Effect of the Substrate to Inoculum Ratios on the Kinetics of Biogas Production during the Mesophilic Anaerobic Digestion of Food Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum
2.2. Experimental Setup
2.3. Analytical Methods
- VSTP = Gas volume of standard temperature and pressure (L);
- VT = Volume of gas measured at temperature T (L);
- T = Temperature of the gas or ambient space (°C); and
- PW = Vapor pressure of the water as a function of temperature (mm Hg).
2.4. Kinetic Study
2.4.1. First-Order Kinetics
- G(t) = Cumulative biogas yield at digestion time of t days;
- Go = Methane potential of the substrate (mL/gVS added);
- k = Biogas production rate constant (first order disintegration rate constant) (1/day); and
- t = Time (days).
2.4.2. Modified Gompertz Model
- M(t) = Cumulative biogas yield at digestion time of t days (mL/g VS);
- P = Methane production potential (mL/g VS);
- Rmax = Maximum methane production rate (mL/g VS.d);
- λ = Lag phase (day);
- t = Time (day); and
- e = exp (1) = 2.7183.
- m = number of data pairs;
- j = jth values;
- Y = measured biogas yield (mL/gVS); and
- d = deviations (differences) between experimental and predicted biogas yield.
- P = Biogas production potential (mL/g VS);
- Rm = Maximum methane production rate (mL/g VS.d); and
- e = exp (1) = 2.7183.
3. Results and Discussions
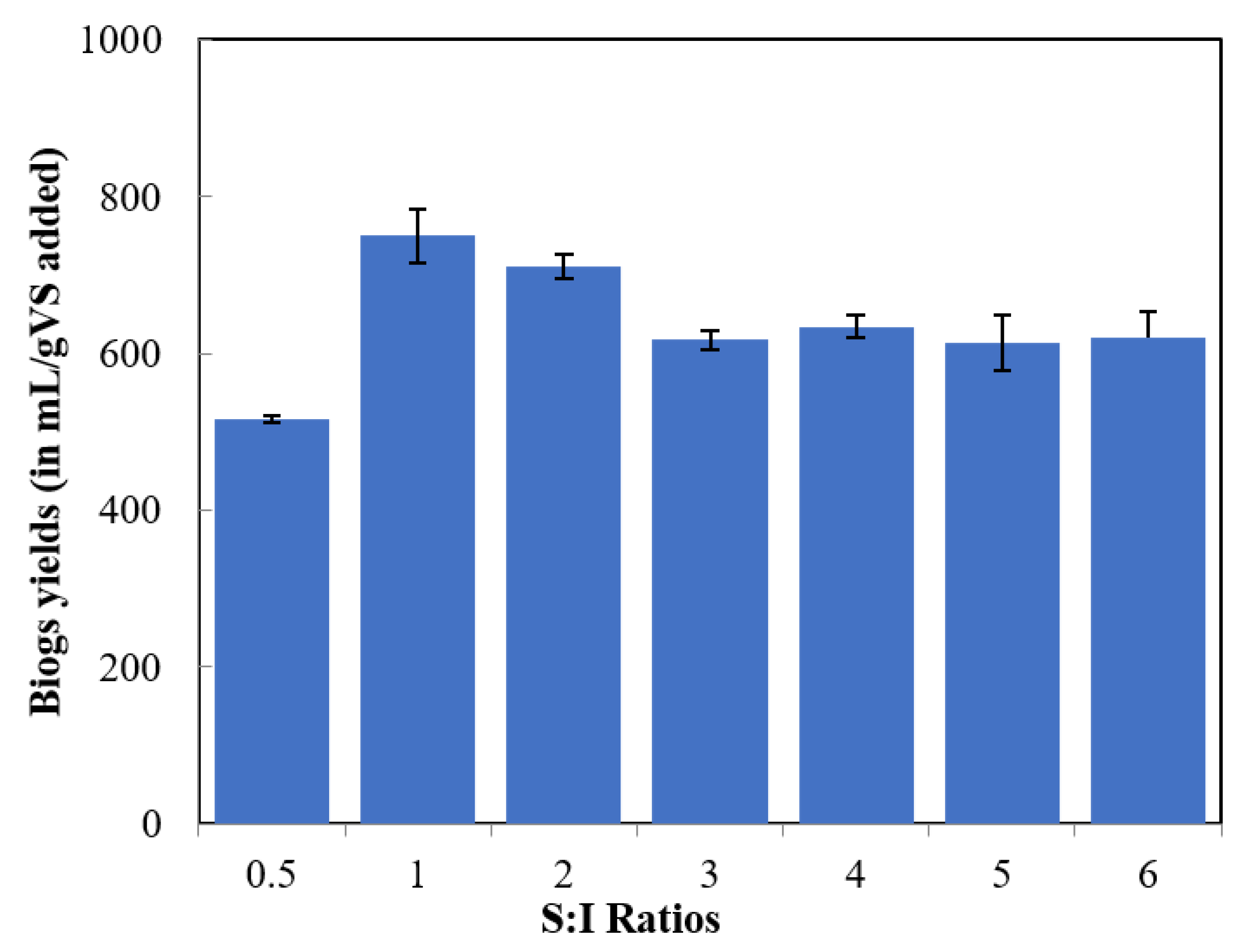
3.1. Effect of S:I Ratios on Biogas Yields
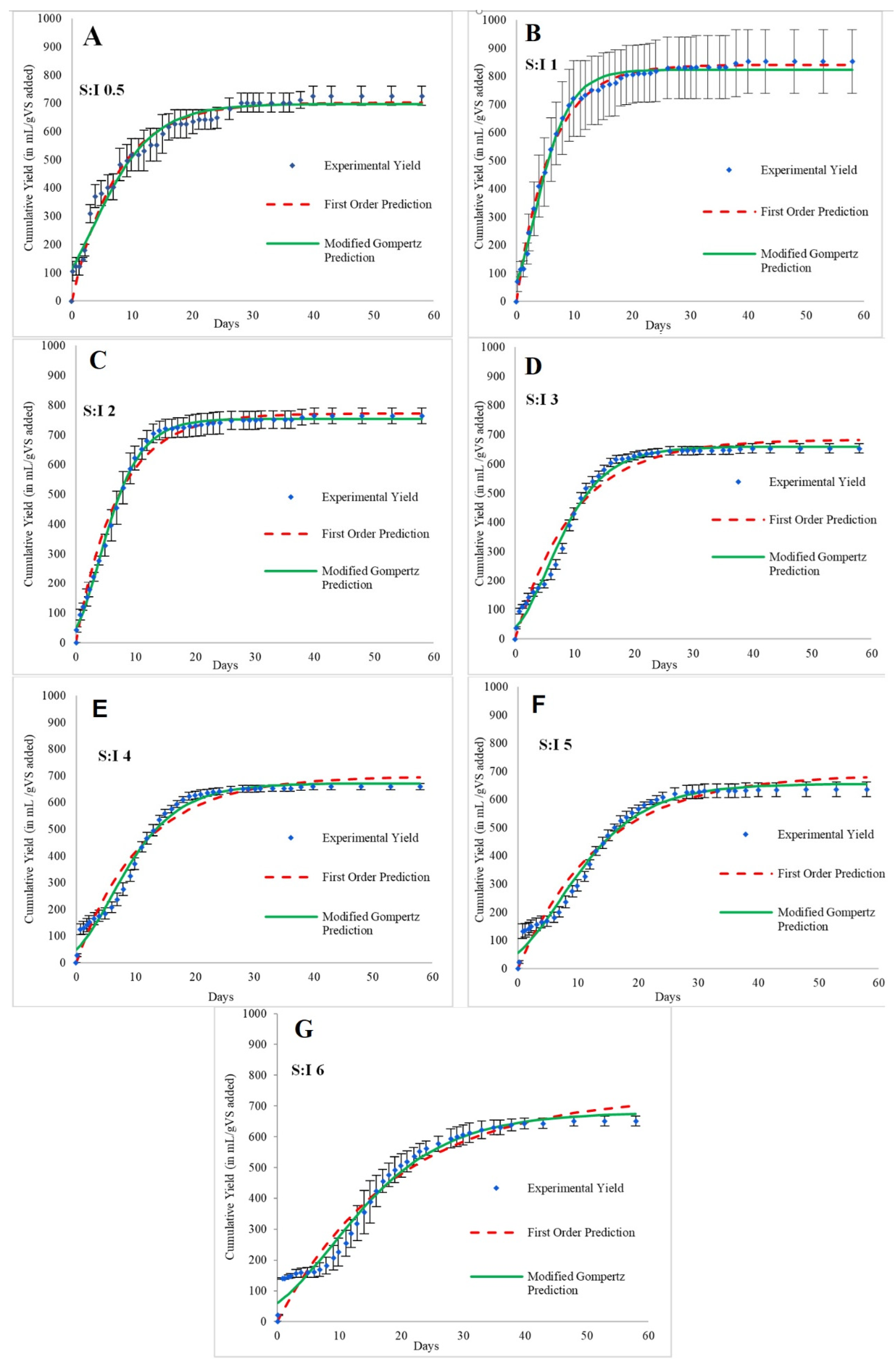
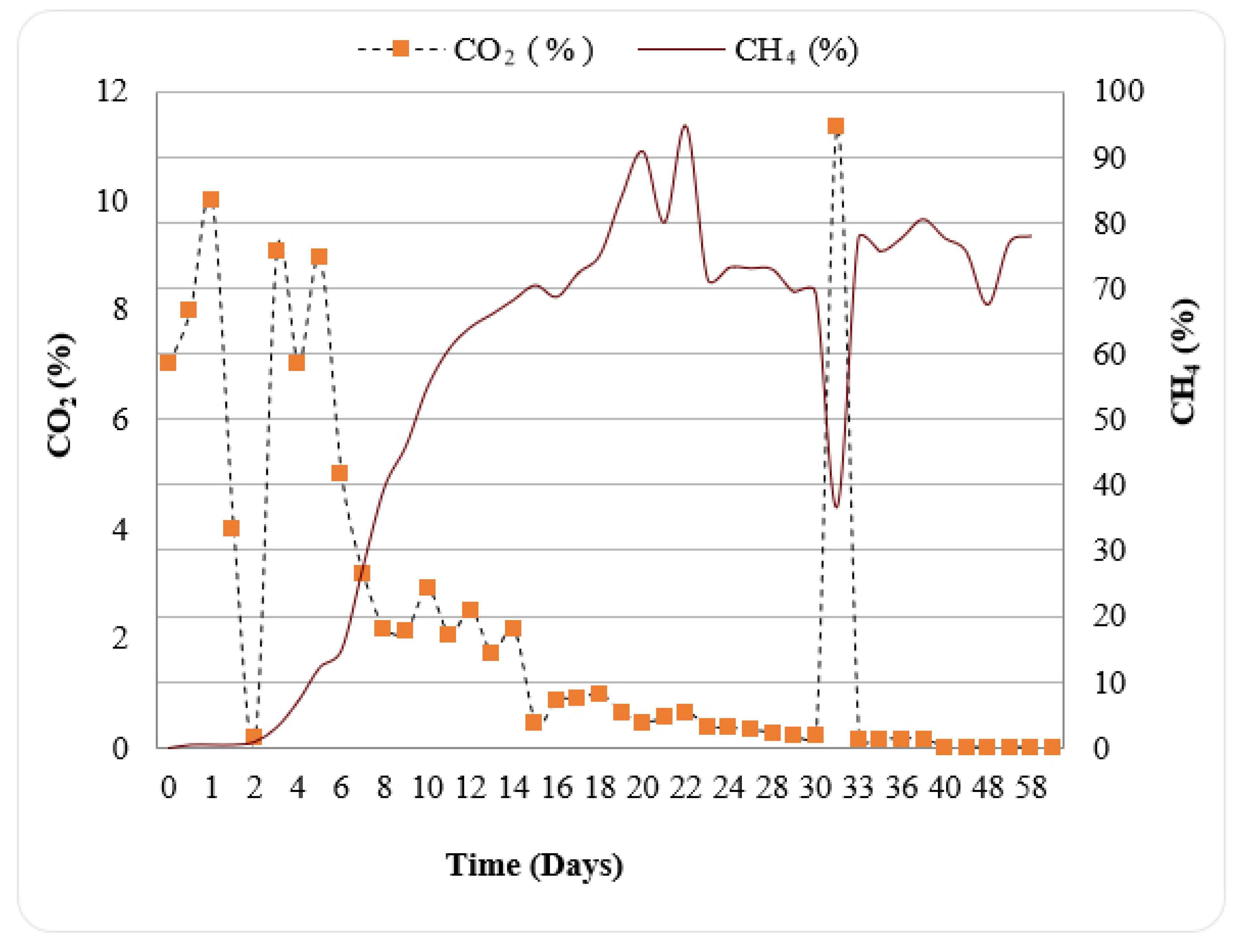
3.2. Effect of S:I Ratios on Biogas Production Kinetics
3.3. Considerations of S:I Ratios in the Context of a Circular Economy Scenario
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bedoić, R.; Smoljanić, G.; Pukšec, T.; Čuček, L.; Ljubas, D.; Duić, N. Geospatial Analysis and Environmental Impact Assessment of a Holistic and Interdisciplinary Approach to the Biogas Sector. Energies 2021, 14, 5374. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Borrion, A.; Li, H.; Li, J. Effects of Organic Composition on Mesophilic Anaerobic Digestion of Food Waste. Bioresour. Technol. 2017, 244, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Dhamodharan, K.; Kumar, V.; Kalamdhad, A.S. Effect of Different Livestock Dungs as Inoculum on Food Waste Anaerobic Digestion and Its Kinetics. Bioresour. Technol. 2015, 180, 237–241. [Google Scholar] [CrossRef]
- Amani, T.; Nosrati, M.; Sreekrishnan, T.R. Anaerobic Digestion from the Viewpoint of Microbiological, Chemical, and Operational Aspects—A Review. Environ. Rev. 2010, 18, 255–278. [Google Scholar] [CrossRef]
- Slimane, K.; Fathya, S.; Assia, K.; Hamza, M. Influence of Inoculums/Substrate Ratios (ISRs) on the Mesophilic Anaerobic Digestion of Slaughterhouse Waste in Batch Mode: Process Stability and Biogas Production. Energy Procedia 2014, 50, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Zeb, I.; Ma, J.; Mehboob, F.; Kafle, G.K.; Amin, B.A.Z.; Nazir, R.; Ndegwa, P.; Frear, C. Kinetic and Microbial Analysis of Methane Production from Dairy Wastewater Anaerobic Digester under Ammonia and Salinity Stresses. J. Clean. Prod. 2019, 219, 797–808. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Kinetic Study on the Effect of Temperature on Biogas Production Using a Lab Scale Batch Reactor. Ecotoxicol. Environ. Saf. 2015, 121, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Piechota, G. Multi-Step Biogas Quality Improving by Adsorptive Packed Column System as Application to Biomethane Upgrading. J. Environ. Chem. Eng. 2021, 9, 105944. [Google Scholar] [CrossRef]
- Piechota, G. Removal of Siloxanes from Biogas Upgraded to Biomethane by Cryogenic Temperature Condensation System. J. Clean. Prod. 2021, 308, 127404. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Zieliński, M.; Dębowski, M. Influence of the Heating Method on the Efficiency of Biomethane Production from Expired Food Products. Fermentation 2021, 7, 12. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dzienis, L.; Dębowski, M.; Zieliński, M. Optimisation of Methane Fermentation as a Valorisation Method for Food Waste Products. Biomass Bioenergy 2021, 144, 105913. [Google Scholar] [CrossRef]
- Córdoba, V.; Fernández, M.; Santalla, E. The Effect of Substrate/Inoculum Ratio on the Kinetics of Methane Production in Swine Wastewater Anaerobic Digestion. Environ. Sci. Pollut. Res. 2018, 25, 21308–21317. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jiang, T.; Chang, J.; Tang, Q.; Luo, T.; Cui, Z. Effect of Substrate to Inoculum Ratio on Biogas Production and Microbial Community During Hemi-Solid-State Batch Anaerobic Co-Digestion of Rape Straw and Dairy Manure. Appl. Biochem. Biotechnol. 2019, 189, 884–902. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Borrion, A.; Li, J. Influence of Feed/Inoculum Ratios and Waste Cooking Oil Content on the Mesophilic Anaerobic Digestion of Food Waste. Waste Manag. 2018, 73, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Han, S.K.; Shin, H.S. The Optimisation of Food Waste Addition as a Co-Substrate in Anaerobic Digestion of Sewage Sludge. Waste Manag. Res. 2003, 21, 515–526. [Google Scholar] [CrossRef]
- Raposo, F.; Borja, R.; Martín, M.A.; Martín, A.; de la Rubia, M.A.; Rincón, B. Influence of Inoculum–Substrate Ratio on the Anaerobic Digestion of Sunflower Oil Cake in Batch Mode: Process Stability and Kinetic Evaluation. Chem. Eng. J. 2009, 149, 70–77. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, R.; Li, K.; Ma, R. A Review of Crop Straw Pretreatment Methods for Biogas Production by Anaerobic Digestion in China. Renew. Sustain. Energy Rev. 2019, 107, 51–58. [Google Scholar] [CrossRef]
- Mu, Y.; Wang, G.; Yu, H.-Q. Kinetic Modeling of Batch Hydrogen Production Process by Mixed Anaerobic Cultures. Bioresour. Technol. 2006, 97, 1302–1307. [Google Scholar] [CrossRef]
- Ghimire, A.; Luongo, V.; Frunzo, L.; Lens, P.N.L.; Pirozzi, F.; Esposito, G. Biohythane Production from Food Waste in a Two-Stage Process: Assessing the Energy Recovery Potential. Environ. Technol. 2021, 1–7. [Google Scholar] [CrossRef]
- Park, Y.; Hong, F.; Cheon, J.; Hidaka, T.; Tsuno, H. Comparison of Thermophilic Anaerobic Digestion Characteristics between Single-Phase and Two-Phase Systems for Kitchen Garbage Treatment. J. Biosci. Bioeng. 2008, 105, 48–54. [Google Scholar] [CrossRef]
- Baird, R.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association Water Works Association (APHA), American Water Works Association (AWWA), Environment Federation (WEF): Washington, DC, USA, 2017. [Google Scholar]
- Iglesias Jiménez, E.; Pérez García, V. Relationships between Organic Carbon and Total Organic Matter in Municipal Solid Wastes and City Refuse Composts. Bioresour. Technol. 1992, 41, 265–272. [Google Scholar] [CrossRef]
- Mota, V.T.; Santos, F.S.; Araújo, T.A.; Amaral, M.C.S. Evaluation of Titration Methods for Volatile Fatty Acids Measurement: Effect of the Bicarbonate Interference and Feasibility for the Monitoring of Anaerobic Reactors. Water Pract. Technol. 2015, 10, 486–495. [Google Scholar] [CrossRef]
- Esposito, G.; Frunzo, L.; Panico, A.; Pirozzi, F. Enhanced Bio-Methane Production from Co-Digestion of Different Organic Wastes. Environ. Technol. 2012, 33, 2733–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, T.; Chatterjee, B.K.; Majumdar, D.; Chakrabarti, P. Antibacterial Effect of Silver Nanoparticles and the Modeling of Bacterial Growth Kinetics Using a Modified Gompertz Model. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 299–306. [Google Scholar] [CrossRef]
- Gibson, A.M.; Bratchell, N.; Roberts, T.A. Predicting Microbial Growth: Growth Responses of Salmonellae in a Laboratory Medium as Affected by PH, Sodium Chloride and Storage Temperature. Int. J. Food Microbiol. 1988, 6, 155–178. [Google Scholar] [CrossRef]
- Gompertz, B. On the Nature of the Function Expressive of the Law of Human Mortality, and on a New Mode of Determining the Value of Life Contingencies. R. Soc. 1825, 115, 1–23. [Google Scholar]
- Ghimire, A.; Sposito, F.; Frunzo, L.; Trably, E.; Escudié, R.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Effects of Operational Parameters on Dark Fermentative Hydrogen Production from Biodegradable Complex Waste Biomass. Waste Manag. 2016, 50, 55–64. [Google Scholar] [CrossRef]
- Cheah, Y.K.; Vidal-Antich, C.; Dosta, J.; Mata-Álvarez, J. Volatile Fatty Acid Production from Mesophilic Acidogenic Fermentation of Organic Fraction of Municipal Solid Waste and Food Waste under Acidic and Alkaline pH. Environ. Sci. Pollut. Res. 2019, 26, 35509–35522. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; He, Q.; Zhao, X.; Wu, D.; Wang, X.; Peng, X. Anaerobic Digestion of Food Waste: Correlation of Kinetic Parameters with Operational Conditions and Process Performance. Biochem. Eng. J. 2018, 130, 1–9. [Google Scholar] [CrossRef]
- Yin, D.; Liu, W.; Zhai, N.; Li, J. Influence of pH Controlling on Fermentation Performance in Kitchen Waste and Cow Manure. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 43, 2339–2351. [Google Scholar] [CrossRef]
- Souli, I.; Liu, X.; Lendormi, T.; Chaira, N.; Ferchichi, A.; Lanoisellé, J.L. Anaerobic Digestion of Waste Tunisian Date (Phoenix Dactylifera L.): Effect of Biochemical Composition of Pulp and Seeds from Six Varieties. Environ. Technol. 2020, 102, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, Y.; Chen, C.; Liu, X.; Xiao, X.; Ma, X.; Zhang, R.; He, Y.; Liu, G. Biochemical Methane Potential (BMP) of Vinegar Residue and the Influence of Feed to Inoculum Ratios on Biogas Production. BioResources 2013, 8, 2487–2498. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, J.; Yan, Y.; Feng, L. Enhanced Production of Short-Chain Fatty Acid by Co-Fermentation of Waste Activated Sludge and Kitchen Waste under Alkaline Conditions and Its Application to Microbial Fuel Cells. Appl. Energy 2013, 102, 1197–1204. [Google Scholar] [CrossRef]
- Blasius, J.P.; Contrera, R.C.; Maintinguer, S.I.; Alves de Castro, M.C.A. Effects of Temperature, Proportion and Organic Loading Rate on the Performance of Anaerobic Digestion of Food Waste. Biotechnol. Rep. 2020, 27, e00503. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, R.; Liu, X.; Chen, C.; Xiao, X.; Feng, L.; He, Y.; Liu, G. Evaluating Methane Production from Anaerobic Mono- and Co-Digestion of Kitchen Waste, Corn Stover, and Chicken Manure. Energy Fuels 2013, 27, 2085–2091. [Google Scholar] [CrossRef]
- Shahbaz, M.; Ammar, M.; Zou, D.; Korai, R.M.; Li, X.J. An Insight into the Anaerobic Co-Digestion of Municipal Solid Waste and Food Waste: Influence of Co-Substrate Mixture Ratio and Substrate to Inoculum Ratio on Biogas Production. Appl. Biochem. Biotechnol. 2019, 187, 1356–1370. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile Fatty Acids Production from Food Waste: Effects of PH, Temperature, and Organic Loading Rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Liang, Y.; Yin, S.; Si, Y.; Zheng, Z.; Yuan, S.; Nie, E.; Luo, X. Effect of Pretreatment and Total Solid Content on Thermophilic Dry Anaerobic Digestion of Spartina Alterniflora. Chem. Eng. J. 2014, 237, 209–216. [Google Scholar] [CrossRef]
- Parra-Orobio, B.; Donoso-Bravo, A.; Torres-Lozada, P. Anaerobic Digestion of Food Waste. Predicting of Methane Production by Comparing Kinetic Models. Ing. y Compet. 2017, 19, 219–227. [Google Scholar]
- Srisowmeya, G.; Chakravarthy, M.; Nandhini Devi, G. Critical Considerations in Two-Stage Anaerobic Digestion of Food Waste—A Review. Renew. Sustain. Energy Rev. 2020, 119, 109587. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chai, W.S.; Lay, C.H.; Chen, C.C.; Lee, C.Y.; Show, P.L. Optimization of Hydrolysis-Acidogenesis Phase of Swine Manure for Biogas Production Using Two-Stage Anaerobic Fermentation. Processes 2021, 9, 1324. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Tsigkou, K.; Kornaros, M. Assessment of Single-vs. Two-Stage Process for the Anaerobic Digestion of Liquid Cow Manure and Cheese Whey. Energies 2021, 14, 5423. [Google Scholar] [CrossRef]
- Rivière, D.; Desvignes, V.; Pelletier, E.; Chaussonnerie, S.; Guermazi, S.; Weissenbach, J.; Li, T.; Camacho, P.; Sghir, A. Towards the Definition of a Core of Microorganisms Involved in Anaerobic Digestion of Sludge. ISME J. 2009, 3, 700–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owamah, H.I.; Ikpeseni, S.C.; Alfa, M.I.; Oyebisi, S.O.; Gopikumar, S.; David Samuel, O.; Ilabor, S.C. Influence of Inoculum/Substrate Ratio on Biogas Yield and Kinetics from the Anaerobic Co-Digestion of Food Waste and Maize Husk. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100558. [Google Scholar] [CrossRef]
- Zahan, Z.; Othman, M.Z.; Muster, T.H. Anaerobic Digestion/Co-Digestion Kinetic Potentials of Different Agro-Industrial Wastes: A Comparative Batch Study for C/N Optimisation. Waste Manag. 2018, 71, 663–674. [Google Scholar] [CrossRef]
- López, I.; Benzo, M.; Passeggi, M.; Borzacconi, L. A Simple Kinetic Model Applied to Anaerobic Digestion of Cow Manure. Environ. Technol. 2021, 42, 3451–3462. [Google Scholar] [CrossRef]
| Parameters | Units | FW | Inoculum |
|---|---|---|---|
| Total solids (TS) | % | 15.94 ± 0.04 | 0.22 ± 0.01 |
| Volatile solids (VS) | % | 14.50 ± 0.16 | 0.09 ± 0.01 |
| VS/TS | 90.84 ± 1.20 | 43.05 ± 0.96 | |
| pH | 4.36 ± 0.01 | 8.08 ± 0.01 | |
| Total chemical oxygen demand (TCOD) | mg/L | 1202.00 ± 54.00 | NA |
| Total organic carbon (TOC) | mg/L | 80,432.15 ± 87.00 | 526.99 ± 1.00 |
| Total volatile fatty acids (TVFA) | mg/L | NA | 364.00 ± 7.00 |
| Total alkalinity (TA) | mg/L | NA | 2000.00 ± 16.00 |
| TVFA/TA ratio | NA | 0.18 ± 0.01 | |
| Density | g/cm3 | 1.06 ± 0.02 | 0.99 ± 0.01 |
| S:I | VS Removal % | Initial pH of Digestion Mixture | Final pH of Digestion Mixture | Time Required for 95% of Biogas Yield (in Days) |
|---|---|---|---|---|
| 0.5 | 26.21 ± 0.21 | 7.84 | 7.21 | 20.26 |
| 1 | 51.87 ± 0.90 | 7.79 | 7.19 | 13.27 |
| 2 | 62.97 ± 4.29 | 7.70 | 7.19 | 15.41 |
| 3 | 74.71 ± 0.62 | 7.71 | 7.37 | 20.95 |
| 4 | 70.66 ± 0.11 | 7.58 | 7.52 | 24.24 |
| 5 | 76.97 ± 1.64 | 7.47 | 7.60 | 29.69 |
| 6 | 78.80 ± 1.17 | 7.45 | 7.86 | 38.96 |
| 0 | 10.43 ± 9.69 | 8.08 | 9.12 |
| Parameters | Units | S:I Ratios | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| First-order kinetic model | ||||||||
| Biogas production rate constant (first order disintegration rate constant) (k) | 1/d | 0.14 | 0.17 | 0.14 | 0.10 | 0.09 | 0.07 | 0.05 |
| Standard error | 0.67 | 0.79 | 2.32 | 4.85 | 7.03 | 8.77 | 11.55 | |
| R2 | 0.73 | 0.55 | 0.63 | 0.73 | 0.75 | 0.80 | 0.87 | |
| Experimental biogas yield | NmL/gVS | 464.01 | 674.37 | 638.88 | 555.13 | 570.14 | 551.58 | 556.78 |
| Predicted biogas yield (Go) | NmL/gVS | 455.97 | 668.65 | 650.40 | 584.85 | 605.27 | 601.64 | 645.12 |
| Difference between measured and predicted gas yield | % | 1.73 | 0.85 | 1.80 | 5.35 | 6.16 | 9.08 | 15.87 |
| Modified Gompertz model | ||||||||
| Maximum biogas production rate (Rmax) | NmL/d | 6.43 | 25.20 | 40.75 | 39.60 | 45.58 | 44.83 | 42.03 |
| Rmax | NmL/gVS.d | 45.21 | 88.56 | 71.60 | 46.38 | 40.05 | 31.51 | 24.62 |
| Lag phase (L) | d | −2.76 | −0.31 | 0.030 | 0.20 | −0.25 | −0.72 | −1.15 |
| Standard error | 0.77 | 1.03 | 1.18 | 2.85 | 4.68 | 5.98 | 8.08 | |
| R2 | 0.68 | 0.65 | 0.56 | 0.67 | 0.72 | 0.79 | 0.88 | |
| Experimental biogas yield | NmL/gVS | 464.01 | 674.37 | 638.88 | 555.13 | 570.14 | 551.58 | 556.78 |
| Predicted biogas yield (M) | NmL/gVS | 451.62 | 653.17 | 634.02 | 563.04 | 581.61 | 571.41 | 588.40 |
| Difference between measured and predicted gas yield | % | 2.67 | 3.14 | 0.76 | 1.42 | 2.01 | 3.60 | 5.68 |
| Time to undergo 95% of yield (t95) | d | 20.26 | 13.27 | 15.41 | 20.95 | 24.24 | 29.69 | 38.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khadka, A.; Parajuli, A.; Dangol, S.; Thapa, B.; Sapkota, L.; Carmona-Martínez, A.A.; Ghimire, A. Effect of the Substrate to Inoculum Ratios on the Kinetics of Biogas Production during the Mesophilic Anaerobic Digestion of Food Waste. Energies 2022, 15, 834. https://doi.org/10.3390/en15030834
Khadka A, Parajuli A, Dangol S, Thapa B, Sapkota L, Carmona-Martínez AA, Ghimire A. Effect of the Substrate to Inoculum Ratios on the Kinetics of Biogas Production during the Mesophilic Anaerobic Digestion of Food Waste. Energies. 2022; 15(3):834. https://doi.org/10.3390/en15030834
Chicago/Turabian StyleKhadka, Aakash, Anmol Parajuli, Sheila Dangol, Bijay Thapa, Lokesh Sapkota, Alessandro A. Carmona-Martínez, and Anish Ghimire. 2022. "Effect of the Substrate to Inoculum Ratios on the Kinetics of Biogas Production during the Mesophilic Anaerobic Digestion of Food Waste" Energies 15, no. 3: 834. https://doi.org/10.3390/en15030834
APA StyleKhadka, A., Parajuli, A., Dangol, S., Thapa, B., Sapkota, L., Carmona-Martínez, A. A., & Ghimire, A. (2022). Effect of the Substrate to Inoculum Ratios on the Kinetics of Biogas Production during the Mesophilic Anaerobic Digestion of Food Waste. Energies, 15(3), 834. https://doi.org/10.3390/en15030834








