Synthesis and Characterization of Gadolinium-Doped Zirconia as a Potential Electrolyte for Solid Oxide Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrolyte Powder Synthesis
2.3. Fabrication of the Electrolyte Pellets
2.4. Characterizations of Solid Electrolytes
3. Results
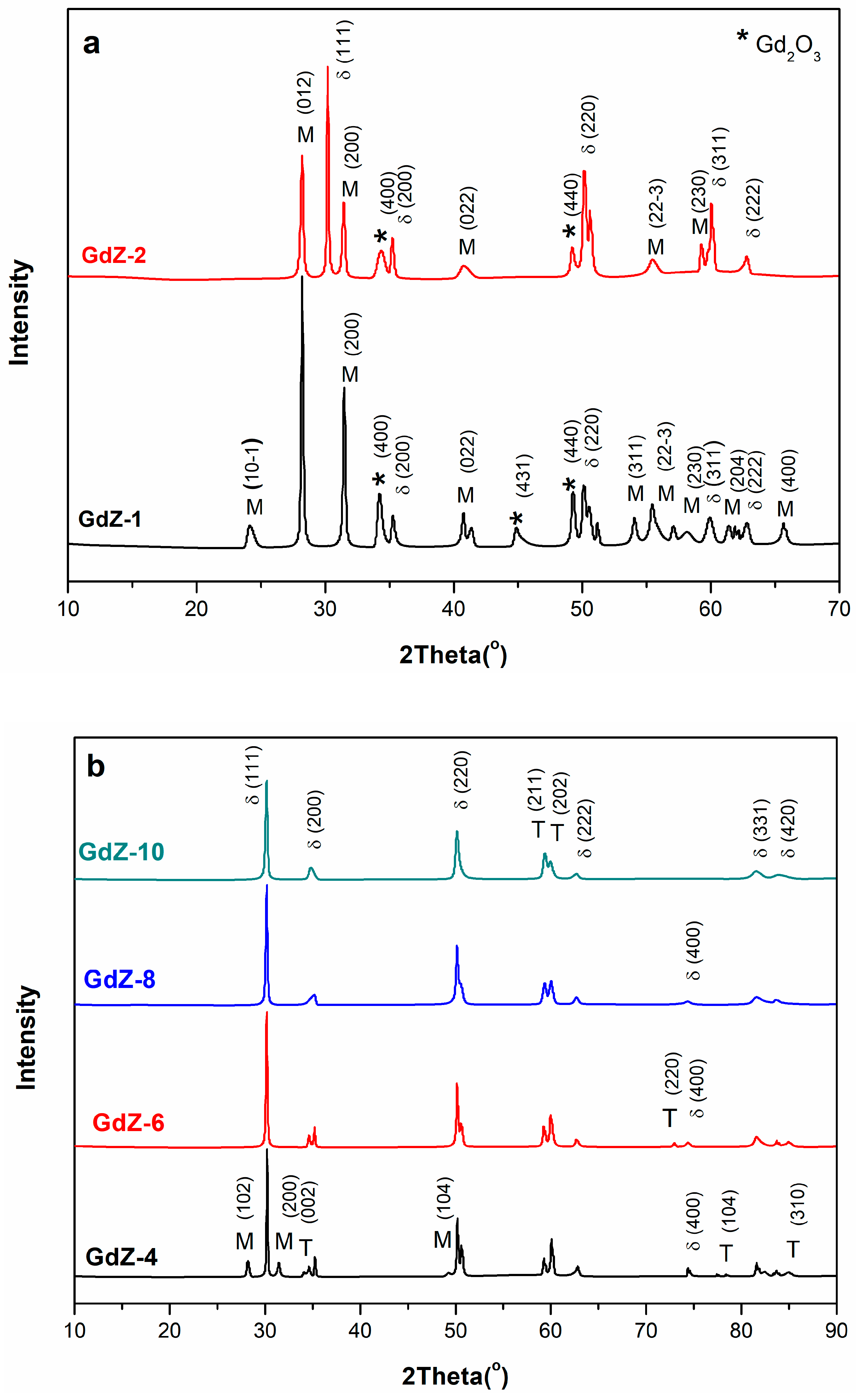
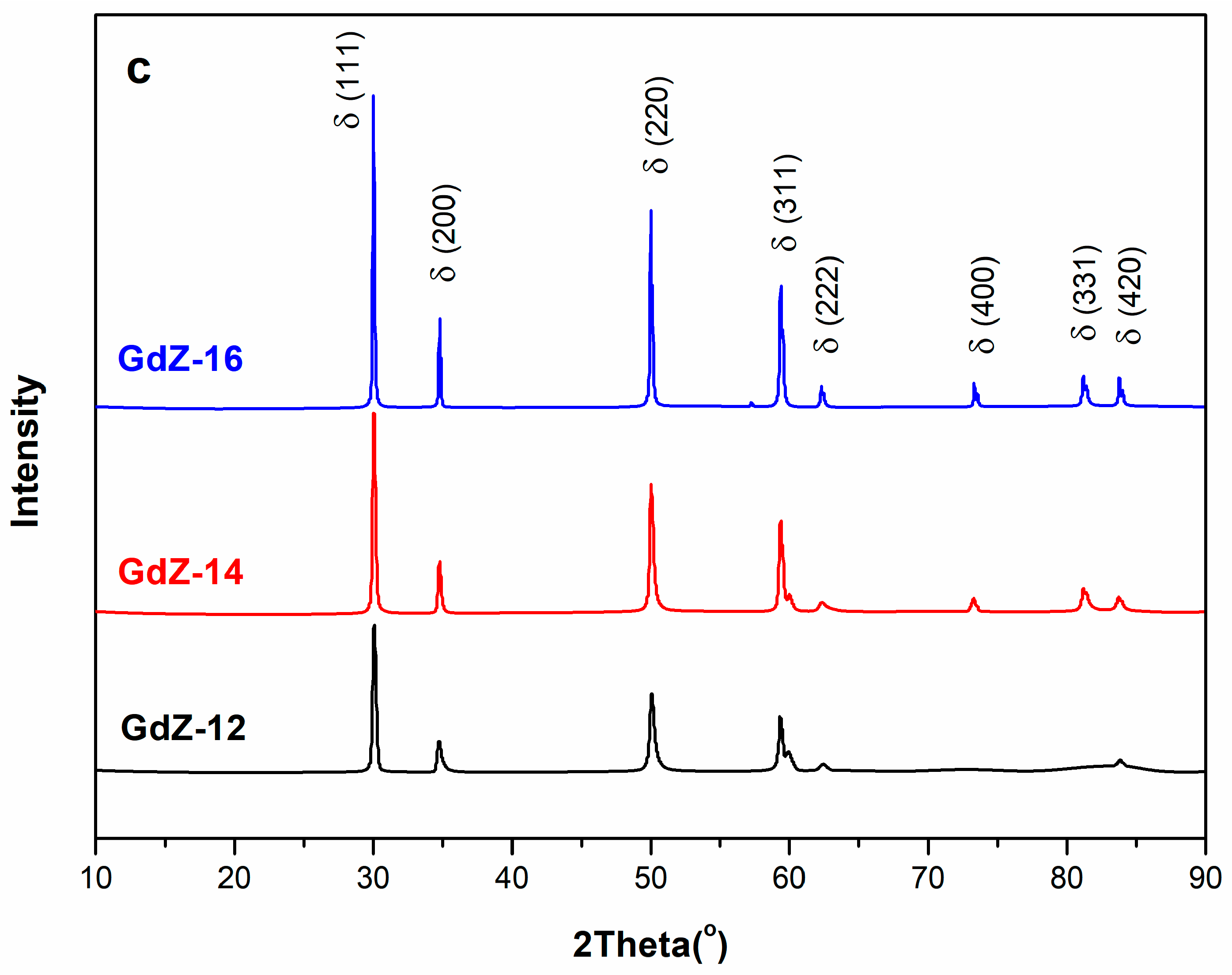
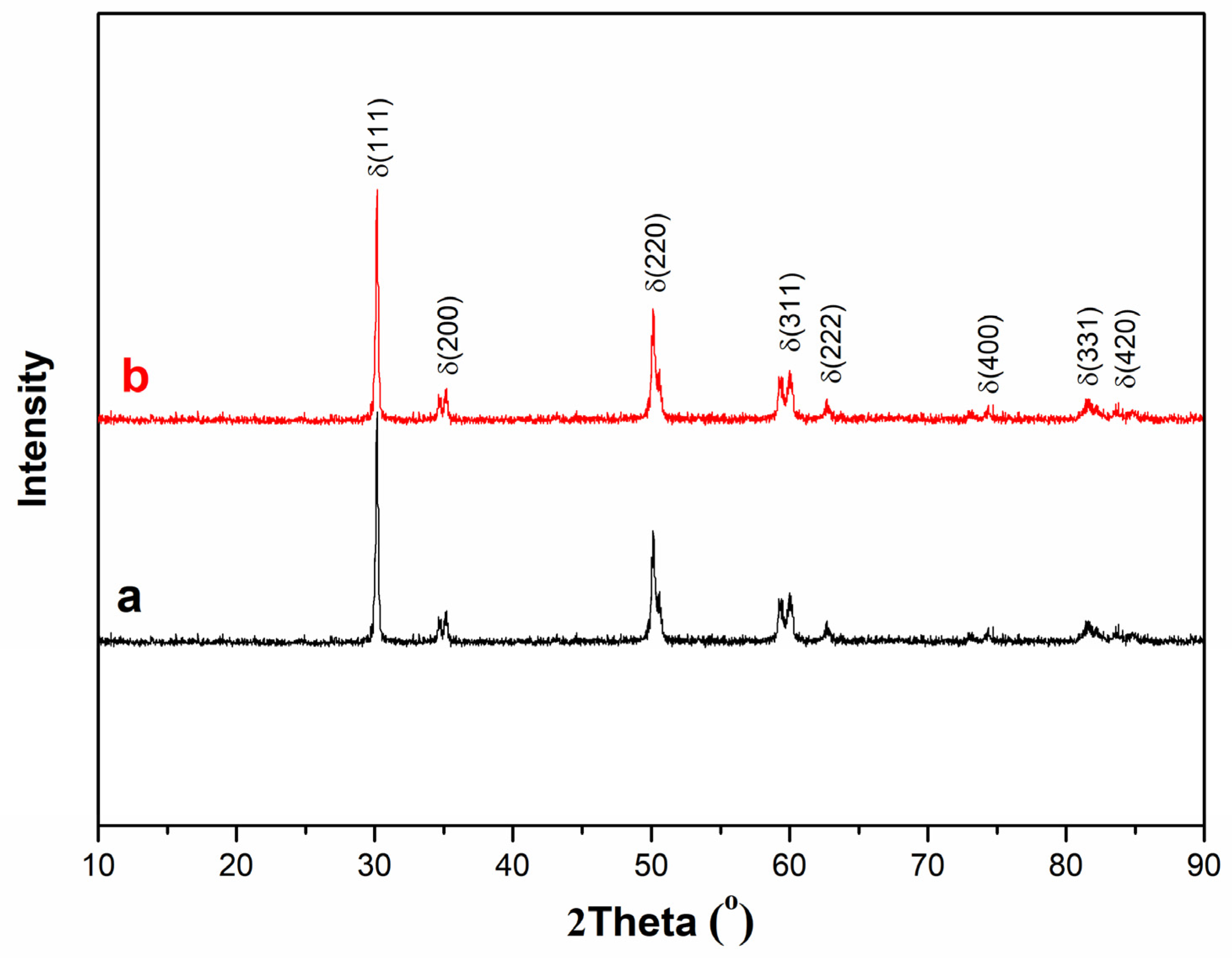
3.1. Crystal Structure Analysis of Powders
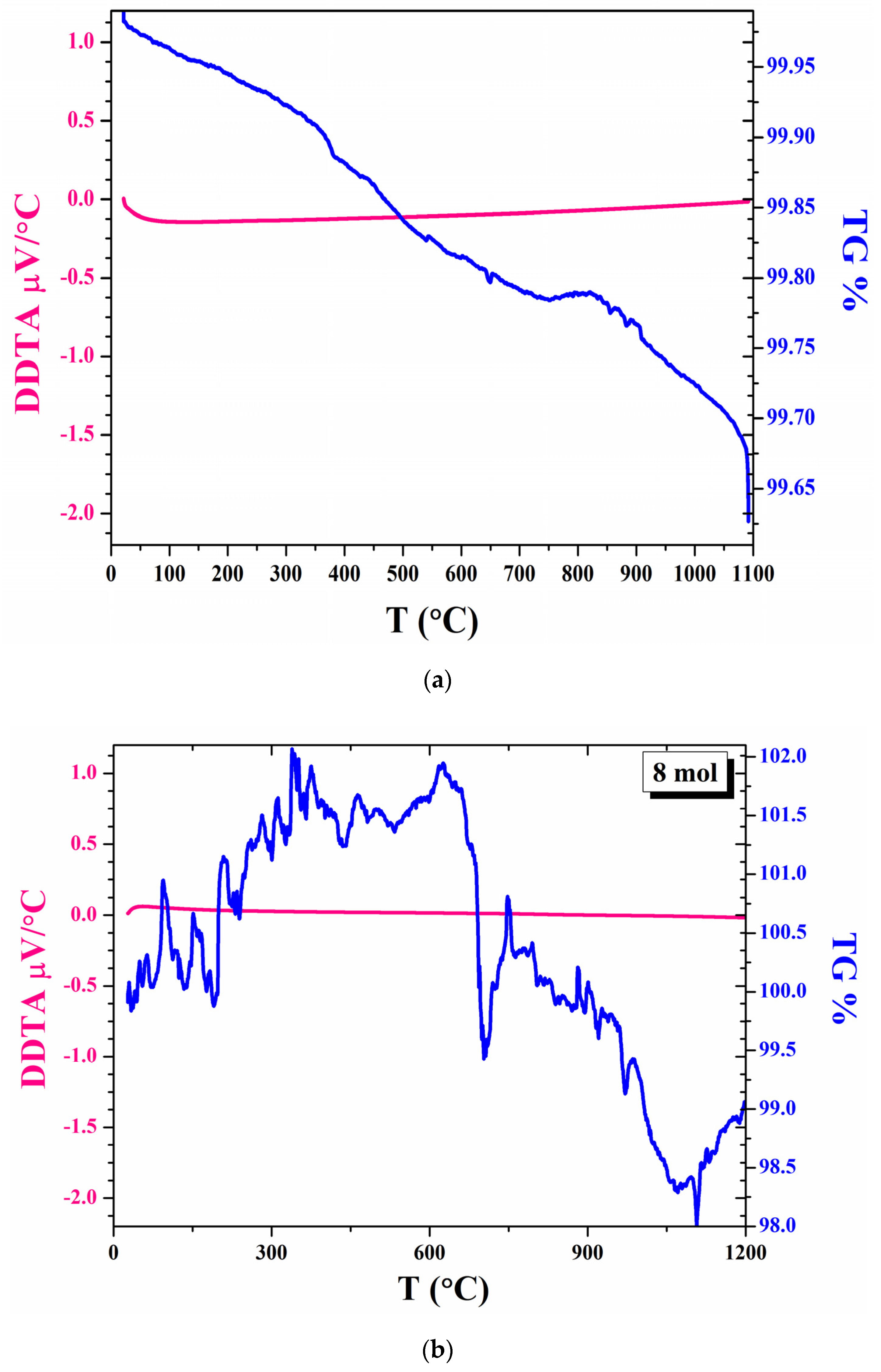
3.2. Thermal Analysis of Powder and Disk Samples
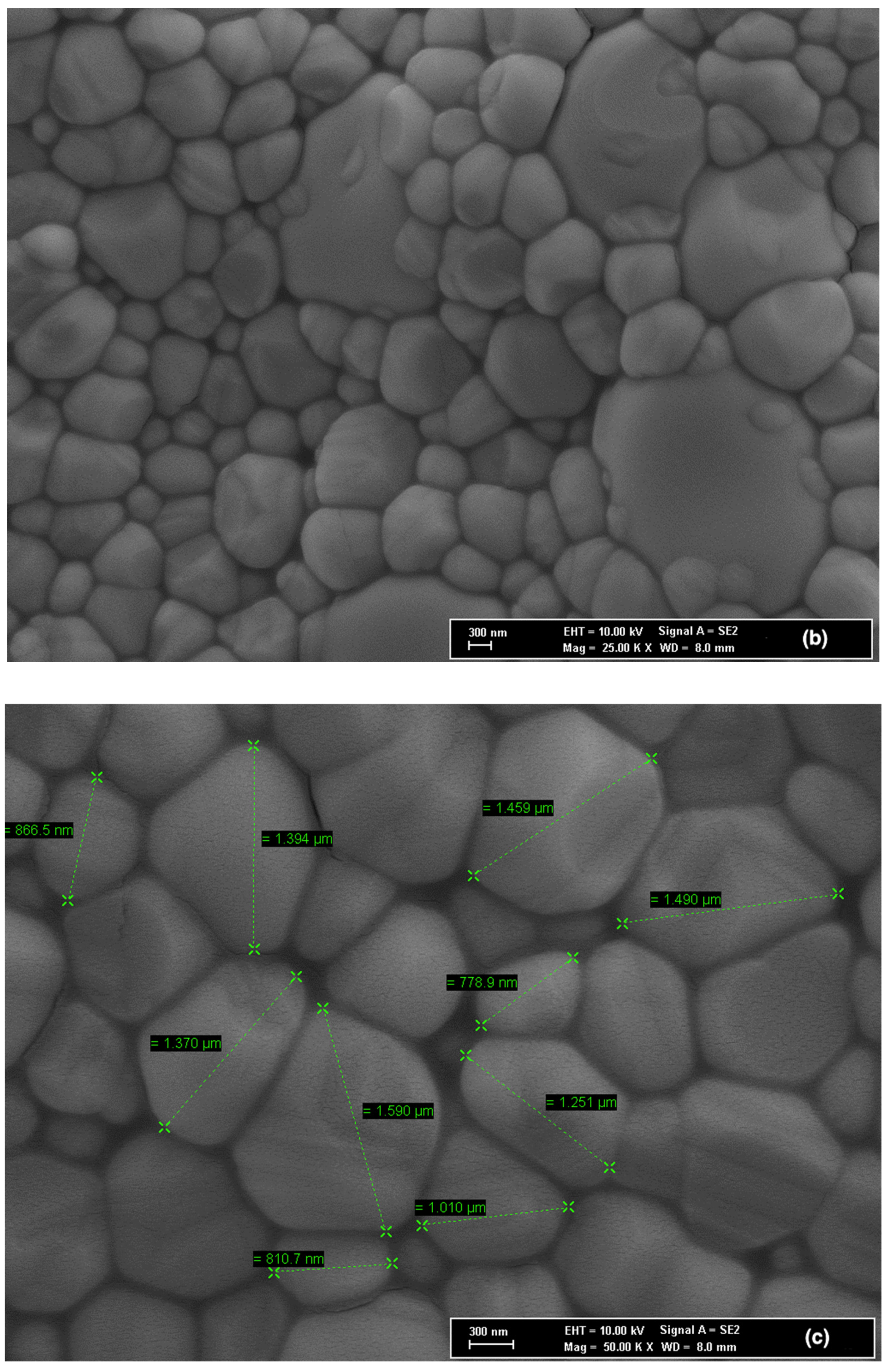
3.3. Microstructure Characterization
3.4. Conductivity Characterisation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Subotić, V.; Napporn, T.W. Nanostructured metal oxides for high-performance solid oxide fuel cells (SOFCs). In Metal Oxide-Based Nanostructured Electrocatalysts for Fuel Cells, Electrolyzers, and Metal-Air Batteries; Napporn, T.W., Holade, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 235–261. [Google Scholar]
- Choolaei, M.; Cai, Q.; Horri, B.A. Green synthesis and characterization of nanocrystalline NiO-GDC powders with low activation energy for solid oxide fuel cells. Ceram. Int. 2021, 47, 32804–32816. [Google Scholar] [CrossRef]
- Alenazey, F.; Alyousef, Y.; AlOtaibi, B.; Almutairi, G.; Minakshi, M.; Cheng, C.K.; Vo, D.-N. Degradation Behaviors of Solid Oxide Fuel Cell Stacks in Steady State and Cycling Conditions. Energy Fuels 2020, 34, 14864–14873. [Google Scholar] [CrossRef]
- Coralli, A.; Sarruf, B.J.M.; de Miranda, P.E.V.; Luigi, O.; Specchia, S.; Minh, N.Q. Fuel Cells. In Science and Engineering of Hydrogen-Based Energy Technologies; Academic Press: Cambridge, MA, USA, 2019; pp. 39–122. [Google Scholar]
- Kumar, V.J.; Karthik, R.; Chen, S.M.; Natarajan, K.; Karuppiah, C.; Yang, C.C.; Muthuraj, V. 3D Flower-Like Gadolinium Molybdate Catalyst for Efficient Detection and Degradation of Organophosphate Pesticide (Fenitrothion). ACS Appl. Mater. Interfaces 2018, 10, 15652–15664. [Google Scholar] [CrossRef] [PubMed]
- Silvaa, C.L.S.; Marchetti, S.G.; Junior, A.C.F.; Silva, T.F.; Assaf, J.M.; Rangel, M.C. Effect of Gadolinium on the Catalytic Properties of Iron Oxides for WGSR. Catal. Today 2013, 213, 127–134. [Google Scholar] [CrossRef][Green Version]
- Madhusudhanaa, H.C.; Shobhadevic, S.N.; Nagabhushanad, B.M.; Krishnad, R.H.; Murugendrappae, M.V.; Nagabhushanaf, H. Structural Characterization and Dielectric studies of Gd doped ZrO2 nano crystals Synthesized by Solution combustion method. Mater. Today 2018, 5, 21195–21204. [Google Scholar] [CrossRef]
- Kang, T.-K.; Nagasaki, T.; Igawa, N.; Il-Hiun, K.; Ohno, H. Electrical Properties of Cubic, Stabilized, Single ZrO2-Gd203 Crystals. Ceram. Soc. 1992, 75, 2297–2299. [Google Scholar] [CrossRef]
- Zinkevich, M.; Wang, C.; Morales, F.M.; Ruhle, M.; Aldinger, F. Phase equilibria in the ZrO2–GdO1.5 system at 1400–1700 °C. J. Alloys Compd. 2005, 398, 261–268. [Google Scholar] [CrossRef]
- Sharma, P.; Sundaram, M.M.; Singh, D.; Ahuja, R. Highly Energetic and Stable Gadolinium/Bismuth Molybdate with a Fast-Reactive Species, Redox Mechanism of Aqueous Electrolyte. ACS Appl. Energy Mater. 2020, 3, 12385–12399. [Google Scholar] [CrossRef]
- Hong, M.; Kwo, J.; Kortan, A.R.; Mannaerts, J.P.; Sergent, A.M. Epitaxial Cubic Gadolinium Oxide as a Dielectric for Gallium Arsenide Passivation. Science 1999, 283, 1897–1900. [Google Scholar] [CrossRef]
- Piconi, C.; Burger, W.; Richter, H.G.; Cittadini, A.; Maccauro, G.; Covacci, V.; Bruzzese, N.; Ricci, G.A.; Marmo, E. Y-TZP ceramics for artificial joint replacements. Biomaterials 1998, 19, 1489–1494. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Vostakola, M.F.; Horri, B.A. Progress in Material Development for Low-Temperature Solid Oxide Fuel Cells: A Review. Energies 2020, 14, 1280. [Google Scholar] [CrossRef]
- Qian, J.; Hou, J.; Tao, Z.; Liu, W. Fabrication of (Sm, Ce)O2−δ interlayer for yttria-stabilized zirconia-based intermediate temperature solid oxide fuel cells. J. Alloys Compd. 2015, 631, 255–260. [Google Scholar] [CrossRef]
- Ghatee, M.; Salihi, H. Electrical and mechanical properties of 25 wt% tetragonal/cubic zirconia based composite thin films prepared by combination of aqueous tape casting and net shape methods. J. Electroceramics 2015, 35, 98–105. [Google Scholar] [CrossRef]
- Yildiz, E.; Yilmaz, S.; Turkoglu, O. The production and characterization of ytterbium-stabilized zirconia films for SOFC applications. Int. J. Appl. Ceram. Technol. 2016, 13, 100–107. [Google Scholar] [CrossRef]
- Bastidas, D.M. High temperature corrosion of metallic interconnects in solid oxide fuel cells. Rev. Metal. 2006, 42, 425–443. [Google Scholar] [CrossRef]
- Karaoglanli, A.C.; Doleker, K.M.; Ozgurluk, Y. Interface failure behavior of yttria stabilized zirconia (YSZ), La2Zr2O7, Gd2Zr2O7, YSZ/La2Zr2O7 and YSZ/Gd2Zr2O7 thermal barrier coatings (TBCs) in thermal cyclic exposure. Mater. Charact. 2020, 159, 110072. [Google Scholar] [CrossRef]
- Lashmi, P.G.; Majithia, S.; Shwetha, V.; Balaji, N.; Aruna, S.T. Improved hot corrosion resistance of plasma sprayed YSZ/Gd2Zr2O7 thermal barrier coating over single layer YSZ. Mater. Charact. 2019, 147, 199–206. [Google Scholar] [CrossRef]
- Liu, Z.-G.; Ouyang, J.-H.; Zhou, Y.; Xia, X.-L. Structure and thermal conductivity of Gd2(TixZr1−x)2O7 ceramics. Mater. Lett. 2008, 62, 4455–4457. [Google Scholar] [CrossRef]
- Díaz-Guillén, J.A.; Díaz-Guillén, M.R.; Padmasree, K.P.; Fuentes, A.F.; Santamaría, J.; León, C. High ionic conductivity in the pyrochlore-type Gd2−yLayZr2O7 solid solution (0 ≤ y ≤ 1). Solid State Ion. 2008, 179, 2160–2164. [Google Scholar] [CrossRef]
- Irshad, M.; Ain, Q.u.; Siraj, K.; Raza, R.; Tabish, A.N.; Rafique, M.; Idrees, R.; Khan, F.; Majeed, S.; Ahsan, M. Evaluation of BaZr0.8X0.2 (X = Y, Gd, Sm) proton conducting electrolytes sintered at low temperature for IT-SOFC synthesized by cost effective combustion method. J. Alloys Compd. 2020, 815, 152389. [Google Scholar] [CrossRef]
- Pechini, M.M. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. U.S. Patent No. 3,330,697, 11 July 1967. [Google Scholar]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Winck, L.B.; Ferreira, J.L.d.; Martinez, J.M.G.; Araujo, J.A.; Rodrigues, A.C.M.; da Silva, C.R.M. Synthesis, sintering and characterization of ceria-based solid electrolytes codoped with samaria and gadolinium using the Pechini method. Ceram. Int. 2017, 43, 16408–16415. [Google Scholar] [CrossRef]
- Boultif, A.; Louër, D. Powder pattern indexing with the dichotomy method. J. Appl. Crystallogr. 2004, 37, 724–731. [Google Scholar] [CrossRef]
- Matović, B.; Bošković, S.; Živković, L.; Vlajić, M.D.; Krstić, V.D. Lattice Parameters of Gd-Doped Ceria Electrolytes. Mater. Sci. Forum 2005, 494, 175–180. [Google Scholar] [CrossRef]
- Basu, S.; Varma, S.; Shirsat, A.N.; Wani, B.N.; Bharadwaj, S.R.; Chakrabarti, A.; Jha, S.N.; Bhattacharyya, D. Extended X-ray absorption fine structure study of Gd doped ZrO2 systems. J. Appl. Phys. 2013, 113, 043508. [Google Scholar] [CrossRef]
- Dutta, S.; Bhattacharya, S.; Agrawal, D.C. Electrical properties of ZrO2-Gd2O3 ceramics. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2003, 100, 191–198. [Google Scholar] [CrossRef]
- Wang, W.; Li, C.; Li, J.; Fan, J.; Zhou, X. Effect of gadolinium doping on phase transformation and microstructure of Gd2O3-Y2O3-ZrO2 composite coatings prepared by electrophoretic deposition. J. Rare Earths 2013, 31, 289–295. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Abdala, P.M.; Fantini, M.C.; Craievich, A.F.; Lamas, D.G. Crystallite size-dependent phases in nanocrystalline ZrO2-Sc2O3. Phys. Chem. Chem. Phys. 2010, 12, 2822–2829. [Google Scholar] [CrossRef]
- del Zotto, L.; Ferrario, A.M.; Hatunoglu, A.; Dell’Era, A.; McPhail, S.; Bocci, E. Experimental Procedures & First Results of an Innovative Solid Oxide Fuel Cell Test Rig: Parametric Analysis and Stability Test. Energies 2021, 14, 2038. [Google Scholar] [CrossRef]
- Sanson, A.; Gondolini, A. Solid Oxide Fuel Cells. In Encyclopedia of Materials: Technical Ceramics and Glasses; Elsevier: Amsterdam, The Netherlands, 2021; pp. 49–58. [Google Scholar]
- Peters, C. Grain-Size Effects in Nanoscaled Electrolyte and Cathode Thin Films for Solid Oxide Fuel Cells (SOFC); KIT Scientific Publishing: Karlsruhe, Germany, 2009. [Google Scholar]


| Slurry Contents | Electrolyte (4.5 g) | |
|---|---|---|
| Dispersant | Fish Oil | 0.05 g |
| Binder | Butvar B-76 | 0.5 g |
| Plasticiser | PEG | 1 mL |
| Solvent | Ethanol | 5 mL |
| x (mol%) | Samples | Phases | Lattice Parameters (Å) | c/a | Lattice Volume (Å3) | Crystal Size (nm) | ||
|---|---|---|---|---|---|---|---|---|
| a | b | c | ||||||
| 1 | GDZ-1 | M | 5.1507 | 5.2028 | 5.3156 | 1.032 | - | 22.3273 |
| 2 | GDZ-2 | M | 5.1523 | 5.2128 | 5.3293 | 1.034 | - | 23.9475 |
| 4 | GDZ-4 | T | 3.6035 | 5.1874 | 1.440 | 67.36 | 38.8600 | |
| 6 | GDZ-6 | T | 3.6062 | 5.1883 | 1.439 | 67.47 | 31.1278 | |
| 8 | GDZ-8 | T | 3.5957 | 5.1850 | 1.442 | 67.03 | 21.0943 | |
| 10 | GDZ-10 | T | 3.6211 | 5.1672 | 1.427 | 67.75 | 32.9000 | |
| 12 | GDZ-12 | C | 5.1497 | 1 | - | 20.2114 | ||
| 14 | GDZ-14 | C | 5.1280 | 1 | - | 24.2144 | ||
| 16 | GDZ-16 | C | 5.1280 | 1 | - | 60.0344 | ||
| Temperature (°C) | σb (10−2 S/cm) | |||
|---|---|---|---|---|
| GDZ-4 | GDZ-6 | GDZ-8 | GDZ-10 | |
| 600 | 4.83 | 5.19 | 5.26 | 5.48 |
| 650 | 5.35 | 5.51 | 5.89 | 5.61 |
| 700 | 5.87 | 5.65 | 6.09 | 5.94 |
| 750 | 6.33 | 6.12 | 6.27 | 6.46 |
| 800 | 7.19 | 6.23 | 7.23 | 6.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, S.; Cobaner, S.; Yalaz, E.; Amini Horri, B. Synthesis and Characterization of Gadolinium-Doped Zirconia as a Potential Electrolyte for Solid Oxide Fuel Cells. Energies 2022, 15, 2826. https://doi.org/10.3390/en15082826
Yilmaz S, Cobaner S, Yalaz E, Amini Horri B. Synthesis and Characterization of Gadolinium-Doped Zirconia as a Potential Electrolyte for Solid Oxide Fuel Cells. Energies. 2022; 15(8):2826. https://doi.org/10.3390/en15082826
Chicago/Turabian StyleYilmaz, Serdar, Senel Cobaner, Emine Yalaz, and Bahman Amini Horri. 2022. "Synthesis and Characterization of Gadolinium-Doped Zirconia as a Potential Electrolyte for Solid Oxide Fuel Cells" Energies 15, no. 8: 2826. https://doi.org/10.3390/en15082826
APA StyleYilmaz, S., Cobaner, S., Yalaz, E., & Amini Horri, B. (2022). Synthesis and Characterization of Gadolinium-Doped Zirconia as a Potential Electrolyte for Solid Oxide Fuel Cells. Energies, 15(8), 2826. https://doi.org/10.3390/en15082826








