Significance and Challenges of Poultry Litter and Cattle Manure as Sustainable Fuels: A Review
Abstract
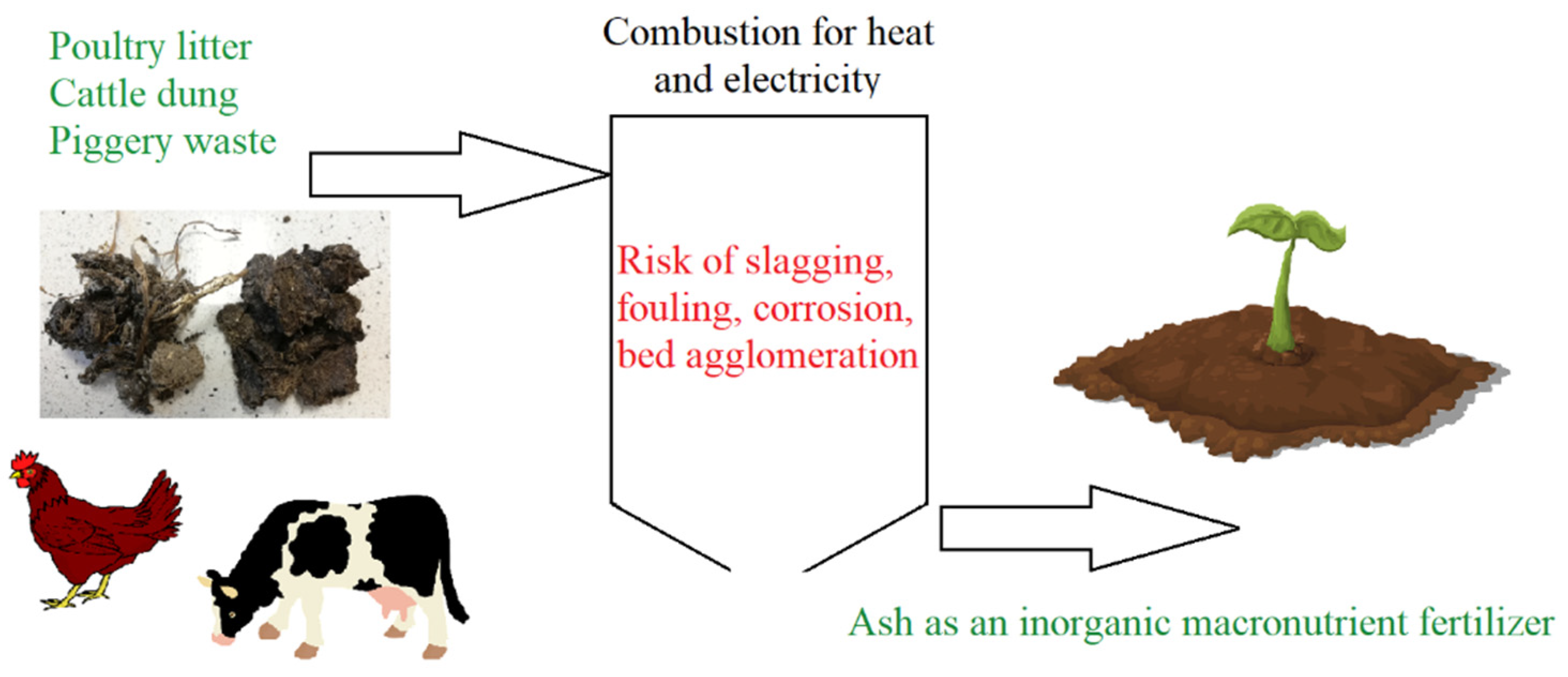
1. Introduction
2. Characteristics of Animal Waste
2.1. Poultry Litter
2.1.1. Moisture Content
2.1.2. Ash Content
2.1.3. Calorific Value
2.1.4. Chlorine Content
2.2. Cattle and Piggery Waste
2.2.1. Moisture Content
2.2.2. Ash Content
2.2.3. Calorific Value
2.2.4. Chlorine Content
3. Combustion and Co-Combustion: State of the Art
3.1. Direct Combustion
3.2. Co-Combustion with Fossil Fuels or Plant-Origin Biomass
3.3. Existing Full-Scale Combustion Units
3.4. The Use of Animal-Origin Biomass in the Metallurgy and Cement Industry
4. Ash Composition and Possible Application
5. Problems and Challenges
6. Conclusions
- (1)
- The thermal utilization of animal waste has the following advantages:
- Reduction in fossil fuel consumption;
- Minimization of the waste stream, reduction in landfilling;
- Availability on a small scale, for local heat and power generation, even in rural areas;
- Reduction in pathogens;
- Decrease in the anaerobic release of GHG: CH4, NH3, and H2S.
- (2)
- Both poultry and cattle waste are highly heterogeneous materials. The ash content in displayed samples varied significantly from 9.31 to 30.10%, while moisture content varied from 2.60 to 91.26%. These properties indicate possible difficulties associated with direct combustion. Therefore, the co-combustion with plant-origin biomass (such as wood) or fuel pre-processing (such as palletization or torrefaction) may be promising solutions.
- (3)
- When considering animal-origin waste as fuel, usually only animal species is given (poultry litter, cattle manure, etc.). However, according to the analyzed literature data, this is not sufficient information. Several more factors may influence the fuel and ash properties of animal waste. The most important are:
- Housing system (with bedding or bedding-less);
- Type of bedding (plant-origin such as straw, wood, etc., or alternative);
- Range style (free-range or industrial farming).
- (4)
- Some animal-origin biomass types are not as prone to combustion-related issues as others. The ash fusion temperatures may exceed 1500 °C, indicating low deposition and slagging risks. The chlorine content of displayed samples was in the range of 0.086–1.16%, placing some of them as low or moderately risky for combustion. In some points, manure addition can even mitigate the unwanted issue of ash deposition during the thermal conversion of low-rank coal.
- (5)
- Low sulfur and high calcium contents in animal waste are beneficial for combustion. The SO2 emission is reduced as a result of ash-derived natural desulfurization of flue gas.
- (6)
- The ash from animal waste combustion has the potential for soil conditioning. It may be applied as a fertilizer due to the high levels of macro- and micro-nutrients. Nevertheless, the possible elevated concentration of metals and metalloids (such as Zn and Cu) should be taken into account. The land application of animal-origin ash is still not recognized enough and requires further investigation.
Funding
Data Availability Statement
Conflicts of Interest
References
- Torchio, M.F.; Lucia, U.; Grisolia, G. Economic and Human Features for Energy and Environmental Indicators: A Tool to Assess Countries’ Progress towards Sustainability. Sustainability 2020, 12, 9716. [Google Scholar] [CrossRef]
- Grisolia, G.; Fino, D.; Lucia, U. Biomethanation of Rice Straw: A Sustainable Perspective for the Valorisation of a Field Residue in the Energy Sector. Sustainability 2022, 14, 5679. [Google Scholar] [CrossRef]
- Dhungana, B.; Lohani, S.P.; Marsolek, M. Anaerobic Co-Digestion of Food Waste with Livestock Manure at Ambient Temperature: A Biogas Based Circular Economy and Sustainable Development Goals. Sustainability 2022, 14, 3307. [Google Scholar] [CrossRef]
- Rahman, K.M.; Melville, L.; Edwards, D.J.; Fulford, D.; Thwala, W.D. Determination of the Potential Impact of Domestic Anaerobic Digester Systems: A Community Based Research Initiative in Rural Bangladesh. Processes 2019, 7, 512. [Google Scholar] [CrossRef]
- Fasake, V.; Dashora, K. A Sustainable Potential Source of Ruminant Animal Waste Material (Dung Fiber) for Various Industrial Applications: A Review. Bioresour. Technol. Rep. 2021, 15, 100693. [Google Scholar] [CrossRef]
- Anderson, K.; Moore, P.A.; Martin, J.; Ashworth, A.J. Evaluation of a Novel Poultry Litter Amendment on Greenhouse Gas Emissions. Atmosphere 2021, 12, 563. [Google Scholar] [CrossRef]
- Szablewski, T.; Stuper-Szablewska, K.; Cegielska-Radziejewska, R.; Tomczyk, Ł.; Szwajkowska-Michałek, L.; Nowaczewski, S. Comprehensive Assessment of Environmental Pollution in a Poultry Farm Depending on the Season and the Laying Hen Breeding System. Animals 2022, 12, 740. [Google Scholar] [CrossRef]
- Billen, P.; Costa, J.; van der Aa, L.; van Caneghem, J.; Vandecasteele, C. Electricity from Poultry Manure: A Cleaner Alternative to Direct Land Application. J. Clean. Prod. 2015, 96, 467–475. [Google Scholar] [CrossRef]
- Pokrant, E.; Yévenes, K.; Trincado, L.; Terraza, G.; Galarce, N.; Maddaleno, A.; Martín, B.S.; Lapierre, L.; Cornejo, J. Evaluation of Antibiotic Dissemination into the Environment and Untreated Animals, by Analysis of Oxytetracycline in Poultry Droppings and Litter. Animals 2021, 11, 853. [Google Scholar] [CrossRef] [PubMed]
- Moffo, F.; Mouiche, M.M.M.; Djomgang, H.K.; Tombe, P.; Wade, A.; Kochivi, F.L.; Dongmo, J.B.; Mbah, C.K.; Mapiefou, N.P.; Ngogang, M.P.; et al. Poultry Litter Contamination by Escherichia Coli Resistant to Critically Important Antimicrobials for Human and Animal Use and Risk for Public Health in Cameroon. Antibiotics 2021, 10, 402. [Google Scholar] [CrossRef]
- Furtula, V.; Jackson, C.; Farrell, E.; Barrett, J.; Hiott, L.; Chambers, P. Antimicrobial Resistance in Enterococcus Spp. Isolated from Environmental Samples in an Area of Intensive Poultry Production. Int. J. Environ. Res. Public Health 2013, 10, 1020–1036. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jiang, X. Microbiological Safety of Chicken Litter or Chicken Litter-Based Organic Fertilizers: A Review. Agriculture 2014, 4, 1–29. [Google Scholar] [CrossRef]
- Lucia, U.; Grisolia, G. Biofuels Analysis Based on the THDI Indicator of Sustainability. Front. Energy Res. 2021, 9, 794682. [Google Scholar] [CrossRef]
- Kelleher, B.P.; Leahy, J.J.; Henihan, A.M.; O’Dwyer, T.F.; Sutton, D.; Leahy, M.J. Advances in Poultry Litter Disposal Technology—A Review. Bioresour. Technol. 2002, 83, 27–36. [Google Scholar] [CrossRef]
- Perera, P.; Bandara, W. Potential of Using Poultry Litter as a Feedstock for Energy Production; Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2010. [Google Scholar]
- Santos Dalólio, F.; da Silva, J.N.; Carneiro de Oliveira, A.C.; Ferreira Tinôco, I.D.F.; Christiam Barbosa, R.; Resende, M.D.O.; Teixeira Albino, L.F.; Teixeira Coelho, S. Poultry Litter as Biomass Energy: A Review and Future Perspectives. Renew. Sustain. Energy Rev. 2017, 76, 941–949. [Google Scholar] [CrossRef]
- Tańczuk, M.; Junga, R.; Kolasa-Więcek, A.; Niemiec, P. Assessment of the Energy Potential of Chicken Manure in Poland. Energies 2019, 12, 1244. [Google Scholar] [CrossRef]
- Baldé, H.; VanderZaag, A.C.; Burtt, S.D.; Wagner-Riddle, C.; Crolla, A.; Desjardins, R.L.; MacDonald, D.J. Methane Emissions from Digestate at an Agricultural Biogas Plant. Bioresour. Technol. 2016, 216, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Orlando, M.-Q.; Borja, V.-M. Pretreatment of Animal Manure Biomass to Improve Biogas Production: A Review. Energies 2020, 13, 3573. [Google Scholar] [CrossRef]
- Font-Palma, C. Methods for the Treatment of Cattle Manure—A Review. J. Carbon Res. 2019, 5, 27. [Google Scholar] [CrossRef]
- Mazurkiewicz, J. Energy and Economic Balance between Manure Stored and Used as a Substrate for Biogas Production. Energies 2022, 15, 413. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef]
- Akor, C.I.; Osman, A.I.; Farrell, C.; McCallum, C.S.; John Doran, W.; Morgan, K.; Harrison, J.; Walsh, P.J.; Sheldrake, G.N. Thermokinetic Study of Residual Solid Digestate from Anaerobic Digestion. Chem. Eng. J. 2021, 406, 127039. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Ryan, D.; Murphy, R.; Enright, A.M. A Comprehensive Review of Green Policy, Anaerobic Digestion of Animal Manure and Chicken Litter Feedstock Potential—Global and Irish Perspective. Renew. Sustain. Energy Rev. 2022, 154, 111884. [Google Scholar] [CrossRef]
- Dairy Energy. Available online: https://www.dairyenergy.co.uk/ (accessed on 10 September 2022).
- Host Bioenergy Systems. Available online: https://www.Host.Nl/En/Biogas-Plants/Manure-Digestion-Microferm/?Gclid=EAIaIQobChMI-Ajpm77y1AIVFBbTCh1nOAluEAAYBCAAEgIJm_D_BwE (accessed on 10 September 2022).
- Sharara, M.; Sadaka, S. Opportunities and Barriers to Bioenergy Conversion Techniques and Their Potential Implementation on Swine Manure. Energies 2018, 11, 957. [Google Scholar] [CrossRef]
- Miedema, J.; van der Windt, H.; Moll, H. Opportunities and Barriers for Biomass Gasification for Green Gas in the Dutch Residential Sector. Energies 2018, 11, 2969. [Google Scholar] [CrossRef]
- Barry, F.; Sawadogo, M.; Bologo (Traoré), M.; Ouédraogo, I.W.K.; Dogot, T. Key Barriers to the Adoption of Biomass Gasification in Burkina Faso. Sustainability 2021, 13, 7324. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Rosen, M.A.; Tyagi, S.K. Global Challenges in the Sustainable Development of Biomass Gasification: An Overview. Renew. Sustain. Energy Rev. 2017, 80, 23–43. [Google Scholar] [CrossRef]
- Mohammadi, A.; Anukam, A. The Technical Challenges of the Gasification Technologies Currently in Use and Ways of Optimizing Them: A Review. In Energy Recovery; IntechOpen: London, UK, 2022. [Google Scholar]
- Najser, J.; Buryan, P.; Skoblia, S.; Frantik, J.; Kielar, J.; Peer, V. Problems Related to Gasification of Biomass—Properties of Solid Pollutants in Raw Gas. Energies 2019, 12, 963. [Google Scholar] [CrossRef]
- Poultry Health Today. Available online: https://Poultryhealthtoday.Com/Case-Built-Litter-Us-Broiler-Complexes/ (accessed on 10 September 2022).
- Diarra, S.; Lameta, S.; Amosa, F.; Anand, S. Alternative Bedding Materials for Poultry: Availability, Efficacy, and Major Constraints. Front. Vet. Sci. 2021, 8, 669504. [Google Scholar] [CrossRef]
- Prišenk, J.; Brus, M. Economic Viability of Alternative Bedding Material in Broiler Chicken Farming. Agriculture 2022, 12, 375. [Google Scholar] [CrossRef]
- Topal, H.; Amirabedin, E. Determination of Some Important Emissions of Poultry Waste Co-Combustion. Environ. Clim. Technol. 2012, 8, 12–17. [Google Scholar] [CrossRef]
- Maj, I.; Kalisz, S.; Ciukaj, S. Properties of Animal-Origin Ash—A Valuable Material for Circular Economy. Energies 2022, 15, 1274. [Google Scholar] [CrossRef]
- Pandey, D.S.; Katsaros, G.; Lindfors, C.; Leahy, J.J.; Tassou, S.A. Fast Pyrolysis of Poultry Litter in a Bubbling Fluidised Bed Reactor: Energy and Nutrient Recovery. Sustainability 2019, 11, 2533. [Google Scholar] [CrossRef]
- Li, S.; Wu, A.; Deng, S.; Pan, W. Effect of Co-Combustion of Chicken Litter and Coal on Emissions in a Laboratory-Scale Fluidized Bed Combustor. Fuel Process. Technol. 2008, 89, 7–12. [Google Scholar] [CrossRef]
- Qian, X.; Lee, S.; Chandrasekaran, R.; Yang, Y.; Caballes, M.; Alamu, O.; Chen, G. Electricity Evaluation and Emission Characteristics of Poultry Litter Co-Combustion Process. Appl. Sci. 2019, 9, 4116. [Google Scholar] [CrossRef]
- Isemin, R.; Mikhalev, A.; Milovanov, O.; Klimov, D.; Kokh-Tatarenko, V.; Brulé, M.; Tabet, F.; Nebyvaev, A.; Kuzmin, S.; Konyakhin, V. Comparison of Characteristics of Poultry Litter Pellets Obtained by the Processes of Dry and Wet Torrefaction. Energies 2022, 15, 2153. [Google Scholar] [CrossRef]
- Jia, L.; Anthony, E.J. Combustion of Poultry-Derived Fuel in a Coal-Fired Pilot-Scale Circulating Fluidized Bed Combustor. Fuel Process. Technol. 2011, 92, 2138–2144. [Google Scholar] [CrossRef]
- Abelha, P. Combustion of Poultry Litter in a Fluidised Bed Combustor. Fuel 2003, 82, 687–692. [Google Scholar] [CrossRef]
- Bowen, B.; Lynch, D.; Lynch, D.; Henihan, A.M.; Leahy, J.J.; McDonnell, K. Biosecurity on Poultry Farms from On-Farm Fluidized Bed Combustion and Energy Recovery from Poultry Litter. Sustainability 2010, 2, 2135–2143. [Google Scholar] [CrossRef]
- Turzyński, T.; Kluska, J.; Ochnio, M.; Kardaś, D. Comparative Analysis of Pelletized and Unpelletized Sunflower Husks Combustion Process in a Batch-Type Reactor. Materials 2021, 14, 2484. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Thengane, S.K.; Gupta, A.; Mahajani, S.M. Co-Gasification of High Ash Biomass and High Ash Coal in Downdraft Gasifier. Bioresour. Technol. 2019, 273, 159–168. [Google Scholar] [CrossRef]
- Hupa, M. Ash-Related Issues in Fluidized-Bed Combustion of Biomasses: Recent Research Highlights. Energy Fuels 2012, 26, 4–14. [Google Scholar] [CrossRef]
- Royo, J.; Canalís, P.; Zapata, S.; Gómez, M.; Bartolomé, C. Ash Behaviour during Combustion of Agropellets Produced by an Agro-Industry—Part 2: Chemical Characterization of Sintering and Deposition. Energies 2022, 15, 1499. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Preto, F.; Zhu, J.; Xu, C. Ash Deposition in Biomass Combustion or Co-Firing for Power/Heat Generation. Energies 2012, 5, 5171–5189. [Google Scholar] [CrossRef]
- Gocławski, J.; Korzeniewska, E.; Sekulska-Nalewajko, J.; Kiełbasa, P.; Dróżdż, T. Method of Biomass Discrimination for Fast Assessment of Calorific Value. Energies 2022, 15, 2514. [Google Scholar] [CrossRef]
- Naik, S.; Goud, V.V.; Rout, P.K.; Jacobson, K.; Dalai, A.K. Characterization of Canadian Biomass for Alternative Renewable Biofuel. Renew. Energy 2010, 35, 1624–1631. [Google Scholar] [CrossRef]
- Morissette, R.; Savoie, P.; Villeneuve, J. Combustion of Corn Stover Bales in a Small 146-KW Boiler. Energies 2011, 4, 1102–1111. [Google Scholar] [CrossRef]
- Vusić, D.; Vujanić, F.; Pešić, K.; Šafran, B.; Jurišić, V.; Zečić, Ž. Variability of Normative Properties of Wood Chips and Implications to Quality Control. Energies 2021, 14, 3789. [Google Scholar] [CrossRef]
- Barišić, I.; Netinger Grubeša, I.; Hackenberger, D.K.; Palijan, G.; Glavić, S.; Trkmić, M. Multidisciplinary Approach to Agricultural Biomass Ash Usage for Earthworks in Road Construction. Materials 2022, 15, 4529. [Google Scholar] [CrossRef]
- Nielsen, H.P.; Frandsen, F.J.; Dam-Johansen, K.; Baxter, L.L. The Implications of Chlorine-Associated Corrosion on the Operation of Biomass-Fired Boilers. Prog. Energy Combust. Sci. 2000, 26, 283–298. [Google Scholar] [CrossRef]
- Król, D.; Motyl, P.; Poskrobko, S. Chlorine Corrosion in a Low-Power Boiler Fired with Agricultural Biomass. Energies 2022, 15, 382. [Google Scholar] [CrossRef]
- Maj, I.; Kalisz, S.; Wejkowski, R.; Pronobis, M.; Gołombek, K. High-Temperature Corrosion in a Multifuel Circulating Fluidized Bed (CFB) Boiler Co-Firing Refuse Derived Fuel (RDF) and Hard Coal. Fuel 2022, 324, 124749. [Google Scholar] [CrossRef]
- Henderson, P.; Szakálos, P.; Pettersson, R.; Andersson, C.; Högberg, J. Reducing Superheater Corrosion in Wood-Fired Boilers. Mater. Corros. 2006, 57, 128–134. [Google Scholar] [CrossRef]
- Broström, M.; Kassman, H.; Helgesson, A.; Berg, M.; Andersson, C.; Backman, R.; Nordin, A. Sulfation of Corrosive Alkali Chlorides by Ammonium Sulfate in a Biomass Fired CFB Boiler. Fuel Process. Technol. 2007, 88, 1171–1177. [Google Scholar] [CrossRef]
- Sandberg, J.; Karlsson, C.; Fdhila, R.B. A 7year Long Measurement Period Investigating the Correlation of Corrosion, Deposit and Fuel in a Biomass Fired Circulated Fluidized Bed Boiler. Appl. Energy 2011, 88, 99–110. [Google Scholar] [CrossRef]
- Sorell, G. The Role of Chlorine in High Temperature Corrosion in Waste-to-Energy Plants. Mater. High Temp. 1997, 14, 207–220. [Google Scholar] [CrossRef]
- Quiroga, G.; Castrillón, L.; Fernández-Nava, Y.; Marañón, E. Physico-Chemical Analysis and Calorific Values of Poultry Manure. Waste Manag. 2010, 30, 880–884. [Google Scholar] [CrossRef]
- Garcia-Maraver, A.; Mata-Sanchez, J.; Carpio, M.; Perez-Jimenez, J.A. Critical Review of Predictive Coefficients for Biomass Ash Deposition Tendency. J. Energy Inst. 2017, 90, 214–228. [Google Scholar] [CrossRef]
- Tortosa Masiá, A.A.; Buhre, B.J.P.; Gupta, R.P.; Wall, T.F. Characterising Ash of Biomass and Waste. Fuel Process. Technol. 2007, 88, 1071–1081. [Google Scholar] [CrossRef]
- Pronobis, M. Evaluation of the Influence of Biomass Co-Combustion on Boiler Furnace Slagging by Means of Fusibility Correlations. Biomass Bioenergy 2005, 28, 375–383. [Google Scholar] [CrossRef]
- Gupta, K.K.; Aneja, K.R.; Rana, D. Current Status of Cow Dung as a Bioresource for Sustainable Development. Bioresour. Bioprocess. 2016, 3, 28. [Google Scholar] [CrossRef]
- Ashraf, M.; Ramzan, N.; Khan, R.U.; Durrani, A.K. Analysis of Mixed Cattle Manure: Kinetics and Thermodynamic Comparison of Pyrolysis and Combustion Processes. Case Stud. Therm. Eng. 2021, 26, 101078. [Google Scholar] [CrossRef]
- Shen, X.; Huang, G.; Yang, Z.; Han, L. Compositional Characteristics and Energy Potential of Chinese Animal Manure by Type and as a Whole. Appl. Energy 2015, 160, 108–119. [Google Scholar] [CrossRef]
- Maglinao, A.L.; Capareda, S.C.; Nam, H. Fluidized Bed Gasification of High Tonnage Sorghum, Cotton Gin Trash and Beef Cattle Manure: Evaluation of Synthesis Gas Production. Energy Convers. Manag. 2015, 105, 578–587. [Google Scholar] [CrossRef]
- Maj, I.; Kalisz, S.; Szymajda, A.; Łaska, G.; Gołombek, K. The Influence of Cow Dung and Mixed Straw Ashes on Steel Corrosion. Renew. Energy 2021, 177, 1198–1211. [Google Scholar] [CrossRef]
- Vankát, A.; Krepl, V.; Kára, J. Animal Dung as a Source of Energy in Remote Areas of Indian Himalayas. Agric. Trop. Subtrop. 2009, 43, 140–142. [Google Scholar]
- Fernandez-Lopez, M.; Puig-Gamero, M.; Lopez-Gonzalez, D.; Avalos-Ramirez, A.; Valverde, J.; Sanchez-Silva, L. Life Cycle Assessment of Swine and Dairy Manure: Pyrolysis and Combustion Processes. Bioresour. Technol. 2015, 182, 184–192. [Google Scholar] [CrossRef]
- Choi, H.L.; Sudiarto, S.I.A.; Renggaman, A. Prediction of Livestock Manure and Mixture Higher Heating Value Based on Fundamental Analysis. Fuel 2014, 116, 772–780. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of Pyrolysis Temperature and Manure Source on Physicochemical Characteristics of Biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Ferraz, P.F.P.; e Silva Ferraz, G.A.; Leso, L.; Klopčič, M.; Barbari, M.; Rossi, G. Properties of Conventional and Alternative Bedding Materials for Dairy Cattle. J. Dairy Sci. 2020, 103, 8661–8674. [Google Scholar] [CrossRef] [PubMed]
- Wzorek, M.; Junga, R.; Yilmaz, E.; Niemiec, P. Combustion Behavior and Mechanical Properties of Pellets Derived from Blends of Animal Manure and Lignocellulosic Biomass. J. Environ. Manag. 2021, 290, 112487. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Maglinao, A.L.; Capareda, S.C.; Rodriguez-Alejandro, D.A. Enriched-Air Fluidized Bed Gasification Using Bench and Pilot Scale Reactors of Dairy Manure with Sand Bedding Based on Response Surface Methods. Energy 2016, 95, 187–199. [Google Scholar] [CrossRef]
- Kim, D.; Park, D.; Lim, Y.; Park, S.; Park, Y.-S.; Kim, K. Combustion Melting Characterisation of Solid Fuel Obtained from Sewage Sludge. Energies 2021, 14, 805. [Google Scholar] [CrossRef]
- Sakiewicz, P.; Piotrowski, K.; Rajca, M.; Maj, I.; Kalisz, S.; Ober, J.; Karwot, J.; Pagilla, K. Innovative Technological Approach for the Cyclic Nutrients Adsorption by Post-Digestion Sewage Sludge-Based Ash Co-Formed with Some Nanostructural Additives under a Circular Economy Framework. Int. J. Environ. Res. Public Health 2022, 19, 11119. [Google Scholar] [CrossRef]
- Netzer, C.; Løvås, T. Chemical Model for Thermal Treatment of Sewage Sludge. ChemEngineering 2022, 6, 16. [Google Scholar] [CrossRef]
- Latosińska, J.; Żygadło, M.; Czapik, P. The Influence of Sewage Sludge Content and Sintering Temperature on Selected Properties of Lightweight Expanded Clay Aggregate. Materials 2021, 14, 3363. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Cempa, M.; Białecka, B. Phosphorus-Rich Ash from Poultry Manure Combustion in a Fluidized Bed Reactor. Minerals 2021, 11, 785. [Google Scholar] [CrossRef]
- Lucia, U.; Grisolia, G. Irreversible Thermodynamics and Bioeconomy: Toward a Human-Oriented Sustainability. Front. Phys. 2021, 9, 659342. [Google Scholar] [CrossRef]
- Sweeten, J.M.; Korenberg, J.; LePori, W.A.; Annamalai, K.; Parnell, C.B. Combustion of Cattle Feedlot Manure for Energy Production. Energy Agric. 1986, 5, 55–72. [Google Scholar] [CrossRef]
- Liukkonen, M.; Hiltunen, T.; Hälikkä, E.; Hiltunen, Y. Modeling of the Fluidized Bed Combustion Process and NOx Emissions Using Self-Organizing Maps: An Application to the Diagnosis of Process States. Environ. Model. Softw. 2011, 26, 605–614. [Google Scholar] [CrossRef]
- Grochowalski, J.; Jachymek, P.; Andrzejczyk, M.; Klajny, M.; Widuch, A.; Morkisz, P.; Hernik, B.; Zdeb, J.; Adamczyk, W. Towards Application of Machine Learning Algorithms for Prediction Temperature Distribution within CFB Boiler Based on Specified Operating Conditions. Energy 2021, 237, 121538. [Google Scholar] [CrossRef]
- Katsaros, G.; Sommersacher, P.; Retschitzegger, S.; Kienzl, N.; Tassou, S.A.; Pandey, D.S. Combustion of Poultry Litter and Mixture of Poultry Litter with Woodchips in a Fixed Bed Lab-Scale Batch Reactor. Fuel 2021, 286, 119310. [Google Scholar] [CrossRef]
- Osman, A.I. Mass Spectrometry Study of Lignocellulosic Biomass Combustion and Pyrolysis with NOx Removal. Renew. Energy 2020, 146, 484–496. [Google Scholar] [CrossRef]
- Yurdakul, S. Determination of Co-Combustion Properties and Thermal Kinetics of Poultry Litter/Coal Blends Using Thermogravimetry. Renew. Energy 2016, 89, 215–223. [Google Scholar] [CrossRef]
- Jiang, C.; Lin, Q.; Wang, C.; Jiang, X.; Bi, H.; Bao, L. Experimental Study of the Ignition and Combustion Characteristics of Cattle Manure under Different Environmental Conditions. Energy 2020, 197, 117143. [Google Scholar] [CrossRef]
- Pu, X.; Wei, M.; Chen, X.; Wang, L.; Deng, L. Thermal Decomposition Characteristics and Kinetic Analysis of Chicken Manure in Various Atmospheres. Agriculture 2022, 12, 607. [Google Scholar] [CrossRef]
- Atimtay, A.; Yurdakul, S. Combustion and Co-Combustion Characteristics of Torrefied Poultry Litter with Lignite. Renew. Energy 2020, 148, 1292–1301. [Google Scholar] [CrossRef]
- Henihan, A. Emissions Modeling of Fluidised Bed Co-Combustion of Poultry Litter and Peat. Bioresour. Technol. 2003, 87, 289–294. [Google Scholar] [CrossRef]
- Turzyński, T.; Kluska, J.; Kardaś, D. Study on Chicken Manure Combustion and Heat Production in Terms of Thermal Self-Sufficiency of a Poultry Farm. Renew. Energy 2022, 191, 84–91. [Google Scholar] [CrossRef]
- Polesek-Karczewska, S.; Turzyński, T.; Kardaś, D.; Heda, Ł. Front Velocity in the Combustion of Blends of Poultry Litter with Straw. Fuel Process. Technol. 2018, 176, 307–315. [Google Scholar] [CrossRef]
- Qian, X.; Lee, S.W.; Yang, Y. Heat Transfer Coefficient Estimation and Performance Evaluation of Shell and Tube Heat Exchanger Using Flue Gas. Processes 2021, 9, 939. [Google Scholar] [CrossRef]
- Heating with Chicken Litter. Available online: https://www.Binder-Gmbh.at/En/Heating-with-Chicken-Litter/ (accessed on 1 September 2022).
- BMC Moerdijk. Available online: https://www.Bmcmoerdijk.Nl/En/Adding-Value-to-Poultry-Manure/ (accessed on 8 October 2022).
- Bolhar-Nordenkampf, M.; Gartnar, F.; Tschanun, I.; Kaiser, S. Combustion of Poultry Litter in BubblingFluidised Beds—Results from a New 120 MWth Unit. In Proceedings of the 17th European Biomass Conference & Exhibition, Hamburg, Germany, 29 June–3 July 2009. [Google Scholar]
- Luyckx, L.; de Leeuw, G.H.J.; van Caneghem, J. Characterization of Poultry Litter Ash in View of Its Valorization. Waste Biomass Valorization 2020, 11, 5333–5348. [Google Scholar] [CrossRef]
- Luostarinen, S.; Kuligowski, K. Examples of Good Practices on Existing Manure Energy Use: Biogas, Combustion and Thermal Gasification; Report from a Project: Baltic Forum for Innovative Technologies for Sustainable Manure Management (BALTIC MANURE). 2011. Available online: https://www.researchgate.net/publication/338502494_Examples_of_Good_Practices_on_Existing_Manure_Energy_Use_Biogas_Combustion_and_Thermal_Gasification (accessed on 9 October 2022).
- Rejdak, M.; Wojtaszek-Kalaitzidi, M.; Gałko, G.; Mertas, B.; Radko, T.; Baron, R.; Książek, M.; Yngve Larsen, S.; Sajdak, M.; Kalaitzidis, S. A Study on Bio-Coke Production—The Influence of Bio-Components Addition on Coke-Making Blend Properties. Energies 2022, 15, 6847. [Google Scholar] [CrossRef]
- Koveria, A.; Kieush, L.; Svietkina, O.; Perkov, Y. Metallurgical Coke Production with Biomass Additives. Part 1. A Review of Existing Practices. Can. Metall. Q. 2020, 59, 417–429. [Google Scholar] [CrossRef]
- Rath, S.S.; Rao, D.S.; Mishra, B.K. A Novel Approach for Reduction Roasting of Iron Ore Slime Using Cow Dung. Int. J. Miner. Process. 2016, 157, 216–226. [Google Scholar] [CrossRef]
- Reuters. Available online: https://www.Reuters.Com/Article/Us-Emirates-Energy-Camels-IdUSKCN1UG0GR (accessed on 8 October 2022).
- Osman, A.I.; Abdelkader, A.; Farrell, C.; Rooney, D.; Morgan, K. Reusing, Recycling and up-Cycling of Biomass: A Review of Practical and Kinetic Modelling Approaches. Fuel Process. Technol. 2019, 192, 179–202. [Google Scholar] [CrossRef]
- Ladwig, K.J.; Blythe, G.M. Flue-Gas Desulfurization Products and Other Air Emissions Controls. In Coal Combustion Products (CCP’s); Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–95. [Google Scholar]
- Lee, K.T.; Mohamed, A.R.; Bhatia, S.; Chu, K.H. Removal of Sulfur Dioxide by Fly Ash/CaO/CaSO4 Sorbents. Chem. Eng. J. 2005, 114, 171–177. [Google Scholar] [CrossRef]
- Hernik, B.; Pronobis, M.; Wejkowski, R.; Wojnar, W. Experimental Verification of a CFD Model Intended for the Determination of Restitution Coefficients Used in Erosion Modelling. E3S Web Conf. 2017, 13, 05001. [Google Scholar] [CrossRef]
- Park, M.-H.; Kumar, S.; Ra, C. Solid Waste from Swine Wastewater as a Fuel Source for Heat Production. Asian-Australas J. Anim. Sci. 2012, 25, 1627–1633. [Google Scholar] [CrossRef]
- Lundgren, J.; Pettersson, E. Combustion of Horse Manure for Heat Production. Bioresour. Technol. 2009, 100, 3121–3126. [Google Scholar] [CrossRef]
- Codling, E.E.; Chaney, R.L.; Sherwell, J. Poultry Litter Ash as a Potential Phosphorus Source for Agricultural Crops. J. Environ. Qual. 2002, 31, 954–961. [Google Scholar] [CrossRef]
- Pagliari, P.H.; Rosen, C.J.; Strock, J.S. Turkey Manure Ash Effects on Alfalfa Yield, Tissue Elemental Composition, and Chemical Soil Properties. Commun. Soil. Sci. Plant Anal. 2009, 40, 2874–2897. [Google Scholar] [CrossRef]
- Isemin, R.; Mikhalev, A.; Milovanov, O.; Nebyvaev, A. Some Results of Poultry Litter Processing into a Fertilizer by the Wet Torrefaction Method in a Fluidized Bed. Energies 2022, 15, 2414. [Google Scholar] [CrossRef]
- Szogi, A.A.; Vanotti, M.B. Prospects for Phosphorus Recovery from Poultry Litter. Bioresour. Technol. 2009, 100, 5461–5465. [Google Scholar] [CrossRef]
- Tan, Z.; Lagerkvist, A. Phosphorus Recovery from the Biomass Ash: A Review. Renew. Sustain. Energy Rev. 2011, 15, 3588–3602. [Google Scholar] [CrossRef]
- Codling, E.E. Laboratory Characterization of Extractable Phosphorus in Poultry Litter and Poultry Litter Ash. Soil Sci. 2006, 171, 858–864. [Google Scholar] [CrossRef]
- Bogush, A.A.; Stegemann, J.A.; Williams, R.; Wood, I.G. Element Speciation in UK Biomass Power Plant Residues Based on Composition, Mineralogy, Microstructure and Leaching. Fuel 2018, 211, 712–725. [Google Scholar] [CrossRef]
- Chastain, J.P.; Coloma-del Valle, A.; Moore, K.P. Using Broiler Litter as an Energy Source: Energy Content and Ash Composition. Appl. Eng. Agric. 2012, 28, 513–522. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, H.; Shang, B.; Xin, H.; Zhu, Z. Characterization of Animal Manure and Cornstalk Ashes as Affected by Incineration Temperature. Appl. Energy 2011, 88, 947–952. [Google Scholar] [CrossRef]
- Bauer, P.J.; Szogi, A.A.; Shumaker, P.D. Fertilizer Efficacy of Poultry Litter Ash Blended with Lime or Gypsum as Fillers. Environments 2019, 6, 50. [Google Scholar] [CrossRef]
- Yu, C.-H.; Wang, S.-L.; Tongsiri, P.; Cheng, M.-P.; Lai, H.-Y. Effects of Poultry-Litter Biochar on Soil Properties and Growth of Water Spinach (Ipomoea Aquatica Forsk.). Sustainability 2018, 10, 2536. [Google Scholar] [CrossRef]
- Netherway, P.; Gascó, G.; Méndez, A.; Surapaneni, A.; Reichman, S.; Shah, K.; Paz-Ferreiro, J. Using Phosphorus-Rich Biochars to Remediate Lead-Contaminated Soil: Influence on Soil Enzymes and Extractable P. Agronomy 2020, 10, 454. [Google Scholar] [CrossRef]
- Osman, A.I.; Fawzy, S.; Farghali, M.; El-Azazy, M.; Elgarahy, A.M.; Fahim, R.A.; Maksoud, M.I.A.A.; Ajlan, A.A.; Yousry, M.; Saleem, Y.; et al. Biochar for Agronomy, Animal Farming, Anaerobic Digestion, Composting, Water Treatment, Soil Remediation, Construction, Energy Storage, and Carbon Sequestration: A Review. Environ. Chem. Lett. 2022, 20, 2385–2485. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Yang, M.; Li, W. Content of Heavy Metals in Animal Feeds and Manures from Farms of Different Scales in Northeast China. Int. J. Environ. Res. Public Health 2012, 9, 2658–2668. [Google Scholar] [CrossRef]
- Lan, W.; Yao, C.; Luo, F.; Jin, Z.; Lu, S.; Li, J.; Wang, X.; Hu, X. Effects of Application of Pig Manure on the Accumulation of Heavy Metals in Rice. Plants 2022, 11, 207. [Google Scholar] [CrossRef]
- Nordin, A.; Strandberg, A.; Elbashir, S.; Åmand, L.-E.; Skoglund, N.; Pettersson, A. Co-Combustion of Municipal Sewage Sludge and Biomass in a Grate Fired Boiler for Phosphorus Recovery in Bottom Ash. Energies 2020, 13, 1708. [Google Scholar] [CrossRef]
- Byrne, L.; Murphy, R.A. Relative Bioavailability of Trace Minerals in Production Animal Nutrition: A Review. Animals 2022, 12, 1981. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Composition and Application of Biomass Ash. Fuel 2013, 105, 19–39. [Google Scholar] [CrossRef]
- Díaz-Ramírez, M.; Frandsen, F.J.; Glarborg, P.; Sebastián, F.; Royo, J. Partitioning of K, Cl, S and P during Combustion of Poplar and Brassica Energy Crops. Fuel 2014, 134, 209–219. [Google Scholar] [CrossRef]
- Zhai, M.; Li, X.; Yang, D.; Ma, Z.; Dong, P. Ash Fusion Characteristics of Biomass Pellets during Combustion. J. Clean Prod. 2022, 336, 130361. [Google Scholar] [CrossRef]
- Horák, J.; Kuboňová, L.; Dej, M.; Laciok, V.; Tomšejová, Š.; Hopan, F.; Krpec, K.; Koloničný, J. Effects of the Type of Biomass and Ashing Temperature on the Properties of Solid Fuel Ashes. Pol. J. Chem. Technol. 2019, 21, 43–51. [Google Scholar] [CrossRef]
- Lynch, D.; Henihan, A.M.; Kwapinski, W.; Zhang, L.; Leahy, J.J. Ash Agglomeration and Deposition during Combustion of Poultry Litter in a Bubbling Fluidized-Bed Combustor. Energy Fuels 2013, 27, 4684–4694. [Google Scholar] [CrossRef]
- Li, F.; Zhao, C.; Guo, Q.; Li, Y.; Fan, H.; Guo, M.; Wu, L.; Huang, J.; Fang, Y. Exploration in Ash-Deposition (AD) Behavior Modification of Low-Rank Coal by Manure Addition. Energy 2020, 208, 118293. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Zhao, C.; Fan, H.; Xu, M.; Guo, Q.; Guo, M.; Wang, Z.; Huang, J.; Fang, Y. Investigation on Ash-Fusion Characteristics of Livestock Manure and Low-Rank Coals. Energy Fuels 2020, 34, 5804–5812. [Google Scholar] [CrossRef]
- Grimm, A.; Skoglund, N.; Boström, D.; Boman, C.; Öhman, M. Influence of Phosphorus on Alkali Distribution during Combustion of Logging Residues and Wheat Straw in a Bench-Scale Fluidized Bed. Energy Fuels 2012, 26, 3012–3023. [Google Scholar] [CrossRef]
- Li, H.; Han, K.; Wang, Q.; Lu, C. Influence of Ammonium Phosphates on Gaseous Potassium Release and Ash-Forming Characteristics during Combustion of Biomass. Energy Fuels 2015, 29, 2555–2563. [Google Scholar] [CrossRef]
| Parameter | Basis | Unit | Poultry Litter with Sawdust [36] | Poultry Litter with Rice Husk [36] | Free-Range Chicken Litter [37] | Industrial Farming Chicken Litter [37] | Poultry Litter [38] | Chicken Litter [39] | Chicken Litter [40] | Poultry Litter Pellets [41] | Poultry-Derived Fuel [42] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | a.r. | wt% | 18.16 | 32.57 | 12.10 | 11.10–38.8 | 8.5 | 11.3 | 21.20 | n.d. | 7.34 |
| Ash | d.b. | wt% | 16.64 (a.r.) | 10.85 | 30.10 | 9.31–17.09 | 14.3 | 24.8 | 24.06 | 16.6 | 25.07 (a.r.) |
| HHV | d.b. | MJ/kg | n.d. | n.d. | 12.22 | 15.60–17.22 | 18.00 | n.d. | 14.34 | 16.7 (LHV) | n.d. |
| a.r | MJ/kg | 12.53 | 11.31 | 10.97 | 11.40–15.31 | n.d. | n.d. | 11.30 | n.d. | 12.77 | |
| C | d.b. | wt% | 37.41 | 39.9 | 31.19 | 37.7–41.85 | 42.72 | 28.2 | 34.11 | 41.4 | 31.84 |
| H | d.b. | wt% | 4.41 | 4.79 | 3.91 | 5.10–5.50 | 5.50 | 5.0 | 5.64 | 5.7 | 3.98 |
| N | d.b. | wt% | 9.96 | 8.70 | 2.90 | 4.70–4.89 | 3.93 | 3.4 | 4.16 | 4.8 | 3.52 |
| S | d.b. | wt% | 0.73 | n.d. | 0.50 | 0.31–0.97 | 0.64 | 0.9 | 1.32 | 0.8 | 0.75 |
| Cl | d.b. | wt% | n.d. | n.d. | 0.11 | 0.66–0.99 | 0.3 | 1.16 | n.d. | n.d. | 0.4 (a.r.) |
| Parameter | Basis | Unit | Dairy Cattle Manure a [69] | Beef Cattle Manure b [69] | Beef Cattle Manure 2 [70] | Free-Range Cow Manure (Straw Bedding) [37] | Industrial Farming Cow Manure (Straw Bedding) [37,71] | Yak Manure [72] | Pig Manure [69] | Pig Manure 2 [73,74,75] |
|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | a.r. | wt% | 75.59 ± 9.22 | 75.66 ± 7.82 | 13.08 ± 0.5 | 8.4–11.4 | 11.1–15.5 | 2.60–4.36 | 71.99 ± 9.65 | 91.26 |
| Ash | d.b. | wt% | 28.20 ± 16.28 | 22.64 ± 11.88 | 29.80 ± 2.8 | 21.4–22.06 | 13.86–16.99 | 11.62–31.16 | 24.18 ± 11.14 | 19.72–20.90 |
| HHV | d.b. | MJ/kg | n.d. | 15.21 | 15.93 ± 0.3 | 16.93–17.26 | 17.91–19.04 | 13.41–17.59 | n.d. | n.d. |
| C | d.b. | wt% | 34.42 ± 8.96 | 37.64 ± 6.16 | 50.43 | 38.93–41.94 | 44.07–45.26 | 32.52–42.87 | 37.74 ± 6.43 | 32.35–47.42 |
| H | d.b. | wt% | 4.91 ± 1.39 | 5.26 ± 1.12 | 7.18 | 4.89–5.38 | 5.45–5.53 | 3.85–5.69 | 5.62 ± 1.00 | 4.82–6.40 |
| N | d.b. | wt% | 1.92 ± 0.50 | 2.16 ± 0.64 | 2.55 | 2.59–1.61 | 2.5–2.79 | 1.60–1.84 | 2.79 ± 0.71 | 1.90–4.11 |
| S | d.b. | wt% | 0.65 ± 0.4 | 0.59 ± 0.28 | 0.57 | 0.32–0.34 | 0.32–0.47 | 0.18 | 0.63 ± 0.30 | 0.71–0.94 |
| Cl | d.b. | wt% | n.d. | n.d. | n.d. | 0.086–0.33 | 0.54–1.02 | 0.14–0.20 | n.d. | n.d. |
| Parameter | Unit | Poultry Litter with Sawdust [36] | Free-Range Chicken Litter [37] | Industrial Farming Chicken Litter [37] | Chicken Litter [39] | Free-Range Cow Manure [37] | Industrial Farming Cow Manure [37,71] | Beef Cattle Manure a [69] | Dairy Cattle Manure b [69] | Pig Manure [69] | Pig (Swine) Solid Waste Bottom Ash [111] | Horse Manure [112] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SO3 | wt% | 6.0 | 1.02 | 0.82–9.68 | 5.8 | 0.88–1.18 | 2.63–4.45 | n.d. | n.d. | n.d. | 8.95 | n.d. |
| K2O | wt% | 10.86 | 2.61 | 13.04–25.20 | 12.2 | 3.19–8.64 | 5.56–18.6 | 7.02 | 4.79 | 8.99 | 12.5 | 11.5 |
| SiO2 | wt% | n.d. | 57.11 | 3.66–13.26 | 35.6 | 59.63–75.60 | 18.30–33.60 | n.d. | n.d. | n.d. | 6.24 | 42.6 |
| Fe2O3 | wt% | 7.42 | 1.94 | 0.92–4.11 | 2.1 | 1.11–1.52 | 1.06–1.24 | 1.89 | 2.03 | 2.11 | 3.92 | 4.24 |
| Al2O3 | wt% | 2.28 | 4.15 | 0.48–2.81 | 4.9 | 2.65–4.28 | 1.31–1.95 | n.d. | n.d. | n.d. | 1.38 | 7.75 |
| Mn3O4 | wt% | n.d. | 0.13 | 0.63–1.92 | n.d. | 0.12–0.18 | 0.16–0.51 | n.d. | n.d. | n.d. | n.d. | n.d. |
| TiO2 | wt% | n.d. | 0.23 | 0.04–0.63 | 0.2 | 0.21–0.24 | 0.09–0.15 | n.d. | n.d. | n.d. | 0.14 | 0.44 |
| CaO | wt% | 15.82 | 13.55 | 18.30–34.07 | 13.5 | 2.11–11.85 | 13.6–30.60 | 7.95 | 8.90 | 11.46 | 25.70 | 15.4 |
| MgO | wt% | 3.28 | 2.15 | 5.72–7.45 | 4.6 | 1.56–2.72 | 5.55–8.14 | 5.42 | 6.24 | 9.50 | 7.42 | 8.85 |
| P2O5 | wt% | 10.19 | 7.81 | 19.23–23.74 | 15.3 | 4.09–8.21 | 10.8–17.50 | 7.19 | 19.13 | 21.49 | 24.00 | 4.27 |
| Na2O | wt% | 1.68 | 3.21 | 3.87–6.80 | 5.8 | 0.73–3.57 | 2.66–3.20 | 2.29 | 1.44 | 1.63 | 2.08 | 2.59 |
| BaO | wt% | n.d. | 0.02 | 0.03–0.17 | n.d. | 0.04–0.02 | 0.03–0.05 | n.d. | n.d. | n.d. | n.d. | n.d. |
| SrO | wt% | n.d. | 0.02 | 0.05–0.39 | n.d. | 0.02 | 0.03–0.04 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cl | wt% | n.d. | 0.90 | 5.67 | n.d. | 0.65–7.56 | 2.57–6.55 | n.d. | n.d. | n.d. | 3.29 | n.d. |
| Parameter | Unit | Poultry Litter with Sawdust [36] | Poultry Litter with Rice Husk [36] | Free-Range Cow Manure [37] | Industrial Farming Cow Manure [37,71] | Free-Range Chicken Litter [37] | Industrial Farming Chicken Litter [37] | Yak Dung [72] |
|---|---|---|---|---|---|---|---|---|
| Initial deformation temperature (IDT) | °C | 1258 | 1360 | 910–1260 | 1130–1230 | 1060–1140 | 1303–1400 | n.d. |
| Softening temperature (ST) | °C | 1417 | >1500 | 1150–1310 | 1170–1270 | 1170–1210 | 1380–1500 | 1110–1120 |
| Hemisphere temperature (HT) | °C | >1500 | >1500 | 1340–1460 | 1200–1320 | 1300–1330 | 1430–1500 | 1130–1160 |
| Flow temperature (FT) | °C | >1500 | >1500 | 1370–1500 | 1310–1440 | 1320–1360 | 1490–1500 | 1140–1210 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maj, I. Significance and Challenges of Poultry Litter and Cattle Manure as Sustainable Fuels: A Review. Energies 2022, 15, 8981. https://doi.org/10.3390/en15238981
Maj I. Significance and Challenges of Poultry Litter and Cattle Manure as Sustainable Fuels: A Review. Energies. 2022; 15(23):8981. https://doi.org/10.3390/en15238981
Chicago/Turabian StyleMaj, Izabella. 2022. "Significance and Challenges of Poultry Litter and Cattle Manure as Sustainable Fuels: A Review" Energies 15, no. 23: 8981. https://doi.org/10.3390/en15238981
APA StyleMaj, I. (2022). Significance and Challenges of Poultry Litter and Cattle Manure as Sustainable Fuels: A Review. Energies, 15(23), 8981. https://doi.org/10.3390/en15238981






