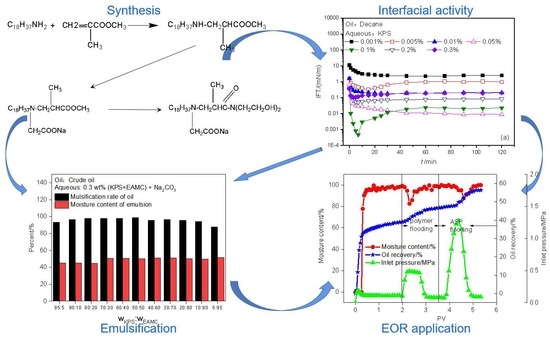
Study on the Synergistic Effects between Petroleum Sulfonate and a Nonionic–Anionic Surfactant for Enhanced Oil Recovery
Abstract
:1. Introduction
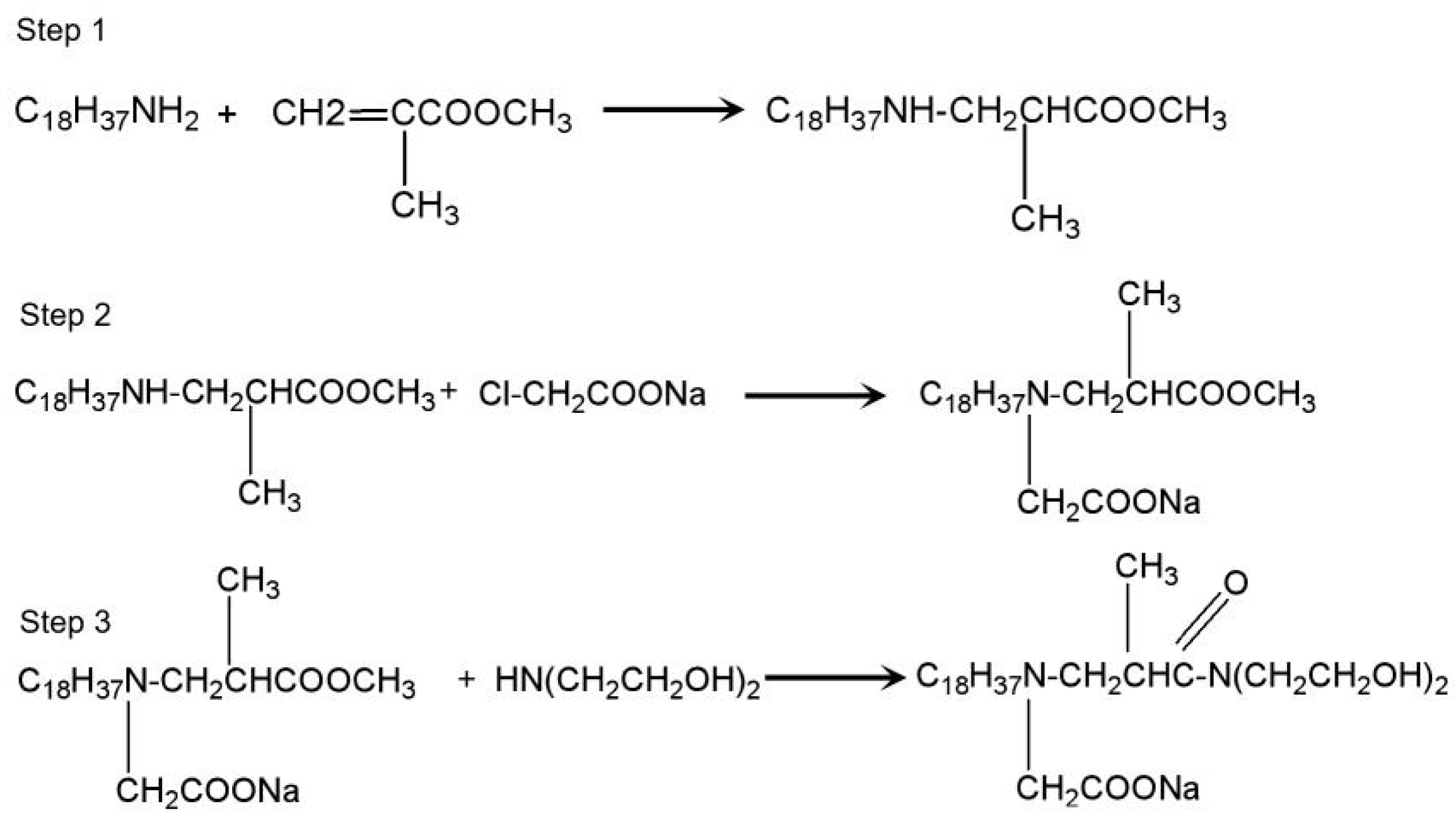
2. Materials and Methods
2.1. Materials
2.2. Apparatus and Methods
2.2.1. Interfacial Tension (IFT) Measurements
2.2.2. Emulsification Measurements
2.2.3. Oil Displacement Measurements
3. Results and Discussion
3.1. Interfacial Properties
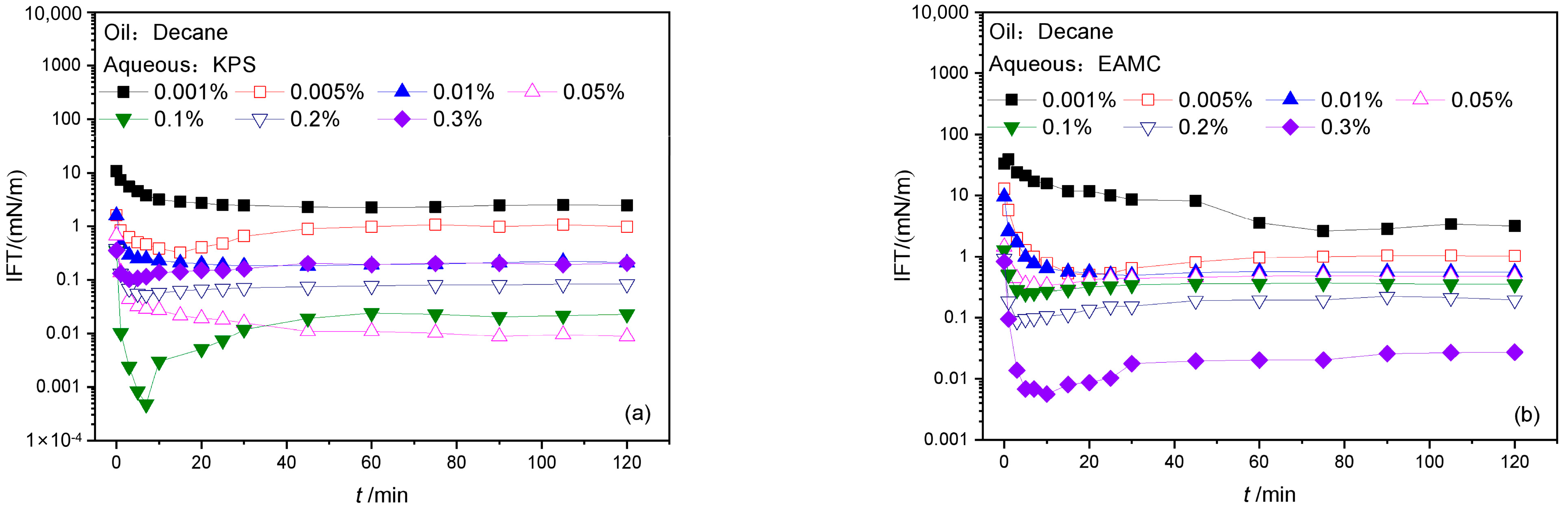
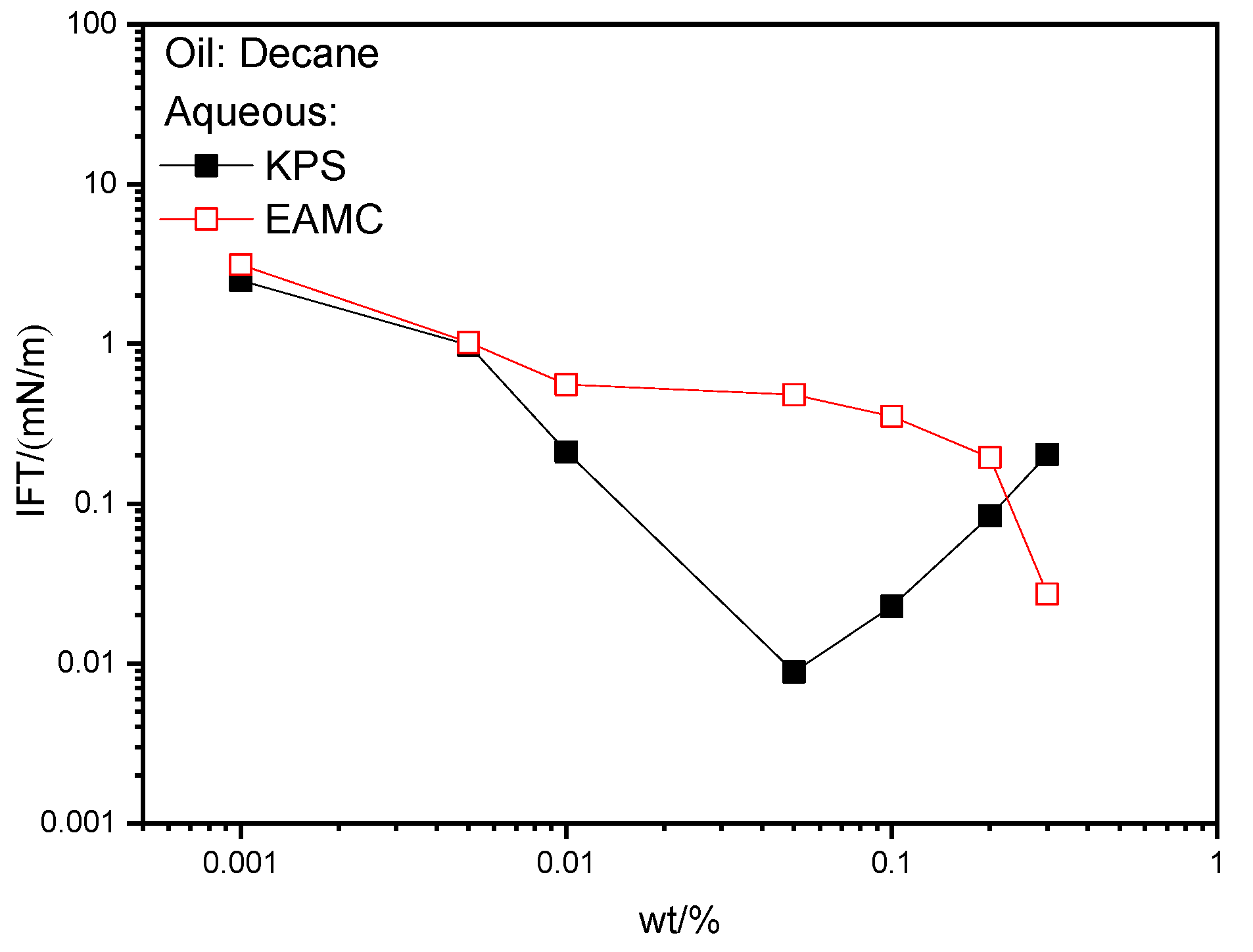
3.1.1. The IFTs of Surfactant Solutions against N-decane
3.1.2. The IFTs of Chemical Flooding Solutions against Crude Oil
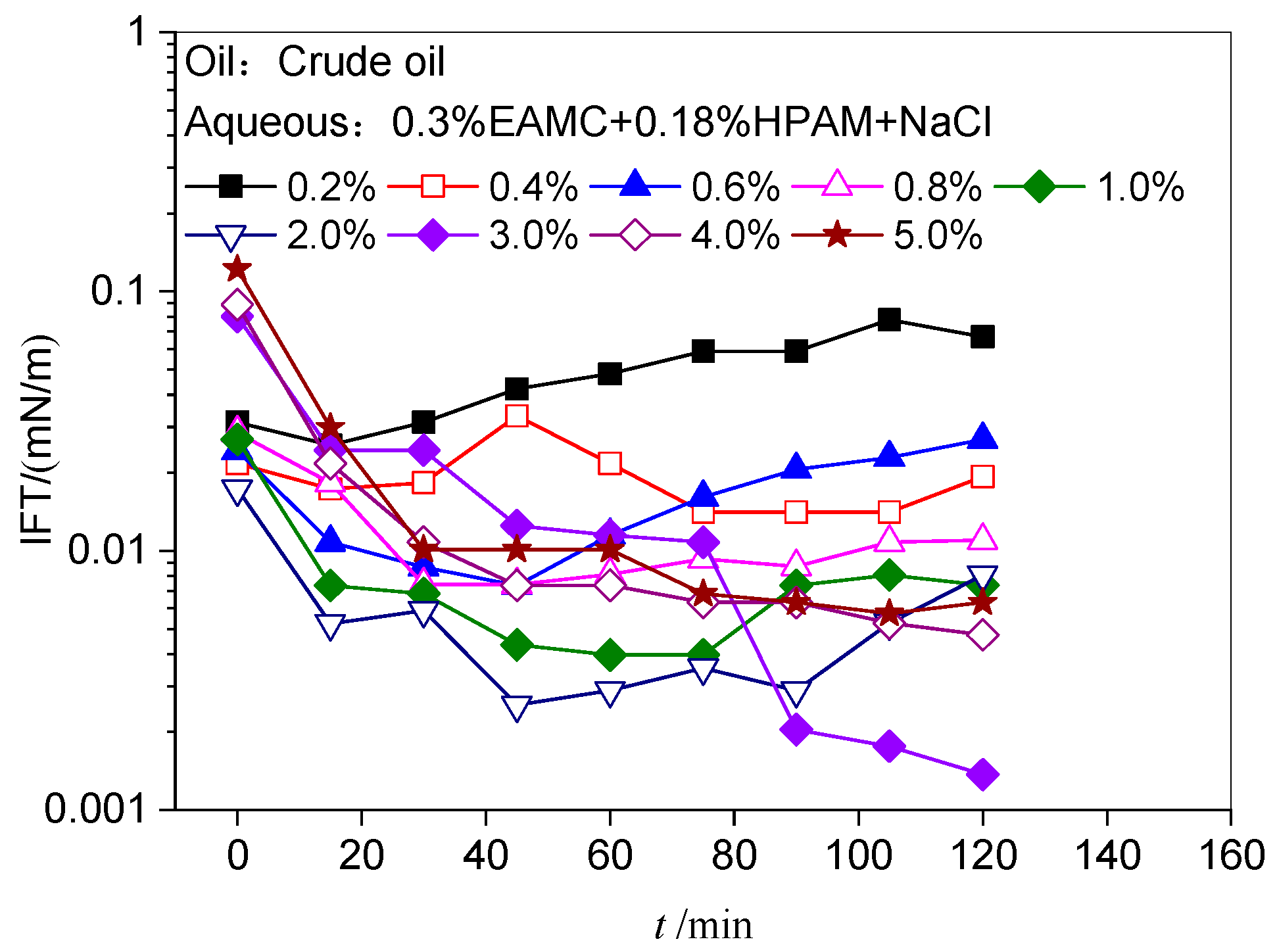
The Salt Tolerance of EAMC and HPAM Solutions
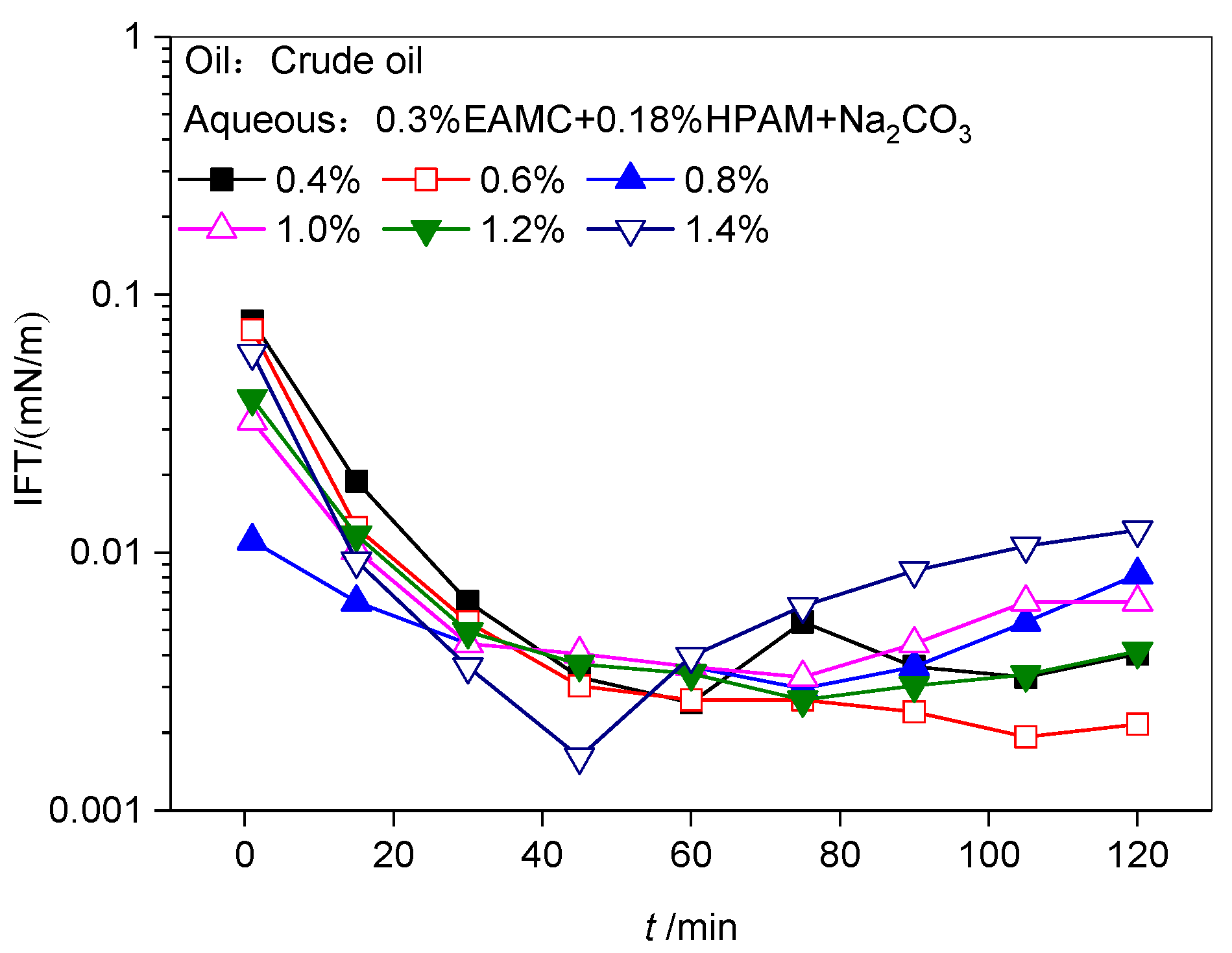
The Alkali Tolerance of EAMC and HPAM Solutions
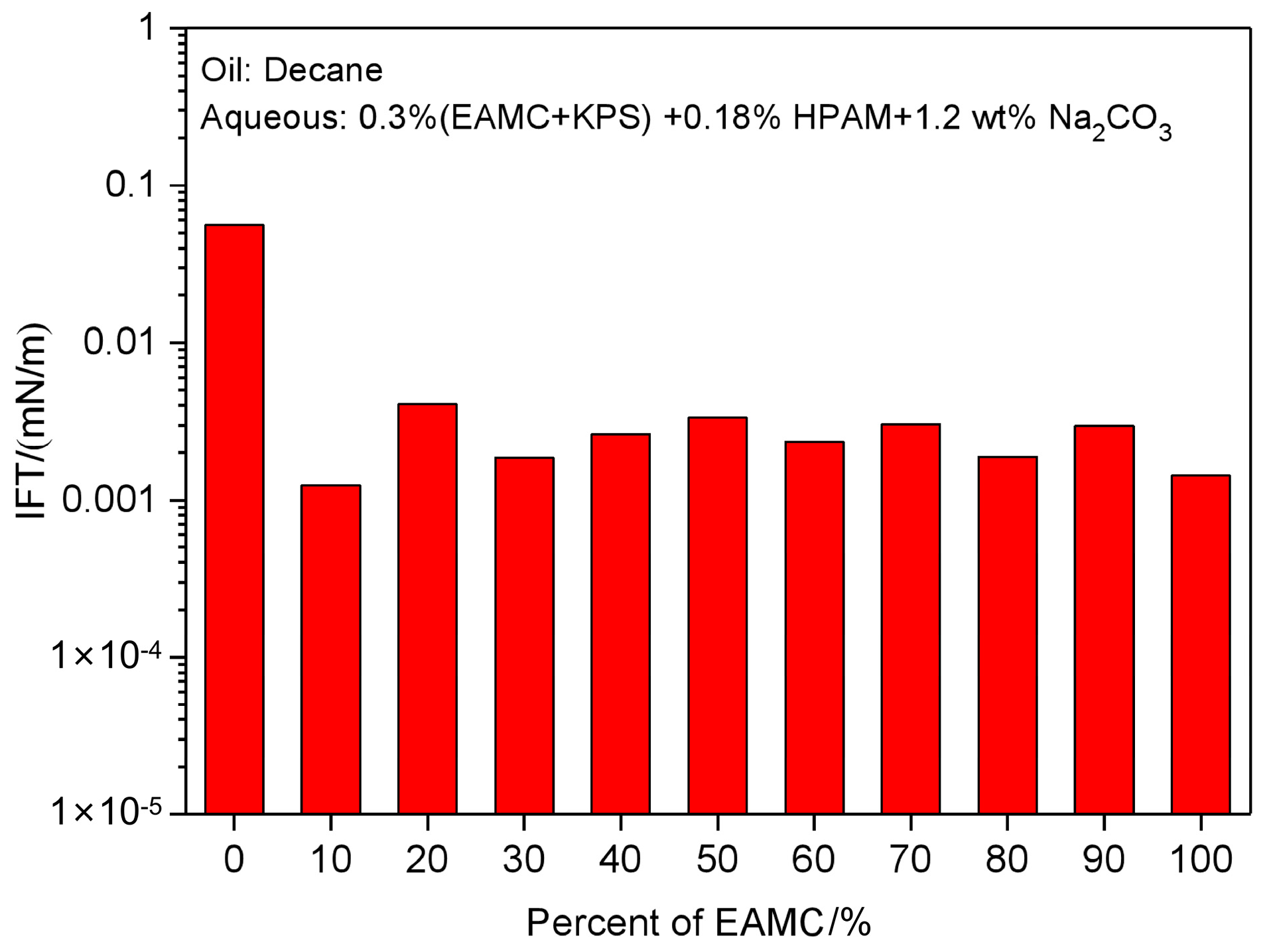
The Effect of EAMC on the IFTs of KPS and HPAM Solutions
The Alkali Tolerance of KPS and EAMC, and HPAM Solutions
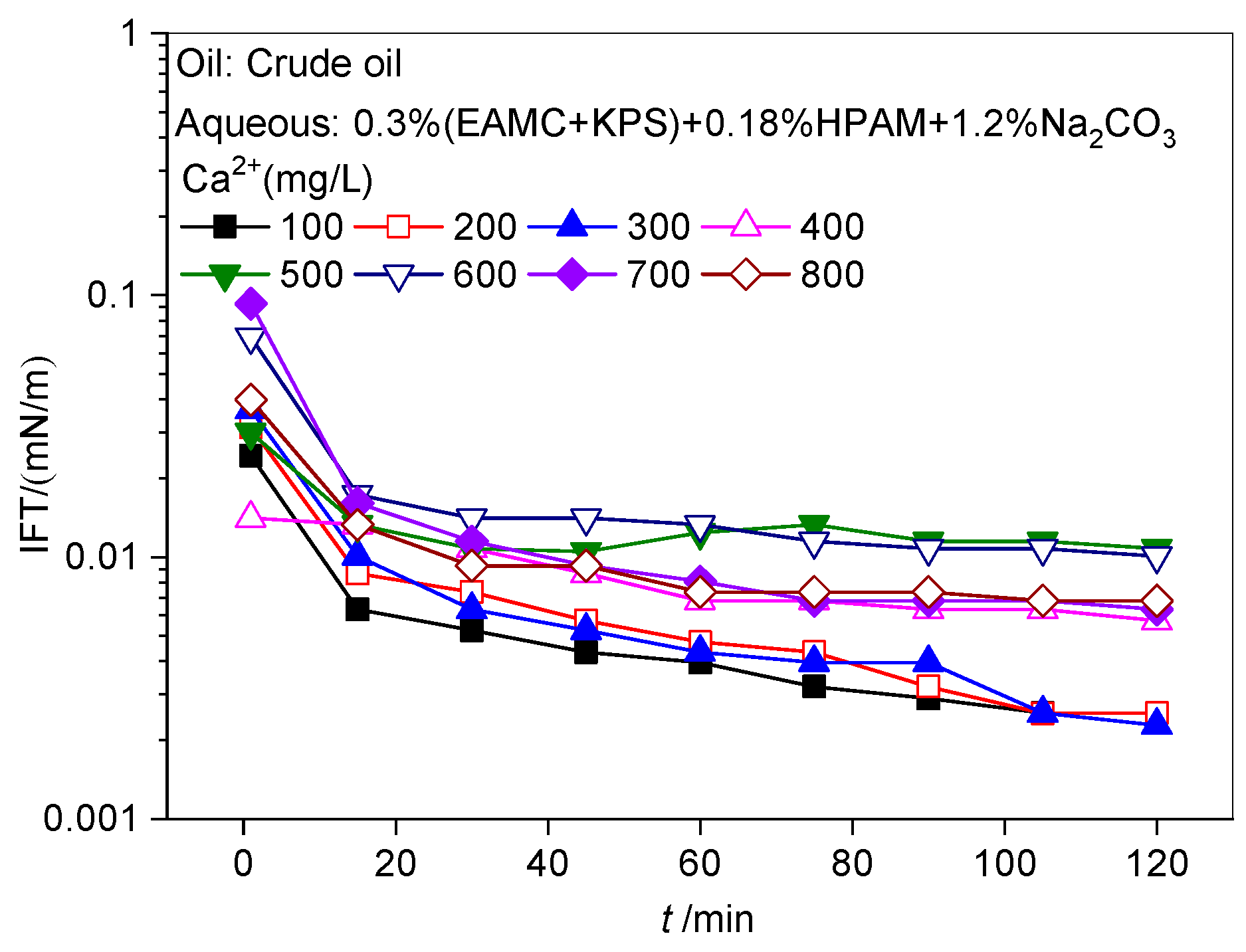
The Effect of Ca2+ on the Dynamic IFTs of Chemical Flooding Solutions
3.2. Emulsification Properties
3.2.1. Effect of Alkali Concentration
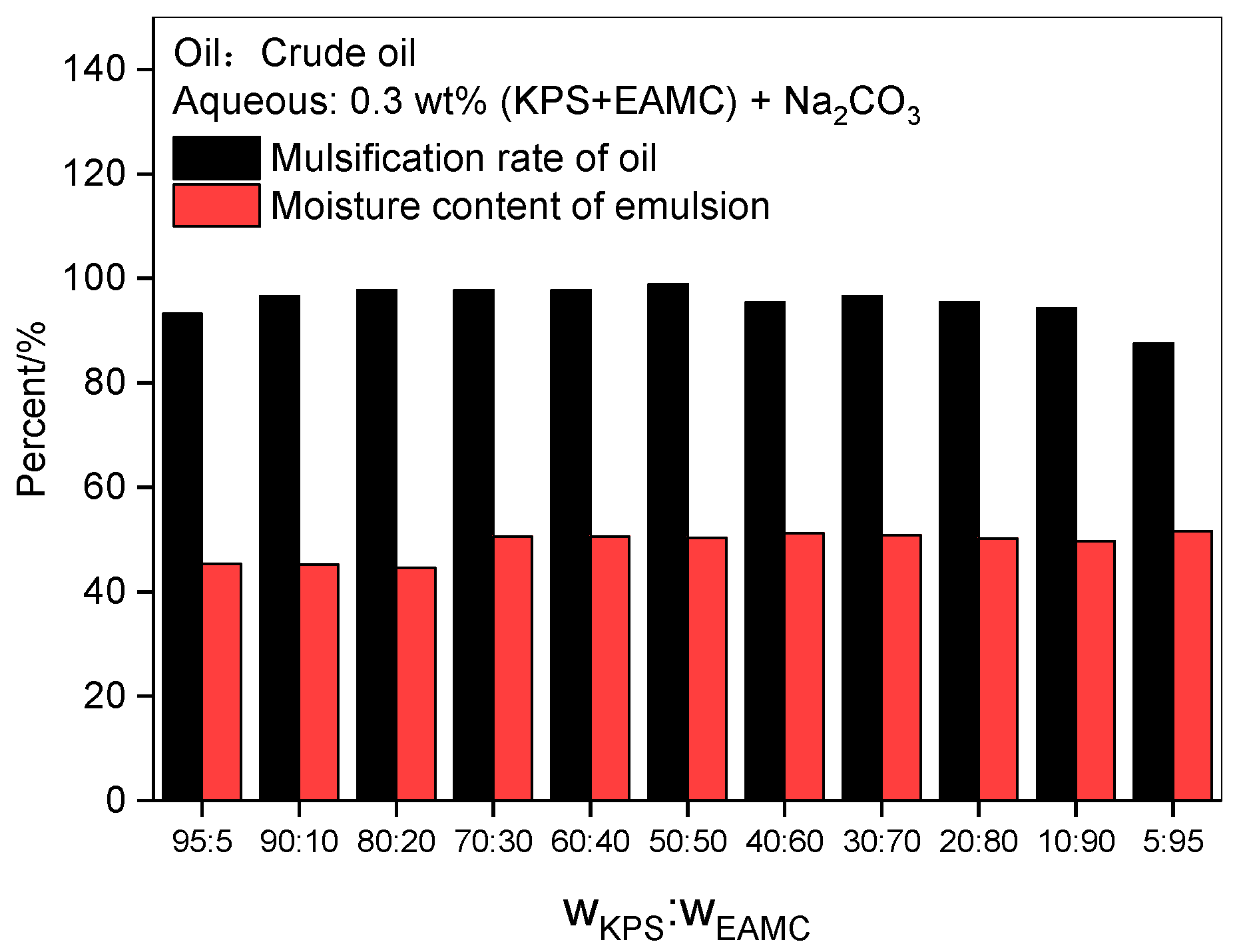
3.2.2. Effect of Mixed Surfactant Ratio
3.3. Oil Displacement Test
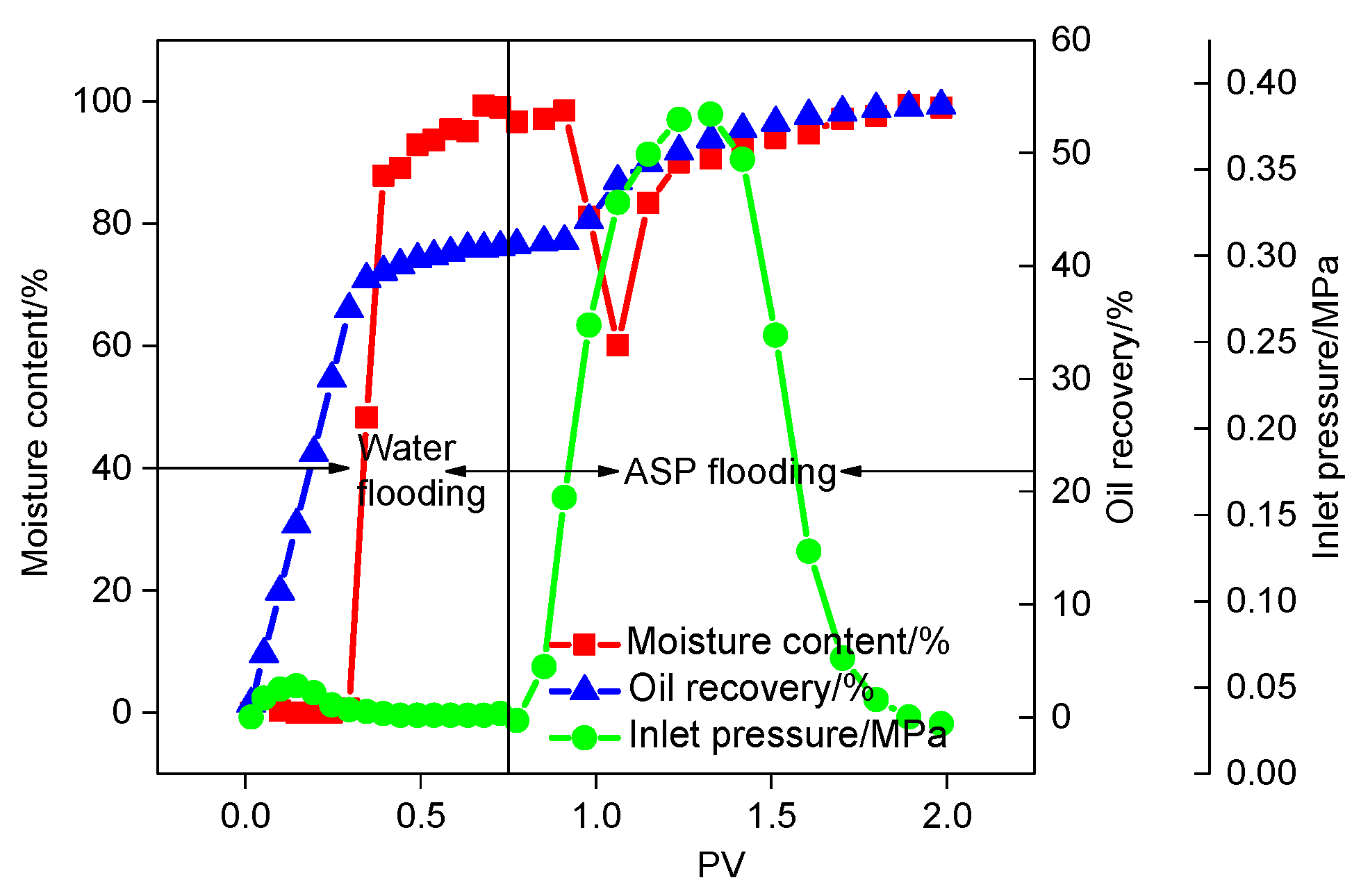
3.3.1. ASP Flooding
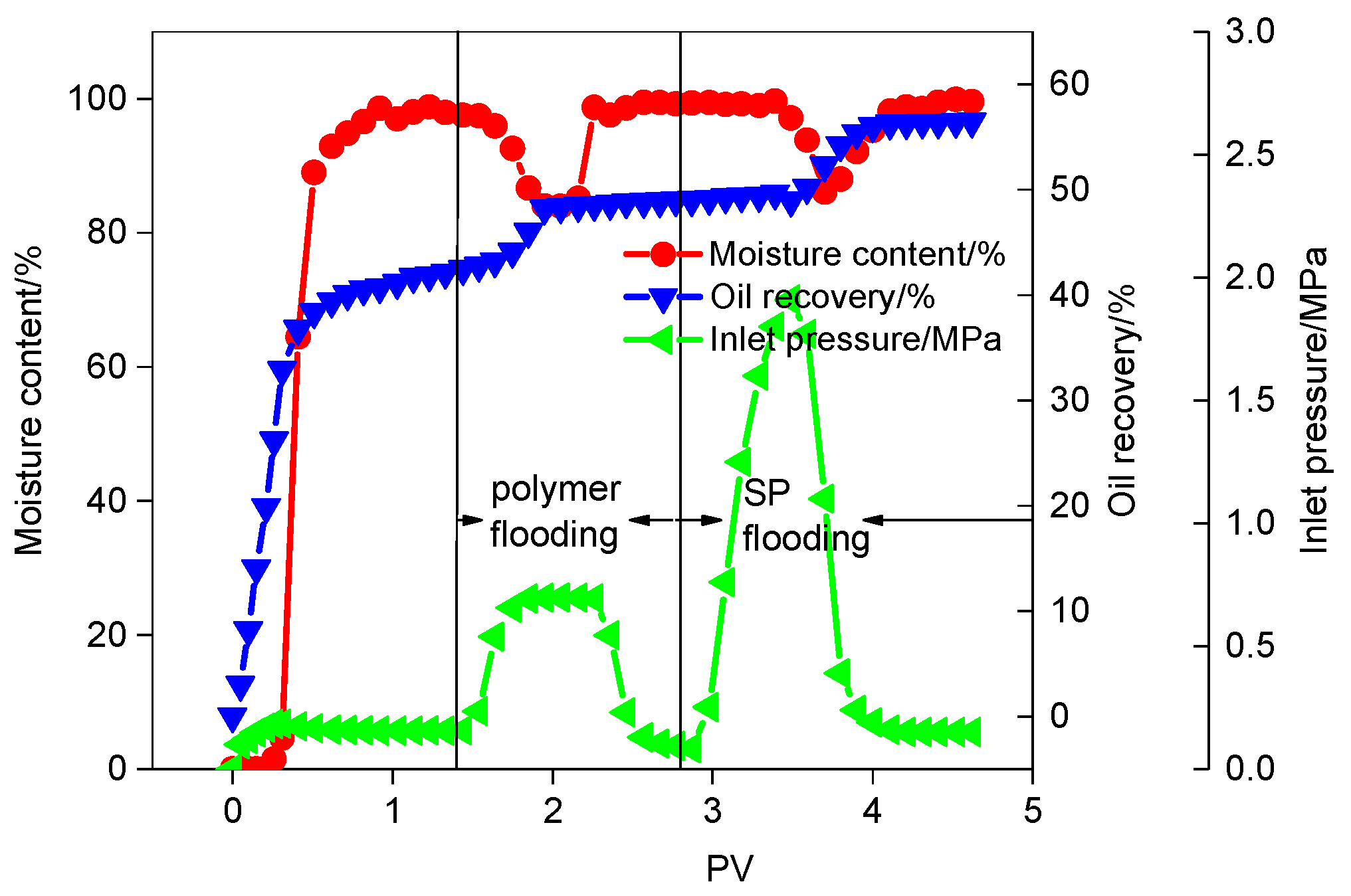
3.3.2. SP Flooding after Polymer Flooding
3.3.3. ASP Flooding after Polymer Flooding
4. Conclusions
- Both EAMC and KPS show high interfacial activity and can reduce IFTs to about 0.01 mN/m order of magnitude against decane at optimized concentrations.
- The area occupied by the hydrophilic group of EAMC on the interface is smaller than that of its own hydrophobic group. The interfacial film formed by EAMC is relatively loose. The IFTs of KPS containing different structure petroleum sulfonates is affected by the difference in the adsorption rate of petroleum sulfonates to the interface, which shows that both the dynamic and equilibrium interfacial tensions may have the lowest values.
- The IFTs of EAMC solutions against crude oil can be reduced to ultralow values because the mixed tight adsorption film will be formed by EAMC and crude oil fraction molecules. On the other hand, the KPS molecule has a hydrophobic part with a large size, and no synergism with crude oil fractions can be observed in the solutions containing only KPS.
- When EAMC has been added to a KPS solution to fill the vacancies of the KPS interface film, even 10 percent of EAMC can reduce the IFT of KPS to an ultralow value. The combination of EAMC and KPS shows ultralow IFT values, good emulsification properties, high alkali tolerance and good salt and Ca2+ tolerance during a wide range of EAMC percent.
- The best formula of a EAMC and KPS system can be applied for EOR after polymer flooding. The total EOR value of polymer flooding and following SP flooding is 14.62%. The total EOR value of polymer flooding and following ASP flooding is 17.49%.
Author Contributions
Funding
Conflicts of Interest
References
- Atilhan, M.; Aparicio, S. Review on Chemical Enhanced Oil Recovery: Utilization of Ionic Liquids and Deep Eutectic Solvents. J. Pet. Sci. Eng. 2021, 205, 108746. [Google Scholar] [CrossRef]
- Olajire, A.A. Review of ASP EOR (Alkaline Surfactant Polymer Enhanced Oil Recovery) Technology in the Petroleum Industry: Prospects and Challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- Cayias, J.L.; Schechter, R.S.; Wade, W.H. Modeling Crude Oils for Low Interfacial Tension. Soc. Pet. Eng. J. 1976, 16, 351–357. [Google Scholar] [CrossRef]
- Cash, L.; Cayias, J.L.; Fournier, G.; Macallister, D.; Schares, T.; Schechter, R.S.; Wade, W.H. The Application of Low Interfacial Tension Scaling Rules to Binary Hydrocarbon Mixtures. J. Colloid Interface Sci. 1977, 59, 39–44. [Google Scholar] [CrossRef]
- Chan, K.S.; Shah, D.O. The Molecular Mechanism for Achieving Ultra Low Interfacial Tension Minimum in a Petroleum Sulfonate/Oil/Brine System. J. Dispers. Sci. Technol. 1980, 1, 55–95. [Google Scholar] [CrossRef]
- Deng, X.; Tariq, Z.; Murtaza, M.; Patil, S.; Mahmoud, M.; Kamal, M.S. Relative Contribution of Wettability Alteration and Interfacial Tension Reduction in EOR: A Critical Review. J. Mol. Liq. 2021, 325, 115175. [Google Scholar] [CrossRef]
- Acevedo, S.; Ranaudo, M.A.; Escobar, G.; Gutiérrez, X. Dynamic Interfacial Tension Measurement of Heavy Crude Oil–Alkaline Systems: The Role of the Counterion in the Aqueous Phase. Fuel 1999, 78, 309–317. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, F.; Qiao, W.; Li, Z.; Cheng, L. Novel Alkyl Methylnaphthalene Sulfonate Surfactants: A Good Candidate for Enhanced Oil Recovery. Fuel 2006, 85, 1815–1820. [Google Scholar] [CrossRef]
- Sharma, H.; Dufour, S.; Arachchilage, G.W.P.P.; Weerasooriya, U.; Pope, G.A.; Mohanty, K. Alternative Alkalis for ASP Flooding in Anhydrite Containing Oil Reservoirs. Fuel 2015, 140, 407–420. [Google Scholar] [CrossRef]
- Alvarado, V.; Manrique, E. Enhanced Oil Recovery: An Update Review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Touhami, Y.; Hornof, V.; Neale, G.H. Dynamic Interfacial Tension Behavior of Acidified Oil/Surfactant-Enhanced Alkaline Systems 1. Experimental Studies. Colloids Surf. A Physicochem. Eng. Asp. 1998, 132, 61–74. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, L.; Zhao, S.; Xu, Z.; An, J.; Yu, J. Effect of Different Acidic Fractions in Crude Oil on Dynamic Interfacial Tensions in Surfactant/Alkali/Model Oil Systems. J. Pet. Sci. Eng. 2004, 41, 189–198. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Mohammadi, A.H.; Manshad, A.K. Characterization and Evaluation of a Natural Surfactant Extracted from Soapwort Plant for Alkali-Surfactant-Polymer (ASP) Slug Injection into Sandstone Oil Reservoirs. J. Mol. Liq. 2020, 318, 114369. [Google Scholar] [CrossRef]
- Zhao, R.; Huang, H.; Wang, H.; Zhang, J.; Zhang, L.; Zhang, L.; Zhao, S. Effect of Organic Additives and Crude Oil Fractions on Interfacial Tensions of Alkylbenzene Sulfonates. J. Dispers. Sci. Technol. 2013, 34, 623–631. [Google Scholar] [CrossRef]
- Aoudia, M.; Al-Shibli, M.N.; Al-Kasimi, L.H.; Al-Maamari, R.; Al-bemani, A. Novel Surfactants for Ultralow Interfacial Tension in a Wide Range of Surfactant Concentration and Temperature. J. Surfact. Deterg. 2006, 9, 287–293. [Google Scholar] [CrossRef]
- Zhao, Z.; Bi, C.; Qiao, W.; Li, Z.; Cheng, L. Dynamic Interfacial Tension Behavior of the Novel Surfactant Solutions and Daqing Crude Oil. Colloids Surf. A Physicochem. Eng. Asp. 2007, 294, 191–202. [Google Scholar] [CrossRef]
- Li, G.; Wang, K.; Lu, C. Effect of Particle Aggregates on the Surface Properties of Amphiphilic SiO2 Particles in Anhydrous Foam. Chem. J. Chin. Univ.-Chin. 2020, 41, 2038–2045. [Google Scholar] [CrossRef]
- Rosen, M.J.; Zhou, Q. Surfactant−Surfactant Interactions in Mixed Monolayer and Mixed Micelle Formation. Langmuir 2001, 17, 3532–3537. [Google Scholar] [CrossRef]
- Wang, K.; Li, G.; Zhang, B. Opposite Results of Emulsion Stability Evaluated by the TSI and the Phase Separation Proportion. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 402–409. [Google Scholar] [CrossRef]
- Bergström, M.; Eriksson, J.C. A Theoretical Analysis of Synergistic Effects in Mixed Surfactant Systems. Langmuir 2000, 16, 7173–7181. [Google Scholar] [CrossRef]
- Witthayapanyanon, A.; Acosta, E.J.; Harwell, J.H.; Sabatini, D.A. Formulation of Ultralow Interfacial Tension Systems Using Extended Surfactants. J. Surfact. Deterg. 2006, 9, 331–339. [Google Scholar] [CrossRef]
- Aoudia, M.; Al-Maamari, R.S.; Nabipour, M.; Al-Bemani, A.S.; Ayatollahi, S. Laboratory Study of Alkyl Ether Sulfonates for Improved Oil Recovery in High-Salinity Carbonate Reservoirs: A Case Study. Energy Fuels 2010, 24, 3655–3660. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Li, Z.-Q.; Song, X.-W.; Zhang, J.-C.; Zhang, L.; Zhang, L.; Zhao, S. Dynamic Interfacial Tensions of Binary Nonionic–Anionic and Nonionic Surfactant Mixtures at Water–Alkane Interfaces. Fuel 2014, 135, 91–98. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Azzam, E.M.S.; Mahmoud, S.A.; Saleh, N.E.A. Synthesis of Ethoxylated Alkyl Sulfosuccinate Surfactants and the Investigation of Mixed Solutions. J. Surfact. Deterg. 2007, 10, 3–8. [Google Scholar] [CrossRef]
- Moslemizadeh, A.; Khayati, H.; Madani, M.; Ghasemi, M.; Shahbazi, K.; Zendehboudi, S.; Hashim Abbas, A. A Systematic Study to Assess Displacement Performance of a Naturally-Derived Surfactant in Flow Porous Systems. Energies 2021, 14, 8310. [Google Scholar] [CrossRef]
- Rezaei, A.; Riazi, M.; Escrochi, M.; Elhaei, R. Integrating Surfactant, Alkali and Nano-Fluid Flooding for Enhanced Oil Recovery: A Mechanistic Experimental Study of Novel Chemical Combinations. J. Mol. Liq. 2020, 308, 113106. [Google Scholar] [CrossRef]
- Sha, O.; Zhang, W.; Lu, R. Synthesis and Properties Evaluation of Nonionic-Anionic Surfactants Suitable for Enhanced Oil Recovery Using Sea Water. Tenside Surfactants Deterg. 2008, 45, 82–86. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Cao, X.; Song, X.; Jin, Z.; Zhang, L.; Zhao, S. Effect of Electrolytes on Interfacial Tensions of Alkyl Ether Carboxylate Solutions. Energy Fuels 2013, 27, 3122–3129. [Google Scholar] [CrossRef]
- Salager, J.-L.; Forgiarini, A.M.; Bullón, J. How to Attain Ultralow Interfacial Tension and Three-Phase Behavior with Surfactant Formulation for Enhanced Oil Recovery: A Review. Part 1. Optimum Formulation for Simple Surfactant–Oil–Water Ternary Systems. J. Surfactants Deterg. 2013, 16, 449–472. [Google Scholar] [CrossRef]
- Goodarzi, F.; Zendehboudi, S. Effects of Salt and Surfactant on Interfacial Characteristics of Water/Oil Systems: Molecular Dynamic Simulations and Dissipative Particle Dynamics. Ind. Eng. Chem. Res. 2019, 58, 8817–8834. [Google Scholar] [CrossRef] [Green Version]
- Riswati, S.S.; Bae, W.; Park, C.; Permadi, A.K.; Efriza, I.; Min, B. Experimental Analysis to Design Optimum Phase Type and Salinity Gradient of Alkaline Surfactant Polymer Flooding at Low Saline Reservoir. J. Pet. Sci. Eng. 2019, 173, 1005–1019. [Google Scholar] [CrossRef]
- Massarweh, O.; Abushaikha, A.S. The Use of Surfactants in Enhanced Oil Recovery: A Review of Recent Advances. Energy Rep. 2020, 6, 3150–3178. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2004; ISBN 978-0-471-47818-8. [Google Scholar]



| Components | TDS | Ca2+ | Mg2+ | SO42− | CO32− | HCO3− | Na+ | Cl− |
|---|---|---|---|---|---|---|---|---|
| Concentration/mg·L−1 | 472.98 | 64.49 | 6.11 | 191.2 | 0 | 98.41 | 70.50 | 42.27 |
| Section | Data | Section | Data |
|---|---|---|---|
| Diameter/cm | 3.8 | Product well number | T71748 |
| Porosity/wt % | 14.33 | Length/cm | 29.3 |
| Kair/μm2 | 0.57802 | Pore volume/mL | 55.6 |
| Koil/μm2 | 0.83 | Kwater/μm2 | 50.0 |
| Crude oil viscosity/mPa·s | 20.81 | Soi/mL | 44.6 |
| T/°C | 34 | Injection flow/mL/min | 0.5 |
| IFT/mN·m−1 | 0.0059 | Oil recovery by water flooding/% | 41.64 |
| Total Oil recovery/% | 54.20 | Oil recovery by ASP flooding/% | 12.56 |
| Section | Data | Section | Data |
|---|---|---|---|
| Diameter/cm | 3.8 | Product well number | ES7013 |
| Porosity/% | 14.32 | Length/cm | 30 |
| Kair/μm2 | 0.5703 | Pore volume/mL | 48.7 |
| Koil/μm2 | 0.82 | Kwater/μm2 | 0.52 |
| Crude oil viscosity/mPa.s | 20.81 | Soi/mL | 36.2 |
| T/°C | 34.3 | Injection flow/mL/min | 0.5 |
| Oil recovery by polymer flooding/% | 7.05 | Oil recovery by water flooding/% | 41.88 |
| EOR/% | 14.62 | Oil recovery by SP flooding/% | 7.57 |
| IFT/mN·m−1 | 0.00462 | Total oil recovery/% | 56.49 |
| Section | Data | Section | Data |
|---|---|---|---|
| Diameter/cm | 3.8 | Product well number | ES7013 |
| Porosity/% | 14.06 | Length/cm | 30 |
| Kair/μm2 | 0.5800 | Pore volume/mL | 47.8 |
| Koil/μm2 | 0.831 | Kwater/μm2 | 0.53 |
| Crude oil viscosity/mPa.s | 20.81 | Soi/mL | 35.9 |
| T/°C | 34.3 | Injection flow/mL·min−1 | 0.50 |
| Oil recovery by polymer flooding/% | 7.71 | Oil recovery by water flooding/% | 38.89 |
| EOR/% | 17.49 | Oil recovery by ASP flooding/% | 9.78 |
| IFT/mN·m−1 | 0.00462 | Total oil recovery/% | 56.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, H.; Zhou, Z.; Xu, C.; Bai, L.; Wang, X.; Han, L.; Zhang, Q.; Li, G. Study on the Synergistic Effects between Petroleum Sulfonate and a Nonionic–Anionic Surfactant for Enhanced Oil Recovery. Energies 2022, 15, 1177. https://doi.org/10.3390/en15031177
Luan H, Zhou Z, Xu C, Bai L, Wang X, Han L, Zhang Q, Li G. Study on the Synergistic Effects between Petroleum Sulfonate and a Nonionic–Anionic Surfactant for Enhanced Oil Recovery. Energies. 2022; 15(3):1177. https://doi.org/10.3390/en15031177
Chicago/Turabian StyleLuan, Huoxin, Zhaohui Zhou, Chongjun Xu, Lei Bai, Xiaoguang Wang, Lu Han, Qun Zhang, and Gen Li. 2022. "Study on the Synergistic Effects between Petroleum Sulfonate and a Nonionic–Anionic Surfactant for Enhanced Oil Recovery" Energies 15, no. 3: 1177. https://doi.org/10.3390/en15031177
APA StyleLuan, H., Zhou, Z., Xu, C., Bai, L., Wang, X., Han, L., Zhang, Q., & Li, G. (2022). Study on the Synergistic Effects between Petroleum Sulfonate and a Nonionic–Anionic Surfactant for Enhanced Oil Recovery. Energies, 15(3), 1177. https://doi.org/10.3390/en15031177