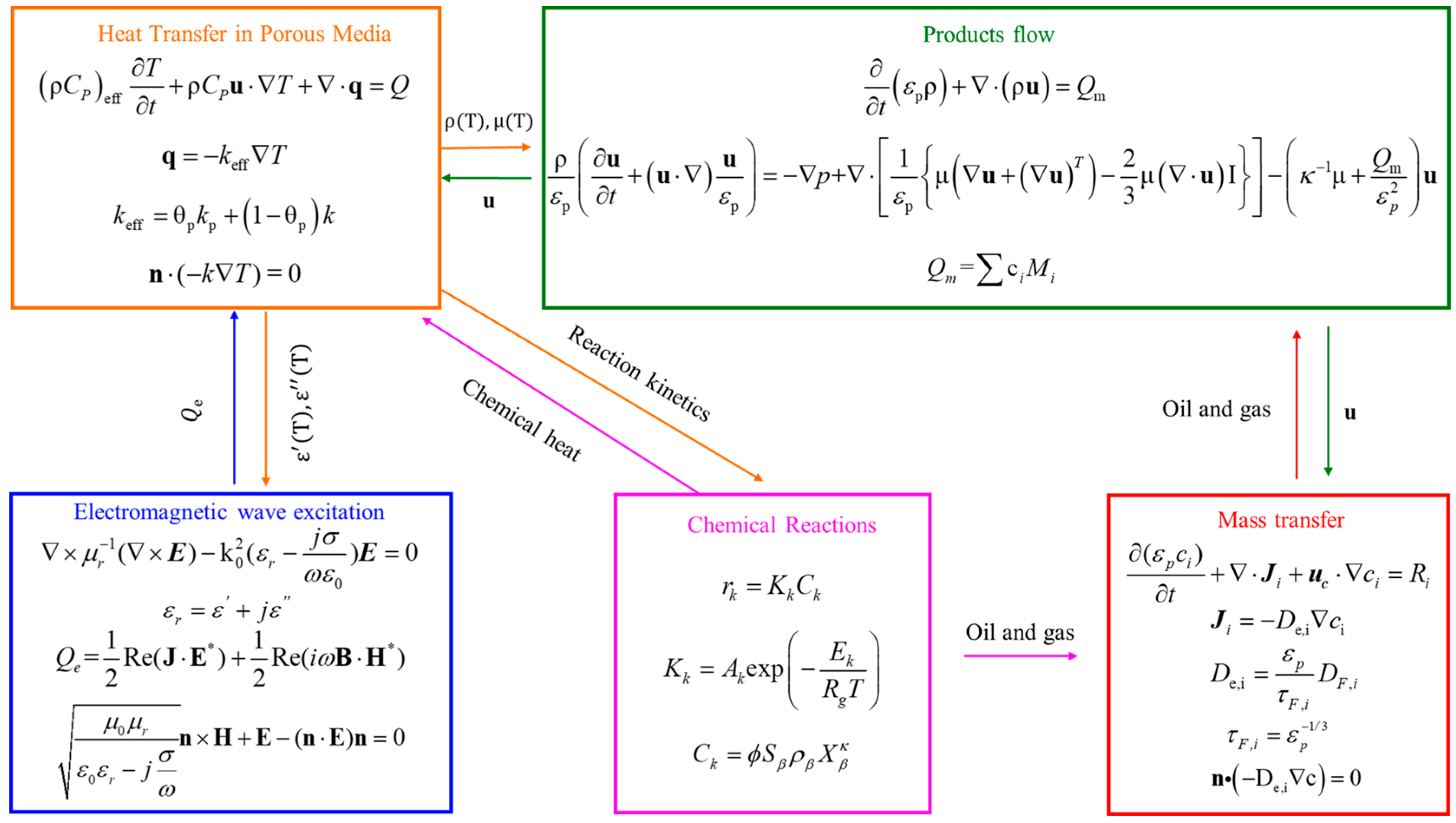
2. Governing Equations
2.1. Electromagnetic Wave Excitation
2.2. Heat Transfer in Porous Media
2.3. Chemical Reactions
2.4. Mass Transfer
2.5. Products Flow
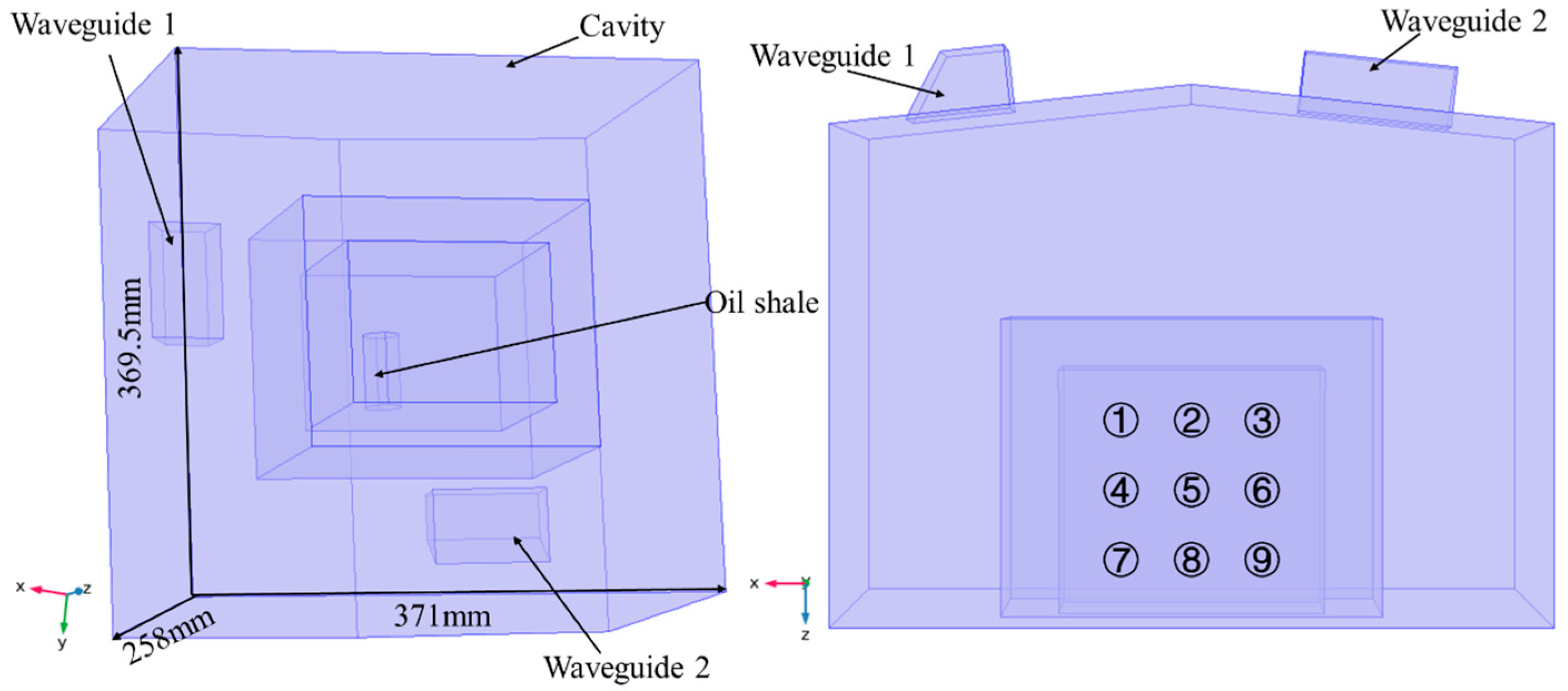
3. Simulation Model
3.1. Model Assumptions
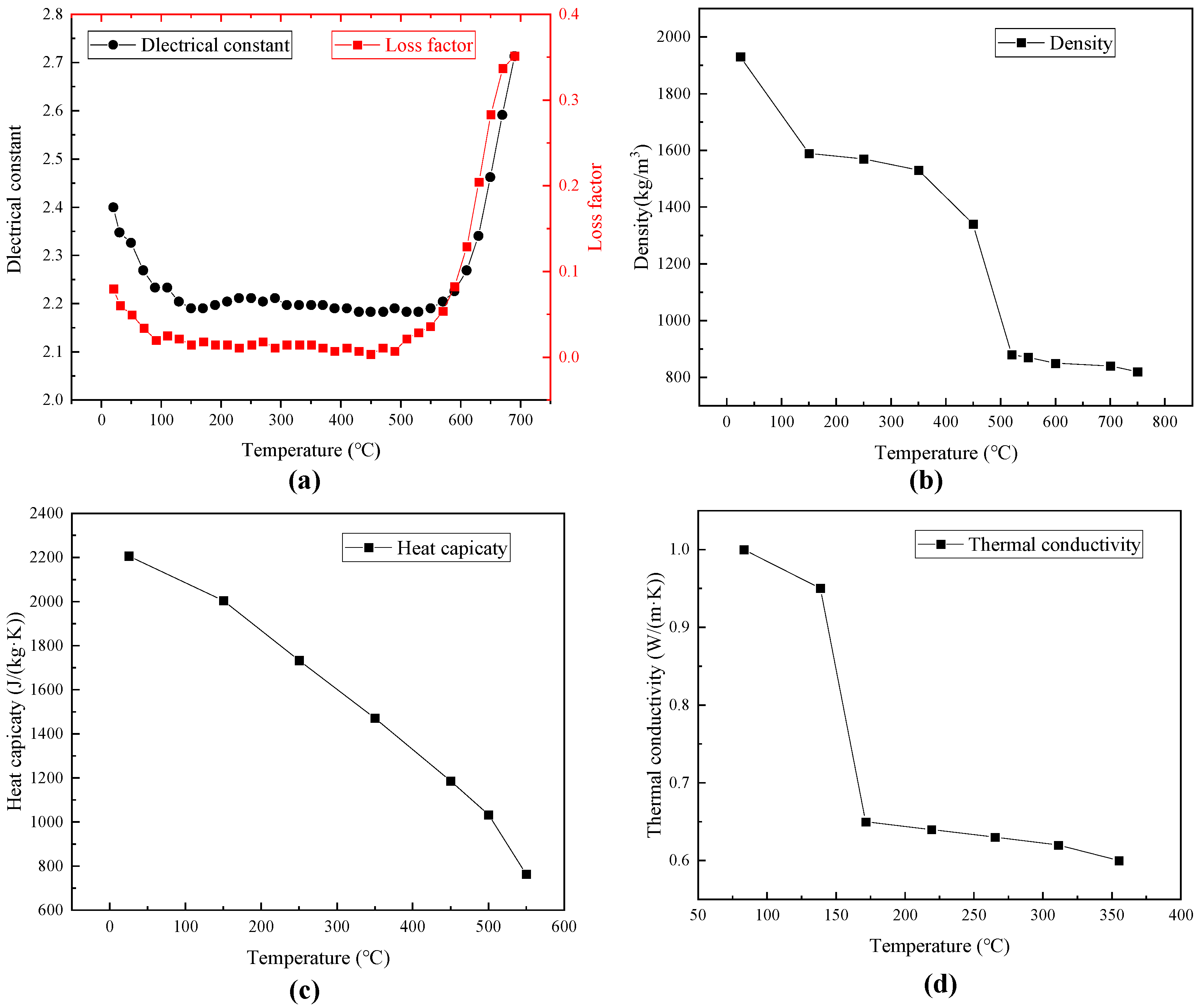
3.2. Geometry Model and Input Parameters
| Parameter | Symbol | Value | Source |
|---|---|---|---|
| Oil shale sample initial temperature | T0 | 20 °C | Given |
| Microwave frequency | f’ | 2.45 GHz | Given |
| Microwave power | P | 1000 W | Given |
| Concentration of kerogen | c_ker | 370 mol/m3 | Ref. [33] |
| Molecular weight of kerogen | M_ker | 647 g/mol | Ref. [34] |
| Molecular weight of heavy oil (C25H50) | M_ho | 352 g/mol | Ref. [34] |
| Molecular weight of light oil (C9H20) | M_lo | 128 g/mol | Ref. [34] |
| Molecular weight of non-hydrocarbon gas (CO2) | M_gas | 44 g/mol | Ref. [34] |
| Molecular weight of methane (CH4) | M_ch | 16 g/mol | Ref. [34] |
| Molecular weight of coke | M_coke | 13 g/mol | Ref. [34] |
| Porosity of oil shale | ϕ | 0.1 | Measured |
| Permeability of oil shale | k | 0.11 mD | Measured |
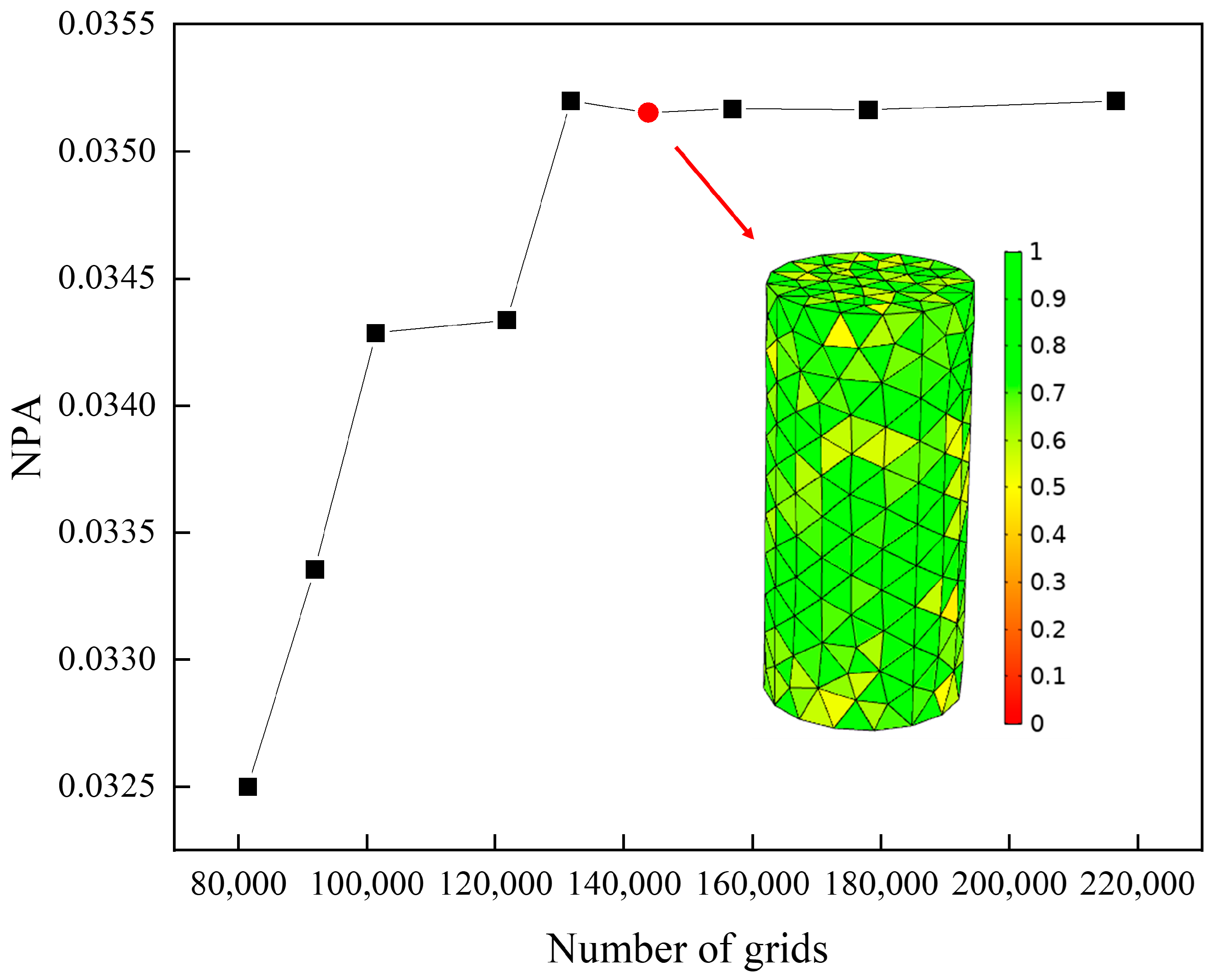
3.3. Grid-Independent Validation
3.4. Experimental Verifications
4. Results and Discussion
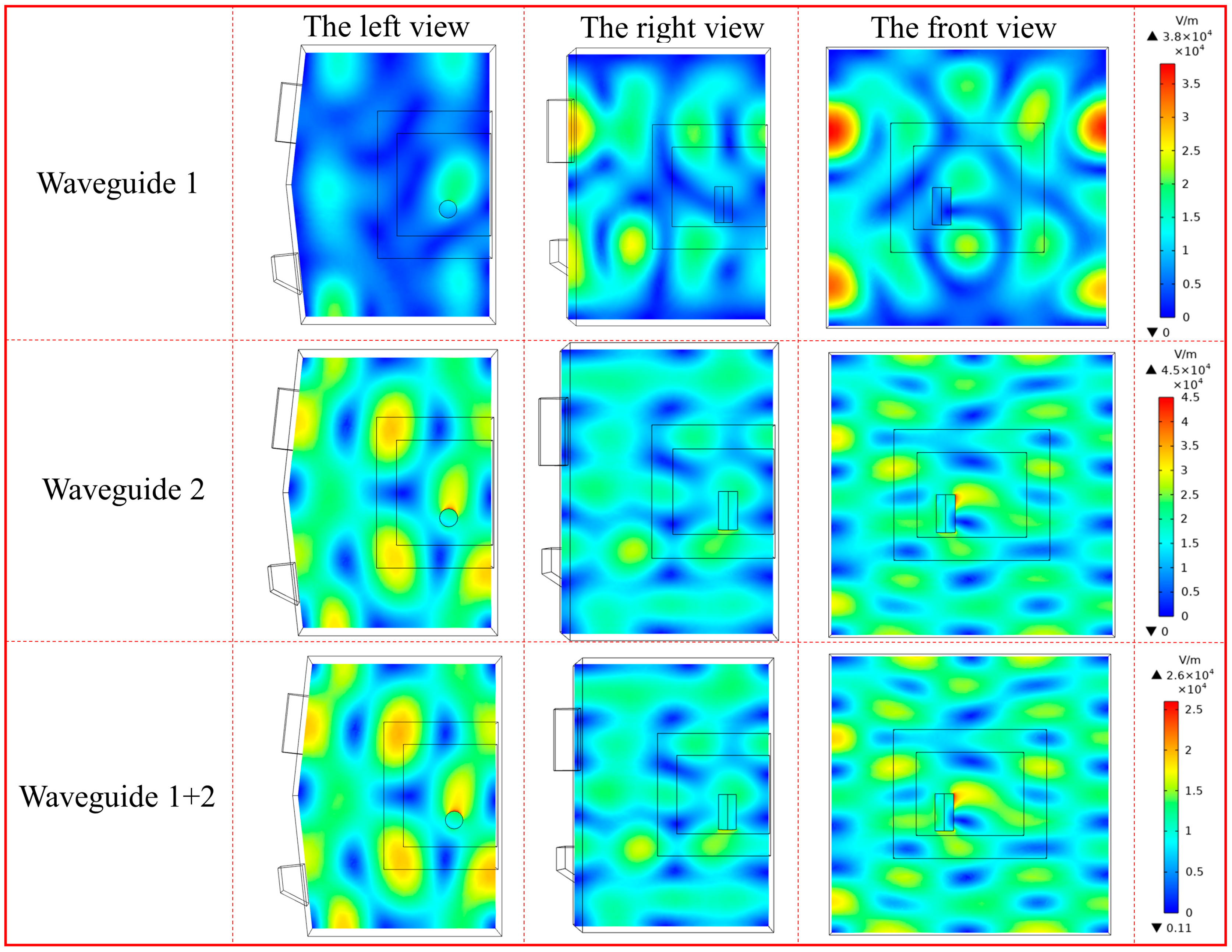
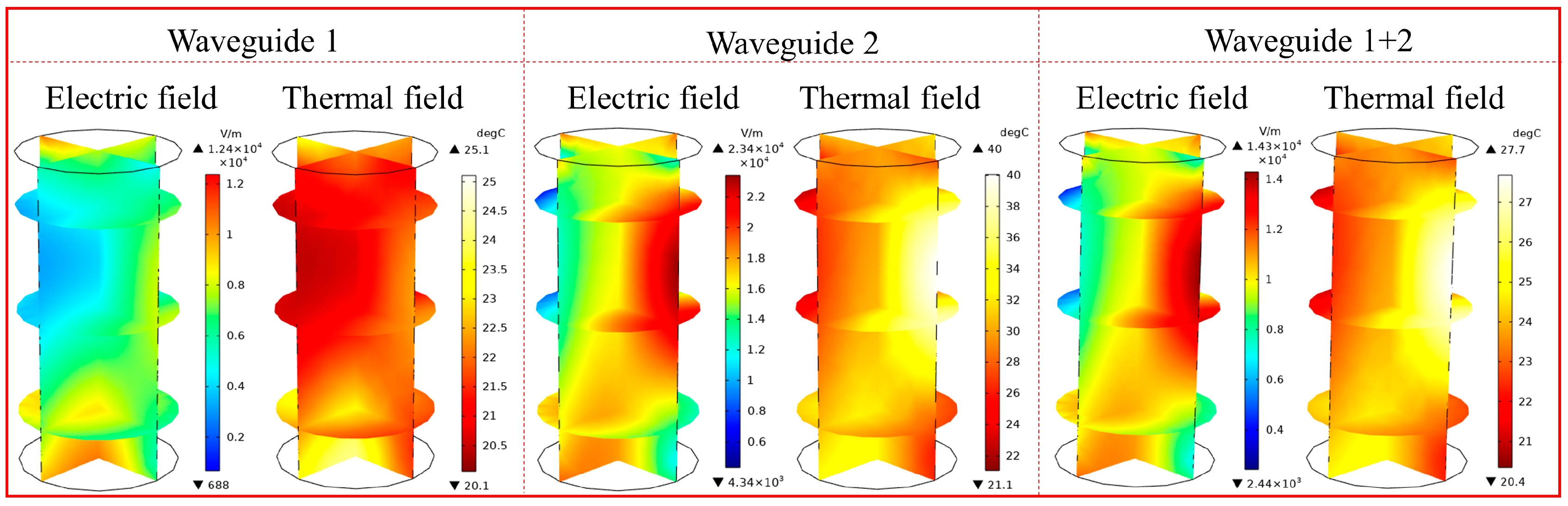
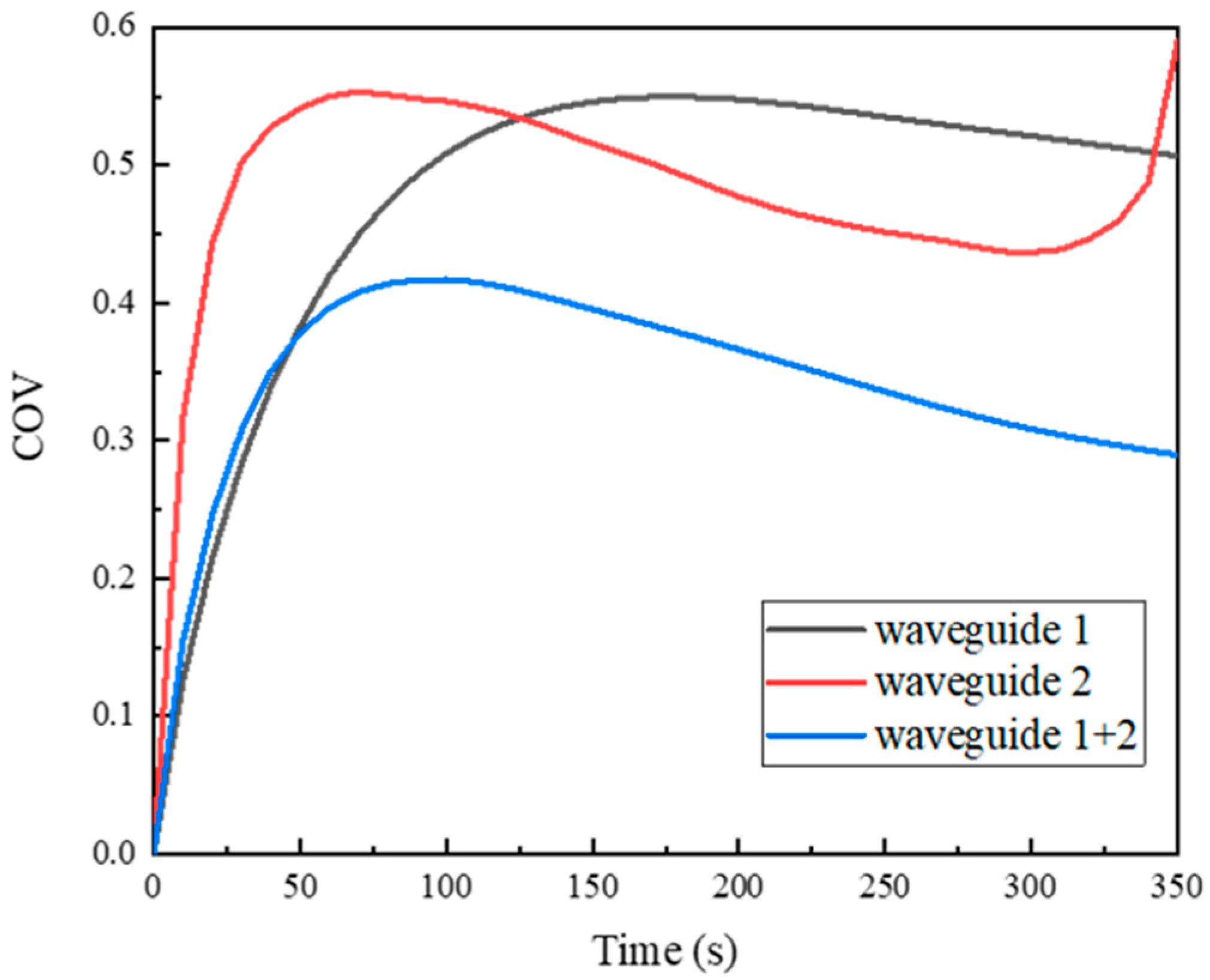
4.1. Effect of Microwave Waveguide
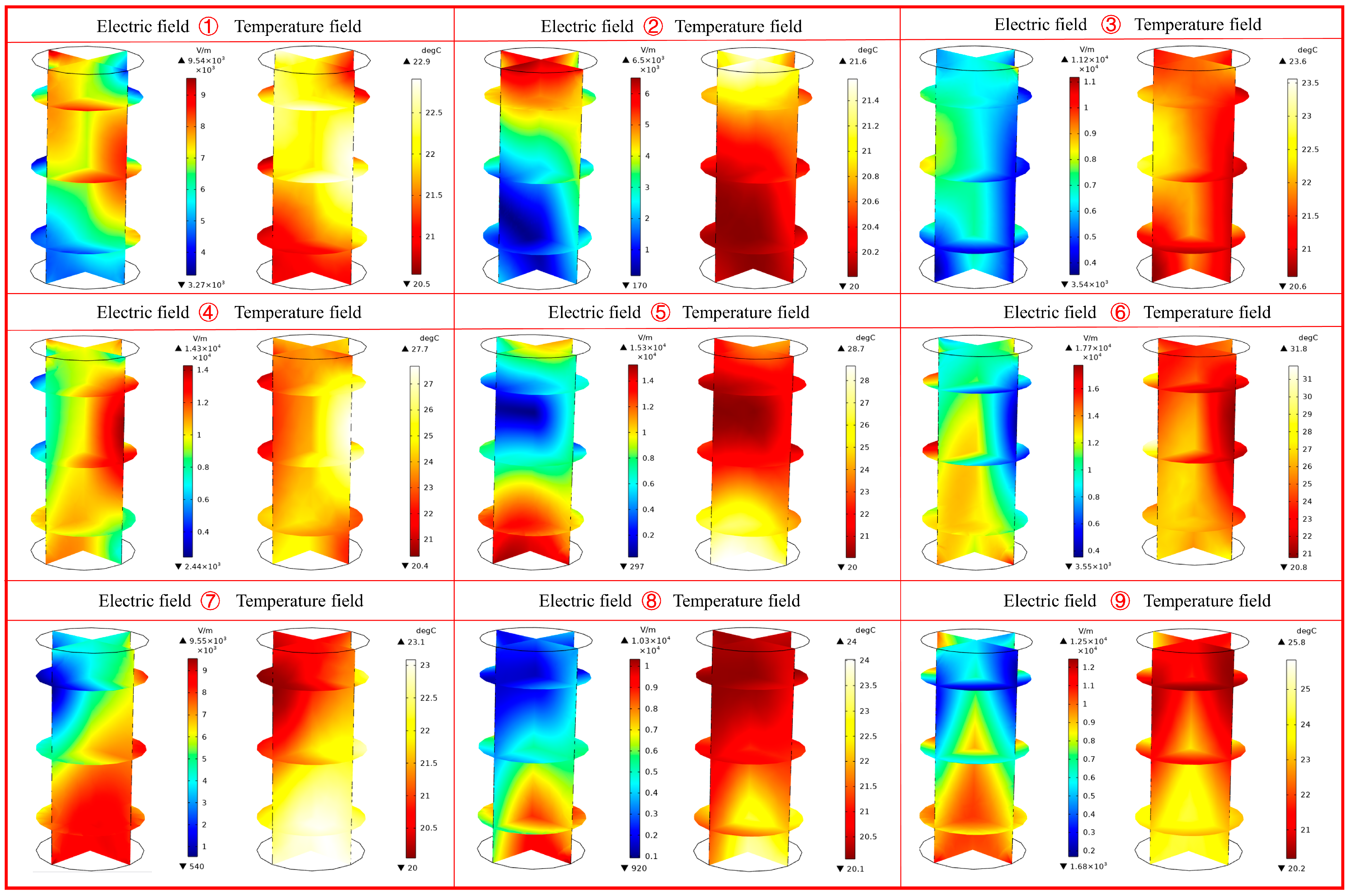
4.2. Effect of Sample Position
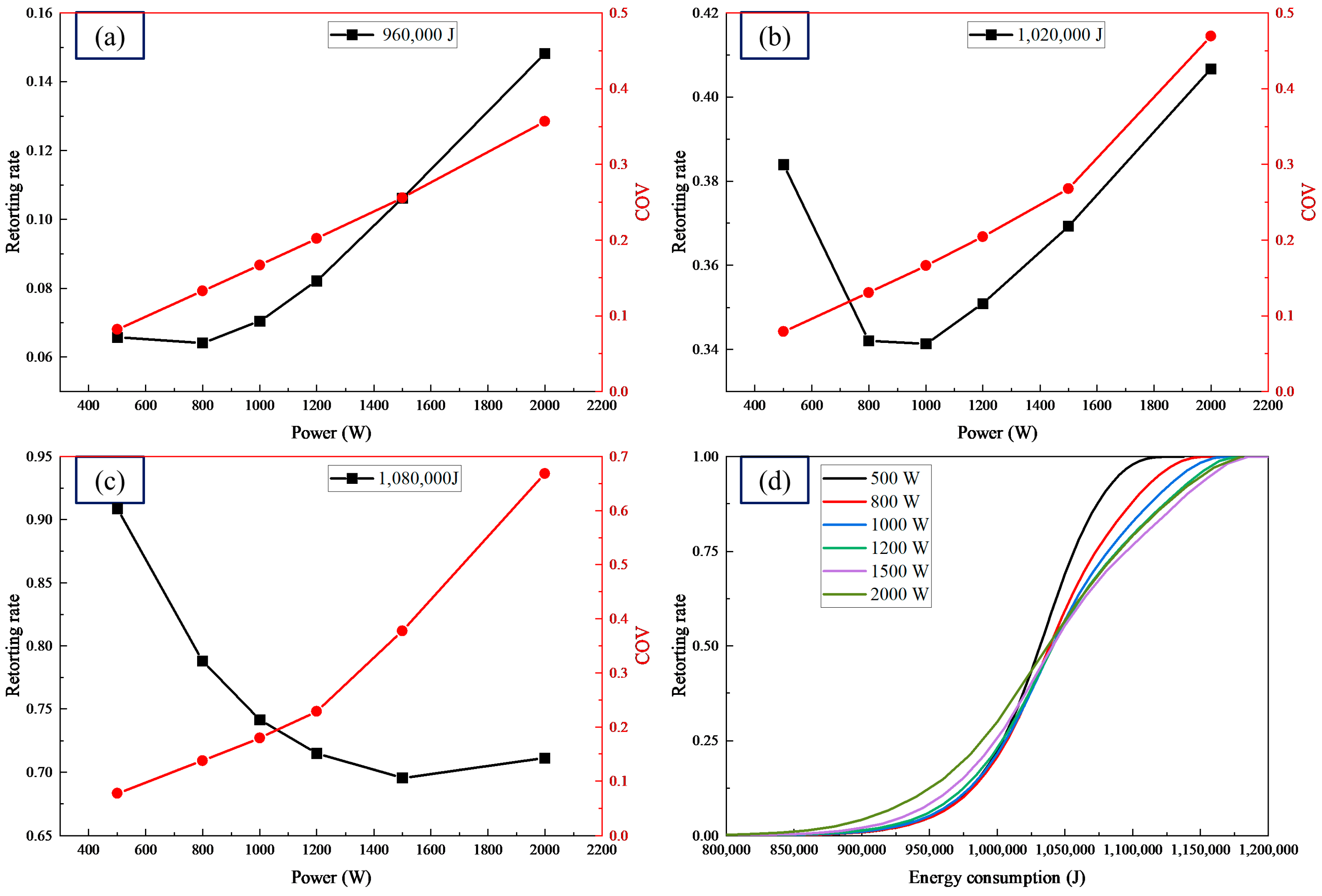
4.3. Effect of Microwave Power
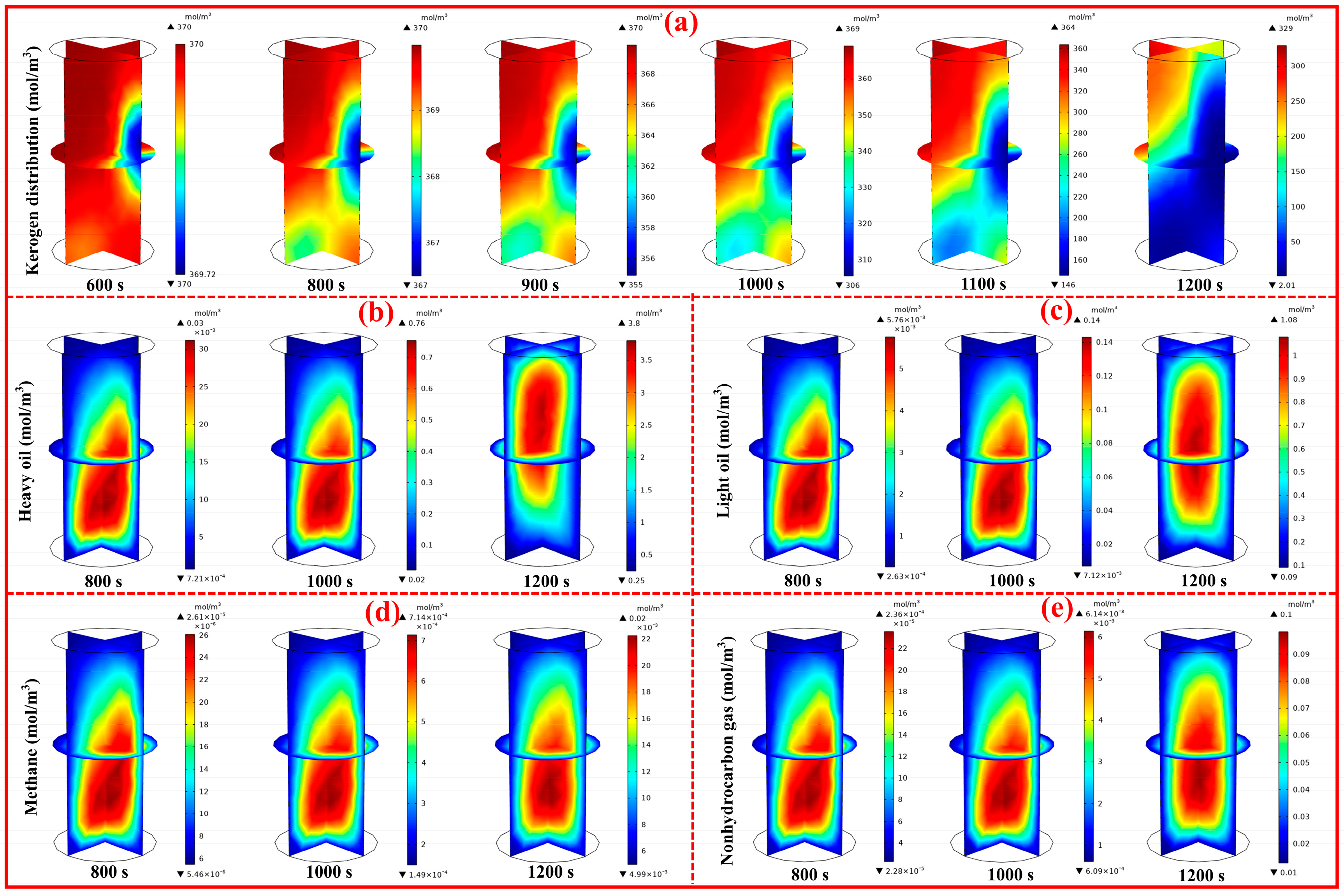
4.4. Analysis of Products Distribution
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Adam, M.; Hart, A.; Wood, J.; Rigby, S.P.; Robinson, J.P. Impact of Oil Composition on Microwave Heating Behavior of Heavy Oils. Energy Fuels 2018, 32, 1592–1599. [Google Scholar] [CrossRef]
- Mead, I. International Energy Outlook 2017; US Energy Information Administration: Washington, DC, USA, 2017. Available online: https://csis-website-prod.s3.amazonaws.com/s3fs-public/event/170914_CSIS_release.pdf (accessed on 9 September 2021).
- Xia, L.; Zhang, H.; Wang, B.; Yu, C. Numerical simulation and experimental validation of oil shale drying in pneumatic conveying dryer. Dry. Technol. 2018, 36, 617–629. [Google Scholar] [CrossRef]
- Dong, F.; Feng, Z.; Yang, D.; Zhao, Y.; Elsworth, D. Permeability Evolution of Pyrolytically-Fractured Oil Shale under In Situ Conditions. Energies 2018, 11, 3033. [Google Scholar] [CrossRef] [Green Version]
- Zou, C. Unconventional Petroleum Geology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Biglarbigi, K.; Crawford, P.; Carolus, M.; Dean, C. Rethinking World Oil-Shale Resource Estimates. In Proceedings of the SPE Annual Technical Conference and Exhibition, Florence, Italy, 20–22 September 2010. [Google Scholar] [CrossRef]
- Song, X.; Zhang, C.; Shi, Y.; Li, G. Production performance of oil shale in-situ conversion with multilateral wells. Energy 2019, 189, 116145. [Google Scholar] [CrossRef]
- Zheng, H.; Shi, W.; Ding, D.; Zhang, C. Numerical Simulation of In Situ Combustion of Oil Shale. Geofluids 2017, 2017, 3028974. [Google Scholar] [CrossRef] [Green Version]
- Kang, Z.; Zhao, Y.; Yang, D. Review of oil shale in-situ conversion technology. Appl. Energy 2020, 269, 115121. [Google Scholar] [CrossRef]
- Zhang, M.; Salazar-Tio, R.; Fager, A.; Crouse, B. A Multiscale Digital Rock Workflow for Shale Matrix Permeability Prediction. In Proceedings of the SPE/AAPG/SEG Unconventional Resources Technology Conference, Virtual, 20–22 July 2020. [Google Scholar] [CrossRef]
- Taheri-Shakib, J.; Shekarifard, A.; Naderi, H. Characterization of the wax precipitation in Iranian crude oil based on Wax Appearance Temperature (WAT): Part 1. The influence of electromagnetic waves. J. Pet. Sci. Eng. 2018, 161, 530–540. [Google Scholar] [CrossRef]
- Taheri-Shakib, J.; Shekarifard, A.; Naderi, H. The experimental study of effect of microwave heating time on the heavy oil properties: Prospects for heavy oil upgrading. J. Anal. Appl. Pyrolysis 2017, 128, 176–186. [Google Scholar] [CrossRef]
- Taheri-Shakib, J.; Shekarifard, A.; Naderi, H. Analysis of the asphaltene properties of heavy crude oil under ultrasonic and microwave irradiation. J. Anal. Appl. Pyrolysis 2018, 129, 171–180. [Google Scholar] [CrossRef]
- Hasanvand, M.Z.; Golparvar, A. A Critical Review of Improved Oil Recovery by Electromagnetic Heating. Pet. Sci. Technol. 2014, 32, 631–637. [Google Scholar] [CrossRef]
- Zhu, J.; Yi, L.; Yang, Z.; Duan, M. Three-dimensional numerical simulation on the thermal response of oil shale subjected to microwave heating. Chem. Eng. J. 2021, 407, 127197. [Google Scholar] [CrossRef]
- Chen, H.; Li, T.; Wang, Z.; Ye, R.; Li, Q. Effect of dielectric properties on heat transfer characteristics of rubber materials via microwave heating. Int. J. Therm. Sci. 2020, 148, 106162. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, S.; Li, Q.; Liu, S.; Han, J. Thermoelectric coupling analysis of high-voltage breakdown industrial frequency pyrolysis in Fuyu oil shale. Int. J. Therm. Sci. 2018, 130, 19–27. [Google Scholar] [CrossRef]
- Li, H.; Shi, S.; Lin, B.; Lu, J.; Ye, Q.; Lu, Y.; Wang, Z.; Hong, Y.; Zhu, X. Effects of microwave-assisted pyrolysis on the microstructure of bituminous coals. Energy 2019, 187, 115986. [Google Scholar] [CrossRef]
- Qi, H.; Jiang, H.; You, Y.; Hu, J.; Wang, Y.; Wu, Z.; Qi, H. Mechanism of Magnetic Nanoparticle Enhanced Microwave Pyrolysis for Oily Sludge. Energies 2022, 15, 1254. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Leung, C.; Gao, F. A Fully Coupled Numerical Model for Microwave Heating Enhanced Shale Gas Recovery. Energies 2018, 11, 1608. [Google Scholar] [CrossRef] [Green Version]
- Amini, A.; Latifi, M.; Chaouki, J. Electrification of materials processing via microwave irradiation: A review of mechanism and applications. Appl. Therm. Eng. 2021, 193, 117003. [Google Scholar] [CrossRef]
- Meng, Y.; Tang, L.; Yan, Y.; Oladejo, J.; Jiang, P.; Wu, T.; Pang, C. Effects of Microwave-enhanced Pretreatment on Oil Shale Milling Performance. Energy Procedia 2019, 158, 1712–1717. [Google Scholar] [CrossRef]
- Al-Gharabli, S.I.; Azzam, M.O.J.; Al-Addous, M. Microwave-assisted solvent extraction of shale oil from Jordanian oil shale. Oil Shale 2015, 32, 240–251. [Google Scholar] [CrossRef] [Green Version]
- Neto, A.; Thomas, S.; Bond, G.; Thibault-Starzyk, F.; Ribeiro, F.; Henriques, C. The Oil Shale Transformation in the Presence of an Acidic BEA Zeolite under Microwave Irradiation. Energy Fuels 2014, 28, 2365–2377. [Google Scholar] [CrossRef] [Green Version]
- El Harfi, K.; Mokhlisse, A.; Chanâa, M.B.; Outzourhit, A. Pyrolysis of the Moroccan (Tarfaya) oil shales under microwave irradiation. Fuel 2000, 79, 733–742. [Google Scholar] [CrossRef]
- Scaar, H.; Franke, G.; Weigler, F.; Delele, M.; Tsotsas, E.; Mellmann, J. Experimental and numerical study of the airflow distribution in mixed-flow grain dryers. Dry. Technol. 2016, 34, 595–607. [Google Scholar] [CrossRef]
- Jamaleddine, T.J.; Ray, M.B. Application of Computational Fluid Dynamics for Simulation of Drying Processes: A Review. Dry. Technol. 2010, 28, 120–154. [Google Scholar] [CrossRef]
- Skuratovsky, I.; Levy, A.; Borde, I. Two-Fluid, Two-Dimensional Model for Pneumatic Drying. Dry. Technol. 2003, 21, 1645–1668. [Google Scholar] [CrossRef]
- Mezhericher, M.; Levy, A.; Borde, I. Three-dimensional modelling of pneumatic drying process. Powder Technol. 2010, 203, 371–383. [Google Scholar] [CrossRef]
- Zhao, S.; Lü, X.; Li, Q.; Sun, Y. Thermal-fluid coupling analysis of oil shale pyrolysis and displacement by heat-carrying supercritical carbon dioxide. Chem. Eng. J. 2020, 394, 125037. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, S.; Bai, J.; Chi, M.; Cui, D.; Wang, Z.; Liu, Q.; Xu, F. Experimental and dynamics simulation studies of the molecular modeling and reactivity of the Yaojie oil shale kerogen. Fuel 2018, 230, 319–330. [Google Scholar] [CrossRef]
- Zhu, J.; Yi, L.; Yang, Z.; Li, X. Numerical simulation on the in situ upgrading of oil shale reservoir under microwave heating. Fuel 2021, 287, 119553. [Google Scholar] [CrossRef]
- Youtsos, M.S.K.; Mastorakos, E.; Cant, R.S. Numerical simulation of thermal and reaction fronts for oil shale upgrading. Chem. Eng. Sci. 2013, 94, 200–213. [Google Scholar] [CrossRef]
- Lee, K.J.; Moridis, G.J.; Ehlig-Economides, C.A. Numerical simulation of diverse thermal in situ upgrading processes for the hydrocarbon production from kerogen in oil shale reservoirs. Energy Explor. Exploit. 2017, 35, 315–337. [Google Scholar] [CrossRef] [Green Version]
- Al-Harahsheh, M.; Kingman, S.; Saeid, A.; Robinson, J.; Dimitrakis, G.; Alnawafleh, H. Dielectric properties of Jordanian oil shales. Fuel Process. Technol. 2009, 90, 1259–1264. [Google Scholar] [CrossRef]
- Wang, L. Experiment and Simulation on Temperature Field during Oil Shale Pyrolysis by Electric-Heating. Master’s Thesis, Jilin University, Changchun, China, 2014. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1014268510.nh&DbName=CMFD2014 (accessed on 17 January 2022).
- Nottenburg, R.; Rajeshwar, K.; Rosenvold, R.; DuBow, J. Measurement of thermal conductivity of Green River oil shales by a thermal comparator technique. Fuel 1978, 57, 789–795. [Google Scholar] [CrossRef]
- Tamang, S.; Aravindan, S. 3D numerical modelling of microwave heating of SiC susceptor. Appl. Therm. Eng. 2019, 162, 114250. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, X.; Ye, J.; Zhu, H.; Yuan, J.; Li, X.; Huang, K. Arbitrary Lagrangian-Eulerian method for computation of rotating target during microwave heating. Int. J. Heat Mass Transf. 2019, 134, 271–285. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.; Zhu, H.; Li, K.; Yao, W.; Huang, K. Microwave heating based on two rotary waveguides to improve efficiency and uniformity by gradient descent method. Appl. Therm. Eng. 2020, 178, 115594. [Google Scholar] [CrossRef]
- Huang, X.; Rudolph, D.L. Coupled model for water, vapour, heat, stress and strain fields in variably saturated freezing soils. Adv. Water Resour. 2021, 154, 103945. [Google Scholar] [CrossRef]
- Abo Seida, O.M. Propagation of electromagnetic waves in a rectangular tunnel. Appl. Math. Comput. 2003, 136, 405–413. [Google Scholar] [CrossRef]
- Huang, J.; Xu, G.; Hu, G.; Kizil, M.; Chen, Z. A coupled electromagnetic irradiation, heat and mass transfer model for microwave heating and its numerical simulation on coal. Fuel Process. Technol. 2018, 177, 237–245. [Google Scholar] [CrossRef]
- Shekarifard, A.; Taheri-Shakib, J. Technical and scientific review on oil shale upgrading. Int. J. Petrochem. Sci. Eng. 2016, 1, 78–83. [Google Scholar] [CrossRef]
- Taheri-Shakib, J.; Kantzas, A. A comprehensive review of microwave application on the oil shale: Prospects for shale oil production. Fuel 2021, 305, 121519. [Google Scholar] [CrossRef]
- Wang, L.; Yang, D.; Kang, Z. Evolution of permeability and mesostructure of oil shale exposed to high-temperature water vapor. Fuel 2021, 290, 119786. [Google Scholar] [CrossRef]
- Wang, G.; Liu, S.; Yang, D.; Fu, M. Numerical study on the in-situ pyrolysis process of steeply dipping oil shale deposits by injecting superheated water steam: A case study on Jimsar oil shale in Xinjiang, China. Energy 2022, 239, 122182. [Google Scholar] [CrossRef]
- Sun, Y.; Bai, F.; Liu, B.; Liu, Y.; Guo, M.; Guo, W.; Wang, Q.; Lü, X.; Yang, F.; Yang, Y. Characterization of the oil shale products derived via topochemical reaction method. Fuel 2014, 115, 338–346. [Google Scholar] [CrossRef]
- Martemyanov, S.; Bukharkin, A.; Koryashov, I.; Ivanov, A. Analysis of applicability of oil shale for in situ conversion. AIP Conf. Proc. 2016, 1772, 020001. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Xiaoqiao, G.; Fansheng, X.; Jialiang, Z.; Yanju, L. Temperature distribution simulation and optimization design of electric heater for in-situ oil shale heating. Oil Shale 2014, 31, 105–120. [Google Scholar] [CrossRef] [Green Version]
- Ryan, R.C.; Fowler, T.D.; Beer, G.L.; Nair, V. Shell’s In Situ Conversion Process—From Laboratory to Field Pilots. In Oil Shale: A Solution to the Liquid Fuel Dilemma; American Chemical Society: Washington, DC, USA, 2010; Volume 1032, pp. 161–183. [Google Scholar] [CrossRef]






| Decomposition Reaction | Frequency Factor (1/s) | Activation Energy (kJ/mol) | H (J/mol) |
|---|---|---|---|
| Kerogen 0.279HO + 0.143LO + 0.018Gas + 0.005Methane + 0.555Coke1 | 1.0 1013 | 213.4 | −46,500 |
| Heavy oil (HO) → 0.373LO + 0.156Gas + 0.03Methane + 0.441Coke2 | 5.0 1011 | 225.9 | −46,500 |
| Light oil (LO) → 0.595Gas + 0.115Methane + 0.290Coke3 | 3.0 1013 | 225.9 | −335,000 |
| Coke1 → 0.031Gas + 0.033Methane + 0.936Coke2 | 1.0 1013 | 225.9 | −46,500 |
| Coke2 → 0.003Gas + 0.033Methane + 0.964Coke3 | 5.0 1011 | 225.9 | −46,500 |
| Item | Proximate Analysis (wt%) | Ultimate Analysis (wt%) | Porous Features | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Volatiles | Ash | Fixed Carbon | H | O | N | S | C | Porosity | Permeability mD | |
| S1 | 0.64 | 18.66 | 75.5 | 5.2 | 2.7 | 5.38 | 0.44 | 1.01 | 15.96 | 0.09 | 0.098 |
| S2 | 0.98 | 17.32 | 75 | 6.7 | 2.88 | 5.98 | 0.66 | 1.11 | 17.32 | 0.08 | 0.087 |
| S3 | 0.74 | 18.66 | 72.5 | 8.1 | 2.74 | 6.11 | 0.59 | 1.09 | 16.36 | 0.1 | 0.11 |
| Energy Consumption of 960,000 J | ||||||
|---|---|---|---|---|---|---|
| Power (W) | 500 | 800 | 1000 | 1200 | 1500 | 2000 |
| Time (s) | 1920 | 1200 | 960 | 800 | 640 | 480 |
| Energy consumption of 1,020,000 J | ||||||
| Power (W) | 500 | 800 | 1000 | 1200 | 1500 | 2000 |
| Time (s) | 2040 | 1275 | 1020 | 850 | 680 | 510 |
| Energy consumption of 1,080,000 J | ||||||
| Power (W) | 500 | 800 | 1000 | 1200 | 1500 | 2000 |
| Time (s) | 2160 | 1350 | 1080 | 900 | 720 | 540 |
| Type of Heating | Benefits | Drawbacks | References |
|---|---|---|---|
| Supercritical carbon dioxide | Supercritical carbon dioxide can effectively extract organic matter from oil shale. | Prolonging the pyrolysis time and increasing the temperature can lead to the aggravation of secondary cracking. | [30] |
| Superheated water steam | Products have high mobility at high temperatures. | Oil shale with very low permeability hinders the entry of high-temperature steam. | [46,47] |
| In situ combustion | This technology conserves energy and decomposes the oil shale more thoroughly. | The pyrolysis and combustion process are extremely difficult to control. | [9,48] |
| Electric heating | Heating by the resistive loss technique is suited to achieving uniform heating. The corresponding research started early and this method is relatively fully studied. | The heating rate is slow and the energy consumption is very high. | [49,50,51] |
| Microwave heating | The temperature rise is fast and pyrolysis efficiency is high. | The uniformity of temperature distribution needs to be improved in the future. | This paper |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, X.; Zhu, J.; Yang, Z.; Zhou, J.; Yi, L. Numerical Simulation of Oil Shale Pyrolysis under Microwave Irradiation Based on a Three-Dimensional Porous Medium Multiphysics Field Model. Energies 2022, 15, 3256. https://doi.org/10.3390/en15093256
Wang H, Li X, Zhu J, Yang Z, Zhou J, Yi L. Numerical Simulation of Oil Shale Pyrolysis under Microwave Irradiation Based on a Three-Dimensional Porous Medium Multiphysics Field Model. Energies. 2022; 15(9):3256. https://doi.org/10.3390/en15093256
Chicago/Turabian StyleWang, Hao, Xiaogang Li, Jingyi Zhu, Zhaozhong Yang, Jie Zhou, and Liangping Yi. 2022. "Numerical Simulation of Oil Shale Pyrolysis under Microwave Irradiation Based on a Three-Dimensional Porous Medium Multiphysics Field Model" Energies 15, no. 9: 3256. https://doi.org/10.3390/en15093256
APA StyleWang, H., Li, X., Zhu, J., Yang, Z., Zhou, J., & Yi, L. (2022). Numerical Simulation of Oil Shale Pyrolysis under Microwave Irradiation Based on a Three-Dimensional Porous Medium Multiphysics Field Model. Energies, 15(9), 3256. https://doi.org/10.3390/en15093256





