Vanadium Oxide–Conducting Polymers Composite Cathodes for Aqueous Zinc-Ion Batteries: Interfacial Design and Enhancement of Electrochemical Performance
Abstract
:1. Introduction
2. Conducting Polymer-Doped Vanadium Oxide-Based Cathodes for AZIBs
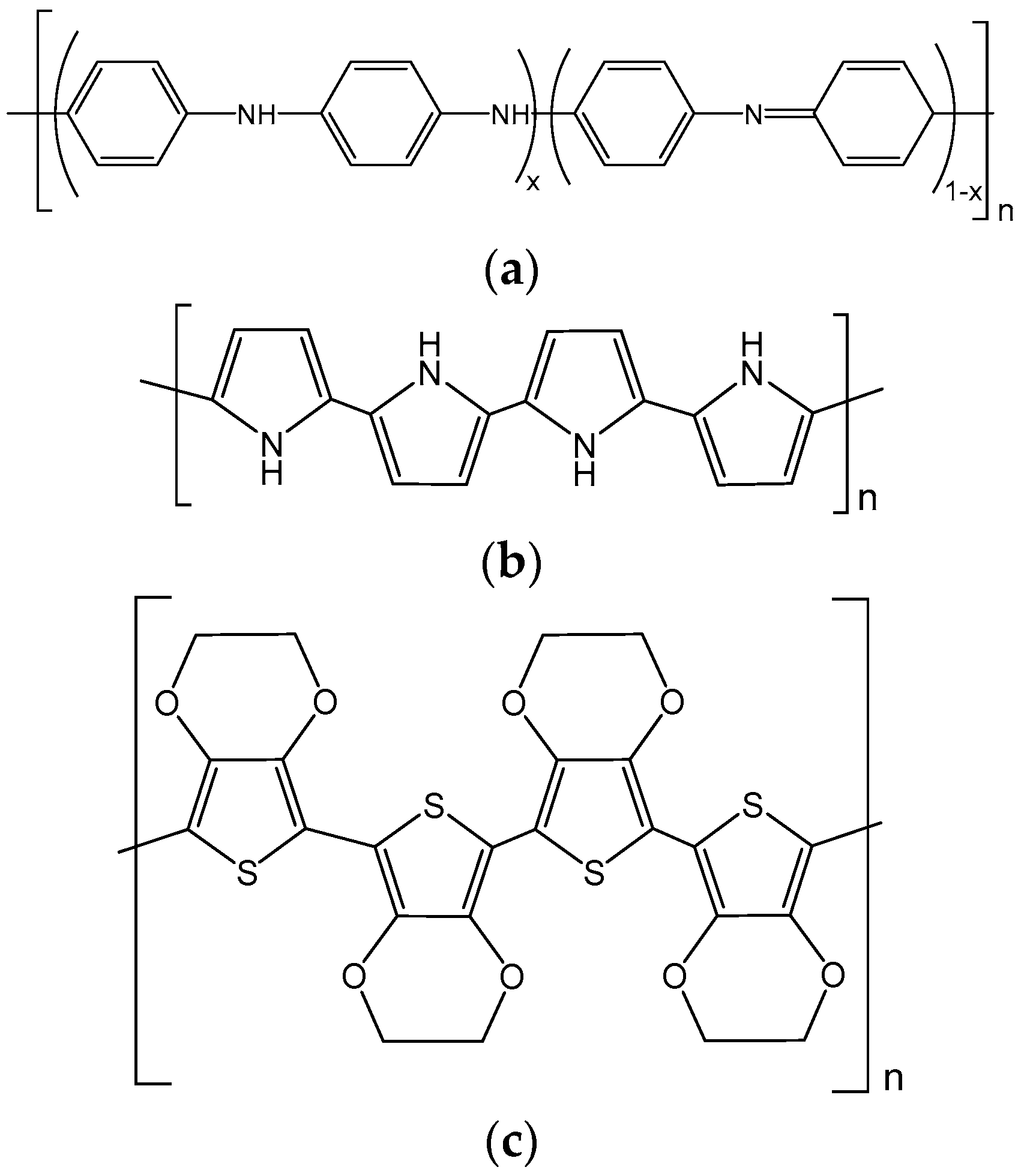
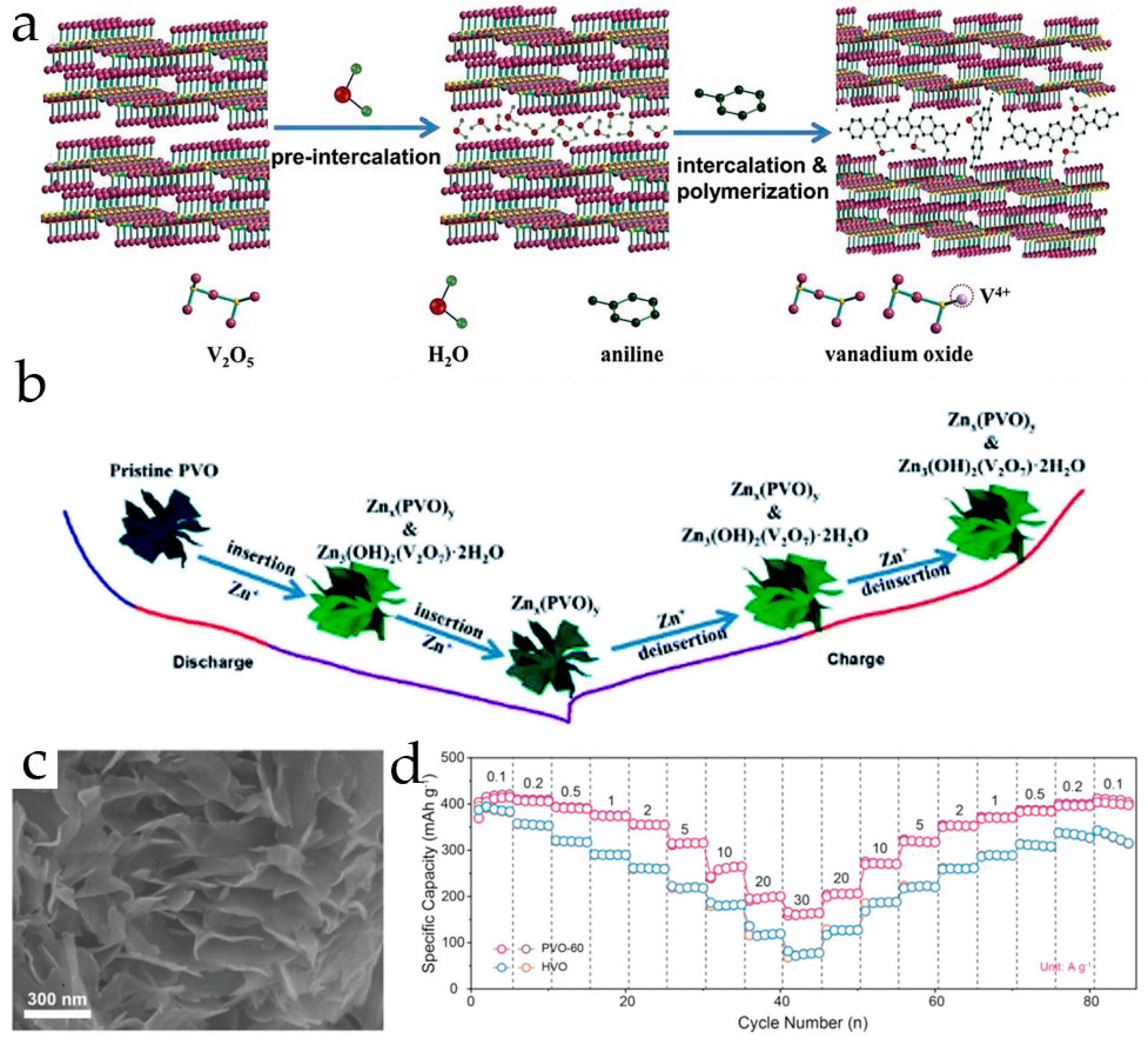
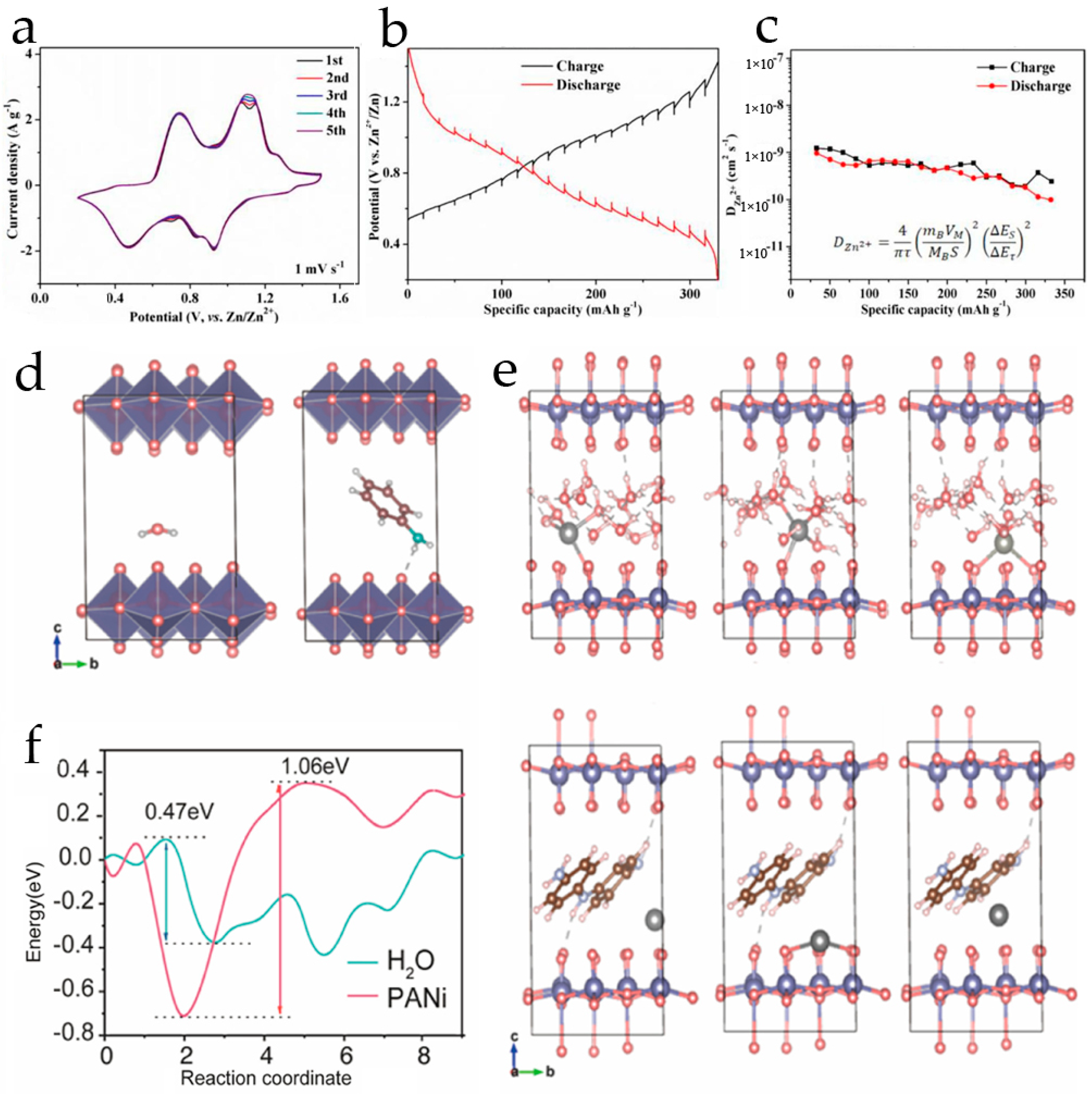
2.1. Polyaniline-Modified Vanadium-Based Cathodes
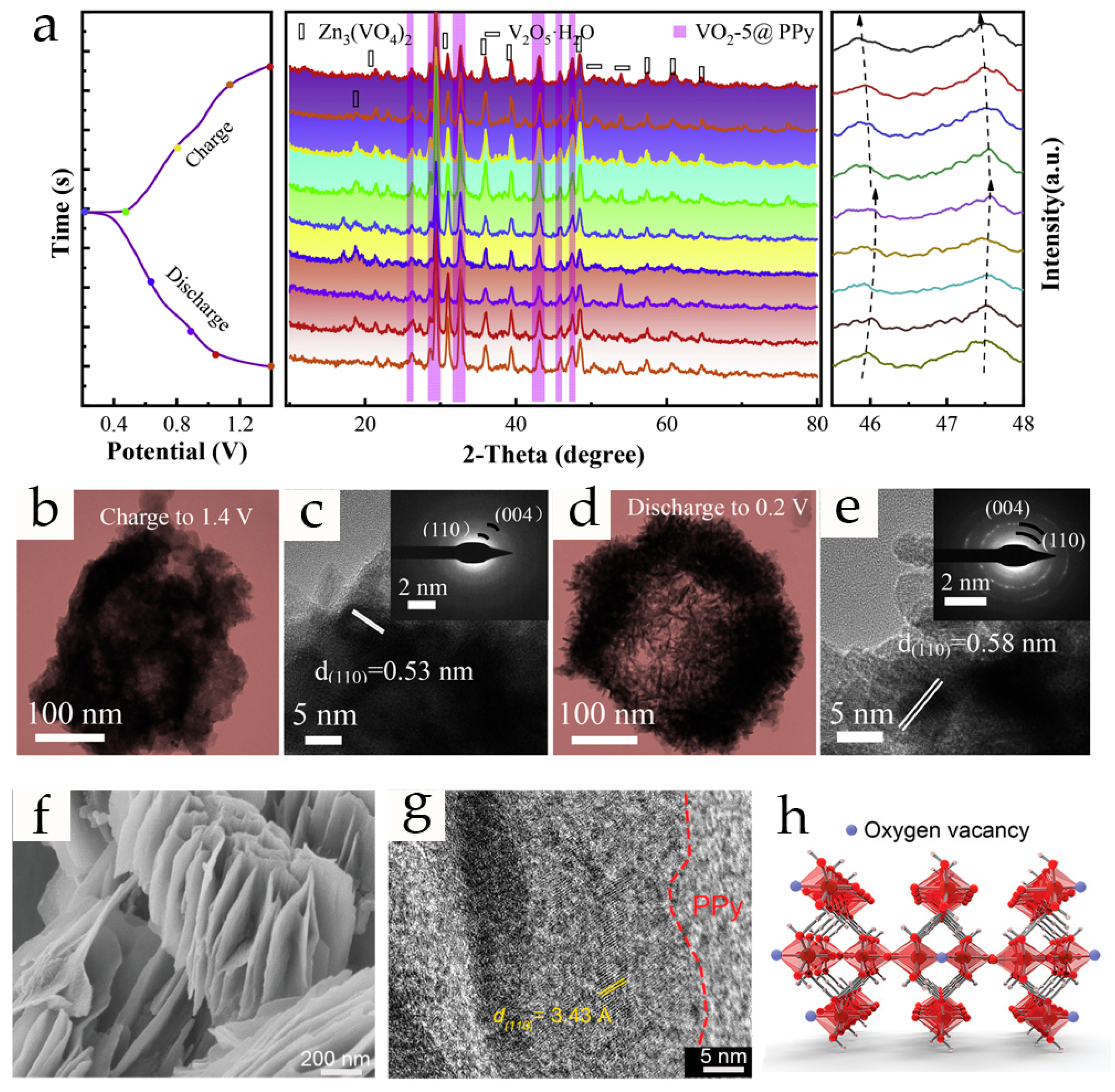
2.2. Polypyrrole-Modified Vanadium-Based Cathodes
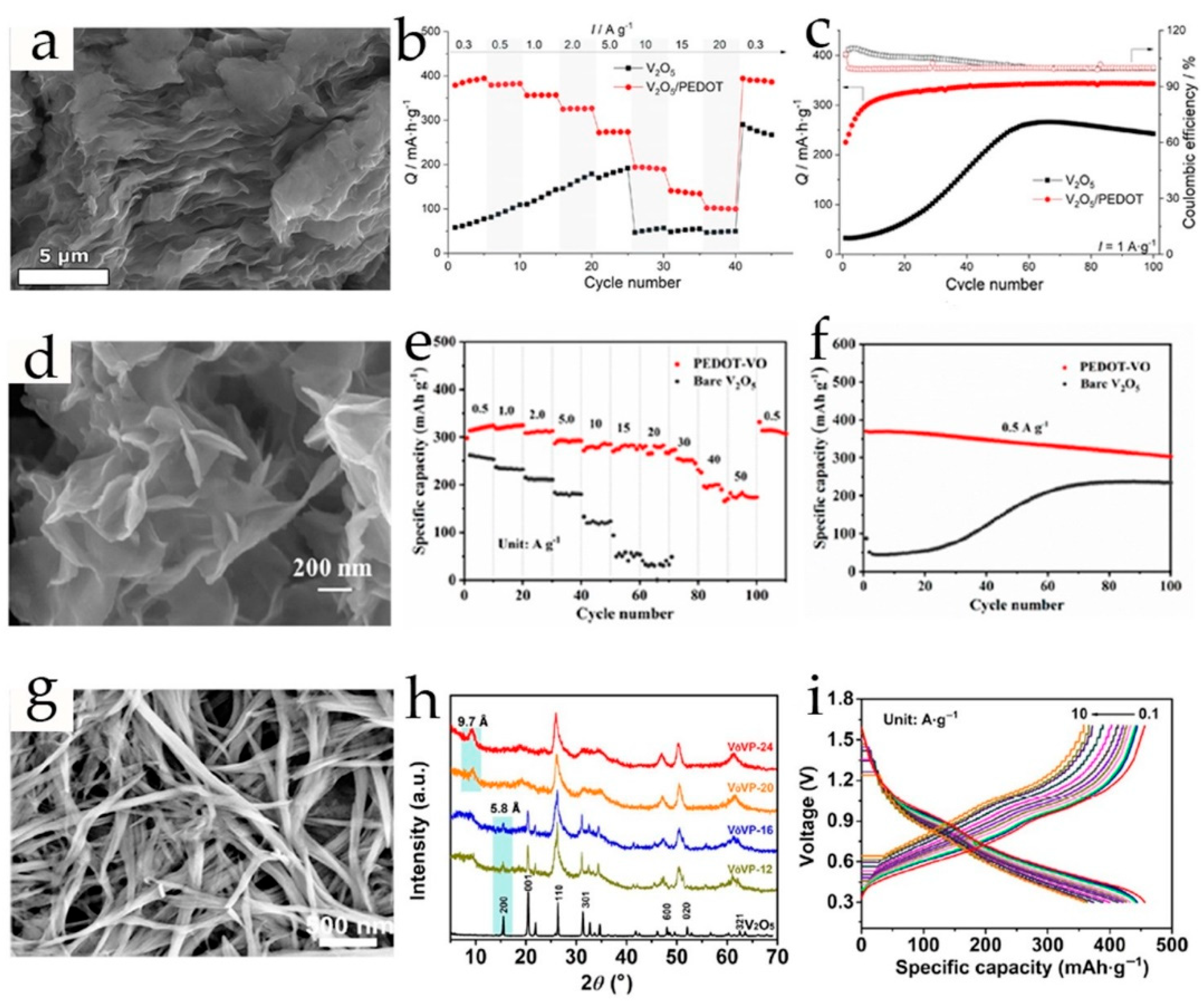
2.3. Poly-3,4-Ethylenedioxythiophene-Modified Vanadium-Based Cathodes
3. Conclusions and Outlook
| Material | Synthesis Method | Morphology/Interlayer Spacing, Å | Electrolyte | Specific Capacity, mAh g−1 (Current Density, A·g−1) | Capacity Retention, (Number of Cycles and Current, A·g−1) | ΔE, V | Ref. |
|---|---|---|---|---|---|---|---|
| PANI-V2O5·nH2O (PVO) | in situ oxidative/intercalative polymerization | rose-like/14.02 | 3 M Zn(CF3SO3)2 | 420.4 (0.5); 400 (5); 288 (20) | 87.5% (600, 5) | 0.4−1.6 | [53] |
| V2O5@PANI | chemical oxidation polymerization | uniform 3D porous | 3 M Zn(CF3SO3)2 | 361 (0.1); 283 (1); 201 (6) | 93.8% (1000, 5) | 0.3−1.6 | [67] |
| PANI-V2O5 | in situ hydrothermal reaction | 13.9 | 3 M Zn(CF3SO3)2 | 375.2 (1); 264.6 (10); 197.1 (20) | 97.6% (2000, 20) | 0.2−1.6 | [60] |
| PANI-V2O5 | one-pot hydrothermal method (140 °C) | nanosheets/14.2 | 3 M Zn(CF3SO3)2 | 360 (0.5); 272 (5); 216 (10) | 90% (2000, 5) | 0.4−1.4 | [76] |
| PANI-V2O5 | one-pot hydrothermal method (120 °C) | nanosheets/14.2 | 3 M Zn(CF3SO3)2 | 297 (0.5); 199 (5); 133 (10) | 85% (100, 5) | 0.4−1.4 | [76] |
| PANI/V2O5 | mild molecule-exchange reaction at room temperature | 3D sponge-like morphology/13.82 | 3 M Zn(CF3SO3)2 | 353.6 (0.1); 278 (4) | 87.5% (100, 0.2) | 0.2−1.5 | [54] |
| PANI-VOH | interface-intercalation method | 3D sponge-like morphology/14.1 | 3 M Zn(CF3SO3)2 | 363 (0.1) | ~49% (2000, 5) | 0.2−1.5 | [77] |
| PANI-VOH | low-temperature hydrothermal process | agglomerated corrugated nanosheets, porous/14.2 | 3 M Zn(CF3SO3)2 + 6 M LiTFSI (water-in-salt) | 346 (0.3); 323 (1); 186 (1) | 80% (800, 1) | 0.4−1.6 | [80] |
| (PANI)x V2O5 | self-assembly process | 3D network of nanosheets/13.9 | 3 M Zn(CF3SO3)2 | 350 (0.1); 250 (1); 190 (5) | ~90% (100, 0.1) | 0.3−1.6 | [63] |
| PANI/V2O5 | hydrothermal method | nanosheets/13.67 | 2 M Zn(CF3SO3)2 | 278 (0.5); 264 (1); 156 (10) | 120.5% (100, 1) | 0.4−1.4 | [104] |
| PANI/V2O5 (PVO) | hydrothermal method | aggregated flakes/14.1 | 2 M Zn(CF3SO3)2 | 356 (0.1); ~290 (5); 235 (20) | 96.3% (1000, 5) | 0.3−1.6 | [62] |
| (V2O5-x)/PANI | intercalation–polymerization method | 2D nanosheet/15.6 | 2 M ZnSO4 | 490 (0.1); 234 (16) | 71% (1000, 1) | 0.4−1.6 | [79] |
| NH4V3O8/PANI | one-step hydrothermal reaction | 10.8 | 2 M Zn(CH3SO3F)2 | 397.5 (1); 300 (10) | 95% (1000, 10) | 0.4−1.6 | [105] |
| PANI/V2O5 | hydrothermal reaction | loosely aggregated nanosheets/ 14.2 | 2 M Zn(CF3SO3)2 | 445 (0.1); 380 (0.5); 340 (1); 247 (2) | 94% (100, 0.1); 80% (600, 2) | 0.4−1.4 | [106] |
| PPy-intercalated V2O5 | hydrothermal reaction | foliated rock/12.38 | 3 M Zn(CF3SO3)2 | 404 (0.1); 241 (5) | 98% (2000, 10) | 0.3−1.5 | [86] |
| PPy/VOH | in situ intercalation | layered nanosheet/14.0 | 3 M Zn(CF3SO3)2 | 383 (0.1); 303 (1); 281 (2) | 72% (2000, 4) | 0.2−1.5 | [85] |
| V2O5-PPy | in situ polymerization at room temperature | fibrous nanobelts/6.9 | 3 M Zn(CF3SO3)2 | 441 (0.1); 291 (5) | 95.92% (2000, 5) | 0.3−1.6 | [64] |
| V2O5/PPy | in situ polymerization at room temperature | nanowires (cable-like)/9.6 | 3 M Zn(CF3SO3)2 | 466 (0.1); 174 (5) | 95% (1000, 5) | 0.3−1.6 | [81] |
| V2O5@PPy | in situ oxidation | grained/2.61 | 2 M ZnSO4 | 186.4 (0.5); 101.8 (1); 65.3 (5) | 95.6% (300, 1) | 0.2−1.8 | [39] |
| VO2@PPy | hydrothermal process at 5 °C | hollow nanospheres/5.6 | 3 M Zn(CF3SO3)2 | 440 (0.1); 330 (1) | ~48% (860, 1) | 0.2–1.4 | [16] |
| Od−HVO@PPy | in situ polymerization | nanosheets/3.43 | 2 M ZnSO4 | 346 (0.1); 206 (10) | 85% (500, 2); 77% (1000, 10) | 0.2−1.6 | [68] |
| V2O5/PEDOT | microwave-assisted in situ polymerization | nanosheets | 3 M ZnSO4 | 390 (0.3); 274 (5); 102 (20) | 93% (200, 5) | 0.3–1.4 | [95] |
| V2O5@PEDOT | one-pot self-polymerization at room temperature | monolithic grains/9.86 | 3 M Zn(CF3SO3)2 | 360 (1); 280 (5); 197 (10) | ~77% (4500, 10) | 0.2−1.6 | [57] |
| PEDOT/V2O5 | synthesis at room temperature | 2D sheet sponge/15.77 | 3 M ZnSO4 saturated with V2O5 | 247 (0.2); 154 (50) | 88% (700, 5) | 0.2−1.6 | [98] |
| PEDOT-VO | hydrothermal method | 3D flower structure/13.95 | 3 M Zn(CF3SO3)2 | 370.5 (0.5); ~290 (5); 175 (50) | 96.9% (1000, 5) | 0.2−1.4 | [65] |
| PEDOT-V2O5 | synthesis at room temperature | nanobelts/9.7 | 3 M Zn(CF3SO3)2 | 449 (0.2); 358 (10) | 94.3% (6000, 10) | 0.3−1.6 | [89] |
| V2O5@PEDOT/CC | electrodeposition | nanosheet array of core-shell particles | 2.5 M Zn(CF3SO3)2 | 360 (0.1); 254 (5); 232 (20) | 89% (1000, 5) | 0.2−1.6 | [70] |
| V2O5·3H2O/ PEDOT: PSS | one-step hydrothermal reaction | wrinkled layers/12.9 | 3 M Zn(CF3SO3)2 | 424 (0.2); 174 (10) | 89.4% (2000, 5) | 0.3−1.6 | [92] |
| PVO/PEDOT/CNTs | wet ball-milling | nanoflakes/10.0 | 3 M Zn(CF3SO3)2 | 440.6 (0.5); 180.0 (30) | 92% (2000, 30) | 0.2−1.6 | [97] |
| VO2/PEDOT | hydrothermal method | ultrathin nanobelts/2.05 | 3 M Zn(CF3SO3)2 | 540 (0.05); 430.4 (1); 231.2 (10) | 84.5% (1000, 5) | 0.3–1.3 | [99] |
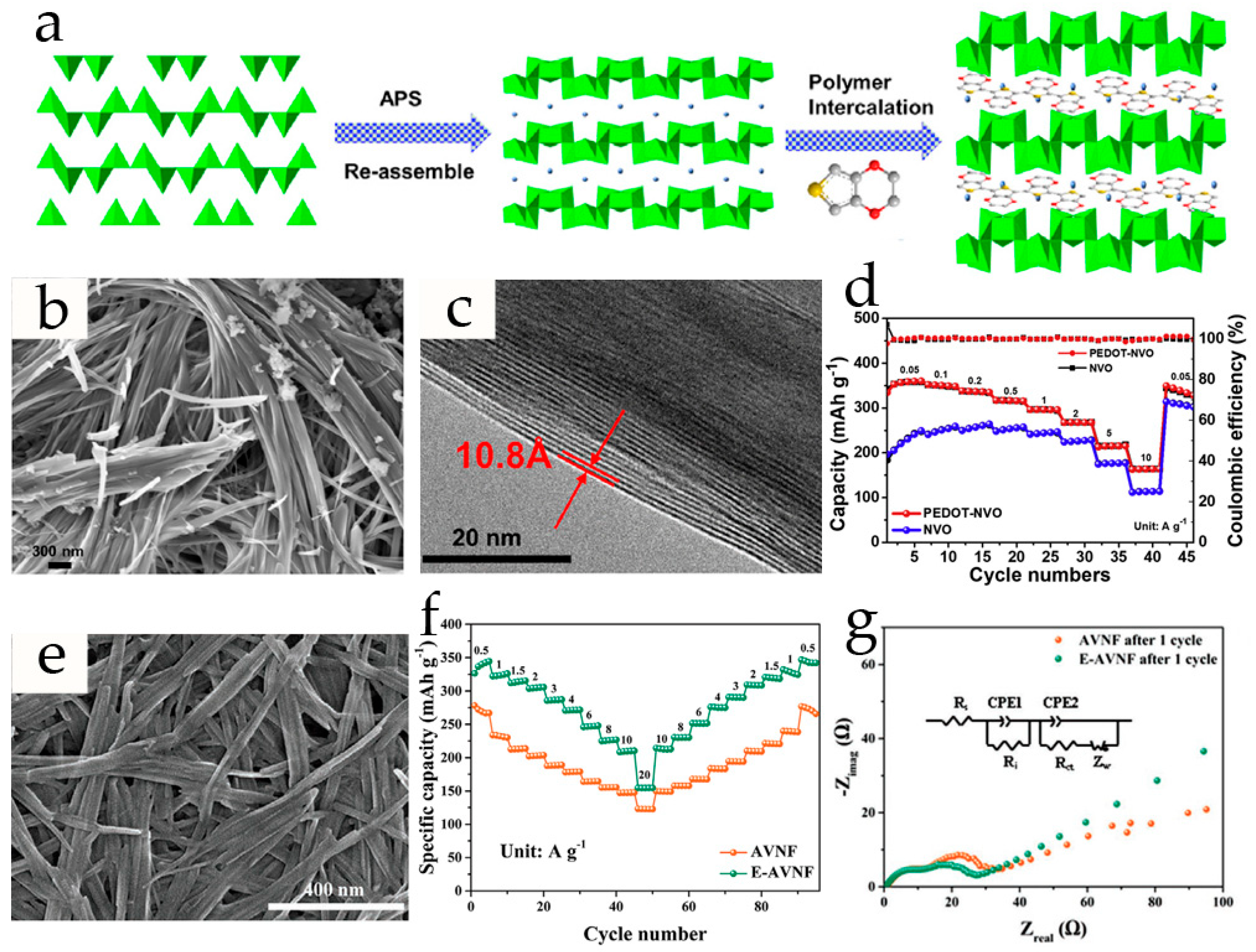
| PEDOT-NH4V3O8 | sonochemical synthesis | nanofiber/11.2 | 2.5 M Zn(CF3SO3)2 | 344 (0.5); 155 (20) | 94% (1000, 10) | 0.2−1.8 | [58] |
| PEDOT-NH4V3O8 | hydrothermal process, intercalation | nanobelts/10.8 | 3 M Zn(CF3SO3)2 | 357 (0.05); 163.6 (10) | 94.1% (5000, 10) | 0.4–1.6 | [87] |
| Na0.76V6O15/PEDOT | vapor polymerization | nanocables | 3 M Zn(CF3SO3)2 | 355 (0.05); 256 (0.1); 165 (4) | 99% (2600, 4) | 0.3−1.5 | [100] |
Author Contributions
Funding
Conflicts of Interest
References
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “beyond Li-Ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Dahbi, M.; Hosaka, T.; Kumakura, S.; Komaba, S. Towards K-Ion and Na-Ion Batteries as “Beyond Li-Ion”. Chem. Rec. 2018, 18, 459–479. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Dong, H.; Aurbach, D.; Yao, Y. Current Status and Future Directions of Multivalent Metal-Ion Batteries. Nat. Energy 2020, 5, 646–656. [Google Scholar] [CrossRef]
- Biemolt, J.; Jungbacker, P.; van Teijlingen, T.; Yan, N.; Rothenberg, G. Beyond Lithium-Based Batteries. Materials 2020, 13, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wang, J.G.; You, Z.; Wei, C.; Kang, F.; Wei, B. Rechargeable Aqueous Zinc-Ion Batteries: Mechanism, Design Strategies and Future Perspectives. Mater. Today 2021, 42, 73–98. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Fu, H. Recent Advances in Rechargeable Zn-Based Batteries. J. Power Sources 2021, 493, 229677. [Google Scholar] [CrossRef]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and Opportunities Facing Aqueous Zinc-Ion Batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Ruan, P.; Xu, X.; Feng, J.; Yu, L.; Gao, X.; Shi, W.; Wu, F.; Liu, W.; Zang, X.; Ma, F.; et al. Boosting Zinc Storage Performance via Conductive Materials. Mater. Res. Bull. 2021, 133, 111077. [Google Scholar] [CrossRef]
- Zhou, J.; Shan, L.; Wu, Z.; Guo, X.; Fang, G.; Liang, S. Investigation of V2O5 as a Low-Cost Rechargeable Aqueous Zinc Ion Battery Cathode. Chem. Commun. 2018, 54, 4457–4460. [Google Scholar] [CrossRef]
- Yan, M.; He, P.; Chen, Y.; Wang, S.; Wei, Q.; Zhao, K.; Xu, X.; An, Q.; Shuang, Y.; Shao, Y.; et al. Water-Lubricated Intercalation in V2O5·nH2O for High-Capacity and High-Rate Aqueous Rechargeable Zinc Batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef]
- Zhang, N.; Jia, M.; Dong, Y.; Wang, Y.; Xu, J.; Liu, Y.; Jiao, L.; Cheng, F. Hydrated Layered Vanadium Oxide as a Highly Reversible Cathode for Rechargeable Aqueous Zinc Batteries. Adv. Funct. Mater. 2019, 29, 1807331. [Google Scholar] [CrossRef]
- Lai, J.; Zhu, H.; Zhu, X.; Koritala, H.; Wang, Y. Interlayer-Expanded V6O13·nH2O Architecture Constructed for an Advanced Rechargeable Aqueous Zinc-Ion Battery. ACS Appl. Energy Mater. 2019, 2, 1988–1996. [Google Scholar] [CrossRef]
- Wang, L.; Huang, K.-W.; Chen, J.; Zheng, J. Ultralong Cycle Stability of Aqueous Zinc-Ion Batteries with Zinc Vanadium Oxide Cathodes. Sci. Adv. 2019, 5, aax4279. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ren, Y.; Mo, L.; Liu, C.; Hsu, K.; Ding, Y.; Zhang, X.; Li, X.; Hu, L.; Ji, D.; et al. Impacts of Oxygen Vacancies on Zinc Ion Intercalation in VO2. ACS Nano 2020, 14, 5581–5589. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Kim, J.M.; Chun, Y.T.; Zhang, J.; Jun, S.C. Recent Advances in Vanadium-Based Aqueous Rechargeable Zinc-Ion Batteries. Adv. Energy Mater. 2020, 10, 2000477. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, P.; Liu, H.; Wu, X.; Zhi, C. Tetragonal VO2 Hollow Nanospheres as Robust Cathode Material for Aqueous Zinc Ion Batteries. Mater. Today Energy 2020, 17, 100431. [Google Scholar] [CrossRef]
- Wang, W.; Yang, C.; Chi, X.; Liu, J.; Wen, B.; Liu, Y. Ultralow-Water-Activity Electrolyte Endows Vanadium-Based Zinc-Ion Batteries with Durable Lifespan Exceeding 30,000 Cycles. Energy Storage Mater. 2022, 53, 774–782. [Google Scholar] [CrossRef]
- Li, R.; Zhang, H.; Zheng, Q.; Li, X. Porous V2O5 Yolk-Shell Microspheres for Zinc Ion Battery Cathodes: Activation Responsible for Enhanced Capacity and Rate Performance. J. Mater. Chem. A 2020, 8, 5186–5193. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Y.; Liang, S.; Wu, Z.; Fang, G.; Cao, X.; Wang, C.; Lin, T.; Pan, A.; Zhou, J. Transition Metal Ion-Preintercalated V2O5 as High-Performance Aqueous Zinc-Ion Battery Cathode with Broad Temperature Adaptability. Nano Energy 2019, 61, 617–625. [Google Scholar] [CrossRef]
- Wu, Y.; Song, T.Y.; Chen, L.N. A Review on Recent Developments of Vanadium-Based Cathode for Rechargeable Zinc-Ion Batteries. Tungsten 2021, 3, 289–304. [Google Scholar] [CrossRef]
- He, P.; Chen, Q.; Yan, M.; Xu, X.; Zhou, L.; Mai, L.; Nan, C.-W. Building Better Zinc-Ion Batteries: A Materials Perspective. EnergyChem 2019, 1, 100022. [Google Scholar] [CrossRef]
- Islam, S.; Lakshmi, G.B.V.S.; Siddiqui, A.M.; Husain, M.; Zulfequar, M. Synthesis, Electrical Conductivity, and Dielectric Behavior of Polyaniline/V2O5 Composites. Int. J. Polym. Sci. 2013, 2013, 307525. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Kim, H.; You, T.S.; Kim, J. Carbon-Coated V2O5 Nanoparticles Derived from Metal-Organic Frameworks as a Cathode Material for Rechargeable Lithium-Ion Batteries. J. Alloys Compd. 2017, 727, 522–530. [Google Scholar] [CrossRef]
- Qin, H.; Chen, L.; Wang, L.; Chen, X.; Yang, Z. V2O5 Hollow Spheres as High Rate and Long Life Cathode for Aqueous Rechargeable Zinc Ion Batteries. Electrochim. Acta 2019, 306, 307–316. [Google Scholar] [CrossRef]
- Chen, H.; Qin, H.; Chen, L.; Wu, J.; Yang, Z. V2O5@CNTs as Cathode of Aqueous Zinc Ion Battery with High Rate and High Stability. J. Alloys Compd. 2020, 842, 155912. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Li, H.; Qin, B.; Passerini, S. High-Voltage Operation of a V2O5 Cathode in a Concentrated Gel Polymer Electrolyte for High-Energy Aqueous Zinc Batteries. ACS Appl. Mater. Interfaces 2020, 12, 15305–15312. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Wang, S.; Zhou, F.; Das, P.; Sun, C.; Zheng, S.; Wu, Z. 2D Amorphous V2O5/Graphene Heterostructures for High-Safety Aqueous Zn-Ion Batteries with Unprecedented Capacity and Ultrahigh Rate Capability. Adv. Energy Mater. 2020, 10, 2000081. [Google Scholar] [CrossRef]
- Javed, M.S.; Lei, H.; Wang, Z.; Liu, B.T.; Cai, X.; Mai, W. 2D V2O5 Nanosheets as a Binder-Free High-Energy Cathode for Ultrafast Aqueous and Flexible Zn-Ion Batteries. Nano Energy 2020, 70, 104573. [Google Scholar] [CrossRef]
- Zhang, W.; Zuo, C.; Tang, C.; Tang, W.; Lan, B.; Fu, X.; Dong, S.; Luo, P. The Current Developments and Perspectives of V2O5 as Cathode for Rechargeable Aqueous Zinc-Ion Batteries. Energy Technol. 2021, 9, 2000789. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X. Review of Vanadium-Based Electrode Materials for Rechargeable Aqueous Zinc Ion Batteries. J. Energy Chem. 2021, 56, 223–237. [Google Scholar] [CrossRef]
- Yang, G.; Li, Q.; Ma, K.; Hong, C.; Wang, C. The Degradation Mechanism of Vanadium Oxide-Based Aqueous Zinc-Ion Batteries. J. Mater. Chem. A 2020, 8, 8084–8095. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Xi, B.; Chen, W.; Jia, Y.; Feng, J.; Xiong, S. Advances and Perspectives of Cathode Storage Chemistry in Aqueous Zinc-Ion Batteries. ACS Nano 2021, 15, 9244–9272. [Google Scholar] [CrossRef] [PubMed]
- Khairy, M.; Tinet, D.; Van Damme, H. The Synthesis of Pillared Vanadium Oxide. J. Chem. Soc. Chem. Commun. 1990, 856–857. [Google Scholar] [CrossRef]
- Jo, J.H.; Sun, Y.K.; Myung, S.T. Hollandite-Type Al-Doped VO1.52(OH)0.77 as a Zinc Ion Insertion Host Material. J. Mater. Chem. A 2017, 5, 8367–8375. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Geiger, D.; Han, J.; Varzi, A.; Kaiser, U.; Moretti, A.; Passerini, S. Calcium Vanadate Sub-Microfibers as Highly Reversible Host Cathode Material for Aqueous Zinc-Ion Batteries. Chem. Commun. 2019, 55, 2265–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Fu, Q.; Yang, D.; Sarapulova, A.; Pang, Q.; Meng, Y.; Wei, L.; Ehrenberg, H.; Wei, Y.; Wang, C.; et al. In Operando Synchrotron Studies of NH4+ Preintercalated V2O5·nH2O Nanobelts as the Cathode Material for Aqueous Rechargeable Zinc Batteries. ACS Nano 2020, 14, 11809–11820. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Chen, A.; Sun, J. Cr3+ Pre-Intercalated Hydrated Vanadium Oxide as an Excellent Performance Cathode for Aqueous Zinc-Ion Batteries. Fundam. Res. 2021, 1, 418–424. [Google Scholar] [CrossRef]
- Kondratiev, V.V.; Holze, R. Intrinsically Conducting Polymers and Their Combinations with Redox-Active Molecules for Rechargeable Battery Electrodes: An Update. Chem. Pap. 2021, 75, 4981–5007. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, T.; Liu, J.; Li, H.; Hu, D.; Liu, X.; Xu, Q. Mechanistic Insight into Polypyrrole Coating on V2O5 Cathode for Aqueous Zinc-Ion Battery. ChemElectroChem 2022, 9, 202101441. [Google Scholar] [CrossRef]
- He, P.; Chen, S. Cathode Strategies to Improve the Performance of Zinc-ion Batteries. Electrochem. Sci. Adv. 2022, 2, 202100090. [Google Scholar] [CrossRef]
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active Materials for Aqueous Zinc Ion Batteries: Synthesis, Crystal Structure, Morphology, and Electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent Progress and Perspectives on Aqueous Zn-Based Rechargeable Batteries with Mild Aqueous Electrolytes. Energy Storage Mater. 2019, 20, 410–437. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Yu, M.; Niu, Z.; Cheng, F.; Chen, J. Materials Chemistry for Rechargeable Zinc-Ion Batteries. Chem. Soc. Rev. 2020, 49, 4203–4219. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Lu, M.; Cai, D.; Yang, H.; Han, W. Recent Advances in Energy Storage Mechanism of Aqueous Zinc-Ion Batteries. J. Energy Chem. 2021, 54, 712–726. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Liu, J.-H.; Gao, Y.; Cao, X.; Zhan, C.; Wang, Y.; Wang, S.; Chou, S.-L.; Dou, S.-X.; et al. Vanadium-Based Cathodes for Aqueous Zinc-Ion Batteries: Mechanism, Design Strategies and Challenges. Energy Storage Mater. 2022, 50, 21–46. [Google Scholar] [CrossRef]
- Pam, M.E.; Yan, D.; Yu, J.; Fang, D.; Guo, L.; Li, X.L.; Li, T.C.; Lu, X.; Ang, L.K.; Amal, R.; et al. Microstructural Engineering of Cathode Materials for Advanced Zinc-Ion Aqueous Batteries. Adv. Sci. 2021, 8, 2002722. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, H.; Qin, L.; Cao, X.; Zhou, J.; Pan, A.; Fang, G.; Liang, S. Interlayer Doping in Layered Vanadium Oxides for Low-cost Energy Storage: Sodium-ion Batteries and Aqueous Zinc-ion Batteries. ChemNanoMat 2020, 6, 1553–1566. [Google Scholar] [CrossRef]
- Karapidakis, E.; Vernardou, D. Progress on V2O5 Cathodes for Multivalent Aqueous Batteries. Materials 2021, 14, 2310. [Google Scholar] [CrossRef]
- Sengodu, P.; Deshmukh, A.D. Conducting Polymers and Their Inorganic Composites for Advanced Li-Ion Batteries: A Review. RSC Adv. 2015, 5, 42109–42130. [Google Scholar] [CrossRef]
- Eliseeva, S.N.; Kamenskii, M.A.; Tolstopjatova, E.G.; Kondratiev, V.V. Effect of Combined Conductive Polymer Binder on the Electrochemical Performance of Electrode Materials for Lithium-Ion Batteries. Energies 2020, 13, 2163. [Google Scholar] [CrossRef]
- Nguyen, V.A.; Kuss, C. Review—Conducting Polymer-Based Binders for Lithium-Ion Batteries and Beyond. J. Electrochem. Soc. 2020, 167, 065501. [Google Scholar] [CrossRef]
- Shin, J.; Choi, D.S.; Lee, H.J.; Jung, Y.; Choi, J.W. Hydrated Intercalation for High-Performance Aqueous Zinc Ion Batteries. Adv. Energy Mater. 2019, 9, 1900083. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, Z.; Guo, X.; Li, G. A Conjugated Polyaniline and Water Co-Intercalation Strategy Boosting Zinc-Ion Storage Performances for Rose-like Vanadium Oxide Architectures. J. Mater. Chem. A 2019, 7, 21079–21084. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Z.; Tian, D.; Hu, T.; Jiang, H.; Yang, J.; Sun, J.; Zheng, J.; Meng, C.; Zhang, Y. Employing “One for Two” Strategy to Design Polyaniline-Intercalated Hydrated Vanadium Oxide with Expanded Interlayer Spacing for High-Performance Aqueous Zinc-Ion Batteries. Chem. Eng. J. 2020, 399, 125842. [Google Scholar] [CrossRef]
- Guan, X.; Sun, Q.; Sun, C.; Duan, T.; Nie, W.; Liu, Y.; Zhao, K.; Cheng, H.; Lu, X. Tremella-like Hydrated Vanadium Oxide Cathode with an Architectural Design Strategy toward Ultralong Lifespan Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 41688–41697. [Google Scholar] [CrossRef]
- Gu, Y.; Han, Y.; Qin, Z.; Li, D.; Wang, L. A Strategy to Control Crystal Water Content in Hydrated Vanadium Oxide Cathode for Promoting Aqueous Rechargeable Zinc-Ion Batteries. J. Alloys Compd. 2022, 911, 165102. [Google Scholar] [CrossRef]
- Yao, Z.; Wu, Q.; Chen, K.; Liu, J.; Li, C. Shallow-Layer Pillaring of a Conductive Polymer in Monolithic Grains to Drive Superior Zinc Storage via a Cascading Effect. Energy Environ. Sci. 2020, 13, 3149–3163. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.H.; Park, C.; Kim, H.; Park, J.; Chung, K.Y.; Ahn, H. Controlling Vanadate Nanofiber Interlayer via Intercalation with Conducting Polymers: Cathode Material Design for Rechargeable Aqueous Zinc Ion Batteries. Adv. Funct. Mater. 2021, 31, 2100005. [Google Scholar] [CrossRef]
- Wu, C.G.; DeGroot, D.C.; Marcy, H.O.; Schindler, J.L.; Kannewurf, C.R.; Liu, Y.J.; Hirpo, W.; Kanatzidis, M.G. Redox Intercalative Polymerization of Aniline in V2O5 Xerogel. The Postintercalative Intralamellar Polymer Growth in Polyaniline/Metal Oxide Nanocomposites Is Facilitated by Molecular Oxygen. Chem. Mater. 1996, 8, 1992–2004. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, H.; Zhang, B.; Li, G.; Zhu, H.; Ren, Y.; Geng, H.; Yang, Y.; Liu, Q.; Li, C.C. Tuning the Kinetics of Zinc-Ion Insertion/Extraction in V2O5 by In Situ Polyaniline Intercalation Enables Improved Aqueous Zinc-Ion Storage Performance. Adv. Mater. 2020, 32, 2001113. [Google Scholar] [CrossRef]
- Kanatzidis, M.G.; Wu, C.G.; Marcy, H.O.; Kannewurf, C.R. Conductive Polymer Bronzes. Intercalated Polyaniline in V2O5 Xerogels. J. Am. Chem. Soc. 1989, 111, 4139–4141. [Google Scholar] [CrossRef]
- Yin, C.; Pan, C.; Liao, X.; Pan, Y.; Yuan, L. Regulating the Interlayer Spacing of Vanadium Oxide by In Situ Polyaniline Intercalation Enables an Improved Aqueous Zinc-Ion Storage Performance. ACS Appl. Mater. Interfaces 2021, 13, 39347–39354. [Google Scholar] [CrossRef]
- Li, R.; Xing, F.; Li, T.; Zhang, H.; Yan, J.; Zheng, Q.; Li, X. Intercalated Polyaniline in V2O5 as a Unique Vanadium Oxide Bronze Cathode for Highly Stable Aqueous Zinc Ion Battery. Energy Storage Mater. 2021, 38, 590–598. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, R.; Wang, X.; Wang, Z.; Song, B.; Du, Y.; Lu, Q.; Chen, X.; Sun, J. Facile Large-Scale Preparation of Vanadium Pentoxide-Polypyrrole Composite for Aqueous Zinc-Ion Batteries. J. Alloys Compd. 2022, 907, 164434. [Google Scholar] [CrossRef]
- Li, S.; Wei, X.; Wu, C.; Zhang, B.; Wu, S.; Lin, Z. Constructing Three-Dimensional Structured V2O5/Conductive Polymer Composite with Fast Ion/Electron Transfer Kinetics for Aqueous Zinc-Ion Battery. ACS Appl. Energy Mater. 2021, 4, 4208–4216. [Google Scholar] [CrossRef]
- Volkov, F.S.; Eliseeva, S.N.; Kamenskii, M.A.; Volkov, A.I.; Tolstopjatova, E.G.; Glumov, O.V.; Fu, L.; Kondratiev, V.V. Vanadium Oxide-Poly(3,4-Ethylenedioxythiophene) Nanocomposite as High-Performance Cathode for Aqueous Zn-Ion Batteries: The Structural and Electrochemical Characterization. Nanomaterials 2022, 12, 3896. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, X.; Man, J.; Sun, J. A Novel Organic-Inorganic Hybrid V2O5@polyaniline as High-Performance Cathode for Aqueous Zinc-Ion Batteries. Mater. Lett. 2020, 272, 127813. [Google Scholar] [CrossRef]
- Zhang, Z.; Xi, B.; Wang, X.; Ma, X.; Chen, W.; Feng, J.; Xiong, S. Oxygen Defects Engineering of VO2·xH2O Nanosheets via In Situ Polypyrrole Polymerization for Efficient Aqueous Zinc Ion Storage. Adv. Funct. Mater. 2021, 31, 2103070. [Google Scholar] [CrossRef]
- Ling, H.; Zhang, R.; Ye, X.; Wen, Z.; Xia, J.; Lu, X. In-Situ Synthesis of Organic-Inorganic Hybrid Thin Film of PEDOT/ V2O5 as Hole Transport Layer for Polymer Solar Cells. Sol. Energy 2019, 190, 63–68. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; Li, F.; Guan, Z.; Wang, R.; He, B.; Gong, Y.; Hu, X. Conformal Conducting Polymer Shells on V2O5 Nanosheet Arrays as a High-Rate and Stable Zinc-Ion Battery Cathode. Adv. Mater. Interfaces 2019, 6, 1801506. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Hou, M.; Dong, X.; Liu, Y.; Wang, Y.; Xia, Y. Polyaniline-Intercalated Manganese Dioxide Nanolayers as a High-Performance Cathode Material for an Aqueous Zinc-Ion Battery. Nat. Commun. 2018, 9, 2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallas, P.; Stamopoulos, D.; Boukos, N.; Tzitzios, V.; Niarchos, D.; Petridis, D. Characterization, Magnetic and Transport Properties of Polyaniline Synthesized through Interfacial Polymerization. Polymer 2007, 48, 3162–3169. [Google Scholar] [CrossRef]
- Li, Z.; Gong, L. Research Progress on Applications of Polyaniline (PANI) for Electrochemical Energy Storage and Conversion. Materials 2020, 13, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Ahn, B.Y.; Wei, T.S.; Jo, Y.; Jeong, S.; Choi, Y.; Kim, I.D.; Lewis, J.A. High-Power Aqueous Zinc-Ion Batteries for Customized Electronic Devices. ACS Nano 2018, 12, 11838–11846. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.; Wang, X.; Bi, S.; Niu, Z.; Chen, J. An Aqueous Rechargeable Zinc-Organic Battery with Hybrid Mechanism. Adv. Funct. Mater. 2018, 28, 1804975. [Google Scholar] [CrossRef]
- Chen, S.; Li, K.; Hui, K.S.; Zhang, J. Regulation of Lamellar Structure of Vanadium Oxide via Polyaniline Intercalation for High-Performance Aqueous Zinc-Ion Battery. Adv. Funct. Mater. 2020, 30, 2003890. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Jiang, H.; Liu, Y.; Meng, C. Polyaniline-Expanded the Interlayer Spacing of Hydrated Vanadium Pentoxide by the Interface-Intercalation for Aqueous Rechargeable Zn-Ion Batteries. J. Colloid Interface Sci. 2021, 603, 641–650. [Google Scholar] [CrossRef]
- Liu, Y.-J.; DeGroot, D.C.; Schindler, J.L.; Kannewurf, C.R.; Kanatzidis, M.G. Stabilization of Anilinium in Vanadium(V) Oxide Xerogel and Its Post-Intercalative Polymerization to Poly(Aniline) in Air. J. Chem. Soc. Chem. Commun. 1993, 593–596. [Google Scholar] [CrossRef]
- Li, W.; Han, C.; Gu, Q.; Chou, S.L.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Electron Delocalization and Dissolution-Restraint in Vanadium Oxide Superlattices to Boost Electrochemical Performance of Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2020, 10, 2001852. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Zhang, L.; Li, J.; Wang, W.; Yang, Z.; Zhang, L.; Wang, Y.; Chen, J.; Huang, Y.; et al. Graphene-like Vanadium Oxygen Hydrate (VOH) Nanosheets Intercalated and Exfoliated by Polyaniline (PANI) for Aqueous Zinc-Ion Batteries (ZIBs). ACS Appl. Mater. Interfaces 2020, 12, 31564–31574. [Google Scholar] [CrossRef]
- Qin, X.; Wang, X.; Sun, J.; Lu, Q.; Omar, A.; Mikhailova, D. Polypyrrole Wrapped V2O5 Nanowires Composite for Advanced Aqueous Zinc-Ion Batteries. Front. Energy Res. 2020, 8, 199. [Google Scholar] [CrossRef]
- Qian, T.; Xu, N.; Zhou, J.; Yang, T.; Liu, X.; Shen, X.; Liang, J.; Yan, C. Interconnected Three-Dimensional V2O5/Polypyrrole Network Nanostructures for High Performance Solid-State Supercapacitors. J. Mater. Chem. A 2015, 3, 488–493. [Google Scholar] [CrossRef]
- Rapi, S.; Bocchi, V.; Gardini, G.P. Conducting Polypyrrole by Chemical Synthesis in Water. Synth. Met. 1988, 24, 217–221. [Google Scholar] [CrossRef]
- Diaz, A.F.; Castillo, J.I.; Logan, J.A.; Lee, W.-Y. Electrochemistry of Conducting Polypyrrole Films. J. Electroanal. Chem. Interfacial Electrochem. 1981, 129, 115–132. [Google Scholar] [CrossRef]
- Feng, Z.; Sun, J.; Liu, Y.; Jiang, H.; Hu, T.; Cui, M.; Tian, F.; Meng, C.; Zhang, Y. Polypyrrole-Intercalation Tuning Lamellar Structure of V2O5·nH2O Boosts Fast Zinc-Ion Kinetics for Aqueous Zinc-Ion Battery. J. Power Sources 2022, 536, 231489. [Google Scholar] [CrossRef]
- Wang, W.; He, D.; Fang, Y.; Wang, S.; Zhang, Z.; Zhao, R.; Xue, W. Pillaring of a Conductive Polymer in Layered V2O5 Boosting Ultra-Fast Zn2+/H+ Storage in Aqueous Media. Electrochim. Acta 2022, 416, 140270. [Google Scholar] [CrossRef]
- Bin, D.; Huo, W.; Yuan, Y.; Huang, J.; Liu, Y.; Zhang, Y.; Dong, F.; Wang, Y.; Xia, Y. Organic-Inorganic-Induced Polymer Intercalation into Layered Composites for Aqueous Zinc-Ion Battery. Chem 2020, 6, 968–984. [Google Scholar] [CrossRef]
- Liao, M.; Wang, J.; Ye, L.; Sun, H.; Wen, Y.; Wang, C.; Sun, X.; Wang, B.; Peng, H. A Deep-Cycle Aqueous Zinc-Ion Battery Containing an Oxygen-Deficient Vanadium Oxide Cathode. Angew. Chemie Int. Ed. 2020, 59, 2273–2278. [Google Scholar] [CrossRef]
- Du, Y.; Wang, X.; Sun, J. Tunable Oxygen Vacancy Concentration in Vanadium Oxide as Mass-Produced Cathode for Aqueous Zinc-Ion Batteries. Nano Res. 2021, 14, 754–761. [Google Scholar] [CrossRef]
- Heywang, G.; Jonas, F. Poly(Alkylenedioxythiophene)s—New, Very Stable Conducting Polymers. Adv. Mater. 1992, 4, 116–118. [Google Scholar] [CrossRef]
- Petsagkourakis, I.; Kim, N.; Tybrandt, K.; Zozoulenko, I.; Crispin, X. Poly(3,4-ethylenedioxythiophene): Chemical Synthesis, Transport Properties, and Thermoelectric Devices. Adv. Electron. Mater. 2019, 5, 1800918. [Google Scholar] [CrossRef]
- Du, Y.; Chen, Y.; Yang, M.; Zou, S.; Song, X.; Fu, Y.; Li, J.; Li, Y.; He, D. Poly(3,4-Ethylenedioxythiophene)-Polystyrenesulfonate-Added Layered Vanadium Oxide Cathode for High-Performance Zinc-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 14582–14589. [Google Scholar] [CrossRef]
- Murugan, A.V.; Kale, B.B.; Kwon, C.; Campet, G.; Vijayamohanan, K. Synthesis and Characterization of a New Organo–Inorganic Poly(3,4-Ethylene Dioxythiophene) PEDOT/ V2O5 Nanocomposite by Intercalation. J. Mater. Chem. 2001, 11, 2470–2475. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Sun, J. A Strategy Associated with Conductive Binder and 3D Current Collector for Aqueous Zinc-Ion Batteries with High Mass Loading. J. Electroanal. Chem. 2020, 873, 114395. [Google Scholar] [CrossRef]
- Volkov, F.S.; Tolstopjatova, E.G.; Eliseeva, S.N.; Kamenskii, M.A.; Vypritskaia, A.I.; Volkov, A.I.; Kondratiev, V.V. Vanadium(V) Oxide Coated by Poly(3,4-Ethylenedioxythiophene) as Cathode for Aqueous Zinc-Ion Batteries with Improved Electrochemical Performance. Mater. Lett. 2022, 308, 131210. [Google Scholar] [CrossRef]
- Liu, C.; Lu, Q.; Omar, A.; Mikhailova, D. A Facile Chemical Method Enabling Uniform Zn Deposition for Improved Aqueous Zn-Ion Batteries. Nanomaterials 2021, 11, 764. [Google Scholar] [CrossRef]
- Liu, X.; Ni, W.; Wang, Y.; Liang, Y.; Wu, B.; Xu, G.; Wei, X.; Yang, L. Water-Processable and Multiscale-Designed Vanadium Oxide Cathodes with Predominant Zn2+ Intercalation Pseudocapacitance toward High Gravimetric/Areal/Volumetric Capacity. Small 2022, 18, 2105796. [Google Scholar] [CrossRef]
- Wang, B.; Dai, S.; Zhu, Z.; Hu, L.; Su, Z.; Jin, Y.; Xiong, L.; Gao, J.; Wan, J.; Li, Z.; et al. A Two-Dimensional Conductive Polymer/V2O5 Composite with Rapid Zinc-Ion Storage Kinetics for High-Power Aqueous Zinc-Ion Batteries. Nanoscale 2022, 14, 12013–12021. [Google Scholar] [CrossRef]
- Liu, X.; Xu, G.; Zhang, Q.; Huang, S.; Li, L.; Wei, X.; Cao, J.; Yang, L.; Chu, P.K. Ultrathin Hybrid Nanobelts of Single-Crystalline VO2 and Poly(3,4-Ethylenedioxythiophene) as Cathode Materials for Aqueous Zinc Ion Batteries with Large Capacity and High-Rate Capability. J. Power Sources 2020, 463, 228223. [Google Scholar] [CrossRef]
- Bi, W.; Gao, G.; Wu, G.; Atif, M.; AlSalhi, M.S.; Cao, G. Sodium Vanadate/PEDOT Nanocables Rich with Oxygen Vacancies for High Energy Conversion Efficiency Zinc Ion Batteries. Energy Storage Mater. 2021, 40, 209–218. [Google Scholar] [CrossRef]
- Yan, X.; Feng, X.; Hao, B.; Liu, J.; Yu, Y.; Qi, J.; Wang, H.; Wang, Z.; Hu, Y.; Fan, X.; et al. Enhancing the Kinetics of Vanadium Oxides via Conducting Polymer and Metal Ions Co-Intercalation for High-Performance Aqueous Zinc-Ions Batteries. J. Colloid Interface Sci. 2022, 628, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, Y.; Song, B.; Wang, Z.; Wang, X.; Wan, F.; Ma, X. Manganese-Ions and Polyaniline Co-Intercalation into Vanadium Oxide for Stable Zinc-Ion Batteries. J. Power Sources 2022, 545, 231920. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, Y.; Zhao, Y.; Sun, J.; Liu, Y.; Jiang, H.; Cui, M.; Hu, T.; Meng, C. Dual Intercalation of Inorganics–Organics for Synergistically Tuning the Layer Spacing of V2O5·nH2O to Boost Zn2+ Storage for Aqueous Zinc-Ion Batteries. Nanoscale 2022, 14, 8776–8788. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ru, Q.; Gao, P.; Shi, Z.; Gao, Y.; Chen, F.; Chi-Chun Ling, F.; Wei, L. Organic Pillars Pre-Intercalated V4+-V2O5·3H2O Nanocomposites with Enlarged Interlayer and Mixed Valence for Aqueous Zn-Ion Storage. Appl. Surf. Sci. 2020, 534, 147608. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Chen, J.; Zheng, Q.; Huo, Y.; Xie, F.; Lin, D. Polyaniline Intercalation Induced Great Enhancement of Electrochemical Properties in Ammonium Vanadate Nanosheets as an Advanced Cathode for High-Performance Aqueous Zinc-Ion Batteries. Chem. Eng. J. 2022, 448, 137681. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, X.; Yuan, S.; Bai, M.; Wang, H.; Liu, S.; Zhang, M.; Ma, Y. Engineering Vanadium Pentoxide Cathode for the Zero-Strain Cation Storage via a Scalable Intercalation-Polymerization Approach. Adv. Funct. Mater. 2021, 31, 2100164. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolstopyatova, E.G.; Kamenskii, M.A.; Kondratiev, V.V. Vanadium Oxide–Conducting Polymers Composite Cathodes for Aqueous Zinc-Ion Batteries: Interfacial Design and Enhancement of Electrochemical Performance. Energies 2022, 15, 8966. https://doi.org/10.3390/en15238966
Tolstopyatova EG, Kamenskii MA, Kondratiev VV. Vanadium Oxide–Conducting Polymers Composite Cathodes for Aqueous Zinc-Ion Batteries: Interfacial Design and Enhancement of Electrochemical Performance. Energies. 2022; 15(23):8966. https://doi.org/10.3390/en15238966
Chicago/Turabian StyleTolstopyatova, Elena G., Mikhail A. Kamenskii, and Veniamin V. Kondratiev. 2022. "Vanadium Oxide–Conducting Polymers Composite Cathodes for Aqueous Zinc-Ion Batteries: Interfacial Design and Enhancement of Electrochemical Performance" Energies 15, no. 23: 8966. https://doi.org/10.3390/en15238966
APA StyleTolstopyatova, E. G., Kamenskii, M. A., & Kondratiev, V. V. (2022). Vanadium Oxide–Conducting Polymers Composite Cathodes for Aqueous Zinc-Ion Batteries: Interfacial Design and Enhancement of Electrochemical Performance. Energies, 15(23), 8966. https://doi.org/10.3390/en15238966








