Efficient Combustion of Low Calorific Industrial Gases: Opportunities and Challenges
Abstract
1. Introduction
2. Composition and Combustion Characteristics of LCIG
2.1. Composition Characteristics
2.2. Fundamental Combustion Characteristics
3. Combustion Strategies of LCIG
3.1. Porous Media Combustion
- (1)
- The complex and diverse inner surface of the porous medium results in efficient heat transfer between the reactant flow and the inert solid;
- (2)
- Dispersion of the reactant flowing through a porous media promotes effective heat transfer and diffusion between the two phases.
3.2. Flameless Combustion
- (1)
- Produce a stable flame and operate the combustor in the classic flame mode. Continuously increase the combustor temperature until it exceeds the fuel’s auto-ignition temperature;
- (2)
- Increase the velocity of inflowing reactants, in order to raise the recirculation ratio, which causes the flame front to vanish and the mean temperature of the combustor to drop;
- (3)
- Diminish the visible and audible flame, and the reaction region spreads towards the downstream of the combustor. The whole combustion chamber enters flameless combustion mode.
3.3. Oxy-Fuel Combustion
3.4. Dual-Fuel Combustion
4. Technical Challenges
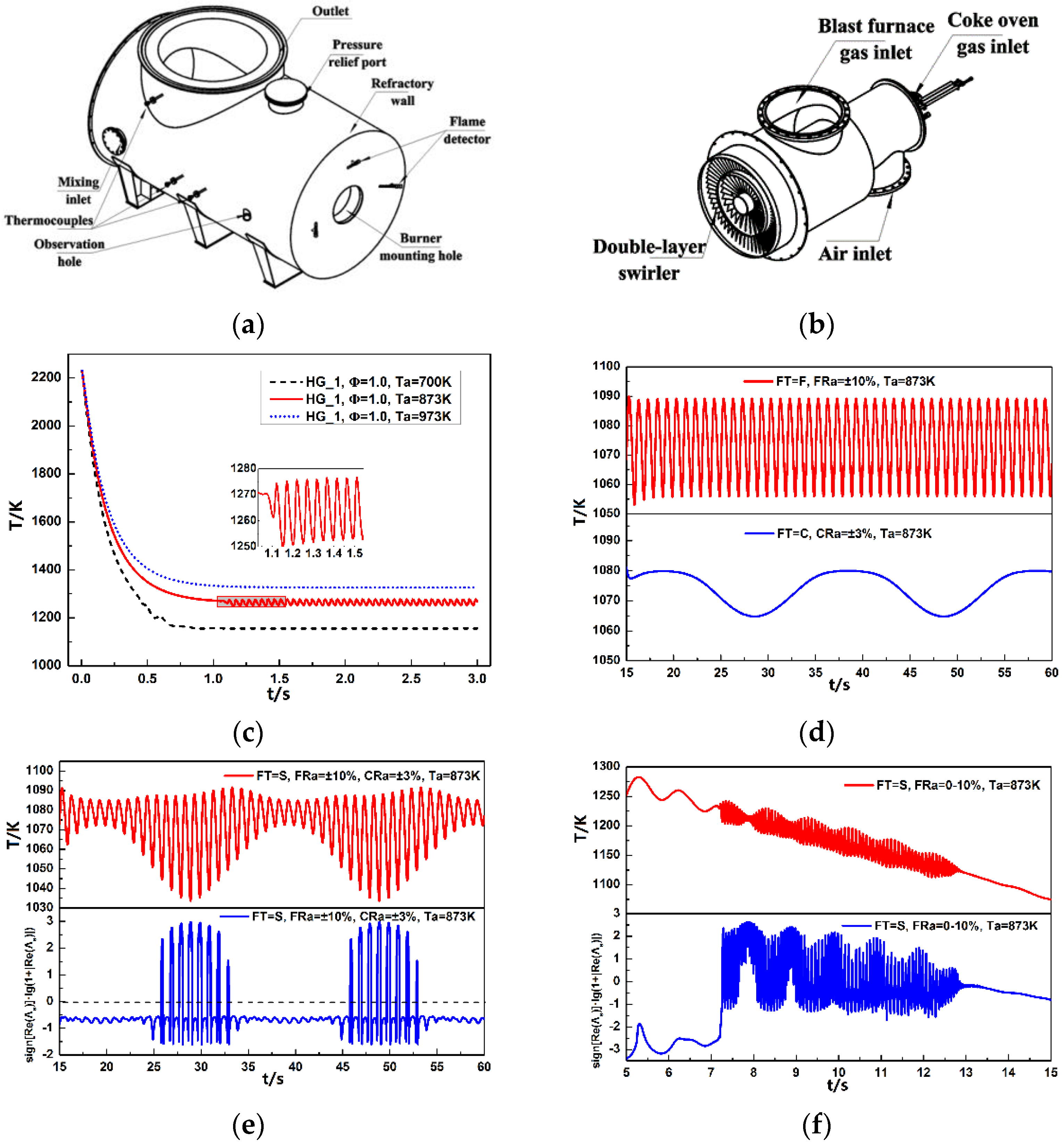
4.1. Oscillating Combustion
4.2. Pollutant Emissions
4.3. System Optimization
5. Conclusions
- (1)
- Low combustion efficiency;
- (2)
- Narrow flammable range;
- (3)
- High combustion instability.
- (1)
- Porous media combustion;
- (2)
- Flameless combustion;
- (3)
- Oxy-fuel combustion;
- (4)
- Dual-fuel combustion.
- (1)
- Mitigation of oscillating combustion;
- (2)
- Reduction in pollutant emissions;
- (3)
- Optimization of operating conditions for fuel flexibility.
- (1)
- Fundamental combustion characteristics under the industrial operating conditions;
- (2)
- In situ adaptive control for burning LCIG;
- (3)
- Optimization of LCIG combustion during operation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajiyev, N.; Abdimomynova, A.; Trukhan, D. Global and local aspects of world energy consumption: Forecast and risks. Proc. Inst. Civ. Eng. Energy 2022, 7, 1–32. [Google Scholar] [CrossRef]
- Li, S.; Zhou, H.; Hou, L.; Ren, Z. An analytic model for the effects of nitrogen dilution and premixing characteristics on NOx formation in turbulent premixed hydrogen flames. Int. J. Hydrogen Energy 2017, 42, 7060–7070. [Google Scholar] [CrossRef]
- Shu, T.; Xue, Y.; Zhou, Z.; Ren, Z. An experimental study of laminar ammonia/methane/air premixed flames using expanding spherical flames. Fuel 2021, 290, 120003. [Google Scholar] [CrossRef]
- Lu, J.; Gao, X. Biogas: Potential, challenges, and perspectives in a changing China. Biomass-Bioenergy 2021, 150, 106127. [Google Scholar] [CrossRef]
- Jung, S.; Lee, J.; Moon, D.H.; Kim, K.H.; Kwon, E.E. Upgrading biogas into syngas through dry reforming. Renew. Sustain. Energy Rev. 2021, 143, 110949. [Google Scholar] [CrossRef]
- Pena, J.G.C.; de Oliveira, V.B.; Salles, J.L.F. Optimal scheduling of a by-product gas supply system in the iron- and steel-making process under uncertainties. Comput. Chem. Eng. 2019, 125, 351–364. [Google Scholar] [CrossRef]
- Jiang, X.; Sommer, S.G.; Christensen, K.V. A review of the biogas industry in China. Energy Policy 2011, 39, 6073–6081. [Google Scholar] [CrossRef]
- Yao, X.; Zhao, Z.; Chen, S.; Zhou, H.; Xu, K. Migration and transformation behaviours of ash residues from a typical fixed-bed gasification station for biomass syngas production in China. Energy 2020, 201, 117646. [Google Scholar] [CrossRef]
- Zhou, D.; Cheng, S.; Wang, Y.; Jiang, X. The production of large blast furnaces during 2016 and future development of ironmaking in China. Ironmak. Steelmak. 2017, 44, 714–720. [Google Scholar] [CrossRef]
- Blakey, S.; Rye, L.; Wilson, C.W. Aviation gas turbine alternative fuels: A review. Proc. Combust. Inst. 2011, 33, 2863–2885. [Google Scholar] [CrossRef]
- Wright, I.G.; Gibbons, T.B. Recent developments in gas turbine materials and technology and their implications for syngas firing. Int. J. Hydrogen Energy 2007, 32, 3610–3621. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, R.; Sharma, S.; Kumar, S. Effect of engine parameters on the performance of dual-fuel CI engines with producer gas—A review. Energy Fuels 2021, 35, 16377–16402. [Google Scholar] [CrossRef]
- Pradhan, A.; Baredar, P.; Kumar, A. Syngas as an alternative fuel used in internal combustion engines: A review. J. Pure Appl. Sci. Technol. 2015, 5, 51–66. [Google Scholar]
- Huang, R.; Cheng, L.; Qiu, K.; Zheng, C.; Luo, Z. Low-calorific gas combustion in a two-layer porous burner. Energy Fuels 2016, 30, 1364–1374. [Google Scholar] [CrossRef]
- Song, F.; Wen, Z.; Dong, Z.; Wang, E.; Liu, X. Ultra-low calorific gas combustion in a gradually-varied porous burner with annular heat recirculation. Energy 2017, 119, 497–503. [Google Scholar] [CrossRef]
- Nurmukan, D.; Chen, T.J.M.; Hung, Y.M.; Ismadi, M.; Chong, C.T.; Tran, M. Enhancement of biogas/air combustion by hydrogen addition at elevated temperatures. Int. J. Energy Res. 2020, 44, 1519–1534. [Google Scholar] [CrossRef]
- Chen, J.; Chen, G.; Zhang, A.; Deng, H.; Wen, X.; Wang, F.; Sheng, W.; Zheng, H. Numerical simulation of the effect of CH4/CO concentration on combustion characteristics of low calorific value syngas. ACS Omega 2021, 6, 5754–5763. [Google Scholar] [CrossRef]
- de Castro, R.R.; Brequigny, P.; Dufitumukiza, J.P.; Mounaim-Rousselle, C. Laminar flame speed of different syngas compositions for varying thermodynamic conditions. Fuel 2021, 301, 121025. [Google Scholar] [CrossRef]
- Benaissa, S.; Adouane, B.; Ali, S.M.; Mohammad, A. Effect of hydrogen addition on the combustion characteristics of premixed biogas/hydrogen-air mixtures. Int. J. Hydrogen Energy 2021, 46, 18661–18677. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Zhang, J.; Hou, X. Experimental study on self-acceleration characteristics of unstable flame of low calorific value gas blended with hydrogen. Int. J. Hydrogen Energy 2019, 44, 25248–25256. [Google Scholar] [CrossRef]
- Gong, X.; Ren, Z. Flame speed scaling in autoignition-assisted freely propagating n-heptane/air flames. Proc. Combust. Inst. 2021, 38, 2153–2161. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, G.; Li, H.; Zhang, G.; Lv, J. Study on the effect of hydrogen fraction on the premixed combustion characteristics of syngas/air mixtures. Energy 2020, 200, 117592. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Yu, T.; Liu, H.; Cai, W.; Weng, S. Effect of carbon dioxide content in biogas on turbulent combustion in the combustor of micro gas turbine. Renew. Energy 2020, 147, 1299–1311. [Google Scholar] [CrossRef]
- Devi, S.; Sahoo, N.; Muthukumar, P. Effect of combustion zone material on the thermal performance of a biogas-fuelled porous media burner: Experimental studies. Biomass Convers. Biorefin. 2022, 12, 1555–1563. [Google Scholar] [CrossRef]
- Ding, C.; Li, P.; Shi, G.; Liu, Y.; Wang, F.; Hu, F.; Huang, S.; Liu, Z. Comparative study between flameless combustion and swirl flame combustion using low preheating temperature air for homogeneous fuel no reduction. Energy Fuels 2021, 35, 8181–8193. [Google Scholar] [CrossRef]
- Rao, A.G.; Levy, Y. A new combustion methodology for low emission gas turbine engines. In Proceedings of the 8th HiTACG Conference, Poznan, Poland, 5–7 July 2010; p. 13. [Google Scholar]
- Romero-Anton, N.; Huang, X.; Bao, H.; Maitin-Eskudero, K.; Salazar-Herran, E.; Roekaerts, D. New extended eddy dissipation concept model for flameless combustion in furnaces. Combust. Flame 2020, 220, 49–62. [Google Scholar] [CrossRef]
- Mardani, A.; Mahalegi, H.K.M. Hydrogen enrichment of methane and syngas for MILD combustion. Int. J. Hydrogen Energy 2019, 44, 9423–9437. [Google Scholar] [CrossRef]
- Singh, H.; Mohapatra, S.K. Production of producer gas from sugarcane bagasse and carpentry waste and its sustainable use in a dual fuel CI engine: A performance, emission, and noise investigation. J. Energy Inst. 2018, 91, 43–54. [Google Scholar] [CrossRef]
- Hagos, F.Y.; Aziz, A.R.A.; Sulaiman, S.A. Effect of injection timing on combustion, performance and emissions of lean-burn syngas (H2/CO) in spark-ignition direct injection engine. Int. J. Engine Res. 2016, 17, 921–933. [Google Scholar] [CrossRef]
- Gentillon, P.; Singh, S.; Lakshman, S.; Zhang, Z.; Paduthol, A.; Ekins-Daukes, N.J.; Chan, Q.N.; Taylor, R.A. A comprehensive experimental characterisation of a novel porous media combustion-based thermophotovoltaic system with controlled emission. Appl. Energy 2019, 254, 113721. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, W.; Jiaqiang, E.; Xu, H.; Li, Z.; Tay, K.; Zeng, G.; Yu, W. Investigation on premixed H2/C3H8/air combustion in porous medium combustor for the micro thermophotovoltaic application. Appl. Energy 2020, 260, 114352. [Google Scholar] [CrossRef]
- Arrieta, C.E.; García, A.; Yepes, H.A.; Bedoya, I.; Amell, A. An experimental study of combustion stability and emissions characteristics of a surface-stabilized combustion burner fueled with natural gas-syngas blends. J. Phys. Conf. Ser. 2019, 1257, 012018. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Diezinger, S.; Talukdar, P.; Issendorff, F.V.; Trimis, D. Combustion of low calorific gases from landfills and waste pyrolysis using porous medium burner technology. Process Saf. Environ. Protect. 2006, 84, 297–308. [Google Scholar] [CrossRef]
- Wang, G.; Tang, P.; Li, Y.; Xu, J.; Durst, F. Flame front stability of low calorific fuel gas combustion with preheated air in a porous burner. Energy 2018, 170, 1279–1288. [Google Scholar] [CrossRef]
- Kim, N.; Kim, Y.; Jaafar, M.N.M.; Rahim, M.R.; Said, M. Effects of hydrogen addition on structure and NO formation of highly co-rich syngas counterflow nonpremixed flames under MILD combustion regime. Int. J. Hydrogen Energy 2021, 46, 10518–10534. [Google Scholar] [CrossRef]
- Bâ, A.; Cessou, A.; Marcano, N.; Panier, F.; Tsiava, R.; Cassarino, G.; Ferrand, L.; Honore, D. Oxyfuel combustion and reactants preheating to enhance turbulent flame stabilization of low calorific blast furnace gas. Fuel 2019, 242, 211–221. [Google Scholar] [CrossRef]
- Kim, D.; Ahn, H.; Yang, W.; Huh, K.Y.; Lee, Y. Experimental analysis of CO/H2 syngas with NOx and SOx reactions in pressurized oxy-fuel combustion. Energy 2021, 219, 119550. [Google Scholar] [CrossRef]
- Berenjestanaki, A.V.; Kawahara, N.; Tsuboi, K.; Tomita, E. Performance, emissions and end-gas autoignition characteristics of premier combustion in a pilot fuel-ignited dual-fuel biogas engine with various CO2 ratios. Fuel 2021, 286, 119330. [Google Scholar] [CrossRef]
- Feroskhan, M.; Thangavel, V.; Subramanian, B.; Sankaralingam, R.K.; Ismail, S.; Chaudhary, A. Effects of operating parameters on the performance, emission and combustion indices of a biogas fuelled HCCI engine. Fuel 2021, 298, 120799. [Google Scholar] [CrossRef]
- Bui, V.G.; Tran, V.N.; Hoang, A.T.; Bui, T.M.T.; Vo, A.V. A simulation study on a port-injection SI engine fueled with hydroxy-enriched biogas. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 8, 1–17. [Google Scholar] [CrossRef]
- Meng, F.; Wang, H.; Ma, Q.; Wang, D.; Lin, J. Emission characteristics of vehicles fueled by hydrogen-enriched syngas under no-load condition. Int. J. Hydrogen Energy 2020, 45, 3840–3845. [Google Scholar] [CrossRef]
- Hagos, F.Y.; Aziz, A.R.A.; Sulaiman, S.A. Methane enrichment of syngas (H2/CO) in a spark-ignition direct-injection engine: Combustion, performance and emissions comparison with syngas and compressed natural gas. Energy 2015, 90, 2006–2015. [Google Scholar] [CrossRef]
- Mustafi, N.N.; Raine, R.R.; Bansal, P.K. The use of biogas in internal combustion engines: A review. Int. Combust. Engine Div. Spring Tech. Conf. 2006, 42061, 225–234. [Google Scholar] [CrossRef]
- Qian, Y.; Sun, S.; Ju, D.; Shan, X.; Lu, X. Review of the state-of-the-art of biogas combustion mechanisms and applications in internal combustion engines. Renew. Sustain. Energy Rev. 2017, 69, 50–58. [Google Scholar] [CrossRef]
- Zhang, K.; Lupo, G.; Duwig, C. Investigation of wet combustion instability due to bio-syngas fuel variability. Fuel 2021, 285, 119120. [Google Scholar] [CrossRef]
- Lee, M.C.; Yoon, J.; Joo, S.; Kim, J.; Hwang, J.; Yoon, Y. Investigation into the cause of high multi-mode combustion instability of H2/CO/CH4 syngas in a partially premixed gas turbine model combustor. Proc. Combust. Inst. 2015, 35, 3263–3271. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, W.; Ren, Z. Combustion stability analysis for non-standard low-calorific gases: Blast furnace gas and coke oven gas. Fuel 2020, 278, 118216. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, Y.; Xie, Q.; Ren, Z. Analysis and neural network prediction of combustion stability for industrial gases. Fuel 2021, 287, 119507. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, N.; Wei, J.; Ren, Z. Exploring active subspace for neural network prediction of oscillating combustion. Combust. Theory Model. 2021, 25, 570–587. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Kaneko, S. Prediction of Ignition and Combustion Development in an HCCI Engine Fueled by Syngas; SAE Technical Paper; No. 2014-32-0002; SAE Technical Paper Series; SAE: Tokyo, Japan, 2014. [Google Scholar] [CrossRef]
- Daniele, S.; Jansohn, P.; Boulouchos, K. Flashback propensity of syngas flames at high pressure: Diagnostic and control. Turbo Expo Power Land Sea Air 2010, 43970, 1169–1175. [Google Scholar] [CrossRef]
- Jaramillo, J.; Zapata, J.; Bedoya, I.D. Interactive control of combustion stability and operating limits in a biogas-fueled spark ignition engine with high compression ratio. Int. J. Interact. Des. Manuf. 2018, 12, 929–942. [Google Scholar] [CrossRef]
- Whitty, K.J.; Zhang, H.R.; Eddings, E.G. Emissions from syngas combustion. Combust. Sci. Technol. 2008, 180, 1117–1136. [Google Scholar] [CrossRef]
- Wei, L.; Geng, P. A review on natural gas/diesel dual fuel combustion, emissions and performance. Fuel Proc. Technol. 2016, 142, 264–278. [Google Scholar] [CrossRef]
- de Castro, R.R.; Brequigny, P.; Mounaïm-Rousselle, C. A multiparameter investigation of syngas/diesel dual-fuel engine performance and emissions with various syngas compositions. Fuel 2022, 318, 123736. [Google Scholar] [CrossRef]
- Prabhu, A.V.; Avinash, A.; Brindhadevi, K.; Pugazhendhi, A. Performance and emission evaluation of dual fuel CI engine using preheated biogas-air mixture. Sci. Total Environ. 2021, 754, 142389. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Minale, M.; Unich, A. Use of biogas containing CH4, H2 and CO2 in controlled auto-ignition engines to reduce NOx emissions. Fuel 2021, 301, 120925. [Google Scholar] [CrossRef]
- Mehregan, M.; Moghiman, M. A numerical investigation of preheated diluted oxidizer influence on NOx emission of biogas flameless combustion using Taguchi approach. Fuel 2018, 227, 1–5. [Google Scholar] [CrossRef]
- Dai, H.; Dai, H.; Zhu, H.; Zhang, B.; He, S.; Wang, Z. Combustion characteristics of a low calorific gas burner with ceramic foam enclosed by alumina pellets. Heat Mass Transf. 2022, 58, 221–231. [Google Scholar] [CrossRef]
- Habib, R.; Yadollahi, B.; Saeed, A.; Doranehgard, M.H.; Li, L.K.B.; Karimi, N. Unsteady ultra-lean combustion of methane and biogas in a porous burner—An experimental study. Appl. Therm. Eng. 2021, 182, 116099. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Yao, M.; Zhou, H.; Ren, Z. Analysis of operating limits and combustion state regulation for low-calorific value gases in industrial burners. Int. J. Hydrog. Energy 2022, 47, 1306–1318. [Google Scholar] [CrossRef]
- Shinoda, M.; Tanaka, R.; Arai, N. Optimization of heat transfer performances of a heat-recirculating ceramic burner during methane/air and low-calorific-fuel/air combustion. Energy Convers. Manag. 2002, 43, 1479–1491. [Google Scholar] [CrossRef]
- Al-Attab, K.A.; Zainal, Z.A. Design and performance of a pressurized cyclone combustor (PCC) for high and low heating value gas combustion. Appl. Energy 2011, 88, 1084–1095. [Google Scholar] [CrossRef]
- Smith, J.D.; Sreedharan, V.; Landon, M.; Smith, Z.P. Advanced design optimization of combustion equipment for biomass combustion. Renew. Energy 2020, 145, 1597–1607. [Google Scholar] [CrossRef]
- Duan, X.; Lai, M.C.; Jansons, M.; Guo, G.; Liu, J. A review of controlling strategies of the ignition timing and combustion phase in homogeneous charge compression ignition (HCCI) engine. Fuel 2021, 285, 119142. [Google Scholar] [CrossRef]




| Fuel | Methane | Biogas | Syngas | COG | BFG | |
|---|---|---|---|---|---|---|
| Volume fraction (%) | H2 | / | / | 9 | 62 | 5 |
| CH4 | 100 | 52 | 7 | 28 | / | |
| CO | / | / | 14 | 6 | 23 | |
| CO2 | / | 40 | 20 | 4 | 23 | |
| N2 | / | 8 | 50 | / | 49 | |
| Calorific Value (kWh/m3) | 9.94 | 5.17 | 1.44 | 4.75 | 0.95 | |
| Density (kg/m3) | 0.67 | 1.20 | 1.18 | 0.38 | 1.27 | |
| Stoichiometric mixture fraction Zs (/) | 0.34 | 0.48 | 0.20 | 0.24 | 0.88 | |
| Ignition delay time at 1200 K (ms) | 45.50 | 51.90 | 0.93 | 0.32 | 0.15 | |
| Laminar flame speed (cm/s) | 38.28 | 21.82 | 14.85 | 80.07 | 8.95 | |
| Fuel | Biogas | Syngas | COG | BFG | |
|---|---|---|---|---|---|
| Volume fraction (%) | CH4 | 55–65 | 8–12 | 20–30 | 0–3 |
| H2 | 0–1 | 35–45 | 50–70 | 1–5 | |
| CO | / | 20–30 | 9–20 | 20–30 | |
| CO2 | 35–45 | 15–25 | 0–5 | 15–25 | |
| N2 | 0–3 | 3–5 | 1–11 | 60–75 | |
| Ref. | Strategy | Fuel | Operating Conditions | Findings |
|---|---|---|---|---|
| [24] | Porous media combustion | Biogas | Material: SiC, ZrO2; Porosity: 10 ppi. | SiC foam offered: Wider working conditions; Higher radiation efficiency; Lower emissions. |
| [15] | Porous media combustion | Syngas | Porosity: 10–50 ppi; Heat recirculation. | Gradually varied porous media enlarged the flame stability limits and decreased CO emissions. |
| [25] | Flameless combustion | Syngas | Inlet Reynolds number: 10,000–15,000; H2 content: 10–80%. | Syngas enriched with H2 in the flameless regime was insensitive to the oxidizer dilution and inlet Reynolds number. |
| [26] | Flameless combustion | Syngas | H2 content: 0–20%; O2 content: 3–21%. | H2 enrichment and O2 augmentation influenced the NO emission characteristics and the dominant NO production route. |
| [27] | Oxy-fuel combustion | BFG | Load: 25–180 kW; Preheat temperature: 300–850 K; O2 content: 10–100%. | Flame instability decreased by reactant preheating, and flame structure was affected by O2 content. |
| [28] | Oxy-fuel combustion | Syngas | Load: 15–21 kW; Pressure: 0–10 barg; Flue gas recirculation. | Heat flow rate increased by a higher H2O fraction, and radical H had a significant impact on the NOx and SOx emissions. |
| [29] | Dual-fuel combustion | Biogas | Compression ignition engine; Compression ratio: 12–18; Dual fuel: diesel. | Brake power, brake thermal efficiency, and NOx emissions were reduced, and noise increased slightly. |
| [30] | Dual-fuel combustion | Syngas | Direct injection spark ignition engine; Engine speed: 1500–2400 r/min; Dual fuel: CNG. | Late injection of syngas improved combustion performance and emissions. Low calorific value resulted in operational limitations for direct injection system |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhang, S.; Zhou, H.; Ren, Z.; Wang, H.; Wang, X. Efficient Combustion of Low Calorific Industrial Gases: Opportunities and Challenges. Energies 2022, 15, 9224. https://doi.org/10.3390/en15239224
Zhang L, Zhang S, Zhou H, Ren Z, Wang H, Wang X. Efficient Combustion of Low Calorific Industrial Gases: Opportunities and Challenges. Energies. 2022; 15(23):9224. https://doi.org/10.3390/en15239224
Chicago/Turabian StyleZhang, Long, Shanshan Zhang, Hua Zhou, Zhuyin Ren, Hongchuan Wang, and Xiuxun Wang. 2022. "Efficient Combustion of Low Calorific Industrial Gases: Opportunities and Challenges" Energies 15, no. 23: 9224. https://doi.org/10.3390/en15239224
APA StyleZhang, L., Zhang, S., Zhou, H., Ren, Z., Wang, H., & Wang, X. (2022). Efficient Combustion of Low Calorific Industrial Gases: Opportunities and Challenges. Energies, 15(23), 9224. https://doi.org/10.3390/en15239224






