Superheated Steam Spray Drying as an Energy-Saving Drying Technique: A Review
Abstract
1. Introduction
2. Superheated Steam Drying
2.1. Advantages of Superheated Steam Drying
2.2. Application of Superheated Steam Drying
- Kiln dryers—wood drying
- Rotary drum dryers—lignite, fish press cake and brewer’s spent grain drying
- Fluidized bed dryers—parboiled rice, Thai native rice cultivars, sawdust, paddy and seeds, pulp and biomass
- Flash dryers—fishmeal drying
- Impingement dryers—fish press-cake and seeds drying.
3. Theoretical Background of Superheated Steam Drying
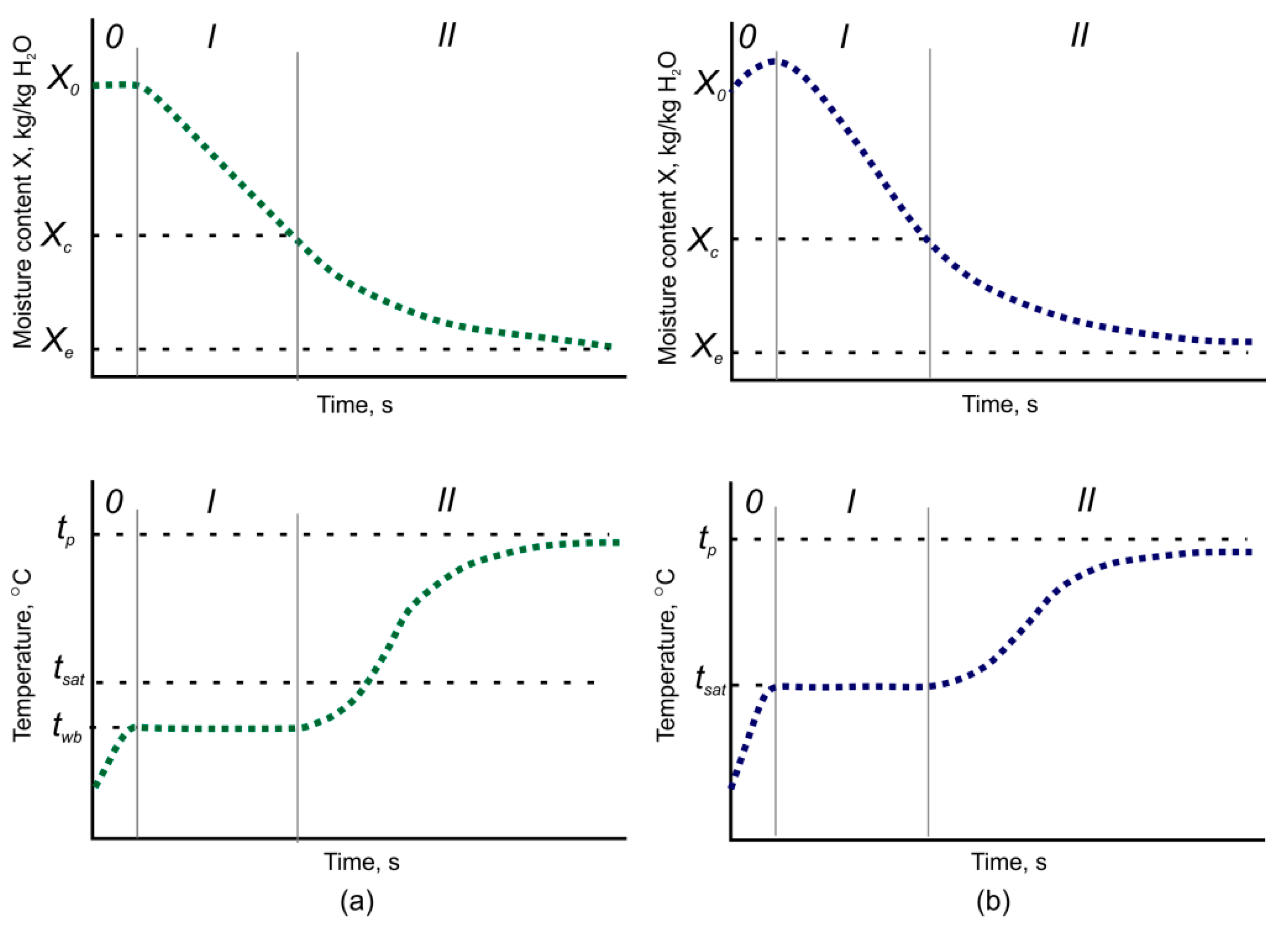
3.1. Drying Kinetics of Superheated Steam Drying
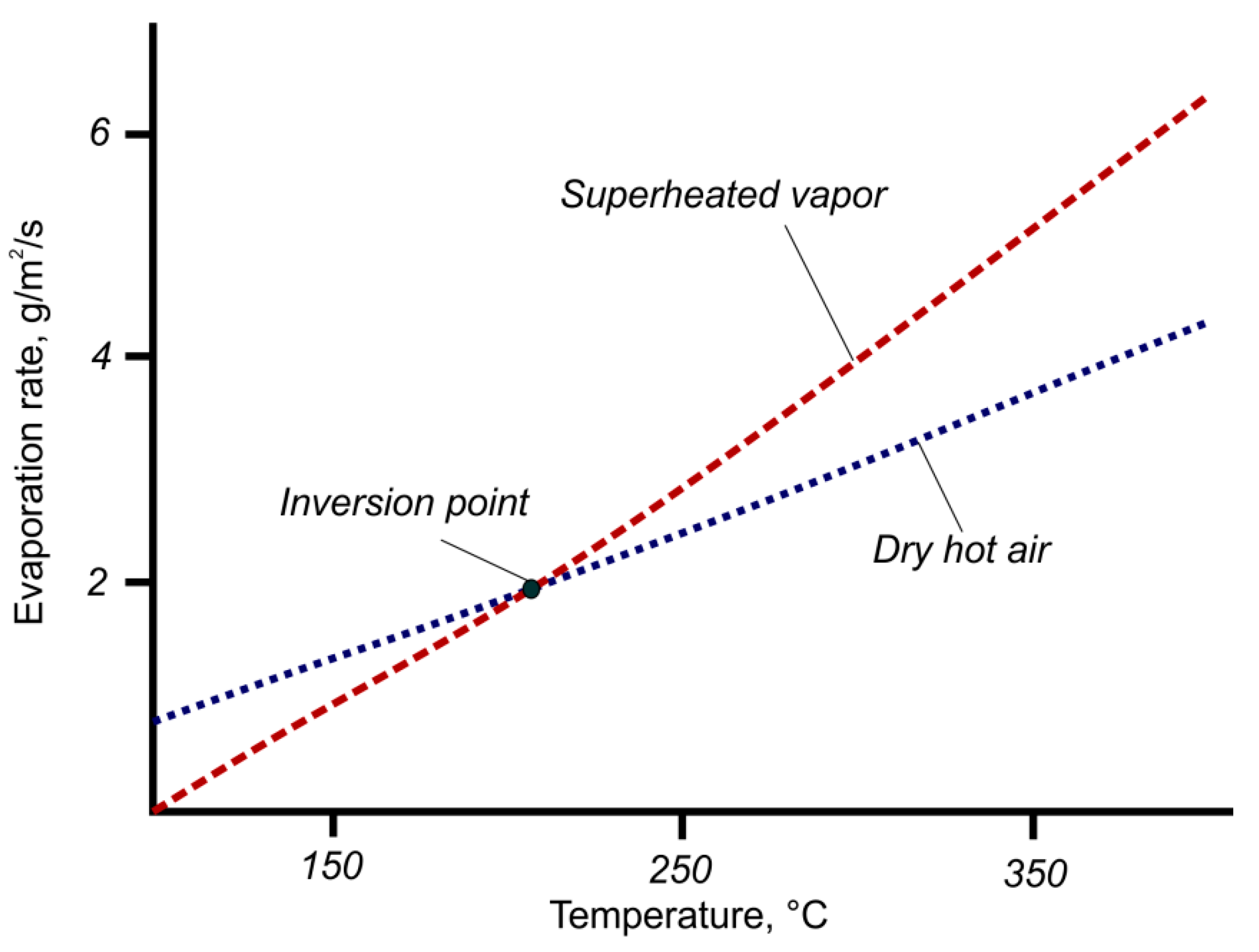
3.2. Inversion Temperature
3.3. Maximum Drying Rate as a Function of SHS Pressure
4. Modeling of Superheated Steam Spray Drying
4.1. Modeling of Isolated Solid Particles Drying in SHS
4.2. Modeling of Superheated Spray Drying in Laboratory and Pilot Plant Dryers
- Continuity equation for steam:
- Momentum balance equation for steam:
- Energy balance for steam:
- Droplet trajectories:
- Heat and mass transfer between steam and droplets:
5. Experimental Studies of Superheated Spray Drying
5.1. Single Droplet Drying in Superheated Steam
5.2. Spray Drying by Superheated Steam in Laboratory and Pilot Plant Scale
6. Perspectives of Superheated Steam Spray Drying
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Ap | particles area (m2) |
| Cdrag | drag coefficient |
| cp | specific heat of liquid phase (J kg−1 K−1) |
| cpg | mean superheated steam heat capacity (J kg−1 K−1) |
| cps | specific heat of solid phase (J kg−1 K−1) |
| D | dryer diameter (m) |
| Dd | droplet diameter (m) |
| F | body force (N) |
| g | gravitational acceleration (m s−2) |
| ΔH | latent heat of vaporization (J kg−1) |
| H | dryer height (m) |
| Hg | specific enthalpy of steam (J kg−1) |
| K | proportion factor in eq. 2 |
| kg | thermal conductivity of steam (W m−1 K−1) |
| mmax | maximum drying rate (kg s−1) |
| mp | mass of particle (kg) |
| ms | mass of solid phase (kg) |
| N | dimensionless parameter, relative variation of heat transfer coefficient with pressure |
| Nq | dimensionless number, ratio of amount of heat transferred by convection to the amount of heat introduced by superheated steam as a sensible heat |
| Nu | Nusselt number |
| P | total pressure (Pa) |
| Pop | optimum superheated steam pressure (Pa) |
| RNq | dimensionless function |
| SH | source term for heat production (J s−1 m−3) |
| Si | inlet sectional area (m2) |
| SM | source term of heat production (kg s−1 m−3) |
| t | time (s) |
| Teq | equilibrium temperature (K) |
| Tg | steam temperature (K) |
| Ti | inlet temperature (K) |
| Tp | particle temperature (K) |
| Ts | saturation temperature (K) |
| Vg | steam velocity (m s−1) |
| vi | inlet velocity (m s−1) |
| Vp | particles velocity (m s−1) |
| W | moisture content (kg kg−1H2O) |
| Wc | critical moisture content (kg kg−1H2O) |
| ∇ | nabla vector differential operator |
| Greek symbols: | |
| α | heat transfer coefficient (W m−2 K−1) |
| θ | parameter in Equation (4), θ = (Tp–Ts)/24.6 |
| ρg | steam density (kg m−3) |
| ρp | particles density (kg m−3) |
| τ | stress tensor (Pa) |
| φ | heat transfer coefficient factor |
References
- Filkova, I.; Huang, L.X.; Mujumdar, A.S. Industrial spray drying systems. In Handbook of Industrial Drying; Mujumdar, A.S., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 215–256. [Google Scholar]
- Land, C.M.V. Spray Drying. In Drying in the Process Industry; Wiley: Hoboken, NJ, USA, 2012; pp. 133–162. [Google Scholar] [CrossRef]
- Bellinghausen, R. Spray drying from yesterday to tomorrow: An industrial perspective. Dry. Technol. 2019, 37, 612–622. [Google Scholar] [CrossRef]
- Wawrzyniak, P.; Jaskulski, M.; Piatkowski, M.; Sobulska, M.; Zbicinski, I.; Egan, S. Experimental detergent drying analysis in a counter-current spray dryer with swirling air flow. Dry. Technol. 2020, 38, 108–116. [Google Scholar] [CrossRef]
- Jaskulski, M.; Wawrzyniak, P.; Zbiciński, I. CFD model of particle agglomeration in spray drying. Dry. Technol. 2015, 33, 1971–1980. [Google Scholar] [CrossRef]
- Oakley, D.E. Spray Dryer Modeling in Theory and Practice. Dry. Technol. 2004, 22, 1371–1402. [Google Scholar] [CrossRef]
- Razmi, R.; Jubaer, H.; Krempski-Smejda, M.; Jaskulski, M.; Xiao, J.; Chen, X.D.; Woo, M.W. Recent initiatives in effective modeling of spray drying. Dry. Technol. 2021, 39, 1614–1647. [Google Scholar] [CrossRef]
- Zbicinski, I. Modeling and Scaling Up of Industrial Spray Dryers: A Review. J. Chem. Eng. Jpn. 2017, 50, 757–767. [Google Scholar] [CrossRef]
- Samborska, K.; Poozesh, S.; Barańska, A.; Sobulska, M.; Jedlińska, A.; Arpagaus, C.; Malekjani, N.; Jafari, S.M. Innovations in spray drying process for food and pharma industries. J. Food Eng. 2022, 321, 110960. [Google Scholar] [CrossRef]
- Mujumdar, A.S. Superheated Steam Drying. In Handbook of Industrial Drying; CRC Press: Boca Raton, FL, USA, 2006; pp. 439–452. [Google Scholar]
- Romdhana, H.; Bonazzi, C.; Esteban-Decloux, M. Superheated Steam Drying: An Overview of Pilot and Industrial Dryers with a Focus on Energy Efficiency. Dry. Technol. 2015, 33, 1255–1274. [Google Scholar] [CrossRef]
- Van Deventer, H.C.; Heijmans, R.M.H. Drying with superheated steam. Dry. Technol. 2001, 19, 2033–2045. [Google Scholar] [CrossRef]
- Nygaard, H.; Hostmark, O. Microbial inactivation during superheated steam drying of fish meal. Dry. Technol. 2008, 26, 222–230. [Google Scholar] [CrossRef]
- Erkinbaev, C.; Ramachandran, R.P.; Cenkowski, S.; Paliwal, J. A comparative study on the effect of superheated steam and hot air drying on microstructure of distillers’ spent grain pellets using X-ray micro-computed tomography. J. Food Eng. 2019, 241, 127–135. [Google Scholar] [CrossRef]
- Brar, N.K.; Ramachandran, R.P.; Cenkowski, S.; Paliwal, J. Effect of Superheated Steam- and Hot Air-Assisted Processing on Functional and Nutritional Properties of Yellow Peas. Food Bioprocess Technol. 2021, 14, 1684–1699. [Google Scholar] [CrossRef]
- Bao, Y.; Zhou, Y. Comparative study of moisture absorption and dimensional stability of Chinese cedar wood with conventional drying and superheated steam drying. Dry. Technol. 2017, 35, 860–866. [Google Scholar] [CrossRef]
- Liu, J.; Xue, J.; Xu, Q.; Shi, Y.; Wu, L.; Li, Z. Drying Kinetics and Quality Attributes of White Radish in Low Pressure Superheated Steam. Int. J. Food Eng. 2017, 13, 20160365. [Google Scholar] [CrossRef]
- Mujumdar, A.S. An overview of innovation in industrial drying: Current status and R&D needs. Transp. Porous Media 2007, 66, 3–18. [Google Scholar] [CrossRef]
- Alfy, A.; Kiran, B.V.; Jeevitha, G.C.; Hebbar, H.U. Recent Developments in Superheated Steam Processing of Foods—A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2191–2208. [Google Scholar] [CrossRef]
- Li, J.; Liang, Q.-C.; Bennamoun, L. Superheated steam drying: Design aspects, energetic performances, and mathematical modeling. Renew. Sustain. Energy Rev. 2016, 60, 1562–1583. [Google Scholar] [CrossRef]
- Jensen, A.S.; Larsen, K. The Development of Large Pressurized Fluid Bed Steam Dryers from Fundamental Research to Industrial Plants. Dry. Technol. 2015, 33, 1631–1643. [Google Scholar] [CrossRef]
- Chryat, Y.; Esteban-Decloux, M.; Labarde, C.; Romdhana, H. A concept and industrial testing of a superheated steam rotary dryer demonstrator: Cocurrent-triple pass design. Dry. Technol. 2019, 37, 468–474. [Google Scholar] [CrossRef]
- Jaszczur, M.; Dudek, M.; Rosen, M.A.; Kolenda, Z. An analysis of integration of a power plant with a lignite superheated steam drying unit. J. Clean. Prod. 2020, 243, 118635. [Google Scholar] [CrossRef]
- Chantasiriwan, S.; Charoenvai, S. Bagasse saving and water recovery in cogeneration system using superheated steam dryer. Chem. Eng. Commun. 2019, 206, 919–926. [Google Scholar] [CrossRef]
- Pakowski, Z. Projektowanie Suszarek do Suszenia Parą Przegrzaną; Monografie Politechniki Łódzkiej: Łódź, Poland, 2011; p. 143. [Google Scholar]
- Iyota, H.; Nishimura, N.; Yoshida, M.; Nomura, T. Simulation of superheated steam drying considering initial steam condensation. Dry. Technol. 2001, 19, 1425–1440. [Google Scholar] [CrossRef]
- Inoue, T.; Iyota, H.; Nishimura, N. Prediction method for drying time of wet porous material in humid hot air and superheated steam. Dry. Technol. 2010, 28, 608–614. [Google Scholar] [CrossRef]
- Pakowski, Z.; Adamski, R. On Prediction of the Drying Rate in Superheated Steam Drying Process. Dry. Technol. 2011, 29, 1492–1498. [Google Scholar] [CrossRef]
- Yoshida, T.; Hyõdõ, T. Evaporation of Water in Air, Humid Air, and Superheated Steam. Ind. Eng. Chem. Process Des. Dev. 1970, 9, 207–214. [Google Scholar] [CrossRef]
- Schwartze, J.P.; Bröcker, S. A theoretical explanation for the inversion temperature. Chem. Eng. J. 2002, 86, 61–67. [Google Scholar] [CrossRef]
- Elustondo, D.M.; Elustondo, M.P.; Urbicain, M. Drying with superheated steam: Maximum drying rate as a linear function of pressure. Chem. Eng. J. 2002, 86, 69–74. [Google Scholar] [CrossRef]
- Hamawand, I. Drying Steps under Superheated Steam: A Review and Modeling. Energy Environ. Res. 2013, 3, 107–125. [Google Scholar] [CrossRef]
- Kiriyama, T.; Sasaki, H.; Hashimoto, A.; Kaneko, S.; Maeda, M. Experimental Observations and Numerical Modeling of a Single Coarse Lignite Particle Dried in Superheated Steam. Mater. Trans. 2013, 54, 1725–1734. [Google Scholar] [CrossRef]
- Kiriyama, T.; Sasaki, H.; Hashimoto, A.; Kaneko, S.; Maeda, M. Size Dependence of the Drying Characteristics of Single Lignite Particles in Superheated Steam. Met. Mater. Trans. E 2014, 1, 349–363. [Google Scholar] [CrossRef]
- Kiriyama, T.; Sasaki, H.; Hashimoto, A.; Kaneko, S.; Maeda, M. Evaluation of Drying Rates of Lignite Particles in Superheated Steam Using Single-Particle Model. Met. Mater. Trans. E 2016, 3, 308–316. [Google Scholar] [CrossRef]
- Le, K.H.; Hampel, N.; Kharaghani, A.; Bück, A.; Tsotsas, E. Superheated steam drying of single wood particles: A characteristic drying curve model deduced from continuum model simulations and assessed by experiments. Dry. Technol. 2018, 36, 1866–1881. [Google Scholar] [CrossRef]
- Hampel, N.; Le, K.H.; Kharaghani, A.; Tsotsas, E. Continuous modeling of superheated steam drying of single rice grains. Dry. Technol. 2019, 37, 1583–1596. [Google Scholar] [CrossRef]
- Le, K.H.; Tran, T.T.H.; Kharaghani, A.; Tsotsas, E. Modeling of superheated steam drying of wood particles. J. Mech. Eng. Res. Dev. 2020, 43, 160–170. [Google Scholar]
- Khan, M.I.H.; Kumar, C.; Joardder, M.U.H.; Karim, M.A. Determination of appropriate effective diffusivity for different food materials. Dry. Technol. 2017, 35, 335–346. [Google Scholar] [CrossRef]
- Karim, M.A.; Hawlader, M.N.A. Mathematical modelling and experimental investigation of tropical fruits drying. Int. J. Heat Mass Transf. 2005, 48, 4914–4925. [Google Scholar] [CrossRef]
- Tran, T.T.H. Modelling of drying in packed bed by super heated steam. J. Mech. Eng. Res. Dev. 2020, 43, 135–142. [Google Scholar]
- Gauvin, W.H.; Costin, M. Spray drying in superheated steam: A technoeconomic study. In Proceedings of the Drying’80 vol. 2; Mujumdar, A.S., Ed.; Hemisphere: New York, NY, USA, 1980; pp. 320–335. [Google Scholar]
- Crowe, C.T.; Chow, C.; Chung, J.N. An assessment of steam operated spray dryers. In Proceedings of the Drying’ 85; Mujumdar, A.S., Ed.; Hemisphere: New York, NY, USA, 1985; pp. 221–229. [Google Scholar]
- Frydman, A.; Vasseur, J.; Ducept, F.; Sionneau, M.; Moureh, J. Simulation of spray drying in superheated steam using computational fluid dynamics. Dry. Technol. 1999, 17, 1313–1326. [Google Scholar] [CrossRef]
- Chauhan, V.; Gudjonsdottir, M.; Saevarsdottir, G. Computational modeling and experimental investigation of aqueous potassium carbonate droplets in superheated steam flow. Heat Mass Transf. 2020, 56, 1307–1316. [Google Scholar] [CrossRef]
- Ducept, F.; Sionneau, M.; Vasseur, J. Superheated steam dryer: Simulations and experiments on product drying. Chem. Eng. J. 2002, 86, 75–83. [Google Scholar] [CrossRef]
- Techni-Process: Overheated Steam Drying. Available online: https://www.techni-process.com/en/overheated-steam-principle (accessed on 16 June 2021).
- Trommelen, A.M.; Crosby, E.J. Evaporation and drying of drops in superheated vapors. AIChE J. 1970, 16, 857–867. [Google Scholar] [CrossRef]
- Lum, A.; Cardamone, N.; Beliavski, R.; Mansouri, S.; Hapgood, K.; Woo, M.W. Unusual drying behaviour of droplets containing organic and inorganic solutes in superheated steam. J. Food Eng. 2019, 244, 64–72. [Google Scholar] [CrossRef]
- Lum, A.; Mansouri, S.; Hapgood, K.; Woo, M.W. Single droplet drying of milk in air and superheated steam: Particle formation and wettability. Dry. Technol. 2018, 36, 1802–1813. [Google Scholar] [CrossRef]
- Lum, A. Superheated Steam in Spray Drying for Particle Functionality Engineering. PhD Thesis, Monash University, Melbourne, Australia, 2018. [Google Scholar]
- Lum, A.; Cardamone, N.; Beliavski, R.; Mansouri, S.; Hapgood, K.; Woo, M.W. Role of Steam as a Medium for Droplet Crystallization. Ind. Eng. Chem. Res. 2019, 58, 8517–8524. [Google Scholar] [CrossRef]
- Gauvin, W.H. Spray Drying with a Plasma of Superheated Steam. U.S. Patent 4,376,010, 8 March 1983. [Google Scholar]
- Raehse, W.; Bauer, V. Process for Spray-Drying Materials and Mixtures Thereof using Superheated Steam. U.S. Patent 5,431,780, 11 July 1995. [Google Scholar]
- Islam, M.Z.; Kitamura, Y.; Kokawa, M.; Monalisa, K. Degradation Kinetics and Storage Stability of Vacuum Spray-Dried Micro Wet-Milled Orange Juice (Citrus unshiu) Powder. Food Bioprocess Technol. 2017, 10, 1002–1014. [Google Scholar] [CrossRef]
- Fuengfoo, M.; Devahastin, S.; Niumnuy, C.; Soponronnarit, S. Preliminary study of superheated steam spray drying: A case study with maltodextrin. In Proceedings of the 21st International Drying Symposium, Valencia, Spain, 11–14 September 2018. [Google Scholar] [CrossRef]
- Linke, T.; Happe, J.; Kohlus, R. Laboratory-scale superheated steam spray drying of food and dairy products. Dry. Technol. 2022, 40, 1703–1714. [Google Scholar] [CrossRef]
- Islam, M.; Kitamura, Y.; Yamano, Y.; Kitamura, M. Effect of vacuum spray drying on the physicochemical properties, water sorption and glass transition phenomenon of orange juice powder. J. Food Eng. 2016, 169, 131–140. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Datta, N.; Howes, T. Problems Associated with Spray Drying Of Sugar-Rich Foods. Dry. Technol. 1997, 15, 671–684. [Google Scholar] [CrossRef]
- Van Deventer, H.C. Industrial Superheated Steam Drying; TNO Environment, Energy and Process Innovation: Apeldoorn, The Netherlands, 2004. [Google Scholar]
- Rähse, W.; Dicoi, O. Produktdesign disperser Stoffe: Industrielle Sprühtrocknung. Chem. Ing. Tech. 2009, 81, 699–716. [Google Scholar] [CrossRef]
- Zbiciński, I. Equipment, technology, perspectives and modeling of pulse combustion drying. Chem. Eng. J. 2002, 86, 33–46. [Google Scholar] [CrossRef]
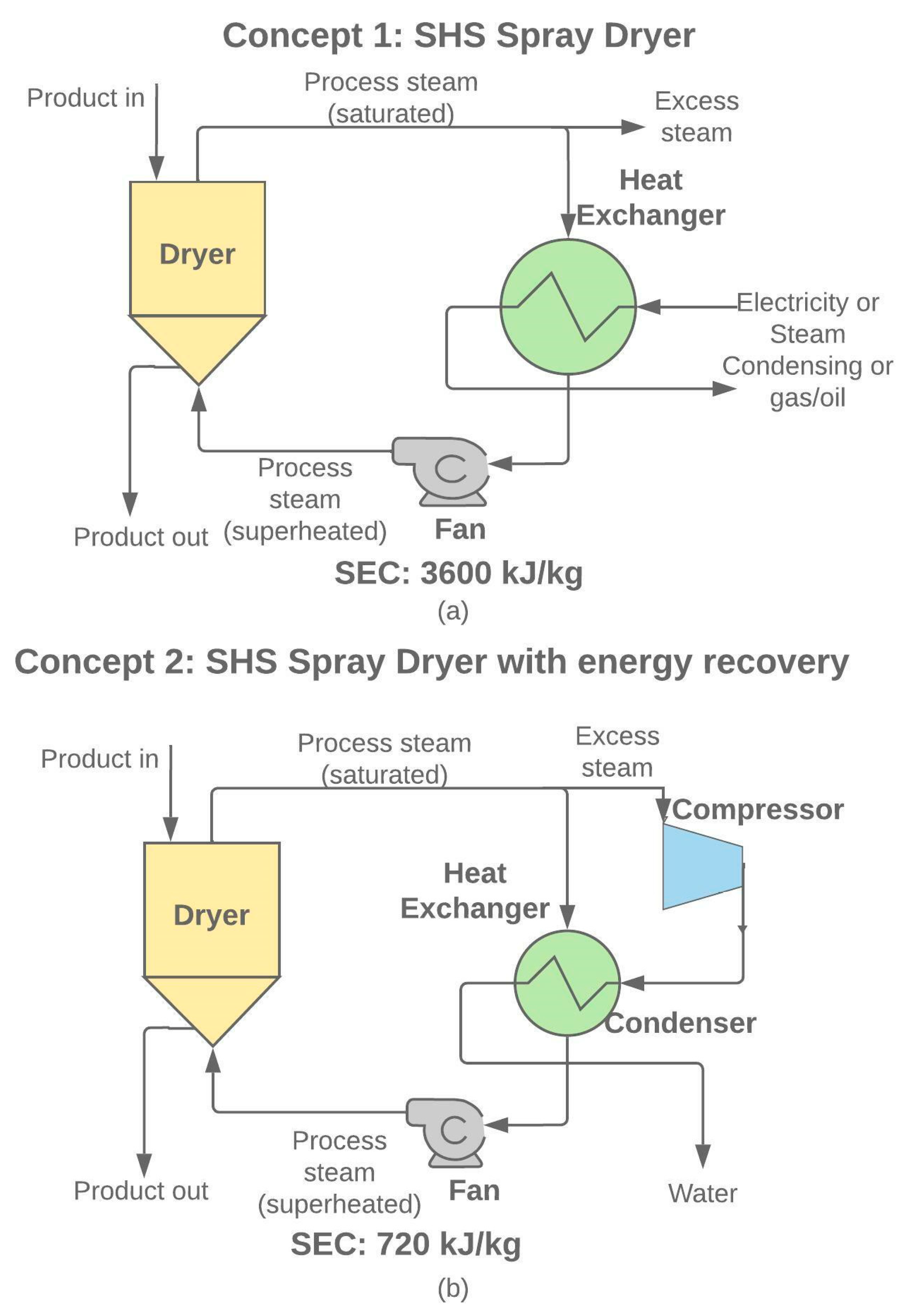
- Baker, C.G.J.; McKenzie, K.A. Energy Consumption of Industrial Spray Dryers. Dry. Technol. 2005, 23, 365–386. [Google Scholar] [CrossRef]
- Bantle, M.; Tolstorebrov, I.; Eikevik, T.M. Possibility for Mechanical Vapor Re-Compression for Steam Based Drying Processes. In Proceedings of the 1st Nordic Baltic Drying Conference, Gdansk, Poland, 17–19 June 2015; pp. 1–8. [Google Scholar]
- Bantle, M. Turbo-Compressors: Prototype Tests of Mechanical Vapour Re-Compression for Steam Driers. In Proceedings of the 12th IEA Heat Pump Conference, Rotterdam, The Netherlands, 11–14 May 2017; pp. 1–7. [Google Scholar]
- Brunetti, L.; Giametta, F.; Catalano, P.; Villani, F.; Fioralba, J.; Fucci, F.; La Fianza, G. Energy Consumption and Analysis of Industrial Drying Plants for Fresh Pasta Process. J. Agric. Eng. 2015, 46, 167–171. [Google Scholar] [CrossRef]

| Product | Equipment | Process Parameters | Product Properties | Reference |
|---|---|---|---|---|
| Concentrated orange juice (C. sinensis) + Maltodextrin DE12 Solids content: 33 Brix% Feed rate: 300 mL/h Ratio of juice solids to maltodextrin solids: 60:40, 50:50, 40:60, and 30:70 | Nozzle type: 2 two-fluid nozzles; Flow direction: co-current; Drying chamber wall temperature: 50 °C; Steam/product separation: in double wall cyclone, heated up by hot water (50 °C); Product collection: in the double wall receivers with entrance of air at temperature 45 °C | SHS Vacuum Spray drying: Atomization air flow rate: 40 NL/min; Vacuum in the drying chamber: 5 kPa; Saturated steam temperature: 40 °C; |
| [55,58] |
| Instant skim milk, Solid content: 25 wt% | SHS Spray dryer size: D = 0.32 m, H = 3.1 m; Flow direction: counter current; Drying chamber: wall temperature: >100 °C; opening at the top and bottom of the dryer to allow for air entrance; Product collection: via bleeding opening at bottom outlet of the dryer; | SHS Spray drying: Inlet steam temperature: 140 °C; Steam mass flow rate: 1.4 kg/h; Atomization pressure: 3 bar; |
| [51] |
| Full cream milk Solid content: 25 wt% | As above | As above |
| [51] |
| D—Mannitol Solid content: 15 wt% | As above | SHS Spray drying: Inlet steam temperature: 140 °C and 180 °C; Steam mass flow rate: 1.4 kg/h; Atomization pressure: 3 bar; |
| [52] |
| Sodium Chloride, NaCl Solid content: 15 wt% | As above | As above |
| [52] |
| Maltodextrin DE 10 Solid content: 30% w/v; feed flow rate: 5 mL/min; | SHS Spray dryer size: D = 0.25 m, H = 0.8 m (Cylinder = 0.5 m, Conical = 0.3 m); Nozzle type: two-fluid nozzle Flow direction: co-current Product/steam separation: glass cyclone with powder collector equipped with cylindrical stainless-still filter, auxiliary heater and steam outlet. | SHS Spray drying: Inlet steam temperature: 160, 170, 180 °C; Steam velocity: 14–15 ms; Steam pressure: 20 kPa (gauge); Atomization pressure: 2 bar; Product discharge: Temperature 105 °C; |
| [56] |
| Skim milk concentrate Solid content: 0.27 kg/kg; Feed flow rate: 1.14 kg/h | SHS Spray dryer size: D = 0.2 m, H = 0.65 m (Cylinder = 0.45 m, Conical = 0.2 m); Flow direction: co-current; Nozzle type: pressure; Product/steam separation: dilution by air in the two stage cyclones equipped with annular Venturi nozzle | SHS Spray drying: inlet steam temperature: 250 °C; outlet steam temperature: 130–160 °C; Steam flow rate:10 kg/h; Atomization pressure: 1 MPa; Product/steam separation: Relative humidity: 73.8–89.6%; Temperature: 31.5–37.9 °C; Product discharge: Relative humidity: <30%; Temperature: <35 °C; |
| [57] |
| Lactose Solid content: 0.10 kg/kg; Feed flow rate: 0.58 kg/h | As above | As above |
| [57] |
| Micellar casein concentrateSolid content: 0.10 kg/kg; Feed flow rate: 0.72 kg/h | As above | As above |
| [57] |
| Whey Protein Isolate Solid content: 0.10 kg/kg; Feed flow rate: 0.71 kg/h | As above | As above |
| [57] |
| Maltodextrin DE < 3 Solid content: 0.15 kg/kg; Feed flow rate: 0.93 kg/h | As above | As above |
| [57] |
| Gum Arabic Seyal Solid content: 0.10 kg/kg; Feed flow rate: 0.87 kg/h | As above | As above |
| [57] |
| Soy Protein Isolate Solid content: 0.05 kg/kg; Feed flow rate: 1.05 kg/h | As above | As above |
| [57] |
| Coffee Extract Solid content: 0.05 kg/kg; Feed flow rate: 1.05 kg/h | As above | As above |
| [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobulska, M.; Wawrzyniak, P.; Woo, M.W. Superheated Steam Spray Drying as an Energy-Saving Drying Technique: A Review. Energies 2022, 15, 8546. https://doi.org/10.3390/en15228546
Sobulska M, Wawrzyniak P, Woo MW. Superheated Steam Spray Drying as an Energy-Saving Drying Technique: A Review. Energies. 2022; 15(22):8546. https://doi.org/10.3390/en15228546
Chicago/Turabian StyleSobulska, Mariia, Pawel Wawrzyniak, and Meng Wai Woo. 2022. "Superheated Steam Spray Drying as an Energy-Saving Drying Technique: A Review" Energies 15, no. 22: 8546. https://doi.org/10.3390/en15228546
APA StyleSobulska, M., Wawrzyniak, P., & Woo, M. W. (2022). Superheated Steam Spray Drying as an Energy-Saving Drying Technique: A Review. Energies, 15(22), 8546. https://doi.org/10.3390/en15228546








