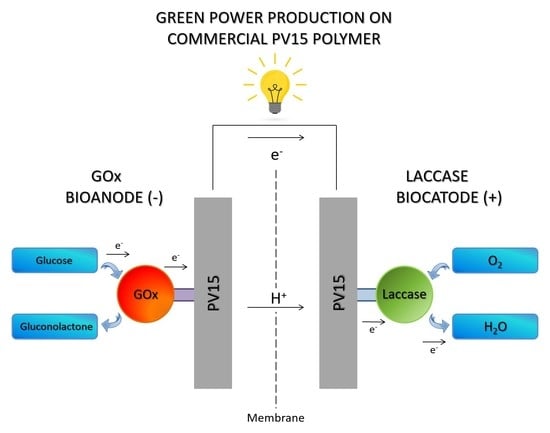
Immobilizing Enzymes on a Commercial Polymer: Performance Analysis of a GOx-Laccase Based Enzymatic Biofuel Cell Assembly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Bioanode Immobilization Technique
2.2.2. Biocathode Immobilization Technique
2.2.3. Determination of GOx Enzymatic Activity
2.2.4. Determination of Laccase Enzymatic Activity
2.3. Experimental Test Rig Description
- Aluminum end plates;
- Copper plates as current collectors;
- Bioelectrodes (PV15 Sigracell, SGL Carbon GmbH, Meitingen, Germany);
- Teflon frames;
- Nafion™ 211 (Ion Power GmbH, München, Germany) membrane as half-cells separator.
- -
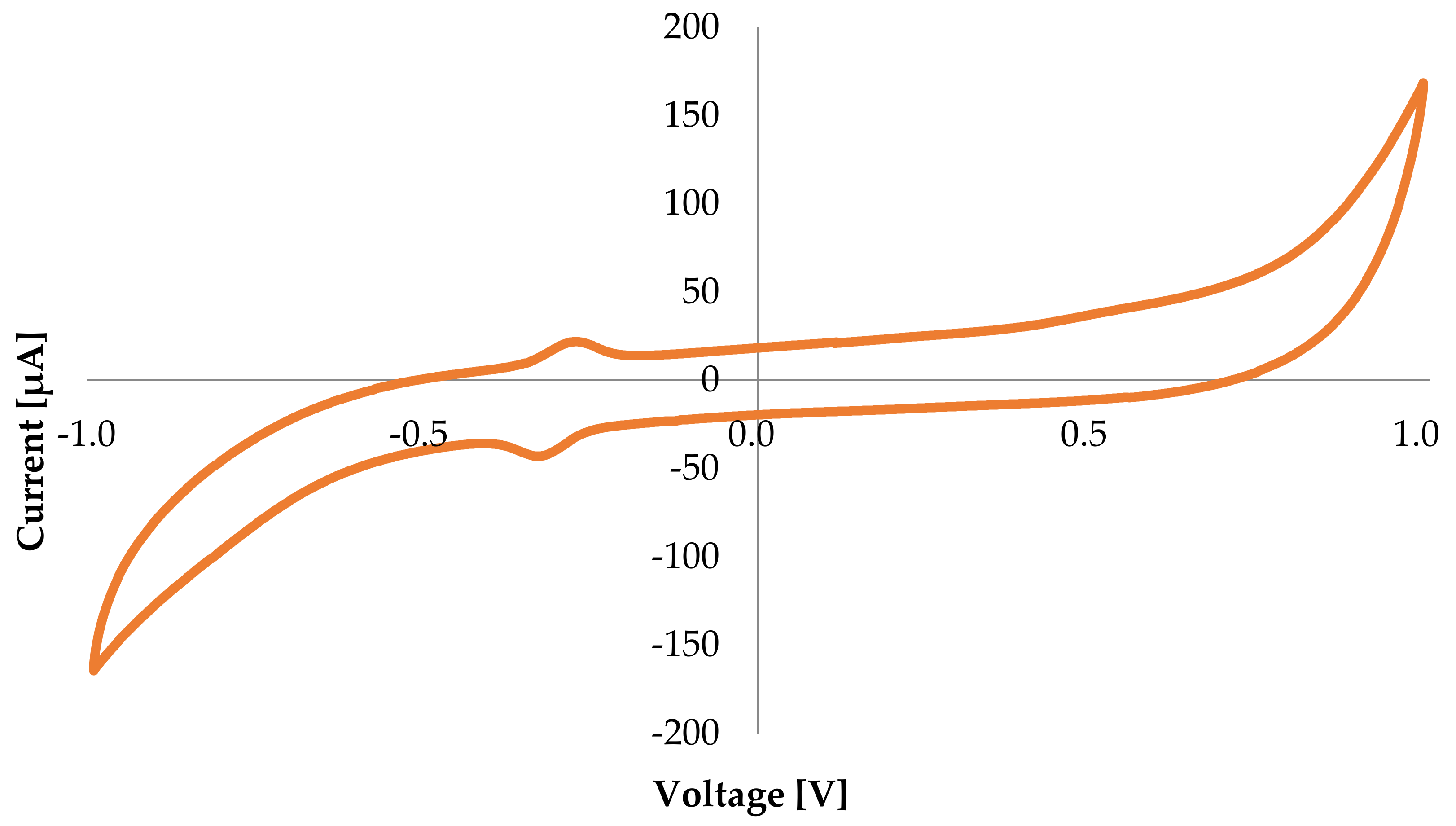
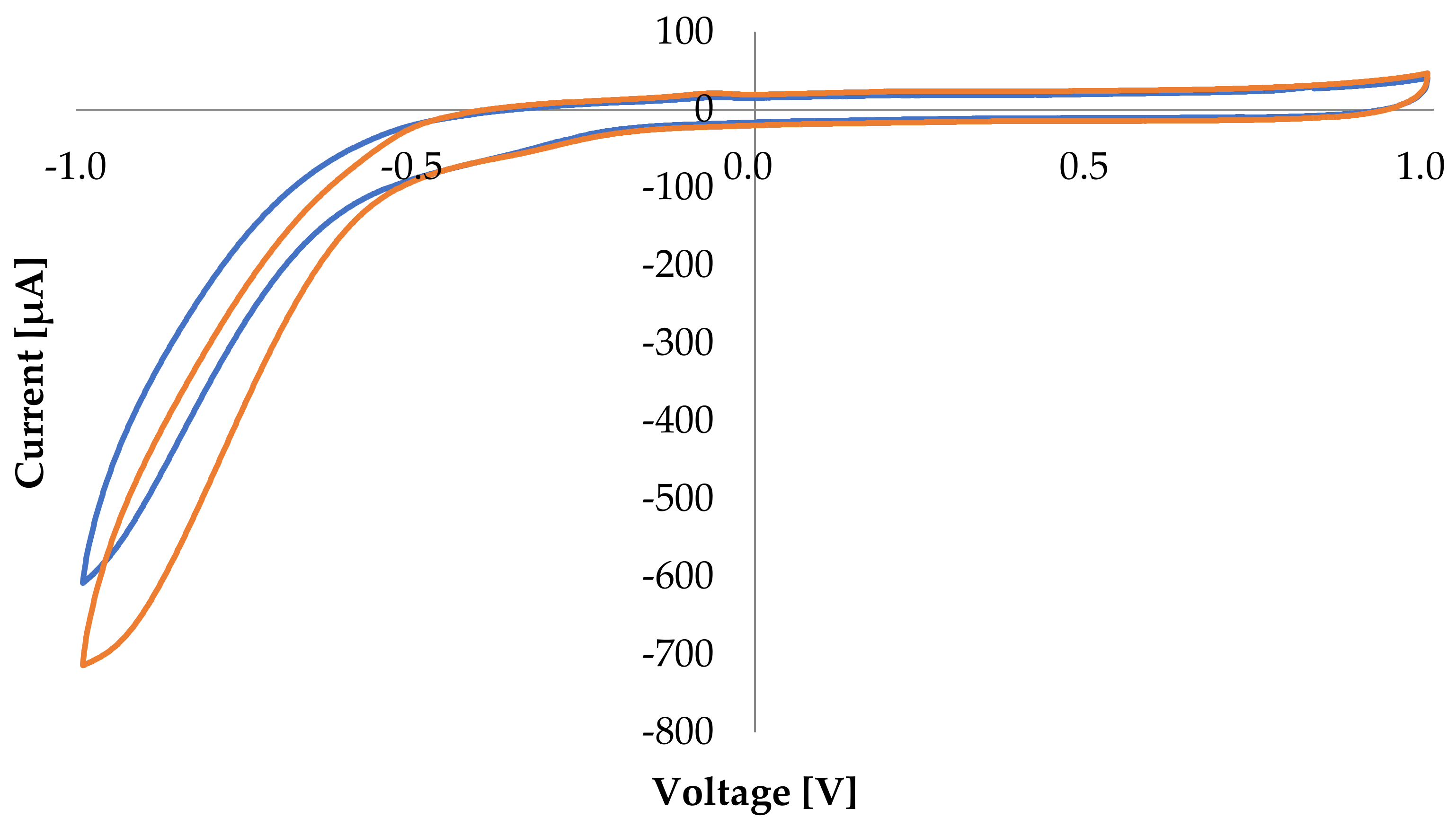
- Cyclic Voltammetry (CV) performed for bioanode and biocathode, in presence of glucose at the bioanode and both in absence and presence of air in the NaOAc buffer solution at the biocathode side. CV tests were carried out with the galvanostat/potentiostat BioLogic SP-240 in a three electrodes setup, using the investigated bioelectrode of 2 cm2 as working electrode, an Ag/AgCl KCl saturated as reference electrode, and a platinum wire as counter electrode.
- -
- Open circuit voltage, in order to evaluate the stability of the enzymes over time;
- -
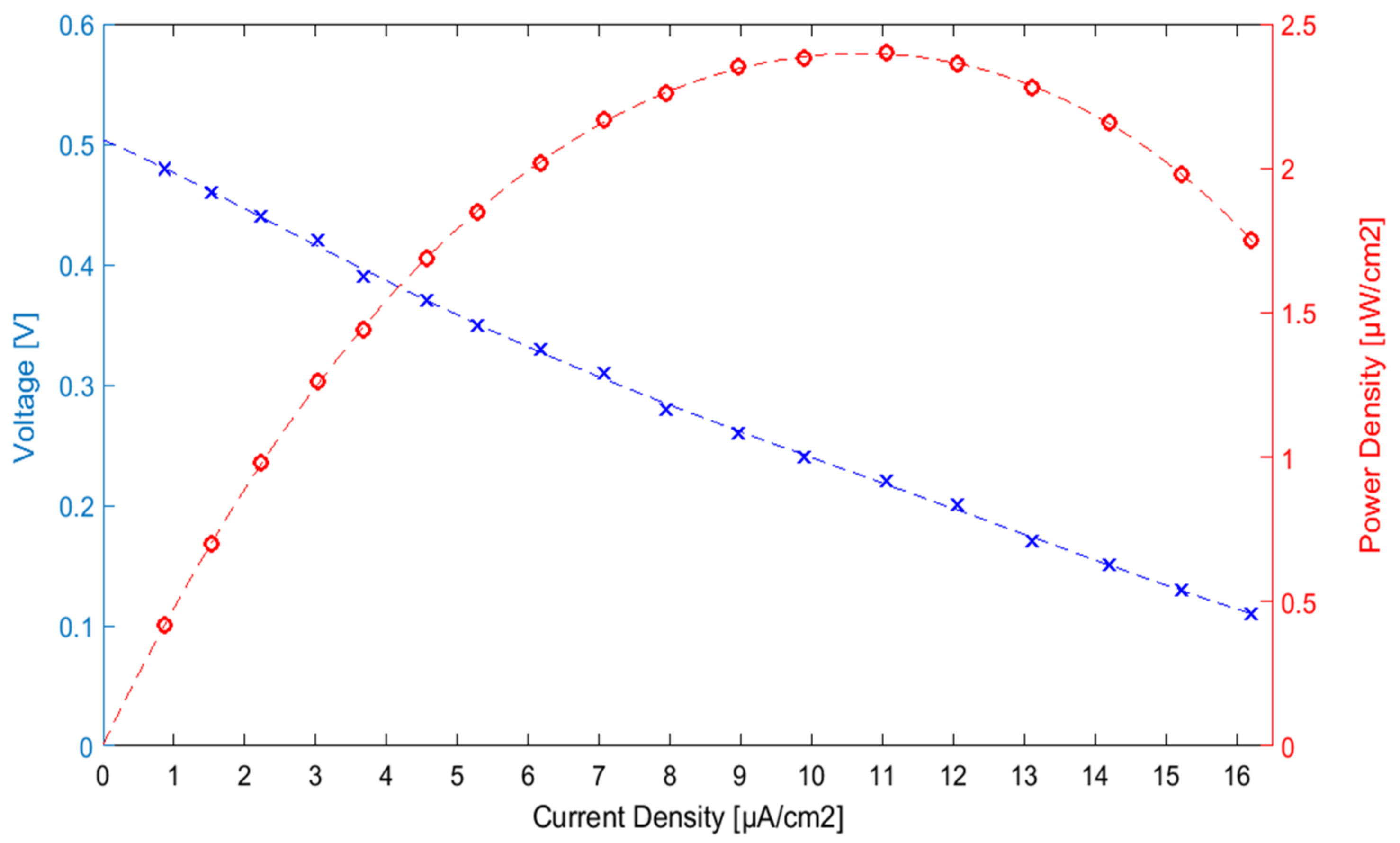
- Polarization curve, aiming to determine the maximum power output. A voltage cut-off of 0.1 V was set for this experiment.
3. Results and Discussion
3.1. Cyclic Voltammetry Preliminary Tests
3.2. Enzymatic Activity of Bioelectrodes
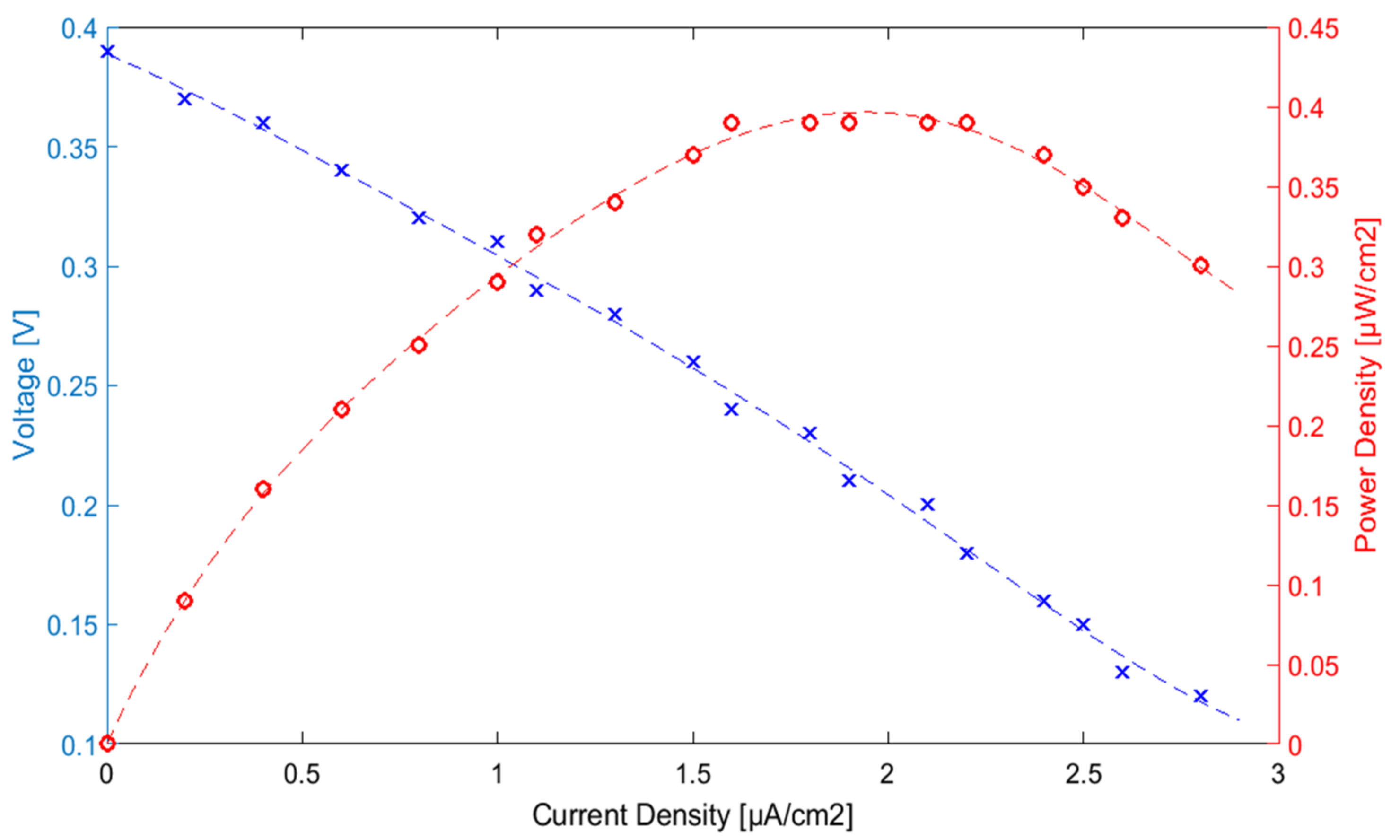
3.3. EBC Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, X.; Ren, L.; Jiang, C.; Han, X.; Yin, X.; Liu, Y.; Yang, W.; Chen, Y. Design of a Novel Photoelectrochemical Enzymatic Biofuel Cell with High Power Output Under Visible Light. Chem. Eng. J. 2022, 431, 134037. [Google Scholar] [CrossRef]
- Tang, J.; Yan, X.; Engelbrekt, C.; Ulstrup, J.; Magner, E.; Xiao, X.; Zhang, J. Development of Graphene-Based Enzymatic Biofuel Cells: A Minireview. Bioelectrochemistry 2020, 134, 107537. [Google Scholar] [CrossRef] [PubMed]
- Barelli, L.; Bidini, G.; Pelosi, D.; Sisani, E. Enzymatic Biofuel Cells: A Review on Flow Designs. Energies 2021, 14, 910. [Google Scholar] [CrossRef]
- Andoralov, V.; Falk, M.; Suyatin, D.B.; Granmo, M.; Sotres, J.; Ludwig, R.; Popov, V.O.; Schouenborg, J.; Blum, Z.; Shleev, S. Biofuel Cell Based on Microscale Nanostructured Electrodes with Inductive Coupling to Rat Brain Neurons. Sci. Rep. 2013, 3, 3270. [Google Scholar] [CrossRef]
- Gonzalez-Solino, C.; Di Lorenzo, M. Enzymatic Fuel Cells: Towards Self-Powered Implantable and Wearable Diagnostics. Biosensors 2018, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Cosnier, S.; Gross, A.J.; Giroud, F.; Holzinger, M. Beyond the Hype Surrounding Biofuel Cells: What’s the Future of Enzymatic Fuel Cells? Curr. Opin. Electrochem. 2018, 12, 148–155. [Google Scholar] [CrossRef]
- Xiao, X.; Xia, H.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A.; et al. Tackling the Challenges of Enzymatic (Bio) Fuel Cells. Chem. Rev. 2019, 119, 9509–9558. [Google Scholar] [CrossRef]
- Joseph, J.; Ponnuchamy, M.; Kapoor, A.; Sivaraman, P. Applications of biofuel cells. In Biofuel Cells: Materials and Challenges; Wiley: Hoboken, NJ, USA, 2021; pp. 465–482. [Google Scholar]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Carbon Nanotube/Enzyme Biofuel Cells. Electrochim. Acta 2012, 82, 179–190. [Google Scholar] [CrossRef]
- Gellett, W.; Kesmez, M.; Schumacher, J.; Akers, N.; Minteer, S.D. Biofuel Cells for Portable Power. Electroanalysis 2010, 22, 727–731. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.R.; Wu, X.; Zhu, J.J. Advances in the Enzymatic Biofuel Cell Powered Sensing Systems for Tumor Diagnosis and Regulation. TrAC-Trends Anal. Chem. 2022, 146, 116476. [Google Scholar] [CrossRef]
- Jin, X.; Bandodkar, A.J.; Fratus, M.; Asadpour, R.; Rogers, J.A.; Alam, M.A. Modeling, Design Guidelines, and Detection Limits of Self-Powered Enzymatic Biofuel Cell-Based Sensors. Biosens. Bioelectron. 2020, 168, 112493. [Google Scholar] [CrossRef]
- Li, X.; Feng, Q.; Wu, D.; Mensah, A.; Li, W.; Cai, Y.; Li, D.; Wei, Q. A Stretchable Electrode for Single Enzymatic Biofuel Cells. Mater. Today Energy 2021, 22, 100886. [Google Scholar] [CrossRef]
- Jayapiriya, U.S.; Rewatkar, P.; Goel, S. Miniaturized Polymeric Enzymatic Biofuel Cell with Integrated Microfluidic Device and Enhanced Laser Ablated Bioelectrodes. Int. J. Hydrogen Energy 2021, 46, 3183–3192. [Google Scholar] [CrossRef]
- Rasmussen, M.; Abdellaoui, S.; Minteer, S.D. Enzymatic Biofuel Cells: 30 Years of Critical Advancements. Biosens. Bioelectron. 2016, 76, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Vasilenko, V.; Arkadeva, I.; Bogdanovskaya, V.; Sudarev, G.; Kalenov, S.; Vocciante, M.; Koltsova, E. Glucose-Oxygen Biofuel Cell with Biotic and Abiotic Catalysts: Experimental Research and Mathematical Modeling. Energies 2020, 13, 5630. [Google Scholar] [CrossRef]
- Cosnier, S.; Le Goff, A.; Holzinger, M. Towards Glucose Biofuel Cells Implanted in Human Body for Powering Artificial Organs: Review. Electrochem. Commun. 2014, 38, 19–23. [Google Scholar] [CrossRef]
- Tortolini, C.; Rea, S.; Carota, E.; Cannistraro, S.; Mazzei, F. Influence of the Immobilization Procedures on the Electroanalytical Performances of Trametes Versicolor Laccase Based Bioelectrode. Microchem. J. 2012, 100, 8–13. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Tacchi, S.; Caponi, S.; Pellegrino, R.M.; Luzi, F.; Cottone, F.; Fioretto, D.; Emiliani, C.; Di Michele, A. Covalent immobilization of proteases on polylactic acid for proteins hydrolysis and waste biomass protein content valorization. Catalysts 2021, 11, 167. [Google Scholar] [CrossRef]
- De Poulpiquet, A.; Ciaccafava, A.; Lojou, E. New Trends in Enzyme Immobilization at Nanostructured Interfaces for Efficient Electrocatalysis in Biofuel Cells. Electrochim. Acta 2014, 126, 104–114. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme Immobilization: An Overview on Techniques and Support Materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Minteer, S.D.; Atanassov, P.; Luckarift, H.R.; Johnson, G.R. New Materials for Biological Fuel Cells. Mater. Today 2012, 15, 166–173. [Google Scholar] [CrossRef]
- Ivanov, I.; Vidaković-Koch, T.; Sundmacher, K. Recent Advances in Enzymatic Fuel Cells: Experiments and Modeling. Energies 2010, 3, 803–846. [Google Scholar] [CrossRef] [Green Version]
- Olszewski, B.; Stolarczyk, K. Laccase-Catalyzed Reduction of Oxygen at Electrodes Modified by Carbon Nanotubes with Adsorbed Promazine or Acetosyringone. Catalysts 2018, 8, 414. [Google Scholar] [CrossRef] [Green Version]
- Palanisamy, S.; Ramaraj, S.K.; Chen, S.M.; Yang, T.C.K.; Pan, Y.F.; Chen, T.W.; Velusamy, V.; Selvam, S. A Novel Laccase Biosensor Based on Laccase Immobilized Graphene-Cellulose Microfiber Composite Modified Screen-Printed Carbon Electrode For Sensitive Determination of Catechol. Sci. Rep. 2017, 7, 41214. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Han, Y.; Yu, Y.; Xu, M.; Zhang, X.; Huang, L.; Dong, S. RGO/Au Nps/N-Doped Cnts Supported on Nickel Foam as an Anode for Enzymatic Biofuel Cells. Biosens. Bioelectron. 2017, 97, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Sánchez, C.; Pita, M.; Vaz-Domínguez, C.; Shleev, S.; De Lacey, A.L. Gold Nanoparticles as Electronic Bridges for Laccase-Based Biocathodes. J. Am. Chem. Soc. 2012, 134, 17212–17220. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ji, P. Enzymes Immobilized on Carbon Nanotubes. Biotechnol. Adv. 2011, 29, 889–895. [Google Scholar] [CrossRef]
- Flexer, V.; Brun, N. Fundamentals of enzymatic electrochemical systems. In Functional Electrodes for Enzymatic and Microbial Electrochemical Systems; WSPC: Singapore, 2017; pp. 3–50. ISBN 9781786343543. [Google Scholar]
- Rewatkar, P.; Hitaishi, V.P.; Lojou, E.; Goel, S. Enzymatic Fuel Cells in a Microfluidic Environment: Status and Opportunities. A Mini Review. Electrochem. Commun. 2019, 107, 106533. [Google Scholar] [CrossRef]
- Kim, M.; Kwon, Y.; Ahn, Y. Paper-Based Mediatorless Enzymatic Microfluidic Biofuel Cells. Biosens. Bioelectron. 2021, 190, 113391. [Google Scholar] [CrossRef]
- Hui, Y.; Wang, H.; Zuo, W.; Ma, X. Spider Nest Shaped Multi-Scale Three-Dimensional Enzymatic Electrodes for Glucose/Oxygen Biofuel Cells. Int. J. Hydrogen Energy 2022, 47, 6187–6199. [Google Scholar] [CrossRef]
- Du Toit, H.; Di Lorenzo, M. Continuous Power Generation from Glucose with Two Different Miniature Flow-Through Enzymatic Biofuel Cells. Biosens. Bioelectron. 2015, 69, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Worthington, C. Enzyme and related biochemical. In Enzyme Manual; Worthington Biochemical Cooperation: Lakewood, NJ, USA, 1988; pp. 155–158. [Google Scholar]
- More, S.S.; Renuka, P.S.; Pruthvi, K.; Swetha, M.; Malini, S.; Veena, S.M. Isolation, Purification, and Characterization of Fungal Laccase from Pleurotus sp. Enzym. Res. 2011, 2011, 248735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, K.; Chaturvedi, V.; Verma, P. Fungal Laccase Discovered but yet Undiscovered. Bioresour. Bioprocess. 2018, 5, 4. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Leech, D.; Paice, M.G. Electrochemical Analysis of the Interactions of Laccase Mediators with Lignin Model Compounds. Biochim. Biophys. Acta-Gen. Subj. 1998, 1379, 381–390. [Google Scholar] [CrossRef]
- Rincón, R.A.; Lau, C.; Luckarift, H.R.; Garcia, K.E.; Adkins, E.; Johnson, G.R.; Atanassov, P. Enzymatic Fuel Cells: Integrating Flow-Through Anode and Air-Breathing Cathode into a Membrane-Less Biofuel Cell Design. Biosens. Bioelectron. 2011, 27, 132–136. [Google Scholar] [CrossRef]
- De Poulpiquet, A.; Ciaccafava, A.; Gadiou, R.; Gounel, S.; Giudici-Orticoni, M.T.; Mano, N.; Lojou, E. Design of a H2/O2 Biofuel Cell Based on Thermostable Enzymes. Electrochem. Commun. 2014, 42, 72–74. [Google Scholar] [CrossRef]
- Abreu, C.; Nedellec, Y.; Gross, A.J.; Ondel, O.; Buret, F.; Le Goff, A.; Holzinger, M.; Cosnier, S. Assembly and Stacking of Flow-through Enzymatic Bioelectrodes for High Power Glucose Fuel Cells. ACS Appl. Mater. Interfaces 2017, 9, 23836–23842. [Google Scholar] [CrossRef]
- Zebda, A.; Gondran, C.; Le Goff, A.; Holzinger, M.; Cinquin, P.; Cosnier, S. Mediatorless High-Power Glucose Biofuel Cells Based on Compressed Carbon Nanotube-Enzyme Electrodes. Nat. Commun. 2011, 2, 370. [Google Scholar] [CrossRef] [Green Version]
- Karim, N.A.; Yang, H. Mini-Review: Recent Technologies of Electrode and System in the Enzymatic Biofuel Cell (EBFC). Appl. Sci. 2021, 11, 5197. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelosi, D.; Barelli, L.; Montegiove, N.; Calzoni, E.; Cesaretti, A.; Di Michele, A.; Emiliani, C.; Gammaitoni, L. Immobilizing Enzymes on a Commercial Polymer: Performance Analysis of a GOx-Laccase Based Enzymatic Biofuel Cell Assembly. Energies 2022, 15, 2182. https://doi.org/10.3390/en15062182
Pelosi D, Barelli L, Montegiove N, Calzoni E, Cesaretti A, Di Michele A, Emiliani C, Gammaitoni L. Immobilizing Enzymes on a Commercial Polymer: Performance Analysis of a GOx-Laccase Based Enzymatic Biofuel Cell Assembly. Energies. 2022; 15(6):2182. https://doi.org/10.3390/en15062182
Chicago/Turabian StylePelosi, Dario, Linda Barelli, Nicolò Montegiove, Eleonora Calzoni, Alessio Cesaretti, Alessandro Di Michele, Carla Emiliani, and Luca Gammaitoni. 2022. "Immobilizing Enzymes on a Commercial Polymer: Performance Analysis of a GOx-Laccase Based Enzymatic Biofuel Cell Assembly" Energies 15, no. 6: 2182. https://doi.org/10.3390/en15062182
APA StylePelosi, D., Barelli, L., Montegiove, N., Calzoni, E., Cesaretti, A., Di Michele, A., Emiliani, C., & Gammaitoni, L. (2022). Immobilizing Enzymes on a Commercial Polymer: Performance Analysis of a GOx-Laccase Based Enzymatic Biofuel Cell Assembly. Energies, 15(6), 2182. https://doi.org/10.3390/en15062182