Influences of Liquid Viscosity and Relative Velocity on the Head-On Collisions of Immiscible Drops
Abstract
1. Introduction
2. Model and Numerical Algorithm
- (a)
- = 0: there is no P-phase fluid in the current grid;
- (b)
- = 1: the current grid is wholly occupied by the P-phase fluid;
- (c)
- 0 < < 1: the current grid has a boundary between the P-phase fluid and one or both of the other phases.
2.1. Governing Equations
2.2. Model and Boundary Conditions
2.3. Simulation Strategies
2.4. Mesh Independence
3. Simulation Results
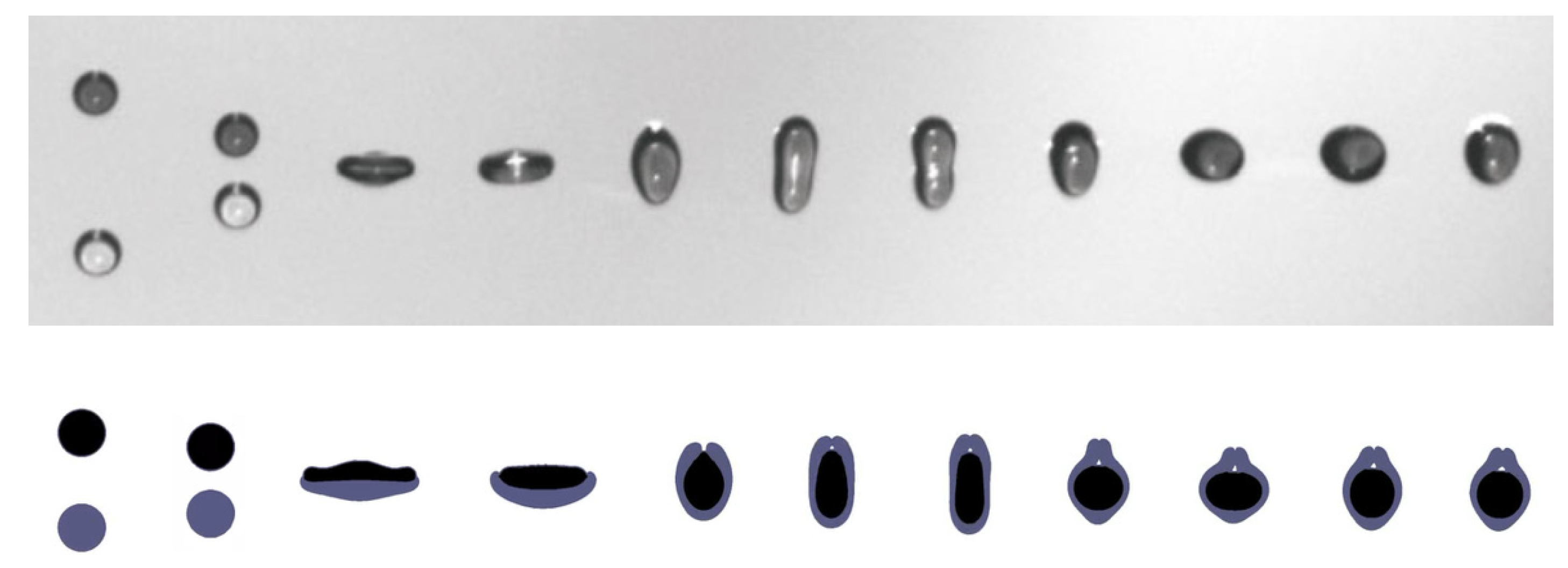
3.1. Comparison with Experiments
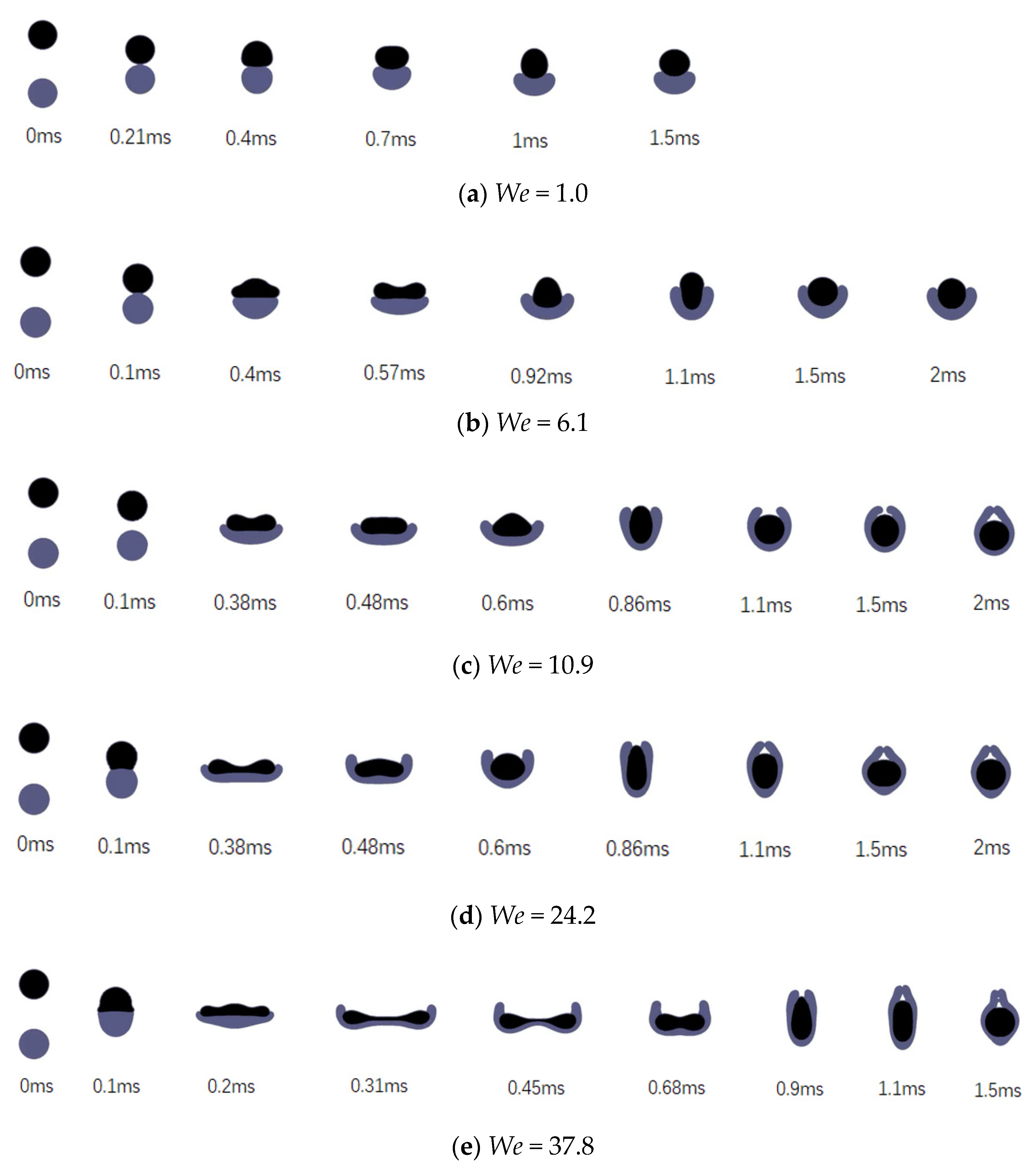
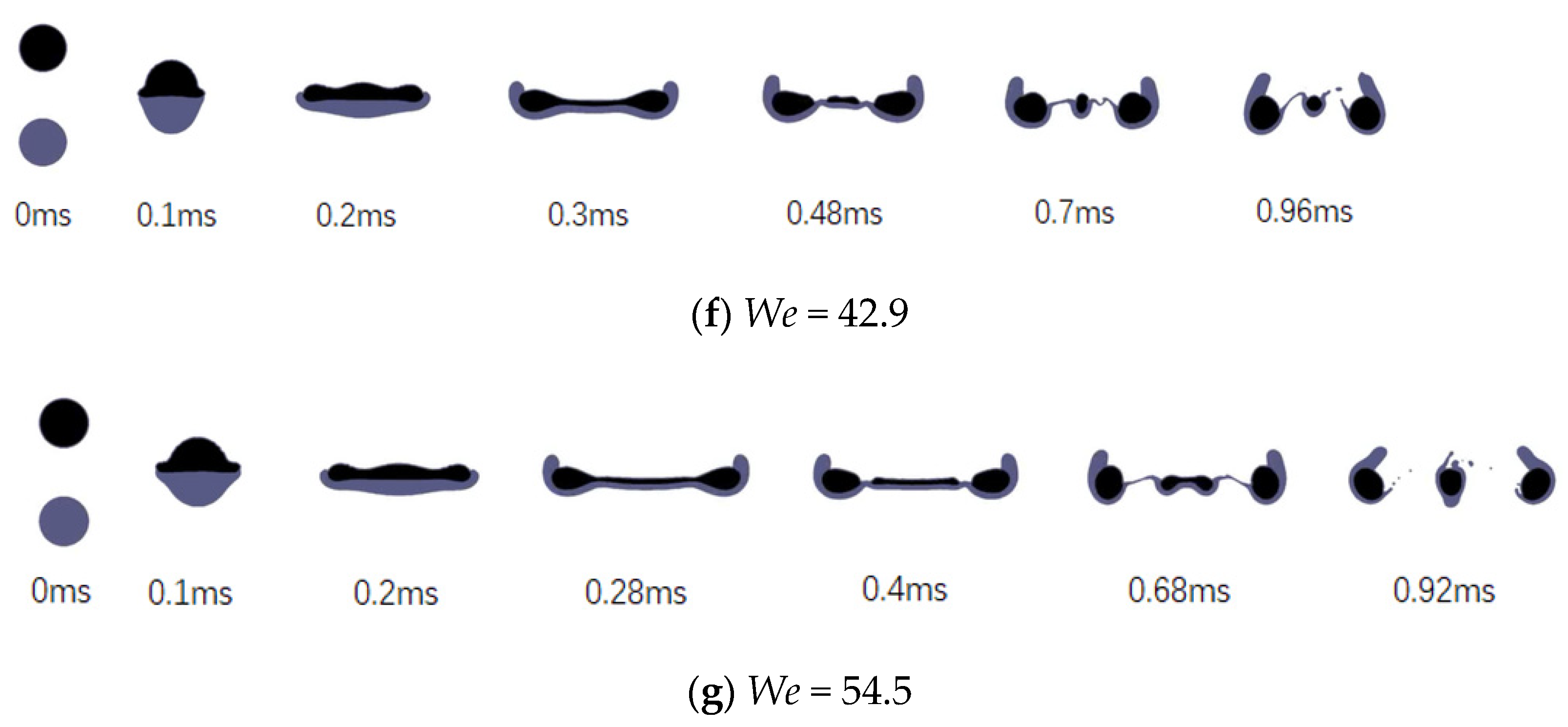
3.2. Effects of the Weber Number
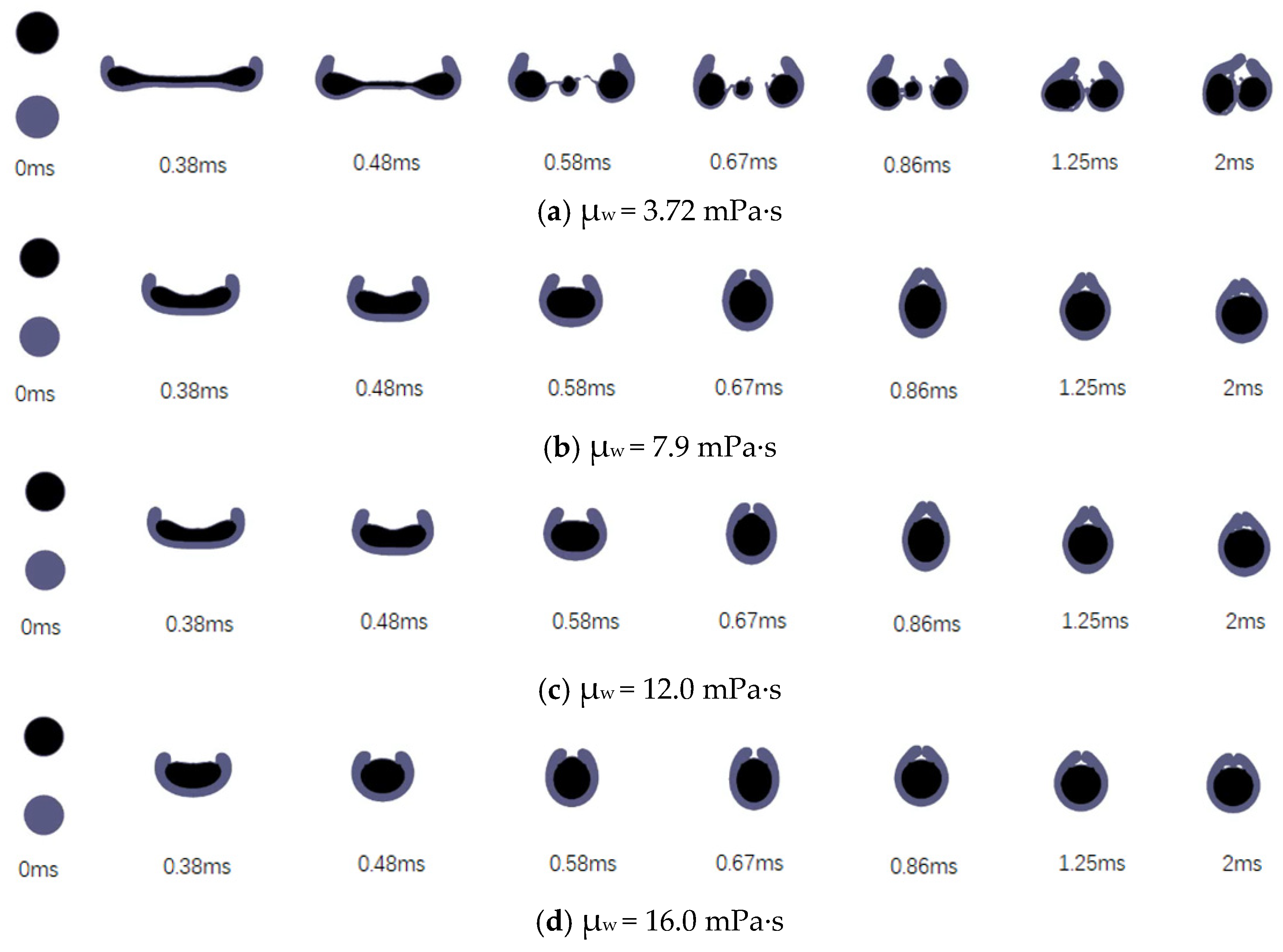
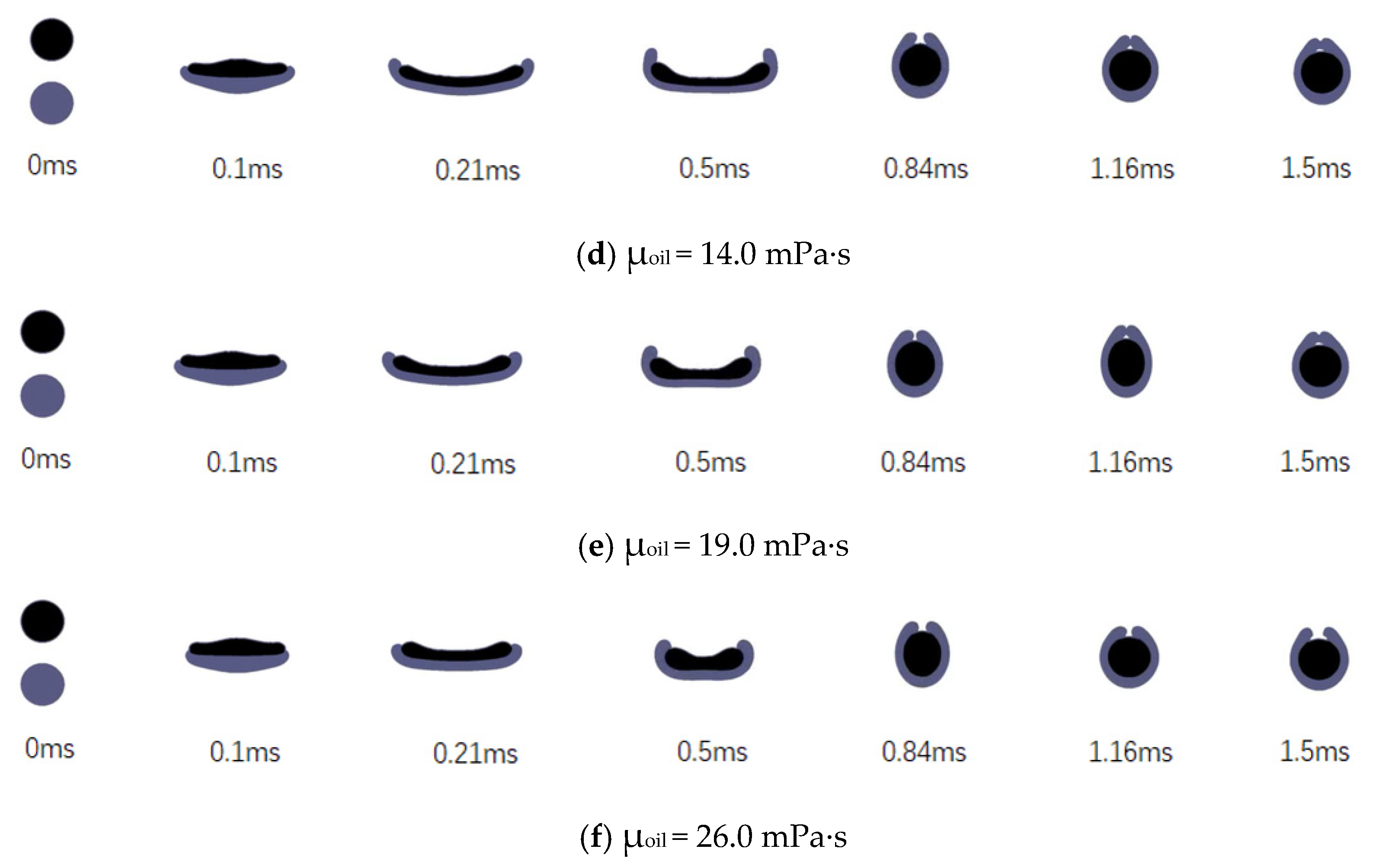
3.3. Effects of the Liquid Viscosity
3.3.1. Effects of the Viscosity of the Encapsulated Phase
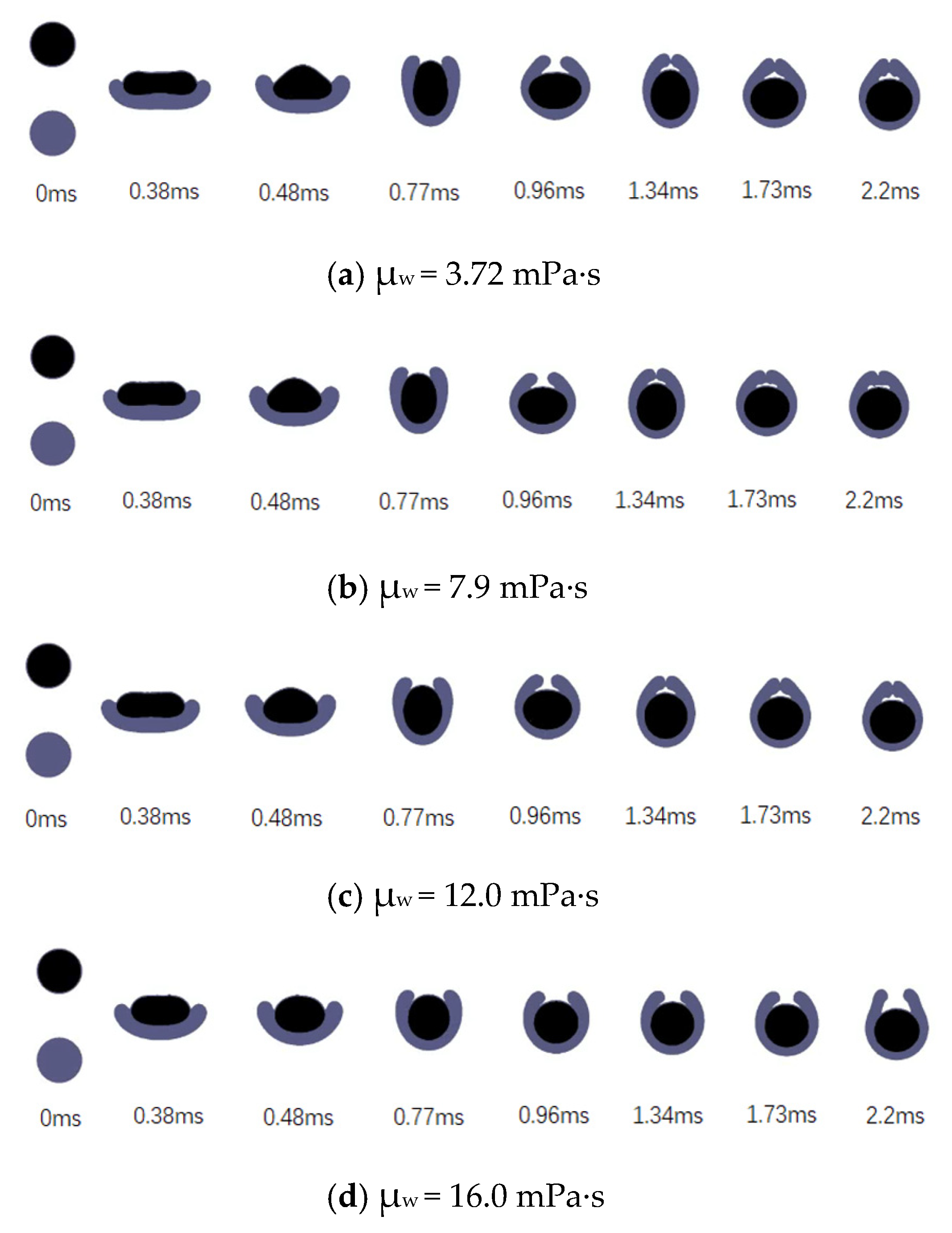
3.3.2. Effects of the Viscosity of the Encapsulating Phase
4. Discussions
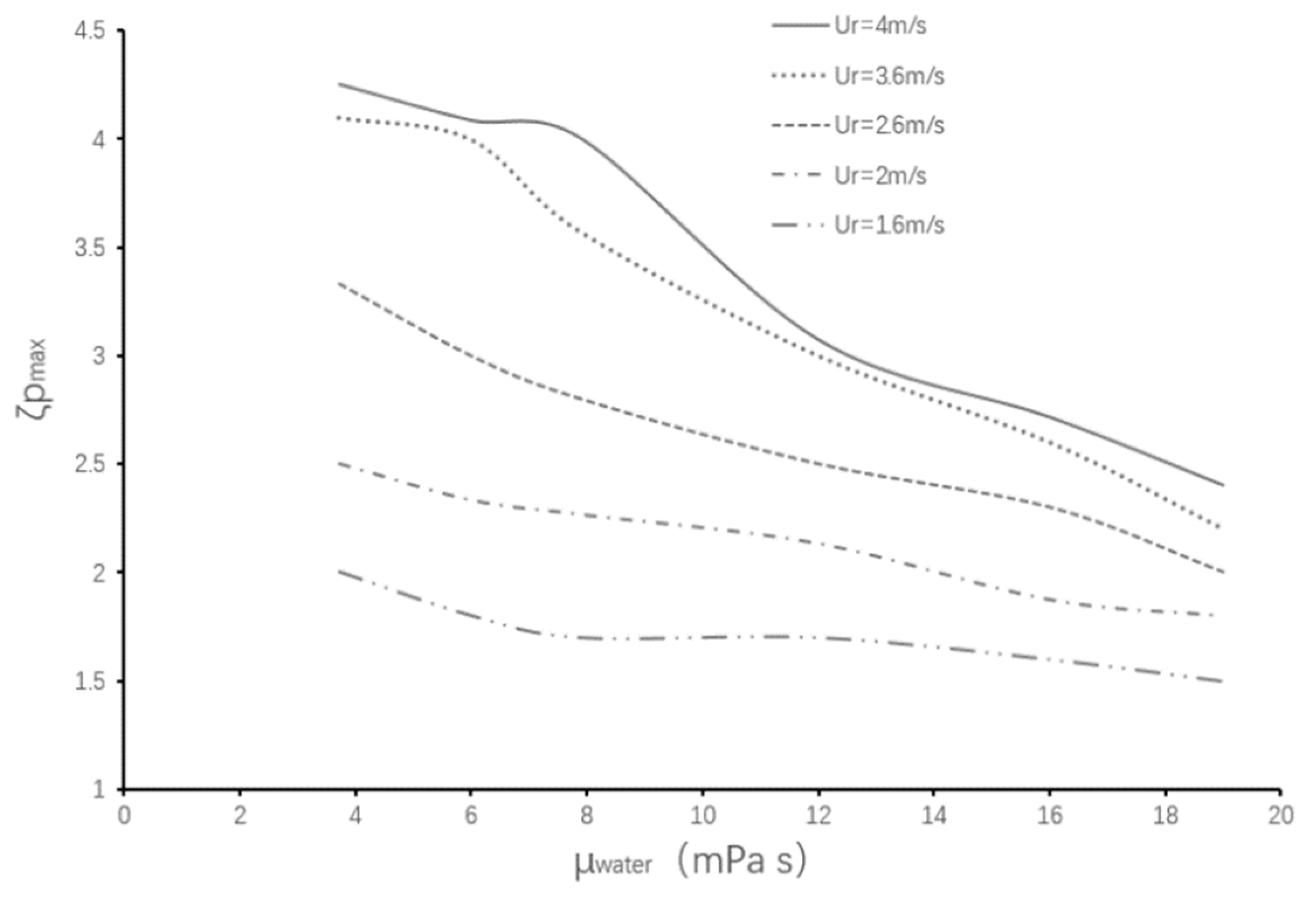
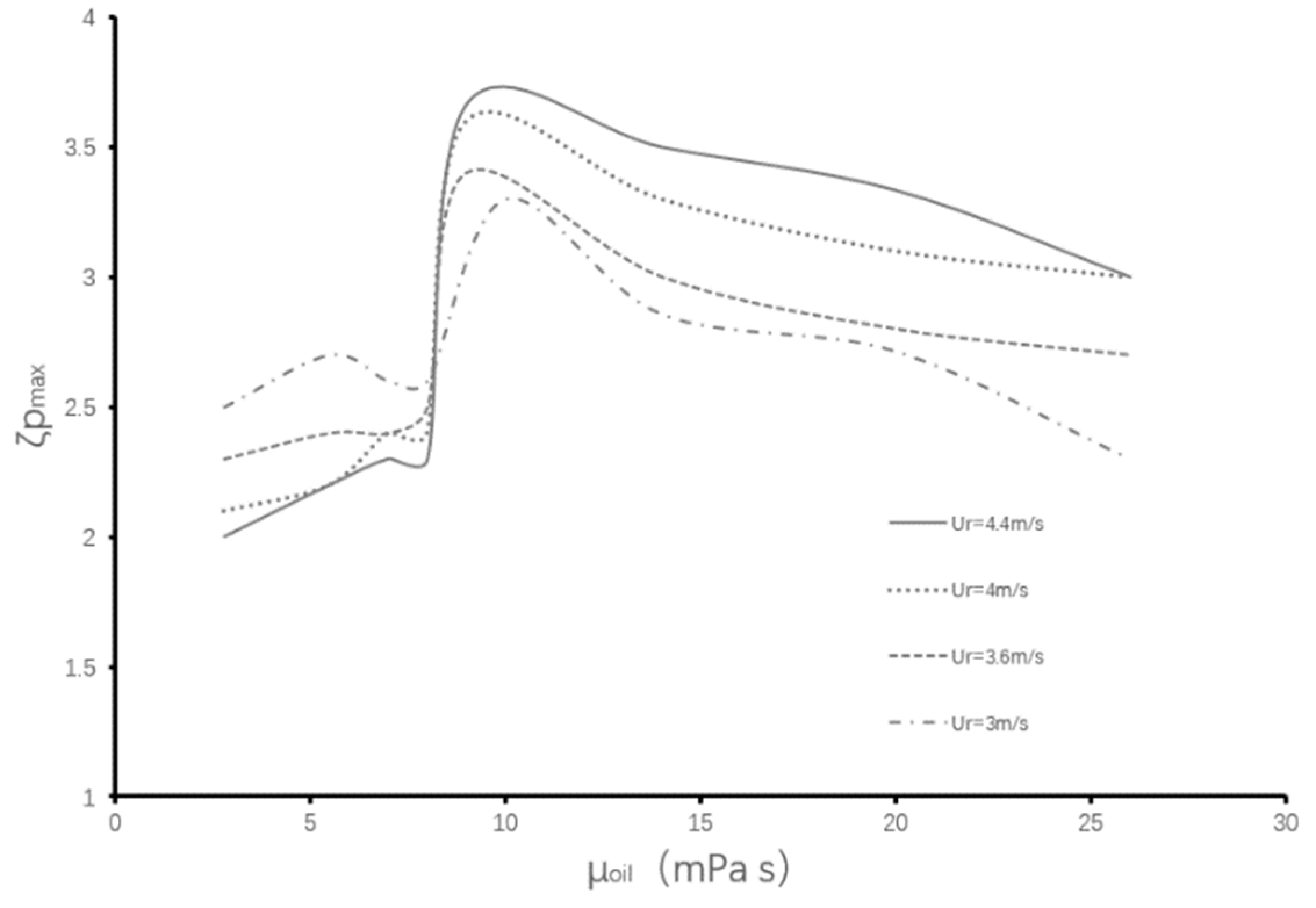
4.1. Maximum Aspect Ratio
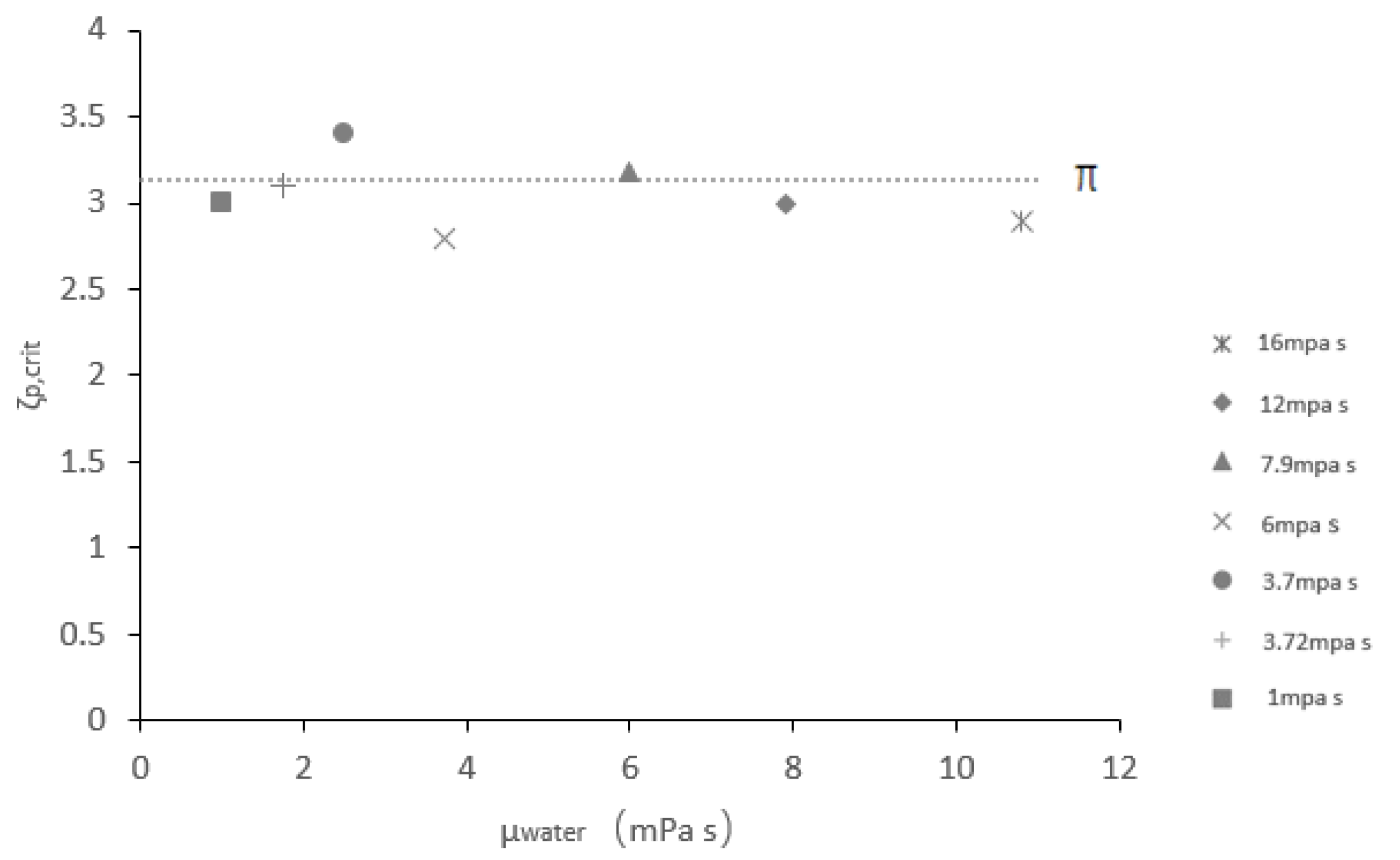
4.2. Critical Aspect Ratio
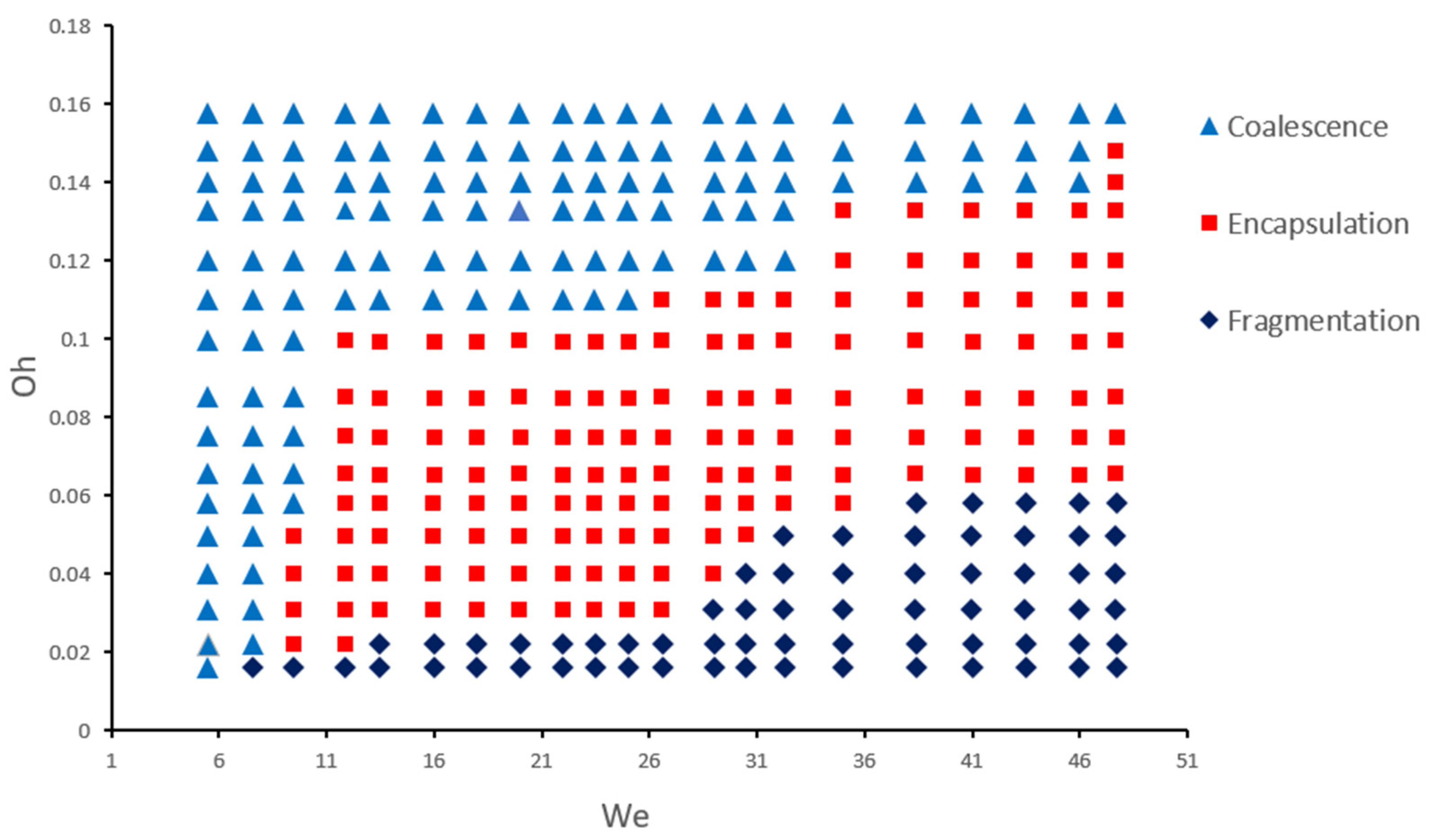
4.3. Phase Diagram
5. Conclusions
- (1)
- Simply increasing the Weber number by increasing the colliding velocity will accelerate the collision process and intensify the degree of deformation.
- (2)
- Under the condition of low colliding velocities (We ≤ 11.9), the variation of the viscosity of the encapsulated phase (the aqueous droplet) has little effect on the deformation degree of the droplets during the collision process. Comparatively, the variation of the viscosity of the encapsulating phase (the oil droplet) has a significant effect on the deformation degree of the droplets.
- (3)
- The critical aspect ratio of the coalescence or fragmentation of the composite droplet is independent of the viscosity of the encapsulated phase (aqueous phase).
Author Contributions
Funding
Conflicts of Interest
References
- Brazier-Smith, P.R.; Jennings, S.G.; Latham, J. Accelerated rates of rainfall. Nature 1971, 232, 112–113. [Google Scholar] [PubMed]
- Whelpdale, D.M.; List, R. The coalescence process in raindrop growth. J. Geophys. Res. 1971, 76, 2836–2856. [Google Scholar]
- List, R.; Gillespie, J.R. Evolution of raindrop spectra with collision-induced breakup. J. Atmos. Sci. 1976, 33, 2007–2013. [Google Scholar]
- Bradley, S.G.; Stow, C.D. On the production of satellite droplets during collisions between water drops falling in still air. J. Atmos. Sci. 1979, 36, 494–500. [Google Scholar] [CrossRef]
- Low, T.B.; List, R. Collision, coalescence, and breakup of raindrops. Part I: Experimentally established coalescence efficiencies and fragment size distributions in breakup. J. Atmos. Sci. 1982, 39, 1591–1606. [Google Scholar] [CrossRef]
- Tan, Y.C.; Ho, Y.; Lee, A. Droplet coalescence by geometrically mediated flow in microfluidic channels. Microfluid. Nanofluidics 2007, 3, 495–499. [Google Scholar]
- Moreira, A.L.N.; Moita, A.S.; Panão, M.R. Advances and challenges in explaining fuel spray impingement: How much of single droplet impact research is useful? Prog. Energy Combust. Sci. 2010, 36, 554–580. [Google Scholar]
- Sun, K.; Zhang, P.; Law, C.K.; Wang, T. Collision dynamics and internal mixing of droplets of non-Newtonian liquids. Phys. Rev. Appl. 2015, 4, 054013. [Google Scholar] [CrossRef]
- Doston, S.; Dmitry, E.; Boris, V.B.; Svyatoslav, C.; Stein, T.J.; Iskander, A. Modeling water droplet freezing and collision with a solid surface. Energies 2021, 14, 1020. [Google Scholar]
- Qian, J.; Law, C.K. Regimes of coalescence and separation in droplet collision. J. Fluid Mech. 1997, 331, 59–80. [Google Scholar]
- Brenn, G.; Kalenderski, S.; Ivanov, I. Investigation of the stochastic collisions of drops produced by Rayleigh breakup of two laminar liquid jets. Phys. Fluids 1997, 9, 349–364. [Google Scholar]
- Willis, K.D.; Orme, M. Experiments on the dynamics of droplet collisions in a vacuum. Exp. Fluids 2000, 29, 347–358. [Google Scholar]
- Planchette, C.; Lorenceau, E.; Brenn, G. The onset of fragmentation in binary liquid drop collisions. J. Fluid Mech. 2012, 702, 5–25. [Google Scholar] [CrossRef]
- Demidovich, A.V.; Kralinova, S.S.; Tkachenko, P.P.; Shlegel, N.E.; Volkov, R.S. Interaction of liquid droplets in gas and vapor flows. Energies 2019, 12, 4256. [Google Scholar]
- Huang, K.L.; Pan, K.L. Transitions of bouncing and coalescence in binary droplet collisions. J. Fluid Mech. 2021, 928, A7. [Google Scholar]
- Kropotova, S.; Strizhak, P. Collisions of liquid droplets in a gaseous medium under conditions of intense phase transformations: Review. Energies 2021, 14, 6150. [Google Scholar] [CrossRef]
- Planchette, C.; Lorenceau, E.; Brenn, G. Liquid encapsulation by binary collisions of immiscible liquid drops. Colloids Surf. A Physicochem. Eng. Asp. 2010, 365, 89–94. [Google Scholar]
- Roisman, I.V.; Planchette, C.; Lorenceau, E.; Brenn, G. Binary collisions of drops of immiscible liquids. J. Fluid Mech. 2012, 690, 512–535. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, P.; Law, C.K. Bouncing, coalescence, and separation in head-on collision of unequal-size droplets. Phys. Fluids 2012, 24, 022101. [Google Scholar]
- Kavehpour, H.P. Coalescence of drops. Annu. Rev. Fluid Mech. 2015, 47, 245–268. [Google Scholar] [CrossRef]
- Planchette, C.; Hinterbichler, H.; Liu, M.; Bothe, D.; Brenn, G. Colliding drops as coalescing and fragmenting liquid springs. J. Fluid Mech. 2017, 814, 277–300. [Google Scholar] [CrossRef]
- Kamp, J.; Villwock, J.; Kraume, M. Drop coalescence in technical liquid/liquid applications: A review on experimental techniques and modeling approaches. Rev. Chem. Eng. 2017, 33, 1–47. [Google Scholar]
- Sommerfeld, M.; Pasternak, L. Advances in modelling of binary droplet collision outcomes in sprays: A review of available knowledge. Int. J. Multiph. Flow 2019, 117, 182–205. [Google Scholar]
- Shlegel, N.; Tkachenko, P.; Strizhak, P. Influence of viscosity, surface and interfacial tensions on the liquid droplet collisions. Chem. Eng. Sci. 2020, 220, 115639. [Google Scholar]
- Focke, C.; Kuschel, M.; Sommerfeld, M.; Bothe, D. Collision between high and low viscosity droplets: Direct numerical simulations and experiments. Int. J. Multiph. Flow 2013, 56, 81–92. [Google Scholar]
- Choi, S.B.; Lee, J.S. Film drainage mechanism between two immiscible droplets. Microfluid. Nanofluidics 2014, 17, 675–681. [Google Scholar]
- Zhang, J.T.; Liu, H.R.; Ding, H. Head-on collision of two immiscible droplets of different components. Phys. Fluids 2020, 32, 082106. [Google Scholar] [CrossRef]
- Leclaire, S.; Reggio, M.; Trépanier, J.Y. Progress and investigation on lattice Boltzmann modeling of multiple immiscible fluids or components with variable density and viscosity ratios. J. Comput. Phys. 2013, 246, 318–342. [Google Scholar]
- Sussman, M.; Smereka, P.; Osher, S. A leverl set approach for computing solutions to incompressible two-phase flow. J. Comput. Phys. 1994, 114, 146–159. [Google Scholar] [CrossRef]
- Ko, G.H.; Ryou, H.S. Modeling of droplet collision-induced breakup process. Int. J. Multiph. Flow 2005, 31, 723–738. [Google Scholar]
- Wörner, M. Numerical modeling of multiphase flows in microfluidics and micro process engineering: A review of methods and applications. Microfluid. Nanofluid. 2012, 12, 841–886. [Google Scholar] [CrossRef]
- Mohammadi, M.; Shahohosseini, S.; Bayat, M. Direct numerical simulation of water droplet coalescence in the oil. Int. J. Heat Fluid Flow 2012, 36, 58–71. [Google Scholar] [CrossRef]
- Yuan, S.; Dabirian, R.; Shoham, O.; Mohan, R.S. Numerical simulation of liquid droplet coalescence and breakup. J. Energy Resour. Technol. 2020, 142, 102101. [Google Scholar]
- Inamuro, T.; Tajima, S.; Ogino, F. Lattice Boltzmann simulation of droplet collision dynamics. Int. J. Heat Mass Transf. 2004, 47, 4649–4657. [Google Scholar]
- Lycett-Brown, D.; Luo, K.H.; Liu, R.; Lv, P. Binary droplet collision simulations by a multiphase cascaded lattice Boltzmann method. Phys. Fluids 2014, 26, 023303. [Google Scholar] [CrossRef]
- Liang, H.; Shi, B.; Chai, Z. Lattice Boltzmann modeling of three-phase incompressible flows. Phys. Rev. E 2016, 93, 013308. [Google Scholar]
- Ebadi, A.; Hosseinalipour, S.M. The collision of immiscible droplets in three-phase liquid systems: A numerical study using phase-field lattice Boltzmann method. Chem. Eng. Res. Des. 2022, 178, 289–314. [Google Scholar] [CrossRef]
- Morris, J.P.; Fox, P.J.; Zhu, Y. Modeling low Reynolds number incompressible flows using SPH. J. Comput. Phys. 1997, 136, 214–226. [Google Scholar]
- Hirschler, M.; Oger, G.; Nieken, U.; Touzé, D.L. Modeling of droplet collisions using smoothed particle hydrodynamics. Int. J. Multiph. Flow 2017, 95, 175–187. [Google Scholar] [CrossRef]
- Nishio, Y.; Komori, K.; Izawa, S.; Fukunishi, Y. Simulation of collisions of two droplets containing two different liquids using incompressible smoothed particle hydrodynamics method. Theor. Comput. Fluid Dyn. 2020, 34, 105–117. [Google Scholar]
- Gao, T.C.; Chen, R.H.; Pu, J.Y.; Lin, T.H. Collision between an ethanol drop and a water drop. Exp. Fluids 2005, 38, 731–738. [Google Scholar] [CrossRef]
- Chen, R.H.; Chen, C.T. Collision between immiscible drops with large surface tension difference: Diesel oil and water. Exp. Fluids 2006, 41, 453–461. [Google Scholar]
- Chen, R.H. Diesel–diesel and diesel–ethanol drop collisions. Appl. Therm. Eng. 2007, 27, 604–610. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Zhao, H.; Xu, J.H.; Luo, G.S. Controlled coalescence of two immiscible droplets for Janus emulsions in a microfluidic device. RSC Adv. 2015, 5, 32768–32774. [Google Scholar]
- Piskunov, M.V.; Shlegel, N.E.; Strizhak, P.A.; Volkov, R.S. Experimental research into collisions of homogeneous and multi-component liquid droplets. Chem. Eng. Res. Des. 2019, 150, 84–98. [Google Scholar] [CrossRef]
- Ashgriz, N.; Poo, J.Y. Coalescence and separation in binary collisions of liquid drops. J. Fluid Mech. 1990, 221, 183–204. [Google Scholar] [CrossRef]
- Freitas, L.G.; Pereira, L.A.A.; Silva, A.L.F.L. Comparison among drag coefficient models of single bubbles under high and low Morton number regimes. Chem. Eng. Sci. 2021, 236, 116473. [Google Scholar] [CrossRef]
- Rivière, A.; Ruth, D.J.; Mostert, W.; Deike, L.; Perrard, S. Caillary driven fragmentation of large gas bubbles in turbulence. Phys. Rev. Fluids 2022, 7, 083602. [Google Scholar]




| Authors | Modeling or Experimental Study | Miscible or Immiscible | Droplet Size or Size Ratio | Equal or Different Properties | Ambient Medium | Dimensionless Numbers or Relative Velocity [m/s] |
|---|---|---|---|---|---|---|
| Focke et al. [25] | Modeling and experiment | Miscible | 380–750 μm; Ratio = 0.66, 1.0. | Only different viscosity | Air | Urel = 1.0–3.0; 15 < We < 133 (app.) |
| Choi et al. [26] | Modeling | Immiscible | 40 μm (equal-sized) | Only different surface tension | Liquid | Re = 0.25 |
| Nishio et al. [40] | Modeling | Immiscible | ~200 μm | All different | Air | Urel = 2.0, 3.0, 3.2 |
| Zhang et al. [27] | Modeling | Immiscible | Equal-sized | Only different surface tension | Air | 3 < We < 600 |
| Ebadi et al. [37] | Modeling | Immiscible | 50–200 μm (app.) | All different | Liquid | We > 40 & Ca > 1 & 0.2 < Oh < 0.3 |
| Gao et al. [41] | Experiment | Miscible | 400–600 μm (equal-sized) | All different | Air | 10 < We < 100 |
| Chen et al. [42,43] | Experiment | Immiscible | 700–800 μm (equal-sized) | All different | Air | 0 < We < 100 & 30 < Re < 500 |
| Roisman et al. [18] | Experiment | Immiscible | 180–210 μm (equal-sized) | All different | Air | Urel = 2.0–7.0 (app.) |
| Planchette et al. [13,17,21] | Experiment | Immiscible | 150–350 μm (equal-size) | All different | Air | 50 < Re < 400 & 0.02 < Oh < 0.5 (app.) |
| Zhang et al. [44] | Experiment | Immiscible | ~160 μm (equal-sized) | All different | Silicone oil | Not provided |
| Piskunov et al. [45] | Experiment | Both | 0.2–10 mm (equal-sized) | All different | Air | Urel = 0.5–10.0 (app.) |
| Liquid | Density kg m−3 | Dynamic Viscosity mPa·s | Surface Tension mN/m | Interfacial Tension mN/m |
|---|---|---|---|---|
| Water | 998 | 1.002 | 72.7 | 18 |
| Diesel oil | 817 | 3.16 | 28.85 |
| Droplet 1 | Droplet 2 | |
|---|---|---|
| Liquid | Water/glycerol mixture | Silicone oil |
| Viscosity | ||
| Density | kg/m3 | kg/m3 |
| Surface Tension | ||
| D0 | 200.0 μm | 200.0 μm |
| Ur | 2.0 m/s | |
| Droplet 1 | Droplet 2 | |
|---|---|---|
| Liquid | Water/glycerol mixture | Silicone oils |
| Viscosity | mPa·s | mPa·s |
| Density | kg/m3 | kg/m3 |
| Surface Tension | ||
| D0 | 200 μm | 200 μm |
| Ur | 4.0 m/s | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.; Xu, R.; Cui, J.; Song, Q.; Shen, T. Influences of Liquid Viscosity and Relative Velocity on the Head-On Collisions of Immiscible Drops. Energies 2022, 15, 8544. https://doi.org/10.3390/en15228544
Chang J, Xu R, Cui J, Song Q, Shen T. Influences of Liquid Viscosity and Relative Velocity on the Head-On Collisions of Immiscible Drops. Energies. 2022; 15(22):8544. https://doi.org/10.3390/en15228544
Chicago/Turabian StyleChang, Jiaqing, Rongchang Xu, Jinsheng Cui, Qiaolin Song, and Teng Shen. 2022. "Influences of Liquid Viscosity and Relative Velocity on the Head-On Collisions of Immiscible Drops" Energies 15, no. 22: 8544. https://doi.org/10.3390/en15228544
APA StyleChang, J., Xu, R., Cui, J., Song, Q., & Shen, T. (2022). Influences of Liquid Viscosity and Relative Velocity on the Head-On Collisions of Immiscible Drops. Energies, 15(22), 8544. https://doi.org/10.3390/en15228544








