Experimental Performance Analysis of Adsorption Modules with Sintered Aluminium Fiber Heat Exchangers and SAPO-34-Water Working Pair for Gas-Driven Heat Pumps: Influence of Evaporator Size, Temperatures, and Half Cycle Times
Abstract
:1. Introduction
- Downsize the module to fit into a wall-hung gas adsorption heat pump. The target volume for such an application is between 10 and 15 dm3.
- Downsize the thermal capacity of the evaporator–condenser without having a negative impact on the power density.
2. Materials and Methods
2.1. Adsorption Module
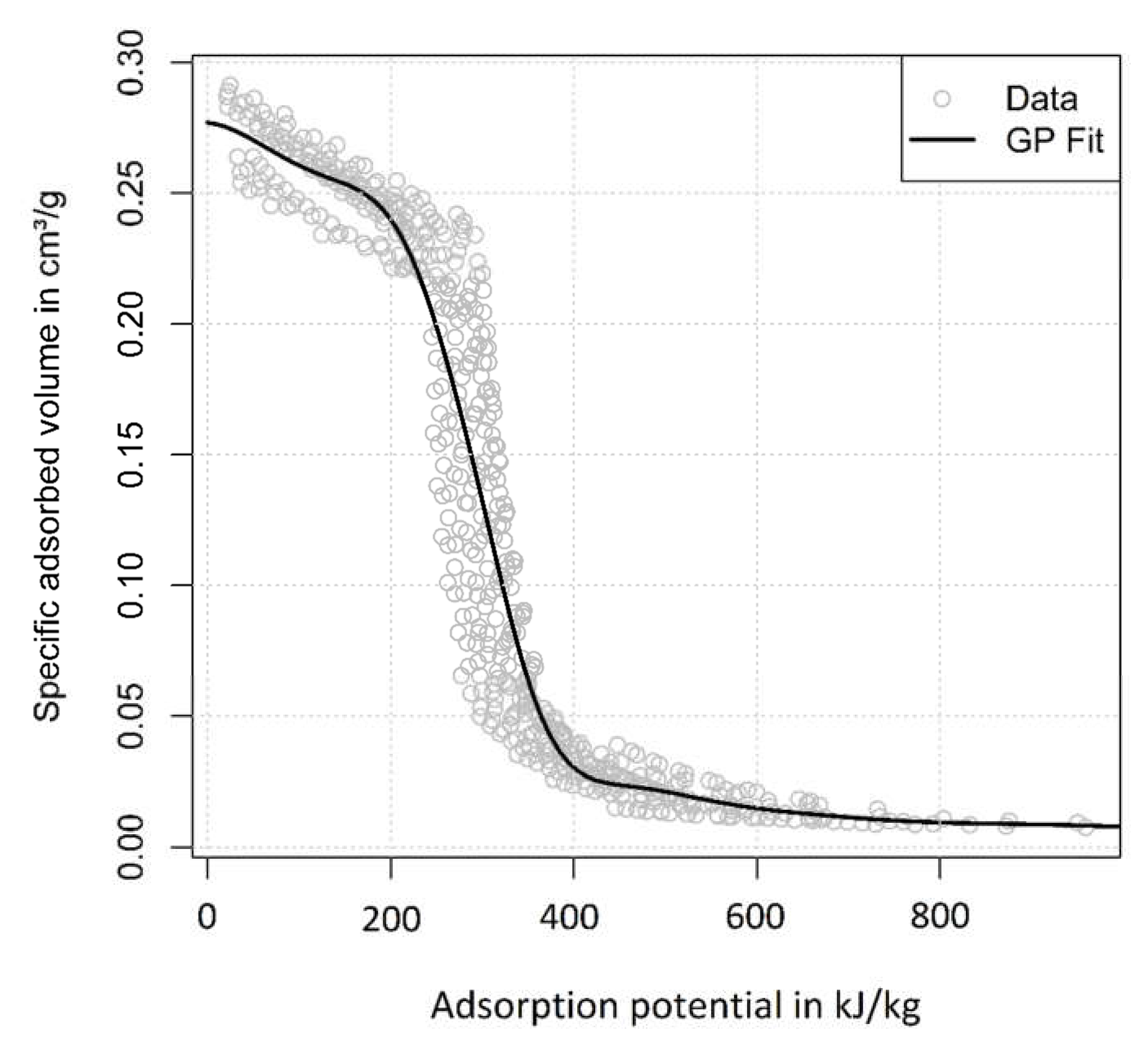
2.2. Adsorption Equilibrium Data
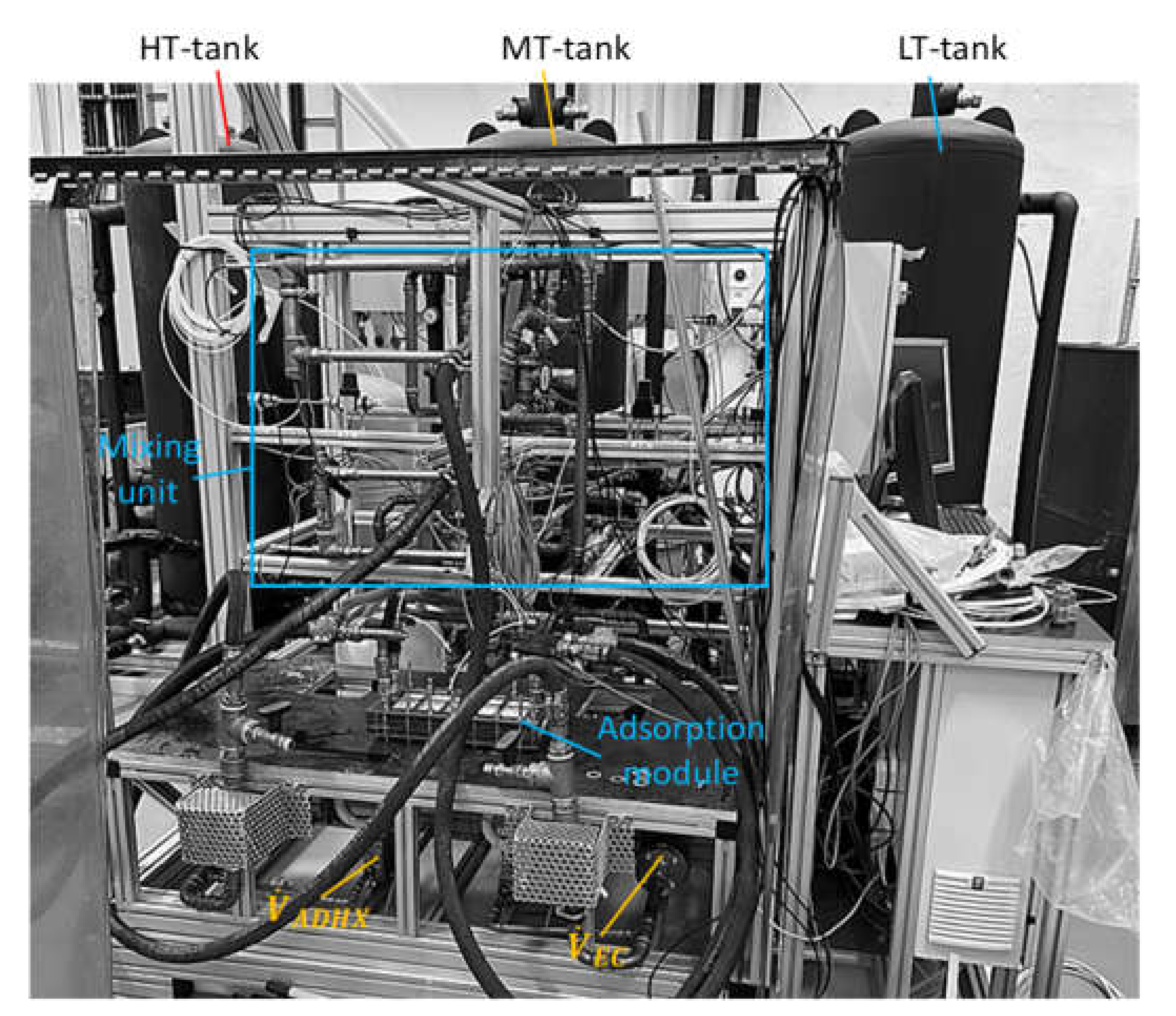
2.3. Test Rig
- The inlet and outlet temperatures of the adsorption heat exchanger and the evaporator-condenser. These temperatures are measured with pairwise calibrated Pt-100 sensors, TMG, with a measurement uncertainty of ±0.01 K.
- The volume flow rates of the evaporator-condenser and the adsorption heat exchanger are measured with volume flow sensors (Proline Promag 50P, Endress + Hauser, Germany, DN25), the uncertainty of the measured velocity is ±0.5% of the measured value ±1 mm/s.
- The pressure of the module with a capacitive pressure sensor (MKS Baratron 627B, Andover, MA, USA).
- The temperature of the housing at three different locations is measured with Pt-100 thin film sensors, ±0.3 K.
2.4. Evaluation of Efficiency and Power Density
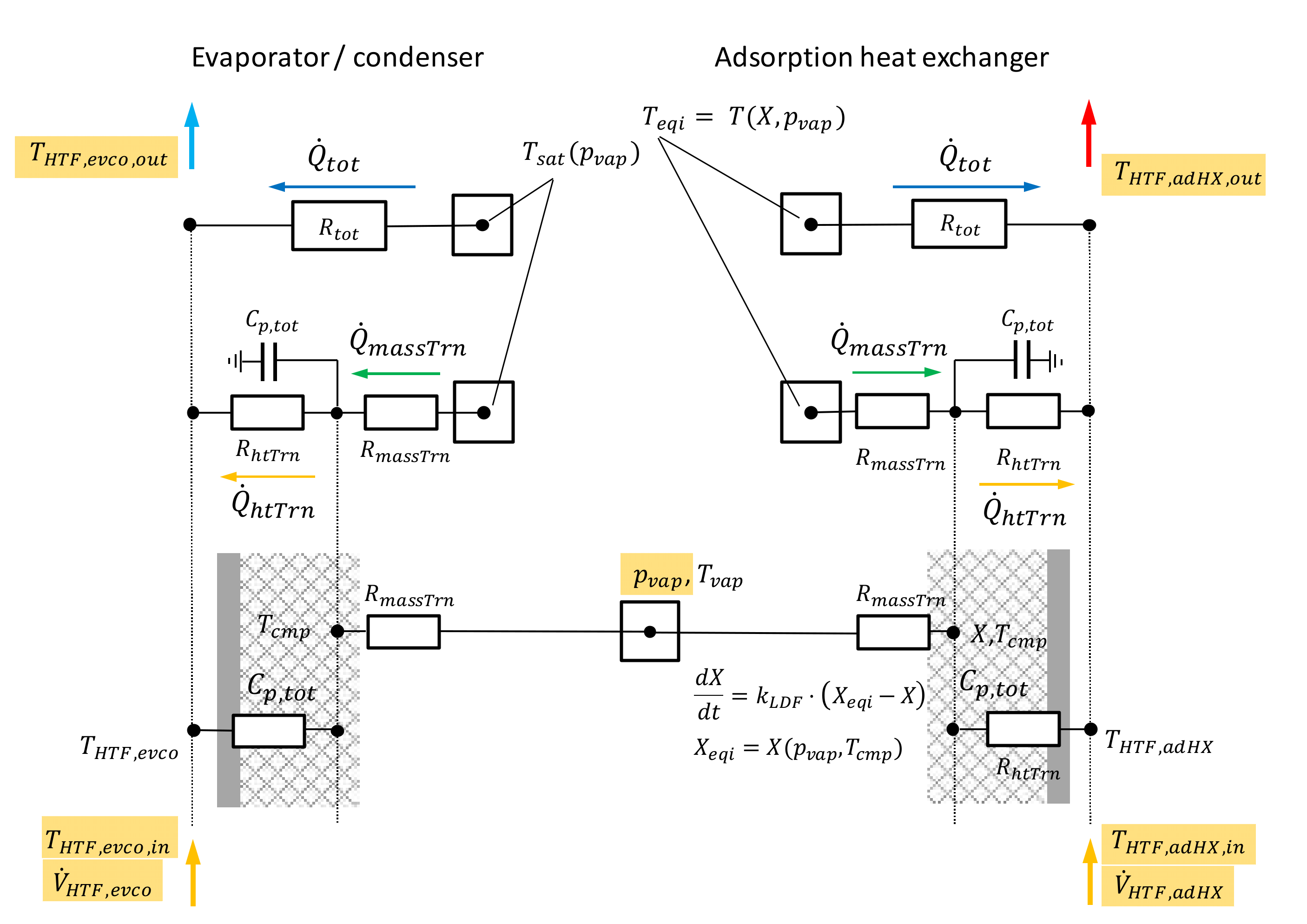
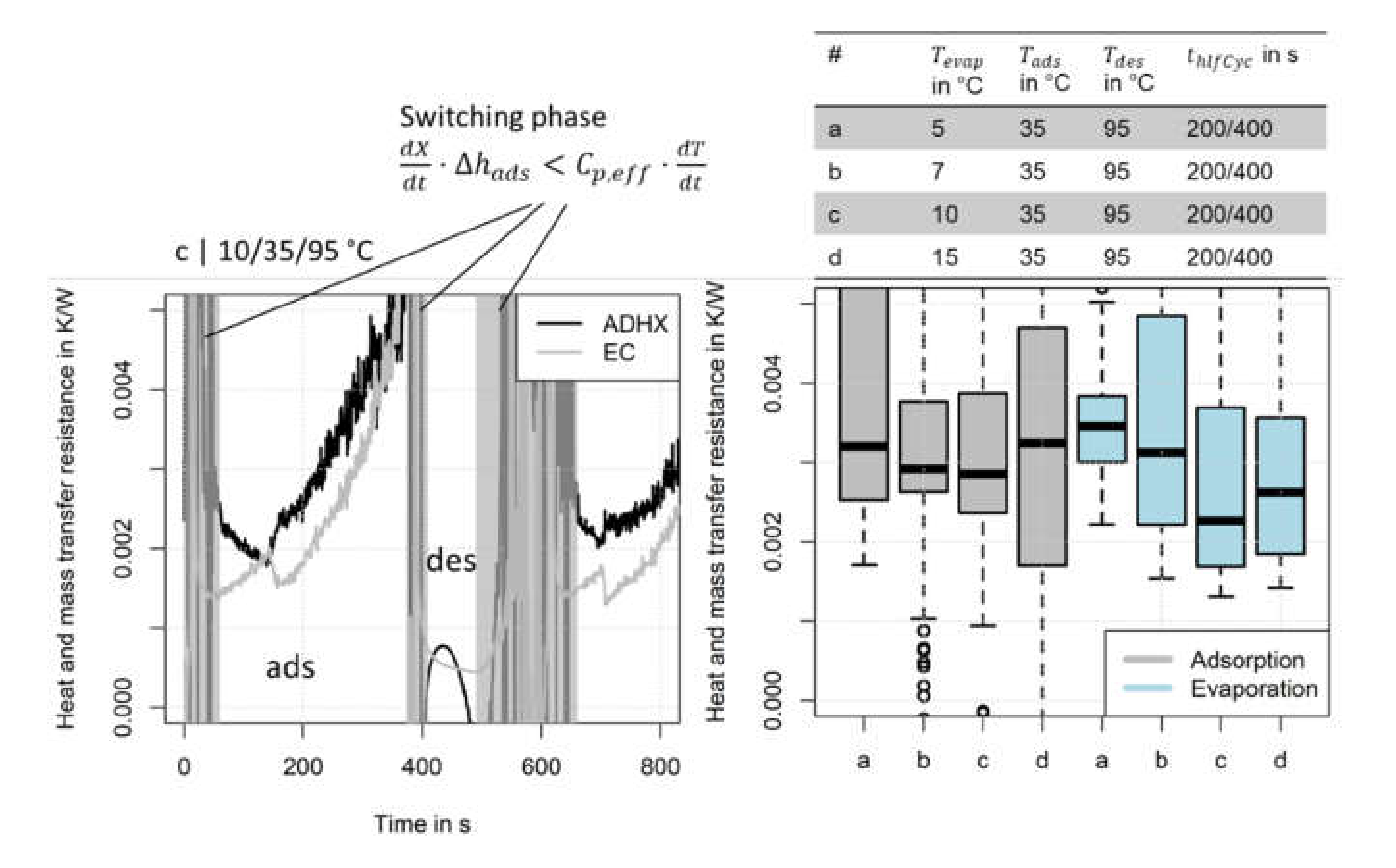
2.5. Evaluation of Heat and Mass Transfer Resistances
- If the in- and outlet temperatures and the pressure within the module is known, we can calculate an overall heat and mass transfer resistance for the evaporator-condenser and the adsorption heat exchanger. A differentiation between heat and mass transfer is not possible from the experimental data if the actual temperature of the component remains unknown.
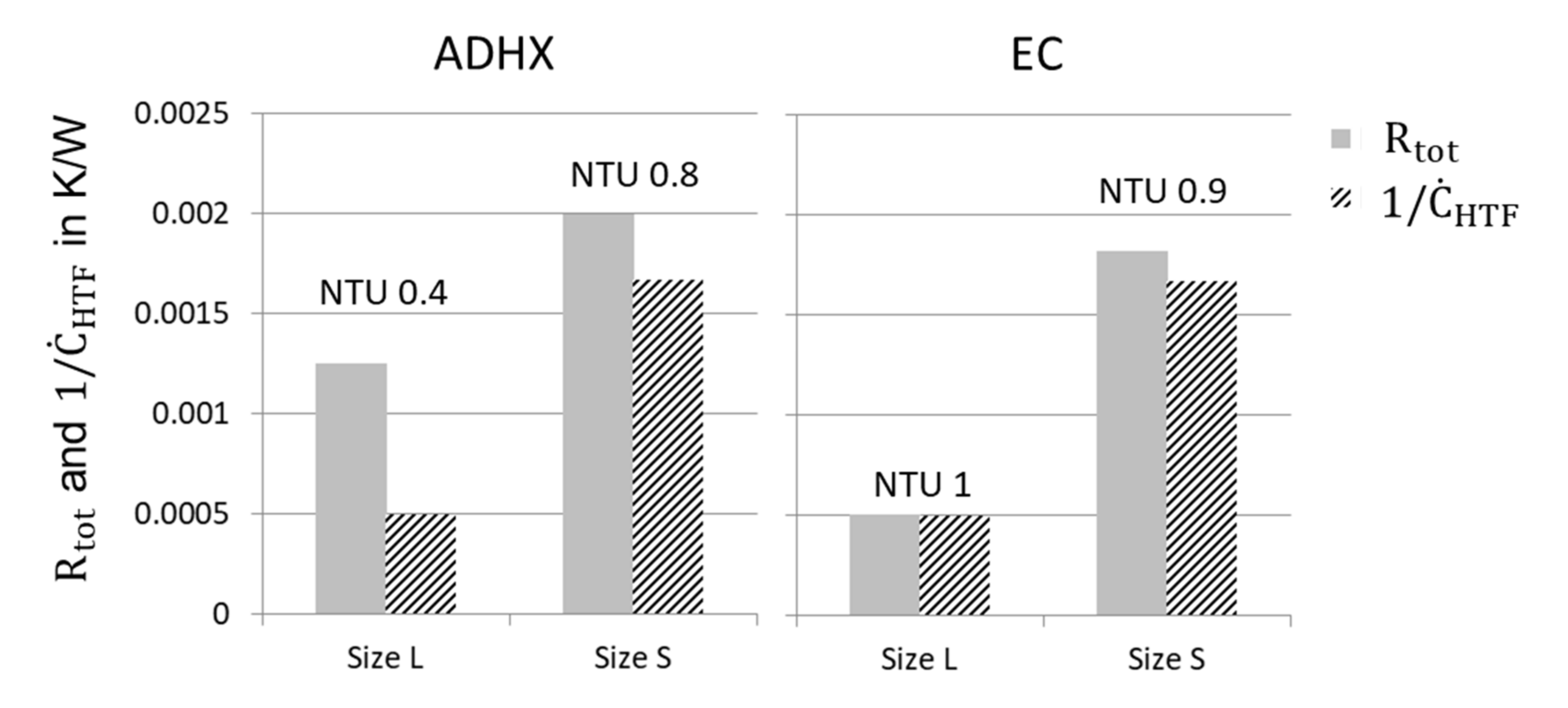
- Besides the heat and mass transfer resistance also the mass flow rate of the HTF is a relevant quantity which can limit the heat transfer within a component. Thus, the evaluation of the NTU and accordingly, the choice of the mass flow rate is decisive.
3. Results and Discussion
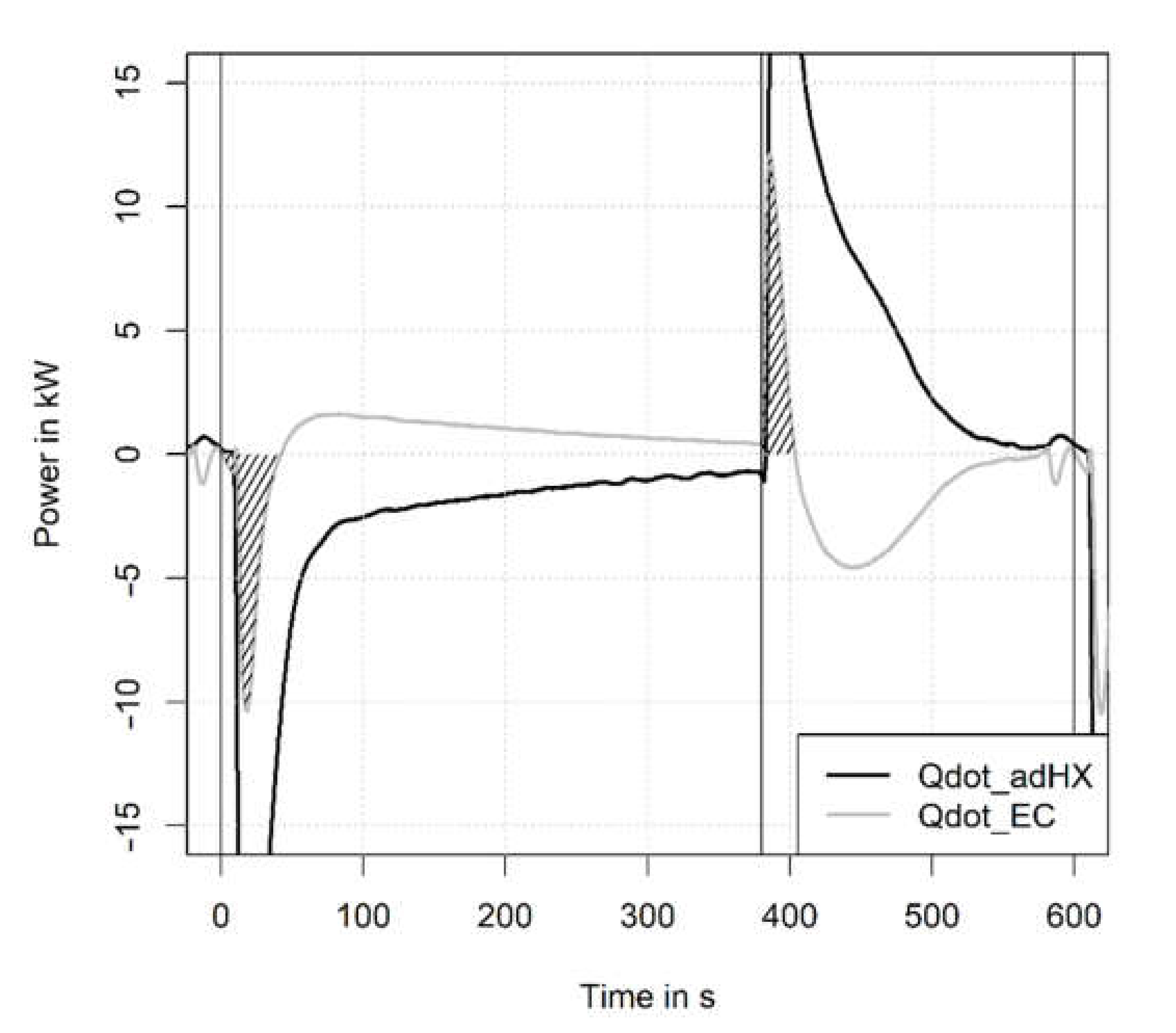
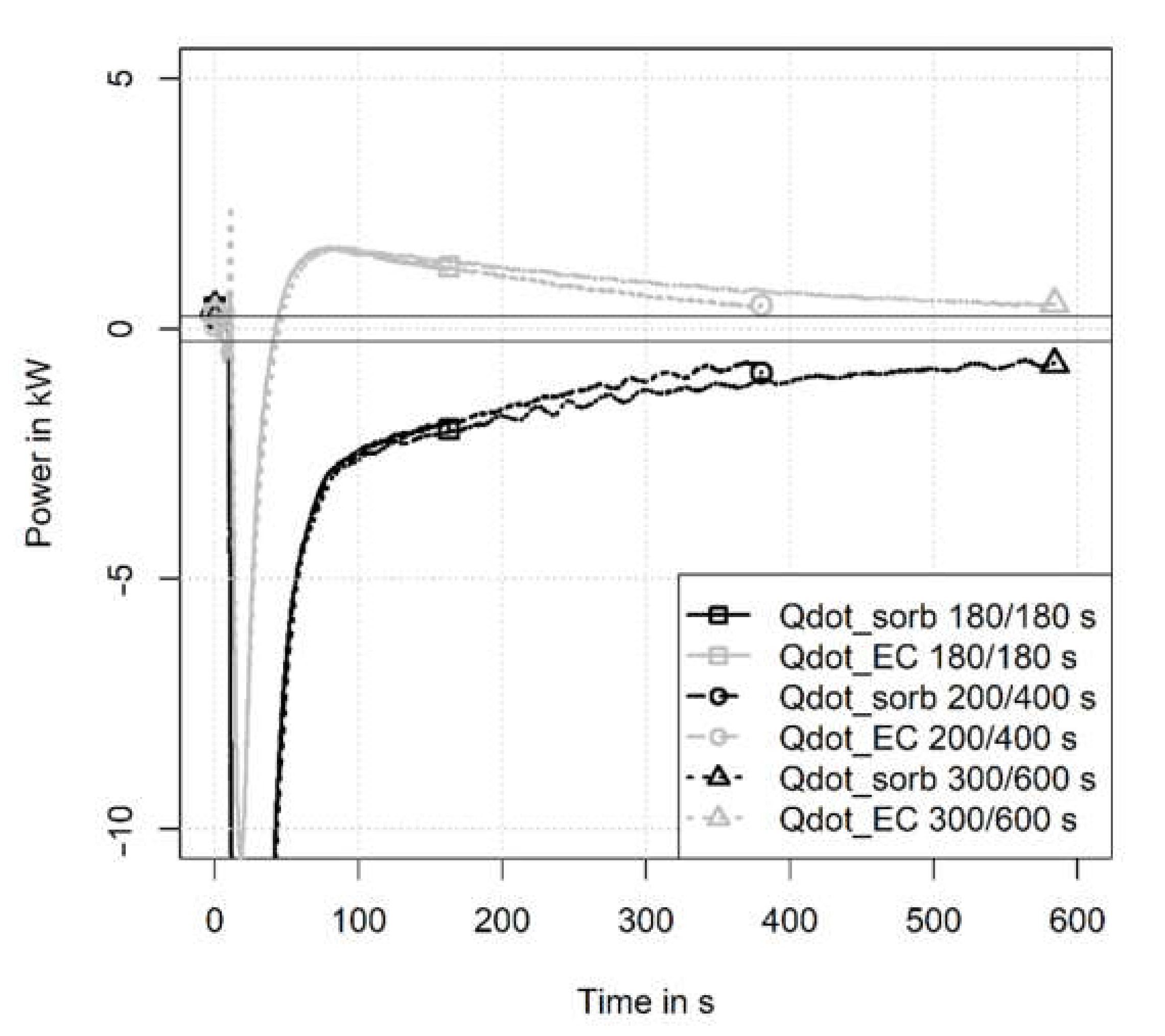
3.1. Temperature and Pressure Curves
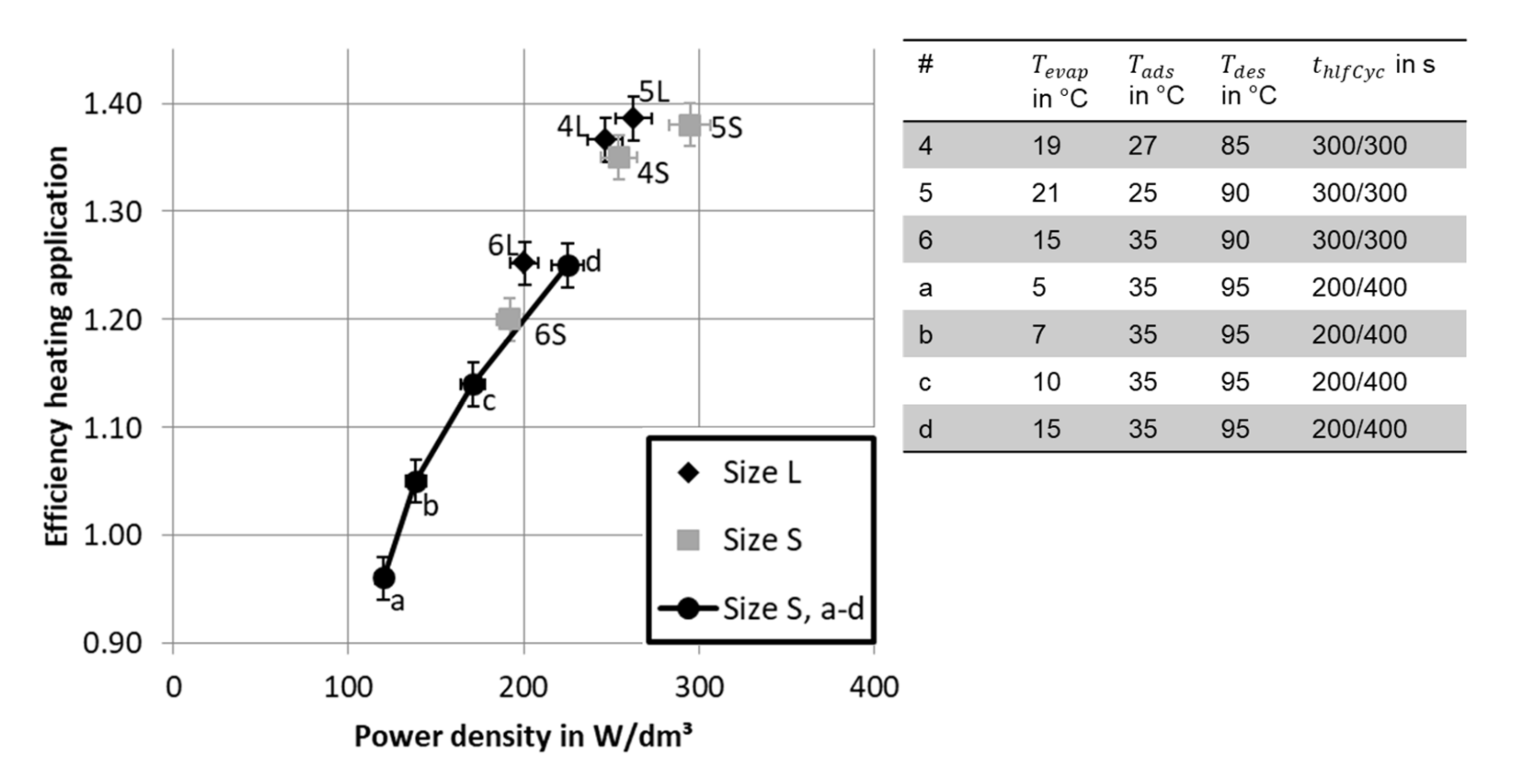
3.2. Efficiency and Power Density
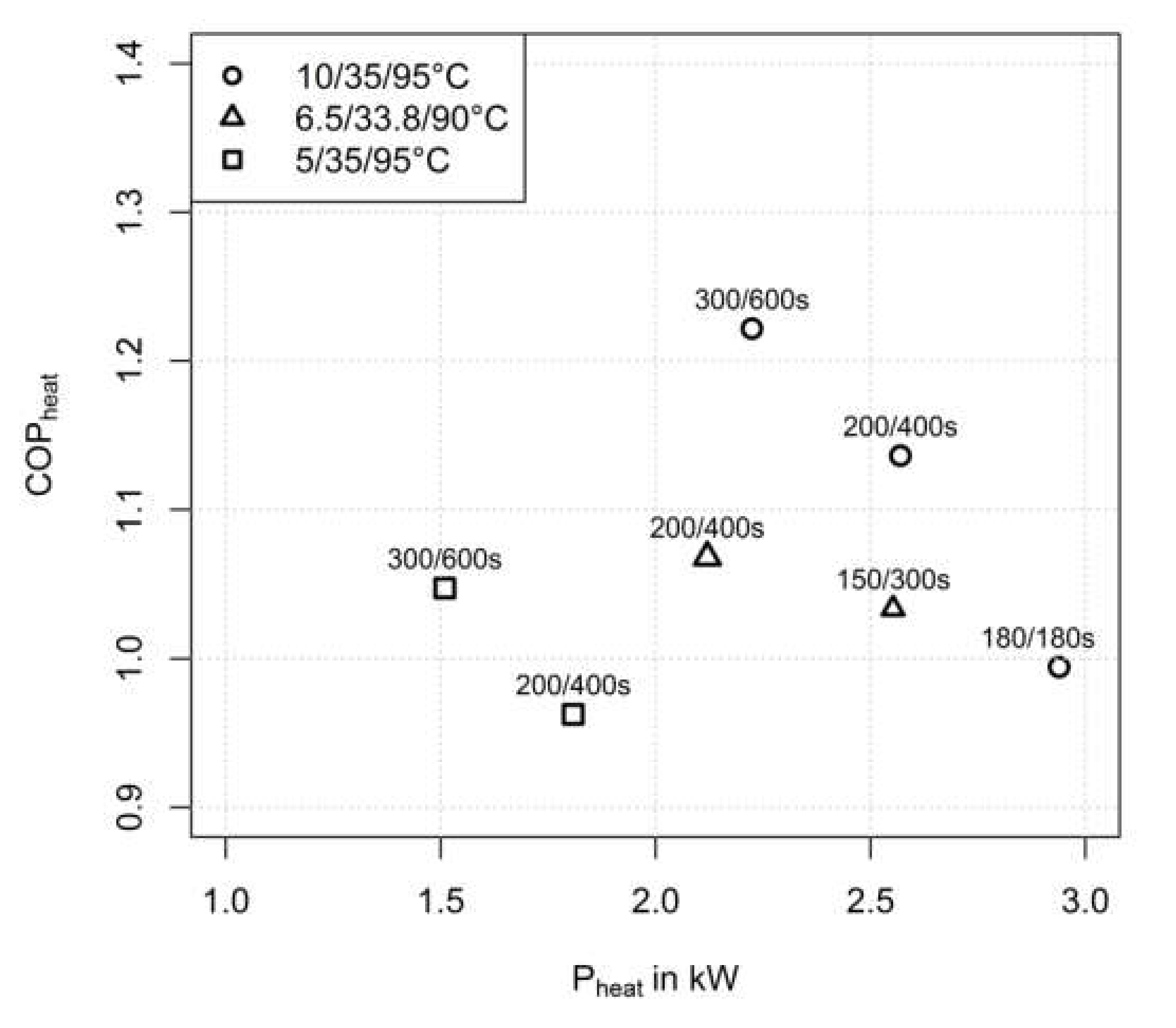
3.2.1. Influence of Half Cycle Time
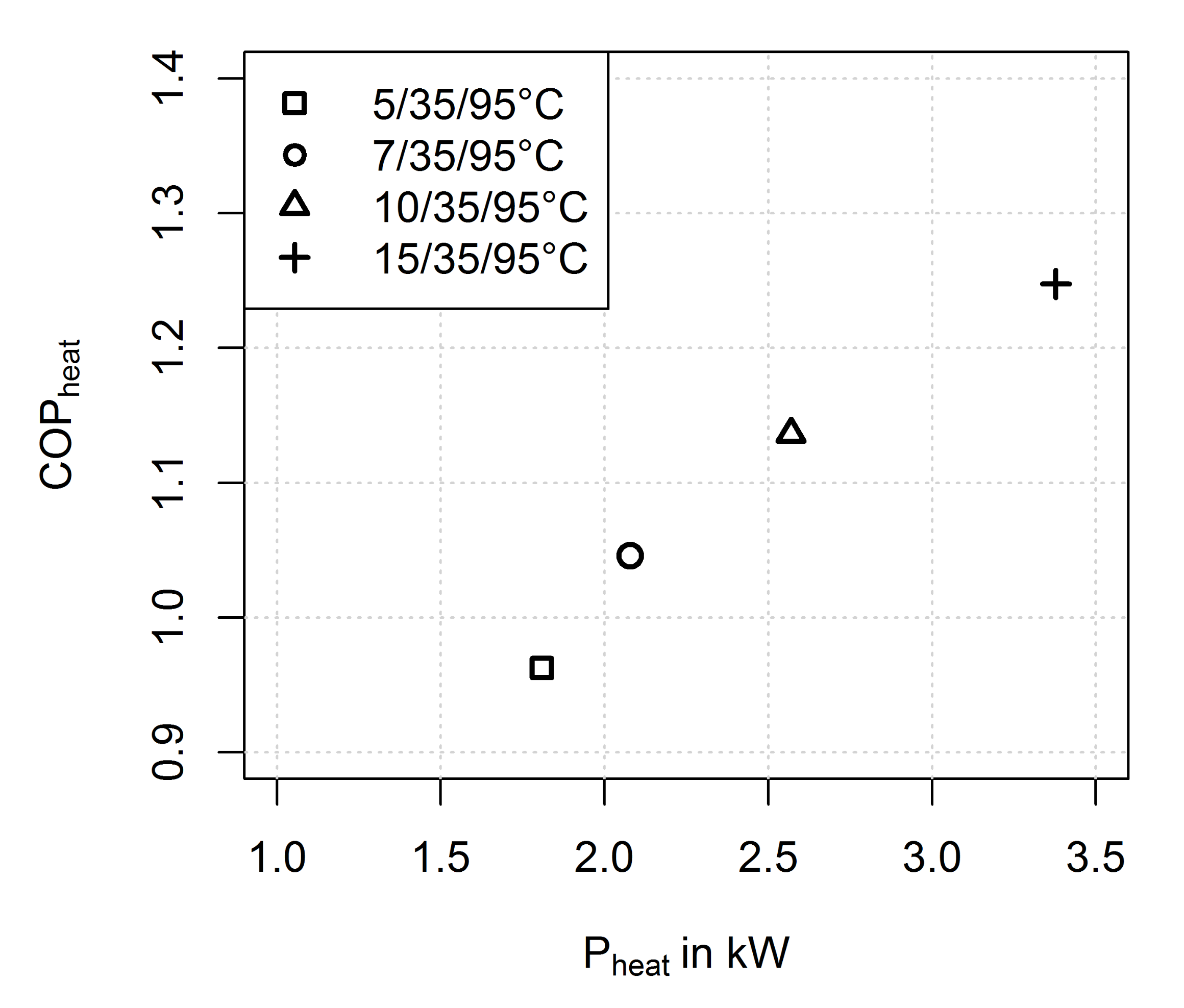
3.2.2. Influence of Evaporator Temperature
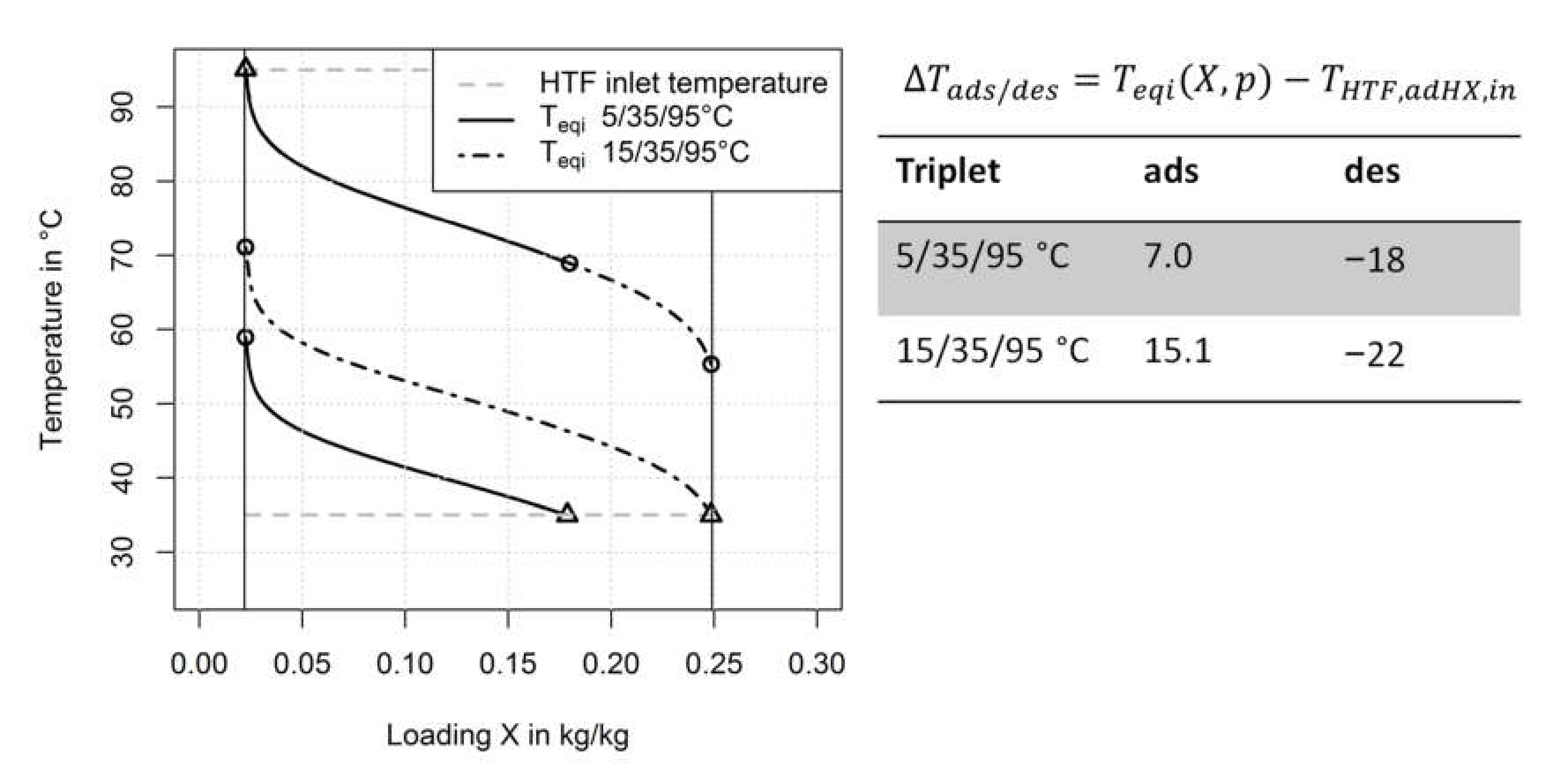
- The heat and mass transfer resistance for adsorption and evaporation increases with a decreasing evaporator temperature (and thus, decreasing pressure of the water vapour)
- The driving temperature differences for the heat and mass transfer processes decrease with decreasing evaporator temperature (and thus, decreasing pressure of the water vapour) as shown in Figure 13. If we compare the average values for the driving temperature differences in the adsorption half cycle it becomes clear that the loading difference of the 5/35/95 °C measurement can be at maximum half the loading difference of the 15/35/95 °C measurement.
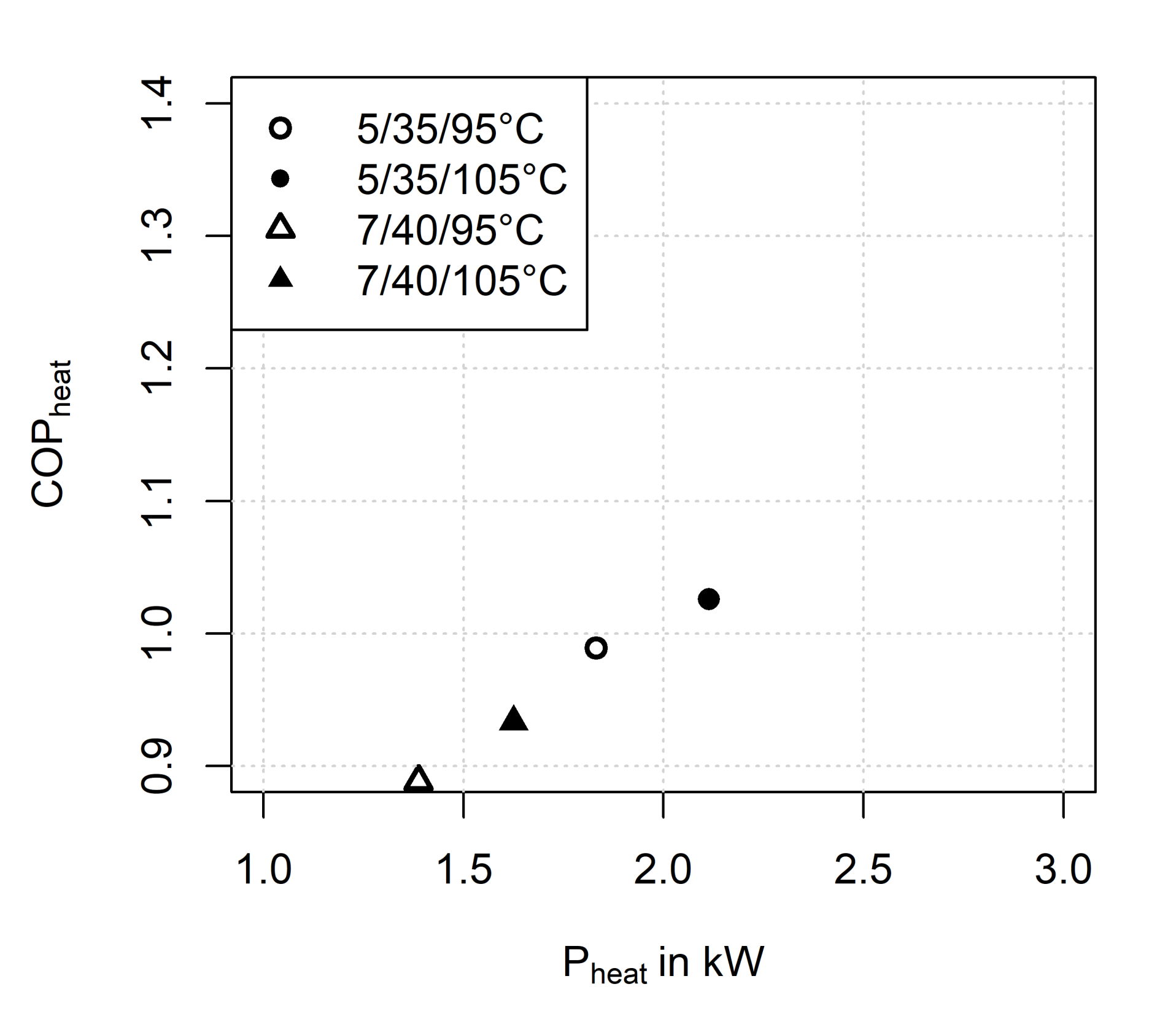
3.2.3. Influence of Adsorption/Condensation Temperature
3.2.4. Influence of Driving Temperature
3.3. Performance Evaluation of the Module
3.3.1. Heat and Mass Transfer Resistances
3.3.2. Comparison with Other Studies
3.4. Reproducibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature and Indices
| Index | Description | |
| ADHX | adsorption heat exchanger | |
| ads | Adsorption | |
| cllt | collector | |
| cmp | Adsorbent-metal-composite | |
| cond | Condensation | |
| COP | Coefficient of Performance/Efficiency | |
| des | Desorption | |
| EC | evaporator-condenser | |
| eff | effective | |
| eqi | sorption equilibrium | |
| evap | Evaporation | |
| ext | external | |
| fib | fibrous structure | |
| heat | Heating case | |
| hlfCyc | Half cycle | |
| hsg | housing | |
| HT | high temperature | |
| HTF | Heat transfer fluid | |
| htTrn | heat transfer | |
| int | internal | |
| loss | thermal loss | |
| LT | low temperature | |
| lv | liquid-vapour | |
| masstRn | mass transfer | |
| mod | Adsorption module | |
| MT | medium temperature | |
| mt | metal | |
| sat | saturation | |
| snk | sink | |
| sorb | adsorbent | |
| src | source | |
| str | structure | |
| tbs | tubes | |
| tot | total | |
| V | Volume | |
| vap | vapour | |
| vl | vapour-liquid | |
| VSHP | Volume specific heating power | |
| wf | working fluid | |
| xpr | experimental | |
| Symbol | Unit | Description |
| m2 | Area | |
| J/(kg∙K) | Specific heat capacity | |
| J/kg | Effective heat capacity | |
| m | Diameter, thickness | |
| 1 | Factor | |
| J/kg | Specific enthalpy of liquid phase | |
| m | Length | |
| kg/s | Mass flow rate | |
| 1 | Number of transfer units | |
| Pa | Pressure | |
| W | Thermal power output | |
| J | Amount of heat | |
| W | Heat flow rate | |
| K/W | Heat (and/or) mass transfer resistance | |
| T | K, °C | Temperature |
| J | Inner energy | |
| UA | W/K | Heat conductance |
| dm3/min | Volume flow rate | |
| kg/kg | Loading | |
| W/(m2∙K) | Heat transfer coefficient | |
| W/(m∙K) | Heat conductivity | |
| kg/m3 | Density of liquid phase | |
References
- Kemna, R.; van Elburg, M.; Corso, A.; Vigand, J.; Maagoe, V. Space and Combination Heaters—Market Analysis: Ecodesign and Energy Labelling; European Commision: Brussels, Belgium, 2019. [Google Scholar]
- Pinheiro, J.M.; Salústio, S.; Rocha, J.; Valente, A.A.; Silva, C.M. Adsorption heat pumps for heating applications. Renew. Sustain. Energy Rev. 2020, 119, 109528. [Google Scholar] [CrossRef]
- Dawoud, B. On the development of an innovative gas-fired heating appliance based on a zeolite-water adsorption heat pump; system description and seasonal gas utilization efficiency. Appl. Therm. Eng. 2014, 72, 323–330. [Google Scholar] [CrossRef]
- Kühn, A. (Ed.) Thermally Driven Heat Pumps for Heating and Cooling; Universitätsverlag der TU Berlin: Berlin, Germany, 2013; ISBN 978-3-7983-2596-8. [Google Scholar]
- Rivero-Pacho, A.M.; Critoph, R.E.; Metcalf, S.J. Modelling and development of a generator for a domestic gas-fired carbon-ammonia adsorption heat pump. Renew. Energy 2017, 110, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Metcalf, S.J.; Critoph, R.E.; Tamainot-Telto, Z. Optimal cycle selection in carbon-ammonia adsorption cycles. Int. J. Refrig. 2012, 35, 571–580. [Google Scholar] [CrossRef]
- Mikhaeil, M.; Gaderer, M.; Dawoud, B. On the development of an innovative adsorber plate heat exchanger for adsorption heat transformation processes; An experimental and numerical study. Energy 2020, 207, 118272. [Google Scholar] [CrossRef]
- Saleh, M.M.; AL-Dadah, R.; Mahmoud, S.; Elsayed, E.; El-Samni, O. Wire fin heat exchanger using aluminium fumarate for adsorption heat pumps. Appl. Therm. Eng. 2020, 164, 114426. [Google Scholar] [CrossRef]
- Seol, S.-H.; Nagano, K.; Togawa, J. Modeling of adsorption heat pump system based on experimental estimation of heat and mass transfer coefficients. Appl. Therm. Eng. 2020, 171, 115089. [Google Scholar] [CrossRef]
- Bendix, P.; Füldner, G.; Möllers, M.; Kummer, H.; Schnabel, L.; Henninger, S.; Henning, H.-M. Optimization of power density and metal-to-adsorbent weight ratio in coated adsorbers for adsorptive heat transformation applications. Appl. Therm. Eng. 2017, 124, 83–90. [Google Scholar] [CrossRef]
- Mohammadzadeh Kowsari, M.; Niazmand, H.; Tokarev, M.M. Bed configuration effects on the finned flat-tube adsorption heat exchanger performance: Numerical modeling and experimental validation. Appl. Energy 2018, 213, 540–554. [Google Scholar] [CrossRef]
- Calabrese, L.; Bruzzaniti, P.; Palamara, D.; Freni, A.; Proverbio, E. New SAPO-34-SPEEK composite coatings for adsorption heat pumps: Adsorption performance and thermodynamic analysis. Energy 2020, 203, 117814. [Google Scholar] [CrossRef]
- Hinze, M.; Ranft, F.; Drummer, D.; Schwieger, W. Reduction of the heat capacity in low-temperature adsorption chillers using thermally conductive polymers as heat exchangers material. Energy Convers. Manag. 2017, 145, 378–385. [Google Scholar] [CrossRef]
- Schnabel, L.; Füldner, G.; Velte, A.; Laurenz, E.; Bendix, P.; Kummer, H.; Wittstadt, U. Innovative Adsorbent Heat Exchangers: Design and Evaluation. In Innovative Heat Exchangers; Bart, H.-J., Scholl, S., Eds.; Springer: Cham, Switzerland, 2018; pp. 363–394. ISBN 978-3-319-71641-1. [Google Scholar]
- Caprì, A.; Frazzica, A.; Calabrese, L. Recent Developments in Coating Technologies for Adsorption Heat Pumps: A Review. Coatings 2020, 10, 855. [Google Scholar] [CrossRef]
- Wittstadt, U.; Füldner, G.; Andersen, O.; Herrmann, R.; Schmidt, F. A New Adsorbent Composite Material Based on Metal Fiber Technology and Its Application in Adsorption Heat Exchangers. Energies 2015, 8, 8431–8446. [Google Scholar] [CrossRef]
- Wittstadt, U.; Füldner, G.; Laurenz, E.; Warlo, A.; Große, A.; Herrmann, R.; Schnabel, L.; Mittelbach, W. A novel adsorption module with fiber heat exchangers: Performance analysis based on driving temperature differences. Renew. Energy 2017, 110, 154–161. [Google Scholar] [CrossRef]
- Velte, A. Experimentelle Arbeiten und Entwicklung von numerischen Modellen zur Analyse und Optimierung von Erweiterten Adsorptionskreisläufen für die Wärmeversorgung von Gebäuden. Ph.D. Thesis, Albert-Ludwigs-Universität Freiburg, Freiburg, Germany, 2018. [Google Scholar]
- Bauer, J.; Herrmann, R.; Mittelbach, W.; Schwieger, W. Zeolite/aluminum composite adsorbents for application in adsorption refrigeration. Int. J. Energy Res. 2009, 33, 1233–1249. [Google Scholar] [CrossRef]
- Wittstadt, U.; Laurenz, E.; Velte, A.; Füldner, G. Using Driving Temperature Difference Equivalents to Compare Heat and Mass Transport Limitations in Sorption Modules and Adsorption Heat Exchangers: Poster at the Sorption Friends Conference, Milazzo, Italy, 14–16 September 2015. Available online: http://www.sorptionfriends.org/poster/ (accessed on 24 April 2017).
- Velte, A.; Herrmann, R.; Andersen, O.; Wittstadt, U.; Füldner, G.; Schnabel, L. Experimental Characterization of Aluminum Fibre/SAPO-34 Composites for Adsorption Heat Pump Applications. In Proceedings of the Heat Powered Cycles, Nottingham, UK, 26–29 June 2016. [Google Scholar]
- Wittstadt, U. Experimentelle und Modellgestützte Charakterisierung von Adsorptionswärmeübertragern. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, 2018. [Google Scholar]
- Ammann, J.; Ruch, P.; Michel, B.; Studart, A.R. Quantification of heat and mass transport limitations in adsorption heat exchangers: Application to the silica gel/water working pair. Int. J. Heat Mass Transf. 2018, 123, 331–341. [Google Scholar] [CrossRef]
- Ammann, J.; Michel, B.; Ruch, P.W. Characterization of transport limitations in SAPO-34 adsorbent coatings for adsorption heat pumps. Int. J. Heat Mass Transf. 2019, 129, 18–27. [Google Scholar] [CrossRef]
- Shah, R.K.; Sekulić, D.P. Fundamentals of Heat Exchanger Design; Wiley-Interscience: Hoboken, NJ, USA, 2003; ISBN 0-471-32171-0. [Google Scholar]
- Baehr, H.D.; Stephan, K. Wärme- und Stoffübertragung; 6, bearb. Aufl.; Springer Berlin: Berlin, Germany, 2008; ISBN 978-3-540-87688-5. [Google Scholar]
- Miyazaki, T.; Akisawa, A. The influence of heat exchanger parameters on the optimum cycle time of adsorption chillers. Appl. Therm. Eng. 2009, 29, 2708–2717. [Google Scholar] [CrossRef]
- Velte, A.; Joos, L.; Kostmann, C.; Fink, M.; Füldner, G. Experimental investigation of an adsorption module based on heat exchangers with fibrous aluminium structures and the SAPO-34/water working pair for gas-driven adsorption heat pumps. In Proceedings of the ISHPC 2021 Proceedings, Online Pre-Conference 2020, Berlin, Germany, 22–25 August 2021; Meyer, T., Ed.; Technische Universität Berlin: Berlin, Germany, 2020. [Google Scholar]
- Gluesenkamp, K.R.; Frazzica, A.; Velte, A.; Metcalf, S.; Yang, Z.; Rouhani, M.; Blackman, C.; Qu, M.; Laurenz, E.; Rivero-Pacho, A.; et al. Experimentally Measured Thermal Masses of Adsorption Heat Exchangers. Energies 2020, 13, 1150. [Google Scholar] [CrossRef] [Green Version]
- Joos, L. Vermessung von Adsorptionsmodulen und Charakterisierung Mittels Dynamischer Simulationen. Master’s Thesis, Technische Universität Berlin, Berlin, Germany, 2019. [Google Scholar]
- Desai, A.; Schwamberger, V.; Herzog, T.; Jänchen, J.; Schmidt, F.P. Modeling of Adsorption Equilibria through Gaussian Process Regression of Data in Dubinin’s Representation: Application to Water/Zeolite Li-LSX. Ind. Eng. Chem. Res. 2019, 58, 17549–17554. [Google Scholar] [CrossRef]
- Wagner, W.; Cooper, J.R.; Dittmann, A.; Kijima, J.; Kretzschmar, H.-J.; Kruse, A.; Mares, R.; Oguchi, K.; Sato, H.; Stöcker, I.; et al. The IAPWS Industrial Formulation 1997 for the Thermodynamic Properties of Water and Steam. J. Eng. Gas Turbines Power 2000, 122, 150–184. [Google Scholar] [CrossRef]
- Holmgren, M. XSteam. Available online: https://www.academia.edu/download/39720574/X_Steam_for_Matlab.pdf (accessed on 10 March 2020).
- Lanzerath, F.; Bau, U.; Seiler, J.; Bardow, A. Optimal design of adsorption chillers based on a validated dynamic object-oriented model. Sci. Technol. Built Environ. 2015, 21, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Fink, M.; Andersen, O.; Seidel, T.; Schlott, A. Strongly Orthotropic Open Cell Porous Metal Structures for Heat Transfer Applications. Metals 2018, 8, 554. [Google Scholar] [CrossRef] [Green Version]
- Schwamberger, V. Thermodynamische und Numerische Untersuchung Eines Neuartigen Sorptionszyklus zur Anwendung in Adsorptionswärmepumpen und -kältemaschinen = Thermodynamic and Numerical Investigation of a Novel Sorption Cycle for Application in Adsorption Heat Pumps and Chillers. Ph.D. Thesis, Karlsruher Institut für Technologie, Karlsruher, Germany, 2016. [Google Scholar]







| Quantity | Size L | Size S |
|---|---|---|
| Adsorption heat exchanger | ||
| Adsorbent mass in kg | 3.3 ± 0.3 | 1.5 ± 0.2 |
| Heat exchanger dimensions in mm with headers w/o headers | 700 × 313 × 45 600 × 313 × 45 | 450 × 185 × 80 400 × 185 × 80 |
| Volume in dm3 with headers | 9.9 ± 0.1 | 5.7 ± 0.1 |
| Evaporator–condenser | ||
| Heat exchanger primary area in m2 | 43 ± 2 | 14 ± 1 |
| Heat exchanger dimensions in mm with headers w/o headers | 700 × 313 × 45 600 × 313 × 45 | 450 × 158 × 45 400 × 158 × 45 |
| Volume in dm3 with headers | 9.9 ± 0.1 | 3.2 ± 0.1 |
| Module | ||
| Dimensions in mm (w/o insulation) | 730 × 320 × 147 | 474 × 169 × 188 |
| Volume in dm3 (w/o insulation) | 34 ± 0.2 | 15 ± 0.2 |
| Quantity | Value | |
|---|---|---|
| Adsorption heat exchanger | ||
| Inner area of flat tube in m2 | 1.17 | |
| Contact area between fibres and tubes in m2 | 0.63 | |
| Thickness of fibrous structure between two flat tubes in mm | 10 | |
| Number of flat tubes | 12 | |
| Height of the component (=width of flat tube) in mm | 80 | |
| Evaporator-condenser | ||
| Inner area of flat tube in m2 | 0.58 | |
| Contact area between fibres and tubes in m2 | 0.39 | |
| Thickness of fibrous structure between two flat tubes in mm | 10 | |
| Number of flat tubes | 12 | |
| Height of the component (=width of flat tube) in mm | 45 | |
| Term | Adsorption Heat Exchanger | Evaporator-Condenser |
|---|---|---|
| , | , | |
| , | , | |
| , | , | |
| , | , | |
| , | , |
| Parameter | Unit | Equation | Value | ||
|---|---|---|---|---|---|
| Heat transfer coefficient HTF-metal *1 | W/(m2K) | - | - | 6000…7500 | |
| Heat conductance HTF-metal | W/K | , | (11) | 7000…8800 | |
| Heat conductivity fibrous structure | W/(m∙K) | - | - | 5…8 *2 | |
| Heat conductance fibrous structure *3 | W/K | (12) | 1900…3100 | ||
| Overall heat transfer resistance | 10−3 K/W | (13) | 0.47…0.67 |
| Parameter | Unit | Equation | Value Evap | Value Cond | ||
|---|---|---|---|---|---|---|
| Heat transfer coefficient HTF-metal *1 | W/(m2K) | - | - | 6000 | 7500 | |
| Heat conductance HTF-metal | W/K | (14) | 3500 | 4300 | ||
| Heat conductivity fibrous structure | W/(m∙K) | - | - | 5…8 *2 | ||
| Mean path length heat transfer | mm | - | - | 1…3 | 1.7 | |
| Ratio of wetted area | 1 | - | 1…0.1 | 1 | ||
| Heat conductance fibrous structure | W/K | (15) | 200… 5800 | 3500 | ||
| Overall heat transfer resistance | 10−3 K/W | (16) | 0.4… 5.5 | 0.4… 0.5 | ||
| No. | Date | COPheat | Pheat in kW | Comment |
|---|---|---|---|---|
| 1 | 06.06.2019 | 1.02+/−0.01 | 2.9 | Original measurement |
| 2 | 20.01.2020 | 1.02+/−0.01 | 2.9 | Reproducibility after 6 months (few other measurements during this period) |
| 3 | 22.01.2020 | 1.02+/−0.01 | 3.0 | Increased the filling level (+150 g) |
| 4 | 04.02.2020 | 1.02+/−0.01 | 3.0 | Final check of reproducibility |
| No. | Date | RMSD(TEC) in K | RMSD(TADHX) in K | RMSD pmod in mbar | ||
|---|---|---|---|---|---|---|
| in | out | in | out | |||
| 1 → 2 | 20.01.2020 | 1 | 0.6 | 1.9 | 0.9 | 1.4 |
| 1 → 3 | 22.01.2020 | 0.7 | 0.5 | 0.9 | 0.8 | 1.2 |
| 1 → 4 | 04.02.2020 | 1.0 | 0.6 | 1.9 | 1.0 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velte, A.; Joos, L.; Füldner, G. Experimental Performance Analysis of Adsorption Modules with Sintered Aluminium Fiber Heat Exchangers and SAPO-34-Water Working Pair for Gas-Driven Heat Pumps: Influence of Evaporator Size, Temperatures, and Half Cycle Times. Energies 2022, 15, 2823. https://doi.org/10.3390/en15082823
Velte A, Joos L, Füldner G. Experimental Performance Analysis of Adsorption Modules with Sintered Aluminium Fiber Heat Exchangers and SAPO-34-Water Working Pair for Gas-Driven Heat Pumps: Influence of Evaporator Size, Temperatures, and Half Cycle Times. Energies. 2022; 15(8):2823. https://doi.org/10.3390/en15082823
Chicago/Turabian StyleVelte, Andreas, Lukas Joos, and Gerrit Füldner. 2022. "Experimental Performance Analysis of Adsorption Modules with Sintered Aluminium Fiber Heat Exchangers and SAPO-34-Water Working Pair for Gas-Driven Heat Pumps: Influence of Evaporator Size, Temperatures, and Half Cycle Times" Energies 15, no. 8: 2823. https://doi.org/10.3390/en15082823
APA StyleVelte, A., Joos, L., & Füldner, G. (2022). Experimental Performance Analysis of Adsorption Modules with Sintered Aluminium Fiber Heat Exchangers and SAPO-34-Water Working Pair for Gas-Driven Heat Pumps: Influence of Evaporator Size, Temperatures, and Half Cycle Times. Energies, 15(8), 2823. https://doi.org/10.3390/en15082823






