Vapour Sorption on Coal: Influence of Polarity and Rank
Abstract
1. Introduction
1.1. Sorption of Water and Methane
1.2. Sorption of Hydrocarbons
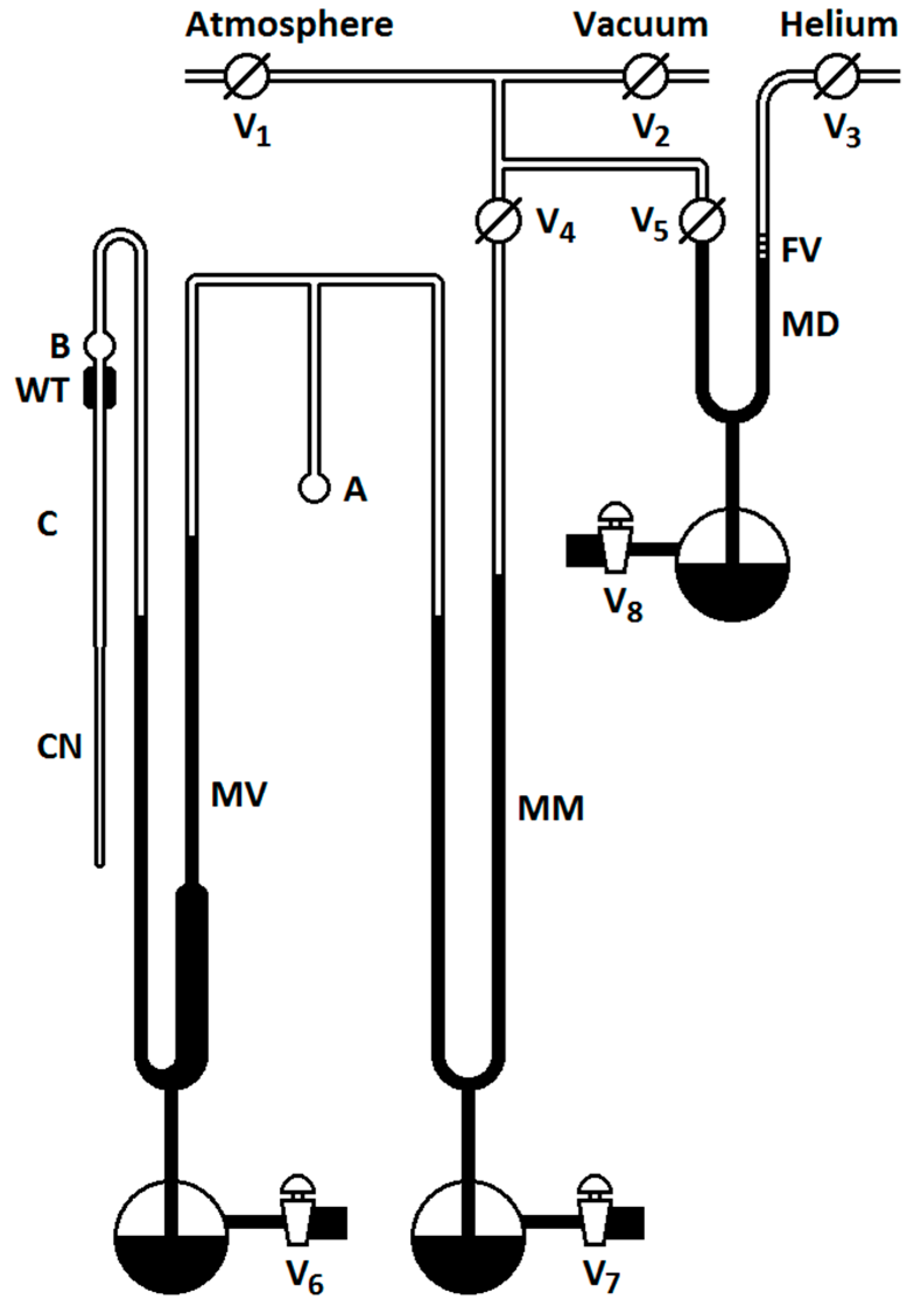
2. Materials and Methods
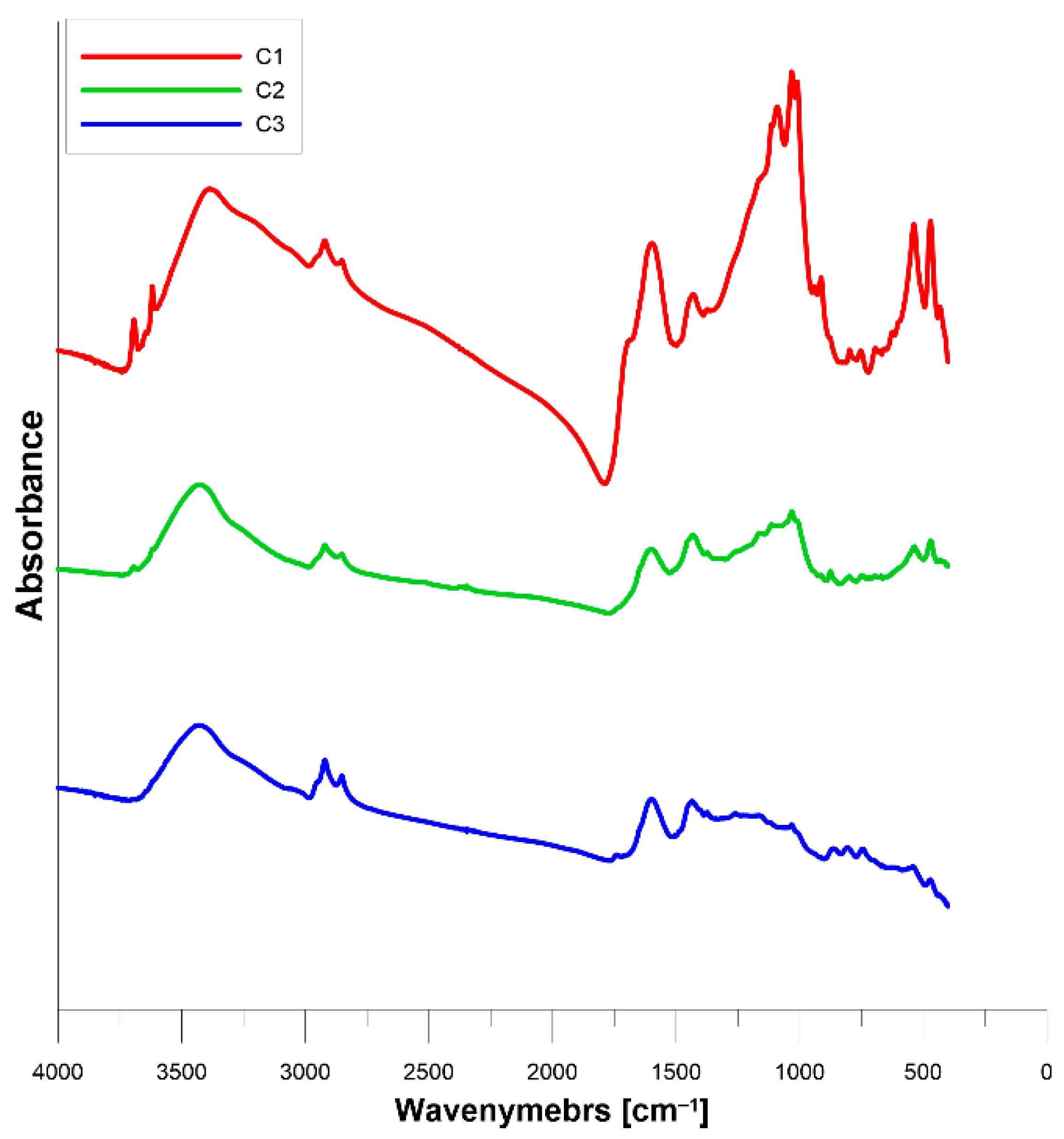
- a broad hydroxyl stretching region, with peak at 3425 cm−1 for C1 and C2 and shifted peak at 3390 for C3,
- a clearly visible aliphatic stretching region, with two peaks for asymmetric bonds at 2920 cm−1 and symmetric bonds at 2855 cm−1; the latter is most pronounced for C1 and least pronounced for C2,
- a prominently displayed aromatic carbon region with a peak at 1600 cm−1, most pronounced for C1,
- an aliphatic bending region with peak at 1435 cm−1 for C1 and C2 and peak at 1430 cm−1 for C3,
- peaks at 1032 cm−1 assigned to the stretching vibration of minerals, such as Si-O-Si or Si-O-C, most pronounced for C3 and almost indistinct for C1,
- noticeable three aromatic out-of-plane peaks within 900–650 cm−1 region,
- peaks at 535 and 460 cm−1 region assigned to the Si-O bending vibration of feldspar and quartz minerals, most pronounced for C3 and indistinct for C1.
3. Results
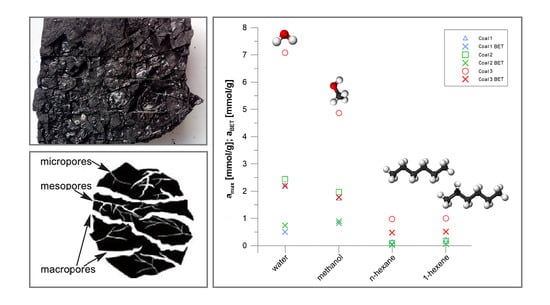
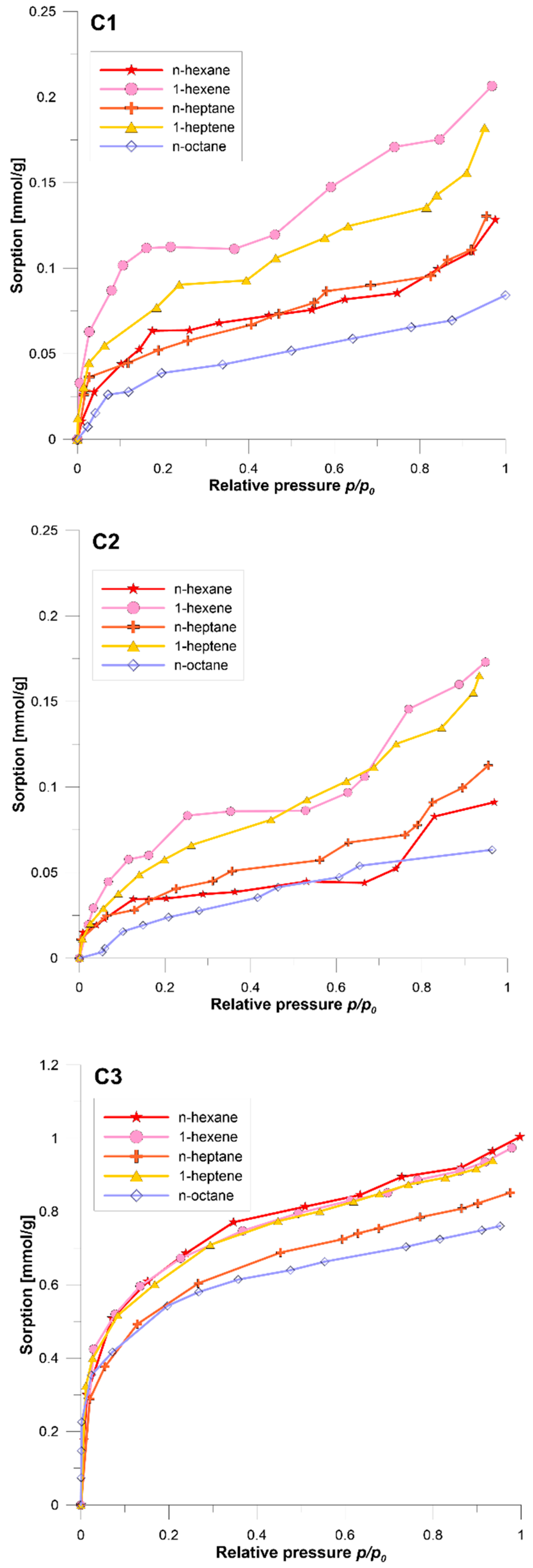
3.1. Sorption Isotherms
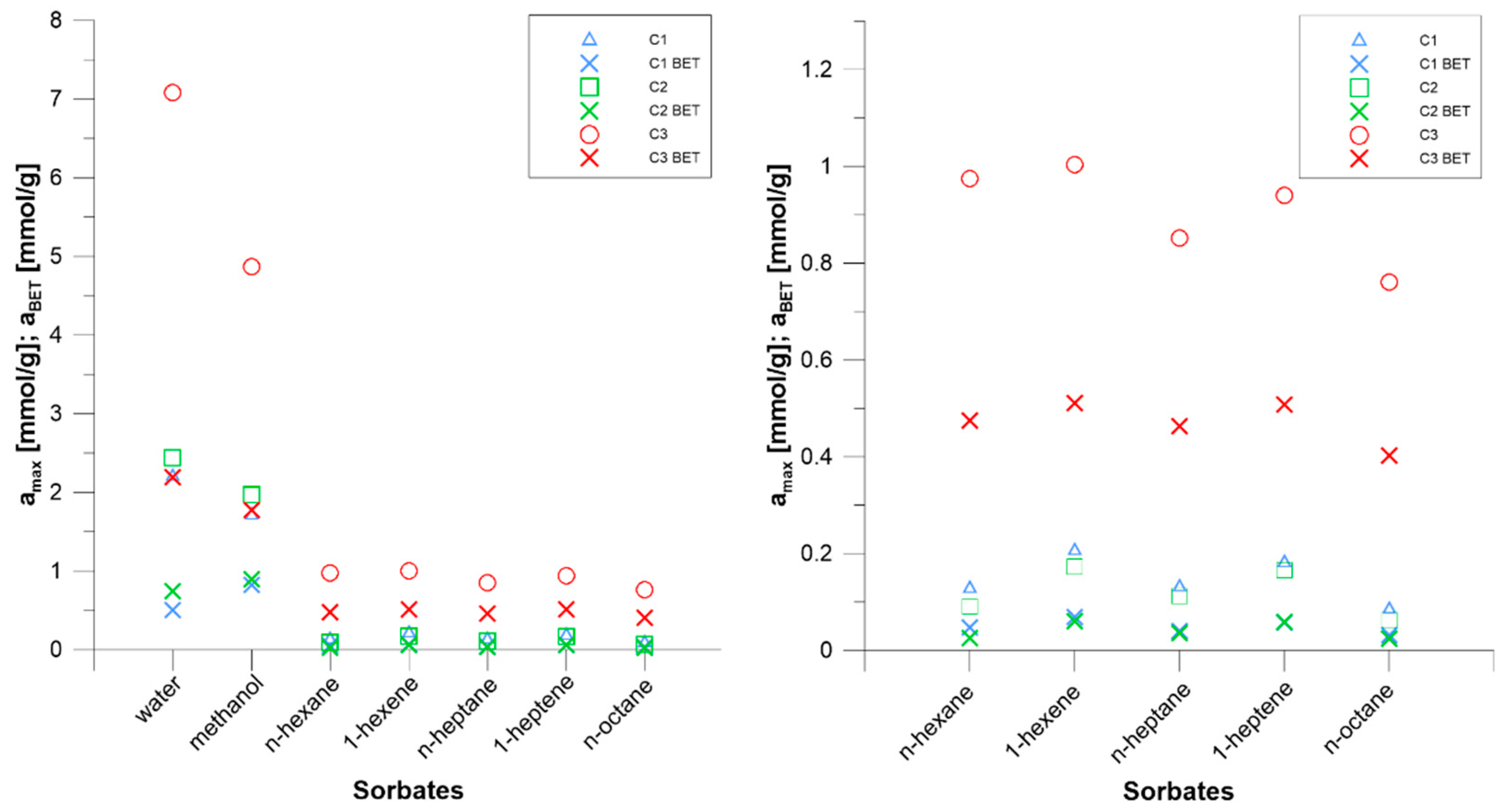
3.2. BET Parameters
4. Discussion
5. Conclusions
- The shape of water sorption isotherms corresponds to type II according to the IUPAC classification, while the shape of methanol sorption isotherms corresponds to type I according to the IUPAC classification. The sorption in the coal-water system follows a course characteristic for sorbents containing micro- and mesopores with the formation of a monolayer and then a multilayer structure. Sorption in the coal-methanol system follows a course characteristic for microporous adsorbents, including formation of the monomolecular adsorbent layer.
- The difference in the maximum sorption value between low-rank coal C3 coal and higher rank coals C2 and C1 is greater than between C2 coal and C1 coal. The parameter that corresponds better to the sorption properties of coal toward water and methanol is the content of element O in the coal.
- The sorption of methanol vapour is higher than that of water vapour in the initial part of the relative pressure scale. This tendency remains longer for lower-rank coals. The maximum sorption capacity is higher for coal-water systems than for coal-methanol systems. The open-pore structure of coal is responsible for this diversity. It enables the formation of association of water molecules with dipole-dipole and hydrogen bonding interactions. In the case of methanol, these interactions are weaker.
- The factors that determine the sorption capacity of coal toward water are (1) the capillary structure of the sorbent, in terms of diffusion in pores and conditions for the occurrence of multilayer sorption and condensation of sorbate vapours, and (2) the availability of polar adsorption sites, associated with oxygen functional groups. In the case of methanol, the second aspect also includes the availability of an apolar coal surface, since methanol molecules can bond to specific polar sites and nonspecific apolar sites. In addition, one methanol molecule can screen for more than one sorption site.
- The shape of the sorption isotherms of unsaturated hydrocarbons is close to type II according to the IUPAC classification. In the case of saturated hydrocarbons, the shapes of the sorption isotherms change from type II to type I as the length of the aliphatic chain in the molecule increases and the degree of coal coalification decreases. The sorption of vapours of nonpolar substances on coal has a surface character. The pores present in coal act as a molecular sieve in this system.
- The sorption capacity of the applied hydrocarbon sorbates depends on (1) the presence or absence of a double bond and (2) the size of the molecule. The sorption capacity of these sorbates increases in the order: n-octane < n-heptane/n-hexane < 1-heptene < 1-hexene.
- The effect of differentiating the sorption amounts of the studied sorbates increases with the degree of coal coalification. Coal with a higher degree of coalification shows greater differentiation of the course of isotherms of alkane sorption, due to higher microporosity, and greater sorption of alkane and their unsaturated counterparts, due to the presence of π-electrons in aromatic structures of coal. It is significant that the double bond effect is dominant over the influence of the length and shape of the hydrocarbon molecule.
- Based on the analysis of the data description with the BET sorption isotherm equation, it was found that the water monolayer capacity is higher than that of methanol in low-rank C3 coal. The sorption of water and methanol takes place mainly at numerous polar sorption sites. Smaller water molecules are more efficient at using the available adsorption sites.
- Analysis of data using the BET sorption isotherm showed that the water monolayer sorption capacity is lower than that of methanol on the higher-rank coals C1 and C2, although the maximum sorption capacity of water is higher than that of methanol. The affinity of methanol for both polar and nonpolar sorption sites results in a higher monolayer capacity. Multilayer sorption based on strong dipole-dipole interactions and hydrogen bonds between water molecules results in a higher sorption capacity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhattacharyya, K. The Role of Sorption of Water Vapour in the Spontaneous Heating of Coal. Fuel 1971, 50, 367–380. [Google Scholar] [CrossRef]
- Ceglarska-Stefańska, G.; Brzóska, K.; Winnicki, J. Sorption of Water Vapour on Exinite Concentrates. Adsorption 1998, 4, 313–319. [Google Scholar] [CrossRef]
- Charrière, D.; Behra, P. Water Sorption on Coals. J. Colloid Interface Sci. 2010, 344, 460–467. [Google Scholar] [CrossRef]
- Chen, M.; Yang, Y.; Gao, C.; Cheng, Y.; Wang, J.; Wang, N. Investigation of the Fractal Characteristics of Adsorption-Pores and Their Impact on the Methane Adsorption Capacity of Various Rank Coals via N2 and H2O Adsorption Methods. Energy Sci. Eng. 2020, 8, 3228–3243. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, D.; Lun, Z.; Zhao, C.; Wang, H. Occurrence of Water within Different Rank Coals: A Review. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–19. [Google Scholar] [CrossRef]
- Mo, Q.; Liao, J.; Zhang, Y.; Chang, L.; Han, Y.; Bao, W. Kinetic Analysis on Water Adsorption of Thermally Upgraded Lignite. Fuel Processing Technol. 2021, 211, 106603. [Google Scholar] [CrossRef]
- Švábová, M.; Weishauptová, Z.; Přibyl, O. Water Vapour Adsorption on Coal. Fuel 2011, 90, 1892–1899. [Google Scholar] [CrossRef]
- Baran, P.; Jodłowski, G.S.; Krzyżanowski, A.; Zarębska, K. Experimental Testing of Methanol Sorption on Selected Coal Samples from Upper Silesian Basin. Geol. Geophys. Environ. 2014, 40, 261–269. [Google Scholar] [CrossRef][Green Version]
- Krzyżanowski, A.; Żyła, M. Characteristics of Water, Methanol and Benzene Vapours Sorption Properties of Selected Metamorphic Types of Hard Coal. Gospod. Surowcami Miner. 2007, 23, 139–147. [Google Scholar]
- Spitzer, Z.; Bíba, V.; Boháč, F.; Málková, E. Micropore Structure Analysis of Coal from Adsorption Isotherms of Methanol. Fuel 1977, 56, 313–318. [Google Scholar] [CrossRef]
- Takanohashi, T.; Terao, Y.; Iino, M. Sorption Behaviors of Methanol Vapor by Coal Extracts and Residues. Fuel 2000, 79, 349–353. [Google Scholar] [CrossRef]
- Baran, P.; Cygankiewicz, J.; Krzyżanowski, A.; Zarębska, K. Sorption of Saturated and Unsaturated Hydrocarbons on Selected Coal Sample from the Pniówek Mine. Geol. Geophys. Environ. 2013, 39, 341–349. [Google Scholar] [CrossRef][Green Version]
- Dudzińska, A.; Cygankiewicz, J.; Włodarek, M. Natural Content of Gases: Carbon Monoxide, Carbon Dioxide, Hydrogen and Unsaturated Hydrocarbons of Ethylene, Propylene and Acetylene in Selected Bituminous Coal Seams. Int. J. Coal Geol. 2017, 178, 110–121. [Google Scholar] [CrossRef]
- Dudzińska, A.; Howaniec, N.; Smoliński, A. Effect of Coal Grain Size on Sorption Capacity with Respect to Propylene and Acetylene. Energies 2017, 10, 1919. [Google Scholar] [CrossRef]
- Dudzińska, A. Analysis of Sorption and Desorption of Unsaturated Hydrocarbons: Ethylene, Propylene and Acetylene on Hard Coals. Fuel 2019, 246, 232–243. [Google Scholar] [CrossRef]
- Wojtacha-Rychter, K.; Smoliński, A. Multi-Component Gas Mixture Transport through Porous Structure of Coal. Fuel 2018, 233, 37–44. [Google Scholar] [CrossRef]
- Wojtacha-Rychter, K.; Smoliński, A. Selective Adsorption of Ethane, Ethylene, Propane, and Propylene in Flammable Gas Mixtures on Different Coal Samples and Implications for Fire Hazard Assessments. Int. J. Coal Geol. 2019, 202, 38–45. [Google Scholar] [CrossRef]
- Allardice, D.; Evans, D. The-Brown Coal/Water System: Part 2. Water Sorption Isotherms on Bed-Moist Yallourn Brown Coal. Fuel 1971, 50, 236–253. [Google Scholar] [CrossRef]
- Czuchajowski, L.; Lasoń, M.; Żyła, M. Tlenowe Grupy Reaktywne Węgla Kamiennego w Świetle Badań Sorpcji Par Polarnych. Chem. Stosow. 1960, 1, 3–13. [Google Scholar]
- Lasoń, M.; Żyła, M. Wielokrotna Sorpcja i Desorpcja Par Wody i Alkoholu Metylowego Na Węglach Kamiennych. Zesz. Nauk. Akad. Górniczo-Hutniczej. Górnictwo 1973, 366, 21–32. [Google Scholar]
- Krevelen, D.W.; Schuyer, J. Coal Science: Aspects of Coal Constitution; Elsevier: Amsterdam, The Netherlands, 1957. [Google Scholar]
- Liu, Z.; Zhang, Z.; Choi, S.K.; Lu, Y. Surface Properties and Pore Structure of Anthracite, Bituminous Coal and Lignite. Energies 2018, 11, 1502. [Google Scholar] [CrossRef]
- Korta, A.; Laskowski, T.; Lasoń, M.; Żyła, M. Sorpcja Par Metanolu i Wody Na Petrograficznych Odmianach Węgli Kamiennych. Koks-Smoła-Gaz 1962, 7, 1–6. [Google Scholar]
- Orzechowska-Zięba, A.; Baran, P.; Zarębska, K.; Cygankiewicz, J. Sorpcja Pary Wodnej Na Próbkach Wytypowanych Węgli Kamiennych w Aspekcie Określenia Potencjału Magazynowego Złoża. Zesz. Nauk. Inst. Gospod. Surowcami Miner. I Energią PAN 2017, 97, 71–82. [Google Scholar]
- Kotarba, M.J. Composition and Origin of Coalbed Gases in the Upper Silesian and Lublin Basins, Poland. Org. Geochem. 2001, 32, 163–180. [Google Scholar] [CrossRef]
- Dudzińska, A. Analiza Sorpcji Gazów Współistniejących w Atmosferze Kopalnianej. Przegląd Górniczy 2016, 72, 10–16. [Google Scholar]
- Dudzińska, A.; Cygankiewicz, J.; Włodarek, M. Gaseous Emissions from Freshly Extracted Coal in the Inert and Air Atmosphere in Terms of Natural Desorption and Early Coal Oxidation. Fuel 2021, 285, 119066. [Google Scholar] [CrossRef]
- Zhao, H.; Lai, Z.; Firoozabadi, A. Sorption Hysteresis of Light Hydrocarbons and Carbon Dioxide in Shale and Kerogen. Sci. Rep. 2017, 7, 16209. [Google Scholar] [CrossRef]
- Krzyżanowski, A.; Zarębska, K. Sorpcja Par Cieczy Apolarnych Na Węglu Kamiennym o Różnym Składzie Petrograficznym. Gospod. Surowcami Miner. 2007, 23, 175–181. [Google Scholar]
- Saha, S.; Sharma, B.K.; Kumar, S.; Sahu, G.; Badhe, Y.; Tambe, S.; Kulkarni, B. Density Measurements of Coal Samples by Different Probe Gases and Their Interrelation. Fuel 2007, 86, 1594–1600. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]


| Coal | C1 | C2 | C3 |
|---|---|---|---|
| Proximate analysis | |||
| Wa [%] | 1.75 | 1.85 | 11.11 |
| Aa [%] | 3.01 | 14.18 | 14.45 |
| Vdaf [%] | 27.12 | 29.88 | 35.63 |
| Ultimate analysis | |||
| Cdaf [%] | 84.24 | 70.82 | 57.83 |
| Hdaf [%] | 4.58 | 3.35 | 3.37 |
| Ndaf [%] | 1.52 | 1.28 | 0.87 |
| Sdaf [%] | 0.39 | 3.50 | 1.10 |
| Odaf [%] | 4.58 | 6.29 | 11.30 |
| Petrographic analysis and vitrinite reflectance | |||
| vitrinite [%] | 73 | 60 | 67 |
| liptinite [%] | 7 | 9 | 5 |
| inertinite [%] | 20 | 31 | 28 |
| mineral matter [%] | 1 | 14 | 11 |
| R0 [%] | 0.92 | 0.78 | 0.51 |
| Structural properties | |||
| dreal [g/cm3] | 1.26 | 1.27 | 1.37 |
| dbulk [g/cm3] | 1.22 | 1.23 | 1.16 |
| porosity [%] | 3.20 | 3.40 | 15.90 |
| Vmicro [cm3/kg] | 0.070 | 0.063 | 0.229 |
| Smicro [m2/g] | 115.8 | 103.2 | 419.6 |
| Sorbate | TC [°C] | pC [MPa] | M [g/mol] | dkin [nm] | p0 [Pa] | μ [D] |
|---|---|---|---|---|---|---|
| water | 373.99 | 22.06 | 18.02 | 0.265 | 4240 | 1.85 |
| methanol | 240.00 | 78.50 | 32.04 | 0.380 | 21,065 | 1.70 |
| n-hexane | 234.64 | 3.04 | 86.18 | 0.430 | 24,598 | 0.00 |
| 1-hexene | 230.83 | 3.21 | 84.16 | 0.430 | 30,507 | 0.44 |
| n-heptane | 266.87 | 2.74 | 100.20 | 0.430 | 7786 | 0.00 |
| 1-heptene | 264.08 | 2.92 | 98.19 | 0.430 | 9533 | 0.44 |
| n-octane | 295.75 | 2.49 | 114.23 | 0.430 | 2240 | 0.00 |
| Coal | R0 [%] | Cdaf [%] | Odaf [%] | TV [%] | aw [mg/g] | am [mmol/g] | aw,prim [%] |
|---|---|---|---|---|---|---|---|
| C1 | 0.92 | 84.24 | 4.6 | 73 | 39.7 | 1.715 | - |
| C2 | 0.78 | 70.82 | 6.29 | 60 | 44.0 | 1.97 | 31 |
| C3 | 0.51 | - | - | 67 | 127.6 | - | - |
| Vitrinite [7] | 0.88 | - | - | 93 | 43 | - | - |
| Huminite [7] | 0.37 | - | - | n.a. | 170 | - | - |
| YZG2 [4] | 0.65 | - | n.a. | - | 82 | - | - |
| LH7 [4] | 1.16 | - | n.a. | - | 21 | - | - |
| Upper Freeport [11] | n.a. | 86.2 | 4.6 | - | - | 1.2 | - |
| Beulah-Zap [11] | n.a. | 71.6 | 21.7 | - | - | 6.5 | - |
| Albert [3] | 0.75 | - | 10.7 | - | - | - | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerw, K.; Krzyżanowski, A.; Baran, P.; Zarębska, K. Vapour Sorption on Coal: Influence of Polarity and Rank. Energies 2022, 15, 3065. https://doi.org/10.3390/en15093065
Czerw K, Krzyżanowski A, Baran P, Zarębska K. Vapour Sorption on Coal: Influence of Polarity and Rank. Energies. 2022; 15(9):3065. https://doi.org/10.3390/en15093065
Chicago/Turabian StyleCzerw, Katarzyna, Andrzej Krzyżanowski, Paweł Baran, and Katarzyna Zarębska. 2022. "Vapour Sorption on Coal: Influence of Polarity and Rank" Energies 15, no. 9: 3065. https://doi.org/10.3390/en15093065
APA StyleCzerw, K., Krzyżanowski, A., Baran, P., & Zarębska, K. (2022). Vapour Sorption on Coal: Influence of Polarity and Rank. Energies, 15(9), 3065. https://doi.org/10.3390/en15093065