Abstract
This study aims to investigate, from a technical and an environmental perspective, various alternatives for acetic acid concentration for maximizing acetic acid production, its purity, and in the meantime, minimizing the energy usage and the environmental impact. Liquid–liquid extraction followed by azeotropic distillation using different solvents such as: (i) ethyl acetate, (ii) isopropyl acetate, and (iii) a mixture containing isopropyl acetate and isopropanol were first explored, using process flow modeling software. The three cases were compared considering various technical key performance indicators (i.e., acetic acid flow-rate, acetic acid purity, acetic acid recovery, power consumption, thermal energy used, and number of equipment units involved) leading to the conclusion that the usage of the isopropyl acetate—isopropanol mixture leads to better technical results. The isopropanol-isopropyl acetate mixture was furthermore investigated in other two cases where process intensification methods, based on thermally coupled respectively the double-effect distillation process, are proposed. The highest quantity of pure acetic acid (e.g., 136 kmol/h) and the highest recovery rate (e.g., 97.74%) were obtained using the double-effect method. A cradle-to-gate life cycle assessment, involving ReCiPe method, was used to calculate and compare various environmental impact indicators (i.e., climate change, freshwater toxicity potential, human toxicity, etc.). Several steam sources (i.e., hard coal, heavy fuel oil, light fuel oil, natural gas, and biomass) were considered in the environmental evaluation. The results of the life cycle assessment show a reduction, by almost half, in all the environmental impact indicators when the double effect method is compared to the thermally coupled process. The usage of biomass for steam generation lead to lower impacts compared to steam generation using fossil fuels (i.e., hard coal, heavy fuel oil, light fuel oil, natural gas).
1. Introduction
Acetic acid, also called ethanoic acid, is an organic compound with the chemical formula CH3COOH used in several domains such as chemical, pharmaceutical, polymer, paint, textile and food industries [1].
Around 15 million tons of acetic acid was used in the past year all over the world. It is expected that in the following years the demand will grow up to 18 million tons and there on to increase with 5% every year due to the enlarging range of utilization in the industrial domain. The biggest quantity of this liquid is produced in the Asia-Pacific region, where it is also mostly consumed [2]. The demand for the acetic acid in 2020 was 11.9 tons and during the forecasted period, it is projected to grow with 4.8% in the following years [3]. At the beginning of 2022, it has been noticed that the Asia Pacific area is the biggest producer and consumer of this substance, and the vinyl acetate monomer is using one-third of the total acetic acid obtained. In order to meet the growing request of the market for this product, new plants will be built in China. It is expected that the North American and European markets will decrease their demand since more production lines will be replaced with those from Asia [4]. The biggest producers of this substance are China, where 55% of the total acetic acid is produced and the USA where 17% is produced [4], and the producers are represented by companies such as British Petroleum, Lyondell Basell, and Eastman and they ship it all over the globe [3]. The main consumers are located in China, the USA, Japan, Europe, and the Asia Pacific region with the main representatives as Jiangsu Sopo Co.Ltd., Shanghai Wujing Chemical, Indian Oil Corporation, DuPont. Lyondell Basel is involved in the production of polymers and plastics since it produces high amounts of household objects, construction materials, and car accessories [3].
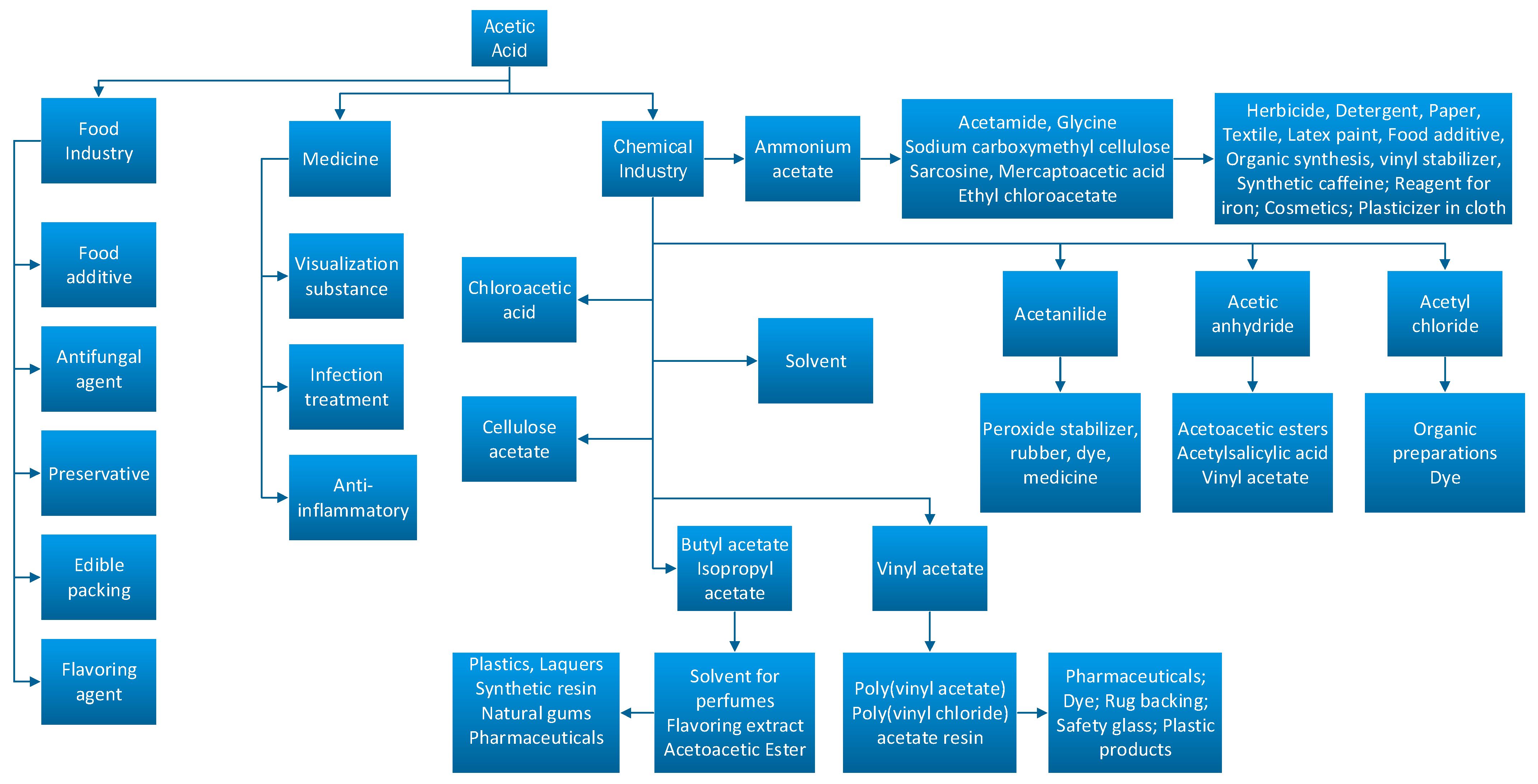
Due to its broad spectrum of applications, acetic acid is a vastly used substance in the food, pharmaceutical, and chemical industry as it can be seen in Figure 1.
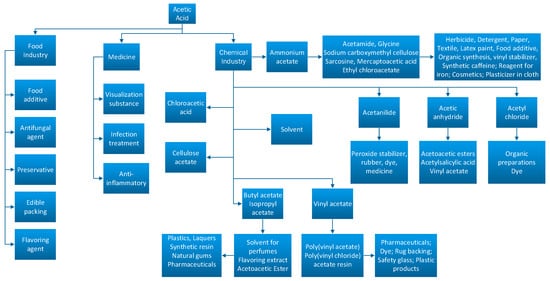
Figure 1.
Acetic acid conversion routes in the industry [5,6].
In countries such as China, the United States, Japan, and European countries, the major part of this substance is transformed into polymers obtained from vinyl acetate monomer which is used for synthetizing coatings, dyes, or adhesives [5,6]. Ethanoic acid is also used in synthesis plants in order to obtain polymers, dyes, paints, disinfectants, processing fibers, or textiles [5]. A share of 18% of the total quantity of acetic acid is transformed into acetic anhydride, 17% of the acid is used for obtaining terephthalate acid, which is further used for obtaining polyethylene terephthalate, resins, or textile fibers [1]. When mixing acetic acid with olefins, acetate esters can be easily obtained and those can be used for obtaining soaps, lubricants, and adhesive paste. Acetate ester is used as a solvent for obtaining inks, coatings for ceramics and paints [5].
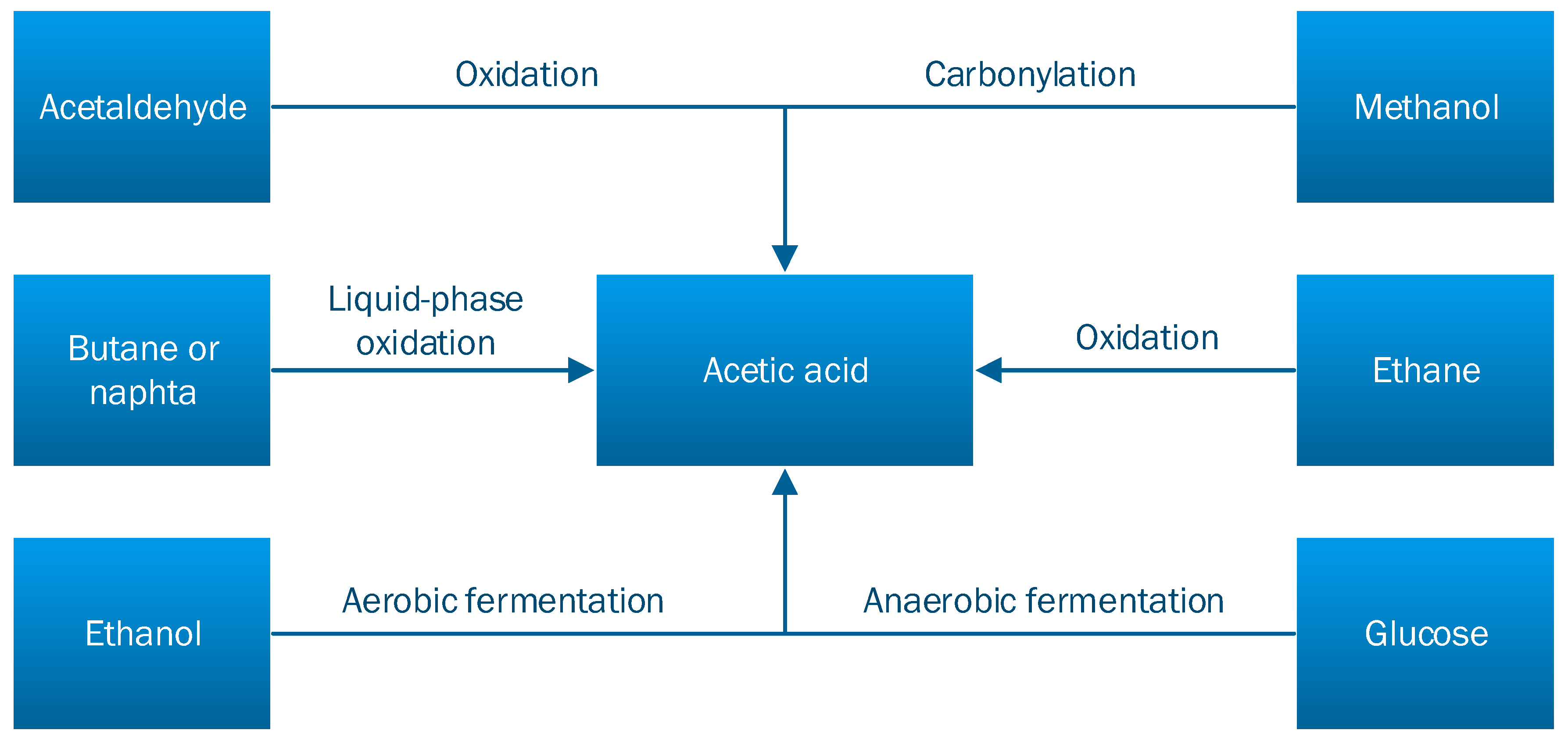
Acetic acid can be obtained using various industrial methods and various raw-materials such as synthesis gas, oil, and algae. The synthetic routes have been explored on a large scale since 1913 by the BASF Company, later by Monsanto, Celanese, and the Showa Denko companies [2,7]. The main methods of obtaining acetic acid are: methanol carbonylation, acetaldehyde oxidation, ethylene oxidation, fermentation (i.e., anaerobic, aerobic), and lignocellulosic matter distillation, being obtained in the biomass pretreatment phase [8]. These methods are schematically represented in Figure 2 [5].
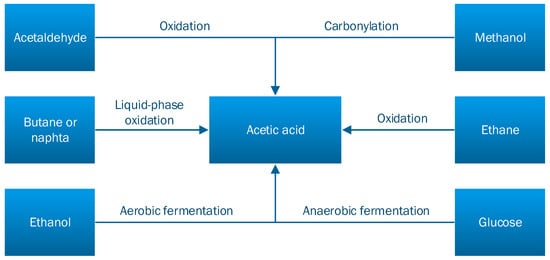
Figure 2.
The main methods for obtaining acetic acid [5].
Acetic acid being such an indispensable substance for the industry, the concentration and separation of this substance from its aqueous solution has been highly studied. Since the global industry is trying to make its way to a more sustainable and green future, the eco-friendly approaches are taken into consideration. Unfortunately, one of the main problems of obtaining acetic acid via the environmental-friendly methods is the fact that the final product is obtained in a diluted solution and it must be concentrated in order to be further processed [2]. The fact that the acetic acid has low volatility compared to the water saturated solution is one of the main problems of the concentration process [2]. Conventional distillation of acetic acid and water is an energy intensive process which is not used in practical applications due to the fact that acetic acid is the heavy component forcing large quantities of water to be condensed overhead. The conventional distillation of acetic acid and water requires also high boil-up ratios for high acetic acid purity [9]. As mentioned by Javed and co-authors, distillation has relatively low thermodynamic efficiency, so it is a prime target for process intensification studies. A small improvement in the thermodynamic efficiency of distillation columns can make a large difference in the overall process efficiency and profitability [10].
In the above-mentioned context, several methods have been proposed and developed to separate the main product using extractive distillation process [11], a salt-effect distillation process [12], or extraction of the acetic acid using the azeotropic distillation method [2].
The liquid–liquid extraction plays an important role in the separation methods since it implies low process costs. The key aspect of this extraction is the equilibrium relationship between the components; these systems need to be very well studied before choosing a suitable extraction component. Compared to the single solvent method, it has been noticed that a synergetic relationship is formed when a dual solvent is used and the process has better results than the classical method. The process of separating acetic acid and water from solution by the method of simple rectification requires a high reflux ratio implying the use of columns with many stages, which lead automatically to higher prices. In practice, the extractive distillation method is used for the acid acetic solution that has a concentration between 50 and 70%. It has been noticed that a ternary system has a high separation ratio using less energy than in the classical method. For mixtures which contain less than 40% acetic acid, the single solvent method is optimum. For choosing the right extractant, the scientists need to take into account the extractive capacity of the solvent and the equilibrium characteristics of the system [13].
In the azeotropic distillation method, a compound that in combination with water can decrease the boiling temperature of the mixture is used. Usually, if the substance is not miscible with water, the distillation can be performed easily with high perspectives for recycling [2]. Even though acetic acid and water solution does not form an azeotropic mixture atmospheric pressure, the liquid system has a pinch tangent where they intercalate on the pure water end. Another issue with this system is that acetic acid and water have close boiling temperatures and that implies using multiple equilibrium stages and a huge reflux ratio which automatically leads to increased difficulty of operating and controlling the heterogenous azeotropic column. For these reasons, when separating the acetic acid from water, an entrainer is usually used such as ethanol or iso propylic alcohols [14] or a system formed from alcohol, cyclohexane, and water [15].
Hybrid separation is one of the methods for acetic acid recovery at industrial level. It involves the liquid–liquid extraction followed by distillation. The purpose of the extraction is to first remove large amounts of water. The extraction is followed by two distillation columns, one column for acetic acid recovery and another column for solvent recovery. According to Angelo and co-authors, some important aspects should be taken into account when hybrid separation schemas are proposed. These aspects refer to the number of stages involved in the extraction column and in the distillation columns, but also to the extraction efficiency in order to minimize the amount of energy involved in the distillation and in the meantime to produce the desired acetic acid quantity and purity [9].
In the above-mentioned context, new methods for acetic acid concentration are investigated. These new methods are based on the process intensification (PI) principles. PI is a new area for research that has helped the chemical process industry gain new and innovative ideas to solve processing problems such as using more efficient technology, designing smaller plants, assuring the safety and sustainability of a chemical process. Its work is oriented to use as few devices as possible (by integrating more utilizations for a single equipment), use mixing technologies so that heat and mass transfer are improved by developing shorter pathways to ensure the diffusion, use new separation approaches, new energy saving methods, new methods for better controlling, and having an optimized plant. The objective of PI is to obtain sustainable flow-sheet possibilities that include unit operations that were subject to intensification methods and who are subject to: already mentioned process limitations, design objectives, and performances as verifying criteria. When it comes to PI in the workplace, the main objective is to boost productivity, described as the relationship created connecting all of the resource inputs necessary to complete the activity and the production of an output [16,17,18,19]. PI has the ability to bring significant benefits in terms of: obtaining a better efficiency of the system, increase the energy savings of the plant, lower the costs that are implied by the buying of assets and the operation of the plant, reduce the quantity of byproducts and waste, ensure the safety of the process. An example of the PI use in the industry is the development and integration of the microreactors which allow a careful selection of the products from the chemical transformation by offering a precise process control, which ends up in decreasing the amount of waste that is produced and usage of raw materials [20]. PI has been proved to be very helpful in the technologies involving CO2 capture and utilization, resulting in lower energy consumption [21].
Distillation is a critical component of PI because it is an often-used separation technology involved in the industry of synthetizing new chemicals. The biggest problem regarding the distillation process is the amount of energy that is required [19]. This method uses around 40–60% of the total amount of energy that is used in the chemical plants [22,23]. For example, in the production of furfural, the reactive distillation column proved to be the most energy demanding part of the simulation with a 69.82% of exergy efficiency, which led to an overall exergy efficiency of 56.41%, even though the substance was obtained with an yield of 97.4% and a purity of 99.96%. It was analyzed that by a 10% reduction of the reboiler duty resulted in a 3.6% cost rate depletion [8].
Over time, several methods were developed (i.e., thermal coupling (TC), double effect distillation (DED), vapor recompression) for reducing the amount of energy used for this process [24]. As an alternative for using the TC method with similar energy savings, a PI alternative solution would be the use of a side stream configuration which allows a better government of the production [25,26].
Moreover, the process of reactive distillation is complex since the analysis and the designing of the process can become challenging by introducing auxiliary reactions into the system. These problems were solved by Park and all (2020) by rigorously analyzing the simulations and taking into account a residue curve map [27].
A series of TC configurations can be realized by associating the reboilers or the condensers from the system. The advantages of this technique are: a higher energy efficiency of the process by using only a single reboiler and condenser in the whole system, there is also zero external reflux and reboiler operation. All in all, this method has high potential and effectiveness for reducing the energy consumption of the chemical plant [28]. Researchers have shown that by using the TC configurations, the system could save up to 30% of its energy usage, in comparison to the conventional methods. Overall, the PI approach of distillation and the use of thermally coupled units open the door to numerous opportunities in multicomponent distillation. The TC systems have the advantage of providing the same results or better ones using fewer units of processing and it helps increase the suitability of a plant [29]. Unfortunately, the energy that is served in the process needs to be supplied to the reboiler at high temperatures and extracted from the condenser at low values of temperature. Operation of the plant is made more difficult since there are more connections made between columns. Another issue is the increased number of trays and columns that should be used in the distillation column for obtaining the same results as in an ordinary system [29].
Another method for reducing the amount of energy used for a process is by using a distillation column that utilizes the heat integration technique. This means that a distillation column has a special configuration that contains an internal heat integration. This is created using the stripping sections of the column, which will act as the heat sink, and the rectifying ones, that will produce the necessary heat used [30]. DED is usually used in combination with other energy efficient methods with the scope of reducing the amount of energy that is consumed. In order to create a double-effect distillation, the heat used in a column is driven to the precedent column so that the heat of the vapor can be used for the process of the first column, thus saving a reboiler or condenser regarding the heating and cooling utilities [31]. Scientists tried to experiment with the use of heat pumps for reducing the amount of resources used for the separation. For example, Gao and co-authors proposed a new methodology to obtain isopropyl acetate by using a heat pump reactive distillation process resulting in a decrease of the CO2 emissions by up to 13.25%, while the total annual cost of production was 13.46% of the original process [32].
One way of obtaining acetic acid is concentrating it from diluted solutions obtained using industrial or fermentation processes. Acetic acid is obtained and needs to be removed from the solution in order to be processed further into other compounds. This paper aims to simulate, using ChemCAD and COCO COFE software, different solvents and methods of concentrating acetic acid from a diluted water solution in order to have a high concentrated acetic acid and reduce the amount of energy consumed. The environmental evaluation is also performed in the present research using the life cycle assessment (LCA) methodology in order to find relevant ways to reduce the impact of the proposed systems.
The technical and environmental evaluations of the proposed cases for acetic acid concentration represent the novelty this research is bringing up.
2. Material and Methods
In order to reach the proposed goal, the following approach was used:
- (i)
- Perform the simulations for the various extractants (i.e., ethyl acetate, isopropanol, and an isopropyl acetate—isopropanol mixture);
- (ii)
- Calculate the technical key performance indicators (i.e., acetic acid recovery, acetic acid purity, extractant quantity, power consumption, energy consumption) based on the material and energy balances derived from simulations;
- (iii)
- Screening of the best solvent, from the solvents listed in step (i), based on the results obtained in step (ii);
- (iv)
- Apply process intensification techniques (thermally coupling and double effect distillation) for the solvent with the best technical key performance indicators (solvent decided in step (iii));
- (v)
- Perform the environmental evaluation, using a cradle-to-gate LCA, on the cases decided on step (iv);
- (vi)
- Investigate, from environmental point of view, different scenarios for various steam generation sources (i.e., hard coal, heavy fuel oil, light fuel oil, natural gas and biomass);
- (vii)
- Discuss the obtained results and draw the conclusions.
Details about process modeling and simulation as well as environmental evaluation are provided in Section 2.1 and Section 2.2.
2.1. Process Modelling and Simulation
The initial acetic acid solution, containing 0.139 mole fraction of acetic acid, the rest being water, needs to be concentrated. The most suitable way to separate acetic acid from water, with an optimum amount of energy, is investigated considering the following case studies.
- Case 1—Acetic acid concentration using ethyl acetate;
- Case 2—Acetic acid concentration using isopropyl acetate;
- Case 3—Acetic acid concentration using isopropyl acetate and isopropanol mixture;
- Case 4—Acetic acid concentration using isopropyl acetate and isopropanol mixture by TC;
- Case 5—Acetic acid concentration using isopropyl acetate and isopropanol mixture by DED.
In order to simulate the above-mentioned processes, ChemCAD process simulator software (version 6.5.7) [33] and COCO COFE Cape Open software (version 3.5) [34] were used. COCO (CAPE-OPEN to CAPE-OPEN) is a free-of-charge CAPE-OPEN compliant steady-state simulation environment. COFE—the CAPE-OPEN Flowsheet Environment is an intuitive graphical user interface to chemical flow sheeting. COFE has sequential solution algorithm using automatic tear streams. It displays properties of streams, deals with unit-conversion, and provides plotting facilities. COFE flowsheets can be used as CAPE-OPEN unit operations; COFE flowsheets can be used as unit operation inside COFE (flowsheets in flowsheets) or inside other simulators.
The desired production capacity is 135 kmol/h (8095 kg/h) acetic acid (equivalent to an annual production of 65,000 tons/year); the desired purity of the acetic acid being 0.99 (by weight).
UNIQUAC thermodynamic package was set for the five cases. It is assumed that all the water streams that leave the system are blended and processed in a waste–water treatment plant. The cases have been compared using technical KPIs indicators such as: acetic acid flow-rate, acetic acid purity, power consumption, thermal energy consumption, number of unit operations, acetic acid recovery, etc. The description of each case as well as the main assumptions used in the process modelling and simulation are presented in the next section.
2.1.1. Case 1—Acetic Acid Concentration Using Ethyl Acetate
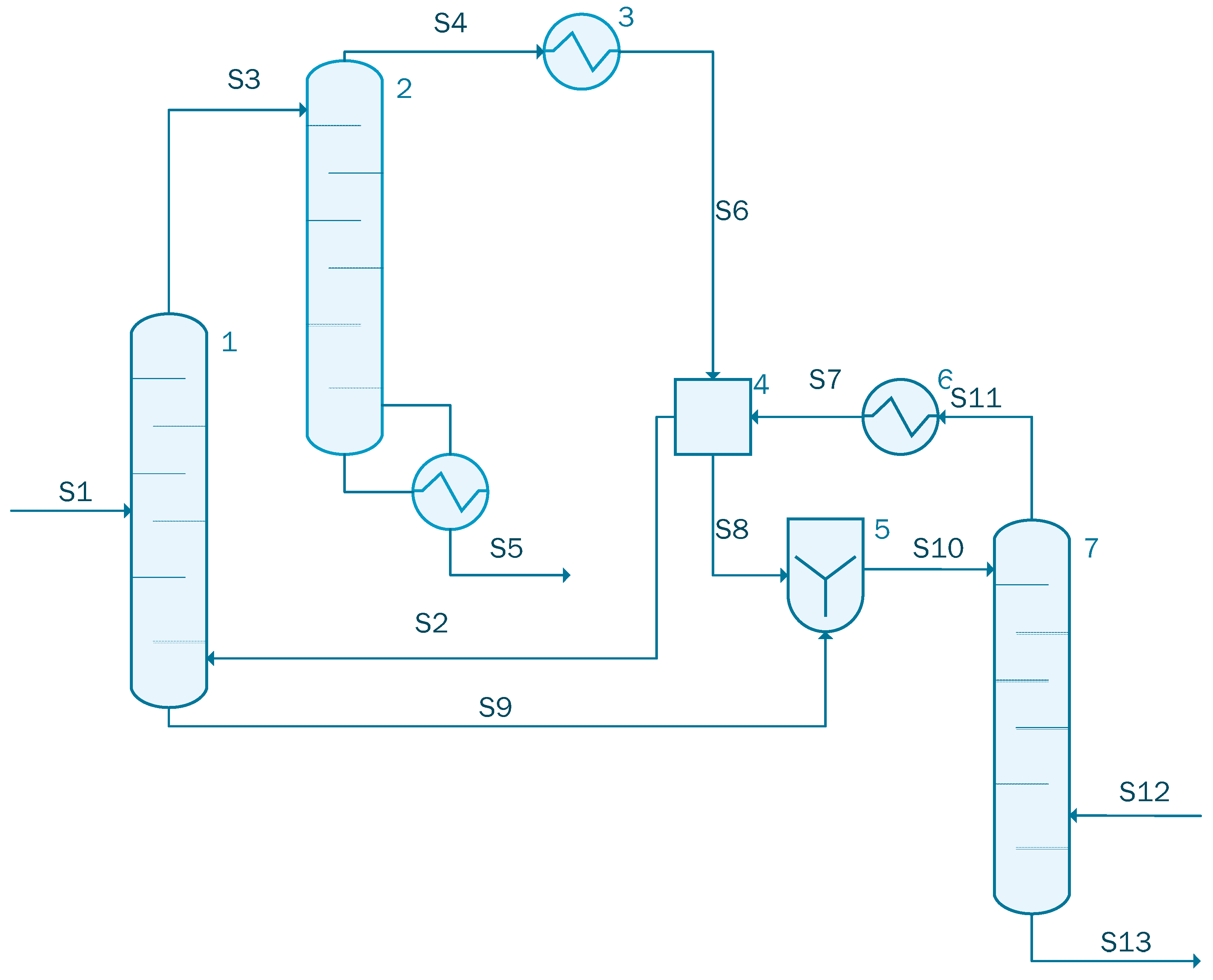
Acetic acid concentration using ethyl acetate is presented in Figure 3. In this case, the simulation contains an extraction column (unit 1), an acid recovery column (unit 2), and a solvent recovery column (unit 7). The simulation also contains two heat exchangers, one cooler (unit 3) and one heater (unit 6), a decanter (unit 4) where the extractant (i.e., ethyl acetate) is collected.
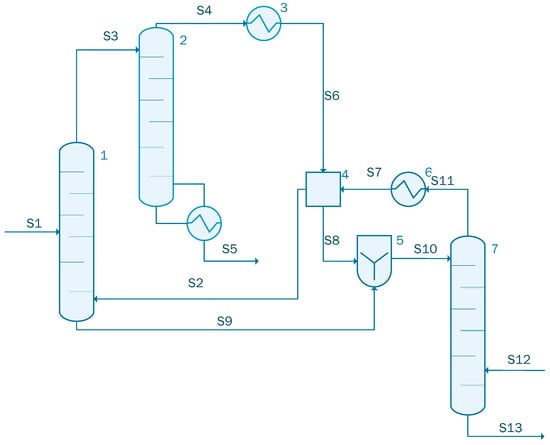
Figure 3.
Process flow diagram for the acetic acid concentration process (case 1).
The stream S1 containing 1000 kmol/h of acetic acid solution with the following component flow-rate: 861 kmol/h of water and 139 kmol/h acetic acid is sent to the extraction column (unit 1) together with the recycled stream S2 containing 740 kmol/h ethyl acetate, 176.53 kmol/h water, and 22.11 kmol/h acetic acid. The extracted acetic acid leaves the extraction column as stream S3 having a flow-rate of 1262.18 kmol/h. This stream is sent to the acid recovery column represented in Figure 3 as unit 2. The raffinate stream from the extractor, stream S9, containing water and some small quantities of acetic acid and ethyl acetate is sent to the mixer (unit 5) situated before the solvent recovery column (unit 7). The outputs stream of acid recovery column (unit 2) are: the acetic acid stream (the main product) defined as S5 having a flow-rate of 113.39 kmol/h and a purity of 0.94%, and the distillate stream, S4, containing 393.09 kmol/h water, 731.45 kmol/h ethyl acetate, and 24.28 kmol/h acetic acid. S4 stream is sent to the heat exchanger where the temperature is decreased from 77.17 °C to 25 °C. In the last distillation column, unit 7 in Figure 3, the ethyl acetate is recovered in order to be recycled. The role of the decanter is to store and recycle the solvent. The main design assumptions used in the present simulation are summarized in Table 1.

Table 1.
Design assumptions of the main units for case 1.
2.1.2. Case 2—Acetic Acid Concentration Using Isopropyl Acetate
Figure 4 presents the process flow diagram of the acetic acid concentration process using isopropyl acetate. The simulation contains four columns: one absorption column (unit 1), followed by an azeotropic distillation column (unit 3) and two dehydration columns (unit 5) and (unit 8).
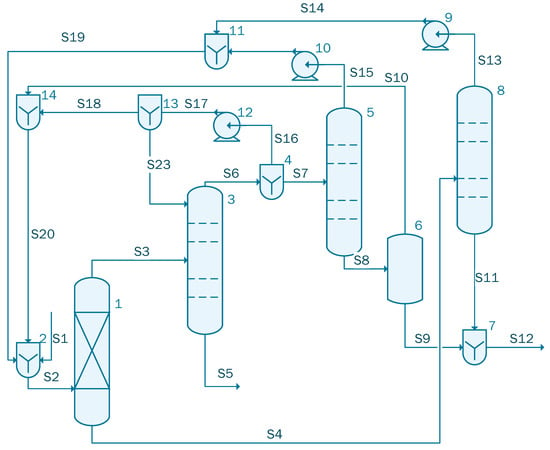
Figure 4.
Process flow diagram for the acetic acid concentration process (case 2).
The stream S1 containing 1000 kmol/h of acetic acid solution with the following component flow-rate: 861 kmol/h of Water and 139 kmol/h acetic acid is mixed in the unit 2 with the recycle (S19 containing 17.79 kmol/h water, and 4.89 kmol/h isopropyl acetate) and the extractant (S20 with 124.01 kmol/h water, 7.86 kmol/h acetic acid, and 337.96 kmol/h isopropyl acetate) streams. In the azeotropic column (unit 3), a two phases separation takes place: from the bottom part of the azeotropic column, 135.06 kmol/h of acetic acid with 0.99 mole fraction purity is extracted (S5 in Figure 4). The organic solution obtained from the top of the azeotropic column contains isopropyl acetate and water (S6 in Figure 4). The divider (unit 4) splits the stream in two parts. A flow-ratio of 0.72 of solution is recycled as an extractant via Pump 12. Stream S18 and S10 are mixed and reintroduced into the process, as Stream S20, while the rest of the flow-rate that comes out of the divider 4 is passed through the dehydration columns (unit 5 and unit 8). Stream S9 with a flow-rate of 96.55 kmol/h of water (purity 99.14%), respectively stream S11 with 679.29 kmol/h (purity 98.01%) of water are obtained from the bottom of the dehydration columns. These streams are mixed leading to Stream S12 which is sent to the wastewater treatment plant (total flow-rate 866.69 kmol/h). The top streams from both the dehydration columns are mixed leading to Stream 19 having a flow-rate of 22.69 kmol/h (containing 17.769 kmol/h water, 1 and 4.89 kmol/h of isopropyl acetate) which is sent to the mixer (unit 2) for recycling. The main assumptions used in the process modelling and simulation of this case are presented in Table 2.

Table 2.
Design assumptions of the main units for case 2.
2.1.3. Case 3—Acetic Acid Concentration Using Isopropyl Acetate and Isopropanol Mixture
The process is based on the information provided by Eun Joo Lee et al. [2]. The figure looks similar to Figure 4, only the extractant used is different. In this case, a mixture of isopropyl acetate and isopropanol was used.
The stream S1 containing 1000 kmol/h of acetic acid solution with the same component flow-rate, 861 kmol/h of water and 139 kmol/h acetic acid considered also in the previous cases, is mixed in unit 2 with the recycle (S19 containing 45.48 kmol/h water, 1.26 × 10−2 kmol/h acetic acid, 9.52 kmol/h isopropanol and 5.29 kmol/h isopropyl acetate) and the extractant streams (S20 with 173.58 kmol/h water, 15.38 kmol/h acetic acid, 5.54 kmol/h isopropanol and 6.51 kmol/h isopropyl acetate). The obtained mixed stream, Stream S2, is sent to column 1 where the mixture is split in two fractions; the pressure of these streams is 152 kPa. In the azeotropic column (unit 3), a two-phase separation takes place: from the bottom part of the azeotropic column 134.89 kmol/h of acetic acid with 0.99 mole fraction purity is extracted (S5 in Figure 4). The organic solution obtained in the top of the azeotropic column contains isopropanol and isopropyl acetate (S6 in Figure 4). The divider (unit 4) splits the stream into two parts: 387.42 kmol/h of solution is recycled as an extractant via Pump 12. Stream S18 and S10 are mixed and reintroduced into the process, as Stream S20, while the rest of 151.77 kmol/h that comes out of the divider 4 is passed through the dehydration columns (unit 5 and 8). Stream S9 with a flow-rate of 97.84 kmol/h of water (purity 99.99%), respectively stream S11 with 767.27 kmol/h (purity 99.45%) of water are obtained from the bottom of the dehydration columns. These streams are mixed leading to Stream S12 which is sent to the wastewater treatment plant. The top streams from both the dehydration columns are mixed leading to Stream 19 of 60.29 kmol/h (containing 45.48 kmol/h water, 1.26 × 10−2 kmol/h acetic acid, 9.51 kmol/h of isopropanol and 5.29 kmol/h of isopropyl acetate) which is sent to the mixer (unit 2) for recycling. The main assumptions used in the process modelling and simulation of this case are presented in Table 3.

Table 3.
Design assumptions of the main units for case 3.
2.1.4. Case 4—Acetic Acid Concentration Using Isopropyl Acetate and Isopropanol Mixture by TC
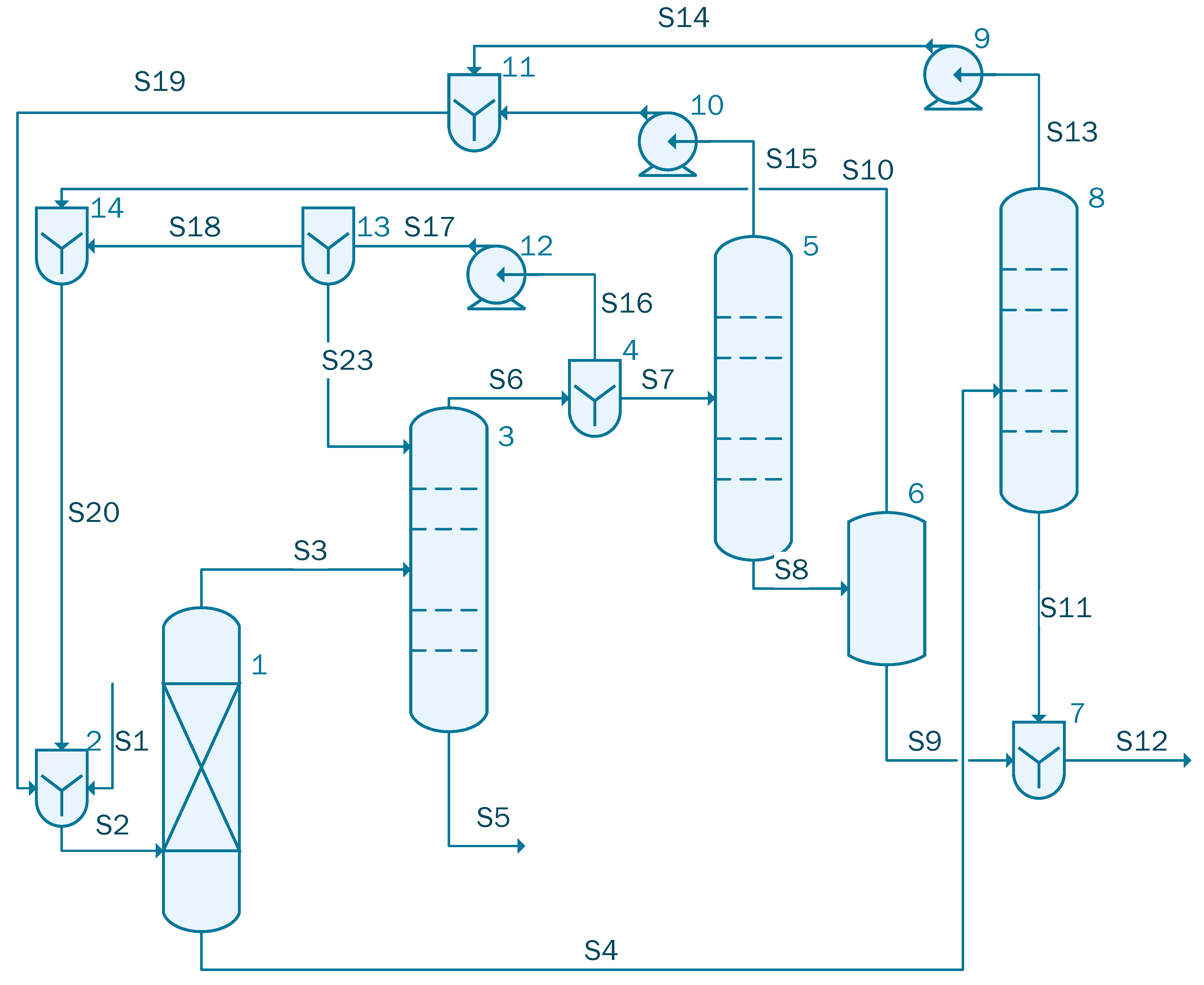
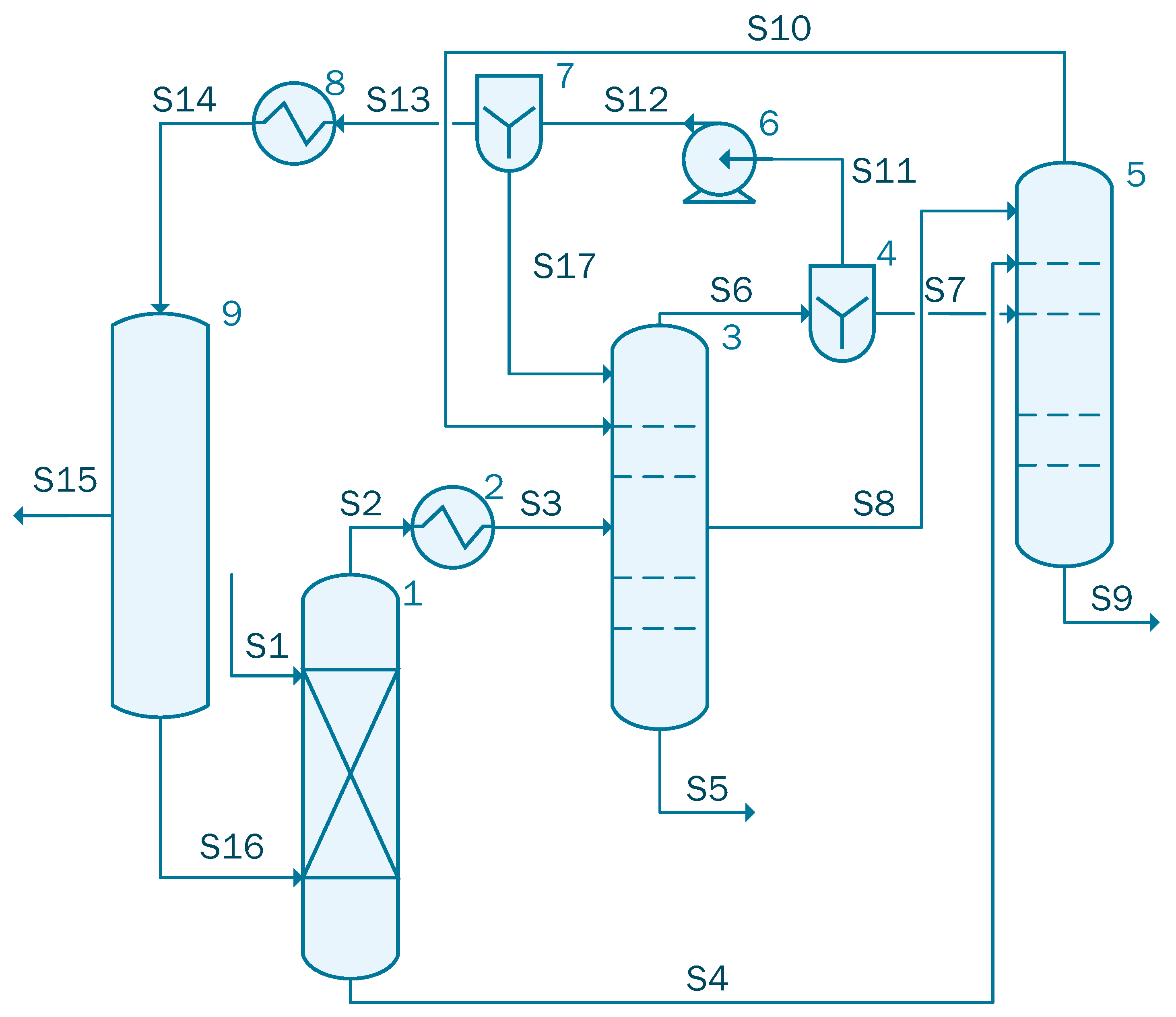
Since both the dehydration columns presented in case 2 and case 3 have similar bottom compositions, the columns can be combined so that they work as a single side stripper azeotropic column as proposed by Caxiano et al. [35]. The simulation for case 4 contains three columns: one absorption column, followed by an azeotropic distillation column and a side stripper (see Figure 5).
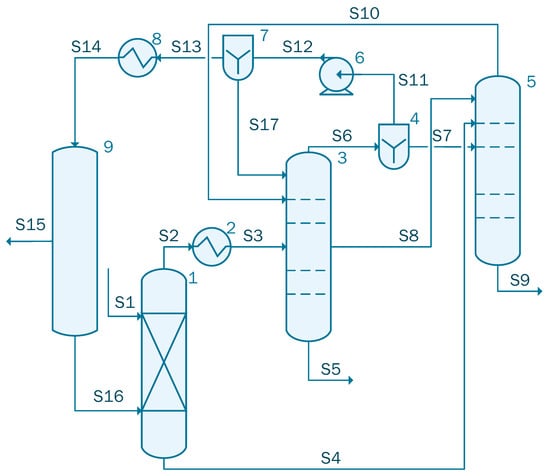
Figure 5.
Process flow diagram of the acetic acid concentration with TC (case 4).
The stream S1 containing 1000 kmol/h of acetic acid solution with the following mole flow-rate: 861 kmol/h of water and 139 kmol/h acetic acid is sent to unit 1 where it is blended with Stream S16 that contains 64.12 kmol/h water, 3.74 × 10−2 kmol/h acetic acid, 5.52 kmol/h isopropanol, and 420.55 kmol/h isopropyl acetate. Stream 2 is cooled in the heat exchanger (unit 2) from 120 °C to 92 °C and then sent to the Column 3. In the azeotropic column (unit 3), 134.05 kmol/h of acetic acid solution is extracted. The organic solution extracted from the top part of the unit 3, is split into two streams by the divider (unit 4): S7 is sent to the distillation column, unit 5. A flow-rate of 823.17 kmol/h of mixture containing 813.82 kmol/h of water, Stream S9, that will be processed in the waste water treatment facility was obtained at the bottom of column 5. Stream S11, after being transported by pump 6, will be split in two streams: S17 that is reintroduced in column 3 and S13 that is cooled to 70 °C in the heat exchanger, unit 8. The cooled stream, S14, is sent to the column 9, where 47.20 kmol/h of water are extracted from Stream 14 and the rest of the solution is recycled and reintroduced in the system through S16.
Table 4 summarizes the main assumptions used in case 4.

Table 4.
Design assumptions for the main units for case 4.
2.1.5. Case 5—Acetic Acid Concentration Using Isopropyl Acetate and Isopropanol Mixture by DED
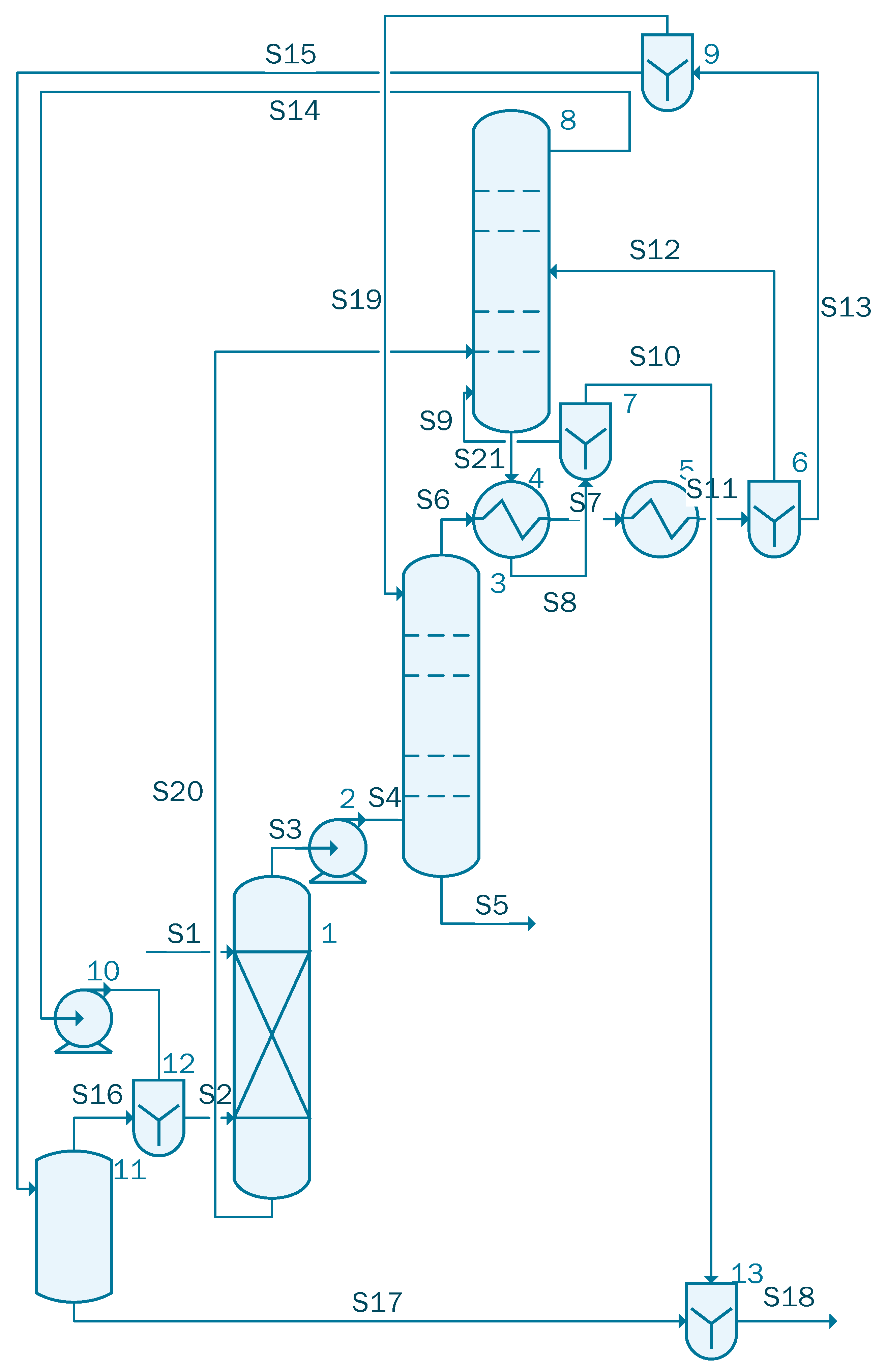
The fifth case contains three columns: one absorption column, followed by an azeotropic distillation column and a side stripper (Figure 6).
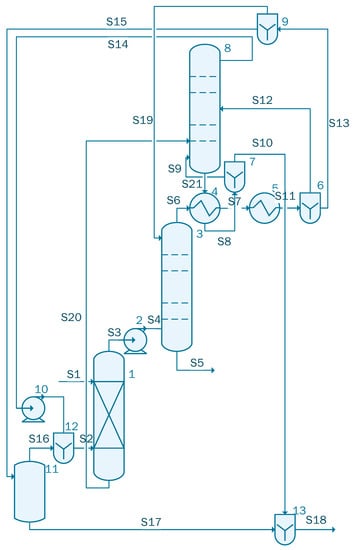
Figure 6.
Process flow diagram of the acetic acid concentration process with DED (case 5).
The stream S1 containing 1000 kmol/h of acetic acid solution having the same composition as in the previous cases is sent to unit 1, which is a liquid–liquid extractor. Stream 2, containing the extractant with the following component flow-rates: 114.57 kmol/h water, 0.38 kmol/ acetic acid, 49.81 kmol/h isopropanol and 255.15 kmol/h isopropyl acetate, is also sent to unit 1. The output streams of the unit are S3 and S20. Stream 3 is sent to the azeotropic distillation column, unit 3, where the solution is separated as Stream 5 containing 135.86 kmol/h of acetic acid and an organic solution, stream S6, that is cooled to 110 °C in heat exchanger 4. Unit 4 uses the flow of cold solution that comes out of unit 8 and will be divided in S9 that is reused in the column 8 and S10 that is sent to the waste water treatment plant. S7 stream, further cooled by the heat exchanger 5, is split in S12 that is reused in column 8 and S13 that is sent to the divider 9. The remaining solution, that is removed from unit 8, is pumped using unit 10 in order to be recycled. The separator 11 removes 124 kmol/h of water from Stream 15; the resulting mixture containing isopropanol and isopropyl acetate is reused in the system.
The main assumptions made for this simulation are presented in Table 5.

Table 5.
Design assumptions for the main units for case 5.
2.2. Life Cycle Assessment
LCA is a standard methodology used for the environmental impact evaluation. According to ISO 14040 and 14044, the methodology involves the compilation and quantification of inputs and outputs for a given product/technology through its life cycle [36,37]. Data about raw-materials, auxiliary materials (i.e., catalysts, solvents), energy, infrastructure, product(s), by-product(s), various waste, and emissions have to be collected, quantified, and used within LCA. LCA methodology contains four main steps as follows: (i) definition of the goal and the scope of the LCA study, (ii) life cycle inventory (LCI), (iii) life cycle impact assessment (LCIA) evaluation, and (iv) interpretation of the results. Details of the above-mentioned steps are provided in the next section.
The goal of the present LCA was to compare the environmental aspects of acetic acid concentration using conventional and PI methods. Since the acetic acid concentration involves distillation processes in all investigated scenarios steam generation is an important parameter consequently, various sources for steam generation have been investigated. The electricity required for the processes is considered from the grid mix. The combination of various types of steam with the electricity grid mix for case 4 and case 5 leads to ten sub-cases which are summarized in Table 6.

Table 6.
Sub-cases considered in the LCA study.
The functional unit considered is one kg of acetic acid obtained.
GaBi software, version 10.5, developed by spheraTM was used in order to perform the LCA calculations [38]. The study is a cradle-to-gate LCA study. The usage of the concentrated acetic acid is not considered in the present research.
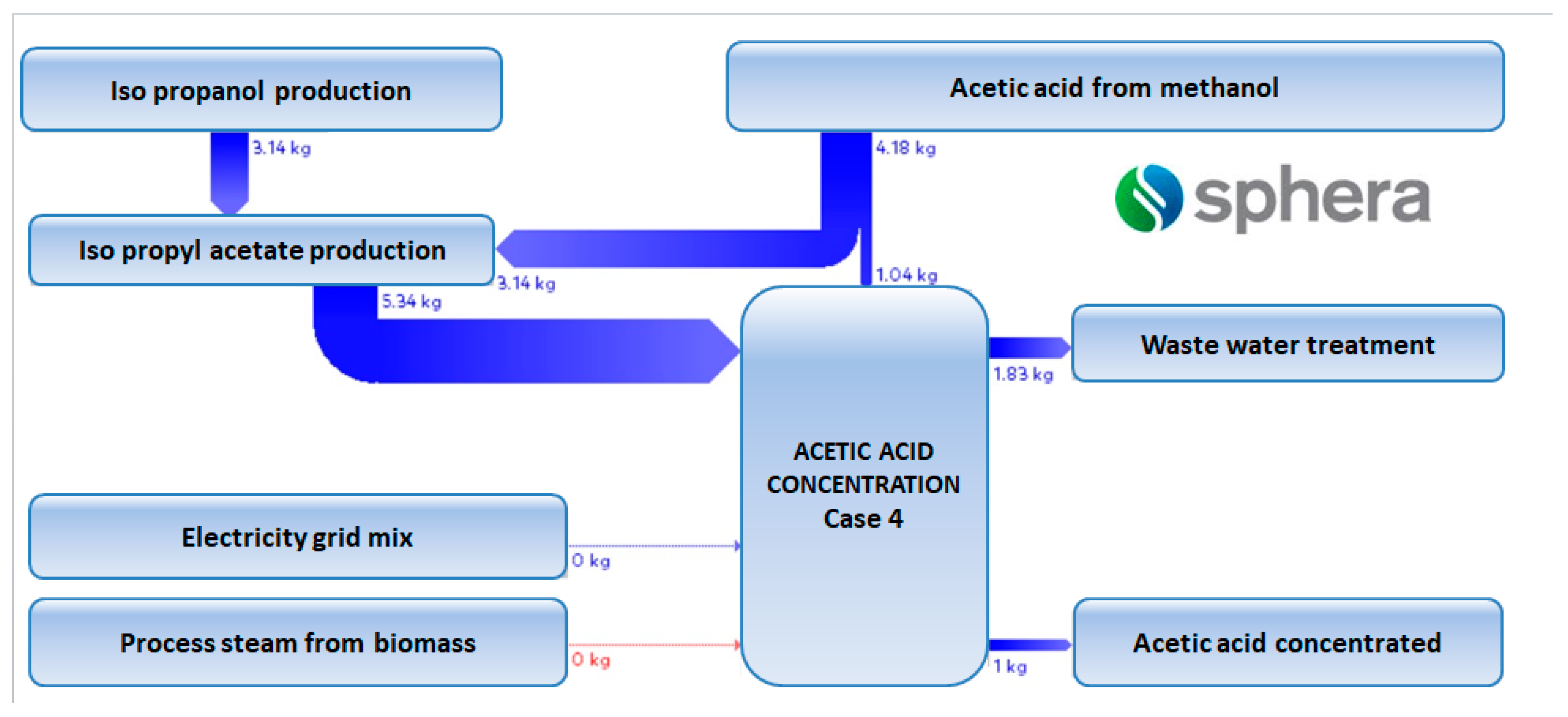
Data derived from process modelling and simulation have been considered as inputs in the LCI. As underlined by Righi and co-authors [39], the usage of process simulators to generate data for the LCI offers a series of advantages such as: (i) detailed mass and energy data about each equipment involved in the process schema; (ii) a quick screening of alternative plant configurations; (iii) the possibility to perform sensitivity analysis in order to understand the influence of the input variables on the outputs; and (iv) a relative ease in studying opportunities of process integration. An example of LCI is provided in Figure 7.
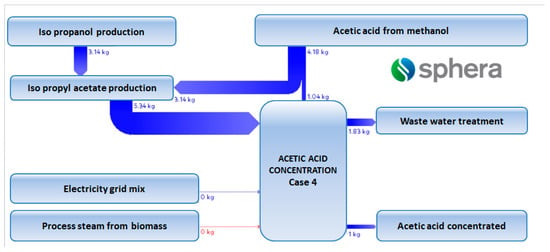
Figure 7.
GaBi diagram for case 4.5.
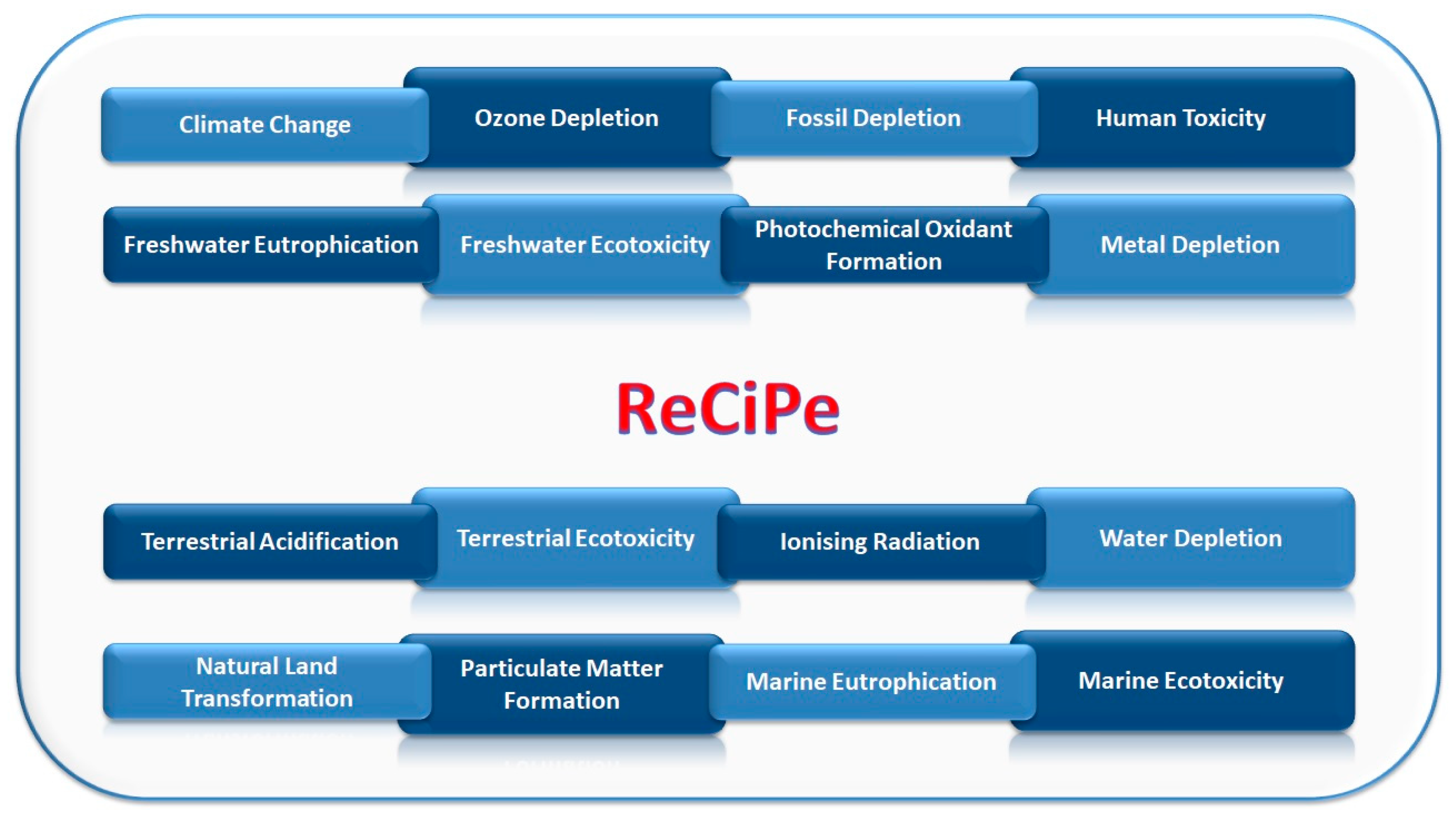
ReCiPe impact assessment method was considered in the LCA for the environmental assessment. ReCiPe has been chosen since it is one of the most recent and updated impact assessment methods available to LCA practitioners [39]. It includes the impact categories listed in Figure 8.
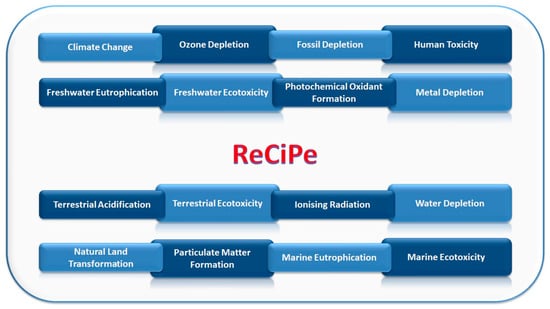
Figure 8.
ReCiPe impact indicators.
The main LCA assumptions considered in the present study are summarized in Table 7.

Table 7.
LCA assumptions [38].
3. Results and Discussion
3.1. Results and Discussions on Process Modelling and Simulation
The first simulation, case 1, illustrated in Figure 3 was performed using the COCO COFE simulator and according to the literature [9]. The second and the third simulation, case 2 and case 3, illustrated in Figure 4, were performed according to the information from the original literature source [35]. In case 4, illustrated in Figure 5, the combination of the two columns into a single side stripper that is connected to the stream that comes out of the azeotropic column so that the TC process can be established between those two units is proposed. This case considered a PI improvement for two reasons: (i) a lower number of unit operations are used in this case compared to case 3 and (ii) according to the literature [40], the TC is a PI Process strategy that should lower the amount of energy consumed by up to 30%. The aim of the simulation for case 5, illustrated in Figure 6, was to reduce the energy consumption even more, using a DED configuration, which is another method of PI. The side stripper from case 4 was redesigned into the thermally equivalent column 8 (case 5) so that a heat integrating system can be obtained with the azeotropic column. The above-mentioned configurations have been proposed considering their potential to reduce the energy and power consumption, to reduce the costs since less equipment pieces are involved and also to reduce the environmental impact of the acetic acid concentration system. The main streams resulting from process modeling and simulation for the five cases under study are summarized in the Supplementary Material as follows: Table S1 for case 1, Table S2 for case 2, Table S3 for case 3, Table S4 for case 4 and Table S5 for case 5). In all the simulations, the quantity of the raw-material that is introduced in the system is 1000 kmol/h containing 0.139 acetic acid and 0.861 water (mole fraction). Beside the raw-material stream and the output streams, other recycled streams are considered for each case, their flow-rates and compositions vary from case to case. The results obtained in the proposed simulations have been validated, being very close to the values from the original literature [9,35].
In order to compare the five cases under investigation, additional information is reported in Table 8.

Table 8.
Comparison between the three five cases regarding key characteristics of the process.
As noticed from Table 8, the highest quantity of acid is obtained in case 5, followed closely by the results of case 3 and case 2 (e.g., 136 kmol/h in case 5 followed by 135.54 in case 3, respectively 135.06 kmol/h in case 2). The purity of the acid from case 1 is 0.94 while in cases 2, 3, 4, 5 it is higher having a value of 0.99. Another parameter which has been calculated is the acetic acid recovery. It is the ratio between the quantity of pure acetic acid obtained and the quantity of acetic acid introduced in the system by Stream 1, the diluted acetic acid solution which should be concentrated. The highest value for this parameter was registered for case 5 (i.e., 97.74%) being followed closely by the other PI case, case 4 with a recovery rate of about 97.42%. The lowest value for this parameter is also registered in the first case when ethyl acetate is used as an extractant.
The flow-rates of extractants have also been reported in Table 8. The lowest quantity of extractant is used in case 5 (e.g., 344.21 kmol/h) while the highest flow-rate is used in case 1 (e.g., 938.65 kmol/h).
By comparing the number of units utilized in the simulations, it can be noticed that the lowest number is registered for case 1 but also the performances of this case (i.e., acetic acid flow-rate, acetic acid purity, acetic acid recovery) are not so high. Compering the other cases in terms of number of units used, it can be noticed that in case 4 a number of 9 units are used, in case 5 a number of 13 units are used while in cases 2 and 3 a number of 14 units are employed. Cases 4 and 5 are favorable because the number of columns is reduced from 3 to 2. For case 2 and case 3, the number of units is determined by the presence of more mixers, pumps, and heat exchangers.
Observing the values from the power consumption parameter and energy parameter, it can be noticed that case 5 consumes the highest quantity of power, but it has the lowest usage of energy. The most power efficient scenario for acetic acid concentration is represented by case 4.
In terms of energy consumption, case 5 is the optimum consumer, followed by case 4. The energy consumption in case 5 is lower than the amount of energy used in case 4 (e.g., 33,640 MJ/h in case 5 vs. 40,646 MJ/h in case 4), which means a reduction of the quantity of used energy of 17.23% which is in the range of the energy saving mentioned in the scientific literature [29]. When comparing case 5 to case 3, we notice that a consumption reduction of 23.37% was registered. The energy consumption between cases 2 and 3 is pretty close as can be observed from Table 8.
As a general conclusion on the technical assessment, case 5 seems to be the most favorable one since: (i) The highest quantity of acetic acid is obtained (e.g., 135.86 kmol/h); (ii) it presents the highest acid recovery rate (e.g., 97.74%); (iii) it uses the lowest quantity of extractant (e.g., 344.21 kmol/h); (iv) a number of two columns are involved; (v) it presents the lowest energy usage (e.g., 33,640 MJ/h).
3.2. Results and Discussion on LCA
After technical screening, the most promising cases, case 4 and case 5, were investigated from the environmental point of view. These cases lead to ten subcases as mentioned in Table 6. The most relevant environmental KPIs derived from the LCA study, using ReCiPe impact assessment method, are reported in Table 9 and Table 10.

Table 9.
LCA results, according to ReCiPe, for the sub-cases correspondent to case 4.

Table 10.
LCA results, according to ReCiPe, for the sub-cases correspondent to case 5.
The LCA results reported in Table 9 and Table 10 can be compared using three strategies as follows: (i) a general comparison (i.e., case 4 vs. case 5) and more detailed comparison referring to (ii) the results of various sub-cases which use the same steam source should be put one against the other (i.e., sub-case 4.1 vs. sub-case 5.1, sub-case 4.2 vs. sub-case 5.2, sub-case 4.3 vs. sub-case 5.3, etc.) and (iii) various sub-cases within the same case study (i.e., sub-case 4.1 vs. sub-cases 4.2, 4.3, 4.4., and 4.5, etc.).
Generally, the values for case 4 are higher than the values correspondent to case 5 except the values for the ozone depletion potential (ODP) indicator. The values are double or more than double for all the impact categories summarized in Table 9 and Table 10. For instance, for the climate change, defined also as global warming potential (GWP) indicator, the ratio of the values for case 4 and the values for case 5 are in the range 2.10–2.35. The highest differences between case 4 and case 5 are registered for the human toxicity potential (HTP) indicator. For this indicator, the values are about 2.34 times higher in case 4 vs. the value registered for case 5.
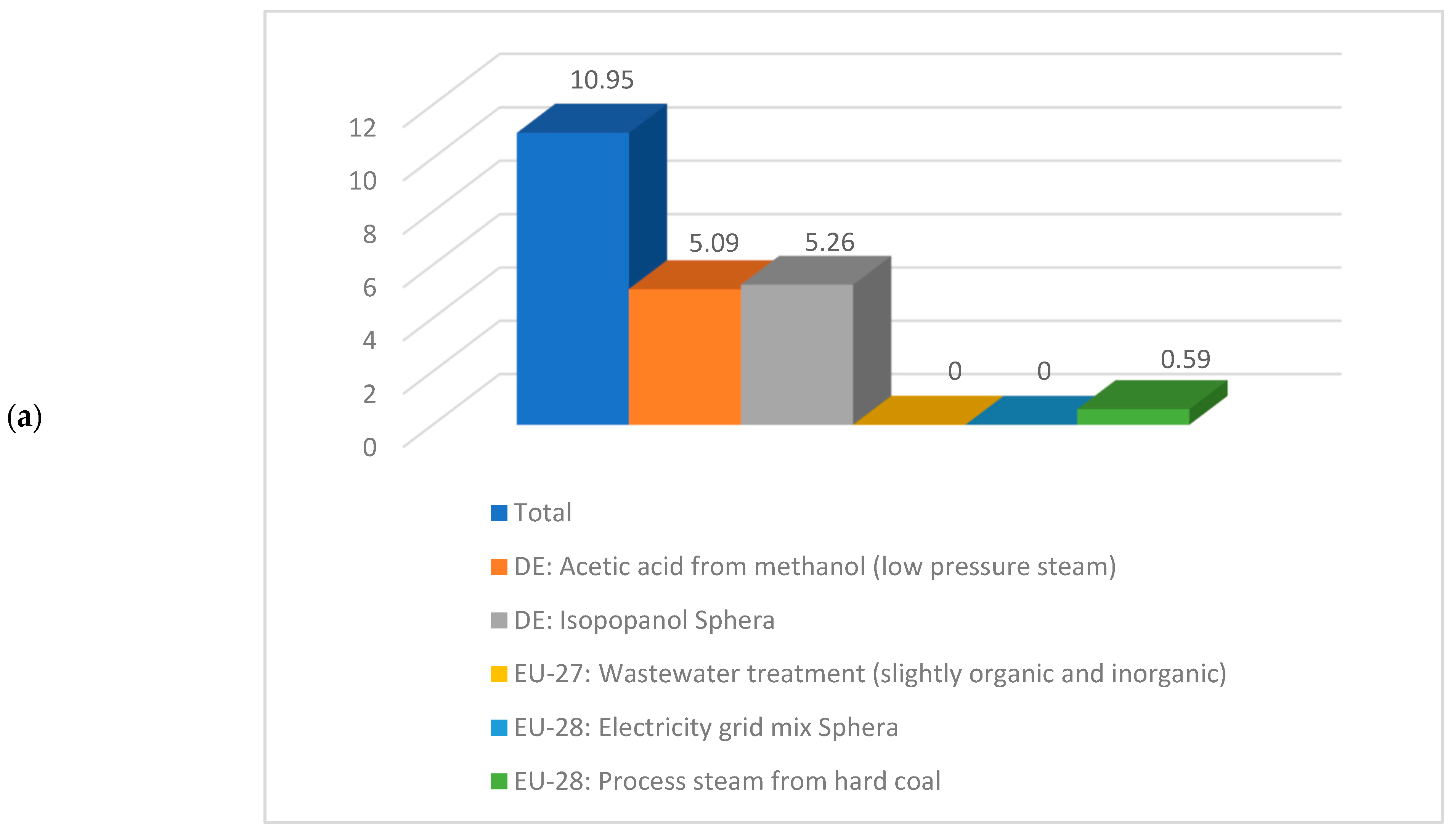
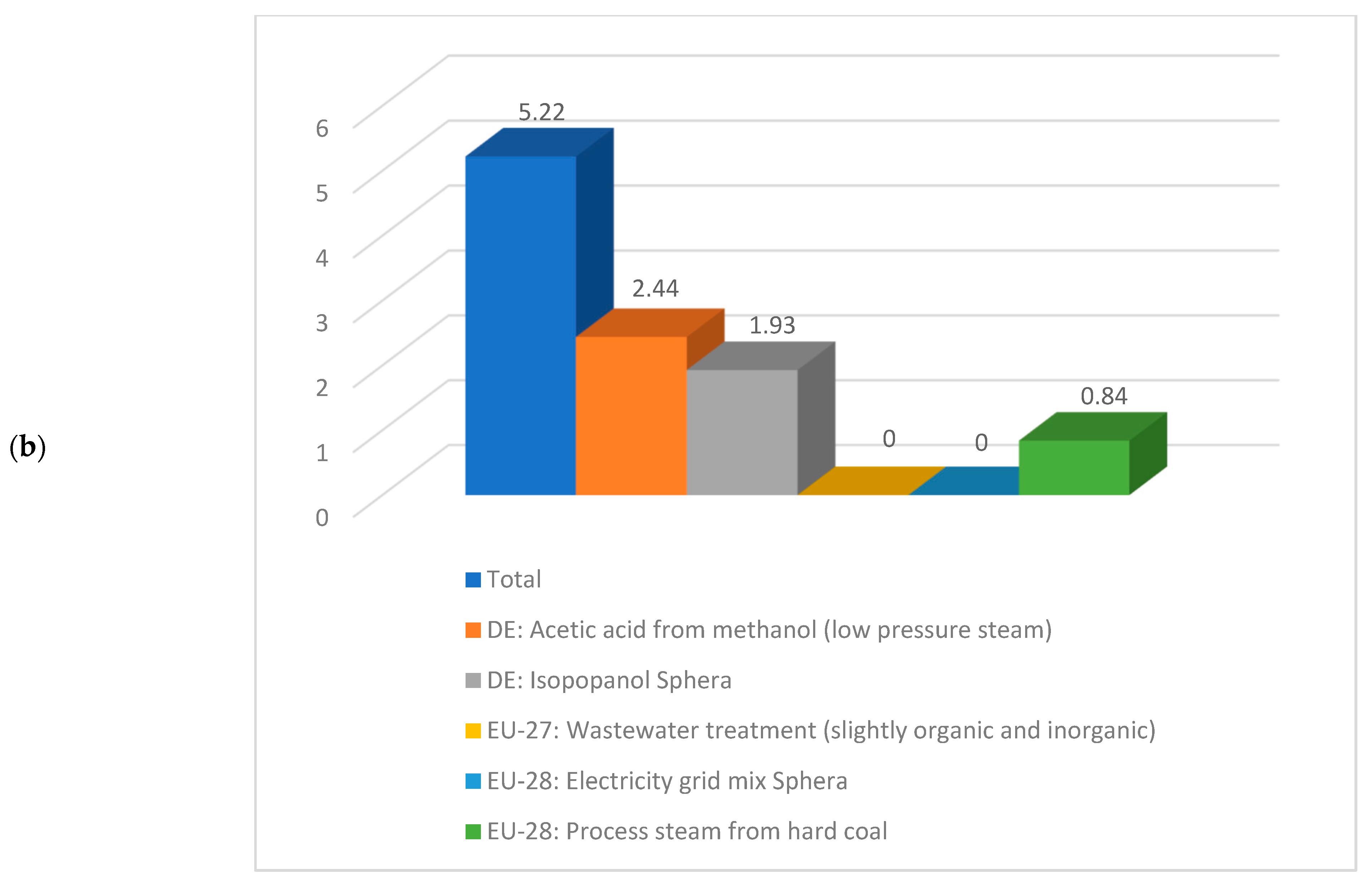
Having a look deeper into detail and comparing the same sub-cases, if for instance sub-case 4.1 is compared to sub-case 5.1, the values correspondent to the climate change are 10.95 kg CO2 eq./kg acetic acid respectively 5.22 kg CO2 eq./kg acetic acid. A detailed contribution to this indicator is illustrated in Figure 9. As noticed from Figure 9, the acetic acid production from methanol, isopropanol production, and steam production are the biggest contributors to the climate change indicator.
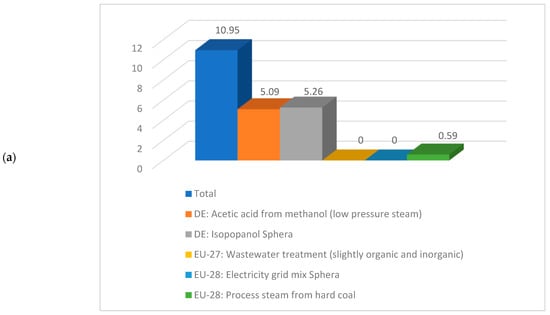
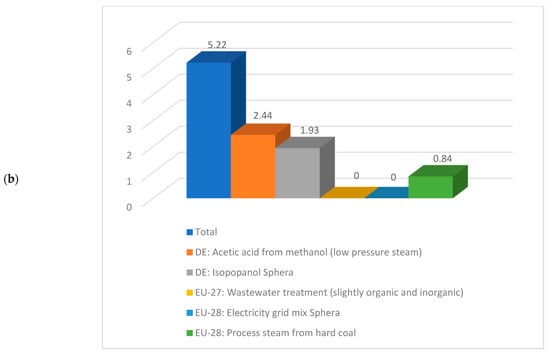
Figure 9.
Details about climate change indicator for (a) sub-process 4.1 and (b) sub-process 5.1.
Analyzing the LCA results within the same case study (i.e., sub-case 4.1, 4.2, 4.3, 4.4, 4.5), it can be observed that the steam source influences the environmental indicators as follows: the highest values for the impact indicators are registered for the usage of fossil fuels while the lowest are registered for the biomass usage as steam source (i.e., climate change indicator (GWP), fossil depletion potential (FDP), terrestrial ecotoxicity potential (TETP)). The exceptions are represented by the freshwater eutrophication potential indicator (FEP) and water depletion potential (WDP) where the opposite situation occurs. Other impact indicators such as ODP, HTP have constant or slightly different values independently of the steam generation source. Between the fossil fuels investigated for the steam generation, the usage of natural gas leads to lower environmental impact values compared to the usage of hard coal, heavy or light fuel oil.
From technical and environmental point of view, it can be concluded that the usage of double effect distillation using isopropanol and isopropyl acetate mixture as extractant coupled with the usage of biomass as a steam source represent the most efficient and environmentally friendly option.
4. Conclusions
The present study aims to investigate, using process modelling and environmental tools (i.e., ChemCAD, COCO COFE and GaBi software), which is the best configuration, from technical and environmental perspectives, to concentrate acetic acid. Five case studies were explored and compared in the present research. The cases under investigation use different extractants (i.e., ethyl acetate, isopropanol and an isopropyl acetate—isopropanol mixture) and different technologies i) classical (i.e., extraction and azeotropic distillation) and ii) intensified (i.e., TC and DED). The five configurations under study are represented by extraction (case 1), azeotropic concentration design (case 2, case 3), and two process intensification designs based on TC (case 4) and DED (case 5).
The main tools used in the present work for process modeling and simulation are ChemCAD version 6.5.7, developed by ChemstationsTM and COCO COFE simulator, version 3.5, developed by AmsterCHEM. GaBi software, version 10.5, developed by spheraTM, was used to perform the LCA.
The main findings derived from the work performed are summarized as follows:
- (i)
- The technical comparison leads to the conclusion that the highest quantity of acetic acid is obtained in case 5. The purity is 0.94 in case 1, while for the rest of the cases it reaches 0.99. For properly comparing the three simulations, the energy consumption should be taken into consideration. In terms of energy consumption, case 5 is the optimum consumer, followed by case 1 and case 4. Even if the energy consumption in case 1 is low, the other technical KPIs (i.e., acetic acid flow-rate, acetic acid purity, quantity of extractant used) are the highest and do not support this scenario.
- (ii)
- The environmental comparison, based on the ReCiPe impact assessment method leads to the conclusion that the most environmentally friendly process is the DED coupled with steam generation from biomass.
As future work, an economic evaluation of the involved technologies should be performed in order to have a complete view of the picture and to decide which technology is the most sustainable one.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en15218119/s1.
Author Contributions
C.-M.C. performed and validated three out of the five simulations, wrote the first draft of the paper, and revised the last version of the manuscript. L.P. deigned the idea, provided guidance, performed two simulations, the LCA study, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
Author Cormos Codruta-Maria is an employee of MDPI; however, she did not work for the journal “Energies” at the time of submission and publication.
Abbreviations
| CHP | Combined Heat and Power |
| DED | Double Effect Distillation Process |
| FDP | Fossil Depletion Potential |
| FEP | Freshwater Eutrophication Potential |
| FETP | Freshwater Ecotoxicity Potential |
| GWP | Global Warming Potential |
| HTP | Human Toxicity Potential |
| IRP | Ionizing Radiation Potential |
| ISO | International Standard Organization |
| LCA | Life Cycle Assessment |
| LCI | Life Cycle Inventory |
| LCIA | Life Cycle Impact Assessment |
| MEP | Marine Eutrophication Potential |
| METP | Marine Ecotoxicity Potential |
| MDP | Metal Depletion Potential |
| NLT | Natural Land Transformation |
| ODP | Ozone Depletion Potential |
| POFP | Photochemical Oxidant Formation Potential |
| PI | Process Intensification |
| PMF | Particulate Matter Formation |
| Sx | Stream no x |
| TAP | Terrestrial Acidification Potential |
| TC | Thermally Coupled Process |
| WDP | Water Depletion Potential |
References
- Deshmukh, G.; Manyar, H. Production Pathways of Acetic Acid and Its Versatile Applications in the Food Industry; Basso, T.P., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, Y.H. Energy saving in acetic acid process using an azeotropic distillation column with a side stripper. Chem. Eng. Commun. 2018, 205, 1311–1322. [Google Scholar] [CrossRef]
- Available online: https://www.chemanalyst.com/industry-report/acetic-acid-market-609 (accessed on 23 April 2022).
- Available online: https://ihsmarkit.com/products/acetic-acid-chemical-economics-handbook.html (accessed on 23 April 2022).
- Cheung, H.; Tanke, R.S.; Torrence, G.P. Acetic Acid. Ullmann’s Encyclopedia of Inustrial Chemistry; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 1–20. [Google Scholar] [CrossRef]
- Malveda, M.; Funada, C. “Acetic Acid”. Chemicals Economic Handbook; SRI International: Menlo Park, CA, USA, 2003; p. 602.5000. [Google Scholar]
- Saka, S.; Minami, E.; Rabemanolontsoa, H.; Kawamoto, H. Advanced biotethanol production process with acetic acid fermentation from lignocellulosics. In Proceedings of the 2014 Grand Renewable Energy International Conference, P-Bm-2014, Tokyo, Japan, 27 July–1 August 2014; pp. 1–9. Available online: https://www.researchgate.net/publication/264894561_Advanced_bioethanol_production_process_with_acetic_acid_fermentation_from_lignocellulosics (accessed on 10 April 2022).
- Wiranarongkorn, K.; Im-orb, K.; Panpranot, J.; Maréchal, F.; Arpornwichanop, A. Exergy and exergoeconomic analyses of sustainable furfural production via reactive distillation. Energy 2021, 226, 120339. [Google Scholar] [CrossRef]
- Lucia, A.; Amale, A.; Taylor, R. Energy efficient Hybrid Separation Processes. Ind. Chem. Res. 2006, 45, 8319–8328. [Google Scholar] [CrossRef]
- Javed, A.; Hassan, A.; Babar, M.; Azhar, U.; Riaz, A.; Mujahid, R.; Ahmad, T.; Mubashir, M.; Lim, H.R.; Show, P.L.; et al. A Comparison of the Exergy Efficiencies of Various Heat-Integrated Distillation Columns. Energies 2022, 15, 6498. [Google Scholar] [CrossRef]
- De Figueiredo, M.F.; Brito, K.D.; Ramos, W.B.; Sales Vasconcelos, L.G.; Brito, R.P. Effect of Solvent Content on the Separation and the Energy Consumption of Extractive Distillation Columns. Chem. Eng. Commun. 2014, 202, 1191–1199. [Google Scholar] [CrossRef]
- Garwin, L.; Hutchison, K.E. Separation of Acetic Acid and Water by Distillation. Effect of Calcium Chloride Addition. Ind. Eng. Chem. 1950, 42, 727–730. [Google Scholar] [CrossRef]
- Kalaichelvi, P.; Perumalsamy, M.; Arunagiri, A.; Sofiya, K. Synergistic Extraction of Acetic Acid from Its Aqueous Solution. J. Univ. Chem. Technol. Metall. 2007, 42, 291–294. [Google Scholar]
- Huang, X.; Zhong, W.; Du, W.; Qian, F. Thermodynamic Analysis and Process. Simulation of an Industrial Acetic Acid Dehydration System via Heterogeneous Azeotropic Distillation. Ind. Eng. Chem. Res. 2013, 52, 2944–2957. [Google Scholar] [CrossRef]
- Wang, S.-J.; Huang, K. Design and control of acetic acid dehydration system via heterogeneous azeotropic distillation using p-xylene as an entrainer. Chem. Eng. Process. Process Intensif. 2012, 60, 65–76. [Google Scholar] [CrossRef]
- Keil, F.J. Process Intensification. Rev. Chem. Eng. 2017, 34, 135–200. [Google Scholar] [CrossRef]
- Becht, S.; Franke, R.; Geißelmann, A.; Hahn, H. An industrial view of process intensification. Chem. Eng. Process. Process Intensif. 2009, 48, 329–332. [Google Scholar] [CrossRef]
- Raghavan, K.V.; Reddy, B.M. Industrial Catalysis and Separations: Innovations for Process Intensification; Apple Academic Press: Waretown, NJ, USA, 2014. [Google Scholar]
- Caballero, J.A. Thermally Coupled Distillation. Comp. Aided Chem. Eng. 2009, 27, 59–64. [Google Scholar] [CrossRef]
- Yue, J. Green process intensification using microreactor technology for the synthesis of biobased chemicals and fuels. Chem. Eng. Process. Process Intensif. 2022, 177, 109002. [Google Scholar] [CrossRef]
- Pahija, E.; Golshan, S.; Blais, B.; Boffito, D.C. Perspectives on the process intensification of CO2 capture and utilization. Chem. Eng. Process. Process Intensif. 2022, 176, 108958. [Google Scholar] [CrossRef]
- Humphrey, J.L. Separation technologies: An. opportunity for energy savings. Chem. Eng. Progress 1992, 88, 32–42. [Google Scholar]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Saidur, R.; Elcevvadi, E.T.; Mekhilef, S.; Safari, A.; Mohammed, H.A. An overview of different distillation methods for small scale applications. Renew. Sustain. Energy Rev. 2011, 15, 4756–4764. [Google Scholar] [CrossRef]
- Kong, Z.Y.; Sánchez-Ramírez, E.; Yang, A.; Shen, W.; Segovia-Hernández, J.G.; Sunarso, J. Process intensification from conventional to advanced distillations: Past, present, and future. Chem. Eng. Res. Des. 2022, 188, 378–392. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Kong, Z.Y.; Yang, A.; Lee, H.-Y.; Sunarso, J. Design and control of an energy intensified side-stream extractive distillation for binary azeotropic separation of nhexane and ethyl acetate. Sep. Purif. Technol. 2022, 294, 121176. [Google Scholar] [CrossRef]
- Park, J.; Jeong, Y.; Han, M. Process intensification of reactive distillation using improved RCMs: Acetic acid production. Chem. Eng. Process. Process Intensif. 2020, 157, 108136. [Google Scholar] [CrossRef]
- Shah, V.H.; Agrawal, R. A matrix method for multicomponent distillation sequences. AIChE J. 2009, 56, 1759–1775. [Google Scholar] [CrossRef]
- Kiss, A.A.; Smith, R. Rethinking energy use in distillation processes for a more sustainable chemical industry. Energy 2020, 203, 117788. [Google Scholar] [CrossRef]
- Kiss, A.A.; Olujic, Z. A review on process intensification in internally heat-integrated distillation columns. Chem. Eng. Process. 2014, 86, 125–144. [Google Scholar] [CrossRef]
- Cui, C.; Sun, J.; Li, X. A hybrid design combining double-effect thermal integration and heat pump to the methanol distillation process for improving energy efficiency. Chem. Eng. Process. Process Intensif. 2017, 119, 81–92. [Google Scholar] [CrossRef]
- Gao, X.; Yang, Y.; Chen, M.; Cheng, Q.; Lu, K. Novel heat pump reactive distillation and dividing-wall column reactive distillation processes for synthesizing isopropyl acetate to save TAC and reduce CO2 emissions. Chem. Eng. Process. Process Intensif. 2022, 171, 108746. [Google Scholar] [CrossRef]
- Chemstations. ChemCAD, Chemical Process Simulation Software. Available online: www.chemstations.com (accessed on 20 September 2022).
- Available online: www.cocosimulator.org (accessed on 20 September 2022).
- Caxiano, I.N.; Junqueira, P.G.; Mangili, P.V.; Prata, D.M. Eco-efficiency analysis and intensification of the acetic acid purification process. Chem. Eng. Process. Process Intensif. 2019, 147, 107784. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO—The International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
- Sphera, GaBi Software. Available online: www.sphera.com (accessed on 15 September 2022).
- Maranghi, S.; Brondi, C. Life Cycle Assessment in the Chemical Product Chain, Challenges, Methodological Approaches and Applications; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Agrawal, R. Multieffect distillation for thermally coupled configurations. AIChE J. 2000, 46, 2211–2224. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).