Abstract
Microbial electrosynthesis is the process of supplying electrons to microorganisms to reduce CO2 and yield industrially relevant products. Such systems are limited by their requirement for high currents, resulting in challenges to cell survival. Electrofermentation is an electron-efficient form of microbial electrosynthesis in which a small cathodic or anodic current is provided to a culture to alter the oxidation–reduction potential of the medium and, in turn, alter microbial metabolism. This approach has been successfully utilised to increase yields of diverse products including biogas, butanediol and lactate. Biomass conversion to lactate is frequently facilitated by ensiling plant biomass with homofermentative lactic acid bacteria. Although most commonly used as a preservative in ensiled animal feed, lactate has diverse industrial applications as a precursor for the production of probiotics, biofuels, bioplastics and platform chemicals. Lactate yields by lactic acid bacteria (LAB) are constrained by a number of redox limitations which must be overcome while maintaining profitability and sustainability. To date, electrofermentation has not been scaled past laboratory- or pilot-stage reactions. The increasing ease of genetic modification in a wide range of LAB species may prove key to overcoming some of the pitfalls of electrofermentation at commercial scale. This review explores the history of electrofermentation as a tool for controlling redox balance within bacterial biocatalysts, and the potential for electrofermentation to increase lactate production from low-value plant biomass.
1. History of Microbial Electrosynthesis
The earliest exploration of microbial electrosynthesis was the use of microbial fuel cells (MFCs) to produce electricity from the microbial oxidation of organic matter, which aimed to scavenge and transfer free electrons produced by electrogenic microbes to an anode. Although small, the resulting currents could theoretically be applied in contexts such as renewable electric car batteries if the yield could be enhanced, the cost decreased and dangerous by-products suppressed [1]. The first discovery of the microbial generation of electrical currents occurred in 1912 in Saccharomyces cerevisiae, but it attracted limited interest. Early research on MFCs suggested that mediators, or electron shuttles, between cell and anode were necessary for current biogenesis but this was later deemed unnecessary if using microorganisms with trans-membrane, redox-active proteins. Since then, the potential of MFCs for remote- and low-power-usage applications has been investigated, such as in conservation monitoring [2], as has the idea of using MFCs for larger-scale power generation. Translation from laboratory-scale experiments to 1–1000 L-scale pilot plants has led to the identification of issues with current generation due to decreased chemical oxygen demand [3] and difficulties in the construction of larger-volume reactors [4]. Utilising smaller interlinked stacked MFCs has been shown to reliably provide power density of 150–200 mA/m3 [5]; however, this technology has still not been adopted at any widespread scale [6].
Microbial electrolysis cells (MECs) are essentially the converse of MFCs; an electrical current is supplied to a culture of electroactive microbes, with the goal of producing H2 at the cathode [7]. While H2 evolution from most typical metabolic end-products is thermodynamically unfavourable (positive ΔG), the addition of energy to the system via a current allows these unfavourable reactions to proceed [7]. In MECs, some of the electrical potential required for the reduction of protons to H2 is supplied by the electrogenic microorganisms, so the input of power required is lower than that of hydrolysis (generally 0.2–1 V) compared to the 2 V required for alkaline water hydrolysis [8]. The increasing use of H2 as a direct fossil fuel replacement and the possibilities offered by using wastewater as a low-cost carbon source for the generation of H2 have therefore attracted renewed interest in MECs [7].
2. Overcoming the Limitations of Electrosynthesis
The main limitations of MES approaches are the lack of product selectivity, the need for electroactive and stress-tolerant biocatalysts, and the requirement for and cost of high current density. MESs can be separated into lithoautotrophic (CO2 reduction) and lithoheterotrophic (organic matter reduction) approaches. While the potential for lithoautotrophic MESs has hard limitations due to the requirement of homoacetogenic biocatalysts to utilise the Wood–Ljungdahl pathway, lithoheterotrophic MESs are theoretically more flexible [9], allowing their limitations to be addressed.
Product selectivity would be easiest to achieve at the microbial level either by selecting organisms with limited outputs, or through metabolic engineering. Limiting metabolic outputs through targeted gene knockouts has been highly successful [10,11] but requires genetically tractable organisms. The requirement for electroactive biocatalysts could theoretically severely limit options for organisms such as Shewanella oneidensis, which has extensive decaheme cytochromes that allow direct electron transfer from an electrode [12,13]. However, genetic engineering has been successful in conferring electroactivity via the heterologous expression of c-type cytochromes [14]. Alternatively, chemical redox mediators may be added to microbial cultures. These mediators, such as methyl viologen and neutral red, are reduced at the electrode, with this reduced form passing through cell membranes and either directly reducing metabolic substrates or altering the NADH:NAD+ balance to redirect pathways [12]. Biological electron shuttles such as phenazines and pyocyanin (which function similarly) are found in naturally electroactive environments, and can be overexpressed in organisms such as Pseudomonas aeruginosa to improve electroactivity, pointing to another potential role of metabolic engineering [15]. Fundamentally, the field of lithoheterotrophic microbial electrosynthesis appears to suffer from seemingly unreasonable expectations of a small number of biocatalysts [16], but metabolic engineering provides a potential escape from this limitation.
Electrofermentation (EF) is an adaptation of MESs wherein small currents are used to alter the redox potential of the medium, in turn, altering the internal redox potential and causing shifts in the balance of existing metabolic pathways. EF can be anodic or cathodic: In anodic EF, the working electrode acts as an electron sink, scavenging any extra electrons produced by microbial metabolism, while in cathodic EF, the working electrode is an electron source donating electrons to the medium. These configurations can feasibly shift metabolism in favour of more oxidised (in anodic) or reduced (in cathodic) end-products. While MES has a high “per-electron” cost [9], EF requires only a small current and provides much more forgiving conditions to support microbial growth because the aim is not for electrons to directly reduce any metabolic compounds; rather EF is a media-based effect. For example, a cathodic study in the electroactive Clostridium pasteurianum showed that just a small current shifted metabolism in favour of reduced outputs. The increase in reduced compounds was stoichiometrically far higher than it would have been if the provided electrons were only being used directly as reducing equivalents. Instead, it seems likely that while cathodic electrons were able to alter the NADH:NAD+ ratio in the cell directly, changes to the media also resulted in increased consumption of the natural metabolic NADH pool [17].
EF has been successfully used in a variety of conditions, with a broad range of study systems [14,18]. Key variations in EF approaches are the apparatus type, direction of the electrical current, target products, O2 conditions, microbial species electroactivity and, connected to this, mediator supplementation and type. Single-chamber, H-cell and single-electrode apparatus can be used, each with different goals. In a single-chamber approach, both electrodes are present in the same chamber. This is arguably more industrially realistic but requires extensive biocatalyst modifications to prevent unwanted (and possibly opposite) effects at the wrong electrode [19]. The most common approach, also used in MFC and other MEC studies, is a two-chamber H-cell, whereby the anode and cathode are separated by an ion exchange membrane to enable a closed circuit (Figure 1). This type of system is useful for elucidating the separate effects of anodic and cathodic currents, especially for EF. However, the presence of the ion exchange membrane and the distance between electrodes means the electrical resistance is higher, thereby limiting the power which can be supplied or produced [18]. H-cell, and to a lesser extent, single-chamber setups translate poorly into industrial applications, serving more as a proof-of-concept. A more industrially realistic setup has been tested, whereby a single, polarised (anodic or cathodic voltage) electrode is submerged in the microbial culture, resulting in an open circuit (no current). This has been used successfully to improve biogas production during anaerobic fermentation [20], and to generate a current in MFCs [21].
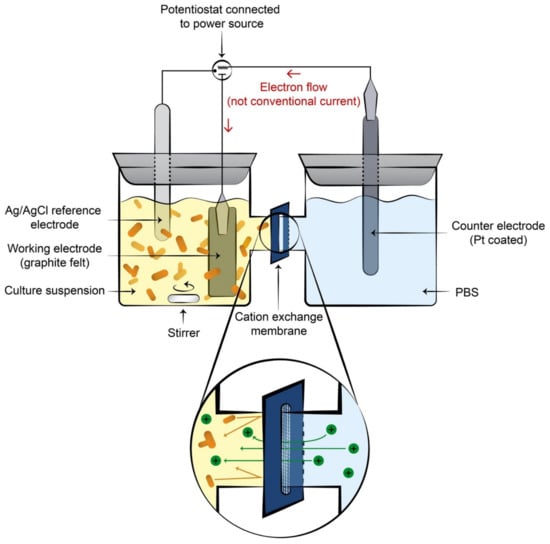
Figure 1.
Representation of an H-cell electrofermentation set up. Culture can be supplied with either an anodic or cathodic electron supply.
Another aspect of apparatus type is the working electrode material, important considerations for which are affordability, pre-treatment requirements, inertness and surface area. By far the most affordable and versatile material is graphite or other carbon-based material, and this is used in most electrofermentation experiments [22]. Carbon electrodes are chemically unreactive, and can be used in the form of rod, felt, brush, and paper electrodes. Graphite felt and brush electrodes are especially easily scaled to improve electrode surface area, and thereby, current density [23]. In small-scale experiments, such electrodes may require chemical pre-treatments to improve initial wettability and permit contact with the culture [23]. Stainless steel electrodes are occasionally used for biogas and H2 evolution [20,24] but are more often integrated with carbon electrodes to improve current collection or provision due to higher conductivity [25]. While platinum-coated electrodes are often used as counter-electrodes [21] the cost of platinum makes industrial applications in working electrodes unfeasible.
EF has been applied to a variety of study systems, with the intended products varying with the microbial species and carbon sources used [14]. Much of the industrial interest in EF currently lies in the production of added-value products from organic waste residue [26,27]. This means that many studies comprise undefined, mixed-culture experiments and it can therefore be difficult to disentangle the community-selection effects of EF from genuine redox-driven metabolic changes. Cathodic electrofermentation in these systems has been highly successful; however, with studies demonstrating up to a 20-fold increase in isobutyrate yield [28]. Cathodic electrofermentation of pure microbial cultures has also been explored for various species, showing impressive shifts from acetate towards a 35-fold increase in lactate and a 3-fold increase in butanediol in Clostridium autoethanogenum [29]. Anodic studies are rarer, but have also shown promise; for example, one study yielded a 1.7-fold increase in the production of 3-hydroxypropionic acid from glycerol by Klebsiella pneumoniae [30]. These enhancements, especially in cathodic treatments, point to impressive potential applications of EF in industry.
EF benefits over other bioelectrochemical systems in that it has less extreme expectations of the biocatalysts used. However, EF research still seems to suffer from viewing biocatalysts as inert machines rather than living organisms, and remains largely theoretical rather than industrially applicable [16]. In the near future, it may be valuable to focus on biocatalysts which naturally produce large amounts of a single metabolite and have established roles in industry. This could reduce the costs of separation and reactor engineering, and prevent the trade-offs often associated with implementing extensively genetically modified bacteria in reactors [31]. A key example of a group of industrial biocatalysts which naturally produce large amounts of a single metabolite are the lactic acid bacteria. Understanding the mechanism by which this output is generated reveals a potential profitable synergy with EF.
In addition to the generation of a current and H2 from microbial populations in a fuel cell setup, microbial electrosynthesis systems (MESs) entail the production of organic chemicals from the reduction of CO2 or organic matter at a cathode. Here, a current is supplied to a culture, driving either lithoautotrophy or lithoheterotrophy. During this electrorespiration, organic compounds can be produced from CO2 reduction via the Wood–Ljungdahl pathway in homoacetogens, or via the direct reduction of other organic compounds already present in the medium [9,12]. MESs have a number of shortcomings which reduce their industrial potential, but alterations to the technology make MES applications more promising. These alterations and their application to lactic acid bacteria as novel fermentative MES biocatalysts are the primary focus of this review.
3. Scalability of Bioelectrochemical Systems: Lessons from MECs
Although it is industrially promising at the laboratory scale, EF research is still in its infancy. Despite overcoming some of the fundamental limitations of MESs, its scalability has not been extensively tested. Scalable setups are essential to assessing the industrial favourability of a process and will therefore be a major challenge in the future of EF research. Currently, the most relevant pilot-scale experiments have been carried out in wastewater treatment MECs which produce H2 or methane. Understanding the relevant pitfalls of moving up from the laboratory scale can help to predict the most industrially realistic conditions for a potential future EF scale-up.
Certain limitations of MECs which arise during pilot-scale experiments may be overcome by inherent features of EF, while others may continue to present issues. Relevant pitfalls of the scale-up are derived from oxygen requirements, the complexity of natural communities and substrates, and size-related system overpotentials. Aeration is a major cost associated with traditional wastewater treatments [32]. At scale, ensuring sufficient aeration can complicate reactor design and often impacts space needs and efficiency in traditional wastewater treatment plants [32,33]. MECs theoretically operate under anaerobic conditions. This hypothetically removes the requirement for aeration, making the technique industrially promising. However, natural microbial communities present in wastewater often include methanogens whose growth thrives under these reducing anaerobic conditions [34]. These organisms metabolise H2 (the intended product) to produce methane, reducing reactor productivity. The laboratory solution to this problem is to expose cultures to oxygen between batches to suppress this [35]. However, pilot-scale MECs must operate with continuous flow, rather than in batches, invalidating this approach [36,37]. In multiple pilot-scale MECs using wastewater, product rerouting towards methanogenesis led to methane being the dominant gaseous product [36,38], ultimately leading the focus of MECs to shift from H2 to biogas production as adding an inhibitor is economically unfeasible [39]. This case study highlights a complication arising both from the scale-up from synthetic to natural substrate and its associated microbes, as well as from scaling operations from batch to continuous flow. The diversity of natural microbial communities is a consistent challenge in MEC scale-up, resulting in unpredictable synergy between species and metabolic bottlenecks [40].
The changes from synthetic to natural substrates and communities, and from batch to continuous flow when shifting from laboratory to pilot scale, are important considerations in designing EF systems for initial prospecting. Laboratory experiments with natural communities are difficult to design; however, there are natural fermentative contexts in which single metabolic groups of organisms dominate (e.g., lactic acid bacteria in silage). Where this is the case in the testing of EF with intended application in contexts such as ensilage, the challenges associated with avoiding certain metabolic outputs may be minimised. As EF fundamentally varies from MEC in that its intended product is not H2, hydrogenotrophic methanogenesis is not a primary concern. Finally, EF has been successfully tested in semi-continuous flow reactors, supporting that the shift from batch to continuous flow reactors is less likely to present a major issue in EF compared to MECs [41].
Bioelectrochemical systems need to compensate for the cost of electrons, so the lower the current for the desired effect, the better. Pilot-scale MECs highlight a further consideration: system overpotentials, which scale with the size of the reactor. As reactor size increases, the difference between the thermodynamically determined reduction potential and the experimental reduction potential also increases [42]. Essentially, the voltage required to supply the energy for the desired reaction to proceed increases. For example, in a pilot-scale, batch-operated MEC using urban wastewater, a potential loss of 0.5 V was observed, requiring the applied voltage to be increased accordingly [43]. Overpotentials increase the cost of MEC operation, and the potential losses will increase as these systems are scaled up further [44]. EF may suffer less from these potential losses than MECs, as one of the key features of EF is that it is very electron-efficient, requiring low potentials to alter the redox potential of the medium rather than being used to directly driving reactions [14]. In this case, overpotentials may, therefore, not be as detrimental but should be considered when determining the maintained profitability of EF at scale. Other successes and pitfalls of MEC scale-up have been reviewed extensively [33,45,46].
4. Metabolism of Lactic Acid Bacteria
The carbohydrate metabolism of lactic acid bacteria (LAB) is crucial to understanding their potential as producers of valuable platform chemicals and precursors, and whether that potential can be expanded and exploited by EF. LAB are ubiquitous industrial biocatalysts, with applications ranging from the food and medicine to biopolymer and biofuel industries. LAB are generally comprised of the Lactobacillus, Lactococcus, Streptococcus, Leuconostoc and Pediococcus genera, although further genera such as Enterococcus and Weisella also share the same physiological and metabolic traits and can be identified as LAB. Carbohydrate metabolism in LAB has been shaped by reductive evolution in nutrient-rich ecological niches such as the human gut, resulting in the uncoupling of carbon metabolism and biomass generation [47]. This feature permits ATP production to occur via stoichiometrically wasteful fermentative hexose metabolism producing large volumes of lactic acid (LA) and yielding just two ATPs per sugar [48]. In turn, this acidic metabolic endpoint has driven the evolution of acid stress tolerance in LAB, allowing them to outcompete other species by acidifying their environment and remain dominant in mixed cultures [49]. The combination of a “wasteful” metabolism producing large quantities of valuable end-product with their maintained ecological dominance in mixed cultures makes these organisms ideal for use in a variety of industrial contexts.
Many fundamental characteristics of LAB make them well suited to industrial and research applications. LAB are mostly non-motile, non-spore-forming Gram-positive bacteria in the order Lactobacillales [50]. Unifying features of their biology include small genomes (2–3.4 Mb) with low GC content (<55%), a facultative anaerobic metabolism, high acid tolerance and an inability to produce ATP via aerobic respiration, instead using fermentative lactic acid metabolism [47]. The large volume of acid produced during lactic acid metabolism means these organisms are acid-tolerant [49]. In addition, LAB species often have large temperature ranges for growth [51]. Finally, LAB are able to grow on a variety of hexoses and pentoses, making them ideal biocatalysts to produce value from a variety of waste products or silage [52].
As a group, LAB can utilise a diverse complement of sugars to produce a relatively constrained number of end-products. Species can be obligately homofermentative, obligately heterofermentative, or facultatively heterofermentative [47,53]. Without specific supplements [54], their metabolism is almost always anaerobic, even in aerobic environments [49], due to incomplete or entirely absent respiratory electron transport chain components [55].
LAB are a diverse group of bacteria and therefore have a variety of sugar metabolism pathways depending on whether they are members of homofermentative or heterofermentative species, and whether they are able to metabolise hexose or pentose sugars (or both). Figure 2 shows a simplified metabolic schematic for the hetero- and homofermentation of C5 and C6 sugars. Generally, homofermentative metabolism yields two ATP molecules and two pyruvate molecules for every glucose, or five pyruvate and five ATP molecules for every three xyloses metabolised; however, this leads to a redox imbalance in the cell, and so pyruvate is reduced to lactate via the oxidisation of NADH [56]. Heterofermentation of both pentoses and hexoses is achieved via the phosphoketolase pathway, with ATP yield depending on the final product [52].
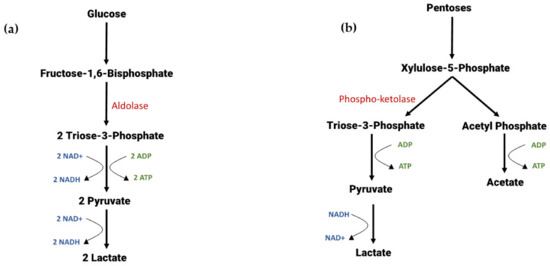
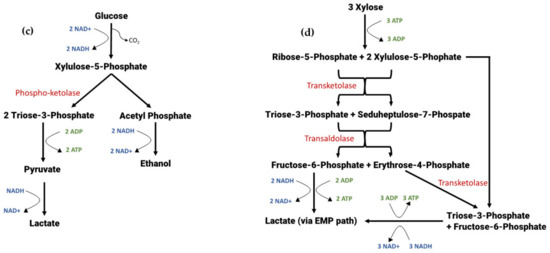
Figure 2.
Simplified metabolic schematic of homo- and heterofermentative metabolism of C5 and C6 sugars in LAB. Key enzymes are shown in red, ATP generation in green and NAD oxidation and reduction reactions in blue. (a) The Embden–Meyerhoff–Parnas Pathway showing homofermentative lactate production from glucose. (b) The pentose phosphoketolase pathway showing heterofermentative metabolism of C5 sugars. (c) Metabolism of glucose via the phosphoketolase pathway. (d) Homofermentative metabolism of pentose sugars via the pentose phosphate pathway.
Despite the relatively small number of compounds generated by LAB sugar metabolism, the bacteria, their metabolic intermediates and their metabolic end-products have diverse industrial applications, either directly or after further chemical and biological conversion. These applications can be separated into those derived from biomass generation and growth and those derived from energy-generating carbohydrate metabolism.
Many LAB can be directly used as dietary supplements, with the cells themselves acting as probiotics and prebiotics, or they can be used to produce nutraceutical supplements such as folic acid [57]. Some LAB also produce antimicrobial bacteriocins which allow further dominance in mixed cultures. These bacteriocins act differently to traditional beta-lactam antibiotics and are therefore of great interest in the pharmaceutical industry [58]. These direct uses are derived from biomass generation, which is uncoupled from carbohydrate metabolism in LAB. This is a result of LAB evolution in nutrient-rich niches which allowed the direct uptake of growth components, rather than the synthesis of biomass building blocks from carbohydrates [47]. LAB can also produce food-grade compounds via their carbohydrate metabolism-including flavourings, sweeteners and exopolysaccharides, the latter of which have medical and pharmaceutical applications [59]. The complete separation of biomass generation and carbohydrate metabolism creates two independent avenues for value addition by LAB.
The potential roles of LAB in the bioenergy and plastics industries are also derived from their sugar metabolism. At present, LAB are primarily used for the production of poly-lactate (PLA) and polyhydroxyalkanoate (PHA) plastic alternatives. PLA can be chemically derived from lactate and is a biodegradable, fossil-fuel-free plastic alternative, while PHAs are naturally produced by many LAB [53]. Potential roles have also been demonstrated for the production of biofuels such as bioalcohols and biohydrogen [53]. LAB in the Lactobacillales order have comparatively high alcohol tolerances, with some species growing healthily in up to 13% v/v ethanol [60] where they can become common contaminants in yeast-based ethanolic fermentations. With the growing capacity to redirect metabolic outputs using synthetic biology, the potential for a LAB-based biorefinery process with ethanol as an output is becoming more realistic [61]. While ethanol is naturally produced by LAB, metabolic engineering expands their potential use to other biofuel compounds such as butanol or 2,3-butanediol, which organisms can be engineered to metabolise from lactic acid [62]. LAB have another potential role in biohydrogen production due to their ability to produce formate through the mixed-acid pathway [53]. Industrially, molecular hydrogen production from formate would then need to be coupled with a second round of fermentation by organisms which are able to oxidise formate and generate H2 [63].
The decoupling of biomass generation and energy production in LAB means that they are able to offset inefficient carbohydrate metabolism with a high glycolytic throughput, as the sugar is not required for biomass generation. Combined with the relative specificity of metabolic outputs, this decoupling of growth and metabolism means that large volumes of a select few compounds are produced by pure cultures of LAB. The main compound, lactate, and the secondary bacteriocins produced by LAB ensure dominance through low pH and antimicrobial activity in mixed cultures, which limits contamination in bioreactors. Waste product reduction and contamination avoidance are key targets of metabolic engineering for industrial applications. The natural metabolic specificity and competitive features of LAB, as well as their serendipitously small genomes and genetic tractability, have therefore allowed them to diversify out of their role in traditional ensiling and maintain dominance in various modern industries.
5. LAB Ensiling and the Potential for Efficiency Gains
The presence of LAB in plant microbiota is essential for the preservation of food and crop nutrients during fermentation [64]. The fermentative preservation of foodstuffs has been a dietary staple for millennia. In the 19th century, this method was scaled to the preservation of seasonal crops for year-round animal feed provision through ensiling. This method was further developed in the 20th century, with innovations by Artturi Virtanen earning him the Nobel Prize in Chemistry in 1945 [65]. More recently, this method has been co-opted in biorefineries, both to reduce biomass loss during feedstock preservation and to generate large volumes of platform chemicals. Homofermentative LAB are therefore vital components of traditional ensiling and the renewable chemical industry, but their metabolism has biochemical limits which are the targets of yield improvement research.
Homofermentative LAB are central to the traditional ensiling process, converting soluble crop carbohydrates to lactic acid, thereby reducing pH and preventing the growth of spoilage microbes such as Clostridium species which convert both free sugar and lactic acid into butyric acid [66]. Additionally, Clostridia are often proteolytic and can convert crop proteins and amino acids into ammoniacal compounds, preventing crop preservation [51]. However, spoilage organisms typically grow above pH 5, while the endpoints of LAB fermentations are usually lower than pH 4 [67]. Successful ensiling therefore requires the rapid establishment of a sufficiently low pH to suppress the establishment, growth and metabolism of spoilage microbes. Homofermentative LAB are well suited to this application due to the large volumes of lactate and relatively small volumes of alternative products produced by their metabolism, which results in rapid pH decrease [68]. Their aerotolerance is also beneficial as this property can improve the aerobic stability of the silage after the opening of silos for feeding [69].
Ensiling has expanded beyond its initial use in animal feedstocks to allow year-round production in green biorefineries that aim to completely convert biomass into a variety of industrially valuable products, including platform chemicals, biofuels, biogas, animal feed pellets and fertiliser [64]. The biorefinery process is intended to mimic that of an oil refinery. Fresh or ensiled wet biomass is separated into solid and liquid fractions through pressing. The solid fraction consists of a press cake which contains lignocellulosic matter (LCM). As is, LCM can either be wrapped and ensiled again to produce animal feed, dried to produce feed pellets, or used to produce fibrous building materials such as insulation [64]. Ideally, however, LCM could be used to generate higher-value products such as biofuels or biogas. LAB are ideal biocatalysts for these processes as organic acids such as lactate can accelerate biogas production at the acetogenesis stage of methane production [70] and, as discussed previously, can also act as an intermediate in biofuel production [71]. The major challenge to this process is the breakdown of LCM, for which an efficient and cost-effective solution has not yet been found. Physico-chemical pre-treatment often produces toxic phenolic compounds, enzymatic breakdown is subject to end-product inhibition, syntrophic co-culture with cellulolytic organisms is high-maintenance at industrial scale, and heterologous cellulase expression in LAB has toxicity issues as well as limited secretion capacity [72].
Currently, higher-value products mainly come from the “green juice” fraction of fresh, plant biomass. In this fraction, which is rich in easily fermentable water-soluble carbohydrates and proteins, it is important to ensure preservation quickly for the same reasons as in ensiling. LAB have multiple functions in this case: to prevent contamination, produce lactic acid, recover proteins through biomass integration, or precipitate proteins through the subsequent pH decrease [73]. The resulting liquid can be separated into a protein concentrate, which can be used as feed supplement, and a “brown juice” which can be treated and used as a further fermentation broth, fed into biogas reactors or used as a fertiliser [64]. At present, many biorefineries focus on the production of polylactic acid, with outputs such as 140,000 tons of bioplastics per year [73]. Yields of PLA would, of course, be amplified if lignocellulosic waste could be efficiently broken down and converted to lactate, but this should also be complemented by attempting to increase yields by increasing the production of lactic acid from soluble carbohydrates.
There are a number of biochemical limitations which govern the conversion efficiency of sugars into lactic acid. As mentioned previously, mixed-acid fermentation can occur in homofermentative LAB under certain pH and temperature conditions, as well as conditions of low glycolytic flux [53]. Mixed- acid fermentation is a detrimental process when lactic acid is the desired output, but in the context of industrial feedstocks, it is common. Mixed-acid production is catalysed by pyruvate formate lyase, and the activity of this enzyme is allosterically and transcriptomically regulated by oxygen, glycolytic flux, pH and temperature [51,74]. Bioreactor conditions must therefore be carefully monitored to prevent significant redirection of pyruvate to acetic and formic acid through this pathway. Natural feedstocks are inherently limiting in this sense, as non-glucose sugars such as galactose can alter glycolytic flux, reducing the inhibition of PFL by GAP and DHAP and resulting in up to 27% of pyruvate being redirected towards formate, ethanol and acetate [74]. The highest outputs of lactic acid are usually found with pure starting sugars, which is irreconcilable with unrefined feedstocks. High glucose metabolism can also inhibit the metabolism of other sugars into lactic acid through catabolite repression [75].
Redox balance is also a factor which constrains LA production by LAB. Due to their obligately anaerobic lifestyle, LAB metabolism is governed by the presence of different (non-O2) electron acceptors and the redox state of the cell [48]. During homolactic fermentation, the energy-production stage occurs via the oxidation of carbohydrates to pyruvate [56]. The subsequent production of lactic acid from pyruvate is carried out to offset the redox debt generated during sugar oxidation, where NAD+ is consumed. Pyruvate is the primary electron acceptor in LAB, being reduced to form lactate, and in turn allowing NAD+ regeneration [48]. Other electron acceptors can also be used, varying based on species differences and environmental availability. Some species generate pyruvate without this NAD+ redox debt through citrate utilisation. In this process, no ATP is produced, but the pyruvate can be rerouted to energetically profitable pathways without needing to regenerate NAD+ [48,76]. Theoretically, because LA is produced to offset this redox debt, LA yield could be increased by artificially increasing this debt.
One obvious consequence of metabolism whose endpoint is a large volume of acid is the constant pH decrease in an enclosed reactor. Although LAB are largely acid-tolerant, most grow optimally between pH 4–6, while LA fermentations can drive reactor pH below 4 [51]. This is part of the reason why ensiling techniques are so successful for biomass preservation. To this end, LA-producing bioreactors currently require the addition of buffering materials such as calcium carbonate to allow continued growth and LA production. This has a number of downsides. Many dissociated (lactate-) forms of lactic acid, such as calcium lactate and ammonium lactate, which are produced when buffering agents are added, still have significant inhibitory effects [77]. Additionally, these compounds add cost to the industrial process, which needs to be low (< USD 0.8 per kg LA for polylactic acid) for LA production to be profitable [72]. Various chemical, synthetic biology and experimental evolution methods have been utilised to improve acid tolerance in LAB [78]. These methods have mostly been in the context of improving the probiotic properties of LAB. Ultimately, on a green biorefinery scale, highly productive mutant strains or constant LA removal/neutralisation are needed to allow the optimal growth-promoting and contamination-preventing conditions around pH 4–5, posing a challenge to the maintained profitability of the industry.
Although lactic acid metabolism is central to traditional ensiling, as well as the biofuel and biomaterial refinery industries, their potential in the latter is largely conceptual at this point. Key issues to be addressed in the coming decade are the breakdown of lignocellulosic material into usable sugars, limiting mixed-acid product formation, and maintaining a constant productive pH to maximise lactic acid production. These will require a combination of synthetic biology and bioprocess engineering to maximise the productivity and maintain the profitability of green bioreactors over fossil-fuel-based refineries.
6. Electrofermentation in Ensiling for Increased Platform-Chemical Yields from Biomass
LAB produce 90% of lactic acid globally [53]. Key factors affecting the scale-up of LA production for green biorefinery applications are the selection of appropriate biocatalysts, bioreactor conditions and the maintenance and suppression of other metabolic pathways. With the decreasing costs of sustainable electricity globally [79], the potential has arisen to integrate bioelectrochemical approaches into bioreactors to both guide biocatalyst metabolism and maintain optimal reactor conditions. As discussed previously, EF is a cost- and electron-efficient technique which can increase metabolic outputs many-fold through changes to the redox potential of the growth media [17,22], making EF an attractive candidate for increasing LA yield by LAB. Additionally, a major challenge for LA-based biorefineries is maintaining high productivity with constant acidification of the media. Cathodic electrofermentation approaches can increase the pH of the media, possibly presenting a solution to this issue. Ultimately, EF should be tested in industrial media over longer timeframes, combined with synthetic biology approaches, and tested in single-electrode reactors to optimise use in LA fermentations. In order to allow this potential to be realised, EF must first be tested in pure LAB cultures to identify the biological and economic viability and potential metabolic targets of the approach.
EF has never been tested in pure cultures of LAB, but its effects on lactic acid production have been observed in a few mixed-culture systems such as wastewater extractions. These studies demonstrated increased lactic acid production in cathodic EF treatments [27] but struggled to differentiate between community selection effects and biochemical mechanisms. Anodic EF experiments often focus on aerobic metabolism with the aim of supplying a non-O2 renewable terminal electron acceptor, but the potential to enhance the production of more oxidised products in anaerobic metabolism has also been proposed [80]. This mechanism has been explored in glycerol fermentations by Escherichia coli and Propionibacterium freudenreichii, where anodic potentials drive the production of more oxidised products [81,82]. In contrast to lactate-specific studies, which are rare, anodic and cathodic EF have been used extensively to alter the production of butanol, ethanol, 1,3-propanediol, glutamate, acetic acid and butyric acid [22], mostly in Gram-negative or mixed-culture study systems [80]. Given that LAB may play a large role in the carboxylate platform especially [83], this potential bioelectrochemical enhancement should be investigated.
An important limitation to exploring EF in LAB is their lack of natural electroactivity [1]. However, microbial electroactivity is not essential to the success of bioelectrochemical systems when mediators are present. Even the original demonstration of electrogenic microbial activity in 1910 used S. cerevisiae and E. coli, which are not considered electroactive but may have benefited from the presence of flavins and B-vitamins in the media, which potentially acted as redox shuttles [1]. As previously mentioned, mediators are molecules which can be reduced at the electrode and shuttle electrons into cells. They can be present in low amounts in media which contains yeast extract, such as the MRS media used for the lab cultures of many LAB. However, it is generally believed that mediators must either be produced by the microbes or added to the fermentation mix separately. Examples of weak mediators produced by fermentative metabolism include formate and acetate [14], both of which are produced via mixed acid fermentation in homofermentative LAB. The ability of heterofermentative products to act as mediators may be of value in avoiding the need for the addition of expensive external mediators; however, in bioreactors where product purity is highly desired, these benefits may be outweighed by reduced yields of the desired output, or the cost of additional purification or product separation. While artificial mediator addition theoretically makes EF possible in a more diverse range of microbial species, the added cost constrains industrial applications. This is especially true when mediators cannot be recycled and need to be continuously added, as is the case for the commonly utilised mediator methyl viologen. Neutral red is a recyclable alternative which has risen in popularity in recent years [84]. It is also worth noting, however, that in many mixed-culture electrofermentations, cathodic biofilms are highly diverse and not usually dominated by electroactive species [85]. This may point to electron shuttles in the media playing a larger role than previously thought.
Although LAB have a variety of industrial applications, the most immediately feasible combination with EF would be deriving platform chemicals from soluble carbohydrates in organic material, including silage. In this context, lactate is currently the main compound of interest. To this end, types of LAB should be selected which are naturally successful in ensiling environments, both as potential inoculants and as a part of the natural microbiome. Success can be measured by high lactate titres, high acid and other stress tolerance, and competitive traits. Morphology may contribute to the success of bioelectrochemical systems [86], and to this end, species should be selected to span the morphological diversity of LAB, including homo- and heterofermentative species, bacilli, coccobacilli and cocci. Other potentially interesting features could be genetic tractability to widen the scope of metabolites which could be produced, and possibly also the applicability of the species to different lactofermenting industries such as food or medicine.
7. Future Prospects
LAB have several metabolic and physiological features making them potentially attractive electrofermentation chassis candidates. As they are already widely used as biofactories, even in the simplest of ensiling paradigms with lactic acid being the sole product of interest as a biomass preservative, a means of increasing product output through the addition of cheap electrons (potentially coming from renewable sources such as photovoltaics) could increase the value of products in more complex bioreactor settings. The opportunity to tune heterofermentative output through current mediation may provide routes to higher-value outputs within the same system. The genetic tractability of several common LAB species also provides the potential for genetic engineering of these chassis to more closely control product output and increase yields through improved electroactivity.
The scalability of electrofermentation systems from the laboratory- or multi-litre pilot-scale to commercially viable industrial-scale fermentation has proven difficult to achieve due to the increasing costs of overpotential, biological diversity in inoculum and the maintenance of the desired hetero- or homofermentative behaviour of the chassis. Regardless of scale, EF benefits over other bioelectrochemical systems in that it has less extreme expectations of the biocatalysts used. However, EF research still seems to suffer from viewing biocatalysts as inert machines rather than living organisms, and remains largely theoretical rather than industrially applicable [16]. The metabolic and community complexity of microbes in MESs is frequently referenced as a limitation of these setups. In the near future, it may be valuable to focus on biocatalysts such as LAB which naturally dominate communities, produce large amounts of a single metabolite, and have established roles in industry. This could reduce the costs of separation and reactor engineering, and may prevent the trade-offs often associated with implementing extensively genetically modified bacteria in reactors [31]. However, the costs associated with engineering a biofactory chassis, and maintaining its dominance in large-scale fermentation, may be offset by the potential for high yields of higher-value metabolic outputs. Many LAB species such as Lactiplantibacillus plantarum, Pediococcus acidilacitici and Lactococcus lactis have been shown to be genetically tractable and, whilst not the most widely used model systems for genetic engineering, the naturally high product yields may provide routes to higher titres of desired outputs, if metabolic pathways can be refined through genetic modification.
Author Contributions
Writing—original draft preparation, J.C.W.; writing—review and editing, J.C.W., M.H., P.L. and J.L; supervision, J.L.; Funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant (PT34767) to J. Love from Shell Research Ltd.
Acknowledgments
The illustration in Figure 1 was created by Daria Chrobok, DC SciArt.
Conflicts of Interest
The authors declare no conflict of interest. Dr Ping Liu is employed at Shell Technology Centre Houston, and reviewed and edited the manuscript; however, there are no patents, products in development or marketed products to declare.
References
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.; Cavanagh, K.; Munnings, C.; Moore, T.; Cheng, K.Y.; Kaksonen, A.H. Application of microbial fuel cells to power sensor networks for ecological monitoring. In Wireless Sensor Networks and Ecological Monitoring; Springer: Berlin/Heidelberg, Germany, 2013; pp. 151–178. [Google Scholar]
- Erable, B.; Etcheverry, L.; Bergel, A. From microbial fuel cell (MFC) to microbial electrochemical snorkel (MES): Maximizing chemical oxygen demand (COD) removal from wastewater. Biofouling 2011, 27, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Blatter, M.; Furrer, C.; Cachelin, C.P.; Fischer, F. Phosphorus, chemical base and other renewables from wastewater with three 168-L microbial electrolysis cells and other unit operations. Chem. Eng. J. 2020, 390, 124502. [Google Scholar] [CrossRef]
- Blatter, M.; Delabays, L.; Furrer, C.; Huguenin, G.; Cachelin, C.P.; Fischer, F. Stretched 1000-L microbial fuel cell. J. Power Sources 2021, 483, 229130. [Google Scholar] [CrossRef]
- Janicek, A.; Fan, Y.; Liu, H. Design of microbial fuel cells for practical application: A review and analysis of scale-up studies. Biofuels 2014, 5, 79–92. [Google Scholar] [CrossRef]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.M.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Rozendal, R.A. Microbial Electrolysis Cells for High Yield Hydrogen Gas Production from Organic Matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef]
- Lu, L.; Hou, D.; Fang, Y.; Huang, Y.; Ren, Z.J. Nickel based catalysts for highly efficient H2 evolution from wastewater in microbial electrolysis cells. Electrochim. Acta 2016, 206, 381–387. [Google Scholar] [CrossRef]
- Rabaey, K.; Girguis, P.; Nielsen, L.K. Metabolic and practical considerations on microbial electrosynthesis. Curr. Opin. Biotechnol. 2011, 22, 371–377. [Google Scholar] [CrossRef]
- Upadhyaya, B.P.; DeVeaux, L.C.; Christopher, L.P. Metabolic engineering as a tool for enhanced lactic acid production. Trends Biotechnol. 2014, 32, 637–644. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Q.; Li, L.; Qin, W.; Yang, J.; Zhang, H.; Jiang, X.; Cheng, T.; Liu, W.; Xu, X.; et al. Metabolic engineering of Escherichia coli for high-specificity production of isoprenol and prenol as next generation of biofuels. Biotechnol. Biofuels 2013, 6, 57. [Google Scholar] [CrossRef]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef]
- Kumar, A.; Hsu, L.H.-H.; Kavanagh, P.; Barrière, F.; Lens, P.N.L.; Lapinsonnière, L.; Lienhard, V.J.H.; Schröder, U.; Jiang, X.; Leech, D. The ins and outs of microorganism–electrode electron transfer reactions. Nat. Rev. Chem. 2017, 1, 24. [Google Scholar] [CrossRef]
- Moscoviz, R.; Toledo-Alarcón, J.; Trably, E.; Bernet, N. Electro-Fermentation: How To Drive Fermentation Using Electrochemical Systems. Trends Biotechnol. 2016, 34, 856–865. [Google Scholar] [CrossRef]
- Yong, X.-Y.; Shi, D.-Y.; Chen, Y.-L.; Jiao, F.; Lin, X.; Zhou, J.; Wang, S.-Y.; Yong, Y.-C.; Sun, Y.-M.; OuYang, P.-K.; et al. Enhancement of bioelectricity generation by manipulation of the electron shuttles synthesis pathway in microbial fuel cells. Bioresour. Technol. 2014, 152, 220–224. [Google Scholar] [CrossRef]
- Prévoteau, A.; Carvajal-Arroyo, J.M.; Ganigué, R.; Rabaey, K. Microbial electrosynthesis from CO2: Forever a promise? Curr. Opin. Biotechnol. 2020, 62, 48–57. [Google Scholar] [CrossRef]
- Choi, O.; Kim, T.; Woo, H.M.; Um, Y. Electricity-driven metabolic shift through direct electron uptake by electroactive heterotroph Clostridium pasteurianum. Sci. Rep. 2014, 4, 6961. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.a.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Awate, B.; Steidl, R.J.; Hamlischer, T.; Reguera, G. Stimulation of electro-fermentation in single-chamber microbial electrolysis cells driven by genetically engineered anode biofilms. J. Power Sources 2017, 356, 510–518. [Google Scholar] [CrossRef]
- Qu, G.; Qiu, W.; Liu, Y.; Zhong, D.; Ning, P. Electropolar effects on anaerobic fermentation of lignocellulosic materials in novel single-electrode cells. Bioresour. Technol. 2014, 159, 88–94. [Google Scholar] [CrossRef]
- Larrosa-Guerrero, A.; Scott, K.; Katuri, K.P.; Godinez, C.; Head, I.M.; Curtis, T. Open circuit versus closed circuit enrichment of anodic biofilms in MFC: Effect on performance and anodic communities. Appl. Microbiol. Biotechnol. 2010, 87, 1699–1713. [Google Scholar] [CrossRef]
- Schievano, A.; Pepé Sciarria, T.; Vanbroekhoven, K.; De Wever, H.; Puig, S.; Andersen, S.J.; Rabaey, K.; Pant, D. Electro-Fermentation—Merging Electrochemistry with Fermentation in Industrial Applications. Trends Biotechnol. 2016, 34, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Alonso, R.M.; Escapa, A.; Morán, A. Methodology for Fast and Facile Characterisation of Carbon-Based Electrodes Focused on Bioelectrochemical Systems Development and Scale Up. Materials 2017, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.; Zhu, X.; Zhang, L.; Tao, Y.; He, X.; Li, D.; Yan, Z. Short communication. Electrochem. Commun. 2014, 45, 67–70. [Google Scholar] [CrossRef]
- Sharma, M.; Jain, P.; Varanasi, J.L.; Lal, B.; Rodríguez, J.; Lema, J.M.; Sarma, P.M. Enhanced performance of sulfate reducing bacteria based biocathode using stainless steel mesh on activated carbon fabric electrode. Bioresour. Technol. 2013, 150, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Nevin, K.P. A shift in the current: New applications and concepts for microbe-electrode electron exchange. Curr. Opin. Biotechnol. 2011, 22, 441–448. [Google Scholar] [CrossRef]
- Xue, G.; Lai, S.; Li, X.; Zhang, W.; You, J.; Chen, H.; Qian, Y.; Gao, P.; Liu, Z.; Liu, Y. Efficient bioconversion of organic wastes to high optical activity of l-lactic acid stimulated by cathode in mixed microbial consortium. Water Res. 2018, 131, 1–10. [Google Scholar] [CrossRef]
- Villano, M.; Paiano, P.; Palma, E.; Miccheli, A.; Majone, M. Electrochemically Driven Fermentation of Organic Substrates with Undefined Mixed Microbial Cultures. ChemSusChem 2017, 10, 3091–3097. [Google Scholar] [CrossRef]
- Kracke, F.; Virdis, B.; Bernhardt, P.V.; Rabaey, K.; Krömer, J.O. Redox dependent metabolic shift in Clostridium autoethanogenum by extracellular electron supply. Biotechnol. Biofuels 2016, 9, 249. [Google Scholar] [CrossRef]
- Kim, C.; Kim, M.Y.; Michie, I.; Jeon, B.-H.; Premier, G.C.; Park, S.; Kim, J.R. Anodic electro-fermentation of 3-hydroxypropionic acid from glycerol by recombinant Klebsiella pneumoniae L17 in a bioelectrochemical system. Biotechnol. Biofuels 2017, 10, 199. [Google Scholar] [CrossRef]
- Kumar, P.K.R.; Maschke, H.E.; Friehs, K.; Schügerl, K. Strategies for improving plasmid stability in genetically modified bacteria in bioreactors. Trends Biotechnol. 1991, 9, 279–284. [Google Scholar] [CrossRef]
- Drewnowski, J.; Remiszewska-Skwarek, A.; Duda, S.; Łagód, G. Aeration Process in Bioreactors as the Main Energy Consumer in a Wastewater Treatment Plant. Review of Solutions and Methods of Process Optimization. Processes 2019, 7, 311. [Google Scholar] [CrossRef]
- Leicester, D.; Amezaga, J.; Heidrich, E. Is bioelectrochemical energy production from wastewater a reality? Identifying and standardising the progress made in scaling up microbial electrolysis cells. Renew. Sustain. Energy Rev. 2020, 133, 110279. [Google Scholar] [CrossRef]
- Vítězová, M.; Kohoutová, A.; Vítěz, T.; Hanišáková, N.; Kushkevych, I. Methanogenic Microorganisms in Industrial Wastewater Anaerobic Treatment. Processes 2020, 8, 1546. [Google Scholar] [CrossRef]
- Call, D.; Logan, B.E. Hydrogen Production in a Single Chamber Microbial Electrolysis Cell Lacking a Membrane. Environ. Sci. Technol. 2008, 42, 3401–3406. [Google Scholar] [CrossRef]
- Cusick, R.D.; Bryan, B.; Parker, D.S.; Merrill, M.D.; Mehanna, M.; Kiely, P.D.; Liu, G.; Logan, B.E. Performance of a pilot-scale continuous flow microbial electrolysis cell fed winery wastewater. Appl. Microbiol. Biotechnol. 2011, 89, 2053–2063. [Google Scholar] [CrossRef]
- Rader, G.K.; Logan, B.E. Multi-electrode continuous flow microbial electrolysis cell for biogas production from acetate. Int. J. Hydrog. Energy 2010, 35, 8848–8854. [Google Scholar] [CrossRef]
- Carmona-Martínez, A.A.; Trably, E.; Milferstedt, K.; Lacroix, R.; Etcheverry, L.; Bernet, N. Long-term continuous production of H2 in a microbial electrolysis cell (MEC) treating saline wastewater. Water Res. 2015, 81, 149–156. [Google Scholar] [CrossRef]
- Sun, R.; Zhou, A.; Jia, J.; Liang, Q.; Liu, Q.; Xing, D.; Ren, N. Characterization of methane production and microbial community shifts during waste activated sludge degradation in microbial electrolysis cells. Bioresour. Technol. 2015, 175, 68–74. [Google Scholar] [CrossRef]
- Ferrera, I.; Sánchez, O. Insights into microbial diversity in wastewater treatment systems: How far have we come? Biotechnol. Adv. 2016, 34, 790–802. [Google Scholar] [CrossRef]
- Ma, H.; Wu, W.; Yu, Z.; Zhao, J.; Fu, P.; Xia, C.; Lam, S.S.; Wang, Q.; Gao, M. Medium-chain fatty acid production from Chinese liquor brewing yellow water by electro-fermentation: Division of fermentation process and segmented electrical stimulation. Bioresour. Technol. 2022, 360, 127510. [Google Scholar] [CrossRef]
- Clauwaert, P.; Aelterman, P.; Pham, T.H.; De Schamphelaire, L.; Carballa, M.; Rabaey, K.; Verstraete, W. Minimizing losses in bio-electrochemical systems: The road to applications. Appl. Microbiol. Biotechnol. 2008, 79, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Baeza, J.A.; Martínez-Miró, À.; Guerrero, J.; Ruiz, Y.; Guisasola, A. Bioelectrochemical hydrogen production from urban wastewater on a pilot scale. J. Power Sources 2017, 356, 500–509. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Escapa, A.; San-Martín, M.I.; Mateos, R.; Morán, A. Scaling-up of membraneless microbial electrolysis cells (MECs) for domestic wastewater treatment: Bottlenecks and limitations. Bioresour. Technol. 2015, 180, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Escapa, A.; Mateos, R.; Martínez, E.J.; Blanes, J. Microbial electrolysis cells: An emerging technology for wastewater treatment and energy recovery. From laboratory to pilot plant and beyond. Renew. Sustain. Energy Rev. 2016, 55, 942–956. [Google Scholar] [CrossRef]
- Sauer, M.; Russmayer, H.; Grabherr, R.; Peterbauer, C.K.; Marx, H. The Efficient Clade: Lactic Acid Bacteria for Industrial Chemical Production. Trends Biotechnol. 2017, 35, 756–769. [Google Scholar] [CrossRef]
- Hansen, E.B. Redox reactions in food fermentations. Curr. Opin. Food Sci. 2018, 19, 98–103. [Google Scholar] [CrossRef]
- Hofvendahl, K.; Hahn–Hägerdal, B. Factors affecting the fermentative lactic acid production from renewable resources1. Enzym. Microb. Technol. 2000, 26, 87–107. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Wood, B.J.B. Lactic Acid Bacteria: Biodiversity and Taxonomy; John Wiley and Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Fitzsimons, A.; Duffner, F.; Curtin, D.; Brophy, G.; O’Kiely, P.; O’Connell, M. Assessment of Pediococcus acidilactici as a Potential Silage Inoculant. Appl. Environ. Microbiol. 1992, 58, 3047–3052. [Google Scholar] [CrossRef]
- Kandler, O. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 1983, 49, 209–224. [Google Scholar] [CrossRef]
- Mazzoli, R.; Bosco, F.; Mizrahi, I.; Bayer, E.A.; Pessione, E. Towards lactic acid bacteria-based biorefineries. Biotechnol. Adv. 2014, 32, 1216–1236. [Google Scholar] [CrossRef]
- Brooijmans, R.J.W.; De Vos, W.M.; Hugenholtz, J. Lactobacillus plantarum WCFS1 Electron Transport Chains. Appl. Environ. Microbiol. 2009, 75, 3580–3585. [Google Scholar] [CrossRef]
- Brooijmans, R.J.W.; Poolman, B.; Schuurman-Wolters, G.K.; Vos, W.M.d.; Hugenholtz, J. Generation of a Membrane Potential by Lactococcus lactis through Aerobic Electron Transport. J. Bacteriol. 2007, 189, 5203–5209. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Hugenholtz, J.; Kleerebezem, M. Metabolic engineering of lactic acid bacteria: Overview of the approaches and results of pathway rerouting involved in food fermentations. Curr. Opin. Biotechnol. 1999, 10, 492–497. [Google Scholar] [CrossRef]
- Porto, M.C.W.; Kuniyoshi, T.M.; Azevedo, P.O.S.; Vitolo, M.; Oliveira, R.P.S. Pediococcus spp.: An important genus of lactic acid bacteria and pediocin producers. Biotechnol. Adv. 2017, 35, 361–374. [Google Scholar] [CrossRef]
- Moscovici, M. Present and future medical applications of microbial exopolysaccharides. Front. Microbiol. 2015, 6, 1012. [Google Scholar] [CrossRef]
- G-Alegría, E.; López, I.; Ruiz, J.I.; Sáenz, J.; Fernández, E.; Zarazaga, M.; Dizy, M.; Torres, C.; Ruiz-Larrea, F. High tolerance of wild Lactobacillus plantarum and Oenococcus oeni strains to lyophilisation and stress environmental conditions of acid pH and ethanol. FEMS Microbiol. Lett. 2004, 230, 53–61. [Google Scholar] [CrossRef]
- Moraïs, S.; Shterzer, N.; Grinberg, I.R.; Mathiesen, G.; Eijsink, V.G.H.; Axelsson, L.; Lamed, R.; Bayer, E.A.; Mizrahi, I. Establishment of a Simple Lactobacillus plantarum Cell Consortium for Cellulase-Xylanase Synergistic Interactions. Appl. Environ. Microbiol. 2013, 79, 5242–5249. [Google Scholar] [CrossRef]
- Liu, J.; Chan, S.H.J.; Brock-Nannestad, T.; Chen, J.; Lee, S.Y.; Solem, C.; Jensen, P.R. Combining metabolic engineering and biocompatible chemistry for high-yield production of homo-diacetyl and homo-(S,S)-2,3-butanediol. Metab. Eng. 2016, 36, 57–67. [Google Scholar] [CrossRef]
- Oh, H.; Wee, Y.-J.; Yun, J.-S.; Ho Han, S.; Jung, S.; Ryu, H.-W. Lactic acid production from agricultural resources as cheap raw materials. Bioresour. Technol. 2005, 96, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Lübeck, M.; Lübeck, P.S. Application of lactic acid bacteria in green biorefineries. FEMS Microbiol. Lett. 2019, 366, fnz024. [Google Scholar] [CrossRef] [PubMed]
- NobelPrize. The Nobel Prize in Chemistry 1945. Nobel Prize Outreach AB. 2022. Available online: https://www.nobelprize.org/prizes/chemistry/1945/summary/ (accessed on 13 October 2022).
- Guo, X.S.; Ke, W.C.; Ding, W.R.; Ding, L.M.; Xu, D.M.; Wang, W.W.; Zhang, P.; Yang, F.Y. Profiling of metabolome and bacterial community dynamics in ensiled Medicago sativa inoculated without or with Lactobacillus plantarum or Lactobacillus buchneri. Sci. Rep. 2018, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Ramirez, J.M.; Haagenson, D.M.; Pryor, S.W.; Wiesenborn, D.P. Beet tissue ensiling: An alternative for long-term storage of sugars in industrial beets for nonfood use. Biomass Bioenergy 2016, 85, 135–143. [Google Scholar] [CrossRef]
- Schmidt, J.; Sipocz, J.; Kaszás, I.; Szakács, G.; Gyepes, A.; Tengerdy, R.P. Preservation of sugar content in ensiled sweet sorghum. Bioresour. Technol. 1997, 60, 9–13. [Google Scholar] [CrossRef]
- Danner, H.; Holzer, M.; Mayrhuber, E.; Braun, R. Acetic Acid Increases Stability of Silage under Aerobic Conditions. Appl. Environ. Microbiol. 2003, 69, 562–567. [Google Scholar] [CrossRef]
- Klang, J.; Theuerl, S.; Szewzyk, U.; Huth, M.; Tölle, R.; Klocke, M. Dynamic variation of the microbial community structure during the long-time mono-fermentation of maize and sugar beet silage. Microb. Biotechnol. 2015, 8, 764–775. [Google Scholar] [CrossRef]
- Petrus, L.; Noordermeer, M.A. Biomass to biofuels, a chemical perspective. Green Chem. 2006, 8, 861–867. [Google Scholar] [CrossRef]
- Tarraran, L.; Mazzoli, R. Alternative strategies for lignocellulose fermentation through lactic acid bacteria: The state of the art and perspectives. FEMS Microbiol. Lett. 2018, 365, fny126. [Google Scholar] [CrossRef]
- Kamm, B.; Schönicke, P.; Hille, C. Green biorefinery—Industrial implementation. Food Chem. 2016, 197, 1341–1345. [Google Scholar] [CrossRef]
- Melchiorsen, C.V.K.J.R. The level of pyruvate-formate lyase controls the shift from homolactic to mixed-acid product formation in Lactococcus lactis. Appl. Microbiol. Biotechnol. 2002, 58, 338–344. [Google Scholar]
- Yun, J.-S.; Ryu, H.-W. Lactic acid production and carbon catabolite repression from single and mixed sugars using Enterococcus faecalis RKY1. Process. Biochem. 2001, 37, 235–240. [Google Scholar] [CrossRef]
- Konings, W.N. Microbial transport: Adaptations to natural environments. Antonie Van Leeuwenhoek 2006, 90, 325–342. [Google Scholar] [CrossRef]
- Hetényi, K.; Németh, Á.; Sevella, B. Role of pH-regulation in lactic acid fermentation: Second steps in a process improvement. Chem. Eng. Process. Process. Intensif. 2011, 50, 293–299. [Google Scholar] [CrossRef]
- Wang, C.; Cui, Y.; Qu, X. Mechanisms and improvement of acid resistance in lactic acid bacteria. Arch. Microbiol. 2018, 200, 195–201. [Google Scholar] [CrossRef]
- International Renewable Energy Agency IRENA. Renewable Power Generation Costs in 2019; International Renewable Energy Agency IRENA: Abu Dhabi, United Arab Emirates, 2020. [Google Scholar]
- Vassilev, I.; Gießelmann, G.; Schwechheimer, S.K.; Wittmann, C.; Virdis, B.; Krömer, J.O. Anodic electro-fermentation: Anaerobic production of L-Lysine by recombinant Corynebacterium glutamicum. Biotechnol. Bioeng. 2018, 115, 1499–1508. [Google Scholar] [CrossRef]
- Emde, R.; Schink, B. Oxidation of glycerol, lactate, and propionate by Propionibacterium freudenreichii in a poised-potential amperometric culture system. Arch. Microbiol. 1990, 153, 506–512. [Google Scholar] [CrossRef]
- Emde, R.; Swain, A.; Schink, B. Anaerobic oxidation of glycerol by Escherichia coli in an amperometric poised-potential culture system. Appl. Microbiol. Biotechnol. 1989, 32, 170–175. [Google Scholar] [CrossRef]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef]
- Choi, O.; Um, Y.; Sang, B.-I. Butyrate production enhancement by Clostridium tyrobutyricum using electron mediators and a cathodic electron donor. Biotechnol. Bioeng. 2012, 109, 2494–2502. [Google Scholar] [CrossRef]
- Wrighton, K.C.; Virdis, B.; Clauwaert, P.; Read, S.T.; Daly, R.A.; Boon, N.; Piceno, Y.; Andersen, G.L.; Coates, J.D.; Rabaey, K. Bacterial community structure corresponds to performance during cathodic nitrate reduction. ISME J. 2010, 4, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Li, M.; Wang, Y.; Wu, Y.; Zhu, L.; Wang, X.; Zhao, Y. Enhancement of hydrogen production and energy recovery through electro-fermentation from the dark fermentation effluent of food waste. Environ. Sci. Ecotechnol. 2020, 1, 100006. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).