Abstract
The hydrolysis of aluminum (Al) is a promising method for on-demand hydrogen generation for low-power proton exchange membrane fuel cell (PEMFC) applications. In this study, Al composites were mechanochemically activated using bismuth (Bi) and nickel (Ni) as activation compounds. The main objective was to determine the effects of Bi and Ni on Al particles during mechanochemical processing, and the hydrolysis activity of the Al-Bi-Ni composites. Successfully formulated ternary Al-Bi-Ni composites were hydrolyzed with de-ionized water under standard ambient conditions to determine the reactivity of the composite (extent of hydrogen production). Scanning electron microscopy (SEM) showed that Bi and Ni were distributed relatively uniformly throughout the Al particles, resulting in numerous micro-galvanic interactions between the anodic Al and cathodic Bi/Ni during hydrolysis reaction. The addition of >1 wt% Ni resulted in incomplete activation of Al, and such composites were non-reactive. All successfully prepared composites had near-complete hydrogen yields. X-ray diffraction (XRD) showed that no mineralogical interaction occurred between Al, Bi, and/or Ni. The main phases detected were Al, Bi, and minute traces of Ni (ascribed to low Ni content). In addition, the effect of the mass ratio (mass Al:mass water) and water quality were also determined.
Keywords:
aluminum; bismuth; nickel; hydrolysis; hydrogen generation; ball-milling; mechanochemical activation 1. Introduction
The growing society places more pressure on the use of carbon-based fuels as an energy carrier due to environmental implications [1,2,3]. This has increased the necessity to replace carbon-based fuels with renewable energy sources such as hydrogen. Hydrogen is considered as an ideal replacement due to its high mass-specific energy (lower heating and higher heating values of 120 and 142 MJ/kg, respectively) and environmentally friendly properties [4,5]. Furthermore, hydrogen is not destroyed when reacted with oxygen and only yields water and energy [6,7,8,9,10,11], unlike hydrocarbon energy carriers that decompose when oxidized [12].
Hydrogen can be generated by numerous processes: formic acid and ammonia decomposition [13,14,15,16,17,18,19,20,21,22,23], photochemical [24,25,26], photocatalytic [27,28,29,30], photo-electrochemical [31,32,33,34], electrochemical [35,36,37,38,39,40,41,42], thermochemical [43,44,45,46], metal and metal hydride hydrolysis [47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75], and various biological processes [76,77,78,79,80]. In addition, numerous niche energy sources and processes can be coupled to generate hydrogen. For instance, coupling a photochemical and thermoelectric system with an electrolyzer [81,82], combining a thermoelectric generator with a photoelectrochemical cell [81,83], and combining the photocatalytic activity of perovskite and the piezoelectric effect to obtain a synergetic piezo-photocatalyst [84].
Non-renewable sources are, however, predominantly used to produce hydrogen [85,86]. Only 4% of hydrogen is produced from water electrolysis, while non-renewable sources, such as natural gas, oil, and coal, account for the balance [86]. The storage and transportation of hydrogen are difficult processes. It is known that steel tanks used to store hydrogen are susceptible to hydrogen embrittlement, which can cause hydrogen to leak from the tank [87,88,89]. The storage and transport of hydrogen are further complicated due to its relatively high liquidous density (70.9 kg/m3) and low gaseous density (0.09 kg/m3) [4], flammability (over a concentration range of 4–75 vol%), and low ignition energy (0.02 to 0.03 mJ) [90]. Here, it is proposed that hydrogen be generated on-site, as it is required, to alleviated perplexities related to its storage and transport.
Several studies have shown that aluminum (Al) can react with an aqueous solution to produce on-demand hydrogen while achieving a high theoretical hydrogen yield of 1360 mL/g of Al under standard ambient conditions. For certain applications, reacting Al with an aqueous solution to generate hydrogen can curtail the issues related to hydrogen storage and transportation [74,75,91,92,93,94].
In addition, Al has several attractive properties, such as its high energy density (29 MJ kg−1), low cost, abundance, recyclability, and light weight (2700 kg m−3 density value). The light weight of Al enables it to be used in portable energy systems where it can supply hydrogen as an energy carrier through hydrolysis [95,96,97]. Furthermore, no CO2 is generated during this hydrolysis reaction, thus having a minor impact on the environment. More so, Pt poisoning of CO is also absent [74,98]. During the spontaneous Al hydrolysis reaction, hydrogen and heat are generated. Reactions (1) and (2) show the hydrolysis reaction of Al:
2Al + 6H2O → 2Al(OH)3 + 3H2 + 16.3 MJ/kg Al
2Al + 4H2O → 2AlOOH + 3H2 + 15.5 MJ/kg Al
In addition to hydrogen generation, the Al–hydroxides formed by the hydrolysis of Al can be used in various industrial processes [99]. More so, the water-insoluble Al–hydroxides can be transformed into alumina (Al2O3), which can be metalized to Al through the energy-intensive Hall–Héroult process [93,100]. Reactions (1) and (2) can be performed in different water types such as seawater, NaCl concentrated water, tap, filtered, de-ionized water, and urine [47,101,102,103].
Nevertheless, the surface of Al is easily oxidized to form a thin and coherent alumina layer. This alumina layer limits the progression of the Al hydrolysis reaction as it blocks the interaction between Al and water [104,105,106]. Several studies have focused on finding a suitable physical or chemical method to disrupt the alumina layer on the Al surface. The Al can be activated by removing the alumina layer through amalgamation. During amalgamation, the Al surface is moistened by mercury (Hg) or gallium (Ga). In addition, Hg and Ga spontaneously induce structural embrittlement of Al particles/structures. Such action will disintegrate the metal; this is known as the Rehbinder effect. Nevertheless, Hg is hazardous, as it is a toxic element, and the utilization of Ga is expensive [107].
Al can also be activated through high-temperature oxidation; however, the method is expensive, and complex equipment is needed for the oxidation process [107]. Another approach is to disrupt the alumina layer by submerging it in alkaline [88] or acidic [108] solutions. This method is perplexed by the corrosiveness of the solutions, and the reaction depends on the solution’s pH value (pH outside 5–9 is ideal for Al de-passivation) [106]. Continuous hydrogen generation through Al hydrolysis can also be achieved by the processes of oxidation of Al electro-explosive ultra-disperse powders (UDP). During this process, Al particles are reduced to tens and hundreds of nm to increase the specific surface area, which subsequently increases the reactivity of Al during hydrolysis. However, electro-explosive UDP is extremely reactive at temperatures between 200–500 °C and highly reactive at <200 °C when in contact with liquid reagents [109,110].
Of particular importance in this study was the use of mechanochemical processing, i.e., ball-milling, to enable Al’s reactivity with neutral pH waters under standard ambient conditions. Combining Al with various activation compounds (e.g., inorganic salts, low melting point metals) has received attention in recent years to enable Al’s spontaneous hydrolysis. Several activated Al composites are listed here: binary composites, Al-x (x = combinations of Ga, Bi, In, Sn, Zn, Mg, Cu, Li, Fe, Ti) [74,99,102,111,112,113,114], Al-BaCl2 [115], Al-KCl [116], Al-BiOCl [117], Al-NaBH4 [118], Al-NaMgH3, Al-Al(OH)3 [119], Al-Fe [120]; ternary composites, Al-Bi-hydride/salt [121], Al-Ni-NaCl [122], Al-Bi-C [123], Al-InCl3-(Ni-Bi-B) [124], Al-Zn-B [125], Al-Bi-Sn [74], Al-Bi-Zn [126], Bi-Ga-Zn [127], Al-Ga-In [102]; quaternary composites, Al-Ga-In-SnCl2 [128], Al-In-Bi-Sn [73], Al-NaMgH3-Bi-Li3AlH6 [129], Ga-In-Sn-Zn [107], and technical grade Al, Al-6061 (contains mainly Mg and Si) [130], and Al-Ga-In-Sn-KCl [92]. A comprehensive summary of mechanochemically activated Al composites was presented by Du Preez and Bessarabov (2021) [12].
Under certain conditions, activated Al can systematically generate hydrogen by the Al hydrolysis reaction under standard ambient conditions. For this reason, the hydrolysis of Al is an attractive approach to supply PEMFCs powered devices with a steady stream of hydrogen. In addition, the generated hydrogen is pure, allowing such PEMFCs to retain their performance (i.e., absence of CO(g) in hydrogen stream) [131]. PEMFCs are typically used for road vehicles, mobile energy applications (e.g., unmanned aerial vehicles), low-power portable electronic devices, and stationary power generation [131,132,133]. It is, however, proposed that activated Al be used for niche, low-power (<1 kW) applications.
Significant research and development are necessary to employ Al composites for hydrogen generation in real-world applications. Nevertheless, generalized goals are to avoid the use of expensive (e.g., Ga) and toxic (e.g., Hg) activation compounds and corrosive hydrolysis environments (acidic or alkaline), while preserving the stability of the Al composite (e.g., prolonged shelf-life). In addition, rapid hydrogen generation may also be considered adverse, as excessively high hydrogen generation rates may result in complexities during PEMFC operation. It is proposed that hydrogen release be controlled by the incorporation of certain activation compounds and/or by varying the reaction conditions, e.g., water temperature and water:composite ratio [88,134].
Wang et al. (2013) focused on hydrogen production from the mechanochemically prepared Al-3% Ga-3% In-5% Sn composite and concluded that a near-100% hydrogen yield was achieved at a hydrogen generation rate of 1080 mL/g min [102]. Wang et al. (2016) used an Al-1% Ga-3% In-6% Sn composite, containing less Ga, while also achieving high hydrogen yields [135]. These studies showed appreciable hydrogen yields at systematic rates; however, the addition of even the slightest amount of Ga in the Al composite will elevate the production cost. This promotes the necessity to investigate less expensive activation compounds such as Bi, Ni, Sn.
Several studies investigated the exclusion of expensive activation compounds, such as Ga, from Al composites for hydrogen production. Du Preez et al. (2017) investigated the addition of Bi into Al-In and Al-In-Sn composites and achieved hydrogen yields of 80–95% and >90%, respectively [73,94]. Du Preez et al. (2018) used Sn and In as activation compounds in ternary Al composites and achieved hydrogen yields of >95% [75]. Du Preez et al. (2019) conducted a study with Al-Bi-Sn composites containing 5 and 10 wt % activation compounds and obtained a hydrogen yield of >95% [74]. Davies et al. (2022) showed the hydrolysis of Al-Bi-Zn composites containing 7.5 wt% Bi and 2.5 wt% Zn achieved a hydrogen yield of 99.5% [126].
Hence, it is known that Bi (a low-cost activation compound) is a suitable activation compound [73,127,134,136,137,138,139]. However, the addition of a secondary activation compound, in addition to Bi, is relatively unexplored. Therefore, in this investigation, ball-milled Al-Bi-Ni composites were prepared and hydrolyzed to evaluate composite reactivity in neutral pH water under standard ambient conditions. The effects of Bi and Ni on the morphological and chemical characteristics of Al were considered.
2. Material and Methods
2.1. Materials
The materials used in this study was purchased from Sigma–Aldrich (Johannesburg, South Africa): Al powder (<200 mm, 95% purity), Bi granules (>99.9% purity), and Ni powder (<50 mm, >99.7% purity). Hydrolysis reactions were conducted using de-ionized (18.2 MΩ cm−1 resistivity), filtered, or tap water. Filtered water was generated by a five-stage water filtration system. De-ionized water was generated by a Milli-Q water purification system. Pure nitrogen gas (99.9%; Afrox, Potchefstroom, South Africa) was used for all purging procedures.
2.2. Composite Constituency and Mechanochemical Activation
All ternary composites comprised of 90 wt% Al, and varying amounts of Bi and Ni collectively accounted for 10 wt% of the composite. During milling procedures, the total amount of composite was kept to 5 g. Table 1 summarizes the compositions prepared in this study.

Table 1.
Binary (Al-Bi) and ternary Al composite (Al-Bi-Ni) compositions (wt%).
A high-energy mechanochemical activation process was employed using a Retsch Emax ball mill (Retsch, Düsseldorf, Germany) to prepare the Al composites shown in Table 1. Milling was performed in a nitrogen atmosphere. The Al, Bi, and Ni were weighed and placed in a 250 mL stainless steel jar with 5 mm diameter stainless steel milling balls. A mass ratio (defined as mass milling balls per mass composite) of 30:1 was employed. The jar was purged with nitrogen after enclosing the jar with an aerated stainless steel lid. Milling was performed at 1500 rpm for 30 min. After milling, the jar was allowed to cool to room temperature before recovering the composite.
After retrieving the composite from the jar, the hydrolysis reactions were performed to avoid unwanted atmospheric oxidation. All successfully milled (defined as a composite that was in a powder form after milling) composites were prepared in duplicate. It is noted here that the Al-8% Bi-2% Ni composite was not recovered as a powder, showed no hydrolysis reactivity, and was excluded from further experimentation.
2.3. Hydrolysis Set-Up and Hydrogen Measurements
The composites’ reactivity was established by performing hydrolysis reactions in a three-neck flask (250 mL) under standard ambient conditions. The flask ports were used as a thermocouple port, a hydrogen escape, and to add water for the hydrolysis reaction. Generated hydrogen was first dried by passing it through a gas drier comprised of DrieriteTM before hydrogen measurements. The volume of dried generated hydrogen was measured using a digital gas mass flow meter (Model GM-32654-12; Cole-Parmer, Johannesburg, South Africa). Unless specified otherwise, hydrogen measurements were carried out by reacting 1 g composite and 50 mL of water. During hydrolysis, the water was not stirred or ultrasonicated.
Generated hydrogen was expressed as a yield %, defined as the measured hydrogen over a theoretical volume of obtainable hydrogen. By applying the ideal gas law, approximately 1360 mL hydrogen per gram of Al is obtainable after complete hydrolysis under standard ambient conditions.
All hydrolysis reactions were performed in triplicate. To avoid overpopulating hydrogen yield graphs, the standard deviations are not included as part of the respective graphs, and only the average values are reported. It is, however, noted here that the maximum hydrogen yield standard deviation did not exceed 1.9%.
2.4. Composite Characterization
A Röntgen diffraction system (PW3040/60 X’Pert Pro) was employed for X-ray diffraction (XRD) analysis. A back-loading preparation method was applied to investigate the crystalline phases present in the Al composites and hydrolysis residues. Measurements were performed using a multi-purpose X-ray diffractometer D8-Advance from Bruker operated in a continuous θ–θ scan in locked coupled mode with Cu-Ka radiation. The sample was mounted in the center of the sample holder on a glass slide and leveled up to the correct height. A range of 2θ with a typical step size of 0.034° was used to run measurements. A Lyn-Eye (position-sensitive detector) was used to record diffraction data at a usual speed of 0.5 s/step, which is equivalent to an effective time of 92 s/step for a scintillation counter.
A X’Pert HighScore Plus software (PANalytical, Malvern, UK) was used to identify the detected phases. The morphology and/or chemical characterization of the as-received Al powder and Al composites were analyzed by using scanning electron microscopy (SEM) equipped with an energy dispersive X-ray spectrometer. An FEI Quanta 250 FEG scanning electron microscope incorporated with an Oxford X-map energy dispersive X-ray spectrometer system, operating at 7 kV and a working distance of approximately 10 mm, was used. Samples chosen for investigation were mounted on a sample stub using a carbon-based adhesive tape and coated with a thin layer of gold–palladium of about 3 nm. SEM micrographs were detected at various magnifications (µm scale), which is indicated on all micrographs presented. SEM–EDS was employed to determine the distribution of activation compounds on the surface.
3. Results and Discussion
3.1. Effects of Bi and Ni on Al during Ball-Milling
During the ball-milling of ductile, malleable, and/or brittle metal particles, these particles are subjected to repetitive forces that induce a variety of deformation and reformation mechanics, during which radical changes in the particle shape, residual stresses, and re-distribution of the metal constituents occur. For the major composite constituent, Al, the mechanics initiate with the onset of ball-milling as particles are caught between the impacts of the ball–ball and ball-milling chamber (milling equipment), which cause the particles to cold weld. As a result, larger coagulated particles, comprising of an unequal and random distribution of the initial constituents (Al, Bi, and Ni), are formed. As the milling process continues, the coagulated particles will undergo work hardening as a result of repetitive plastic deformation. After a certain period of milling, the work-hardened particles reach a stress-to-strain peak. Hereafter, particles no longer deform and start to fracture into non-agglomerating particles. In addition to this, the average particle size decreases to a certain size without undergoing further particle size reduction, regardless of milling time [73,74,75,140,141].
The different activation compounds added during the ball-milling of Al each have a different effect on the behavior of Al particles. Of importance here was the joint effect of Bi and Ni on Al’s morphological changes (Figure 1).

Figure 1.
Secondary electron SEM micrographs of as-received Al (a) and mechanochemically processed Al-10% Bi (b), Al-9.5% Bi-0.5% Ni (c), Al-9.25% Bi-0.75% Ni (d), and Al-9% Bi-1% Ni (e) composite particles.
It is evident from Figure 1 that the as-received Al particles (Figure 1a) transformed from an uneven, strand-like morphology to a platelet morphology with a decrease in the average particle size when Bi and Ni were added during ball-milling (Figure 1b–e). The as-received Al had a particle size between 100–300 µm, while the Al-10% Bi composite consisted of small, fractured particles of 90–258 µm. The addition of Ni, and subsequent reduction in Bi addition, to the Al composite, yielded composites with particle sizes smaller than that of the Al-10% Bi composites. The majority of particles observed for the Ni-containing composites were ≤94 µm. The reduction in particle size is ascribed to the increase in average particle size during cold welding, followed by the decrease in average particle size during fracturing.
Though some large particles were observed (marked with red circle in Figure 1c), these particles were aggregates of smaller particles. The presence of these aggregates in the composites is indicative of the presence of an equilibrium between particle fracturing and fractured particle aggregation—which can be considered as an indication that it is unlikely that further ball-milling will reduce the average particle size further [12,73,74].
In all cases, the ball-milled particles remained in their solid states, and no traces of particle softening occurred. This was, however, expected, as the use of diffusive chemicals, e.g., Hg and Ga, were avoided.
It is worth noting that the reduction in particle size alone will not ensure Al’s hydrolysis activity. For the hydrolysis reaction to proceed spontaneously, the protective oxide layer has to be disrupted or removed to expose the underlying fresh Al to water. This is made possible when the activation compounds (Bi and Ni) are dispersed equally over the entire surface of the Al particles, which prevents contact between freshly exposed metallic Al and ambient O, as well as the formation of micro-galvanic cells between anodic Al and cathodic activation compounds. These cells are responsible for maintaining the Al hydrolysis reaction until the available Al is hydrolyzed, as explained in the following text.
Micro-galvanic cells are formed when the standard electrode potentials, i.e., E° (V), of the activation compounds are more positive than the E° (V) of Al (−1.662 V). Hence galvanic coupling between anodic Al and cathodic Bi and Ni will be possible due to the E° of Bi and Ni being 0.308 V and −0.260 V, respectively. The formation of these micro-galvanic cells will cause Al0 to oxidize to Al3+, from where it will be released into the aqueous phase during hydrolysis.
Reboul et al. (1984) proposed a mechanism explaining the dissolution of Al by activation compounds. Reaction 3 shows the oxidation of the activation compound through galvanic coupling [142]:
Al(M) → Al3+ + Mn+, M = activation compound
The Mn+ has a cathodic nature relative to Al, causing the cathodic activation compound to redeposit onto fresh Al surfaces via an electrochemical exchange reaction. The repositioning occurs as the following reaction:
Al + Mn+ → Al3+ + M
Hence, the oxidation of Al to Al3+ causes the Al ions to migrate from the solid matrix into the hydrolysis solution.
The distributions of Bi and Ni in Al particles were determined by SEM–EDS mapping of the cross-sectioned Al-9.25% Bi-0.75% Ni composite particles (Figure 2).
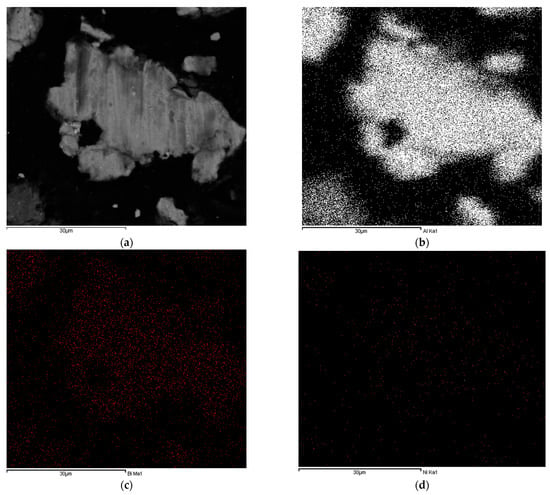
Figure 2.
Backscatter SEM micrograph of a cross-sectioned Al-9.25% Bi-0.75% Ni composite particle (a) and the corresponding EDS mappings for Al (b), Bi (c), and Ni (d).
It is evident from Figure 2 that Bi and Ni were present in Al particles and that they were relatively distributed throughout the Al particles. Therefore, the formation of micro-galvanic cells in the composite is likely due to the intimate proximity of Al and the activation compounds. The occurrence of uniformly distributed activation compounds throughout the Al particles ensures continuous galvanic couplings between anodic Al and cathodic Bi/Ni. Furthermore, no Bi or Ni agglomerates or chunks were observed in Figure 2c, d. It is, however, worthy to note that the intensity of the Ni mapping (d) is not as significant as for Bi (b) due to the relatively low Ni wt% (i.e., 0.75 wt%) content of the composite.
The solid solubility of Ni in Al is very low (i.e., <0.04%) at room temperature, while Bi has no appreciable solid solubility in Al [143,144,145]. Nevertheless, mechanochemical processing does not rely on such solubilities, as Bi and Ni are physically distributed in the Al particles due to the composite constituency homogenization mechanisms discussed earlier in this section. Therefore, mechanochemical processing foregoes the limitations of element–element solubilities.
3.2. Hydrolysis of Ternary Al-Bi-Ni Composites
The Al-Bi-Ni composites (see Table 1) were hydrolyzed to explore the effects of Bi and Ni on the hydrolysis activity of Al. The hydrogen yields of these ternary composites are presented in Figure 3. The effect of Ni addition was compared to the reference composite Al-10% Bi in Figure 3. Standard deviations are not included in the experimental results to avoid overpopulating the graph. The maximum hydrogen yield standard deviation did not exceed 1.9%.
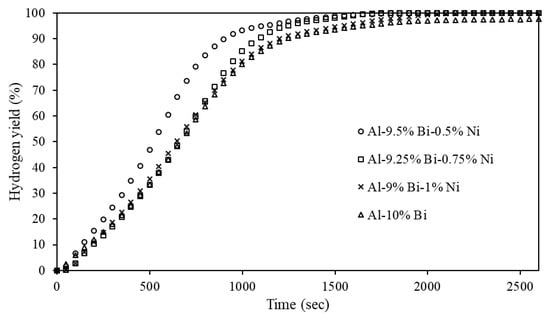
Figure 3.
Hydrogen yields of Al-10% Bi and Al-Bi-Ni composites.
Figure 3 shows that the addition of Ni to the Al composite increased the hydrogen yield from 97.4% (for the Al-10% Bi reference) to >99% (in all cases). A study by Du Preez and Bessarabov showed that binary Al-Bi composites had an optimal Bi addition (i.e., 15 wt%) to obtained composites with high hydrolysis activity; Bi additions above or below this amount reduced the hydrolysis activity of the composite [94]. In addition to showing that Ni accelerates the corrosion of Al, Jia et al. (2014) made a similar observation to Du Preez and Bessarabov (2017) for binary Al-Ni composites, i.e., adding Ni beyond a certain value reduces the composite’s hydrolysis reactivity [122]. Du Preez and Bessarabov (2017) showed that increasing or decreasing the Bi and Sn contents of 7.5 and 2.5 wt%, respectively, yielded composites with decreased hydrolysis activity [84]. Similarly, Du Preez and Bessarabov (2018) showed that increasing or decreasing the Sn and In contents of 3.5 and 6.5 wt%, respectively, decreased the hydrolysis activity of the respective composites [69].
It can be seen from Figure 3 that a 0.5 wt% Ni addition (and 0.5 wt% Bi reduction) accelerated the hydrolysis reaction. Further increasing the Ni content >0.5 wt% reverted the hydrolysis activity to a rate similar to Al-10% Bi while maintaining a >99% hydrogen yield.
It is noted that composites containing >1 wt% Ni (i.e., Al-8% Bi-2% Ni) yielded inactive composites due to the inability of the activation compounds to induce structural degradation of the Al particles. Considering this, it is clear that a certain degree of synergy exists between Bi and Ni as activation compounds and that an optimal Bi and Ni addition exists to obtain a composite with appreciable hydrolysis activity. This observation is comparable to results presented by Du Preez and Bessarabov (2017; 2018) [69,84].
However, it is currently unclear if the effect of Bi and Ni on the morphological changes of Al or if the galvanic activity of the various Bi/Ni additions is the determining factor in the hydrolysis activity of composites. It is therefore proposed that future research determines the effect of Bi:Ni ratios on the galvanic activity of Al-Bi-Ni composites.
Nevertheless, Ni-containing ternary Al composites, mechanochemically activated and intended for on-demand hydrogen generation via hydrolysis using neutral pH waters under standard ambient conditions, are relatively unexplored. For a general comparison, the composites shown in Figure 3 had comparable or improved hydrolysis activity when compared to other Bi-containing ternary composites (Al-Bi-x; x = Sn, Ga, Zn, In) hydrolyzed in neutral pH waters under standard ambient conditions [47,73,74,127]. It is therefore evident that up to 1 wt% Ni can be employed as an Al activation metal.
3.3. XRD Analysis of Ternary Al Composites
XRD analysis was performed on ternary Al composites to investigate the mineralogical composition of the Al composites (Figure 4). According to Figure 4, the Al composites had similar XRD patterns: the presence of the Ni-rich phase and the intensity of the Bi-rich phases was all that set the patterns apart from one another. This was expected considering the various amounts of Bi and Ni added to the composite. However, this is not all that influences the peak intensity, but also the particle size and degree of crystallinity.
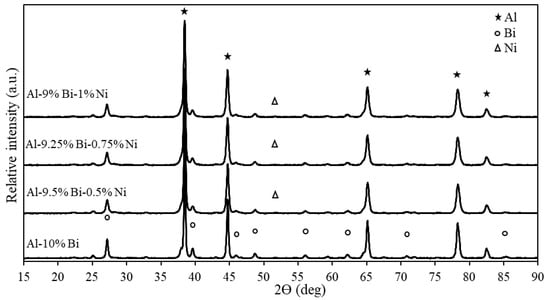
Figure 4.
XRD patterns of binary Al-Bi and ternary Al-Bi-Ni composites.
It is evident from Figure 4 that Al, Bi, and Ni were the only detected phases, indicating that no intermetallic phases formed between Al, Bi, and Ni during the mechanochemical processing of the Al composites. This could be ascribed to the low solubility of Ni and Bi in Al [143,144,145]. Furthermore, the small Ni-rich phase in the 2θ peaks could be due to the small amount of Ni (≤1 wt%) added to the Al composite. A similar small Ni-rich phase was observed by Jia et al. (2014) while investigating hydrogen production through ball-milled Al-Ni-NaCl mixtures. However, the Ni-rich phase peak intensified as the Ni addition to the Al-Ni-NaCl composite increased from 1–4 wt% [122].
It is further noted that due to the lack of inter-elemental activity, the prepared composites are not susceptible to chemical decomposition. More so, when jointly considering Figure 2 and Figure 4, the composite particles comprise of homogenized starting elements in a solid phase. If proper storage precautions are in place, e.g., contact with ambient atmospheric moisture is prevented, the composite particles can be stored for extensive periods.
3.4. Effects of Mass Ratio on the Hydrolysis Reaction
When considering Reactions (1) and (2), it can be seen that the hydrolysis reaction is exothermic, and a relatively large amount of heat is generated during the reaction. If the reaction heat accumulates (i.e., reaction heat is not managed/displaced), it will over-accelerate the hydrolysis reaction and, subsequently, the hydrogen generation rate.
As shown in Figure 3, the hydrolysis reactivity of Al-9.25% Bi-0.75% Ni was between that of the Al-9.5% Bi-0.5% Ni and Al-9% Bi-1% Ni composites. The Al-9.25% Bi-0.75% Ni composite was therefore selected to determine the effects of mass ratio on the composite reactivity (Figure 5). The mass ratios considered ranged from 1:20 to 1:125.
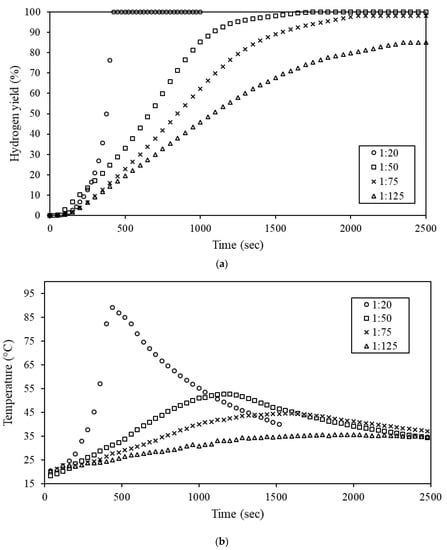
Figure 5.
Effect of mass ratio (1:20 to 1:125) on the hydrogen yield of Al-9.25% Bi-0.75% Ni (a) and the change in reaction temperature during hydrolysis (b).
It is evident from Figure 5a that elevating the mass ratio from 1:20 to 1:125 caused a steady decrease in the reactivity of the Al-9.25% Bi-0.75% Ni composite. Mass ratios of 1:20 and 1:50 had hydrogen yields of >99%, whereas 1:75 and 1:125 achieved a hydrogen yield of 98.1% and 85.1%, respectively. Therefore, it is clear that reducing the mass ratio to 1:20 did not hold any benefit in increasing the hydrogen yield, whereas increasing the ratio to 1:75 did not have an appreciable effect (i.e., approximately 1.8% decrease) on the yield. Further increase in the mass ratio to 1:125 showed a significant reduction in the hydrogen yield when compared to the 1:50 mass ratio. Therefore, when considering the hydrogen yield (and not the reaction temperature), mass ratios of 1:20 to 1:75 may be considered. Figure 5b shows a significantly higher increase in reaction temperature for the 1:20 mass ratio than for the 1:125 mass ratio; the reaction temperature increase for the 1:20 and 1:125 mass ratios was approximately 68.0 ± 9.3 °C and 15.5 ± 1.6 °C, respectively. Studies by Du Preez et al. (2017) and Du Preez et al. (2018) showed similar results during the hydrolysis of Al-In-Sn and Al-Bi-In composites under comparable experimental conditions [73].
When jointly considering Figure 5a, b, it is evident that the hydrolysis reaction rate can be controlled to a certain degree by varying the mass ratio. This is ascribed to in situ reaction heat being dispersed into a larger volume of water, e.g., at a mass ratio of 1:125, a relatively large volume of water is available for reaction heat to be dispersed in when compared to a 1:25 mass ratio. The dispersion of reaction heat slows the hydrolysis reaction, as it is highly dependent on the reaction temperature [73]. Gai et al. (2012) stated that during hydrolysis, hydrogen bubbles accumulate between an Al2O3 layer (passivation layer) and the underlying Al. The hydrogen bubbles rupture the passivation layer when they exceed the critical gas pressure that the passivation layer can sustain. The bubbles are only released from the Al particle when the bubbles rupture the passivation layer [146]. The hydrolysis reaction proceeds when the passivation layer is ruptured as fresh Al is exposed to water. However, if the rate of passivation exceeds the hydrogen generation rate (i.e., the passivation layer becomes too thick for the hydrogen bubbles to rupture it), the hydrolysis reaction ceases. Therefore, by increasing the mass ratio, the hydrogen generation rate is slowed to the point where the particles are pacified before they are consumed by the hydrolysis reaction.
The large increase in reaction temperature did lead to an increase in hydrogen generation rate, which can be seen in Figure 6. The effect of mass ratio on the hydrogen generation rate of the Al-9.25% Bi-0.75% Ni composite was determined and illustrated in Figure 6.
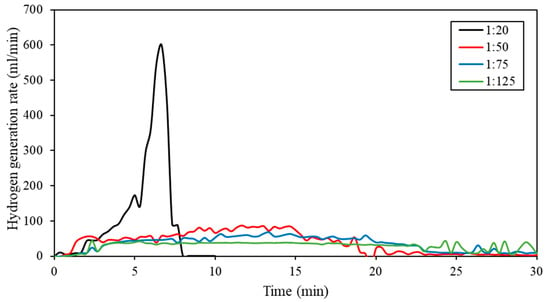
Figure 6.
Hydrogen generation rates of Al-9.25% Bi-0.75% Ni composite hydrolyzed at different mass ratios (1:20 to 1:125).
Figure 6 shows that the maximum hydrogen generation rate at a 1:20 mass ratio was 600 mL/min, which was significantly higher compared to the larger mass ratios of 1:50 (87.5 mL/min), 1:75 (67 mL/min), and 1:125 (43.3 mL/min). The rapid hydrogen generation rate at the 1:20 mass ratio is ascribed to the significant increase in reaction temperatures, which accelerated the hydrolysis reaction. The maximum hydrogen generation rate at a 1:20 mass ratio was achieved at approximately 6.5 min, which is significantly faster than at a 1:125 mass ratio where the maximum hydrogen generation rate was obtained at 24 min. Mass ratios of 1:50, 1:75, and 1:125 all had similar curves, which were significantly flatter than the curve observed for the mass ratio of 1:20. The flatter curve suggests a systematic hydrogen generation rate, which is, in general, preferred over a rapid hydrogen generation rate.
Nevertheless, the hydrogen generation rate at a 1:20 mass ratio was slightly higher than the rate reported by Xiao et al. (2018) of 570 mL/min for a 3 h Al-7.5% Bi-2.5% Sn. The authors did, however, perform the hydrolysis reaction at a mass ratio of 1:100 and a starting temperature of 35 °C [41]. Wang et al. (2013) achieved a maximum hydrogen generation rate of 1080 mL/min for an Al-3% Ga-3% In-5% Sn composite at a mass ratio of 1:300 [92]. A recent review article by Xiao et al. (2022) presented a comprehensive summary of numerous Al composites used for hydrogen generation [147].
It is worth noting that the thermal energy released during Al hydrolysis should not be discarded, as it accounts for a relatively significant fraction of the overall energy balance. This is discussed in-depth in the following section.
3.5. Proposed Applications
Hydrogen generated by solid materials, either via its decomposition (e.g., sodium borohydride, NaBH4) or hydrolysis (e.g., Al) can essentially only be considered for niche applications. These materials can, however, be regenerated indefinitely, which technically classifies them as renewable sources. For instance, spent NaBH4 can be regenerated by ball-milling its by-products (i.e., NaBO2.4H2O or NaBO2.2H2O) with Mg2Si or Mg [148,149,150]. However, the re-metallization of hydrolyzed Al by the Hall–Héroult requires approximately 1.9 kg Al2O3, 50 g cryolite, 20 g aluminum fluoride, 500 g carbon, and 13–15 kWh electrical energy to produce 1 kg of Al [151,152]. Therefore, the use of Al is not truly a green approach to hydrogen generation. Additionally, during Al’s hydrolysis, 1 kg of Al generates 0.11 kg hydrogen and approximately 4.4 kWh of heat [104]. Though coupling the generated hydrogen with a PEMFC is attractive due to the aforementioned properties, current PEMFCs have an efficiency of approximately 50%, and between 1.6–2.2 kWh of electrical energy can be produced from the hydrogen generated by the hydrolysis of 1 kg of Al [101,104]. Thus, for an Al hydrolysis energy system, only an approximate 25% of energy is recoverable as electrical energy, with thermal energy as the balance.
Therefore, thermal energy should not be foregone. Godart and Hart (2020) proposed driving seawater through a semi-permeable membrane, allowing drinkable water to permeate via reverse osmosis using a piston driven by the thermal energy released during the hydrolysis reaction [153]. In addition, biologically contaminated drinking water can be flocculated by Al–hydroxides and partially heated by reaction heat (additional heating would be required to achieve a water temperature of 100 °C for some time). Nevertheless, The Russian Skolkovo Foundation announced that the PEMFC portable charging devices market has a value of $34 bn [154]. Other sectors include unmanned aerial vehicles (UAV), military and emergency applications, and hydrogen sources for underground mining equipment.
The Al composites presented in this investigation may be employed for PEMFC or thermal applications under certain conditions. For example, 1 g Al-9.25% Bi-0.75% Ni hydrolyzed at a 1:20 mass ratio achieved a >99% hydrogen yield, a reaction temperature increase of 68.0 °C, and a maximum hydrogen generation rate of approximately 600 mL/min. These extreme conditions may cause complications during PEMFC applications while being attractive for applications proposed by Godart and Hart (2020) [153]. Nevertheless, the same hydrolysis reaction performed at a 1:50 mass ratio will result in a reaction temperature increase of 34.3 °C and a peak hydrogen generation rate of 87.5 mL/min (see Figure 6), while achieving a >99% hydrogen yield.
It is noted here that currently, the overall energy efficiency of the Al–hydrolysis energy system is not at its optimum due to the underutilization of produced thermal energy [104] and that an application that can effectively utilize both hydrogen and thermal energy is unexplored. This formulates the necessity to further research and develop possible applications.
3.6. Effects of Water Quality on Hydrolysis
De-ionized water, which was the water quality used thus far, is not as easily accessible as lower quality water sources. Therefore, the effects of water quality on the hydrolysis reaction were investigated by hydrolyzing Al-9.25% Bi-0.75% Ni in de-ionized, filter, and tap water (Figure 7).
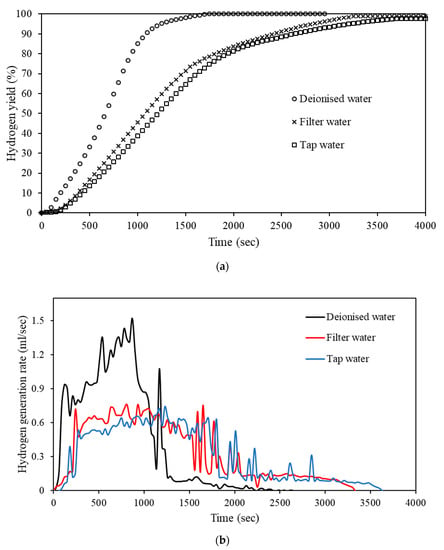
Figure 7.
Hydrogen yield curves (a) and hydrogen generation rates (b) of Al-9.25% Bi-0.75% Ni hydrolyzed using different water qualities.
According to Figure 7a, the use of different water qualities had a slight influence on the percentage hydrogen yield of the Al-9.25% Bi-0.75% Ni composite. The highest hydrogen yield was obtained in de-ionized water, i.e., >99%, while a hydrogen yield of 98.8% and 97.6% was achieved in filter and tap water, respectively.
Furthermore, is it evident from Figure 7b that a reduction in water quality led to a decrease in the hydrogen generation rate. Hydrolysis performed in de-ionized water achieved a maximum hydrogen generation rate of 1.5 mL/s, and the hydrolysis reaction was complete after approximately 869 s. Filter and tap water had similar hydrogen generation rates, obtaining a maximum hydrogen peak of 0.76 and 0.75 mL/s, respectively. Hydrolysis performed in filter and tap water ended after approximately 3300 and 3650 s, respectively.
The high hydrogen yield and generation rate observed for the hydrolysis using de-ionized water is ascribed to the absence of ions generally associated with tap water, e.g., Na+, K+, Mg2+, Ca2+, whereas the presence of such ions likely interfered with the hydrolysis reaction. When considering the hydrolysis similarities for both tap and filtered water, it is clear that these ions were present in the filtered water, which was expected. Nevertheless, considering the results obtained in Figure 7 and that de-ionized water is generated via specialized equipment, the use of filtered or tap water will be the most suited water quality for hydrolysis. More so, for PEMFC applications, the retarded hydrogen generation rate associated with filtered and tap water-based hydrolysis would be preferred over that of de-ionized water.
3.7. Hydrolysis Residue Characterization
XRD analysis was performed on the hydrolysis residues of Al-9.25% Bi-0.75% Ni at mass ratios of 1:20, 1:50, and 1:125 (Figure 8). From Figure 8, it can be seen that the hydrolysis residue of the 1:20 mass ratio only consisted of boehmite (AlOOH), indicating complete Al hydrolysis and that the boehmite peaks overlapped the activation metal peaks.
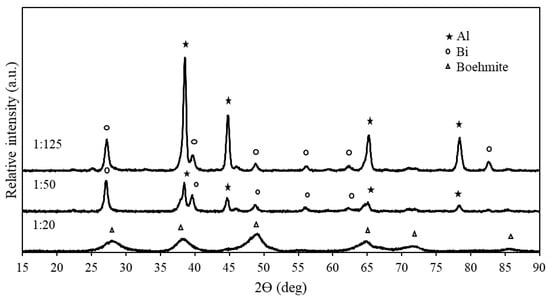
Figure 8.
Hydrolysis residues of ternary composite Al-9.25% Bi-0.75% Ni at different mass ratios.
In the XRD pattern of 1:50 and 1:125, several small boehmite peaks were detected; however, the peaks were too small to indicate in Figure 8. Similar results were presented by Du Preez et al. (2017) and Davies et al. (2022) [94,126]. The mass ratios of 1:50 and 1:125 had peaks that mainly consisted of Al-rich phases, suggesting that some of the Al did not hydrolyze during the hydrolysis reaction. Furthermore, Bi peaks were also observed in the XRD patterns of the 1:50 and 1:125 mass ratios, showing that Bi was present as segregated phases and was unaffected during the hydrolysis reaction. When considering that minute Ni peaks were observed in Figure 4, the total absence thereof here is jointly ascribed to small peak size and the amorphousness of the hydrolyzed composites.
In addition, the higher intensity Al-rich peaks in the mass ratio of 1:125 than those in that of 1:50 suggest less Al hydrolyzed during the hydrolysis reaction. This was expected, as a higher mass ratio causes a lower reaction temperature, leading to a lower Al hydrolysis reaction rate.
4. Conclusions
Several Al-Bi-Ni composites were prepared through a mechanochemical activation process and hydrolyzed in de-ionized water under standard ambient conditions. Various amounts of Bi and Ni (collectively, 10 wt%) were added to the Al composite to determine the effect thereof on the hydrogen yield and hydrogen generation rate during hydrolysis. SEM analysis revealed an overall decrease in Al particle size after the ball-milling process. Furthermore, the SEM–EDS analysis showed a relatively uniform distribution of Bi and Ni throughout the Al particle, which suggests the formation of galvanic cells between anodic Al and cathodic Bi/Ni during hydrolysis reactions.
Composites Al-9% Bi-1% Ni, Al-9.25% Bi-0.75% Ni, and Al-9.5% Bi-0.5% Ni obtained a hydrogen yield of >99%, while the Al-10% Bi composite achieved 97.4%. Thus, the addition of Ni (and reduction in Bi) to the Al composite resulted in a minor increase in the hydrogen yield. The Al-9% Bi-1% Ni, Al-9.25% Bi-0.75% Ni composites had comparable hydrogen generation rates than Al-10% Bi, whereas Al-9.5% Bi-0.5% Ni had an accelerated hydrogen generation rate. XRD analysis of the composites indicated no inter-metallic phase formation during the ball-milling process; major phases detected were Al, Bi, and minor Ni peaks. The XRD analysis of hydrolysis residues showed boehmite as the hydrolysis by-product.
Increasing the mass ratio from 1:20 to 1:125 resulted in a significant decrease in the change in reaction temperatures, i.e., 68 °C and 15 °C for 1:20 and 1:125 mass ratios, respectively. In addition, the hydrogen yield decreased from >99% to 85% with the increase in mass ratio. The water quality (de-ionized, filtered, tap) had a slight influence on the hydrogen yield, and the hydrogen yield ranged from 97.6 to >99%. The water quality did, however, affect the hydrogen generation rate, as the generation rate was 1.5 mL/s for de-ionized water and approximately 0.75 mL/s for filtered and tap waters.
Author Contributions
Conceptualization, S.P.D.P., D.G.B.; methodology, J.D.; formal analysis, J.D., S.P.D.P.; investigation, J.D.; resources, D.G.B.; data curation, J.D.; writing—original draft preparation, J.D.; writing, review and editing, S.P.D.P., D.G.B.; visualization, J.D.; supervision, S.P.D.P.; project administration, D.G.B.; funding acquisition, D.G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Department of Science and Innovation (DSI) HySA Infrastructure Center of Competence at the North-West University, South Africa, through the KP5 program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bockris, J.O.M. The hydrogen economy: Its history. Int. J. Hydrogen Energy 2013, 38, 2579–2588. [Google Scholar] [CrossRef]
- Neef, H.-J. International overview of hydrogen and fuel cell research. Energy 2009, 34, 327–333. [Google Scholar] [CrossRef]
- Kothari, R.; Buddhi, D.; Sawhney, R. Comparison of environmental and economic aspects of various hydrogen production methods. Renew. Sustain. Energy Rev. 2008, 12, 553–563. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Najjar, Y.S. Hydrogen safety: The road toward green technology. Int. J. Hydrogen Energy 2013, 38, 10716–10728. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Jones, D.R.; Bessarabov, D.G.; Falch, A.; Mota das Neves Quaresma, C.; Dunnill, C.W. Development of a Pt/stainless steel mesh catalyst and its application in catalytic hydrogen combustion. Int. J. Hydrogen Energy 2019, 44, 27094–27106. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Shuro, I.; Bessarabov, D.G. Development of a low purity aluminum alloy (Al6082) anodization process and its application as a platinum-based catalyst in catalytic hydrogen combustion. Surf. Coat. Technol. 2020, 404, 126483. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Jones, D.R.; Warwick, M.E.A.; Falch, A.; Sekoai, P.T.; Mota das Neves Quaresma, C.; Bessarabov, D.G.; Dunnill, C.W. Thermally stable Pt/Ti mesh catalyst for catalytic hydrogen combustion. Int. J. Hydrogen Energy 2020, 45, 16851–16864. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Bessarabov, D.G. Catalytic Hydrogen Combustion for Domestic and Safety Applications: A Critical Review of Catalyst Materials and Technologies. Energies 2021, 14, 4897. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Malakhov, A.A.; Bessarabov, D.G. A Thermally Conductive Pt/AAO Catalyst for Hydrogen Passive Autocatalytic Recombination. Catalysts 2021, 11, 491. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Bessarabov, D.G. Preparation of Pt/Ce–Zr–Y mixed oxide/anodized aluminium oxide catalysts for hydrogen passive autocatalytic recombination. Int. J. Hydrogen Energy 2022, 47, 12726–12738. [Google Scholar] [CrossRef]
- du Preez, S.; Bessarabov, D. On-demand hydrogen generation by the hydrolysis of ball-milled aluminum composites: A process overview. Int. J. Hydrogen Energy 2021, 46, 35790–35813. [Google Scholar] [CrossRef]
- Chiuta, S.; Everson, R.C.; Neomagus, H.W.J.P.; Bessarabov, D.G. Hydrogen production from ammonia decomposition over a commercial Ru/Al2O3 catalyst in a microchannel reactor: Experimental validation and CFD simulation. Int. J. Hydrogen Energy 2016, 41, 3774–3785. [Google Scholar] [CrossRef]
- Chiuta, S.; Everson, R.C.; Neomagus, H.W.J.P.; Bessarabov, D.G. Performance evaluation of a high-throughput microchannel reactor for ammonia decomposition over a commercial Ru-based catalyst. Int. J. Hydrogen Energy 2015, 40, 2921–2926. [Google Scholar] [CrossRef]
- Chiuta, S.; Everson, R.C.; Neomagus, H.W.J.P.; van der Gryp, P.; Bessarabov, D.G. Reactor technology options for distributed hydrogen generation via ammonia decomposition: A review. Int. J. Hydrogen Energy 2013, 38, 14968–14991. [Google Scholar] [CrossRef]
- Bi, Q.-Y.; Du, X.-L.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Efficient Subnanometric Gold-Catalyzed Hydrogen Generation via Formic Acid Decomposition under Ambient Conditions. J. Am. Chem. Soc. 2012, 134, 8926–8933. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, X.; Yin, M.; Liu, C.; Xing, W. Novel PdAu@Au/C Core−Shell Catalyst: Superior Activity and Selectivity in Formic Acid Decomposition for Hydrogen Generation. Chem. Mater. 2010, 22, 5122–5128. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Ping, Y.; Yan, J.-M.; Wang, H.-L.; Jiang, Q. Hydrogen generation from formic acid decomposition at room temperature using a NiAuPd alloy nanocatalyst. Int. J. Hydrogen Energy 2014, 39, 4850–4856. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Q. Metal-Nanoparticle-Catalyzed Hydrogen Generation from Formic Acid. Acc. Chem. Res. 2017, 50, 1449–1458. [Google Scholar] [CrossRef]
- Czaun, M.; Goeppert, A.; May, R.; Haiges, R.; Prakash, G.K.S.; Olah, G.A. Hydrogen Generation from Formic Acid Decomposition by Ruthenium Carbonyl Complexes. Tetraruthenium Dodecacarbonyl Tetrahydride as an Active Intermediate. ChemSusChem 2011, 4, 1241–1248. [Google Scholar] [CrossRef]
- Mukherjee, S.; Devaguptapu, S.V.; Sviripa, A.; Lund, C.R.F.; Wu, G. Low-temperature ammonia decomposition catalysts for hydrogen generation. Appl. Catal. B Environ. 2018, 226, 162–181. [Google Scholar] [CrossRef]
- Podila, S.; Driss, H.; Zaman, S.F.; Alhamed, Y.A.; AlZahrani, A.A.; Daous, M.A.; Petrov, L.A. Hydrogen generation by ammonia decomposition using Co/MgO–La2O3 catalyst: Influence of support calcination atmosphere. J. Mol. Catal. A Chem. 2016, 414, 130–139. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Xu, Z.P.; Zhu, Z. Catalytic ammonia decomposition for CO-free hydrogen generation over Ru/Cr2O3 catalysts. Appl. Catal. A Gen. 2013, 467, 246–252. [Google Scholar] [CrossRef]
- Amao, Y.; Tomonou, Y.; Okura, I. Highly efficient photochemical hydrogen production system using zinc porphyrin and hydrogenase in CTAB micellar system. Solar Energy Mater. Solar Cells 2003, 79, 103–111. [Google Scholar] [CrossRef]
- Ziming, C.; Fuqiang, W.; Huaxu, L.; Shengpeng, H.; Bo, L.; Jianyu, T.; Hongyang, L. Photon-absorption-based explanation of ultrasonic-assisted solar photochemical splitting of water to improve hydrogen production. Int. J. Hydrogen Energy 2018, 43, 14439–14450. [Google Scholar] [CrossRef]
- Liu, W.-S.; Perng, T.-P. Ta2O5 hollow fiber composed of internal interconnected mesoporous nanotubes and its enhanced photochemical H2 evolution. Int. J. Hydrogen Energy 2019, 44, 17688–17696. [Google Scholar] [CrossRef]
- Nada, A.A.; Barakat, M.H.; Hamed, H.A.; Mohamed, N.R.; Veziroglu, T.N. Studies on the photocatalytic hydrogen production using suspended modified TiO2 photocatalysts. Int. J. Hydrogen Energy 2005, 30, 687–691. [Google Scholar] [CrossRef]
- Falch, A.; Kriek, R.J. Laser induced H2 production employing Pt-TiO2 photocatalysts. J. Photochem. Photobiol. A: Chem. 2013, 271, 117–123. [Google Scholar] [CrossRef]
- Li, F.; Yang, J.; Gao, J.; Liu, Y.; Gong, Y. Enhanced photocatalytic hydrogen production of CdS embedded in cationic hydrogel. Int. J. Hydrogen Energy 2020, 45, 1969–1980. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, C.; Shi, F. MoS2/Ti3C2 heterostructure for efficient visible-light photocatalytic hydrogen generation. Int. J. Hydrogen Energy 2020, 45, 6291–6301. [Google Scholar] [CrossRef]
- Sediroglu, V.; Eroglu, İ.; Yücel, M.; Türker, L.; Gündüz, U. The biocatalytic effect of Halobacterium halobium on photoelectrochemical hydrogen production. J. Biotechnol. 1999, 70, 115–124. [Google Scholar] [CrossRef]
- Patel, P.P.; Ghadge, S.D.; Hanumantha, P.J.; Datta, M.K.; Gattu, B.; Shanthi, P.M.; Kumta, P.N. Active and robust novel bilayer photoanode architectures for hydrogen generation via direct non-electric bias induced photo-electrochemical water splitting. Int. J. Hydrogen Energy 2018, 43, 13158–13176. [Google Scholar] [CrossRef]
- Casallas, C.; Dincer, I.; Zamfirescu, C. Experimental investigation and analysis of a novel photo-electrochemical hydrogen production cell with polymeric membrane photocathode. Int. J. Hydrogen Energy 2016, 41, 7968–7975. [Google Scholar] [CrossRef]
- Manwar, N.R.; Borkar, R.G.; Khobragade, R.; Rayalu, S.S.; Jain, S.L.; Bansiwal, A.K.; Labhsetwar, N.K. Efficient solar photo-electrochemical hydrogen generation using nanocrystalline CeFeO3 synthesized by a modified microwave assisted method. Int. J. Hydrogen Energy 2017, 42, 10931–10942. [Google Scholar] [CrossRef]
- Levchenko, A.V.; Dobrovolsky, Y.A.; Bukun, N.G.; Leonova, L.S.; Zyubina, T.S.; Neudachina, V.S.; Yashina, L.V.; Tarasov, A.B.; Shatalova, T.B.; Shtanov, V.I. Chemical and electrochemical processes in low-temperature superionic hydrogen sulfide sensors. Russ. J. Electrochem. 2007, 43, 552–560. [Google Scholar] [CrossRef]
- Bessarabov, D.G.; Human, G.; Kruger, A.J.; Chiuta, S.; Modisha, P.M.; du Preez, S.P.; Oelofse, S.P.; Vincent, I.; Van Der Merwe, J.; Langmi, H.W.; et al. South African hydrogen infrastructure (HySA infrastructure) for fuel cells and energy storage: Overview of a projects portfolio. Int. J. Hydrogen Energy 2017, 42, 13568–13588. [Google Scholar] [CrossRef]
- Phillips, R.; Dunnill, C.W. Zero gap alkaline electrolysis cell design for renewable energy storage as hydrogen gas. RSC Adv. 2016, 6, 100643–100651. [Google Scholar] [CrossRef] [Green Version]
- Falch, A.; Badets, V.A.; Labrugère, C.; Kriek, R.J. Co-sputtered PtxPdyAlz thin film electrocatalysts for the production of hydrogen via SO2(aq) electro-oxidation. Electrocatalysis 2016, 7, 376–390. [Google Scholar] [CrossRef]
- Choi, P.; Bessarabov, D.G.; Datta, R. A simple model for solid polymer electrolyte (SPE) water electrolysis. Solid State Ion. 2004, 175, 535–539. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Vincent, I.; Kruger, A.; Bessarabov, D. Development of efficient membrane electrode assembly for low cost hydrogen production by anion exchange membrane electrolysis. Int. J. Hydrogen Energy 2017, 42, 10752–10761. [Google Scholar] [CrossRef]
- Merwe, J.v.d.; Uren, K.; Schoor, G.v.; Bessarabov, D. A study of the loss characteristics of a single cell PEM electrolyser for pure hydrogen production. In Proceedings of the 2013 IEEE International Conference on Industrial Technology (ICIT), Cape Town, South Africa, 25–28 February 2013; pp. 668–672. [Google Scholar]
- Funk, J.E. Thermochemical hydrogen production: Past and present. Int. J. Hydrogen Energy 2001, 26, 185–190. [Google Scholar] [CrossRef]
- Kaneko, H.; Gokon, N.; Hasegawa, N.; Tamaura, Y. Solar thermochemical process for hydrogen production using ferrites. Energy 2005, 30, 2171–2178. [Google Scholar] [CrossRef]
- Pandey, B.; Prajapati, Y.K.; Sheth, P.N. Recent progress in thermochemical techniques to produce hydrogen gas from biomass: A state of the art review. Int. J. Hydrogen Energy 2019, 44, 25384–25415. [Google Scholar] [CrossRef]
- Canavesio, C.; Nassini, D.; Nassini, H.E.; Bohé, A.E. Study on an original cobalt-chlorine thermochemical cycle for nuclear hydrogen production. Int. J. Hydrogen Energy 2019, 45, 26090–26103. [Google Scholar] [CrossRef]
- Xiao, F.; Guo, Y.; Li, J.; Yang, R. Hydrogen generation from hydrolysis of activated aluminum composites in tap water. Energy 2018, 157, 608–614. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Bessarabov, D.G. Material Aspects Pertaining to Hydrogen Production from Aluminum: Opinion. Res. Dev. Mater. Sci. 2019, 12, 1247–1248. [Google Scholar] [CrossRef]
- Du Preez, S.P. Hydrogen Generation by the Reaction of Mechanochemically Activated Aluminium and Water; North-West University: Potchefstroom, South Africa, 2019. [Google Scholar]
- Tan, Z.; Ouyang, L.; Huang, J.; Liu, J.; Wang, H.; Shao, H.; Zhu, M. Hydrogen generation via hydrolysis of Mg2Si. J. Alloys Compd. 2019, 770, 108–115. [Google Scholar] [CrossRef]
- Ma, M.; Yang, L.; Ouyang, L.; Shao, H.; Zhu, M. Promoting hydrogen generation from the hydrolysis of Mg-Graphite composites by plasma-assisted milling. Energy 2019, 167, 1205–1211. [Google Scholar] [CrossRef]
- Tan, Z.; Ouyang, L.; Liu, J.; Wang, H.; Shao, H.; Zhu, M. Hydrogen generation by hydrolysis of Mg-Mg2Si composite and enhanced kinetics performance from introducing of MgCl2 and Si. Int. J. Hydrogen Energy 2018, 43, 2903–2912. [Google Scholar] [CrossRef]
- Huang, M.; Ouyang, L.; Chen, Z.; Peng, C.; Zhu, X.; Zhu, M. Hydrogen production via hydrolysis of Mg-oxide composites. Int. J. Hydrogen Energy 2017, 42, 22305–22311. [Google Scholar] [CrossRef]
- Grosjean, M.-H.; Roué, L. Hydrolysis of Mg–salt and MgH2–salt mixtures prepared by ball milling for hydrogen production. J. Alloys Compd. 2006, 416, 296–302. [Google Scholar] [CrossRef]
- Grosjean, M.-H.; Zidoune, M.; Roué, L.; Huot, J.-Y. Hydrogen production via hydrolysis reaction from ball-milled Mg-based materials. Int. J. Hydrogen Energy 2006, 31, 109–119. [Google Scholar] [CrossRef]
- Grosjean, M.-H.; Zidoune, M.; Roué, L. Hydrogen production from highly corroding Mg-based materials elaborated by ball milling. J. Alloys Compd. 2005, 404, 712–715. [Google Scholar] [CrossRef]
- Zou, H.; Chen, S.; Zhao, Z.; Lin, W. Hydrogen production by hydrolysis of aluminum. J. Alloys Compd. 2013, 578, 380–384. [Google Scholar] [CrossRef]
- Retnamma, R.; Novais, A.Q.; Rangel, C.M. Kinetics of hydrolysis of sodium borohydride for hydrogen production in fuel cell applications: A review. Int. J. Hydrogen Energy 2011, 36, 9772–9790. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, L.Z.; Liu, J.W.; Yao, X.D.; Wang, H.; Liu, Z.W.; Zhu, M. Hydrolysis and regeneration of sodium borohydride (NaBH4)–A combination of hydrogen production and storage. J. Power Sources 2017, 359, 400–407. [Google Scholar] [CrossRef]
- Fernandes, R.; Patel, N.; Miotello, A.; Filippi, M. Studies on catalytic behavior of Co–Ni–B in hydrogen production by hydrolysis of NaBH4. J. Mol. Catal. A Chem. 2009, 298, 1–6. [Google Scholar] [CrossRef]
- Hsieh, C.P.; Ho, C.Y.; Hsu, L.C.; Chang, Y.-J. Synergistic effect on hydrolytic sodium borohydride adding waste Al for hydrogen generation. Int. J. Hydrogen Energy 2020, 45, 10334–10341. [Google Scholar] [CrossRef]
- Choi, B.; Panthi, D.; Nakoji, M.; Kabutomori, T.; Tsutsumi, K.; Tsutsumi, A. Novel hydrogen production and power generation system using metal hydride. Int. J. Hydrogen Energy 2015, 40, 6197–6206. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, H.; Han, S.-C.; Kim, H.-S.; Song, M.-S.; Lee, J.-Y. Production of hydrogen from sodium borohydride in alkaline solution: Development of catalyst with high performance. Int. J. Hydrogen Energy 2004, 29, 263–267. [Google Scholar] [CrossRef]
- Kojima, Y.; Kawai, Y.; Kimbara, M.; Nakanishi, H.; Matsumoto, S. Hydrogen generation by hydrolysis reaction of lithium borohydride. Int. J. Hydrogen Energy 2004, 29, 1213–1217. [Google Scholar] [CrossRef]
- Huang, J.M.; Duan, R.M.; Ouyang, L.Z.; Wen, Y.J.; Wang, H.; Zhu, M. The effect of particle size on hydrolysis properties of Mg3La hydrides. Int. J. Hydrogen Energy 2014, 39, 13564–13568. [Google Scholar] [CrossRef]
- Sevastyanova, L.G.; Klyamkin, S.N.; Bulychev, B.M. Generation of hydrogen from magnesium hydride oxidation in water in presence of halides. Int. J. Hydrogen Energy 2020, 45, 3046–3052. [Google Scholar] [CrossRef]
- Gan, D.; Liu, Y.; Zhang, J.; Zhang, Y.; Cao, C.; Zhu, Y.; Li, L. Kinetic performance of hydrogen generation enhanced by AlCl3 via hydrolysis of MgH2 prepared by hydriding combustion synthesis. Int. J. Hydrogen Energy 2018, 43, 10232–10239. [Google Scholar] [CrossRef]
- Liu, P.; Wu, H.; Wu, C.; Chen, Y.; Xu, Y.; Wang, X.; Zhang, Y. Microstructure characteristics and hydrolysis mechanism of Mg–Ca alloy hydrides for hydrogen generation. Int. J. Hydrogen Energy 2015, 40, 3806–3812. [Google Scholar] [CrossRef]
- Hiroi, S.; Hosokai, S.; Akiyama, T. Ultrasonic irradiation on hydrolysis of magnesium hydride to enhance hydrogen generation. Int. J. Hydrogen Energy 2011, 36, 1442–1447. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, L.Z.; Wen, Y.J.; Xu, Y.J.; Yang, X.S.; Sun, L.X.; Zhu, M. The effect of Ni and Al addition on hydrogen generation of Mg3La hydrides via hydrolysis. Int. J. Hydrogen Energy 2010, 35, 8161–8165. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, C.; Wu, H.; Chen, Y. Hydrogen generation by CaH2-induced hydrolysis of Mg17Al12 hydride. Int. J. Hydrogen Energy 2011, 36, 15698–15703. [Google Scholar] [CrossRef]
- Weng, B.; Wu, Z.; Li, Z.; Yang, H. Hydrogen generation from hydrolysis of NH3BH3/MH (M=Li, Na) binary hydrides. Int. J. Hydrogen Energy 2012, 37, 5152–5160. [Google Scholar] [CrossRef]
- Du Preez, S.; Bessarabov, D. Hydrogen generation by means of hydrolysis using activated Al-In-Bi-Sn composites for electrochemical energy applications. Int. J. Electrochem. Sci. 2017, 12, 8663–8682. [Google Scholar] [CrossRef]
- Du Preez, S.; Bessarabov, D. The effects of bismuth and tin on the mechanochemical processing of aluminum-based composites for hydrogen generation purposes. Int. J. Hydrogen Energy 2019, 44, 21896–21912. [Google Scholar] [CrossRef]
- Du Preez, S.; Bessarabov, D. Hydrogen generation by the hydrolysis of mechanochemically activated aluminum-tin-indium composites in pure water. Int. J. Hydrogen Energy 2018, 43, 21398–21413. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ouma, C.N.M.; du Preez, S.P.; Modisha, P.; Engelbrecht, N.; Bessarabov, D.G.; Ghimire, A. Application of nanoparticles in biofuels: An overview. Fuel 2019, 237, 380–397. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Engelbrecht, N.; du Preez, S.P.; Bessarabov, D. Thermophilic Biogas Upgrading via ex Situ Addition of H2 and CO2 Using Codigested Feedstocks of Cow Manure and the Organic Fraction of Solid Municipal Waste. ACS Omega 2020, 5, 17367–17376. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Daramola, M.O.; Mogwase, B.; Engelbrecht, N.; Yoro, K.O.; du Preez, S.P.; Mhlongo, S.; Ezeokoli, O.T.; Ghimire, A.; Ayeni, A.O.; et al. Revising the dark fermentative H2 research and development scenario–An overview of the recent advances and emerging technological approaches. Biomass Bioenergy 2020, 140, 105673. [Google Scholar] [CrossRef]
- Varella Rodrigues, C.; Oliveira Santana, K.; Nespeca, M.G.; Varella Rodrigues, A.; Oliveira Pires, L.; Maintinguer, S.I. Energy valorization of crude glycerol and sanitary sewage in hydrogen generation by biological processes. Int. J. Hydrogen Energy 2020, 45, 11943–11953. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Mulla, R.; Dunnill, C.W. Powering the Hydrogen Economy from Waste Heat: A Review of Heat-to-Hydrogen Concepts. ChemSusChem 2019, 12, 3882–3895. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, M.; Adamaki, V.; Khanbareh, H.; Bowen, C.R. Control of electro-chemical processes using energy harvesting materials and devices. Chem. Soc. Rev. 2017, 46, 7757–7786. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.-M.; Jung, J.-Y.; Park, M.-J.; Song, J.-W.; Lee, J.-H. Catalyst-free hydrogen evolution of Si photocathode by thermovoltage-driven solar water splitting. J. Power Sources 2015, 279, 151–156. [Google Scholar] [CrossRef]
- Wang, M.; Zuo, Y.; Wang, J.; Wang, Y.; Shen, X.; Qiu, B.; Cai, L.; Zhou, F.; Lau, S.P.; Chai, Y. Remarkably Enhanced Hydrogen Generation of Organolead Halide Perovskites via Piezocatalysis and Photocatalysis. Adv. Energy Mater. 2019, 9, 1901801. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Fierro, J. Ni-based catalysts for reforming of methane with CO2. Int. J. Hydrogen Energy 2012, 37, 15966–15975. [Google Scholar] [CrossRef]
- Balat, M. Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrogen Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Chalk, S.G.; Miller, J.F. Key challenges and recent progress in batteries, fuel cells, and hydrogen storage for clean energy systems. J. Power Sources 2006, 159, 73–80. [Google Scholar] [CrossRef]
- Huang, X.; Gao, T.; Pan, X.; Wei, D.; Lv, C.; Qin, L.; Huang, Y. A review: Feasibility of hydrogen generation from the reaction between aluminum and water for fuel cell applications. J. Power Sources 2013, 229, 133–140. [Google Scholar] [CrossRef]
- Wang, H.; Leung, D.Y.; Leung, M.; Ni, M. A review on hydrogen production using aluminum and aluminum alloys. Renew. Sustain. Energy Rev. 2009, 13, 845–853. [Google Scholar] [CrossRef]
- Tzimas, E.; Filiou, C.; Peteves, S.; Veyret, J. Hydrogen Storage: State-of-the-Art and Future Perspective; JRC Petten, EUR 20995EN; EU Commission: Petten, The Netherlands, 2003. [Google Scholar]
- Prabu, S.; Wang, H.-W. Enhanced hydrogen generation from graphite-mixed aluminum hydroxides catalyzed Al/water reaction. Int. J. Hydrogen Energy 2020, 45, 33419–33429. [Google Scholar] [CrossRef]
- Sheng, P.; Zhang, S.; Guan, C.; Qian, W.; Gao, X.; Wang, Y. Preparation and characterization of the Al-Ga-In-Sn-KCl composites for hydrogen generation. Energy Storage 2021, 3, e241. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Teng, H.-T.; Lee, T.-Y.; Wang, H.-W. Rapid hydrogen generation from aluminum–water system by adjusting water ratio to various aluminum/aluminum hydroxide. Int. J. Energy Environ. Eng. 2014, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Du Preez, S.; Bessarabov, D. Hydrogen generation of mechanochemically activated AlBiIn composites. Int. J. Hydrogen Energy 2017, 42, 16589–16602. [Google Scholar] [CrossRef]
- Kumar, D.; Muthukumar, K. An overview on activation of aluminium-water reaction for enhanced hydrogen production. J. Alloys Compd. 2020, 835, 155189. [Google Scholar] [CrossRef]
- Yang, S.; Knickle, H. Design and analysis of aluminum/air battery system for electric vehicles. J. Power Sources 2002, 112, 162–173. [Google Scholar] [CrossRef]
- Vargel, C. Corrosion of Aluminium; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Reshetenko, T.V.; Bethune, K.; Rocheleau, R. Spatial proton exchange membrane fuel cell performance under carbon monoxide poisoning at a low concentration using a segmented cell system. J. Power Sources 2012, 218, 412–423. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, C.; Wei, H.; Zou, H.; Lin, K.; Guo, Y.; Yang, S.; Liu, X. Mild hydrogen production from the hydrolysis of Al–Bi–Zn composite powder. Int. J. Hydrogen Energy 2021, 46, 9314–9323. [Google Scholar] [CrossRef]
- Haupin, W.E. Electrochemistry of the Hall-Heroult Process for Aluminum Smelting; ACS Publications: New Kensington, PA, USA, 1983. [Google Scholar] [CrossRef]
- Elitzur, S.; Rosenband, V.; Gany, A. Urine and aluminum as a source for hydrogen and clean energy. Int. J. Hydrogen Energy 2016, 41, 11909–11913. [Google Scholar] [CrossRef]
- Wang, H.; Chang, Y.; Dong, S.; Lei, Z.; Zhu, Q.; Luo, P.; Xie, Z. Investigation on hydrogen production using multicomponent aluminum alloys at mild conditions and its mechanism. Int. J. Hydrogen Energy 2013, 38, 1236–1243. [Google Scholar] [CrossRef]
- Zou, M.-S.; Guo, X.-Y.; Huang, H.-T.; Yang, R.-J.; Zhang, P. Preparation and characterization of hydro-reactive Mg–Al mechanical alloy materials for hydrogen production in seawater. J. Power Sources 2012, 219, 60–64. [Google Scholar] [CrossRef]
- Shkolnikov, E.; Zhuk, A.; Vlaskin, M. Aluminum as energy carrier: Feasibility analysis and current technologies overview. Renew. Sustain. Energy Rev. 2011, 15, 4611–4623. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.; Leung, M.K.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Czech, E.; Troczynski, T. Hydrogen generation through massive corrosion of deformed aluminum in water. Int. J. Hydrogen Energy 2010, 35, 1029–1037. [Google Scholar] [CrossRef]
- Parmuzina, A.; Kravchenko, O. Activation of aluminium metal to evolve hydrogen from water. Int. J. Hydrogen Energy 2008, 33, 3073–3076. [Google Scholar] [CrossRef]
- El-Meligi, A. Hydrogen production by aluminum corrosion in hydrochloric acid and using inhibitors to control hydrogen evolution. Int. J. Hydrogen Energy 2011, 36, 10600–10607. [Google Scholar] [CrossRef]
- Ivanov, V.; Safronov, M.; Gavrilyuk, O. Macrokinetics of oxidation of ultradisperse aluminum by water in the liquid phase. Combust. Explos. Shock Waves 2001, 37, 173–177. [Google Scholar] [CrossRef]
- Ivanov, V.; Gavrilyuk, O. Specific features of the oxidation and self-ignition of electroexplosive ultradisperse metal powders in air. Combust. Explos. Shock Waves 1999, 35, 648–655. [Google Scholar] [CrossRef]
- Ilyukhina, A.; Kravchenko, O.; Bulychev, B. Studies on microstructure of activated aluminum and its hydrogen generation properties in aluminum/water reaction. J. Alloys Compd. 2017, 690, 321–329. [Google Scholar] [CrossRef]
- Baniamerian, M.; Moradi, S. Al–Ga doped nanostructured carbon as a novel material for hydrogen production in water. J. Alloys Compd. 2011, 509, 6307–6310. [Google Scholar] [CrossRef]
- Kravchenko, O.; Semenenko, K.; Bulychev, B.; Kalmykov, K. Activation of aluminum metal and its reaction with water. J. Alloys Compd. 2005, 397, 58–62. [Google Scholar] [CrossRef]
- Tan, S.-C.; Gui, H.; Yang, X.-H.; Yuan, B.; Zhan, S.-H.; Liu, J. Comparative study on activation of aluminum with four liquid metals to generate hydrogen in alkaline solution. Int. J. Hydrogen Energy 2016, 41, 22663–22667. [Google Scholar] [CrossRef]
- Irankhah, A.; Fattahi, S.M.S.; Salem, M. Hydrogen generation using activated aluminum/water reaction. Int. J. Hydrogen Energy 2018, 43, 15739–15748. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.; Szpunar, J. Effect of addition of water-soluble salts on the hydrogen generation of aluminum in reaction with hot water. J. Alloys Compd. 2016, 679, 364–374. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, F.; Sun, L.; Chen, J.; Guo, X.; Yan, E.; Yu, F.; Chu, H.; Peng, H.; Zou, Y.; et al. A novel AlBiOCl composite for hydrogen generation from water. Int. J. Hydrogen Energy 2019, 44, 6655–6662. [Google Scholar] [CrossRef]
- Liu, J.; Fei, Y.; Pan, H.; Fan, M.-Q.; Wang, L.L.; Yao, J. Controllable hydrogen generation performance from Al/NaBH4 composite activated by La metal and CoCl2 salt in pure water. J. Rare Earths 2012, 30, 548–551. [Google Scholar] [CrossRef]
- Newell, A.; Thampi, K.R. Novel amorphous aluminum hydroxide catalysts for aluminum–water reactions to produce H2 on demand. Int. J. Hydrogen Energy 2017, 42, 23446–23454. [Google Scholar] [CrossRef]
- López-Miranda, J.; Rosas, G. Hydrogen generation by aluminum hydrolysis using the Fe2Al5 intermetallic compound. Int. J. Hydrogen Energy 2016, 41, 4054–4059. [Google Scholar] [CrossRef]
- Fan, M.-Q.; Xu, F.; Sun, L.-X.; Zhao, J.-N.; Jiang, T.; Li, W.-X. Hydrolysis of ball milling Al–Bi–hydride and Al–Bi–salt mixture for hydrogen generation. J. Alloys Compd. 2008, 460, 125–129. [Google Scholar] [CrossRef]
- Jia, Y.; Shen, J.; Meng, H.; Dong, Y.; Chai, Y.; Wang, N. Hydrogen generation using a ball-milled Al/Ni/NaCl mixture. J. Alloys Compd. 2014, 588, 259–264. [Google Scholar] [CrossRef]
- Zhang, F.; Edalati, K.; Arita, M.; Horita, Z. Fast hydrolysis and hydrogen generation on Al-Bi alloys and Al-Bi-C composites synthesized by high-pressure torsion. Int. J. Hydrogen Energy 2017, 42, 29121–29130. [Google Scholar] [CrossRef]
- Chen, J.; Xu, F.; Sun, L.; Zhang, K.; Xia, Y.; Guo, X.; Zhang, H.; Yu, F.; Yan, E.; Peng, H. Effect of doped Ni-Bi-B alloy on hydrogen generation performance of Al-InCl3. J. Energy Chem. 2019, 39, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Kaya, M.F.; Kahveci, O.; Erol, H.; Akkaya, A. Effect of low B addition on Al-Zn alloy’s hydrogen production performance. Int. J. Hydrogen Energy 2021, 46, 15192–15202. [Google Scholar] [CrossRef]
- Davies, J.; Du Preez, S.; Bessarabov, D. On-Demand Hydrogen Generation by the Hydrolysis of Ball-Milled Aluminum-Bismuth-Zinc Composites. Materials 2022, 15, 1197. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.-Q.; Xu, F.; Sun, L.-X. Studies on hydrogen generation characteristics of hydrolysis of the ball milling Al-based materials in pure water. Int. J. Hydrogen Energy 2007, 32, 2809–2815. [Google Scholar] [CrossRef]
- Liu, K.; Luo, P.; Deng, Y.; Zuo, Y.; Xu, X.; Yi, S.; Dong, S. Hydrogen production from hydrolysis of Al–Ga–In–SnCl2 composites. Mater. Res. Express 2019, 6, 085515. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, X.; Sun, L.; Yu, F.; Li, P.; Chen, J.; Wu, Y.; Cao, L.; Xu, C.; Yang, X.; et al. Hydrogen generation of a novel AlNaMgH3 composite reaction with water. Int. J. Hydrogen Energy 2017, 42, 30535–30542. [Google Scholar] [CrossRef]
- Katsoufis, P.; Doukas, E.; Politis, C.; Avgouropoulos, G.; Lianos, P. Enhanced rate of hydrogen production by corrosion of commercial aluminum. Int. J. Hydrogen Energy 2020, 45, 10729–10734. [Google Scholar] [CrossRef]
- Ghenciu, A.F. Review of fuel processing catalysts for hydrogen production in PEM fuel cell systems. Curr. Opin. Solid State Mater. Sci. 2002, 6, 389–399. [Google Scholar] [CrossRef]
- Kreuer, K. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Guo, Y.-F.; Chen, H.-C.; Wang, F.-C. The development of a hybrid PEMFC power system. Int. J. Hydrogen Energy 2015, 40, 4630–4640. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Beukes, J.P.; Paktunc, D.; Van Zyl, P.G.; Jordaan, A. Recycling pre-oxidized chromite fines in the oxidative sintered pellet production process. J. South. Afr. Inst. Min. Metall. 2019, 119, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.-Q.; Wang, H.-H.; Jian, W.; Jia, L.; Ping, L.; Chang, Y.; Dong, S.-J. Effects of low melting point metals (Ga, In, Sn) on hydrolysis properties of aluminum alloys. Trans. Nonferrous Met. Soc. China 2016, 26, 152–159. [Google Scholar] [CrossRef]
- Mahmoodi, K.; Alinejad, B. Enhancement of hydrogen generation rate in reaction of aluminum with water. Int. J. Hydrogen Energy 2010, 35, 5227–5232. [Google Scholar] [CrossRef]
- Liu, S.; Fan, M.-Q.; Wang, C.; Huang, Y.-X.; Chen, D.; Bai, L.-Q.; Shu, K.-Y. Hydrogen generation by hydrolysis of Al–Li–Bi–NaCl mixture with pure water. Int. J. Hydrogen Energy 2012, 37, 1014–1020. [Google Scholar] [CrossRef]
- Fan, M.Q.; Sun, L.X.; Xu, F. Experiment assessment of hydrogen production from activated aluminum alloys in portable generator for fuel cell applications. Energy 2010, 35, 2922–2926. [Google Scholar] [CrossRef]
- Fan, M.-Q.; Xu, F.; Sun, L.-X. Hydrogen generation by hydrolysis reaction of ball-milled Al− Bi alloys. Energy Fuels 2007, 21, 2294–2298. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.; Szpunar, J. Effect of structural evolution of aluminum powder during ball milling on hydrogen generation in aluminum–water reaction. Int. J. Hydrogen Energy 2013, 38, 795–806. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.; Szpunar, J. Role of ball milling of aluminum powders in promotion of aluminum-water reaction to generate hydrogen. Metall. Mater. Trans. E 2014, 1, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Reboul, M.; Gimenez, P.; Rameau, J. A proposed activation mechanism for Al anodes. Corrosion 1984, 40, 366–371. [Google Scholar] [CrossRef]
- Weiss, D. Improved high-temperature aluminum alloys containing cerium. J. Mater. Eng. Perform. 2019, 28, 1903–1908. [Google Scholar] [CrossRef]
- Papworth, A.; Fox, P. The disruption of oxide defects within aluminium alloy castings by the addition of bismuth. Mater. Lett. 1998, 35, 202–206. [Google Scholar] [CrossRef]
- Sagar, K.; Radhakrishna, L. The Influence of Nickel Addition on the Mechanical Properties of AA6061 Fabricated By Stir Casting. Int. J. Mater. Sci. 2017, 12, 617–625. [Google Scholar]
- Gai, W.-Z.; Liu, W.-H.; Deng, Z.-Y.; Zhou, J.-G. Reaction of Al powder with water for hydrogen generation under ambient condition. Int. J. Hydrogen Energy 2012, 37, 13132–13140. [Google Scholar] [CrossRef]
- Xiao, F.; Yang, R.; Liu, Z. Active aluminum composites and their hydrogen generation via hydrolysis reaction: A review. Int. J. Hydrogen Energy 2022, 47, 365–386. [Google Scholar] [CrossRef]
- Ouyang, L.; Chen, W.; Liu, J.; Felderhoff, M.; Wang, H.; Zhu, M. Enhancing the Regeneration Process of Consumed NaBH4 for Hydrogen Storage. Adv. Energy Mater. 2017, 7, 1700299. [Google Scholar] [CrossRef]
- Zhu, Y.; Ouyang, L.; Zhong, H.; Liu, J.; Wang, H.; Shao, H.; Huang, Z.; Zhu, M. Closing the Loop for Hydrogen Storage: Facile Regeneration of NaBH4 from its Hydrolytic Product. Angew. Chem. Int. Ed. 2020, 59, 8623–8629. [Google Scholar] [CrossRef]
- Zhong, H.; Ouyang, L.Z.; Ye, J.S.; Liu, J.W.; Wang, H.; Yao, X.D.; Zhu, M. An one-step approach towards hydrogen production and storage through regeneration of NaBH4. Energy Storage Mater. 2017, 7, 222–228. [Google Scholar] [CrossRef]
- Kvande, H.; Drabløs, P.A. The aluminum smelting process and innovative alternative technologies. J. Occup. Environ. Med. 2014, 56, S23–S32. [Google Scholar] [CrossRef] [Green Version]
- Grjotheim, K. Introduction to Aluminium Electrolysis: Understanding the Hall-Hérloult Process; Aluminium-Verlag: Düsseldorf, Germany, 1993; pp. 199–217. [Google Scholar]
- Godart, P.; Hart, D. Aluminum-powered climate change resiliency: From aluminum debris to electricity and clean water. Appl. Energy 2020, 275, 115316. [Google Scholar] [CrossRef]
- Foundation, R.S. SK Nuclear. Available online: https://sk.ru/news/m/wiki/14838/download.aspx (accessed on 15 November 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).