In Situ Transesterification of Microbial Biomass for Biolubricant Production Catalyzed by Heteropolyacid Supported on Niobium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of Microalgae
2.2. Cultivation of Microalgae-Fungi Consortium
2.3. Catalyst: Synthesis and Surface Acidity
2.4. Direct Transesterification Reactions and Biolubricant Production
2.5. Purification of Biolubricants
2.6. Analytical Procedures
3. Results
3.1. Growth Parameters and Characterization of Microalgae Oil
3.2. Catalyst Activity
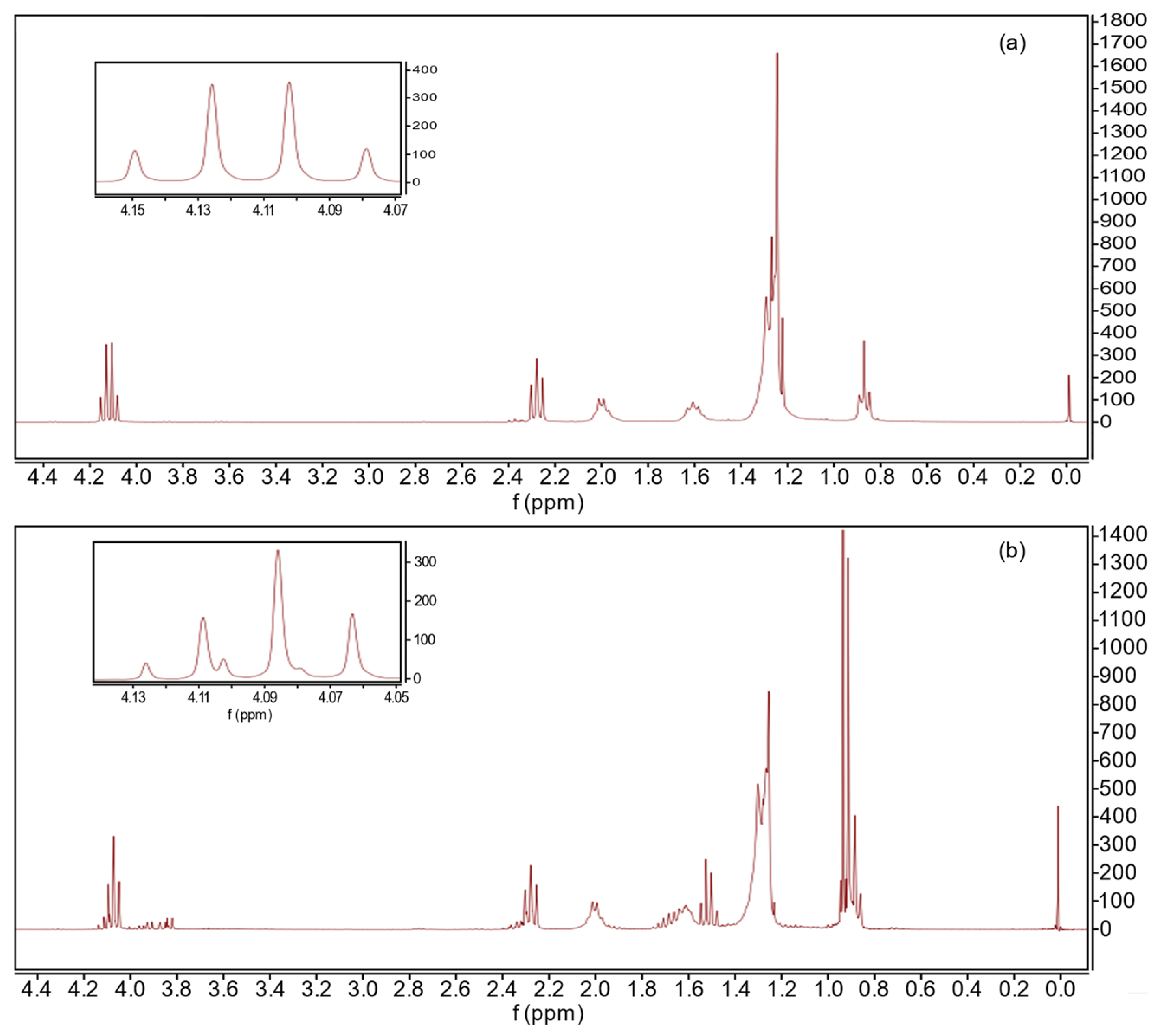
3.3. Biolubricant Synthesis Catalyzed by Keggin-Structure Heteropolyacid Supported on Niobia
| Acyl Receptor | Conversion (%) | * MAG (m/m%) | * DAG (m/m%) | Viscosity (mm2 s−1) |
|---|---|---|---|---|
| Ethanol | 98.14 | 1.86 | ** ND | 4.9 |
| Fusel oil | 99.10 | 0.81 | ** ND | 5.5 |

| Alcohol | Conversion (%) | * MAG (m/m%) | * DAG (m/m%) | Viscosity (mm2 s−1) | |
|---|---|---|---|---|---|
| Dunaliella salina | Ethanol | 92.94 | 1.55 | 5.51 | 6.2 |
| Butanol | 97.60 | 1.14 | 1.26 | 4.8 | |
| Isoamyl alcohol | 97.40 | 2.60 | ** ND | 4.9 | |
| Simulated fusel oil | 97.58 | 2.42 | ** ND | 5.0 | |
| Microalgae-fungus consortium | Simulated fusel oil | 96.80 | 2.30 | 0.90 | 5.1 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolina, I.C.A.; Gomes, R.A.B.; Mendes, A.A. Biolubricant Production from Several Oleaginous Feedstocks Using Lipases as Catalysts: Current Scenario and Future Perspectives. BioEnergy Res. 2021, 14, 1039–1057. [Google Scholar] [CrossRef]
- Mobarak, H.; Mohamad, E.N.; Masjuki, H.; Kalam, A.; Al Mahmud, K.; Habibullah, M.; Ashraful, A. The prospects of biolubricants as alternatives in automotive applications. Renew. Sustain. Energy Rev. 2014, 33, 34–43. [Google Scholar] [CrossRef]
- GVC Lubricants Market Size, Share & Trends Analysis Report by Application (Industrial, Marine, Automotive, Aerospace), by Region (Asia Pacific, North America, Europe, MEA), and Segment Forecasts, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/lubricants-market (accessed on 10 December 2021).
- Da Silva, A.P.T.; Bredda, E.H.; de Castro, H.F.; Da Rós, P.C.M. Enzymatic catalysis: An environmentally friendly method to enhance the transesterification of microalgal oil with fusel oil for production of fatty acid esters with potential application as biolubricants. Fuel 2020, 273, 117786. [Google Scholar] [CrossRef]
- De Souza, E.C.; Belinato, G.; Otero, R.L.S.; Simêncio, É.A.; Augustinho, S.C.M.; Capelupi, W.; Conconi, C.; Canale, L.C.F.; Totten, G.E.; Rhee, I.-S.; et al. Thermal Oxidative Stability of Vegetable Oils as Metal Heat Treatment Quenchants. J. ASTM Int. 2012, 9, 1–30. [Google Scholar] [CrossRef]
- Qiao, S.; Shi, Y.; Wang, X.; Lin, Z.; Jiang, Y. Synthesis of Biolubricant Trimethylolpropane Trioleate and Its Lubricant Base Oil Properties. Energy Fuels 2017, 31, 7185–7190. [Google Scholar] [CrossRef]
- Bredda, E.H.; Da Rós, P.; Pedro, G.A.; De Castro, H.F.; Silva, M.B. Nannochloropsis gaditana and Dunaliella salina as Feedstock for Biodiesel Production: Lipid Production and Biofuel Quality. J. Adv. Biol. Biotechnol. 2018, 20, 1–10. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Ratomski, P.; Koniuszy, A.; Golimowski, W.; Teleszko, M.; Grygier, A. Fatty Acid Profile of Microalgal Oils as a Criterion for Selection of the Best Feedstock for Biodiesel Production. Energies 2021, 14, 7334. [Google Scholar] [CrossRef]
- Patel, A.; Mu, L.; Shi, Y.; Rova, U.; Christakopoulos, P.; Matsakas, L. Single-Cell Oils from Oleaginous Microorganisms as Green Bio-Lubricants: Studies on Their Tribological Performance. Energies 2021, 14, 6685. [Google Scholar] [CrossRef]
- Bredda, E.H.; da Silva, A.F.; Silva, M.B.; Da Rós, P.C.M. Mixture design as a potential tool in modeling the effect of light wavelength on Dunaliella salina cultivation: An alternative solution to increase microalgae lipid productivity for biodiesel production. Prep. Biochem. Biotechnol. 2020, 50, 379–389. [Google Scholar] [CrossRef]
- Zorn, S.M.F.E.; Reis, C.E.R.; Silva, M.B.; Hu, B.; De Castro, H.F. Consortium Growth of Filamentous Fungi and Microalgae: Evaluation of Different Cultivation Strategies to Optimize Cell Harvesting and Lipid Accumulation. Energies 2020, 13, 3648. [Google Scholar] [CrossRef]
- Valdovinos-García, E.M.; Barajas-Fernández, J.; de los Ángeles Olán-Acosta, M.; Petriz-Prieto, M.A.; Guzmán-López, A.; Bravo-Sánchez, M.G.; Patel, A.; Mu, L.; Shi, Y.; Rova, U.; et al. Isolation and characterization of two microalgal isolates from Vietnam with potential for food, feed, and biodiesel production. Energies 2020, 13, 1–19. [Google Scholar]
- Kowal, P.; Ciesielski, S.; Otieno, J.; Majtacz, J.B.; Czerwionka, K.; Mąkinia, J. Denitrification Process Enhancement and Diversity of the Denitrifying Community in the Full Scale Activated Sludge System after Adaptation to Fusel Oil. Energies 2021, 14, 5225. [Google Scholar] [CrossRef]
- Simsek, S.; Ozdalyan, B. Improvements to the Composition of Fusel Oil and Analysis of the Effects of Fusel Oil–Gasoline Blends on a Spark-Ignited (SI) Engine’s Performance and Emissions. Energies 2018, 11, 625. [Google Scholar] [CrossRef]
- Da Conceição, L.R.V.; Reis, C.E.R.; de Lima, R.; Cortez, D.V.; de Castro, H.F. Keggin-structure heteropolyacid supported on alumina to be used in trans/esterification of high-acid feedstocks. RSC Adv. 2019, 9, 23450–23458. [Google Scholar] [CrossRef] [Green Version]
- Da Conceição, L.R.V.; Carneiro, L.M.; Giordani, D.S.; de Castro, H.F. Synthesis of biodiesel from macaw palm oil using mesoporous solid catalyst comprising 12-molybdophosphoric acid and niobia. Renew. Energy 2017, 113, 119–128. [Google Scholar] [CrossRef]
- Chu, R.; Li, S.; Zhu, L.; Yin, Z.; Hu, D.; Liu, C.; Mo, F. A review on co-cultivation of microalgae with filamentous fungi: Efficient harvesting, wastewater treatment and biofuel production. Renew. Sustain. Energy Rev. 2021, 139, 110689. [Google Scholar] [CrossRef]
- Darki, B.Z.; Seyfabadi, J.; Fayazi, S. Effect of nutrients on total lipid content and fatty acids profile of Scenedesmus obliquus. Braz. Arch. Biol. Technol. 2017, 60, e17160304. [Google Scholar] [CrossRef] [Green Version]
- Cheung, S.L.; Allen, D.G.; Short, S.M. Specific quantification of Scenedesmus obliquus and Chlorella vulgaris in mixed-species algal biofilms. Bioresour. Technol. 2020, 295, 122251. [Google Scholar] [CrossRef]
- Gatamaneni Loganathan, B.; Orsat, V.; Lefsrud, M.; Wu, B.S. A comprehensive study on the effect of light quality imparted by light-emitting diodes (LEDs) on the physiological and biochemical properties of the microalgal consortia of Chlorella variabilis and Scenedesmus obliquus cultivated in dairy wastewater. Bioprocess Biosyst. Eng. 2020, 43, 1445–1455. [Google Scholar] [CrossRef]
- Rajendran, A.; Hu, B. Mycoalgae biofilm: Development of a novel platform technology using algae and fungal cultures. Biotechnol. Biofuels 2016, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Guillard, R.R.L.; Lorenzen, C.J. Yellow-Green Algae with Chlorophyllide C1,2. J. Phycol. 1972, 8, 10–14. [Google Scholar] [CrossRef]
- Santos, F.D.; da Conceição, L.R.V.; Ceron, A.; de Castro, H.F. Chamotte clay as potential low cost adsorbent to be used in the palm kernel biodiesel purification. Appl. Clay Sci. 2017, 149, 41–50. [Google Scholar] [CrossRef]
- Carvalho, A.K.F.; Bento, H.B.S.; Rivaldi, J.D.; de Castro, H.F. Direct transesterification of Mucor circinelloides biomass for biodiesel production: Effect of carbon sources on the accumulation of fungal lipids and biofuel properties. Fuel 2018, 234, 789–796. [Google Scholar] [CrossRef]
- Cerón, A.A.; Boas, R.N.V.; Biaggio, F.C.; de Castro, H.F. Synthesis of biolubricant by transesterification of palm kernel oil with simulated fusel oil: Batch and continuous processes. Biomass Bioenergy 2018, 119, 166–172. [Google Scholar] [CrossRef]
- Carvalho, A.K.F.; Rivaldi, J.D.; Barbosa, J.C.; de Castro, H.F. Biosynthesis, characterization and enzymatic transesterification of single cell oil of Mucor circinelloides—A sustainable pathway for biofuel production. Bioresour. Technol. 2015, 181, 47–53. [Google Scholar] [CrossRef]
- Cui, Y.; Liang, Y. Direct transesterification of wet Cryptococcus curvatus cells to biodiesel through use of microwave irradiation. Appl. Energy 2014, 119, 438–444. [Google Scholar] [CrossRef]
- Indarti, E. Preparation of Biodiesel from Microalgae and Palm Oil by Direct Transesterification in a Batch Microwave Reactor. J. Phys. Conf. Ser. 2015, 622, 012040. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, A.K.F.; da Conceição, L.R.V.; Silva, J.P.V.; Perez, V.H.; de Castro, H.F. Biodiesel production from Mucor circinelloides using ethanol and heteropolyacid in one and two-step transesterification. Fuel 2017, 202, 503–511. [Google Scholar] [CrossRef]
| Growth Parameters | D. Salina | Microbial Consortium |
|---|---|---|
| Biomass concentration (g L−1) | 1.15 ± 0.40 | 1.27 ± 0.7 |
| Lipid concentration (mg L−1) | 219 ± 0.30 | 482.6 ± 0.3 |
| Lipid yield (mg lipids mg−1 biomass) | 0.19 ± 0.10 | 0.38 ± 0.3 |
| Volumetric lipid production rate (mg L−1 day−1) | 31.28 ± 0.30 | 64.4 ± 0.5 |
| Volumetric biomass production rate (mg L−1 day−1) | 164.28 ± 0.10 | 169.3 ± 0.4 |
| Fatty Acid Groups (wt%) | ||
| SFA (saturated fatty acids) | 34.27 | 33.69 |
| MUFA (monounsaturated fatty acids) | 46.74 | 34.23 |
| PUFA (polyunsaturated fatty acids) | 18.99 | 32.08 |
| Catalyst | Lipid Source | Time (h) | Conversion (%) | Reference |
|---|---|---|---|---|
| LBC/Nb2O5 | D. salina microalgal oil | 120 | 90.00 | da Silva et al. [4] |
| H3PMo12O40/ Nb2O5 | D. salina microalgal biomass | 6 | 97.58 | This work |
| Microalgae-fungus biomass | 6 | 96.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorn, S.M.F.E.; da Silva, A.P.T.; Bredda, E.H.; Bento, H.B.S.; Pedro, G.A.; Carvalho, A.K.F.; Silva, M.B.; Da Rós, P.C.M. In Situ Transesterification of Microbial Biomass for Biolubricant Production Catalyzed by Heteropolyacid Supported on Niobium. Energies 2022, 15, 1591. https://doi.org/10.3390/en15041591
Zorn SMFE, da Silva APT, Bredda EH, Bento HBS, Pedro GA, Carvalho AKF, Silva MB, Da Rós PCM. In Situ Transesterification of Microbial Biomass for Biolubricant Production Catalyzed by Heteropolyacid Supported on Niobium. Energies. 2022; 15(4):1591. https://doi.org/10.3390/en15041591
Chicago/Turabian StyleZorn, Savienne M. F. E., Ana Paula T. da Silva, Eduardo H. Bredda, Heitor B. S. Bento, Guilherme A. Pedro, Ana Karine F. Carvalho, Messias Borges Silva, and Patrícia C. M. Da Rós. 2022. "In Situ Transesterification of Microbial Biomass for Biolubricant Production Catalyzed by Heteropolyacid Supported on Niobium" Energies 15, no. 4: 1591. https://doi.org/10.3390/en15041591
APA StyleZorn, S. M. F. E., da Silva, A. P. T., Bredda, E. H., Bento, H. B. S., Pedro, G. A., Carvalho, A. K. F., Silva, M. B., & Da Rós, P. C. M. (2022). In Situ Transesterification of Microbial Biomass for Biolubricant Production Catalyzed by Heteropolyacid Supported on Niobium. Energies, 15(4), 1591. https://doi.org/10.3390/en15041591









