Enhanced Bio-Oil Yield from Thermal Decomposition of Peanut Shells Using Termite Hill as the Catalyst
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Characterization of Catalyst
2.3. Pyrolysis-GC/MS
2.4. Kinetic Study
3. Results and Discussion
3.1. Catalyst Characterization
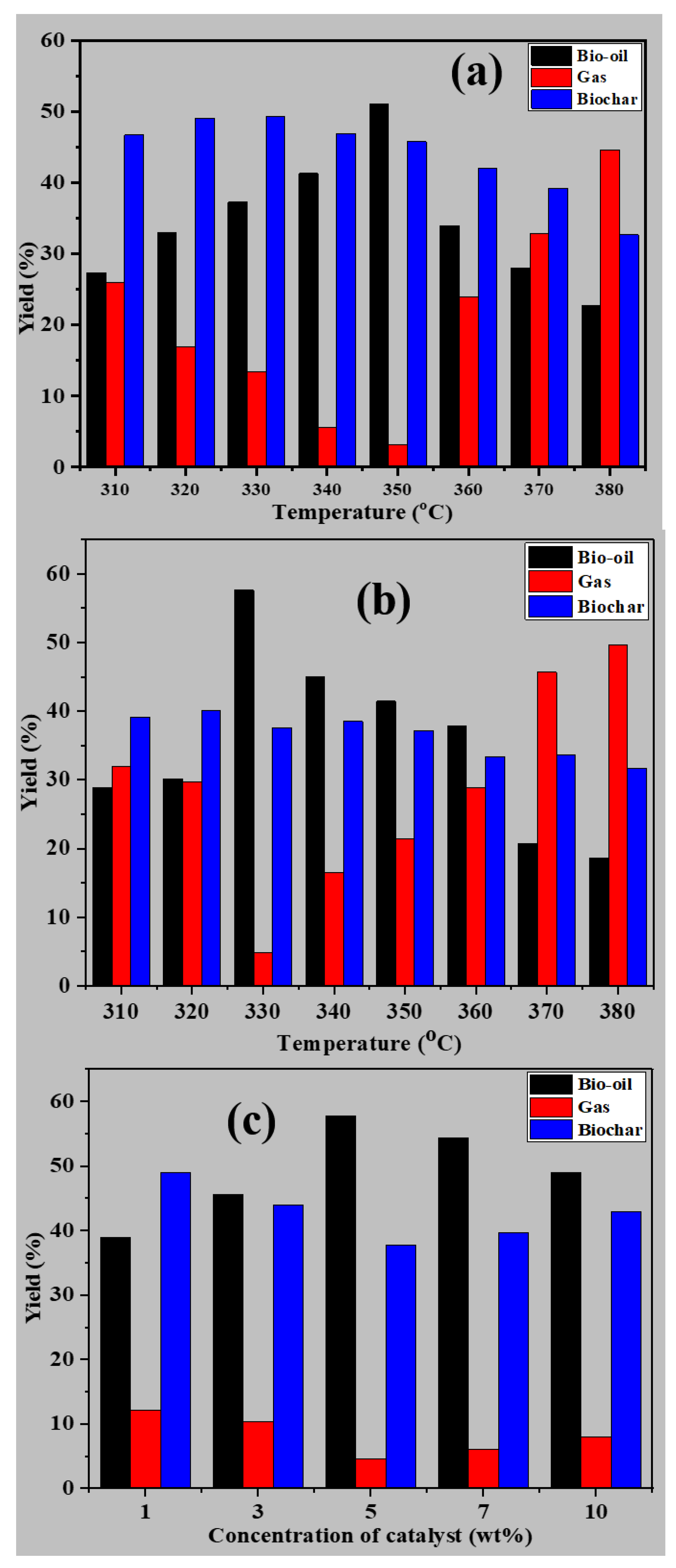
3.2. Pyrolysis of Peanut Shells and Bio-Oil Characterization
3.3. Kinetic Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Demirbas, A. Recent advances in biomass conversion technologies. Energy Edu. Sci. Technol. 2000, 6, 19–40. [Google Scholar]
- Alhumade, H.; da Silva, J.C.G.; Ahmad, M.S.; Çakman, G.; Yıldız, A.; Ceylan, S.; Elkamel, A. Investigation of pyrolysis kinetics and thermal behavior of Invasive Reed Canary (Phalaris arundinacea) for bioenergy potential. J. Anal. Appl. Pyrolysis 2019, 140, 385–392. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, G.K.; Mondal, M.K. Slow pyrolysis of chemically treated walnut shell for valuable products: Effect of process parameters and in-depth product analysis. Energy 2019, 181, 665–676. [Google Scholar] [CrossRef]
- Farhad, S.; Saffar-Avval, M.; Younessi-Sinaki, M. Efficient design of feedwater heaters network in steam power plants using pinch technology and exergy analysis. Int. J. Energy Res. 2008, 32, 1–11. [Google Scholar] [CrossRef]
- Danje, S. Fast Pyrolysis of Corn Residues for Energy Production; Stellenbosch University: Stellenbosch, South Africa, 2011. [Google Scholar]
- Anspaugh, L.R.; Catlin, R.J.; Goldman, M. The global impact of the Chernobyl reactor accident. Science 1988, 242, 1513–1519. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Was, G. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Rathore, N.S.; Panwar, N. Renewable Energy Sources for Sustainable Development; New India Publishing: Delhi, India, 2007. [Google Scholar]
- Lusas, E. Food uses of peanut protein. J. Am. Oil Chem. Soc. 1979, 56, 425–430. [Google Scholar] [CrossRef]
- Torres-García, E.; Ramírez-Verduzco, L.; Aburto, J. Pyrolytic degradation of peanut shell: Activation energy dependence on the conversion. Waste Manag. 2020, 106, 203–212. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, R.; Li, X.; Li, Y.; Shi, W.; Ren, X.; Xu, X. Bench-scale fluidized-bed fast pyrolysis of peanut shell for bio-oil production. Environ. Prog. Sustain. Energy 2011, 30, 11–18. [Google Scholar] [CrossRef]
- Messina, L.G.; Bonelli, P.R.; Cukierman, A.L. Effect of acid pretreatment and process temperature on characteristics and yields of pyrolysis products of peanut shells. Renew. Energy 2017, 114, 697–707. [Google Scholar] [CrossRef]
- Mamaeva, A.; Tahmasebi, A.; Yu, J. The effects of mineral salt catalysts on selectivity of phenolic compounds in bio-oil during microwave pyrolysis of peanut shell. Korean J. Chem. Eng. 2017, 34, 672–680. [Google Scholar] [CrossRef]
- Nisar, J.; Rahman, A.; Ali, G.; Shah, A.; Farooqi, Z.H.; Bhatti, I.A.; Iqbal, M.; Ur Rehman, N. Pyrolysis of Almond Shells Waste: Effect of Zinc Oxide on Kinetics and Product Distribution. Biomass Convers. Biorefinery 2020, 1–13. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Millogo, Y.; Hajjaji, M.; Morel, J.C. Microstructure, physical and mechanical properties of clayey material from termite mound: A stabilized material for adobe building. Appl. Clay Sci. 2011, 51, 160–164. [Google Scholar] [CrossRef]
- Ayanda, O.S.; Amodu, O.S.; Adubiaro, H.; Olutona, G.O.; Ebenezer, O.T.; Nelana, S.M.; Naidoo, E.B. Effectiveness of termite hill as an economic adsorbent for the adsorption of alizarin red dye. J. Water Reuse Desalination 2019, 9, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Mahamat, A.A.; Azeko, S.T. Mechanical and structural properties of termite soil as a partial replacement to cement for different applications. Mater. Sci. 2018, 2, 1. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Azeez, T.M.; Babatunde, E.O. Titania-termite hill composite as a heterogeneous catalyst: Preparation, characterization, and performance in transesterification of waste frying oil. Int. J. Chem. React. Eng. 2020, 18, 10–11. [Google Scholar] [CrossRef]
- Yusuff, A.S. Photocatalytic degradation of cationic dye in aqueous solution by TiO2 nanoparticle immobilized on termite hill soil. React. Kinet. Mech. Catal. 2020, 131, 979–995. [Google Scholar] [CrossRef]
- Folorunso, D.; Aribo, S.; Olaniran, O. Performance Evaluation of Insulating Firebricks Produced from Hydrometallurgically Purified Termite Hill Clay Reinforced with Alumina. Am. J. Eng. Res. 2015, 4, 1–7. [Google Scholar]
- Yusuff, A.S.; Bello, K.A.; Azeez, T.M. Photocatalytic degradation of an anionic dye in aqueous solution by visible light responsive zinc oxide-termite hill composite. Reaction Kinetics. Mech. Catal. 2020, 131, 537–554. [Google Scholar]
- Zanardi, S.; Cruciani, G.; Alberti, A.; Galli, E. Dehydration and rehydration process in boggsite: An in situ X-ray single-crystal study. Am. Mineral. 2004, 89, 1033–1042. [Google Scholar] [CrossRef]
- Dent, L.; Smith, J. Crystal structure of chabazite, a molecular sieve. Nature 1958, 181, 1794–1796. [Google Scholar] [CrossRef]
- Burt, J.B.; Ross, N.L.; Angel, R.J.; Koch, M. Equations of state and structures of andalusite to 9.8 GPa and sillimanite to 8.5 GPa. Am. Mineral. 2006, 91, 319–326. [Google Scholar] [CrossRef]
- Gao, M.Y.; Wang, F.; Gu, Z.G.; Zhang, D.X.; Zhang, L.; Zhang, J. Fullerene-like Polyoxotitanium cage with high solution stability. J. Am. Chem. Soc. 2016, 138, 2556–2559. [Google Scholar] [CrossRef]
- Zachariasen, W. Skrifter utgitt av det Norske Videnskaps Akademi i Oslo 1: Matematisk Naturvidenskapelig Klasse. 1928. [Google Scholar]
- Swamy, V.; Dubrovinsky, L.S.; Dubrovinskaia, N.A.; Langenhorst, F.; Simionovici, A.S.; Drakopoulos, M.; Dmitriev, V.; Weber, H.P. Size effects on the structure and phase transition behavior of baddeleyite TiO2. Solid State Commun. 2005, 134, 541–546. [Google Scholar] [CrossRef]
- Pecharromán, C.; Gonzalez-Carreno, T.; Iglesias, J.E. The infrared dielectric properties of maghemite, γ-Fe2O3, from reflectance measurement on pressed powders. Phys. Chem. Miner. 1995, 22, 21–29. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Inneren Struktur und der Größe von Kolloidteilchen Mittels Röntgenstrahlen, Kolloidchemie Ein Lehrbuch; Springer: Berlin/Heidelberg, Germany, 1912; pp. 387–409. [Google Scholar]
- Millogo, Y.; Hajjaji, M.; Morel, J. Physical properties, microstructure and mineralogy of termite mound material considered as construction materials. Appl. Clay Sci. 2011, 52, 160–164. [Google Scholar] [CrossRef]
- Mujinya, B.B.; Mees, F.; Erens, H.; Dumon, M.; Baert, G.; Boeckx, P.; Ngongo, M.; Van Ranst, E. Clay composition and properties in termite mounds of the Lubumbashi area, DR Congo. Geoderma 2013, 192, 304–315. [Google Scholar] [CrossRef]
- Pütün, E.; Uzun, B.B.; Pütün, A.E. Production of bio-fuels from cottonseed cake by catalytic pyrolysis under steam atmosphere. Biomass Bioenergy 2006, 30, 592–598. [Google Scholar] [CrossRef]
- Aho, A.; Kumar, N.; Eränen, K.; Salmi, T.; Hupa, M.; Murzin, D.Y. Catalytic pyrolysis of woody biomass in a fluidized bed reactor: Influence of the zeolite structure. Fuel 2008, 87, 2493–2501. [Google Scholar] [CrossRef]
- Messina, L.G.; Bonelli, P.R.; Cukierman, A.L. In-situ catalytic pyrolysis of peanut shells using modified natural zeolite. FuelProcessing Technol. 2017, 159, 160–167. [Google Scholar]
- Lopez-Velazquez, M.; Santes, V.; Balmaseda, J.; Torres-Garcia, E. Pyrolysis of orange waste: A thermo-kinetic study. J. Anal. Appl. Pyrolysis 2013, 99, 170–177. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-depth investigation of biomass pyrolysis based on three major components: Hemicellulose, cellulose and lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Xiang, Z.; Liang, J.; Morgan, H.M., Jr.; Liu, Y.; Mao, H.; Bu, Q. Thermal behavior and kinetic study for co-pyrolysis of lignocellulosic biomass with polyethylene over Cobalt modified ZSM-5 catalyst by thermogravimetric analysis. Bioresour. Technol. 2018, 247, 804–811. [Google Scholar] [CrossRef]
- Loy, A.C.M.; Gan, D.K.W.; Yusup, S.; Chin, B.L.F.; Lam, M.K.; Shahbaz, M.; Unrean, P.; Acda, M.N.; Rianawati, E. Thermogravimetric kinetic modelling of in-situ catalytic pyrolytic conversion of rice husk to bioenergy using rice hull ash catalyst. Bioresour. Technol. 2018, 261, 213–222. [Google Scholar] [CrossRef] [PubMed]
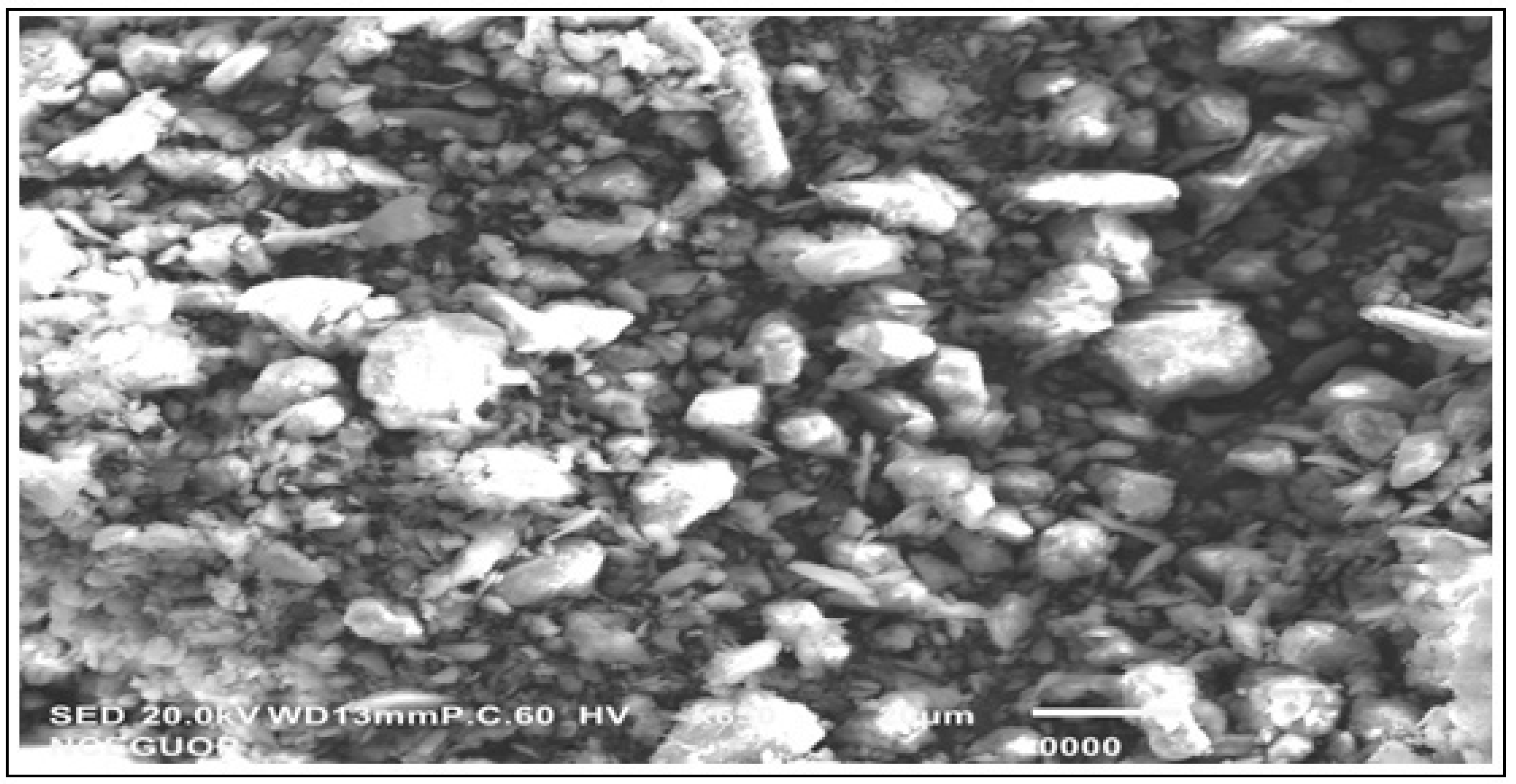


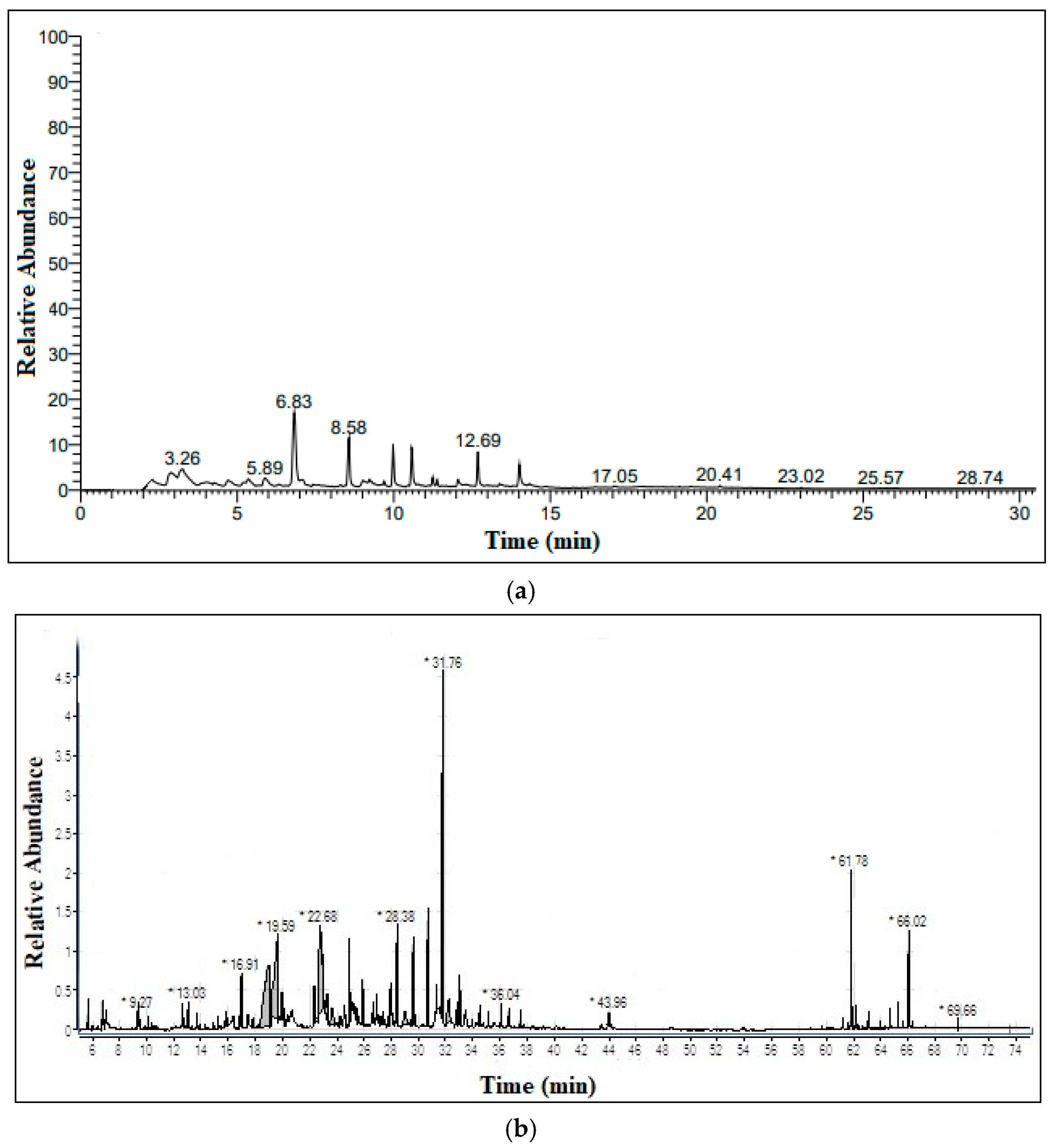

| S.No | Compound | %Age |
|---|---|---|
| 01 | SiO2 | 69.030 |
| 02 | Al2O3 | 21.990 |
| 03 | K2O | 4.154 |
| 04 | CaO | 3.342 |
| 05 | TiO | 1.273 |
| 06 | SO3 | 0.120 |
| 07 | V2O5 | 0.053 |
| 08 | Cr2O3 | 0.037 |
| S.No | R/Time | Component | Chem. Formula | M.wt. | %Area |
|---|---|---|---|---|---|
| (a) | |||||
| 1 | 2.26 | Pentanoic acid, 4-methyl- | C6H12O2 | 116 | 2.081833 |
| 2 | 2.89 | Furfural | C5H4O2 | 96 | 8.101473 |
| 3 | 3.26 | 2-Furanmethanol | C5H6O2 | 98 | 12.11129 |
| 4 | 4.05 | Cyclohexanol, 2,4-dimethyl- | C8H16O | 128 | 2.864157 |
| 5 | 4.71 | 2-Furancarboxaldehyde, 5-methyl- | C6H6O2 | 110 | 2.536825 |
| 6 | 5.36 | 1-Hydroxy-2-pentanone | C5H10O2 | 102 | 3.436989 |
| 7 | 5.89 | 1,2-Cyclopentanedione, 3-methyl- | C6H8O2 | 112 | 3.191489 |
| 8 | 6.34 | 1,2-Butanediol, 1-phenyl- | C10H14O2 | 166 | 1.163666 |
| 9 | 6.83 | Phenol, 2-methoxy- | C7H8O2 | 124 | 24.05892 |
| 10 | 7.44 | Undecanoic acid, hydroxy-, lactone | C11H20O2 | 184 | 1.572831 |
| 11 | 8.58 | Phenol, 2-methoxy-4-methyl- | C8H10O2 | 138 | 9.001637 |
| 12 | 9.23 | Lactose | C12H22O11 | 342 | 5.237316 |
| 13 | 9.98 | Phenol, 4-ethyl-2-methoxy- | C9H12O2 | 152 | 6.382979 |
| 14 | 10.57 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150 | 6.95581 |
| 15 | 11.25 | Phenol, 2-methoxy-6-(2-propenyl)- | C10H12O2 | 164 | 1.14566 |
| 16 | 12.06 | Phenol, 2-methoxy-5-(1-propenyl)- | C10H12O2 | 164 | 2.209493 |
| 17 | 12.69 | Phenol, 2-methoxy-4-(1-propenyl)- | C10H12O2 | 164 | 4.746318 |
| 18 | 13.38 | Ethanone, 1-(4-hydroxy-3-methoxyphenyl)- | C9H10O3 | 166 | 0.490998 |
| 19 | 14.34 | 3-Lauramidobenzoic acid | C19H29NO3 | 319 | 0.736498 |
| 20 | 17.05 | Tertubryn | C10H19N5S | 241 | 0.163666 |
| 21 | 20.41 | 10-Octadecenoic acid, methyl ester | C19H36O2 | 296 | 0.163666 |
| 22 | 23.02 | 1,2-Benzenedicarboxylic acid, diisooctyl ester | C24H38O4 | 390 | 0.081833 |
| (b) | |||||
| 1 | 5.6 | p-Dioxane-2,3-diol | C4H8O4 | 120 | 0.57 |
| 2 | 9.27 | Ethane, 1,2-bis[(4-amino-3-furazanyl)oxy]- | C6H8N6O4 | 228 | 0.37 |
| 3 | 12.57 | Cyclopentanone | C5H8O | 84 | 0.56 |
| 4 | 13.03 | 1,2-Cyclopentanedione | C5H6O2 | 98 | 0.6 |
| 5 | 13.65 | 2,5-Furandione, 3-methyl- | C5H4O3 | 112 | 0.31 |
| 6 | 15.82 | Pyridine, 3-methoxy- | C6H7NO | 109 | 0.26 |
| 7 | 16.91 | 1,2-Cyclopentanedione, 3-methyl- | C6H8O2 | 112 | 2.53 |
| 8 | 18.84 | Tetrahydro[2,2’]bifuranyl-5-one | C8H12O3 | 156 | 13.22 |
| 9 | 19.59 | Cyclopropyl carbinol | C4H8O | 72 | 11.36 |
| 10 | 19.9 | Maltol | C6H6O3 | 126 | 0.75 |
| 11 | 22.27 | Phenol, 2-methoxy-4-methyl- | C8H10O2 | 138 | 0.74 |
| 12 | 22.68 | 1,2-Benzenediol | C6H6O2 | 110 | 12.72 |
| 13 | 23.21 | 1,4:3,6-Dianhydro-α-d-glucopyranose | C6H8O4 | 144 | 1.25 |
| 14 | 23.58 | Furancarboxaldehyde, 5-(hydroxymethyl)- | C6H6O3 | 126 | 1.62 |
| 15 | 24.84 | Phenol, 4-ethyl-2-methoxy- | C9H12O2 | 152 | 2 |
| 16 | 25.86 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150 | 1 |
| 17 | 26.62 | 2(3H)-Furanone, 5-heptyldihydro- | C11H20O2 | 184 | 0.72 |
| 18 | 26.86 | 2,4-Dimethoxyphenol | C8H10O3 | 154 | 1.01 |
| 19 | 27.3 | Benzenemethanol, α-ethyl-4-methoxy- | C10H14O2 | 166 | 0.22 |
| 20 | 27.89 | 4-Ethylcatechol | C8H10O2 | 138 | 1.13 |
| 21 | 28.38 | Benzaldehyde, 3-hydroxy-4-methoxy- | C8H8O3 | 152 | 3.79 |
| 22 | 28.95 | d-Mannose | C6H12O6 | 180 | 1.74 |
| 23 | 29.54 | Phenol, 2-methoxy-4-(1-propenyl)-, (Z)- | C10H12O2 | 164 | 2.22 |
| 24 | 29.64 | Ascaridole epoxide | C10H16O3 | 184 | 0.33 |
| 25 | 30.63 | Ethanone, 1-(4-hydroxy-3-methoxyphenyl)- | C9H10O3 | 166 | 4.65 |
| 26 | 31.26 | Benzoic acid, 4-hydroxy-3-methoxy-, methyl ester | C9H10O4 | 182 | 0.5 |
| 27 | 31.76 | Propanone, 1-(4-hydroxy-3-methoxyphenyl)- | C10H12O3 | 180 | 16.87 |
| 28 | 32.19 | Guanosine | C10H13N5O5 | 283 | 2.42 |
| 29 | 32.74 | 9-Ethoxy-10-oxatricyclo[7.2.1.0(1,6)]dodecan-11-one | C13H20O3 | 224 | 0.32 |
| 30 | 32.92 | 1,2-Dimethoxy-4-n-propylbenzene | C11H16O2 | 180 | 1.1 |
| 31 | 33.02 | 3-Benzofuranmethanol, 2,3-dihydro-2-(4-hydroxy-3-methoxyphenyl)-5-(3-hydroxy-1-propenyl)-7-methoxy- | C20H22O6 | 358 | 0.74 |
| 32 | 34.46 | Phenylacetylformic acid, 4-hydroxy-3-methoxy- | C10H10O5 | 210 | 0.73 |
| 33 | 35.1 | Benzenesulfonamide, 2-nitro-N-[2-(4-pyridinyl)ethyl]- | C13H13N3O4S | 307 | 0.27 |
| 34 | 36.04 | 4-Hydroxy-2-methoxycinnamaldehyde | C10H10O3 | 178 | 0.65 |
| 35 | 36.59 | 1,2-Cycloheptanedione, 3,3,7,7-tetramethyl-, dihydrazone | C11H22N4 | 210 | 0.55 |
| 36 | 37.44 | 5,10-Diethoxy-2,3,7,8-tetrahydro-1H,6H-dipyrrolo[1,2-a;1’,2’-d]pyrazine | C14H22N2O2 | 250 | 0.54 |
| 37 | 43.96 | Tetradecanoic acid, 3,3a,4,6a,7,8,9,10,10a,10b-decahydro-3a,10a-dihydroxy-5-(hydroxymethyl)-2,10-dimethyl-3-oxobenz[e]azulen-8-yl ester, [3aR-(3aα,6aα,8α,10β,10aβ,10bβ)]- | C31H50O6 | 518 | 0.95 |
| 38 | 61.18 | Ethyl homovanillate | C11H14O4 | 210 | 0.25 |
| 39 | 61.78 | Hydroxy-4-(1-methoxycyclopropyl)-3,3,5,8,10,10-hexamethyltricyclo[6.2.2.0(2,7)]dodeca-5,11-dien-9-one | C22H32O3 | 344 | 4.17 |
| 40 | 62.11 | Phenol, 2,2’-methylenebis[6-(1,1-dimethylethyl)-4-methyl- | C23H32O2 | 340 | 0.48 |
| 41 | 63.1 | 6,7-Epoxypregn-4-ene-9,11,18-triol-3,20-dione, 11,18-diacetate | C25H32O8 | 460 | 0.46 |
| 42 | 64.64 | 6,7-Epoxypregn-4-ene-9,11,18-triol-3,20-dione, 11,18-diacetate | C25H32O8 | 460 | 0.37 |
| 43 | 65.21 | 10,11-Dihydro-10-hydroxy-2,3-dimethoxydibenz(b,f)oxepin | C16H16O4 | 272 | 0.51 |
| 44 | 66.02 | 1,2-Benzenedicarboxylic acid, diisooctyl ester | C24H38O4 | 390 | 2.14 |
| Component | Without Catalyst | With Catalyst | ||
|---|---|---|---|---|
| E (kJ/mol) | A (min−1) | E (kJ/mol) | A (min−1) | |
| Hemicellulose | 108.082 | 1.9 × 108 | 66.512 | 5.835 × 106 |
| Cellulose | 116.396 | 2.42 × 109 | 74.826 | 2.852 × 107 |
| Lignin | 182.908 | 2.98 × 1011 | 133.024 | 1.460 × 109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisar, J.; Ahmad, A.; Ali, G.; Rehman, N.U.; Shah, A.; Shah, I. Enhanced Bio-Oil Yield from Thermal Decomposition of Peanut Shells Using Termite Hill as the Catalyst. Energies 2022, 15, 1891. https://doi.org/10.3390/en15051891
Nisar J, Ahmad A, Ali G, Rehman NU, Shah A, Shah I. Enhanced Bio-Oil Yield from Thermal Decomposition of Peanut Shells Using Termite Hill as the Catalyst. Energies. 2022; 15(5):1891. https://doi.org/10.3390/en15051891
Chicago/Turabian StyleNisar, Jan, Ali Ahmad, Ghulam Ali, Nafees Ur Rehman, Afzal Shah, and Iltaf Shah. 2022. "Enhanced Bio-Oil Yield from Thermal Decomposition of Peanut Shells Using Termite Hill as the Catalyst" Energies 15, no. 5: 1891. https://doi.org/10.3390/en15051891
APA StyleNisar, J., Ahmad, A., Ali, G., Rehman, N. U., Shah, A., & Shah, I. (2022). Enhanced Bio-Oil Yield from Thermal Decomposition of Peanut Shells Using Termite Hill as the Catalyst. Energies, 15(5), 1891. https://doi.org/10.3390/en15051891







