Effect of Bipolar Plate Material on Proton Exchange Membrane Fuel Cell Performance
Abstract
1. Introduction
2. Materials and Methods
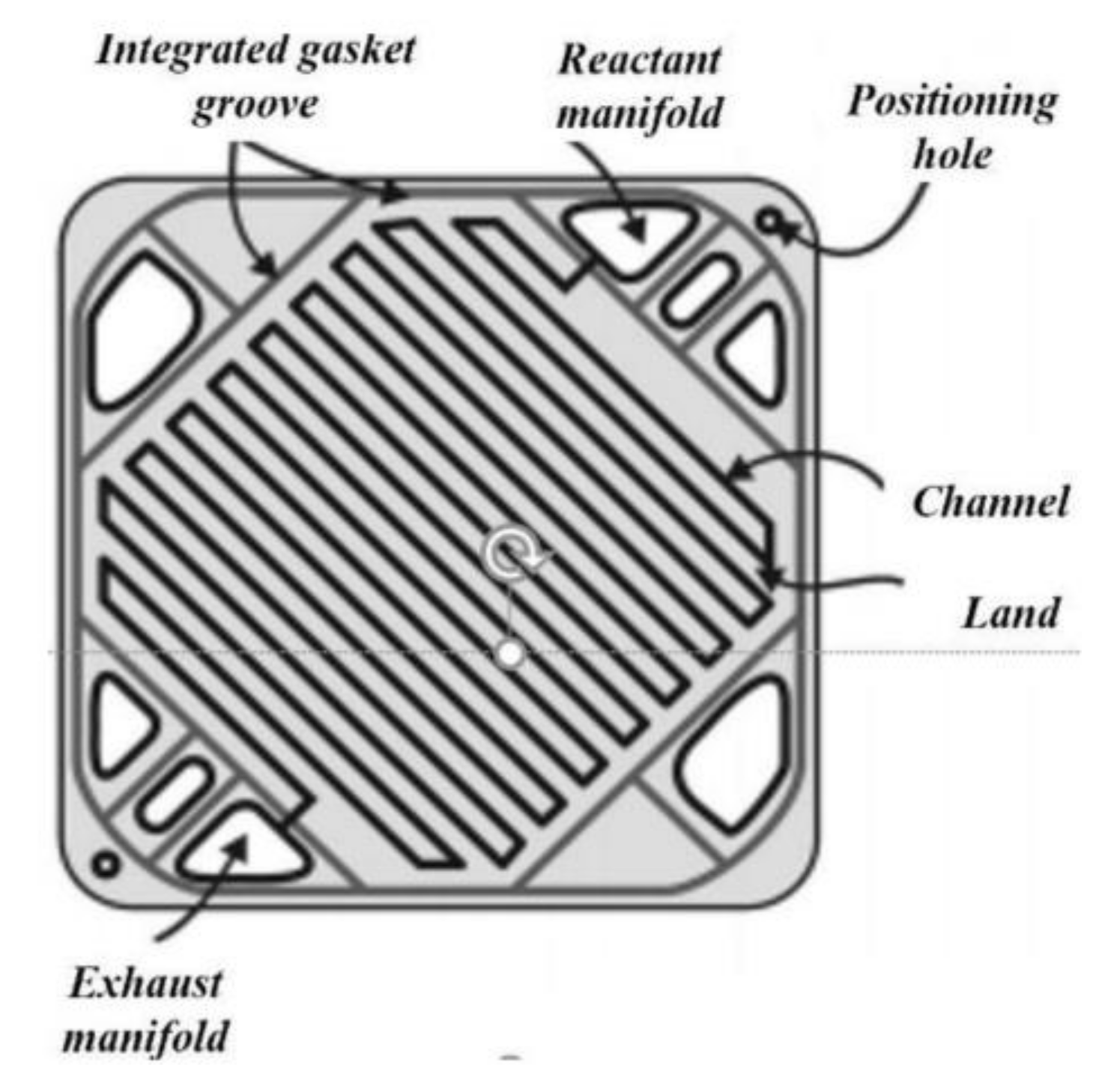
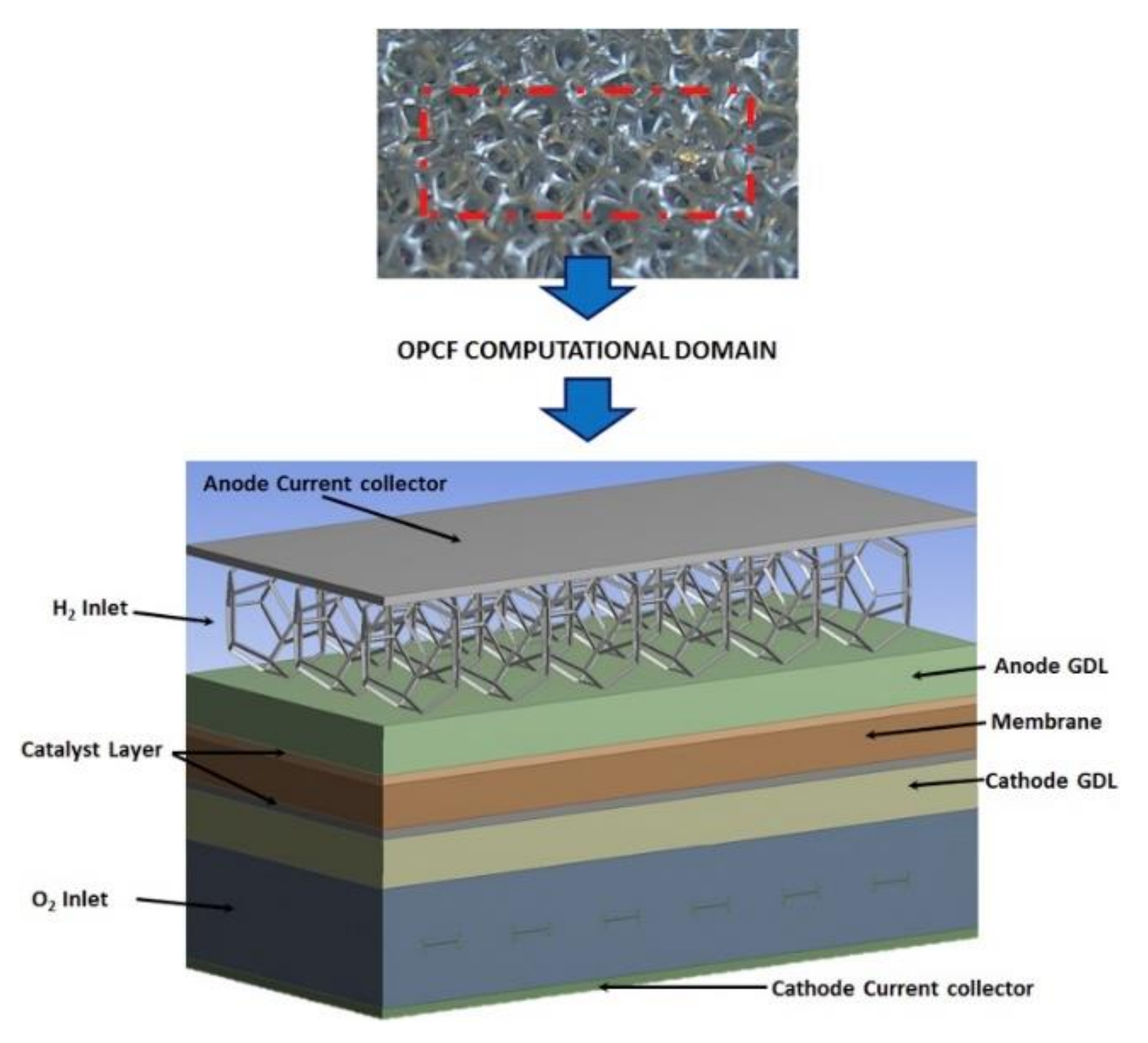
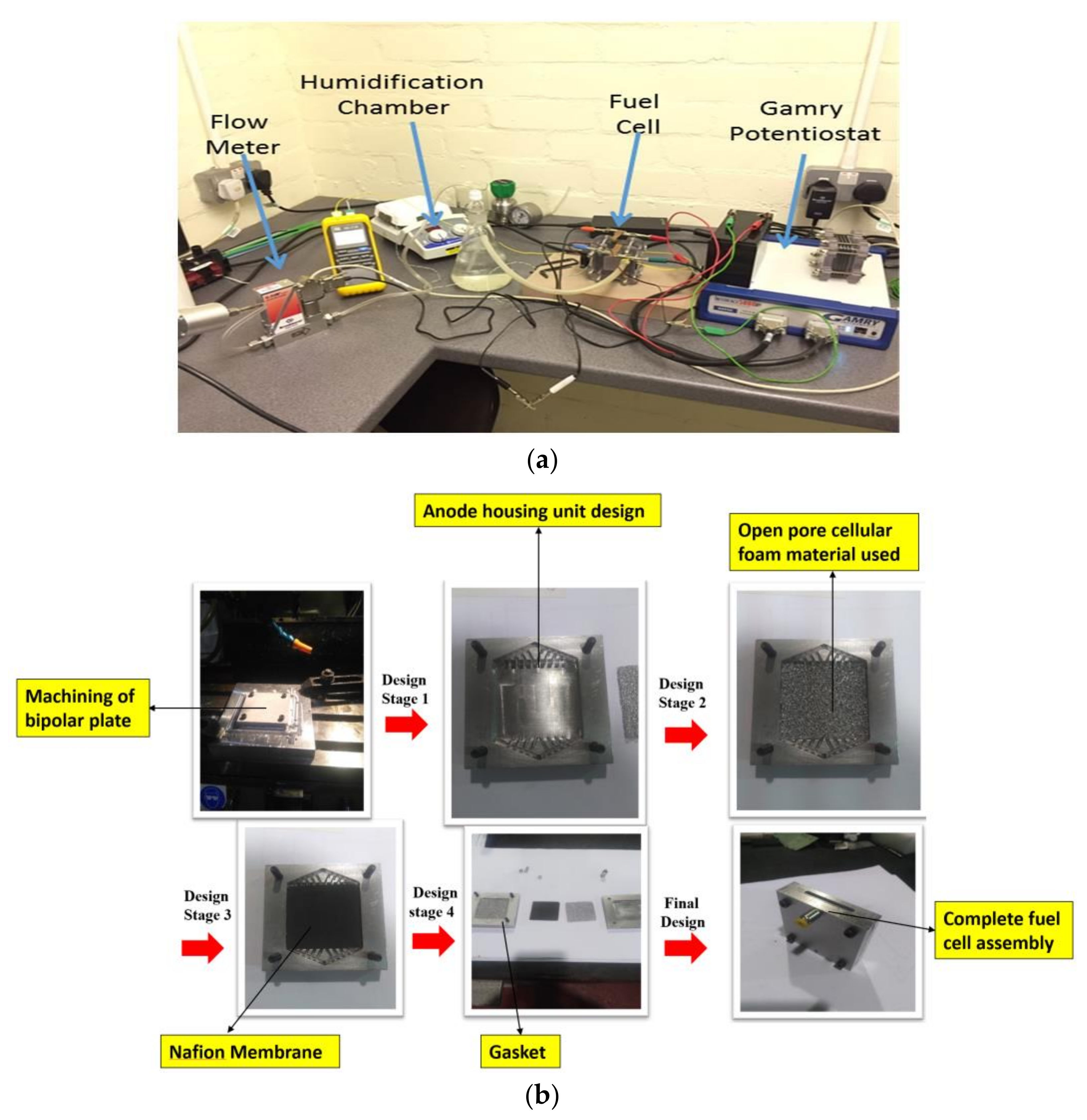
2.1. Experimental Set-Up
2.2. Procedure
3. Experimental Results
3.1. Effects of Humidification Temperature
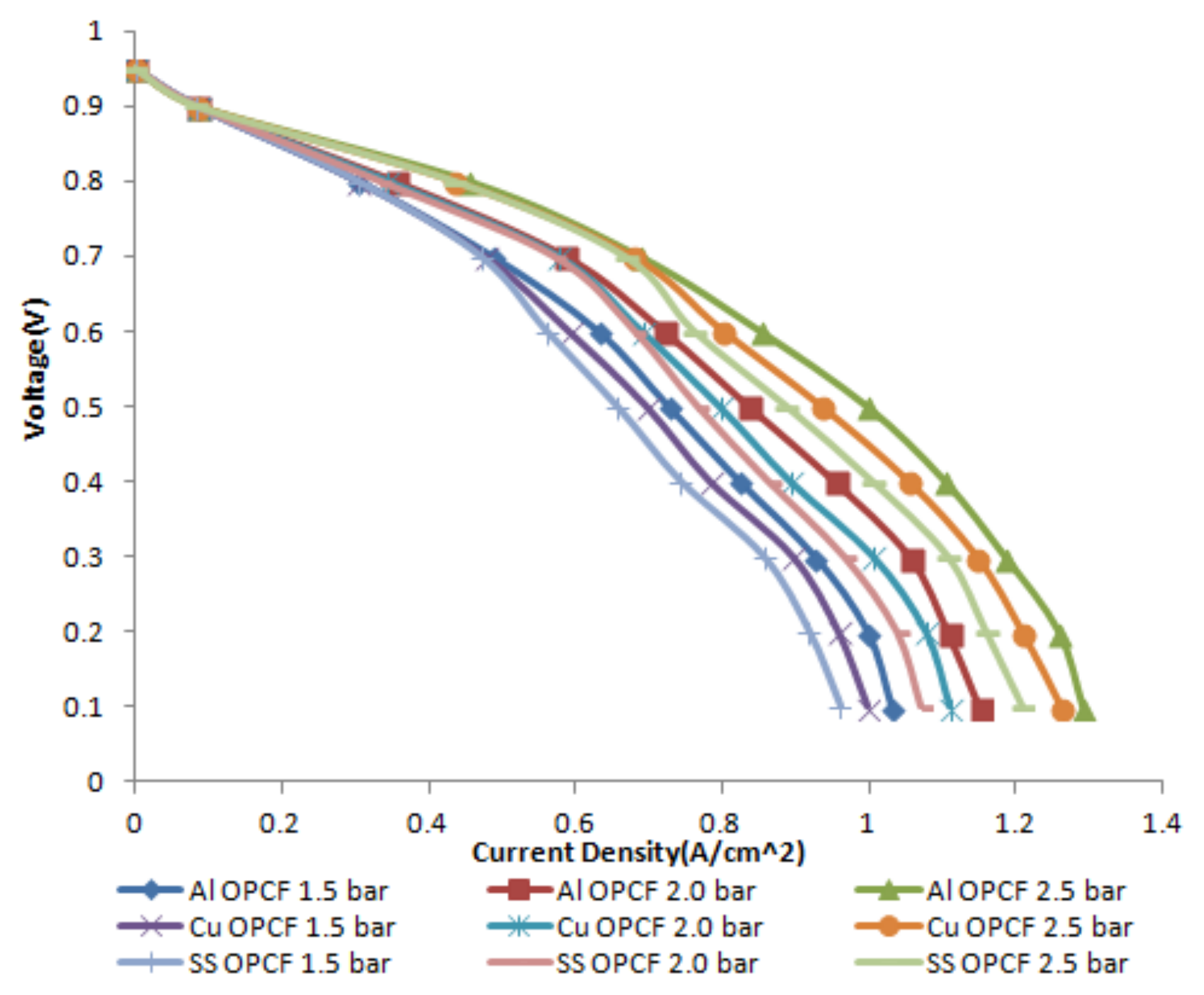
3.2. Effect of an Operating Pressure
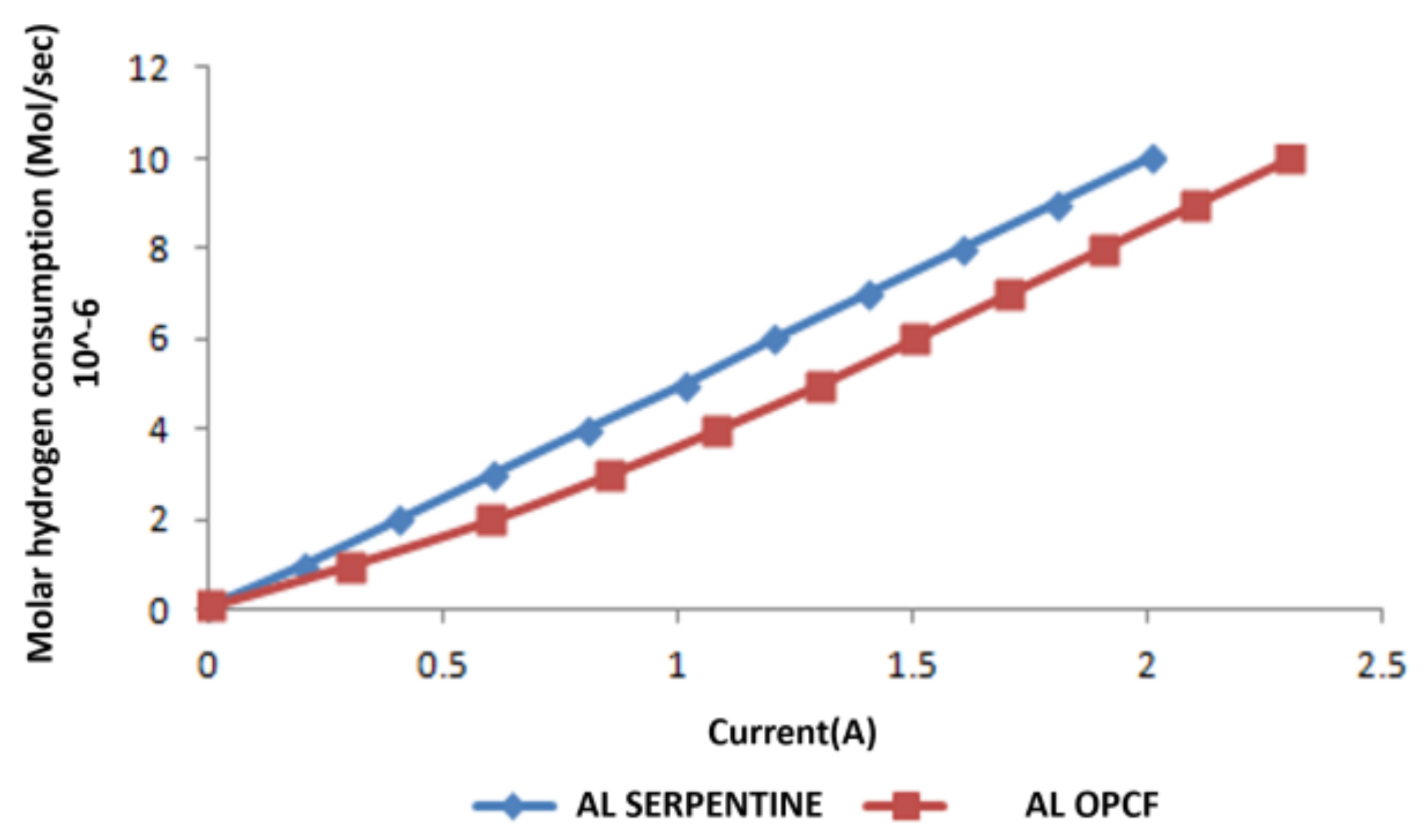
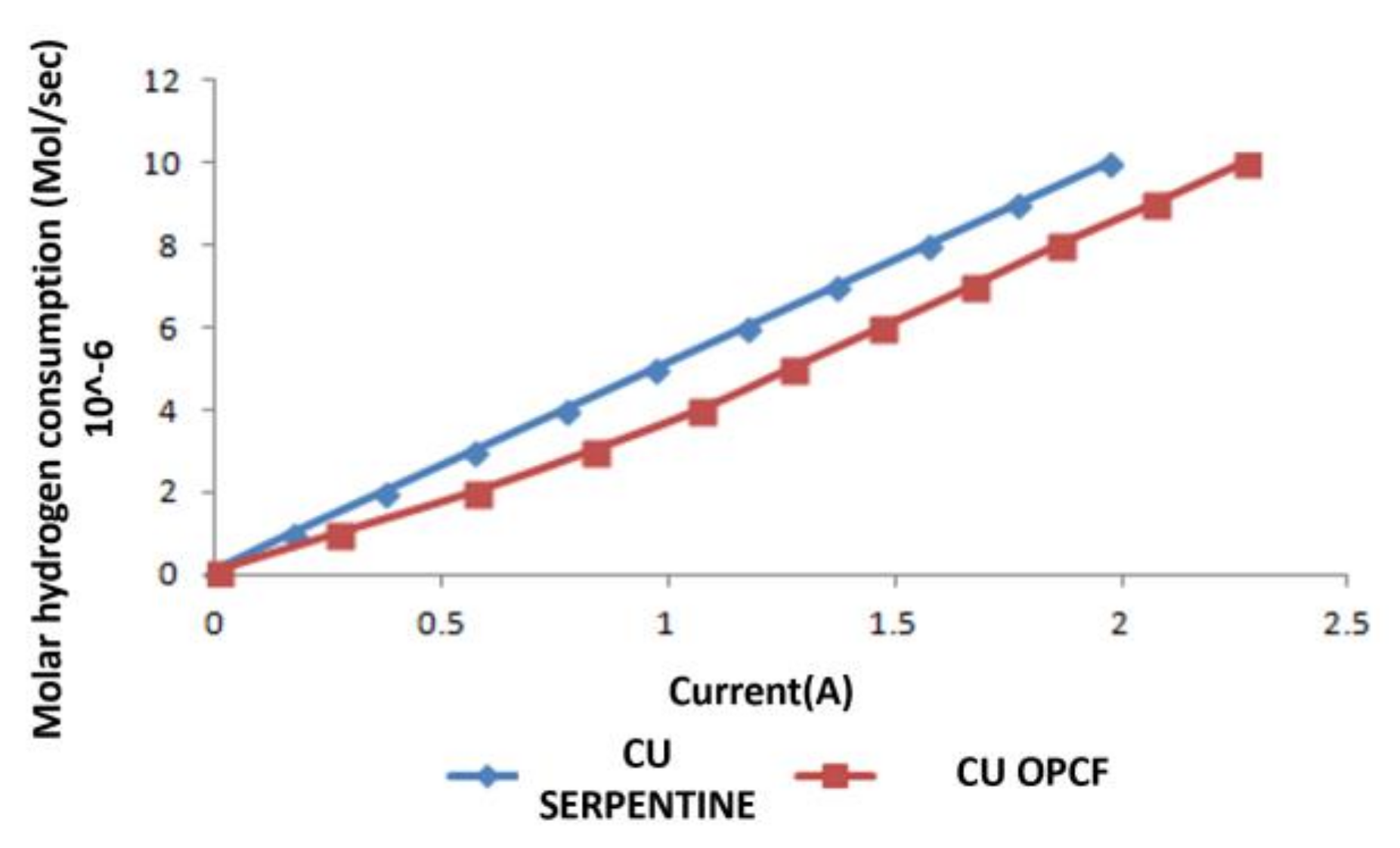
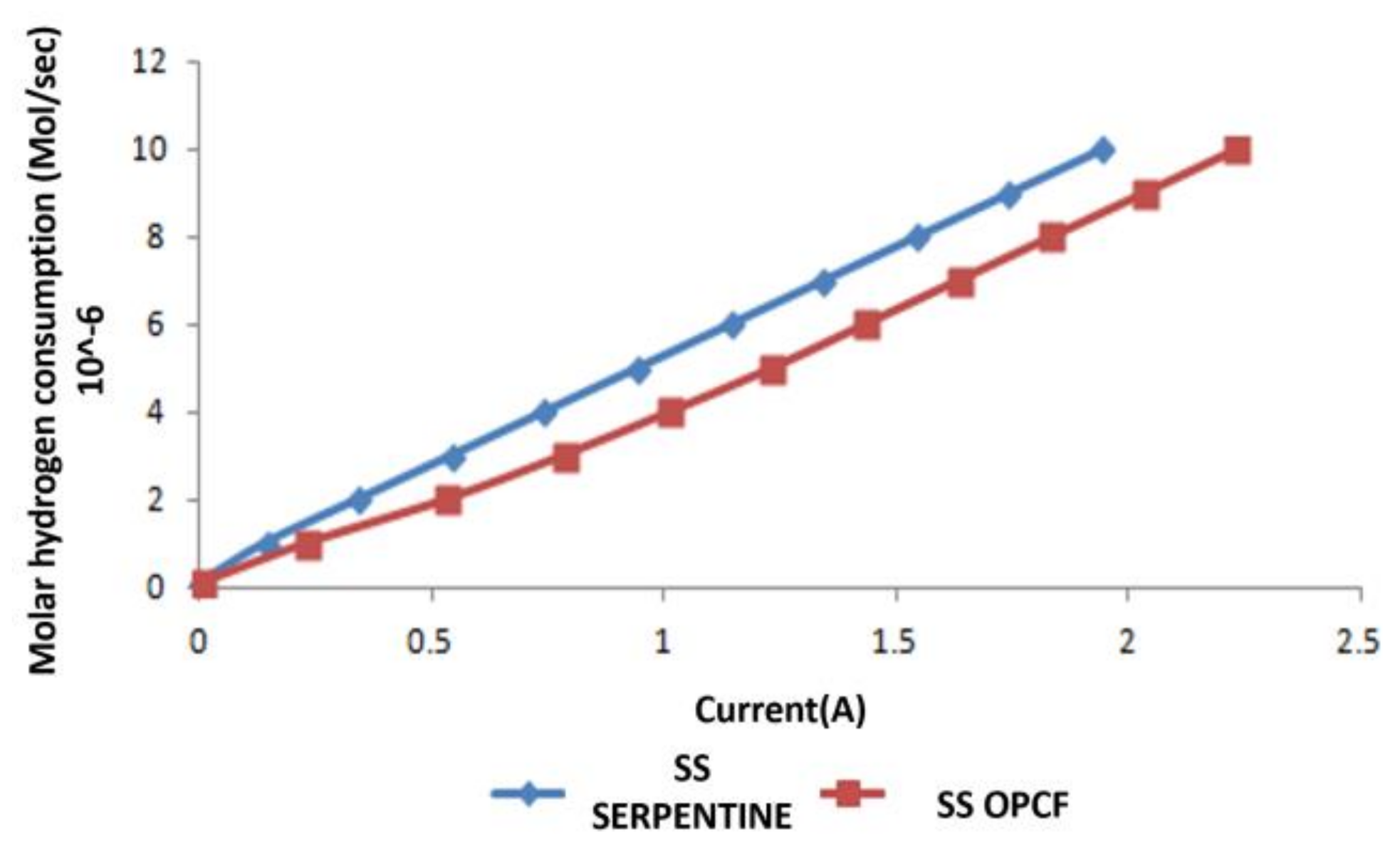
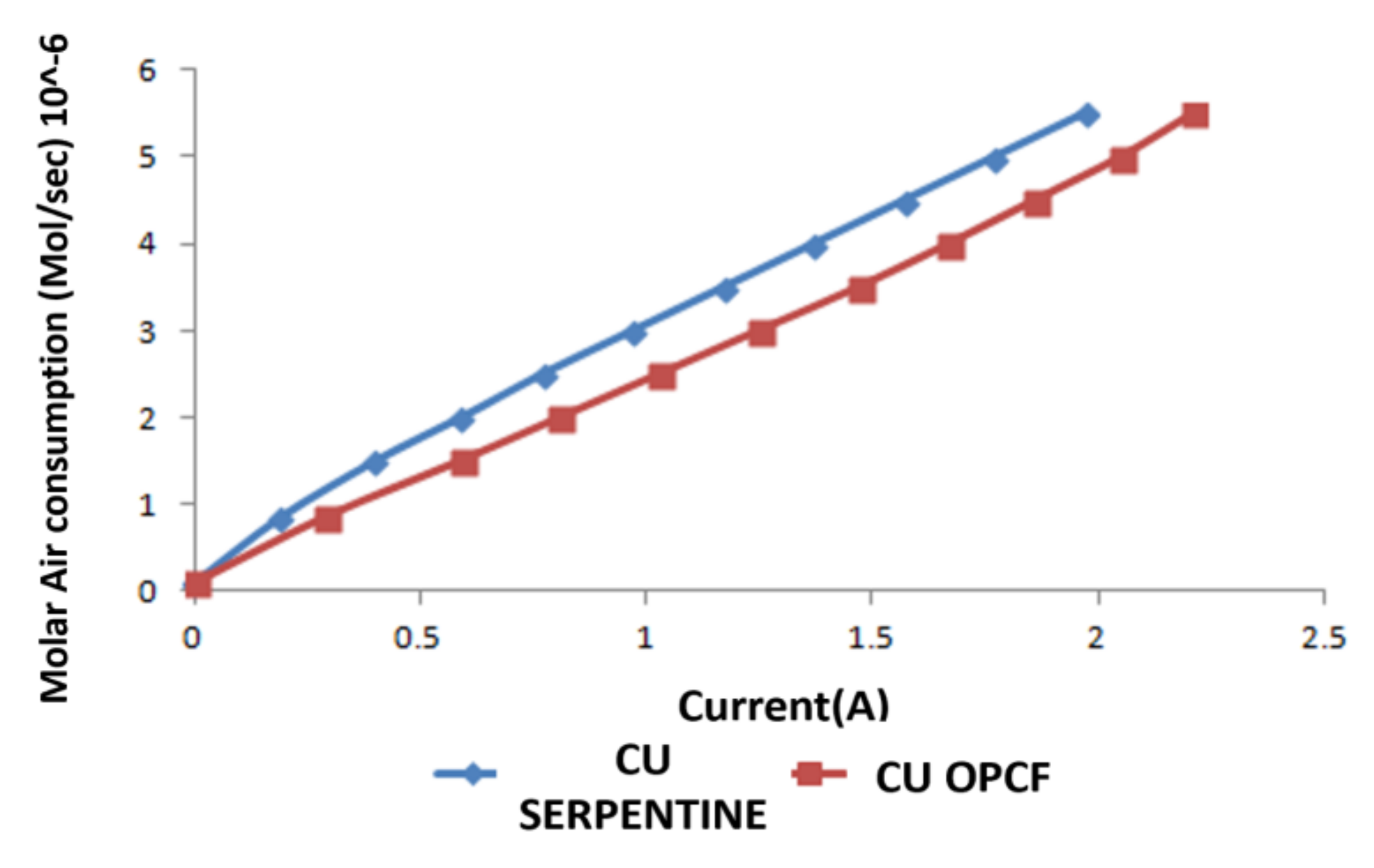
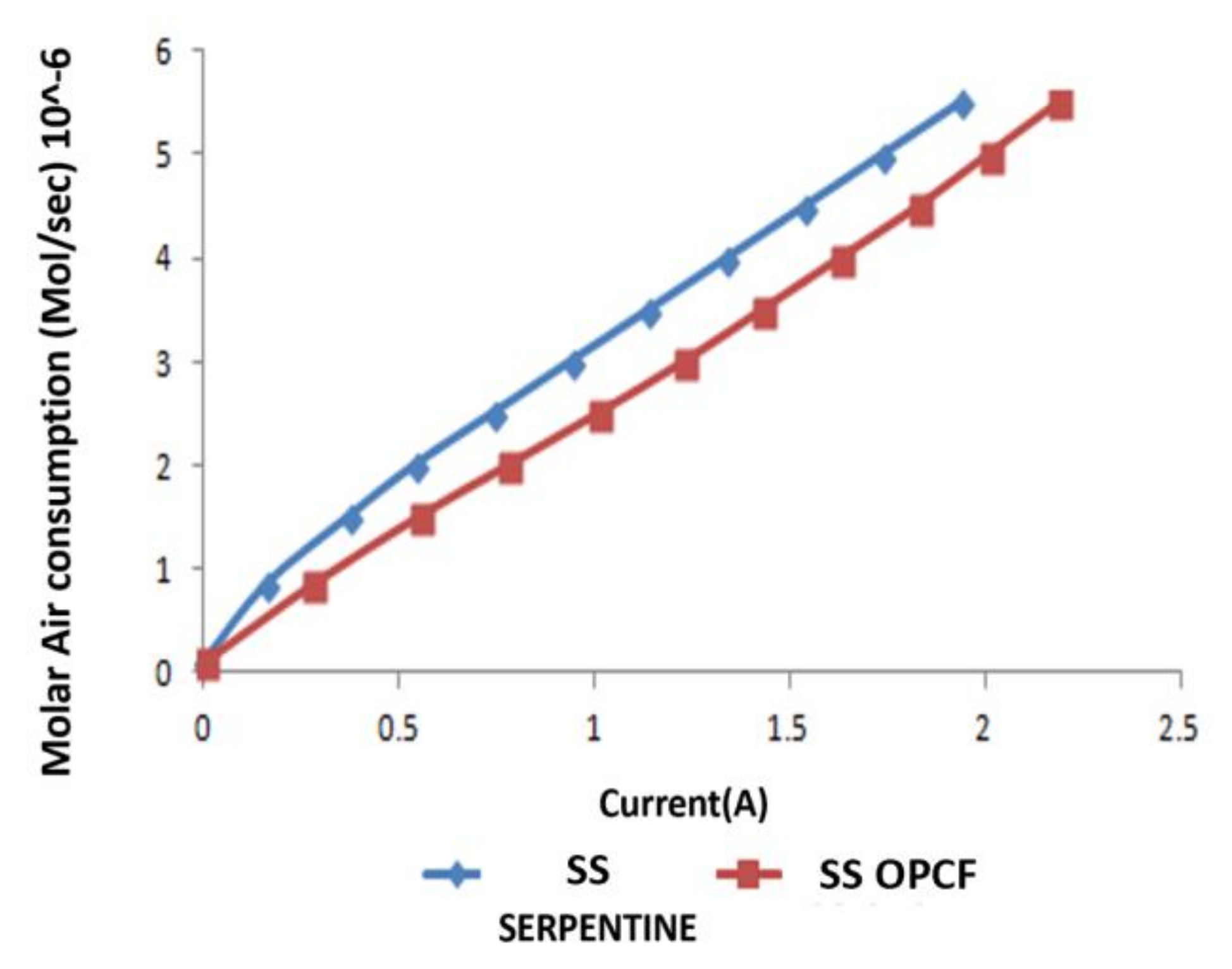
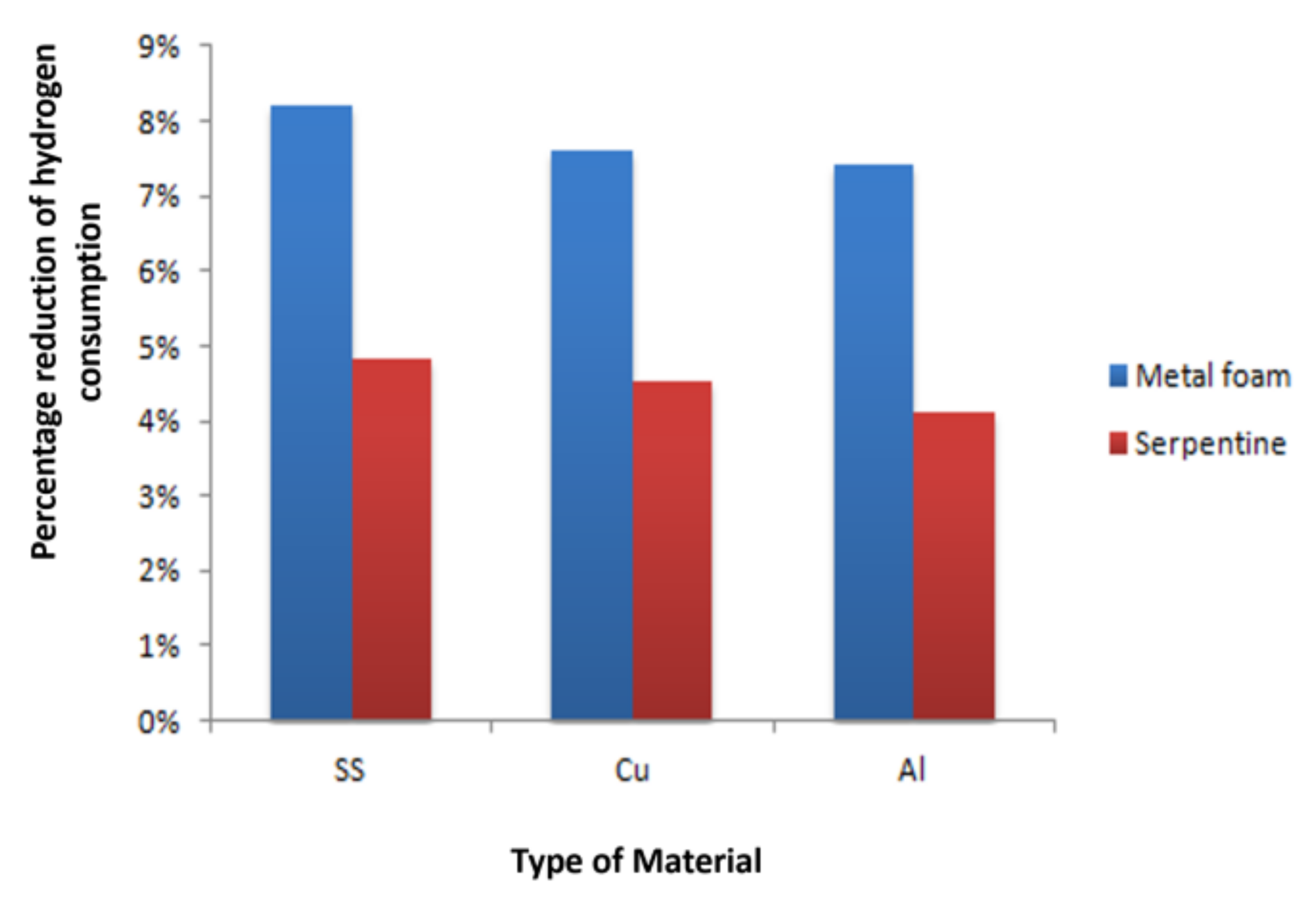
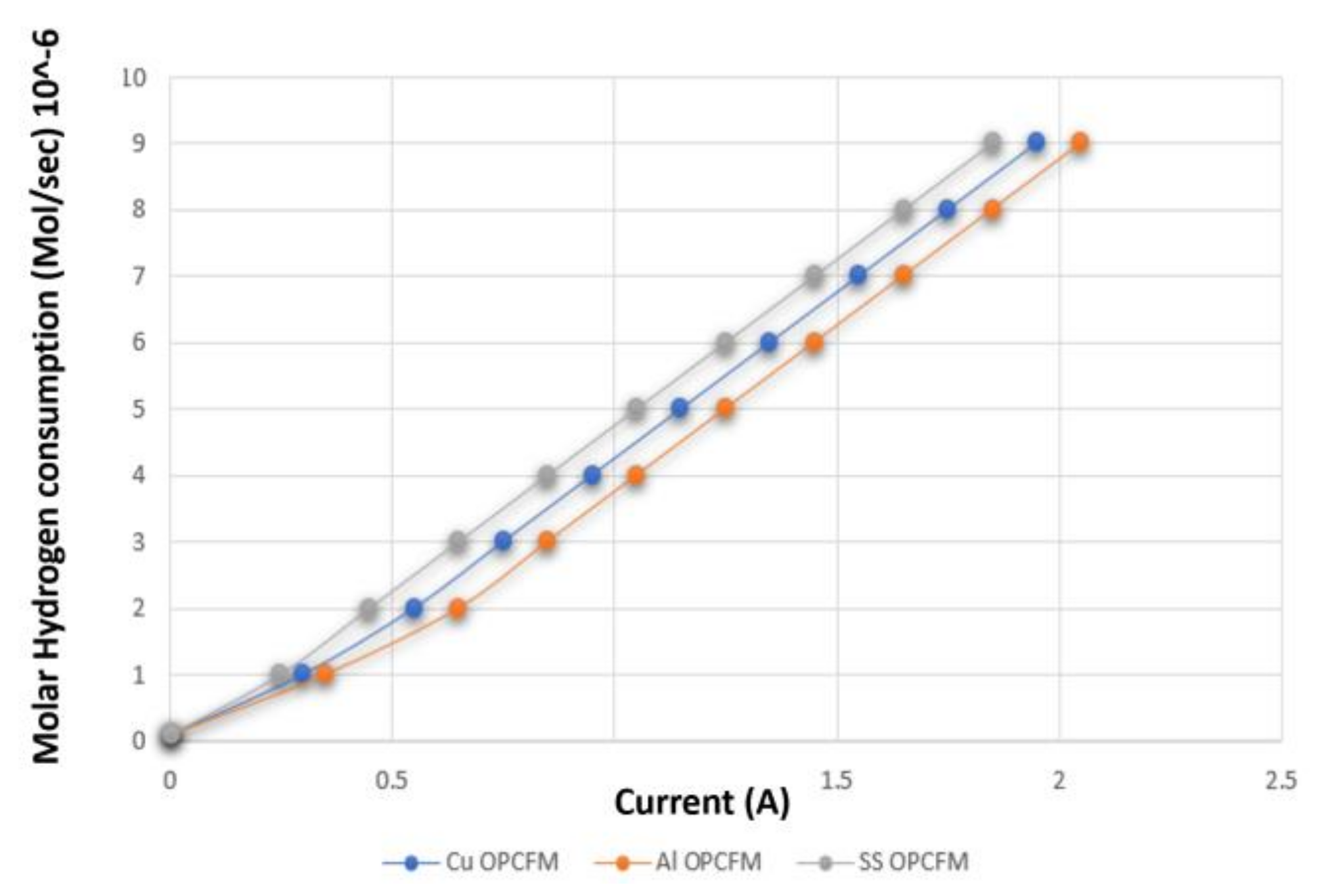
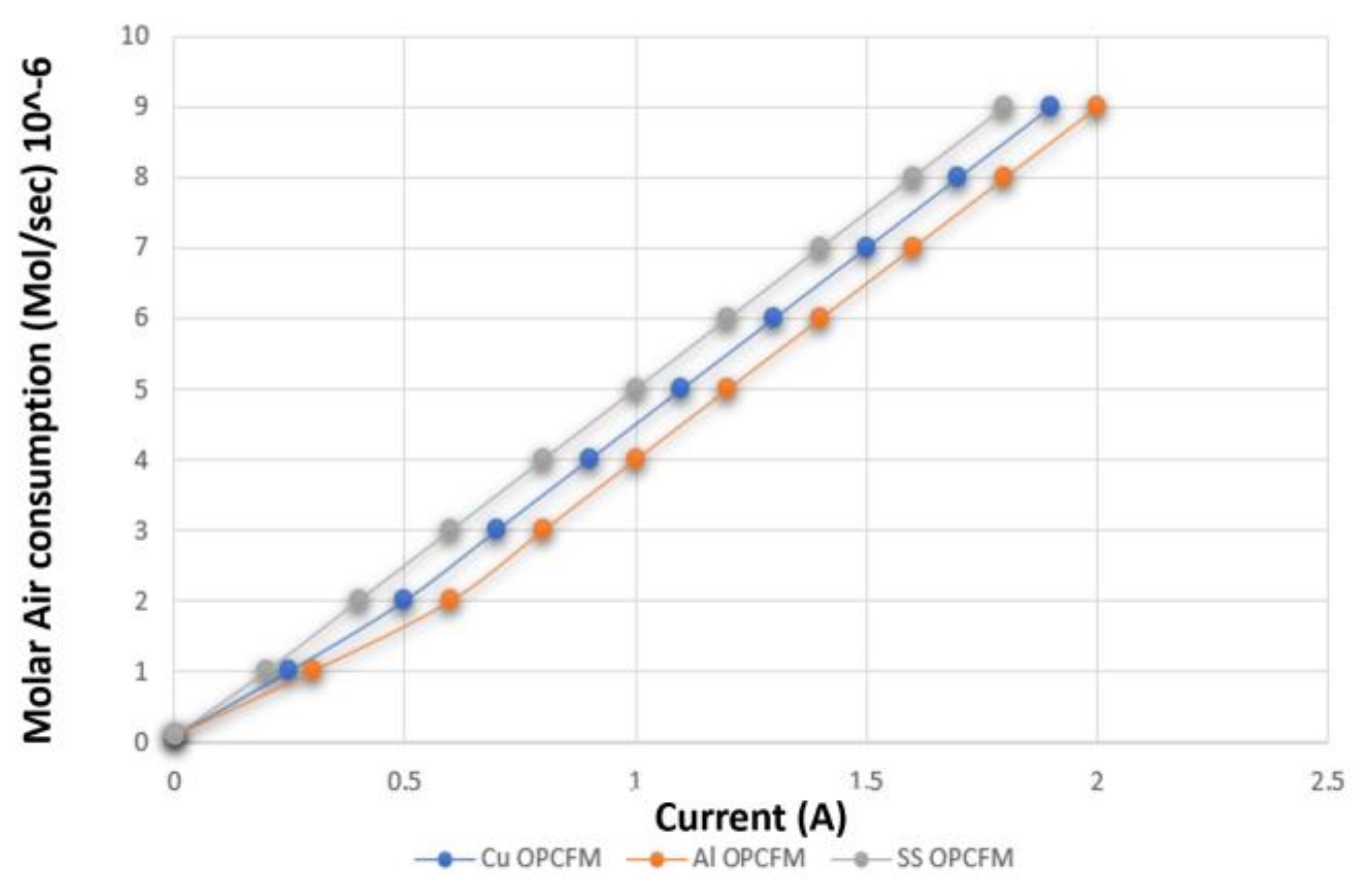
3.3. Hydrogen and Oxygen Consumption
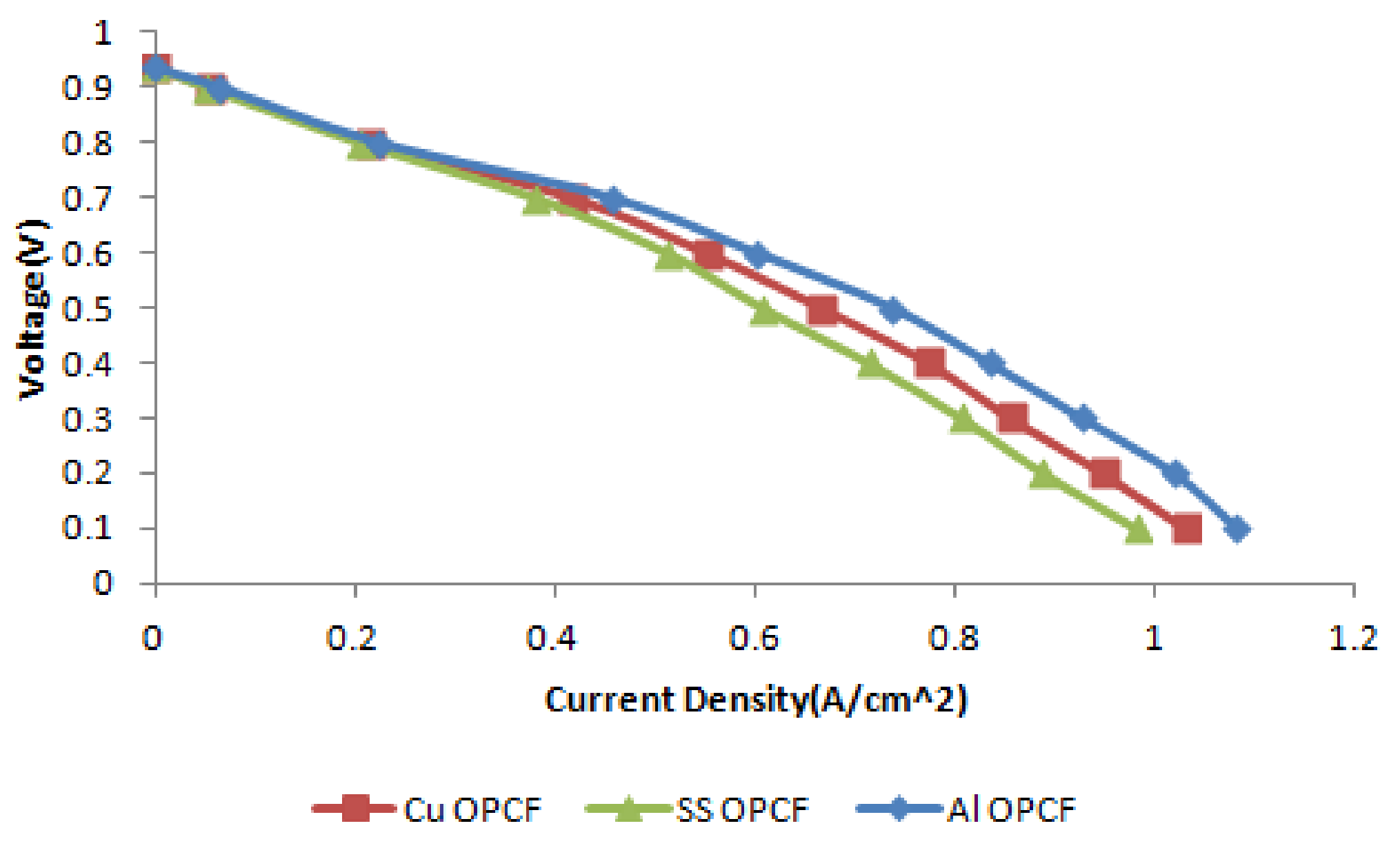
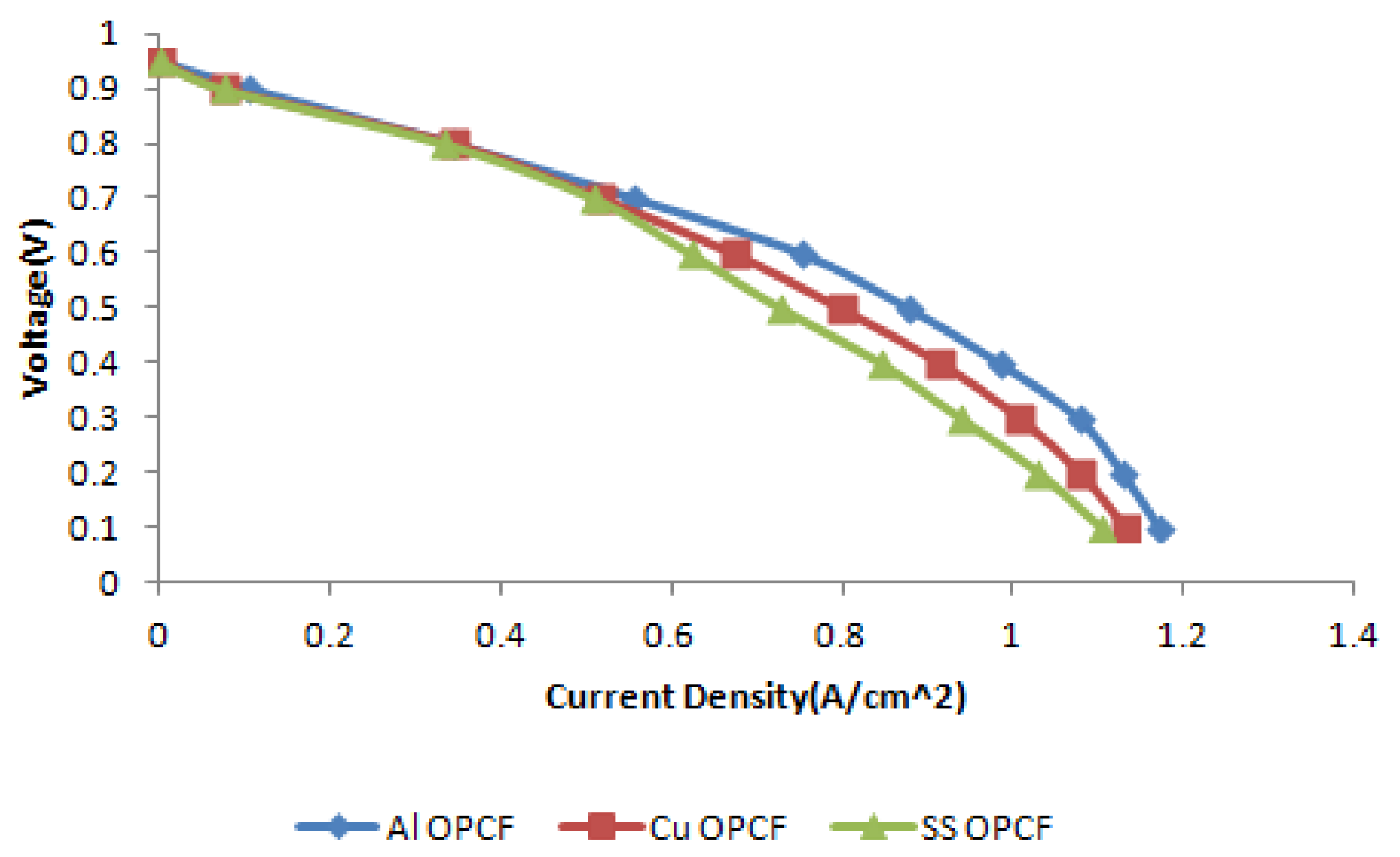
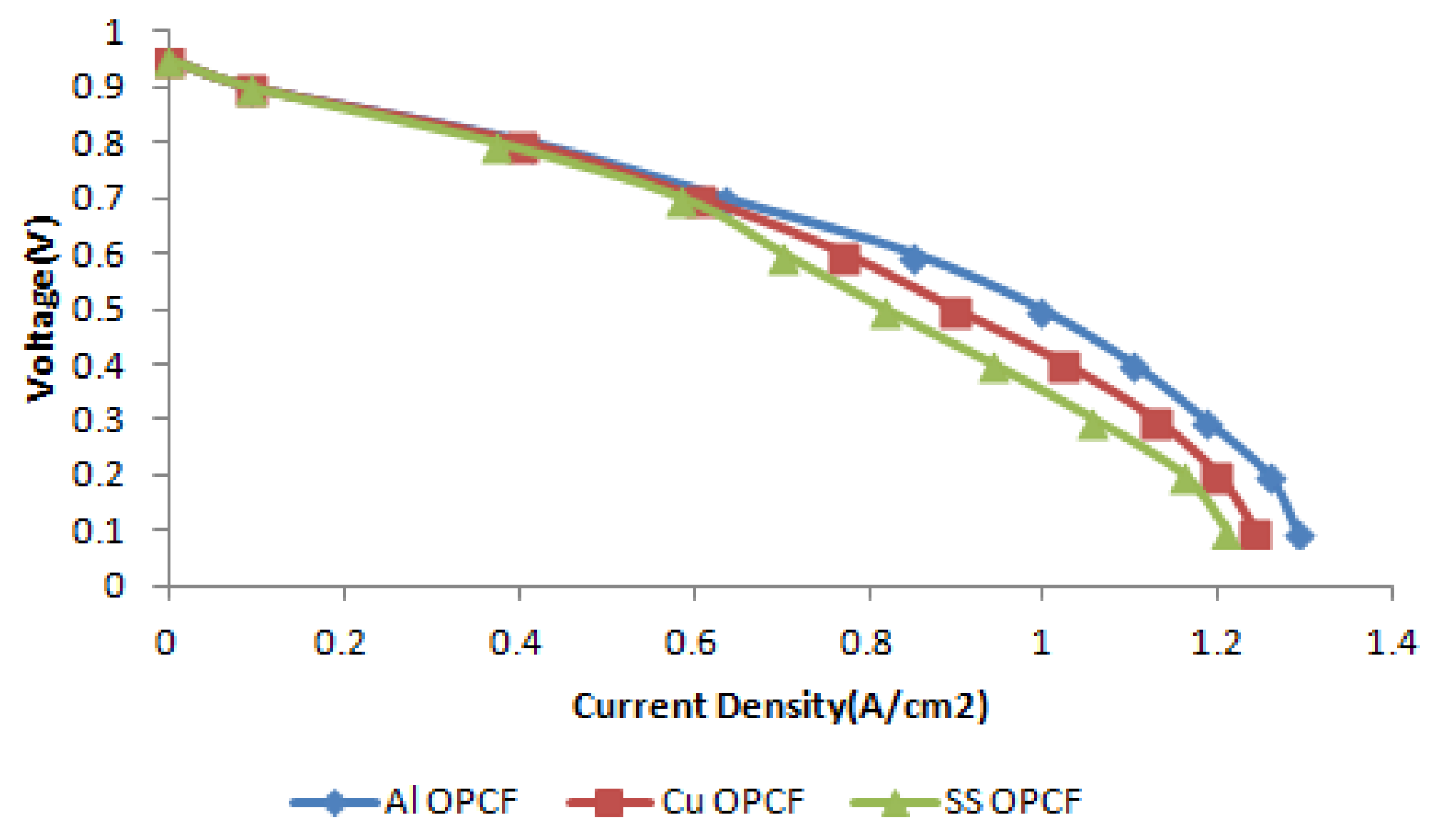
3.4. Comparison between Al, Cu and SS as OPCF Material
4. Experimental Validation of PEM Fuel Cell Results Using Machine Learning Algorithms
4.1. Machine Learning Models
4.2. Linear Regression
- They express the relationship accuracy;
- They signify if there is the relationship between variables;
- If a relationship exists, they indicate the nature of it;
- If various variables are used, they evaluate each variables contributions.
4.3. Ridge Regression
4.4. Polynomial Regression Algorithm
4.5. Data Preparation
4.6. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olabi, A.; Wilberforce, T.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Prospects of Fuel Cell Combined Heat and Power Systems. Energies 2020, 13, 4104. [Google Scholar] [CrossRef]
- Ijaodola, O.; Ogungbemi, E.; Khatib, F.N.; Wilberforce, T.; Ramadan, M.; El-Hassan, Z.; Thompson, J. Energy efficiency improvements by investigating the water flooding management on proton exchange membrane fuel cell (PEMFC). Energy 2019, 179, 246–267. [Google Scholar] [CrossRef]
- Ijaodola, O.; Ogungbemi, E.; Khatib, F.N.; Wilberforce, T.; Ramadan, M.; El Hassan, Z.; Thompson, J.; Olabi, A.G. Evaluating the Effect of Metal Bipolar Plate Coating on the Performance of Proton Exchange Membrane Fuel Cells. Energies 2018, 11, 3203. [Google Scholar] [CrossRef]
- Ogungbemi, E.; Ijaodola, O.; Khatib, F.N.; Wilberforce, T.; El Hassan, Z.; Thompson, J.; Ramadan, M.; Olabi, A.G. Fuel cell membranes—Pros and cons. Energy 2019, 172, 155–172. [Google Scholar] [CrossRef]
- Wilberforce, T.; Ijaodola, O.; Ogungbemi, E.; El Hassan, Z.; Thompson, J.; Olabi, A.G. Effect of bipolar plate materials on performance of fuel cells. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Alaswad, A.; Palumbo, A.; Dassisti, M.; Olabi, A.G. Fuel cell technologies, applications, and state of the art: A reference guide. In Encyclopedia of Smart Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 315–333. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A. Solid polymer electrolyte membranes for fuel cell applications—A review. J. Membr. Sci. 2005, 259, 10–26. [Google Scholar] [CrossRef]
- Yun, W.; Ken, S.C.; Jeffrey, M.; Sung, C.C.; Xavier, C.A. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Shahrukh, S.; Sudhakar, K.; Brajesh, C.; Jamil, A. A review on recent advances in proton exchange membrane fuel cells: Materials, technology and applications. Pelagia Res. Libr. Adv. Appl. Sci. Res. 2015, 6, 89–100. [Google Scholar]
- Lin, W.; Attila, H.; Tianhong, Z.; Hongtan, L. A parametric study of PEM fuel cell performances. Int. J. Hydrog. Energy 2003, 28, 1263–1272. [Google Scholar] [CrossRef]
- Barreto, L.; Makihira, A.; Riahi, K. The hydrogen economy in the 21st century: A sustainable development scenario. Int. J. Hydrog. Energy 2003, 28, 267–284. [Google Scholar] [CrossRef]
- Abdin, Z.; Webb, C.; Gray, E. PEM fuel cell model and simulation in Matlab–Simulink based on physical parameters. Energy 2016, 116, 1131–1144. [Google Scholar] [CrossRef]
- Yafei, C.; Yanzhou, Q.; Yan, Y.; Junfeng, Z.; Xianguo, L. Humidification strategy for polymer electrolyte membrane fuel cells. A review. Appl. Energy 2018, 230, 643–662. [Google Scholar] [CrossRef]
- Wilberforce, T.; El-Hassan, Z.; Khatib, F.N.; Al Makky, A.; Baroutaji, A.; Carton, J.G.; Olabi, A.G. Developments of electric cars and fuel cell hydrogen electric cars. Int. J. Hydrog. Energy 2017, 42, 25695–25734. [Google Scholar] [CrossRef]
- Khazaee, I.; Sabadbafan, H. Effect of humidity content and direction of the flow of reactant gases on water management in the 4-serpentine and 1-serpentine flow channel in a PEM (proton exchange membrane) fuel cell. Energy 2016, 101, 252–265. [Google Scholar] [CrossRef]
- Yousfi-Steiner, N.; Moçotéguy, P.; Candusso, D.; Hissel, D.; Hernandez, A.; Aslanides, A. A review on PEM voltage degradation associated with water management: Impacts, influent factors and characterization. J. Power Sources 2008, 183, 260–274. [Google Scholar] [CrossRef]
- Judith, O.; Manikandan, R.; Murat, A. In situ detection of anode flooding of a PEM fuel cell. Int. J. Hydrog. Energy 2009, 34, 6765–6770. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Liu, X.; Wu, W.-T. Modeling of PEM fuel cell with thin MEA under low humidity operating condition. Appl. Energy 2019, 242, 1513–1527. [Google Scholar] [CrossRef]
- Kumar, A.; Reddy, R.G. Polymer Electrolyte Membrane Fuel Cell with Metal Foam in the Gas Flow-Field of Bipolar/End Plates. J. New Mat. Electrochem. Syst. 2003, 6, 231–236. [Google Scholar]
- Sierra, J.; Figueroa-Ramírez, S.; Díaz, S.; Vargas, J.; Sebastian, P. Numerical evaluation of a PEM fuel cell with conventional flow fields adapted to tubular plates. Int. J. Hydrog. Energy 2014, 39, 16694–16705. [Google Scholar] [CrossRef]
- Ogungbemi, E.; Wilberforce, T.; Ijaodola, O.; Thompson, J.; Olabi, A.G. Review of operating condition, design parameters and material properties for proton exchange membrane fuel cells. Int. J. Energy Res. 2021, 45, 1227–1245. [Google Scholar] [CrossRef]
- Carton, J.; Olabi, A.G. Three-dimensional proton exchange membrane fuel cell model: Comparison of double channel and open pore cellular foam flow plates. Energy 2017, 136, 185–195. [Google Scholar] [CrossRef]
- Tseng, C.; Heush, Y.; Chiang, C.; Lee, Y.; Lee, K. Application of metal foams to high temperature PEM fuel cells. Int. J. Hydrog. Energy 2016, 41, 16196–16204. [Google Scholar] [CrossRef]
- Shimpalee, S.; Lilavivat, V.; McCrabb, H.; Khunatorn, Y.; Lee, H.-K.; Lee, W.-K.; Weidner, J. Investigation of bipolar plate materials for proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2016, 41, 13688–13696. [Google Scholar] [CrossRef]
- Wilberforce, T.; Khatib, F.; Ijaodola, O.; Ogungbemi, E.; El-Hassan, Z.; Durrant, A.; Thompson, J.; Olabi, A. Numerical modelling and CFD simulation of a polymer electrolyte membrane (PEM) fuel cell flow channel using an open pore cellular foam material. Sci. Total Environ. 2019, 678, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-J.; Tsai, B.T.; Liu, Z.-S.; Cheng, T.-C.; Chang, W.-C.; Lo, S.-K. A PEM fuel cell with metal foam as flow distributor. Energy Convers. Manag. 2012, 62, 14–21. [Google Scholar] [CrossRef]
- Mohammad, S.H.; Bahman, S. Metal foams application to enhance cooling of open cathode polymer electrolyte membrane fuel cells. J. Power Sources 2015, 295, 275–291. [Google Scholar] [CrossRef]
- Wilberforce, T.; Ijaodola, O.; Khatib, F.; Ogungbemi, E.; El Hassan, Z.; Thompson, J.; Olabi, A. Effect of humidification of reactive gases on the performance of a proton exchange membrane fuel cell. Sci. Total Environ. 2019, 688, 1016–1035. [Google Scholar] [CrossRef]
- Yupeng, Y.; Xu, Z.; Liejin, G.; Hongtan, L. Overall and local effects of operating conditions in PEM fuel cells with dead-ended anode. Int. J. Hydrog. Energy 2017, 42, 4690–4698. [Google Scholar] [CrossRef]
- Yussef, A.; Nihad, D. Experimental performance assessment of metal-foam flow fields for proton exchange membrane fuel cells. Appl. Energy 2019, 252, 113458. [Google Scholar] [CrossRef]
- Ding, S.; Jiang, W.; Shen, Z. Linear-regression models and algorithms based on the Total-Least-Squares principle. Geod. Geodyn. 2012, 3, 42–46. [Google Scholar] [CrossRef][Green Version]
- Shiu-tong, T.N.; Martin, S.; Keung, F.W. Using genetic algorithms and linear regression analysis for private housing demand forecast. Build. Environ. 2008, 43, 1171–1184. [Google Scholar] [CrossRef]
- Arthur, E.H.; Robert, W.K. Ridge Regression: Biased Estimation for Nonorthogonal Problems. American Statistical Association and American Society for Quality. Technometrics 1970, 12, 55–67. [Google Scholar] [CrossRef]
- Zaixu, C.; Gaolang, G. The effect of machine learning regression algorithms and sample size on individualized behavioral prediction with functional connectivity features. NeuroImage 2018, 178, 622–637. [Google Scholar] [CrossRef]
- Yani, Z.; Chaoyang, Z.; Xiaoxin, L. Improved principal component analysis and linear regression classification for face recognition. Signal Process. 2018, 145, 175–182. [Google Scholar] [CrossRef]
- Dhruva, G.; Saraju, P.M.; Garima, T. Fast optimization of nano-CMOS voltage-controlled oscillator using polynomial regression and genetic algorithm. Microelectron. J. 2013, 44, 631–641. [Google Scholar] [CrossRef]
- Zhong, S.; Zhang, K.; Bagheri, M.; Burken, J.G.; Gu, A.; Li, B.; Ma, X.; Marrone, B.L.; Ren, Z.J.; Schrier, J.; et al. Machine Learning: New Ideas and Tools in Environmental Science and Engineering. Environ. Sci. Technol. 2021, 55, 12741–12754. [Google Scholar] [CrossRef]
- Ding, R.; Ding, Y.; Zhang, H.; Wang, R.; Xu, Z.; Liu, Y.; Yin, W.; Wang, J.; Li, J.; Liu, J. Applying machine learning to boost the development of high-performance membrane electrode assembly for proton exchange membrane fuel cells. J. Mater. Chem. A 2021, 9, 6841–6850. [Google Scholar] [CrossRef]


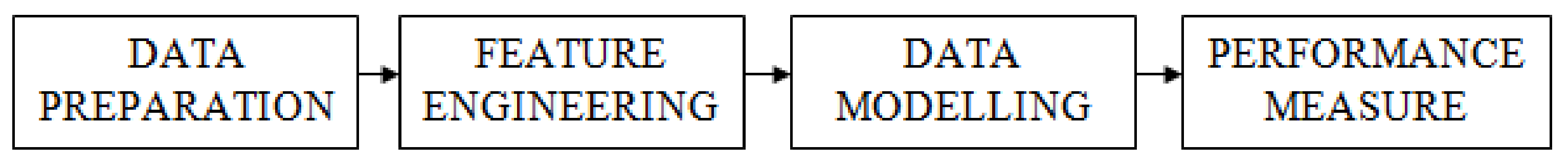
| Category | Temperatures (°C) | Electrical Performance | Charge Carrier | Fuel | Electrolyte |
|---|---|---|---|---|---|
| Molten carbonate fuel cells (MCFCs) | (600–700 °C) | (65–70%) | CO32− | Methane | Molten carbonate salt immerse in a porous matrix |
| Solid oxide fuel cells (SOFCs), | (800–1000 °C) | (50–65%) | O2− | Methane | Yttria-stabilized zirconia (YSZ) |
| Alkaline fuel cells (AFCs), | (80–150 °C) | (40–70%) | OH− | Hydrogen | Potassium hydroxide(KOH) |
| phosphoric acid fuel cells (PAFCs) | (150–200 °C) | (37–42%). | H+ | Hydrogen | Phosphoric acid(H₃PO₄) immerse in a porous mix |
| Proton exchange membrane (PEM) fuel cells | (60–80 °C) | (40–55%) | H+ | Hydrogen | Polymer membrane electrolyte (Nafion) |
| Reports | Unit |
|---|---|
| Maximum current | A |
| Maximum voltage | V |
| Maximum voltage efficiency | % |
| Current intercept (I–V graph) | A |
| Hydrogen consumption | gs−1 |
| Hydrogen fuel efficiency | % |
| Oxygen consumption | gs−1 |
| Oxygen fuel efficiency | % |
| Machine Learning Algorithm (MLA) | Algorithm Accuracy | |
|---|---|---|
| 0 | Linear Regression | 0.72 |
| 1 | Polynomial Regression | 0.54 |
| 2 | Ridge Regression | 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilberforce, T.; Ijaodola, O.; Baroutaji, A.; Ogungbemi, E.; Olabi, A.G. Effect of Bipolar Plate Material on Proton Exchange Membrane Fuel Cell Performance. Energies 2022, 15, 1886. https://doi.org/10.3390/en15051886
Wilberforce T, Ijaodola O, Baroutaji A, Ogungbemi E, Olabi AG. Effect of Bipolar Plate Material on Proton Exchange Membrane Fuel Cell Performance. Energies. 2022; 15(5):1886. https://doi.org/10.3390/en15051886
Chicago/Turabian StyleWilberforce, Tabbi, Oluwatosin Ijaodola, Ahmad Baroutaji, Emmanuel Ogungbemi, and Abdul Ghani Olabi. 2022. "Effect of Bipolar Plate Material on Proton Exchange Membrane Fuel Cell Performance" Energies 15, no. 5: 1886. https://doi.org/10.3390/en15051886
APA StyleWilberforce, T., Ijaodola, O., Baroutaji, A., Ogungbemi, E., & Olabi, A. G. (2022). Effect of Bipolar Plate Material on Proton Exchange Membrane Fuel Cell Performance. Energies, 15(5), 1886. https://doi.org/10.3390/en15051886








