RETRACTED: Gas Hydrate-Based CO2 Capture: A Journey from Batch to Continuous
Abstract
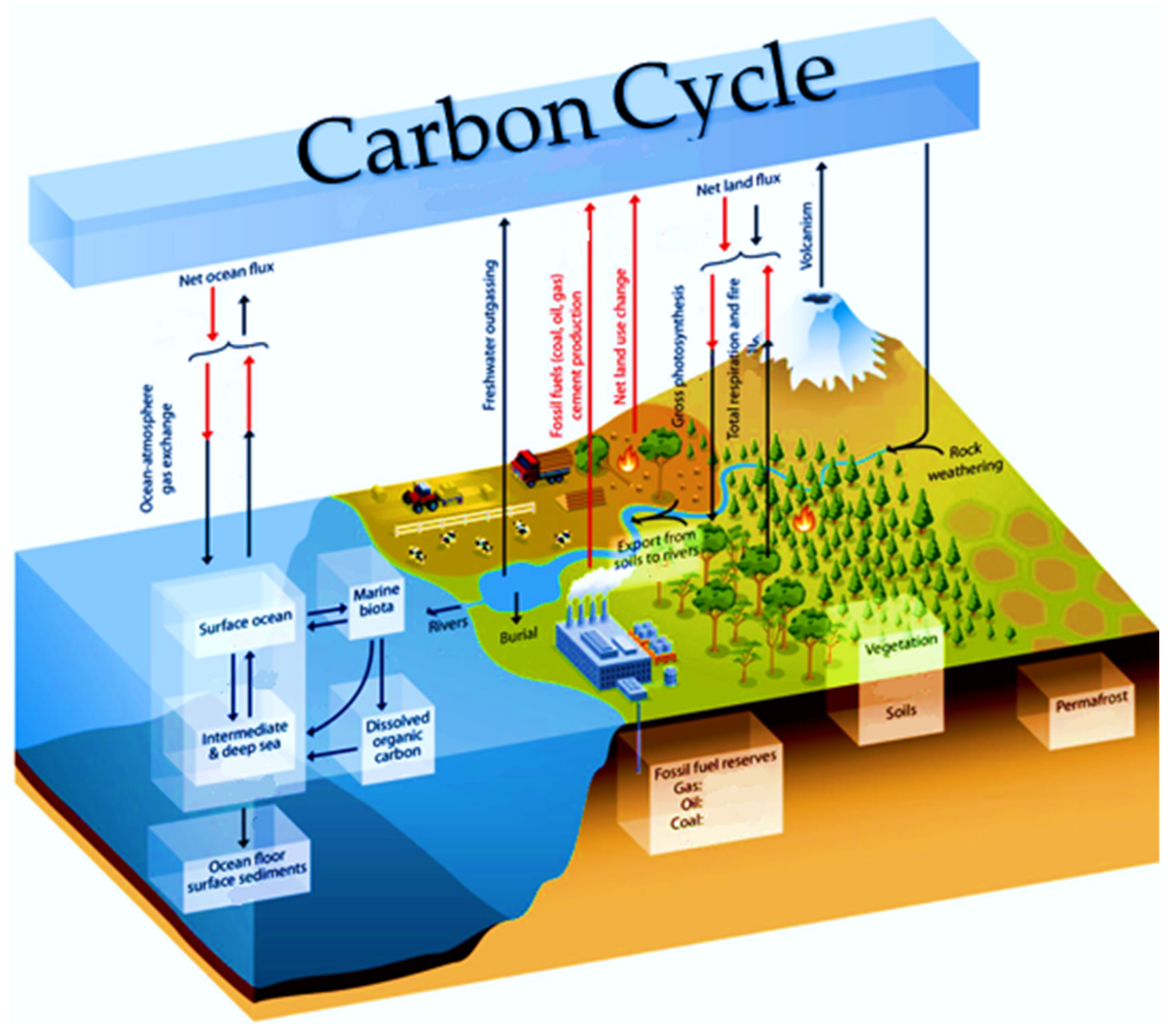
1. Introduction
- Cutting down on greenhouse gas output instantly. A number of outcomes are included in this, such as:
1.1. Absorption
1.2. Adsorption
1.3. Membrane
| Method | Advantages | Drawbacks | Ref. |
|---|---|---|---|
| Absorption |
|
| [48,49,50,51,52,53,54] |
| Adsorption |
|
| [55,56,57,58] |
| Membrane |
|
| [54,59,60,61] |
| Cryogenic distillation |
|
| [62,63,64] |
1.4. Cryogenic Distillation
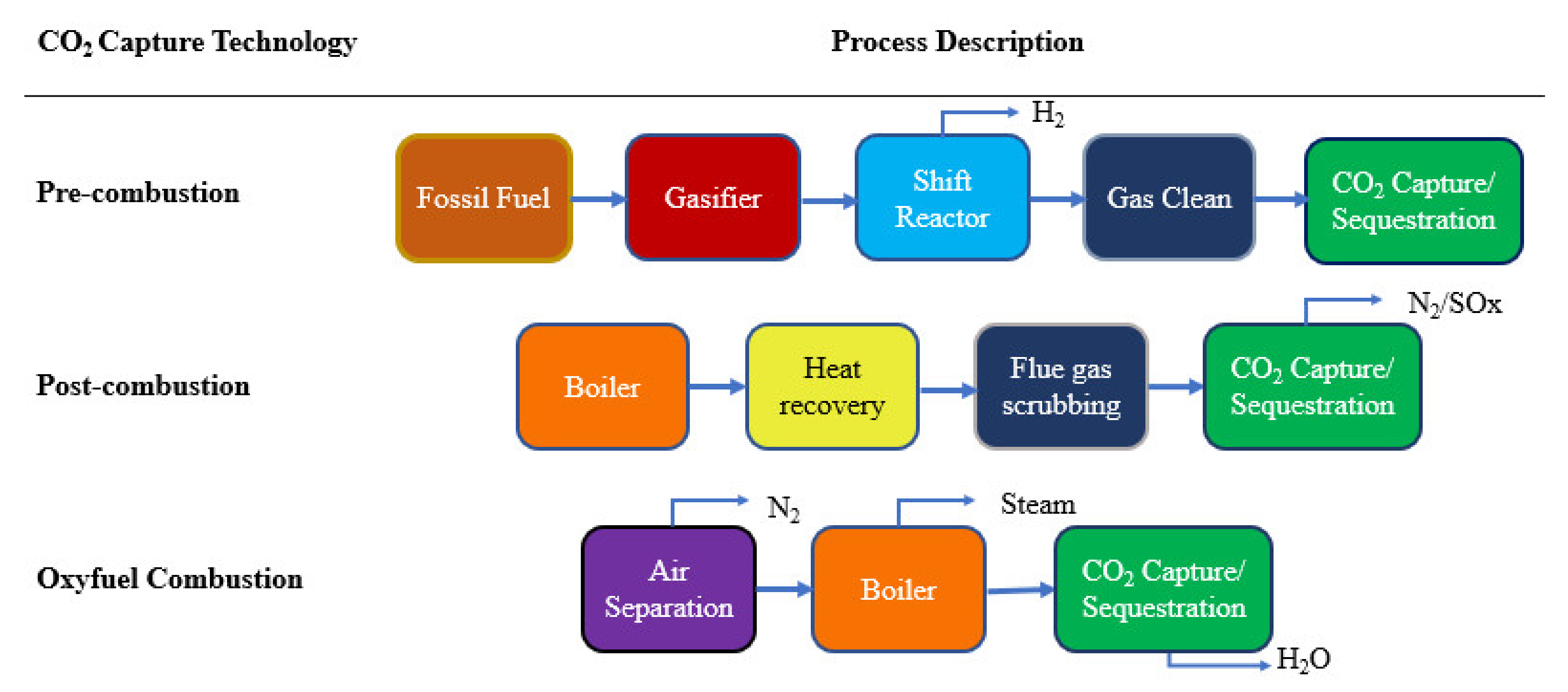
2. CO2 Capture Challenges and Outlooks
Gas Hydrate-Based Technology for Separation of CO2
| Ref. No. | Authors | Gas Mixture | Additive | Reactor Type/Volume | Year |
|---|---|---|---|---|---|
| [107] | Kang and Lee | CO2/N2 | THF 1 | Stirrer/70 cm3 | 2000 |
| [44] | Seo et al. | CO2/N2 CO2/CH4 | N/A | Stirrer/50 cm3 | 2000 |
| [10] | Seo et al. | CO2/N2 | Silica gel | Stirrer, 50 cm3 | 2005 |
| [108] | Duc et al. | CO2/N2 | TBAB 2 | Stirrer/1820 cm3 | 2007 |
| [109] | Linga et al. | CO2/N2 CO2/H2 | THF | Stirrer/323 cm3 | 2007 |
| [110] | Linga et al. | CO2/N2 | THF | Stirrer/323 cm3 | 2008 |
| [111] | Li et al. | CO2/N2 | TBAB | Stirrer/1000 cm3 | 2009 |
| [112] | Seo and Kang | CO2/H2 | Silica gel | Stirrer or fluidized bed/500 cm3 | 2010 |
| [12] | Li et al. | CO2/N2 | TBAB, DTAC 3 | Stirrer/1350 cm3 | 2010 |
| [15] | Li et al. | CO2/H2 | TBAB, CP 4 | Stirrer/1350 cm3 | 2011 |
| [113] | Gholinezhad et al. | CO2/H2 | TBAB | Stirrer/570 cm3 | 2011 |
| [114] | Kim et al. | CO2/H2 | TBAB | Stirrer/N/A | 2011 |
| [115] | Xu et al. | CO2/H2 | TBAB | Bubble reactor/40 L | 2012 |
| [116] | Xu et al. | CO2/H2 | TBAB | Stirrer/336 cm3 | 2012 |
| [117] | Babu et al. | CO2/H2 | Silica gel | Fixed bed/1240 cm3 | 2013 |
| [118] | Sun et al. | CO2/N2 CH4/N2 CH4/H2 | THF | Stirrer/1500 cm3 | 2014 |
| [119] | Zhou et al. | CO2/CH4 | N/A | Stirrer/5.7 L | 2014 |
| Reactor Type | Advantages | Drawbacks |
|---|---|---|
| Stirred-tank reactors | Suitable for lab-scale Simple operation Design is well established | High torque power for mixer High fabrication cost Risk of leakage is high Difficult scaling up |
| Bubble column | Effective heat removal Capability for internal cooling Heat and mass transfer can increase with addition of mixer Can convert all water to hydrate | Requires high-pressure compatible compressor to recycle extra gas Use of stirrer to bring up the stirrer-tank disadvantages |
| Spray Tower | Fast hydrate formation of a large contact area Can eliminate induction time Simple design No mechanical stirrer is needed Easy scaling up | Non-effective heat removal Hydrate slurry is inevitable |
3. Economic Limitations to Hydrate-Based CCS
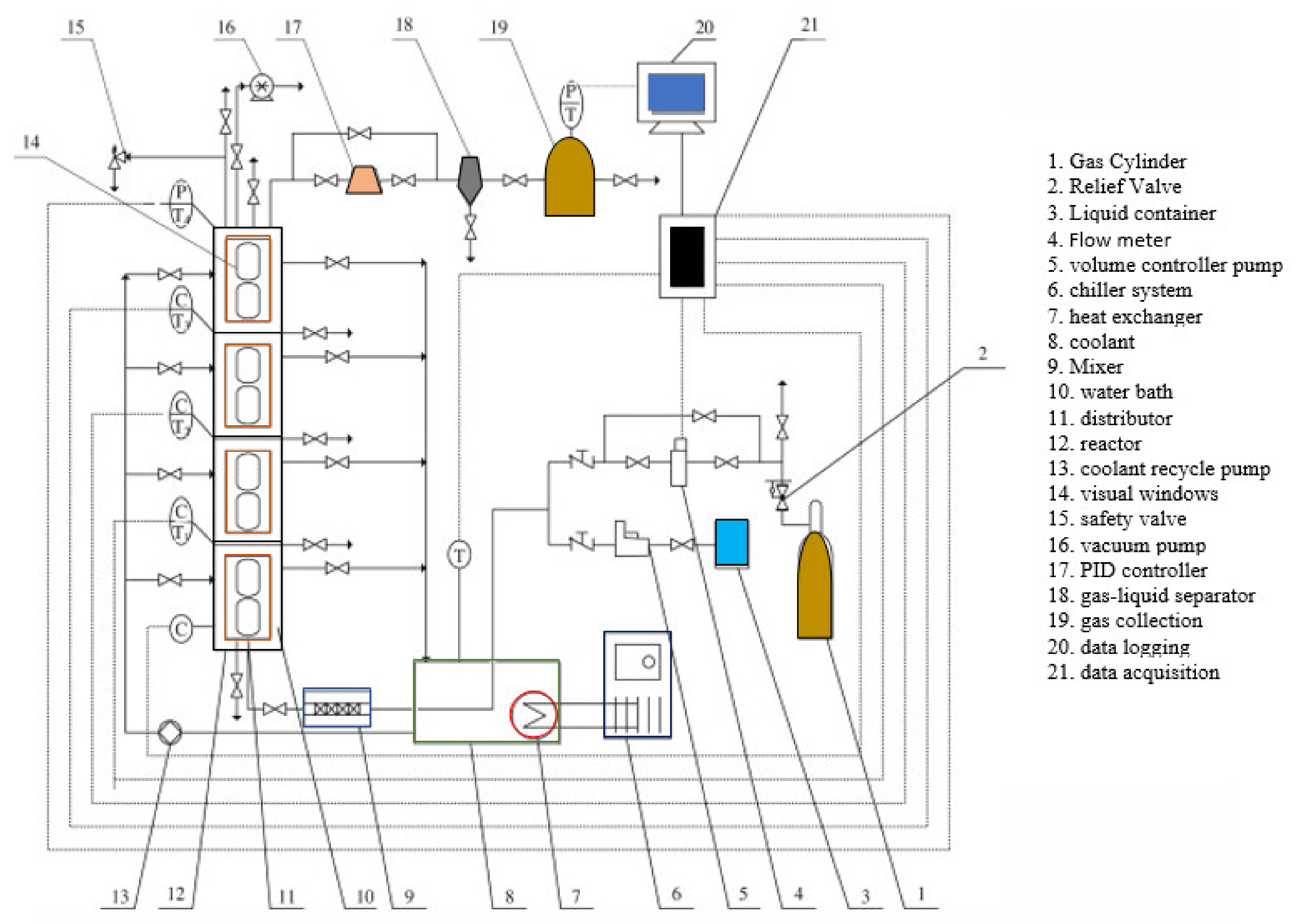
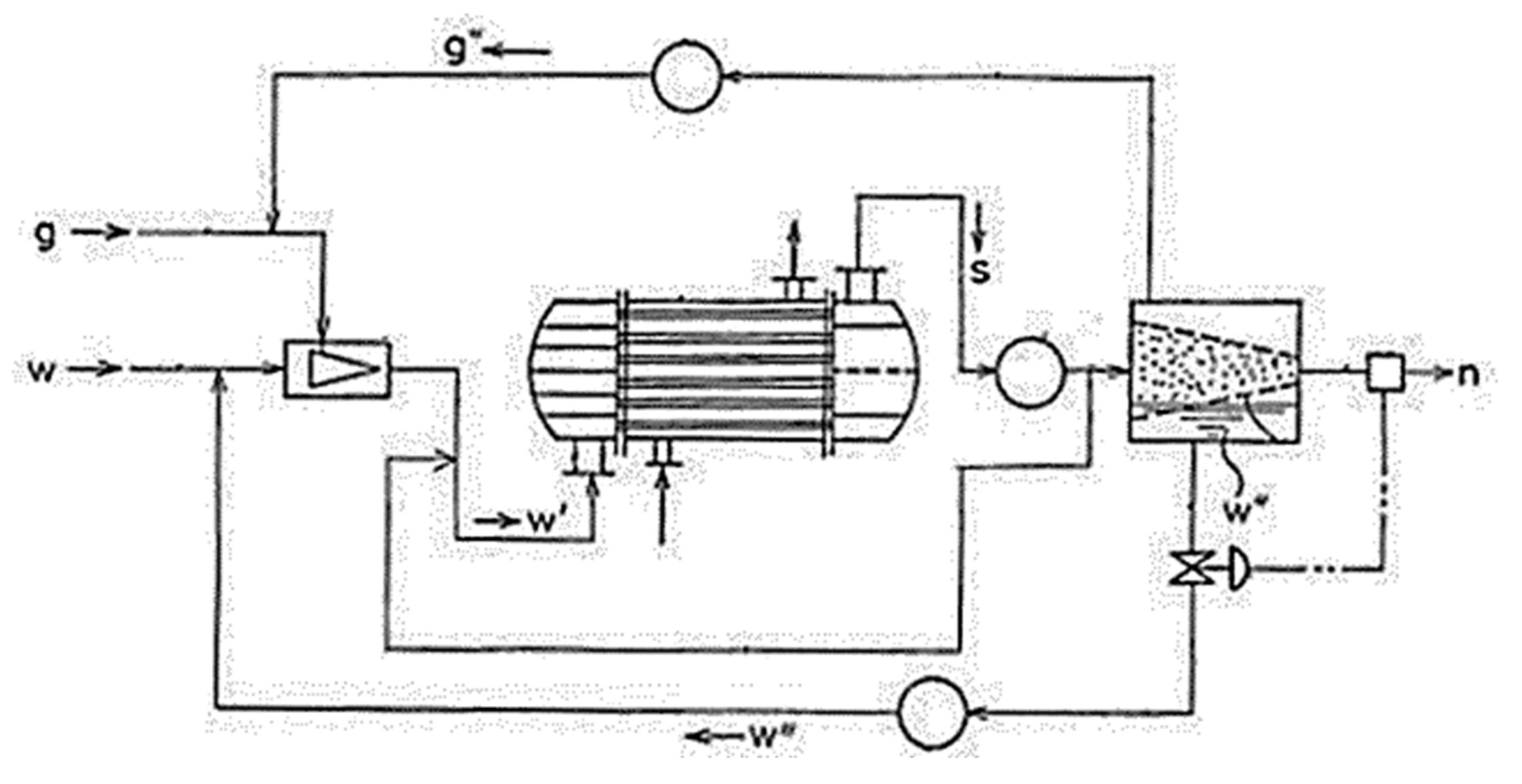
4. Gas Hydrate Crystallizers
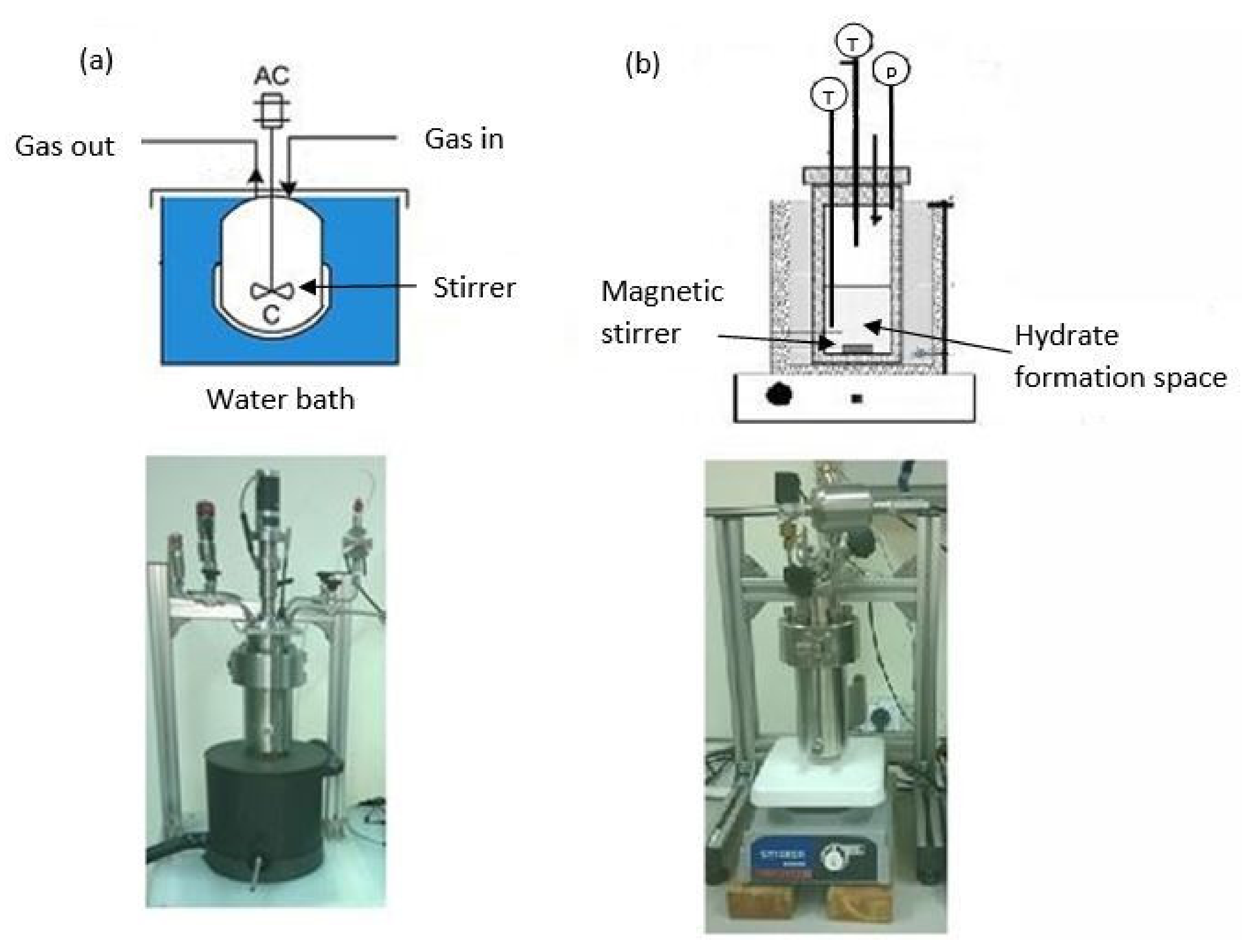
4.1. Stirrer-Tank Reactors (STRs)
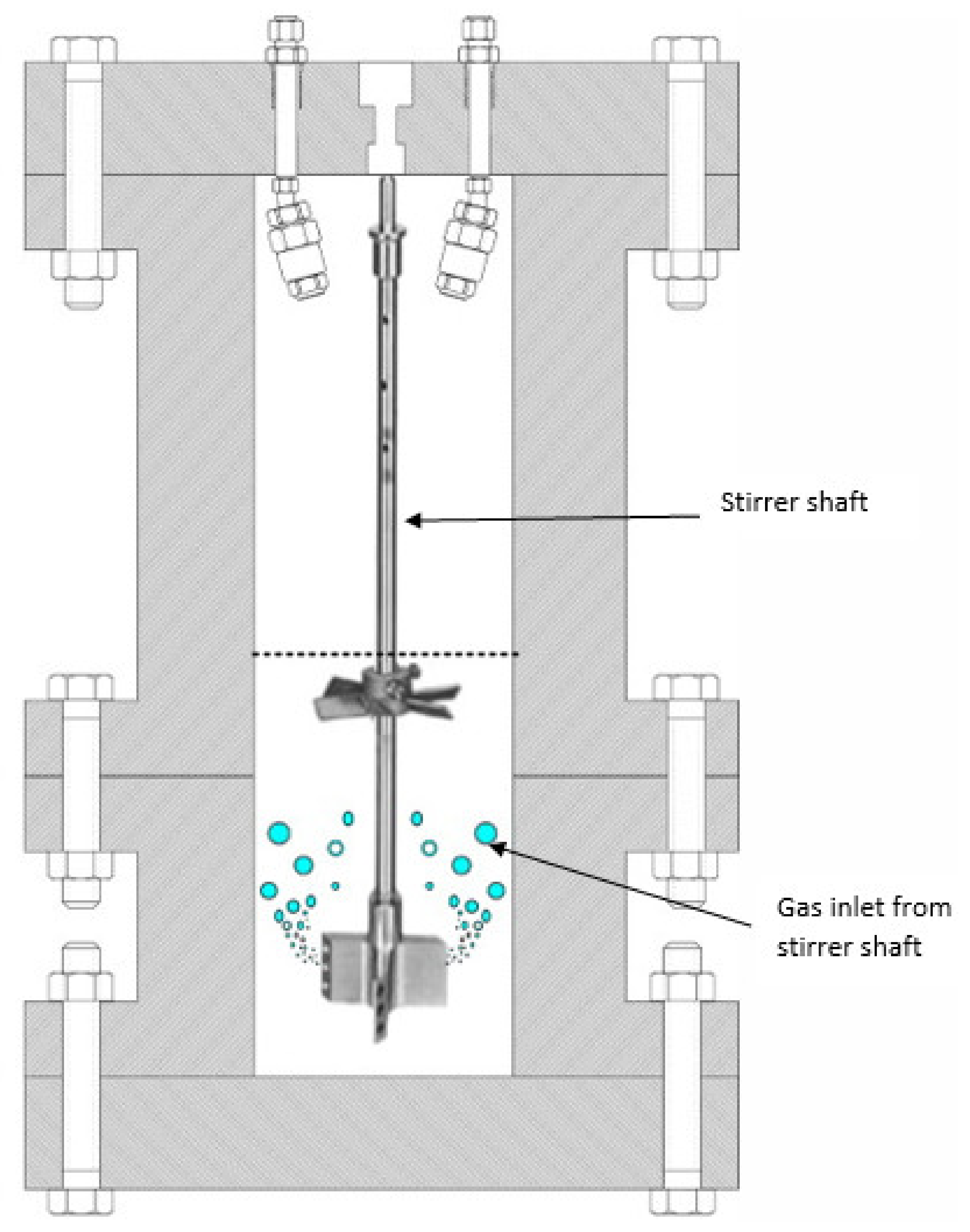
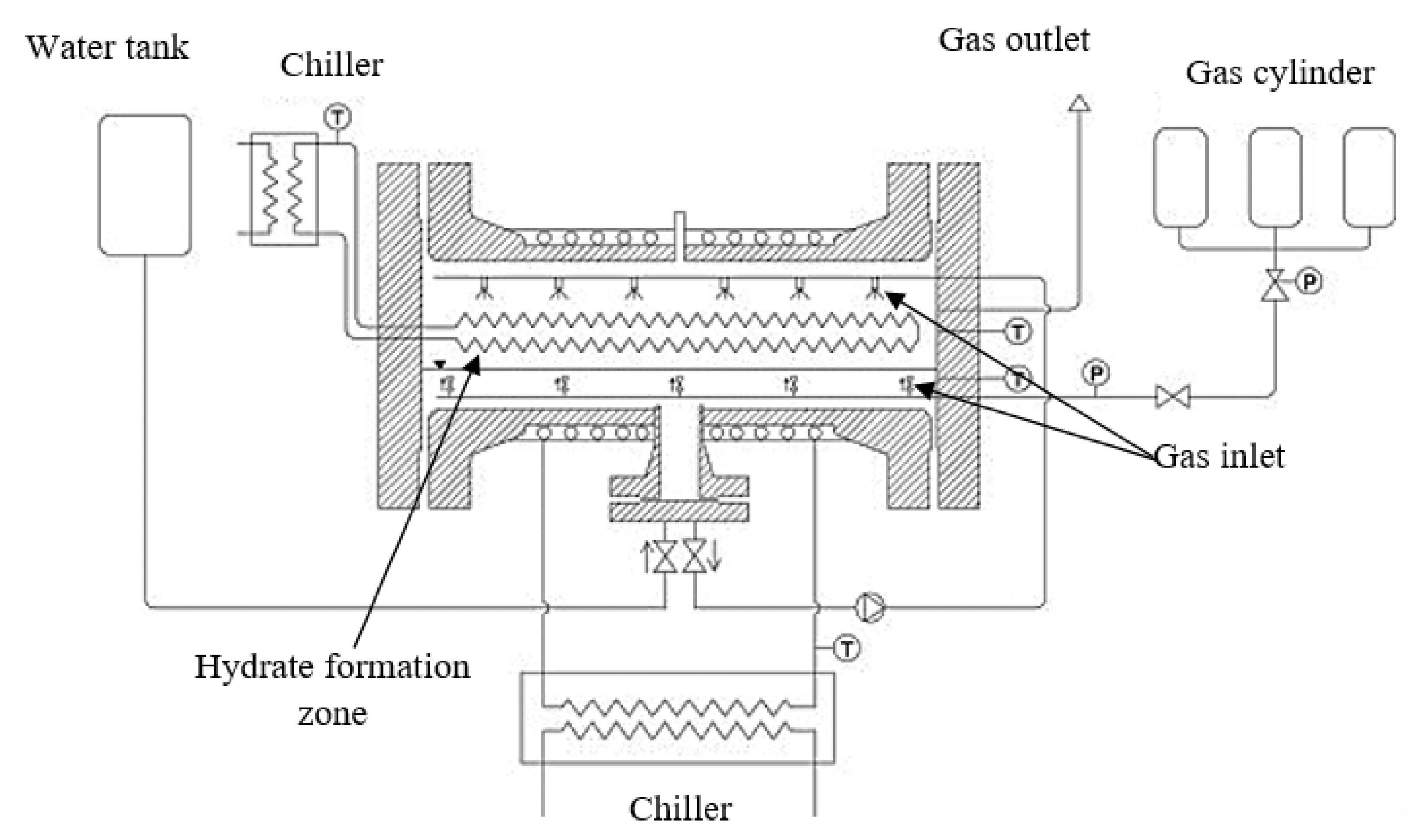
4.2. Bubble Reactors
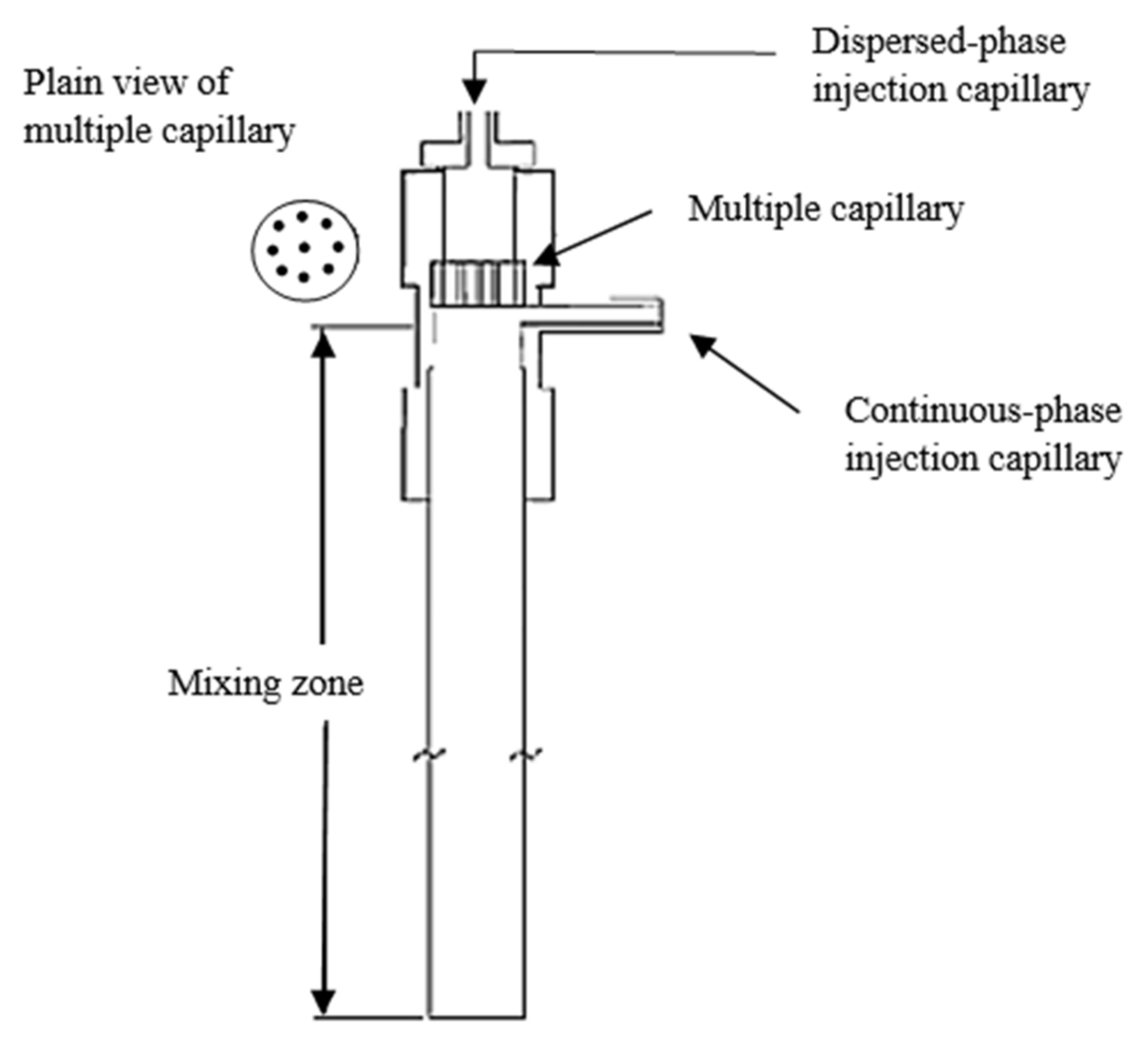
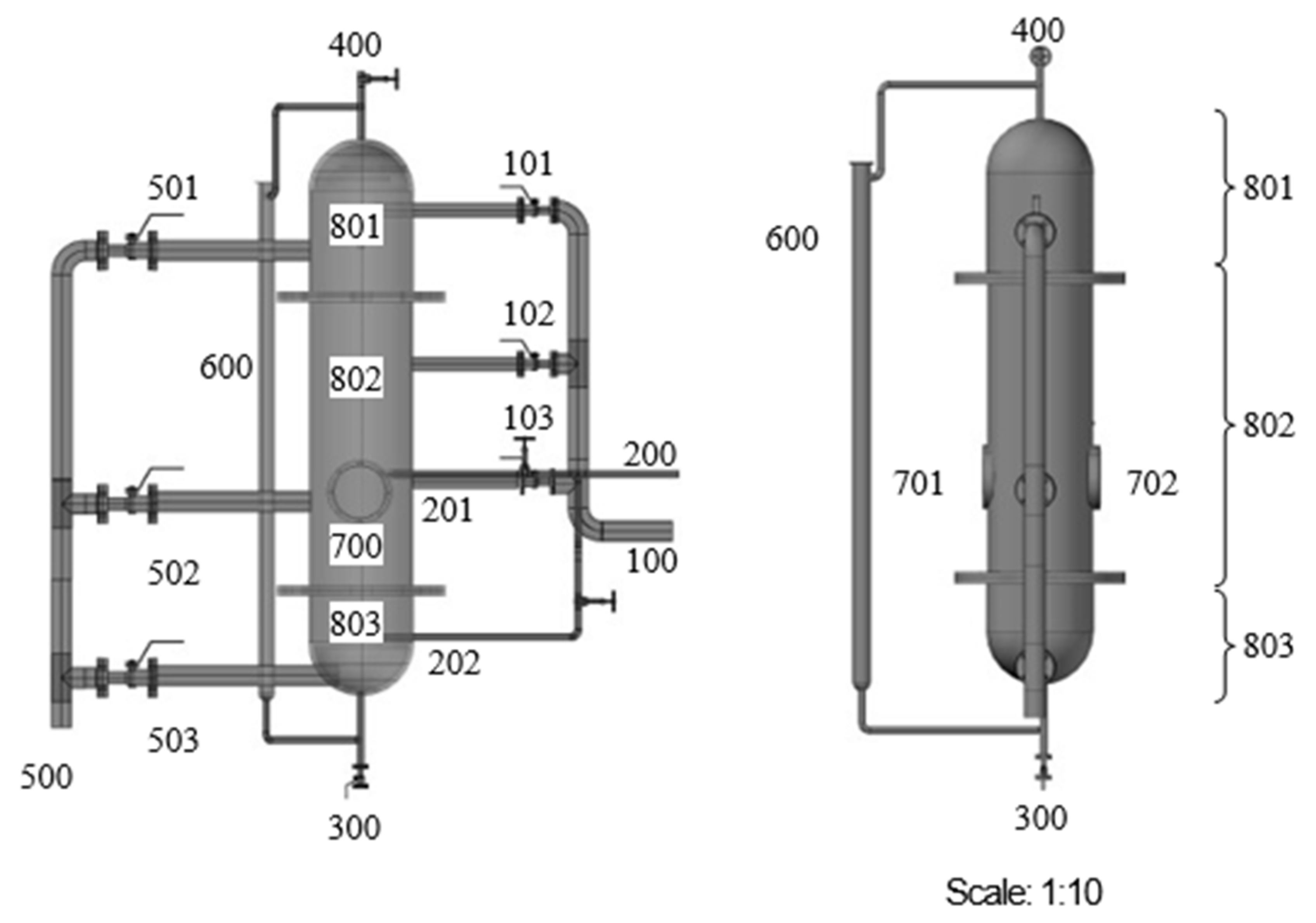
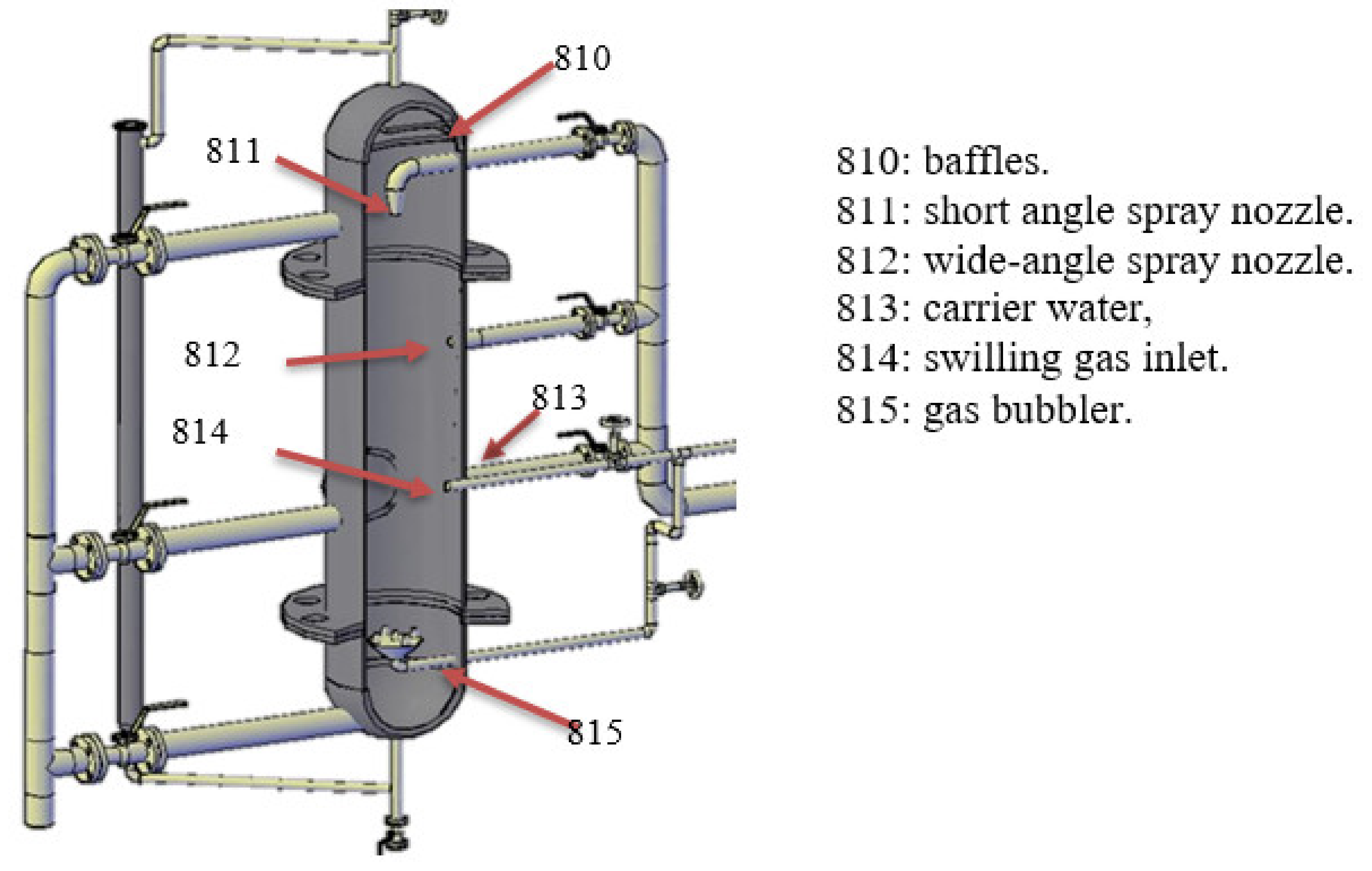
4.3. Spray Reactors
Miscellaneous Types
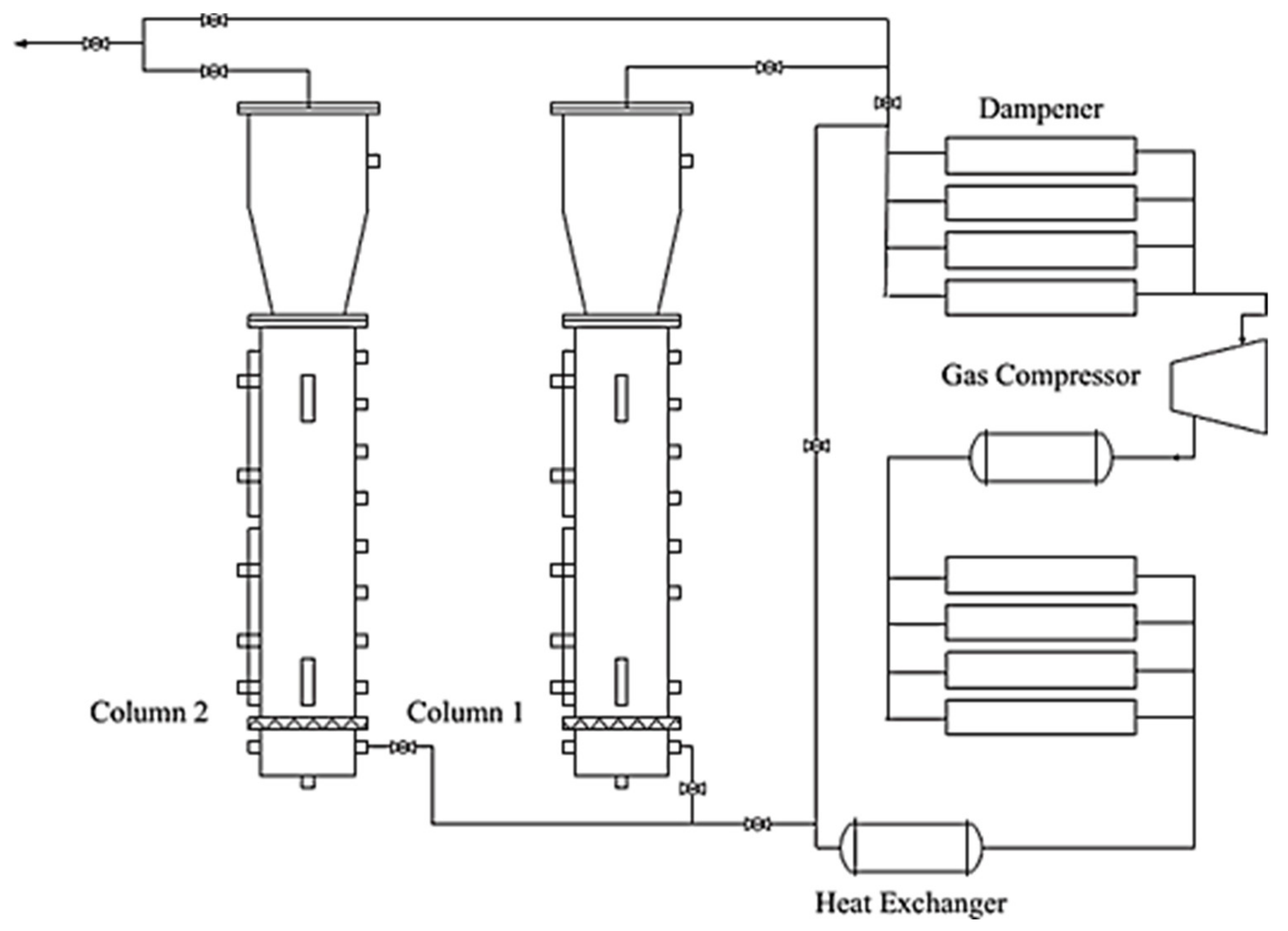
4.4. Recent Development in the Gas Hydrate-Based CO2 Capture and Sequestration
5. Conclusions
Funding
Conflicts of Interest
References
- Bachu, S. CO2 storage in geological media: Role, means, status and barriers to deployment. Prog. Energy Combust. Sci. 2008, 34, 254–273. [Google Scholar] [CrossRef]
- Sarkodie, S.A.; Strezov, V. Effect of foreign direct investments, economic development and energy consumption on greenhouse gas emissions in developing countries. Sci. Total Environ. 2019, 646, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Ciais, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; Canadell, J.; Chhabra, A.; DeFries, R.; Galloway, J.; Heimann, M.; et al. Carbon and other biogeochemical cycles. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 465–570. [Google Scholar]
- Harley, C.D.G.; Anderson, K.M.; Demes, K.W.; Jorve, J.P.; Kordas, R.L.; Coyle, T.A.; Graham, M.H. Effects of Climate Change on Global Seaweed Communities. J. Phycol. 2012, 48, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.R.; Frame, D.J.; Huntingford, C.; Jones, C.D.; Lowe, J.A.; Meinshausen, M.; Meinshausen, N. Warming caused by cumulative carbon emissions towards the trillionth tonne. Nature 2009, 458, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Frieler, K.; Meinshausen, M.; Golly, A.; Mengel, M.; Lebek, K.; Donner, S.D.; Hoegh-Guldberg, O. Limiting global warming to 2 C is unlikely to save most coral reefs. Nat. Clim. Chang. 2013, 3, 165–170. [Google Scholar] [CrossRef]
- Oliver, J.E. Kyoto protocol. In Encyclopedia of World Climatology; Springer: Berlin/Heidelberg, Germany, 2005; p. 443. [Google Scholar]
- Kaya, Y. The role of CO2 removal and disposal. Energy Convers. Manag. 1995, 36, 375–380. [Google Scholar] [CrossRef]
- Watts, R.G. Innovative Energy Strategies for CO2 Stabilization; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Parag, Y.; Darby, S. Consumer–supplier–government triangular relations: Rethinking the UK policy path for carbon emissions reduction from the UK residential sector. Energy Policy 2009, 37, 3984–3992. [Google Scholar] [CrossRef]
- Anable, J.; Brand, C.; Eyre, N.; Layberry, R.; Bergman, N.; Strachan, N.; Fawcett, T.; Tran, M. Lifestyle and Energy Consumption. UKERC Report. 2011. Available online: https://ukerc.rl.ac.uk/UCAT/cgi-bin/ucat_query.pl?GoButton=DisplayLanding&ucatID=459&URadio=P_12&GoButton=Find+Publications (accessed on 12 October 2022).
- Huaman, R.N.E.; Jun, T.X. Energy related CO2 emissions and the progress on CCS projects: A review. Renew. Sustain. Energy Rev. 2014, 31, 368–385. [Google Scholar] [CrossRef]
- Stigka, E.K.; Paravantis, J.A.; Mihalakakou, G.K. Social acceptance of renewable energy sources: A review of contingent valuation applications. Renew. Sustain. Energy Rev. 2014, 32, 100–106. [Google Scholar] [CrossRef]
- Gullberg, A.T.; Ohlhorst, D.; Schreurs, M. Towards a low carbon energy future–Renewable energy cooperation between Germany and Norway. Renew. Energy 2014, 68, 216–222. [Google Scholar] [CrossRef]
- Aslani, A.; Wong, K.-F.V. Analysis of renewable energy development to power generation in the United States. Renew. Energy 2014, 63, 153–161. [Google Scholar] [CrossRef]
- Abdullah, M.A.; Agalgaonkar, A.P.; Muttaqi, K.M. Climate change mitigation with integration of renewable energy resources in the electricity grid of New South Wales, Australia. Renew. Energy 2014, 66, 305–313. [Google Scholar] [CrossRef]
- Ludig, S.; Haller, M.; Schmid, E.; Bauer, N. Fluctuating renewables in a long-term climate change mitigation strategy. Energy 2011, 36, 6674–6685. [Google Scholar] [CrossRef]
- Van der Zwaan, B. The role of nuclear power in mitigating emissions from electricity generation. Energy Strateg. Rev. 2013, 1, 296–301. [Google Scholar] [CrossRef]
- Akashi, O.; Hijioka, Y.; Masui, T.; Hanaoka, T.; Kainuma, M. GHG emission scenarios in Asia and the world: The key technologies for significant reduction. Energy Econ. 2012, 34, S346–S358. [Google Scholar] [CrossRef]
- Raadal, H.L.; Vold, B.I.; Myhr, A.; Nygaard, T.A. GHG emissions and energy performance of offshore wind power. Renew. energy 2014, 66, 314–324. [Google Scholar] [CrossRef]
- Andress, D.; Nguyen, T.D.; Das, S. Reducing GHG emissions in the United States’ transportation sector. Energy Sustain. Dev. 2011, 15, 117–136. [Google Scholar] [CrossRef]
- Díaz, J.J.V.; Wilby, M.R.; González, A.B.R. Setting up GHG-based energy efficiency targets in buildings: The Ecolabel. Energy Policy 2013, 59, 633–642. [Google Scholar] [CrossRef]
- Du, J.D.; Han, W.J.; Peng, Y.H.; Gu, C.C. Potential for reducing GHG emissions and energy consumption from implementing the aluminum intensive vehicle fleet in China. Energy 2010, 35, 4671–4678. [Google Scholar] [CrossRef]
- Goldberg, D.S.; Takahashi, T.; Slagle, A.L. Carbon dioxide sequestration in deep-sea basalt. Proc. Natl. Acad. Sci. USA 2008, 105, 9920–9925. [Google Scholar] [CrossRef]
- Giavarini, C.; Maccioni, F.; Santarelli, M.L. CO2 sequestration from coal fired power plants. Fueln 2010, 89, 623–628. [Google Scholar] [CrossRef]
- Riestenberg, D.E.; Tsouris, C.; Brewer, P.G.; Peltzer, E.T.; Walz, P.; Chow, A.C.; Adams, E.E. Field studies on the formation of sinking CO2 particles for ocean carbon sequestration: Effects of injector geometry on particle density and dissolution rate and model simulation of plume behavior. Environ. Sci. Technol. 2005, 39, 7287–7293. [Google Scholar] [CrossRef]
- Herzog, H. What future for carbon capture and sequestration? Environ. Sci. Technol. 2001, 35, 148A. [Google Scholar] [CrossRef]
- Huesemann, M.H. The limits of technological solutions to sustainable development. Clean Technol. Environ. Policy 2003, 5, 21–34. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, H. Carbon capture and storage—Solidification and storage of carbon dioxide captured on ships. Ocean Eng. 2014, 91, 172–180. [Google Scholar] [CrossRef]
- Bove, R.; Lunghi, P. Electric power generation from landfill gas using traditional and innovative technologies. Energy Convers. Manag. 2006, 47, 1391–1401. [Google Scholar] [CrossRef]
- Burgers, W.F.J.; Northrop, P.S.; Kheshgi, H.S.; Valencia, J.A. Worldwide development potential for sour gas. Energy Procedia 2011, 4, 2178–2184. [Google Scholar] [CrossRef]
- Darman, N.H.; Harun, A.R. Technical challenges and solutions on natural gas development in Malaysia. In Proceedings of the Petroleum Policy and Management (PPM) Project 4th Workshop of the China—Sichuan Basin Case Study, Beijing, China, 30 May–3 June 2006. [Google Scholar]
- Yadav, S.; Mondal, S.S. A review on the progress and prospects of oxy-fuel carbon capture and sequestration (CCS) technology. Fuel 2022, 308, 122057. [Google Scholar] [CrossRef]
- Olabi, A.G.; Obaideen, K.; Elsaid, K.; Wilberforce, T.; Sayed, E.T.; Maghrabie, H.M.; Abdelkareem, M.A. Assessment of the pre-combustion carbon capture contribution into sustainable development goals SDGs using novel indicators. Renew. Sustain. Energy Rev. 2022, 153, 111710. [Google Scholar] [CrossRef]
- Chao, C.; Deng, Y.; Dewil, R.; Baeyens, J.; Fan, X. Post-combustion carbon capture. Renew. Sustain. Energy Rev. 2021, 138, 110490. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, X.; Ji, X. Carbon Dioxide Capture. In Deep Eutectic Solvents; Wiley: Hoboken, NJ, USA, 2019; pp. 297–319. [Google Scholar]
- Dragos, L.; Nada, O.; Flueraru, C.; Scarlat, N. Romanian researches for CO2 recovery. Energy Convers. Manag. 1996, 37, 923–928. [Google Scholar] [CrossRef]
- Maddox, R.N. Gas conditioning and processing. In Gas and Liquid Sweetening; Campbell Petroleum Series: Norman, OK, USA, 1982; Volume 4. Available online: https://www.osti.gov/biblio/6399050 (accessed on 12 October 2022).
- Jones, J.H.; Froning, H.R.; Claytor, E.E., Jr. Solubility of Acidic Gases in Aqueous Monoethanolamine. J. Chem. Eng. Data 1959, 4, 85–92. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef]
- Desideri, U. Advanced absorption processes and technology for carbon dioxide (CO2) capture in power plants. In Developments and Innovation in Carbon Dioxide (CO2) Capture and Storage Technology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 155–182. [Google Scholar]
- Zennaro, R.; Tagliabue, M.; Bartholomew, C.H. Kinetics of Fischer–Tropsch synthesis on titania-supported cobalt. Catal. Today 2000, 58, 309–319. [Google Scholar] [CrossRef]
- Campbell, J.M. Gas Conditioning and Processing-Volume 1: The Basic Principles; Campbell Pertroleum Series: Norman, OK, USA, 1992. [Google Scholar]
- Yong, Z.; Mata, V.; Rodrigues, A.E. Adsorption of carbon dioxide at high temperature—A review. SePurif. Technol. 2022, 26, 195–205. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Adams, J.U.; Fairman, J. Essentials of cell biology. Camb. MA NPG Educ. 2010, 1, 54. [Google Scholar]
- Glater, J. The early history of reverse osmosis membrane development. Desalination 1998, 117, 297–309. [Google Scholar] [CrossRef]
- Sridhar, S.; Smitha, B.; Aminabhavi, T.M. Separation of carbon dioxide from natural gas mixtures through polymeric membranes—A review. SePurif. Rev. 2007, 36, 113–174. [Google Scholar] [CrossRef]
- Vora, S.; Brickett, L.; Indrikanti, P.; Munson, R.; Murphy, J.; Rife, T.; Strock, J.; Zaremsky, C. DOE/NETL Advanced Carbon Dioxide Capture R&D Program: Technology Update; US Department of Energy/National Energy Technology Laboratory: Washington, DC, USA, 2013.
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas: A review. SeSci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Zhao, B.; Su, Y.; Tao, W.; Li, L.; Peng, Y. Post-combustion CO2 capture by aqueous ammonia: A state-of-the-art review. Int. J. Greenh. Gas Control 2012, 9, 355–371. [Google Scholar] [CrossRef]
- Granite, E.J.; O’Brien, T. Review of novel methods for carbon dioxide separation from flue and fuel gases. Fuel Process. Technol. 2005, 86, 1423–1434. [Google Scholar] [CrossRef]
- Gouedard, C.; Picq, D.; Launay, F.; Carrette, P.-L. Amine degradation in CO2 capture. I. A review. Int. J. Greenh. Gas Control. 2012, 10, 244–270. [Google Scholar] [CrossRef]
- Aghel, B.; Janati, S.; Wongwises, S.; Shadloo, M.S. Review on CO2 capture by blended amine solutions. Int. J. Greenh. Gas Control. 2022, 119, 103715. [Google Scholar] [CrossRef]
- Chang, P.T.; Ng, Q.H.; Ahmad, A.L.; Low, S.C. A critical review on the techno-economic analysis of membrane gas absorption for CO2 capture. Chem. Eng. Commun. 2022, 209, 1553–1569. [Google Scholar] [CrossRef]
- Siegelman, R.L.; Milner, P.J.; Kim, E.J.; Weston, S.C.; Long, J.R. Challenges and opportunities for adsorption-based CO2 capture from natural gas combined cycle emissions. Energy Environ. Sci. 2019, 12, 2161–2173. [Google Scholar] [CrossRef]
- Raganati, F.; Chirone, R.; Ammendola, P. CO2 capture by temperature swing adsorption: Working capacity as affected by temperature and CO2 partial pressure. Ind. Eng. Chem. Res. 2020, 59, 3593–3605. [Google Scholar] [CrossRef]
- Aghel, B.; Behaein, S.; Alobiad, F. CO2 capture from biogas by biomass-based adsorbents: A review. Fuel 2022, 328, 125276. [Google Scholar] [CrossRef]
- Kaur, B.; Gupta, R.K.; Bhunia, H. Chemically activated nanoporous carbon adsorbents from waste plastic for CO2 capture: Breakthrough adsorption study. Microporous Mesoporous Mater. 2019, 282, 146–158. [Google Scholar] [CrossRef]
- Kárászová, M.; Zach, B.; Petrusová, Z.; Červenka, V.; Bobák, M.; Šyc, M.; Izák, P. Post-combustion carbon capture by membrane separation, Review. SePurif. Technol. 2020, 238, 116448. [Google Scholar] [CrossRef]
- Han, Y.; Ho, W.S.W. Recent advances in polymeric membranes for CO2 capture. Chin. J. Chem. Eng. 2018, 26, 2238–2254. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and techniques for CO2 capture: Review of potential solutions and applications in modern energy technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Shen, M.; Tong, L.; Yin, S.; Liu, C.; Wang, L.; Feng, W.; Ding, Y. Cryogenic technology progress for CO2 capture under carbon neutrality goals: A review. SePurif. Technol. 2022, 299, 121734. [Google Scholar] [CrossRef]
- Naquash, A.; Qyyum, M.A.; Haider, J.; Bokhari, A.; Lim, H.; Lee, M. State-of-the-art assessment of cryogenic technologies for biogas upgrading: Energy, economic, and environmental perspectives. Renew. Sustain. Energy Rev. 2022, 154, 111826. [Google Scholar] [CrossRef]
- Bi, Y.; Ju, Y. Review on cryogenic technologies for CO2 removal from natural gas. Front. Energy 2022, 1–19. [Google Scholar] [CrossRef]
- CCS “Red Flag?” World’s Sole Coal Project Hits Snag—E&E News. Available online: https://www.eenews.net/articles/ccs-red-flag-worlds-sole-coal-project-hits-snag/ (accessed on 14 October 2022).
- Sabil, K.M.; Witkamp, G.-J.; Peters, C.J. Estimations of enthalpies of dissociation of simple and mixed carbon dioxide hydrates from phase equilibrium data. Fluid Phase Equilib. 2010, 290, 109–114. [Google Scholar] [CrossRef]
- Rehman, A.U.; Zaini, D.B.; Lal, B. Gas Hydrates in Wastewater Treatment. Gas Hydrate Water Treat. Technol. Econ. Ind. Asp. 2022, 113–137. [Google Scholar] [CrossRef]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of carbon dioxide for post-combustion capture: A review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Kargari, A.; Rezaeinia, S. State-of-the-art modification of polymeric membranes by PEO and PEG for carbon dioxide separation: A review of the current status and future perspectives. J. Ind. Eng. Chem. 2020, 84, 1–22. [Google Scholar] [CrossRef]
- Ziobrowski, Z.; Rotkegel, A. Comparison of CO2 Separation Efficiency from Flue Gases Based on Commonly Used Methods and Materials. Materials 2022, 15, 460. [Google Scholar] [CrossRef]
- Pérez-Calvo, J.-F.; Sutter, D.; Gazzani, M.; Mazzotti, M. Advanced configurations for post-combustion CO2 capture processes using an aqueous ammonia solution as absorbent. SePurif. Technol. 2021, 274, 118959. [Google Scholar] [CrossRef]
- Mazari, S.A.; Ghalib, L.; Sattar, A.; Bozdar, M.M.; Qayoom, A.; Ahmed, I.; Muhammad, A.; Abro, R.; Abdulkareem, A.; Nizamuddin, S.; et al. Review of modelling and simulation strategies for evaluating corrosive behavior of aqueous amine systems for CO2 capture. Int. J. Greenh. Gas Control. 2020, 96, 103010. [Google Scholar] [CrossRef]
- Hou, R.; Fong, C.; Freeman, B.D.; Hill, M.R.; Xie, Z. Current status and advances in membrane technology for carbon capture. SePurif. Technol. 2022, 300, 121863. [Google Scholar] [CrossRef]
- Tu, Z.; Liu, P.; Zhang, X.; Shi, M.; Zhang, Z.; Luo, S.; Zhang, L.; Wu, Y.; Hu, X. Highly-selective separation of CO2 from N2 or CH4 in task-specific ionic liquid membranes: Facilitated transport and salting-out effect. SePurif. Technol. 2021, 254, 117621. [Google Scholar] [CrossRef]
- Rodger, P.M. Stability of gas hydrates. J. Phys. Chem. 1990, 94, 6080–6089. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Gas Hydrates of Natural Gases; CRC Press LLC: Boca Raton, FL, USA, 2008. [Google Scholar]
- Jensen, L. Experimental Investigation and Molecular Simulation of Gas Hydrates; Technical University of Denmark: Lyngby, Denmark, 2010. [Google Scholar]
- Englezos, P. Clathrate hydrates. Ind. Eng. Chem. Res. 1993, 32, 1251–1274. [Google Scholar] [CrossRef]
- Ur Rehman, A.; Abdulwahab, A.; Kaur, A.; Khan, M.S.; Zaini, D.B.; Shariff, A.M.; Lal, B. Experimental investigation and modelling of synergistic thermodynamic inhibition of Diethylene Glycol and glycine mixture on CO2 gas hydrates. Chemosphere 2022, 308, 136181. [Google Scholar] [CrossRef]
- Iitaka, T.; Ebisuzaki, T. Methane hydrate under high pressure. Phys. Rev. B 2003, 68, 172105. [Google Scholar] [CrossRef]
- Nallakukkala, S.; Rehman, A.u.; Zaini, D.B.; Lal, B. Gas Hydrate-Based Heavy Metal Ion Removal from Industrial Wastewater: A Review. Water 2022, 14, 1171. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, S.; Liu, Y.; Zhang, Y.; Ling, Z.; Yang, M.; Jiang, L.; Song, Y. Post-combustion CO2 capture and separation in flue gas based on hydrate technology: A review. Renew. Sustain. Energy Rev. 2022, 154, 111806. [Google Scholar] [CrossRef]
- Wei, W.-N.; Li, B.; Gan, Q.; Li, Y.-L. Research progress of natural gas hydrate exploitation with CO2 replacement: A review. Fuel 2022, 312, 122873. [Google Scholar] [CrossRef]
- Von Solms, N. Gas Hydrates for CO2 Capture. In Sustainable Carbon Capture; CRC Press: Boca Raton, FL, USA, 2022; pp. 143–159. [Google Scholar]
- Haq, I.U.; Qasim, A.; Lal, B.; Zaini, D.B. Mini review on environmental issues concerning conventional gas hydrate inhibitors. Process Saf. Prog. 2021, 41, 1–6. [Google Scholar] [CrossRef]
- Haq, I.U.; Lal, B.; Zaini, D.B. Applicability of Deep Eutectic Solvents in Oil and Gas Processing Fields for CO2 Control. Chem. Eng. Technol. 2022, 45, 1439–1447. [Google Scholar] [CrossRef]
- Lal, B.; Nashed, O. Chemical Additives for Gas Hydrates; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Almashwali, A.A.; Bavoh, C.B.; Lal, B.; Khor, S.F.; Jin, Q.C.; Zaini, D. Gas Hydrate in Oil-Dominant Systems: A Review. ACS Omega 2022, 7, 27021–27037. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008; p. 119. [Google Scholar]
- Lal, B.; Qasim, A.; Shariff, A.M. Ionic Liquids in Flow Assurance; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Khan, M.S.; Yaqub, S.; Manner, N.; Karthwathi, N.A.; Qasim, A.; Mellon, N.B.; Lal, B. Experimental equipment validation for methane (CH4) and carbon dioxide (CO2) hydrates. IOP Conf. Ser. Mater. Sci. Eng. 2018, 344, 12025. [Google Scholar] [CrossRef]
- Wang, P.; Teng, Y.; Zhu, J.; Bao, W.; Han, S.; Li, Y.; Zhao, Y.; Xie, H. Review on the synergistic effect between metal–organic frameworks and gas hydrates for CH4 storage and CO2 separation applications. Renew. Sustain. Energy Rev. 2022, 167, 112807. [Google Scholar] [CrossRef]
- Partoon, B.; Sabil, K.M.; Lau, K.K.; Nasrifar, K.; Shariff, A.M. Selective separation of methane from carbon dioxide through sII hydrates formation in a semibatch process. Ind. Eng. Chem. Res. 2019, 58, 16834–16842. [Google Scholar] [CrossRef]
- Ul Haq, I.; Qasim, A.; Lal, B.; Zaini, D.B.; Foo, K.S.; Mubashir, M.; Khoo, K.S.; Vo, D.V.N.; Leroy, E.; Show, P.L. Ionic liquids for the inhibition of gas hydrates. A review. Environ. Chem. Lett. 2022, 20, 2165–2188. [Google Scholar] [CrossRef]
- Xijia, L.; Miles, P.; Brock, F.; Mike, M. A Novel Power Generation System Utilizing Un-treated Sour Gas Fuel. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 12–15 November 2018. [Google Scholar]
- Kulišić, B.; Par, V.; Metzler, R. Calculation of on-farm biogas potential: A Croatian case study. Biomass Bioenergy 2015, 74, 66–78. [Google Scholar] [CrossRef]
- Qasim, A.; Khan, M.S.; Lal, B.; Shariff, A.M.; Ismail, M.C. Evaluation of tetramethylammonium acetate as corrosion suppressor for flow assurance applications. Mater. Today Proc. 2020, 47, 1355–1358. [Google Scholar] [CrossRef]
- Khan, M.S.; Bavoh, C.B.; Partoon, B.; Lal, B.; Bustam, M.A.; Shariff, A.M. Thermodynamic effect of ammonium based ionic liquids on CO2 hydrates phase boundary. J. Mol. Liq. 2017, 238, 533–539. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Partoon, B.; Lal, B.; Gonfa, G.; Khor, S.F.; Sharif, A.M. Inhibition effect of amino acids on carbon dioxide hydrate. Chem. Eng. Sci. 2017, 171, 331–339. [Google Scholar] [CrossRef]
- Heidaryan, E.; Filho, P.d.P.; Fuentes, M.D.R. Molecular Dynamic Simulations of Clathrate Hydrate Structures I: Lattice Constant and Thermal Expansion. J. Low TemPhys. 2022, 207, 227–240. [Google Scholar] [CrossRef]
- Heidaryan, E.; Fuentes, M.D.R.; Filho, P.D.P. Equilibrium of Methane and Carbon Dioxide Hydrates Below the Freezing Point of Water: Literature Review and Modeling. J. Low TemPhys. 2019, 194, 27–45. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, F.; Li, Y.; Lü, X.; Yang, M. Progress and trends in hydrate based desalination (HBD) technology: A review. Chin. J. Chem. Eng. 2019, 27, 2037–2043. [Google Scholar] [CrossRef]
- Nallakukkala, S.; Lal, B. Seawater and produced water treatment via gas hydrate: Review. J. Environ. Chem. Eng. 2021, 9, 105053. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Keong, L.K.; Ahmed, I. Tetramethyl ammonium chloride as dual functional inhibitor for methane and carbon dioxide hydrates. Fuel 2019, 236, 251–263. [Google Scholar] [CrossRef]
- McElligott, A.; Guerra, A.; Du, C.Y.; Rey, A.D.; Meunier, J.-L.; Servio, P. Dynamic viscosity of methane hydrate systems from non-Einsteinian, plasma-functionalized carbon nanotube nanofluids. Nanoscale 2022, 14, 10211–10225. [Google Scholar] [CrossRef]
- Guerra, A.; McElligott, A.; Du, C.Y.; Marić, M.; Rey, A.D.; Servio, P. Dynamic viscosity of methane and carbon dioxide hydrate systems from pure water at high-pressure driving forces. Chem. Eng. Sci. 2022, 252, 117282. [Google Scholar] [CrossRef]
- Kang, S.-P.; Lee, H. Recovery of CO2 from flue gas using gas hydrate: Thermodynamic verification through phase equilibrium measurements. Environ. Sci. Technol. 2000, 34, 4397–4400. [Google Scholar] [CrossRef]
- Duc, N.H.; Chauvy, F.; Herri, J.-M. CO2 capture by hydrate crystallization–A potential solution for gas emission of steelmaking industry. Energy Convers. Manag. 2007, 48, 1313–1322. [Google Scholar] [CrossRef]
- Linga, P.; Kumar, R.; Englezos, P. Gas hydrate formation from hydrogen/carbon dioxide and nitrogen/carbon dioxide gas mixtures. Chem. Eng. Sci. 2007, 62, 4268–4276. [Google Scholar] [CrossRef]
- Linga, P.; Adeyemo, A.; Englezos, P. Medium-pressure clathrate hydrate/membrane hybrid process for postcombustion capture of carbon dioxide. Environ. Sci. Technol. 2008, 42, 315–320. [Google Scholar] [CrossRef]
- Li, S.; Fan, S.; Wang, J.; Lang, X.; Liang, D. CO2 capture from binary mixture via forming hydrate with the help of tetra-n-butyl ammonium bromide. J. Nat. Gas Chem. 2009, 18, 15–20. [Google Scholar] [CrossRef]
- Seo, Y.; Kang, S.-P. Enhancing CO2 separation for pre-combustion capture with hydrate formation in silica gel pore structure. Chem. Eng. J. 2010, 161, 308–312. [Google Scholar] [CrossRef]
- Gholinezhad, J.; Chapoy, A.; Tohidi, B. Separation and capture of carbon dioxide from CO2/H2 syngas mixture using semi-clathrate hydrates. Chem. Eng. Res. Des. 2011, 89, 1747–1751. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, J.D.; Lee, H.J.; Lee, E.K.; Kim, Y. Gas hydrate formation method to capture the carbon dioxide for pre-combustion process in IGCC plant. Int. J. Hydrogen Energy 2011, 36, 1115–1121. [Google Scholar] [CrossRef]
- Li, X.-S.; Xu, C.-G.; Chen, Z.-Y.; Wu, H.-J. Tetra-n-butyl ammonium bromide semi-clathrate hydrate process for post-combustion capture of carbon dioxide in the presence of dodecyl trimethyl ammonium chloride. Energy 2010, 35, 3902–3908. [Google Scholar] [CrossRef]
- Xu, C.; Li, X.; Cai, J.; Chen, Z. Hydrate-based carbon dioxide capture from simulated integrated gasification combined cycle gas. J. Nat. Gas Chem. 2012, 21, 501–507. [Google Scholar] [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. Pre-combustion capture of carbon dioxide in a fixed bed reactor using the clathrate hydrate process. Energy 2013, 50, 364–373. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, G.; Guo, X.; Liu, A. Experiments on the continuous separation of gas mixtures via dissolution and hydrate formation in the presence of THF. Fluid Phase Equilib. 2014, 361, 250–256. [Google Scholar] [CrossRef]
- Zhou, X.; Liang, D.; Yi, L. Experimental study of mixed CH4/CO2 hydrate formation kinetics and modeling. Asia-Pac. J. Chem. Eng. 2014, 9, 886–894. [Google Scholar] [CrossRef]
- Mori, H. Recent advances in hydrate-based technologies for natural gas storage—A review. Chin. J. Chem. Eng. 2003, 1, 1–17. [Google Scholar]
- Iwasaki, T.; Katoh, Y.; Nagamori, S.; Takahashi, S.; Oya, N. Continuous Natural Gas Hydrate Pellet Production (NGHP) by Process Development Unit (PDU); DOE: Trondheim, Norway, 2005. [Google Scholar]
- Luo, Y.T.; Zhu, J.H.; Fan, S.S.; Chen, G.J. Study on the kinetics of hydrate formation in a bubble column. Chem. Eng. Sci. 2007, 62, 1000–1009. [Google Scholar] [CrossRef]
- Hashemi, S.; Macchi, A.; Servio, P. Gas–liquid mass transfer in a slurry bubble column operated at gas hydrate forming conditions. Chem. Eng. Sci. 2009, 64, 3709–3716. [Google Scholar] [CrossRef]
- Nguyen, N.N.; La, V.T.; Huynh, C.D.; Nguyen, A.V. Technical and economic perspectives of hydrate-based carbon dioxide capture. Appl. Energy 2022, 307, 118237. [Google Scholar] [CrossRef]
- Babu, P.; Linga, P.; Kumar, R.; Englezos, P. A review of the hydrate based gas separation (HBGS) process for carbon dioxide pre-combustion capture. Energy 2015, 85, 261–279. [Google Scholar] [CrossRef]
- Dashti, H.; Lou, X. Gas hydrate-based CO2 separation process: Quantitative assessment of the effectiveness of various chemical additives involved in the process. In TMS Annual Meeting & Exhibition; Springer: Cham, Switzerland, 2018; pp. 3–16. [Google Scholar]
- Spencer, D.F. Integration of An Advanced CO2 Separation Process with Methods for Disposing of CO2 in Oceans and Terrestrial Deep Aquifers; ABB Corporate Research Ltd.: Baden-Daettwil, Switzerland, 1999. [Google Scholar]
- Sundramoorthy, J.D.; Sabil, K.M.; Lal, B.; Hammonds, P. Catastrophic crystal growth of clathrate hydrate with a simulated natural gas system during a pipeline shut-in condition. Cryst. Growth Des. 2015, 15, 1233–1241. [Google Scholar] [CrossRef]
- Daraboina, N.; Malmos, C.; von Solms, N. Synergistic kinetic inhibition of natural gas hydrate formation. Fuel 2013, 108, 749–757. [Google Scholar] [CrossRef]
- Jerbi, S.; Delahaye, A.; Fournaison, L.; Haberschill, P. Characterization of CO2 hydrate formation and dissociation kinetics in a flow loop. Int. J. Refrig. 2010, 33, 1625–1631. [Google Scholar] [CrossRef]
- Sarshar, M.; Fathikalajahi, J.; Esmaeilzadeh, F. Experimental and theoretical study of gas hydrate formation in a high-pressure flow loop. Can. J. Chem. Eng. 2010, 88, 751–757. [Google Scholar] [CrossRef]
- Partoon, B.; Javanmardi, J. Effect of mixed thermodynamic and kinetic hydrate promoters on methane hydrate phase boundary and formation kinetics. J. Chem. Eng. Data 2013, 58, 501–509. [Google Scholar] [CrossRef]
- Sabil, K.M.; Witkamp, G.J.; Peters, C.J. Phase equilibria of mixed carbon dioxide and tetrahydrofuran hydrates in sodium chloride aqueous solutions. Fluid Phase Equilib. 2009, 284, 38–43. [Google Scholar] [CrossRef]
- Partoon, B.; Malik, S.N.A.; Azemi, M.H.; Sabil, K.M. Experimental investigations on the potential of SDS as low-dosage promoter for carbon dioxide hydrate formation. Asia-Pac. J. Chem. Eng. 2013, 8, 916–921. [Google Scholar] [CrossRef]
- Seo, Y.-T.; Lee, H. Multiple-phase hydrate equilibria of the ternary carbon dioxide, methane, and water mixtures. J. Phys. Chem. B 2001, 105, 10084–10090. [Google Scholar] [CrossRef]
- Clarke, M.A.; Bishnoi, P.R. Determination of the intrinsic kinetics of CO2 gas hydrate formation using in situ particle size analysis. Chem. Eng. Sci. 2005, 60, 695–709. [Google Scholar] [CrossRef]
- Vysniauskas, A.; Bishnoi, P.R. A kinetic study of methane hydrate formation. Chem. Eng. Sci. 1983, 38, 1061–1072. [Google Scholar] [CrossRef]
- Linga, P.; Kumar, R.; Lee, J.D.; Ripmeester, J.; Englezos, P. A new apparatus to enhance the rate of gas hydrate formation: Application to capture of carbon dioxide. Int. J. Greenh. Gas Control. 2010, 4, 630–637. [Google Scholar] [CrossRef]
- Xu, C.-G.; Li, X.-S.; Lv, Q.-N.; Chen, Z.-Y.; Cai, J. Hydrate-based CO2 (carbon dioxide) capture from IGCC (integrated gasification combined cycle) synthesis gas using bubble method with a set of visual equipment. Energy 2012, 44, 358–366. [Google Scholar] [CrossRef]
- Murakami, T.; Kuritsuka, H.; Fujii, H.; Mori, Y.H. Forming a structure-H hydrate using water and methylcyclohexane jets impinging on each other in a methane atmosphere. Energy Fuels 2009, 23, 1619–1625. [Google Scholar] [CrossRef]
- Fukumoto, K.; Tobe, J.; Ohmura, R.; Mori, Y.H. Hydrate formation using water spraying in a hydrophobic gas: A preliminary study. Am. Inst. Chem. Eng. AIChE J. 2001, 47, 1899. [Google Scholar] [CrossRef]
- Ohmura, R.; Kashiwazaki, S.; Shiota, S.; Tsuji, H.; Mori, Y.H. Structure-I and structure-H hydrate formation using water spraying. Energy Fuels 2002, 16, 1141–1147. [Google Scholar] [CrossRef]
- Tsuji, H.; Kobayashi, T.; Ohmura, R.; Mori, Y.H. Hydrate formation by water spraying in a methane+ ethane+ propane gas mixture: An attempt at promoting hydrate formation utilizing large-molecule guest substances for structure-H hydrates. Energy Fuels 2005, 19, 869–876. [Google Scholar] [CrossRef]
- Tsuji, H.; Ohmura, R.; Mori, Y.H. Forming structure-H hydrates using water spraying in methane gas: Effects of chemical species of large-molecule guest substances. Energy Fuels 2004, 18, 418–424. [Google Scholar] [CrossRef]
- Li, G.; Liu, D.; Xie, Y.; Xiao, Y. Study on effect factors for CO2 hydrate rapid formation in a water-spraying apparatus. Energy Fuels 2010, 24, 4590–4597. [Google Scholar] [CrossRef]
- Kikuo, N. Hydrate Production Equipment. Japanese Patent JP 2006-111774 A, 27 April 2006. [Google Scholar]
- Lucia, B.; Castellani, B.; Rossi, F.; Cotana, F.; Morini, E.; Nicolini, A.; Filipponi, M. Experimental investigations on scaled-up methane hydrate production with surfactant promotion: Energy considerations. J. Pet. Sci. Eng. 2014, 120, 187–193. [Google Scholar] [CrossRef]
- Heinemann, R.F.; Huang, D.D.-T.; Long, J.; Saeger, R.B. Process for Making Gas Hydrates. U.S. Patent US6028234A, 22 February 2000. [Google Scholar]
- West, O.R.; Tsouris, C.; Lee, S.; McCallum, S.D.; Liang, L. Negatively buoyant CO (2)-hydrate composite for ocean carbon sequestration. Am. Inst. Chem. Eng. AIChE J. 2003, 49, 283. [Google Scholar] [CrossRef]
- Szymcek, P.; McCallum, S.D.; Taboada-Serrano, P.; Tsouris, C. A pilot-scale continuous-jet hydrate reactor. Chem. Eng. J. 2008, 135, 71–77. [Google Scholar] [CrossRef]
- Tsouris, C.; Szymcek, P.; Taboada-Serrano, P.; McCallum, S.D.; Brewer, P.; Peltzer, E.; Walz, P.; Adams, E.; Chow, A.; Johnson, W.K.; et al. Scaled-up ocean injection of CO2–hydrate composite particles. Energy Fuels 2007, 21, 3300–3309. [Google Scholar] [CrossRef]
- Horiguchi, K.; Tokinosu, A. Gas Hydrate Production Apparatus. United States Patent US 8.420,018 B2, 16 April 2013. [Google Scholar]
- Partoon, B.; Sabil, K.M.; Lau, K.K.; Lal, B.; Nasrifar, K. Production of gas hydrate in a semi-batch spray reactor process as a means for separation of carbon dioxide from methane. Chem. Eng. Res. Des. 2018, 138, 168–175. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, A.u.; Lal, B. RETRACTED: Gas Hydrate-Based CO2 Capture: A Journey from Batch to Continuous. Energies 2022, 15, 8309. https://doi.org/10.3390/en15218309
Rehman Au, Lal B. RETRACTED: Gas Hydrate-Based CO2 Capture: A Journey from Batch to Continuous. Energies. 2022; 15(21):8309. https://doi.org/10.3390/en15218309
Chicago/Turabian StyleRehman, Adeel ur, and Bhajan Lal. 2022. "RETRACTED: Gas Hydrate-Based CO2 Capture: A Journey from Batch to Continuous" Energies 15, no. 21: 8309. https://doi.org/10.3390/en15218309
APA StyleRehman, A. u., & Lal, B. (2022). RETRACTED: Gas Hydrate-Based CO2 Capture: A Journey from Batch to Continuous. Energies, 15(21), 8309. https://doi.org/10.3390/en15218309







