Abstract
The current development of chemical looping combustion (CLC) technology is presented in this paper. This technique of energy conversion enables burning of hydrocarbon fuels with dramatically reduced CO2 emission into the atmosphere, since the inherent separation of carbon dioxide takes place directly in a combustion unit. In the beginning, the general idea of the CLC process is described, which takes advantage of solids (so-called oxygen carriers) being able to transport oxygen between combustion air and burning fuel. The main groups of oxygen carriers (OC) are characterized and compared, which are Fe-, Mn-, Cu-, Ni-, and Co-based materials. Moreover, different constructions of reactors tailored to perform the CLC process are described, including fluidized-bed reactors, swing reactors, and rotary reactors. The whole systems are based on the chemical looping concept, such as syngas CLC (SG-CLC), in situ Gasification CLC (iG-CLC), chemical looping with oxygen uncoupling (CLOU), and chemical looping reforming (CLR), are discussed as well. Finally, a comparison with other pro-CCS (carbon capture and storage) technologies is provided.
1. Introduction
Carbon dioxide emission from anthropogenic sources, including the power industry, has attracted more and more attention over the last 15 years. This interest brings rapid development of so-called pro-CCS (carbon capture and storage) technologies, which offer the ability to reduce significantly the emission of CO2 into the atmosphere. As a result, three of these methods, such as pre-combustion capture, post-combustion capture, and oxy-fuel combustion [1,2,3,4], have achieved satisfied technological maturity. Nevertheless, commercial implementation on a large scale is still very limited, mainly due to the high energy-efficiency penalty associated among others with ASU (air separation unit) operation [5]. This problem does not concern, however, the innovative technique of chemical looping combustion CLC [6], where solid particles are used to transport oxygen from combustion air to the burning fuel.
The CLC process is usually conducted in a system of two reactors (Figure 1), which consists of the fuel reactor (FR), where combustion takes place, and the air reactor (AR), where oxygen carriers (OC) are regenerated. Thus, oxygen carriers are subjected to continuous oxidation and reduction (Equations (1) and (2)) [7] while they circulate throughout the combined CLC unit. There are two crucial benefits of OCs employment in a power generation system, which are the elimination of conventional energy-consuming air separation units and the inherent separation of forming flue gases (mainly CO2 and H2O) from the air supplied to the combustion process. As a result, the flue gas that leaves the fuel reactor is free of atmospheric nitrogen and thus it becomes almost ready for geological sequestration—CCS (carbon capture and storage) and/or commercial utilization—CCU (carbon capture and utilization) [8]. Concurrently, the exhaust gas from the air reactor consists essentially of nitrogen and the remaining oxygen, which are both environmentally friendly gases.
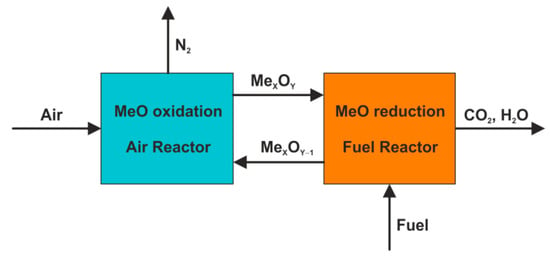
Figure 1.
Chemical looping combustion concept.
The combined reaction can be shown as Equation (3), where the total amount of heat released in the CLC process is equal to the heat obtained from conventional combustion with gaseous oxygen.
Therefore, it is obvious that subsequent CO2 storage [9,10] and/or utilization [11,12] are inseparable from the CLC process since this is the only way to ultimately restrain the greenhouse gas emission into the atmosphere. Generally, there are two major pathways to utilizing CO2, which include direct and indirect methods. Technologies such as enhanced oil recovery (EOR) [13,14] and enhanced gas recovery (EGR) [15,16], where CO2 is directly injected underground while enhancing oil or gas recovery, are fully mature and commercially in use. CO2 can also be utilized in concrete building materials via mineral carbonation, which additionally increases the mechanical strength of these materials. There is also a continuous demand for CO2 in the food industry (e.g., dry ice, food preservative, and drinks carbonation). However, CO2 conversion to fuels (methanol, ethanol, syngas, and methane) and chemicals (formic acid and oxalic acid), which is already sufficiently developed technology for full-scale demonstration, seems to be the most promising alternative for the near future. Detailed studies on CCSU (carbon capture, storage, and utilization) technologies can be found elsewhere [17,18,19,20,21,22].
2. Oxygen Carriers
Solid oxygen carriers behave like red blood cells transporting oxygen in a “blood circulation” of the CLC system where the air reactor acts as “lungs”, whereas the fuel reactor resembles “tissue” in which the “organic matter conversion” takes place. Therefore, they become indispensable for proper functioning of the “body” and as their oxygen transport abilities run down the “metabolic” capacities of the whole CLC system disappear.
As described above, solid oxygen carriers (OC) are essential for chemical looping combustion technology. The key features of oxygen carriers, which determine the performance of CLC process include:
- Reactivity with combustibles and oxygen;
- Oxygen transport capacity (OTC);
- Chemical lifetime;
- Mechanical resistance (including melting temperature);
- Tendency to carbon deposition on oxygen carrier surface;
- Toxicity and environmental impact;
- Price and availability.
Oxygen carriers can be classified into several categories. The first one is based on the origin, which means natural OCs (e.g., ilmenite) and synthetic OCs (e.g., Cu0.95Fe1.05AlO4). Another criterion takes into account the number of active compounds (e.g., NiO or (Mn,Fe)2O3) and their structure (e.g., perovskites [23,24]). Finally, the ability of oxygen uncoupling in an environment of a fuel reactor (CLOU-type OC) is taken into consideration. Recently, very promising novel synthetic oxygen carriers based on geopolymer composites were proposed [25]. The main advantages of geopolymer OCs are, among others, low cost, green synthesis, non-toxicity, and CLOU feature [26]. Furthermore, a good mechanical resistance makes them suitable for fluidized bed applications [27]. In the search for potential oxygen carriers, steel converter slag was also tested with success under circulating fluidized bed (CFB) conditions [28]. Usually, however, solid oxygen carriers are simply divided into five groups, which are: Fe−, Cu−, Mn−, Co−, and Ni-based OC. Moreover, in addition to metal oxides the supports of different kinds are used, which enriched their inherent properties, among others are: Al2O3, MgAl2O4, TiO2, SiO2, ZrO2, and bentonite.
Key properties of basic oxygen carriers and hence their usefulness in the CLC systems are presented in Table 1. A wide variety of different features can be seen in this chart; therefore, it seems reasonable to use mixed oxygen carriers with selected supports, which should be tailored individually for each chemical looping application. In addition to these data, the comparison of oxygen transport capacity (OTC) is provided for all aforementioned materials as well as for two natural oxygen carriers such as ilmenite and anhydrite (Figure 2). Significant differences can be seen in this figure where the values of OTC vary up to an order of magnitude in general. Much bigger differences relate to the market prices of basic metals (Figure 3), provided by the London Metal Exchange LME (average data for the years 2013–2017). It can be found, for instance, that cobalt is about a hundred times more expensive than iron or manganese and even much more expensive than gypsum compounds. Another crucial factor, which is melting temperature [29], varies from 1084 °C for copper up to 1538 °C for iron [30]. The values of Tm for manganese, nickel, and cobalt are 1244 °C, 1455 °C, and 1495 °C [30], respectively.
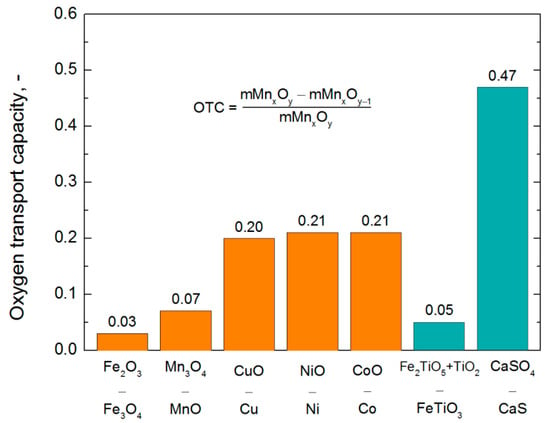
Figure 2.
Oxygen transport capacity.
An interesting issue involves the thermal balance over the CLC unit, since the process of oxygen carriers regeneration that takes place in the air reactor appears to be much more exothermic compared with the combustion reactions, which proceed concurrently in the fuel reactor. Furthermore, the standard heat of reduction reactions with methane and carbon is negative only for Cu-based oxygen carriers [7]. This exposes another crucial function of solid oxygen carriers, which naturally take part in the heat transfer not only to the working fluid but also between the reactors in the CLC system. Safety and environmental issues related to the use of solid oxygen carriers in power generation systems are discussed in detail elsewhere [31,32].

Table 1.
Properties of oxygen carriers (based on [33,34,35,36]).
Table 1.
Properties of oxygen carriers (based on [33,34,35,36]).
| Fe2O3/Fe3O4 | Mn3O4/MnO | CuO/Cu | NiO/Ni | CoO/Co | |
|---|---|---|---|---|---|
| Reactivity with CH4 | low | average | high | very high | low |
| Reactivity with CO and H2 | average | high | very high | average | low |
| Oxygen transport capacity | low | average | high | high | high |
| CLOU effect | no | no 1 | yes | no | no 2 |
| Cost | low | average | high | very high | very high |
| Melting temperature | high | average | low | high | high |
| Health and environment | safe | safe | harmful | very harmful | very harmful |
1 yes, for Mn2O3 only; 2 yes, for Co3O4 only.
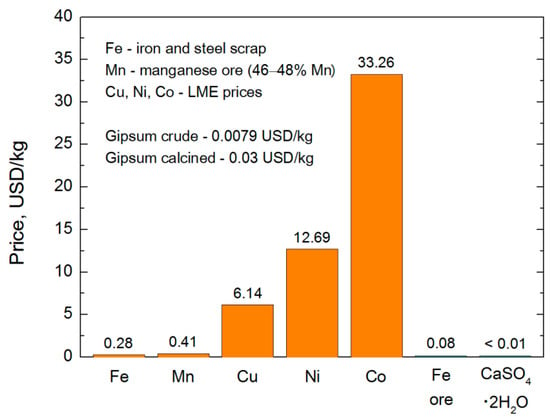
Figure 3.
Market prices of feedstocks for oxygen carriers production (based on [37]).
3. Chemical Looping Systems
Several different systems based on chemical looping principles are developed concurrently in order to burn fossil and other fuels in a clean and efficient manner. In a first approach, the whole group of various systems with oxygen carriers [38,39,40,41,42,43] and so-called carbonate looping systems (with CO2 carriers) [44,45] can be distinguished. Further classification is presented in Figure 4, which takes into account a feature of oxygen carriers and place of fuel gasification. A somewhat different idea is applied in the chemical looping reforming (CLR) systems [46,47], where fuel conversion is oriented towards hydrogen production.
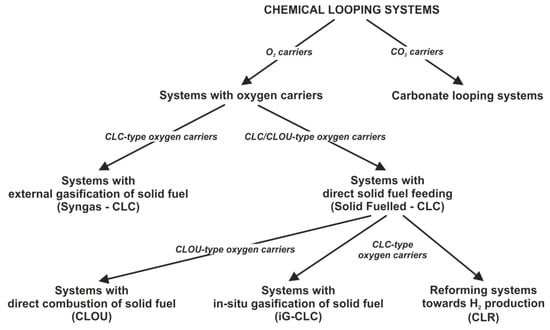
Figure 4.
Classification of chemical looping systems.
3.1. SG-CLC System
Originally, the CLC concept was developed for the combustion of gaseous fuel [48,49]. Therefore, for the purpose of solid fuel combustion, the preliminary gasification in an external unit was initially considered, and hence the fuel reactor might be fed directly with a produced syngas. Thus, the proposed Syngas CLC (SG-CLC) system [38,39] exhibits the technical ability of coal conversion in a chemical looping environment (Figure 5), however, it still needs the detached unit for air separation, which is crucial from the economic point of view.
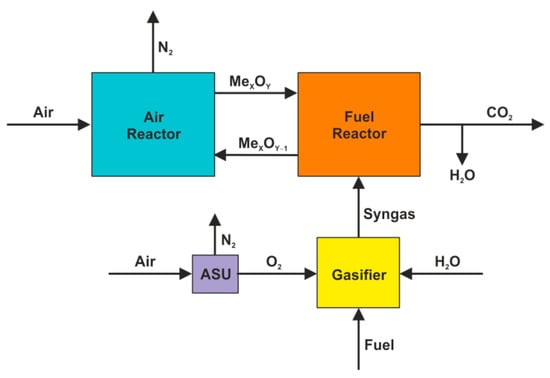
Figure 5.
SG-CLC system.
The thermal decomposition of fuel and the gasification of char in a mixture of steam and oxygen proceed according to the following Equations (4)–(6):
whereas subsequent combustion of volatiles and syngas both being in contact with solid oxygen carriers can be described by the reactions (7) and (8):
Concurrently, the reduced oxygen carriers undergo regeneration (Equation (9)) in the air reactor, before they are returned to the combustion zone, which makes the chemical loop complete.
3.2. iG-CLC System
For the improved concept called iG-CLC (in situ gasification CLC) [40,41], it was assumed that the gasification process would take place inside the fuel reactor, whereas the recirculated CO2 might be used as a gasification agent (Figure 6). Thereby, a huge cost associated with both the construction and the operation of an external air separation unit can be eliminated. A weak point appears, however, in a low rate of gasification reactions (6, 10), which slow down the whole combustion process with the use of oxygen carriers.
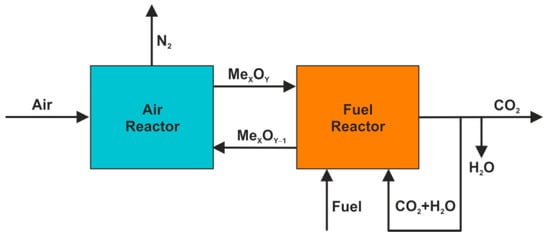
Figure 6.
iG-CLC system.
It is also worth mentioning that the exothermic reaction (5) is replaced in the presented system by a Boudouard reaction (10) which, similar to the reaction (6) and the devolatilization process (Equation (4)), has an endothermic nature. In the following stages, the burn out of combustible gases and the oxidation of oxygen carriers run according to the above-given Equations (7)–(9), respectively.
3.3. CLOU System
New opportunities appeared with the second generation of oxygen carriers (CLOU-type OC), which have the ability to release gaseous oxygen in an environment of the fuel reactor ((Equation (11)). Thus, the initial gasification became less important and the CLOU (chemical looping with oxygen uncoupling) combustion [42,43] (Equations (4), (12) and (13)) starts to resemble essentially the oxy-fuel combustion technology. Therefore, the recirculated carbon dioxide or the mixture of CO2 and H2O (in case of “wet” recirculation) fulfils the function of fluidizing gas in the unit (Figure 7) rather than the gasification agent as described previously.
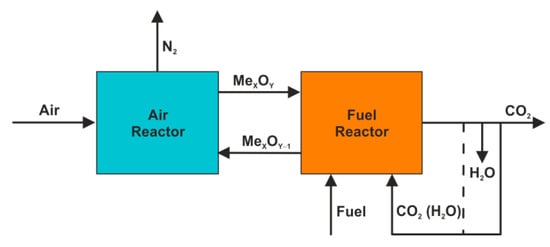
Figure 7.
CLOU system.
It can be claimed thereby that the intensive investigations focused on inexpensive oxygen carriers with increased O2 transport capacity, improved reactivity, and high mechanical strength are currently of great importance for further accelerated development of the chemical looping combustion technology.
3.4. CLR System
Another interesting concept based on the chemical looping idea is oriented towards hydrogen production and it is widely known as chemical looping reforming (CLR) [46,47]. Two different techniques can be found under this name, which are Dry CLR and Steam CLR, where the fuel reactor is supplied with CO2 or H2O, respectively (Figure 8). Concurrently, oxygen is provided to the fuel reactor with the use of solid oxygen carriers and hence the whole gasification process can be described by the Equations (4) and (14) together with Equation (10) in the case of Dry CLR or Equation (6) for the steam-reforming method.
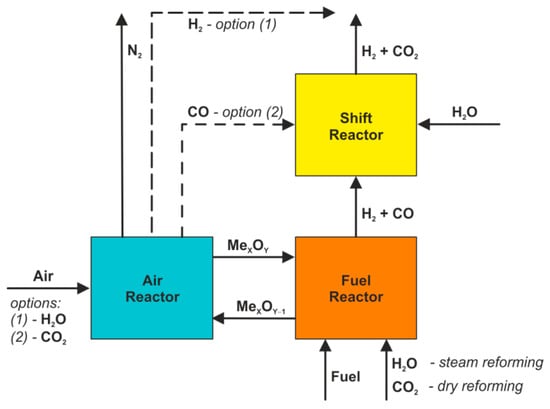
Figure 8.
CLR system.
In the second stage, the products of aforementioned Equations (6), (10) and (14) are directed from the fuel reactor to the shift reactor (SR), where CO is converted into CO2 according to the following Equation (15):
Moreover, two additional options are considered for the regeneration of oxygen carriers, which take into account the use of steam (Equation (16)) or CO2 (Equation (17)) instead of air (Equation (9)) that is usually utilized for this purpose in the air reactor (see: Figure 8).
The biggest challenge seems to be, however, the selective oxygen carriers, which react very easily with carbon (conversion to CO) rather than with hydrogen and initially generated carbon monoxide. A very narrow group of such materials includes, among others, some ferrites (e.g., CaFe2O4 or BaFe2O4) [50,51]. It is also worth mentioning that the gasification reactions can be accelerated by the catalysts (e.g., Ni-Fe composites) [52,53] introduced directly into the fuel reactor, which is distinguished as a catalyst-assisted CLR technique. The results of the experimental investigations on CLR can be found in [54,55,56,57].
4. Chemical Looping Reactors
Generally, three basic constructions of reactors are considered in order to put the idea of chemical looping combustion into practice, which are as follows:
- Dual fluidized-bed reactors [33,58,59];
- Swing reactors or fixed (packed) bed reactors [60,61];
- Rotary (rotating bed) reactors [62,63].
Each aforementioned design certainly has its advantages and disadvantages. Similarly, one has a facility for gaseous fuel combustion while the other can be easily adapted to burn solid fuels. Nevertheless, every construction should meet three common criteria, which finally affect the operation efficiency of the CLC unit. The first one concerns maintaining the oxygen carriers-to-fuel ratio by means of an appropriate recirculation rate (fluidized-bed reactors), oxidation–reduction sequence (swing reactors), and rotational speed (rotary reactors). Another issue involves the sufficient residence times of oxygen carriers in both air and fuel reactors, which influence the combustion process as well. The last question relates to the efficient capture of CO2, which is executed by the effective sealing of the sections with fuel conversion and oxygen carriers regeneration. This prevents gas (particularly CO2) from leaking between AR and FR as well as from char slipping into the air reactor.
4.1. Dual Fluidized-Bed Reactor
Most of the CLC reactors presented in the literature are dual fluidized-bed (DFB) reactors [33,58,59] of peculiar design. This is due to the high efficiency of fuel conversion of these reactors and their ability to burn solid fuels. Other advantages of this kind of reactor is the suitability for scaling-up [64,65], high throughout output capacity, and smooth operation [66]. On the other hand, the construction of DFB reactors is more sophisticated and hence the operation appears to be more demanding compared with other designs mentioned above. Moreover, the mechanical attrition caused by moving particles may lead to serious problems, which are common for circulating fluidized bed units in general.
A dual fluidized-bed reactor consists of two combined reactors, which are the fuel reactor and the air reactor (Figure 9). The fuel reactor is fed with fuel being burnt with the help of oxygen carriers, which are further regenerated in the air reactor. Solids circulation becomes possible due to the cyclone separator and two loop-seals installed between the reactors. Usually, the air reactor is operated under fast-fluidized bed (FFB) conditions, whereas the fuel reactor works in a regime of bubbling fluidized bed (BFB), however, there are known constructions with FFB [67,68] or BFB [69] in both reactors. Moreover, CLC reactors are often equipped with a so-called carbon stripper (CS) system and/or oxygen polishing (OP) system, both shown in Figure 9. The carbon stripper [70,71,72] is used to return unburned char back to the fuel reactor, whereas oxygen polishing [73,74] enables to burn out all combustibles remaining in the exhaust gases.
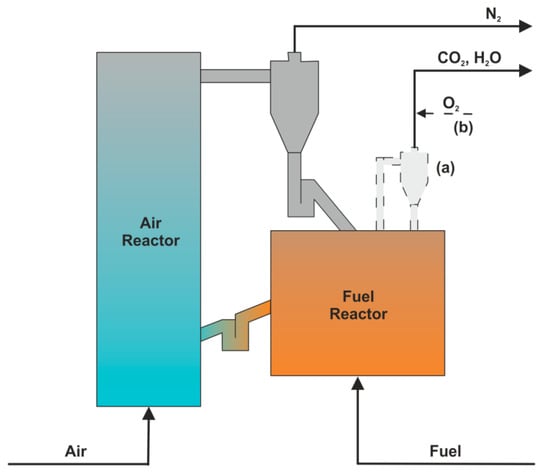
Figure 9.
Dual-fluidized bed reactor for chemical looping combustion; optional systems: carbon stripper (a) and oxygen polishing (b).
Whitty et al. [75] preformed CLOU experiments in a 200 kWth dual-fluidized bed reactor. Authors point to the main challenges as balancing oxygen supply to the fuel reactor, heat generation, and temperatures, as well as global and internal circulation rates. Low CO2 capture efficiency was noticed on a 1 MWth pilot-scale CLC unit with two interconnected CFB reactors using ilmenite as oxygen carrier and hard coal as fuel [76]. Authors found that unconverted char left the fuel reactor and burned in the air reactor. The reason for this is that no carbon stripper was used during these investigations. A different operational strategy based on the fuel reactor staged fluidization without a carbon stripper is proposed by Shen et al. [77,78]. It was proved that internal distributors in a multistage fuel reactor sufficiently improve carbon conversion and CO2 capture efficiencies. The main difficulties with biomass fuel utilization in CLC reactors are mentioned by Gogolev et al. [79]. The authors indicate the bed agglomeration, oxygen carrier deactivation, fouling, and corrosion as the most harmful effects associated with biomass alkali release. The problem of gas leakages between fluidized bed reactors via a loop seal was investigated by Lindmuller et al. [80] on a 25 kWth CLC facility. Loop seal design ideas and operation condition proposals are suggested in this work. Very useful guidelines were provided by Zhao et al. [81], who described long-term operational experiences from the interconnected fluidized bed reactors of different scales. Further details on the performance of a 50 kWth coal-fueled chemical-looping combustor can be found in [82]. Another excellent work of Lyngfelt et al. [83] was published recently, which summarizes over 10,000 hours of chemical looping combustion tests carried out around the world. The authors identify a major difficulty with the scaling-up of the CLC process to commercial use, which is the lack of incentives for CO2 capture and the risk in general. Therefore, the multipurpose dual fluidized bed (MDFB) concept for maximum flexibility is proposed among others. Operational experiences and practical challenges of the fluidized-bed units are also presented in other research works [84,85,86,87,88,89,90,91,92,93,94,95,96,97,98].
4.2. Swing Reactor
Another type of reactor that can be employed for chemical looping combustion is a swing reactor (fixed/packed bed reactor) [60,61]. Simple design and easy operation are the main advantages of this construction. Moreover, the ability of chemical looping performance under elevated pressure [99,100] seems to be an additional benefit as well. A weak point of swing reactors is, however, the readiness for gaseous fuel combustion only, therefore, in the case of solid fuels they need to be gasified in advance (see: SG-CLC system).
The single module of the swing reactor consists of two columns placed in parallel, both filled up with solid oxygen carriers (Figure 10). While the first column (FR) is fed with fuel, the second one acts as the air reactor. After the bed in the fuel reactor is depleted and the oxygen carriers in AR are re-oxidized, the roles are reversed. Thus, the CLC process becomes cyclical. Between the following stages, the columns are purged, which consumes energy and causes interruptions. Therefore, the system of several smaller modules rather than one large unit is recommended for the continuous and smooth operation of the whole CLC system.
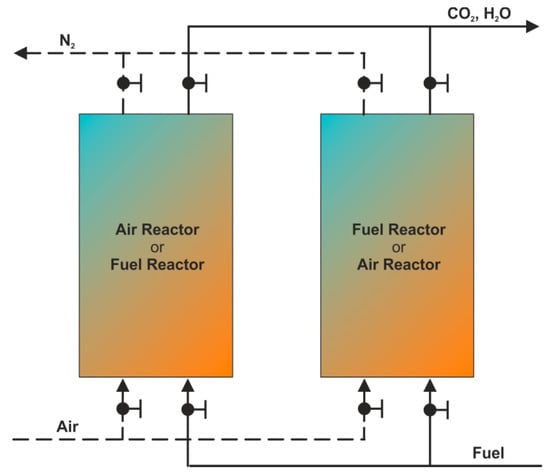
Figure 10.
Swing reactor.
Han and Bollas [101] explored the use of a reverse-flow in a fixed-bed reactor fueled with methane and syngas. The authors found that periodic reversal of gas flow direction enhances the contact between the fuel and unconverted oxygen carriers, improves temperature distribution in a bed and conversion uniformity, as well as suppresses carbon deposition on NiO oxygen carriers. It was also emphasized that fixed-bed design avoids the issue of attrition and gas–solid separation typically found in circulating fluidized bed units. The problem with excessive carbon deposition on ilmenite oxygen carriers was also mentioned by Gallucci et al. [102]. The experimental results showed, however, that the use of wet syngas as fuel and the increase in operating pressure enables to eliminate this unfavorable phenomenon. It was concurrently demonstrated that pressurized CLC can be easily accommodated in fixed-bed reactors. In another work [103], a fixed-bed reactor was employed for the tests with bimetallic Cu–Fe–Si oxygen carriers at various operating conditions. The authors state that optimized OC to fuel ratio is the key to avoiding solids agglomeration caused by copper at higher temperatures. The combustion of CO and CH4 with the use of ilmenite as the oxygen carrier in packed fluidized bed reactor was recently investigated by Nemati and Ryden [104]. The results show that random packings in a packed fluidized bed can significantly improve fuel conversion in comparison with an ordinary bubbling bed without packings. The authors believe that this improvement results from more efficient gas–solid mass transfer due to the reduced bubble size. Detailed results from experimental tests on fixed bed reactors are reported in [105,106,107,108,109,110].
4.3. Rotary Reactor
Rotary reactors [62,63] are not investigated often compared with dual fluidized-bed reactors and swing reactors, despite a relatively high efficiency of fuel conversion. The biggest challenge in construction and operation of rotary reactors appears to be, however, the sealing of the following sections and parts being in continuous motion. These difficulties lead primarily to lower efficiency of CO2 capture as well as lower purity of exhaust gases. Moreover, this type of CLC unit is destined for gaseous fuel only.
A rotary reactor (Figure 11) is made of three coaxial rings, from which internal and external rings are fixed to each other while the central ring revolves incessantly in one initially set direction. Moreover, the whole unit is divided into four sections, including the air section, fuel section, and two curtain sections. The central ring is packed with solid oxygen carriers. Fuel and air together with purging gas are fed into the appropriate sections of the internal ring, whereas flue gas and exhaust gases are taken off from the external ring, respectively. Thereby, the concept of the rotary reactor combines certain features of both the dual-fluidized bed reactor (continuous operation, oxygen carriers “circulation”) and swing reactor (fixed/packed bed).
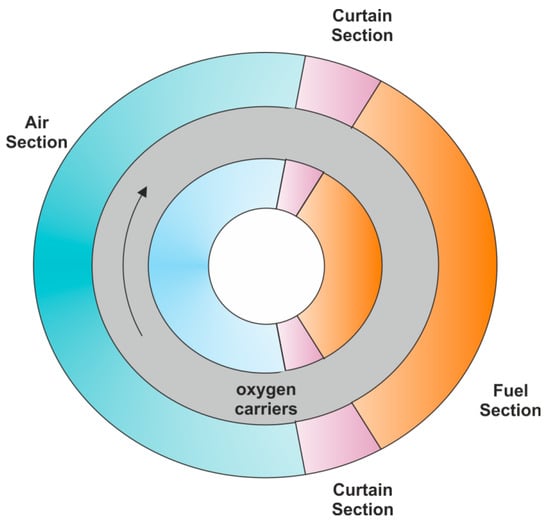
Figure 11.
Rotary reactor.
Iloeje et al. [111] noticed that one of the main operational problems associated with solid oxygen carriers’ circulation in DFB constructions, which is the mechanical attrition, is naturally eliminated in rotary reactors. In another work [112], the authors evaluated the rotary reactor design for copper, nickel, and iron-based oxygen carriers. Researchers define practical challenges as proper selection of reactor geometry, rotating speed, and gas flow rates, which influence efficient fuel conversion and CO2 separation. The authors claim, however, that low rotation speed and appropriately selected seals can minimize gas leakages during reactor operation. The problem with air slipping to the fuel section was also observed by Hakonsen and Blom [113] in their prototype rotating bed reactor, which finally results in low purity of captured CO2. Other results presented by Zhao and Ghoniem [114] confirm that the geometry of the rotary reactor and the operating conditions will depend preliminary on the oxygen carrier properties. The OC kinetics together with the inlet gas temperature and the operating pressure are identified as the most important factors in this work.
5. Competitiveness of CLC Technology
Chemical looping combustion technology offers almost pure carbon dioxide at the outlet of the reactor, without any additional installations for gas separation. Therefore, dramatic reduction in CO2 emission into the atmosphere becomes possible with lower operating and capital expenditures (OPEX and CAPEX, respectively) related to the whole power system [115,116]. Near-zero emission takes place when captured CO2 is subsequently stored or utilized, which is described at the begging of this paper, however, even negative CO2 emission is possible if only biomass is used as fuel [117,118]. Thus, the CLC technique with its inherent separation appears to be competitive for other pro-CCS technologies, which need advanced air separation units (oxy-fuel combustion and pre-combustion capture) or post-processing CO2 capture unit (post-combustion capture). The comparison of selected factors determined for both the CLC reactor and conventional circulating fluidized bed (CFB) boiler integrated with post-combustion CO2 scrubber using monoethanolamine (MEA) is presented in Table 2 [119,120]. Moreover, the authors claim that the results of analyses carried out for the CFB-MEA case and the OXY-CFB case are very similar. The key difference between a reference case (conventional CFB unit) and other examined pro-CCS technologies is the CO2 capture facility, which significantly affects the net efficiency and other indices of the so-called near-zero emission power plants. A considerable discrepancy (more than 5 percentage points) between the net efficiencies of the CLC reactor and the CFB-MEA unit can be seen, which results mainly from the operation of the CO2 scrubbing system. Consequently, the energy penalty affects the cost of electricity production, which is also shown in this chart. Another issue is the CO2 avoidance cost, which is higher by 16 EUR/tCO2 for the CFB-MEA unit which means a significant increase of about 43%.

Table 2.
Comparison of chemical looping combustion technology (CLC) with the amine absorption method (MEA30%) with reference to a 630 MWe power unit with a fluidized bed boiler (CFB) (based on [119,120]).
The comparison of the SG-CLC concept with the pre-combustion CO2 capture technology can be found in [116]. The net efficiency of 39.7% was determined for the CLC unit, which is about 2.6 percentage points more compared with the plant with the pre-combustion CO2 capture. Furthermore, the CO2 capture efficiency significantly exceeds the level of 90% in both evaluated cases. As a reference, a conventional integrated gasification combined cycle (IGCC) of the same fuel input energy (1127 MWth) was established, for which the net efficiency reaches 44.3% as it is not burdened by any CO2 capture systems. Very promising results can be also found in [121], where the achieved net efficiency penalty is less than one percentage point for the integrated system of the CLOU reactor and CHP plant in comparison with the conventional reference plant with the same fuel input. Furthermore, the use of biomass as fuel allows the combined production of heat and power with the so-called negative emission of CO2. The potential of biomass combustion in the CLC plant with CCS is also noticed by Keller et al. [122]. In this study, chemical looping combustion is benchmarked against a CFB process with post-combustion CO2 capture by amine scrubbing. The authors estimate, among other things, that for the plant of 50 MWth HHV fuel input, a cost reduction in the entire CLC-CCS chain can reach 17% over the CFB reference plant with an amine-scrubbing post-combustion capture process. Another interesting evaluation of a poly-generation solar-assisted natural gas-fired CLC plant with waste heat utilization using absorption chilling for cooling purposes was carried out by Ogidiama et al. [123]. The authors claim that exhaust heat utilization significantly improves the overall efficiency of the combined power and cooling plant, which can exceed 63%. Furthermore, the integration with the solar system results in higher flexibility of the entire CLC plant. Other comprehensive techno-economic analyses of chemical looping combustion technologies can be found elsewhere [124,125,126,127,128,129,130,131,132,133]. The most recent study on a 550 MWe brown coal-fired CLC power plant is provided by Sayeed et al. [134]. The net efficiency penalty of slightly more than five percentage points was obtained for a deeply integrated CLC system in comparison with a conventional supercritical pulverized coal-fired power station of the same net output. In conclusion, the authors state that for a considered commercial-scale CLC power plant with 90% CO2 capture, an increase in the cost of electricity can be lower than 35%, which enables to meet the USDOE’s (United States Department of Energy) target.
6. Conclusions
A rising technology of chemical looping combustion (CLC) has untapped potential to be used in zero CO2 emission power generation systems. The inherent separation that takes place in the CLC reactors results in a highly reduced internal load of the plant, and hence the CLC technology can compete with other pro-CCS technologies such as oxy-fuel combustion, pre-combustion capture, and post-combustion capture. However, it is still not mature enough for commercial implementation.
Key properties of currently considered oxygen carriers are provided, from which the CLOU feature seems to be crucial regarding direct solid fuel combustion. Innovative OCs as geopolymer composites are also mentioned. Various kinds of chemical looping systems are presented including those with external and internal gasification as well as one with direct combustion of solid fuels. Moreover, different designs of CLC reactors are shown including dual fluidized-bed construction, which gives the best prospects for further development of chemical looping combustion technology.
It is also noticed that ultimate utilization of the generated CO2 is essential for this concept, however, geological sequestration seems to be also necessary due to the global volume of greenhouse gas emissions. The greatest capabilities have EOR and EGR technologies, but the production of carbon-based fuels and other chemicals appears to be of great importance at present.
Author Contributions
Conceptualization, T.C.; resources, T.C., J.K., A.Z. and W.N.; writing—original draft preparation, T.C.; writing—review and editing, T.C., J.K. and A.Z.; visualization, T.C.; supervision, W.N.; project administration, T.C.; funding acquisition, T.C. and W.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded from Norway Grants in the Polish-Norwegian Research Programme operated by the National Centre for Research and Development, project: Innovative Idea for Combustion of Solid Fuels via Chemical Looping Technology, agreement number POL-NOR/235083/104/2014. The support is gratefully acknowledged. The work was also funded by the statute subvention of Czestochowa University of Technology, Faculty of Infrastructure and Environment.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Nomenclature
| AR | Air reactor |
| ASU | Air separation unit |
| BFB | Bubbling fluidized bed |
| CAPEX | Capital expenditures |
| CCS | Carbon capture and storage |
| CCSU | Carbon capture, storage, and utilization |
| CCU | Carbon capture and utilization |
| CFB | Circulating fluidized bed |
| CLC | Chemical looping combustion |
| CLOU | Chemical looping with oxygen uncoupling |
| CLR | Chemical looping reforming |
| CS | Carbon stripper |
| DFB | Dual fluidized bed |
| EGR | Enhanced gas recovery |
| EOR | Enhanced oil recovery |
| FFB | Fast fluidized bed |
| FR | Fuel reactor |
| IGCC | Integrated gasification combined cycle |
| iG-CLC | Incsitu gasification chemical looping combustion |
| MDFB | Multipurpose dual fluidized bed |
| MEA | Monoethanolamine |
| OC | Oxygen carrier |
| OP | Oxygen polishing |
| OPEX | Operating expenditures |
| OTC | Oxygen transport capacity |
| OXY | Oxy-fuel combustion |
| SG-CLC | Syngas chemical looping combustion |
| SR | Shift reactor |
| USDOE | United States Department of Energy |
References
- Sifat, N.S.; Haseli, Y. A critical review of CO2 capture technologies and prospects for clean power generation. Energies 2019, 12, 4143. [Google Scholar] [CrossRef]
- Cannone, S.F.; Lanzini, A.; Santarelli, M. A review on CO2 capture technologies with focus on CO2-enhanced methane recovery from hydrates. Energies 2021, 14, 387. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Alminshid, A.H. CO2 capturing methods: Chemical looping combustion (CLC) as a promising technique. Sci. Total Environ. 2021, 788, 147850. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Naveneethan, S.; Kus, T. Methods and techniques for CO2 capture: Review of potential solutions and applications in modern energy technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Czakiert, T.; Zuwala, J.; Lasek, J. Oxy-fuel combustion: The state of the art. In Proceedings of the 12th International Conference on Fluidized Bed Technology, Cracow, Poland, 23–27 May 2017; Nowak, W., Sciazko, M., Mirek, P., Eds.; Printing House of the Redemptorists: Tuchow, Poland, 2017. [Google Scholar]
- Hossain, M.M.; de Lasa, H.I. Chemical-looping combustion (CLC) for inherent CO2 separations—A review. Chem. Eng. Sci. 2008, 63, 4433–4451. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Anthony, E.J. Chemical-looping combustion systems and technology for carbon dioxide (CO2) capture in power plants. In Developments and Innovation in Carbon Dioxide (CO2) Capture and Storage Technology; Maroto-Valer, M.M., Ed.; Woodhead Publishing: Cambridge, UK, 2010; Volume 1, pp. 358–379. [Google Scholar]
- Cao, C.; Liu, H.; Hou, Z.; Mehmood, F.; Liao, J.; Feng, W. A review of CO2 storage in view of safety and cost-effectiveness. Energies 2020, 13, 600. [Google Scholar] [CrossRef]
- Zheng, F.; Jahandideh, A.; Jha, B.; Jafarpour, B. Geologic CO2 storage optimization under geomechanical risk using coupled-physics models. Int. J. Greenh. Gas Control 2021, 110, 103385. [Google Scholar] [CrossRef]
- Valluri, S.; Claremboux, V.; Kawatra, S. Opportunities and challenges in CO2 utilization. J. Environ. Sci. 2022, 113, 322–344. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, S.-Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Liu, Z.-X.; Liang, Y.; Wang, Q.; Guo, Y.-J.; Gao, M.; Wang, Z.-B.; Liu, W.-L. Status and progress of worldwide EOR field applications. J. Pet. Sci. Eng. 2020, 193, 107449. [Google Scholar] [CrossRef]
- Alvarado, V.; Manrique, E. Enhanced oil recovery: An update review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Lui, S.-Y.; Ren, B.; Li, H.-Y.; Yang, Y.-Z.; Wang, Z.-Q.; Wang, B.; Xu, J.-C.; Agarwal, R. CO2 storage with enhanced gas recovery (CSEGR): A review of experimental and numerical studies. Pet. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Hamza, A.; Hussein, I.A.; Al-Marri, M.J.; Mahmoud, M.; Shawabkeh, R.; Aparicio, S. CO2 enhanced gas recovery and sequestration in depleted gas reservoirs: A review. J. Pet. Sci. Eng. 2021, 196, 107685. [Google Scholar] [CrossRef]
- Gur, T.M. Carbon dioxide emissions, capture, storage and utilization: Review of materials, processes and technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Eide, L.I.; Batum, M.; Dixon, T.; Elamin, Z.; Graue, A.; Hagen, S.; Havorka, S.; Nazarian, B.; Nokleby, P.H.; Olsen, G.I.; et al. Enabling large-scale carbon capture, utilization, and storage (CCUS) using offshore carbon dioxide (CO2) infrastructure developments—A review. Energies 2019, 12, 1945. [Google Scholar] [CrossRef]
- Kamkeng, A.D.N.; Wang, M.; Hu, J.; Du, W.; Qian, F. Transformation technologies for CO2 utilisation: Current status, challenges and future prospects. Chem. Eng. J. 2021, 409, 128138. [Google Scholar] [CrossRef]
- Alok, A.; Shrestha, R.; Ban, S.; Devkota, S.; Uprety, B.; Joshi, R. Technological advances in the transformative utilization of CO2 to value-added products. J. Environ. Chem. Eng. 2022, 10, 106922. [Google Scholar] [CrossRef]
- Atsbha, T.A.; Yoon, T.; Seongho, P.; Lee, C.-J. A review on the catalytic conversion of CO2 using H2 for synthesis of CO, methanol, and hydrocarbons. J. CO2 Util. 2021, 44, 101413. [Google Scholar] [CrossRef]
- Park, J.H.; Yang, J.; Kim, D.; Gim, H.; Choi, W.Y.; Lee, J.W. Review of recent technologies for transforming carbon dioxide to carbon materials. Chem. Eng. J. 2022, 427, 130980. [Google Scholar] [CrossRef]
- Lee, M.; Kim, Y.; Suk Lim, H.; Jo, A.; Kang, D.; Lee, J.W. Reverse water-gas shift chemical looping using a core-shell structured perovskite oxygen carrier. Energies 2020, 13, 5324. [Google Scholar] [CrossRef]
- Gorke, R.H.; Marek, E.J.; Donat, F.; Scott, S.A. Reduction and oxidation behavior of strontium perovskites for chemical looping air separation. Int. J. Greenh. Gas Control 2020, 94, 102891. [Google Scholar] [CrossRef]
- Miccio, F.; Bendoni, R.; Piancastelli, A.; Medri, V.; Landi, E. Geopolymer composites for chemical looping combustion. Fuel 2018, 225, 436–442. [Google Scholar] [CrossRef]
- Bendoni, R.; Miccio, F.; Medri, V.; Landi, E. Chemical looping combustion using geopolymer-based oxygen carriers. Chem. Eng. J. 2018, 341, 187–197. [Google Scholar] [CrossRef]
- Murri, A.N.; Miccio, F.; Medri, V.; Landi, E. Geopolymer-composites with thermomechanical stability as oxygen carriers for fluidized bed chemical looping combustion with oxygen uncoupling. Chem. Eng. J. 2020, 393, 124756. [Google Scholar] [CrossRef]
- Hildor, F.; Mattisson, T.; Leion, H.; Linderholm, C.; Ryden, M. Steel converter slag as an oxygen carrier in a 12 MWth CFB boiler—Ash interaction and material evolution. Int. J. Greenh. Gas Control 2019, 88, 321–331. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.; Li, W.; Cai, N. The melting characteristics of Vietnamese ilmenite and manganese ores used in chemical looping combustion. Int. J. Greenh. Gas Control 2019, 90, 102792. [Google Scholar] [CrossRef]
- Rumble, J.R. Handbook of Chemistry and Physics, 99th ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2018. [Google Scholar]
- Idziak, K.; Czakiert, T.; Krzywanski, J.; Zylka, A.; Kozlowska, M.; Nowak, W. Safety and environmental reasons for the use of Ni-, Co-, Cu-, Mn- and Fe-based oxygen carriers in CLC/CLOU applications: An overview. Fuel 2020, 268, 117245. [Google Scholar] [CrossRef]
- Thorne, R.J.; Bouman, E.A.; Sundseth, K.; Aranda, A.; Czakiert, T.; Pacyna, J.M.; Pacyna, E.G.; Krauz, M.; Celinska, A. Environmental impacts of a chemical looping combustion power plant. Int. J. Greenh. Gas Control 2019, 86, 101–111. [Google Scholar] [CrossRef]
- Mattisson, T.; Keller, M.; Linderholm, C.; Moldenhauer, P.; Ryden, M.; Leion, H.; Lyngfelt, A. Chemical-looping technologies using circulating fluidized bed systems: Status of development. Fuel Process. Technol. 2018, 172, 1–12. [Google Scholar] [CrossRef]
- Cho, P.; Mattisson, T.; Lyngfelt, A. Comparison of iron-, nickel-, copper- and manganese-based oxygen carriers for chemical-looping combustion. Fuel 2004, 83, 1215–1225. [Google Scholar] [CrossRef]
- Lyngfelt, A. Oxygen carriers for chemical-looping combustion. In Calcium and Chemical Looping Technology for Power Generation and Carbon Dioxide (CO2) Capture; Fennell, P., Anthony, B., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 221–254. [Google Scholar]
- Adanez, J.; de Diego, L.F.; Garcia-Labiano, F.; Gayan, P.; Abad, A.; Palacios, J.M. Selection of oxygen carriers for chemical combustion. Energy Fuels 2004, 18, 371–377. [Google Scholar] [CrossRef]
- U.S. Department of the Interior and U.S. Geological Survey. Mineral Commodity Summaries 2018. U.S. Geological Survey; U.S. Department of the Interior and U.S. Geological Survey: Reston, VA, USA, 2018. [Google Scholar]
- Dueso, C.; Garcia-Labiano, F.; Adanez, J.; de Diego, L.F.; Gayan, P.; Abad, A. Syngas combustion in a chemical-looping combustion system using an impregnated Ni-based oxygen carrier. Fuel 2009, 88, 2357–2364. [Google Scholar] [CrossRef]
- Tian, H.; Chaudhari, K.; Simonyi, T.; Poston, J.; Liu, T.; Sanders, T.; Veser, G.; Siriwardane, R. Chemical-looping combustion of coal-derived synthesis gas over copper oxide oxygen carriers. Energy Fuels 2008, 22, 3744–3755. [Google Scholar] [CrossRef]
- Cuadrat, A.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F.; Adanez, J. Relevance of the coal rank on the performance of the in situ gasification chemical-looping combustion. Chem. Eng. J. 2012, 195–196, 91–102. [Google Scholar] [CrossRef]
- Shen, Z.; Huang, Z. High-efficiency and pollution-controlling in-situ gasification chemical looping combustion system by using CO2 instead of steam as gasification agent. Chin. J. Chem. Eng. 2018, 26, 2368–2376. [Google Scholar] [CrossRef]
- Adanez-Rubio, I.; Abad, A.; Gayan, P.; de Diego, L.F.; Garcia-Labiano, F.; Adanez, J. Performance of CLOU process in the combustion of different types of coal with CO2 capture. Int. J. Greenh. Gas Control 2013, 12, 430–440. [Google Scholar] [CrossRef]
- Mattisson, T.; Lyngfelt, A.; Leion, H. Chemical-looping with oxygen uncoupling for combustion of solid fuels. Int. J. Greenh. Gas Control 2009, 3, 11–19. [Google Scholar] [CrossRef]
- Strohle, J.; Galloy, A.; Epple, B. Feasibility study on the carbonate looping process for post-combustion CO2 capture from coal-fired power plants. Energy Procedia 2009, 1, 1313–1320. [Google Scholar] [CrossRef]
- Martinez, A.; Lisbona, P.; Lara, Y.; Romeo, L.M. Carbonate looping cycle for CO2 capture: Hydrodynamic of complex CFB systems. Energy Procedia 2011, 4, 410–416. [Google Scholar] [CrossRef][Green Version]
- Luo, M.; Yi, Y.; Wang, S.; Wang, Z.; Du, M.; Pan, J.; Wang, Q. Review of hydrogen production using chemical-looping technology. Renew. Sustain. Energy Rev. 2018, 81, 3186–3214. [Google Scholar] [CrossRef]
- Ryden, M.; Lyngfelt, A. Using steam reforming to produce hydrogen with carbon dioxide capture by chemical-looping combustion. Int. J. Hydrogen Energy 2006, 31, 1271–1283. [Google Scholar] [CrossRef]
- Mattisson, T.; Lyngfelt, A.; Cho, P. The use of iron oxide as an oxygen carrier in chemical-looping combustion of methane with inherent separation of CO2. Fuel 2001, 80, 1953–1962. [Google Scholar] [CrossRef]
- de Diego, L.F.; Garcia-Labiano, F.; Adanez, J.; Gayan, P.; Abad, A.; Corbella, B.M.; Palacios, J.M. Development of Cu-based oxygen carriers for chemical-looping combustion. Fuel 2004, 83, 1749–1757. [Google Scholar] [CrossRef]
- Siriwardane, R.; Monazam, E.; Miller, D.; Poston, J.; Richards, G. Production of CO and hydrogen via chemical looping gasification of coal with calcium ferrite and oxidation with steam. In Proceedings of the 5th International Conference on Chemical Looping, Park City, UT, USA, 24–27 September 2018. [Google Scholar]
- Abad, A. Chemical looping for hydrogen production. In Calcium and Chemical Looping Technology for Power Generation and Carbon Dioxide (CO2) Capture; Fennell, P., Anthony, B., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 327–374. [Google Scholar]
- Hu, J.; Galvita, V.V.; Poelman, H.; Marin, G.B. Catalyst-assisted chemical looping auto-thermal dry reforming: The effect of operating pressure. In Proceedings of the 5th International Conference on Chemical Looping, Park City, UT, USA, 24–27 September 2018. [Google Scholar]
- Tang, M.; Liu, K.; Roddick, D.M.; Fan, M. Enhanced lattice oxygen reactivity over Fe2O3/Al2O3 redox catalyst for chemical-looping dry (CO2) reforming of CH4: Synergistic La-Ce effect. J. Catal. 2018, 368, 38–52. [Google Scholar] [CrossRef]
- De Vos, Y.; Vamvakeros, A.; Matras, D.; Jacobs, M.; Van Der Voort, P.; Van Driessche, I.; Jacques, S.; Middelkoop, V.; Verberckmoes, A. Sustainable iron-based oxygen carriers for hydrogen production—Real-time operando investigation. Int. J. Greenh. Gas Control 2019, 88, 393–402. [Google Scholar] [CrossRef]
- Ugwu, A.; Zaabout, A.; Amini, S. An advancement in CO2 utilization through novel gas switching dry reforming. Int. J. Greenh. Gas Control 2019, 90, 102791. [Google Scholar] [CrossRef]
- Shen, T.; Ge, H.; Shen, L. Characterization of combined Fe-Cu oxides as oxygen carrier in chemical looping gasification of biomass. Int. J. Greenh. Gas Control 2018, 75, 63–73. [Google Scholar] [CrossRef]
- Wu, J.; Bai, L.; Tian, H.; Riley, J.; Siriwardane, R.; Wang, Z.; He, T.; Li, J.; Zhang, J.; Wu, J. Chemical looping gasification of lignin with bimetallic oxygen carriers. Int. J. Greenh. Gas Control 2020, 93, 102897. [Google Scholar] [CrossRef]
- Song, T.; Shen, L. Review of reactor for chemical looping combustion of solid fuels. Int. J. Greenh. Gas Control 2018, 76, 92–110. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, L.; Lu, Y.; Hu, X.; Luo, X.; Chen, H.; Wang, J.; Yang, Y. Control of pressure balance and solids circulation characteristics in DCFB reactors. Powder Technol. 2018, 328, 114–121. [Google Scholar] [CrossRef]
- Noorman, S.; van Sint Annaland, M.; Kuipers, H. Packed bed reactor technology for chemical-looping combustion. Ind. Eng. Chem. Res. 2007, 46, 4212–4220. [Google Scholar] [CrossRef]
- Fernandez, J.R.; Alarcon, J.M. Chemical looping combustion process in fixed-bed reactors using ilmenite as oxygen carrier: Conceptual design and operation strategy. Chem. Eng. J. 2015, 264, 797–806. [Google Scholar] [CrossRef]
- Zhao, Z.; Iloeje, C.O.; Chen, T.; Ghoniem, A.F. Design of a rotary reactor for chemical-looping combustion. Part 1: Fundamentals and design methodology. Fuel 2014, 121, 327–343. [Google Scholar] [CrossRef]
- Hakonsen, S.F.; Grande, C.A.; Blom, R. Rotating bed reactor for CLC: Bed characteristics dependences on internal gas mixing. Appl. Energy 2014, 113, 1952–1957. [Google Scholar] [CrossRef]
- Kolbitsch, P.; Bolhar-Nordenkampf, J.; Proll, T.; Hofbauer, H. Operating experience with chemical looping combustion in a 120 kW dual circulating fluidized bed (DCFB) unit. Int. J. Greenh. Gas Control 2010, 4, 180–185. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Leckner, B. A 1000 MWth boiler for chemical-looping combustion of solid fuels—Discussion of design and costs. Appl. Energy 2015, 157, 475–487. [Google Scholar] [CrossRef]
- Bayham, S.; Straub, D.; Weber, J. Operation of a 50-kWth chemical looping combustion test facility under autothermal conditions. Int. J. Greenh. Gas Control 2019, 87, 211–220. [Google Scholar] [CrossRef]
- Kolbitsch, P.; Bolhar-Nordenkampf, J.; Proll, T.; Hofbauer, H. Design of a chemical looping combustor using a dual circulating fluidized bed (DCFB) reactor system. In Proceedings of the 9th International Conference on Circulating Fluidized Beds, Hamburg, Germany, 13–16 May 2008; Werther, J., Nowak, W., Wirth, K.-E., Hartge, E.-U., Eds.; TuTech Innovation GmbH: Hamburg, Germany, 2008. [Google Scholar]
- Chen, X.; Ma, J.; Tian, X.; Wan, J.; Zhao, H. CPFD simulation and optimization of a 50 kWth dual circulating fluidized bed reactor for chemical looping combustion of coal. Int. J. Greenh. Gas Control 2019, 90, 102800. [Google Scholar] [CrossRef]
- Adanez, J.; Gayan, P.; Celaya, J.; de Diego, L.F.; Garcia-Labiano, F.; Abad, A. Chemical looping combustion in a 10 kWth prototype using a CuO/Al2O3 oxygen carrier: Effect of operating conditions on methane combustion. Ind. Eng. Chem. Res. 2006, 45, 6075–6080. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, M.; Liu, L.; Li, Y.; Li, Z.; Cai, N. Coal-fired chemical looping combustion coupled with a high-efficiency annual carbon stripper. Int. J. Greenh. Gas Control 2020, 93, 102889. [Google Scholar] [CrossRef]
- Nasah, J.; Jensen, B.; Dyrstad-Cincotta, N.; Gerber, J.; Laudal, D.; Mann, M.; Srinivasachar, S. Method for separation of coal conversion products from oxygen carriers. Int. J. Greenh. Gas Control 2019, 88, 361–370. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, X.; Chen, D.; Al-Qadri, B.M.H. Retrospect and prospect of carbon stripper technology in solid-fuel chemical looping combustion. Fuel Process. Technol. 2021, 221, 106920. [Google Scholar] [CrossRef]
- Mei, D.; Linderholm, C.; Lyngfelt, A. Performance on an oxy-polishing step in the 100 kWth chemical looping combustion prototype. Chem. Eng. J. 2021, 409, 128202. [Google Scholar] [CrossRef]
- Perez-Vega, R.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F.; Adanez, J. Coal combustion in a 50 kWth chemical looping combustion unit: Seeking operating conditions to maximize CO2 capture and combustion efficiency. Int. J. Greenh. Gas Control 2016, 50, 80–92. [Google Scholar] [CrossRef]
- Whitty, K.J.; Hamilton, M.A.; Merrett, K.M.; Lighty, J.S. Performance of a dual fluidized bed reactor for chemical looping combustion with oxygen uncoupling. In Proceedings of the 12th International Conference on Fluidized Bed Technology, Cracow, Poland, 23–27 May 2017; Nowak, W., Sciazko, M., Mirek, P., Eds.; Printing House of the Redemptorists: Tuchow, Poland, 2017. [Google Scholar]
- Ohlemuller, P.; Busch, J.P.; Reitz, M.; Strohle, J.; Epple, B. Chemical-looping combustion of hard coal: Autothermal operation of a 1 MWth pilot plant. J. Energy Resour. Technol. 2016, 137, 042203. [Google Scholar] [CrossRef]
- Shen, T.; Wang, S.; Yan, J.; Shen, L.; Tian, H. Performance improvement of chemical looping combustion with coal by optimizing operational strategies in a 3 kWth interconnected fluidized bed. Int. J. Greenh. Gas Control 2020, 98, 103060. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, T.; Bollas, G.; Shen, L. Design and operation of a multi-stage reactor system for chemical looping combustion process. Fuel Process. Technol. 2021, 215, 106748. [Google Scholar] [CrossRef]
- Gogolev, I.; Linderholm, C.; Gall, D.; Schmitz, M.; Mattisson, T.; Pettersson, J.B.C.; Lyngfelt, A. Chemical-looping combustion in a 100 kW unit using a mixture of synthetic and natural oxygen carriers—Operational results and fate of biomass fuel alkali. Int. J. Greenh. Gas Control 2019, 88, 371–382. [Google Scholar] [CrossRef]
- Lindmuller, L.; Haus, J.; Nair, A.R.K.; Heinrich, S. Minimizing gas leakages in a system of coupled fluidized bed reactors for chemical looping combustion. Chem. Eng. Sci. 2022, 250, 117366. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, X.; Ma, J.; Su, M.; Wang, B.; Mei, D. Development of tailor-made oxygen carriers and reactors for chemical looping processes at Huazhong University of Science & Technology. Int. J. Greenh. Gas Control 2020, 93, 102898. [Google Scholar] [CrossRef]
- Ma, J.; Tian, X.; Wang, C.; Chen, X.; Zhao, H. Performance of a 50 kWth coal-fuelled chemical looping combustor. Int. J. Greenh. Gas Control 2018, 75, 98–106. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Brink, A.; Langorgen, O.; Mattisson, T.; Ryden, M.; Linderholm, C. 11,000 h of chemical-looping combustion operation—Where are we and where do we want to go? Int. J. Greenh. Gas Control 2019, 88, 38–56. [Google Scholar] [CrossRef]
- Hallberg, P.; Hanning, M.; Ryden, M.; Mattisson, T.; Lyngfelt, A. Investigation of a calcium manganite as oxygen carrier during 99 h of operation of chemical-looping combustion in a 10 kWth reactor unit. Int. J. Greenh. Gas Control 2016, 53, 222–229. [Google Scholar] [CrossRef]
- Moldenhauer, P.; Ryden, M.; Mattisson, T.; Lyngfelt, A. Chemical-looping combustion and chemical-looping reforming of kerosene in a circulating fluidized-bed 300 W laboratory reactor. Int. J. Greenh. Gas Control 2012, 9, 1–9. [Google Scholar] [CrossRef]
- Vilches, T.B.; Lind, F.; Ryden, M.; Thunman, H. Experience of more than 1000 h of operation with oxygen carriers and solid biomass at large scale. Appl. Energy 2017, 190, 1174–1183. [Google Scholar] [CrossRef]
- Siriwardane, R.; Benincosa, W.; Riley, J.; Tian, H.; Richards, G. Investigation of reactions in a fluidized bed reactor during chemical looping combustion of coal/steam with copper oxide-iron oxide-alumina oxygen carrier. Appl. Energy 2016, 183, 1550–1564. [Google Scholar] [CrossRef]
- Xu, L.; Sun, H.; Li, Z.; Cai, N. Experimental study of copper modified manganese ores as oxygen carriers in a dual fluidized bed reactor. Appl. Energy 2016, 162, 940–947. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, H.; Tian, X.; Wei, Y.; Rajendran, S.; Zhang, Y.; Bhattacharya, S.; Zheng, C. Chemical looping combustion of coal in a 5 kWth interconnected fluidized bed reactor using hematite as oxygen carrier. Appl. Energy 2015, 157, 304–313. [Google Scholar] [CrossRef]
- Cuadrat, A.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F.; Adanez, J. Effect of operating conditions in chemical-looping combustion of coal in a 500 Wth unit. Int. J. Greenh. Gas Control 2012, 6, 153–163. [Google Scholar] [CrossRef]
- Schmitz, M.; Linderholm, C.J.; Lyngfelt, A. Chemical looping combustion of four different solid fuels using a manganese-silicon-titanium oxygen carrier. Int. J. Greenh. Gas Control 2018, 70, 88–96. [Google Scholar] [CrossRef]
- Abad, A.; Adanez-Rubio, I.; Gayan, P.; Garcia-Labiano, F.; de Diego, L.F.; Adanez, J. Demonstration of chemical-looping with oxygen uncoupling (CLOU) process in a 1.5 kWth continuously operating unit using a Cu-based oxygen-carrier. Int. J. Greenh. Gas Control 2012, 6, 189–200. [Google Scholar] [CrossRef]
- Strohle, J.; Orth, M.; Epple, B. Chemical looping combustion of hard coal in a 1 MWth pilot plant using ilmenite as oxygen carrier. Appl. Energy 2015, 157, 288–294. [Google Scholar] [CrossRef]
- Mei, D.; Soleimanisalim, A.H.; Linderholm, C.; Lyngfelt, A.; Mattisson, T. Reactivity and lifetime assessment of an oxygen releasable manganese ore with biomass fuels in a 10 kWth pilot rig for chemical looping combustion. Fuel Process. Technol. 2021, 215, 106743. [Google Scholar] [CrossRef]
- Moldenhauer, P.; Ryden, M.; Mattisson, T.; Younes, M.; Lyngfelt, A. The use of ilmenite as oxygen carrier with kerosene in a 300 W CLC laboratory reactor with continuous circulation. Appl. Energy 2014, 113, 1846–1854. [Google Scholar] [CrossRef]
- Pishahang, M.; Larring, Y.; Stensrod, R.E.; Andreassen, K.A.; Spjelkavik, A.I. 3 kW circulating fluidized bed chemical looping reactor—A thermochemical and chemomechanical investigation on the performance of Cu- impregnated Al2O3 as an oxygen carrier material. Int. J. Greenh. Gas Control 2021, 109, 103384. [Google Scholar] [CrossRef]
- Idziak, K.; Czakiert, T.; Krzywanski, J.; Zylka, A.; Nowak, W. Studies on solids flow in a cold model of a dual fluidized bed reactor for chemical looping combustion of solid fuels. J. Energy Resour. Technol. 2020, 142, 022102. [Google Scholar] [CrossRef]
- Abad, A.; Gayan, P.; Garcia-Labiano, F.; de Diego, L.F.; Adanez, J. Relevance of plant design on CLC process performance using a Cu-based oxygen carrier. Fuel Process. Technol. 2018, 171, 78–88. [Google Scholar] [CrossRef]
- Xiao, R.; Song, Q.; Song, M.; Lu, Z.; Zhang, S.; Shen, L. Pressurized chemical-looping combustion of coal with an iron ore-based oxygen carrier. Combust. Flame 2010, 157, 1140–1153. [Google Scholar] [CrossRef]
- Zaabout, A.; Cloete, S.; Tolchard, J.R.; Amini, S. A pressurized gas switching combustion reactor: Autothermal operation with a CaMnO3-δ-based oxygen carrier. Chem. Eng. Res. Des. 2018, 137, 20–32. [Google Scholar] [CrossRef]
- Han, L.; Bollas, G.M. Chemical-looping combustion in a reverse-flow fixed bed reactor. Energy 2016, 102, 669–681. [Google Scholar] [CrossRef]
- Gallucci, F.; Hamers, H.P.; van Zanten, M.; van Sint Annaland, M. Experimental demonstration of chemical-looping combustion of syngas in packed bed reactors with ilmenite. Chem. Eng. J. 2015, 274, 156–168. [Google Scholar] [CrossRef]
- Wang, P.; Howard, B.; Means, N. Investigation of reactivities of bimetallic Cu-Fe oxygen carriers with coal in high temperature in-situ gasification chemical-looping combustion (iG-CLC) and chemical-looping with oxygen uncoupling (CLOU) using a fixed bed reactor. Fuel 2021, 285, 119012. [Google Scholar] [CrossRef]
- Nemati, N.; Ryden, M. Chemical-looping combustion in packed-fluidized beds: Experiments with random packings in bubbling bed. Fuel Process. Technol. 2021, 222, 106978. [Google Scholar] [CrossRef]
- Tian, Q.; Che, L.; Ding, B.; Wang, Q.; Su, Q. Performance of Cu-based oxygen carrier in a CLC process based on fixed bed reactors. Greenh. Gases Sci. Technol. 2017, 7, 731–744. [Google Scholar] [CrossRef]
- Tian, Q.; Che, L.; Ding, B.; Wang, Q.; Su, Q. Performance of a Cu–Fe-based oxygen carrier combined with a Ni-based oxygen carrier in a chemical-looping combustion process based on fixed-bed reactors. Greenh. Gases Sci. Technol. 2018, 8, 542–556. [Google Scholar] [CrossRef]
- Kooiman, R.F.; Hamers, H.P.; Gallucci, F.; van Sint Annaland, M. Experimental demonstration of two-stage packed bed chemical-looping combustion using syngas with CuO/Al2O3 and NiO/CaAl2O4 as oxygen carriers. Ind. Eng. Chem. Res. 2015, 54, 2001–2011. [Google Scholar] [CrossRef]
- Wang, K.; Yu, Q.; Qin, Q.; Zuo, Z.; Wu, T. Evaluation of Cu-based oxygen carrier for chemical looping air separation in a fixed-bed reactor. Chem. Eng. J. 2016, 287, 292–301. [Google Scholar] [CrossRef]
- Tilland, A.; Prieto, J.; Petitjean, D.; Schaer, E. Study and analyses of a CLC oxygen carrier degradation mechanism in a fixed bed reactor. Chem. Eng. J. 2016, 302, 619–632. [Google Scholar] [CrossRef]
- Hamers, H.P.; Gallucci, F.; Williams, G.; van Sint Annaland, M. Experimental demonstration of CLC and the pressure effect in packed bed reactors using NiO/CaAl2O4 as oxygen carrier. Fuel 2015, 159, 828–836. [Google Scholar] [CrossRef]
- Iloeje, C.O.; Zhao, Z.; Ghoniem, A.F. Design and techno-economic optimization of a rotary chemical looping combustion power plant with CO2 capture. Appl. Energy 2018, 231, 1179–1190. [Google Scholar] [CrossRef]
- Iloeje, C.O.; Zhao, Z.; Ghoniem, A.F. A reduced fidelity model for the rotary chemical looping combustion. Appl. Energy 2017, 190, 725–739. [Google Scholar] [CrossRef]
- Hakonsen, S.F.; Blom, R. Chemical looping combustion in a rotating bed reactor—Finding optimal process conditions for prototype reactor. Environ. Sci. Technol. 2011, 45, 9611–9626. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ghoniem, A.F. Design of a rotary reactor for chemical-looping combustion. Part 2: Comparison of copper-, nickel-, and iron-based oxygen carriers. Fuel 2014, 121, 344–360. [Google Scholar] [CrossRef]
- Ekstrom, C.; Schwendig, F.; Biede, O.; Franco, F.; Haupt, G.; de Koeijer, G.; Papapavlou, C.; Rokke, P.E. Techno-economic evaluations and benchmarking of pre-combustion CO2 capture and oxy-fuel processes developed in the European ENCAP project. Energy Procedia 2009, 1, 4233–4240. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kumar, P.; Yang, A.; Fennell, P. Energy and exergy analysis of chemical looping combustion technology and comparison with pre-combustion and oxy-fuel combustion technologies for CO2 capture. J. Environ. Chem. Eng. 2015, 3, 2104–2114. [Google Scholar] [CrossRef]
- Mendiara, T.; Garcia-Labiano, F.; Abad, A.; Gayan, P.; de Diego, L.F.; Izquierdo, M.T.; Adanez, J. Negative CO2 emissions through the use of biofuels in chemical looping technology: A review. Appl. Energy 2018, 232, 657–684. [Google Scholar] [CrossRef]
- Coppola, A.; Scala, F. Chemical looping for combustion of solid biomass: A review. Energy Fuels 2021, 35, 19248–19265. [Google Scholar] [CrossRef]
- Gauthier, T.; Yazdanpanah, M.; Forret, A.; Amblard, B.; Lambert, A.; Bertholin, S. CLC, a promising concept with challenging development issues. In Proceedings of the 15th International Conference on Fluidization, Fluidization XV, Montebello, QC, Canada, 22–27 May 2016; Chaouki, J., Berruti, F., Bi, X., Cocco, R., Eds.; ECI Symposium Series: New York, NY, USA, 2016. [Google Scholar]
- Bourgeon, S.; Gauthier, T.; Guillou, F.; Stainton, H. Chemical looping combustion: Development status and perspectives. In Proceedings of the 2th International Conference on Chemical Looping, Darmstadt, Germany, 26–28 September 2012. [Google Scholar]
- Saari, J.; Peltola, P.; Tynjala, T.; Hyppanen, T.; Kaikko, J.; Vakkilainen, E. High-efficiency bioenergy carbon capture integrating chemical looping combustion with oxygen uncoupling and a large cogeneration plant. Energies 2020, 13, 3075. [Google Scholar] [CrossRef]
- Keller, M.; Kaibe, K.; Hatano, H.; Otomo, J. Techno-economic evaluation of BECCS via chemical looping combustion of Japanese woody biomass. Int. J. Greenh. Gas Control 2019, 83, 69–82. [Google Scholar] [CrossRef]
- Ogidiama, O.V.; Abu-Zahra, M.R.M.; Shamim, T. Techno-economic analysis of a poly-generation solar-assisted chemical looping combustion power plant. Appl. Energy 2018, 228, 724–735. [Google Scholar] [CrossRef]
- Ogidiama, O.V.; Zahra, M.A.; Shamim, T. Techno-economic analysis of a carbon capture chemical looping combustion power plant. J. Energy Resour. Technol. 2018, 140, 112004. [Google Scholar] [CrossRef]
- Sethi, S.S.; Dutta, S.K.; Singh, J.; Kumar, M. Techno economic analysis of chemical looping system for Indian power plants. Environ. Technol. Innov. 2018, 9, 16–29. [Google Scholar] [CrossRef]
- Diego, M.E.; Abanades, J.C. Techno-economic analysis of a low carbon back-up power system using chemical looping. Renew. Sustain. Energy Rev. 2020, 132, 110099. [Google Scholar] [CrossRef]
- Spinelli, M.; Peltola, P.; Bischi, A.; Ritvanen, J.; Hyppanen, T.; Romano, M.C. Process integration of chemical combustion with oxygen uncoupling in a coal-fired power plant. Energy 2016, 103, 646–659. [Google Scholar] [CrossRef]
- Mantripragada, H.C.; Rubin, E.S. Chemical looping for pre-combustion and post-combustion CO2 capture. Energy Procedia 2017, 114, 6403–6410. [Google Scholar] [CrossRef]
- Erlach, B.; Schmidt, M.; Tsatsaronis, G. Comparison of carbon capture IGCC with pre-combustion decarbonisation and with chemical-looping combustion. Energy 2011, 36, 3804–3815. [Google Scholar] [CrossRef]
- Bartocci, P.; Abad, A.; Cabello, A.; Zampilli, M.; Buia, G.; Serra, A.; Calantoni, S.; Taiana, A.; Bidini, G.; Fantozzi, F. Technical economic and environmental analysis of chemical looping versus oxyfuel combustion for NGCC power plant. E3S Web Conf. 2021, 312, 08019. [Google Scholar] [CrossRef]
- Stenberg, V.; Spallina, V.; Mattisson, T.; Ryden, M. Techno-economic analysis of H2 production processes using fluidized bed heat exchangers with steam reforming—Part 1: Oxygen carrier aided combustion. Int. J. Hydrogen Energy 2020, 45, 6059–6081. [Google Scholar] [CrossRef]
- Stenberg, V.; Spallina, V.; Mattisson, T.; Ryden, M. Techno-economic analysis of processes with integration of fluidized bed heat exchangers for H2 production—Part 2: Chemical-looping combustion. Int. J. Hydrogen Energy 2021, 46, 25355–25375. [Google Scholar] [CrossRef]
- Sun, Z.; Aziz, M. Comparative thermodynamic and techno-economic assessment of green methanol production from biomass through direct chemical looping processes. J. Clean. Prod. 2021, 321, 129023. [Google Scholar] [CrossRef]
- Sayeed, I.; Kibria, M.A.; Bhattacharya, S. Process modelling and techno-economic analysis of a 550 MWe chemical looping power plant with victorian brown coal. Int. J. Greenh. Gas Control 2022, 113, 103547. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).