Review of Closed SCO2 and Semi-Closed Oxy–Fuel Combustion Power Cycles for Multi-Scale Power Generation in Terms of Energy, Ecology and Economic Efficiency
Abstract
1. Introduction
- The problem of coolant leakage, which can increase the carbon footprint of a plant;
- The need for the compression of carbon dioxide in the near-critical region near the saturation boundary of the coolant (fraught with sharp jumps in thermophysical properties up to gravitational effects on the inlet blade of the compressor);
- The large heating surfaces required by the carbon dioxide heat exchangers because of the large capacity of the regenerators, which generate increasing capital costs;
- The low critical temperature of CO2, which creates the need for a low-temperature cold source (10–15 °C) for closed cycles, especially cycles with condensation.
2. Closed SCO2 Cycles
- Cycles without condensation, with the working fluid compression by a compressor (Brayton cycles);
- Cycles with condensation, with the working fluid compression by a pump (Rankine cycles).
2.1. Closed SCO2 Brayton Cycles
- Supercritical CO2 Brayton simple cycle;
- Supercritical CO2 regenerative Brayton cycle;
- Supercritical CO2 regenerative Brayton cycle with recompression;
- Supercritical CO2 Brayton cascade cycle.
2.2. Closed SCO2 Rankine Cycles
- Rankine cycle with regeneration;
- Cascade Rankine cycle;
- Split Rankine cycle with regeneration.
2.3. Prospective Closed Cycles
- Supercritical carbon dioxide Brayton cycle with liquefied natural gas;
- Supercritical carbon dioxide Brayton cycle with trigeneration;
- Supercritical carbon dioxide Rankine cycle with ejection of the working fluid;
- Supercritical carbon dioxide Brayton cycle with partial condensation of the working fluid.
3. Semi-Closed Oxy–Fuel Combustion Power Cycles with CO2 Recirculation
- Optimization of the structure of the metal consumption of the main equipment due to the absence of massive boiler units;
- Minimization of greenhouse gas emissions into the atmosphere through carbon dioxide capture and deposition.
- Semi-closed cycle with oxygen–fuel combustion (SCOC-CC);
- MATIANT and E-MATIANT cycles;
- The Allam cycle.
3.1. SCOC-CC
3.2. MATIANT Cycles
3.3. Allam Cycle
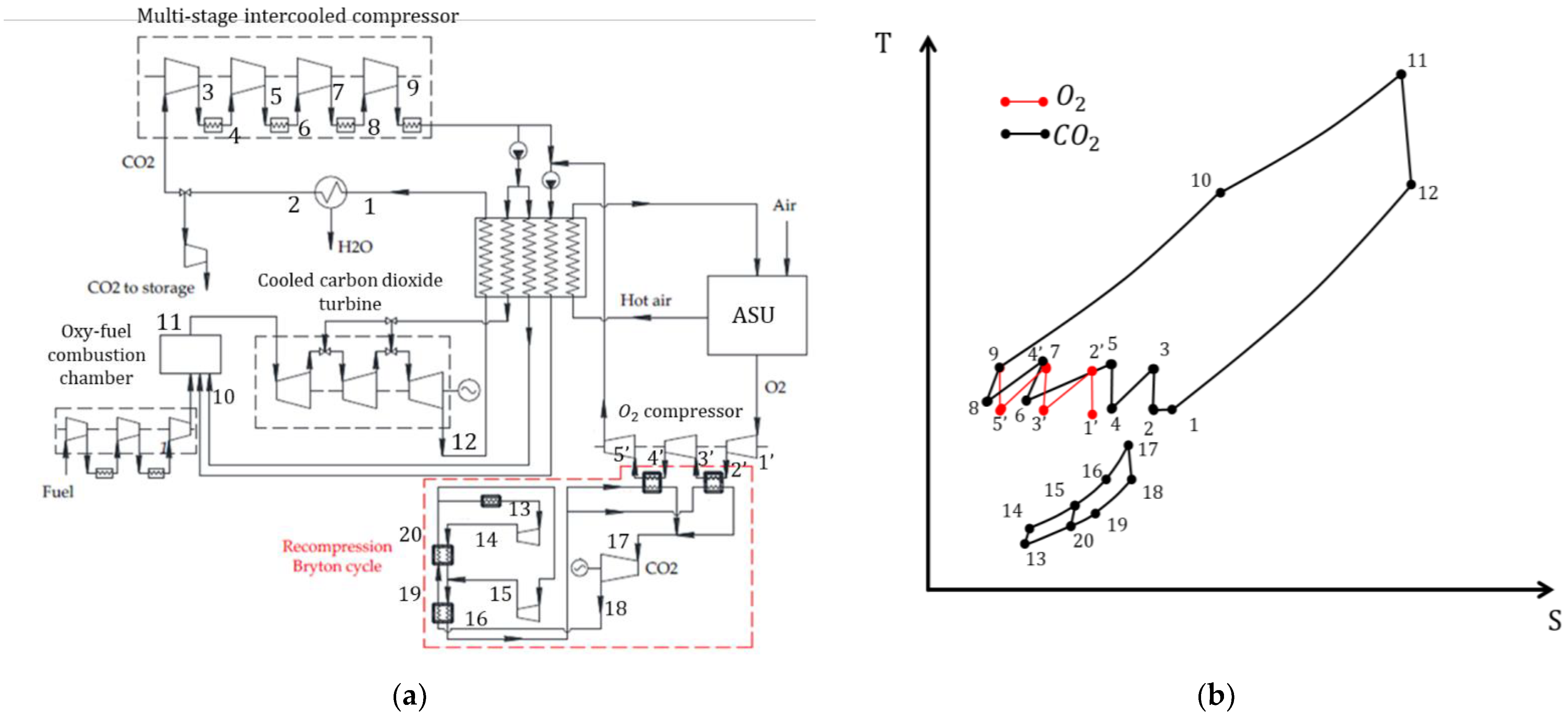
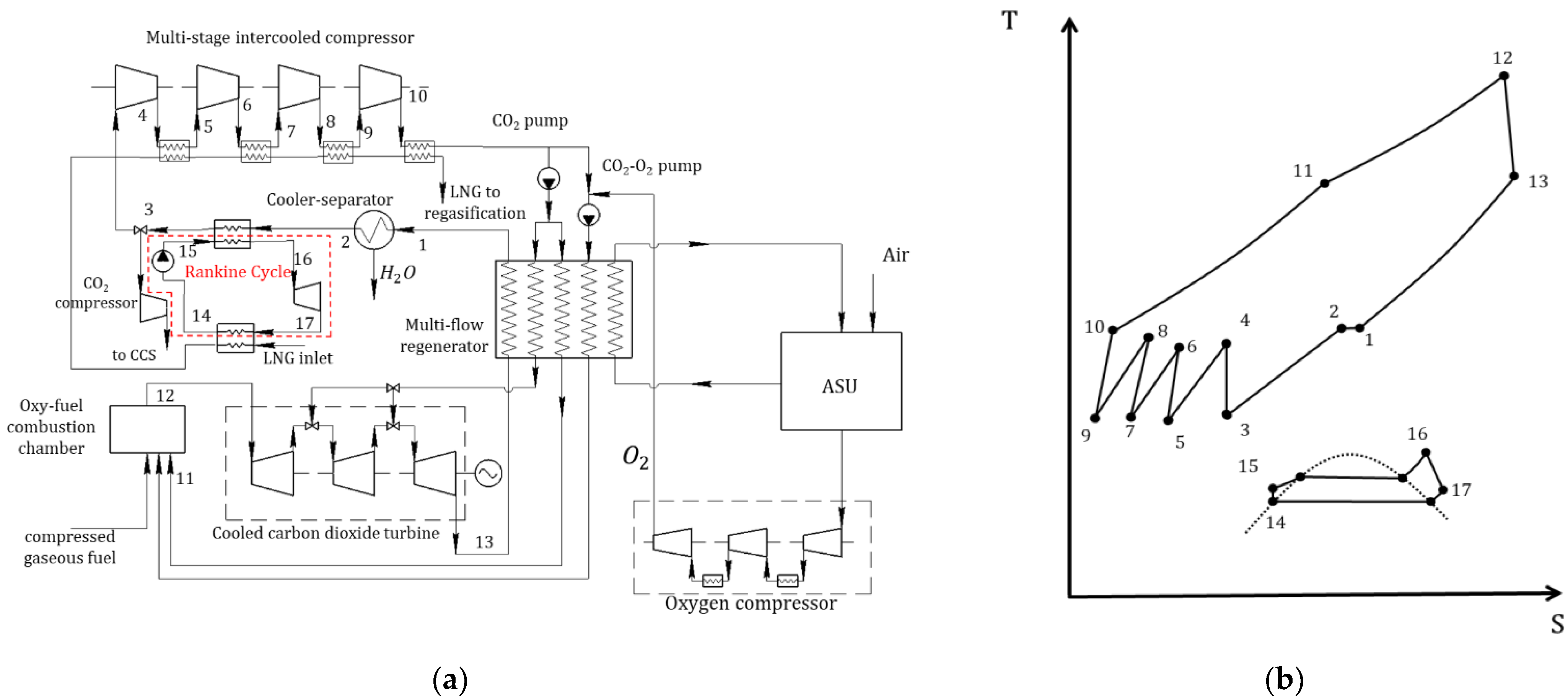
3.4. Modified Semi-Closed Oxy–Fuel Combustion Power Cycles with CO2 Recirculation
- SCOC-CC cycle with refrigerant cooling;
- SCOC-CC cycle with water injection;
- SCOC-CC cycle with WHR;
- Allam cycle using liquefied natural gas for cooling;
- Allam cycle with WHR;
- MATIANT cycle with production of electric power and methane in Sabatier reactors.
4. Efficiency and Environment
- Efficiency is a metric of energy conversion efficiency;
- Specific capital costs—technology cost metric;
- Power is a metric of the technology applicability for generating electric power at different scales;
- Temperature at the turbine input is a metric reflecting potential sources of thermal energy;
- Specific carbon dioxide emission per unit of energy produced—a metric of the environmental safety of the cycle;
- Cost of generated energy in current and future conditions is a key metric reflecting total economic efficiency for an electric power producer.
- For high-power cycles with a reference power of over 20 MW, the power for calculating specific capital costs was 300 MW;
- For cycles with a low reference power from 0.2 MW to 20 MW, the power for calculating specific capital costs was 10 MW;
- For cycles with a reference power less than 0.2 MW, the power for calculating specific capital costs was assumed to be equal to 0.1 MW.
- NGCC plants;
- NGCC plants with carbon dioxide capturing;
- NGCC plants with a gas turbine and a Brayton CO2 power cycle.
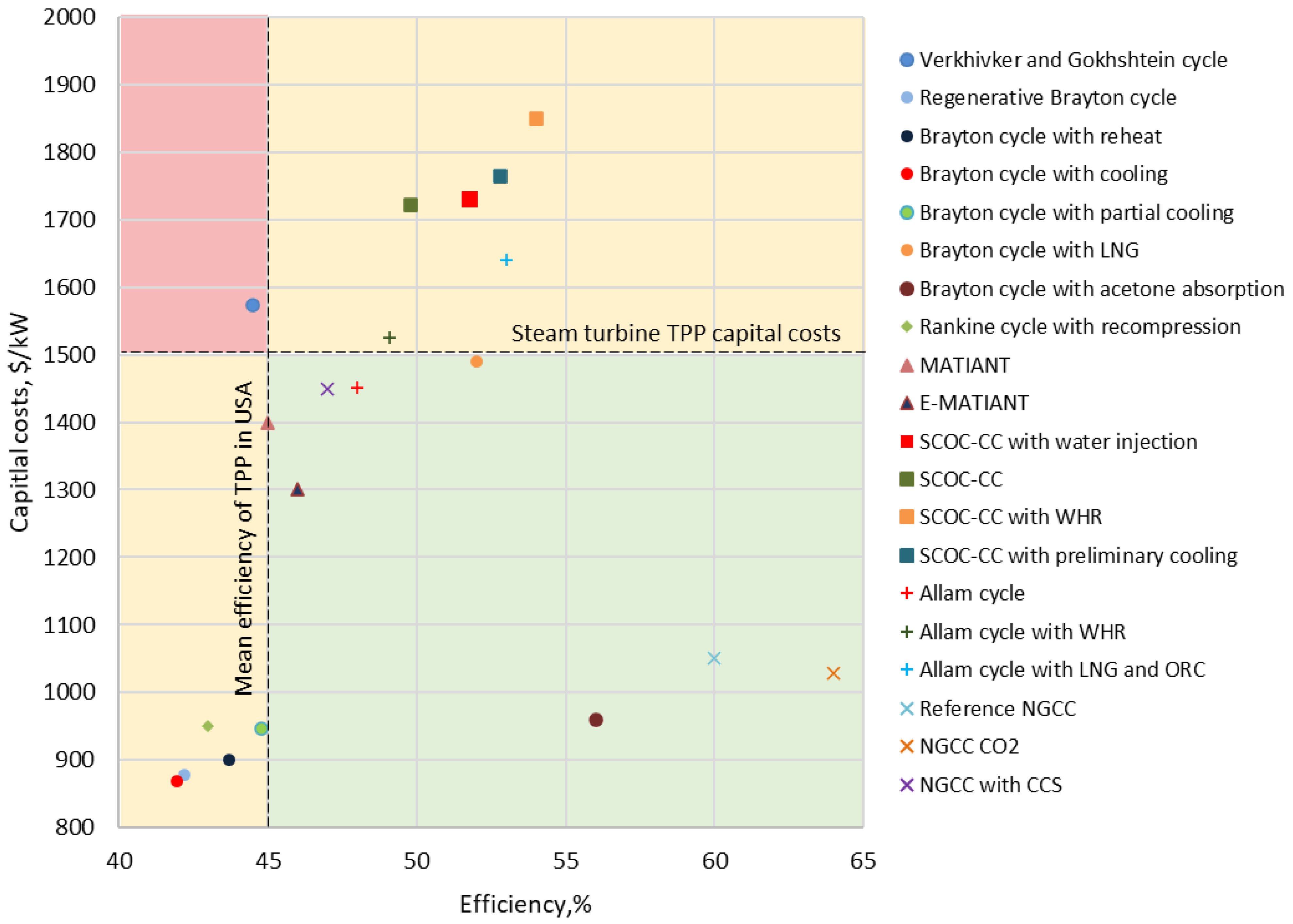
- Efficiency of at least 45%, which is the average level of steam turbine plant efficiency in the USA;
- Specific capital construction costs of not more than 1500 $/kW, reflecting the average capital intensity of traditional steam turbine power units.
- MATIANT;
- E-MATIANT;
- Brayton cycle using LNG;
- Brayton cycle with acetone absorption;
- Basic Allam cycle.
- Efficiency not less than 22%;
- Specific capital costs not more than 4000 $/kW.
5. Economics
- —net costs of energy, $/kWh;
- —cycle efficiency, %;
- —specific heat of combustion, kWh/kg;
- —fuel costs, $/kg;
- —specific CO2 emissions, g/kWh;
- —cost of CO2 allowances, $/ton;
- —additional costs coefficient;
- —specific capital costs, $/kW;
- —term of operating, hour;
- —specific staffing, people/kW;
- —staff hourly rate, $/hour.
6. Summary
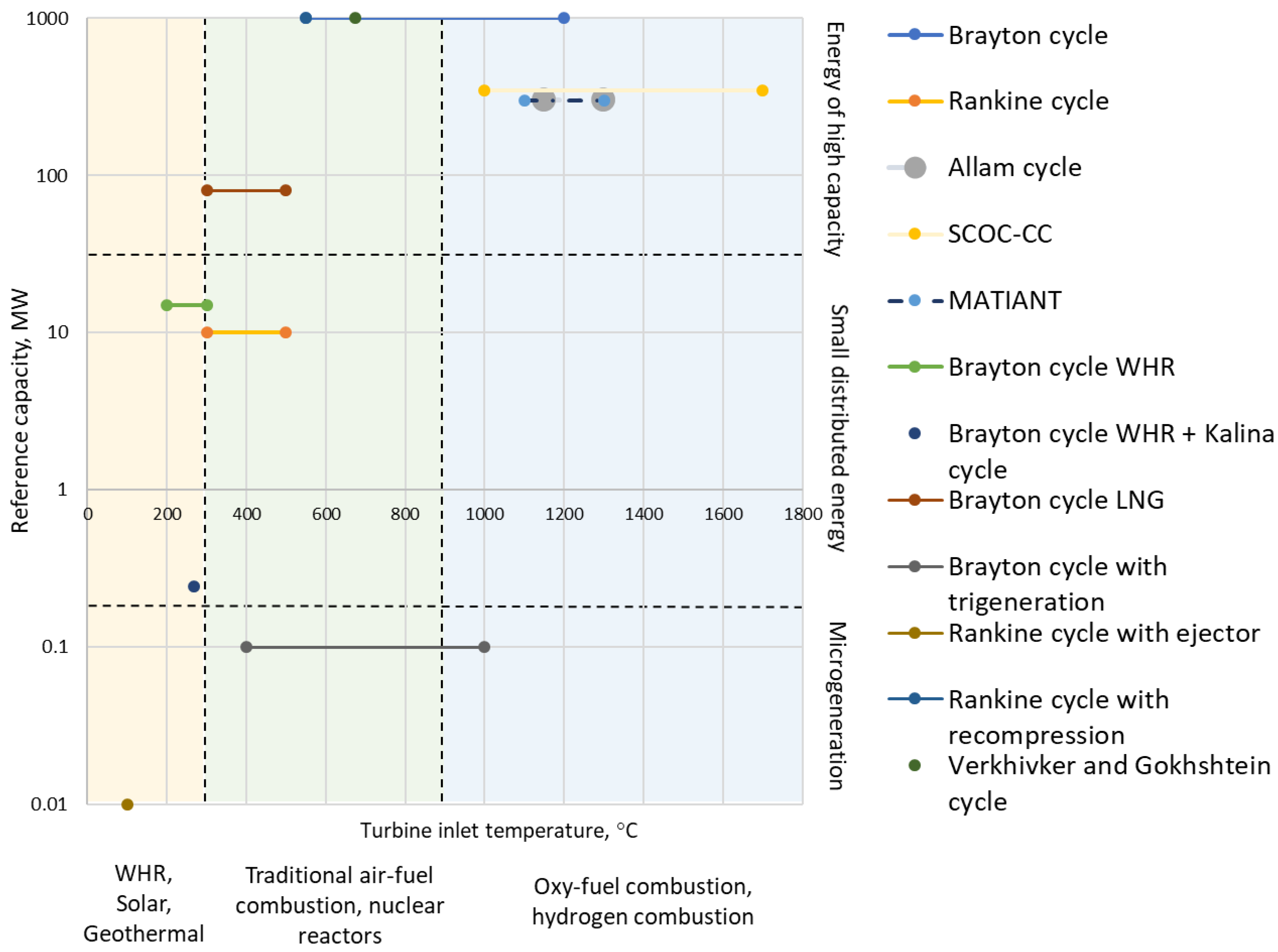
- Less than 300 °C: WHR sources, including solar energy collectors and geothermal sources;
- 300–900 °C: traditional burning of solid, liquid and gaseous fuels in boilers, as well as nuclear reactors;
- More than 900 °C: burning of gaseous fuel, including hydrogen, in pure oxygen.
- For large-capacity energy production, with an increase in the cost of CO2 emission quotas, oxygen–fuel cycles with carbon dioxide capturing become significantly more attractive from an economic point of view. In particular, at a quota cost of 50 $/ton, the NGCC with a gas turbine and supercritical Brayton cycle has the lowest cost of generating electric power, but, if the cost of quota is equal to 100 $/ton, the SCOC-CC cycle with WHR has the lowest cost of generating electric power;
- Among the closed CO2 power cycles for large-capacity power generation, the Brayton cycle with acetone absorption is the most attractive in terms of the entire set of technical and economic parameters, and, among semi-closed oxygen–fuel cycles, the SCOC-CC cycles with high efficiency and relatively low specific capital costs are the most effective;
- For CO2 power cycles used in small, distributed generation, the Brayton cycle with WHR and recompression is the most effective, which is achieved due to high efficiency and low specific capital costs;
- For microgeneration cycles, the most effective among those considered is the Brayton cycle with trigeneration, which has the lowest cost of energy generation for all the considered values of CO2 emission quota.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Symbol | Description | Unit |
| Nref | net cycle power | MW |
| Tinlet | turbine inlet temperature | °C |
| Tref | reference turbine inlet temperature | °C |
| η | cycle efficiency | % |
| Cc | specific capital costs | $/kW |
| Es | specific CO2 emissions | g/kWh |
| net costs of energy | $/kWh | |
| specific heat of combustion | kWh/kg | |
| fuel costs | $/kg | |
| cost of CO2 allowances | $/ton | |
| additional costs coefficient | ||
| term of operating | hour | |
| specific staffing | people/kW | |
| staff hourly rate | $/hour | |
| Abbreviations | ||
| WHR | Waste Heat Recovery | |
| HFC | Hydrofluorocarbon | |
| GWP | Global Warming Potential | |
| ORC | Organic Rankine Cycle | |
| TPP | Thermal Power Plant | |
| AGR | Advanced Gas-Cooled Reactor | |
| SCO2 | Supercritical Carbon Dioxide | |
| LNG | Liquefied Natural Gas | |
| SCOC-CC | Semiclosed Oxy–Fuel Combustion Combined Cycle | |
| SCOC | Semiclosed Oxy–Fuel Combustion | |
| CES | Clean-Energy Systems | |
| AZEP | Advanced Zero-Emissions Plant | |
| ZEITMOP | Zero-Emission Ion Transport Membrane Oxygen Power | |
| GT | Gas Turbine | |
| CC | Combustion Chamber | |
| ST | Steam Turbine | |
| NGCC | Natural Gas Combined Cycle |
References
- Kotowicz, J.; Job, M.; Brzęczek, M. The characteristics of ultramodern combined cycle power plants. Energy 2015, 92, 197–211. [Google Scholar] [CrossRef]
- Pronobis, M. Reduction of nitrogen oxide emissions. In Environmentally Oriented Modernization of Power Boilers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 79–133. ISBN 978-0-12-819921-3. [Google Scholar]
- Shi, L.; Shu, G.; Tian, H.; Deng, S. A Review of Modified Organic Rankine Cycles (ORCs) for Internal Combustion Engine Waste Heat Recovery (ICE-WHR). Renew. Sustain. Energy Rev. 2018, 92, 95–110. [Google Scholar] [CrossRef]
- Braimakis, K.; Karellas, S. Energetic optimization of regenerative Organic Rankine Cycle (ORC) configurations. Energy Convers. Manag. 2018, 159, 353–370. [Google Scholar] [CrossRef]
- Sun, W.; Yue, X.; Wang, Y. Exergy efficiency analysis of ORC (Organic Rankine Cycle) and ORC-based combined cycles driven by low-temperature waste heat. Energy Convers. Manag. 2017, 135, 63–73. [Google Scholar] [CrossRef]
- Siddiqui, M.E. Thermodynamic performance improvement of recompression Brayton cycle utilizing CO2-C7H8 binary mixture. Mechanics 2021, 27, 259–264. [Google Scholar] [CrossRef]
- Bianchi, M.; Branchini, L.; De Pascale, A.; Melino, F.; Ottaviano, S.; Peretto, A.; Torricelli, N. Performance modelling and greenhouse impact assessment of a micro-orc energy system working with HFCs, low GWP fluids and mixtures. E3S Web Conf. 2021, 238, 10002. [Google Scholar] [CrossRef]
- Bahrami, M.; Pourfayaz, F.; Kasaeian, A. Low Global Warming Potential (GWP) Working Fluids (WFs) for Organic Rankine Cycle (ORC) applications. Energy Rep. 2022, 8, 2976–2988. [Google Scholar] [CrossRef]
- Liu, C.; He, C.; Gao, H.; Xie, H.; Li, Y.; Wu, S.; Xu, J. The environmental impact of organic Rankine cycle for waste heat recovery through life-cycle assessment. Energy 2013, 56, 144–154. [Google Scholar] [CrossRef]
- Allam, R.; Martin, S.; Forrest, B.; Fetvedt, J.; Lu, X.; Freed, D.; Brown, G.W.; Sasaki, T.; Itoh, M.; Manning, J. Demonstration of the Allam cycle: An update on the development status of a high efficiency supercritical carbon dioxide power process employing full carbon capture. Energy Procedia 2017, 114, 5948–5966. [Google Scholar] [CrossRef]
- Gokhshtein, D.P.; Verkhivker, G.P. Use of carbon dioxide as a heat carrier and working substance in atomic power stations. Sov. Energy 1969, 18, 430–432. [Google Scholar] [CrossRef]
- Rogalev, N.; Rogalev, A.; Kindra, V.; Komarov, I.; Zlyvko, O. Structural and parametric optimization of S–CO2 nuclear power plants. Entropy 2021, 23, 1079. [Google Scholar] [CrossRef] [PubMed]
- Angelino, G. Carbon dioxide condensation cycles for power production. J. Eng. Gas Turbines Power 1968, 90, 287–295. [Google Scholar] [CrossRef]
- Huang, M.; Sonwane, C. Thermodynamics of conventional and non-conventional SCO2 recompression brayton cycles with direct and indirect heating. In Proceedings of the 4-th International Symposium-Supercritical CO2 Power Cycles, Pittsburgh, PA, USA, 9–10 September 2014. [Google Scholar]
- Angelino, G. Real gas effects in carbon dioxide cycles. In Proceedings of the International Gas Turbine Conference and Products Show, Cleveland, OH, USA, 10–13 March 1969. [Google Scholar]
- Bonalumi, D.; Giuffrida, A.; Sicali, F. A case study of cascade supercritical CO2 power cycle for waste heat recovery from a small gas turbine. Energy Convers. Manag. X 2022, 14, 100212. [Google Scholar] [CrossRef]
- Astolfi, M.; Alfani, D.; Lasala, S.; Macchi, E. Comparison between ORC and CO2 power systems for the exploitation of low-medium temperature heat sources. Energy 2018, 161, 1250–1261. [Google Scholar] [CrossRef]
- Feng, Y.; Du, Z.; Shreka, M.; Zhu, Y.; Zhou, S.; Zhang, W. Thermodynamic analysis and performance optimization of the supercritical carbon dioxide Brayton cycle combined with the Kalina cycle for waste heat recovery from a marine low-speed diesel engine. Energy Convers. Manag. 2020, 206, 112483. [Google Scholar] [CrossRef]
- Kim, Y.M.; Sohn, J.L.; Yoon, E.S. Supercritical CO2 Rankine cycles for waste heat recovery from gas turbine. Energy 2017, 118, 893–905. [Google Scholar] [CrossRef]
- Invernizzi, C.M.; Iora, P. The exploitation of the physical exergy of liquid natural gas by closed power thermodynamic cycles. An overview. Energy 2016, 105, 2–15. [Google Scholar] [CrossRef]
- Angelino, G.; Invernizzi, C.M. Carbon dioxide power cycles using liquid natural gas as heat sink. Appl. Therm. Eng. 2009, 29, 2935–2941. [Google Scholar] [CrossRef]
- Bellos, E.; Tzivanidis, C. Parametric analysis of a polygeneration system with CO2 working fluid. Appl. Sci. 2021, 11, 3215. [Google Scholar] [CrossRef]
- Li, X.; Huang, H.; Zhao, W. A supercritical or transcritical Rankine cycle with ejector using low-grade heat. Energy Convers. Manag. 2014, 78, 551–558. [Google Scholar] [CrossRef]
- McClung, A.; Portnoff, M. Absorption/Desorption Based High Efficiency Supercritical Carbon Dioxide Power Cycles—Final Scientific/Technical Report; Southwest Research Institute: San Antonio, TX, USA, 2018; pp. DOE-SwRI-25348, 1474410. [Google Scholar]
- Climent Barba, F.; Martínez-Denegri Sánchez, G.; Soler Seguí, B.; Gohari Darabkhani, H.; Anthony, E.J. A technical evaluation, performance analysis and risk assessment of multiple novel oxy-turbine power cycles with complete CO2 capture. J. Clean. Prod. 2016, 133, 971–985. [Google Scholar] [CrossRef]
- Cau, G.; Tola, V.; Ferrara, F.; Porcu, A.; Pettinau, A. CO2-free coal-fired power generation by partial oxy-fuel and post-combustion CO2 capture: Techno-economic analysis. Fuel 2018, 214, 423–435. [Google Scholar] [CrossRef]
- Bolland, O.; Sæther, S. New concepts for natural gas fired power plants which simplify the recovery of carbon dioxide. Energy Convers. Manag. 1992, 33, 467–475. [Google Scholar] [CrossRef]
- Chiesa, P.; Lozza, G. CO2 emission abatement in IGCC power plant by semiclosed cycles. Part A: With Oxygen-Blown Combustion. Asme 1999, 121, 635–641. [Google Scholar] [CrossRef]
- Dahlquist, A.; Genrup, M.; Sjoedin, M.; Jonshagen, K. Optimization of an oxyfuel combined cycle regarding performance and complexity level. In Proceedings of the ASME Turbo Expo 2013: Turbine Technical Conference and Exposition, San Antonio, TX, USA, 3–7 June 2013; Volume 2: Aircraft Engine; Coal, Biomass and Alternative Fuels; Cycle Innovations. American Society of Mechanical Engineers: New York City, NY, USA, 2013; p. V002T07A011. [Google Scholar]
- Bolland, O.; Mathieu, P. Comparison of two CO2 removal options in combined cycle power plants. Energy Convers. Manag. 1998, 39, 1653–1663. [Google Scholar] [CrossRef]
- Yang, H.J.; Kang, D.W.; Ahn, J.H.; Kim, T.S. Evaluation of design performance of the semi-closed oxy-fuel combustion combined cycle. In Proceedings of the ASME Turbo Expo 2012, Turbine Technical Conference and Exposition, Copenhagen, Denmark, 11–15 June 2012; Volume 3: Cycle Innovations; Education; Electric Power; Fans and Blowers; Industrial and Cogeneration. American Society of Mechanical Engineers: New York City, NY, USA, 2012; pp. 227–238. [Google Scholar]
- Mathieu, P.; Nihart, R. Sensitivity analysis of the MATIANT cycle. Energy Convers. Manag. 1999, 15–16, 1687–1700. [Google Scholar] [CrossRef]
- Mathieu, P.; Nihart, R. Zero-emission MATIANT cycle. J. Eng. Gas Turbines Power 1999, 121, 116–120. [Google Scholar] [CrossRef]
- Mathieu, P.; Dubuisson, R.; Houyou, S.; Nihart, R. New concept of CO2 removal technologies in power generation, combined with fossil fuel recovery and long term CO2 sequestration. In Proceedings of the ASME Turbo Expo 2000: Power for Land, Sea, and Air, Munich, Germany, 8–11 May 2000. Paper No: 2000-GT-0160. [Google Scholar]
- Allam, R.J.; Palmer, M.; JR, G.W.B. System and Method for High Efficiency Power Generation Using a Carbon Dioxide Circulating Working Fluid. U.S. Patent 8,596,075, 3 December 2013. [Google Scholar]
- Kirubakaran, V.; Bhatt, D. Effect of different turbulence model on prediction of lean blowout limit for micro gas turbine combustor. Adv. Mater. Process. Technol. 2020, 8, 1324–1335. [Google Scholar] [CrossRef]
- Klara, J.M.; Plunkett, J.E. The potential of advanced technologies to reduce carbon capture costs in future IGCC power plants. Int. J. Greenh. Gas Control 2010, 4, 112–118. [Google Scholar] [CrossRef]
- Rogalev, A.N.; Kindra, V.O.; Rogalev, N.D.; Sokolov, V.P.; Milukov, I.A. Methods for efficiency improvement of the semi-closed oxy-fuel combustion combined cycle. J. Phys. Conf. Ser. 2018, 1111, 012003. [Google Scholar] [CrossRef]
- Kindra, V.O.; Zlyvko, O.V.; Zonov, A.S.; Kaplanovich, I.B. Oxygen-Fuel Power Plant. RU Patent 2,749,081, 3 June 2021. [Google Scholar]
- Rogalev, A.; Rogalev, N.; Kindra, V.; Zlyvko, O.; Vegera, A. A study of low-potential heat utilization methods for oxy-fuel combustion power cycles. Energies 2021, 14, 3364. [Google Scholar] [CrossRef]
- Yu, H.; Gundersen, T.; Gençer, E. Optimal Liquified Natural Gas (LNG) cold energy utilization in an allam cycle power plant with carbon capture and storage. Energy Convers. Manag. 2021, 228, 113725. [Google Scholar] [CrossRef]
- Beyrami, J.; Jalili, M.; Ziyaei, M.; Chitsaz, A.; Rosen, M.A. A novel system for electricity and synthetic natural gas production from captured CO2: Techno-economic evaluation and multi-objective optimization. J. CO2 Util. 2022, 63, 102116. [Google Scholar] [CrossRef]
- Driscoll, M.J.; Driscoll, P.M.J.; Paul, D.; Pickard, S. Supercritical CO2 Plant Cost Assessment; Report No: MIT-GFR-019 Interim Topical Report; Center for Advanced Nuclear Energy Systems, MIT Nuclear Engineering Department, Massachusetts Institute of Technology: Cambridge, MA, USA, 2004. [Google Scholar]
- Weiland, N.T.; Lance, B.W.; Pidaparti, S.R. sCO2 power cycle component cost correlations from DOE data spanning multiple scales and applications. In Proceedings of the ASME Turbo Expo 2019: Turbomachinery Technical Conference and Exposition; American Society of Mechanical Engineers: New York City, NY, USA, 2019. [Google Scholar]
- US Energy Information Administration. Capital Cost Estimates for Utility Scale Electricity Generating Plants; U.S. Department of Energy: Washington, DC, USA, 2016; 142p.
- Sharifzadeh, M.; Shah, N. Carbon capture from natural gas combined cycle power plants: Solvent performance comparison at an industrial scale. AIChE J. 2016, 62, 166–179. [Google Scholar] [CrossRef]
- Khadse, A.; Blanchette, L.; Kapat, J.; Vasu, S.; Ahmed, K. Optimization of supercritical CO2 Brayton cycle for simple cycle gas turbines exhaust heat recovery using genetic algorithm. In Proceedings of the ASME Turbo Expo 2017: Turbomachinery Technical Conference and Exposition, Charlotte, NC, USA, 26–30 June 2017; American Society of Mechanical Engineers: New York City, NY, USA, 2017. [Google Scholar]
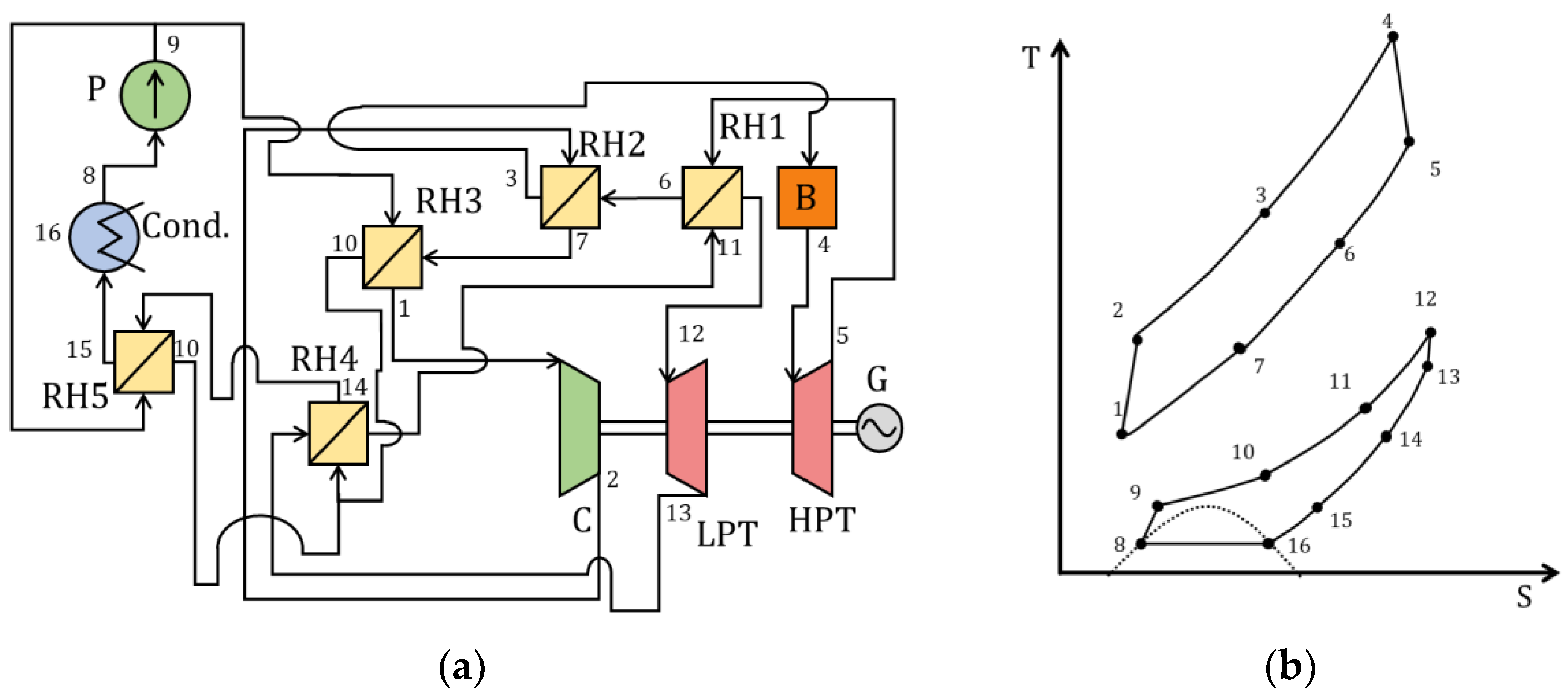

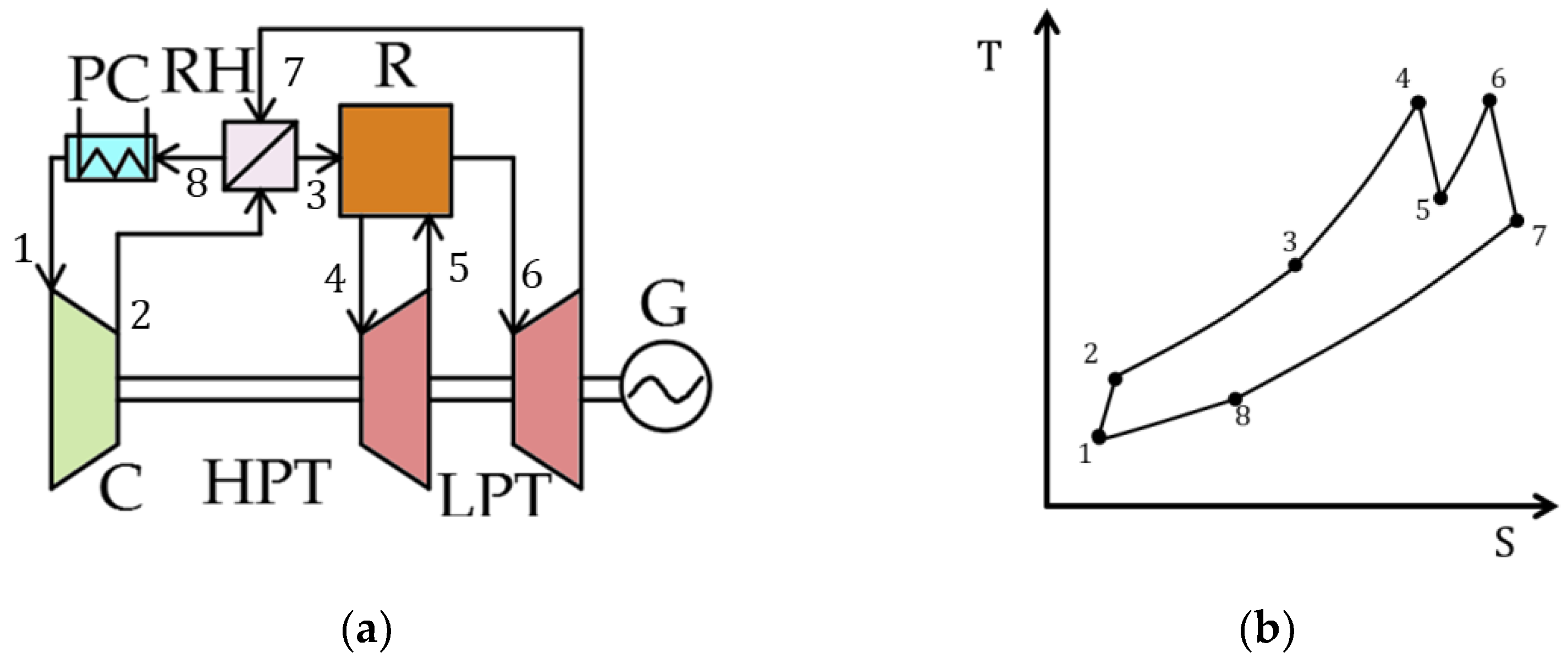
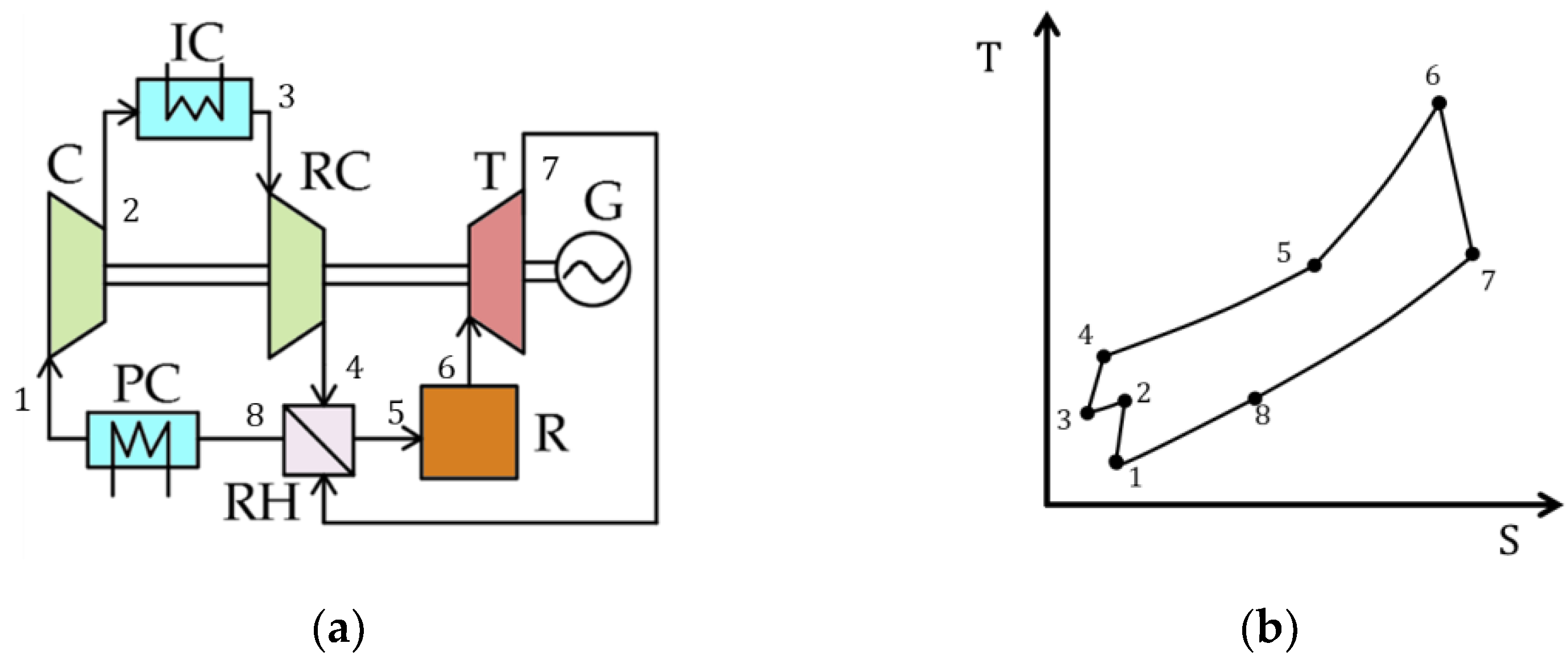
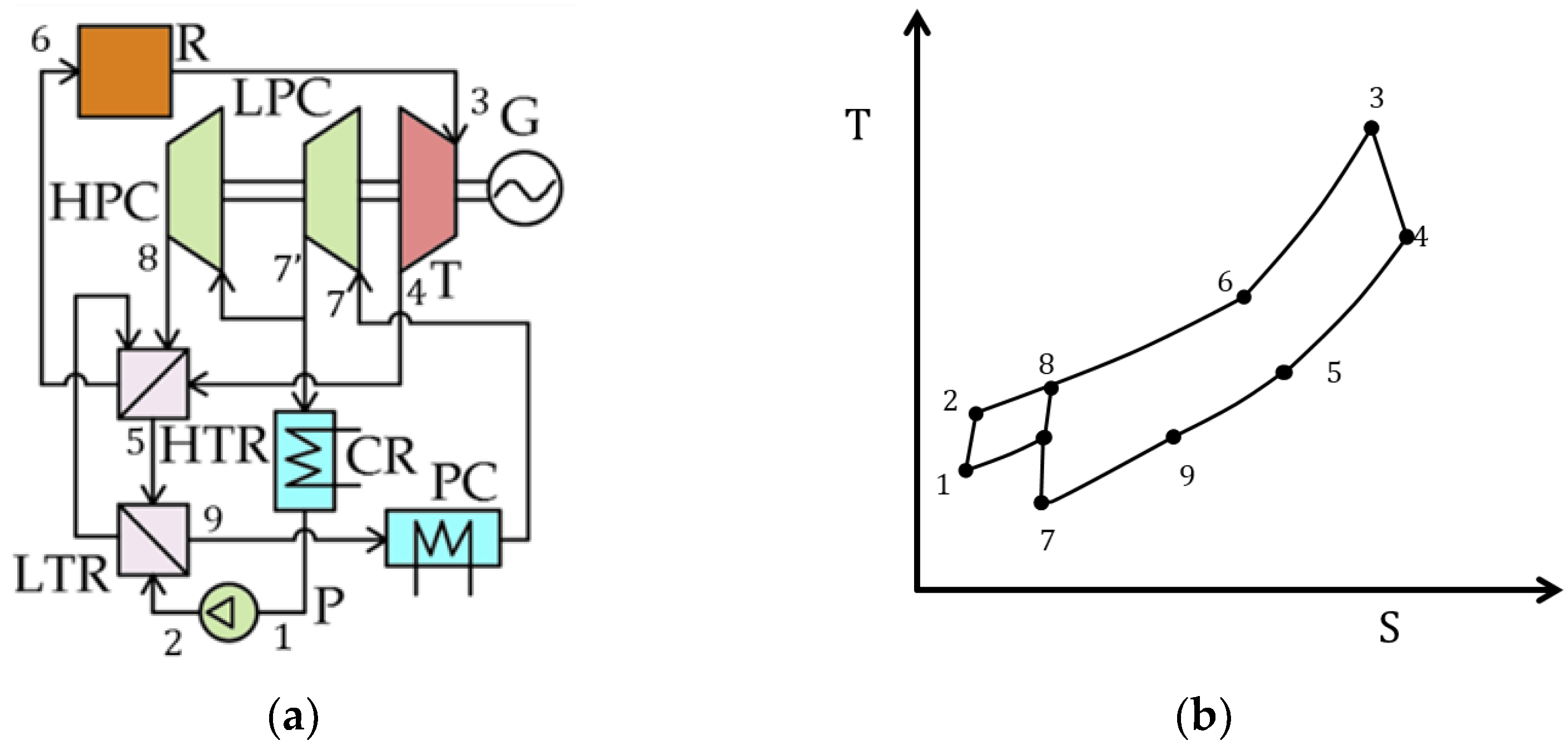

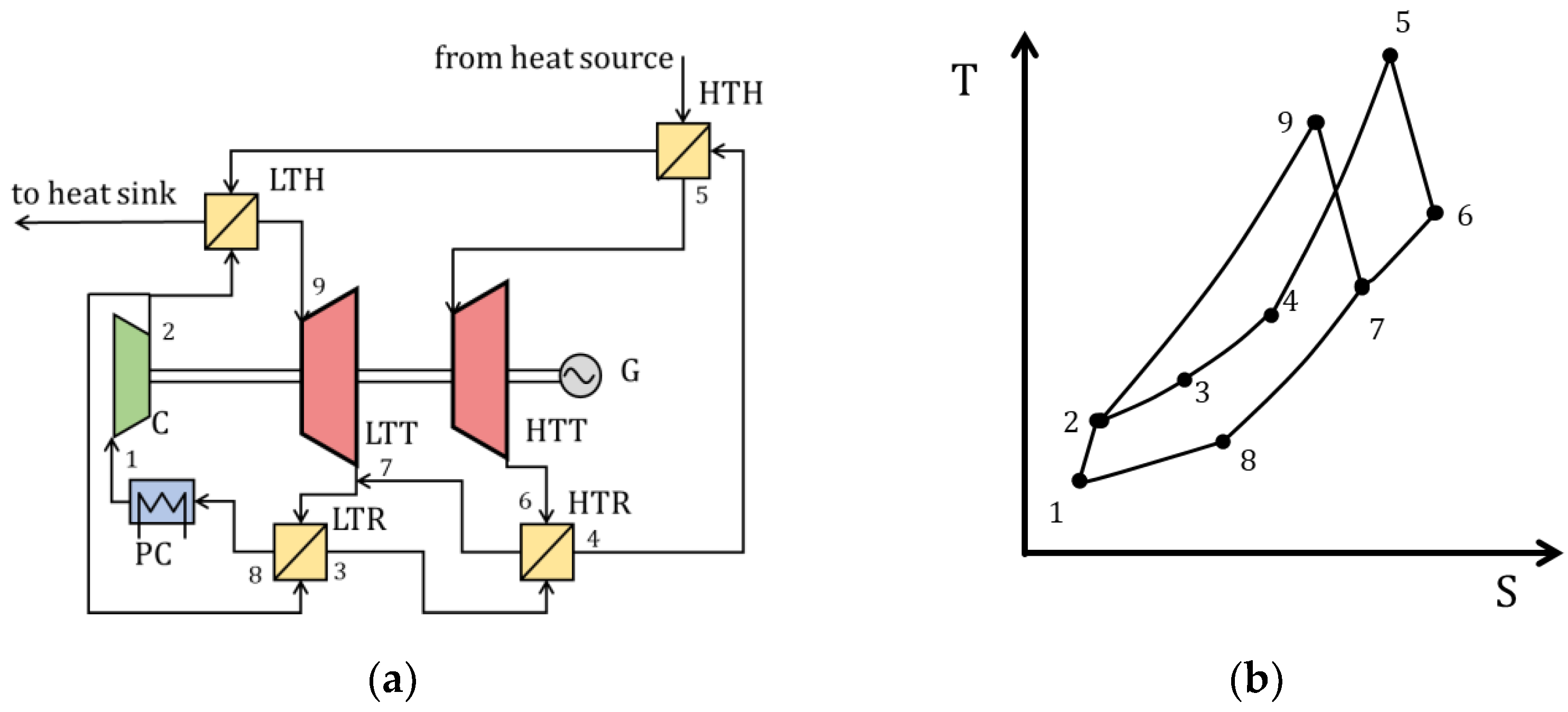
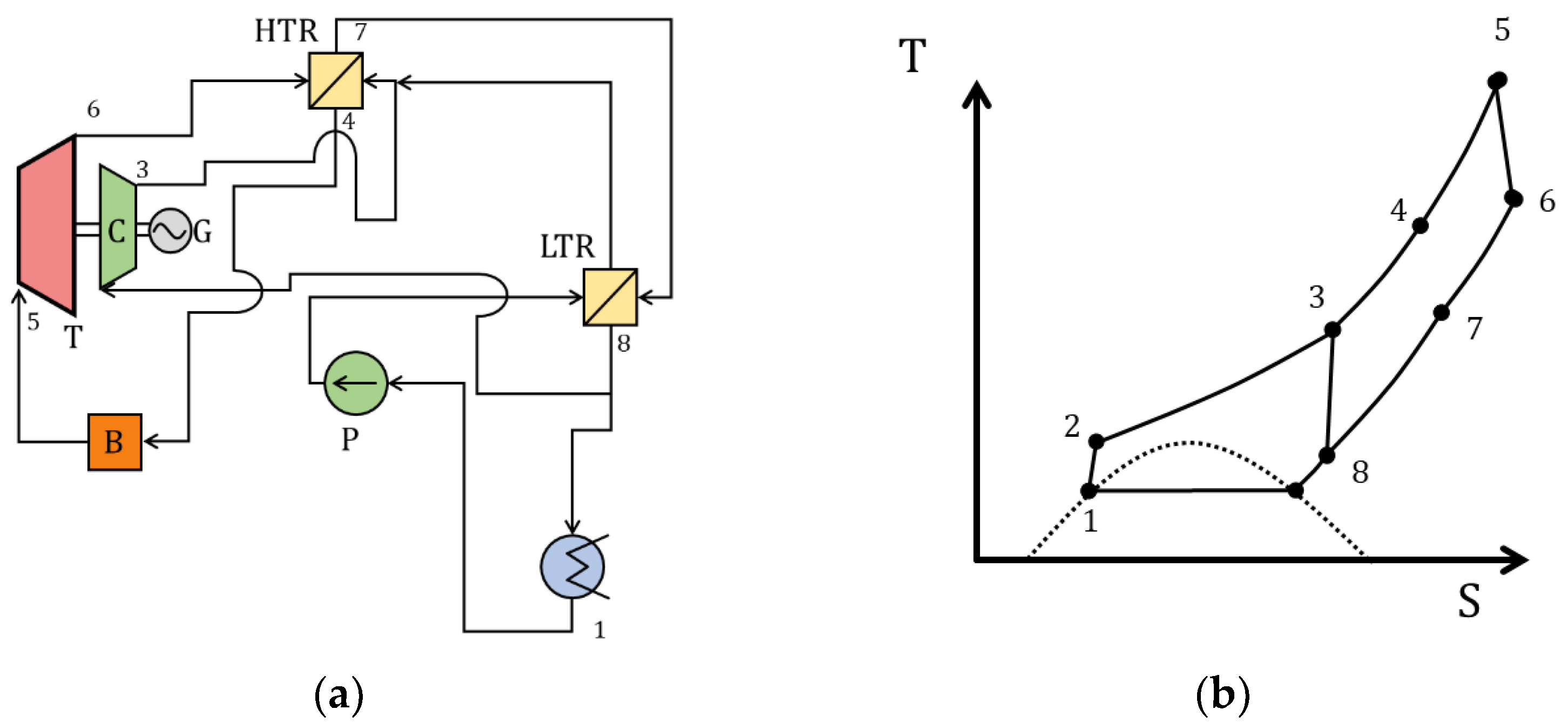

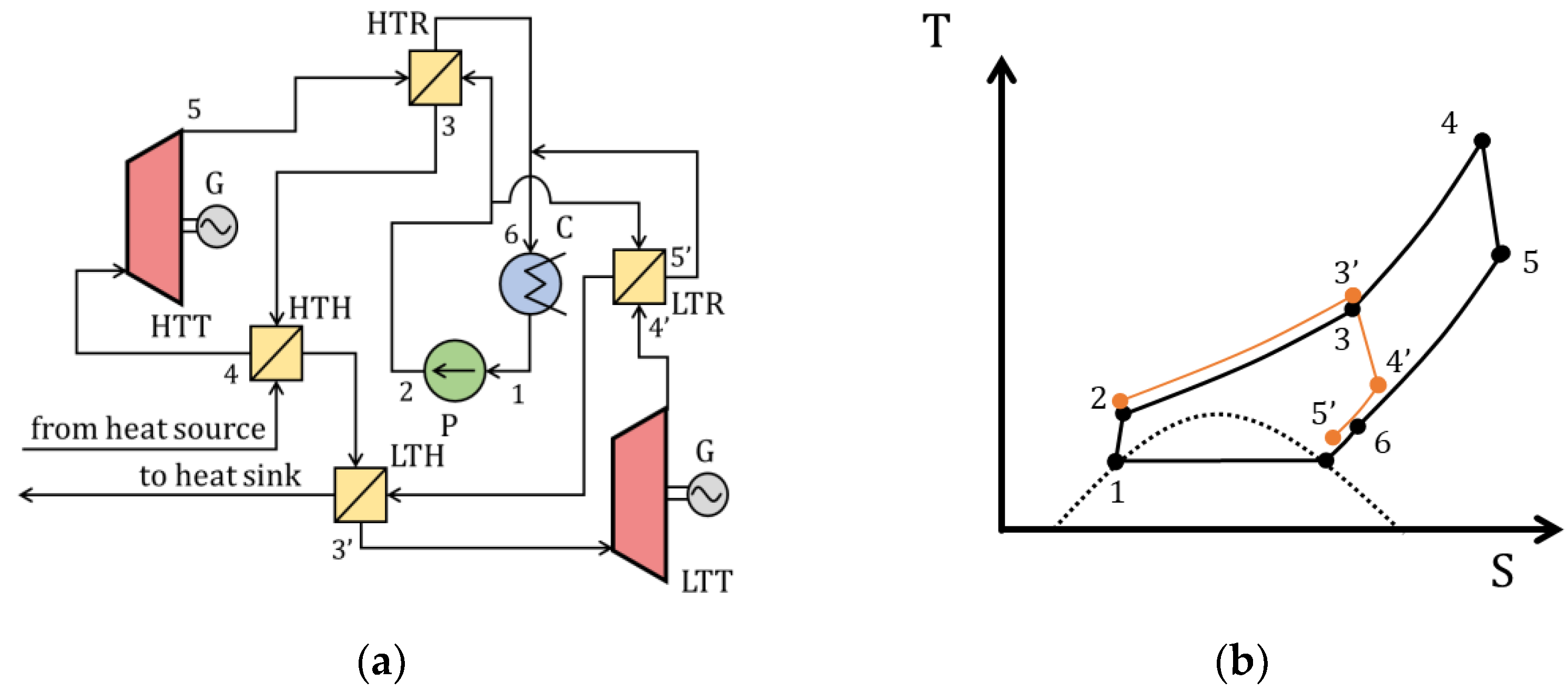
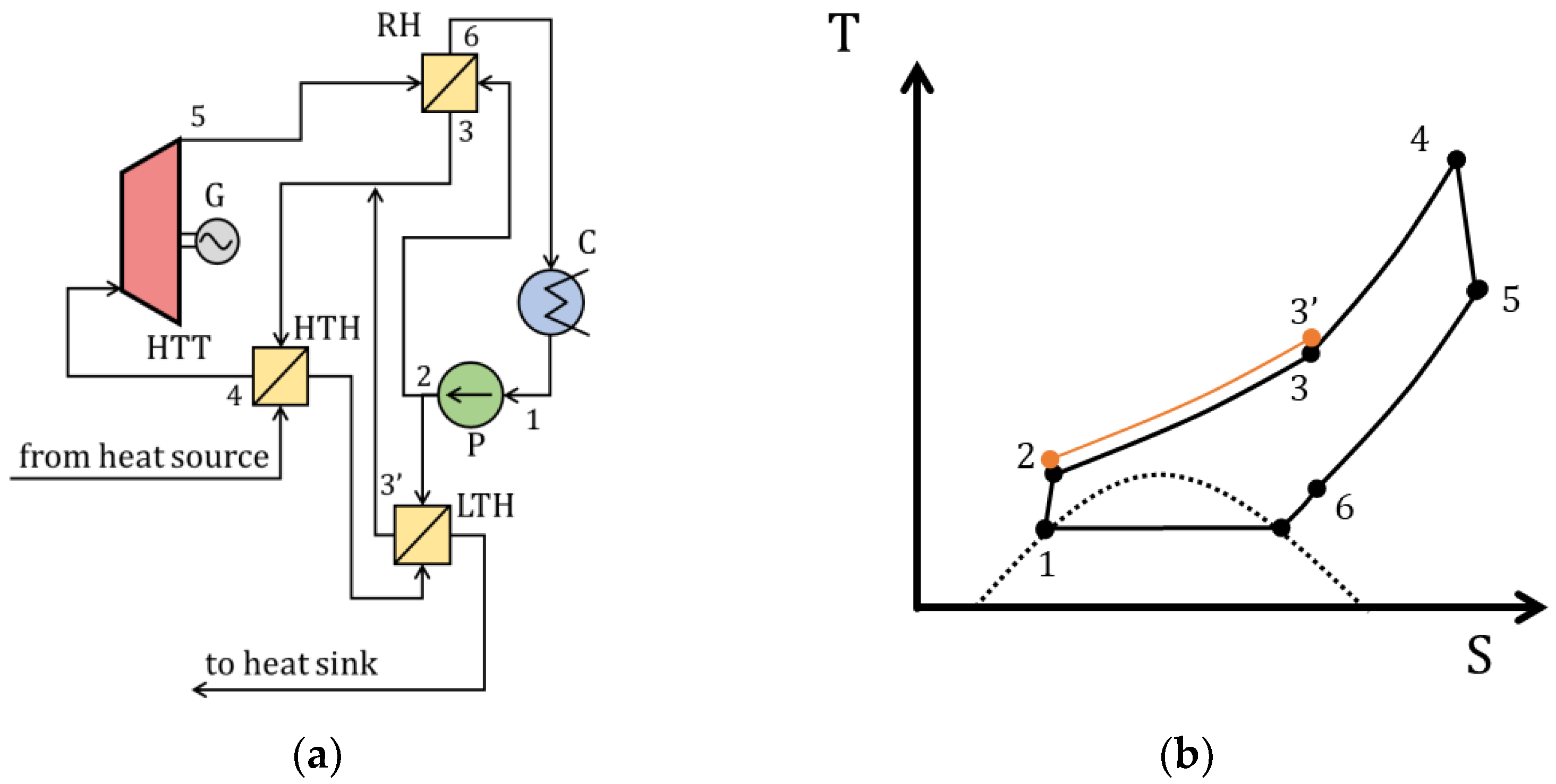

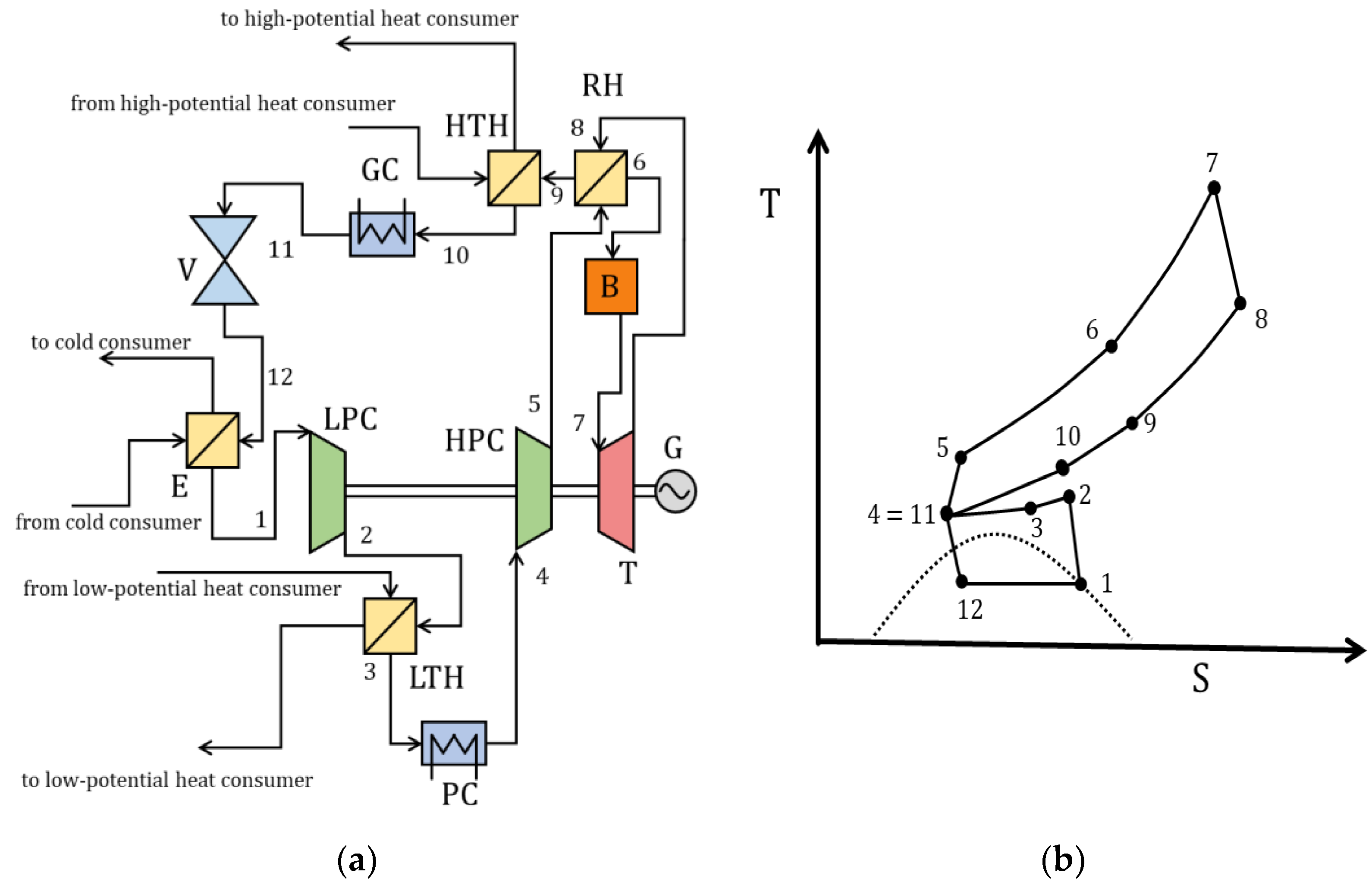
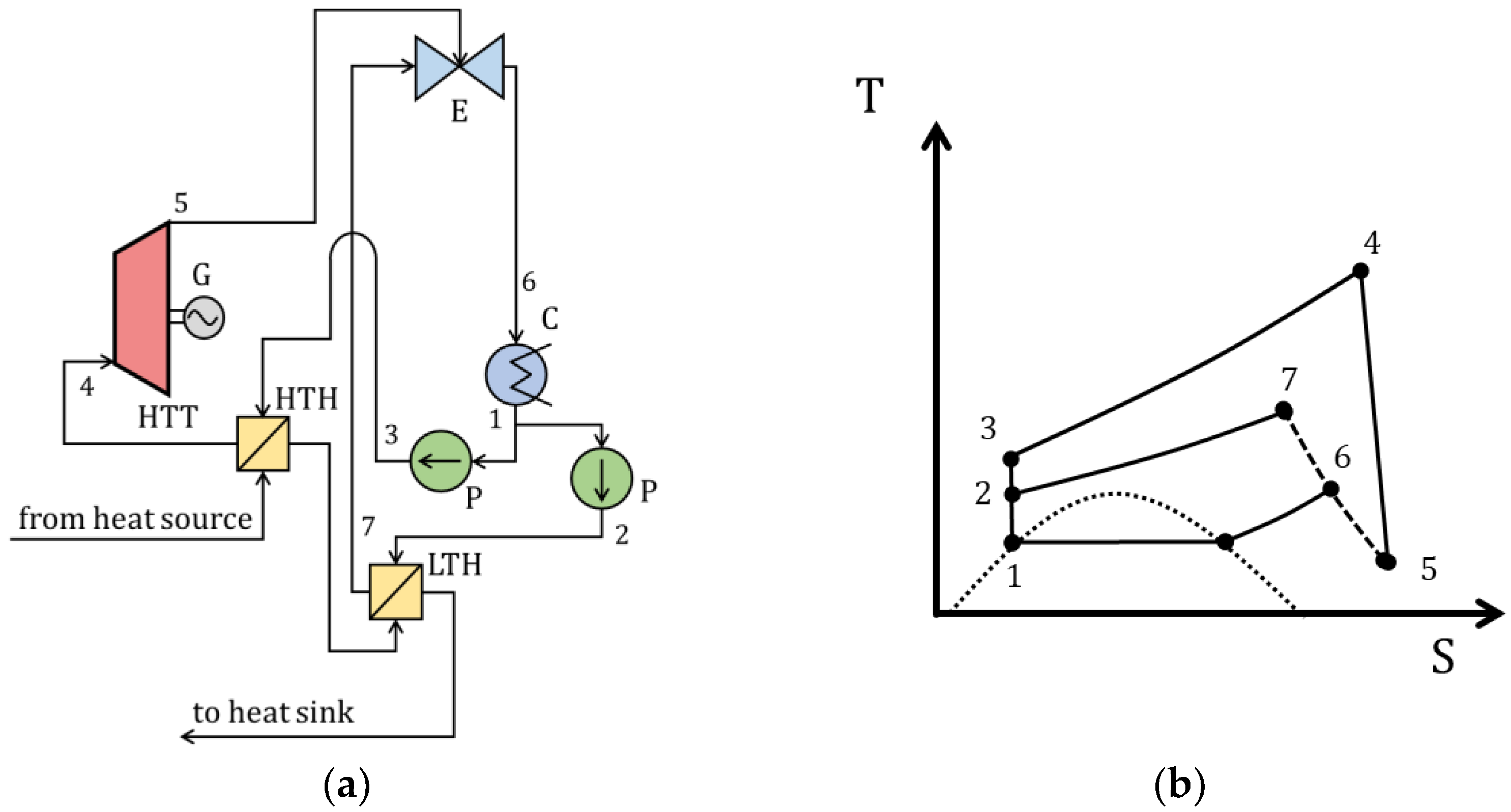
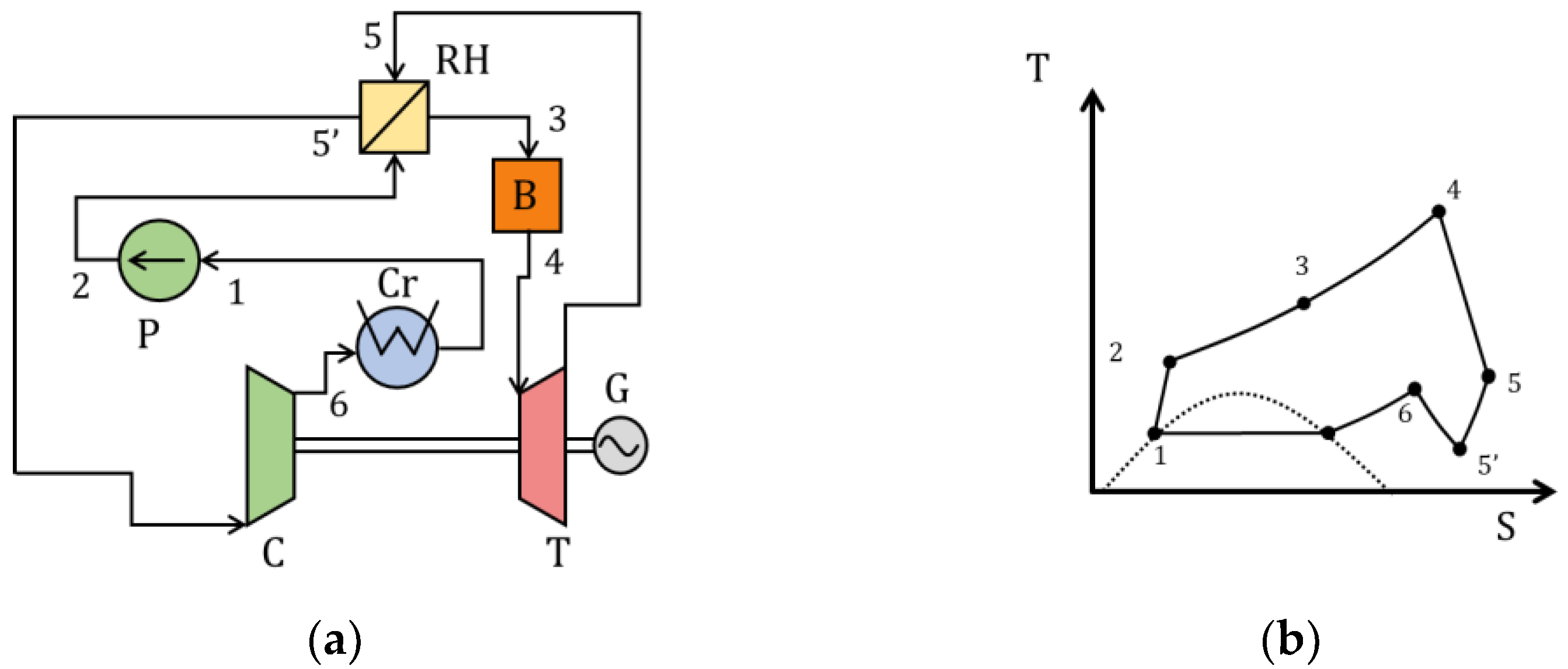
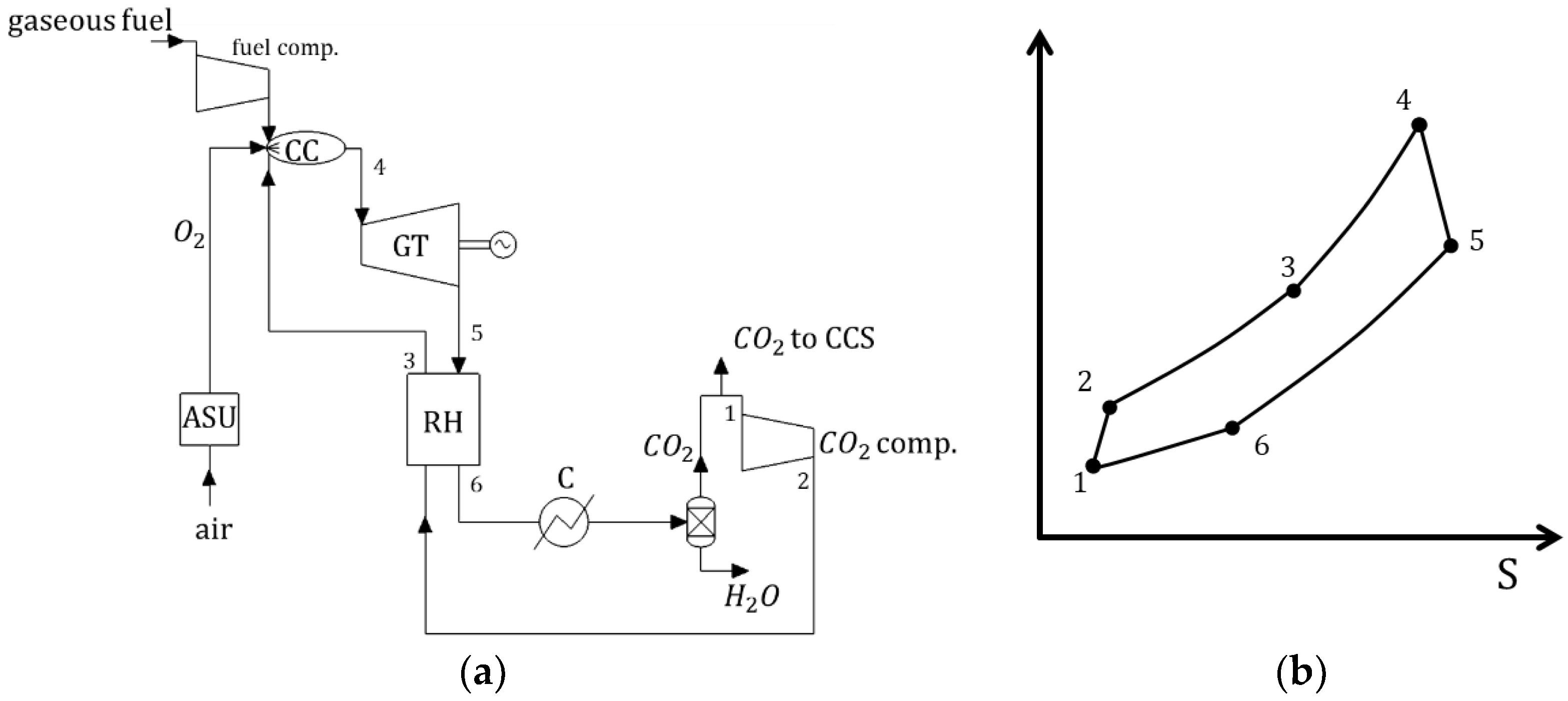
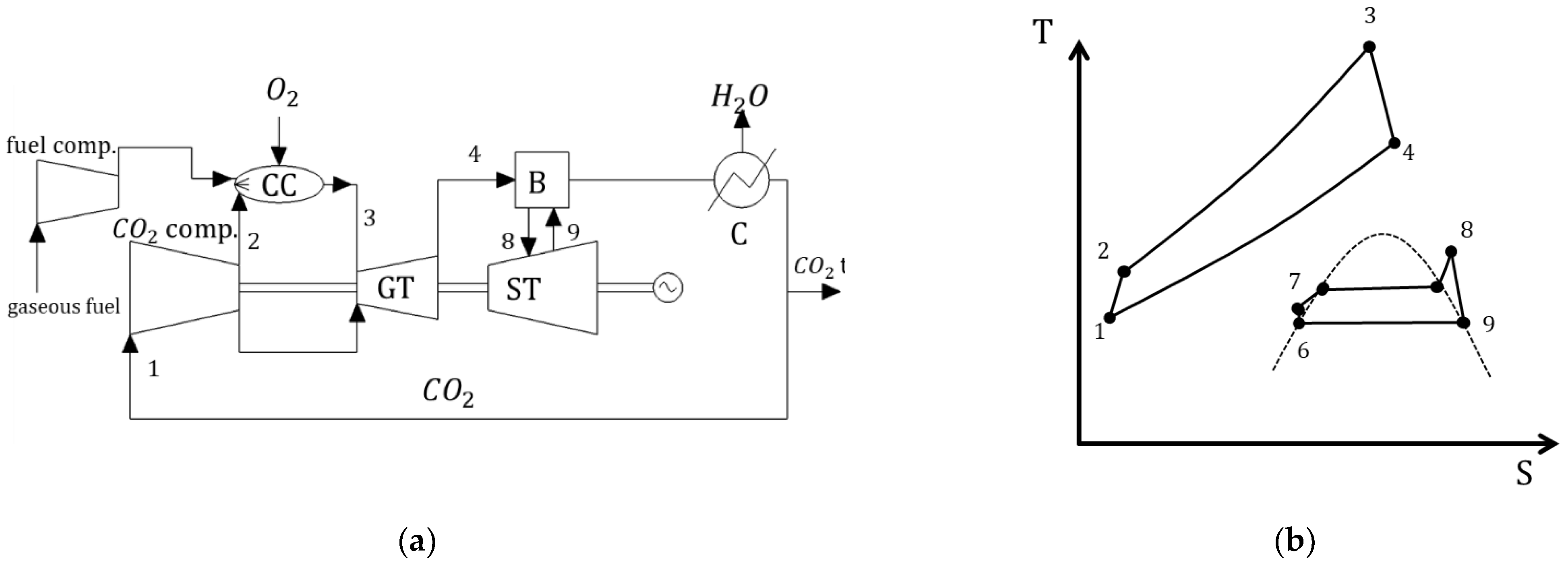

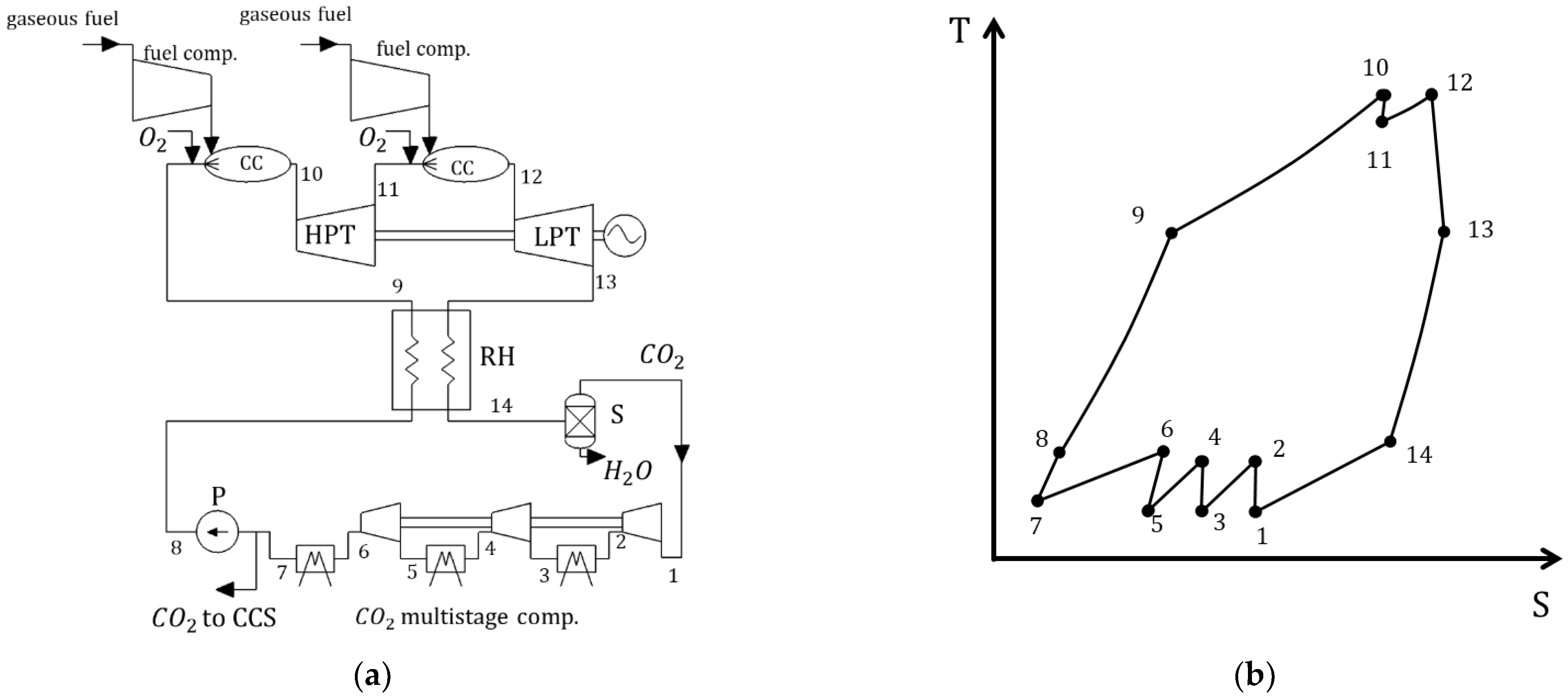
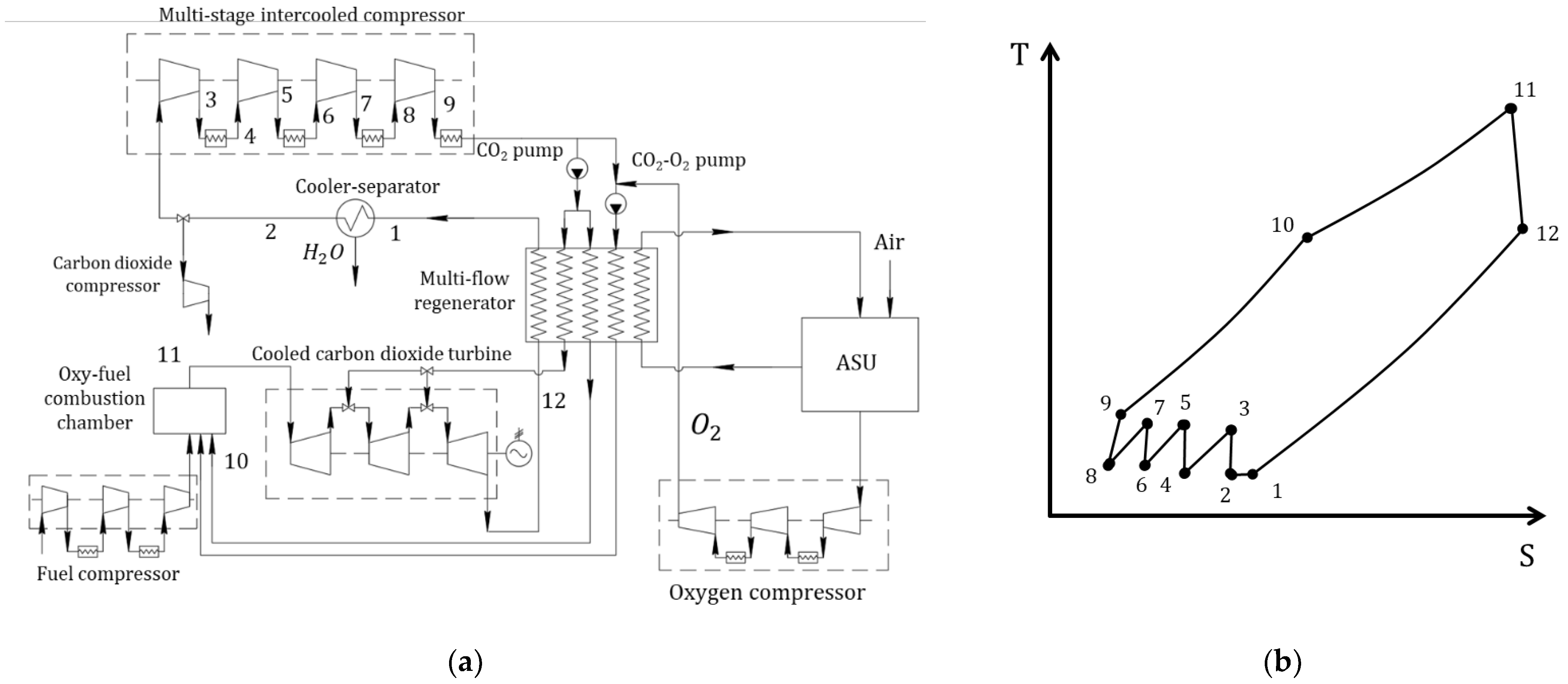
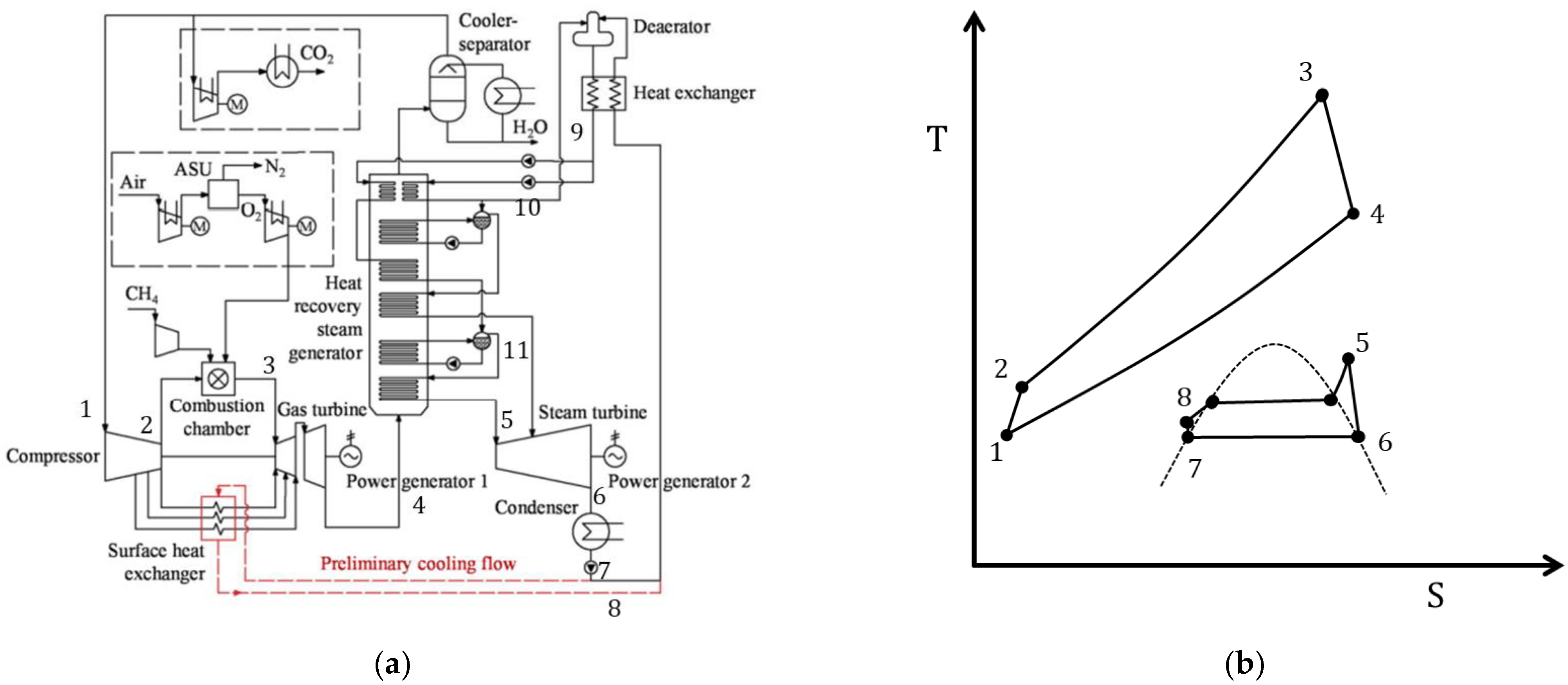



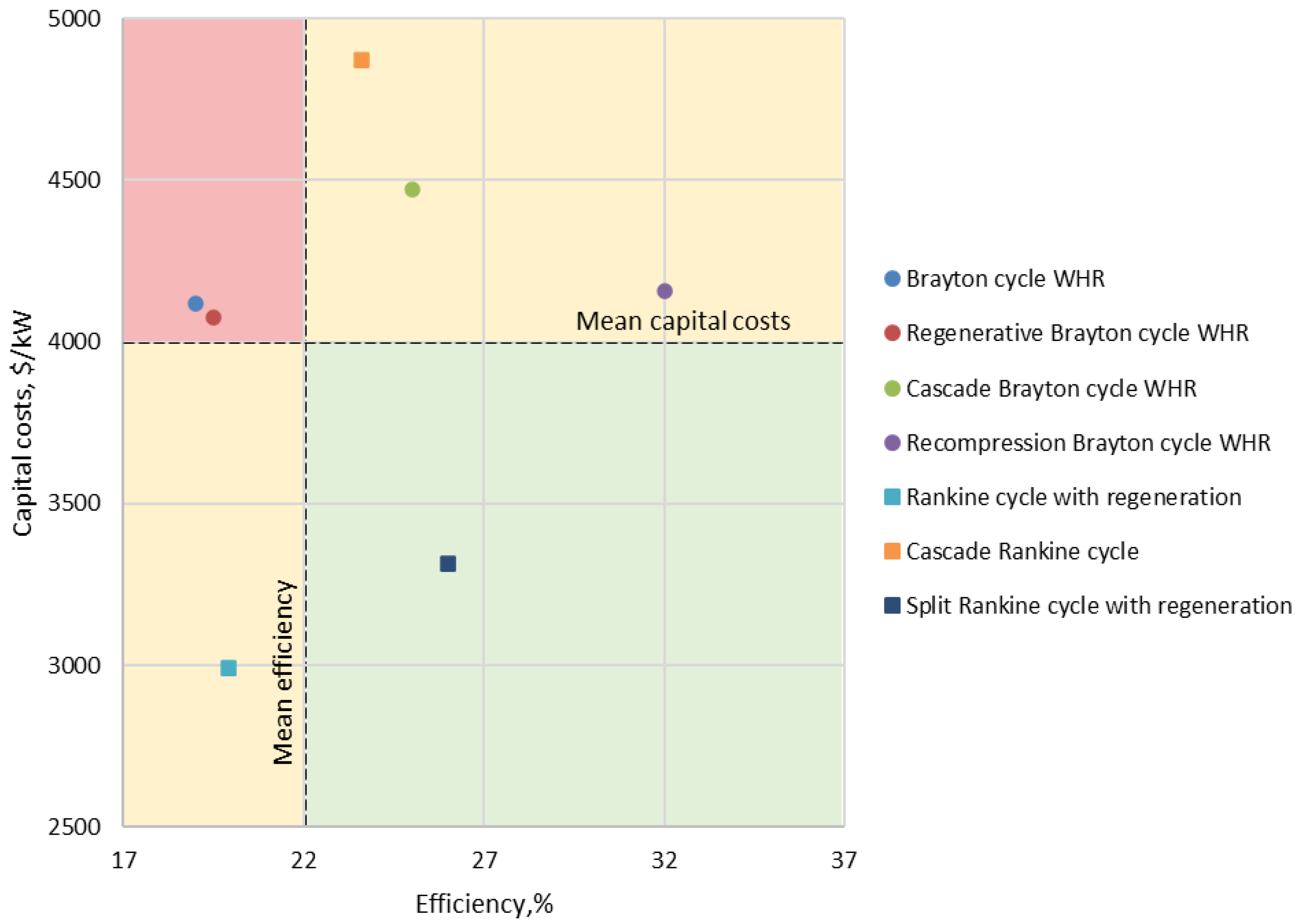
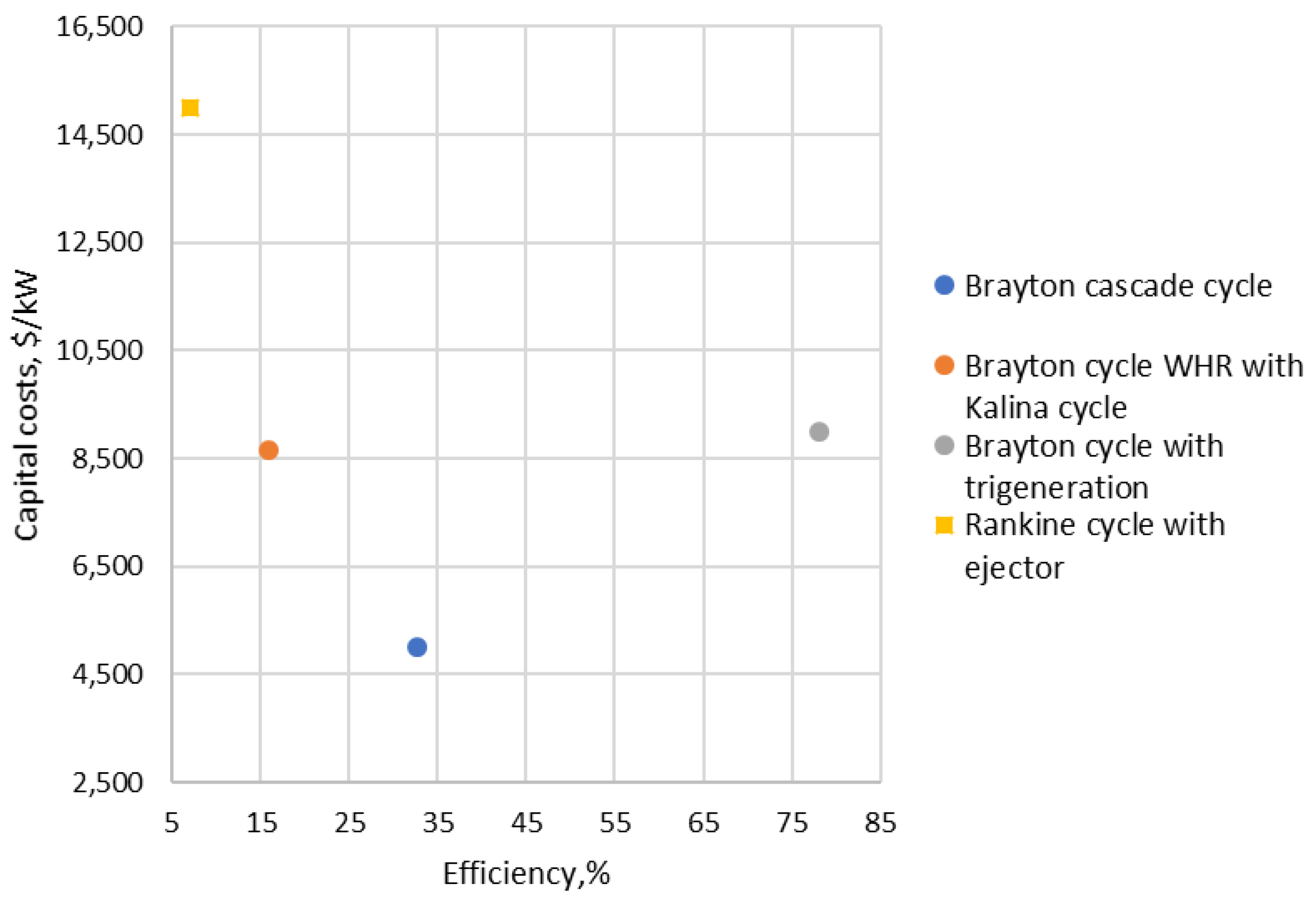

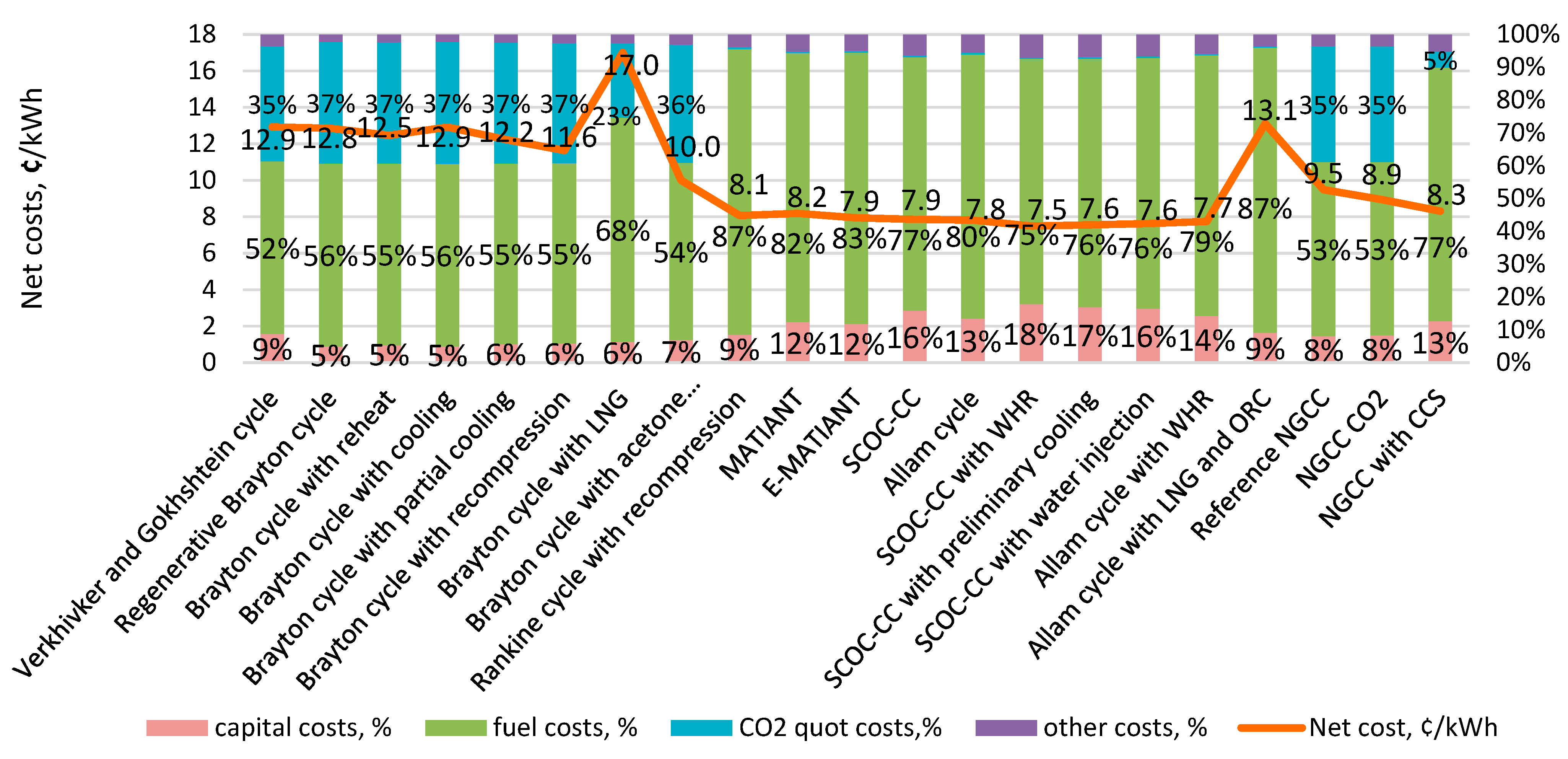
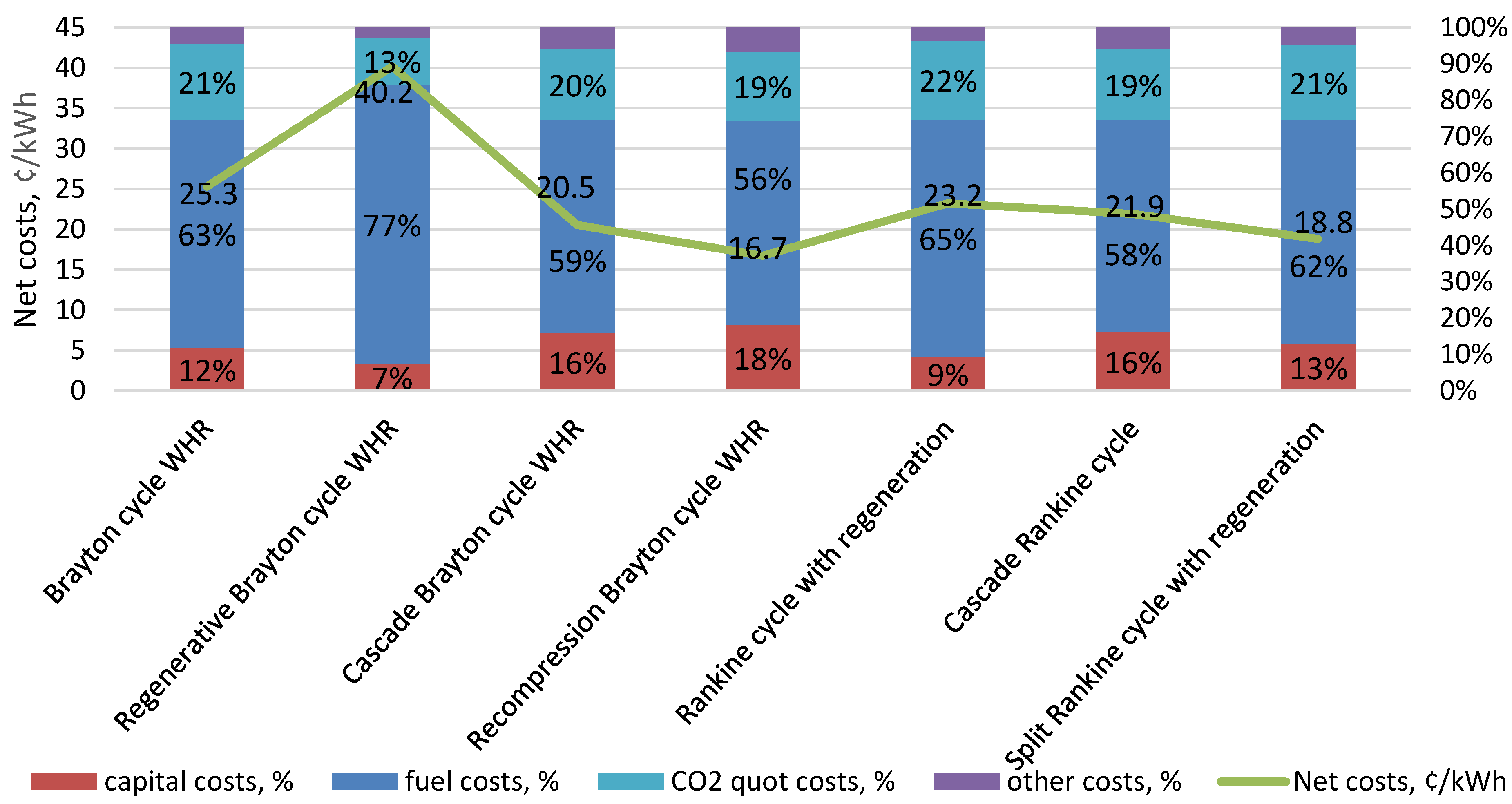

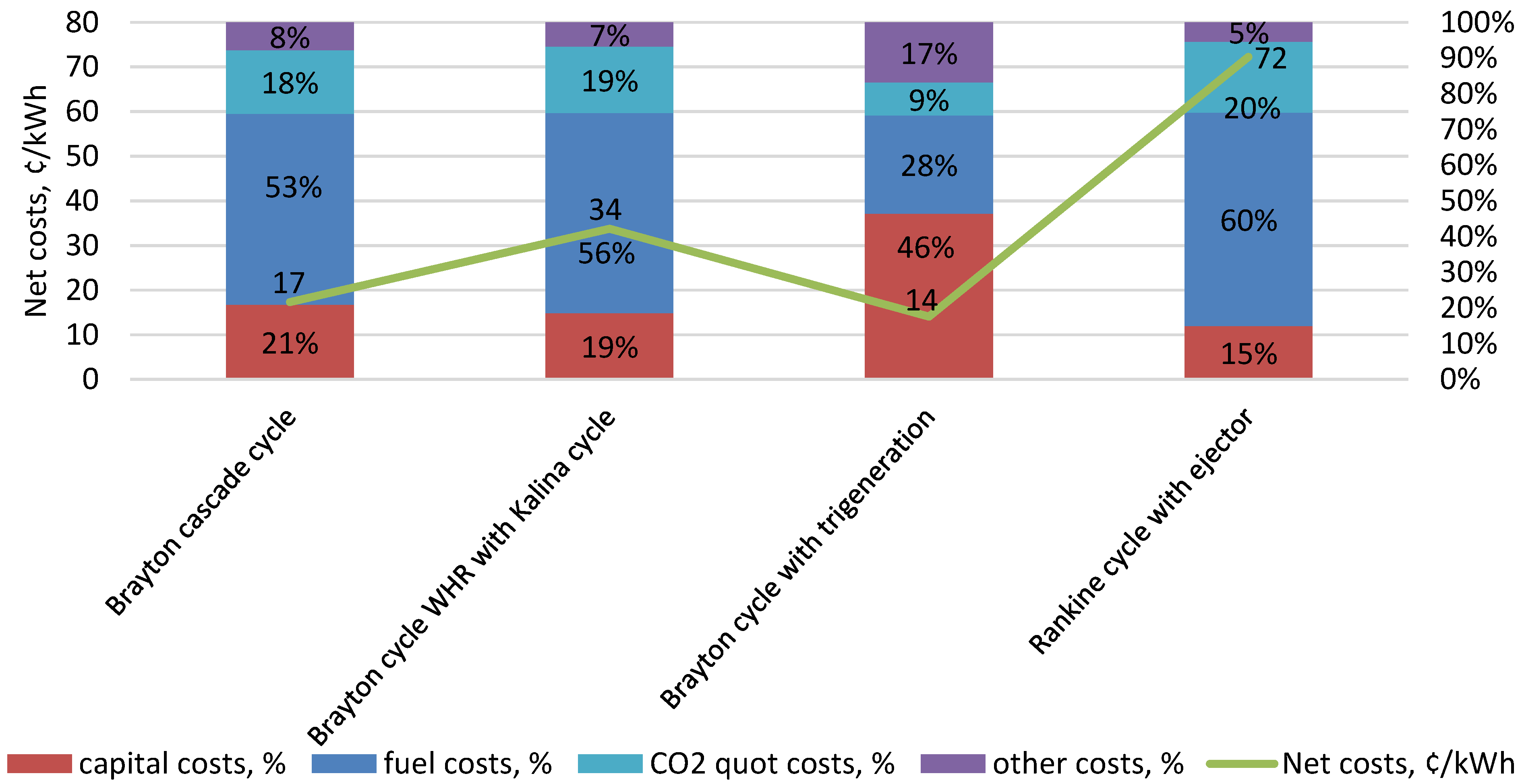

| Type of Thermal Power Plant | Temperature and Pressure of Working Fluid for Turbine Inlet/Outlet | Density of Working Fluid for Turbine Inlet/Outlet, kg/m3 | Dynamic Viscosity of Working Fluid for Turbine Inlet/Outlet, ·106 Pa·s | Specific Enthalpy of Working Fluid for Turbine Inlet/Outlet, kJ/kg |
|---|---|---|---|---|
| Steam turbine | 540 °C, 23.5 MPa/29 °C, 4 kPa | 74.6/0.004 | 320.8/0.11 | 3324.3/120.9 |
| NGCC | 1100 °C, 1.3 MPa/515 °C, 0.1 MPa | 3.3/0.44 | 535.9/369.8 | 1610.4/935.2 |
| Supercritical CO2 power plant | 540 °C, 25 MPa/407 °C, 8 MPa | 155.8/70.8 | 373.9/318.6 | 1019.1/875.3 |
| № | Cycle | Nref, MW | Tinlet, Tref, °C | η, % | Cc, $/kW | Es, g/kWh | Links |
|---|---|---|---|---|---|---|---|
| 1 | Verkhivker and Gokhshtein cycle | 1000 | 675, 675 | 44.5 | 1573 | 453 | [11] |
| 2 | Regenerative Brayton cycle | 1000 | 550–1200, 550 | 42.20 | 878 | 477 | [12] |
| 3 | Brayton cycle with reheating | 1000 | 550–1200, 550 | 43.70 | 901 | 461 | [12] |
| 4 | Brayton cycle with cooling | 1000 | 550–1200, 550 | 41.96 | 869 | 480 | [12] |
| 5 | Brayton cycle with partial cooling | 1000 | 550–1200, 550 | 44.80 | 946 | 450 | [12] |
| 6 | Brayton cycle with recompression | 1000 | 550–1200, 550 | 47.28 | 940 | 426 | [12] |
| 7 | Brayton cascade cycle | 1.4 | 500, 500 | 32.60 | 5000 | 618 | [16] |
| 8 | Brayton cycle WHR | 15 | 200–600, 400 | 19.00 | 4119 | 1060 | [17] |
| 9 | Regenerative Brayton cycle WHR | 15 | 200–600, 400 | 19.50 | 4077 | 1033 | [17] |
| 10 | Cascade Brayton cycle WHR | 15 | 200–600, 400 | 25.00 | 4469 | 806 | [17] |
| 11 | Recompression Brayton cycle WHR | 15 | 200–600, 400 | 32.00 | 4155 | 629 | [17] |
| 12 | Brayton cycle WHR with Kalina cycle | 0.242 | 268, 268 | 16.00 | 8660 | 1259 | [18] |
| 13 | Brayton cycle with LNG | 80 | 300–600, 550 | 52 | 1491 | 387 | [20,21] |
| 14 | Brayton cycle with acetone absorption | 1000 | 1400, 1400 | 56.00 | 960 | 360 | [24] |
| 15 | Brayton cycle with trigeneration | 0.1 | 400–1000, 1000 | 78.00 | 9000 | 258 | [22] |
| 16 | Rankine cycle with recompression | 1000 | 550, 550 | 43.00 | 950 | 5 | [13] |
| 17 | Rankine cycle with regeneration | 10 | 300–500, 400 | 19.90 | 2994 | 1012 | [19] |
| 18 | Cascade Rankine cycle | 10 | 300–500, 400 | 23.60 | 4870 | 853 | [19] |
| 19 | Split Rankine cycle with regeneration | 10 | 300–500, 400 | 26.00 | 3316 | 775 | [19] |
| 20 | Rankine cycle with ejector | 0.01 | 100, 100 | 7.00 | 15,000 | 2877 | [23] |
| 21 | MATIANT | 300 | 1100–1300, 1300 | 45.00 | 1400 | 4 | [32] |
| 22 | E-MATIANT | 300 | 1100–1700, 1300 | 46.00 | 1300 | 4 | [34] |
| 23 | SCOC-CC | 300 | 1000–1700, 1700 | 49.80 | 1722 | 4 | [27] |
| 24 | Allam cycle | 300 | 1100–1300, 11100 | 48.00 | 1452 | 4 | [35] |
| 25 | SCOC-CC with WHR | 300 | 1000–1700, 1700 | 54 | 1849 | 2 | [39] |
| 26 | SCOC-CC with preliminary cooling | 300 | 1100–1700, 1700 | 52.80 | 1764 | 4 | [38] |
| 27 | SCOC-CC with water injection | 300 | 1100–1700, 1700 | 51.80 | 1730 | 4 | [38] |
| 28 | Allam cycle with WHR | 300 | 1100–1300, 1100 | 49.10 | 1524.6 | 4 | [40] |
| 29 | Allam cycle with LNG and ORC | 300 | 1100–1300, 1100 | 53.00 | 1640.76 | 4 | [41] |
| 30 | MATIANT with methane synthesis | 100 | 1300, 1300 | 43.37 | 3800 | 5 | [42] |
| Cycle | Nref, MW | η, % | Cc, $/kW | Es, g/kWh | Links |
|---|---|---|---|---|---|
| Reference NGCC | 300 | 60 | 1050 | 336 | [45] |
| NGCC with CCS | 300 | 47 | 1029 | 315 | [46] |
| NGCC with CO2 cycle | 300 | 64 | 1449 | 40 | [47] |
| , kWh/kg | 13.8 |
| for natural gas, $/kg | 0.4 |
| for LNG, $/kg | 0.8 |
| , $/ton | 50;100 |
| 0.1 | |
| , year | 23 |
| , people/kW | 0.0002 |
| , $/hour | 12.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogalev, N.; Rogalev, A.; Kindra, V.; Zlyvko, O.; Bryzgunov, P. Review of Closed SCO2 and Semi-Closed Oxy–Fuel Combustion Power Cycles for Multi-Scale Power Generation in Terms of Energy, Ecology and Economic Efficiency. Energies 2022, 15, 9226. https://doi.org/10.3390/en15239226
Rogalev N, Rogalev A, Kindra V, Zlyvko O, Bryzgunov P. Review of Closed SCO2 and Semi-Closed Oxy–Fuel Combustion Power Cycles for Multi-Scale Power Generation in Terms of Energy, Ecology and Economic Efficiency. Energies. 2022; 15(23):9226. https://doi.org/10.3390/en15239226
Chicago/Turabian StyleRogalev, Nikolay, Andrey Rogalev, Vladimir Kindra, Olga Zlyvko, and Pavel Bryzgunov. 2022. "Review of Closed SCO2 and Semi-Closed Oxy–Fuel Combustion Power Cycles for Multi-Scale Power Generation in Terms of Energy, Ecology and Economic Efficiency" Energies 15, no. 23: 9226. https://doi.org/10.3390/en15239226
APA StyleRogalev, N., Rogalev, A., Kindra, V., Zlyvko, O., & Bryzgunov, P. (2022). Review of Closed SCO2 and Semi-Closed Oxy–Fuel Combustion Power Cycles for Multi-Scale Power Generation in Terms of Energy, Ecology and Economic Efficiency. Energies, 15(23), 9226. https://doi.org/10.3390/en15239226






