Functional Properties of Pineapple Plant Stem for Enhanced Glucose Recovery in Amino Acids Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Characterisation of Pineapple Plant Stem
2.3. Gelatinisation and Functional Analysis of Pineapple Plant Stem
2.3.1. Gelatinisation Properties Using Differential Scanning Calorimetry
2.3.2. Water Solubility, Swelling Power, and Water Absorption of Pineapple Plant Stem
2.4. Enzymatic Hydrolysis of Pineapple Plant Stem
2.5. Amino Acids Production by B. subtilis ATCC 6051
2.5.1. Culture Preparation
2.5.2. Fermentation for Amino Acids Production
2.6. Analytical Analysis
3. Results and Discussion
3.1. Carbohydrate Content of Pineapple Plant Stem
3.2. Enzymatic Hydrolysis of Pineapple Plant Stem
3.3. Enhancement of Glucose Recovery from Pineapple Plant Stem
3.3.1. Gelatinisation Properties of Pineapple Plant Stem
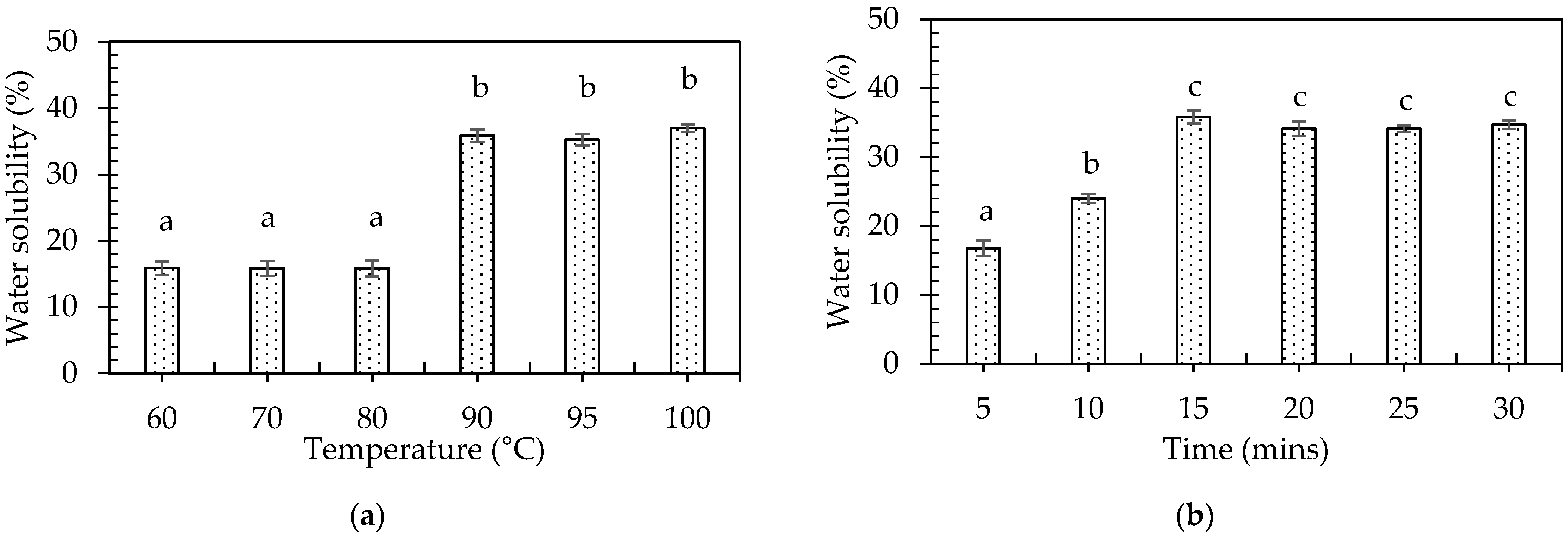
3.3.2. Effects of Temperature and Time on Water Solubility of Pineapple Plant Stem
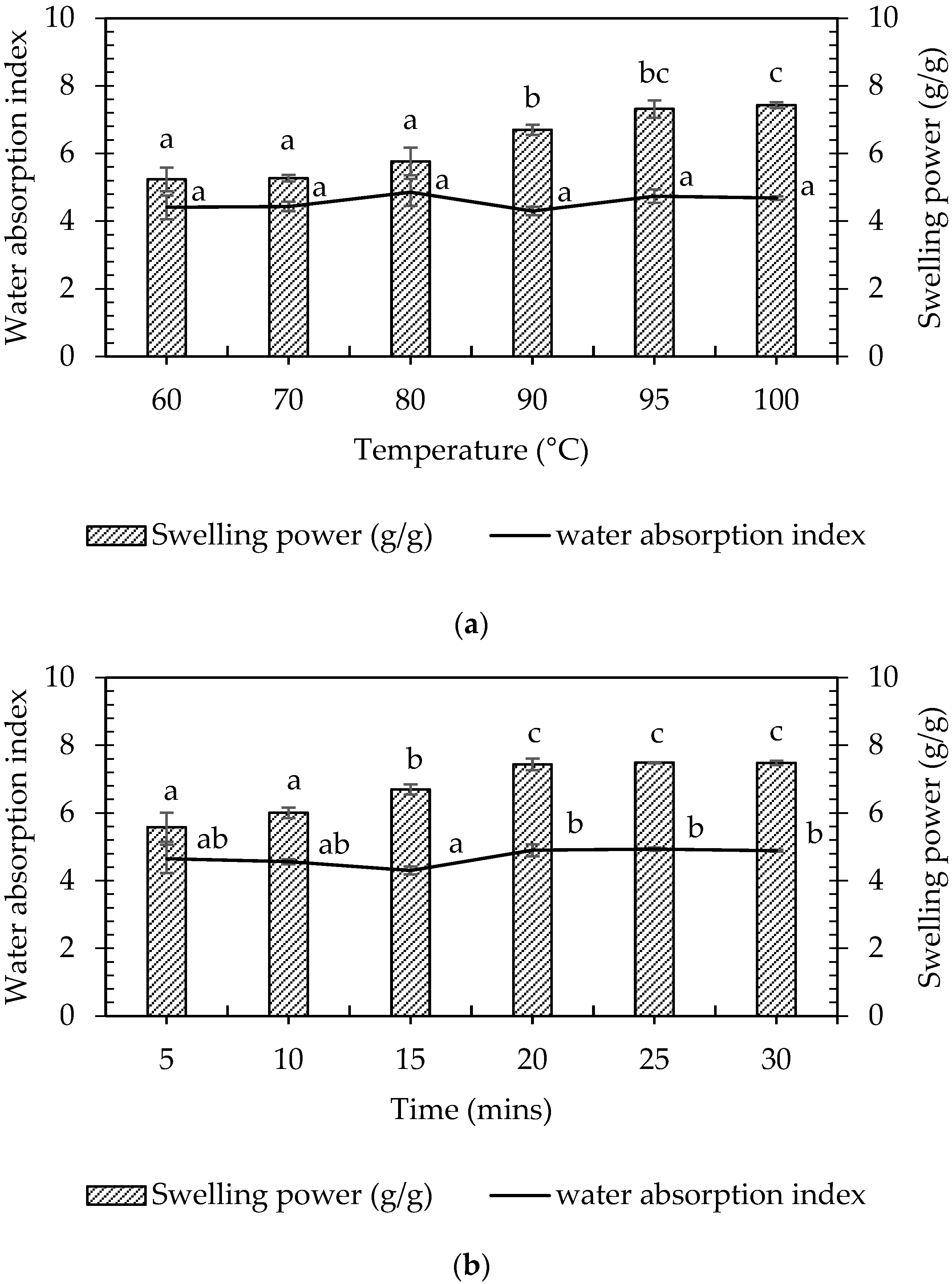
3.3.3. Effects of Temperature and Time on Swelling Power of Pineapple Plant Stem
3.3.4. Effects of Temperature and Time on Water Absorption of Pineapple Plant Stem
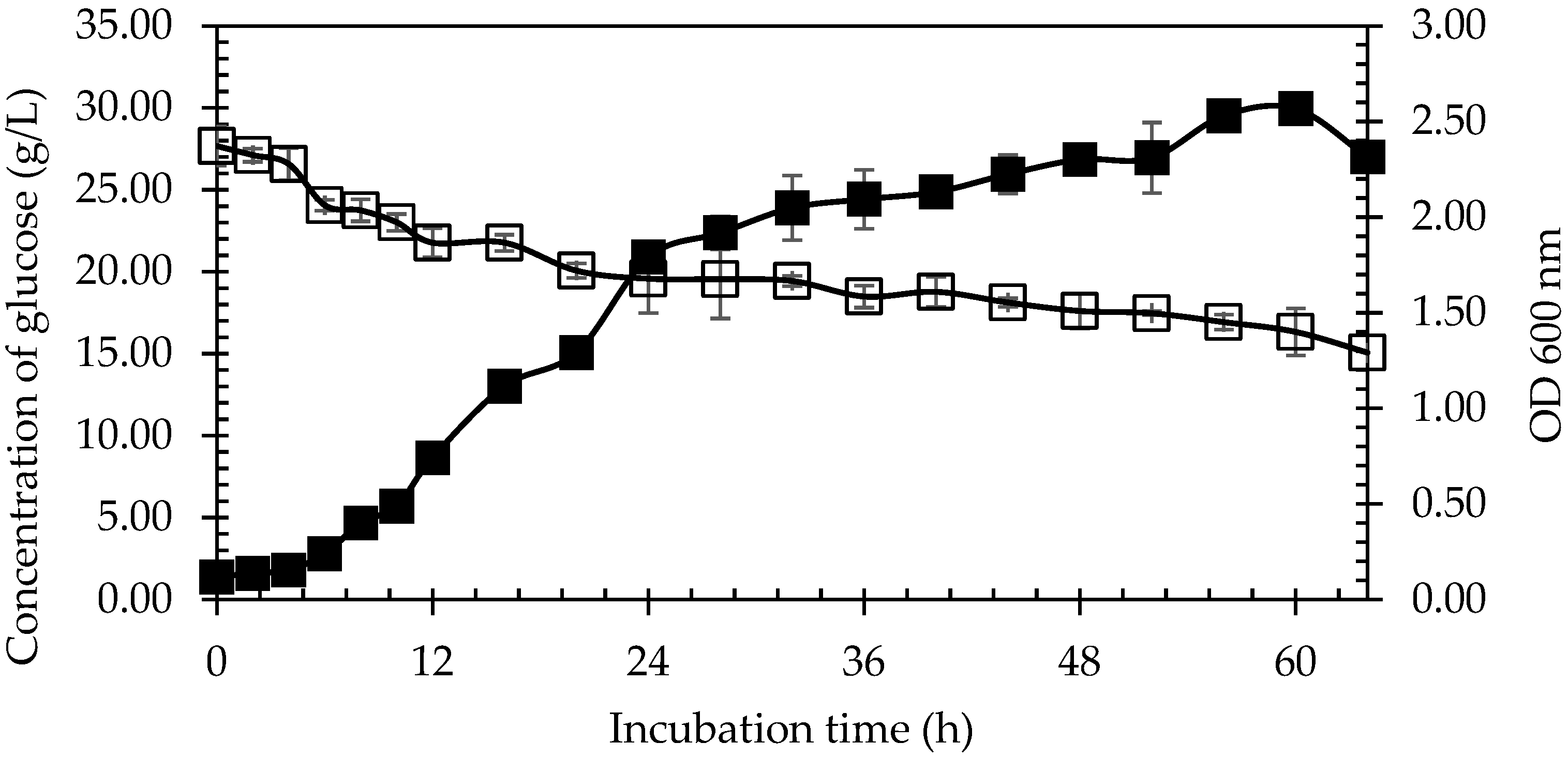
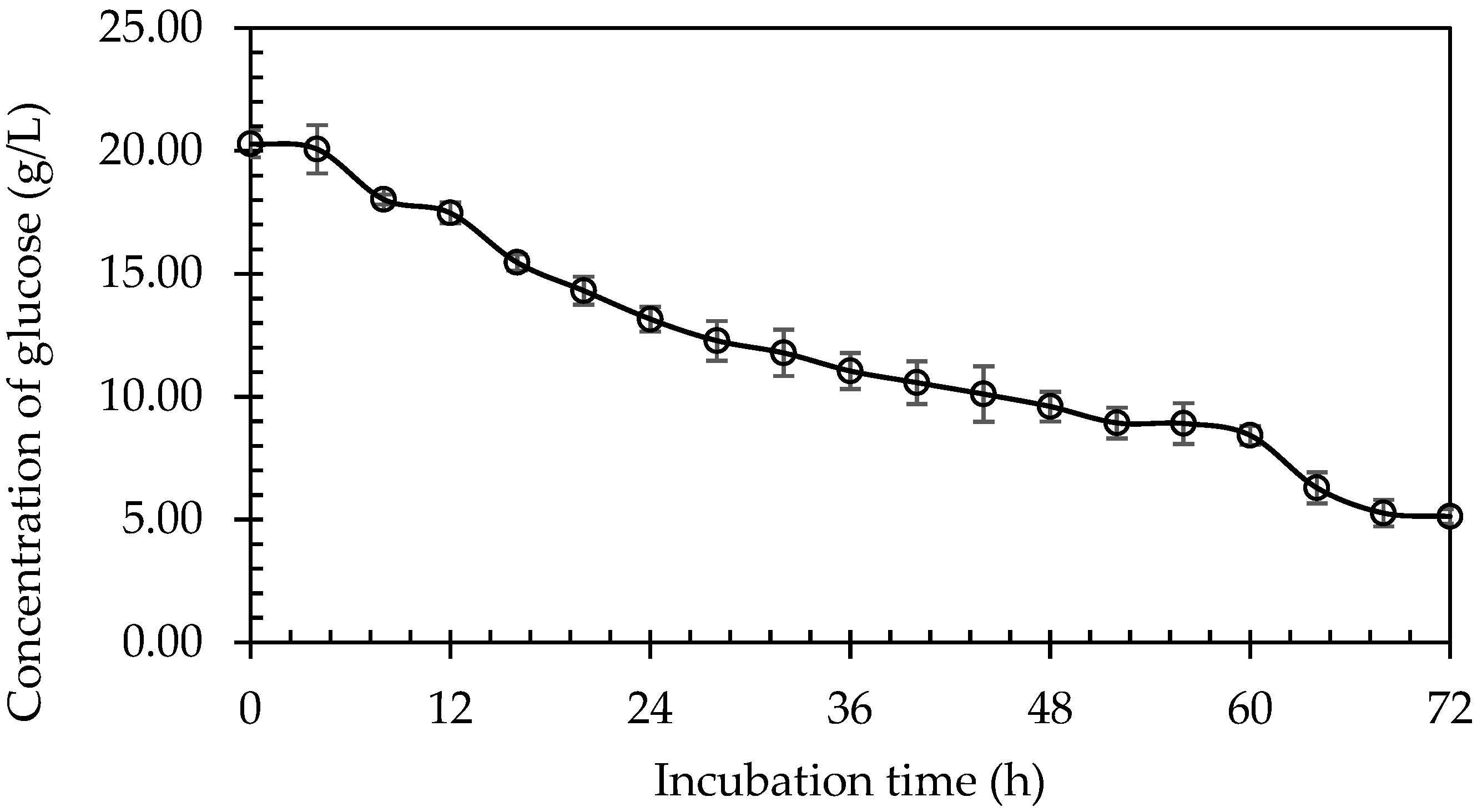
3.3.5. Glucose Recovery from Pineapple Plant Stem at Optimised Gelatinisation Condition
3.4. Amino Acids Production by B. subtilis ATCC 6051 from Pineapple Plant Stem Hydrolysate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khongpradit, A.; Boonsaen, P.; Homwong, N.; Suzuki, Y.; Koike, S.; Sawanon, S.; Kobayashi, Y. Effect of Pineapple Stem Starch Feeding on Rumen Microbial Fermentation, Blood Lipid Profile, and Growth Performance of Fattening Cattle. Anim. Sci. J. 2020, 91, e13459. [Google Scholar] [CrossRef] [PubMed]
- Rinju, R.; Harikumaran-Thampi, B.-S. Characteristics of Starch Extracted from the Stem of Pineapple Plant (Ananas comosus)—An Agro Waste from Pineapple Farms. Braz. Arch. Biol. Technol. 2021, 64, 1–14. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Available online: http://www.fao.org (accessed on 21 July 2021).
- Susmitha, A.; Sasikumar, K.; Rajan, D.; Padmakumar, M.A.; Nampoothiri, K.M. Development and Characterization of Corn Starch-Gelatin Based Edible Films Incorporated with Mango and Pineapple for Active Packaging. Food Biosci. 2021, 41, 100977. [Google Scholar] [CrossRef]
- Chu, P.H.; Jenol, M.A.; Phang, L.Y.; Ibrahim, M.F.; Prasongsuk, S.; Bankeeree, W.; Punnapayak, H.; Lotrakul, P.; Abd-Aziz, S. Starch Extracted from Pineapple (Ananas comosus) Plant Stem as a Source for Amino Acids Production. Chem. Biol. Technol. Agric. 2021, 8, 29. [Google Scholar] [CrossRef]
- Nakthong, N.; Wongsagonsup, R.; Amornsakchai, T. Characteristics and Potential Utilizations of Starch from Pineapple Stem Waste. Ind. Crop. Prod. 2017, 105, 74–82. [Google Scholar] [CrossRef]
- Chisenga, S.M.; Workneh, T.S.; Bultosa, G.; Alimi, B.A. Progress in Research and Applications of Cassava Flour and Starch: A Review. J. Food Sci. Technol. 2019, 56, 2799–2813. [Google Scholar] [CrossRef]
- Bashir, K.; Aggarwal, M. Physicochemical, Structural and Functional Properties of Native and Irradiated Starch: A Review. J. Food Sci. Technol. 2019, 56, 513–523. [Google Scholar] [CrossRef]
- Uthumporn, U.; Shariffa, Y.N.; Karim, A.A. Hydrolysis of Native and Heat-Treated Starches at Sub-Gelatinization Temperature Using Granular Starch Hydrolyzing Enzyme. Appl. Biochem. Biotechnol. 2012, 166, 1167–1182. [Google Scholar] [CrossRef]
- Kusumayanti, H.; Handayani, N.A.; Santosa, H. Swelling Power and Water Solubility of Cassava and Sweet Potatoes Flour. Procedia Environ. Sci. 2015, 23, 164–167. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Retnowati, D.S.; Budiyati, C.S.; Manurung, T. Siswanto Water Solubility, Swelling and Gelatinization Properties of Raw and Ginger Oil Modified Gadung (Dioscorea hispida Dennst) Flour. Res. J. Appl. Sci. Eng. Technol. 2012, 4, 2854–2860. [Google Scholar]
- Rahma, A.; Adriani, M.; Rahayu, P.; Tjandrawinata, R.R.; Rachmawati, H. Green Isolation and Physical Modification of Pineapple Stem Waste Starch as Pharmaceutical Excipient. Drug Dev. Ind. Pharm. 2019, 45, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Burgos, V.E.; Armada, M. Characterization and Nutritional Value of Precooked Products of Kiwicha Grains (Amaranthus caudatus). Food Sci. Technol. 2015, 35, 531–538. [Google Scholar] [CrossRef][Green Version]
- Awg-Adeni, D.S.; Bujang, K.B.; Hassan, M.A.; Abd-Aziz, S. Recovery of Glucose from Residual Starch of Sago Hampas for Bioethanol Production. Biomed Res. Int. 2013, 2013, 935852. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Guo, X.Y.; Zhang, B.; Jiang, Y.; Ye, B.C. Recent Advances of L-Ornithine Biosynthesis in Metabolically Engineered Corynebacterium glutamicum. Front. Bioeng. Biotechnol. 2020, 7, 440. [Google Scholar] [CrossRef] [PubMed]
- Wendisch, V.F. Metabolic Engineering Advances and Prospects for Amino Acid Production. Metab. Eng. 2019, 58, 17–34. [Google Scholar] [CrossRef]
- Sturm, V.; Banse, M.; Salamon, P. The Role of Feed-Grade Amino Acids in the Bioeconomy: Contribution from Production Activities and Use in Animal Feed. Clean. Environ. Syst. 2022, 4, 100073. [Google Scholar] [CrossRef]
- D’Este, M.; Alvarado-Morales, M.; Angelidaki, I. Amino Acids Production Focusing on Fermentation Technologies—A Review. Biotechnol. Adv. 2018, 36, 14–25. [Google Scholar] [CrossRef]
- Sgobba, E.; Blöbaum, L.; Wendisch, V.F. Production of Food and Feed Additives from Non-Food-Competing Feedstocks: Valorizing N-Acetylmuramic Acid for Amino Acid and Carotenoid Fermentation with Corynebacterium glutamicum. Front. Microbiol. 2018, 9, 2046. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, H.W.; Park, J.H.; Ahn, J.O.; Jung, J.K.; Hwang, Y. Il Improved L-Threonine Production of Escherichia coli Mutant by Optimization of Culture Conditions. J. Biosci. Bioeng. 2006, 101, 127–130. [Google Scholar] [CrossRef]
- Toe, C.J.; Foo, H.L.; Loh, T.C.; Mohamad, R. Extracellular Proteolytic Activity and Amino Acid Production by Lactic Acid Bacteria Isolated from Malaysian Foods. Int. J. Mol. Sci. 2019, 20, 1777. [Google Scholar] [CrossRef]
- Zhang, K.; Su, L.; Wu, J. Enhancing Extracellular Pullulanase Production in Bacillus subtilis through DltB Disruption and Signal Peptide Optimization. Appl. Biochem. Biotechnol. 2022, 194, 1206–1220. [Google Scholar] [CrossRef]
- Mhatre, A.; Kalscheur, B.; Mckeown, H.; Bhakta, K.; Sarnaik, A.P.; Flores, A.; Nielsen, D.R.; Wang, X.; Soundappan, T.; Varman, A.M. Consolidated Bioprocessing of Hemicellulose to Fuels and Chemicals through an Engineered Bacillus subtilis-Escherichia coli Consortium. Renew. Energy 2022, 193, 288–298. [Google Scholar] [CrossRef]
- Ji, M.; Liu, Y.; Xie, S.; Fu, C.; Liu, M.; Shi, J. De Novo Synthesis of 2’-Fucosyllactose in Engineered Bacillus subtilis ATCC 6051a. Process Biochem. 2022, 120, 178–185. [Google Scholar] [CrossRef]
- Raul, D.; Biswas, T.; Mukhopadhyay, S.; Kumar Das, S.; Gupta, S. Production and Partial Purification of Alpha Amylase from Bacillus subtilis (Mtcc 121) using Solid State Fermentation. Biochem. Res. Int. 2014, 2014, 568141. [Google Scholar] [CrossRef]
- Rajagopalan, G.; Krishnan, C. Optimization of Agro-Residual Medium for α-Amylase Production from a Hyper-Producing Bacillus subtilis KCC103 in Submerged Fermentation. J. Chem. Technol. Biotechnol. 2009, 84, 618–625. [Google Scholar] [CrossRef]
- Jeong, W.H.; Harada, K.; Yamada, T.; Abe, J.; Kitamura, K. Establishment of New Method for Analysis of Starch Contents and Varietal Differences in Soybean Seeds. Breed. Sci. 2010, 60, 160–163. [Google Scholar] [CrossRef]
- Zeng, J.; Li, G.; Gao, H.; Ru, Z. Comparison of A and B Starch Granules from Three Wheat Varieties. Molecules 2011, 16, 10570–10591. [Google Scholar] [CrossRef]
- Atukuri, J.; Odong, B.B.; Muyonga, J.H. Multi-Response Optimization of Extrusion Conditions of Grain Amaranth Flour by Response Surface Methodology. Food Sci. Nutr. 2019, 7, 4147–4162. [Google Scholar] [CrossRef]
- Kaur, M.; Oberoi, D.P.S.; Sogi, D.S.; Gill, B.S. Physicochemical, Morphological and Pasting Properties of Acid Treated Starches from Different Botanical Sources. J. Food Sci. Technol. 2011, 48, 460–465. [Google Scholar] [CrossRef]
- Chisenga, S.M.; Workneh, T.S.; Bultosa, G.; Laing, M. Characterization of Physicochemical Properties of Starches from Improved Cassava Varieties Grown in Zambia. AIMS Agric. Food 2019, 4, 939–966. [Google Scholar] [CrossRef]
- Jenol, M.A.; Ibrahim, M.F.; Yee, P.L.; Md Salleh, M.; Abd-Aziz, S. Sago Biomass as a Sustainable Source for Biohydrogen Production by Clostridium butyricum A1. BioResources 2014, 9, 1007–1026. [Google Scholar] [CrossRef]
- Leaes, E.X.; Lima, D.; Miklasevicius, L.; Ramon, A.P.; Pra, V.D.; Bassaco, M.M.; Terra, L.M.; Mazutti, M.A. Effect of Ultrasound-Assisted Irradiation on the Activities of α-Amylase and Amyloglucosidase. Biocatal. Agric. Biotechnol. 2013, 2, 21–25. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Linggang, S.; Phang, L.Y.; Wasoh, M.H.; Abd-Aziz, S. Sago Pith Residue as an Alternative Cheap Substrate for Fermentable Sugars Production. Appl. Biochem. Biotechnol. 2012, 167, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ao, Z.; Han, J.A.; Jane, J.L.; BeMiller, J.N. Waxy Maize Starch Subpopulations with Different Gelatinization Temperatures. Carbohydr. Polym. 2004, 57, 177–190. [Google Scholar] [CrossRef]
- Juarez, G.F.Y.; Pabiloña, K.B.C.; Manlangit, K.B.L.; Go, A.W. Direct Dilute Acid Hydrolysis of Spent Coffee Grounds: A New Approach in Sugar and Lipid Recovery. Waste Biomass Valorization 2018, 9, 235–246. [Google Scholar] [CrossRef]
- Pezzotti, G.; Zhu, W.; Chikaguchi, H.; Marin, E.; Masumura, T.; Sato, Y.-I.; Nakazaki, T. Raman Spectroscopic Analysis of Polysaccharides in Popular Japanese Rice Cultivars. Food Chem. 2021, 354, 129434. [Google Scholar] [CrossRef]
- Edwards, C.H.; Veerabahu, A.S.; Mason, A.J.; Butterworth, P.J.; Ellis, P.R. α-Amylase Action on Starch in Chickpea Flour Following Hydrothermal Processing and Different Drying, Cooling and Storage Conditions. Carbohydr. Polym. 2021, 259, 117738. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Structure-Function Relationships of Starch Components. Starch/Staerke 2015, 67, 55–68. [Google Scholar] [CrossRef]
- An, H.E.; Lee, K.H.; Jang, Y.W.; Kim, C.B.; Yoo, H.Y. Improved Glucose Recovery from Sicyos angulatus by NaOH Pretreatment and Application to Bioethanol Production. Processes 2021, 9, 245. [Google Scholar] [CrossRef]
- Uthumporn, U.; Nadiah, I.; Izzuddin, I.; Cheng, L.H.; Aida, H. Physicochemical Characteristics of Non-Starch Polysaccharides Extracted from Cassava Tubers. Sains Malays. 2017, 46, 223–229. [Google Scholar] [CrossRef]
- Bisinella, R.Z.B.; Beninca, C.; Bet, C.D.; de Oliveira, C.S.; Demiate, I.M.; Schnitzler, E. Thermal, Structural and Morphological Characterisation of Organic Rice Starch after Physical Treatment. J. Therm. Anal. Calorim. 2022, 147, 3615–3623. [Google Scholar] [CrossRef]
- Sirivongpaisal, P. Structure and Functional Properties of Starch and Flour from Bambarra Groundnut. Songklanakarin J. Sci. Technol. 2008, 30, 51–56. [Google Scholar]
- Singh, S.; Raina, C.S.; Bawa, A.S.; Saxena, D.C. Effect of Heat-Moisture Treatment and Acid Modification on Rheological, Textural, and Differential Scanning Calorimetry Characteristics of Sweetpotato Starch. J. Food Sci. 2005, 70, e373–e378. [Google Scholar] [CrossRef]
- Zheng, M.-Z.; Xiao, Y.; Yang, S.; Liu, H.-M.; Liu, M.-H.; Yaqoob, S.; Xu, X.-Y.; Liu, J.-S. Effects of Heat–Moisture, Autoclaving, and Microwave Treatments on Physicochemical Properties of Proso Millet Starch. Food Sci. Nutr. 2020, 8, 735–743. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Retnowati, D.S.; Ratnawati, R.; Widiyanti, M. Effect of Temperature and Reaction Time on the Swelling Power and Solubility of Gadung (Dioscorea hispida Dennst) Tuber Starch during Heat Moisture Treatment Process. J. Phys. Conf. Ser. 2019, 1295, 012062. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, H.; Ma, S.; Xue, M.; Li, J.; Yang, J. Development of a Water Solubility Model of Extruded Feeds by Utilizing a Starch Gelatinization Model. Int. J. Food Prop. 2022, 25, 463–476. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Huang, Q. Effects of Hydrothermal Pretreatment on Subsequent Octenylsuccinic Anhydride (OSA) Modification of Cornstarch. Carbohydr. Polym. 2014, 101, 493–498. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, H.; Cornacchia, L.; Sala, G.; Scholten, E. Effect of Gelatinization and Swelling Degree on the Lubrication Behavior of Starch Suspensions. Carbohydr. Polym. 2022, 291, 119523. [Google Scholar] [CrossRef]
- Gujral, H.S.; Sharma, P.; Kaur, H.; Singh, J. Physiochemical, Pasting, and Thermal Properties of Starch Isolated from Different Barley Cultivars. Int. J. Food Prop. 2013, 16, 1494–1506. [Google Scholar] [CrossRef]
- Morales-Sánchez, E.; Cabrera-Ramírez, A.H.; Gaytán-Martínez, M.; Mendoza-Zuvillaga, A.L.; Velázquez, G.; Méndez-Montealvo, M.G.; Rodríguez-García, M.E. Heating-Cooling Extrusion Cycles as a Method to Improve the Physicochemical Properties of Extruded Corn Starch. Int. J. Biol. Macromol. 2021, 188, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Roumeliotis, S.; Eglinton, J. Relationships between Swelling Power, Water Solubility and Near-Infrared Spectra in Whole Grain Barley: A Feasibility Study. Food Bioprocess Technol. 2013, 6, 2732–2738. [Google Scholar] [CrossRef]
- Neder-Suárez, D.; Amaya-Guerra, C.A.; Báez-González, J.G.; Quintero-Ramos, A.; Aguilar-Palazuelos, E.; Galicia-García, T.; Ramírez-Wong, B.; Campos-Venegas, K.; de Zazueta-Morales, J.J. Resistant Starch Formation from Corn Starch by Combining Acid Hydrolysis with Extrusion Cooking and Hydrothermal Storage. Starch/Staerke 2018, 70, 1700118. [Google Scholar] [CrossRef]
- Laovachirasuwan, P.; Peerapattana, J.; Srijesdaruk, V.; Chitropas, P.; Otsuka, M. The Physicochemical Properties of a Spray Dried Glutinous Rice Starch Biopolymer. Colloids Surf. B Biointerfaces 2010, 78, 30–35. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.; Park, S. Amino Acids Analysis during Lactic Acid Fermentation by Single Strain Cultures of Lactobacilli and Mixed Culture Starter Made from Them. Afr. J. Biotechnol. 2014, 13, 2867–2873. [Google Scholar] [CrossRef]
- Anusree, M.; Nampoothiri, K.M. Biosynthesis, Recovery and Purification of l-Lysine from Jackfruit Seed (JFS) Hydrolysate by Corynebacterium glutamicum DM 1729. Biocatal. Agric. Biotechnol. 2015, 4, 506–513. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, G.; Zhang, J.; Bao, J. A Preliminary Study on L-Lysine Fermentation from Lignocellulose Feedstock and Techno-Economic Evaluation. Bioresour. Technol. 2019, 271, 196–201. [Google Scholar] [CrossRef]
- Leuchtenberger, W.; Huthmacher, K.; Drauz, K. Biotechnological Production of Amino Acids and Derivatives: Current Status and Prospects. Appl. Microbiol. Biotechnol. 2005, 60, 1–8. [Google Scholar] [CrossRef]
- Sanchez, S.; Rodríguez-Sanoja, R.; Ramos, A.; Demain, A.L. Our Microbes Not Only Produce Antibiotics, They Also Overproduce Amino Acids. J. Antibiot. 2018, 71, 26–36. [Google Scholar] [CrossRef]
- Lim, Y.H.; Foo, H.L.; Loh, T.C.; Mohamad, R.; Abdullah, N. Comparative Studies of Versatile Extracellular Proteolytic Activities of Lactic Acid Bacteria and Their Potential for Extracellular Amino Acid Productions as Feed Supplements. J. Anim. Sci. Biotechnol. 2019, 10, 15. [Google Scholar] [CrossRef]
- Lee, K.; Lee, J.; Kim, Y.H.; Moon, S.H.; Park, Y.H. Unique Properties of Four Lactobacilli in Amino Acid Production and Symbiotic Mixed Culture for Lactic Acid Biosynthesis. Curr. Microbiol. 2001, 43, 383–390. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Value |
|---|---|
| Start Temperature, Ts (°C) | 95 |
| Onset Temperature, To (°C) | 111 |
| Peak Temperature, Tp (°C) | 116 |
| Conclusion Temperature, Tc (°C) | 161 |
| End Temperature, Te (°C) | 167 |
| Gelatinisation Enthalpy, ΔH (J/g) | 263.58 |
| Amino Acid | Maximum Increment | ||
|---|---|---|---|
| Amount (mg/mL) | Time (h) | Productivity (mg/L/h) | |
| Essential Amino Acid | |||
| Histidine | 0.00 a | 72 | 0.00 a |
| Threonine | 0.25 h | 24 | 10.44 j |
| Valine | 0.16 f | 72 | 2.23 e |
| Methionine | 0.03 ab | 72 | 0.42 b |
| Lysine | 20.81 m | 48 | 433.57 n |
| Isoleucine | 0.11 e | 72 | 1.53 c |
| Leucine | 0.07 cd | 24 | 2.92 g |
| Phenylalanine | 0.00 a | 48 | 0.00 a |
| Non-Essential Amino Acid | |||
| Hydroxyproline | 0.00 a | 72 | 0.00 a |
| Aspartic Acid | 0.34 j | 48 | 7.10 h |
| Serine | 0.29 i | 24 | 12.11 l |
| Glutamic Acid | 0.52 k | 48 | 10.86 k |
| Glycine | 0.06 bc | 24 | 2.51 f |
| Arginine | 0.00 a | 24 | 0.00 a |
| Alanine | 1.36 l | 48 | 28.39 m |
| Proline | 0.21 g | 24 | 8.77 i |
| Tyrosine | 0.10 de | 48 | 2.09 d |
| Total Amino Acid | 23.53 | 48 | 490.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, P.H.; Jenol, M.A.; Phang, L.-Y.; Syed Muhammad, S.K.; Abd-Aziz, S. Functional Properties of Pineapple Plant Stem for Enhanced Glucose Recovery in Amino Acids Production. Energies 2022, 15, 9155. https://doi.org/10.3390/en15239155
Chu PH, Jenol MA, Phang L-Y, Syed Muhammad SK, Abd-Aziz S. Functional Properties of Pineapple Plant Stem for Enhanced Glucose Recovery in Amino Acids Production. Energies. 2022; 15(23):9155. https://doi.org/10.3390/en15239155
Chicago/Turabian StyleChu, Pei Hsia, Mohd Azwan Jenol, Lai-Yee Phang, Sharifah Kharidah Syed Muhammad, and Suraini Abd-Aziz. 2022. "Functional Properties of Pineapple Plant Stem for Enhanced Glucose Recovery in Amino Acids Production" Energies 15, no. 23: 9155. https://doi.org/10.3390/en15239155
APA StyleChu, P. H., Jenol, M. A., Phang, L.-Y., Syed Muhammad, S. K., & Abd-Aziz, S. (2022). Functional Properties of Pineapple Plant Stem for Enhanced Glucose Recovery in Amino Acids Production. Energies, 15(23), 9155. https://doi.org/10.3390/en15239155







