Lignin Gasification: Current and Future Viability
Abstract
1. Introduction
1.1. Sources of Lignin
1.2. Lignin Conversion Methods
2. Extraction and Sorting of the Relevant Literature
3. Lignin Gasification
- Drying (100–200 °C). It is the initial stage of the gasification process for solid lignin. The starting moisture content of lignin depends on the source and pretreatment.
- Primary pyrolysis reactions (200–400 °C). Pyrolysis reactions begin to occur in this temperature range and primarily sever the α- and β-ether bonds of the lignin structure. This was confirmed both in real lignin, and with model molecules [35]; however, C-C bonds remain stable in this temperature range. It is also during this temperature processing window where re-polymerization reactions could begin to occur, with volatile aromatic monomers produced, such as coniferyl aldehyde, isoeugenol, guaiacol, 4-vinylguaiacol, vanillic acid, and vanillin interacting with each other to form new C-C bonds.
- Combustion (400 °C~). Lignin oxidation reactions could happen at lower temperatures. However, the complete or partial combustion towards CO, CO2, and H2 begins to happen mostly after passing the ignition point of lignin [36]. H2O could also be formed through the combustion of H2 at this stage. The degree of combustion that happens depends entirely on the stoichiometric ratio of the oxidant to lignin, with low values resulting in a slower conversion and higher selectivity towards CO and H2.
- Secondary pyrolysis reactions (400 °C~). Demethoxylation reactions targeting the aromatic ring begin to occur at approximately 450 °C [35], resulting in the formation of catechols with the methoxy group hydrogenating into CH4 or further oxidizing into CO/CO2. The intramolecular H abstraction from the methyl groups could also lead to the formation of phenols. These reactions compete with combustion reactions.
- Coking and PAH formation (550 °C~). Lignin-derived monomers could begin to convert to coke and polyaromatic hydrocarbons (PAHs) at this temperature range, with PAHs forming from ~600 °C and their production intensifying as the temperature approaches ~700 °C [37].
- Reduction (800–1000 °C). In the presence of sub-stoichiometric concentrations of the oxidizing agent, reduction reactions involving single-carbon molecules could begin to take place. Some of these reactions are endothermic and often involve H2O as a product or reactant; they are shown as follows:
3.1. Lignin Gasification in Oxidative Media
| Ref. | Conditions | Reactor Type | Catalyst | Results | Notes |
|---|---|---|---|---|---|
| [43] | 1000 °C, 0.24 kg lignin/h | Entrained flow gasifier | Dolomite/Na2CO3 | 8% vol yield of H2 and 13.5% vol yield of CO, at 46% C conversion for the Na2CO3 catalyst |
|
| [42] | 740–860 °C, 0.3 kg lignin/h | Fluidized bed | Lime, olivine, and dolomite | 35% H2 yield v/v, 1.25 NI/g of gas yield |
|
| [38] | 550–850 °C, 20–30 kg lignin/h | Updraft gasifier | None | Max H2 yield of 49 g/kg lignin along with 330 g/kg of CO, another notable run was 35 g/kg lignin H2 with 842 g/kg lignin of CO, both of them were under O2 and steam |
|
| [40] | 1000 °C 0.25 ER for O2, 0.24 kg lignin/h | Entrained flow gasifier | None/ashes | H2 yield of 9 vol% |
|
| [44] | 500–900 °C, batch operation of 12 g lignin/run | Fixed bed reactor | None | Maximum gas yield of 57% at 900 °C |
|
| [41] | 835.8 °C at bed temperature, lignin feeding rate not available | Circulating fluidized bed reactor | None | 17.62 vol% yield of H2 at 5 bar pressure with 21.16 vol% CO |
|
3.2. Lignin Gasification in Water or Steam
| Ref. | Conditions | Reactor Type | Catalyst | Results | Notes |
|---|---|---|---|---|---|
| [47] | 900 °C, 10–20 s residence time, lignin feeding rate not available | Entrained flow reactor | None | 0.89 mol H2 per mol of C and 0.1 mol of CO per mol of °C at 900 C |
|
| [48] | 900–1500 °C, 1 to 8 min residence time, 1.08 kg lignin/h | Fixed bed reactor | None | 49.37 vol% H2 yield and 35.07 vol% CO yield at 1500 °C also 54.11 vol% H2 yield and 11.07 vol% CO yield at 900 C |
|
| [50] | 650 °C, 26 MPa, 0.65 g lignin/run | Batch reactor | NiMgAl | 12.9% highest gas yield, max 39.06 % mol selectivity towards H2 |
|
| [51] | 650 °C, 50 min residence time, 0.65 g lignin/run | Batch reactor | K2CO3, NiCe/Al2O3 | 2.86 mmol H2/g lignin with K2CO3, 18.0 mmol/g lignin overall gas yield, and 2.15 mmol H2/g lignin with NiCe/alumina with an overall 12.9 mmol gas/g lignin yield |
|
| [54] | 300–600 °C 90–410 bar, 1.2 g lignin/run | Batch reactor | None/K2CO3 | 18.99 mol H2/kg C in feedstock without catalyst, 23.47 mol H2/kg C in feedstock with K2CO3, both at 600 C |
|
| [55] | 550 °C in the pyrolysis stage, 750 °C in the second stage, batch operation with 2 g lignin/run | Two-stage fixed bed reactor | None (sand) or 10% Ni/Al2O3 | 25.5 mmol/g feedstock of H2, 6.44 mmol/g feedstock of CO with catalyst at conditions in the left |
|
| [56] | 400 °C, 1 h reaction time, batch operation with 0.1 g lignin/run | Batch reactor | Ru/C | 73.5% overall gas yield, 7% H2, no CO reported |
|
| [57] | 399–651 °C 50 min reaction time, 0.65 g lignin/run | Batch reactor | None | 1.59 mmol/g lignin at 651 °C 26 MPa, with an overall gas yield of 16.1 |
|
3.3. Black Liquor Gasification
4. Economic, Energetic, and Technological Perspectives of Lignin Gasification
4.1. Economic and Energetic Perspective
- Syngas production via the SCWG of lignin; the focus of the process is purely the production of H2-rich syngas that would then be used as feedstock for other processes, ideally at high lignin concentrations to minimize the degree of endothermicity of the process.
- Syngas and heat co-production via oxidative lignin gasification; this process is focused on the production of syngas in addition to heat by integrating the gasification process with heat exchangers to valorize the residual heat of the products.
- The gasification of black liquor; this process only applies to pulping processes and prioritizes the production of H2 gas over other co-products. This process is possibly the easiest to integrate with existing pulping facilities, as it overcomes the need to precipitate the lignin from black liquor and could be more seamlessly integrated into the rest of the process.
4.2. Comparison to Other Lignin Conversion Methods and Recommendations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moriarty, P.; Honnery, D. Can Renewable Energy Power the Future? Energy Policy 2016, 93, 3–7. [Google Scholar] [CrossRef]
- Henry, R.J. Evaluation of Plant Biomass Resources Available for Replacement of Fossil Oil. Plant Biotechnol. J. 2010, 8, 288–293. [Google Scholar] [CrossRef]
- Lynd, L.R.; Liang, X.; Biddy, M.J.; Allee, A.; Cai, H.; Foust, T.; Himmel, M.E.; Laser, M.S.; Wang, M.; Wyman, C.E. Cellulosic Ethanol: Status and Innovation. Curr. Opin. Biotechnol. 2017, 45, 202–211. [Google Scholar] [CrossRef]
- Martinelli, L.A.; Filoso, S. Expansion of Sugarcane Ethanol Production in Brazil: Environmental and Social Challenges. Ecol. Appl. 2008, 18, 885–898. [Google Scholar] [CrossRef]
- Issariyakul, T.; Dalai, A.K. Biodiesel from Vegetable Oils. Renew. Sustain. Energy Rev. 2014, 31, 446–471. [Google Scholar] [CrossRef]
- Wenger, J.; Haas, V.; Stern, T. Why Can We Make Anything from Lignin Except Money? Towards a Broader Economic Perspective in Lignin Research. Curr. For. Rep. 2020, 6, 294–308. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin Utilization: A Review of Lignin Depolymerization from Various Aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Beckham, G.T.; Johnson, C.W.; Karp, E.M.; Salvachúa, D.; Vardon, D.R. Opportunities and Challenges in Biological Lignin Valorization. Curr. Opin. Biotechnol. 2016, 42, 40–53. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, N.; Li, H.; Deng, W.; Cheng, Y.; Wang, Y.; Liu, Y.; Cobb, K.; Lei, H.; Chen, P.; et al. Recent Advances in Improving Lignocellulosic Biomass-Based Bio-Oil Production. J. Anal. Appl. Pyrolysis 2020, 149, 104845. [Google Scholar] [CrossRef]
- Yoon, H.C.; Pozivil, P.; Steinfeld, A. Thermogravimetric Pyrolysis and Gasification of Lignocellulosic Biomass and Kinetic Summative Law for Parallel Reactions with Cellulose, Xylan, and Lignin. Energy Fuels 2012, 26, 357–364. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen Energy, Economy and Storage: Review and Recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Chaube, A.; Chapman, A.; Shigetomi, Y.; Huff, K.; Stubbins, J. The Role of Hydrogen in Achieving Long Term Japanese Energy System Goals. Energies 2020, 13, 4539. [Google Scholar] [CrossRef]
- Padilha, C.E.d.A.; Nogueira, C.d.C.; Alencar, B.R.A.; de Abreu, Í.B.S.; Dutra, E.D.; Ruiz, J.A.C.; Souza, D.F.d.S.; dos Santos, E.S. Production and Application of Lignin-Based Chemicals and Materials in the Cellulosic Ethanol Production: An Overview on Lignin Closed-Loop Biorefinery Approaches. Waste Biomass Valorization 2021, 12, 6309–6337. [Google Scholar] [CrossRef]
- Novaes, E.; Kirst, M.; Chiang, V.; Winter-Sederoff, H.; Sederoff, R. Lignin and Biomass: A Negative Correlation for Wood Formation and Lignin Content in Trees. Plant Physiol. 2010, 154, 555–561. [Google Scholar] [CrossRef]
- Hatfield, R.; Vermerris, W. Lignin Formation in Plants. The Dilemma of Linkage Specificity. Plant Physiol. 2001, 126, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Seshan, K.; Li, Y. A Review on Thermal Chemical Reactions of Lignin Model Compounds. Catal. Today 2017, 298, 276–297. [Google Scholar] [CrossRef]
- Garcia, A.C.; Cheng, S.; Cross, J.S. Solvolysis of Kraft Lignin to Bio-Oil: A Critical Review. Clean Technol. 2020, 2, 513–528. [Google Scholar] [CrossRef]
- Gellerstedt, G. Softwood Kraft Lignin: Raw Material for the Future. Ind. Crops Prod. 2015, 77, 845–854. [Google Scholar] [CrossRef]
- Wei Kit Chin, D.; Lim, S.; Pang, Y.L.; Lam, M.K. Fundamental Review of Organosolv Pretreatment and Its Challenges in Emerging Consolidated Bioprocessing. Biofuels Bioprod. Biorefining 2020, 14, 808–829. [Google Scholar] [CrossRef]
- Kilpeläinen, I.; Xie, H.; King, A.; Granstrom, M.; Heikkinen, S.; Argyropoulos, D.S. Dissolution of Wood in Ionic Liquids. J. Agric. Food Chem. 2007, 55, 9142–9148. [Google Scholar] [CrossRef]
- Pettersen, R.C. The Chemical Composition of Wood. Chem. Solid Wood 1984, 207, 57–126. [Google Scholar]
- Maksimuk, Y.; Antonava, Z.; Krouk, V.; Korsakova, A.; Kursevich, V. Prediction of Higher Heating Value Based on Elemental Composition for Lignin and Other Fuels. Fuel 2020, 263, 116727. [Google Scholar] [CrossRef]
- Yi, L.; Feng, J.; Qin, Y.-H.; Li, W.-Y. Prediction of Elemental Composition of Coal Using Proximate Analysis. Fuel 2017, 193, 315–321. [Google Scholar] [CrossRef]
- Gong, X.; Meng, Y.; Lu, J.; Tao, Y.; Cheng, Y.; Wang, H. A Review on Lignin-Based Phenolic Resin Adhesive. Macromol. Chem. Phys. 2022, 223, 2100434. [Google Scholar] [CrossRef]
- Verrillo, M.; Savy, D.; Cangemi, S.; Savarese, C.; Cozzolino, V.; Piccolo, A. Valorization of Lignins from Energy Crops and Agro-industrial Byproducts as Antioxidant and Antibacterial Materials. J. Sci. Food Agric. 2022, 102, 2885–2892. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Wang, H.; Tucker, M.; Ji, Y. Recent Development in Chemical Depolymerization of Lignin: A Review. J. Appl. Chem. 2013, 2013, 838645. [Google Scholar] [CrossRef]
- Funkenbusch, L.T.; Mullins, M.E.; Vamling, L.; Belkhieri, T.; Srettiwat, N.; Winjobi, O.; Shonnard, D.R.; Rogers, T.N. Technoeconomic Assessment of Hydrothermal Liquefaction Oil from Lignin with Catalytic Upgrading for Renewable Fuel and Chemical Production. WIREs Energy Environ. 2019, 8, e319. [Google Scholar] [CrossRef]
- Asadullah, M. Barriers of Commercial Power Generation Using Biomass Gasification Gas: A Review. Renew. Sustain. Energy Rev. 2014, 29, 201–215. [Google Scholar] [CrossRef]
- Slivka, R.M.; Chinn, M.S.; Grunden, A.M. Gasification and Synthesis Gas Fermentation: An Alternative Route to Biofuel Production. Biofuels 2011, 2, 405–419. [Google Scholar] [CrossRef]
- Baruah, D.; Baruah, D.C. Modeling of Biomass Gasification: A Review. Renew. Sustain. Energy Rev. 2014, 39, 806–815. [Google Scholar] [CrossRef]
- Khwanjaisakun, N.; Amornraksa, S.; Simasatitkul, L.; Charoensuppanimit, P.; Assabumrungrat, S. Techno-Economic Analysis of Vanillin Production from Kraft Lignin: Feasibility Study of Lignin Valorization. Bioresour. Technol. 2020, 299, 122559. [Google Scholar] [CrossRef]
- Tijmensen, M.J.A.; Faaij, A.P.C.; Hamelinck, C.N. Exploration of the Possibilities for Production of Fischer Tropsch Liquids and Power via Biomass Gasiÿcation. Biomass Bioenergy 2002, 23, 129–152. [Google Scholar] [CrossRef]
- Yin, X.; Leung, D.Y.C.; Chang, J.; Wang, J.; Fu, Y.; Wu, C. Characteristics of the Synthesis of Methanol Using Biomass-Derived Syngas. Energy Fuels 2005, 19, 305–310. [Google Scholar] [CrossRef]
- Kawamoto, H. Lignin Pyrolysis Reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef]
- Cao, W.; Li, J.; Martí-Rosselló, T.; Zhang, X. Experimental Study on the Ignition Characteristics of Cellulose, Hemicellulose, Lignin and Their Mixtures. J. Energy Inst. 2019, 92, 1303–1312. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, C.; Onwudili, J.A.; Meng, A.; Zhang, Y.; Williams, P.T. Polycyclic Aromatic Hydrocarbon Formation from the Pyrolysis/Gasification of Lignin at Different Reaction Conditions. Energy Fuels 2014, 28, 6371–6379. [Google Scholar] [CrossRef]
- Cerone, N.; Zimbardi, F. Effects of Oxygen and Steam Equivalence Ratios on Updraft Gasification of Biomass. Energies 2021, 14, 2675. [Google Scholar] [CrossRef]
- Wiinikka, H.; Johansson, A.-C.; Wennebro, J.; Carlsson, P.; Öhrman, O.G.W. Evaluation of Black Liquor Gasification Intended for Synthetic Fuel or Power Production. Fuel Process. Technol. 2015, 139, 216–225. [Google Scholar] [CrossRef]
- Yu, H.; Yang, H.; Chao, H. Influence of the Interaction among Three Biomass Components on Gasification Characteristics. J. Renew. Sustain. Energy 2022, 14, 023101. [Google Scholar] [CrossRef]
- Szul, M.; Iluk, T.; Zuwała, J. Use of CO2 in Pressurized, Fluidized Bed Gasification of Waste Biomasses. Energies 2022, 15, 1395. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.N.; Carolino, C.; Miranda, M.; Abelha, P.; Direito, D.; Dohrup, J.; Sørensen, H.R.; Girio, F. Effects of Experimental Conditions and of Addition of Natural Minerals on Syngas Production from Lignin by Oxy-Gasification: Comparison of Bench- and Pilot Scale Gasification. Fuel 2015, 140, 62–72. [Google Scholar] [CrossRef]
- Yu, H.; Wu, Z.; Chen, G. Catalytic Gasification Characteristics of Cellulose, Hemicellulose and Lignin. Renew. Energy 2018, 121, 559–567. [Google Scholar] [CrossRef]
- Zhou, B.; Dichiara, A.; Zhang, Y.; Zhang, Q.; Zhou, J. Tar Formation and Evolution during Biomass Gasification: An Experimental and Theoretical Study. Fuel 2018, 234, 944–953. [Google Scholar] [CrossRef]
- Weingärtner, H.; Franck, E.U. Supercritical Water as a Solvent. Angew. Chem. Int. Ed. 2005, 44, 2672–2692. [Google Scholar] [CrossRef]
- Salierno, G.; Marinelli, F.; Likozar, B.; Ghavami, N.; De Blasio, C. Supercritical Water Gasification of Glycerol: Continuous Reactor Kinetics and Transport Phenomena Modeling. Int. J. Heat Mass Transf. 2022, 183, 122200. [Google Scholar] [CrossRef]
- Ogi, T.; Nakanishi, M.; Fukuda, Y. Effect of Lignin Components on Gasification of Japanese Cedar (Cryptopmeria Japonica) Wood and Bark Using an Entrained-Flow-Type Gasification Reactor. Energy Fuels 2016, 30, 7867–7877. [Google Scholar] [CrossRef]
- Tian, T.; Li, Q.; He, R.; Tan, Z.; Zhang, Y. Effects of Biochemical Composition on Hydrogen Production by Biomass Gasification. Int. J. Hydrog. Energy 2017, 42, 19723–19732. [Google Scholar] [CrossRef]
- Wang, R.; Guo, L.; Jin, H.; Lu, L.; Yi, L.; Zhang, D.; Chen, J. DFT Study of the Enhancement on Hydrogen Production by Alkaline Catalyzed Water Gas Shift Reaction in Supercritical Water. Int. J. Hydrog. Energy 2018, 43, 13879–13886. [Google Scholar] [CrossRef]
- Kang, K.; Azargohar, R.; Dalai, A.K.; Wang, H. Hydrogen Generation via Supercritical Water Gasification of Lignin Using Ni-Co/Mg-Al Catalysts: Hydrogen, Supercritical Water Gasification, Lignin, Ni-Co/Mg-Al. Int. J. Energy Res. 2017, 41, 1835–1846. [Google Scholar] [CrossRef]
- Kang, K.; Azargohar, R.; Dalai, A.K.; Wang, H. Hydrogen Production from Lignin, Cellulose and Waste Biomass via Supercritical Water Gasification: Catalyst Activity and Process Optimization Study. Energy Convers. Manag. 2016, 117, 528–537. [Google Scholar] [CrossRef]
- Pinkard, B.R.; Gorman, D.J.; Tiwari, K.; Rasmussen, E.G.; Kramlich, J.C.; Reinhall, P.G.; Novosselov, I.V. Supercritical Water Gasification: Practical Design Strategies and Operational Challenges for Lab-Scale, Continuous Flow Reactors. Heliyon 2019, 5, e01269. [Google Scholar] [CrossRef]
- Guan, Q.; Wei, C.; Chai, X.; Ning, P.; Tian, S.; Gu, J.; Chen, Q.; Miao, R. Energetic Analysis of Gasification of Biomass by Partial Oxidation in Supercritical Water. Chin. J. Chem. Eng. 2015, 23, 205–212. [Google Scholar] [CrossRef]
- Güngören Madenoğlu, T.; Sağlam, M.; Yüksel, M.; Ballice, L. Hydrothermal Gasification of Biomass Model Compounds (Cellulose and Lignin Alkali) and Model Mixtures. J. Supercrit. Fluids 2016, 115, 79–85. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Pyrolysis-Catalytic Steam Reforming of Agricultural Biomass Wastes and Biomass Components for Production of Hydrogen/Syngas. J. Energy Inst. 2019, 92, 1987–1996. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Mimura, N.; Segawa, A.; Mazaki, H.; Sato, O. Lignin Depolymerization into Aromatic Monomers Using Supported Metal Catalysts in Supercritical Water. J. Jpn. Pet. Inst. 2020, 63, 221–227. [Google Scholar] [CrossRef]
- Kang, K.; Azargohar, R.; Dalai, A.K.; Wang, H. Noncatalytic Gasification of Lignin in Supercritical Water Using a Batch Reactor for Hydrogen Production: An Experimental and Modeling Study. Energy Fuels 2015, 29, 1776–1784. [Google Scholar] [CrossRef]
- Sricharoenchaikul, V. Assessment of Black Liquor Gasification in Supercritical Water. Bioresour. Technol. 2009, 100, 638–643. [Google Scholar] [CrossRef]
- Cao, C.; Xu, L.; He, Y.; Guo, L.; Jin, H.; Huo, Z. High-Efficiency Gasification of Wheat Straw Black Liquor in Supercritical Water at High Temperatures for Hydrogen Production. Energy Fuels 2017, 31, 3970–3978. [Google Scholar] [CrossRef]
- Andeme Ela, R.C.; Spahn, L.; Safaie, N.; Ferrier, R.C., Jr.; Ong, R.G. Understanding the Effect of Precipitation Process Variables on Hardwood Lignin Characteristics and Recovery from Black Liquor. ACS Sustain. Chem. Eng. 2020, 8, 13997–14005. [Google Scholar] [CrossRef]
- Curmi, H.; Chirat, C.; Roubaud, A.; Peyrot, M.; Haarlemmer, G.; Lachenal, D. Extraction of Phenolic Compounds from Sulfur-Free Black Liquor Thanks to Hydrothermal Treatment before the Production of Syngas for Biofuels. J. Supercrit. Fluids 2022, 181, 105489. [Google Scholar] [CrossRef]
- De Blasio, C.; De Gisi, S.; Molino, A.; Simonetti, M.; Santarelli, M.; Björklund-Sänkiaho, M. Concerning Operational Aspects in Supercritical Water Gasification of Kraft Black Liquor. Renew. Energy 2019, 130, 891–901. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Valerio, V.; Rimauro, J.; Marino, T.; Casella, P.; Cerbone, A.; Arcieri, G.; Viola, E. Supercritical Water Gasification of Lignin Solution Produced by Steam Explosion Process on Arundo Donax after Alkaline Extraction. Fuel 2018, 221, 513–517. [Google Scholar] [CrossRef]
- De Blasio, C.; Lucca, G.; Özdenkci, K.; Mulas, M.; Lundqvist, K.; Koskinen, J.; Santarelli, M.; Westerlund, T.; Järvinen, M. A Study on Supercritical Water Gasification of Black Liquor Conducted in Stainless Steel and Nickel-Chromium-Molybdenum Reactors: A Study on Supercritical Water Gasification. J. Chem. Technol. Biotechnol. 2016, 91, 2664–2678. [Google Scholar] [CrossRef]
- Cao, C.; Guo, L.; Chen, Y.; Guo, S.; Lu, Y. Hydrogen Production from Supercritical Water Gasification of Alkaline Wheat Straw Pulping Black Liquor in Continuous Flow System. Int. J. Hydrog. Energy 2011, 36, 13528–13535. [Google Scholar] [CrossRef]
- Hawangchu, Y.; Atong, D.; Sricharoenchaikul, V. The Effect of Alkali on the Product Distribution from Black Liquor Conversion under Supercritical Water. Environ. Technol. 2017, 38, 1742–1750. [Google Scholar] [CrossRef]
- Malladi, K.T.; Sowlati, T. Biomass Logistics: A Review of Important Features, Optimization Modeling and the New Trends. Renew. Sustain. Energy Rev. 2018, 94, 587–599. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A Concise Review of Current Lignin Production, Applications, Products and Their Environmental Impact. Ind. Crop. Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Fonseca, G.C.; Costa, C.B.B.; Cruz, A.J.G. Economic Analysis of a Second-Generation Ethanol and Electricity Biorefinery Using Superstructural Optimization. Energy 2020, 204, 117988. [Google Scholar] [CrossRef]
- Obydenkova, S.V.; Kouris, P.D.; Hensen, E.J.M.; Smeulders, D.M.J.; van der Meer, Y.; Boot, M.D. Industrial Lignin from 2G Biorefineries—Assessment of Availability and Pricing Strategies. Bioresour. Technol. 2019, 291, 121805. [Google Scholar] [CrossRef] [PubMed]
- Seçer, A.; Küçet, N.; Fakı, E.; Hasanoğlu, A. Comparison of Co–Gasification Efficiencies of Coal, Lignocellulosic Biomass and Biomass Hydrolysate for High Yield Hydrogen Production. Int. J. Hydrog. Energy 2018, 43, 21269–21278. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Luo, H.-L.; Wang, C. Progress and Trends of Global Carbon Neutrality Pledges. Adv. Clim. Chang. Res. 2021, 17, 88–97. [Google Scholar]
- Muradov, N.Z.; Veziroğlu, T.N. “Green” Path from Fossil-Based to Hydrogen Economy: An Overview of Carbon-Neutral Technologies. Int. J. Hydrog. Energy 2008, 33, 6804–6839. [Google Scholar] [CrossRef]
- Field, C.B.; Campbell, J.E.; Lobell, D.B. Biomass Energy: The Scale of the Potential Resource. Trends Ecol. Evol. 2008, 23, 65–72. [Google Scholar] [CrossRef]
- Nässén, J.; Hedenus, F.; Karlsson, S.; Holmberg, J. Concrete vs. Wood in Buildings—An Energy System Approach. Build. Environ. 2012, 51, 361–369. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as Renewable Raw Material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Meister, J.J. Modification of Lignin*. J. Macromol. Sci. Part C 2002, 42, 235–289. [Google Scholar] [CrossRef]
- Mainka, H.; Täger, O.; Körner, E.; Hilfert, L.; Busse, S.; Edelmann, F.T.; Herrmann, A.S. Lignin—An Alternative Precursor for Sustainable and Cost-Effective Automotive Carbon Fiber. J. Mater. Res. Technol. 2015, 4, 283–296. [Google Scholar] [CrossRef]
- Kalami, S.; Arefmanesh, M.; Master, E.; Nejad, M. Replacing 100% of Phenol in Phenolic Adhesive Formulations with Lignin. J. Appl. Polym. Sci. 2017, 134, 45124. [Google Scholar] [CrossRef]
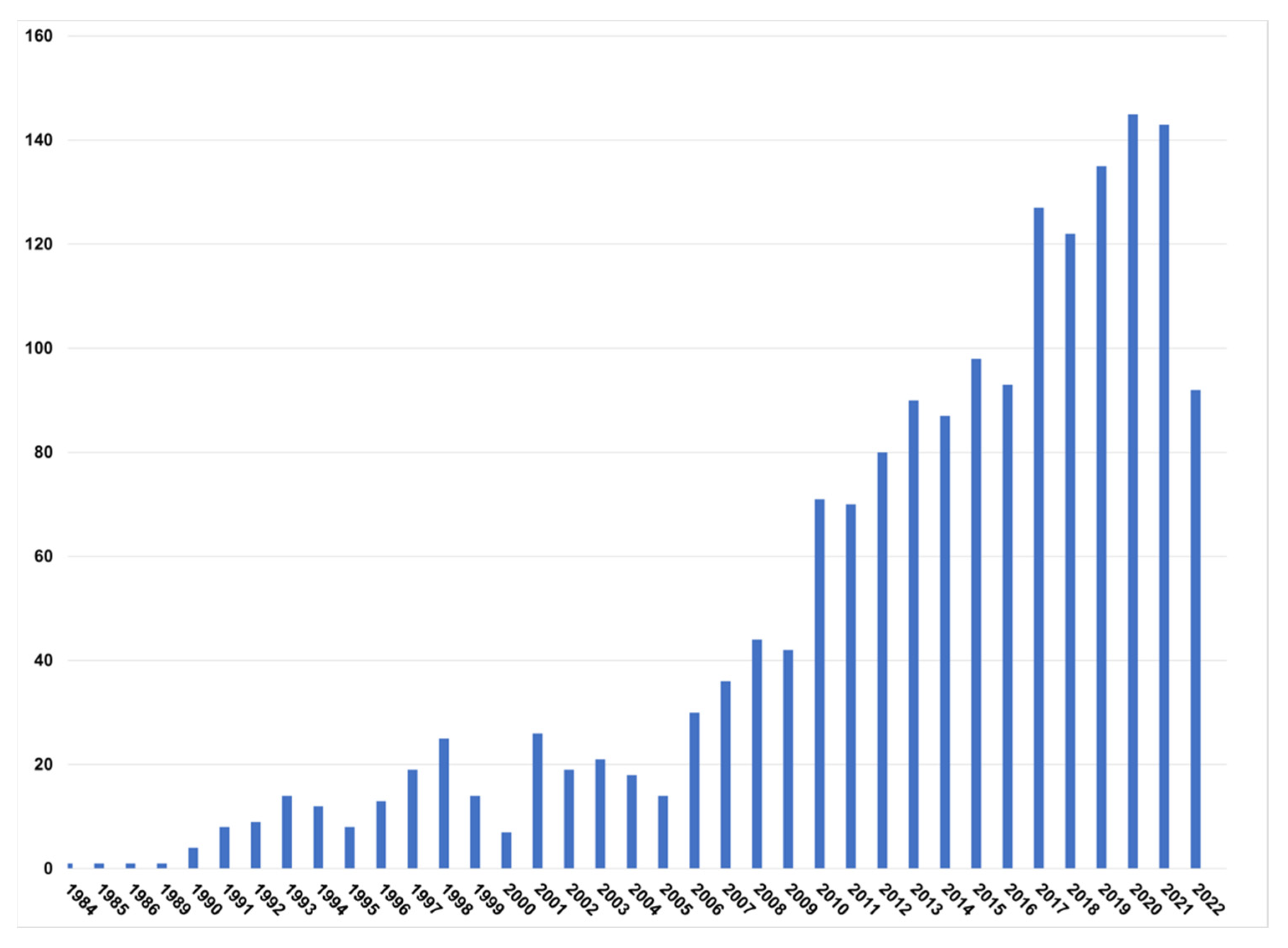
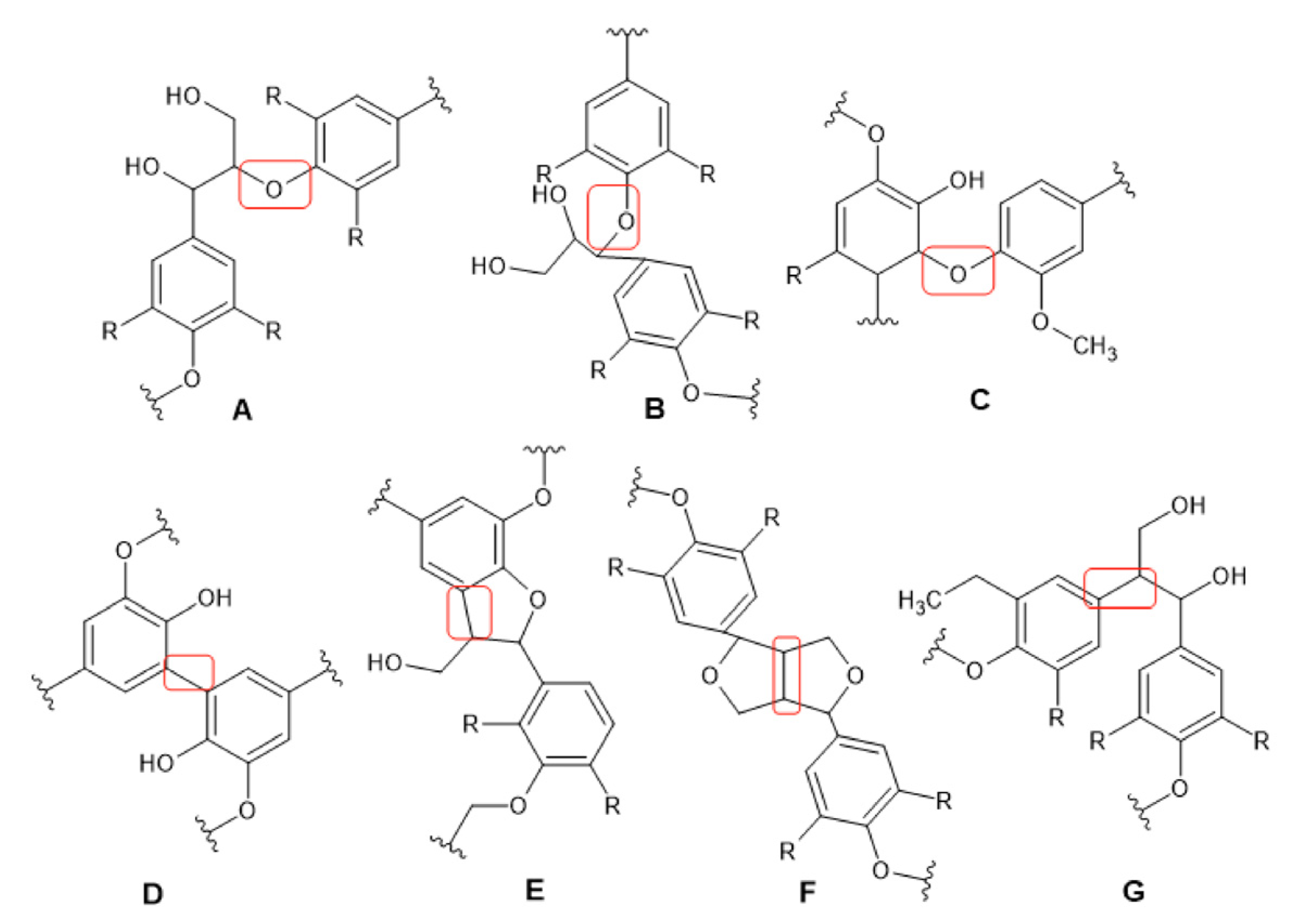
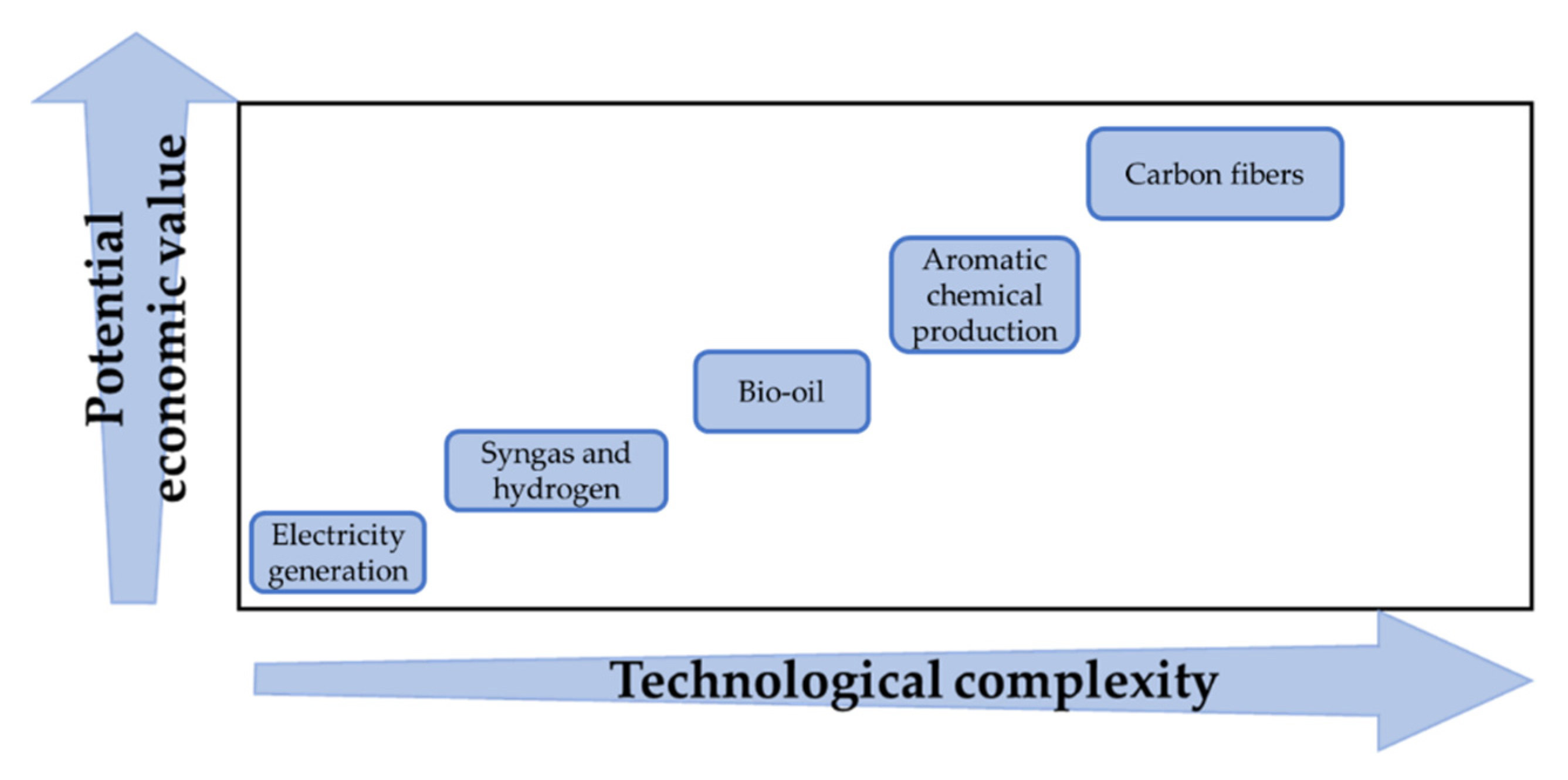
| Feedstock | C% | H% | O% | N% | S% | Ash% | Reference |
|---|---|---|---|---|---|---|---|
| Wood | 50 | 6 | 42 | 1 | - | 4–10 | [21] |
| Cellulose | 44 | 6 | 49 | - | - | - | |
| Lignin | 42~66 | 4.6~6.18 | 23~41.4 | 0.07~2.9 | 0.06~5.27 | 0.4~27.2 | [22] |
| Lignite coal | 63.55 | 5.25 | 15.74 | 1.20 | 0.26 | 14.0 | [23] |
| Anthracite Coal | 89.07 | 3.53 | 1.49 | 0.69 | 0.20 | 5.01 | [23] |
| Method | Temperature | Products | Reference |
|---|---|---|---|
| Gasification | 400–1000+ °C | Syngas | [26] |
| Pyrolysis | 300–600 °C | Bio-oil, gaseous hydrocarbons, and char | [27] |
| Solvolysis | 200–400 °C | Bio-oil and char | [28] |
| Ref. | Conditions | Reactor Type | Catalyst | Results | Notes |
|---|---|---|---|---|---|
| [64] | 500–700 °C, BL flowrate specified as higher than [66] | Tubular-flow through reactor | Ni/None (Inconel 625) | 50.32% vol H2 yield without a catalyst at 700 °C; 60.18% vol H2 yield with a catalyst at 600 °C |
|
| [58] | 375–650 °C 5–120 s reaction time, approximately 1.87 g lignin/run | Quartz capillary | None | 75.86% gas yield, 23.78 % of hydrogen in the feedstock as H2 gas, 12.78% of C as CO as gas (optimal conditions) |
|
| [61] | 400–600 °C 30 min reaction time, 26–103 g lignin/run | Batch reactor | None | 5.03% gas yield at 600 °C 25% H2 yield, no CO yield |
|
| [59] | 600–750 °C, 10–50 min reaction time, amount of BL not specified | Batch reactor | Inconel 625, no catalyst | Max H2 yield of 75%, negligible CO yield, no mention of the actual conversion |
|
| [62] | 500–700 °C, 36.18 g BL/h | Flow through reactor | SS 316 reactor, no catalyst | Max H2 yield of 49%, CO concentration dropped to nearly 0% at 700 °C from 27% to 500 °C |
|
| [63] | 550 °C, 10–60 mL BL/min | Flow through reactor | No catalyst | Max H2 yield of 66.11% and CO yield of 5.86% at 550 °C with a 10 mL/min flow rate |
|
| [66] | 400–600 °C, 10 min reaction time, amount of BL not specified | Batch quartz reactor | 57.8 mol/kg max H2 yield for Soda BL at 600 °C and 0.5 mmol/kg at this value too |
| |
| [65] | 400–600 °C 25 MPa, 4.94 to 13.71 s residence time, 52 mL BL/h | Flow through reactor | None | 11.26 mol/kg of H2 at 600 °C no CO at this temperature. |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro Garcia, A.; Cheng, S.; Cross, J.S. Lignin Gasification: Current and Future Viability. Energies 2022, 15, 9062. https://doi.org/10.3390/en15239062
Castro Garcia A, Cheng S, Cross JS. Lignin Gasification: Current and Future Viability. Energies. 2022; 15(23):9062. https://doi.org/10.3390/en15239062
Chicago/Turabian StyleCastro Garcia, Abraham, Shuo Cheng, and Jeffrey S. Cross. 2022. "Lignin Gasification: Current and Future Viability" Energies 15, no. 23: 9062. https://doi.org/10.3390/en15239062
APA StyleCastro Garcia, A., Cheng, S., & Cross, J. S. (2022). Lignin Gasification: Current and Future Viability. Energies, 15(23), 9062. https://doi.org/10.3390/en15239062








