Electrode/Electrolyte Interphases of Sodium-Ion Batteries
Abstract
1. Introduction
2. SEI on Sodium Metal
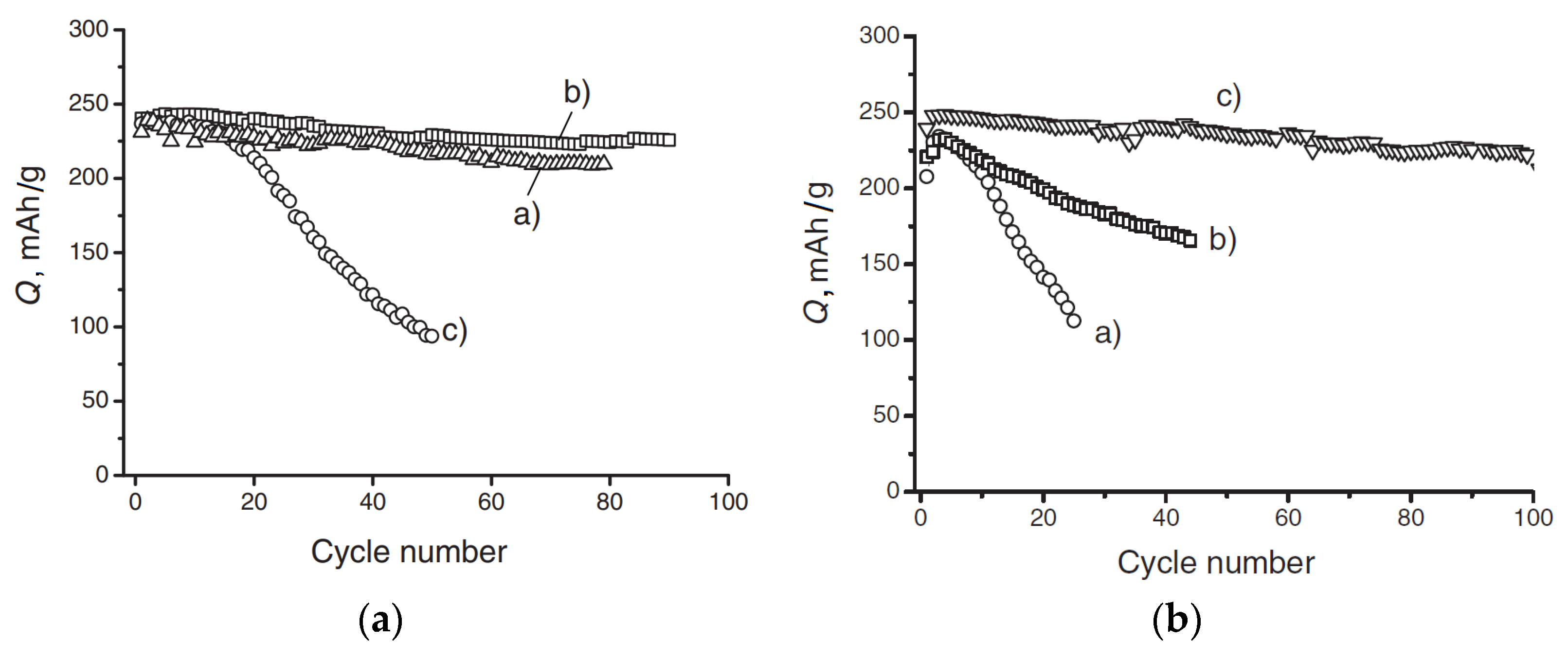
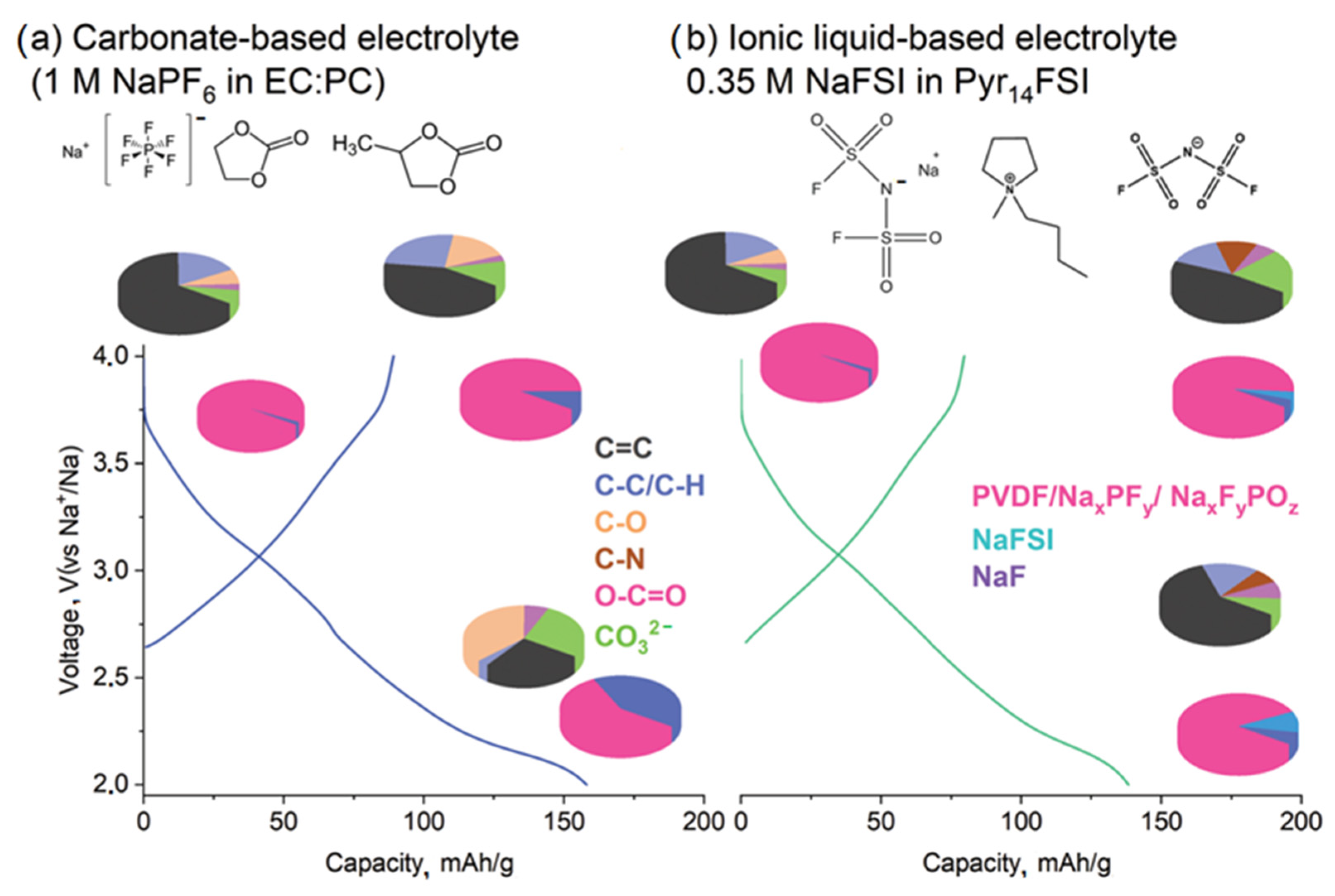
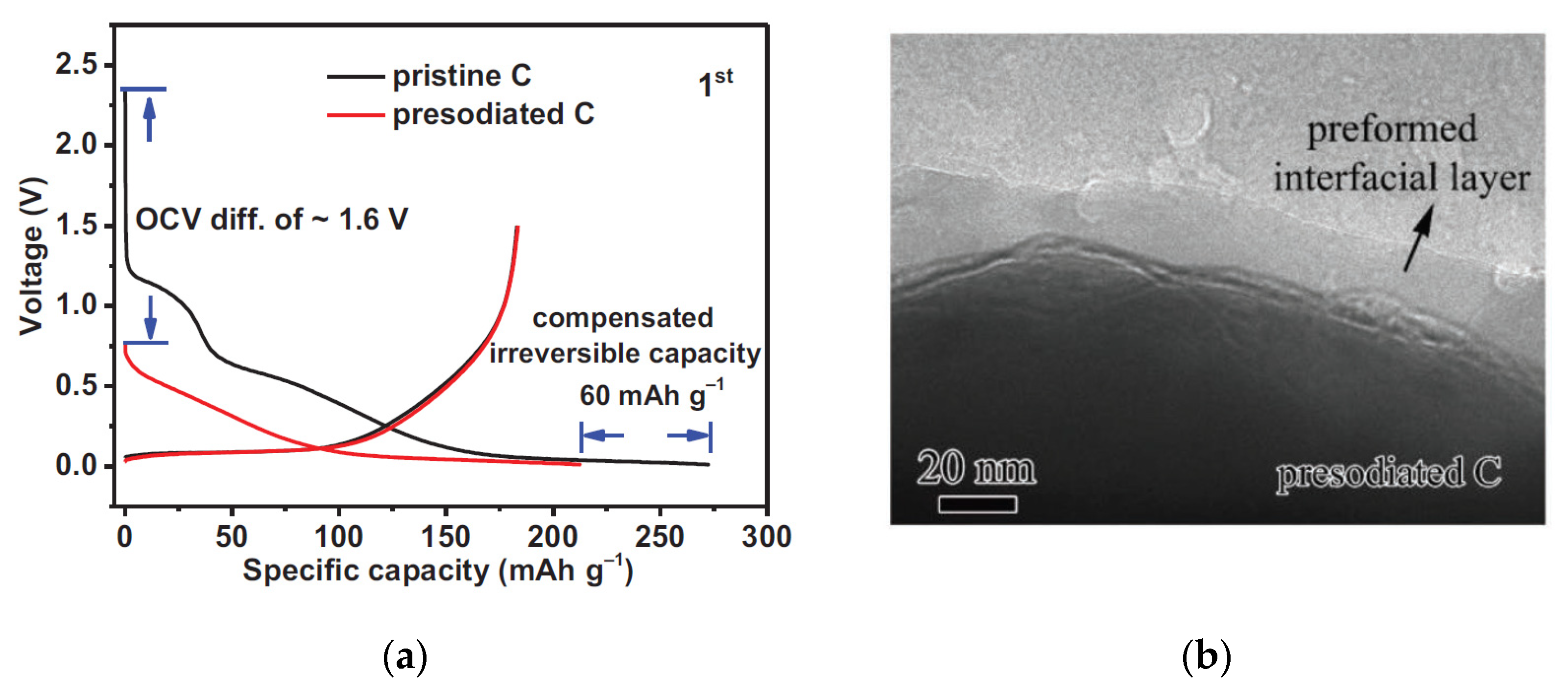
3. SEI on Hard Carbon
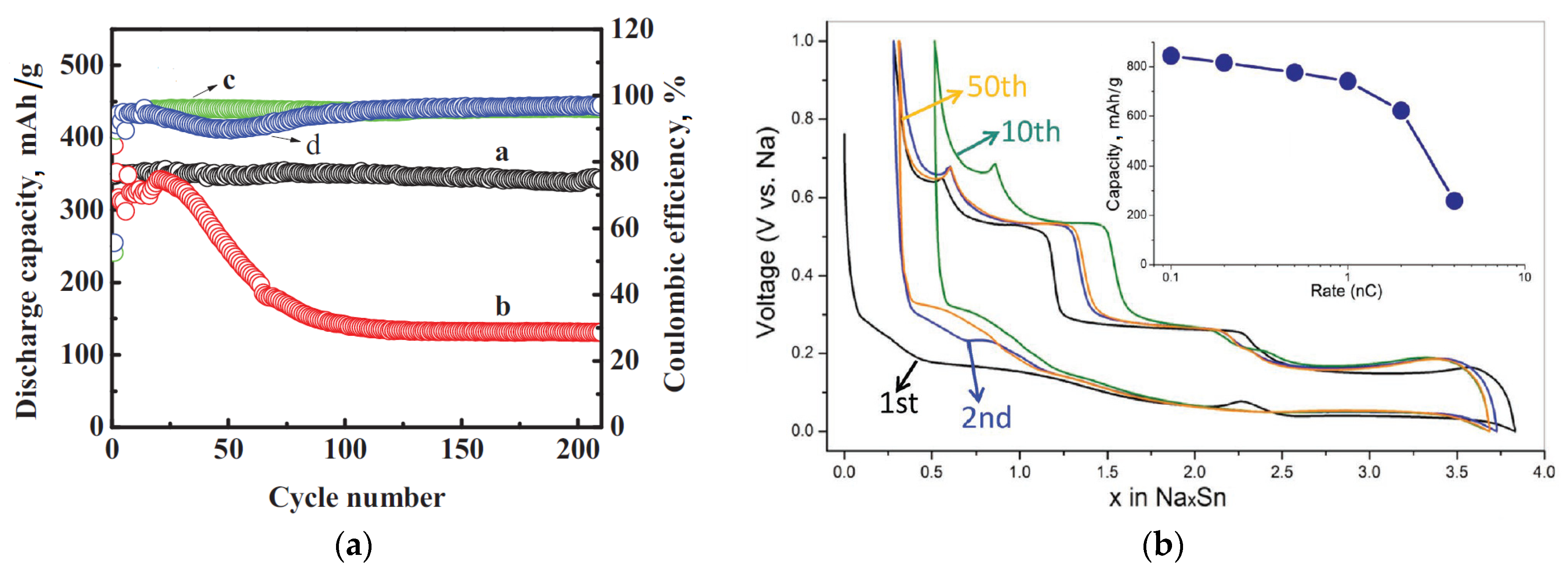
4. SEI on Other Anode Materials
5. CEI on Cathode Materials
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SEI | solid electrolyte interphase |
| CEI | cathode electrolyte interphase |
| EC | ethylene carbonate |
| PC | propylene carbonate |
| DEC | diethyl carbonate |
| DMC | dimethyl carbonate |
| RCH2ONa, RONa | sodium alkoxides |
| NaFSI | sodium bisfluorosulfonylimide |
| DME | 1,2-dimethoxyethane |
| BTFE | bis(2,2,2-trifluoroethyl) ether |
| FEC | fluoroethylene carbonate |
| PVdF–HFP | polyvinylidene fluoride with hexafluoropropylene |
| Qrev | reversible capacity |
| Qirr | irreversible capacity |
| NaTFSI | sodium bis(trifluoromethanesulfonyl)imide |
| NaFTFSI | sodium fluorosulfonyl-(trifluoromethanesulfonyl)imide |
| XPS | photoelectron spectroscopy |
| ROCO3Na | sodium alkyl carbonates |
| VC | vinylene carbonate |
| DFT | density functional theory |
| TMSP | tris(trimethylsilyl)phosphite |
| NaODFB | sodium-difluoro(oxalate)borate |
| ROSO2Na | sodium alkyl sulfates |
| RSO3Na | sodium alkyl and sulfites |
| DEGDME | diethylene glycol dimethyl ether, diglyme |
| TEGDME | tetraethylene glycol dimethyl ether |
| PMP–FSI | N-propyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide |
| PMP-TFSI | N-propyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide |
| TFP | tris (2,2,2-trifluoroethyl) phosphate |
References
- Balbuena, P.B.; Wang, Y. (Eds.) Lithium-Ion Batteries: Solid-Electrolyte Interface; Imperial College Press: London, UK, 2004; 407p. [Google Scholar]
- Peled, E. The Electrochemical Behavior of Alkali and Alkaline Earth Metals in Nonaqueous Battery Systems—The Solid Electrolyte Interphase Model. J. Electrochem. Soc. 1979, 126, 2047–2051. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Ardel, G. Advanced Model for Solid Electrolyte Interphase Electrodes in Liquid and Polymer Electrolytes. J. Electrochem. Soc. 1997, 144, L208–L210. [Google Scholar] [CrossRef]
- Qian, Y.; Hu, S.; Zou, X.; Deng, Z.; Xu, Y.; Cao, Z.; Kang, Y.; Deng, Y.; Shi, Q.; Xu, K.; et al. How electrolyte additives work in Li-ion batteries. Energy Storage Mater. 2019, 20, 208–215. [Google Scholar] [CrossRef]
- Haregewoin, A.M.; Wotango, A.S.; Hwang, B.-J. Electrolyte additives for lithium ion battery electrodes: Progress and perspectives. Energy Environ. Sci. 2016, 9, 1955–1988. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, L.; Li, L.; Xie, M.; Wu, F.; Chen, R. Electrolytes and Electrolyte/Electrode Interfaces in Sodium-Ion Batteries: From Scientific Research to Practical Application. Adv. Mater. 2019, 31, 1808393. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Elia, G.A.; Armand, M.; Forsyth, M.; Komaba, S.; Rojo, T.; Passerini, S. Electrolytes and Interphases in Sodium-Based Rechargeable Batteries: Recent Advances and Perspectives. Adv. Energy Mater. 2020, 10, 2000093. [Google Scholar] [CrossRef]
- Wang, E.; Niu, Y.; Yin, Y.-X.; Guo, Y.-G. Manipulating Electrode/Electrolyte Interphases of Sodium-Ion Batteries: Strategies and Perspectives. ACS Mater. Lett. 2021, 3, 18–41. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Feng, X.; Li, Y.; Wu, F.; Bai, Y.; Wu, C. How Can the Electrode Influence the Formation of the Solid Electrolyte Interface? ACS Energy Lett. 2021, 6, 3307–3320. [Google Scholar] [CrossRef]
- Bao, C.; Wang, B.; Liu, P.; Wu, H.; Zhou, Y.; Wang, D.; Liu, H.; Dou, S. Solid Electrolyte Interphases on Sodium Metal Anodes. Adv. Funct. Mater. 2020, 30, 2004891. [Google Scholar] [CrossRef]
- Zhang, J.; Gai, J.; Song, K.; Chen, W. Advances in electrode/electrolyte interphase for sodium-ion batteries from half cells to full cells. Cell Rep. Phys. Sci. 2022, 3, 100868. [Google Scholar] [CrossRef]
- Lee, B.; Paek, E.; Mitlin, D.; Lee, S.W. Sodium metal anodes: Emerging solutions to dendrite growth. Chem. Rev. 2019, 119, 5416–5460. [Google Scholar] [CrossRef]
- Ma, L.; Cui, J.; Yao, S.; Liu, X.; Luo, Y.; Shen, X.; Kim, J.-K. Dendrite-Free Lithium Metal and Sodium Metal Batteries. Energy Storage Mater. 2020, 27, 522–554. [Google Scholar] [CrossRef]
- Fang, L.; Bahlawane, N.; Sun, W.; Pan, H.; Xu, B.B.; Yan, M.; Jiang, Y. Conversion-Alloying Anode Materials for Sodium Ion Batteries. Small 2021, 17, 2101137. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xu, R.; Mu, D.; Tan, G.; Gao, H.; Li, N.; Chen, R.; Wu, F. Progress in electrolyte and interface of hard carbon and graphite anode for sodium-ion battery. Carbon Energy 2022, 4, 458–479. [Google Scholar] [CrossRef]
- Yadegari, H.; Sun, Q.; Sun, S. Sodium-Oxygen Batteries: A Comparative Review from Chemical and Electrochemical Fundamentals to Future Perspective. Adv. Mater. 2016, 28, 7065–7093. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X. Ambient Temperature Sodium–Sulfur Batteries. Small 2015, 11, 2108–2114. [Google Scholar] [CrossRef]
- Hu, X.; Sun, J.; Li, Z.; Zhao, Q.; Chen, C.; Chen, J. Rechargeable Room-Temperature Na–CO2 Batteries. Angew. Chem. Int. Ed. 2016, 55, 6482–6486. [Google Scholar] [CrossRef]
- Rodriguez, R.; Loeffler, K.E.; Nathan, S.S.; Sheavly, J.K.; Dolocan, A.; Heller, A.; Mullins, C.B. In Situ Optical Imaging of Sodium Electrodeposition: Effects of Fluoroethylene Carbonate. ACS Energy Lett. 2017, 2, 2051–2057. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.-W.; Lv, W.; Qin, L.; Niu, S.; Zhang, S.; Cao, T.; Kang, F.; Yang, Q.-H. Ethers Illume Sodium-Based Battery Chemistry: Uniqueness; Surprise; and Challenges. Adv. Energy Mater. 2018, 8, 1801361. [Google Scholar] [CrossRef]
- Mandl, M.; Becherer, J.; Kramer, D.; Mönig, R.; Diemant, T.; Behm, R.J.; Hahn, M.; Böse, O.; Danzer, M.A. Sodium metal anodes: Deposition and dissolution behaviour and SEI formation. Electrochim. Acta 2020, 354, 136698. [Google Scholar] [CrossRef]
- She, Z.W.; Sun, J.; Sun, Y.; Cui, Y. A Highly Reversible Room-Temperature Sodium Metal Anode. ACS Cent. Sci. 2015, 1, 449–455. [Google Scholar] [CrossRef]
- Dugas, R.; Ponrouch, A.; Gachot, G.; David, R.; Palacin, M.R.; Tarascon, J.M. Na Reactivity toward Carbonate-Based Electrolytes: The Effect of FEC as Additive. J. Electrochem. Soc. 2016, 163, A2333–A2339. [Google Scholar] [CrossRef]
- Mogensen, R.; Brandell, D.; Younesi, R. Solubility of the Solid Electrolyte Interphase (SEI) in Sodium Ion Batteries. ACS Energy Lett. 2016, 1, 1173–1178. [Google Scholar] [CrossRef]
- Iermakova, D.I.; Dugas, R.; Palacín, M.R.; Ponrouch, A. On the Comparative Stability of Li and Na Metal Anode Interfaces in Conventional Alkyl Carbonate Electrolytes. J. Electrochem. Soc. 2015, 162, A7060–A7066. [Google Scholar] [CrossRef]
- Rudola, A.; Aurbach, D.; Balaya, P. A new phenomenon in sodium batteries: Voltage step due to solvent interaction. Electrochem. Commun. 2014, 46, 56–59. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Wang, W.; Choi, D.; Nie, Z.; Yu, J.; Saraf, L.V.; Yang, Z.; Liu, J. Reversible Sodium Ion Insertion in Single Crystalline Manganese Oxide Nanowires with Long Cycle Life. Adv. Mater. 2011, 23, 3155–3160. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Wang, C.; Porter, S.; Wang, B.; Lie, W.; Liu, H.K. Sodium-difluoro(oxalato)borate (NaDFOB): A new electrolyte salt for Na-ion batteries. Chem. Commun. 2015, 51, 9809–9812. [Google Scholar] [CrossRef]
- Gao, L.; Chen, J.; Liu, Y.; Yamauchi, Y.; Huang, Z.; Kong, X. Revealing the chemistry of an anode-passivating electrolyte salt for high rate and stable sodium metal batteries. J. Mater. Chem. A 2018, 6, 12012–12017. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Lee, L.; Lee, S.-M.; Choi, J.-H.; Kim, H.; Kwon, M.-S.; Kang, K.; Lee, K.T.; Choi, N.-S. Ultraconcentrated Sodium Bis(fluorosulfonyl)imide-Based Electrolytes for High-Performance Sodium Metal Batteries. ACS Appl. Mater. Interfaces 2017, 9, 3723–3732. [Google Scholar] [CrossRef]
- Cao, R.; Mishra, K.; Li, X.; Qian, J.; Engelhard, M.H.; Bowden, M.E.; Han, K.S.; Mueller, K.T.; Henderson, W.A.; Zhang, J.-G. Enabling room temperature sodium metal batteries. Nano Energy 2016, 30, 825–830. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, S.; Zhao, W.; Song, J.; Engelhard, M.; Zhang, J.-G. Extremely Stable Sodium Metal Batteries Enabled by Localized High Concentration Electrolytes. ACS Energy Lett. 2018, 3, 315–321. [Google Scholar] [CrossRef]
- Basile, A.; Makhlooghiazad, F.; Yunis, R.; MacFarlane, D.R.; Forsytha, M.; Howlett, P.C. Extensive sodium metal plating and stripping in a highly concentrated inorganic-organic ionic liquid electrolyte via surface pretreatment. ChemElectroChem 2017, 4, 986–991. [Google Scholar] [CrossRef]
- Ruiz-Martínez, D.; Kovacs, A.; Gómez, R. Development of novel inorganic electrolytes for room temperature rechargeable sodium metal batteries. Energy Environ. Sci. 2017, 10, 1936–1941. [Google Scholar] [CrossRef]
- Badoz-Lambling, J.; Bardin, M.; Bernard, C.; Fahys, B.; Herlem, M.; Thiebault, A.; Robert, G. New Battery Electrolytes for Low and High Temperatures: Liquid and Solid Ammoniates for High Energy Batteries I. Sodium Iodide and Lithium Perchlorate Ammoniates. J. Electrochem. Soc. 1988, 135, 578–591. [Google Scholar] [CrossRef]
- Song, J.; Jeong, G.; Lee, A.; Park, J.H.; Kim, H.; Kim, Y.-J. Dendrite-Free Polygonal Sodium Deposition with Excellent Interfacial Stability in a NaAlCl4–2SO2 Inorganic Electrolyte. ACS Appl. Mater. Interfaces. 2015, 7, 27206–27214. [Google Scholar] [CrossRef]
- Komaba, S.; Ishikawa, T.; Yabuuchi, N.; Murata, W.; Ito, A.; Ohsawa, Y. Fluorinated Ethylene Carbonate as Electrolyte Additive for Rechargeable Na Batteries. ACS Appl. Mater. Interfaces 2011, 3, 4165–4168. [Google Scholar] [CrossRef]
- Xu, C.; Lindgren, F.; Philippe, B.; Gorgoi, M.; Björefors, F.; Edström, K.; Gustafsson, T. Improved Performance of the Silicon Anode for Li-Ion Batteries: Understanding the Surface Modification Mechanism of Fluoroethylene Carbonate as an Effective Electrolyte Additive. Chem. Mater. 2015, 27, 2591–2599. [Google Scholar] [CrossRef]
- Han, M.; Zhu, C.; Ma, T.; Pan, Z.; Tao, Z.; Chen, J. In situ atomic force microscopy study of nano–micro sodium deposition in ester-based electrolytes. Chem. Commun. 2018, 54, 2381–2384. [Google Scholar] [CrossRef]
- Shiraz, M.H.A.; Zhao, P.; Liu, J. High-performance sodium–selenium batteries enabled by microporous carbon/selenium cathode and fluoroethylene carbonate electrolyte additive. J. Power Sources 2020, 453, 227855. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, C.; Lv, X.; Lu, Y.; Lin, K.; Zhang, S.; Kang, F.; Hu, Y.-S.; Li, B. Stabilizing a sodium-metal battery with the synergy effects of a sodiophilic matrix and fluorine-rich interface. J. Mater. Chem. A 2019, 7, 24857–24867. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Lee, J.; Kim, K.; Cha, A.; Kang, S.; Wi, T.; Kang, S.J.; Lee, H.-W.; Choi, N.-S. Fluoroethylene Carbonate-Based Electrolyte with 1 M Sodium Bis(fluorosulfonyl)imide Enables High-Performance Sodium Metal Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 15270–15280. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Lu, Y.; Sun, X.; Zhang, H.; Yu, H.; Sun, C. Fluorinated Ether Based Electrolyte Enabling Sodium-Metal Batteries with Exceptional Cycling Stability. ACS Appl. Mater. Interfaces 2019, 11, 46965–46972. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Mu, D.; Wu, B.; Wang, L.; Gai, L.; Wu, F. Density Functional Theory Research into the Reduction Mechanism for the Solvent/Additive in a Sodium-Ion Battery. ChemSusChem 2016, 10, 786–796. [Google Scholar] [CrossRef]
- Åvall, G.; Mindemark, J.; Brandell, D.; Johansson, P. Sodium-Ion Battery Electrolytes: Modeling and Simulations. Adv. Energy Mater. 2018, 8, 1703036. [Google Scholar] [CrossRef]
- Takenaka, N.; Sakai, H.; Suzuki, Y.; Uppula, P.; Nagaoka, M. A Computational Chemical Insight into Microscopic Additive Effect on Solid Electrolyte Interphase Film Formation in Sodium-Ion Batteries: Suppression of Unstable Film Growth by Intact Fluoroethylene Carbonate. J. Phys. Chem. C 2015, 119, 18046–18055. [Google Scholar] [CrossRef]
- Kumar, H.; Detsi, E.; Abraham, D.P.; Sheno, V.B. Fundamental Mechanisms of Solvent Decomposition Involved in Solid-Electrolyte Interphase Formation in Sodium Ion Batteries. Chem. Mater. 2016, 28, 8930–8941. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Matios, E.; Li, W. Facile Stabilization of Sodium Metal Anode with Additives: Unexpected Key Role of Sodium Polysulfide and Adverse Effect of Sodium Nitrate. Angew. Chem. Int. Ed. 2018, 57, 7734–7737. [Google Scholar] [CrossRef]
- Shi, Q.; Zhong, Y.; Wu, M.; Wang, H.; Wang, H. High performance sodium metal anodes enabled by a bifunctional potassium salt. Angew. Chem. Int. Ed. 2018, 57, 9069–9072. [Google Scholar] [CrossRef]
- Fang, W.; Jiang, H.; Zheng, Y.; Zheng, H.; Liang, X.; Sun, Y.; Chen, C.; Xiang, H. A bilayer interface formed in high concentration electrolyte with SbF3 additive for long-cycle and high-rate sodium metal battery. J. Power Sources 2020, 455, 227956. [Google Scholar] [CrossRef]
- Wang, S.; Cai, W.; Sun, Z.; Huang, F.; Jie, Y.; Liu, Y.; Chen, Y.; Peng, B.; Cao, R.; Zhang, G.; et al. Stable cycling of Na metal anodes in a carbonate electrolyte. Chem. Commun. 2019, 55, 14375–14378. [Google Scholar] [CrossRef]
- Doi, K.; Yamada, Y.; Okoshi, M.; Ono, J.; Chou, C.-P.; Nakai, H.; Yamada, A. Reversible Sodium Metal Electrodes: Is Fluorine an Essential Interphasial Component? Angew. Chem. Int. Ed. 2019, 58, 8024–8028. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Fu, H.; Hu, C.; Xu, H.; Huang, Y.; Wen, J.; Sun, H.; Luo, W.; Huang, Y. Toward a Stable Sodium Metal Anode in Carbonate Electrolyte: A Compact; Inorganic Alloy Interface. J. Phys. Chem. Lett. 2019, 10, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; She, Z.W.; Yan, K.; Fu, Z.; Tang, P.; Lu, Y.; Zhang, R.; Legut, D.; Cui, Y.; Zhang, Q. Theoretical Investigation of 2D Layered Materials as Protective Films for Lithium and Sodium Metal Anodes. Adv. Energy Mater. 2017, 7, 1602528. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Matios, E.; Li, W. Critical Role of Ultrathin Graphene Films with Tunable Thickness in Enabling Highly Stable Sodium Metal Anodes. Nano Lett. 2017, 17, 6808–6815. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, H.; Noh, H.; Lee, J.; Kim, S.; Ryou, M.-H.; Lee, Y.M.; Kim, H.-T. Enhancing the Cycling Stability of Sodium Metal Electrode by Building an Inorganic/Organic Composite Protective Layer. ACS Appl. Mater. Interfaces 2017, 9, 6000–6006. [Google Scholar] [CrossRef]
- Zhao, Y.; Goncharova, L.V.; Zhang, Q.; Kaghazchi, P.; Sun, Q.; Lushington, A.; Wang, B.; Li, R.; Sun, X. Inorganic–Organic Coating via Molecular Layer Deposition Enables Long Life Sodium Metal Anode. Nano Lett. 2017, 17, 5653–5659. [Google Scholar] [CrossRef]
- Zhang, D.; Li, B.; Wang, S.; Yang, S. Simultaneous formation of artificial SEI film and 3D host for stable metallic sodium anodes. ACS Appl. Mater. Interfaces 2017, 9, 40265–40272. [Google Scholar] [CrossRef]
- Wang, A.; Hu, X.; Tang, H.; Zhang, C.; Liu, S.; Yang, Y.-W.; Yang, Q.-H.; Luo, J. Processable and Moldable Sodium Metal Anodes. Angew. Chem. Int. Ed. 2017, 56, 11921–11926. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. High Capacity Anode Materials for Rechargeable Sodium-Ion Batteries. J. Electrochem. Soc. 2000, 147, 1271–1273. [Google Scholar] [CrossRef]
- Barker, J.; Saidi, M.Y.; Swoyer, J.L. A Sodium-Ion Cell Based on the Fluorophosphate Compound NaVPO4F. Electrochem. Solid-State Lett. 2003, 6, A1–A4. [Google Scholar] [CrossRef]
- Irisarri, E.; Ponrouch, A.; Palacin, M.R. Review—Hard Carbon Negative Electrode Materials for Sodium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A2476–A2482. [Google Scholar] [CrossRef]
- Ponrouch, A.; Goñi, A.R.; Palacín, M.R. High capacity hard carbon anodes for sodium ion batteries in additive free electrolyte. Electrochem. Commun. 2013, 27, 85–88. [Google Scholar] [CrossRef]
- Alptekin, H.; Au, H.; Olsson, E.; Cottom, J.; Jensen, A.C.; Headen, T.F.; Cai, Q.; Drew, A.J.; Crespo-Ribadeneyra, M.; Titirici, M.-M. Elucidation of the Solid Electrolyte Interphase Formation Mechanism in Micro-Mesoporous Hard-Carbon Anodes. Adv. Mater. Interfaces 2022, 9, 2101267. [Google Scholar] [CrossRef]
- Alptekin, H.; Au, H.; Jensen, A.; Olsson, E.; Goktas, M.; Headen, T.F.; Adelhelm, P.; Cai, Q.; Drew, A.J.; Titirici, M.-M. Sodium Storage Mechanism Investigations Through Structural Changes in Hard Carbons. ACS Appl. Energy Mater. 2020, 3, 9918–9927. [Google Scholar] [CrossRef]
- Komaba, S.; Murata, W.; Ishikawa, T.; Yabuuchi, N.; Ozeki, T.; Nakayama, T.; Ogata, A.; Gotoh, K.; Fujiwara, K. Electrochemical Na Insertion and Solid Electrolyte Interphase for Hard-Carbon Electrodes and Application to Na-Ion Batteries. Adv. Funct. Mater. 2011, 21, 3859–3867. [Google Scholar] [CrossRef]
- Muñoz-Márquez, M.A.; Zarrabeitia, M.; Passerini, S.; Rojo, T. Structure; Composition; Transport Properties; and Electrochemical Performance of the Electrode-Electrolyte Interphase in Non-Aqueous Na-Ion Batteries. Adv. Mater. Interfaces 2022, 9, 2101773. [Google Scholar] [CrossRef]
- Song, X.; Zhou, X.; Zhou, Y.; Deng, Y.; Meng, T.; Gao, A.; Nan, J.; Shu, D.; Yi, F. Reaction Mechanisms of Sodium-Ion Batteries under Various Charge and Discharge Conditions in a Three-Electrode Setup. ChemElectroChem 2018, 5, 2475–2481. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Diemant, T.; Hekmatfar, M.; Grugeon, S.; Behm, R.J.; Laruelle, S.; Armand, M.; Passerini, S. Impact of the electrolyte salt anion on the solid electrolyte interphase formation in sodium ion batteries. Nano Energy 2019, 55, 327–340. [Google Scholar] [CrossRef]
- Fondard, J.; Irisarri, E.; Courrèges, C.; Palacin, M.R.; Ponrouch, A.; Dedryvère, R. SEI Composition on Hard Carbon in Na-Ion Batteries After Long Cycling: Influence of Salts (NaPF6; NaTFSI) and Additives (FEC; DMCF). J. Electrochem. Soc. 2020, 167, 070526. [Google Scholar] [CrossRef]
- Morikawa, Y.; Yamada, Y.; Doi, K.; Nishimura, S.; Yamada, A. Reversible and High-rate Hard Carbon Negative Electrodes in a Fluorine-free Sodium-salt Electrolyte. Electrochemistry 2020, 88, 151–156. [Google Scholar] [CrossRef]
- Che, H.; Chen, S.; Xie, Y.; Wang, H.; Amine, K.; Liao, X.-Z.; Ma, Z.-F. Electrolyte design strategies and research progress for room-temperature sodium-ion batteries. Energy Environ. Sci. 2017, 10, 1075–1101. [Google Scholar] [CrossRef]
- Bommier, C.; Ji, X. Electrolytes; SEI Formation; and Binders: A Review of Nonelectrode Factors for Sodium-Ion Battery Anodes. Small 2018, 14, 1703576. [Google Scholar] [CrossRef]
- Du, K.; Wang, C.; Subasinghe, L.U.; Gajella, S.R.; Law, M.; Rudola, A.; Balaya, P. A comprehensive study on the electrolyte; anode and cathode for developing commercial type non-flammable sodium-ion battery. Energy Storage Mater. 2020, 29, 287–299. [Google Scholar] [CrossRef]
- Zhao, D.; Lu, H.; Li, S.; Wang, P.; Fan, X. Boosting the cycling stability of hard carbon with NaODFB-based electrolyte at high temperature. Mater. Today Chem. 2022, 24, 100866. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Grugeon, S.; Kim, H.; Jeong, S.; Wu, L.; Gachot, G.; Laruelle, S.; Armand, M.; Passerini, S. Comprehensive Insights into the Reactivity of Electrolytes Based on Sodium Ions. ChemSusChem 2016, 9, 462–471. [Google Scholar] [CrossRef]
- Ponrouch, A.; Dedryvère, R.; Monti, D.; Demet, A.E.; Mba, J.M.A.; Croguennec, L.; Masquelier, C.; Johansson, P.; Palacín, M.R. Towards high energy density sodium ion batteries through electrolyte optimization. Energy Environ. Sci. 2013, 6, 2361–2369. [Google Scholar] [CrossRef]
- Carboni, M.; Manzi, J.; Armstrong, A.R.; Billaud, J.; Brutti, S.; Younesi, R. Analysis of the Solid Electrolyte Interphase on Hard Carbon Electrodes in Sodium-Ion Batteries. ChemElectroChem 2019, 6, 1745–1753. [Google Scholar] [CrossRef]
- Ponrouch, A.; Monti, D.; Boschin, A.; Steen, B.; Johansson, P.; Palacín, M.R. Non-aqueous electrolytes for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 22–42. [Google Scholar] [CrossRef]
- Bhawana, K.; Roy, A.; Chakrabarty, N.; Gautam, M.; Dutta, D.P.; Mitra, S. Sodium-ion batteries: Chemistry of biomass derived disordered carbon in carbonate and ether-based electrolytes. Electrochim. Acta 2022, 425, 140744. [Google Scholar] [CrossRef]
- Dahbi, M.; Nakano, T.; Yabuuchi, N.; Fujimura, S.; Chihara, K.; Son, J.-Y.; Cui, Y.-T.; Oji, H.; Komaba, S. Effect of Hexafluorophosphate and Fluoroethylene Carbonate on Electrochemical Performance and Surface Layer of Hard Carbon for Sodium-Ion Batteries. ChemElectroChem 2016, 3, 1856–1867. [Google Scholar] [CrossRef]
- Bai, P.; Han, X.; He, Y.; Xiong, P.; Zhao, Y.; Sun, J.; Xu, Y. Solid electrolyte interphase manipulation towards highly stable hard carbon anodes for sodium ion batteries. Energy Storage Mater. 2020, 25, 324–333. [Google Scholar] [CrossRef]
- Bouibes, A.; Takenaka, N.; Fujie, T.; Kubota, K.; Komaba, S.; Nagaoka, M. Concentration Effect of Fluoroethylene Carbonate on Formation of Solid Electrolyte Interphase Layer in Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 28525–28532. [Google Scholar] [CrossRef]
- Ponrouch, A.; Marchante, E.; Courty, M.; Tarascon, J.-M.; Palacín, M.R. In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 2012, 5, 8572–8583. [Google Scholar] [CrossRef]
- Purushotham, U.; Takenaka, N.; Nagaoka, M. Additive Effect of Fluoroethylene and Difluoroethylene Carbonates for the Solid Electrolyte Interphase Film Formation in Sodium-Ion Batteries: A Quantum Chemical Study. RSC Adv. 2016, 6, 65232–65242. [Google Scholar] [CrossRef]
- Cometto, C.; Yan, G.; Mariyappan, S.; Tarascon, J.-M. Means of Using Cyclic Voltammetry to Rapidly Design a Stable DMC-Based Electrolyte for Na-Ion Batteries. J. Electrochem. Soc. 2019, 166, A3723–A3730. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Li, X.; Guo, H.; Peng, W.; Wang, J.; Yan, G. Comparative study of 1;3-propane sultone; prop-1-ene-1;3-sultone and ethylene sulfate as film-forming additives for sodium ion batteries. J. Power Sources 2022, 541, 231726. [Google Scholar] [CrossRef]
- Monti, D.; Ponrouch, A.; Palacín, M.R.; Johansson, P. Towards safer sodium-ion batteries via organic solvent/ionic liquid based hybrid electrolytes. J. Power Sources 2016, 324, 712–721. [Google Scholar] [CrossRef]
- Fukunaga, A.; Nohira, T.; Hagiwara, R.; Numata, K.; Itani, E.; Sakai, S.; Nitta, K. Performance validation of sodium-ion batteries using an ionic liquid electrolyte. J. Appl. Electrochem. 2016, 46, 487–496. [Google Scholar] [CrossRef]
- Benchakar, M.; Naéjus, R.; Damas, C.; Santos-Peña, J. Exploring the use of EMImFSI ionic liquid as additive or co-solvent for room temperature sodium ion battery electrolytes. Electrochim. Acta 2020, 330, 135193. [Google Scholar] [CrossRef]
- Lee, D.J.; Im, D.; Ryu, Y.-G.; Lee, S.; Yoon, J.; Lee, J.; Choi, W.; Jung, I.; Lee, S.; Doo, S.-G. Phosphorus derivatives as electrolyte additives for lithium-ion battery: The removal of O2 generated from lithium-rich layered oxide cathode. J. Power Sources 2013, 243, 831–835. [Google Scholar] [CrossRef]
- Yim, T.; Woo, S.-G.; Lim, S.H.; Cho, W.; Song, J.H.; Han, Y.-K.; Kim, Y.-J. 5V-Class High-Voltage Batteries with Over-Lithiated Oxide and a Multi-Functional Additive. J. Mater. Chem. A 2015, 3, 6157–6167. [Google Scholar] [CrossRef]
- Ledwoch, D.; Robinson, J.B.; Gastol, D.; Smith, K.; Shearing, P.; Brett, D.J.L.; Kendrick, E. Hard Carbon Composite Electrodes for Sodium-Ion Batteries with Nano-Zeolite and Carbon Black Additives. Batter. Supercaps 2021, 4, 163–172. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Yang, C.; Li, N.; Ji, T.; Yan, K.; Zhu, B.; Yin, J.; Zhao, J.; Li, Y.A. V2O5-nanosheets-coated hard carbon fiber fabric as high-performance anode for sodium ion battery. Surf. Coat. Technol. 2019, 358, 661–666. [Google Scholar] [CrossRef]
- Lu, H.; Chen, X.; Jia, Y.; Chen, H.; Wang, Y.; Ai, X.; Yang, H.; Cao, Y. Engineering Al2O3 atomic layer deposition: Enhanced hard carbon-electrolyte interface towards practical sodium ion batteries. Nano Energy 2019, 64, 103903. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Wu, X.; Yu, J.; Wang, Y.; Hu, Y.-S.; Li, H.; Chen, L.; Huang, X. Amorphous monodispersed hard carbon micro-spherules derived from biomass as a high performance negative electrode material for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 71–77. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, Y.; Pinyi, Z.; Yuan, C.; Chen, M.; Wang, C. Commercial activated carbon as a novel precursor of the amorphous carbon for high performance sodium-ion batteries anode. Carbon 2018, 129, 85–94. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Li, Z.; Kohandehghan, A.; Cui, K.; Xu, Z.; Zahiri, B.; Tan, X.; Lotfabad, E.M.; Olsen, B.C.; et al. Carbon Nanosheet Frameworks Derived from Peat Moss as High Performance Sodium Ion Battery Anodes. ACS Nano 2013, 7, 11004–11015. [Google Scholar] [CrossRef]
- Bommier, C.; Luo, W.; Gao, W.-Y.; Greaney, A.; Ma, S.; Ji, X. Predicting capacity of hard carbon anodes in sodium-ion batteries using porosity measurements. Carbon 2014, 76, 165–174. [Google Scholar] [CrossRef]
- Xiao, L.; Lu, H.; Fang, Y.; Sushko, M.L.; Cao, Y.; Ai, X.; Yang, H.; Liu, J. Low-Defect and Low-Porosity Hard Carbon with High Coulombic Efficiency and High Capacity for Practical Sodium Ion Battery Anode. Adv. Energy Mater. 2018, 8, 1703238. [Google Scholar] [CrossRef]
- Wang, X.-K.; Shi, J.; Mi, L.-W.; Zhai, Y.-P.; Zhang, J.-Y.; Feng, X.-M.; Wu, Z.-J.; Chen, W.-H. Hierarchical porous hard carbon enables integral solid electrolyte interphase as robust anode for sodium-ion batteries. Rare Met. 2020, 39, 1053–1062. [Google Scholar] [CrossRef]
- Xie, H.; Wu, Z.; Wang, Z.; Qin, N.; Li, Y.; Cao, Y.; Lu, Z. Solid electrolyte interface stabilization via surface oxygen species functionalization in hard carbon for superior performance sodium-ion batteries. J. Mater. Chem. A 2020, 8, 3606–3612. [Google Scholar] [CrossRef]
- Evans, J.F.; Kuwana, T. Introduction of Functional Groups onto Carbon Electrodes via Treatment with Radio-Frequency Plasmas. Anal. Chem. 1979, 51, 358–365. [Google Scholar] [CrossRef]
- Besenhard, J.O.; Fritz, H.P. The Electrochemistry of Black Carbons. Angew. Chem. Int. Ed. Engl. 1983, 22, 950–975. [Google Scholar] [CrossRef]
- Lin, X.; Du, X.; Tsui, P.S.; Huang, J.-Q.; Tan, H.; Zhang, B. Exploring room- and low-temperature performance of hard carbon material in half and full Na-ion batteries. Electrochim. Acta 2019, 316, 60–68. [Google Scholar] [CrossRef]
- Rangom, Y.; Gaddam, R.R.; Duignan, T.T.; Zhao, X.S. Improvement of Hard Carbon Electrode Performance by Manipulating SEI Formation at High Charging Rates. ACS Appl. Mater. Interfaces 2019, 11, 34796–34804. [Google Scholar] [CrossRef]
- Liu, X.; Tan, Y.; Liu, T.; Wang, W.; Li, C.; Lu, J.; Sun, Y. A Simple Electrode-Level Chemical Presodiation Route by Solution Spraying to Improve the Energy Density of Sodium-Ion Batteries. Adv. Funct. Mater. 2019, 29, 1903795. [Google Scholar] [CrossRef]
- Thomas, P.; Billaud, D. Electrochemical insertion of sodium into hard carbons. Electrochim. Acta 2002, 47, 3303–3307. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Parimalam, B.S.; Nguyen, C.C.; Wang, G.; Lucht, B.L. Investigation of the solid electrolyte interphase on hard carbon electrode for sodium ion batteries. J. Electroanalyt. Chem. 2017, 799, 181–186. [Google Scholar] [CrossRef]
- Thomas, P.; Ghanbaja, J.; Billaud, D. Electrochemical insertion of sodium in pitch-based carbon fibres in comparison with graphite in NaClO4–ethylene carbonate electrolyte. Electrochim. Acta 1999, 45, 423–430. [Google Scholar] [CrossRef]
- Schafzahl, L.; Ehmann, H.; Kriechbaum, M.; Sattelkow, J.; Ganner, T.; Plank, H.; Wilkening, M.; Freunberger, S.A. Long-Chain Li and Na Alkyl Carbonates as Solid Electrolyte Interphase Components: Structure; Ion Transport; and Mechanical Properties. Chem. Mater. 2018, 30, 3338–3345. [Google Scholar] [CrossRef]
- Bommier, C.; Leonard, D.; Jian, Z.; Stickle, W.F.; Greaney, P.A.; Ji, X. New Paradigms on the Nature of Solid Electrolyte Interphase Formation and Capacity Fading of Hard Carbon Anodes in Na-Ion Batteries. Adv. Mater. Interfaces 2016, 3, 1600449. [Google Scholar] [CrossRef]
- Skundin, A.M.; Kulova, T.L.; Yaroslavtsev, A.B. Sodium-ion Batteries (a Review). Russ. J. Electrochem. 2018, 54, 113–152. [Google Scholar] [CrossRef]
- Alcántara, R.; Lavela, P.; Ortiz, G.F.; Tirado, J.L. Carbon Microspheres Obtained from Resorcinol-Formaldehyde as High-Capacity Electrodes for Sodium-Ion Batteries. Electrochem. Solid-State Lett. 2005, 8, A222–A225. [Google Scholar] [CrossRef]
- Yan, X.; Liang, S.; Shi, H.; Hu, Y.; Liu, L.; Xu, Z. Nitrogen-enriched carbon nanofibers with tunable semi-ionic CAF bonds as a stable long cycle anode for sodium-ion batteries. J. Colloid Interface Sci. 2021, 583, 535–543. [Google Scholar] [CrossRef]
- Jin, J.; Shi, Z.; Wang, C. Electrochemical Performance of Electrospun carbon nanofibers as free-standing and binder-free anodes for Sodium-Ion and Lithium-Ion Batteries. Electrochim. Acta 2014, 141, 302–310. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.; Liu, J. Sodium Ion Insertion in Hollow Carbon Nanowires for Battery Applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef]
- Luo, W.; Schardt, J.; Bommier, C.; Wang, B.; Razink, J.; Simonsen, J.; Ji, X. Carbon nanofibers derived from cellulose nanofibers as a long-life anode material for rechargeable sodium-ion batteries. J. Mater. Chem. A 2013, 1, 10662–10666. [Google Scholar] [CrossRef]
- Cui, X.-Y.; Wang, Y.-J.; Wu, H.-D.; Lin, X.-D.; Tang, S.; Xu, P.; Liao, H.-G.; Zheng, M.-S.; Dong, Q.-F. A Carbon Foam with Sodiophilic Surface for Highly Reversible; Ultra-Long Cycle Sodium Metal Anode. Adv. Sci. 2021, 8, 2003178. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Liu, Y.; Bai, Y.; Chen, G.; Li, Y.; Wang, X.; Xu, H.; Wu, C.; Lu, J. Analysis of the Stable Interphase Responsible for the Excellent Electrochemical Performance of Graphite Electrodes in Sodium-Ion Batteries. Small 2020, 16, 2003268. [Google Scholar] [CrossRef]
- Wang, C.; Huang, J.; Li, Q.; Cao, L.; Li, J.; Kajiyoshi, K. Catalyzing carbon surface by Ni to improve initial coulombic efficiency of sodium-ion batteries. J. Energy Storage 2020, 32, 101868. [Google Scholar] [CrossRef]
- Cabello, M.; Chyrka, T.; Klee, R.; Aragón, M.J.; Bai, X.; Lavela, P.; Vasylchenko, G.M.; Alcántara, R.; Tirado, J.L.; Ortiz, G.F. Treasure Na-ion anode from trash coke by adept electrolyte selection. J. Power Sources 2017, 347, 127–135. [Google Scholar] [CrossRef]
- Cao, B.; Liu, H.; Xu, B.; Lei, Y.; Chen, X.; Song, H. Mesoporous soft carbon as an anode material for sodium ion batteries with superior rate and cycling performance. J. Mater. Chem. A 2016, 4, 6472–6478. [Google Scholar] [CrossRef]
- Cuesta, N.; Cameán, I.; Arenillas, A.; García, A.B. Exploring the application of carbon xerogels as anodes for sodium-ion batteries. Microporous Mesoporous Mater. 2020, 308, 110542. [Google Scholar] [CrossRef]
- Jo, C.; Park, Y.; Jeong, J.; Lee, K.T.; Lee, J. Structural effect on electrochemical performance of ordered porous carbon electrodes for Na-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 11748–11754. [Google Scholar] [CrossRef]
- Mehmood, A.; Ali, G.; Koyutürk, B.; Pampel, J.; Chung, K.Y.; Fellinger, T.-P. Nanoporous nitrogen doped carbons with enhanced capacity for sodium ion battery anodes. Energy Storage Mater. 2020, 28, 101–111. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Yoon, G.; Kim, H.; Park, K.-Y.; Park, M.-S.; Yoon, W.-S.; Kang, K. Sodium intercalation chemistry in graphite. Energy Environ. Sci. 2015, 8, 2963–2969. [Google Scholar] [CrossRef]
- Jache, B.; Adelhelm, P. Use of Graphite as a Highly Reversible Electrode with Superior Cycle Life for Sodium-Ion Batteries by Making Use of Co-Intercalation Phenomena. Angew. Chem. 2014, 126, 10333–10337. [Google Scholar] [CrossRef]
- Seidl, L.; Bucher, N.; Chu, E.; Hartung, S.; Martens, S.; Schneider, O.; Stimming, U. Intercalation of solvated Na-ions into graphite. Energy Environ. Sci. 2017, 10, 1631–1642. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Park, Y.-U.; Kim, J.; Hwang, I.; Kang, K. Sodium Storage Behavior in Natural Graphite using Ether-based Electrolyte Systems. Adv. Funct. Mater. 2015, 25, 534–541. [Google Scholar] [CrossRef]
- Goktas, M.; Bolli, C.; Berg, E.J.; Novák, P.; Pollok, K.; Langenhorst, F.; Roeder, M.V.; Lenchuk, O.; Mollenhauer, D.; Adelhelm, P. Graphite as Cointercalation Electrode for Sodium-Ion Batteries: Electrode Dynamics and the Missing Solid Electrolyte Interphase (SEI). Adv. Energy Mater. 2018, 8, 1702724. [Google Scholar] [CrossRef]
- Lin, Z.; Tan, X.; Ge, L.; Lin, Y.; Yang, W.; Lin, J.; Fu, H.; Ying, S.; Huang, Z. Ultrathin 2D hexagon CoP/N-doped carbon nanosheets for robust sodium storage. J. Alloys Comp. 2022, 921, 166075. [Google Scholar] [CrossRef]
- Li, J.; Qi, H.; Wang, Q.; Xu, Z.; Liu, Y.; Li, Q.; Kong, X.; Huang, J. Constructing graphene-like nanosheets on porous carbon framework for promoted rate performance of Li-ion and Na-ion storage. Electrochim. Acta 2018, 271, 92–102. [Google Scholar] [CrossRef]
- Wang, C.; Huang, J.; Li, J.; Cao, L.; Chen, Q.; Qian, C.; Chen, S. Revealing the effect of nanopores in biomass-derived carbon on its sodium-ion storage behavior. ChemElectroChem 2020, 7, 201–211. [Google Scholar] [CrossRef]
- Wang, C.; Huang, J.; Qi, H.; Cao, L.; Xu, Z.; Cheng, Y.; Zhao, X.; Li, J. Controlling pseudographtic domain dimension of dandelion derived biomass carbon for excellent sodium-ion storage. J. Power Sources 2017, 358, 85–92. [Google Scholar] [CrossRef]
- Wang, C.; Huang, J.; Li, J.; Cao, L.; Xi, Q.; Chen, S. Exposing inner defects of porous carbon sheets to enhance rate performance of sodium-ion batteries. J. Electroanalyt. Chem. 2020, 860, 113924. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, J.; Kong, D.; Cao, T.; Zhang, S.-W.; Chen, X.; Tao, Y.; Lv, W.; Kang, F.; Yang, Q.-H. Deactivating Defects in Graphenes with Al2O3 Nanoclusters to Produce Long-Life and High-Rate Sodium-Ion Batteries. Adv. Energy Mater. 2019, 9, 1803078. [Google Scholar] [CrossRef]
- Kang, J.; Kim, D.-Y.; Chae, S.-A.; Saito, N.; Choid, S.-Y.; Kim, K.-H. Maximization of sodium storage capacity of pure carbon material used in sodium-ion batteries. J. Mater. Chem. A 2019, 7, 16149–16160. [Google Scholar] [CrossRef]
- Zhang, S.-W.; Lv, W.; Qiu, D.; Cao, T.; Zhang, J.; Lin, Q.; Chen, X.; He, Y.-B.; Kang, F.; Yang, Q.-H. An ion-conducting SnS–SnS2 hybrid coating for commercial activated carbons enabling their use as high performance anodes for sodium-ion batteries. J. Mater. Chem. A 2019, 7, 10761–10768. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D.S.; Um, J.H.; Yoon, J.; Kim, J.M.; Kim, H.; Won-Sub Yoon, W.-S. Revisiting Solid Electrolyte Interphase on the Carbonaceous Electrodes Using Soft X-ray Absorption Spectroscopy. ACS Appl. Mater. Interfaces 2018, 10, 29992–29999. [Google Scholar] [CrossRef]
- Maibach, J.; Jeschull, F.; Brandell, D.; Edström, K.; Valvo, M. Surface Layer Evolution on Graphite During Electrochemical Sodium-tetraglyme Co-intercalation. ACS Appl. Mater. Interfaces 2017, 9, 12373–12381. [Google Scholar] [CrossRef]
- Doeff, M.M.; Ma, Y.; Visco, S.J.; De Jonghe, L.C. Electrochemical Insertion of Sodium into Carbon. J. Electrochem. Soc. 1993, 140, L169–L170. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.-W.; Lv, W.; Zhang, S.; Liang, Q.; Zheng, D.; Kang, F.; Yang, Q.-H. Achieving Superb Sodium Storage Performance on Carbon Anodes through Ether-derived Solid Electrolyte Interphase. Energy Environ. Sci. 2017, 10, 370–376. [Google Scholar] [CrossRef]
- Ye, L.; Liao, M.; Zhao, T.; Sun, H.; Zhao, Y.; Sun, X.; Wang, B.; Peng, H. A Sodiophilic Interphase-Mediated; Dendrite-Free Anode with Ultrahigh Specific Capacity for Sodium-Metal Batteries. Angew. Chem. 2019, 131, 17210–17216. [Google Scholar] [CrossRef]
- Luo, X.-F.; Helal, A.S.; Hsieh, C.-T.; Li, J.; Chang, J.-K. Three-dimensional carbon framework anode improves sodiation–desodiation properties in ionic liquid electrolyte. Nano Energy 2018, 49, 515–522. [Google Scholar] [CrossRef]
- Darwiche, A.; Bodenes, L.; Madec, L.; Monconduit, L.; Martinez, H. Impact of the salts and solvents on the SEI formation in Sb/Na batteries: An XPS analysis. Electrochim. Acta 2016, 207, 284–292. [Google Scholar] [CrossRef]
- Darwiche, A.; Marino, C.; Sougrati, M.T.; Fraisse, B.; Stievano, L.; Monconduit, L. Better Cycling Performances of Bulk Sb in Na-Ion Batteries Compared to Li-Ion Systems: An Unexpected Electrochemical Mechanism. J. Am. Chem. Soc. 2012, 134, 20805–20811. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Kravchyk, K.; Walter, M.; Kovalenko, M.V. Monodisperse Antimony Nanocrystals for High-Rate Li-ion and Na-ion Battery Anodes: Nano versus Bulk. Nano Lett. 2014, 14, 1255–1262. [Google Scholar] [CrossRef]
- Baggetto, L.; Ganesh, P.; Sun, C.-N.; Meisner, R.A.; Zawodzinski, T.A.; Veith, G.M. Intrinsic thermodynamic and kinetic properties of Sb electrodes for Li-ion and Na-ion batteries: Experiment and theory. J. Mater. Chem. A 2013, 1, 7985–7994. [Google Scholar] [CrossRef]
- Zhou, X.; Dai, Z.; Bao, J.; Guo, Y. Wet milled synthesis of an Sb/MWCNT nanocomposite for improved sodium storage. J. Mater. Chem. A 2013, 1, 13727–13731. [Google Scholar] [CrossRef]
- Ji, L.; Gu, M.; Shao, Y.; Li, X.; Engelhard, M.H.; Arey, B.W.; Wang, W.; Nie, Z.; Xiao, J.; Wang, C.; et al. Controlling SEI Formation on SnSb-Porous Carbon Nanofibers for Improved Na Ion Storage. Adv. Mater. 2014, 26, 2901–2908. [Google Scholar] [CrossRef]
- Zheng, X.-M.; You, J.-H.; Fan, J.-J.; Tu, G.-P.; Rong, W.-Q.; Li, W.-J.; Wang, Y.-X.; Tao, S.; Zhang, P.-Y.; Zhang, S.-Y.; et al. Electrodeposited binder-free Sb/NiSb anode of sodium-ion batteries with excellent cycle stability and rate capability and new insights into its reaction mechanism by operando XRD analysis. Nano Energy 2020, 77, 105123. [Google Scholar] [CrossRef]
- Bian, X.; Dong, Y.; Zhao, D.; Ma, X.; Qiu, M.; Xu, J.; Jiao, L.; Cheng, F.; Zhang, N. Microsized Antimony as a Stable Anode in Fluoroethylene Carbonate Containing Electrolytes for Rechargeable Lithium-/Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 3554–3562. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.A.; Hu, V.W.; Yoo, T.B.; Affandy, M.; Opie, C.; Paradis, E.K.; Holmberg, V.C. Temperature-Dependent Electrochemical Characteristics of Antimony Nanocrystal Alloying Electrodes for Na-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 6741–6750. [Google Scholar] [CrossRef]
- Song, J.; Xiao, D.; Jia, H.; Zhu, G.; Engelhard, M.; Xiao, B.; Feng, S.; Li, D.; Reed, D.; Sprenkle, V.L.; et al. A comparative study of pomegranate Sb@C yolk–shell microspheres as Li and Na-ion battery anodes. Nanoscale 2019, 11, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Guo, X.; Wang, S.; Zang, R.; Li, X.; Man, Z.; Li, P.; Liu, S.; Wu, Y.; Wang, G. Two-dimensional Sb@TiO2−x nanoplates as a high-performance anode material for sodium-ion batteries. J. Mater. Chem. A 2019, 7, 2553–2559. [Google Scholar] [CrossRef]
- Bodenes, L.; Darwiche, A.; Monconduit, L.; Martinez, H. The Solid Electrolyte Interphase a key parameter of the high performance of Sb in sodium-ion batteries: Comparative X-ray Photoelectron Spectroscopy study of Sb/Na-ion and Sb/Li-ion batteries. J. Power Sources 2015, 273, 14–24. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Y.; Wu, L.; Cao, Y.; Ai, X.; Yang, H. High capacity Na-storage and superior cyclability of nanocomposite Sb/C anode for Na-ion batteries. Chem. Commun. 2012, 48, 7070–7072. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Z.; Wang, J.; Li, W.; Gu, L.; Yu, Y. Sb Nanoparticles Encapsulated in a Reticular Amorphous Carbon Network for Enhanced Sodium Storage. Small 2015, 11, 5381–5387. [Google Scholar] [CrossRef]
- Ko, Y.N.; Kang, Y.C. Electrochemical properties of ultrafine Sb nanocrystals embedded in carbon microspheres for use as Na-ion battery anode materials. Chem. Commun. 2014, 50, 12322–12324. [Google Scholar] [CrossRef]
- Zhu, Y.; Han, X.; Xu, Y.; Liu, Y.; Zheng, S.; Xu, K.; Hu, L.; Wang, C. Electrospun Sb/C Fibers for a Stable and Fast Sodium-Ion Battery Anode. ACS Nano 2013, 7, 6378–6386. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Du, X.; Lin, X.; Huang, J.-Q.; Tan, H.; Zhu, Y.; Zhang, B. Nanostructures of solid electrolyte interphases and their consequences for microsized Sn anodes in sodium ion batteries. Energy Environ. Sci. 2019, 12, 1550–1557. [Google Scholar] [CrossRef]
- Song, M.; Wang, C.; Du, D.; Li, F.; Chen, J. A high-energy-density sodium-ion full battery based on tin anode. Sci. China Chem. 2019, 62, 616–621; [Google Scholar] [CrossRef]
- Prosini, P.P.; Carewska, M.; Cento, C.; Tarquini, G.; Maroni, F.; Birrozzi, A.; Nobili, F. Tin-Decorated Reduced Graphene Oxide and NaLi0.2Ni0.25Mn0.75Oδ as Electrode Materials for Sodium-Ion Batteries. Materials 2019, 12, 1074. [Google Scholar] [CrossRef]
- Zhang, B.; Rousse, G.; Foix, D.; Dugas, R.; Corte, D.A.D.; Tarascon, J.-M. Microsized Sn as Advanced Anodes in Glyme-Based Electrolyte for Na-Ion Batteries. Adv. Mater. 2016, 28, 9824–9830. [Google Scholar] [CrossRef]
- Baggetto, L.; Ganesh, P.; Meisner, R.P.; Unocic, R.R.; Jumas, J.-C.; Bridges, C.A.; Veith, G.M. Characterization of sodium ion electrochemical reaction with tin anodes: Experiment and theory. J. Power Sources 2013, 234, 48–59. [Google Scholar] [CrossRef]
- Komaba, S.; Matsuura, Y.; Ishikawa, T.; Yabuuchi, N.; Murata, W.; Kuze, S. Redox reaction of Sn-polyacrylate electrodes in aprotic Na cell. Electrochem. Commun. 2012, 21, 65–68. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, K.; Wang, D.; Luo, S.; Liu, Y.; Wang, Q.; Zhang, Y.; Hao, A.; Shid, C.; Zhao, N. A nanosized SnSb alloy confined in N-doped 3D porous carbon coupled with ether-based electrolytes toward high-performance potassium-ion batteries. J. Mater. Chem. A 2019, 7, 14309–14318. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, F.; Wei, X.; Lan, J.-L.; Liu, Y.; Yu, Y.; Yang, X.; Park, H.S. Ionic-Conducting and Robust Multilayered Solid Electrolyte Interphases for Greatly Improved Rate and Cycling Capabilities of Sodium Ion Full Cells. Adv. Energy Mater. 2020, 10, 2001418. [Google Scholar] [CrossRef]
- Yang, H.; Xu, R.; Yao, Y.; Ye, S.; Zhou, X.; Yu, Y. Multicore–Shell Bi@N-doped Carbon Nanospheres for High Power Density and Long Cycle Life Sodium- and Potassium-Ion Anodes. Adv. Funct. Mater. 2019, 29, 1809195. [Google Scholar] [CrossRef]
- Long, H.; Yin, X.; Wang, X.; Zhao, Y.; Yan, L. Bismuth nanorods confined in hollow carbon structures for high performance sodium- and potassium-ion batteries. J. Energy Chem. 2022, 67, 787–796. [Google Scholar] [CrossRef]
- Xiong, P.; Bai, P.; Li, A.; Li, B.; Cheng, M.; Chen, Y.; Huang, S.; Jiang, Q.; Bu, X.-H.; Xu, Y. Bismuth Nanoparticle@Carbon Composite Anodes for Ultralong Cycle Life and High-Rate Sodium-Ion Batteries. Adv. Mater. 2019, 31, 1904771. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, D.; Wu, Y.; Xu, R.; Yu, Y. Bismuth Nanospheres Embedding in Three-Dimensional (3D) Porous Graphene Frameworks as Highly Performance Anodes for Sodium and Potassium-Ion Batteries. J. Mater. Chem. A 2019, 7, 4913–4921. [Google Scholar] [CrossRef]
- Xue, P.; Wang, N.; Fang, Z.; Lu, Z.; Xu, X.; Wang, L.; Du, Y.; Ren, X.; Bai, Z.; Dou, S.; et al. Rayleigh-Instability-Induced Bismuth Nanorod@Nitrogen-Doped Carbon Nanotubes as A Long Cycling and High Rate Anode for Sodium-Ion Batteries. Nano Lett. 2019, 19, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Li, S.; Su, X.; Wang, Y.; Xua, L.; Yuan, S.; Li, H.; Zhang, S. Bismuth nano-spheres encapsulated in porous carbon network for robust and fast sodium storage. Chem. Eng. J. 2017, 320, 300–307. [Google Scholar] [CrossRef]
- Weadock, N.; Varongchayakul, N.; Wan, J.; Lee, S.; Seog, J.; Hun, L. Determination of mechanical properties of the SEI in sodium ion batteries via colloidal probe microscopy. Nano Energy 2013, 2, 713–719. [Google Scholar] [CrossRef]
- Li, X.; Steirer, K.X.; Krishna, L.; Xiao, C.; Fink, K.; Santhanagopalan, S. Electrochemical Properties and Challenges of Type II Silicon Clathrate Anode in Sodium Ion Batteries. J. Electrochem. Soc. 2019, 166, A3051–A3058. [Google Scholar] [CrossRef]
- Xu, Y.; Swaans, E.; Basak, S.; Zandbergen, H.W.; Borsa, D.M.; Mulder, F.M. Reversible Na-Ion Uptake in Si Nanoparticles. Adv. Energy Mater. 2016, 6, 1501436. [Google Scholar] [CrossRef]
- Gavrilin, I.M.; Smolyaninov, V.A.; Dronov, A.A.; Gavrilov, S.A.; Trifonov, A.Y.; Kulova, T.L.; Kuz’mina, A.A.; Skundin, A.M. Electrochemical insertion of sodium into nanostructured materials based on germanium. Mendeleev Commun. 2018, 28, 659–660. [Google Scholar] [CrossRef]
- Lebedev, E.A.; Gavrilin, I.M.; Kudryashova, Y.O.; Martynova, I.K.; Volkov, R.L.; Kulova, T.L.; Skundin, A.M.; Borgardt, N.I.; Gavrilov, S.A. Effect of Vinylene Carbonate Electrolyte Additive on the Process of Insertion/Extraction of Na into Ge Microrods Formed by Electrodeposition. Batteries 2022, 8, 109. [Google Scholar] [CrossRef]
- Oh, S.-M.; Myung, S.-T.; Yoon, C.S.; Lu, J.; Hassoun, J.; Scrosati, B.; Amine, K.; Sun, Y.-K. Advanced Na[Ni0.25Fe0.5Mn0.25]O2/C–Fe3O4 Sodium-Ion Batteries Using EMS Electrolyte for Energy Storage. Nano Lett. 2014, 14, 1620–1626. [Google Scholar] [CrossRef]
- Ahuja, V.; Vengarathody, R.; Singh, S.; Senguttuvan, P. Realization of high cycle life bismuth oxychloride Na-ion anode in glyme-based electrolyte. J. Power Sources 2022, 529, 231227. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Wang, M.-Q.; Niu, Y.; Liu, S.; Li, Y.; Wu, X.; Bao, S.-J.; Xu, M. Bismuth oxychloride ultrathin nanoplates as an anode material for sodium-ion batteries. Mater. Lett. 2016, 178, 44–47. [Google Scholar] [CrossRef]
- Bai, Z.; Lv, X.; Liu, D.-H.; Dai, D.; Gu, J.; Yang, L.; Chen, Z. Two-Dimensional NiO@C-N Nanosheets Composite as a Superior Low-Temperature Anode Material for Advanced Lithium-/Sodium-Ion Batteries. ChemElectroChem 2020, 7, 3616–3622. [Google Scholar] [CrossRef]
- Cha, C.; Mohajernia, S.; Nguyen, N.T.; Mazare, A.; Denisov, N.; Hwang, I.; Schmuki, P. Li+ Pre-Insertion Leads to Formation of Solid Electrolyte Interface on TiO2 Nanotubes That Enables High-Performance Anodes for Sodium Ion Batteries. Adv. Energy Mater. 2020, 10, 1903448. [Google Scholar] [CrossRef]
- Kong, H.; Wu, Y.; Hong, W.; Yan, C.; Zhao, Y.; Chen, G. Structure-designed synthesis of Cu-doped Co3O4@N-doped carbon with interior void space for optimizing alkali-ion storage. Energy Storage Mater. 2020, 24, 610–617. [Google Scholar] [CrossRef]
- Rubio, S.; Maça, R.R.; Aragón, M.J.; Cabello, M.; Castillo-Rodríguez, M.; Lavela, P.; Tirado, J.L.; Etacheri, V.; Ortiz, G.F. Superior electrochemical performance of TiO2 sodium-ion battery anodes in diglyme-based electrolyte solution. J. Power Sources 2019, 432, 82–91. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, X.; Liu, X.; Wang, G.; Wang, H.; Bai, J. Rational design of Fe3O4@C yolk-shell nanorods constituting a stable anode for high-performance Li/Na-ion batteries. J. Colloid Interface Sci. 2018, 528, 225–236. [Google Scholar] [CrossRef]
- Philippe, B.; Valvo, M.; Lindgren, F.; Rensmo, H.; Edström, K. Investigation of the Electrode/Electrolyte Interface of Fe2O3 Composite Electrodes: Li vs Na Batteries. Chem. Mater. 2014, 26, 5028–5041. [Google Scholar] [CrossRef]
- Komaba, S.; Mikumo, T.; Yabuuchi, N.; Ogata, A.; Yoshida, H.; Yamada, Y. Electrochemical Insertion of Li and Na Ions into Nanocrystalline Fe3O4 and α-Fe2O3 for Rechargeable Batteries. J. Electrochem. Soc. 2010, 157, A60–A65. [Google Scholar] [CrossRef]
- Valvo, M.; Lindgren, F.; Lafont, U.; Björefors, F.; Edström, K. Towards more sustainable negative electrodes in Na-ion batteries via nanostructured iron oxide. J. Power Sources 2014, 245, 967–978. [Google Scholar] [CrossRef]
- Kim, K.-T.; Ali, G.; Chung, K.Y.; Yoon, C.S.; Yashiro, H.; Sun, Y.-K.; Lu, J.; Amine, K.; Myung, S.-T. Anatase Titania Nanorods as an Intercalation Anode Material for Rechargeable Sodium Batteries. Nano Lett. 2014, 14, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xu, Y.; Wang, C.; Li, Q.; Xiang, J.; Liang, L.; Wu, M.; Zhao, H.; Lei, Y. Amorphous TiO2 Inverse Opal Anode for High-rate Sodium Ion Batteries. Nano Energy 2017, 31, 514–524. [Google Scholar] [CrossRef]
- Bresser, D.; Oschmann, B.; Tahir, M.N.; Mueller, F.; Lieberwirth, I.; Tremel, W.; Zentel, R.; Passerini, S. Carbon-Coated Anatase TiO2 Nanotubes for Li and Na-Ion Anodes. J. Electrochem. Soc. 2015, 162, A3013–A3020. [Google Scholar] [CrossRef]
- Oh, S.-M.; Hwang, J.-Y.; Yoon, C.S.; Lu, J.; Amine, K.; Belharouak, I.; Sun, Y.-K. High Electrochemical Performances of Microsphere C-TiO2 Anode for Sodium-Ion Battery. ACS Appl. Mater. Interfaces 2014, 6, 11295–11301. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lotfabad, E.M.; Wang, H.; Farbod, B.; Xu, Z.; Kohandehghan, A.; Mitlin, D. Nanocrystalline anatase TiO2: A new anode material for rechargeable sodium ion batteries. Chem. Commun. 2013, 49, 8973–8975. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Slater, M.D.; Balasubramanian, M.; Johnson, C.S.; Rajh, T. Amorphous TiO2 Nanotube Anode for Rechargeable Sodium Ion Batteries. J. Phys. Chem. Lett. 2011, 2, 2560–2565. [Google Scholar] [CrossRef]
- Bi, Z.; Paranthaman, M.P.; Menchhofer, P.A.; Dehoff, R.R.; Bridges, C.A.; Chi, M.; Guo, B.; Sun, X.-G.; Dai, S. Self-organized amorphous TiO2 nanotube arrays on porous Ti foam for rechargeable lithium and sodium ion batteries. J. Power Sources 2013, 222, 461–466. [Google Scholar] [CrossRef]
- Wu, L.; Bresser, D.; Buchholz, D.; Passerini, S. Nanocrystalline TiO2(B) as Anode Material for Sodium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A3052–A3058. [Google Scholar] [CrossRef]
- Wu, L.; Buchholz, D.; Bresser, D.; Chagas, L.G.; Passerini, S. Anatase TiO2 nanoparticles for high power sodium-ion anodes. J. Power Sources 2014, 251, 379–385. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, L.; Tan, J.; Zhou, Q.; Huang, Z.; Xia, D.; Shu, H.; Yang, X.; Wang, X. One-pot synthesis of bicrystalline titanium dioxide spheres with a core–shell structure as anode materials for lithium and sodium ion batteries. J. Power Sources 2014, 269, 37–45. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Lee, J.-H.; Abouimrane, A.; Belharouak, I.; Sun, Y.-K. Ultrafast sodium storage in anataseTiO2 nanoparticles embedded on carbon nanotubes. Nano Energy 2015, 16, 218–226. [Google Scholar] [CrossRef]
- Pérez-Flores, J.C.; Baehtz, C.; Kuhn, A.; García-Alvarado, F. Hollandite-type TiO2: A new negative electrode material for sodium-ion batteries. J. Mater. Chem. A 2014, 2, 1825–1833. [Google Scholar] [CrossRef]
- Prutsch, D.; Wilkening, M.; Hanzu, I. Long Cycle-Life Na-ion Anodes Based on Amorphous Titania Nanotubes—Interfaces and Diffusion. ACS Appl. Mater. Interfaces 2015, 7, 25757–25769. [Google Scholar] [CrossRef]
- Usui, H.; Yoshioka, S.; Wasada, K.; Shimizu, M.; Sakaguchi, H. Nb-Doped Rutile TiO2: A Potential Anode Material for Na-Ion Battery. ACS Appl. Mater. Interfaces 2015, 7, 6567–6573. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chen, Y.-M.; Zhu, Y.; Vogt, B.D. Fabrication of porous carbon/TiO2 composites through polymerization induced phase separation and use as an anode for Na-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 21011–21018. [Google Scholar] [CrossRef]
- Wen, J.-W.; Zhang, D.-W.; Zang, Y.; Sun, X.; Cheng, B.; Ding, C.-X.; Yu, Y.; Chen, C.-H. Li and Na storage behavior of bowl-like hollow Co3O4 microspheres as an anode material for lithium-ion and sodium-ion batteries. Electrochim. Acta 2014, 132, 193–199. [Google Scholar] [CrossRef]
- Jian, Z.; Liu, P.; Li, F.; Chen, M.; Zhou, H. Monodispersed hierarchical Co3O4 spheres intertwined with carbon nanotubes for use as anode materials in sodium-ion batteries. J. Mater. Chem. A 2014, 2, 13805–13809. [Google Scholar] [CrossRef]
- Klavetter, K.C.; Garcia, S.; Dahal, N.; Snider, J.L.; de Souza, J.P.; Cell, T.H.; Cassara, M.A.; Heller, A.; Humphrey, S.M.; Mullins, C.B. Li- and Na-reduction products of meso-Co3O4 form high-rate; stably cycling battery anode materials. J. Mater. Chem. A 2014, 2, 14209–14221. [Google Scholar] [CrossRef]
- Rahman, M.M.; Glushenkov, A.M.; Ramireddy, T.; Chen, Y. Electrochemical investigation of sodium reactivity with nanostructured Co3O4 for sodium-ion batteries. Chem. Commun. 2014, 50, 5057–5060. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sultana, I.; Chen, Z.; Srikanth, M.; Li, L.H.; Dai, X.J.; Chen, Y. Ex-situ electrochemical sodiation/desodiation observation of Co3O4 anchored carbon nanotubes: A high performance sodium-ion battery anode produced by a pulsed plasma in liquid. Nanoscale 2015, 7, 13088–13095. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, Z.; Sun, H.; Arandiyan, H.; Li, J.; Ahmad, M. Mesoporous Co3O4 sheets/3D graphene networks nanohybrids for high-performance sodium-ion battery anode. J. Power Sources 2015, 273, 878–884. [Google Scholar] [CrossRef]
- Sun, W.; Rui, X.; Zhu, J.; Yu, L.; Zhang, Y.; Xu, Z.; Madhavi, S.; Yan, Q. Ultrathin nickel oxide nanosheets for enhanced sodium and lithium storage. J. Power Sources 2015, 274, 755–761. [Google Scholar] [CrossRef]
- Sun, Q.; Ren, Q.-Q.; Li, H.; Fu, Z.-W. High capacity Sb2O4 thin film electrodes for rechargeable sodium battery. Electrochem. Commun. 2011, 13, 1462–1464. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Xu, Y.; Liu, Y.; Dai, Z.; Bao, J. An SbOx/Reduced Graphene Oxide Composite as a High-Rate Anode Material for Sodium-Ion Batteries. J. Phys. Chem. C 2014, 118, 23527–23534. [Google Scholar] [CrossRef]
- Hu, M.; Jiang, Y.; Sun, W.; Wang, H.; Jin, C.; Yan, M. Reversible Conversion-Alloying of Sb2O3 as a High-Capacity; High-Rate; and Durable Anode for Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 19449–19455. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liao, S.; Sun, Y.; Song, H.W.; Wang, C.X. Uniformly dispersed self-assembled growth of Sb2O3/Sb@graphene nanocomposites on a 3D carbon sheet network for high Na-storage capacity and excellent stability. J. Mater. Chem. A 2015, 3, 5820–5828. [Google Scholar] [CrossRef]
- Kim, H.; Lim, E.; Jo, C.; Yoon, G.; Hwang, J.; Jeong, S.; Lee, J.; Kang, K. Ordered-mesoporous Nb2O5/carbon composite as a sodium insertion material. Nano Energy 2015, 16, 62–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, J.; Zhang, S.; Zhu, P.; Cao, G.; Zhao, X. Ultrafine tin oxide on reduced graphene oxide as high-performance anode for sodium-ion batteries. Electrochim. Acta 2015, 151, 8–15. [Google Scholar] [CrossRef]
- Wang, Y.; Su, D.; Wang, C.; Wang, G. SnO2@MWCNT nanocomposite as a high capacity anode material for sodium-ion batteries. Electrochem. Commun 2013, 29, 8–11. [Google Scholar] [CrossRef]
- Su, D.; Ahn, H.-Y.; Wang, G. SnO2@graphene nanocomposites as anode materials for Na-ion batteries with superior electrochemical performance. Chem. Commun. 2013, 49, 3131–3133. [Google Scholar] [CrossRef]
- Shimizu, M.; Usui, H.; Sakaguchi, H. Electrochemical Na-insertion/extraction properties of SnO thick-film electrodes prepared by gas-deposition. J. Power Sources 2014, 248, 378–382. [Google Scholar] [CrossRef]
- Su, D.; Xie, X.; Wang, G. Hierarchical Mesoporous SnO Microspheres as High Capacity Anode Materials for Sodium-Ion Batteries. Chem. Eur. J. 2014, 20, 3192–3197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Lim, Y.-G.; Park, M.-S.; Chou, S.-L.; Kim, J.H.; Liu, H.-K.; Dou, S.-X.; Kim, Y.-J. Ultrafine SnO2 nanoparticle loading onto reduced graphene oxide as anodes for sodium-ion batteries with superior rate and cycling performances. J. Mater. Chem. A 2014, 2, 529–534. [Google Scholar] [CrossRef]
- Lu, Y.C.; Ma, C.; Alvarado, J.; Kidera, T.; Dimov, N.; Meng, Y.S.; Okada, S. Electrochemical properties of tin oxide anodes for sodium-ion batteries. J. Power Sources 2015, 284, 287–295. [Google Scholar] [CrossRef]
- Górka, J.; Baggetto, L.; Keum, J.K.; Mahurin, S.M.; Mayes, R.T.; Dai, S.; Veith, G.M. The electrochemical reactions of SnO2 with Li and Na: A study using thin films and mesoporous carbons. J. Power Sources 2015, 284, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, X.; Ge, M.; Rong, J.; Shen, C.; Zhang, A.; Enaya, H.A.; Zhou, C. SnO2 coated carbon cloth with surface modification as Na-ion battery anode. Nano Energy 2015, 16, 399–407. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, J.; Li, J.; Xu, Z.; Cao, L.; Ouyang, H.; Yan, J.; Qi, H. SnO2/super P nanocomposites as anode materials for Na-ion batteries with enhanced electrochemical performance. J. Alloys Compd. 2016, 658, 234–240. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, F.; Wei, X.; Jia, S.; Kang, P.; Yu, Y.; Yang, X.; Lan, J.-L. Engineering fluoride-rich interphase and inhibiting grain coarsening for highly reversible bismuth sulfide anode for sodium storage. Mater. Today Energy 2022, 28, 101084. [Google Scholar] [CrossRef]
- Chen, L.; Song, K.; Shi, J.; Zhang, J.; Mi, L.; Chen, W.; Liu, C.; Shen, C. PAANa-induced ductile SEI of bare micro-sized FeS enables high sodium-ion storage performance. Sci. China Mater. 2021, 64, 105–114. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Han, F.; Wu, H.; Zhang, F.; Zhang, G.; Liu, J. Improved Electrochemical Performance of Sodium/Potassium-Ion Batteries in Ether-Based Electrolyte: Cases Study of MoS2@C and Fe7S8@C Anodes. Adv. Mater. Interfaces 2020, 7, 2000486. [Google Scholar] [CrossRef]
- Wang, T.; Yang, K.; Shi, J.; Zhou, S.; Mi, L.; Li, H.; Chen, W. Simple synthesis of sandwich-like SnSe2/rGO as high initial coulombic efficiency and high stability anode for sodium-ion batteries. J. Energy Chem. 2020, 46, 71–77. [Google Scholar] [CrossRef]
- Ihsan-Ul-Haq, M.; Huang, H.; Wu, J.; Cui, J.; Yao, S.; Chong, W.G.; Huang, B.; Kim, J.-K. Thin solid electrolyte interface on chemically bonded Sb2Te3/CNT composite anodes for high performance sodium ion full cells. Nano Energy 2020, 71, 104613. [Google Scholar] [CrossRef]
- Xu, S.; Gao, X.; Hua, Y.; Neville, A.; Wang, Y.; Zhang, K. Rapid deposition of WS2 platelet thin films as additive-free anode for sodium ion batteries with superior volumetric capacity. Energy Storage Mater. 2020, 26, 534–542. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, X.; Song, K.; Zhang, J.; Liu, C.; Mi, L.; Wang, Y.; Chen, W. Se–C bond and reversible SEI in facile synthesized SnSe2⸦3D carbon induced stable anode for sodium-ion batteries. Electrochim. Acta 2020, 337, 135783. [Google Scholar] [CrossRef]
- Chang, X.; Ma, Y.; Yang, M.; Xing, T.; Tang, L.; Chen, T.; Guo, Q.; Zhu, X.; Liu, J.; Xia, H. In-situ solid-state growth of N; S codoped carbon nanotubes encapsulating metal sulfides for high-efficient-stable sodium ion storage. Energy Storage Mater. 2019, 23, 358–366. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, J.; Cao, C.; Yin, G.; Jiang, Z.; Ge, M.; Xiao, X.; Lee, W.-K.; Wang, J. Insights into enhanced sodium ion storage mechanism in Fe3S4: The coupling of surface chemistry; microstructural regulation and 3D electronic transport. Nano Energy 2019, 62, 384–392. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Feng, Y.; Li, W.; Peng, P.; Yao, J.; Li, M.; Jiang, C. Ultrafine CoSe nano-crystallites confined in leaf-like N-doped carbon for long-cyclic and fast sodium ion storage. Electrochim. Acta 2019, 294, 173–182. [Google Scholar] [CrossRef]
- Dong, C.; Wu, L.; He, Y.; Zhou, Y.; Sun, X.; Du, W.; Sun, X.; Xu, L.; Jiang, F. Willow-Leaf-Like ZnSe@N-Doped Carbon Nanoarchitecture as a Stable and High-Performance Anode Material for Sodium-Ion and Potassium-Ion Batteries. Small 2020, 16, 2004580. [Google Scholar] [CrossRef]
- Shi, Z.-T.; Kang, W.; Xu, J.; Sun, L.-L.; Wu, C.; Wang, L.; Yu, Y.-Q.; Yu, D.Y.W.; Zhang, W.; Lee, C.-S. In Situ Carbon-Doped Mo(Se0.85S0.15)2 Hierarchical Nanotubes as Stable Anodes for High-Performance Sodium-Ion Batteries. Small 2015, 11, 5667–5674. [Google Scholar] [CrossRef]
- Ahmed, B.; Anjum, D.H.; Hedhili, M.N.; Alshareef, H.N. Mechanistic Insight into the Stability of HfO2–Coated MoS2 Nanosheet Anodes for Sodium Ion Batteries. Small 2015, 11, 4341–4350. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, G.; Lin, Y.; Wang, Y.; Ou, X.; Zheng, F.; Yang, C.; Wang, J.-H.; Liu, M. Enhancing Sodium Ion Battery Performance by Strongly Binding Nanostructured Sb2S3 on Sulfur-Doped Graphene Sheets. ACS Nano 2016, 10, 10953–10959. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Pang, W.K.; Zhang, C.; Yang, J.; Chen, Z.; Liu, H.K.; Guo, Z. Enhanced Sodium-Ion Battery Performance by Structural Phase Transition from Two-Dimensional Hexagonal-SnS2 to Orthorhombic-SnS. ACS Nano 2014, 8, 8323–8333. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Lee, Y.; Kim, Y.; Lee, J.; Lee, S.-M.; Lee, K.T.; Choi, N.-S. Interfacial architectures based on a binary additive combination for high-performance Sn4P3 anodes in sodium-ion batteries. J. Mater. Chem. A 2015, 3, 8332–8338. [Google Scholar] [CrossRef]
- Li, W.; Chou, S.-L.; Wang, J.-Z.; Kim, J.H.; Liu, H.-K.; Dou, S.-X. Sn4+xP3@Amorphous Sn-P Composites as Anodes for Sodium-Ion Batteries with Low Cost; High Capacity; Long Life; and Superior Rate Capability. Adv. Mater. 2014, 26, 4037–4042. [Google Scholar] [CrossRef]
- Nazarian-Samani, M.; Nazarian-Samani, M.; Haghighat-Shishavan, S.; Kim, K.-B. Predelithiation-driven ultrastable Na-ion battery performance using Si; P-rich ternary M-Si-P anodes. Energy Storage Mater. 2022, 49, 421–432. [Google Scholar] [CrossRef]
- Li, M.; Feng, N.; Liu, M.; Cong, Z.; Sun, J.; Du, C.; Liu, Q.; Pu, X.; Hua, W. Hierarchically porous carbon/red phosphorus composite for high-capacity sodium-ion battery anode. Sci. Bull. 2018, 63, 982–989. [Google Scholar] [CrossRef]
- Kaushik, S.; Matsumoto, K.; Orikasa, Y.; Katayama, M.; Inada, Y.; Sato, Y.; Gotoh, K.; Ando, H.; Hagiwara, R. Vanadium diphosphide as a negative electrode material for sodium secondary batteries. J. Power Sources 2021, 483, 229182. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, K.; Yang, J.; Lau, V.W.; Lee, G.; Park, M.; Kang, Y.-M. Engineering Solid Electrolyte Interphase on Red Phosphorus for Long-Term and High-Capacity Sodium Storage. Chem. Mater. 2020, 32, 448–458. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, X.; Yu, X. A core–shell structure of polydopamine-coated phosphorus–carbon nanotube composite for high-performance sodium-ion batteries. Nanoscale 2018, 10, 16675–16682. [Google Scholar] [CrossRef]
- Liu, S.; Feng, J.; Bian, X.; Liu, J.; Xu, H.; An, Y. A controlled red phosphorus@Ni–P core@shell nanostructure as an ultralong cycle-life and superior high-rate anode for sodium-ion batteries. Energy Environ. Sci. 2017, 10, 1222–1233. [Google Scholar] [CrossRef]
- Muñoz-Márquez, M.A.; Zarrabeitia, M.; Castillo-Martínez, E.; Eguía-Barrio, A.; Rojo, T.; Casas-Cabanas, M. Composition and Evolution of the Solid-Electrolyte Interphase in Na2Ti3O7 Electrodes for Na-Ion Batteries: XPS and Auger Parameter Analysis. ACS Appl. Mater. Interfaces 2015, 7, 7801–7808. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Manohar, C.V.; Mitra, S. A simple approach to minimize the first cycle irreversible loss of sodium titanate anode towards the development of sodium-ion battery. Nano Energy 2020, 70, 104520. [Google Scholar] [CrossRef]
- Gangaja, B.; Nair, S.; Santhanagopalan, D. Solvent-controlled solid-electrolyte interphase layer composition of a high performance Li4Ti5O12 anode for Na-ion battery applications. Sustain. Energy Fuels 2019, 3, 2490–2498. [Google Scholar] [CrossRef]
- Slamet, P.; Widayatno, W.B.; Subhan, A.; Prihandoko, B. Synthesis of Na2Ti3O7–based anode for sodium-ion battery using solid state reaction method. IOP Conf. Series Mater. Sci. Eng. 2018, 432, 012058. [Google Scholar] [CrossRef]
- Qi, Y.; Gao, W.; Wang, H.; Liu, D.; Deng, J.; Guo, B.; Bao, S.; Xu, M. Na3(TiOPO4)2F microspheres as a long-life anode for Na-ion batteries. Chem. Eng. J. 2020, 402, 126118. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Z.; Mentbayeva, A.; Yuan, G.; Wang, B.; Wang, H.; Wang, G. Enhanced electrochemical performance of hollow heterostructured carbon encapsulated zinc metastanate microcube composite for lithium-ion and sodium-ion batteries. Electrochim. Acta 2019, 312, 31–44. [Google Scholar] [CrossRef]
- Galceran, M.; Rikarte, J.; Zarrabeitia, M.; Pujol, M.C.; Aguilό, M.; Casas-Cabanas, M. Investigation of NaTiOPO4 as Anode for Sodium-Ion Batteries: A Solid Electrolyte Interphase Free Material? ACS Appl. Energy Mater. 2019, 2, 1923–1931. [Google Scholar] [CrossRef]
- Brennhagen, A.; Cavallo, C.; Wragg, D.S.; Vajeeston, P.; Sjåstad, A.O.; Koposov, A.Y.; Fjellvåg, H. Operando XRD studies on Bi2MoO6 as anode material for Na-ion batteries. Nanotechnology 2022, 33, 185402. [Google Scholar] [CrossRef]
- van Dinter, J.; Grantz, D.; Bitter, A.; Bensch, W. A Combined Sodium Intercalation and Copper Extrusion Mechanism in the Thiophosphate Family: CuCrP2S6 as Anode Material in Sodium-Ion Batteries. ChemElectroChem 2022, 9, e202200018. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Li, F.; Cheng, F.; Chen, J. Bulk Bismuth as a High-Capacity and Ultralong Cycle-Life Anode for Sodium-Ion Batteries by Coupling with Glyme-Based Electrolytes. Adv. Mater. 2017, 29, 1702212. [Google Scholar] [CrossRef]
- Chen, H.-C.; Patra, J.; Lee, S.-W.; Tseng, C.-J.; Wu, T.-Y.; Lind, M.-H.; Chang, J.-K. Electrochemical Na+ storage properties of SnO2/graphene anodes in carbonate-based and ionic liquid electrolytes. J. Mater. Chem. A 2017, 5, 13776–13784. [Google Scholar] [CrossRef]
- Li, C.-Y.; Patra, J.; Yang, C.-H.; Tseng, C.-M.; Majumder, S.B.; Dong, Q.-F.; Chang, J.-K. Electrolyte Optimization for Enhancing Electrochemical Performance of Antimony Sulfide/Graphene Anodes for Sodium-Ion Batteries—Carbonate-Based and Ionic Liquid Electrolytes. ACS Sustain. Chem. Eng. 2017, 5, 8269–8276. [Google Scholar] [CrossRef]
- Tang, J.; Kye, D.K.; Pol, V.G. Ultrasound-assisted synthesis of sodium powder as electrode additive to improve cycling performance of sodium-ion batteries. J. Power Sources 2018, 396, 476–482. [Google Scholar] [CrossRef]
- Matsumoto, K.; Hwang, J.; Kaushik, S.; Chen, C.-Y.; Hagiwara, R. Advances in sodium secondary batteries utilizing ionic liquid electrolytes. Energy Environ. Sci. 2019, 12, 3247–3287. [Google Scholar] [CrossRef]
- Wang, C.-H.; Yang, C.-H.; Chang, J.-K. Suitability of ionic liquid electrolytes for roomtemperature sodium-ion battery applications. Chem. Commun. 2016, 52, 10890–10893. [Google Scholar] [CrossRef]
- Sangster, J.; Pelton, A.D. The Na-Si (Sodium-Silicon) System. J. Phase Equil. 1992, 13, 67–69. [Google Scholar] [CrossRef]
- Ellis, L.D.; Wilkes, B.N.; Hatchard, T.D.; Obrovac, M.N. In Situ XRD Study of Silicon; Lead and Bismuth Negative Electrodes in Nonaqueous Sodium Cells. J. Electrochem. Soc. 2014, 161, A416–A421. [Google Scholar] [CrossRef]
- Bian, H.; Zhang, J.; Yuen, M.-F.; Kang, W.; Zhan, Y.; Yu, D.Y.W.; Xu, Z.; Li, Y.Y. Anodic nanoporous SnO2 grown on Cu foils as superior binder-free Na-ion battery anodes. J. Power Sources 2016, 307, 634–640. [Google Scholar] [CrossRef]
- An, C.; Yuan, Y.; Zhang, B.; Tang, L.; Xiao, B.; He, Z.; Zheng, J.; Lu, J. Graphene Wrapped FeSe2 Nano-Microspheres with High Pseudocapacitive Contribution for Enhanced Na-Ion Storage. Adv. Energy Mater. 2019, 9, 1900356. [Google Scholar] [CrossRef]
- Kulova, T.L.; Skundin, A.M. The Use of Phosphorus in Sodium-Ion Batteries (A Review). Russ. J. Electrochem. 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Xu, L.; Ma, L.; Chen, X. Solvothermal preparation of tin phosphide as a long-life anode for advanced lithium and sodium ion batteries. J. Power Sources 2016, 304, 346–353. [Google Scholar] [CrossRef]
- Kulova, T.L.; Kudryashova, Y.O.; Kuz’mina, A.A.; Skundin, A.M.; Stenina, I.A.; Chekannikov, A.A.; Yaroslavtsev, A.B.; Libich, J. Study of degradation of Na2Ti3O7-based electrode during cycling. J. Solid State Electrochem. 2019, 23, 455–463. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, F.; Qian, Y.; Ji, H. High-Performance P2-Na0.70Mn0.80Co0.15Zr0.05O2 Cathode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 42380–42386. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.-Q.; Yu, F.-D.; Zheng, L.-L.; Yin, B.-S.; Wang, Z.-B.; Ke, K. Spinel (Ni0.4Co0.4Mn0.2)3O4 nanoparticles as conversion-type anodes for Li- and Na-ion batteries. Ceram. Int. 2019, 45, 7552–7559. [Google Scholar] [CrossRef]
- Garg, U.; Rexhausen, W.; Smith, N.; Harris, J.; Qu, D.; Guptasarma, P. Crystal structure stabilization; electrochemical properties; and morphology of P2-type Na0.67Mn0.625Fe0.25Ni0.125O2 for Na-ion battery cathodes. J. Power Sources 2019, 431, 105–113. [Google Scholar] [CrossRef]
- Wang, L.; Yan, C.; Wang, Z.; Zhuang, Q. Facile synthesis of Na2FexFe1−x(SO4)2(OH)x material as a cathode for sodium-ion batteries. Ionics 2019, 25, 2509–2518. [Google Scholar] [CrossRef]
- Jin, Y.; Le, P.M.; Gao, P.; Xu, Y.; Xiao, B.; Engelhard, M.H.; Cao, X.; Vo, T.D.; Hu, J.; Zhong, L.; et al. Low-solvation electrolytes for high-voltage sodium-ion batteries. Nat. Energy 2022, 7, 718–725. [Google Scholar] [CrossRef]
- Cheng, Z.; Mao, Y.; Dong, Q.; Jin, F.; Shen, Y.; Chen, L. Fluoroethylene Carbonate as an Additive for Sodium-Ion Batteries: Effect on the Sodium Cathode. Acta Phys.-Chim. Sin. 2019, 35, 868–875. [Google Scholar] [CrossRef]
- Fu, H.; Xia, M.; Qi, R.; Liang, X.; Zhao, M.; Zhang, Z.; Lu, X.; Cao, G. Improved rate performance of Prussian blue cathode materials for sodium ion batteries induced by ion-conductive solid-electrolyte interphase layer. J. Power Sources 2018, 399, 42–48. [Google Scholar] [CrossRef]
- Manohar, C.V.; Forsyth, M.; MacFarlane, D.R.; Mitra, S. Role of N-Propyl-N-Methyl Pyrrolidinium bis(trifluoromethanesulfonyl)imide as an Electrolyte Additive in Sodium Battery Electrochemistry. Energy Technol. 2018, 6, 2232–2237. [Google Scholar] [CrossRef]
- Wu, F.; Zhu, N.; Bai, Y.; Li, Y.; Wang, Z.; Ni, Q.; Wang, H.; Wu, C. Unveil the mechanism of solid electrolyte interphase on Na3V2(PO4)3 formed by a novel NaPF6/BMITFSI ionic liquid electrolyte. Nano Energy 2018, 51, 524–532. [Google Scholar] [CrossRef]
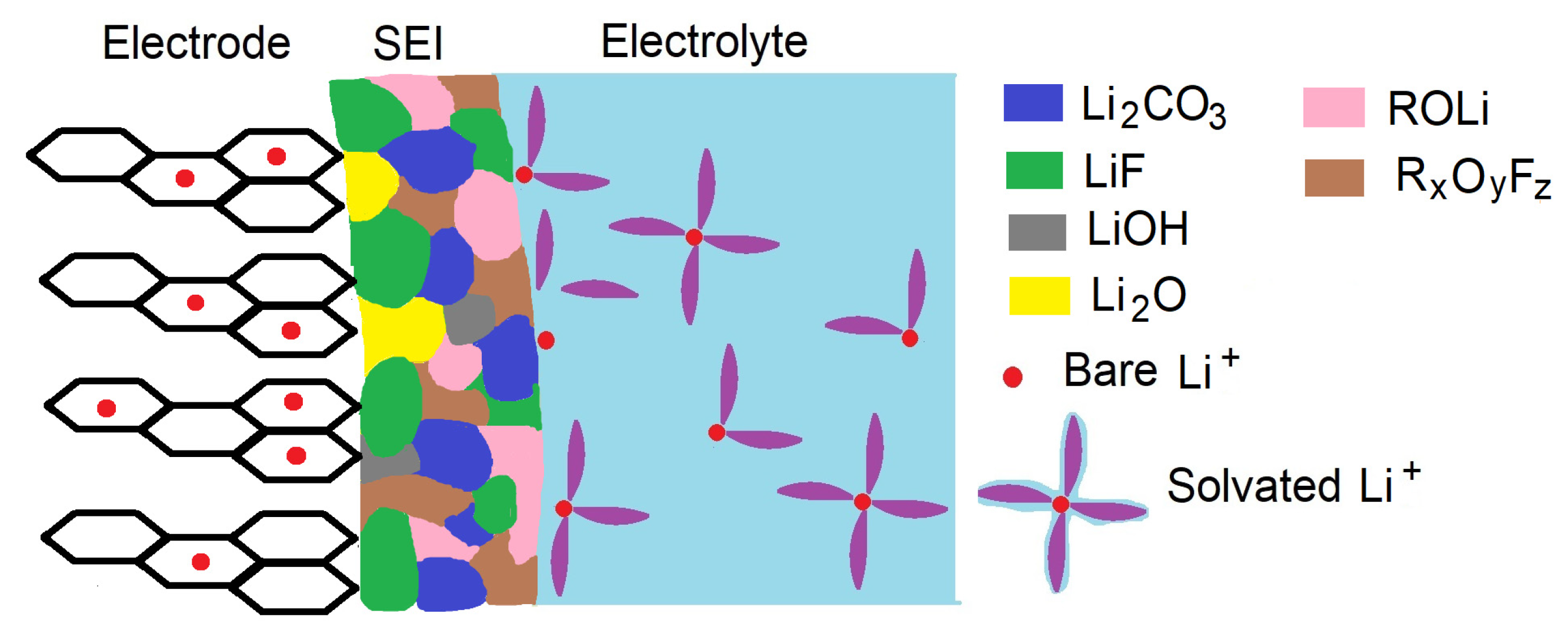
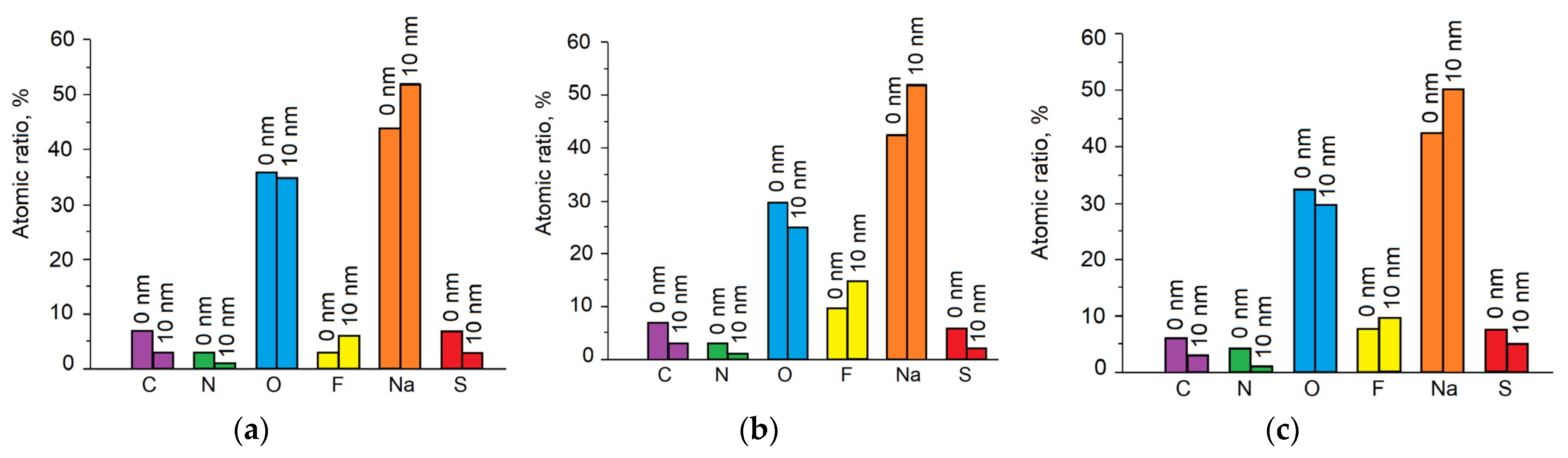
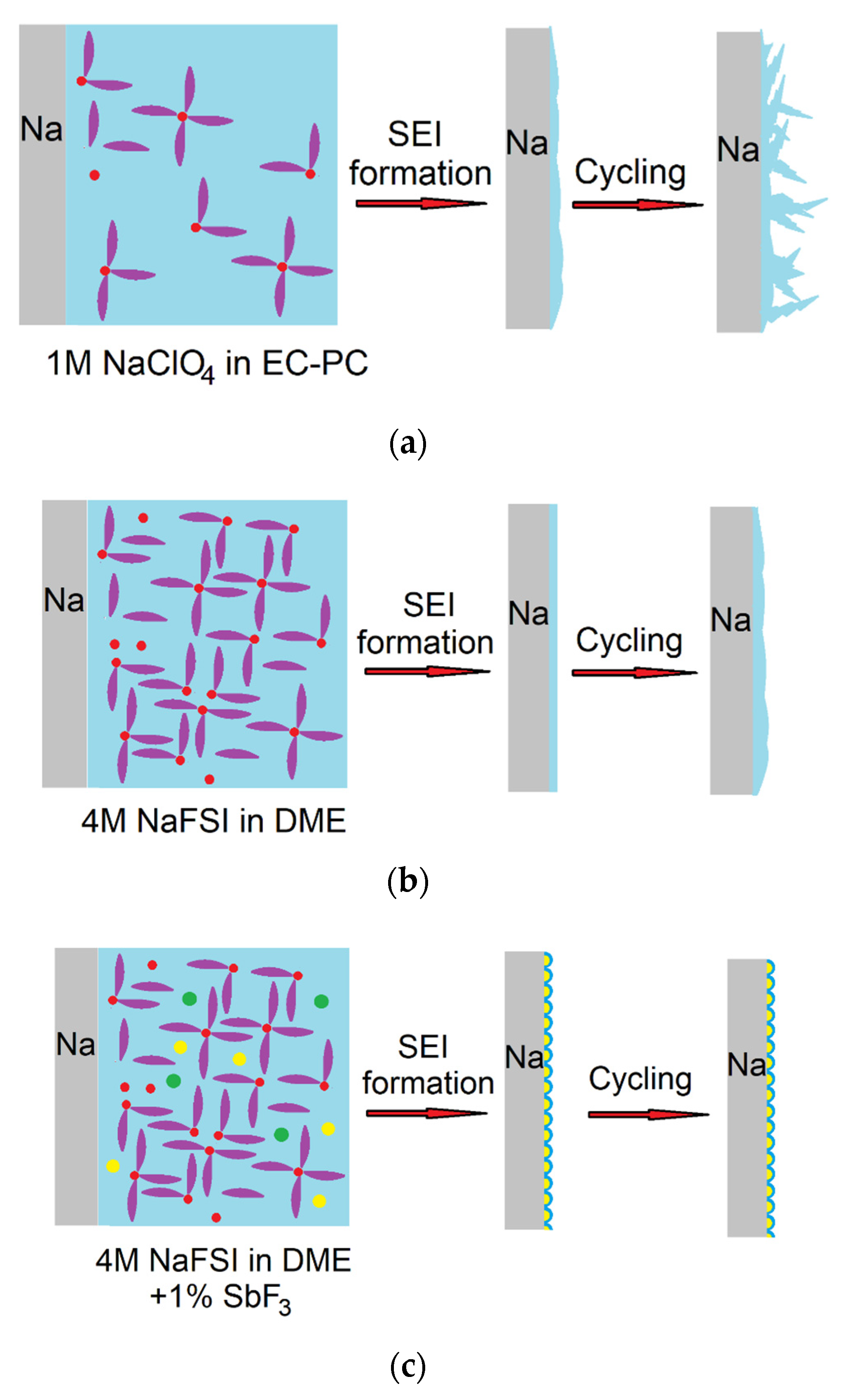
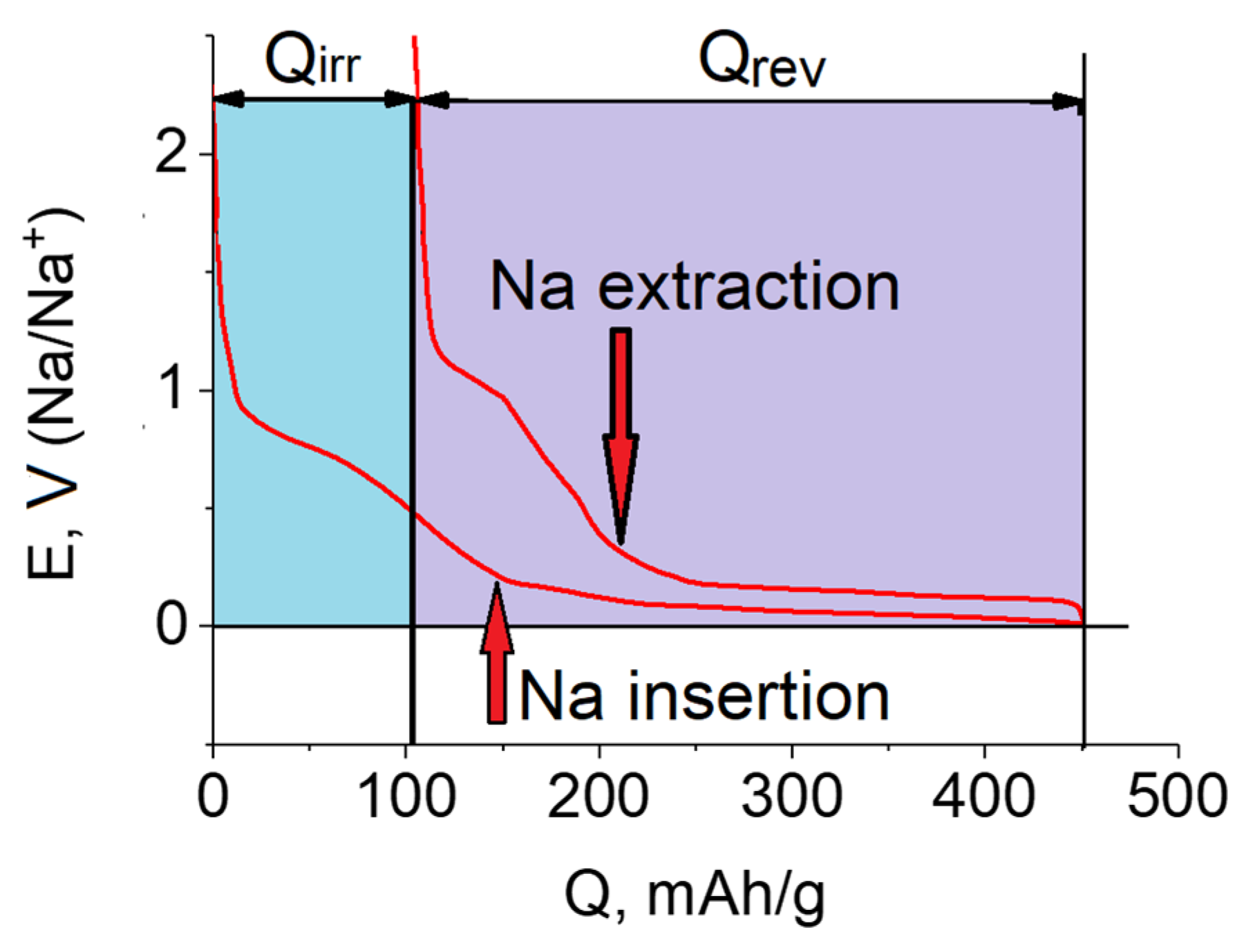


| Electrode Material | Electrolyte | SEI Composition | Qc/Qa 1st Cycle | Ref. |
|---|---|---|---|---|
| HC | 1 M NaClO4 in EC:DEC (3:7) | - | 350/300 | [60] |
| HC | 1 M NaPF6 in DEC:EC (1:1) | NaF, Na2CO3, NaxPFy, ROCO2Na | - | [69] |
| 1 M NaClO4 in DEC:EC (1:1) | NaCl, Na2CO3, NaxPFy, ROCO2Na, PEO | - | ||
| 1 M NaTFSI in DEC:EC (1:1) | NaF, ROCO3Na, RONa, PEO | - | ||
| 1 M NaFTFSI in DEC:EC (1:1) | NaF, ROCO3Na, RONa, PEO | - | ||
| 1 M NaFSI in DEC:EC (1:1) | NaF, Na2SO4, Na2S, ROCO3Na, RONa, PEO | - | ||
| HC | 1 M NaPF6 in EC:DMC (1:1) + 3% FEC | NaF, Na2CO3, R–COONa, NaO2CO-C2H4-OCO2Na | 320/220 | [70] |
| 1 M NaTFSI in EC:DMC (1:1) +3% FEC | Na2CO3, NaF, NaO2CO-C2H4-OCO2Na | 275/175 | ||
| HC | 0.5 M NaBPh4 in DME | NaF | 275/261 | [71] |
| 0.5 M NaPF6 in DME | NaF | 276/261 | ||
| 0.5 M NaFSA in DME | Na2S | 270/244 | ||
| 0.5 M NaTFSA in DME | NaF | 234/207 | ||
| 1 M NaPF6 in EC:DEC (1:1) | NaF | 271/251 | ||
| HC | 1M NaClO4 PC:EC (1:1) | Na2CO3, (CH2OCO2Na)2, Na2CO3, R–OCO2Na | 275/190 | [74] |
| 1 M NaBF4 in tetraglyme | R–ONa, -C-O-C-, CH3OCH2CH2O-, O–CH2– | 320/278 | ||
| HC | 1 M NaODFB in DME | RCH2ONa; groups C=O/B–O, groups B–F; B–O, Na2CO3 | 290/175 | [75] |
| 1 M NaPF6 in DME | NaF; Na2CO3; RCH2ONa | 265/250 | ||
| HC | 1 M NaClO4 in EC:PC:DMC (0.45:0.45:0.1) | Na2CO3, ROCO2Na, R–CH2–OCO2Na, PEO | 560/360 | [77,79] |
| HC | 1 M NaTFSI in PC + 3% FEC | Na2CO3, NaF, sodium organic salts | 570/220 | [78] |
| HC | 1M NaClO4 in EC:PC (1:1) + 5% FEC | PEO; Na2CO3; NaF; groups C–F | 910/301 | [80] |
| 1M NaClO4 in TEGDME | Sodium alkoxides; Na2CO3; Na2CH2R | 1050/363 | ||
| HC | 1 M NaPF6 in PC + 0.5% FEC | Na2CO3, NaF, Na-O-(C=O)-O-CH2-R, Na-O-(C=O)-O-R, Na2CO3, NaF | 280/240 | |
| 1 M NaPF6 in PC:EC + 0.5% FEC | Na-O-(C=O)-O-CH2-R, Na-O-(C=O)-O-R, NaxPFy, NaxPOyFz | 280/240 | ||
| HC | 1 M NaPF6 in DME + 0.5% VC | Na2CO3, RCO3Na, NaF, -OCO2CH=CH)n- | 210/200 | |
| 1 M NaPF6 in DEGDME + 0.5% VC | ||||
| HC | 1 M NaClO4 in EC:DEC (1:1) | Na2CO3, RONa, ROCO2Na, (CH2–CH2–O–)n | 400/325 | [101] |
| HC | 1 M NaPF6 in EC:DEC (1:2) | NaF, Na2CO3, NaxPFyOz | 335/235 | [109] |
| Graphite | 1 M NaPF6 in EC:DEC (1:1) | CH3CH2OCO2Na, (CH2OCO2Na)2), Na2CO3, NaF | 120/30 | [120] |
| 1 M NaPF6 in diglyme | CH3OCH2CH2ONa, CH3CH2OCH2CH2ONa, Na2CO3, NaF | 260/160 | ||
| Sb | 1 M NaClO4 in PC | Na2CO3, NaF, alkylcarbonates | 714/550 | [146] |
| 1 M NaPF6 in EC:DMC | Na2CO3, NaF, alkylcarbonates | 740/620 | ||
| 1 M NaPF6 in PC + 5% FEC | Na2CO3, NaF, alkylcarbonates | 735/580 | ||
| Sn4P3 | 1 M NaClO4 in EC:PC (1:1) + 5% FEC | Na2CO3, NaF | 720/560 | [244] |
| 1 M NaClO4 in EC:PC (1:1) + 5% FEC + 0.5% in TMSP | Na2CO3, NaF, SiF | 880/680 | ||
| Na2Ti3O7 | 1 M NaClO4) in EC:PC (1:1) | Na2CO3, NaCO3R, NaF, NaCl, NaOR, PEO, poly(ethylene oxide)s | 385/170 | [252] |
| FeS | 1 M NaCF3SO3 in diglyme | Na2CO3, Na2CO2R, NaF | 600/500 | [230] |
| MoS2@C | 1 M NaPF6 in EC:DEC (1:1) | RONa, Na2CO3, ROCOONa, RCH2ONa | 500/450 | [231] |
| Na2Ti3O7 | 1 NaClO4 in EC:PC + 1 % FEC | NaF, Na2O, Na2CO3, NaHCO3, ROCO2Na | 250/200 | [253] |
| SnSe2 | 1 M LiPF6 in EC:DMC (1: 1) | NaF, Na2CO3, polyethers, Se-O | 750/525 | [235] |
| TiO2 | 1 M NaClO4 in EC:PC (1:1) | Na2CO3, ROCO2Na | -/200 | [185] |
| Ni3S2@NS-CNTs | 1.0 M NaSO3CF3 in diglyme | NaF, Na2CO3, Na2SO3, organic polyethers, C-Fx components | 470/435 | [236] |
| TiO2 | 1 M NaPF6 EC:DEC (1:1) + 3% VC | Polycarbonates, Alkyl carbonates | 350/160 | [187] |
| 1 M NaPF6 in diglyme | Polyethers | 320/150 | ||
| Fe3S4 | 1.0 M NaClO4 in DMC:EC (1:1) | RCH2ONa, Na2CO3, NaOH | 600/530 | [237] |
| NaTiOPO4 | 1 M NaClO4 in EC:PC (1:1) | Hydrocarbons, alkyl-carbonates, carbonates, NaF, ethers | 110/80 | [258] |
| Li4Ti5O12 | 1 M NaPF6 in diglyme | RCH2ONa, ROCO3Na, NaF, Na2CO3, R–CO3 | 300/160 | [254] |
| 1 M NaPF6 in EC:DMC (3:7) | NaF, CFx, Li2CO3 | 275/140 | ||
| Sn | 1 M NaPF6 in diglyme | RCH2ONa, Na2CO3, NaOH, Na2O, NaF | 850/780 | [165] |
| Sn films | 1 M NaClO4 in PC | Na2CO3, NaCl | 900/800 | [166] |
| Graphite | 1 M NaFSI in TEGDME | NaF, polyethers | 160/110 | [141] |
| Fe2O3 | NaClO4 in EC:DEC (2:1) | NaOH, Na2CO3, alkyl carbonates ROCO2Li | - | [189] |
| Sb | 1 M NaClO4 in PC + 5% FEC | NaOH, Na2O, NaF, NaCl | 770/600 | [157] |
| rGO | 1 M sodium triflate (NaOTf) in (EC-DEC (1:1)) | Na2CO3, Na2CO2R, polyesters, RSO3Na, NaF | 1200/500 | [143] |
| 1 M sodium triflate (NaOTf) in diglym | CF3, Na2CO3, Na2CO2R, polyesters, RSO3Na, NaF | 700/650 | ||
| Bi | 1M NaPF6 in diglyme | R-COO-Na, RCH2ONa, Na2CO3, sodium alkycarbonates, polyesters | 420/400 | [261] |
| meso-porous Co3O4 | 1 M NaPF6 in EC:DEC + 5% FEC | R-CH2-OCO2Na, R-CH2-OCO2Na, Na2CO3, NaOH, NaF | 790/750 | [212] |
| 1 M NaPF6 in FEC:DEC | R-CH2-OCO2Na, R-CH2-OCO2Na, Na2CO3, NaOH, NaF | 820/750 | ||
| Na3V2(PO4)3 | 0.25 M NaPF6-incorporated in 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl) imide (BMITFSI IL) | NaF, NaOH, Na2SO4, Na2S2O7 | 108/135 | [282] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulova, T.L.; Skundin, A.M. Electrode/Electrolyte Interphases of Sodium-Ion Batteries. Energies 2022, 15, 8615. https://doi.org/10.3390/en15228615
Kulova TL, Skundin AM. Electrode/Electrolyte Interphases of Sodium-Ion Batteries. Energies. 2022; 15(22):8615. https://doi.org/10.3390/en15228615
Chicago/Turabian StyleKulova, Tatiana L., and Alexander M. Skundin. 2022. "Electrode/Electrolyte Interphases of Sodium-Ion Batteries" Energies 15, no. 22: 8615. https://doi.org/10.3390/en15228615
APA StyleKulova, T. L., & Skundin, A. M. (2022). Electrode/Electrolyte Interphases of Sodium-Ion Batteries. Energies, 15(22), 8615. https://doi.org/10.3390/en15228615







