Accumulation of Inert Impurities in a Polymer Electrolyte Fuel Cell System with Anode Recirculation and Periodic Purge: A Simple Analytical Model
Abstract
:1. Introduction
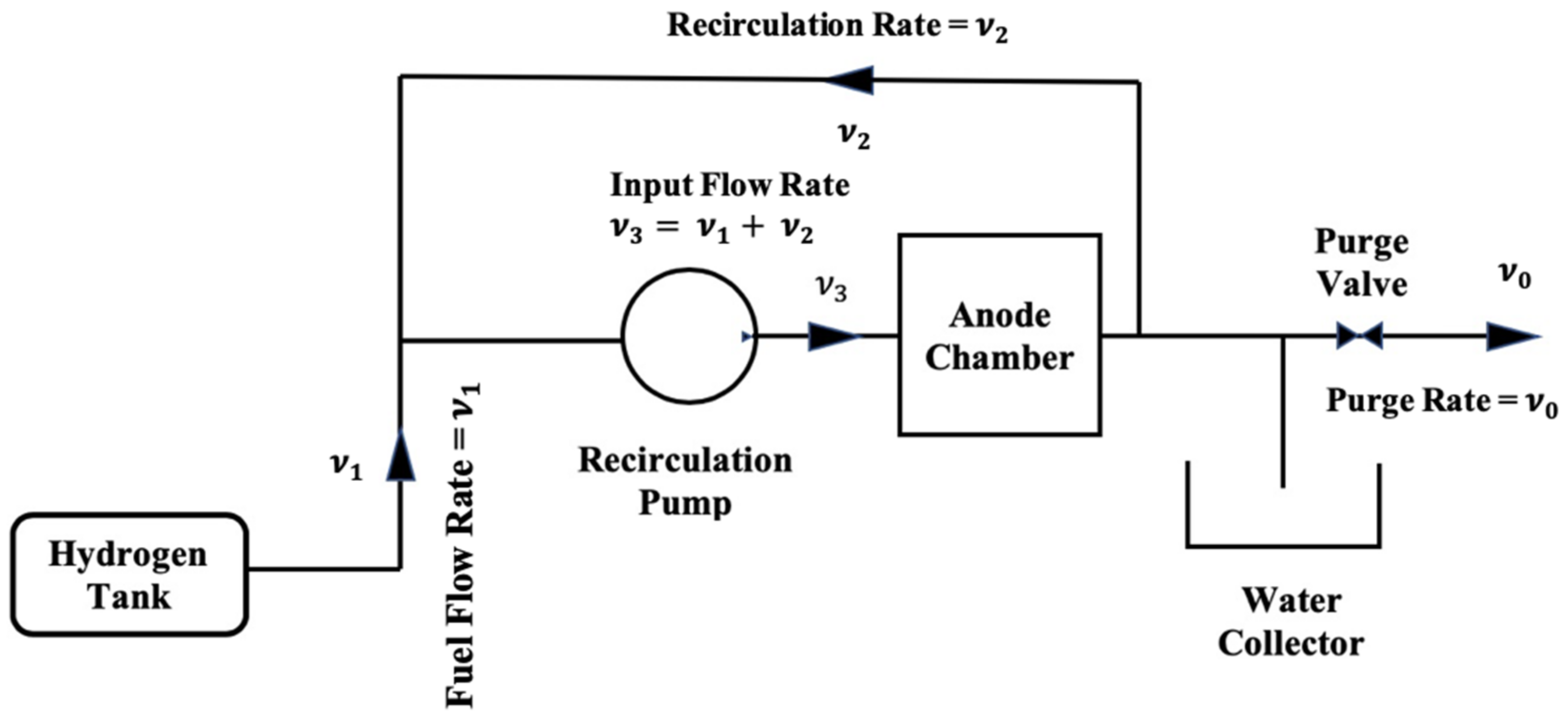
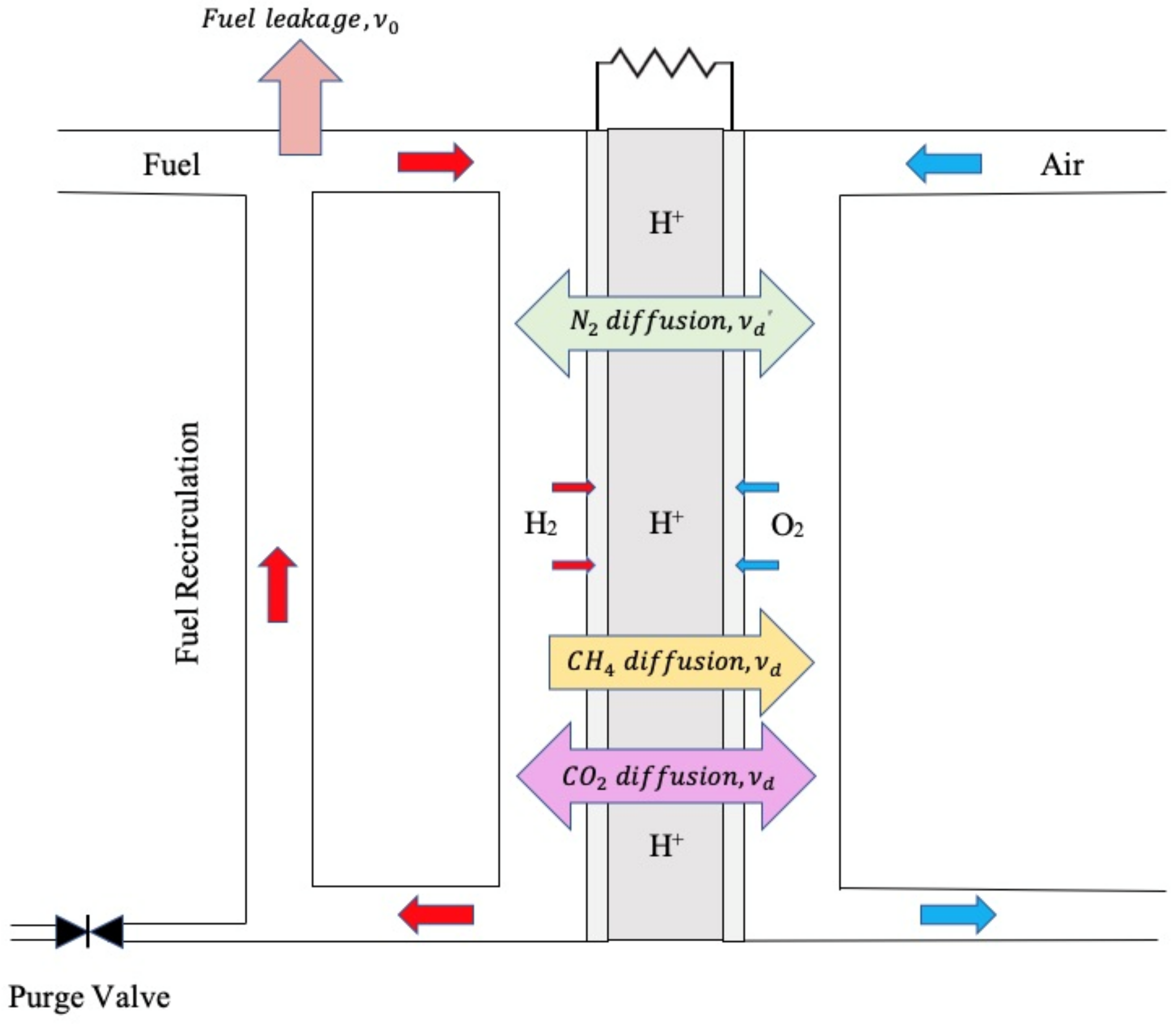
2. Model Development
- (a)
- All reactants and inert species inside the anode recirculation loop are assumed to be well mixed.
- (b)
- Temperatures and pressures across the recirculation plumbing are assumed to be constant.
- (c)
- The system is assumed to be operating at steady state with pure hydrogen flowing before time t = 0. The impure hydrogen stream is assumed to be introduced at time t = 0. The dynamics of the accumulation of impurities in the anode chamber is then followed at times > 0.
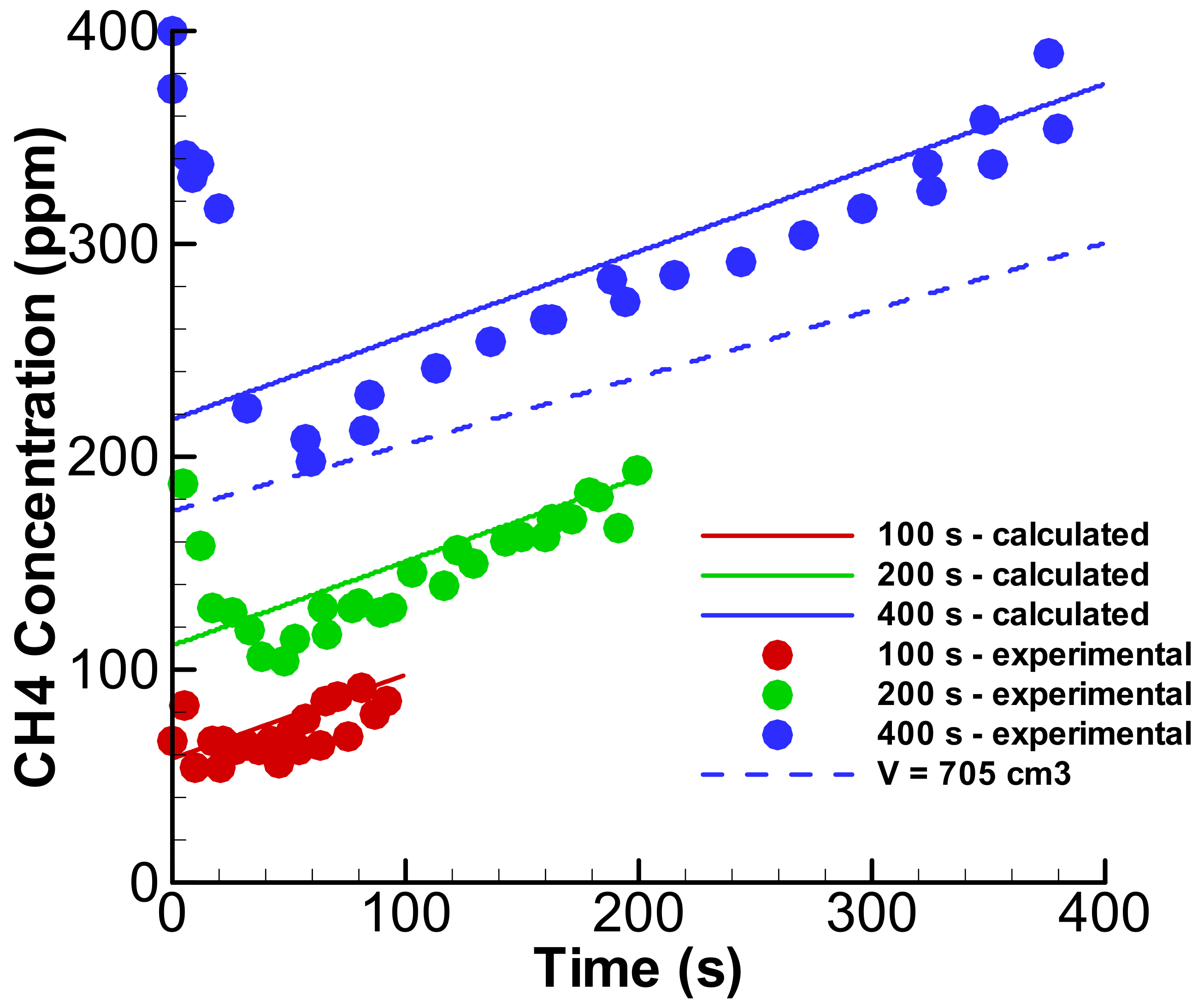
3. Results and Discussion
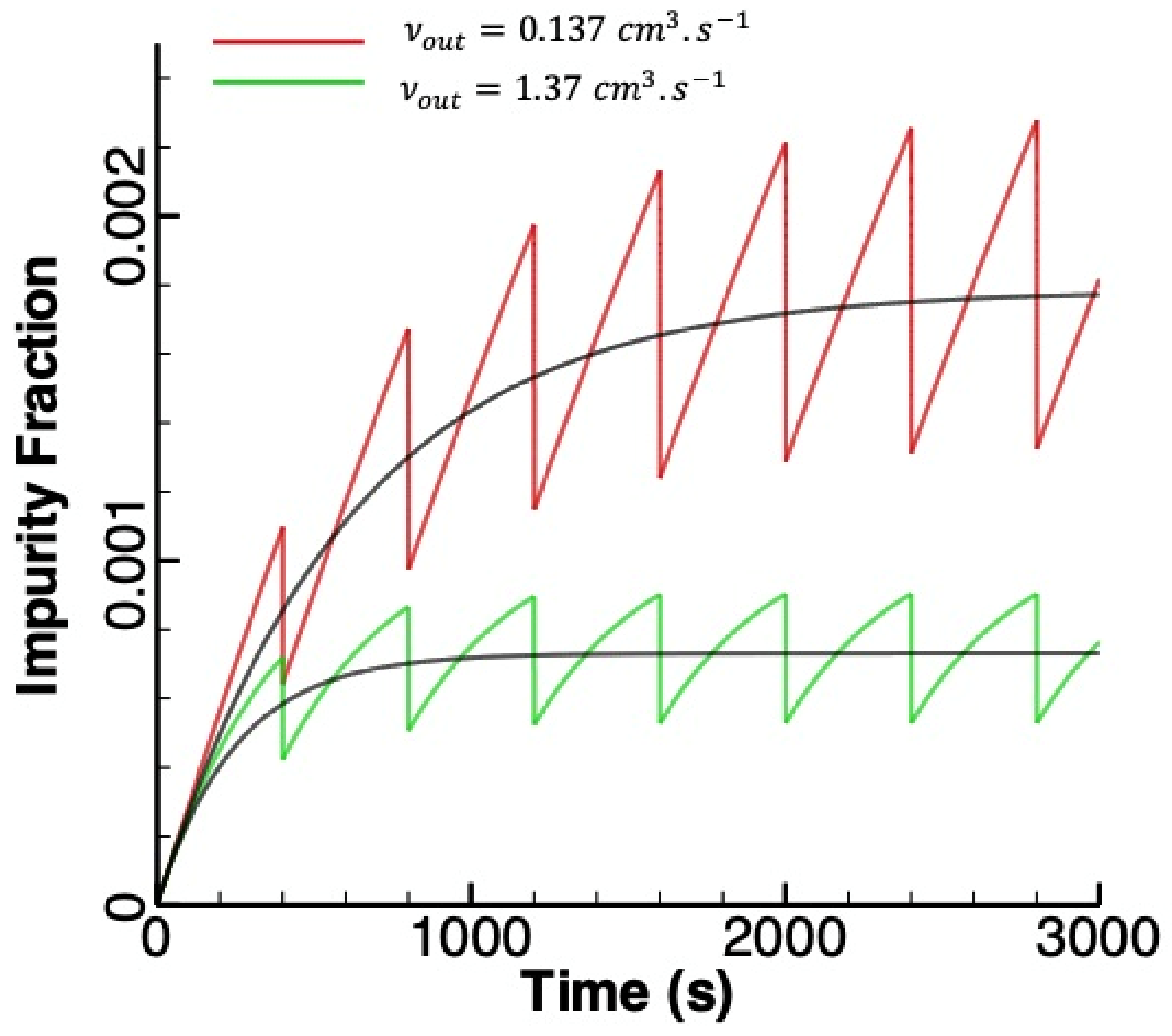
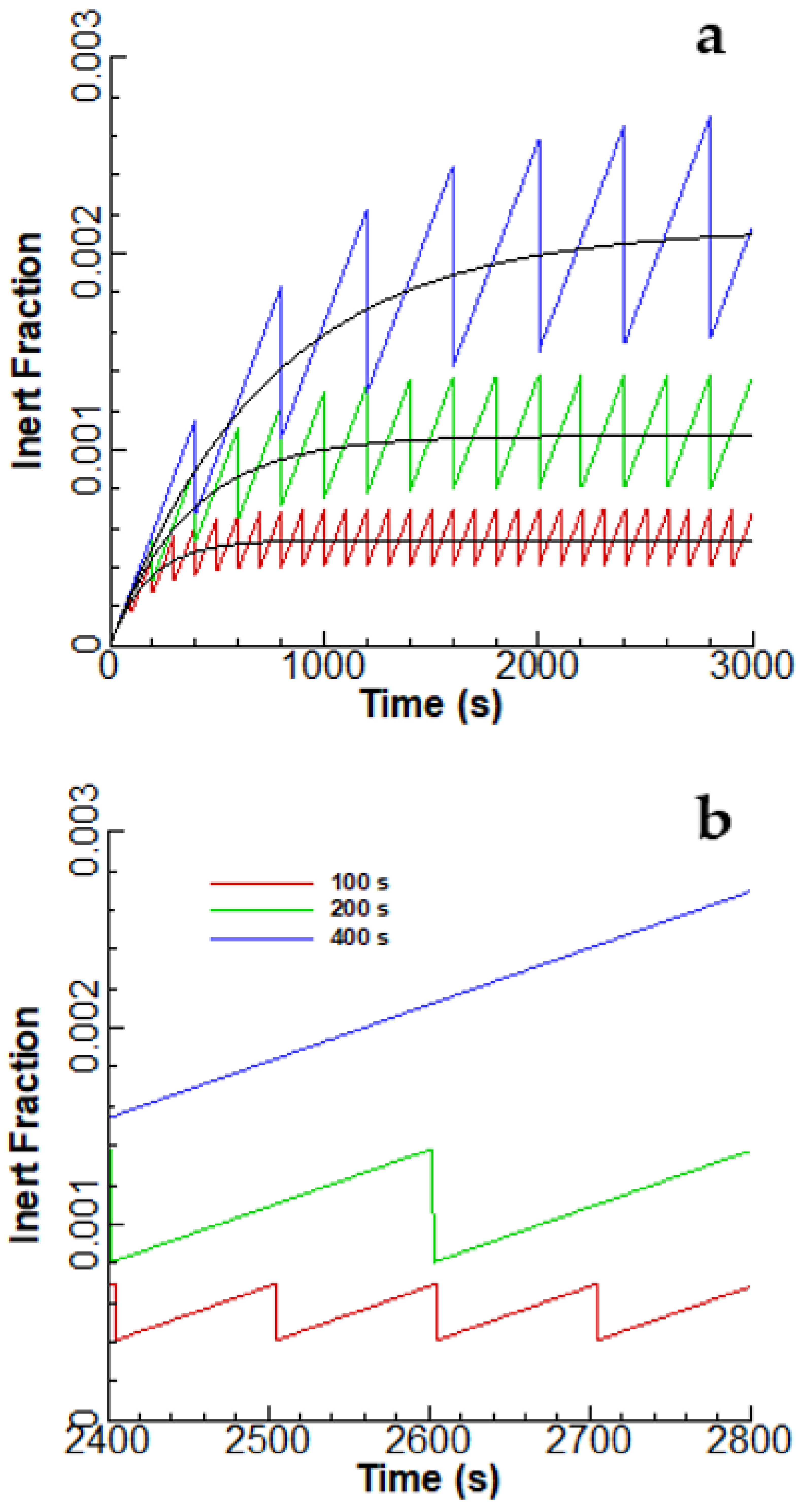
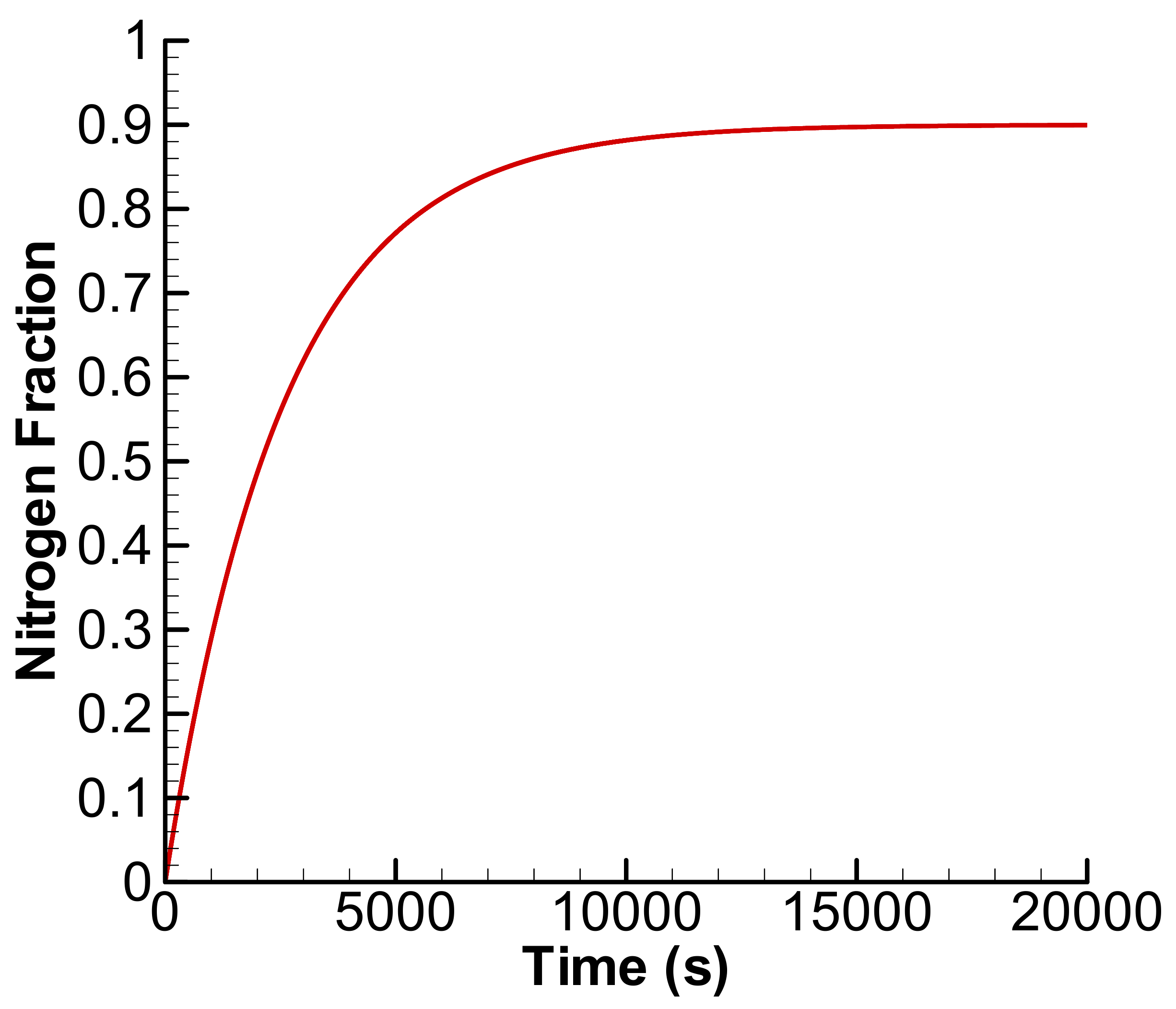
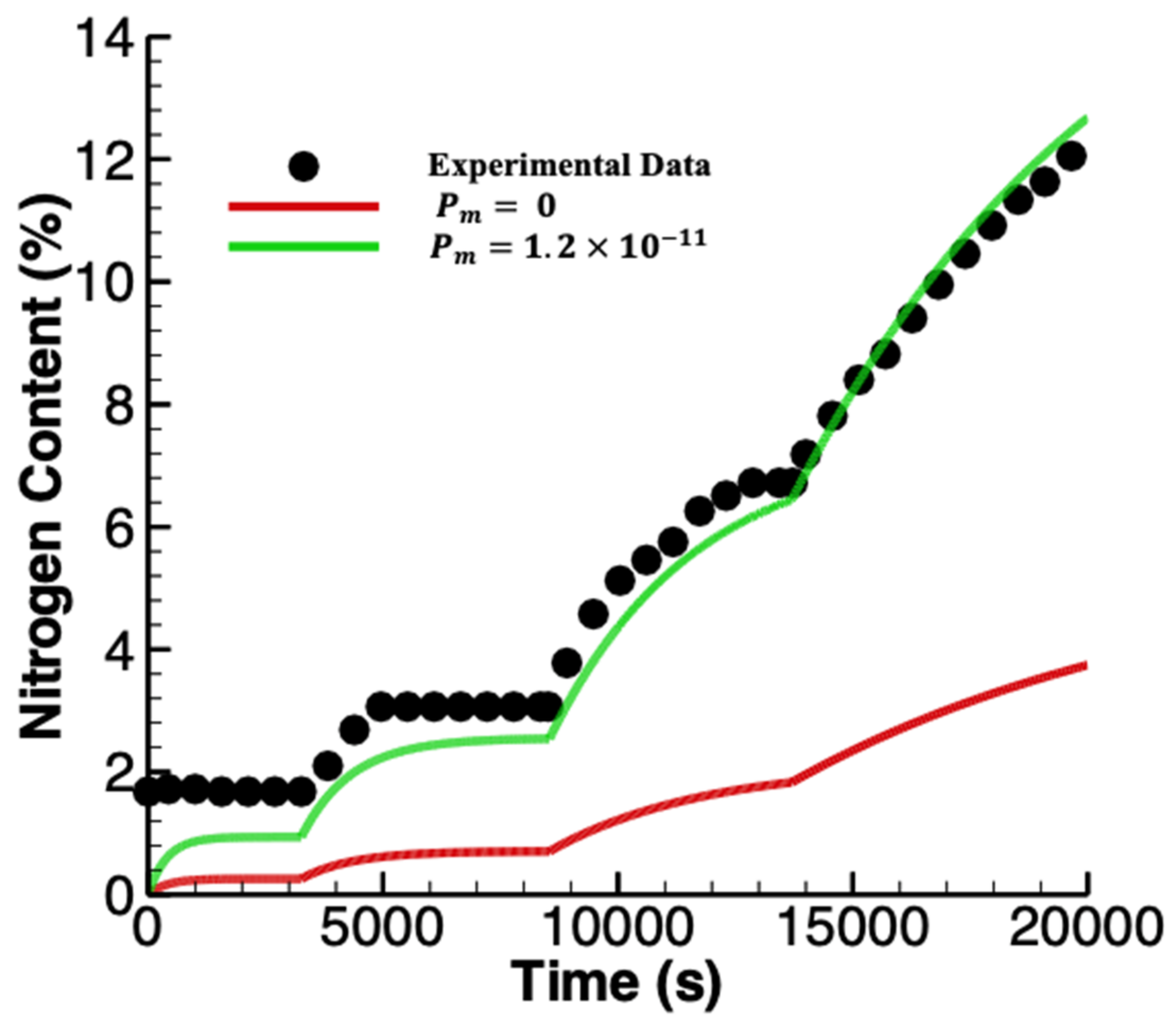
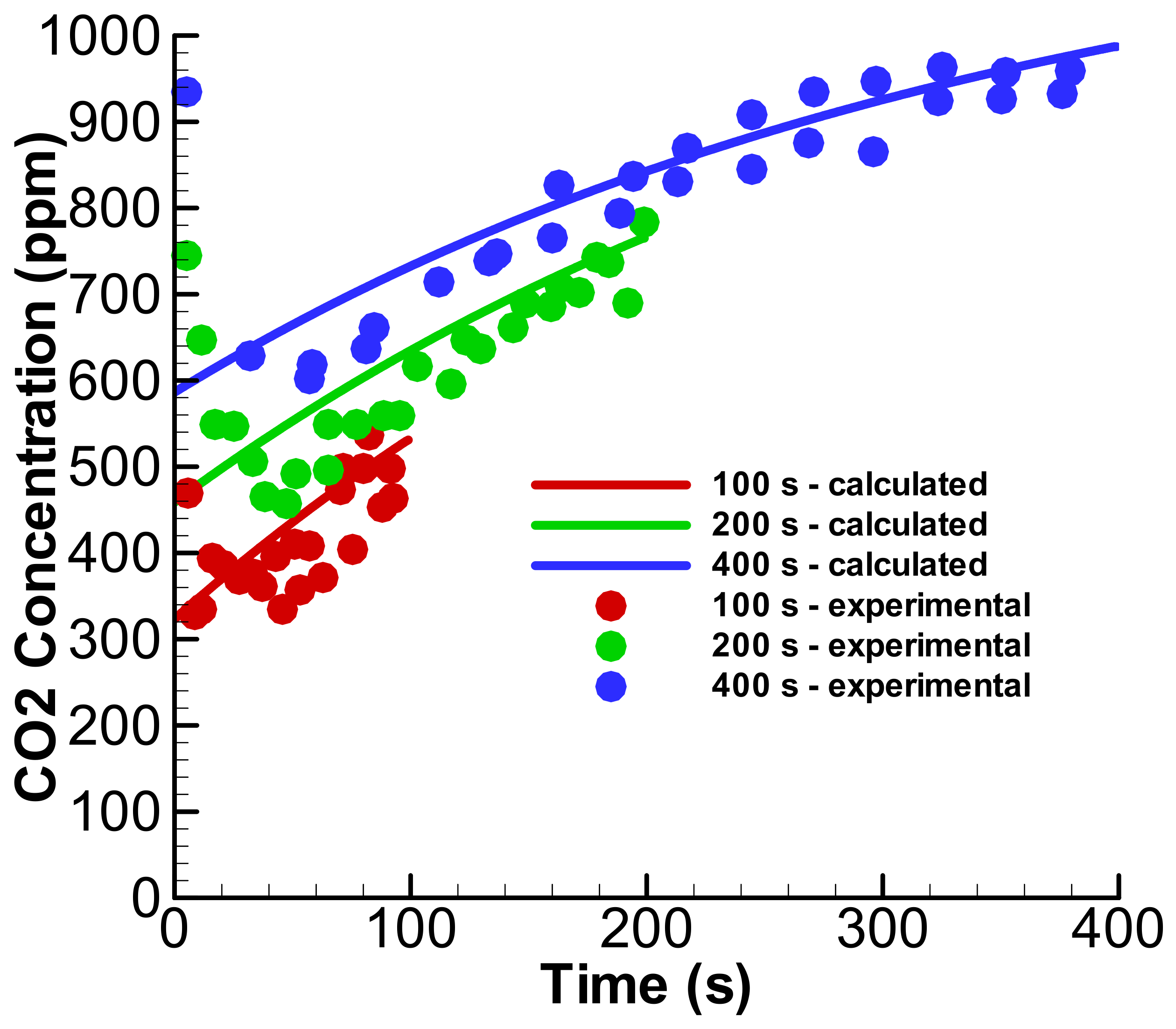
3.1. Linear Accumulation—No Significant Loss from the Anode Chamber
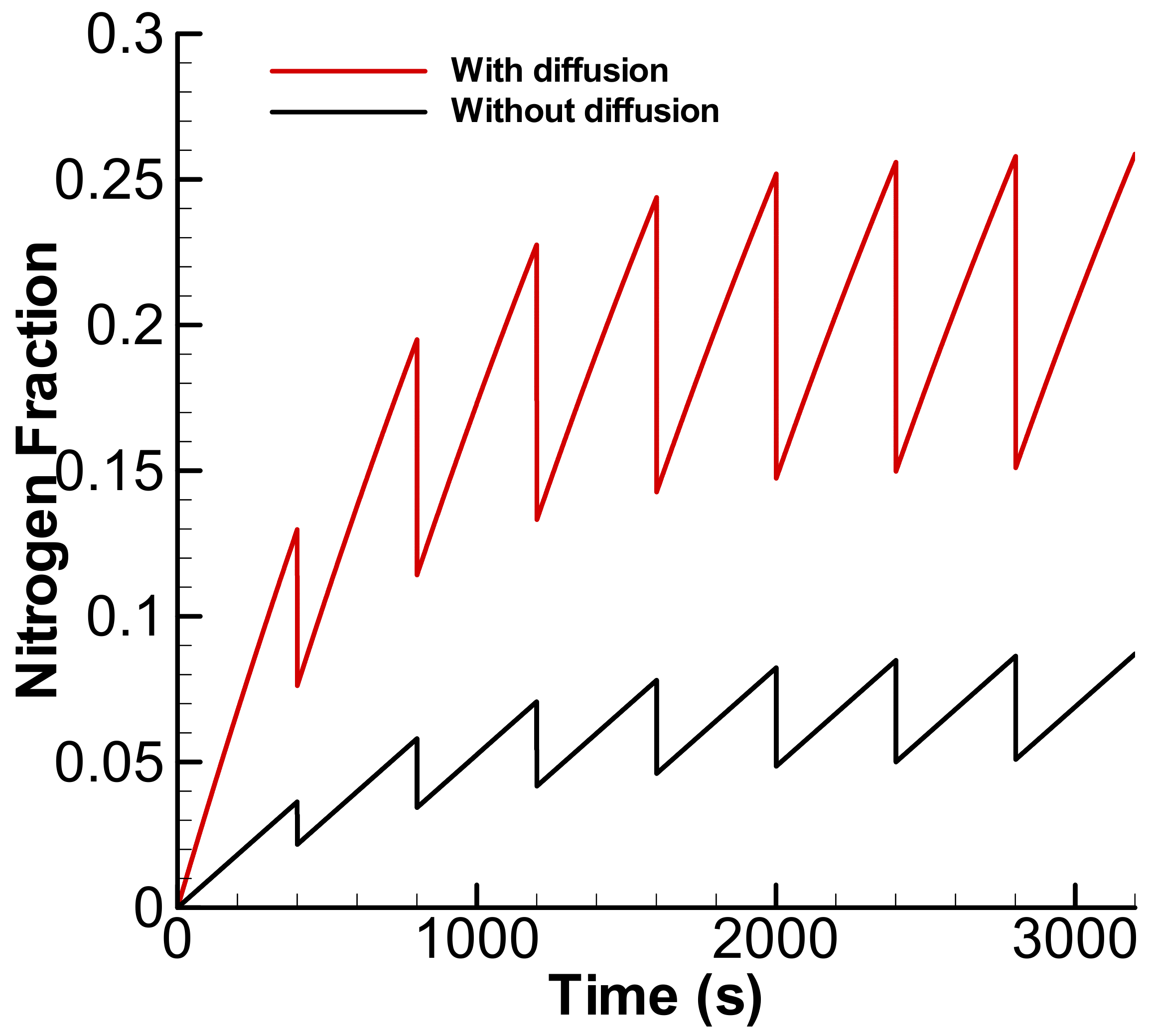
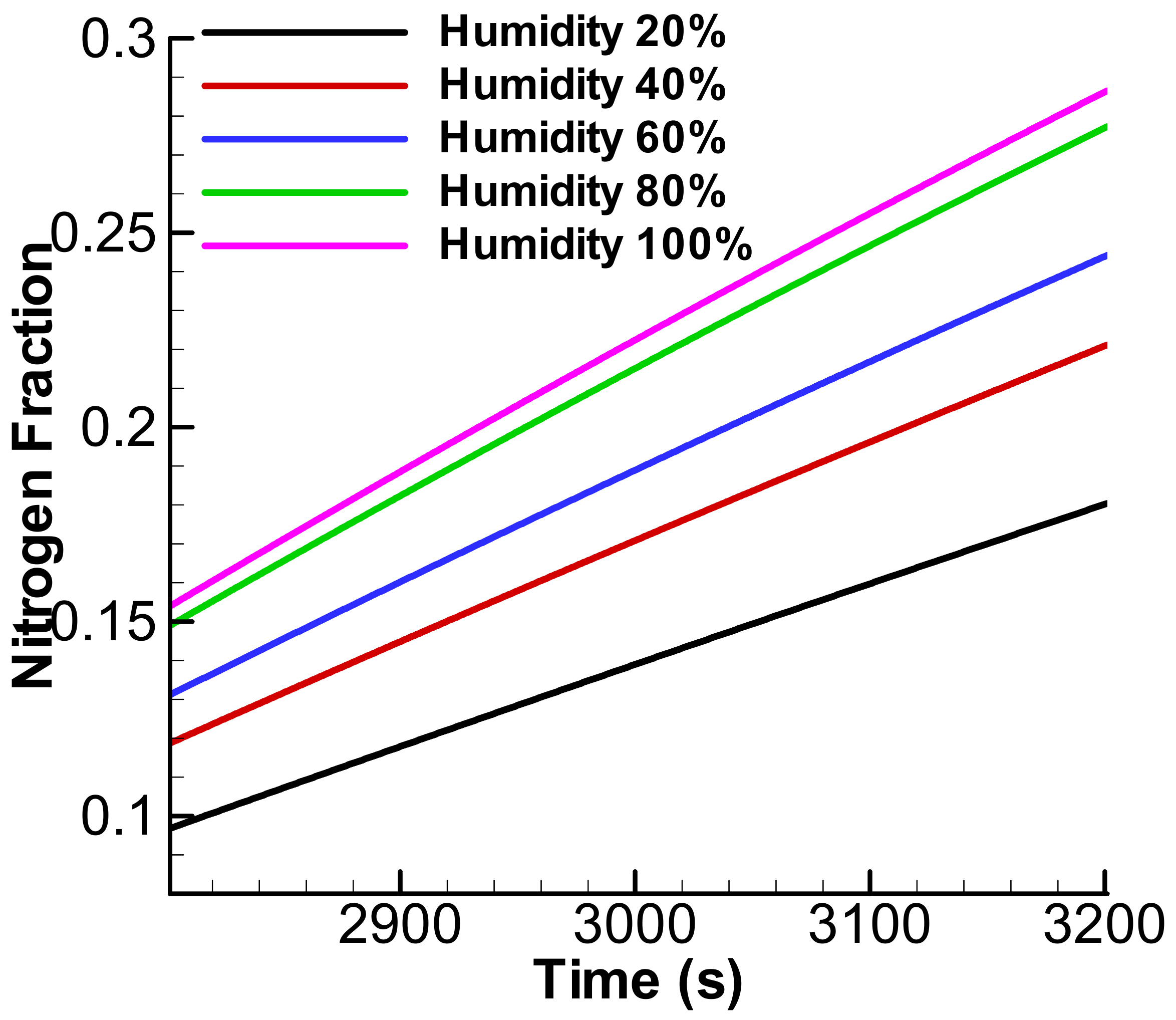
3.2. Exponential Accumulation—Diffusion through the Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurnia, J.C.; Sasmito, A.P.; Shamim, T. Advances in proton exchange membrane fuel cell with dead-end anode operation: A review. Appl. Energy. 2019, 252, 113416. [Google Scholar] [CrossRef]
- Hwang, J.J. Effect of hydrogen delivery schemes on fuel cell efficiency. J. Power Sources 2013, 239, 54–63. [Google Scholar] [CrossRef]
- Meyers, J.P.; Darling, R.M. Model of Carbon Corrosion in PEM Fuel Cells. J. Electrochem. Soc. 2006, 153, A1432–A1442. [Google Scholar] [CrossRef]
- Tang, H.; Qi, Z.; Ramani, M.; Elter, J.F. PEM fuel cell cathode carbon corrosion due to the formation of air/fuel boundary at the anode. J. Power Sources 2006, 158, 1306–1312. [Google Scholar] [CrossRef]
- Matsuura, T.; Chen, J.; Siegel, J.B.; Stefanopoulou, A.G. Degradation phenomena in PEM fuel cell with dead-ended anode. Int. J. Hydrogen Energy 2013, 38, 11346–11356. [Google Scholar] [CrossRef]
- Karimaki, H.; Perez, L.C.; Nikiforow, K.; Keranen, T.M.; Viitakangas, J.; Ihonen, J. The use of on-line hydrogen sensor for studying inert gas effects and nitrogen crossover in PEMFC system. Int. J. Hydrogen Energy 2011, 36, 10179–10187. [Google Scholar] [CrossRef]
- Nikiforow, K.; Karimäki, H.; Keränen, T.M.; Ihonen, J. Optimization study of purge cycle in proton exchange membrane fuel cell system. J. Power Sources 2013, 238, 336–344. [Google Scholar] [CrossRef]
- Matsuda, Y.; Hashimasa, Y.; Imamura, D.; Akai, M.; Watanabe, S. Accumulation Behavior of Impurities in Fuel Cell Hydrogen Circulation System. Rev. Automot. Eng. 2009, 30, 167–172. [Google Scholar] [CrossRef]
- Matsuda, Y.; Shimizu, T.; Hashimasa, Y. Effect of Carbon Monoxide on Polymer Electrolyte Fuel Cell Performance with a Hydrogen Circulation System. J. Electrochem. Soc. 2020, 167, 044509. [Google Scholar] [CrossRef]
- Koski, P.; Pérez, L.C.; Ihonen, J. Comparing Anode Gas Recirculation with Hydrogen Purge and Bleed in a Novel PEMFC Laboratory Test Cell Configuration. Fuel Cells 2015, 15, 494–504. [Google Scholar] [CrossRef]
- Koski, P.; Viitakangas, J.; Ihonen, J. Determination of fuel utilisation and recirculated gas composition in dead-ended PEMFC systems. Int. J. Hydrogen Energy 2020, 45, 23201–23226. [Google Scholar] [CrossRef]
- Chen, J.; Siegel, J.B.; Stefanopoulou, A.G.; Waldecker, J.R. Optimization of purge cycle for dead-ended anode fuel cell operation. Int. J. Hydrogen Energy 2013, 38, 5092–5105. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Wang, X. Buildup of nitrogen in direct hydrogen polymer-electrolyte fuel cell stacks. J. Power Sources 2007, 171, 63–71. [Google Scholar] [CrossRef]
- Promislow, K.; St-Pierre, J.; Wetton, B. A simple, analytic model of polymer electrolyte membrane fuel cell anode recirculation at operating power including nitrogen crossover. J. Power Sources 2011, 196, 10050–10056. [Google Scholar] [CrossRef]
- Gardner, C.L.; Sarma, P.; Mehta, D.; Chugh, S.; Kjeang, E. Accumulation of CO and other fuel impurities in the anode recirculation loop of a fuel cell: A zero dimensional transient model. J. Power Sources 2021, 489, 229420. [Google Scholar] [CrossRef]
- Bhatia, K.K.; Wang, C.-Y. Transient carbon monoxide poisoning of a polymer electrolyte fuel cell operating on diluted hydrogen feed. Electrochim. Acta 2004, 49, 2333–2341. [Google Scholar] [CrossRef]
- Zhang, J. Investigation of CO Tolerance in Proton Exchange Membrane Fuel Cells. Ph.D. Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, June 2004. [Google Scholar]
- Zhang, J.; Datta, R. Sustained Potential Oscillations in Proton Exchange Membrane Fuel Cells with PtRu as Anode Catalyst. J. Electrochem. Soc. 2002, 149, A1423. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Fehribach, J.D.; Datta, R. Mechanistic and Bifurcation Analysis of Anode Potential Oscillations in PEMFCs with CO in Anode Feed. J. Electrochem. Soc. 2004, 151, A689–A697. [Google Scholar] [CrossRef]
- Farrell, C.G.; Gardner, C.L.; Ternan, M. Experimental and modelling studies of CO poisoning in PEM fuel cells. J. Power Sources 2007, 171, 282–293. [Google Scholar] [CrossRef]
- Hanke-Rauschenbach, R.; Weinzierl, C.; Krasnyk, M.; Rihko-Struckmann, L.; Lu, H.; Sundmacher, K. Operating Behavior and Scale-Up of an ECPrOx Unit for CO Removal from Reformate for PEM Fuel Cell Application. J. Electrochem. Soc. 2009, 156, B1267. [Google Scholar] [CrossRef]
- Steinberger, M.; Geiling, J.; Oechsnera, R.; Freya, L. Anode recirculation and purge strategies for PEM fuel cell operation with diluted hydrogen feed gas. Appl. Energy 2018, 232, 572–582. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Bethune, K.; Rubio, M.A.; Rocheleau, R. Study of low concentration CO poisoning of Pt anode in a protonexchange membrane fuel cell using spatial electrochemical impedancspectroscopy. J. Power Sources 2014, 269, 344–362. [Google Scholar] [CrossRef]
- Moore, W.J. Physical Chemistry; Prentice-Hall Inc.: Upper Saddle River, NJ, USA, 1955; p. 533. [Google Scholar]
- Mittelstaedt, C.; Umbrell, M. Gas Permeability in Perfluorinated Sulfonic Acid Polymer Electrolyte Membranes. In Proceedings of the 207th Electrochemical Society Meeting, Abstract # 770, Quebec City, QC, Canada, 15–20 May 2005. [Google Scholar] [CrossRef]
- Springer, T.E.; Rockward, T.; Zawodzinski, T.A.; Gottesfeld, S. Model for Polymer Electrolyte Fuel Cell Operation on Reformate Feed: Effects of CO, H2 Dilution, and High Fuel Utilization. J. Electrochem. Soc. 2001, 148, A11–A23. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lai, W.-H.; Yan, W.-M.; Chen, C.-C.; Hsu, S.-W. Effects of nitrogen and carbon monoxide concentrations on performance of proton exchange membrane fuel cells with Pt–Ru anodic catalyst. J. Power Sources 2013, 243, 138–146. [Google Scholar] [CrossRef]
- Sarma, P.J.; Gardner, C.L.; Chugh, S.; Sharma, A.; Kjeang, E. Strategic implementation of pulsed oxidation for mitigation of CO poisoning in polymer electrolyte fuel cells. J. Power Sources 2020, 468, 228352. [Google Scholar] [CrossRef]
- Ren, X.; Myles, T.D.; Grew, K.N.; Chiu, W.K.S. Carbon Dioxide Transport in Nafion 1100 EW Membrane and in a Direct Methanol Fuel Cell. J. Electrochem. Soc. 2015, 162, F1221–F1230. [Google Scholar] [CrossRef]
- Erbach, S.; Epple, S.; Heinen, M.; Toth, G.; Klages, M.; Gaudreau, D.; Ages, M.; Putz, A. CO2 Enrichment in Anode Loop and Correlation with CO Poisoning of Low Pt Anodes in PEM Fuel Cells. Fuel Cells 2018, 18, 613–618. [Google Scholar] [CrossRef]


| Symbol | Meaning | Value | Unit |
|---|---|---|---|
| Anode volume | 564 | cm3 | |
| Purge volume | 282 | cm3 | |
| Concentration of species i in the anode chamber | Variable | mol/L | |
| Concentration of species i in the cathode chamber | Variable | mol/L | |
| Mole fraction of species i in the anode chamber | Variable | ||
| Mole fraction of species i in the cathode chamber | Variable | ||
| Permeability of species i | Variable | mol·cm−1·s−1.atm | |
| Cell area | 25 | cm2 | |
| Anode pressure | 1.2 | atm | |
| Cathode pressure | 1 | atm | |
| Normal pressure | 1 | atm | |
| Membrane thickness | 0.0018 | cm | |
| Flow rate | Variable | scc/s | |
| Gas constant | 8.314 | Latm/mol | |
| Time | Variable | s | |
| Purge time | 0.2 | s | |
| Current density | 0.6 | A/cm2 | |
| Operating Temperature | 353 | K | |
| Standard temperature | 298 | K |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardner, C.L.; Sarma, P.J.; Kjeang, E. Accumulation of Inert Impurities in a Polymer Electrolyte Fuel Cell System with Anode Recirculation and Periodic Purge: A Simple Analytical Model. Energies 2022, 15, 1968. https://doi.org/10.3390/en15061968
Gardner CL, Sarma PJ, Kjeang E. Accumulation of Inert Impurities in a Polymer Electrolyte Fuel Cell System with Anode Recirculation and Periodic Purge: A Simple Analytical Model. Energies. 2022; 15(6):1968. https://doi.org/10.3390/en15061968
Chicago/Turabian StyleGardner, Christopher L., Paran J. Sarma, and Erik Kjeang. 2022. "Accumulation of Inert Impurities in a Polymer Electrolyte Fuel Cell System with Anode Recirculation and Periodic Purge: A Simple Analytical Model" Energies 15, no. 6: 1968. https://doi.org/10.3390/en15061968
APA StyleGardner, C. L., Sarma, P. J., & Kjeang, E. (2022). Accumulation of Inert Impurities in a Polymer Electrolyte Fuel Cell System with Anode Recirculation and Periodic Purge: A Simple Analytical Model. Energies, 15(6), 1968. https://doi.org/10.3390/en15061968







