Thin-Film Lithium Cobalt Oxide for Lithium-Ion Batteries
Abstract
1. Introduction
1.1. Thin-Film Lithium Battery
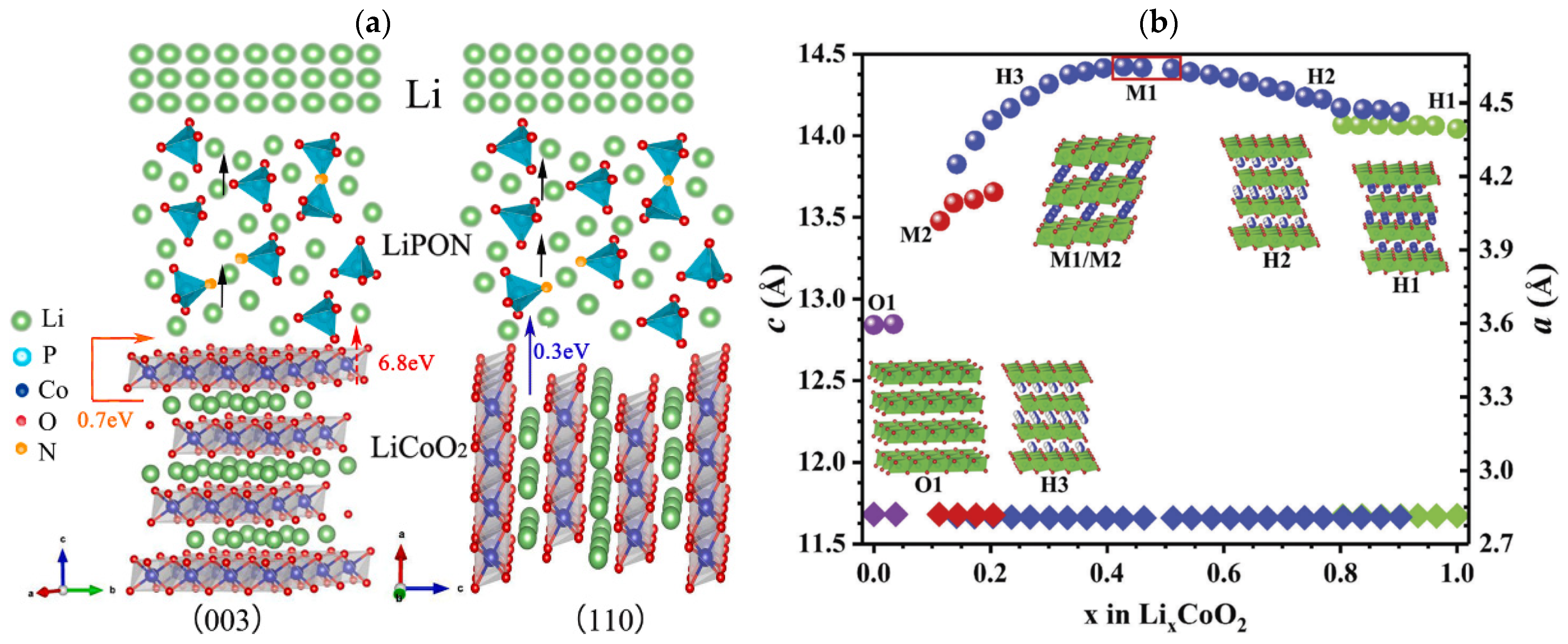
1.2. High-Voltage LiCoO2 Cathode
1.3. Fabrication of LiCoO2 Thin Films
1.4. Outline
2. Sputtering of LiCoO2 Thin Films
2.1. Annealing Process
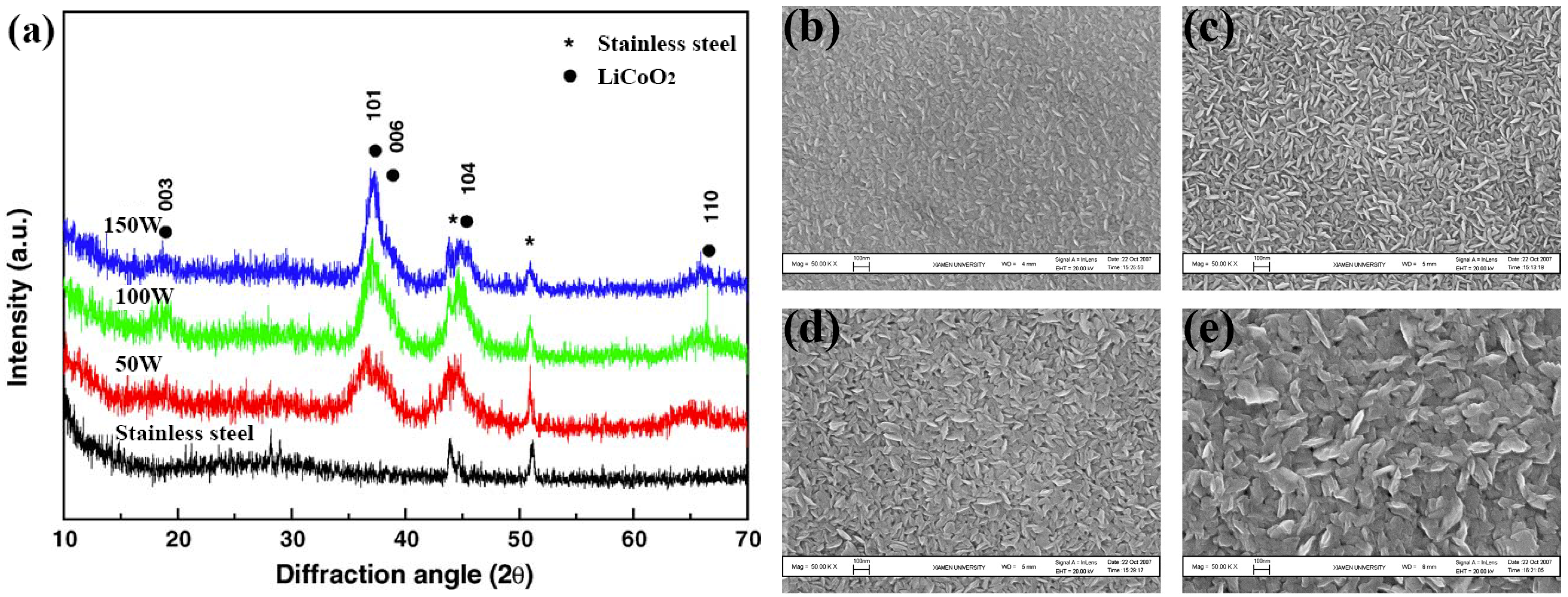
2.2. Substrate Influence
2.3. Sputtering Power
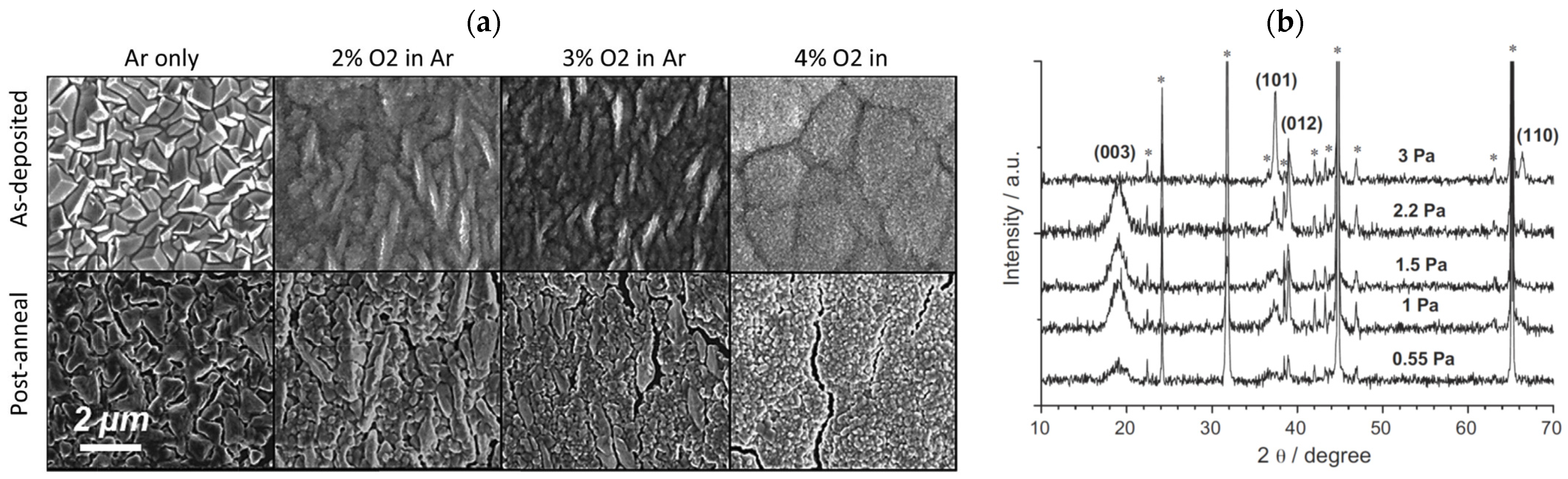
2.4. Sputtering Atmosphere
3. LiCoO2 Thin Films Fabricated by Other Methods
3.1. Spin Coating and Sol–Gel Methods
3.2. Atomic Layer Deposition
3.3. Pulsed Laser Deposition
3.4. Pros and Cons of Each Method
4. Modification of LiCoO2 Cathode
4.1. Bulk Doping
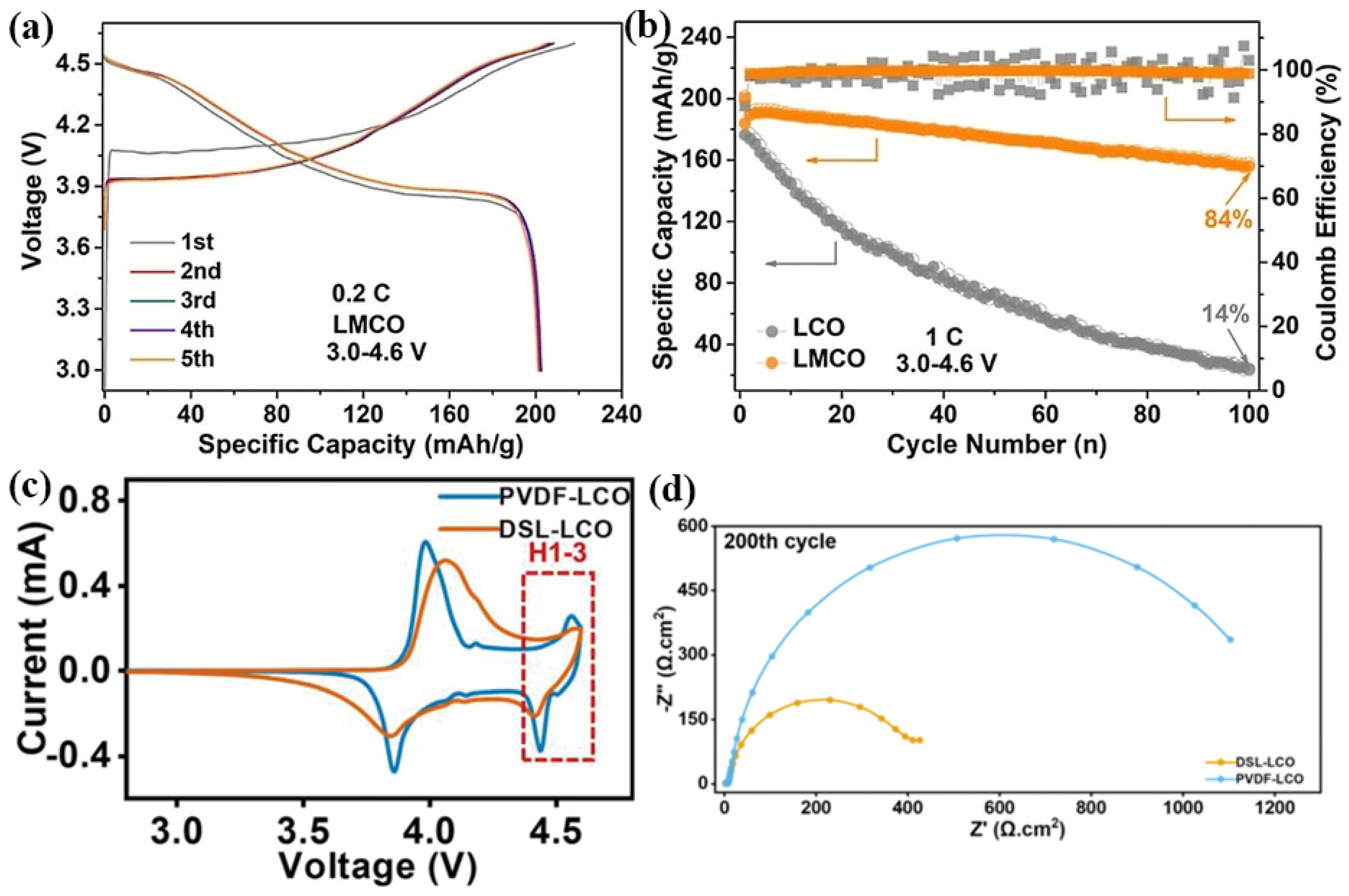
4.2. Surface Coating
5. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Park, H.Y.; Lee, S.R.; Lee, Y.J.; Cho, B.W.; Cho, W.I. Bias Sputtering and Characterization of LiCoO2 Thin Film Cathodes for Thin Film Microbattery. Mater. Chem. Phys. 2005, 93, 70–78. [Google Scholar] [CrossRef]
- Pan, X.; Hong, X.; Xu, L.; Li, Y.; Yan, M.; Mai, L. On-Chip Micro/Nano Devices for Energy Conversion and Storage. Nano Today 2019, 28, 100764. [Google Scholar]
- Mai, L.; Sheng, J.; Xu, L.; Tan, S.; Meng, J. One-Dimensional Hetero-Nanostructures for Rechargeable Batteries. Acc. Chem. Res. 2018, 51, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, K.; Hu, Z.; Tao, Z.; Mai, L.; Kang, Y.-M.; Chou, S.-L.; Chen, J. Recent Developments on and Prospects for Electrode Materials with Hierarchical Structures for Lithium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1701415. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. Lixcoo2 (0 < X < −1): A New Cathode Material for Batteries of High Energy Density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar]
- Muldoon, J.; Bucur, C.B.; Gregory, T. Quest for Nonaqueous Multivalent Secondary Batteries: Magnesium and Beyond. Chem. Rev. 2014, 114, 11683–11720. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wan, J.; Xiang, Y.; Zhu, J.; Leng, Q.; Wang, M.; Xu, L.; Yang, Y. Recent Advances and Historical Developments of High Voltage Lithium Cobalt Oxide Materials for Rechargeable Li-Ion Batteries. J. Power Sources 2020, 460, 228062. [Google Scholar] [CrossRef]
- Smith, A.J.; Dahn, H.M.; Burns, J.C.; Dahn, J.R. Long-Term Low-Rate Cycling of LiCoO2/Graphite Li-Ion Cells at 55 °C. J. Electrochem. Soc. 2012, 159, A705–A710. [Google Scholar] [CrossRef]
- He, P.; Yu, H.; Li, D.; Zhou, H. Layered Lithium Transition Metal Oxide Cathodes Towards High Energy Lithium-Ion Batteries. J. Mater. Chem. 2012, 22, 3680–3695. [Google Scholar] [CrossRef]
- Chikkannanavar, S.B.; Bernardi, D.M.; Liu, L. A Review of Blended Cathode Materials for Use in Li-Ion Batteries. J. Power Sources 2014, 248, 91–100. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A. Solid-State Redox Reactions of LiCoO2 (R3m) for 4 Volt Secondary Lithium Cells. J. Electrochem. Soc. 1994, 141, 2972–2977. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.P.; Fu, L.J.; Liu, H.; Wu, Y.P.; Rahm, E.; Holze, R.; Wu, H.Q. Cathode Materials Modified by Surface Coating for Lithium Ion Batteries. Electrochim. Acta 2006, 51, 3872–3883. [Google Scholar] [CrossRef]
- Park, J.S.; Mane, A.U.; Elam, J.W.; Croy, J.R. Amorphous Metal Fluoride Passivation Coatings Prepared by Atomic Layer Deposition on LiCoO2 for Li-Ion Batteries. Chem. Mater. 2015, 27, 1917–1920. [Google Scholar] [CrossRef]
- Crabtree, G.; Kócs, E.; Trahey, L. The Energy-Storage Frontier: Lithium-Ion Batteries and Beyond. MRS Bull. 2015, 40, 1067–1078. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Chen, Z.; Dahn, J.R. Methods to Obtain Excellent Capacity Retention in LiCoO2 Cycled to 4.5 V. Electrochim. Acta 2004, 49, 1079–1090. [Google Scholar] [CrossRef]
- Choi, N.-S.; Han, J.-G.; Ha, S.-Y.; Park, I.; Back, C.-K. Recent Advances in the Electrolytes for Interfacial Stability of High-Voltage Cathodes in Lithium-Ion Batteries. RSC Adv. 2015, 5, 2732–2748. [Google Scholar] [CrossRef]
- Sharifi-Asl, S.; Lu, J.; Amine, K.; Shahbazian-Yassar, R. Oxygen Release Degradation in Li-Ion Battery Cathode Materials: Mechanisms and Mitigating Approaches. Adv. Energy Mater. 2019, 9, 1900551. [Google Scholar] [CrossRef]
- Zhou, A.; Dai, X.; Lu, Y.; Wang, Q.; Fu, M.; Li, J. Enhanced Interfacial Kinetics and High-Voltage/High-Rate Performance of LiCoO2 Cathode by Controlled Sputter-Coating with a Nanoscale Li4ti5o12 Ionic Conductor. ACS Appl. Mater. Interfaces 2016, 8, 34123–34131. [Google Scholar] [CrossRef]
- Aboulaich, A.; Ouzaouit, K.; Faqir, H.; Kaddami, A.; Benzakour, I.; Akalay, I. Improving Thermal and Electrochemical Performances of Licoo 2 Cathode at High Cut-Off Charge Potentials by Mf 3 (M = Ce, Al) Coating. Mater. Res. Bull. 2016, 73, 362–368. [Google Scholar] [CrossRef]
- Xia, L.; Xia, Y.; Liu, Z. Thiophene Derivatives as Novel Functional Additives for High-Voltage LiCoO2 Operations in Lithium Ion Batteries. Electrochim. Acta 2015, 151, 429–436. [Google Scholar] [CrossRef]
- Hayashi, M.; Takahashi, M.; Sakurai, Y. Preparation of Positive LiCoO2 Films by Electron Cyclotron Resonance (Ecr) Plasma Sputtering Method and Its Application to All-Solid-State Thin-Film Lithium Batteries. J. Power Sources 2007, 174, 990–995. [Google Scholar] [CrossRef]
- Takahashi, M.; Hayashi, M.; Shodai, T. Characterization of All-Solid-State Secondary Batteries with LiCoO2 Thin Films Prepared by Ecr Sputtering as Positive Electrodes. J. Power Sources 2009, 189, 191–196. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, J.Z.; Xin, H.L.; Han, L.; Grillon, N.; Guy-Bouyssou, D.; Bouyssou, E.; Proust, M.; Meng, Y.S. Effects of Cathode Electrolyte Interfacial (Cei) Layer on Long Term Cycling of All-Solid-State Thin-Film Batteries. J. Power Sources 2016, 324, 342–348. [Google Scholar] [CrossRef]
- Kim, H.S.; Oh, Y.; Kang, K.H.; Kim, J.H.; Kim, J.; Yoon, C.S. Characterization of Sputter-Deposited LiCoO2 Thin Film Grown on Nasicon-Type Electrolyte for Application in All-Solid-State Rechargeable Lithium Battery. ACS Appl. Mater. Interfaces 2017, 9, 16063–16070. [Google Scholar] [CrossRef]
- Lidor-Shalev, O.; Leifer, N.; Ejgenberg, M.; Aviv, H.; Perelshtein, I.; Goobes, G.; Noked, M. Rosy, Molecular Layer Deposition of Alucone Thin Film on LiCoO2 to Enable High Voltage Operation. Batter. Supercaps 2021, 4, 1739–1748. [Google Scholar] [CrossRef]
- Kutbee, A.T.; Ghoneim, M.T.; Ahmad, S.M.; Hussain, M.M. Free-Form Flexible Lithium-Ion Microbattery. IEEE Trans. Nanotechnol. 2016, 15, 402–408. [Google Scholar] [CrossRef]
- Lin, J.; Lin, L.; Qu, S.; Deng, D.; Wu, Y.; Yan, X.; Xie, Q.; Wang, L.; Peng, D. Promising Electrode and Electrolyte Materials for High-Energy-Density Thin-Film Lithium Batteries. Energy Environ. Mater. 2021, 5, 133–156. [Google Scholar] [CrossRef]
- Krówka, K.; Wiatrowski, A.; Posadowski, W.M. Magnetron Sputtering Modes During Pulsed Deposition Process Determined by the Analysis of Power Supply Parameter. Thin Solid Film. 2012, 520, 4127–4130. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Hussain, O.M. Sputtered LiCoO2 Cathode Materials for All-Solid-State Thin-Film Lithium Microbatteries. Materials 2019, 12, 2687. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, J.; Xu, J.; Wu, X.; Chen, L. In Situ Visualized Cathode Electrolyte Interphase on LiCoO2 in High Voltage Cycling. ACS Appl. Mater. Interfaces 2017, 9, 19313–19318. [Google Scholar] [CrossRef]
- Liao, C.-L.; Lee, Y.-H.; Fung, K.-Z. The Film Growth and Electrochemical Properties of Rf-Sputtered LiCoO2 Thin Films. J. Alloys Compd. 2007, 436, 303–308. [Google Scholar] [CrossRef]
- HakanYudar, H.; Pat, S.; Özen, S.; Mohammadigharehbagh, R.; Musaoğlu, C.; Korkmaz, Ş.; Pat, Z. Microstructural, Surface and Electrochemical Properties of the Nano Layered LiCoO2 Thin Film Cathode for Li Ion Battery. Vacuum 2018, 152, 248–251. [Google Scholar] [CrossRef]
- Joo, H.; Lee, H.; Cho, G.; Nam, T.; Huh, S.; Choi, B.; Jueong, H.; Noh, J. Influence of the Metal-Induced Crystallization on the Structural and Electrochemical Properties of Sputtered LiCoO2 Thin Films. Thin Solid Film. 2017, 641, 53–58. [Google Scholar] [CrossRef]
- Pan, R.; Rau, D.; Moryson, Y.; Sann, J.; Janek, J. Reversible Capacity Loss of LiCoO2 Thin Film Electrodes. ACS Appl. Energy Mater. 2020, 3, 6065–6071. [Google Scholar] [CrossRef]
- Liao, C.-L.; Fung, K.-Z. Lithium Cobalt Oxide Cathode Film Prepared by Rf Sputtering. J. Power Sources 2004, 128, 263–269. [Google Scholar] [CrossRef]
- Jung, K.-T.; Cho, G.-B.; Kim, K.-W.; Nam, T.-H.; Jeong, H.-M.; Huh, S.-C.; Chung, H.-S.; Noh, J.-P. Influence of the Substrate Texture on the Structural and Electrochemical Properties of Sputtered LiCoO2 Thin Films. Thin Solid Film. 2013, 546, 414–417. [Google Scholar] [CrossRef]
- Jayanth-Babu, K.; Jeevan-Kumar, P.; Hussain, O.M.; Julien, C.M. Influence of Annealing Temperature on Microstructural and Electrochemical Properties of Rf-Sputtered Limn2o4 Film Cathodes. J. Solid State Electrochem. 2012, 16, 3383–3390. [Google Scholar] [CrossRef]
- Noh, J.-P.; Cho, G.-B.; Jung, K.-T.; Kang, W.-G.; Ha, C.-W.; Ahn, H.-J.; Ahn, J.-H.; Nam, T.-H.; Kim, K.-W. Fabrication of LiCoO2 Thin Film Cathodes by Dc Magnetron Sputtering Method. Mater. Res. Bull. 2012, 47, 2823–2826. [Google Scholar] [CrossRef]
- Tintignac, S.; Baddour-Hadjean, R.; Pereira-Ramos, J.P.; Salot, R. Electrochemical Properties of High Rate Bias Sputtered LiCoO2 Thin Films in Liquid Electrolyte. J. Power Sources 2014, 245, 76–82. [Google Scholar] [CrossRef]
- Xia, H.; Wan, Y.; Assenmacher, W.; Mader, W.; Yuan, G.; Lu, L. Facile Synthesis of Chain-Like LiCoO2 Nanowire Arrays as Three-Dimensional Cathode for Microbatteries. NPG Asia Mater. 2014, 6, e126. [Google Scholar] [CrossRef]
- Donders, M.E.; Arnoldbik, W.M.; Knoops, H.C.M.; Kessels, W.M.M.; Notten, P.H.L. Atomic Layer Deposition of LiCoO2 thin-Film Electrodes for All-Solid-State Li-Ion Micro-Batteries. J. Electrochem. Soc. 2013, 160, A3066–A3071. [Google Scholar] [CrossRef]
- Shiraki, S.; Oki, H.; Takagi, Y.; Suzuki, T.; Kumatani, A.; Shimizu, R.; Haruta, M.; Ohsawa, T.; Sato, Y.; Ikuhara, Y.; et al. Fabrication of All-Solid-State Battery Using Epitaxial LiCoO2 Thin Films. J. Power Sources 2014, 267, 881–887. [Google Scholar] [CrossRef]
- Turrell, S.J.; Zekoll, S.; Liu, J.; Grovenor, C.R.M.; Speller, S.C. Optimization of a Potential Manufacturing Process for Thin-Film LiCoO2 Cathodes. Thin Solid Film. 2021, 735, 138888. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, M.; Yan, Y.; Wei, Y.; Liu, W.; Zhang, X.; Li, J.; Fu, Z.; Li, J.; Zhang, X. Annealing of LiCoO2 Films on Flexible Stainless Steel for Thin Film Lithium Batteries. J. Mater. Res. 2019, 35, 31–41. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, Y.; Zhang, X.; Ma, S.; Wei, K.; Wei, Y.; Cui, Y. Improved Solid Interfacial Kinetics and Electrochemical Performance for LiCoO2(1 1 0) Textured Thin-Film Lithium Batteries. Appl. Surf. Sci. 2022, 591, 153174. [Google Scholar] [CrossRef]
- Shiraki, S.; Takagi, Y.; Shimizu, R.; Suzuki, T.; Haruta, M.; Sato, Y.; Ikuhara, Y.; Hitosugi, T. Orientation Control of LiCoO2 Epitaxial Thin Films on Metal Substrates. Thin Solid Film. 2016, 600, 175–178. [Google Scholar] [CrossRef]
- Bohne, L.; Pirk, T.; Jaegermann, W. Investigations on the Influence of the Substrate on the Crystal Structure of Sputtered LiCoO2. J. Solid State Electrochem. 2011, 17, 2095–2099. [Google Scholar] [CrossRef]
- Yoon, Y.; Park, C.; Kim, J.; Shin, D. Lattice Orientation Control of Lithium Cobalt Oxide Cathode Film for All-Solid-State Thin Film Batteries. J. Power Sources 2013, 226, 186–190. [Google Scholar] [CrossRef]
- Wang, L.; Chen, B.; Ma, J.; Cui, G.; Chen, L. Reviving Lithium Cobalt Oxide-Based Lithium Secondary Batteries-toward a Higher Energy Density. Chem. Soc. Rev. 2018, 47, 6505–6602. [Google Scholar] [CrossRef]
- Jeon, S.-W.; Lim, J.-K.; Lim, S.-H.; Lee, S.-M. As-Deposited LiCoO2 Thin Film Cathodes Prepared by Rf Magnetron Sputtering. Electrochim. Acta 2005, 51, 268–273. [Google Scholar] [CrossRef]
- Pan, H.; Yang, Y. Effects of Radio-Frequency Sputtering Powers on the Microstructures and Electrochemical Properties of LiCoO2 Thin Film Electrodes. J. Power Sources 2009, 189, 633–637. [Google Scholar] [CrossRef]
- Tintignac, S.; Baddour-Hadjean, R.; Pereira-Ramos, J.P.; Salot, R. High Rate Bias Sputtered Licoo 2 Thinfilms as Positive Electrode for All-Solid-State Lithium Microbatteries. Electrochim. Acta 2014, 146, 472–476. [Google Scholar] [CrossRef]
- Trask, J.; Anapolsky, A.; Cardozo, B.; Januar, E.; Kumar, K.; Miller, M.; Brown, R.; Bhardwaj, R. Optimization of 10-Μm, Sputtered, Licoo 2 Cathodes to Enable Higher Energy Density Solid State Batteries. J. Power Sources 2017, 350, 56–64. [Google Scholar] [CrossRef]
- Tintignac, S.; Baddour-Hadjean, R.; Pereira-Ramos, J.-P.; Salot, R. High Performance Sputtered LiCoO2 Thin Films Obtained at a Moderate Annealing Treatment Combined to a Bias Effect. Electrochim. Acta 2012, 60, 121–129. [Google Scholar] [CrossRef]
- Kwon, T.; Ohnishi, T.; Mitsuishi, K.; Ozawa, T.C.; Takada, K. Synthesis of LiCoO2 Epitaxial Thin Films Using a Sol–Gel Method. J. Power Sources 2015, 274, 417–423. [Google Scholar] [CrossRef]
- Porthault, H.; Le Cras, F.; Franger, S. Synthesis of LiCoO2 Thin Films by Sol/Gel Process. J. Power Sources 2010, 195, 6262–6267. [Google Scholar] [CrossRef]
- Nandihalli, N. Thermoelectric Films and Periodic Structures and Spin Seebeck Effect Systems: Facets of Performance Optimization. Mater. Today Energy 2022, 25, 100965. [Google Scholar] [CrossRef]
- Shiraki, S.; Oki, H.; Hitosugi, T. Li Diffusion in (110)-Oriented LiCoO2 Thin Films Grown on Au and Pt (110) Substrates. Surface Interface Anal. 2016, 48, 1240–1243. [Google Scholar] [CrossRef]
- Luo, D.; Li, G.; Yu, C.; Yang, L.; Zheng, J.; Guan, X.; Li, L. Low-Concentration Donor-Doped LiCoO2 as a High Performance Cathode Material for Li-Ion Batteries to Operate between −10.4 and 45.4 °C. J. Mater. Chem. 2012, 22, 22233–22241. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Hu, X.; Tian, H.; Zhang, Y.; Yang, X. Realization of Ti Doping by Electrostatic Assembly to Improve the Stability of LiCoO2 Cycled to 4.5 v. J. Electrochem. Soc. 2019, 166, A1793–A1798. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, Y.; Fu, H.; Ou, M.; Hu, C.; Yu, S.; Hu, Z.; Chen, C.T.; Jiang, G.; Gu, H.; et al. Mg-Pillared LiCoO2: Towards Stable Cycling at 4.6 V. Angew. Chem. Int. Ed. Engl. 2021, 60, 4682–4688. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.S.; Reddy, B.P.; Kumar, P.J.; Hussain, O.M. Influence of Zr Dopant on Microstructural and Electrochemical Properties of LiCoO2 Thin Film Cathodes by Rf Sputtering. J. Electroanal. Chem. 2018, 828, 71–79. [Google Scholar] [CrossRef]
- Ganesh, K.S.; Reddy, B.P.; Kumar, P.J.; Jayanthbabu, K.; Rosaiah, P.; Hussain, O.M. Microstructural and Electrochemical Properties of Liti Y Co 1-Y O2 Film Cathodes Prepared by Rf Sputtering. J. Solid State Electrochem. 2015, 19, 3621–3627. [Google Scholar] [CrossRef]
- Fingerle, M.; Späth, T.; Schulz, N.; Hausbrand, R. Adsorption of Ethylene Carbonate on Lithium Cobalt Oxide Thin Films: A Synchrotron-Based Spectroscopic Study of the Surface Chemistry. Chem. Phys. 2017, 498–499, 19–24. [Google Scholar] [CrossRef]
- Noh, J.-P.; Jung, K.-T.; Jang, M.-S.; Kwon, T.-H.; Cho, G.-B.; Kim, K.-W.; Nam, T.-H. Protection Effect of ZrO2 Coating Layer on LiCoO2 Thin Film Fabricated by Dc Magnetron Sputtering. J. Nanosci. Nanotechnol. 2013, 13, 7152–7154. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, J.; Li, Y.; Wang, Y.; Qiu, J.; Zhang, J.; Yu, H.; Yu, X.; Li, H.; Chen, L. Surface-Protected LiCoO2 with Ultrathin Solid Oxide Electrolyte Film for High-Voltage Lithium Ion Batteries and Lithium Polymer Batteries. J. Power Sources 2018, 388, 65–70. [Google Scholar] [CrossRef]
- Nie, K.; Sun, X.; Wang, J.; Wang, Y.; Qi, W.; Xiao, D.; Zhang, J.-N.; Xiao, R.; Yu, X.; Li, H.; et al. Realizing Long-Term Cycling Stability and Superior Rate Performance of 4.5 v–LiCoO2 by Aluminum Doped Zinc Oxide Coating Achieved by a Simple Wet-Mixing Method. J. Power Sources 2020, 470, 228423. [Google Scholar] [CrossRef]
- Huang, H.; Li, Z.; Gu, S.; Bian, J.; Li, Y.; Chen, J.; Liao, K.; Gan, Q.; Wang, Y.; Wu, S.; et al. Dextran Sulfate Lithium as Versatile Binder to Stabilize High-Voltage LiCoO2 to 4.6 V. Adv. Energy Mater. 2021, 11, 2101864. [Google Scholar] [CrossRef]
- Kim, J.; Kang, H.; Go, N.; Jeong, S.; Yim, T.; Jo, Y.N.; Lee, K.T.; Mun, J. Egg-Shell Structured LiCoO2 by Cu2+ Substitution to Li+ Sites Via Facile Stirring in an Aqueous Copper(Ii) Nitrate Solution. J. Mater. Chem. A 2017, 5, 24892–24900. [Google Scholar] [CrossRef]
- Qian, J.; Liu, L.; Yang, J.; Li, S.; Wang, X.; Zhuang, H.L.; Lu, Y. Electrochemical Surface Passivation of LiCoO2 Particles at Ultrahigh Voltage and Its Applications in Lithium-Based Batteries. Nat. Commun. 2018, 9, 4918. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Z.; Wu, Y.; Lin, J.; Wang, L.; Peng, D.-L. Thin-Film Lithium Cobalt Oxide for Lithium-Ion Batteries. Energies 2022, 15, 8980. https://doi.org/10.3390/en15238980
Duan Z, Wu Y, Lin J, Wang L, Peng D-L. Thin-Film Lithium Cobalt Oxide for Lithium-Ion Batteries. Energies. 2022; 15(23):8980. https://doi.org/10.3390/en15238980
Chicago/Turabian StyleDuan, Zeqing, Yunfan Wu, Jie Lin, Laisen Wang, and Dong-Liang Peng. 2022. "Thin-Film Lithium Cobalt Oxide for Lithium-Ion Batteries" Energies 15, no. 23: 8980. https://doi.org/10.3390/en15238980
APA StyleDuan, Z., Wu, Y., Lin, J., Wang, L., & Peng, D.-L. (2022). Thin-Film Lithium Cobalt Oxide for Lithium-Ion Batteries. Energies, 15(23), 8980. https://doi.org/10.3390/en15238980






