Effect of Hydrogen Enhancement on Natural Flame Luminosity of Tri-Fuel Combustion in an Optical Engine
Abstract
1. Introduction
2. Experimental Setup and Methodology
2.1. Specifications of the Optically Accessible Engine
2.2. Operating Conditions
2.3. Fuel Properties
2.4. High-Speed Natural Flame Luminosity Imaging
3. Image Postprocessing
3.1. Raw Image Postprocessing
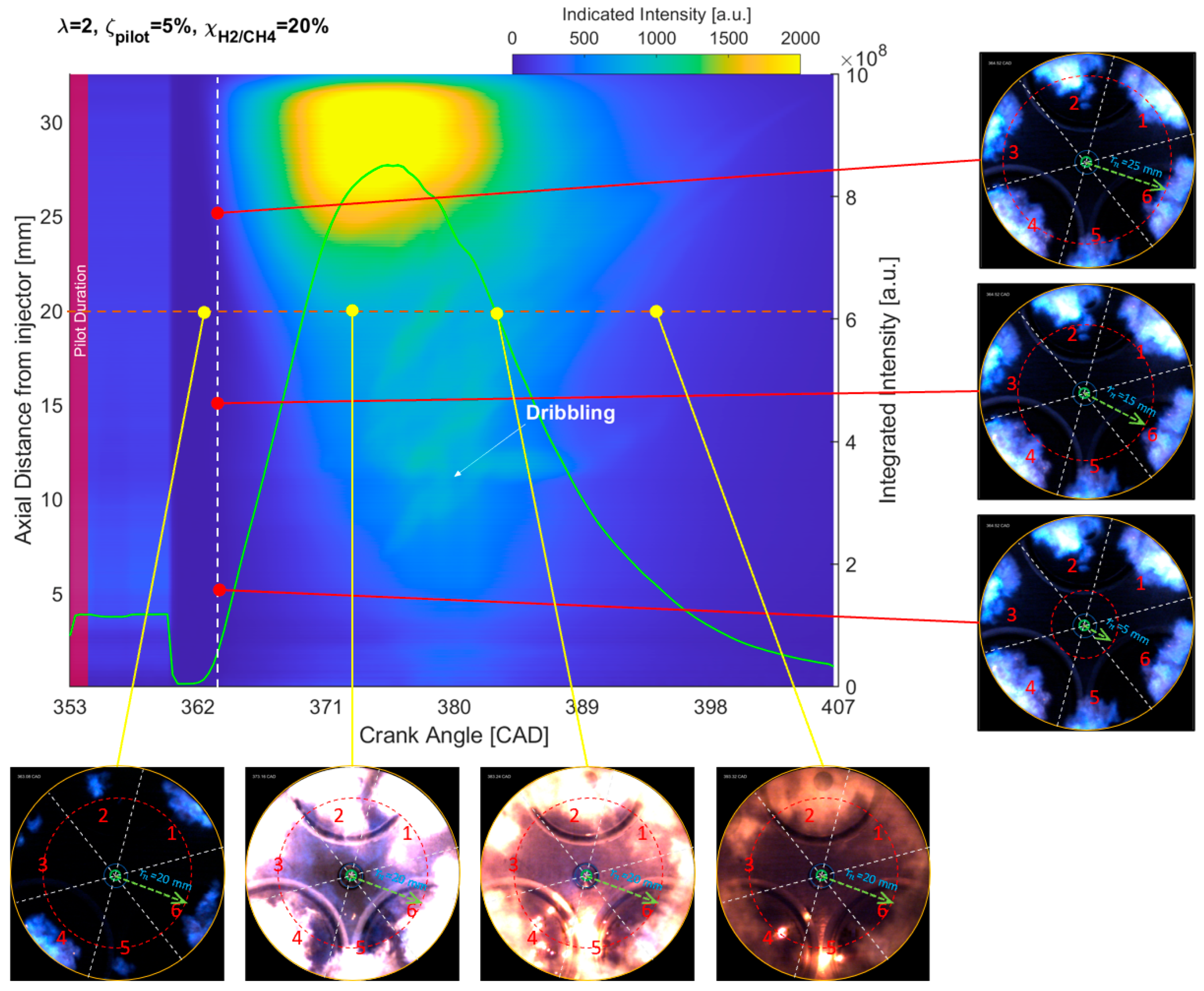
3.2. Spatiotemporal Flame Intensity Integration
3.3. Flame Stability
4. Results and Discussion
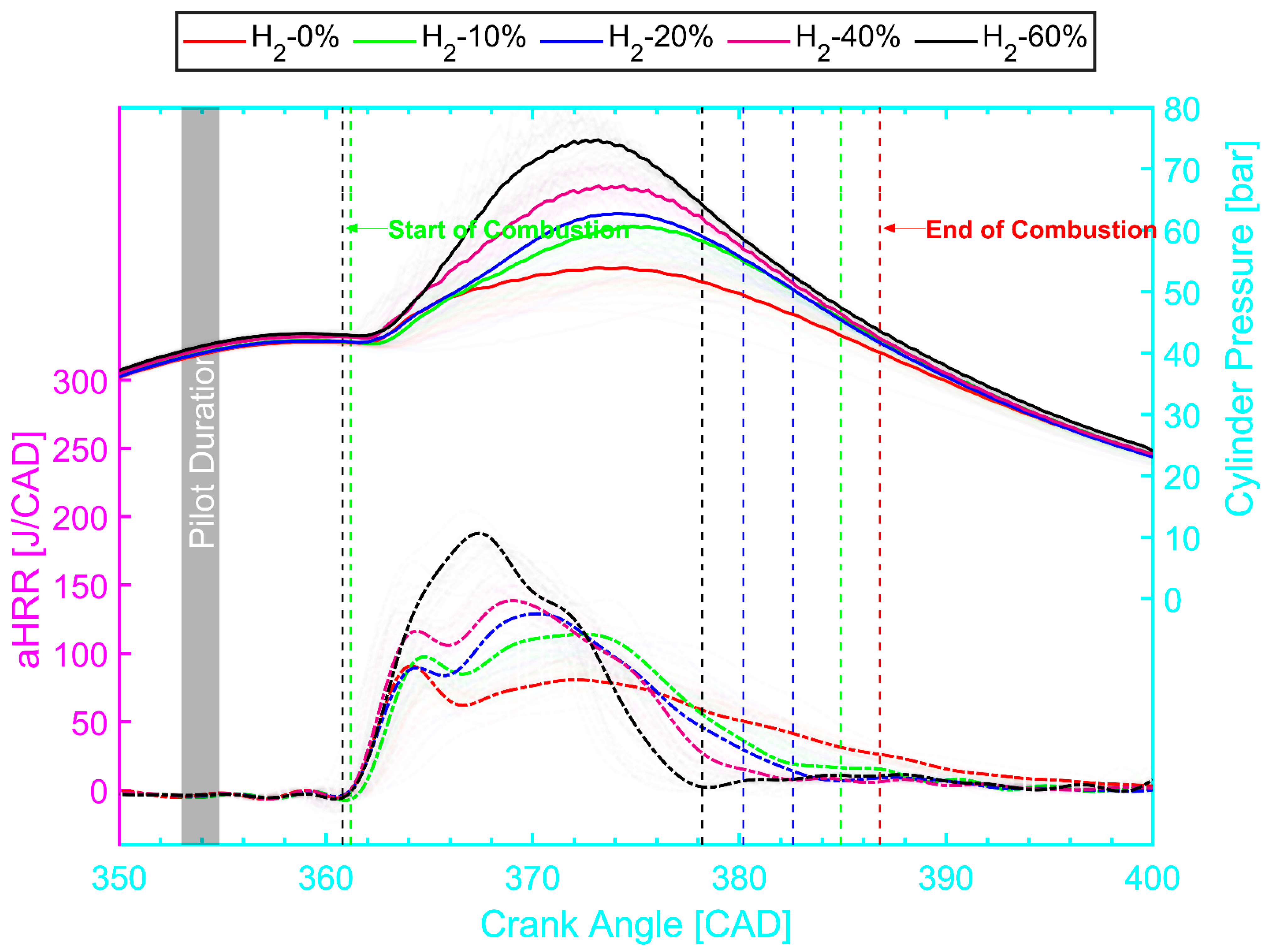
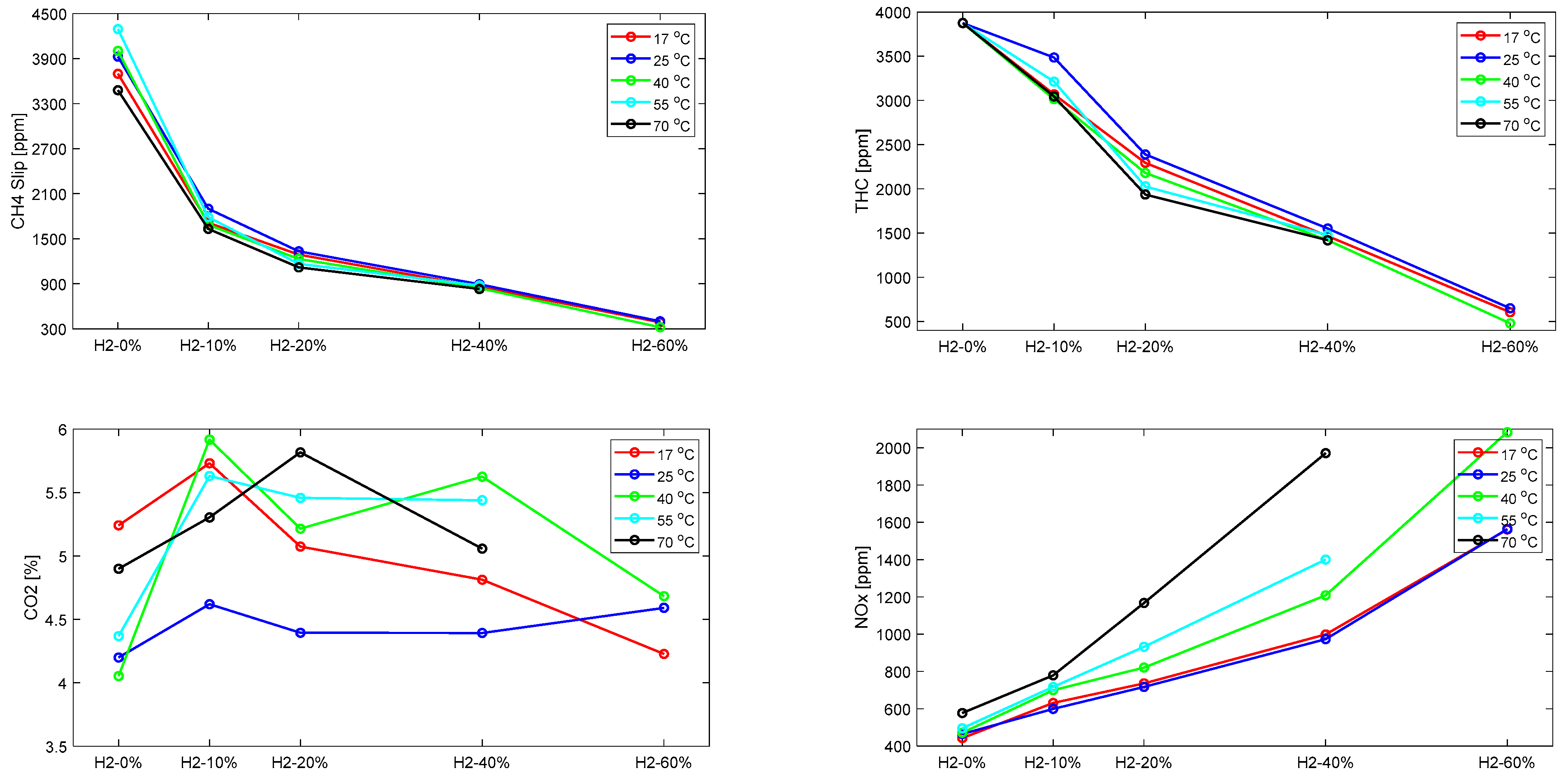
4.1. Effect of H2 Addition on the Engine Performance
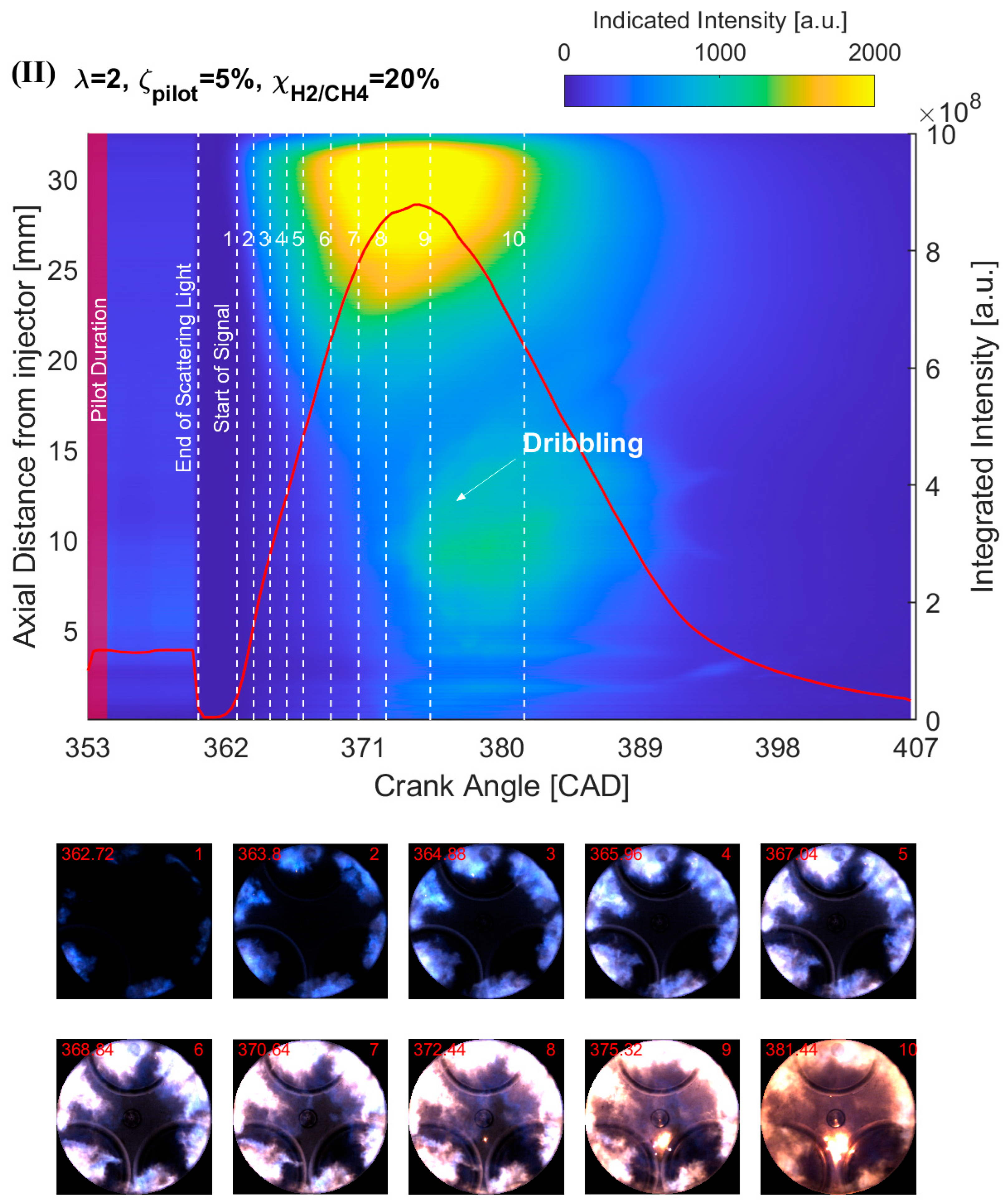
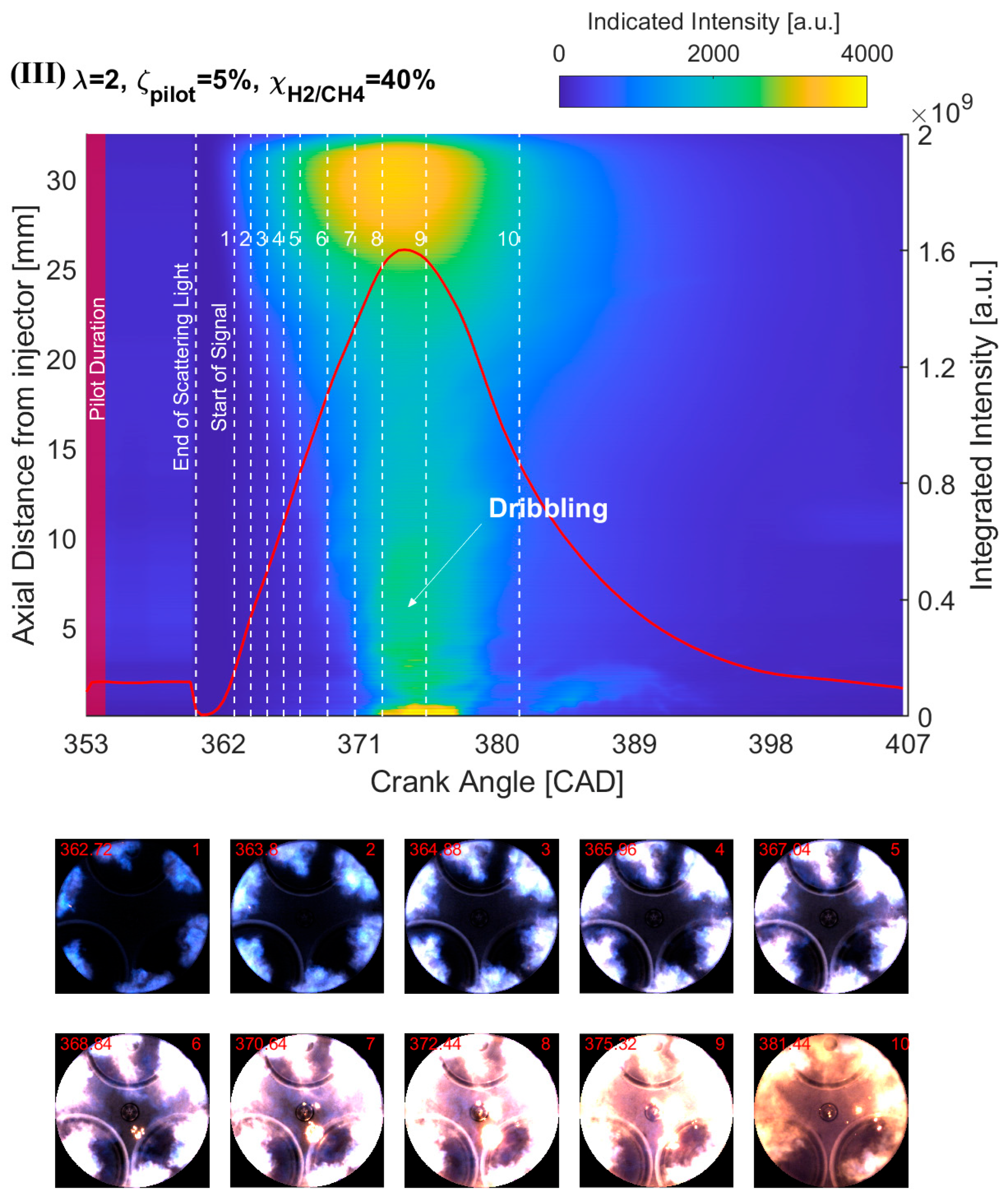
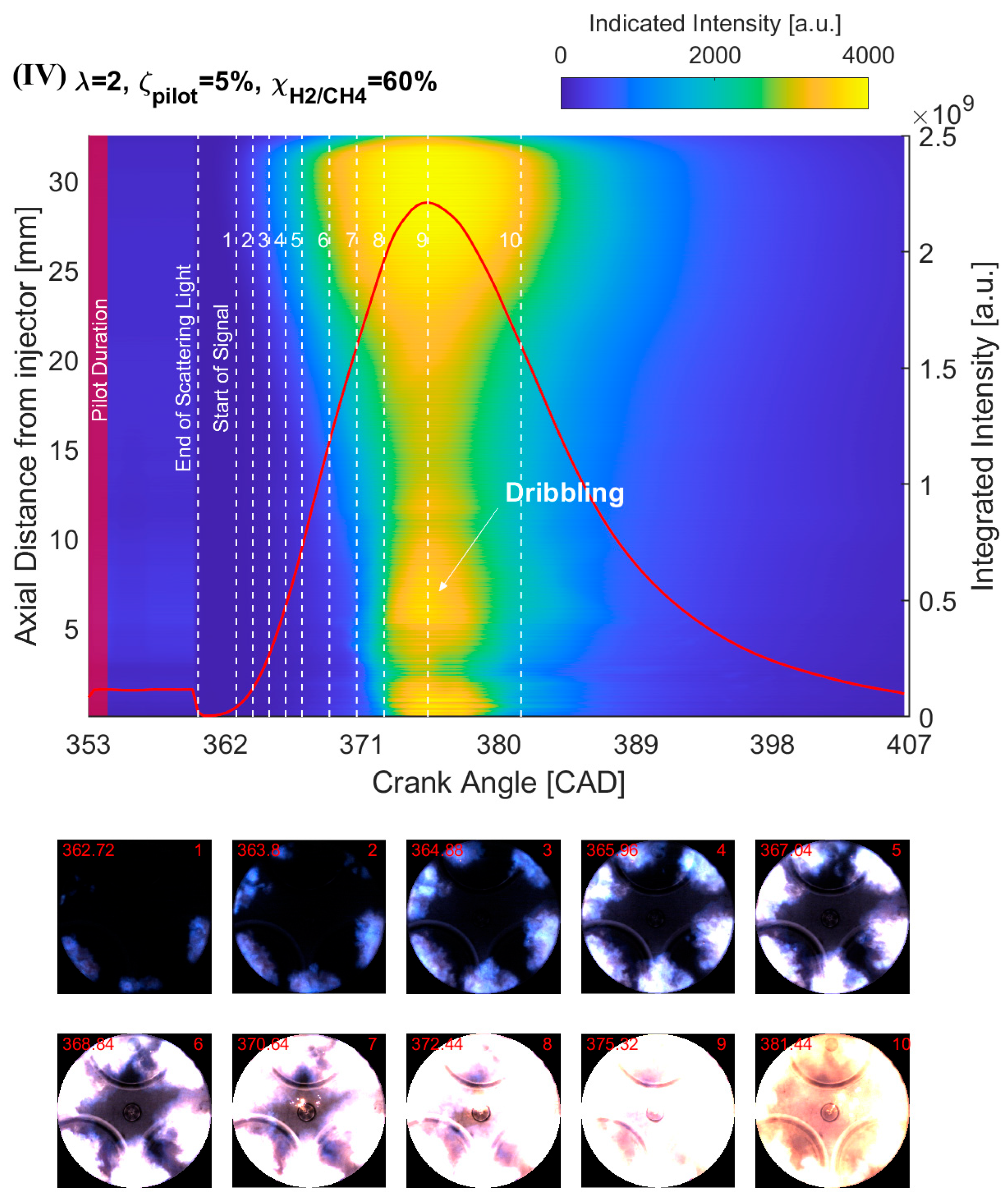
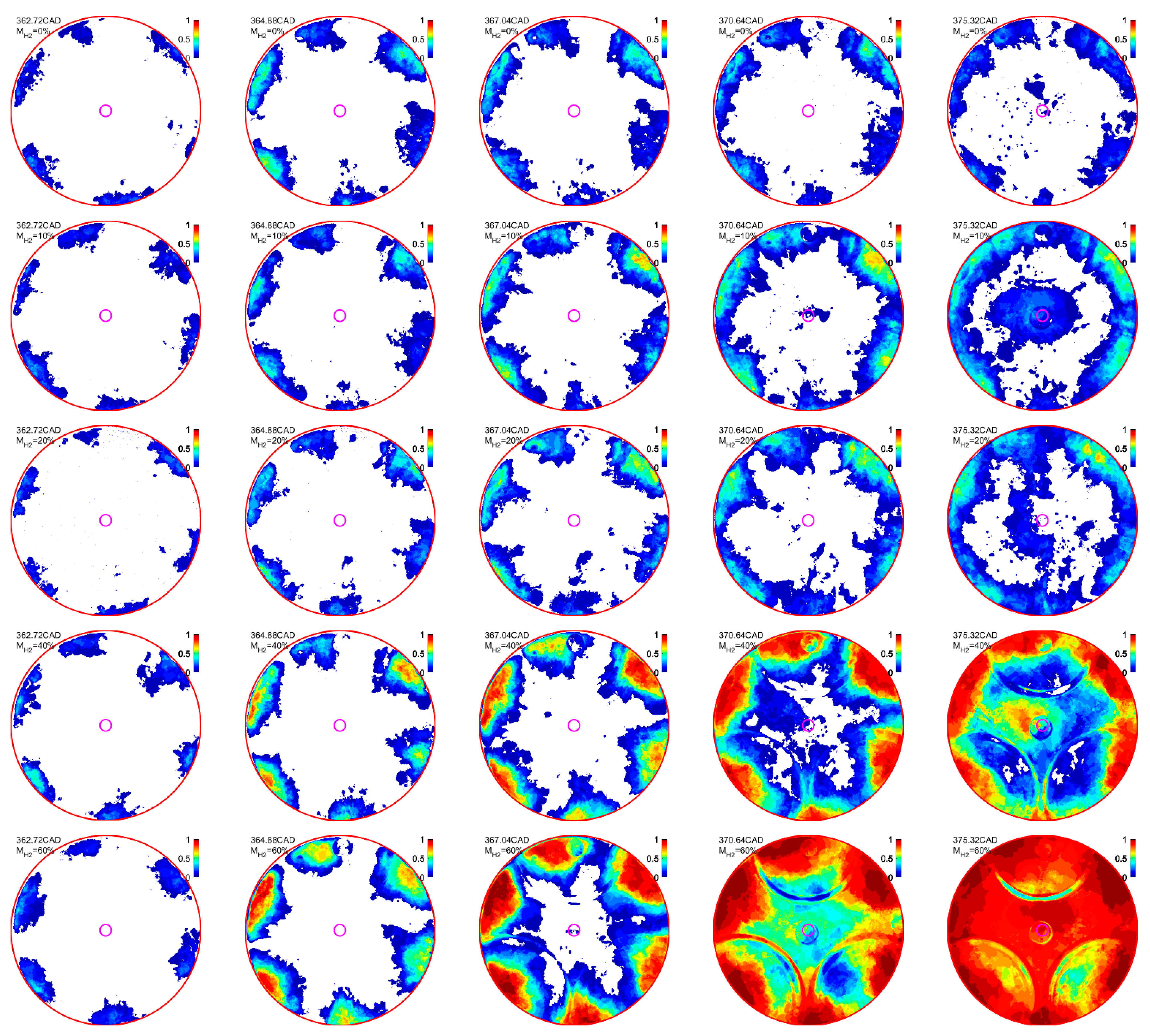
4.2. Effect of H2 Addition on the NFL Intensity
4.3. Effect of H2 Addition on Flame Stability
5. Conclusions
- (1)
- The AHRR and in-cylinder profiles were steeper with the increased H2 concentration. Meanwhile, more combustion happened in the premixed stage rather than in the diffusion stage as the concentration of H2 increased. This explanation is related to the lower ignition energy needed for the H2 and faster flame speed, which resulted in spontaneous multiple flame propagation and a faster burning rate.
- (2)
- Adding H2 into the CH4–air mixture promoted combustion efficiency as well as improved the engine thermal efficiency by up to 6.5% due to the border flammability limit and higher flame velocity of the H2 over CH4. However, increasing the H2 fraction in CH4–air mixture showed an insignificant effect of the H2 on the ignition delay time.
- (3)
- The results from the natural flame luminosity image analysis indicate that tri-fuel combustion can be categorized as a three-stage combustion. The dark-bluish flame can be observed at the initial combustion stage, which represents the low-temperature HRR combustion in-cylinder. With more H2/CH4–air involved in the combustion, the main combustion stage starts with the high-temperature HRR combustion, which exhibits bright-bluish flames in-cylinder. With the in-cylinder temperature increasing and fuel depleting, the large dribbling droplets start to burn, and residual gas starts to glow, which emits bright-yellowish flames.
- (4)
- The effect of H2 enhancement on the natural flame luminosity also indicates that the increase in the H2 fraction in the CH4–air mixture monotonically increases the natural flame luminosity and flame area, especially when 40%.
- (5)
- The flame stability index (FSI) was introduced to estimate the effect of H2 addition on the ignition and flame stability. The results indicate that the H2 induction has an inconsiderable effect on the flame kernel creation, which shows a low FSI value in all cases. Meanwhile, the addition of H2 also shows an insignificant effect on the FSI in the main and tail combustions when 40%, which exhibits a similar FSI distribution and flame area. Nevertheless, when 40%, a significant FSI improvement can be observed in the main and tail combustions.
6. Recommendation for Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | |||
| BTDC | Before top dead center | NFL | Natural flame luminosity |
| CAD | Crank angle degree | LTC | Low-temperature combustion |
| CI | Compression–ignition | PLIF | Planar laser-induced fluorescence |
| DF | Dual fuel | PPC | Partially premixed combustion |
| EHVA | Electrohydraulic valve actuator | Pratio | Pilot fuel ratio |
| FSI | Flame stability index | RCCI | Reactivity-controlled compression ignition |
| HRR | Heat release rate | SoC | Start of combustion |
| IDT | Ignition delay timing | ||
| IMEP | Indicated mean effective pressure | SoI | Start of injection |
| ITE | Indicated thermal efficiency | TF | Tri-fuel |
| Mathematic and Greek Symbols | |||
| λmixture | Gaseous fuel lambda | ||
| Discretized radius of the combustion chamber | Mass flow rate of charge-air, pilot diesel, methane, and hydrogen | ||
| Intensity of a pixel at | Tair | Charge-air temperature | |
| Hydrogen and methane volumetric ratio | |||
| Normalized intensity of a pixel at | Energy share ratio of diesel pilot | ||
References
- International Energy Agency (IEA). Tracking Transport 2020. Available online: https://www.iea.org/reports/tracking-transport-2020 (accessed on 3 March 2022).
- European Commission. Delivering the European Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal/delivering-european-green-deal_en (accessed on 3 March 2022).
- European Commission. A Hydrogen Strategy for a Climate Neutral Europe. Available online: https://www.h2greentech.eu/a-hydrogen-strategy-for-a-climate-neutral-europe/ (accessed on 3 March 2022).
- Verhelst, S.; Wallner, T. Hydrogen-fueled internal combustion engines. Prog. Energy Combust. Sci. 2009, 35, 490–527. [Google Scholar] [CrossRef]
- Nguyen, D.; Choi, Y.; Park, C.; Kim, Y.; Lee, J. Effect of supercharger system on power enhancement of hydrogen-fueled spark-ignition engine under low-load condition. Int. J. Hydrogen Energy 2021, 46, 6928–6936. [Google Scholar] [CrossRef]
- Gürbüz, H.; Akçay, İ.H. Evaluating the effects of boosting intake-air pressure on the performance and environmental-economic indicators in a hydrogen-fueled SI engine. Int. J. Hydrogen Energy 2021, 46, 28801–28810. [Google Scholar] [CrossRef]
- Şöhret, Y.; Gürbüz, H.; Akçay, İ.H. Energy and exergy analyses of a hydrogen fueled SI engine: Effect of ignition timing and compression ratio. Energy 2019, 175, 410–422. [Google Scholar] [CrossRef]
- Mohammadi, A.; Shioji, M.; Nakai, Y.; Ishikura, W.; Tabo, E. Performance and combustion characteristics of a direct injection SI hydrogen engine. Int. J. Hydrogen Energy 2007, 32, 296–304. [Google Scholar] [CrossRef]
- Babayev, R.; Andersson, A.; Dalmau, A.S.; Im, H.G.; Johansson, B. Computational characterization of hydrogen direct injection and nonpremixed combustion in a compression-ignition engine. Int. J. Hydrogen Energy 2021, 46, 18678–18696. [Google Scholar] [CrossRef]
- Takagi, Y.; Oikawa, M.; Sato, R.; Kojiya, Y.; Mihara, Y. Near-zero emissions with high thermal efficiency realized by optimizing jet plume location relative to combustion chamber wall, jet geometry and injection timing in a direct-injection hydrogen engine. Int. J. Hydrogen Energy 2019, 44, 9456–9465. [Google Scholar] [CrossRef]
- Li, H.; Karim, G.A. Knock in spark ignition hydrogen engines. Int. J. Hydrogen Energy 2004, 29, 859–865. [Google Scholar] [CrossRef]
- Li, Y.; Gao, W.; Zhang, P.; Fu, Z.; Cao, X. Influence of the equivalence ratio on the knock and performance of a hydrogen direct injection internal combustion engine under different compression ratios. Int. J. Hydrogen Energy 2021, 46, 11982–11993. [Google Scholar] [CrossRef]
- Dhyani, V.; Subramanian, K.A. Development of online control system for elimination of backfire in a hydrogen fuelled spark ignition engine. Int. J. Hydrogen Energy 2021, 46, 14757–14763. [Google Scholar] [CrossRef]
- Dhyani, V.; Subramanian, K.A. Control of backfire and NOx emission reduction in a hydrogen fueled multi-cylinder spark ignition engine using cooled EGR and water injection strategies. Int. J. Hydrogen Energy 2019, 44, 6287–6298. [Google Scholar] [CrossRef]
- Zhen, X.; Li, X.; Wang, Y.; Liu, D.; Tian, Z. Comparative study on combustion and emission characteristics of methanol/hydrogen, ethanol/hydrogen and methane/hydrogen blends in high compression ratio SI engine. Fuel 2020, 267, 117193. [Google Scholar] [CrossRef]
- Zareei, J.; Rohani, A.; Mazari, F.; Mikkhailova, M.V. Numerical investigation of the effect of two-step injection (direct and port injection) of hydrogen blending and natural gas on engine performance and exhaust gas emissions. Energy 2021, 231, 120957. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, L.; Wei, H.; Pan, J.; Li, J.; Yang, P.; Chen, R. Optical study on the effects of the hydrogen injection timing on lean combustion characteristics using a natural gas/hydrogen dual-fuel injected spark-ignition engine. Int. J. Hydrogen Energy 2021, 46, 20777–20789. [Google Scholar] [CrossRef]
- Taghavifar, H.; Nemati, A.; Salvador, F.J.; De la Morena, J. 1D energy, exergy, and performance assessment of turbocharged diesel/hydrogen RCCI engine at different levels of diesel, hydrogen, compressor pressure ratio, and combustion duration. Int. J. Hydrogen Energy 2021, 46, 22180–22194. [Google Scholar] [CrossRef]
- Liu, X.; Srna, A.; Yip, H.L.; Kook, S.; Chan, Q.N.; Hawkes, E.R. Performance and emissions of hydrogen-diesel dual direct injection (H2DDI) in a single-cylinder compression-ignition engine. Int. J. Hydrogen Energy 2021, 46, 1302–1314. [Google Scholar] [CrossRef]
- Jamrozik, A.; Grab-Rogaliński, K.; Tutak, W. Hydrogen effects on combustion stability, performance and emission of diesel engine. Int. J. Hydrogen Energy 2020, 45, 19936–19947. [Google Scholar] [CrossRef]
- Ikegami, M.; Miwa, K.; Shioji, M. A study of hydrogen fuelled compression ignition engines. Int. J. Hydrogen Energy 1982, 7, 341–353. [Google Scholar] [CrossRef]
- Kavtaradze, R.Z. Improving the ecological indices of a hydrogen diesel engine with direct gaseous hydrogen injection. J. Mach. Manuf. Reliab. 2016, 45, 307–315. [Google Scholar] [CrossRef]
- Cheng, Q.; Ahmad, Z.; Kaario, O.; Vuorinen, V.; Larmi, M. Experimental study on tri-fuel combustion using premixed methane-hydrogen mixtures ignited by a diesel pilot. Int. J. Hydrogen Energy 2021, 46, 21182–21197. [Google Scholar] [CrossRef]
- Karimkashi, S.; Kahila, H.; Kaario, O.; Larmi, M.; Vuorinen, V. Numerical study on tri-fuel combustion: Ignition properties of hydrogen-enriched methane-diesel and methanol-diesel mixtures. Int. J. Hydrogen Energy 2020, 45, 4946–4962. [Google Scholar] [CrossRef]
- Sanli, A.; Yılmaz, I.T.; Gümüş, M. Assessment of combustion and exhaust emissions in a common-rail diesel engine fueled with methane and hydrogen/methane mixtures under different compression ratio. Int. J. Hydrogen Energy 2020, 45, 3263–3283. [Google Scholar] [CrossRef]
- Mansor MR, A.; Abbood, M.M.; Mohamad, T.I. The influence of varying hydrogen-methane-diesel mixture ratio on the combustion characteristics and emissions of a direct injection diesel engine. Fuel 2017, 190, 281–291. [Google Scholar] [CrossRef]
- Rahimi, H.M.; Jazayeri, S.A.; Ebrahimi, M. Hydrogen energy share enhancement in a heavy duty diesel engine under RCCI combustion fueled with natural gas and diesel oil. Int. J. Hydrogen Energy 2020, 45, 17975–17991. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Jazayeri, S.A. Effect of hydrogen addition on RCCI combustion of a heavy duty diesel engine fueled with landfill gas and diesel oil. Int. J. Hydrogen Energy 2019, 44, 7607–7615. [Google Scholar] [CrossRef]
- Kannan, J.; Gadalla, M.; Tekgül, B.; Karimkashi, S.; Kaario, O.; Vuorinen, V. Large-eddy simulation of tri-fuel ignition: Diesel spray-assisted ignition of lean hydrogen–methane–air mixtures. Combust. Theory Model. 2021, 25, 436–459. [Google Scholar] [CrossRef]
- Rui, Z.; Leping, X.; Shiquan, F. Construction of a reduced mechanism for diesel-natural gas -hydrogen using HCCI model with Direct Relation Graph and Sensitivity Analysis. Pol. J. Chem. Technol. 2020, 22, 55–60. [Google Scholar] [CrossRef]
- Zhou, J.H.; Cheung, C.S.; Leung, C.W. Combustion, performance and emissions of a diesel engine with H2, CH4 and H2–CH4 addition. Int. J. Hydrogen Energy 2014, 39, 4611–4621. [Google Scholar] [CrossRef]
- Sabah, O.; Mohammad, T.I.; Mansor, M.R.A. Effect on changing of intake temperature to hydrogen-methane-diesel mixture combustion characteristics. IOP Conf. Ser. Earth Environ. Sci. 2020, 463, 012059. [Google Scholar] [CrossRef]
- Cernat, A.; Pana, C.; Negurescu, N.; Lazaroiu, G.; Nutu, C.; Fuiorescu, D. Hydrogen—An Alternative Fuel for Automotive Diesel Engines Used in Transportation. Sustainability 2020, 12, 9321. [Google Scholar] [CrossRef]
- Talibi, M.; Balachandran, R.; Ladommatos, N. Influence of combusting methane-hydrogen mixtures on compression–ignition engine exhaust emissions and in-cylinder gas composition. Int. J. Hydrogen Energy 2017, 42, 2381–2396. [Google Scholar] [CrossRef]
- Saanum, I.; Bysveen, M.; Hustad, J. Study of Particulate Matter-, NOx- and Hydrocarbon Emissions from a Diesel Engine Fueled with Diesel Oil and Biodiesel with Fumigation of Hydrogen, Methane and Propane; SAE Technical Paper 2008-01-1809; SAE: Warrendale, PA, USA, 2008. [Google Scholar] [CrossRef]
- Abu-Jrai, A.M.; Ala’a, H.; Hasan, A.O. Combustion, performance, and selective catalytic reduction of NOx for a diesel engine operated with combined tri fuel (H2, CH4, and conventional diesel). Energy 2017, 119, 901–910. [Google Scholar] [CrossRef]
- Liu, X.; Kokjohn, S.; Wang, H.; Yao, M. A comparative numerical investigation of reactivity controlled compression ignition combustion using Large Eddy Simulation and Reynolds-Averaged Navier-Stokes approaches. Fuel 2019, 257, 116023. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, X.; Liu, H.; Wang, H.; Yao, M. Investigation on the dual-fuel active-thermal atmosphere combustion strategy based on optical diagnostics and numerical simulations. Fuel 2020, 276, 118023. [Google Scholar] [CrossRef]
- Gehmlich, R.K.; Dumitrescu, C.E.; Wang, Y.; Mueller, C.J. Leaner Lifted-Flame Combustion Enabled by the Use of an Oxygenated Fuel in an Optical CI Engine. SAE Int. J. Engines 2016, 9, 1526–1543. [Google Scholar] [CrossRef]
- Huang, H.W.; Zhang, Y. Digital colour image processing based measurement of premixed CH4+air and C2H4+air flame chemiluminescence. Fuel 2011, 90, 48–53. [Google Scholar] [CrossRef]
- Bedard, M.J.; Fuller, T.L.; Sardeshmukh, S.; Anderson, W.E. Chemiluminescence as a diagnostic in studying combustion instability in a practical combustor. Combust. Flame 2020, 213, 211–225. [Google Scholar] [CrossRef]
- Gupta, S.B.; Bihari, B.P.; Biruduganti, M.S.; Sekar, R.R.; Zigan, J. On use of CO2∗ chemiluminescence for combustion metrics in natural gas fired reciprocating engines. Proc. Combust. Inst. 2011, 33, 3131–3139. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, H.; Ran, X.; Li, M.; Yao, M. Effects of direct-injection fuel types and proportion on late-injection reactivity controlled compression ignition. Combust. Flame 2020, 211, 445–455. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, H.; Yao, M. Simultaneous Measurement of Natural Flame Luminosity and Emission Spectra in a RCCI Engine under Different Fuel Stratification Degrees. SAE Int. J. Engines 2017, 10, 1155–1162. [Google Scholar] [CrossRef]
- Upatnieks, A.; Mueller, C.; Martin, G. The Influence of Charge-Gas Dilution and Temperature on DI Diesel Combustion Processes Using a Short-Ignition-Delay, Oxygenated Fuel; SAE Technical Paper 2005-01-2088; SAE: Warrendale, PA, USA, 2005. [Google Scholar] [CrossRef]
- Upatnieks, A.; Mueller, C. Clean, Controlled DI Diesel Combustion Using Dilute, Cool Charge Gas and a Short-Ignition-Delay, Oxygenated Fuel; SAE Technical Paper 2005-01-0363; SAE: Warrendale, PA, USA, 2005. [Google Scholar] [CrossRef]
- Raman, V.; Tang, Q.; An, Y.; Shi, H.; Sharma, P.; Magnotti, G.; Chang, J.; Johansson, B. Impact of spray-wall interaction on the in-cylinder spatial unburned hydrocarbon distribution of a gasoline partially premixed combustion engine. Combust. Flame 2020, 215, 157–168. [Google Scholar] [CrossRef]
- Liu, H.; Tang, Q.; Ran, X.; Fang, X.; Yao, M. Optical diagnostics on the reactivity controlled compression ignition (RCCI) with micro direct-injection strategy. Proc. Combust. Inst. 2019, 37, 4767–4775. [Google Scholar] [CrossRef]
- Liu, H.; Tang, Q.; Yang, Z.; Ran, X.; Geng, C.; Chen, B.; Feng, L.; Yao, M. A comparative study on partially premixed combustion (PPC) and reactivity controlled compression ignition (RCCI) in an optical engine. Proc. Combust. Inst. 2019, 37, 4759–4766. [Google Scholar] [CrossRef]
- Escofet-Martin, D.; Chien, Y.-C.; Dunn-Rankin, D. PLIF and chemiluminescence in a small laminar coflow methane-air diffusion flame at elevated pressures. Combust. Flame 2022, 243, 112067. [Google Scholar] [CrossRef]
- Ahmad, Z.; Kaario, O.; Qiang, C.; Larmi, M. Effect of pilot fuel properties on lean dual-fuel combustion and emission characteristics in a heavy-duty engine. Applied Energy 2021, 282 Pt A, 116134. [Google Scholar] [CrossRef]
- Dronniou, N.; Kashdan, J.; Lecointe, B.; Sauve, K.; Soleri, D. Optical investigation of dual-fuel CNG/diesel combustion strategies to reduce CO2 emissions. SAE Int. J. Engines 2014, 7, 873–887. [Google Scholar] [CrossRef]
- Nithyanandan, K.; Gao, Y.; Wu, H.; Lee, C.-F.; Liu, F.; Yan, J. An Optical Investigation of Multiple Diesel Injections in CNG/Diesel Dual-Fuel Combustion in a Light-Duty Optical Diesel Engine; SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2017; Volume 1. [Google Scholar] [CrossRef]
- Khosravi, M.; Rochussen, J.; Yeo, J.; Kirchen, P.; McTaggart-Cowan, G.; Wu, N. Effect of Fuelling Control Parameters on Combustion Characteristics of Diesel-Ignited Natural Gas Dual-Fuel Combustion in an Optical Engine; ASME International: New York, NY, USA, 2016; p. V001T03A012. [Google Scholar] [CrossRef]
- Ahmad, Z.; Aryal, J.; Ranta, O.; Kaario, O.; Vuorinen, V.; Larmi, M. An Optical Characterization of Dual-Fuel Combustion in a Heavy-Duty Diesel Engine; SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2018; Volume 1. [Google Scholar] [CrossRef]
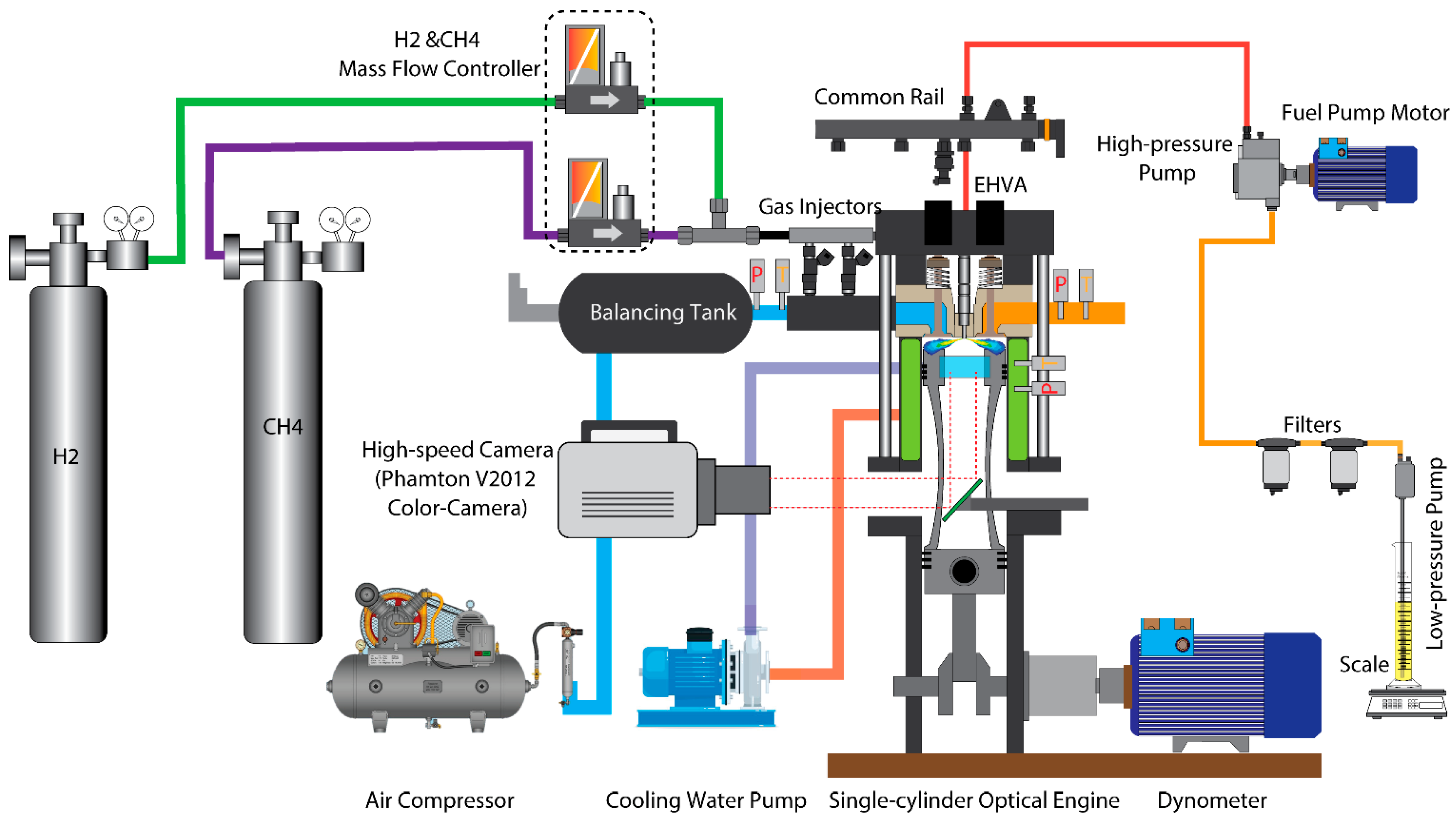
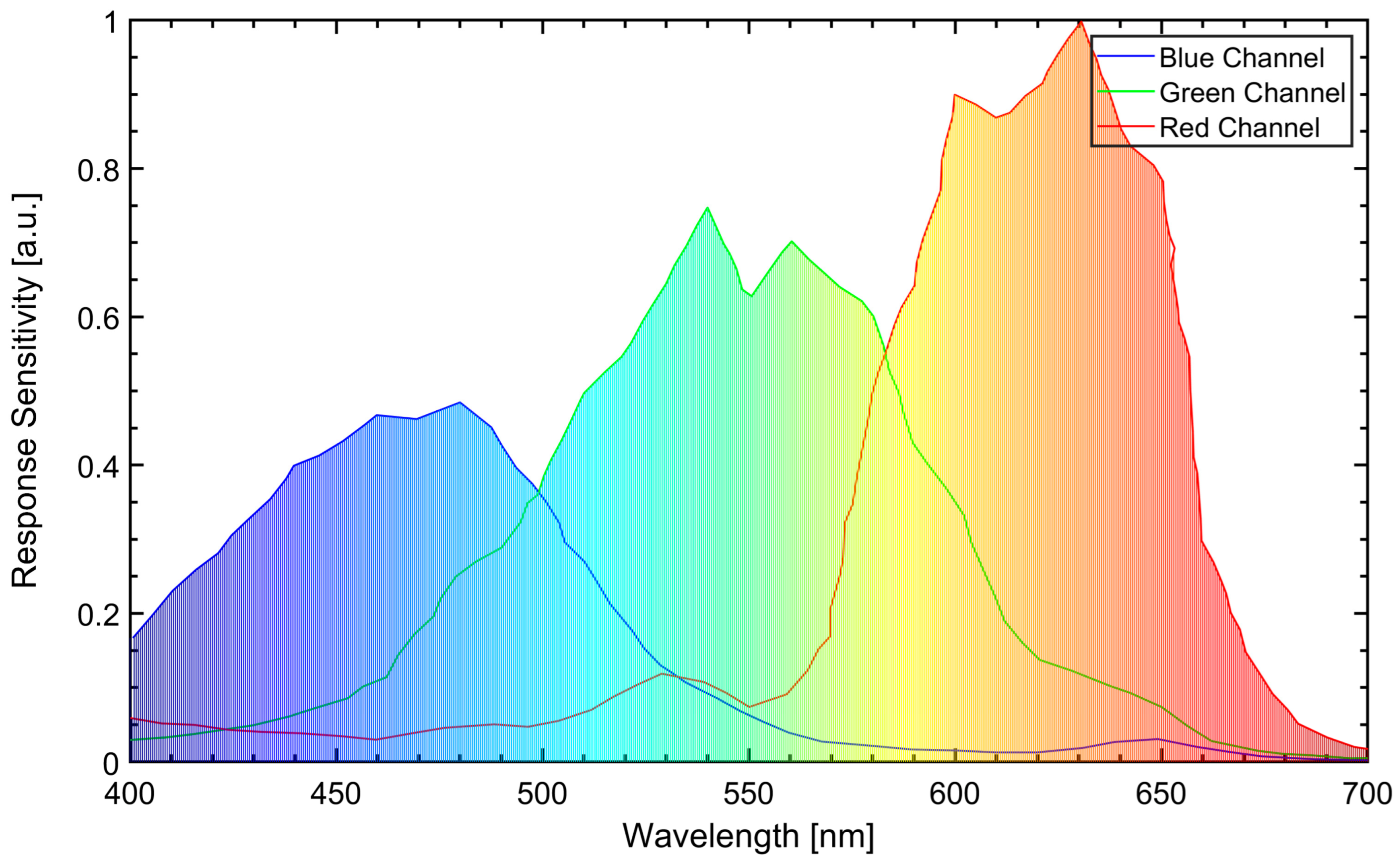
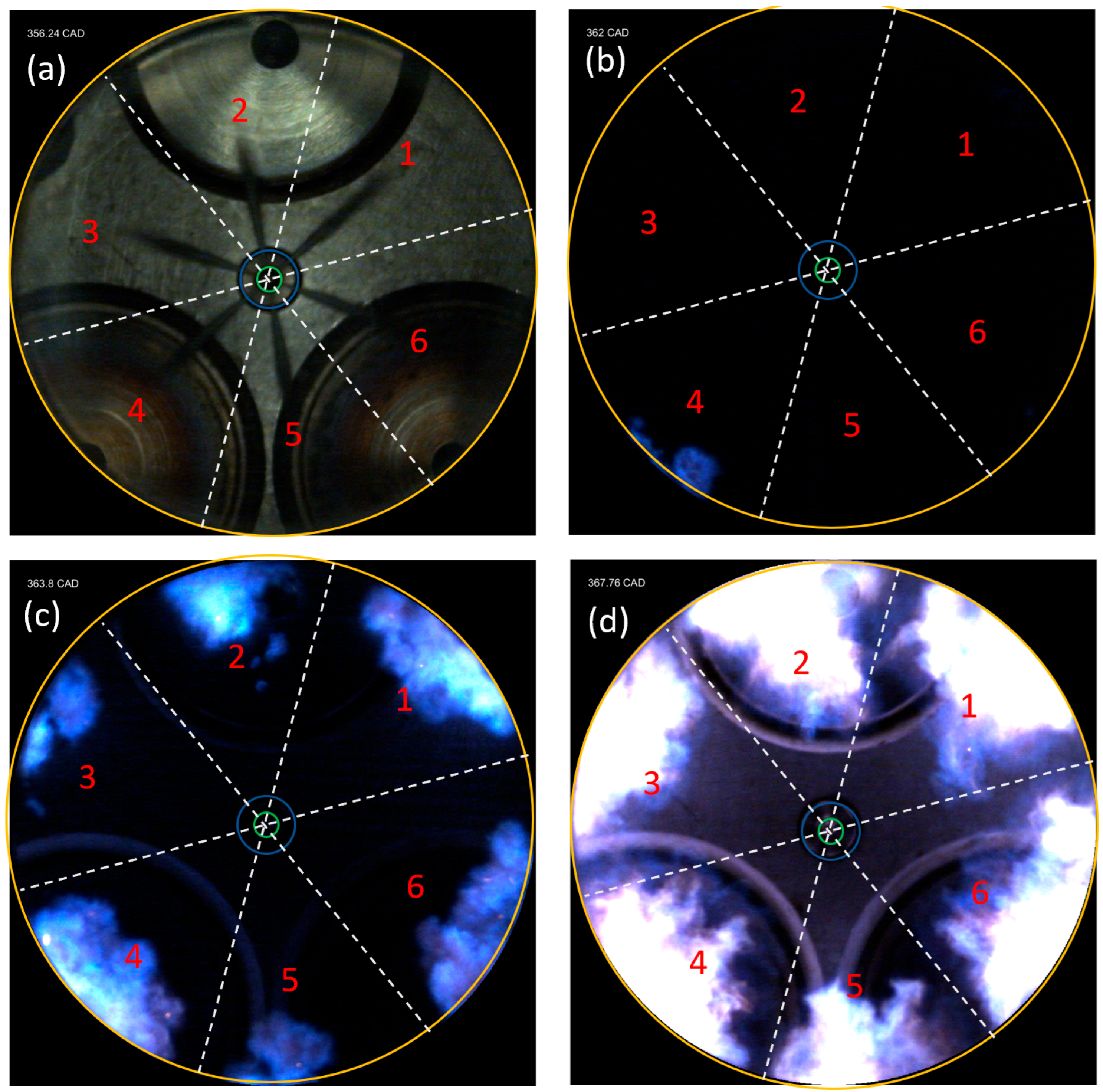


| Parameter | Unit | Value |
|---|---|---|
| Engine model | Modified 6-cylinder AGCO 84AWI engine | |
| Output power | kW | 200–298 @2100 rpm |
| Operating engine speed | rpm | 1200 |
| Bore × stroke | mm × mm | 111 × 145 |
| Connecting rod length | mm | 132 |
| Displacement volume | cm3 | 1402 |
| Combustion bowl volume | cm3 | 89.9 |
| Geometric compression ratio | 16.7:1 | |
| Swirl ratio | <0.1 | |
| Diesel pilot injector | Bosch CRI3 injector (piezo) | |
| Hole number of diesel pilot | 6 | |
| µm | 90 | |
| Diesel pilot pressure | bar | 1000 |
| Gas Injector | 2 × Bosch NGI injectors | |
| Gas pressure | bar | 8 |
| Crank angle of intake opening | CAD BTDC | 2 |
| Crank angle of intake closing | CAD ATDC | 210 |
| Crank angle of exhaust opening | CAD BTDC | 225 |
| Crank angle of exhaust closing | CAD BTDC | 6 |
| Variable | Device Type/Model and Manufacture | Manufacture | Range | Accuracy |
|---|---|---|---|---|
| Load and speed | 45 KW 94 AMP MOTOR with ACS800-11 frequency converter, | ABB, Switzerland | 0–2960 rpm | ±10 rpm |
| Valve Timing | EHVA | Parker, US | 0–12 mm | ±0.05 mm |
| Cooling temperature | Cooling system | Customized, Finland | 0–100 °C | ±1 °C |
| Charge-air pressure | Electric Compressor | Customized, Finland | 0–3 bar | ±0.5% |
| Charge-air mass flow | RHM-08 Coriolis | Rheonik Messtechnik, Germany | 0–200 kg/h | ±0.5% |
| Charge-air temperature | PT100 | TC, UK | 0–200 °C | ±0.1% |
| Charge-air pressure | Piezoelectric/AVL LPD11DA05 | AVL, Austra | 0–5 bar | ±0.1% |
| Cylinder pressure | 6125C sensor and 5011B amplifier | Kistler, Switzerland | 0–300 bar | ≤±0.4% |
| Exhaust temperature | Type K, TC | TC, UK | 0–1000 °C | ±0.5% |
| Exhaust pressure | Piezoelectric/AVL GU21C | AVL, Austra | 0–10 bar | ±0.1% |
| H2 mass flow | MVM-030-PA | EL-FLOW®, Netherland | 1–30 L/min | ±0.3% |
| CH4 mass flow | MVM-060-PA | EL-FLOW®, Netherland | 1–60 L/min | ±0.3% |
| Parameter | Unit | Value |
|---|---|---|
| Pilot fuel | EN590 | |
| Start of injection timing | CAD BTDC | 7 |
| air | kg/h | 80 |
| Lambda | 2 | |
| Pilot energy share ratio | % | 5 |
| Cooling temperature | °C | 70 |
| Pilot energy | MJ/h | 13.26 |
| pilot | g/h | 318.4 |
| Pilot duration | ms | 0.174 |
| Charge-air temperature | K | 298 |
| Case No. | H2 Volume Fraction | H2 Energy Share Ratio | H2 Energy | CH4 Energy | ||
|---|---|---|---|---|---|---|
| Vol.% | % | g/h | g/h | MJ/h | MJ/h | |
| 1 | 0 | 0 | 0 | 1450 | 0 | 72.51 |
| 2 | 10 | 3.1 | 19.7 | 1410.7 | 2.36 | 70.54 |
| 3 | 20 | 6.7 | 42.86 | 1364.4 | 5.14 | 68.22 |
| 4 | 40 | 15.9 | 104.05 | 1242 | 12.49 | 62.1 |
| 5 | 60 | 29.6 | 198.5 | 1053.1 | 23.82 | 52.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Q.; Ahmad, Z.; Kaario, O.; Vuorinen, V.; Larmi, M. Effect of Hydrogen Enhancement on Natural Flame Luminosity of Tri-Fuel Combustion in an Optical Engine. Energies 2022, 15, 9080. https://doi.org/10.3390/en15239080
Cheng Q, Ahmad Z, Kaario O, Vuorinen V, Larmi M. Effect of Hydrogen Enhancement on Natural Flame Luminosity of Tri-Fuel Combustion in an Optical Engine. Energies. 2022; 15(23):9080. https://doi.org/10.3390/en15239080
Chicago/Turabian StyleCheng, Qiang, Zeeshan Ahmad, Ossi Kaario, Ville Vuorinen, and Martti Larmi. 2022. "Effect of Hydrogen Enhancement on Natural Flame Luminosity of Tri-Fuel Combustion in an Optical Engine" Energies 15, no. 23: 9080. https://doi.org/10.3390/en15239080
APA StyleCheng, Q., Ahmad, Z., Kaario, O., Vuorinen, V., & Larmi, M. (2022). Effect of Hydrogen Enhancement on Natural Flame Luminosity of Tri-Fuel Combustion in an Optical Engine. Energies, 15(23), 9080. https://doi.org/10.3390/en15239080








