Biofuel Production from Seaweeds: A Comprehensive Review
Abstract
1. Introduction
2. The Origin of the Biomass
2.1. Cultivation
2.2. Wild Seaweed
Application
2.3. Drift Seaweed
3. Characterization of Seaweeds
3.1. Morphology of Seaweeds
3.2. General Composition
3.2.1. Algal Structure (Focus Carbohydrates)
3.2.2. Biochemical Composition of Seaweeds
4. Energy Production from Seaweeds
4.1. Biofuel Production
4.1.1. Bioethanol
4.1.2. Biobutanol
4.1.3. Bio-Oil
4.1.4. Biodiesel
4.2. Biogas
4.2.1. Parameters Likely to Influence the Quality of AD
4.2.2. Effect of the Sulfur Content
4.2.3. Effect of the ISR
4.2.4. Variation of Methane Yield with Different Species and Components
4.3. Biohydrogen
4.3.1. Biological Conversion Pathway
4.3.2. Thermochemical Conversion Pathway
4.4. Effects of Pretreatment Methods
4.4.1. Physical Pretreatment
4.4.2. Chemical Pretreatment
4.4.3. Biological Pretreatment
4.4.4. Combined Pretreatment
4.5. By-Products Generation and Detoxification Techniques
4.5.1. Biomethane Potential and Experimental Conditions
4.5.2. Biohydrogen Potential and Experimental Conditions
4.6. Remarks on BMP and BHP Evaluation/Assays
- The majority of previous research has applied mechanical pretreatment to reduce the size of algal biomass, whether by chopping, cutting, grounding, milling, or even Hollander beating. Although the size of samples after pretreatments has been specified, only a few studies demonstrate the preservation methods used before pretreatment. It is clear that a fresh sample does not result in the same loss of VS during the pretreatment process as a frozen sample. The question then becomes how best to define this loss of fermentable substrate and how to compensate in case of a considerable loss. Moreover, the various mechanical pretreatment methods could result in a loss of water content and therefore the VS value is biased, which can potentially impact the gas yield results. We highly recommend that these points be considered and worth mentioning.
- Most studies mentioned the pretreatment methods used for inoculum without its characterization (TS, VS, even pH, alkalinity, etc.). However, we considered that the efficiency of pre-treatment strongly depends on the initial property of the inoculum. The choice of temperature and duration may differ between treated inoculums. Moreover, a detailed description of the seed inoculum would facilitate the comparison of different studies performed under various conditions.
- When exploring the BMP and BHP of the substrate, only a few studies within the literature have demonstrated the temperature and pressure conditions of the gas produced. In some circumstances, this may be problematic, for example: when comparing tests conducted under mesophilic and thermophilic conditions, the results would be unusable without conversion to normal condition (298 K, 101,325 Pa).
- In the BMP test, methane yields were calculated by dividing the corrected methane volume (standard pressure and temperature) by the weight of sample (VS) added to each bottle. In this case, to minimize the effect of endogenous gas production (gas produced by the inoculum on total gas production), an important point is to increase the amount of substrate. However, some studies deal with a low amount of substrate, which may decrease the reliability of the test [104,112].
- When choosing pretreatment methods, most studies aimed to achieve maximum methane/hydrogen yield. However, the economic aspect is hardly mentioned: the balance between energy input and output, the profitability of the process and the feasibility of industrialization. These issues remain to be addressed in the future.
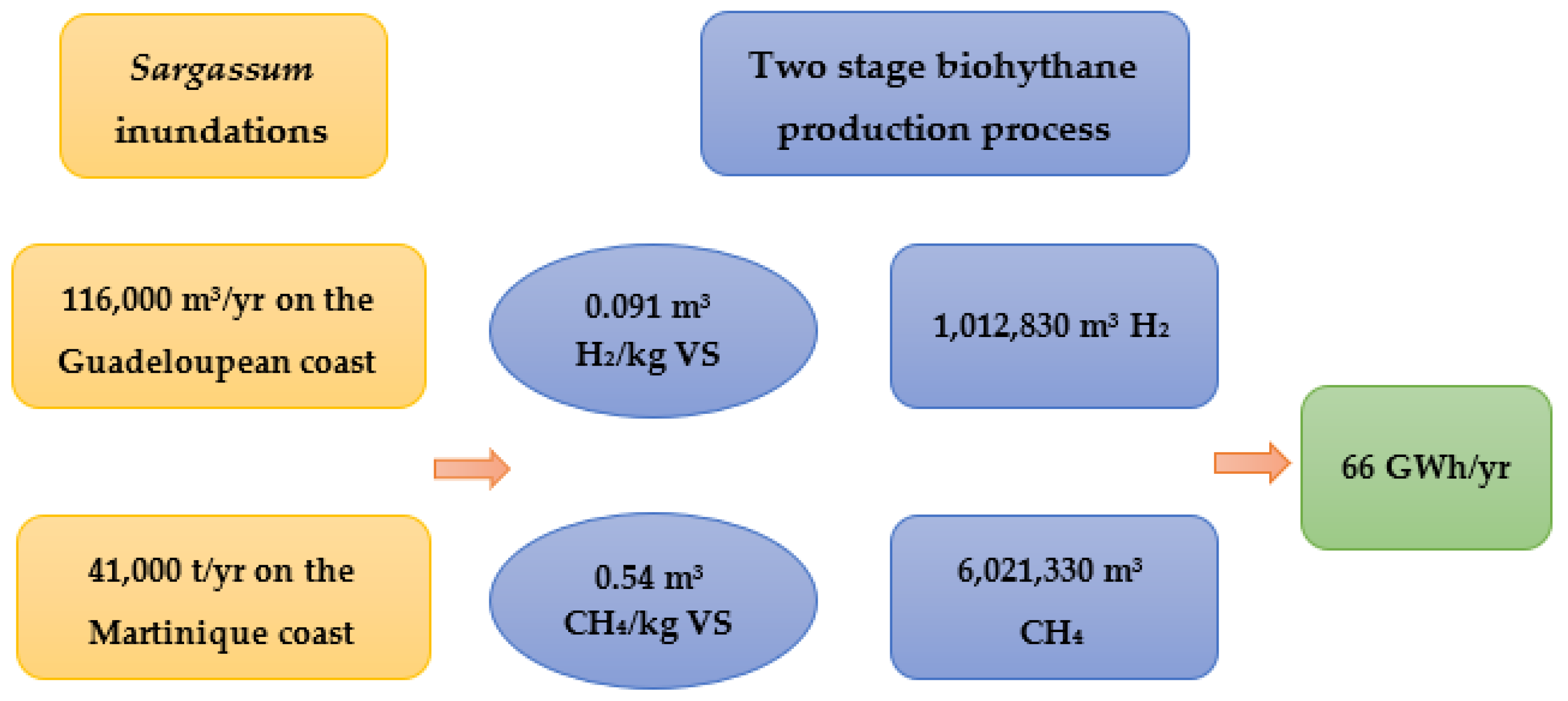
5. Study Example: Potential Energy Estimation of Sargassum in the French West Indies (Guadeloupe and Martinique)
6. Challenges, Constraints, Future Scope
7. Conclusions
- (1)
- Seaweed composition may vary according to the species studied, the geographical region, the harvesting season, biotic/abiotic parameters, the pretreatment methods, the processing of biomass (storage, drying), and analytical methods. Its characterization prior to the bioconversion process is essential.
- (2)
- Attention should be devoted to the presence of heavy metals, marine biotoxins and by-products (i.e., furfural, 5-HMF) during the fermentation process; they can be an obstacle to the further exploitation and valorization of seaweeds and should therefore be carefully considered.
- (3)
- AD and dark fermentation are promising processes suitable for energy production frommacroalgal biomass, with a relatively high yield of BMP (average 0.2~0.3 Nm3/kg) obtained in a manner comparable to terrestrial biomasses. The aim of dark fermentation will be to obtain a stable hydrogen production by adjusting the operating conditions.
- (4)
- Both gasification and anaerobic digestion considered promising methods, the choice of one process over the other should be based on energy balance and economic competitiveness.
- (5)
- Sargassum invasions pose a threat for coastal communities, which at the same time represent an opportunity for energy production, estimation brings a total of 66 GWh of energy per year in the French West Indies.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Anaerobic digestion |
| ALS | Ammonium lauryl sulfate |
| BHP | Biohydrogen potential |
| BMP | Biomethane potential |
| COD | Chemical oxygen demand |
| EU | European Union |
| LHV | Lower heating value |
| OLR | Organic loading rate |
| TS | Total solids |
| HRT | Hydraulic retention time |
| VFA | Volatile fatty acids |
| VS | Volatile solids |
| 5-HMF | 5-(Hydroxymethyl)furfural |
| SCWG | Supercritical water gasification |
| SDS | Sodium dodecyl sulfate |
| SRMs | Sulfate-reducing microorganisms |
References
- Boudouresque, C.-F. Taxonomy and Phylogeny of Unicellular Eukaryotes. In Environmental Microbiology: Fundamentals and Applications: Microbial Ecology; Bertrand, J.-C., Caumette, P., Lebaron, P., Matheron, R., Normand, P., Sime-Ngando, T., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 191–257. ISBN 978-94-017-9118-2. [Google Scholar]
- Davis, T.A.; Ramirez, M.; Mucci, A.; Larsen, B. Extraction, Isolation and Cadmium Binding of Alginate from Sargassum spp. J. Appl. Phycol. 2004, 16, 275–284. [Google Scholar] [CrossRef]
- Balina, K.; Romagnoli, F.; Blumberga, D. Seaweed Biorefinery Concept for Sustainable Use of Marine Resources. Energy Procedia 2017, 128, 504–511. [Google Scholar] [CrossRef]
- Baghel, R.S.; Suthar, P.; Gajaria, T.K.; Bhattacharya, S.; Anil, A.; Reddy, C.R.K. Seaweed Biorefinery: A Sustainable Process for Valorising the Biomass of Brown Seaweed. J. Clean. Prod. 2020, 263, 121359. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s Bioactive Candidate Compounds to Food Industry and Global Food Security. Life 2020, 10, 140. [Google Scholar] [CrossRef]
- Ben Yahmed, N.; Carrere, H.; Marzouki, M.N.; Smaali, I. Enhancement of Biogas Production from Ulva sp. by Using Solid-State Fermentation as Biological Pretreatment. Algal Res. 2017, 27, 206–214. [Google Scholar] [CrossRef]
- European Commission. 2050 Long-Term Strategy. Available online: https://climate.ec.europa.eu/eu-action/climate-strategies-targets/2050-long-term-strategy_en (accessed on 21 September 2022).
- Bouckaert, S.; Pales, A.F.; McGlade, C.; Remme, U.; Wanner, B.; Varro, L.; Abergel, T.; Arsalane, Y.; Bains, P.; Menendez, J.M.B.; et al. Net Zero by 2050—A Roadmap for the Global Energy Sector. p. 224. Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 21 September 2022).
- United Nations Educational, Scientific and Cultural Organization. UNESCO Science Report 2021: The Race Against Time for Smarter Development; World Science Report; United Nations: New York, NY, USA, 2021; ISBN 978-92-1-005857-5. [Google Scholar]
- Ministry of Ecological Transition (France). Programmation Pluriannuelle de L’énergie. 400. Available online: https://www.ecologie.gouv.fr/sites/default/files/20200422%20Programmation%20pluriannuelle%20de%20l%27e%CC%81nergie.pdf (accessed on 16 October 2022).
- Government of Iceland, Ministry of the Environment, Energy and Climate. Available online: https://www.government.is/topics/business-and-industry/energy/ (accessed on 17 October 2022).
- Official Website of the International Trade Administration. Denmark—Country Commercial Guide. Available online: https://www.trade.gov/country-commercial-guides/denmark-renewable-energy-products (accessed on 17 October 2022).
- Federal Ministry for Economic Affairs and Climate Action (Germany). Renewable Energy. Available online: https://www.bmwk.de/Redaktion/EN/Dossier/renewable-energy.html (accessed on 17 October 2022).
- Kumar, A.; Jones, D.; Hanna, M. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Maneein, S.; Milledge, J.J.; Harvey, P.J.; Nielsen, B.V. Methane Production from Sargassum muticum: Effects of Seasonality and of Freshwater Washes. Energy Built Environ. 2021, 2, 235–242. [Google Scholar] [CrossRef]
- Thompson, T.M.; Ramin, P.; Udugama, I.; Young, B.R.; Gernaey, K.V.; Baroutian, S. Techno-Economic and Environmental Impact Assessment of Biogas Production and Fertiliser Recovery from Pelagic Sargassum: A Biorefinery Concept for Barbados. Energy Convers. Manag. 2021, 245, 114605. [Google Scholar] [CrossRef]
- Prestipino, M.; Palomba, V.; Vasta, S.; Freni, A.; Galvagno, A. A Simulation Tool to Evaluate the Feasibility of a Gasification-I.C.E. System to Produce Heat and Power for Industrial Applications. Energy Procedia 2016, 101, 1256–1263. [Google Scholar] [CrossRef]
- Prestipino, M.; Piccolo, A.; Polito, M.F.; Galvagno, A. Combined Bio-Hydrogen, Heat, and Power Production Based on Residual Biomass Gasification: Energy, Exergy, and Renewability Assessment of an Alternative Process Configuration. Energies 2022, 15, 5524. [Google Scholar] [CrossRef]
- Barbot, Y.N.; Al-Ghaili, H.; Benz, R. A Review on the Valorization of Macroalgal Wastes for Biomethane Production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef]
- Singh, J.; Gu, S. Commercialization Potential of Microalgae for Biofuels Production. Renew. Sustain. Energy Rev. 2010, 14, 2596–2610. [Google Scholar] [CrossRef]
- Bird, K.T.; Chynoweth, D.P.; Jerger, D.E. Effects of marine algal proximate composition on methane yields. J. Appl. Phycol. 1990, 2, 207–213. [Google Scholar] [CrossRef]
- Chynoweth, D.P. Renewable Biomethane from Land and Ocean Energy Crops and Organic Wastes. HortScience 2005, 40, 283–286. [Google Scholar] [CrossRef]
- Enquist-Newman, M.; Faust, A.M.E.; Bravo, D.D.; Santos, C.N.S.; Raisner, R.M.; Hanel, A.; Sarvabhowman, P.; Le, C.; Regitsky, D.D.; Cooper, S.R.; et al. Efficient Ethanol Production from Brown Seaweeds Sugars by a Synthetic Yeast Platform. Nature 2014, 505, 239–243. [Google Scholar] [CrossRef]
- Rodriguez, C.; Alaswad, A.; Mooney, J.; Prescott, T.; Olabi, A.G. Pre-Treatment Techniques Used for Anaerobic Digestion of Algae. Fuel Process. Technol. 2015, 138, 765–779. [Google Scholar] [CrossRef]
- Alvarado-Morales, M.; Boldrin, A.; Karakashev, D.B.; Holdt, S.L.; Angelidaki, I.; Astrup, T. Life Cycle Assessment of Biofuel Production from Brown Seaweed in Nordic Conditions. Bioresour. Technol. 2013, 129, 92–99. [Google Scholar] [CrossRef]
- Robledo, D.; Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Vásquez-Elizondo, R.M.; Qui-Minet, Z.N.; Salazar-Garibay, A. Challenges and Opportunities in Relation to Sargassum Events Along the Caribbean Sea. Front. Mar. Sci. 2021, 8, 699664. [Google Scholar] [CrossRef]
- FAO Publication. Report of the Expert Meeting on Food Safety for Seaweed—Current Status and Future Perspectives; FAO Publication: Rome, Italy, 2021; ISBN 978-92-5-136590-8. [Google Scholar]
- West, J.; Calumpong, H.P.; Martin, G. World Ocean Assessment of the Regular Process, Chapter 14 Seaweeds. United Nations. Available online: https://www.un.org/depts/los/global_reporting/WOA_RPROC/Chapter_14.pdf (accessed on 7 October 2022).
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-Year Retrospective Review of Global Aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef]
- FAO Publication. La Situation Mondiale des Pêches et de L’aquaculture 2020. 2020. Available online: https://www.fao.org/documents/card/en/c/ca9229fr (accessed on 15 August 2022).
- Cai, J. Global Status of Seaweed Production, Trade and Utilization. Seaweed Innovation Forum Belize 28 May 2021. Available online: https://www.competecaribbean.org/wp-content/uploads/2021/05/Global-status-of-seaweed-production-trade-and-utilization-Junning-Cai-FAO.pdf (accessed on 7 October 2022).
- FAO Publication. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development; FAO: Rome, Italy, 2021; ISBN 978-92-5-134710-2. Available online: https://www.fao.org/documents/card/fr/c/cb5670en/ (accessed on 7 October 2022).
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-Gómez, M.T.; Piiparinen, J.; Spilling, K.; Yang, B. European Union Legislation on Seaweeds Products. Aquac. Int. 2021, 29, 487–509. [Google Scholar] [CrossRef]
- Mac Monagail, M.; Cornish, L.; Morrison, L.; Araújo, R.; Critchley, A.T. Sustainable Harvesting of Wild Seaweed Resources. Eur. J. Phycol. 2017, 52, 371–390. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Troy, D.J. Chapter 1—Seaweed Sustainability—Food and Nonfood Applications. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1–6. ISBN 978-0-12-418697-2. [Google Scholar]
- Alemañ, A.E.; Robledo, D.; Hayashi, L. Development of Seaweed Cultivation in Latin America: Current Trends and Future Prospects. Phycologia 2019, 58, 462–471. [Google Scholar] [CrossRef]
- Duinker, A.; Kleppe, M.; Fjaere, E.; Biancarosa, I.; Heldal, H.E.; Dahl, L.; Lunestad, B.T. Knowledge Update on Seaweeds Food and Feed Safety; Institute of Marine Research: Bergen, Norway, 2020. [Google Scholar] [CrossRef]
- Algae Technology & Innovation Center. Edible Seaweed and Microalgae—Regulatory Status in France and Europe. Available online: https://www.ceva-algues.com/wp-content/uploads/2020/03/ (accessed on 7 October 2022).
- Smetacek, V.; Zingone, A. Green and Golden Seaweed Tides on the Rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.; Harvey, P. Golden Tides: Problem or Golden Opportunity? The Valorisation of Sargassum from Beach Inundations. J. Mar. Sci. Eng. 2016, 4, 60. [Google Scholar] [CrossRef]
- Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Salazar-Garibay, A.; Serviere-Zaragoza, E.; Méndez-Rodríguez, L.C.; Robledo, D. Species Composition and Chemical Characterization of Sargassum Influx at Six Different Locations along the Mexican Caribbean Coast. Sci. Total Environ. 2021, 795, 148852. [Google Scholar] [CrossRef]
- Bruton, T.; Lyons, H.; Lerat, Y.; Stanley, M.; Rasmussen, M.B. A Review of the Potential of Marine Algae as a Source of Biofuel in Ireland. Sustain. Energy Ireland 2009, 1–88. [Google Scholar]
- Charlier, R.H.; Morand, P.; Finkl, C.W.; Thys, A. Green Tides on the Brittany Coasts. In Proceedings of the 2006 IEEE US/EU Baltic International Symposium, Klaipeda, Lithuania, 23–26 May 2006; pp. 1–13. [Google Scholar]
- Kopp, J. Etude Du Phénomène de Marée Verte Affectant Les Baies de Lannion et de Saint-Brieuc. II—Complément d’étude Portant Sur Les Prédateurs Éventuels de l’algue Verte Ulva lactuca. 18. Available online: https://archimer.ifremer.fr/doc/00018/12925/9887.pdf (accessed on 15 August 2022).
- Pillard, S. Mise au Point sur les Algues Vertes: Risques Environnementaux et Valorisations en 2016. Ph.D. Thesis, Université de Picardie Jules Verne, Amiens, France, 2016. [Google Scholar]
- Peu, P. La Gestion Des Effluents d’élevage et La Production d’hydrogène Sulfuré, Cas Particulier de La Méthanisation. Ph.D. Thesis, Université de Rennes 1, Rennes, France, 2011. [Google Scholar]
- Allen, E.; Wall, D.M.; Herrmann, C.; Murphy, J.D. Investigation of the Optimal Percentage of Green Seaweed That May Be Co-Digested with Dairy Slurry to Produce Gaseous Biofuel. Bioresour. Technol. 2014, 170, 436–444. [Google Scholar] [CrossRef]
- Peu, P.; Sassi, J.-F.; Girault, R.; Picard, S.; Saint-Cast, P.; Béline, F.; Dabert, P. Sulphur Fate and Anaerobic Biodegradation Potential during Co-Digestion of Seaweed Biomass (Ulva sp.) with Pig Slurry. Bioresour. Technol. 2011, 102, 10794–10802. [Google Scholar] [CrossRef]
- Liu, D.; Keesing, J.K.; Xing, Q.; Shi, P. World’s Largest Macroalgal Bloom Caused by Expansion of Seaweed Aquaculture in China. Mar. Pollut. Bull. 2009, 58, 888–895. [Google Scholar] [CrossRef]
- Ye, N.; Zhang, X.; Mao, Y.; Liang, C.; Xu, D.; Zou, J.; Zhuang, Z.; Wang, Q. ‘Green Tides’ Are Overwhelming the Coastline of Our Blue Planet: Taking the World’s Largest Example. Ecol. Res. 2011, 26, 477–485. [Google Scholar] [CrossRef]
- Davies, P. Michoacán Biogas Firm Turns to Sargassum for a New Source. Mex. News Dly. 2022. Available online: https://www.bioenergy-news.com/news/michoacan-biogas-firm-turns-to-sargassum-as-new-source/ (accessed on 3 October 2022).
- Aparicio, E.; Rodríguez-Jasso, R.M.; Lara, A.; Loredo-Treviño, A.; Aguilar, C.N.; Kostas, E.T.; Ruiz, H.A. Chapter 15—Biofuels Production of Third Generation Biorefinery from Macroalgal Biomass in the Mexican Context: An Overview. In Sustainable Seaweed Technologies; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Advances in Green and Sustainable Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 393–446. ISBN 978-0-12-817943-7. [Google Scholar]
- Fleurence, J.; Levine, I.A. Seaweed in Health and Disease Prevention; Elsevier: London, UK; Academic Press: San Diego, CA, USA, 2016; ISBN 978-0-12-802772-1. [Google Scholar]
- Vigie-Nature école. Alamer Bretagne Livret du Participant. Available online: https://depot.vigienature-ecole.fr/ressources/livrets_profs/Alamer_Bretagne.pdf (accessed on 7 October 2022).
- Bourgougnon, N.; Gervois, A. Les Algues Marines: Biologie, Écologie et Utilisation; Ellipses: Paris, France, 2021; ISBN 978-2-340-05654-1. [Google Scholar]
- Usov, A.I.; Smirnova, G.P.; Klochkova, N.G. Polysaccharides of Algae: 55. Polysaccharide Composition of Several Brown Algae from Kamchatka. Russ. J. Bioorg. Chem. 2001, 27, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The Seasonal Variation of Fucoidan within Three Species of Brown Seaweeds. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef]
- Fasahati, P.; Woo, H.C.; Liu, J.J. Industrial-Scale Bioethanol Production from Brown Algae: Effects of Pretreatment Processes on Plant Economics. Appl. Energy 2015, 139, 175–187. [Google Scholar] [CrossRef]
- Desrochers, A.; Cox, S.-N.; Oxenford, H.A.; van Tussenbroek, B. Sargassum Uses Guide: A Resource for Caribbean Researchers, Entrepreneurs and Policy Makers. Available online: https://www.cavehill.uwi.edu/cermes/projects/sargassum/docs/desrochers_et_al_2020_sargassum_uses_guide_advance.aspx (accessed on 15 August 2022).
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Haneji, K.; Matsuda, T.; Tomita, M.; Kawakami, H.; Ohshiro, K.; Uchihara, J.-N.; Masuda, M.; Takasu, N.; Tanaka, Y.; Ohta, T.; et al. Fucoidan Extracted from Cladosiphon Okamuranus Tokida Induces Apoptosis of Human T-Cell Leukemia Virus Type 1-Infected T-Cell Lines and Primary Adult T-Cell Leukemia Cells. Nutr. Cancer 2005, 52, 189–201. [Google Scholar] [CrossRef]
- Teruya, T.; Konishi, T.; Uechi, S.; Tamaki, H.; Tako, M. Anti-Proliferative Activity of Oversulfated Fucoidan from Commercially Cultured Cladosiphon Okamuranus TOKIDA in U937 Cells. Int. J. Biol. Macromol. 2007, 41, 221–226. [Google Scholar] [CrossRef]
- Santoyo, S.; Plaza, M.; Jaime, L.; Ibañez, E.; Reglero, G.; Señorans, J. Pressurized Liquids as an Alternative Green Process to Extract Antiviral Agents from the Edible Seaweed Himanthalia Elongata. J. Appl. Phycol. 2011, 23, 909–917. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of Fucoidan from Sargassum Polycystum Brown Algae: Structural Characterization, in Vitro Antioxidant and Anticancer Activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef]
- Pang, Z.; Otaka, K.; Maoka, T.; Hidaka, K.; Ishijima, S.; Oda, M.; Ohnishi, M. Structure of β-Glucan Oligomer from Laminarin and Its Effect on Human Monocytes to Inhibit the Proliferation of U937 Cells. Biosci. Biotechnol. Biochem. 2005, 69, 553–558. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. A Practical Perspective on Ulvan Extracted from Green Algae. J. Appl. Phycol. 2013, 25, 407–424. [Google Scholar] [CrossRef]
- Morelli, A.; Chiellini, F. Ulvan as a New Type of Biomaterial from Renewable Resources: Functionalization and Hydrogel Preparation: Ulvan as a New Type of Biomaterial from Renewable Resources: Functionalization. Macromol. Chem. Phys. 2010, 211, 821–832. [Google Scholar] [CrossRef]
- Wong, K.H.; Cheung, P.C.K. Nutritional Evaluation of Some Subtropical Red and Green Seaweeds Part I—Proximate Composition, Amino Acid Pro®les and Some Physico-Chemical Properties. Food Chem. 2000, 71, 475–482. [Google Scholar] [CrossRef]
- Chen, H. Seaweeds for Biofuels Production Progress and Perspectives. Renew. Sustain. Energy Rev. 2015, 11, 427–437. [Google Scholar] [CrossRef]
- Kumar, S.; Sahoo, D.; Levine, I. Assessment of Nutritional Value in a Brown Seaweed Sargassum wightii and Their Seasonal Variations. Algal Res. 2015, 9, 117–125. [Google Scholar] [CrossRef]
- Jard, G.; Marfaing, H.; Carrère, H.; Delgenes, J.P.; Steyer, J.P.; Dumas, C. French Brittany Seaweeds Screening: Composition and Methane Potential for Potential Alternative Sources of Energy and Products. Bioresour. Technol. 2013, 144, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Padam, B.S.; Chye, F.Y. Chapter 2—Seaweed Components, Properties, and Applications. In Sustainable Seaweed Technologies; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Advances in Green and Sustainable Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 33–87. ISBN 978-0-12-817943-7. [Google Scholar]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.-P.; Carrère, H. Do Furanic and Phenolic Compounds of Lignocellulosic and Algae Biomass Hydrolyzate Inhibit Anaerobic Mixed Cultures? A Comprehensive Review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef]
- Jensen, A. Present and Future Needs for Algae and Algal Products. Hydrobiologia 1993, 260, 15–23. [Google Scholar] [CrossRef]
- Baghel, R.S.; Mantri, V.A.; Reddy, C.R.K. A New Wave of Research Interest in Marine Seaweeds for Chemicals and Fuels: Challenges and Potentials. In Fuels, Chemicals and Materials from the Oceans and Aquatic Sources; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 43–63. ISBN 978-1-119-11719-3. [Google Scholar]
- Library of French Environment and Energy Management Agency (ADEME). Évaluation Du Gisement Potentiel de Ressources Algales Pour l’énergie et La Chimie En France à Horizon 2030. Available online: https://librairie.ademe.fr/produire-autrement/3062-evaluation-du-gisement-potentiel-de-ressources-algales-pour-l-energie-et-la-chimie-en-france-a-horizon-2030.html (accessed on 14 September 2022).
- Aizawa, M.; Asaoka, K.; Atsumi, M.; Sakou, T. Seaweed Bioethanol Production in Japan—The Ocean Sunrise Project. In Proceedings of the OCEANS 2007, Vancouver, BC, Canada, 29 September–4 October 2007; pp. 1–5. [Google Scholar]
- Milledge, J.; Smith, B.; Dyer, P.; Harvey, P. Seaweeds-Derived Biofuel: A Review of Methods of Energy Extraction from Seaweed Biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Kumar, A. A Short Review on Biobutanol, a Second-Generation Biofuel Production from Lignocellulosic Biomass. J. Clean Energy Technol. 2017, 5, 27–30. [Google Scholar] [CrossRef]
- Huesemann, M.H.; Kuo, L.-J.; Urquhart, L.; Gill, G.A.; Roesijadi, G. Acetone-Butanol Fermentation of Marine Seaweeds. Bioresour. Technol. 2012, 108, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Tobío-Pérez, I.; Alfonso-Cardero, A.; Díaz-Domínguez, Y.; Pohl, S.; Piloto-Rodríguez, R.; Lapuerta, M. Thermochemical Conversion of Sargassum for Energy Production: A Comprehensive Review. BioEnergy Res. 2022, 15, 1872–1893. [Google Scholar] [CrossRef]
- Yanik, J.; Stahl, R.; Troeger, N.; Sinag, A. Pyrolysis of Algal Biomass. J. Anal. Appl. Pyrolysis 2013, 103, 134–141. [Google Scholar] [CrossRef]
- Suganya, T.; Renganathan, S. Optimization and Kinetic Studies on Algal Oil Extraction from Marine Seaweeds Ulva lactuca. Bioresour. Technol. 2012, 107, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Suganya, T.; Nagendra Gandhi, N.; Renganathan, S. Production of Algal Biodiesel from Marine Seaweeds Enteromorpha compressa by Two Step Process: Optimization and Kinetic Study. Bioresour. Technol. 2013, 128, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Moletta, R. La Méthanisation, 2nd ed.; Lavoisier: Paris, France, 2011; ISBN 978-2-7430-1271-7. [Google Scholar]
- Osman, M.M.M.; Shao, X.; Zhao, D.; Basheer, A.K.; Jin, H.; Zhang, Y. Methane Production from Alginate-Extracted and Non-Extracted Waste of Laminaria Japonica: Anaerobic Mono- and Synergetic Co-Digestion Effects on Yield. Sustainability 2019, 17, 1269. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; van Lier, J.B. Defining the Biomethane Potential (BMP) of Solid Organic Wastes and Energy Crops: A Proposed Protocol for Batch Assays. Water Sci. 2009, 8, 927–934. [Google Scholar] [CrossRef]
- Gruduls, A.; Maurers, R.; Romagnoli, F. Baltic Sea Seaweed Biomass Pretreatment: Effect of Combined CO2 and Thermal Treatment on Biomethane Potential. Energy Procedia 2018, 147, 607–613. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Kong, X.; Zhen, F.; Wang, Z.; Sun, Y.; Dong, P.; Lv, P. Inhibition Effect of Sodium Concentrations on the Anaerobic Digestion Performance of Sargassum Species. Energy Fuels 2017, 31, 7101–7109. [Google Scholar] [CrossRef]
- Bird, K.T.; Hanisak, M.D.; Ryther, J.H. Changes in Agar and Other Chemical Constituents of the Seaweed Gracilaria Tikvahiae When Used as a Substrate in Methane Digesters. Resour. Conserv. 1981, 6, 321–327. [Google Scholar] [CrossRef]
- Feijoo, G.; Soto, M.; Méndez, R.; Lema, J.M. Sodium Inhibition in the Anaerobic Digestion Process: Antagonism and Adaptation Phenomena. Enzym. Microb. Technol. 1995, 17, 180–188. [Google Scholar] [CrossRef]
- Stams, A.J.M.; Plugge, C.M.; de Bok, F.A.M.; van Houten, B.H.G.W.; Lens, P.; Dijkman, H.; Weijma, J. Metabolic Interactions in Methanogenic and Sulfate-Reducing Bioreactors. Water Sci. Technol. 2005, 52, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Hao, O.J.; Chen, J.M.; Huang, L.; Buglass, R.L. Sulfate-reducing Bacteria. Crit. Rev. Environ. Sci. Technol. 1996, 26, 155–187. [Google Scholar] [CrossRef]
- Peu, P.; Picard, S.; Diara, A.; Girault, R.; Béline, F.; Bridoux, G.; Dabert, P. Prediction of Hydrogen Sulphide Production during Anaerobic Digestion of Organic Substrates. Bioresour. Technol. 2012, 121, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Wellinger, A.; Lindberg, A. Biogas Upgrading IEA Bioenergy. Available online: http://www.iea-biogas.net/files/daten-redaktion/download/publi-task37/Biogas%20upgrading.pdf (accessed on 3 October 2022).
- Lahaye, M.; Robic, A. Structure and Functional Properties of Ulvan, a Polysaccharide from Green Seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Ghadiryanfar, M.; Rosentrater, K.A.; Keyhani, A.; Omid, M. A Review of Seaweeds Production, with Potential Applications in Biofuels and Bioenergy. Renew. Sustain. Energy Rev. 2016, 54, 473–481. [Google Scholar] [CrossRef]
- Raposo, F.; Borja, R.; Rincon, B.; Jimenez, A.M. Assessment of Process Control Parameters in the Biochemical Methane Potential of Sunflower Oil Cake. Biomass Bioenergy 2008, 32, 1235–1244. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; de Wilde, V.; et al. Towards a Standardization of Biomethane Potential Tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Chynoweth, D.P.; Turick, C.E.; Owens, J.M.; Jerger, D.E.; Peck, M.W. Biochemical Methane Potential of Biomass and Waste Feedstocks. Biomass Bioenergy 1993, 5, 95–111. [Google Scholar] [CrossRef]
- Zeng, S.; Yuan, X.; Shi, X.; Qiu, Y. Effect of Inoculum/Substrate Ratio on Methane Yield and Orthophosphate Release from Anaerobic Digestion of Microcystis spp. J. Hazard. Mater. 2010, 178, 89–93. [Google Scholar] [CrossRef]
- Costa, J.C.; Gonçalves, P.R.; Nobre, A.; Alves, M.M. Biomethanation Potential of Seaweeds Ulva spp. and Gracilaria spp. and in Co-Digestion with Waste Activated Sludge. Bioresour. Technol. 2012, 114, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.C.; Oliveira, J.V.; Pereira, M.A.; Alves, M.M.; Abreu, A.A. Biohythane Production from Marine Seaweeds Sargassum sp. Coupling Dark Fermentation and Anaerobic Digestion. Bioresour. Technol. 2015, 190, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Pierra, M.; Trably, E.; Godon, J.-J.; Bernet, N. Fermentative Hydrogen Production under Moderate Halophilic Conditions. Int. J. Hydrogen Energy 2014, 39, 7508–7517. [Google Scholar] [CrossRef]
- Kamran, M. Chapter 8—Bioenergy. In Renewable Energy Conversion Systems; Kamran, M., Fazal, M.R., Eds.; Academic Press: Amsterdam, The Netherlands, 2021; pp. 243–264. ISBN 978-0-12-823538-6. [Google Scholar]
- Cheonh, P.Y.Y.; Kansedo, J.; Lau, J.S.Y.; Tan, Y.H. 5.13—Renewable Biomass Wastes for Biohydrogen Production. In Comprehensive Renewable Energy, 2nd ed.; Letcher, T.M., Ed.; Elsevier: Oxford, UK, 2022; pp. 273–298. ISBN 978-0-12-819734-9. [Google Scholar]
- Antonopoulou, G.; Ntaikou, I.; Stamatelatou, K.; Lyberatos, G. 13—Biological and Fermentative Production of Hydrogen. In Handbook of Biofuels Production; Luque, R., Campelo, J., Clark, J., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Cambridge, UK, 2011; pp. 305–346. ISBN 978-1-84569-679-5. [Google Scholar]
- Dauptain, K. Impact des Communautés Microbiennes et des Prétraitements de la Matière Organique sur les Performances de la Fermentation Sombre. Ph.D. Thesis, Université de Montpellier, Montpellier, France, 2021. [Google Scholar]
- Castelló, E. Stability Problems in the Hydrogen Production by Dark Fermentation: Possible Causes and Solutions. Renew. Sustain. Energy Rev. 2020, 16, 109602. [Google Scholar] [CrossRef]
- Paillet, F. Optimisation d’un Procédé à Deux étapes pour la Production d’un Mélange Hydrogène/Méthane (Biohythane) à Partir de la Fraction Fermentescible des Ordures Ménagères. Ph.D. Thesis, Université de Montpellier, Montpellier, France, 2017. [Google Scholar]
- Radha, M.; Murugesan, A.G. Enhanced Dark Fermentative Biohydrogen Production from Marine Seaweeds Padina Tetrastromatica by Different Pretreatment Processes. Biofuel Res. J. 2017, 4, 551–558. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, J.; Xia, A.; Jacob, A.; Voelklein, M.; Murphy, J.D. Co-Generation of Biohydrogen and Biomethane through Two-Stage Batch Co-Fermentation of Macro- and Micro-Algal Biomass. Bioresour. Technol. 2016, 218, 224–231. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, J.; Wang, J. Enriching Hydrogen-Producing Bacteria from Digested Sludge by Different Pretreatment Methods. Int. J. Hydrogen Energy 2014, 39, 13550–13556. [Google Scholar] [CrossRef]
- Ljunggren, M.; Zacchi, G. Techno-Economic Analysis of a Two-Step Biological Process Producing Hydrogen and Methane. Bioresour. Technol. 2010, 101, 7780–7788. [Google Scholar] [CrossRef]
- Bolzonella, D. Recent Developments in Biohythane Production from Household Food Wastes—A Review. Bioresour. Technol. 2018, 9, 311–319. [Google Scholar] [CrossRef]
- Calderón, C.; Avagianos, I.; Jossart, J.-M. Biogas—Bioenergy Europe. Available online: https://bioenergyeurope.org/article.html/309 (accessed on 14 September 2022).
- Liu, S.; Yang, Y.; Yu, L.; Li, X. Thermodynamic and Environmental Analysis of Solar-Driven Supercritical Water Gasification of Algae for Ammonia Synthesis and Power Production. Energy Convers. Manag. 2021, 243, 114409. [Google Scholar] [CrossRef]
- Kumar, M.; Oyedun, A.O.; Kumar, A. A Comparative Analysis of Hydrogen Production from the Thermochemical Conversion of Algal Biomass. Int. J. Hydrogen Energy 2019, 44, 10384–10397. [Google Scholar] [CrossRef]
- Liu, J.J.; Dickson, R.; Niaz, H.; Van Hal, J.W.; Dijkstra, J.W.; Fasahati, P. Production of Fuels and Chemicals from Macroalgal Biomass: Current Status, Potentials, Challenges, and Prospects. Renew. Sustain. Energy Rev. 2022, 169, 112954. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L.; Jones, D.D.; Hanna, M.A. Contemporary Issues in Thermal Gasification of Biomass and Its Application to Electricity and Fuel Production. Biomass Bioenergy 2008, 32, 573–581. [Google Scholar] [CrossRef]
- Azadi, P.; Brownbridge, G.P.E.; Mosbach, S.; Inderwildi, O.R.; Kraft, M. Production of Biorenewable Hydrogen and Syngas via Algae Gasification: A Sensitivity Analysis. Energy Procedia 2014, 61, 2767–2770. [Google Scholar] [CrossRef]
- Rahbari, A.; Venkataraman, M.B.; Pye, J. Energy and Exergy Analysis of Concentrated Solar Supercritical Water Gasification of Algal Biomass. Appl. Energy 2018, 228, 1669–1682. [Google Scholar] [CrossRef]
- Raheem, A.; Wan Azlina, W.A.K.G.; Taufiq Yap, Y.H.; Danquah, M.K.; Harun, R. Optimization of the Microalgae Chlorella Vulgaris for Syngas Production Using Central Composite Design. RSC Adv. 2015, 5, 71805–71815. [Google Scholar] [CrossRef]
- Brandenberger, M.; Matzenberger, J.; Vogel, F.; Ludwig, C. Producing Synthetic Natural Gas from Microalgae via Supercritical Water Gasification: A Techno-Economic Sensitivity Analysis. Biomass Bioenergy 2013, 51, 26–34. [Google Scholar] [CrossRef]
- Farobie, O.; Syaftika, N.; Masfuri, I.; Rini, T.P.; Lanank Es, D.P.A.; Bayu, A.; Amrullah, A.; Hartulistiyoso, E.; Moheimani, N.R.; Karnjanakom, S.; et al. Green Algae to Green Fuels: Syngas and Hydrochar Production from Ulva lactuca via Sub-Critical Water Gasification. Algal Res. 2022, 67, 102834. [Google Scholar] [CrossRef]
- Faraji, M.; Saidi, M. Hydrogen-Rich Syngas Production via Integrated Configuration of Pyrolysis and Air Gasification Processes of Various Algal Biomass: Process Simulation and Evaluation Using Aspen Plus Software. Int. J. Hydrogen Energy 2021, 46, 18844–18856. [Google Scholar] [CrossRef]
- Kumar, M.D.; Kavitha, S.; Tyagi, V.K.; Rajkumar, M.; Bhatia, S.K.; Kumar, G.; Banu, J.R. Seaweeds-Derived Biohydrogen Production: Biorefinery and Circular Bioeconomy. Biomass Convers. Biorefinery 2022, 12, 769–791. [Google Scholar] [CrossRef]
- Barbot, Y.N.; Thomsen, C.; Thomsen, L.; Benz, R. Anaerobic Digestion of Laminaria Japonica Waste from Industrial Production Residues in Laboratory- and Pilot-Scale. Mar. Drugs 2015, 13, 5947–5975. [Google Scholar] [CrossRef] [PubMed]
- Barbot, Y.N.; Falk, H.M.; Benz, R. Thermo-Acidic Pretreatment of Marine Brown Algae Fucus Vesiculosus to Increase Methane Production—A Disposal Principle for Seaweeds Waste from Beaches. J. Appl. Phycol. 2015, 27, 601–609. [Google Scholar] [CrossRef]
- Aslanzadeh, S.; Ishola, M.M.; Richards, T.; Taherzadeh, M.J. Chapter 1—An Overview of Existing Individual Unit Operations. In Biorefineries; Qureshi, N., Hodge, D.B., Vertès, A.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–36. ISBN 978-0-444-59498-3. [Google Scholar]
- Moodley, P.; Trois, C. 2—Lignocellulosic Biorefineries: The Path Forward. In Sustainable Biofuels; Ray, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 21–42. ISBN 978-0-12-820297-5. Applied Biotechnology Reviews. [Google Scholar]
- Palmowski, L.M.; Müller, J.A. Influence of the Size Reduction of Organic Waste on Their Anaerobic Digestion. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2000, 41, 155–162. [Google Scholar] [CrossRef]
- Briand, X.; Morand, P. Anaerobic Digestion of Ulva sp. 1. Relationship between Ulva Composition and Methanisation. J. Appl. Phycol. 1997, 9, 511–524. [Google Scholar]
- Sambusiti, C.; Bellucci, M.; Zabaniotou, A.; Beneduce, L.; Monlau, F. Algae as Promising Feedstocks for Fermentative Biohydrogen Production According to a Biorefinery Approach: A Comprehensive Review. Renew. Sustain. Energy Rev. 2015, 44, 20–36. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kumar, G.; Kobayashi, T.; Xu, K.; Kim, S.-H. Effects of Various Dilute Acid Pretreatments on the Biochemical Hydrogen Production Potential of Marine Macroalgal Biomass. Int. J. Hydrogen Energy 2017, 42, 27600–27606. [Google Scholar] [CrossRef]
- Kavitha, S.; Stella, P.B.C.; Kaliappan, S.; Yeom, I.T.; Banu, J.R. Enhancement of Anaerobic Degradation of Sludge Biomass through Surfactant-Assisted Bacterial Hydrolysis. Process Saf. Environ. Prot. 2016, 99, 207–215. [Google Scholar] [CrossRef]
- Ferdeș, M.; Dincă, M.N.; Moiceanu, G.; Zăbavă, B.Ș.; Paraschiv, G. Microorganisms and Enzymes Used in the Biological Pretreatment of the Substrate to Enhance Biogas Production: A Review. Sustainability 2020, 12, 7205. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, J.; Lin, R.; Deng, C.; Zhou, J.; Murphy, J.D. Improving Biohydrogen and Biomethane Co-Production via Two-Stage Dark Fermentation and Anaerobic Digestion of the Pretreated Seaweed Laminaria Digitata. J. Clean. Prod. 2020, 251, 119666. [Google Scholar] [CrossRef]
- Passos, F.; Hom-Diaz, A.; Blanquez, P.; Vicent, T.; Ferrer, I. Improving Biogas Production from Microalgae by Enzymatic Pretreatment. Bioresour. Technol. 2016, 199, 347–351. [Google Scholar] [CrossRef]
- Chikani-Cabrera, K.D.; Fernandes, P.M.B.; Tapia-Tussell, R.; Parra-Ortiz, D.L.; Hernández-Zárate, G.; Valdez-Ojeda, R.; Alzate-Gaviria, L. Improvement in Methane Production from Pelagic Sargassum Using Combined Pretreatments. Life 2022, 12, 1214. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, J. Hydrogen Production and Energy Recovery from Seaweeds Saccharina Japonica by Different Pretreatment Methods. Renew. Energy 2019, 141, 1–8. [Google Scholar] [CrossRef]
- Du, B.; Sharma, L.N.; Becker, C.; Chen, S.-F.; Mowery, R.A.; van Walsum, G.P.; Chambliss, C.K. Effect of Varying Feedstock-Pretreatment Chemistry Combinations on the Formation and Accumulation of Potentially Inhibitory Degradation Products in Biomass Hydrolysates. Biotechnol. Bioeng. 2010, 107, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulos, I.A.; Bakker, R.R.; de Vrije, T.; Koukios, E.G. Effect of Pretreatment Severity on the Conversion of Barley Straw to Fermentable Substrates and the Release of Inhibitory Compounds. Bioresour. Technol. 2011, 102, 11204–11211. [Google Scholar] [CrossRef]
- Naseeruddin, S.; Srilekha Yadav, K.; Sateesh, L.; Manikyam, A.; Desai, S.; Venkateswar Rao, L. Selection of the Best Chemical Pretreatment for Lignocellulosic Substrate Prosopis Juliflora. Bioresour. Technol. 2013, 136, 542–549. [Google Scholar] [CrossRef]
- Monlau, F.; Barakat, A.; Steyer, J.P.; Carrere, H. Comparison of Seven Types of Thermo-Chemical Pretreatments on the Structural Features and Anaerobic Digestion of Sunflower Stalks. Bioresour. Technol. 2012, 120, 241–247. [Google Scholar] [CrossRef]
- Taherzadeh, M.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Park, J.-H.; Cheon, H.-C.; Yoon, J.-J.; Park, H.-D.; Kim, S.-H. Optimization of Batch Dilute-Acid Hydrolysis for Biohydrogen Production from Red Algal Biomass. Int. J. Hydrogen Energy 2013, 38, 6130–6136. [Google Scholar] [CrossRef]
- Hierholtzer, A.; Chatellard, L.; Kierans, M.; Akunna, J.c.; Collier, P.J. The Impact and Mode of Action of Phenolic Compounds Extracted from Brown Seaweed on Mixed Anaerobic Microbial Cultures. J. Appl. Microbiol. 2013, 114, 964–973. [Google Scholar] [CrossRef]
- Quéméneur, M. Inhibition of Fermentative Hydrogen Production by Lignocellulose-Derived Compounds in Mixed Cultures. Int. J. Hydrogen Energy 2012, 37, 3150–3159. [Google Scholar] [CrossRef]
- Wierckx, N.; Koopman, F.; Ruijssenaars, H.J.; de Winde, J.H. Microbial Degradation of Furanic Compounds: Biochemistry, Genetics, and Impact. Appl. Microbiol. Biotechnol. 2011, 92, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, B.; Ezeji, T.C. Biotransformation of Furfural and 5-Hydroxymethyl Furfural (HMF) by Clostridium acetobutylicum ATCC 824 during Butanol Fermentation. New Biotechnol. 2012, 29, 345–351. [Google Scholar] [CrossRef] [PubMed]
- von Sivers, M.; Zacchi, G.; Olsson, L.; Hahn-Haegerdal, B. Cost Analysis of Ethanol Production from Willow Using Recombinant Escherichia Coli. Biotechnol. Prog. 1994, 10, 555–560. [Google Scholar] [CrossRef]
- Chamaa, M.A. Couplage de la Méthanisation et des électrotechnologies: Intentisification de la Production de Biogaz et du Séchage du Digestat. Ph.D. Thesis, Université de Bretagne Sud, Lorient, France, 2017. [Google Scholar]
- Cazier, E.A.; Trably, E.; Steyer, J.P.; Escudie, R. Biomass Hydrolysis Inhibition at High Hydrogen Partial Pressure in Solid-State Anaerobic Digestion. Bioresour. Technol. 2015, 190, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Cresson, R. Etude du Démarrage de Procédés Intensifs de Méthanisation. Impact des Conditions Hydrodynamiques et de la Stratégie de Montée en Charge sur la Formation et l’activité du Biofilm. Ph.D. Thesis, Université Montpellier 2, Montpellier, France, 2006. [Google Scholar]
- Vanegas, C.H.; Bartlett, J. Green Energy from Marine Algae: Biogas Production and Composition from the Anaerobic Digestion of Irish Seaweed Species. Environ. Technol. 2013, 34, 2277–2283. [Google Scholar] [CrossRef]
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S.; Olesen, B.; Arias, C.; Jensen, P.D. Bioenergy Potential of Ulva lactuca: Biomass Yield, Methane Production and Combustion. Bioresour. Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef]
- Allen, E.; Browne, J.; Hynes, S.; Murphy, J.D. The Potential of Algae Blooms to Produce Renewable Gaseous Fuel. Waste Manag. 2013, 33, 2425–2433. [Google Scholar] [CrossRef]
- Herrmann, C. Ensiling of Seaweed for a Seaweed Biofuel Industry. Bioresour. Technol. 2015, 13, 301–313. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Heiske, S. Anaerobic Digestion of Seaweeds: Methane Potentials, Pre-Treatment, Inhibition and Co-Digestion. Water Sci. Technol. 2011, 64, 1723–1729. [Google Scholar] [CrossRef]
- Tedesco, S.; Benyounis, K.Y.; Olabi, A.G. Mechanical Pretreatment Effects on Seaweeds-Derived Biogas Production in Co-Digestion with Sludge in Ireland. Energy 2013, 61, 27–33. [Google Scholar] [CrossRef]
- Oliveira, J.V.; Alves, M.M.; Costa, J.C. Design of Experiments to Assess Pre-Treatment and Co-Digestion Strategies That Optimize Biogas Production from Seaweeds Gracilaria Vermiculophylla. Bioresour. Technol. 2014, 162, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Jard, G.; Dumas, C.; Delgenes, J.P.; Marfaing, H.; Sialve, B.; Steyer, J.P.; Carrère, H. Effect of Thermochemical Pretreatment on the Solubilization and Anaerobic Biodegradability of the Red Macroalga Palmaria Palmata. Biochem. Eng. J. 2013, 79, 253–258. [Google Scholar] [CrossRef]
- Milledge, J.; Nielsen, B.; Sadek, M.; Harvey, P. Effect of Freshwater Washing Pretreatment on Sargassum muticum as a Feedstock for Biogas Production. Energies 2018, 11, 1771. [Google Scholar] [CrossRef]
- Soto, M.; Vázquez, M.A.; de Vega, A.; Vilariño, J.M.; Fernández, G.; de Vicente, M.E.S. Methane Potential and Anaerobic Treatment Feasibility of Sargassum muticum. Bioresour. Technol. 2015, 189, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Maneein, S.; Arribas López, E.; Bartlett, D. Sargassum Inundations in Turks and Caicos: Methane Potential and Proximate, Ultimate, Lipid, Amino Acid, Metal and Metalloid Analyses. Energies 2020, 13, 1523. [Google Scholar] [CrossRef]
- Hanssen, J.F.; Indergaard, M.; Østgaard, K.; Bævre, O.A.; Pedersen, T.A.; Jensen, A. Anaerobic Digestion of Laminaria spp. and Ascophyllum Nodosum and Application of End Products. Biomass 1987, 14, 1–13. [Google Scholar] [CrossRef]
- Montingelli, M.E. Influence of Mechanical Pretreatment and Organic Concentration of Irish Brown Seaweed for Methane Production. Energy 2017, 11, 1079–1089. [Google Scholar] [CrossRef]
- Yazdani, P.; Zamani, A.; Karimi, K.; Taherzadeh, M.J. Characterization of Nizimuddinia Zanardini Seaweeds Biomass Composition and Its Potential for Biofuel Production. Bioresour. Technol. 2015, 176, 196–202. [Google Scholar] [CrossRef]
- Edward, M.; Edwards, S.; Egwu, U.; Sallis, P. Bio-Methane Potential Test (BMP) Using Inert Gas Sampling Bags with Seaweeds Feedstock. Biomass Bioenergy 2015, 83, 516–524. [Google Scholar] [CrossRef]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Comparison of Pre-Treatments to Reduce Salinity and Enhance Biomethane Yields of Laminaria Digitata Harvested in Different Seasons. Energy 2017, 140, 546–551. [Google Scholar] [CrossRef]
- Vivekanand, V.; Eijsink, V.G.H.; Horn, S.J. Biogas Production from the Brown Seaweed Saccharina Latissima: Thermal Pretreatment and Codigestion with Wheat Straw. J. Appl. Phycol. 2012, 24, 1295–1301. [Google Scholar] [CrossRef]
- Rinzema, A.; van Lier, J.; Lettinga, G. Sodium Inhibition of Acetoclastic Methanogens in Granular Sludge from a UASB Reactor. Enzyme Microb. Technol. 1988, 10, 24–32. [Google Scholar] [CrossRef]
- Vivekanand, V.; Olsen, E.F.; Eijsink, V.G.H.; Horn, S.J. Effect of Different Steam Explosion Conditions on Methane Potential and Enzymatic Saccharification of Birch. Bioresour. Technol. 2013, 127, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Nuopponen, M.; Vuorinen, T.; Jämsä, S.; Viitaniemi, P. Thermal Modifications in Softwood Studied by FT-IR and UV Resonance Raman Spectroscopies. J. Wood Chem. Technol. 2005, 24, 13–26. [Google Scholar] [CrossRef]
- Kumar, M.D.; Tamilarasan, K.; Kaliappan, S.; Banu, J.R.; Rajkumar, M.; Kim, S.H. Surfactant Assisted Disperser Pretreatment on the Liquefaction of Ulva Reticulata and Evaluation of Biodegradability for Energy Efficient Biofuel Production through Nonlinear Regression Modelling. Bioresour. Technol. 2018, 255, 116–122. [Google Scholar] [CrossRef]
- Kumar, D.; Eswari, A.P.; Park, J.-H.; Adishkumar, S.; Banu, J.R. Biohydrogen Generation from Macroalgal Biomass, Chaetomorpha Antennina Through Surfactant Aided Microwave Disintegration. Front. Energy Res. 2019, 7, 78. [Google Scholar] [CrossRef]
- Park, J.-H.; Yoon, J.-J.; Park, H.-D.; Kim, Y.J.; Lim, D.J.; Kim, S.-H. Feasibility of Biohydrogen Production from Gelidium Amansii. Int. J. Hydrogen Energy 2011, 36, 13997–14003. [Google Scholar] [CrossRef]
- Shi, X.; Jung, K.-W.; Kim, D.-H.; Ahn, Y.-T.; Shin, H.-S. Direct Fermentation of Laminaria Japonica for Biohydrogen Production by Anaerobic Mixed Cultures. Int. J. Hydrogen Energy 2011, 36, 5857–5864. [Google Scholar] [CrossRef]
- Shi, X.; Kim, D.-H.; Shin, H.-S.; Jung, K.-W. Effect of Temperature on Continuous Fermentative Hydrogen Production from Laminaria Japonica by Anaerobic Mixed Cultures. Bioresour. Technol. 2013, 144, 225–231. [Google Scholar] [CrossRef]
- Park, J.-I.; Lee, J.; Sim, S.J.; Lee, J.-H. Production of Hydrogen from Marine Macro-Algae Biomass Using Anaerobic Sewage Sludge Microflora. Biotechnol. Bioprocess Eng. 2009, 14, 307–315. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, D.-H.; Kim, H.-W.; Shin, H.-S. Optimization of Combined (Acid + Thermal) Pretreatment for Fermentative Hydrogen Production from Laminaria Japonica Using Response Surface Methodology (RSM). Int. J. Hydrogen Energy 2011, 36, 9626–9631. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, D.-H.; Shin, H.-S. Fermentative Hydrogen Production from Laminaria Japonica and Optimization of Thermal Pretreatment Conditions. Bioresour. Technol. 2011, 102, 2745–2750. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.-Y.; Cho, S.-K.; Shin, H.-S.; Jung, K.-W. Application of an Electric Field for Pretreatment of a Feedstock (Laminaria Japonica) for Dark Fermentative Hydrogen Production. Biomass Bioenergy 2015, 72, 184–188. [Google Scholar] [CrossRef]
- Liu, H.; Wang, G. Fermentative Hydrogen Production from Macro-Algae Laminaria Japonica Using Anaerobic Mixed Bacteria. Int. J. Hydrogen Energy 2014, 39, 9012–9017. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Pelagic Sargassum for Energy and Fertiliser Production in the Caribbean: A Case Study on Barbados. Renew. Sustain. Energy Rev. 2020, 118, 109564. [Google Scholar] [CrossRef]
- Blanfuné, A. Le Changement Global en Méditerranée Nord Occidentale: Forêt de Cystoseires, de Sargasses, Encorbellement à Lithophyllum et Bloom d’Ostreopsis. Ph.D. Thesis, Aix-Marseille Université, Marseille, France, 2016. [Google Scholar]
- Martin, L. Pelagic Sargassum and Its Associated Mobile Fauna in the Caribbean, Gulf of Mexico, and Sargasso Sea. Master’s Thesis, Texas A&M University, College Station, TX, USA, 2016. [Google Scholar]
- Lee, R.E. Phycology, 4th ed.; Cambridge University Press: Cambridge, UK, 2008; Available online: http://deskuenvis.nic.in/pdf/PhycologyLee.pdf (accessed on 3 October 2022).
- French Environment and Energy Management Agency (ADEME en Guadeloupe). Algues Sargasses. Available online: https://guadeloupe.ademe.fr/expertises/algues-sargasses (accessed on 1 September 2022).
- French Environment and Energy Management Agency (ADEME en Martinique). Algues Sargasses. Available online: https://martinique.ademe.fr/expertises/algues-sargasses (accessed on 1 September 2022).
- Devault, D.A.; Modestin, E.; Cottereau, V.; Vedie, F.; Stiger-Pouvreau, V.; Pierre, R.; Coynel, A.; Dolique, F. The Silent Spring of Sargassum. Environ. Sci. Pollut. Res. 2021, 28, 15580–15583. [Google Scholar] [CrossRef]
- Vos, B.; Foursoff, W.; de Bruijn, L.; Bruijn, W. Coastal Seaweed Solutions. Available online: https://repository.tudelft.nl/islandora/object/uuid%3A4de9aa1b-a9a9-4dcb-bfef-82fe4ae0584c (accessed on 1 September 2022).
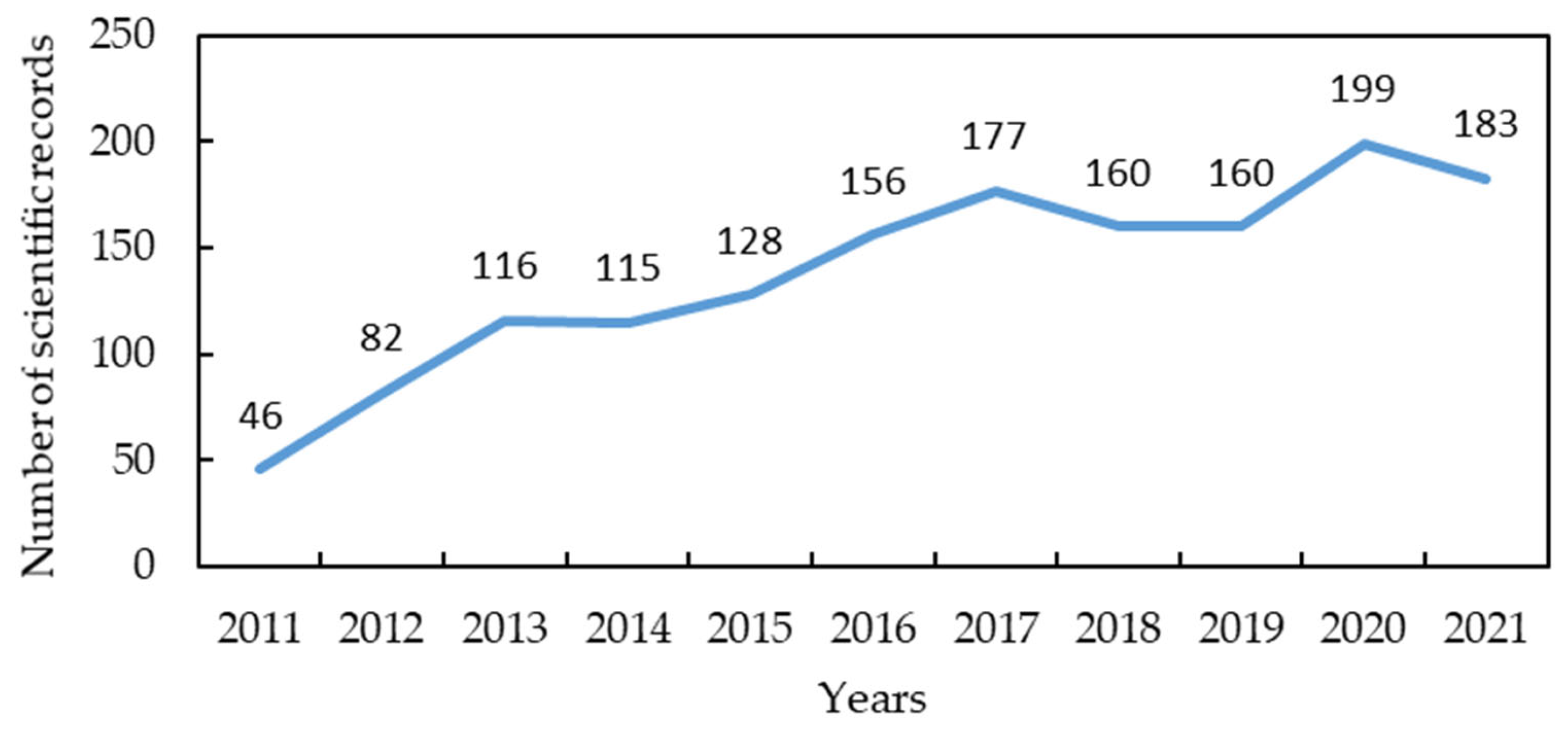

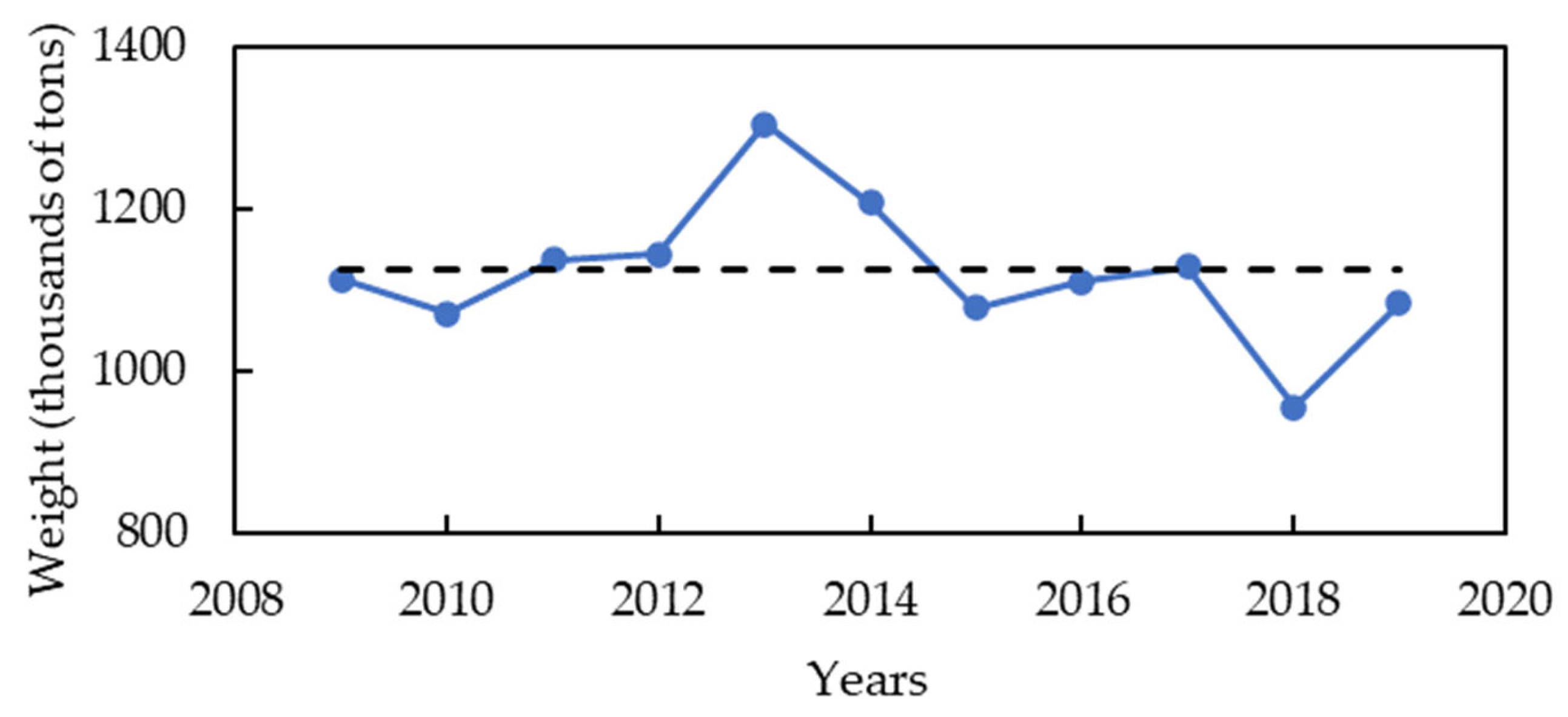



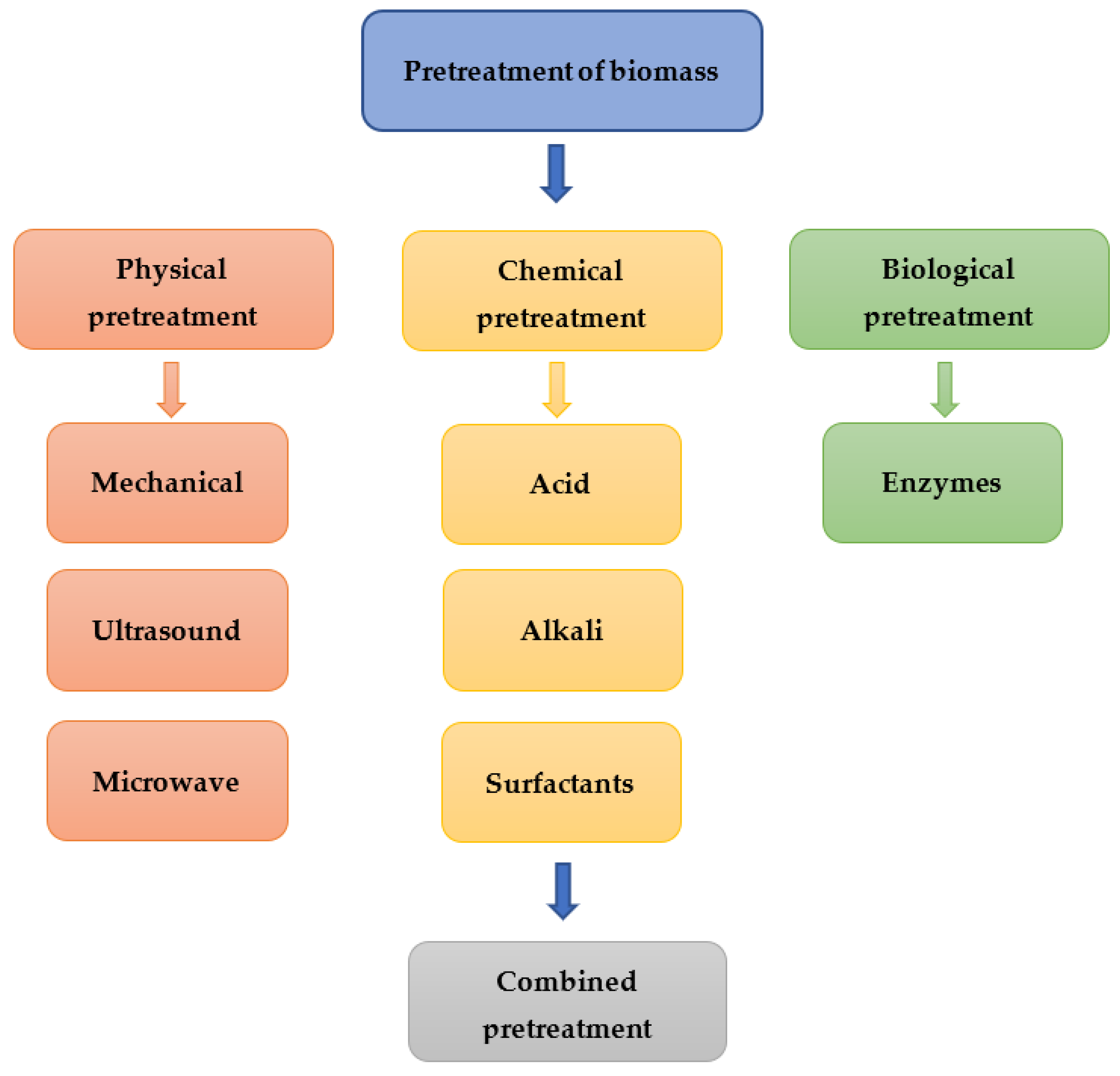
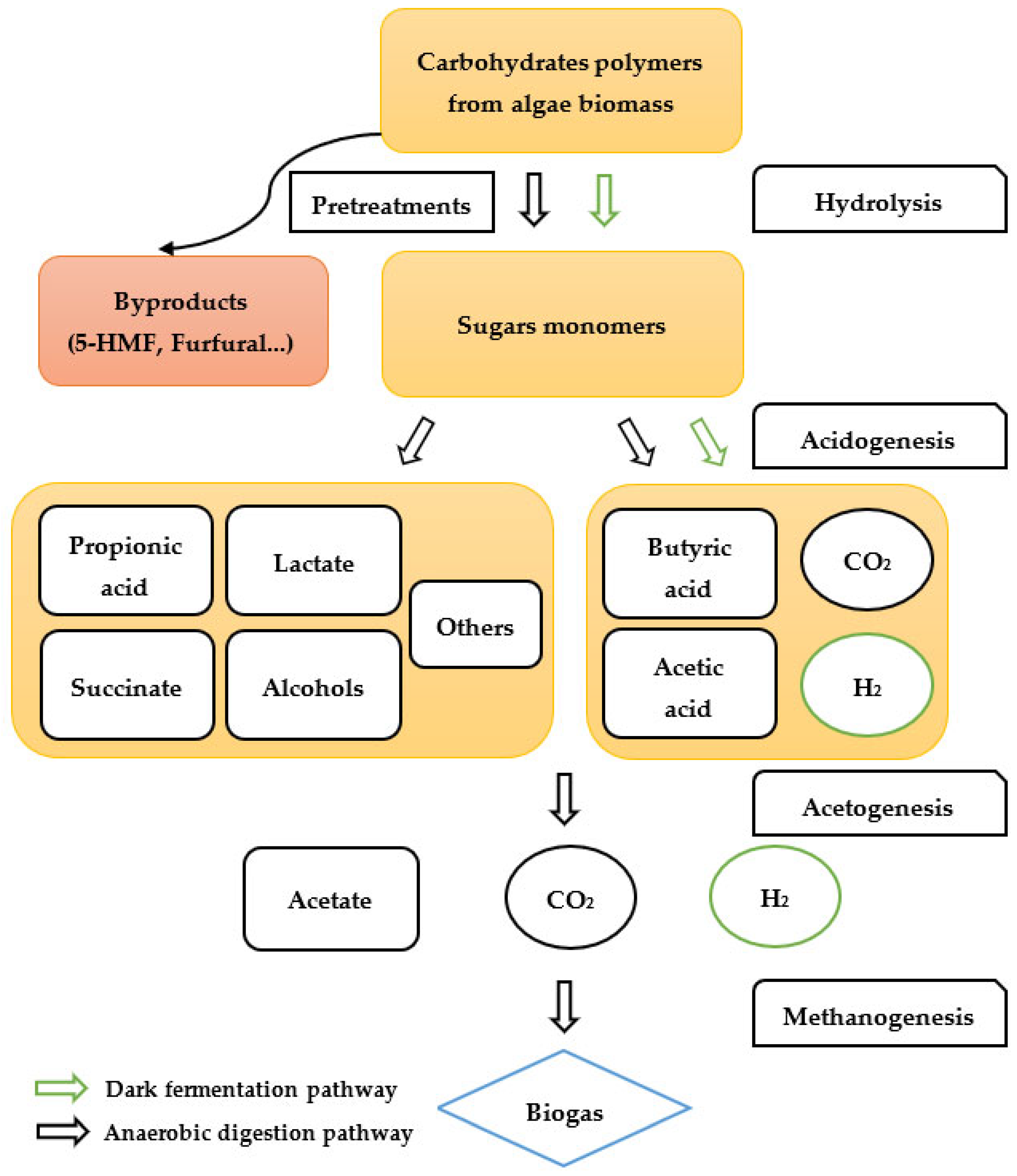
| Compound | Green Algae | Red Algae | Brown Algae | Reference |
|---|---|---|---|---|
| Carbohydrates 1 | 25–50% | 30–60% | 30–60% | [53,70] |
| Protein 1 | 10–20% | 10–25% | 3–15% | [56,71,72] |
| Lipid 1 | 1–4% | 0.6–4% | 0.4–2.4% | [56,73,74] |
| Mineral 1 | 18–53% | 26–48% | 34–55% | [72] |
| Water content 2 | 70–85% | 70–80% | 75–90% | [75] |
| Groups | Seaweeds | Pretreatment | Condition | Methane Yield | Ref. |
|---|---|---|---|---|---|
| Ulva sp. | Ground and centrifuged | B | 0.148 m3/kg VS | [49] | |
| Green algae | Non-washed | B | 0.11 m3/kg VS | [134] | |
| Washed | B | 0.094 m3/kg VS | |||
| Non-ground dried | B | 0.145 m3/kg VS | |||
| Ground dried | B | 0.177 m3/kg VS | |||
| Ground | C (HRT: 15 days OLR: 1.8 kg VS m−3 day−1 T: 35 °C) | 0.203 m3/kg VS | |||
| Ground | C (HRT: 20 days OLR: 1.7 kg VS m−3 day−1 T: 35 °C) | 0.182 m3/kg VS | |||
| Washed, dried, milled | B | 0.191 m3/kg VS | [157] | ||
| Ulva lactuca | Macerated | B | 0.271 m3/kg VS | [158] | |
| Fresh | B | 0.183 m3/kg VS | [159] | ||
| Washed and dried | B | 0.25 m3/kg VS | |||
| Washed, cut, and ensiling | B | 0.256 m3/kg VS | [160] | ||
| Chaetomorpha linum | Frozen, washed, chopped | B | 0.166 m3/kg VS | [161] | |
| Red algae | Gracilaria spp. | Frozen | C (HRT: 15 days OLR: 1.6 kg VS m−3 day−1 T: 35 °C) | 0.28–0.4 m3/kg VS | [21] |
| Gracilaria gracilis | Non-pretreated | B | 0.0818 m3 biogas/kg TS | [162] | |
| Cut by a Hollander beater | B | 0.1718 m3 biogas/kg TS | |||
| Gracilaria vermiculophylla | Frozen, washed, chopped | B | 0.132 m3/kg VS | [161] | |
| Washed, maceration (cut, crushed by a mortar) | B | 0.481 ± 0.009 m3/kg VS | [163] | ||
| Palmaria palmata | Raw alga (dried, chopped) | B | 0.308 m3/kg VS | [164] | |
| Dried, chopped then maceration (20 °C) | B | 0.328 m3/kg VS | |||
| Dried, chopped then thermal treatment (120 °C) | B | 0.296 m3/kg VS | |||
| Dried, chopped then thermal treatment (160 °C) | B | 0.269 m3/kg VS | |||
| Dried, chopped then thermal treatment (180 °C) | B | 0.268 m3/kg VS | |||
| Dried, chopped then thermal treatment (200 °C) | B | 0.211 m3/kg VS | |||
| Dried, chopped then thermal treatment (160 °C) + NaOH | B | 0.282 m3/kg VS | |||
| Dried, chopped then thermal treatment (160 °C) + HCl | B | 0.268 m3/kg VS | |||
| Brown algae | Sargassum | Frozen | C (HRT: 15 days OLR: 1.6 kg VS m−3 day−1 T: 35 °C) | 0.12–0.19 m3/kg VS | [21] |
| S. muticum | Washed | B | 0.177 m3/kg VS | [165] | |
| Non-washed | B | 0.225 m3/kg VS | |||
| Dried | B | 0.13 m3/kg VS | [72] | ||
| Dried, ground/chopped | B | 0.166–0.208 m3/kg VS | [166] | ||
| S. natans VIII | Frozen and freeze-dried | B | 0.145 m3/kg VS | [167] | |
| S. natans I | Frozen and freeze-dried | B | 0.066 m3/kg VS | ||
| S. fluitans | Frozen and freeze-dried | B | 0.113 m3/kg VS | ||
| A. nodosum | Chopped and frozen | B | 0.28 m3 biogas/kg VS | [168] | |
| Chopped and frozen | C (HRT: 24 days OLR: 1.75 kg VS m−3 day−1 T: 35 °C) | 0.11 m3/kg VS | |||
| Cut, 15 min mechanical pretreatment | B | 0.169 m3/kg VS | [169] | ||
| Washed, cut, and ensiling | B | 0.237 m3/kg VS | [160] | ||
| Saccorhiza polyschides | Washed, dried, milled | B | 0.255 m3/kg VS | [157] | |
| Washed, cut, and ensiling | B | 0.277 m3/kg VS | [160] | ||
| Nizimuddinia zanardini | Washed, dried | B | 0.117 m3/kg VS | [170] | |
| Washed, dried, autoclaved (30 min, 121 °C) | B | 0.143 m3/kg VS | |||
| Fucus vesiculosus | Washed, dried, thermochemical pretreatment (200 mol/m3 HCl, 24 h, 80 °C) | B | 0.113 m3/kg VS | [130] | |
| Laminaria digitata | Oven drying (24 h, 104 °C) then pulverized with a blender | B | 0.141 m3/kg VS | [171] | |
| Washed with hot water then macerated | B | 0.282 m3/kg VS | [172] | ||
| Washed, cut, and ensiling | B | 0.354 m3/kg VS | [160] | ||
| Saccharina latissima | Frozen, defrosted, cut, ground | B | 0.223 m3/kg VS | [173] | |
| Frozen, defrosted, cut, ground then steam explosion (10 min, 130 °C) | B | 0.268 m3/kg VS | |||
| Washed, cut, and ensiling | B | 0.33 m3/kg VS | [160] |
| Seaweeds | Pretreatment Methods | BMP | Ref. |
|---|---|---|---|
| Ulva sp. | Ground | +0.032 m3/kg VS (+22%) | [134] |
| Ulva lactuca | Washed and dried | +0.067 m3/kg VS (+37%) | [159] |
| Gracilaria gracilis | Hollander beater | +0.09 m3/kg TS (+110%) | [162] |
| Nizimuddinia zanardini | Autoclaved | +0.026 m3/kg VS (+22%) | [170] |
| Saccharina latissima | Steam explosion | +0.045 m3/kg VS (+20%) | [173] |
| Groups | Seaweeds | Substrate Pretreatment | Inoculum Pretreatment | Condition | pH | Hydrogen Yield | Ref. |
|---|---|---|---|---|---|---|---|
| Green algae | Ulva reticulata | Washed, dried, disperser | 102 °C, 30 min | B | 5.5 ± 0.1 | 0.045 m3/kg COD | [177] |
| Washed, dried, disperser, 21.6 mg/L tween 80 | 102 °C, 30 min | B | 0.063 m3/kg COD | ||||
| Chaetomorpha antennina | Washed, microwave disintegration, 15 min | 100 °C, 30 min | B | — | 0.063 m3/kg COD | [178] | |
| Washed, ammonium dodecyl sulfate + microwave disintegration | 100 °C, 30 min | B | 0.0745 m3/kg COD | ||||
| Red algae | Gelidium amansii | 121 °C, 1% H2SO4, 30 min | 90 °C, 30 min | B | 7 | 0.0528 ± 0.0002 m3/kg TS | [136] |
| 121 °C, 1% HNO3, 30 min | B | 0.016 ± 0.0009 m3/kg TS | |||||
| 121 °C, 1% HCl, 30 min | B | 0.0224 ± 0.0004 m3/kg TS | |||||
| 121 °C, 1% H3PO4, 30 min | B | 0.014 ± 0.0004 m3/kg TS | |||||
| 121 °C, water, 30 min | B | 0.0272 ± 0.0003 m3/kg TS | |||||
| Washed, dried, ground, sieved, then 150 °C, 2% H2SO4, 15 min | 90 °C, 10 min | B | >5.5 | 0.518 m3 kg−1 VS day−1 | [179] | ||
| Washed, milled, then 164 °C, 12.7% S/L, 0.5% H2SO4 * | 90 °C, 20 min | B | >5.3 | 0.037 m3/kg TS | [148] | ||
| Brown algae | Laminaria japonica | Non-pretreated | 90 °C, 20 min | B | 5.5 | 0.0714 m3/kg TS | [180] |
| Washed, dried and ground | 90 °C, 20 min | C (HRT: 6 days OLR: 3.4 kg COD m−3 day−1 T: 35 °C) | 0.0613 ± 0.002 m3/kg TS | [181] | |||
| Washed, dried with a ball mill at 120 °C for 30 min | 65 °C, 20 min | B | 7.5 | 0.028 m3/kg TS | [182] | ||
| Washed, dried and ground, 93 °C, 4.8% HCl, 23 min * | 90 °C, 20 min | B | 5.5 | 0.1596 m3/kg TS | [183] | ||
| Washed, dried and ground, 170 °C, 20 min | 90 °C, 20 min | B | 5.5 | 0.1096 m3/kg CODadded | [184] | ||
| Washed, dried and ground, 11.7 V/cm, 30 min * | 2 V/cm, 10 min | B | 5.5 | 0.1027 m3/kg TS | [185] | ||
| Csub 2%, Washed, oven dried, 105 °C, 4 h, then autoclaved 121 °C, 30 min | 80 °C, 20 min | B | 6 | 0.08345 ± 0.00696 m3/kg TS | [186] | ||
| Washed, oven dried, 105 °C, 4 h, ball milling | B | 7.0 ± 0.1 | 0.01 ± 0.00121 m3/kg TS | ||||
| Washed, oven dried, 105 °C, 4 h, then autoclaved 121°C, 30 min | B | 0.06668 ± 0.00568 m3/kg TS | |||||
| Washed, oven dried, 105 °C, 4 h, then ultrasonic cell breaker, 20 kHz | B | 0.02356 ± 0.00456 m3/kg TS | |||||
| Washed, oven dried, 105 °C, 4 h, then HCl 1000 mol/m3, 30 min | B | 0.04365 ± 0.00687 m3/kg TS | |||||
| Washed, oven dried, 105 °C, 4 h, then NaOH 1000 mol/m3, 30 min | B | 0.015 ± 0.00389 m3/kg TS | |||||
| Sargassum sp. | Dried, milled, autoclaved 121 °C, 1 bar, 15 min | Precultured C. saccharolyticus | B | 7.0–7.2 | 0.0913 ± 0.0033 m3/kg VS | [104] | |
| Padina tetrastromatica | Washed, dried, cut, milled then 1% HCl, 100 °C, 2 h | 60 °C, 10 min | B | 6 ± 0.5 | 0.76 m3/kg VS | [112] | |
| Washed, dried, cut, milled then 1% HNO3, 100 °C, 2 h | B | 0.68 m3/kg VS | |||||
| Washed, dried, cut, milled then 1% H2SO4, 100 °C, 2 h | B | 1.56 m3/kg VS | |||||
| Washed, dried, cut, milled then 2% KOH, 100 °C, 2 h | B | 0.84 m3/kg VS | |||||
| Washed, dried, cut, milled then 2% NaOH, 100 °C, 2 h | B | 1.1 m3/kg VS | |||||
| Laminaria digitata | Washed, cut | 100 °C, 30 min | B | 6.00 ± 0.05 | 0.097 m3/kg VS | [113] | |
| Saccharina japonica | Washed, dried, 80 °C, 24 h, milled, sifted, then 2% NaOH, 121 °C, 30 min | 5 kGy ionizing irradiation | B | - | 0.0175 m3/kg TS | [142] |
| Seaweeds | Pretreatment Methods | BHP | Ref. |
|---|---|---|---|
| Ulva reticulata | Tween 80 | +0.018 m3/kg COD (+40%) | [177] |
| Chaetomorpha antennina | ALS | +0.0115 m3/kg COD (+18%) | [178] |
| Gelidium amansii | 1% H2SO4, 121 °C, 30 min | +0.0256 m3/kg TS (+94%) | [136] |
| Laminaria japonica | Autoclaved | +0.07345 m3/kg dry sample (+735%) | [186] |
| Padina tetrastromatica | 1% H2SO4, 100 °C, 2 h | +1.56 m3/kg VS (-) | [112] |
| Helpful | Harmful | |
|---|---|---|
| Internal | Strengths | Weaknesses |
|
| |
| External | Opportunities | Threats |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Bourgougnon, N.; Lanoisellé, J.-L.; Lendormi, T. Biofuel Production from Seaweeds: A Comprehensive Review. Energies 2022, 15, 9395. https://doi.org/10.3390/en15249395
Zhao Y, Bourgougnon N, Lanoisellé J-L, Lendormi T. Biofuel Production from Seaweeds: A Comprehensive Review. Energies. 2022; 15(24):9395. https://doi.org/10.3390/en15249395
Chicago/Turabian StyleZhao, Yiru, Nathalie Bourgougnon, Jean-Louis Lanoisellé, and Thomas Lendormi. 2022. "Biofuel Production from Seaweeds: A Comprehensive Review" Energies 15, no. 24: 9395. https://doi.org/10.3390/en15249395
APA StyleZhao, Y., Bourgougnon, N., Lanoisellé, J.-L., & Lendormi, T. (2022). Biofuel Production from Seaweeds: A Comprehensive Review. Energies, 15(24), 9395. https://doi.org/10.3390/en15249395









