A Perylenediimide-Based Zinc-Coordination Polymer for Photosensitized Singlet-Oxygen Generation
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Method
2.2. Synthesis
2.3. 1O2 Evolution Experiments
3. Results and Discussion
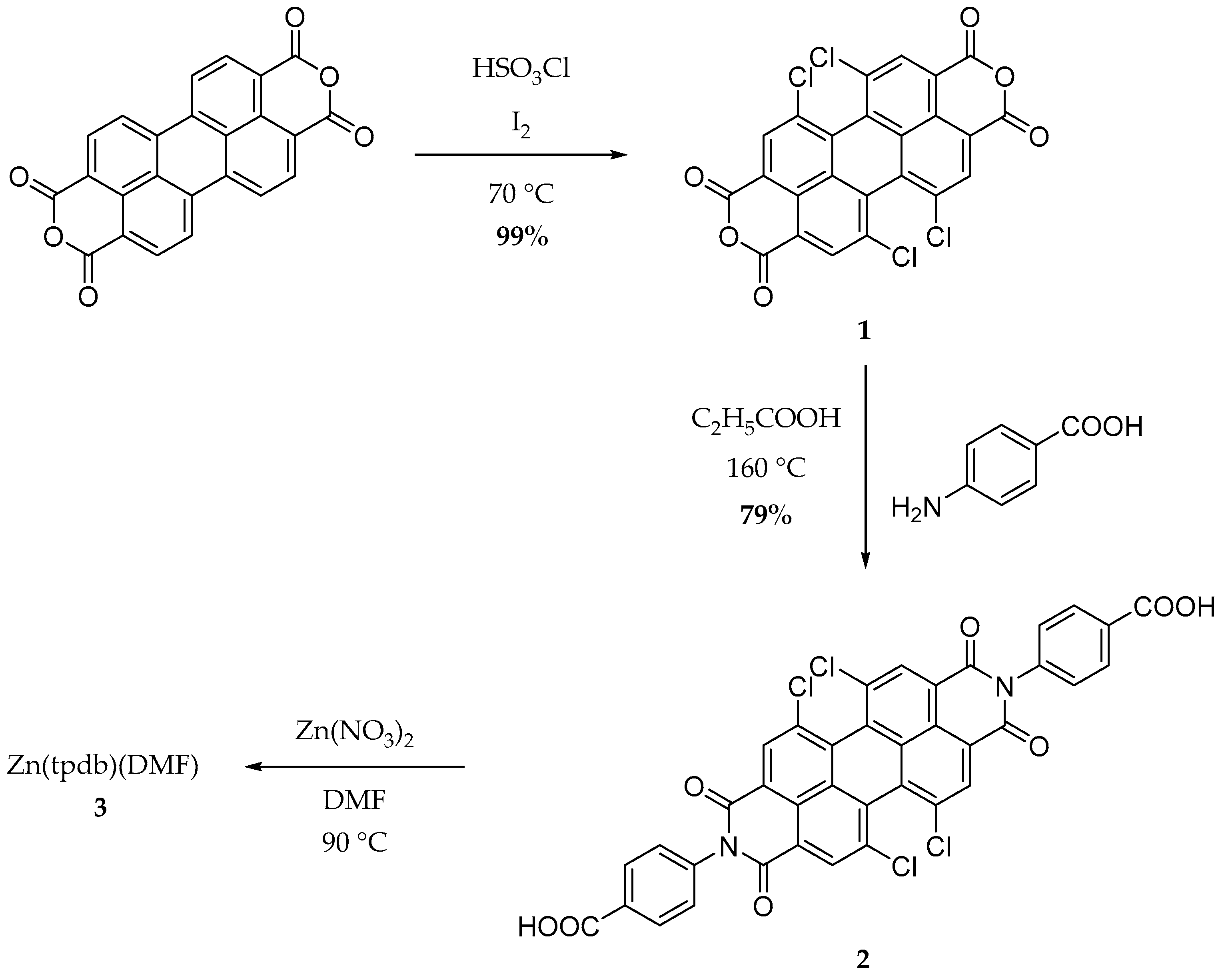
3.1. Linker and CP Synthesis
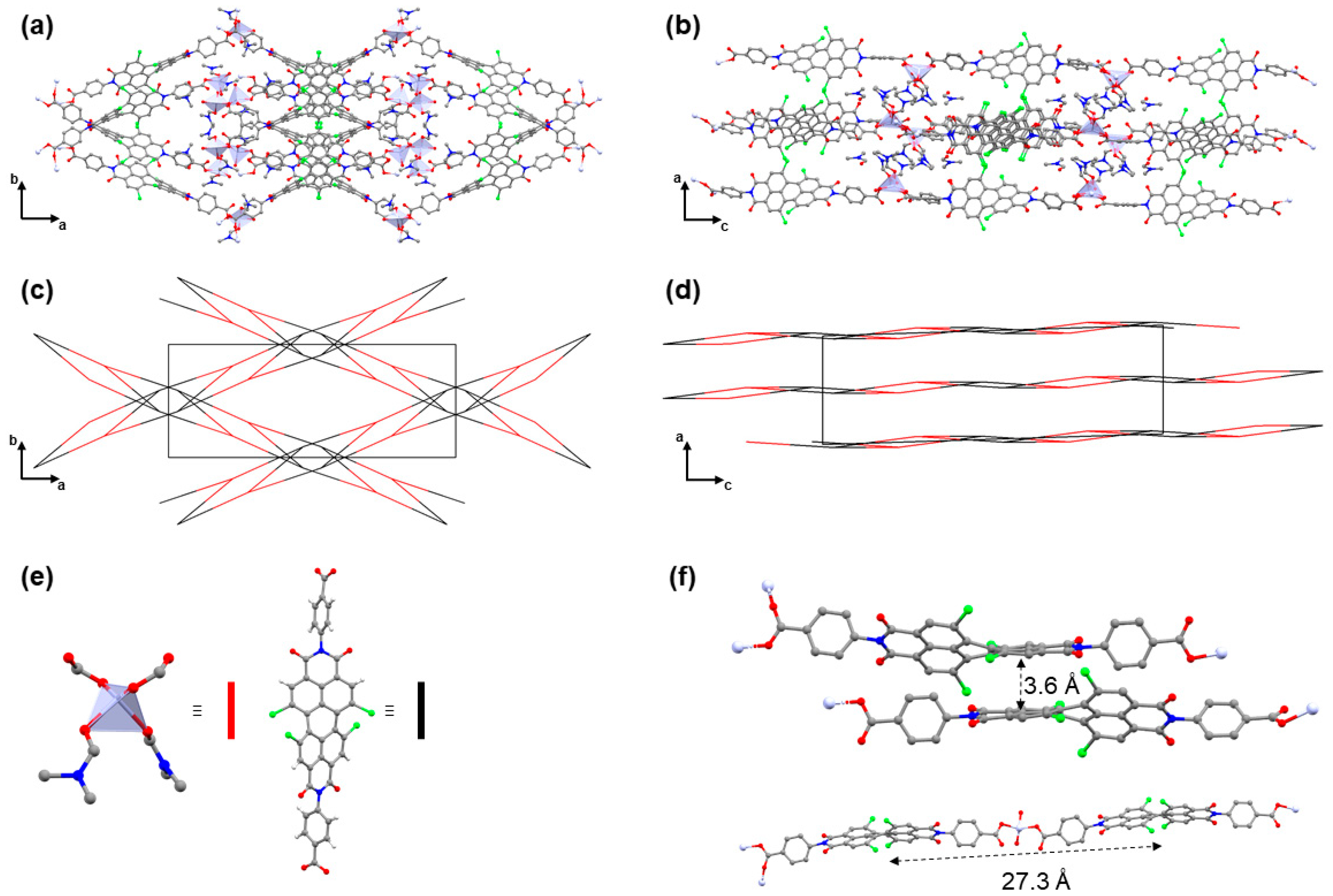
3.2. Crystal Structure Analysis of H2tpbd
3.3. Crystal Structure Analysis of Zn(tpbd)(DMF)3
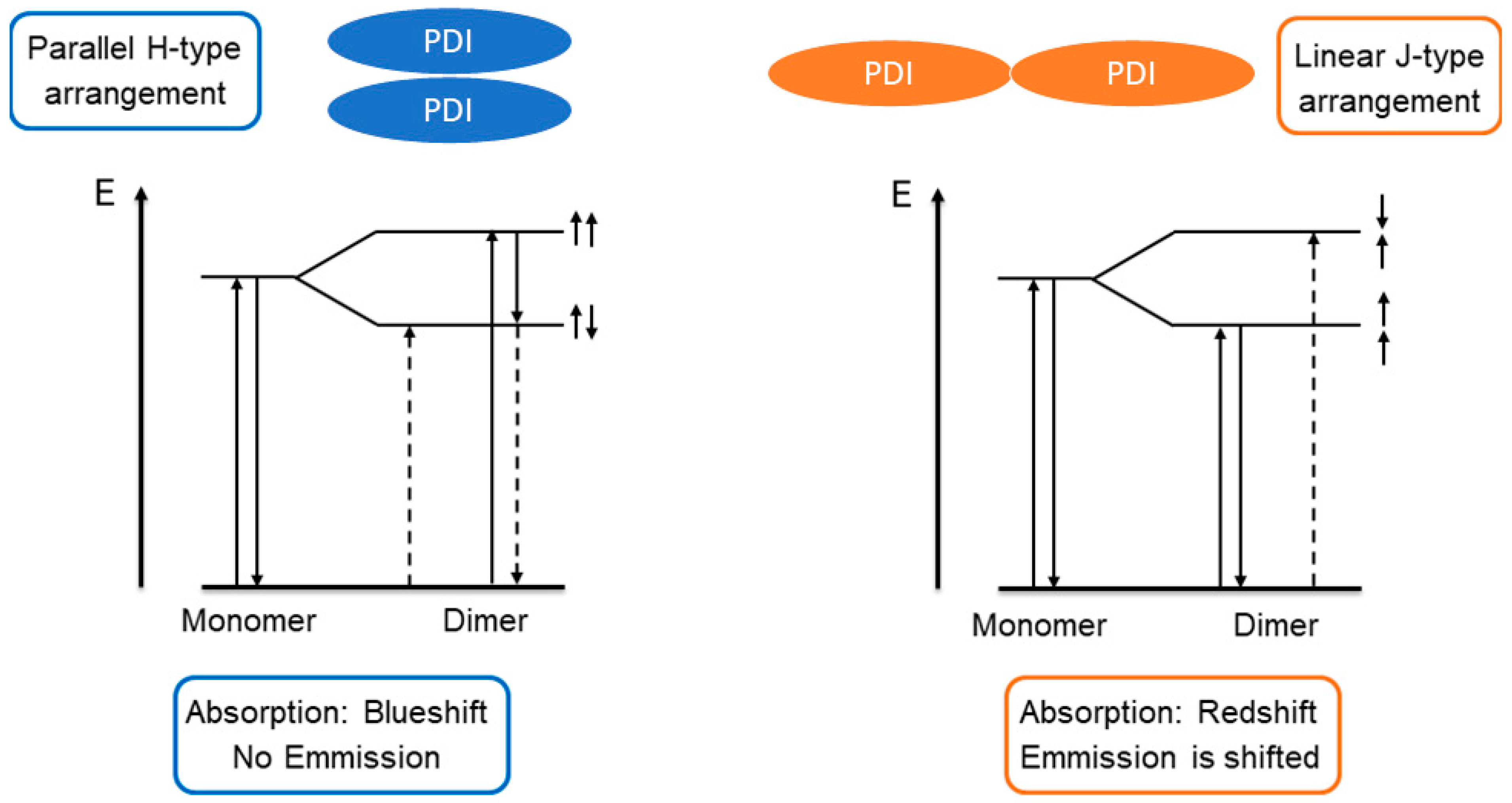
3.4. Photophysical Characterization of H2tpdb and Zn(tpdb)(DMF)3
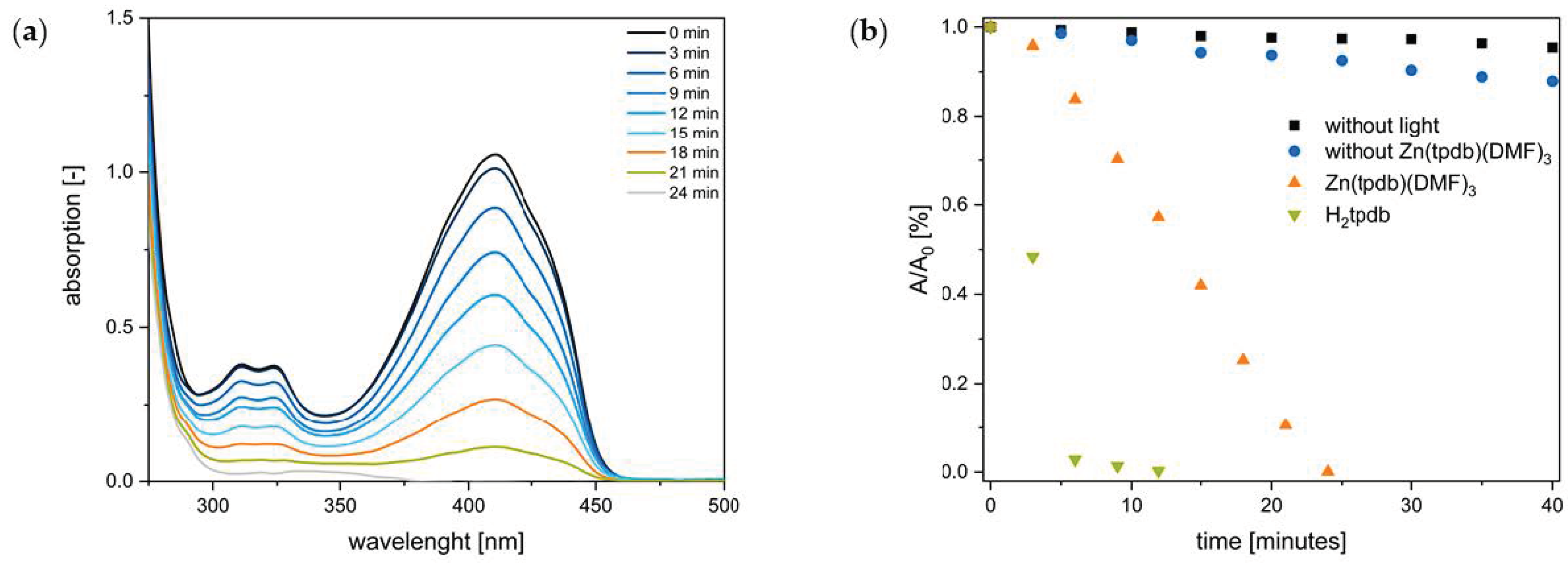
3.5. Photosensitization Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnett, T.P.; Pierce, D.W.; Schnur, R. Detection of Anthropogenic Climate Change in the World’s Oceans. Science 2001, 292, 270–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janna Olmos, J.D.; Kargul, J. A quest for the artificial leaf. Int. J. Biochem. Cell Biol. 2015, 66, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, C.P. The chemical mechanism of photosynthesis. Bot. Rev. 1952, 18, 245–290. [Google Scholar] [CrossRef]
- Michl, J. Towards an artificial leaf? Nat. Chem. 2011, 3, 268–269. [Google Scholar] [CrossRef]
- Nocera, D.G. The Artificial Leaf. Acc. Chem. Res. 2012, 45, 767–776. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metalorganic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Dhakshinamoorthy, A.; Asiri, A.M.; García, H. Metal–Organic Framework (MOF) Compounds: Photocatalysts for Redox Reactions and Solar Fuel Production. Angew. Chem. Int. Ed. 2016, 55, 5414–5445. [Google Scholar] [CrossRef]
- Medishetty, R.; Nemec, L.; Nalla, V.; Henke, S.; Samoć, M.; Reuter, K.; Fischer, R.A. Multi-Photon Absorption in Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2017, 56, 14743–14748. [Google Scholar] [CrossRef]
- Zhu, J.; Shaikh, S.; Mayhall, N.; Morris, A. Energy Transfer in Metal-Organic Frameworks. In Elaboration and Applications of Metal-Organic Frameworks; World Scientific: Singapore, 2018; Volume 2, pp. 581–654. [Google Scholar]
- Lephalala, M.; Kanchi, S.; Sabela, M.I.; Bisetty, K. Electrochemical Enzymatic Biosensing of Neotame Supported by Computational Methods. Electroanalysis 2020, 32, 2669–2680. [Google Scholar] [CrossRef]
- Sajjadi, S.; Khataee, A.; Bagheri, N.; Kobya, M.; Şenocak, A.; Demirbas, E.; Karaoğlu, A.G. Degradation of diazinon pesticide using catalyzed persulfate with Fe3O4@MOF-2 nanocomposite under ultrasound irradiation. J. Ind. Eng. Chem. 2019, 77, 280–290. [Google Scholar] [CrossRef]
- Şenocak, A.; Khataee, A.; Demirbas, E.; Doustkhah, E. Ultrasensitive detection of rutin antioxidant through a magnetic micro-mesoporous graphitized carbon wrapped Co nanoarchitecture. Sens. Actuators B Chem. 2020, 312, 127939. [Google Scholar] [CrossRef]
- Şenocak, A.; Tümay, S.O.; Ömeroğlu, İ.; Şanko, V. Crosslinker polycarbazole supported magnetite MOF@CNT hybrid material for synergetic and selective voltammetric determination of adenine and guanine. J. Electroanal. Chem. 2022, 905, 115963. [Google Scholar] [CrossRef]
- Kent, C.A.; Mehl, B.P.; Ma, L.; Papanikolas, J.M.; Meyer, T.J.; Lin, W. Energy Transfer Dynamics in Metal−Organic Frameworks. J. Am. Chem. Soc. 2010, 132, 12767–12769. [Google Scholar] [CrossRef]
- Würthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 2004, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- Troeger, A.; Ledendecker, M.; Margraf, J.T.; Sgobba, V.; Guldi, D.M.; Vieweg, B.F.; Spiecker, E.; Suraru, S.-L.; Würthner, F. p-Doped Multiwall Carbon Nanotube/Perylene Diimide Derivative Photoelectrochemical Cells for Photocurrent Generation. Adv. Energy Mater. 2012, 2, 536–540. [Google Scholar] [CrossRef]
- Zhao, F.-J.; Zhang, G.; Ju, Z.; Tan, Y.-X.; Yuan, D. The Combination of Charge and Energy Transfer Processes in MOFs for Efficient Photocatalytic Oxidative Coupling of Amines. Inorg. Chem. 2020, 59, 3297–3303. [Google Scholar] [CrossRef] [PubMed]
- Almuhana, A.R.Y.; Langer, P.; Griffin, S.L.; Lodge, R.W.; Rance, G.A.; Champness, N.R. Retention of perylene diimide optical properties in solid-state materials through tethering to nanodiamonds. J. Mater. Chem. C 2021, 9, 10317–10323. [Google Scholar] [CrossRef]
- Haddow, S.L.; Ring, D.J.; Bagha, H.; Pearce, N.; Nowell, H.; Blake, A.J.; Lewis, W.; McMaster, J.; Champness, N.R. Perylene Diimide Triple Helix Formation in the Solid State. Cryst. Growth Des. 2018, 18, 802–807. [Google Scholar] [CrossRef]
- Weissman, H.; Shirman, E.; Ben-Moshe, T.; Cohen, R.; Leitus, G.; Shimon, L.J.W.; Rybtchinski, B. Palladium Complexes of Perylene Diimides: Strong Fluorescence Despite Direct Attachment of Late Transition Metals to Organic Dyes. Inorg. Chem. 2007, 46, 4790–4792. [Google Scholar] [CrossRef]
- Dinçalp, H.; Kızılok, Ş.; İçli, S. Fluorescent macromolecular perylene diimides containing pyrene or indole units in bay positions. Dye. Pigment. 2010, 86, 32–41. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233-234, 351–371. [Google Scholar] [CrossRef]
- Tanaka, F.; Tsumura, K.; Furuta, T.; Iwamoto, K.; Okamoto, M. Efficiencies of singlet oxygen production and rate constants for oxygen quenching in the S1 state of dicyanonaphthalenes and related compounds. Photochem. Photobiol. Sci. 2008, 7, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Pibiri, I.; Buscemi, S.; Palumbo Piccionello, A.; Pace, A. Photochemically Produced Singlet Oxygen: Applications and Perspectives. ChemPhotoChem 2018, 2, 535–547. [Google Scholar] [CrossRef]
- Chen, J.; Keltner, L.; Christophersen, J.; Zheng, F.; Krouse, M.; Singhal, A.; Wang, S.-s. New Technology for Deep Light Distribution in Tissue for Phototherapy. Cancer J. 2002, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Liu, Y.; Wang, Z.; Wang, P.; Zheng, Z.; Cheng, H.; Dai, Y.; Huang, B. Enhanced singlet oxygen production over a photocatalytic stable metal organic framework composed of porphyrin and Ag. J. Colloid Interface Sci. 2021, 602, 300–306. [Google Scholar] [CrossRef]
- Ling, P.; Cheng, S.; Chen, N.; Gao, F. Singlet-oxygen generated by a metal–organic framework for electrochemical biosensing. J. Mater. Chem. B 2021, 9, 4670–4677. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jiang, Q.; Feng, D.; Zhou, H.-C. Controlled Generation of Singlet Oxygen in Living Cells with Tunable Ratios of the Photochromic Switch in Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2016, 55, 7188–7193. [Google Scholar] [CrossRef]
- Nelson, A.P.; Farha, O.K.; Mulfort, K.L.; Hupp, J.T. Supercritical Processing as a Route to High Internal Surface Areas and Permanent Microporosity in Metal−Organic Framework Materials. J. Am. Chem. Soc. 2009, 131, 458–460. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Swager, T.M. Fluorofluorescent perylene bisimides. Synlett 2018, 29, 2509–2514. [Google Scholar]
- Addicott, C.; Oesterling, I.; Yamamoto, T.; Müllen, K.; Stang, P.J. Synthesis of a Bis(pyridyl)-Substituted Perylene Diimide Ligand and Incorporation into a Supramolecular Rhomboid and Rectangle via Coordination Driven Self-Assembly. J. Org. Chem. 2005, 70, 797–801. [Google Scholar] [CrossRef]
- Würthner, F.; Saha-Möller, C.R.; Fimmel, B.; Ogi, S.; Leowanawat, P.; Schmidt, D. Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials. Chem. Rev. 2016, 116, 962–1052. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [Green Version]
- Lv, R.; Li, H.; Su, J.; Fu, X.; Yang, B.; Gu, W.; Liu, X. Zinc Metal–Organic Framework for Selective Detection and Differentiation of Fe(III) and Cr(VI) Ions in Aqueous Solution. Inorg. Chem. 2017, 56, 12348–12356. [Google Scholar] [CrossRef] [PubMed]
- Basu, U.; Otto, S.; Heinze, K.; Gasser, G. Biological Evaluation of the NIR-Emissive Ruby Analogue [Cr(ddpd)2][BF4]3 as a Photodynamic Therapy Photosensitizer. Eur. J. Inorg. Chem. 2019, 2019, 37–41. [Google Scholar] [CrossRef]
- Eder, T.; Stangl, T.; Gmelch, M.; Remmerssen, K.; Laux, D.; Höger, S.; Lupton, J.M.; Vogelsang, J. Switching between H- and J-type electronic coupling in single conjugated polymer aggregates. Nat. Commun. 2017, 8, 1641. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, B.D.; Hontz, E.R.; Yost, S.R.; Van Voorhis, T.; Dincă, M. Charge Transfer or J-Coupling? Assignment of an Unexpected Red-Shifted Absorption Band in a Naphthalenediimide-Based Metal–Organic Framework. J. Phys. Chem. Lett. 2013, 4, 453–458. [Google Scholar] [CrossRef]
- Herbst, S.; Soberats, B.; Leowanawat, P.; Stolte, M.; Lehmann, M.; Würthner, F. Self-assembly of multi-stranded perylene dye J-aggregates in columnar liquid-crystalline phases. Nat. Commun. 2018, 9, 2646. [Google Scholar] [CrossRef] [Green Version]
- Gierschner, J.; Lüer, L.; Milián-Medina, B.; Oelkrug, D.; Egelhaaf, H.-J. Highly Emissive H-Aggregates or Aggregation-Induced Emission Quenching? The Photophysics of All-Trans para-Distyrylbenzene. J. Phys. Chem. Lett. 2013, 4, 2686–2697. [Google Scholar] [CrossRef]
- Feng, X.; Wang, X.; Wang, H.; Wu, H.; Liu, Z.; Zhou, W.; Lin, Q.; Jiang, J. Elucidating J-Aggregation Effect in Boosting Singlet-Oxygen Evolution Using Zirconium–Porphyrin Frameworks: A Comprehensive Structural, Catalytic, and Spectroscopic Study. ACS Appl. Mater. Interfaces 2019, 11, 45118–45125. [Google Scholar] [CrossRef]
- He, L.; Cai, L.-X.; Li, M.-H.; Zhang, G.-L.; Zhou, L.-P.; Chen, T.; Lin, M.-J.; Sun, Q.-F. Designing a highly stable coordination-driven metallacycle for imaging-guided photodynamic cancer theranostics. Chem. Sci. 2020, 11, 7940–7949. [Google Scholar] [CrossRef]
- Blacha-Grzechnik, A.; Drewniak, A.; Walczak, K.Z.; Szindler, M.; Ledwon, P. Efficient generation of singlet oxygen by perylene diimide photosensitizers covalently bound to conjugate polymers. J. Photochem. Photobiol. A Chem. 2020, 388, 112161. [Google Scholar] [CrossRef]
- Meng, A.-N.; Chaihu, L.-X.; Chen, H.-H.; Gu, Z.-Y. Ultrahigh adsorption and singlet-oxygen mediated degradation for efficient synergetic removal of bisphenol A by a stable zirconium-porphyrin metal-organic framework. Sci. Rep. 2017, 7, 6297. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.M.; Silva, P.R.; Vono, L.L.R.; Fernandes, A.U.; Tada, D.B.; Baptista, M.S. Protoporphyrin IX Nanoparticle Carrier: Preparation, Optical Properties, and Singlet Oxygen Generation. Langmuir 2008, 24, 12534–12538. [Google Scholar] [CrossRef] [PubMed]
- Hynek, J.; Chahal, M.K.; Payne, D.T.; Labuta, J.; Hill, J.P. Porous framework materials for singlet oxygen generation. Coord. Chem. Rev. 2020, 425, 213541. [Google Scholar] [CrossRef]
- Wahlen, J.; De Vos, D.E.; Jacobs, P.A.; Alsters, P.L. Solid Materials as Sources for Synthetically Useful Singlet Oxygen. Adv. Synth. Catal. 2004, 346, 152–164. [Google Scholar] [CrossRef]




| MOF Name | Linker | Metal Salt | Temperature | Solvent | Additives | Refs. |
|---|---|---|---|---|---|---|
| MOF-5, IRMOF(2-20) | R1-7-BDC, 2,6-NDC, BPDC, HPDC, PDC, TPDC | Zn(NO3)2 | 85° to 105 °C | (DMF/DEF), chlorobenzene | H2O2, NEt3 | [6,33] |
| [Zn2(TPOM)(NDC)2] | TPOM, H2NDC | Zn(NO3)2 | 100 °C | DMF | H2O | [34] |
| {[Zn(μ-4-hzba)2]2·4(H2O)}n | 4-hydrazinebenzoic acid | Zn(OAc)2 | 110 °C | EtOH | H2O | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deger, S.N.; Weishäupl, S.J.; Pöthig, A.; Fischer, R.A. A Perylenediimide-Based Zinc-Coordination Polymer for Photosensitized Singlet-Oxygen Generation. Energies 2022, 15, 2437. https://doi.org/10.3390/en15072437
Deger SN, Weishäupl SJ, Pöthig A, Fischer RA. A Perylenediimide-Based Zinc-Coordination Polymer for Photosensitized Singlet-Oxygen Generation. Energies. 2022; 15(7):2437. https://doi.org/10.3390/en15072437
Chicago/Turabian StyleDeger, Simon N., Sebastian J. Weishäupl, Alexander Pöthig, and Roland A. Fischer. 2022. "A Perylenediimide-Based Zinc-Coordination Polymer for Photosensitized Singlet-Oxygen Generation" Energies 15, no. 7: 2437. https://doi.org/10.3390/en15072437
APA StyleDeger, S. N., Weishäupl, S. J., Pöthig, A., & Fischer, R. A. (2022). A Perylenediimide-Based Zinc-Coordination Polymer for Photosensitized Singlet-Oxygen Generation. Energies, 15(7), 2437. https://doi.org/10.3390/en15072437






