Abstract
In recent years, the rapid consumption of fossil fuels has brought about the energy crisis and excess CO2 emission, causing a series of environmental problems. Photocatalytic CO2 reduction technology can realize CO2 emission reduction and fuel regeneration, which alleviates the energy crisis and environmental problems. As the most widely used LDH material in commercial application, MgAl-layered double hydroxide (MgAl-LDH) already dominates large-scale production lines and has the potential to be popularized in CO2 photoreduction. The adjustable component, excellent CO2 adsorption performance, and unique layer structure of MgAl-LDH bring specific advantages in CO2 photoreduction. This review briefly introduces the theory and reaction process of CO2 photocatalytic reduction, and summarizes the features and drawbacks of MgAl-LDH. The modification strategies to overcome the drawbacks and improve photocatalytic activity for MgAl-LDH are elaborated in detail and the development perspectives of MgAl-LDH in the field of CO2 photoreduction are highlighted to provide a guidance for future exploration.
1. Introduction
The development of human society is closely related to the utilization of fossil energy, and the consumption of fossil energy has increased sharply accompanied by the advancement of industrialization. The massive consumption of fossil energy has triggered an energy crisis and brought about a large amount of CO2 emissions, which leads to the serious environmental problems, such as climate change [1] and ocean acidification [2]. Therefore, the reduction of CO2 emissions has become one of the common challenges faced by mankind [3], and developing an efficient and sustainable technology for CO2 emissions reduction is of great significance. Pacala and Socolow [4] have proposed the technologies to stabilize the global CO2 concentration at 500 ppm for the next 50 years, including the improvement in energy utilization efficiency, carbon capture, and storage (CCS) technology, the development of nuclear power and renewable energy, etc. The improvement in energy utilization efficiency is limited by technological breakthroughs, and the development of energy technology has currently hit a bottleneck. The development of nuclear power is conducive to the control of CO2 and gas pollutant emissions, but radioactive and thermal pollutions are quite serious. The utilization of renewable energy, including wind, hydrogen, and biomass energy, is mainly limited by the high cost, which is derived from economic or technical deficiencies. Carbon capture and storage (CCS) technology is especially suitable for the control of CO2 emissions from stationary sources and is considered one of the most effective methods for large-scale CO2 emission control in the short term, but it cannot fundamentally solve the CO2 emission problem. In addition, CO2 is the most abundant and economical carbon source on earth, and the above technologies only focus on CO2 emissions reduction without considering the sustainable utilization of CO2. CO2 photocatalytic reduction can utilize solar energy to convert CO2 into carbon-containing fuels or valued chemical products, which achieves solar energy storage and CO2 cyclic utilization simultaneously.
Limited by the stable and inert structure of CO2, CO2 reduction reaction is quite hard to attain [5]. Developing the efficient photocatalysts to promote the reaction process has attracted much attention, and an ideal photocatalyst should be able to overcome the energy loss as far as possible in the process of light absorption, charge separation and reduce the energy barrier of surface reaction. Traditional metal oxides or sulfides are always limited in practical application by their poor solar utilization efficiency due to the wide bandgap, such as TiO2 (~3.2 eV) [6], SnO2 (~3.6 eV) [7] and ZnS (~3.6 eV) [8], which can only utilize the UV light in sunlight. Traditional 2D materials, such as graphene [9] and graphitic carbon nitride [10], have been reported in photocatalysis due to the outstanding optical, electrical and mechanical performance, but the fast charge recombination always limits practical application [11]. Therefore, more novel materials have been explored and various strategies have been proposed to improve catalytic activity, such as surface loading [12], element doping [13], morphology control [14] and adsorption strengthening [15], etc.
Layered double hydroxides (LDHs), a class of 2D anionic inorganic materials, have been wildly reported in heterogeneous catalysis due to their unique layer structure, exclusive physical and chemical properties, and optical characteristics [16]. Due to the abundant hydroxy groups on the surface of LDHs, the superior affinity to CO2 molecule strengthens CO2 adsorption and promotes the photocatalytic reaction [17]. The synthetic methods of LDHs are quite facile, which can expediently regulate and control the components, morphologies, defects, grain size and electrical properties. Generally, the synthetic methods of LDHs can be divided into one-step methods and multistep methods. One-step methods always achieve the synthesis of LDHs by the direct preparation process, including coprecipitation [18] and hydrothermal [19] methods, etc. However, many specific LDHs cannot be synthesized by one-step methods directly, and hence multistep methods have been developed. Multistep methods always include ion exchange [20], structure reconstruction [21], and a sol–gel process with specific colloid as the precursor [22]. Among various LDH materials, MgAl-LDH is the most widely used commercially, and has been used in PVC stabilizer, polymer or asphalt additives and retardant [23,24]. Meanwhile, MgAl-LDH already has scaled up production lines [25], and it has the potential to be promoted in CO2 photoreduction due to the low cost and mature market. Moreover, the synthesis of nanostructured LDHs has been developed in recent years, and the separate nucleation and aging method is the only industrial method for large-scale synthesis of nanoscale LDHs at present [26], which has the advantages of simplicity, speed, and yield amplification. Zhao et al. [27] prepared MgAl-LDH by the separate nucleation and aging method with uniform particle size distribution (60–80 nm), and found that the control of particle size could be simply achieved by adjusting the metal ratio, aging time and temperature. This method has been adapted to the production line of LDHs in China for industrial-scale preparation. Mature industrial production and the development of synthetic methods have indicated that MgAl-LDH has the most promising prospects as a photocatalyst for CO2 photoreduction.
Numerous reviews have been published about the application of LDH materials for CO2 photoreduction, and the universal structural features and development prospects of LDH materials have been introduced comprehensively [28,29,30,31]. However, systematic reviews of specific MgAl-LDH in the field of CO2 photoreduction are still scarce. In this review, the theory and reaction process of CO2 photocatalytic reduction based on LDH materials are introduced and the key steps are summarized. The properties and drawbacks of MgAl-LDH are introduced and summarized. Research on the application of MgAl-LDH in CO2 photoreduction is reviewed and future development trends pointed out. We believe that this review provides guidance for further development of MgAl-LDH in CO2 photoreduction and a direction for the design and construction of MgAl-LDH-based photocatalysts.
2. Theory of CO2 Photoreduction
CO2 is a linear molecule with a stable structure, and the bond energy of C=O reaches 750 kJ/mol (the bond energy of C-C is 336 kJ/mol and the bond energy of C-O is 327 kJ/mol) [32]. The direct decomposition reaction of CO2 usually requires a temperature above 2000 K [33,34], and the hydrogenation reaction of CO2 can occur at a relatively low temperature with the assistance of hydrogen. Kattel et al. [35] realized the hydrogenation of CO2 to methanol in the temperature range of 525–575 K. CO2 photocatalytic reduction can achieve CO2 reduction under mild conditions, while originally needing harsh conditions [36,37]. The main products of CO2 photocatalytic reduction in current reports include CO [38], CH4 [39], HCOOH [40], HCHO [41] and CH3OH [42], but the conversion efficiency is far from the requirement for practical application. The potential advantages of CO2 photocatalytic reduction in CO2 emission control, carbon recycling utilization and solar energy storage are attractive and the exploration of this promising technology is precious and worthy.
2.1. Basic Concepts of Photocatalysis
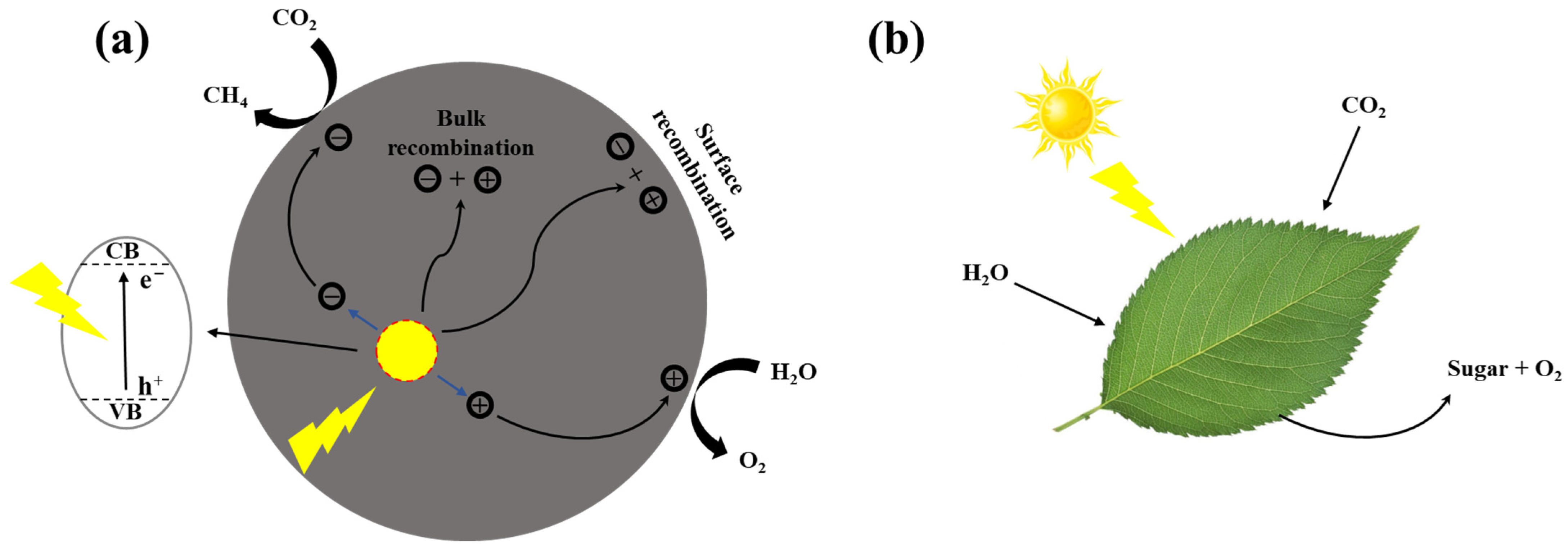
Since Inoue et al. [43] first reported the use of TiO2 and other semiconductors in photocatalytic CO2 conversion into formaldehyde, formic acid and methanol, CO2 photocatalytic reduction over semiconductor catalysts has attracted much attention. As shown in Figure 1a, the reaction process of CO2 photocatalytic reduction utilizes the photogenerated electrons to achieve CO2 reduction, which is inspired by the photosynthesis of plants in Figure 1b.

Figure 1.
Schematic diagram of (a) photocatalytic reaction and (b) photosynthesis [44].
When the photon energy is enough to overcome bandgap, the semiconductor catalysts are activated under light illumination. The electrons transfer to the conduction band (CB) and the holes remain in the valence band (VB), and then the electron–holes pairs overcome the Coulomb force to achieve the separation and transfer to surface reaction sites. The redox reaction of adsorbed reactants occurs on the surface of semiconductor catalysts due to strong redox ability of photogenerated charge. In the process of photocatalysis, the interspace between VB and CB represents the energy state that electrons cannot occupy (forbidden band) and the bandgap of forbidden band determines the difficult degree of electron transfer from VB to CB. The recombination of electron–hole pairs always occurs in the process of charge transfer inside or on the surface of catalysts, which greatly limits the catalytic activity. Meanwhile, the adsorption of reactants on the surface is helpful to break the stable structure of CO2 molecules, which influences the product yield and selectivity.
2.2. Performance Evaluation and Influencing Factors of CO2 Photoreduction
Photocatalysts play an important role in the photocatalytic CO2 reduction and the performance evaluation of photocatalysts is of great significance for estimating the photocatalytic activity and revealing the reaction mechanism. Product evolution rate is a common parameter in the performance evaluation of catalysts, which directly reflects the catalytic activity. The unit of product evolution rate is generally μmol·g−1·h−1 or μmol·h−1. Photocatalytic CO2 reduction is a reaction involving multiple electrons, and different products will be generated due to the various intermediates. Product selectivity is also an important parameter for performance evaluation of photocatalysts, and the regulation of photocatalytic product, to targeting product, is of great significance for practical application. Generally, the competitive H2 evolution reaction is an important factor affecting product selectivity. External quantum efficiency () and turnover frequency (TOF) are always used for performance evaluation of photocatalysts, which reflect the ability of photocatalysts to capture the photons with a particular wavelength and frequency of reaction at specific active sites. In addition, the stability of the catalytic reaction is an important index to evaluate the catalytic performance of catalysts. The long-term photocatalytic reaction is the most direct method to evaluate the reaction stability and the cyclic experiment is also used widely to evaluate the reaction stability. At present, there is still no unified standard for performance evaluation of photocatalysts in CO2 photoreduction. The performance evaluation methods and the defined evaluation parameters in various studies are different, and a unified standard for performance evaluation of photocatalysts is urgently needed.
In addition to the properties of catalysts, the experimental conditions also have an influence on the catalytic performance of photocatalysts, including light intensity, pressure, temperature, reactor structure and sacrificial reagents. Generally, the photocatalytic activity is proportional to light intensity, but the recombination of photogenerated electrons and holes will be aggravated and the catalytic activity will be inhibited when the light intensity is too high [45]. The increase of pressure in the reaction system can improve the solubility of CO2 in the aqueous solution, which accelerates the catalytic reaction rate and affects the product selectivity. Theoretically, the photocatalytic reaction is not sensitive to the temperature change, but the electron collision frequency and diffusion rate will increase at the high temperature and the catalytic activity will also be improved. Reactor structure affects the light absorption and the contact between the catalysts and CO2 molecules, and the catalytic activity is also affected. The addition of sacrificial reagents can effectively trap holes and inhibit the recombination of electron–hole pairs, and the catalytic activity can be greatly improved. Due to the different reaction systems and experimental conditions, it is very difficult to compare the catalytic activities of photocatalysts strictly in different reports, and a unified standard for experimental method also needs to be developed.
2.3. Reaction Process of CO2 Photoreduction
Photocatalytic CO2 reduction involves many complex physical and chemical processes, including the adsorption and activation of CO2 molecules, the generation of electron–hole pairs, the separation and transfer of charge carriers and the surface redox reaction. Electrons will transfer from VB to CB of semiconductor catalysts after absorbing the energy of photons under light irradiation, which is a necessary step for photocatalytic reactions. Only when the energy of incident photon is higher than the bandgap of the semiconductor catalyst can electrons be excited from VB to CB and the photogenerated electron–hole pairs can generate. After the generation of electron–hole pairs, electrons and holes are still bound together due to the effect of Coulomb force and a bound electron–hole pair is called an exciton. The process of overcoming Coulomb force and achieving the separation of electron–hole pairs has an important influence on the photocatalytic activity. The separation efficiency of electron–hole pairs is closely related to the structure of the semiconductor catalysts. For example, layered materials usually have an advantage in the separation of electron–hole pairs because the presence of interlayer electric field can promote the transfer of electrons [46]. Photogenerated electrons and holes will diffuse to the surface of semiconductor catalysts and the recombination of electrons and holes always occurs inside or on the surface of catalysts. The recombination of charge carriers leads to the annihilation of electrons and holes and releases energy through radiation simultaneously. For the same material, the diffusion distance of electrons and holes is fixed and reducing the particle size can reduce the diffusion length, which is beneficial to reduce the recombination rate of electrons and holes.
After the electrons and holes migrate to the active sites on the surface of semiconductor catalysts, the redox reaction will occur between the adsorbed CO2 or H2O molecules and electrons or holes. Generally, H2O molecules will be oxidized by holes to O2 and CO2 molecules will be reduced to different products depending on the number of electrons involved in the reaction. In this process, the electrons in the CB should provide higher energy (more negative) than the reduction potential of CO2 to targeting products and the holes in the VB should provide higher energy (more positive) than the oxidation potential. The standard redox potentials of reactions are shown in Table 1 (vs. NHE, pH = 7). The first step of redox reaction is the activation of CO2 molecule by a single-electron reaction, and CO2 molecule obtain an electron to form •CO2− and break the stable linear structure. The redox potential of •CO2− formation is quite high, and this step is very difficult and needs large energy to promote the progression of subsequent reactions. In view of thermodynamics, the targeting product can be obtained when the electrons and holes in the CB and VB can provide sufficient energy to overcome the redox potential of corresponding product. However, the multiple-electrons-involved reaction is quite hard to achieve compared with the few-electrons-involved reaction in view of kinetics. For example, only two electrons are required for CO production, but eight electrons are required for CH4 production, and more electrons are required for C2+ product production. Therefore, CO is the most readily available product. The selectivity of products can be regulated by improving the band structure, charge separation and surface adsorption.

Table 1.
Standard redox potentials for photocatalytic CO2 reduction to different products [47].
CO2 adsorption is also an important step that affects the catalytic activity and product selectivity. Strengthening the adsorption of CO2 is beneficial to overcome the energy barrier for CO2 activation to •CO2−, and the metal or defect sites on the surface can facilitate the adsorption of CO2 and promote the reduction reaction [48]. Product selectivity is closely related to the adsorption model of the CO2 molecule. If CO2 is absorbed on the surface of catalysts by only bonding with O atoms, it is inclined to form formic acid ions by combining with hydrogen atoms. Formic acid may be the final product if the C-O bond is not further broken. If CO2 is absorbed on the surface of catalysts by bonding with C atom, it is inclined to form carboxyl groups by combining with hydrogen atoms preferentially and C-O bond is broken early to form CO. The adsorbed CO can be further hydrogenated to form CH4 [32]. Zhou et al. [49] found that the introduction of Ru atoms into TiO2 could convert the main product from CO to CH4 in CO2 photoreduction due to the synergy of Ru and oxygen defects. The competitive adsorption of CO2 and H2O also has an influence on the product selectivity, which can be adjusted by changing the surface metal sites. Han et al. [50] found that the adsorption energies of CO2 and H2O could be adjusted by changing the metal species over metal–organic framework monolayers and the product selectivity towards CO was enhanced. Previous research indicated that the metal species in LDHs also greatly affected product selectivity in CO2 photoreduction, while Zn in LDHs is beneficial for CO generation [51] and Cu in LDHs is beneficial for CH3OH generation [52].
3. Structures and Properties of MgAl-LDH
3.1. Component and Characteristic of LDH Materials
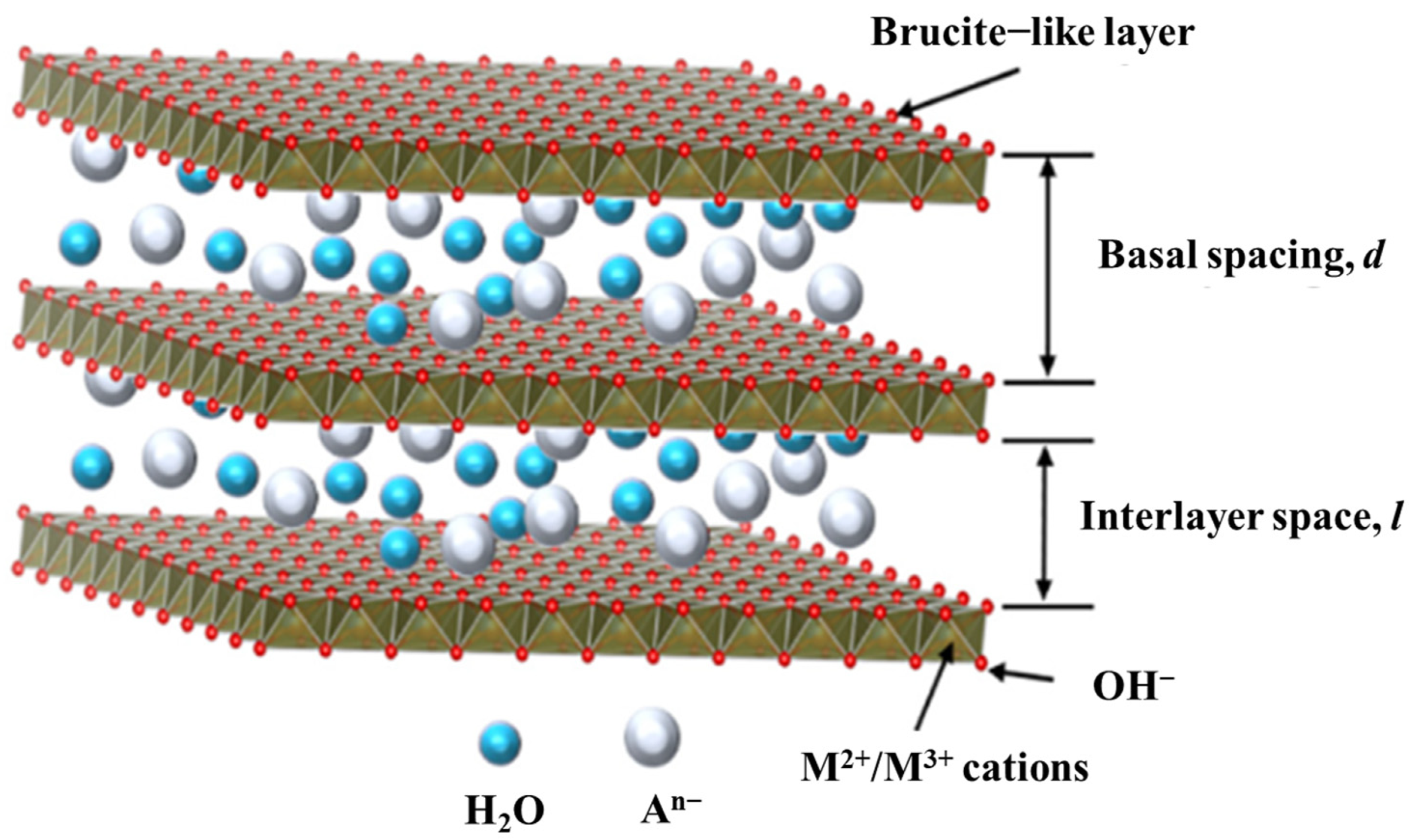
Layered double hydroxides (LDHs), a class of anionic inorganic materials, is a hydrotalcite-like compound with the chemical formula of [M2+1−xM_3+x(OH)2]x+(An−x/n) •yH2O, where M2+ and M3+ represent divalent and trivalent metal cations, x is the molar ratio of trivalent cations/total cations, and y is the number of H2O molecules between layers [53]. As shown in Figure 2, the layered structure of LDHs is composed of the arranged divalent and trivalent metal cations coordinated by OH-octahedrons, while the layered structure is positively charged and the charge-balancing anions (An−) exist in the interlayer. Common divalent metal cations in LDHs include Mg2+, Zn2+, Ni2+, Mn2+, and trivalent metal cations include Al3+, Fe3+, Cr3+, while anions include F−, Cl−, NO3−, CO32−. The basic structural parameters of MgAl-LDH are shown in Table 2. The chemical stability of LDHs is related to the metal species in LDHs. Generally, the metal elements with ionic radii similar to Mg2+ can form stable layer structure [54], which is close to Mg(OH)2, but it is not completely absolute. For example, the ionic radius of Ca2+ (100 pm) has a huge difference compared with that of Mg2+ (72 pm), but it can form stable layer structure with Al3+ [55]. In addition, the properties of specific metal elements also influence the chemical stability. Although the ionic radius of Cu2+ (73 pm) is similar to that of Mg2+, the Jahn–Teller effect causes the distortion of octahedron and the stable layer structure cannot be formed [56]. Pt2+ and Pd2+ have strong tendency to form planar tetra-coordination structures and cannot participate in the construction of stable LDH layers. The chemical stability of photocatalysts is quite important for practical application and it should be considered in the design of LDHs.

Table 2.
Structural parameters of MgAl-LDH [57].
Due to the positive charges of layer structure, anions (An−) in the interlayer play the role of charge compensation for overall electric neutrality. Generally, the volume, number, valence state of anions and the bonding intensity with layer structure influence the interlayer spacing [58]. The neighboring layers are connected with each other by hydrogen bonds originated from H2O molecules and anions. Therefore, the interrelation between neighboring layers can be weakened by changing anions and the monolayer LDH can be exfoliated. LDHs will lose surface OH, interlaminar anions and H2O molecules during the calcining process and the highly dispersed metal oxides can be obtained. When metal oxides derived from the calcination at relatively low temperature get in touch with water or anion solution, it will restore the layer structure of LDHs due to the memory effect of LDHs and the absorbed H2O molecules will convert to OH [59].

Figure 2.
Schematic diagram of LDH structure [60].
Figure 2.
Schematic diagram of LDH structure [60].
3.2. Advantages of MgAl-LDH for CO2 Photoreduction
As 2D materials with unique layer structure, LDHs have advantages for CO2 photoreduction derived from physicochemical properties, morphology characteristics, and layer structure. The advantages of MgAl-LDH for CO2 photoreduction are summarized in this section, while some advantages are common for LDHs and some advantages are unique for MgAl-LDH.
3.2.1. Tunable Composition for Demand
The metal species, element ratio and anion species for the construction of LDHs can be adjusted for demand in the catalytic reaction, which immediately leads to a change in optical absorption and chemical properties. The introduction of specific metal species into LDHs can regulate the bandgap to expand the light absorption range and reduce the energy barrier of CO2 activation. Wang et al. [61] explored the mechanism of d-bands classification in LDHs for CO2 photoreduction by changing metal cations M (Mg2+, Ni2+, Cu2+, Zn2+) in MAl LDHs and NiAl-LDH exhibited the highest CO yield. The upward shift of d-band center position could induce anti-bonding state above the Fermi level and strengthen CO2 adsorption and activation. Different metal species in LDHs also have an influence on the product selectivity due to the change of reaction paths over different active sites. Xiong et al. [62] adjusted the metal cations in Zn-based LDHs for CO2 photoreduction. The results indicated that the main product of LDHs constructed by Ti4+, Ga3+ and Al3+ was CO or CH4, and the main product of LDHs constructed by Fe3+ and Co3+ was H2. The d-band center of specific metal (Ti4+, Ga3+ and Al3+) was close to Fermi level, resulting in the strong CO2 adsorption and the low energy barrier of the conversion from CO2 to CO and CH4.
The metal element ratio of LDHs can be adjusted easily by changing the amount of metal precursors, and the chemical property and charge density of layers also change in this process. When the molar ratio of Mg2+/Al3+ is at the range of 2.0–4.0, the octahedral structure is formed in MgAl-LDH and the positive charge of the layers is disordered. The charge density of layers and the electrostatic force decrease with the increase of the molar ratio of Mg2+/Al3+. Mg(OH)2 and Al(OH)3 maybe appear accompanied with the formation of MgAl-LDH if the molar ratio of Mg2+/Al3+ is beyond the range of 2–4. The molar ratio of metal elements in LDHs influences the bandgap and CO2 adsorption directly. Tao et al. [63] adjusted the molar ratio of Al3+/Ce3+ in NiAlCe-LDH from 4 to 0 and found that the bandgap decreased from 2.07 to 1.81 eV. Yang et al. [28] found that the intensity of CO2 adsorption over LDHs was strongest when the molar ratio of metal elements was in the range of 2–3. Mori et al. realized the isolated single-atom Ru bound on MgAl-LDH and found that the CO2 adsorption capacity could be adjusted near Ru sites by changing the molar ratio of Mg2+/Al3+. When the molar ratio of Mg2+/Al3+ was 5, the CO2 adsorption capacity reached the maximum due to the variation of basicity [64].
The anion species in the interlayer can be adjusted by precursor selecting or ion exchange, resulting in the change of chemical property, stability and the interaction intensity between anions and layers. Generally, the volume, amount, valance state of anions and binding intensity between anions and hydroxyl determine the interlayer distance and space. Hirata et al. [65] synthesized ZnCr-LDH with different interlayer anions (CO32−, Cl−, SO42−, NO3−) for O2 evolution by photocatalytic H2O decomposition and found that Cl− intercalated ZnCr-LDH exhibited the highest photocatalytic activity. Previous studies have shown that CO32− intercalated LDHs had more stable structure and stronger interactions between anions and layers than NO3− intercalated LDHs [66]. The larger interlayer distance of NO3− intercalated LDHs makes the exfoliation of LDHs easier to implement. Xu et al. [67] obtained the exfoliated MgAl-LDH by the mechanical vibration after ion exchange from CO32− to NO3−. However, previous studies indicated that the existence of NO3− was not beneficial to CO2 adsorption due to the occupancy of active sites over MgAl-LDH [68].
3.2.2. Outstanding CO2 Adsorption Performance
The basicity of LDHs can promote the adsorption and activation of CO2 molecules on the surface of catalysts. Meanwhile, the basicity and acidity of LDHs can be adjusted by changing chemical compositions [69]. Generally, the acidity of LDHs is closely related to interlayer anions. When the common anions (CO32−, NO3−) occupy the interlayer space, the acidity of LDHs is always quite weak. The basicity of LDHs is always determined by the metal species and metal element ratio [66]. Therefore, LDHs always exhibit outstanding CO2 adsorption performance. In the photocatalytic reaction over LDHs, the adsorbed CO2 may exist in three forms: (1) dissociative or captured CO32− in the interlayer of LDHs, (2) dissolved CO2 in water, (3) adsorbed CO2 on the surface of LDHs in the form of linear or nonlinear molecules [70,71,72]. Compared with the surrounding free CO2 or CO32−, the interlayer CO32− is easier to be reduced to target products in the photocatalytic reaction, which will be supplemented subsequently by surrounding carbon species [73]. The nonlinear CO2 molecules adsorbed on the surface of LDHs are unstable and easy to be activated under illumination [74]. Extending the interlayer distance of LDHs can increase the CO2 adsorption capacity, promote the diffusion of CO2 and react with hydroxyl groups to form bicarbonate intermediates, resulting in the decrease of energy barrier in photocatalytic reduction [75,76]. Therein, MgAl-LDH shows stronger CO2 adsorption capacity than other LDHs due to the stronger basicity of Mg2+ compared with Zn2+, Ni2+ and Cr3+. Hong et al. [73] synthesized MgAl-LDH modified g-C3N4 for photocatalytic CO2 reduction and found that MgAl-LDH greatly enhanced the CO2 adsorption capacity. MgAl-LDH exhibited much higher CO2 adsorption capacity than NiAl-LDH, ZnAl-LDH and ZnCr-LDH at room temperature. Meanwhile, MgAl-LDH could realize the targeting activation of CO2 in the photothermal reaction [77].
3.2.3. Layer Structure for Construction of Composite Catalysts
The layer structure of LDHs has unique advantages for the construction of composite catalysts. On the one hand, the hierarchical heterostructures containing 2D nanosheets have multiple intrinsic advantages in heterogeneous photocatalysis, including the increased light harvesting capacity, the accelerated charge separation and transfer, and the promotion of surface redox reactions [78]. On the other hand, the intercalation of LDHs can assemble the layer structure and specific guest anions, and the guest anions can overcome the force between the layers of LDHs to insert into the interlayers. Therefore, specific heterogeneous catalysts can be custom-made based on the characteristic of LDHs. Dou et al. [79] fabricated TiO2@CoAl-LDH nanospheres with core–shell structure by in situ growth of LDHs on the surface of TiO2 hollow nanospheres. The interaction between the core–shell structure and the matched band structure are favorable for photogenerated charge separation and transfer. Zhao et al. [80] utilized the confined interlayer space of LDHs to enhance the dispersion of metal active sites and inserted a series of metalloporphyrin molecules (TCPP-M, M = Zn2+, Co2+, Ni2+, Fe2+) into interlayer of CuAl-LDH by ion exchange, and the heterogeneous catalysts with highly dispersed metal sites were obtained after calcination. The framework of metalloporphyrin molecules and the confined interlayer space of LDHs effectively improved the dispersion of active sites on the surface of catalysts. Nayak et al. [81] synthesized g-C3N4/NiFe-LDH catalyst by an impregnation method and the exfoliated g-C3N4 sheets were dispersed on the layer of LDHs. The strong interaction promoted the charge transport and enhanced the lifetime of carriers, and the composite catalyst exhibited high activity in the photocatalytic H2 production from water decomposition.
3.3. Drawbacks of MgAl-LDH
Although MgAl-LDH has many advantages in photocatalytic CO2 reduction, some drawbacks still greatly limit the practical application of MgAl-LDH. The drawbacks related to the properties of MgAl-LDH are summarized below.
3.3.1. Wide Bandgap of MgAl-LDH
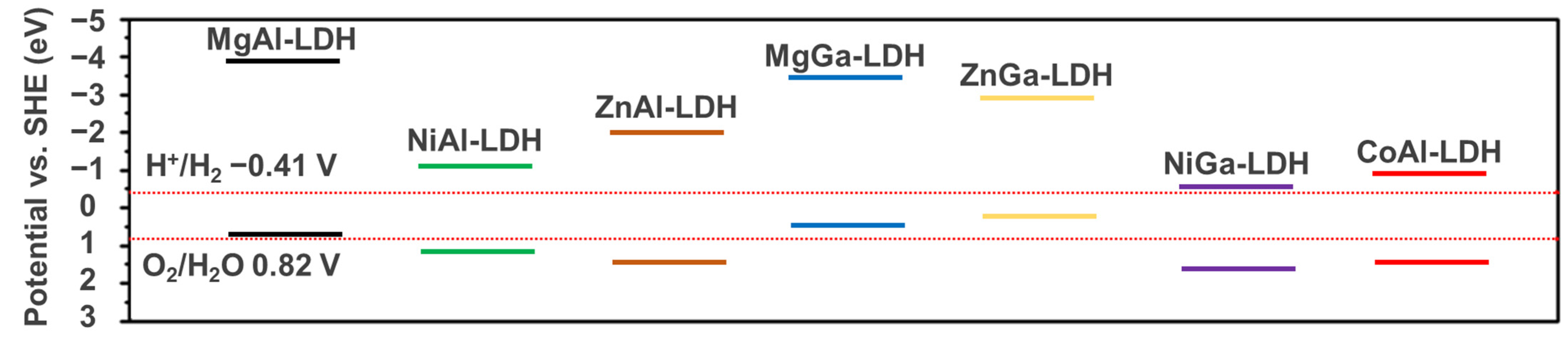
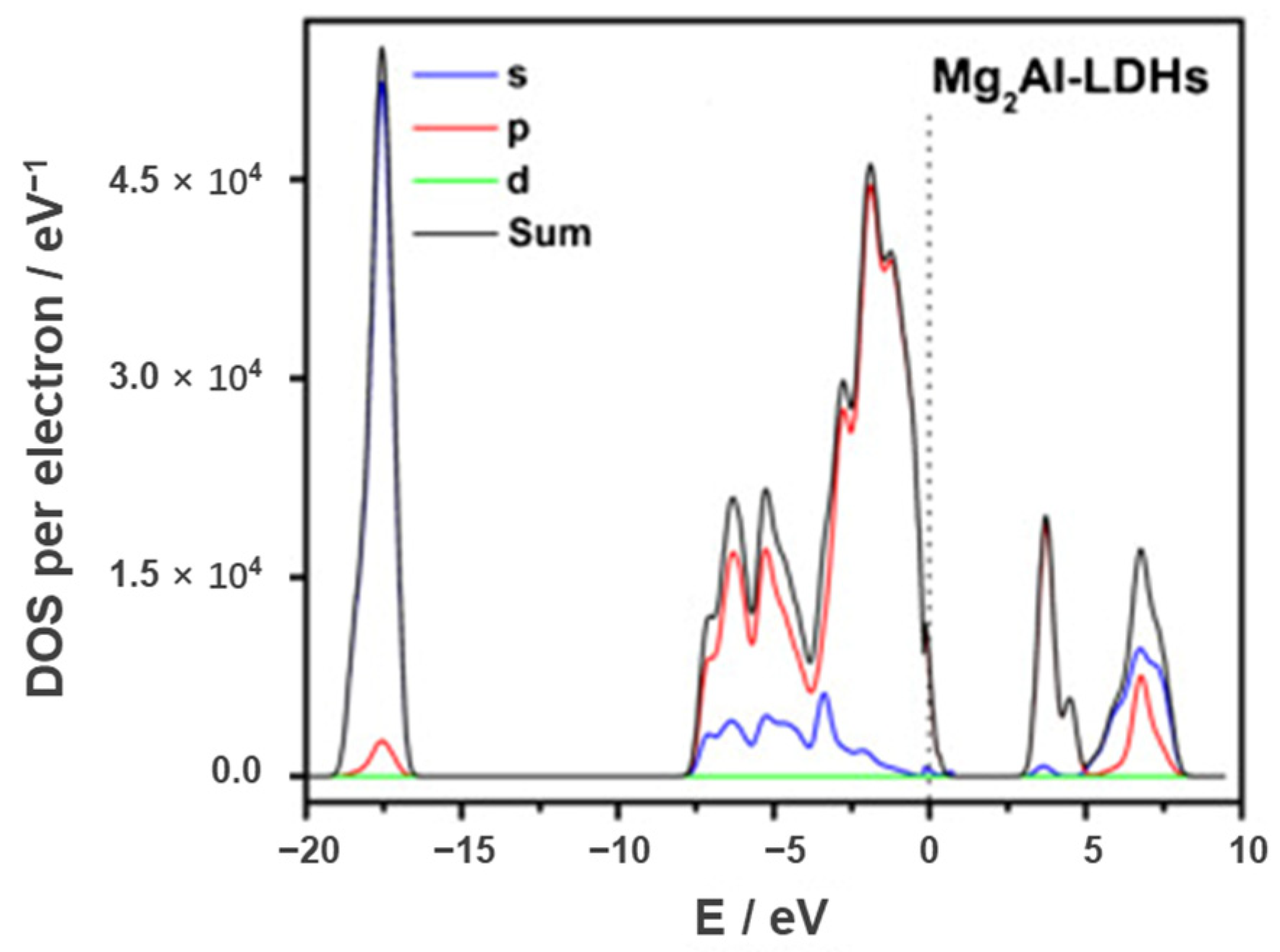
Visible light accounts for 45% in the solar spectrum, while ultraviolet light only accounts for 5%. MgAl-LDH has a wide bandgap and can only absorb the ultraviolet light in photocatalytic reaction. As shown in Figure 3, Xu et al. [57] calculated the bandgap and potentials of Mg-, Zn-, Co-, and Ni based LDHs by DFT calculation. The results indicated that the bandgaps of Co- and Ni-based LDHs were more narrowed than 3.1 eV and could absorb the visible light, but the bandgaps of Mg- and Zn-based LDHs were wider than 3.1 eV and could only absorb ultraviolet light. Therein, the bandgap of MgAl-LDH was 4.63 eV and the visible light could not be utilized in photocatalytic reaction. As the most widely used LDH material, the wide bandgap of MgAl-LDH seriously limits its potential application. In recent reports, the bandgaps of MgAl-LDH synthesized by Nayak et al. [82], Gao et al. [83], Chen et al. [84] and Ning et al. [85] are 3.20, 3.57, 3.66 and 4.18 eV, respectively. The bandgaps of MgAl-LDH in various reports are inconsistent, but none of them can absorb the visible light. The poor visible light absorption performance is ascribed to the lack of electrons in the d orbital. As shown in Figure 4, the lack of electrons in the d orbital leads to the absence of intermediate band and the electron transfer is quite difficult between the metal atoms. Therefore, the electron transition requires high energy and the utilization efficiency of visible light is quite unsatisfied.

Figure 3.
Band potentials of Mg, Zn, Co, Ni based LDHs [57].

Figure 4.
Densities of state for MgAl-LDH [86].
3.3.2. High Recombination Rate of Electron–Hole Pairs
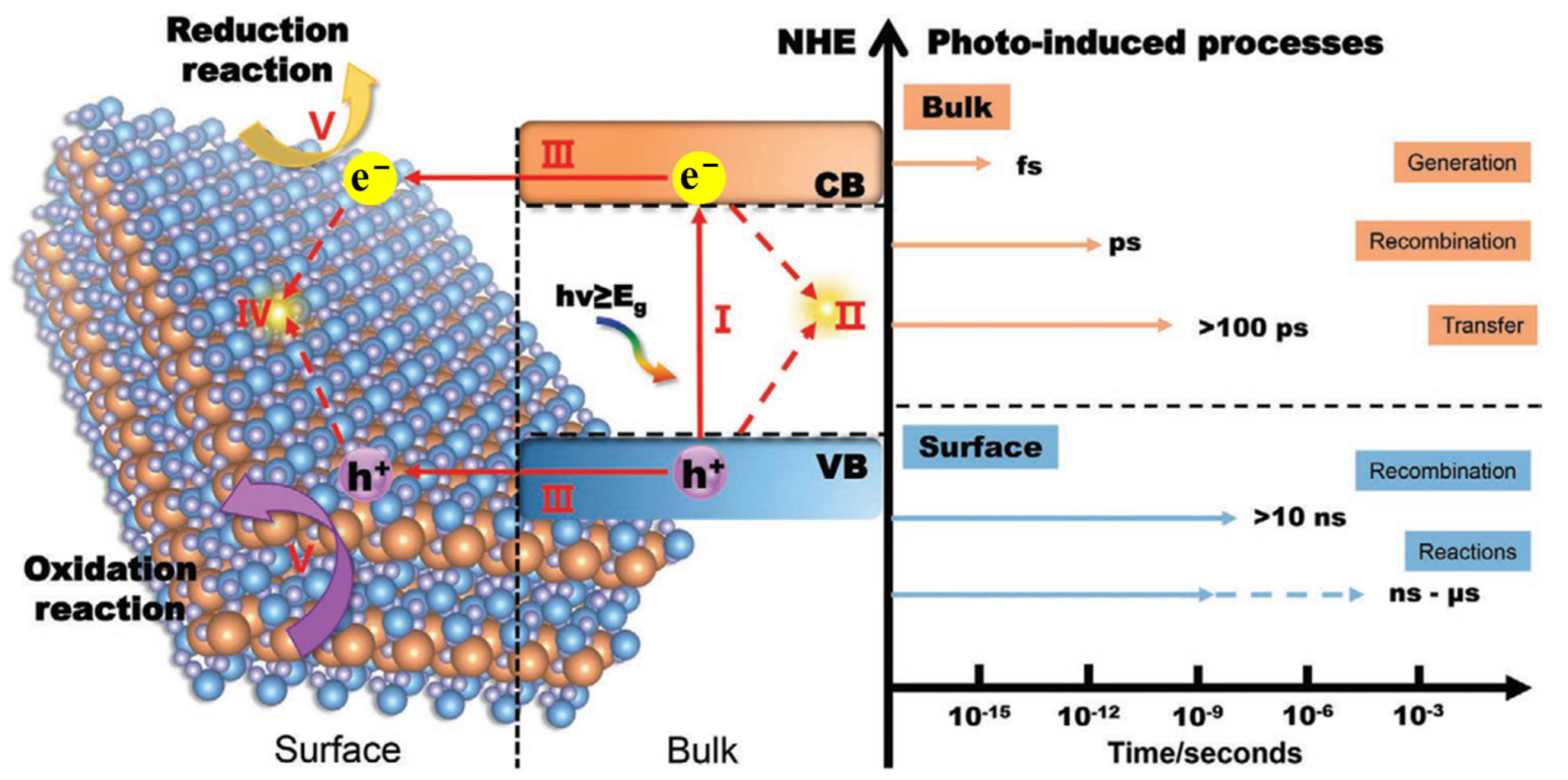
Single LDH materials always have a high recombination rate of electron–hole pairs and the performance of photogenerated charge transfer is poor. As shown in Figure 5, the different steps and time scales in the photocatalytic reaction based on semiconductor catalysts are illustrated. The catalyst will generate high-energy electrons and holes under the excitation of photons (step I). Due to the existence of Coulomb force, some electrons and holes will recombine in the bulk of catalyst and release energy in the form of light or heat (step II). Meanwhile, the uncombined electrons and holes will migrate from the bulk to the surface of catalyst (step III), and some electrons and holes will also recombine on the surface of catalyst (step IV). The remaining electrons and holes finally reach the active sites on the surface and participate in the catalytic reaction (step V). Generally, the generation of electrons and holes is completed at the time scale of femtosecond (fs) and the migration of electrons and holes from bulk to surface is completed within hundreds of picoseconds (ps), while the catalytic reaction between charge carriers and absorbed reactants is completed within a few nanoseconds (ns) to a few microseconds (μs) [87]. In contrast, the recombination of electrons and holes in the bulk of catalyst is always completed within a few picoseconds (ps) while the recombination of electrons and holes on the surface is always completed within ten nanoseconds (ns) [88]. The process of charge recombination is much faster than the process of charge transfer and surface reaction. With the assistance of DFT calculation, Liu et al. [86] demonstrated that the bandgap of ZnCr-LDH is 2–3 eV and has satisfactory visible light absorption performance, but the rapid recombination of charge carriers leads to low separation efficiency of electron–hole pairs and limited photocatalytic activity. Therefore, most of the electrons and holes will be annihilated due to the high recombination rate and only a few electrons and holes can participate in the final surface redox reaction for single MgAl-LDH catalyst.
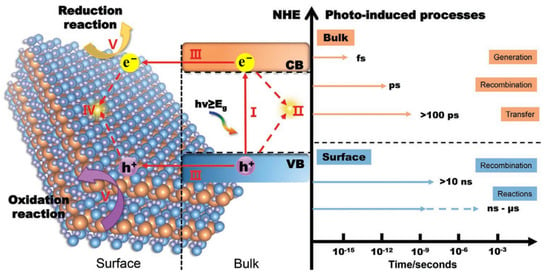
Figure 5.
Different steps and time scales in the photocatalytic reaction based on semiconductor catalysts [89].
4. CO2 Photocatalytic Reduction by MgAl-LDH-Based Materials
Due to the poor visible light absorption and fast charge recombination of MgAl-LDH, its application in photocatalysis is limited and studies on the application of MgAl LDH in photocatalytic CO2 reduction are relatively few. In order to overcome the drawbacks of MgAl-LDH in visible light absorption and charge separation, many efforts have been devoted to developing different modification methods to improve the photocatalytic performance. Several strategies, such as defect engineering, morphology control, component regulation and material coupling, have been reported for the modification of MgAl-LDH. The specific modification methods are summarized in this section and the performance summary of MgAl-LDH based photocatalysts for CO2 photoreduction is listed in Table 3.
4.1. The Introduction of Active Components into MgAl-LDH
Single MgAl-LDH always exhibits poor photocatalytic activity due to its own drawbacks. Iguchi et al. [74] compared the catalytic activity of different LDHs in the photocatalytic CO2 reduction and found that NiAl-LDH exhibited the highest photocatalytic activity in the presence of Cl− as the hole sacrificial agent. In contrast, MgAl-LDH only exhibited the poor CO evolution rate of 0.6 µmol·g−1·h−1 under UV light. Due to the ordered dispersion of metal cations in the layer structure of LDHs and flexible adjustment property, active components can be introduced into the layer structure by element doping to improve the visible light absorption and charge separation of MgAl-LDH.

Table 3.
Summary of MgAl-LDH based photocatalysts for CO2 photoreduction.
Table 3.
Summary of MgAl-LDH based photocatalysts for CO2 photoreduction.
| Catalyst | Synthesized Method | Reaction System | Light Source | Type of Reactor | Reaction Condition | Product Yield (μmol·g−1·h−1) | Selectivity | Ref. |
|---|---|---|---|---|---|---|---|---|
| MgAl-LDH | Coprecipitation | Cl− + H2O | 400 W Hg lamp (UV light) | 220 mL closed circulating system | 7.7 mmol CO2 + 350 mL H2O | CO = 0.6 CH4 = 0.3 H2 = 1.2 | CO: 28.6% CH4: 14.3% H2: 57.1% | [74] |
| MgAl-LDH | Microwave-hydrothermal | - | 150 W Xe lamp (Simulated sunlight) | 200 mL stainless cylindrical reactor | - | CO = 1.3 CH4 = 0.1 | CO: 92.9% CH4: 7.1% | [68] |
| Fluorinated MgAl-LDH | Coprecipitation | Cl− + H2O | 400 W Xe lamp (UV light) | Quartz inner-irradiation reactor | 7.6 mmol CO2 + 350 mL H2O | CO = 4.3 H2 = 10.5 | CO: 29.1% H2: 70.9% | [70] |
| MgAlTi-LDH | Coprecipitation | H2O | 400 W UV lamp (200–1000 nm) | Continuous-flow quartz tube reactor | CO2 + 2.3 vol.% H2O + 150 °C | CO = 10 (per gram of TiO2) | - | [90] |
| CoMgAl-LDH | Coprecipitation | [Ru(bpy)3]Cl2·6H2O + TEOA + MeCN + H2O | 300 W Xe lamp (>400 nm) | 50 mL stainless reactor | 1.8 bar + 30 °C | CO = 7700 H2 = 5808.8 | CO: 57.0% H2: 43.0% | [85] |
| CrMgAl-LDH | Hydrothermal | [Ru(bpy)3]Cl2·6H2O + TEOA + H2O | 300 W Xe lamp (420–780 nm) | 250 mL quartz reactor | 0.1 MPa CO2 + 20 °C | CO = 3.91 H2 = 2.05 | CO: 65.6% H2: 34.4% | [48] |
| Ultrathin MgAl-LDH | Titration | [Ru(bpy)3]Cl2·6H2O + TEOA + MeCN + H2O | 300 W Xe lamp (400–800 nm) | 50 mL stainless reactor | 1.8 bar | CO = 700 H2 = 1700 | CO: 29.2% H2: 70.8% | [91] |
| Ru/ultrathin MgAl-LDH | Impregnation | H2 | 300 W Xe lamp | Flow photoreactor | 5 mL·min−1 CO2 + 20.5 mL·min−1 H2 + ~350 °C | CH4 = 2.77 × 105 | CH4: ~100% | [77] |
| Monolayer MgAl-LDH | SNAS | [Ru(bpy)3]Cl2·6H2O + TEOA + MeCN + H2O | 300 W Xe lamp (>400 nm) | 50 mL stainless reactor | 1.8 bar + 30 °C | CO = 170 H2 = 210 | CO: 44.7% H2: 55.3% | [92] |
| Pt/MgAl-LDH | Electrostatic attraction | H2O | 300 W Xe lamp (UV light) | 250 mL quartz reactor | 0.1 MPa CO2 + 20 °C | CO = 2.64 CH4 = 0.2 | CO: 93.0% CH4: 7.0% | [67] |
| Ag/Ga2O3/MgAl-LDH | Coprecipitation | NaHCO3 | 400 W Hg lamp (UV light) | Flowing batch system | 30 mL·min−1 CO2 + Room temperature | CO = 211.7 H2 = 131.4 | CO: 61.7% H2: 38.3% | [93] |
| CeO2/MgAl-LDH | Hydrothermal | [Ru(bpy)3]Cl2·6H2O + TEOA + MeCN + H2O | 300 W Xe lamp (Visible light) | 1.8 bar + 30 °C | CO = 85 H2 = 110.5 | CO: 42.1% H2: 57.9% | [94] | |
| Fe3O4/MgAl-LDH | Coprecipitation | NaOH + MeCN + H2O | 8 W UV light | 300 mL quartz reactor | 0.1 MPa CO2 + 20 °C | CO = 442.2 CH4 = 223.9 | CO: 68.9% CH4: 31.1% | [83] |
| Co-TCPP@MgAl-LDH | Esterification | H2O | 300 W Xe lamp (420–780 nm) | 250 mL quartz reactor | - | CO = 0.13 CH4 = 0.08 | CO: 62.0% H2: 38.0% | [95] |
| Pd/C3N4/MgAl-LDH | Self-assembly | H2O | 500 W Hg-Xe lamp (UV light) | 0.03 MPa CO2 + Room temperature | CH4 = 0.7 | - | [73] | |
| Pt/MgAl-LDO/TiO2 | Coprecipitation | H2O | 300 W Xe lamp (260–400 nm) | Closed circulating system | 0.1 MPa CO2 + 40 mL H2O + 20 °C | CO = 1.5 CH4 = 2.3 | CO: 39.5% CH4: 60.5% | [96] |
| MgAl-LDO/Nv-CN | In situ deposition | H2O | 300 W Xe arc lamp (>400 nm) | 280 mL double-necked reactor | - | CO = 20.47 | - | [97] |
| Fe/MgAl-LDO | Thermal reduction | H2 | 300 W Xe lamp (200–800 nm) | 50 cm3 stainless reaction chamber | 1.8 bar + CO2/H2/Ar = 15/60/25 + ~275 °C | CO2 conversion = 50.1% | CO: 16.0% CH4: 49.5% C2–4: 31.2% C5: 3.3% | [98] |
| MgAl-LDO/TiO2 | Coprecipitation | H2O | 100 W Hg lamp (<390 nm) | Continuous-flow quartz tube reactor | 4.5 mL·min−1 He with H2O + 200 °C | CO = 99 μmol·g−1 | - | [99] |
| MgAl-LDO/TiO2 | Hydrothermal | H2O | 100 W Hg lamp (<390 nm) | Continuous-flow quartz tube reactor | 4.5 mL·min−1 CO2 with 2.3 vol.% H2O + 150 °C | CO = 4.3 | - | [100] |
| TiMgAl-LDH/GO | Coprecipitation | H2O | 300 W Xe lamp (>420 nm) | 50 cm3 stainless reactor | 0.08 MPa | CO = 4.6 CH4 = 3.8 | CO: 54.8% CH4: 45.2% | [101] |
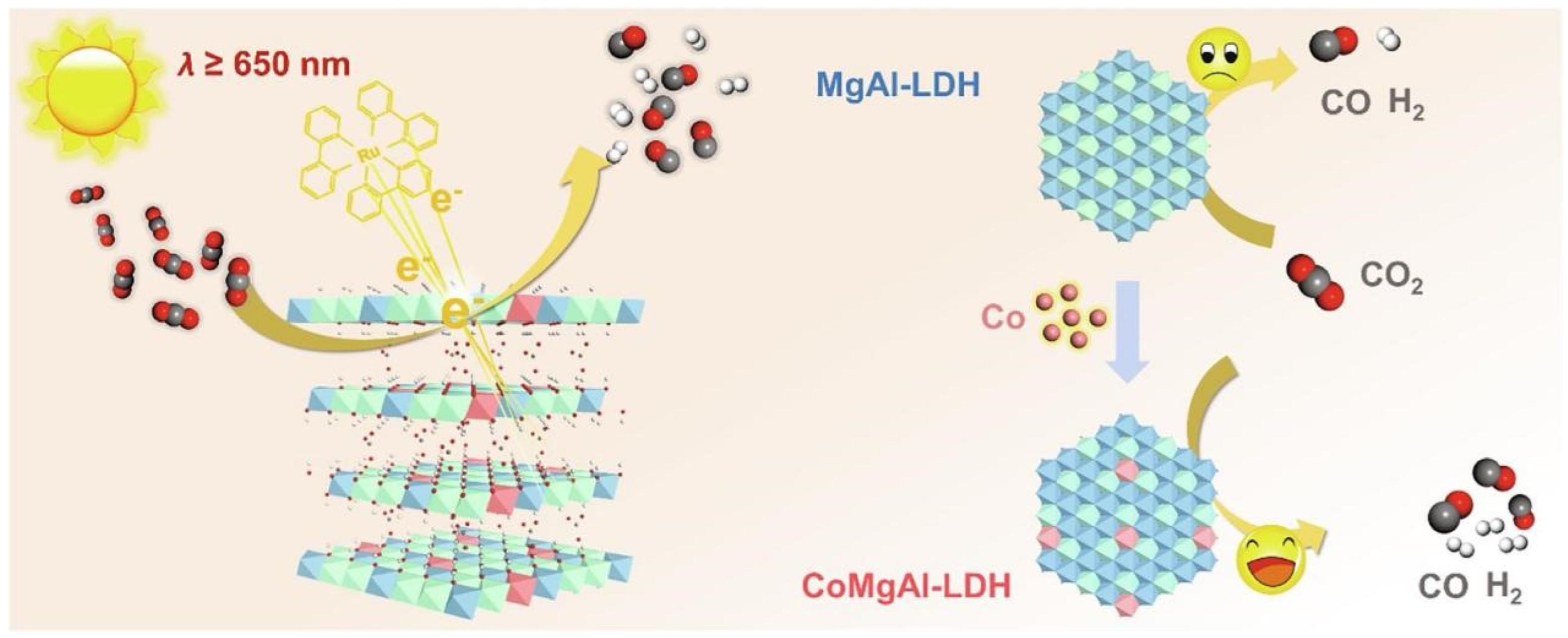
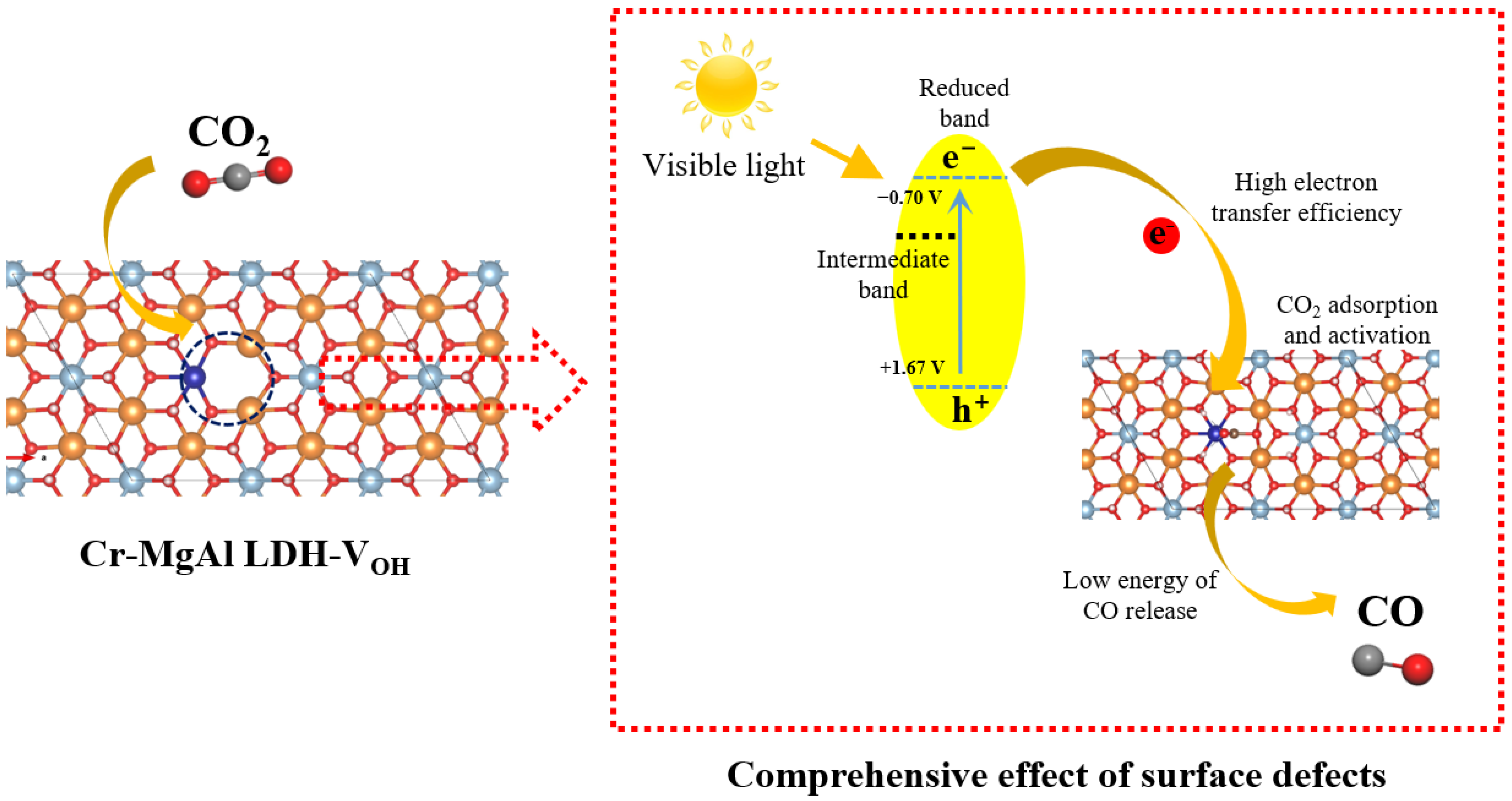
Doping suitable elements into MgAl-LDH to construct ternary LDHs can adjust the band structure, introduce the surface defects, improve the separation of electron–hole pairs and strength the surface reaction [102]. The introduction of Co, Ni, Ti and other metals by element doping is always applied to improve the visible light absorption of LDHs with wide bandgaps, which can enhance d-band electrons and introduce impurity levels in the forbidden band. Meanwhile, the introduced metal atoms are embedded into the layer structure of LDHs, which provide abundant active sites and create defect sites. The active sites and defect sites promote the surface reduction reaction of CO2 and improve the charge separation performance. As shown in Figure 6, Ning et al. [85] synthesized Co-doped MgAl-LDH by a hydrothermal method for CO2 photoreduction and the bandgap was reduced from 4.18 eV to 1.80 eV after the introduction of Co sites. The introduction of Co into MgAl-LDH led to the broad absorption peaks at 520 nm and 610 nm due to the d–d transitions of Co2+. Hydroxyl vacancies (VOH) were created after Co doping and the charge separation was greatly improved. Under the light irradiation above 400 nm, the TOF value of CO production for CoMgAl-LDH came to 11.57 h−1, which was three times that of CoAl-LDH. Although the wavelength was up to 650 nm, CoMgAl-LDH still exhibited high activity with CO evolution rate of 0.35 µmol·h−1. It was noteworthy that the formation energy of hydroxyl vacancy (VOH) in CoMgAl-LDH was lower than that of CoAl-LDH, indicating that the high content of Co made it harder to form unsaturated sites. Xu et al. [48] introduced five kinds of transition metal (Co, Ni, Cu, Fe, Cr) into the layer structure of MgAl-LDH and found that that the introduction of transition metal could effectively reduce bandgap and improve visible light absorption performance. Therein, Cr-doped MgAl-LDH exhibited the highest TOF value of 0.0053 h−1 for CO production under visible light irradiation (420–780 nm). The bandgap of MgAl-LDH was 5.42 eV and the introduction of Co, Ni, Cu, Fe, Cr reduced the bandgap to 2.13, 2.72, 3.94, 2.01 and 2.37 eV, respectively. Except Cu-doped MgAl-LDH, other metal-doped MgAl-LDH all exhibited visible light response ability. As shown in Figure 7, The introduction of metal elements introduced the intermediate bands, which led to the decreased OH vacancy formation energy and the increased defect density. Cr doping into MgAl-LDH provided the ability to continuously generate defects and the high surface defect density, which led to the highest charge transfer efficiency. Meanwhile, Cr-doped MgAl-LDH exhibited competitiveness in the release of CO during the surface reaction, which was confirmed by DFT calculation.

Figure 6.
Schematic diagram for MgAl-LDH and CoMgAl-LDH for CO2 photoreduction to generate CO and H2 under λ ≥ 650 nm combining with a Ru complex [85].

Figure 7.
Mechanism diagram of Cr doping into MgAl-LDH in CO2 photoreduction [48].
In addition to the metal doping into MgAl-LDH, the metal compounds can also be introduced into the layer structure to achieve the substitution of hydroxyl groups. Iguchi et al. [70] prepared a MgAl-LDH photocatalyst containing AlF63− units by a coprecipitation method, which achieved the substitution of hydroxyl with fluorine by changing the precursor species. The fluorination of MgAl-LDH obviously promoted the evolution of CO in the photocatalytic CO2 reduction with Cl− as a hole scavenger and CO yield was twice that of pure MgAl-LDH. Meanwhile, the product selectivity of CO was also increased after the fluorination of MgAl-LDH. UV-vis spectrum indicated that the absorption edge of MgAl-LDH was not influenced by the fluorination of MgAl-LDH. The increased activity was due to the enhanced CO2 adsorption on the surface and the high electronegativity of fluorine units greatly influenced the basicity of MgAl-LDH. In summary, the introduction of active components into MgAl-LDH always obtains the highly dispersed active sites, creates the unsaturated defect sites and changes the surface chemical property, resulting the increased photocatalytic activity.
4.2. Morphology Control of MgAl-LDH
The bulk LDHs always show limited catalytic activity in CO2 photoreduction due to the limited specific surface area, fast recombination of electron–hole pairs and insufficient charge transfer [103]. Morphology control can improve the electronic structure and expose more active sites, which can further improve catalytic activity of bulk MgAl-LDH [104]. Ultrathin or monolayer MgAl-LDH nanosheets can be prepared by a bottom-up or top-down method [16,105], which always leads to the structural distortion and the formation of vacancies. The electronic structure and charge transfer will be modified due to the existence of vacancies, which directly influences the photocatalytic performance.
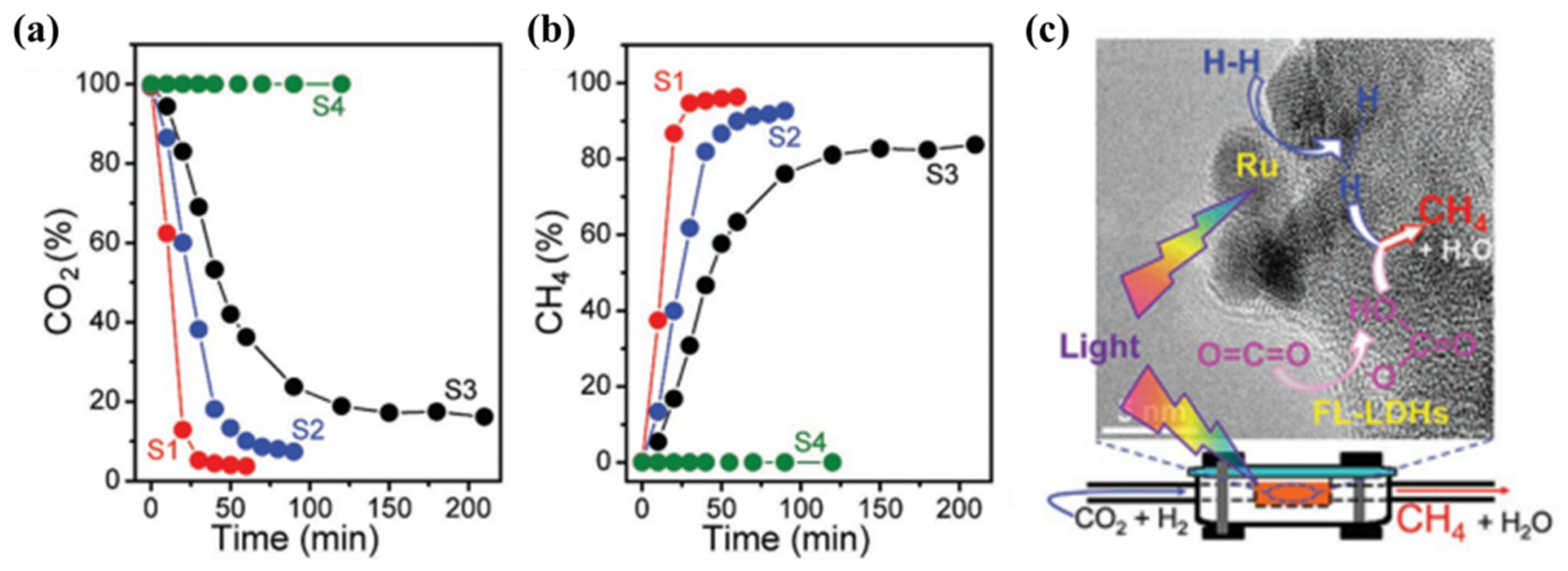
The ultrathin LDHs always show the increased specific surface area and more active sites can be exposed. The surface defects in the ultrathin nanosheets are considered to be important adsorption sites for CO2 molecules and lead to the enhanced conductivity of LDHs [104]. Xu et al. [67] prepared the ultrathin MgAl-LDH by the mechanical exfoliation in formamide solution and obtained the nanosheets with the thickness of 1–2 nm. The exfoliated MgAl-LDH showed the increased specific surface area compared with bulk MgAl-LDH due to the expansion of layer structure. Ren et al. [77] prepared Ru-loaded ultrathin MgAl-LDH (Ru@FL-LDHs) by ultrasonicated exfoliation and impregnation for photothermal CO2 methanation. The thickness of the ultrathin MgAl-LDH was around 8 Å, which is consistent with the single basal spacing of MgAl-LDH. As shown in Figure 8, Ru@FL-LDHs showed the fastest initiation of the reaction within 30 min and the highest CO2 conversion (96.3% after 60 min irradiation). Meanwhile, the highest selectivity of CH4 was also achieved in photothermal CO2 reduction over Ru@FL-LDHs. Compared with Ru-loaded bulk MgAl-LDH (Ru@LDHs), Ru@FL-LDHs exhibited the higher CO2 adsorption capacity due to the stronger basicity of the exfoliated LDHs, while CO2 molecules could be converted into CO32− that was easily activated. The exfoliated MgAl-LDH with abundant basic sites provided the ability to activate CO2 molecules effectively. The excellent photothermal conversion of CO2 was ascribed to the targeting activation of CO2 and H2 over ultrathin MgAl-LDH and Ru nanoparticles. Bai et al. [91] developed the strategy of manipulating d-electron configuration in ultrathin LDHs by changing the selection of divalent metal cations to regulate photocatalytic activity for CO2 reduction. DFT calculation indicated that the bandgap of ultrathin MgAl-LDH was reduced to 3.33 eV effectively after the morphology control.

Figure 8.
Concentration change of (a) CO2 reactant and (b) CH4 product in photothermal CO2 methanation over different catalysts (S1: Ru@FL-LDHs; S2: Ru@LDHs; S3: Ru@SiO2; S4: FL-LDHs). Reaction condition: 150 mg catalysts, H2: 20.5 mL·min−1, CO2: 5.0 mL·min−1, 300 W xenon lamp. (c) Schematic diagram of targeting activation of CO2 and H2 over Ru@FL-LDHs [77].
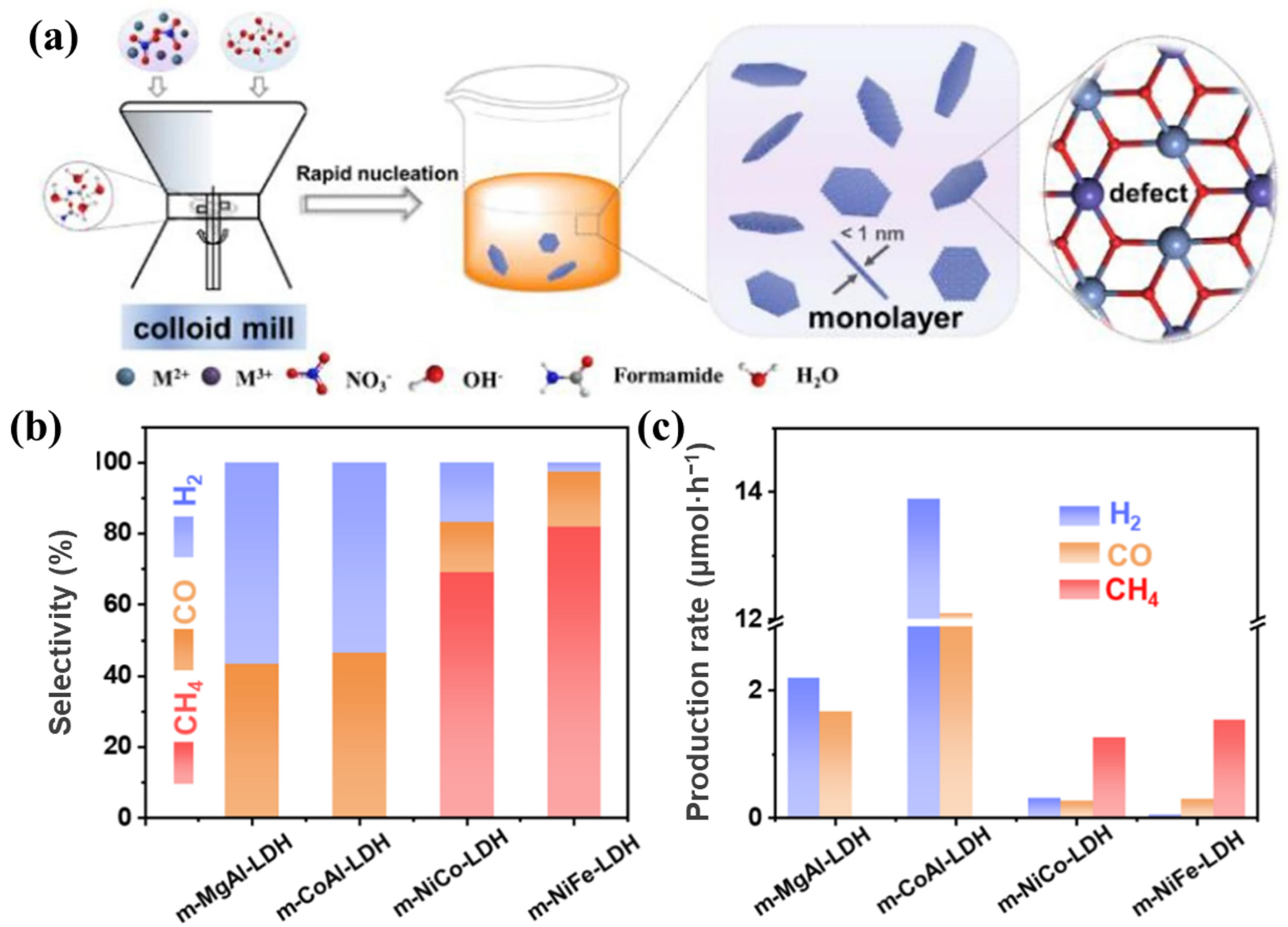
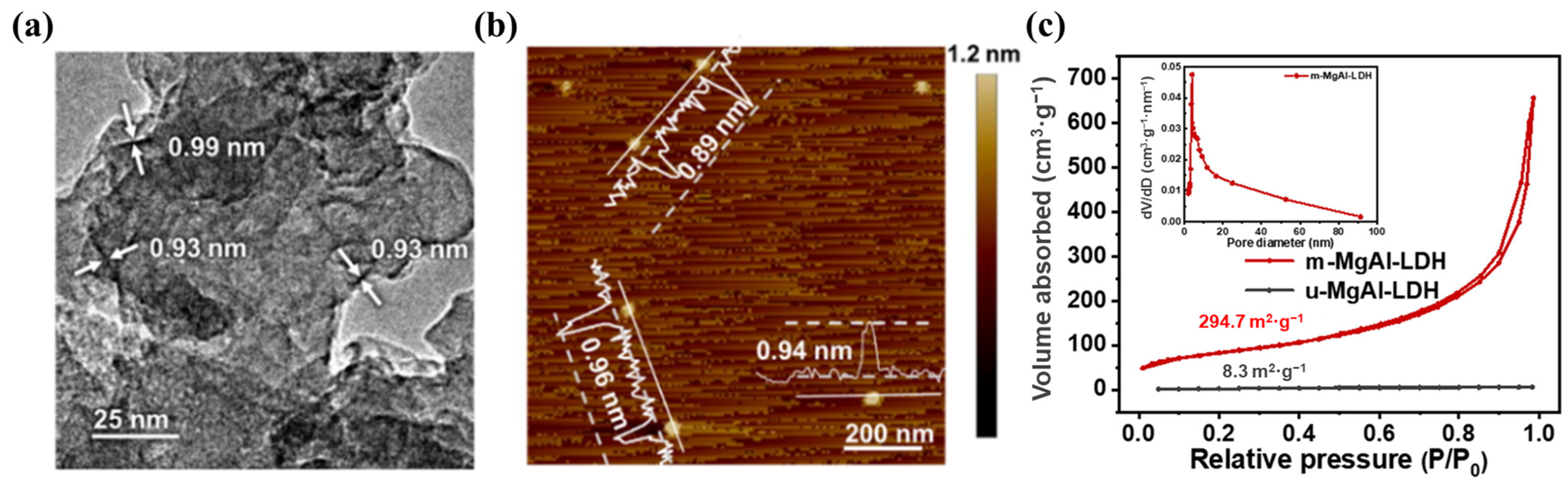
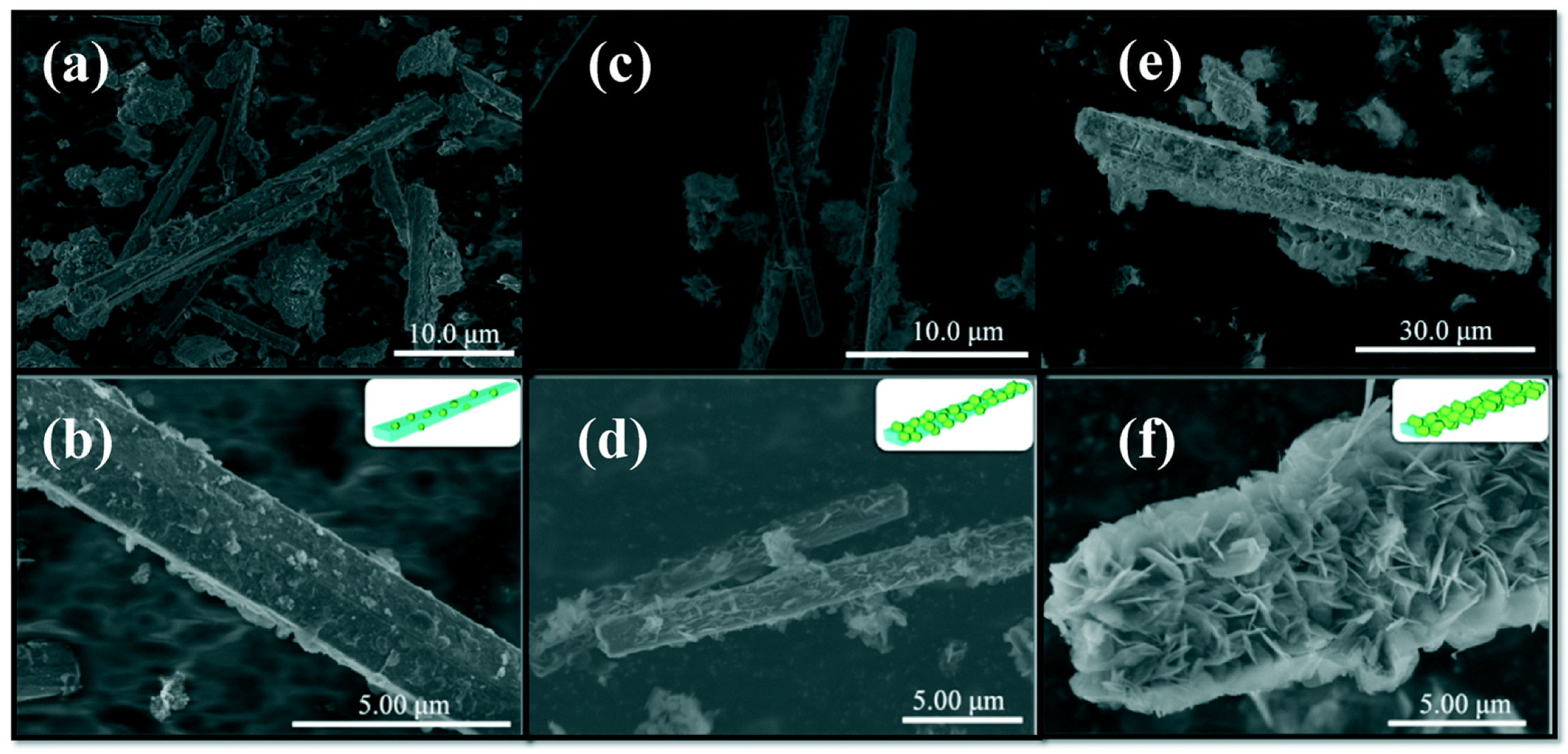
Ulteriorly, the monolayer LDHs have better advantages in charge separation efficiency than ultrathin LDHs benefiting from the thickness at the atomic level. Bai et al. [92] developed a facile method to synthesize monolayer LDHs by adding layer growth inhibitor (formamide) into a colloid mill reactor and obtained uniform monolayer MgAl-LDH with the thickness of ~1 nm. EXAFS results confirmed that lots of vacancies (oxygen and metal vacancies) existed on the surface of monolayer LDHs. The monolayer LDHs exhibited higher photocurrent density than multilayer LDHs, indicating that the vacancies in monolayer LDHs improved the electron transport performance. Meanwhile, as shown in Figure 9, different metal species in monolayer LDHs had an influence on the selectivity and evolution rate of products in CO2 photoreduction. The synthetic method can be applied to prepare different monolayer LDHs and achieve the great increasement of preparation rate, which can be used for large-scale industrial production. Lai et al. [106] prepared monolayer MgAl-LDH by a similar method in colloid mill reactor. As shown in Figure 10, the TEM image and AFM image clearly indicate the atomic thickness of monolayer MgAl-LDH (~1 nm). N2 adsorption–desorption isotherms were used to evaluate the specific surface areas of monolayer and multilayer LDHs. The specific surface areas of monolayer and multilayer MgAl-LDH were 294.7 m2/g and 8.3 m2/g, respectively, indicating that monolayer MgAl-LDH could provide much more active sites.

Figure 9.
(a) Schematic illustration of synthetic process of monolayer LDHs; (b) selectivity and (c) production rate of CH4, CO, and H2 in photocatalytic CO2 reduction over different monolayer LDHs under λ ≥ 400 nm [92].

Figure 10.
(a) HRTEM image and (b) AFM image of monolayer MgAl-LDH; (c) N2 adsorption–desorption isotherms for monolayer MgAl-LDH and multilayer MgAl-LDH and pore size distribution of monolayer MgAl-LDH (inset) [106].
4.3. Supported Active Components over MgAl-LDH
The supporting of active components on the surface of MgAl-LDH is a potential method to improve the photocatalytic activity of catalysts, which provides the highly dispersed active sites and constructs the interaction between the two components [107]. Generally, multiple active components, such as metals, metal compounds and nonmetallic materials, can be loaded on the surface of LDHs to improve the photocatalytic activity. Depending on the different characteristics of various active components, the interface engineering can be designed minutely [108]. As a result, the light response range of composite catalysts can be widened and the transfer efficiency of photogenerated carriers can be improved.
The surface hydroxyl groups of MgAl-LDH have a strong immobilization effect for the loading of noble metal, so it is suitable to be the support to form stable noble-metal-based catalysts [109,110]. For example, Zhu et al. [111] anchored Ru atoms on the surface of MgAl-LDH by an impregnation method and a strong metal-support interaction between Ru and LDHs was constructed through the coordinated Ru species with one OH and three oxygen atoms, which have an important influence on the photocatalytic activity. Generally, the supported noble metal can act as the active sites to capture photogenerated electrons and promote the separation of electron–hole pairs. Xu et al. [67] achieved high dispersion of Pt nanoparticles on the surface of MgAl-LDH by electrostatic attraction for CO2 photocatalytic reduction. The loading of Pt nanoparticles over MgAl-LDH greatly improved the charge transfer efficiency of catalysts, further increasing the photocatalytic activity. 0.1 wt.% Pt-loaded MgAl-LDH exhibited the highest activity with CO evolution rate of 2.64 µmol·g−1·h−1 under UV light, which is about 8.52 times that of 1 wt.% Pt-loaded MgAl-LDH. The high content of Pt species did not form the electron traps but promoted the formation of combination centers, which increased the combination rate of electron–hole pairs. This method provided a strategy that maintained high photocatalytic activity while reducing the dosage of noble metal. Iguchi et al. [93] prepared Ag-loaded MgAl-LDH/Ga2O3 by an impregnation method for CO2 photocatalytic reduction under UV light. 0.25 wt.% Ag-loaded MgAl-LDH/Ga2O3 exhibited the CO evolution rate of 211.7 µmol·h−1 and CO selectivity of 61.7%. The loading of MgAl-LDH improved the CO2 adsorption capacity and Ag nanoparticles acted as co-catalyst, which improved the product selectivity. Photogenerated electrons transferred from Ga2O3 to Ag nanoparticles via MgAl-LDH, which promoted the conversion of CO2 to CO. In addition, the SPR effect of noble metals also improves the visible light response performance, which has been reported over LDHs [112].
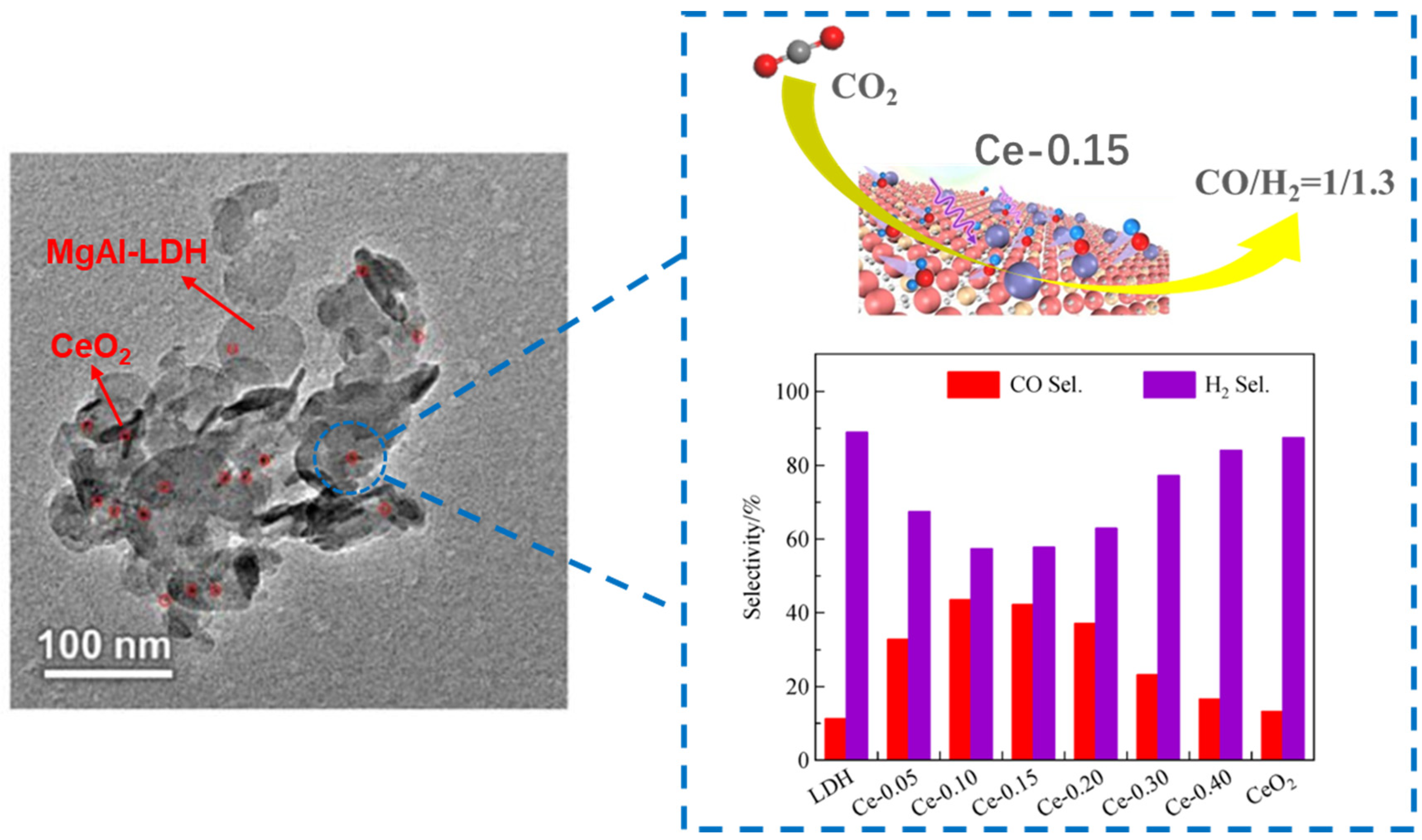
The limitation of noble metals in CO2 photoreduction is the high cost, and transition metal components have been reported to replace noble metals. The coupling of MgAl-LDH with suitable transition metal components can effectively improve the visible light absorption and charge separation. Tan et al. [94] prepared CeO2 decorated MgAl-LDH for photocatalytic CO2 reduction and found that the selectivity of syngas (CO/H2) was closely related to the loading amount of CeO2. Mg6Al0.85Ce0.15-LDH (Ce-0.15) exhibited the CO productivity of 0.85 µmol, which was 4.7 and 9.4 times that of MgAl-LDH and CeO2. As shown in Figure 11, the selectivity and productivity trend of CO achieved the highest point at Ce-0.15 whit the evolution rate of 85 µmol·g−1·h−1 and selectivity of 42.1%. UV-visible absorption indicated that Ce-0.15 exhibited the best light absorption performance in the visible region, which was due to the interaction between CeO2 and MgAl-LDH. EIS spectra proved that the resistance of Ce-0.15 was smaller than that of MgAl-LDH, indicating that the loading of CeO2 effectively promoted the separation and transfer of charge carriers. Gao et al. [83] prepared Fe3O4-loaded ultrathin MgAl-LDH by a coprecipitation method for photocatalytic CO2 reduction, and it exhibited high catalytic activity with CO and CH4 yields of 442.2 μmol·g−1·h−1 and 223.9 µmol·g−1·h−1 in the presence of NaOH and acetonitrile under UV light, which were 1.8 and 1.7 times than those of MgAl-LDH. Fe3O4 with the narrow bandgap and high conductivity, strikingly broadened the absorption edge to ~700 nm and promoted the transfer of f-photogenerated electrons. Ultrathin MgAl-LDH reduced the transfer resistance of charge carriers and provided abundant active sites. Fe3O4 could act as electron traps, which prolonged the lifetime of photogenerated electrons. The direct loading of transition metal components always obtains the weak interfacial interaction and it is difficult to ensure the dispersion of transition metal sites. Xu et al. [95] prepared Co-porphyrin-loaded flower-like MgAl-LDH for photocatalytic CO2 reduction under visible light. The excellent light absorption of metalloporphyrin effectively broadened the absorption edge to visible region. The framework of metalloporphyrin could ensure the high dispersion of transition metal sites and the esterification effect could obtain tight intramolecular interfaces, which further promoted the separation of electrons and holes.

Figure 11.
Schematic diagram of the tunable selectivity of syngas from photocatalytic CO2 reduction by CeO2/LDH [94].
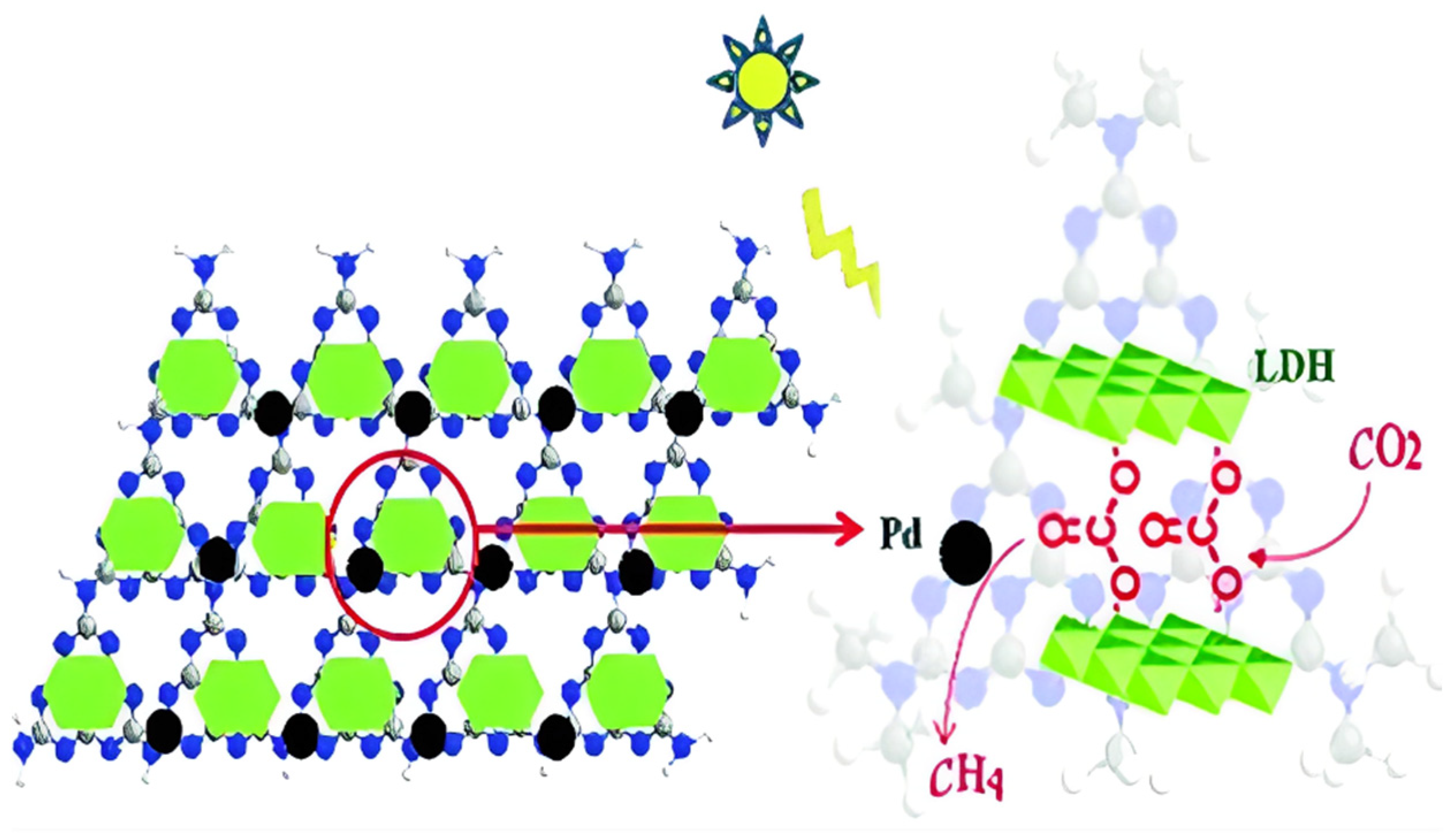
In addition to the loading of metal components, the nonmetal materials are also reported to improve the photocatalytic activity of MgAl-LDH. Hong et al. [73] reported the self-assembling catalyst constructed by carbon nitride (C3N4) and MgAl-LDH for photocatalytic CO2 reduction under UV light. In the presence of Pd cocatalyst, the CH4 yield of C3N4/MgAl-LDH was 0.15 μmol·h−1, but the catalytic activity mainly came from C3N4, and the role of MgAl LDH is mainly to strengthen CO2 adsorption and activation. As shown in Figure 12, the enriched CO2 in the form of CO32− in the interlayer could be reduced easily to CH4 by photogenerated electrons from C3N4 at Pd active sites. The supported active components over MgAl-LDH always provide the visible light absorption ability and promote the separation of charge carriers, while MgAl-LDH always provide the considerable CO2 adsorption performance.

Figure 12.
Schematic diagram of the self-assembly of carbon nitride (C3N4) and MgAl-LDH for CH4 production with Pd as cocatalyst [73].
4.4. MgAl-LDH as Precursor for Catalyst Preparation
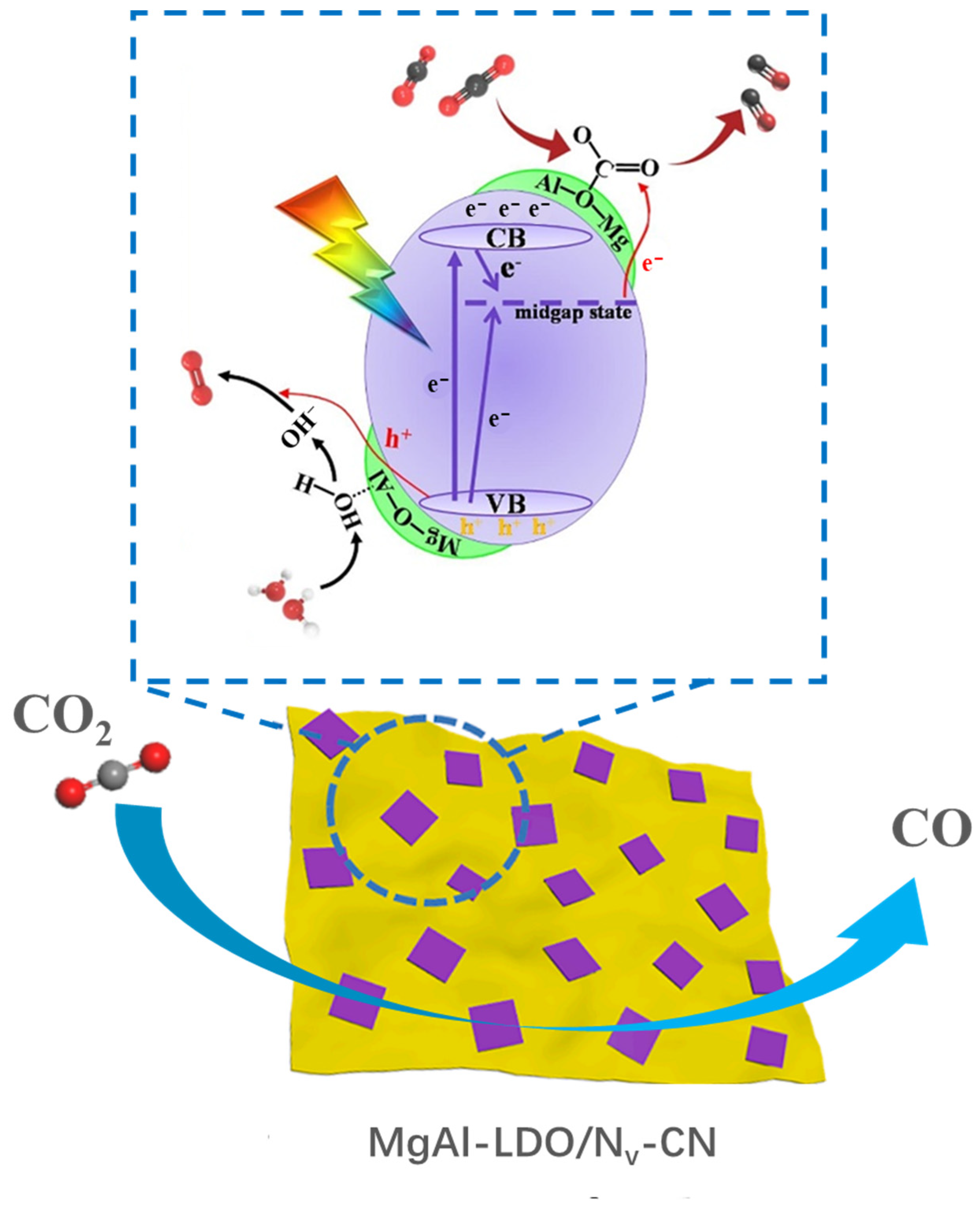
LDO (layer double oxide), which is derived from LDHs precursor, is the mixed metal oxide with high dispersibility of metal sites and high surface area. The component, morphology and surface sites can be adjusted expediently by changing the metal cations of LDHs [113]. As the product after calcination, MgAl-LDO retains the highly dispersed metal sites and provides abundant surface basic or acid sites (OH−, Mg2+-O2− pairs, Al3+-O2− pairs) derived from the diffusion of Al3+ into MgO lattice [114]. The Lewis basic sites are beneficial to adsorb CO2 and form carbonates and bicarbonates, while the Lewis acid sites are beneficial to dissociate H2O and provide abundant protons [100,115]. Chong et al. [96] prepared MgAl-LDO/TiO2 by an in situ deposition method and then Pt cocatalyst was loaded on the surface of catalysts by an in situ photo deposition method for photocatalytic CO2 reduction in the present of water vapor. CO and CH4 evolution rates of Pt/MgAl-LDO/TiO2 were 0.030 and 0.046 μmol·h−1 under UV light, which were 2 and 11 times than those of Pt/TiO2, respectively. The deposition of MgAl-LDO had a negligible effect on the light absorption of TiO2 but changed CO2 adsorption states and the adsorbed species. FTIR spectra indicated that the Lewis basic sites and Lewis acid sites enhanced CO2 adsorption and H2O dissociation. The recombination of electrons and holes was inhibited due to the formation of oxygen vacancy derived from the interaction between MgAl-LDO and TiO2, and Pt cocatalyst effectively promoted the transfer of photogenerated electrons. Song et al. [97] prepared the composite catalyst between MgAl-LDO and nitrogen-deficient g-C3N4 (MgAl-LDO/Nv-CN) by an in situ deposition and calcination transformation method for photoreduction of carbon dioxide. 10% MgAl-LDO/Nv-CN exhibited the highest CO evolution rate (20.47 µmol·g−1·h−1) under visible light, which was 6 times that of CN (3.38 µmol·g−1·h−1) and 3 times that of Nv-CN (5.71 µmol·g−1·h−1). The introduction of MgAl-LDO on Nv-CN led to a slight blue-shift of absorption edge and the bandgap increased from 2.38 eV to 2.42 eV. As shown in Figure 13, Lewis base/acid sites provided the targeting activation for CO2 and H2O molecules. The introduction of nitrogen defects and the interfacial interaction between MgAl-LDO and Nv-CN facilitated the charge transfer and inhibited the recombination of photogenerated electrons and holes.

Figure 13.
Schematic diagram of surface Lewis base/acid and nitrogen defect in MgAl-LDO/Nv-CN for CO2 photoreduction [97].
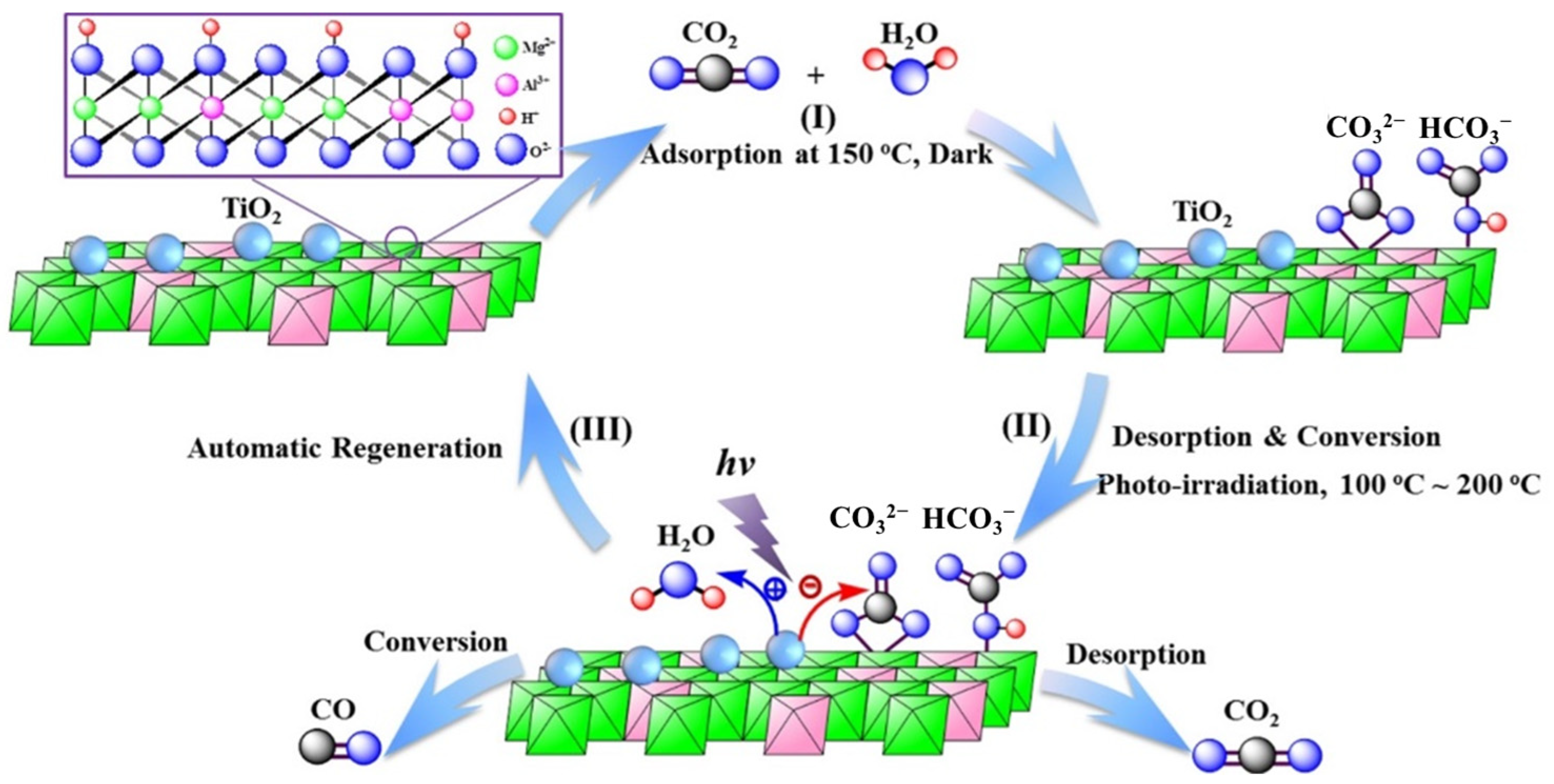
The introduction of active metal components into LDH precursors to prepare the supported metal catalysts is an efficient method to improve the photocatalytic activity. Due to the uniform and ordered distribution of metal cations in the layer structure of LDHs, active metal components will be highly dispersed on the surface of catalysts. The strong interaction between active metal components and supports will be constructed and the confined effect will prevent the sintering and aggregation of active metal components. The memory effect of LDHs can be used for the loading of active metal components with LDHs as the precursor. Zhao et al. [90] prepared Ti-embedded MgAl-LDH for CO2 photoreduction by redispersing calcined MgAlTi-LDH in water to achieve the structural reconstruction. The sample calcined at 400 °C exhibited the increased photocatalytic activity under UV light compared with initial MgAl-LDH and the catalytic activity was closely related to the crystallinity and specific surface areas. The reduction treatment of LDH precursors can be used to prepare the supported metal catalysts. Li et al. [98] prepared Fe-based catalysts by reducing MgFeAl-LDH in the H2/Ar atmosphere for direct photohydrogenation of CO2. The catalyst reduced at 500 °C (Fe-500) exhibited high CO2 conversion (50.1%) and significant C2+ product selectivity (52.9%) in the photohydrogenation reaction. Fe-500 consisted of Fe0 nanoparticles and FeOx supported on MgO-Al2O3. The Fe species suppressed the hydrogenation of -CH2 and -CH3 intermediates and promoted the coupling of C-C bonds, while MgO strengthened the adsorption of CO2 molecules. The supported metal catalysts over MgAl-LDO can be used for the integration of CO2 capture and photocatalytic conversion. Liu et al. [99] prepared MgAl-LDO/TiO2 for CO2 capture and photocatalytic conversion simultaneously. As shown in Figure 14, MgAl-LDO/TiO2 adsorbed CO2 at flue gas temperature, while then the desorption of gas-phase CO2 and the conversion of adsorbed species to CO under UV light took place concurrently at the range of 100–200 °C. The hybrid material can be regenerated automatically for the next cycle. The incorporation of MgAl-LDO and TiO2 enhanced CO2 capture capacity and the sample containing Ti atoms of 43% (MgAl/Ti43) exhibited the capacity of 0.648 mmol/g after 2 h, which was 24 times that of TiO2 and 1.4 times that of MgAl-LDO. There is a trade-off between CO2 capture and CO2 conversion capability, while MgAl/Ti43 exhibited good CO2 capture capacity and conversion efficiency (15.3%) as well.

Figure 14.
Schematic diagram of the integrated CO2 capture and photocatalytic conversion over MgAl-LDO/TiO2 [99].
In addition, the morphology of LDO can be designed by inheriting layer structure of LDHs precursor, which can be applied to develop the catalysts with special morphology [116,117]. Zhao et al. [100] prepared MgAl-LDO-grafted TiO2 cuboids by hydrothermal and coprecipitation methods. As shown in Figure 15, the SEM images showed that the morphology of samples composed of MgAl-LDO grafted on TiO2 cuboids and the platelet shape of MgAl-LDO was almost the same as MgAl-LDH precursor. The graft of MgAl-LDO on TiO2 cuboids did not significantly improve the photocatalytic activity compared with bare TiO2 due to the weak CO2 adsorption of MgAl-LDO at low temperature. In the activity test at 150 °C under UV light, 10% MgAl-LDO/TiO2 exhibited the increased activity with CO production of 4.3 µmol·g−1·h−1, which was 6.1 times that of bare TiO2. The photo-induced electrons on TiO2 transferred to the CO2 adsorption sites at the interfaces and promoted the reduction reaction. The high loading of MgAl-LDO on TiO2 cuboids did not improve the photocatalytic activity at 150 °C, which was ascribed to the weak contact between TiO2 and adsorbed CO2 and the limited light absorption due to the complete covering of TiO2 by MgAl-LDO. In summary, the properties of LDHs endow the derived catalysts the adjustable composition and morphology, excellent CO2 adsorption capacity, highly dispersed metal sites and large surface area, which ensure the good catalytic activity.

Figure 15.
SEM images of MgAl-LDO/TiO2 catalysts: (a,b) 8% MgAl-LDO/TiO2, (c,d) 10% MgAl-LDO/TiO2, and (e,f) 12% MgAl-LDO/TiO2 [100].
4.5. Construction of Heterojunction
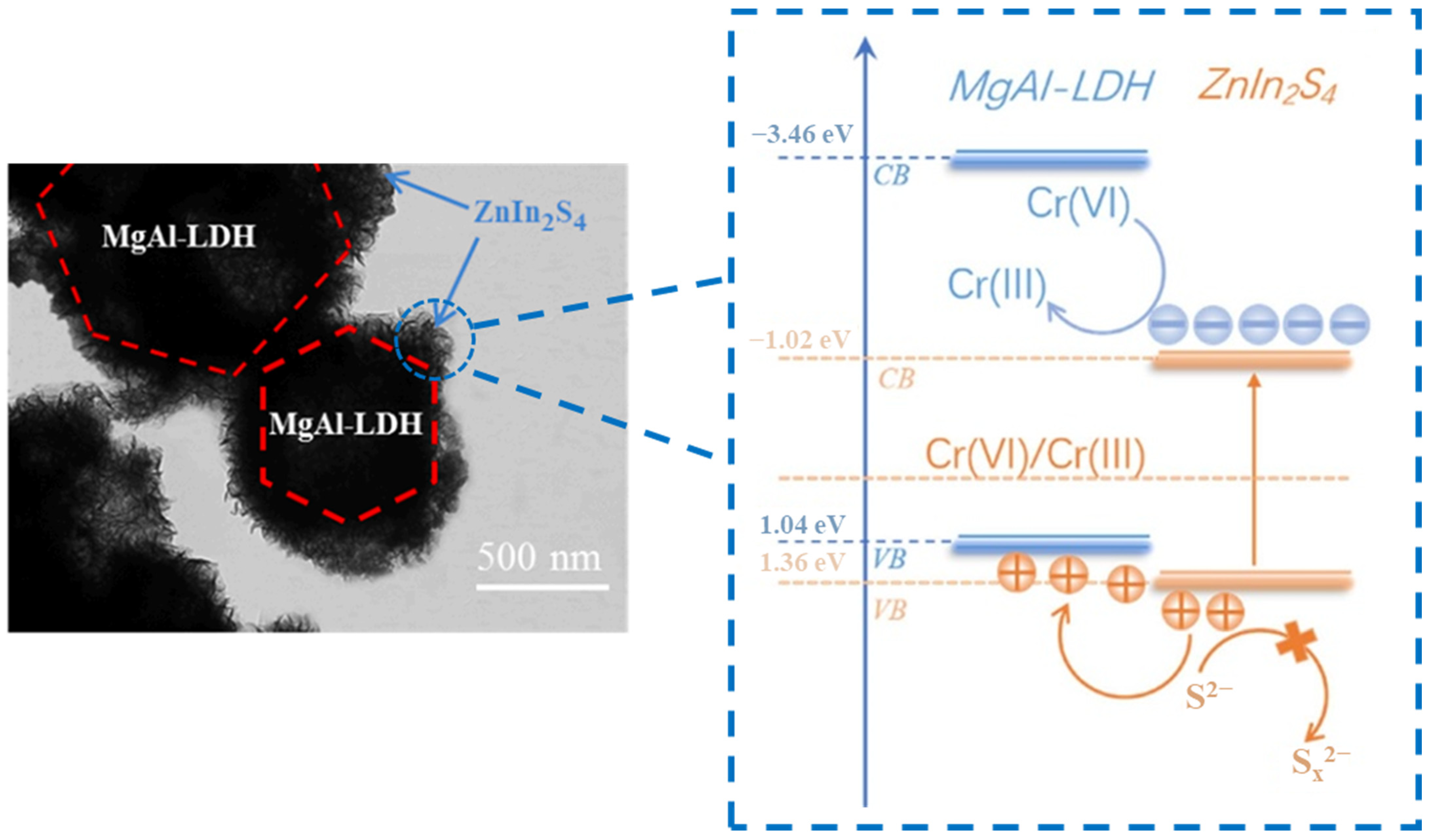
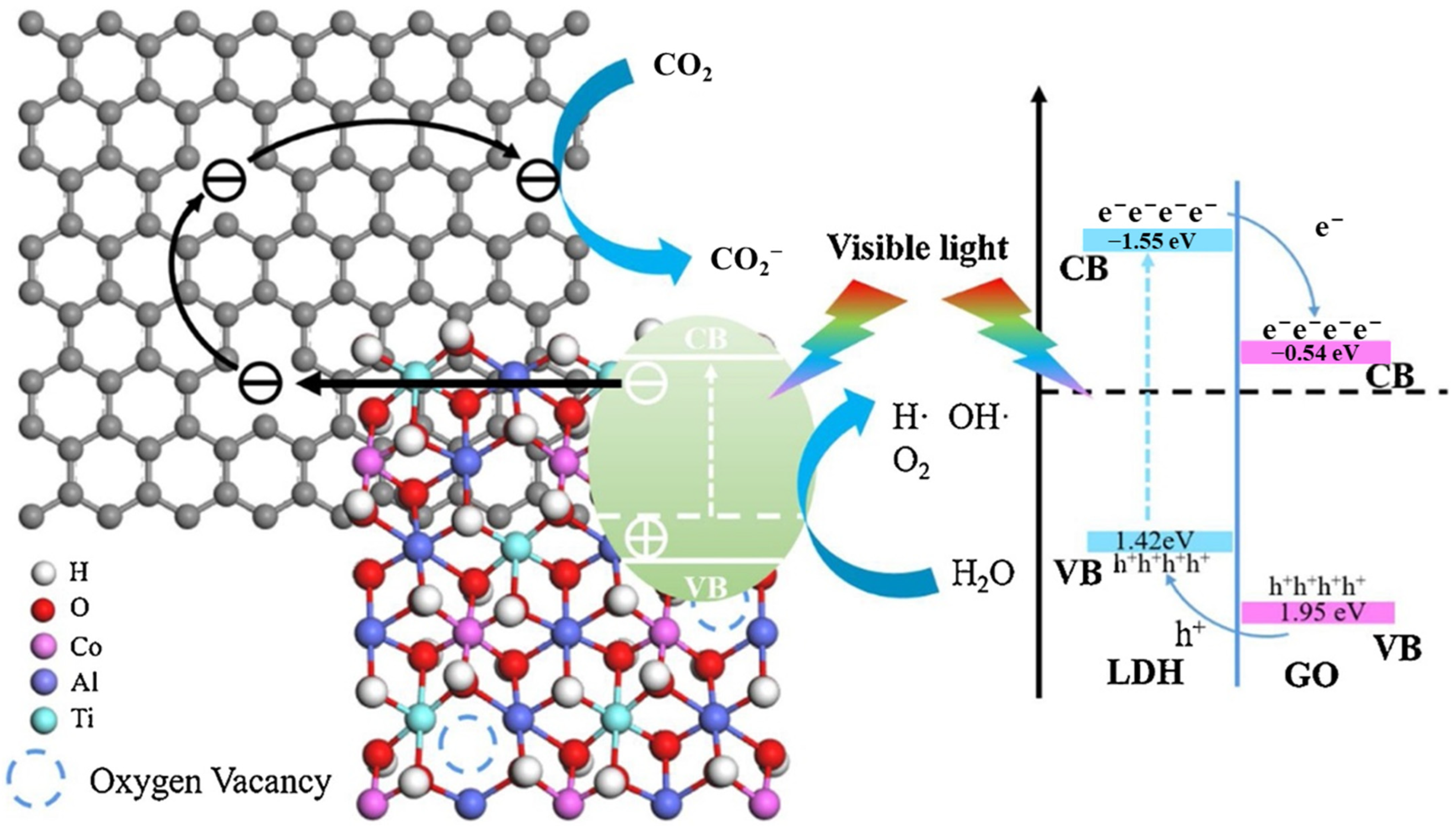
The separation efficiency of photogenerated charge can be effectively improved and the light absorption can be widened by constructing heterojunctions between LDHs and other semiconductors with matched band structures [118]. Studies on LDH-based heterojunctions, including Type-II [119], Z-scheme [120] and S-scheme [121] heterojunctions, have been widely reported for CO2 photoreduction. However, the heterojunctions constructed by MgAl-LDH are rarely reported for CO2 photoreduction due to the wide bandgap and it is difficult to form a stagger band structure. Yang et al. [122] prepared ZnIn2S4/MgAl-LDH heterojunction for photocatalytic Cr (VI) reduction and hydrogen evolution. MgAl-LDH promoted the expose of high-active (001) facets on ZnIn2S4 and acted as a hole storage layer, which realized the spatial separation of electrons and holes. As shown in Figure 16, MgAl-LDH cannot be excited under visible light and the holes in the VB of ZnIn2S4 transferred to the VB of MgAl-LDH to realize the separation of electrons and holes. Due to the wide band of MgAl-LDH, the other semiconductor should possess more positive VB or more negative CB to form stagger band structure, which is strict for the selectivity of semiconductors. Therefore, it is important to improve the wide bandgap of MgAl LDH and further construct efficient heterojunctions. Wang et al. [101] prepared ultrathin GO/TiMgAl-LDH heterojunction by the electrostatic self-assembly method for CO2 photoreduction. When the proportion of GO was 5%, LDH/5GO exhibited the highest photocatalytic activity under visible light with CH4 and CO evolution rate of 3.8 µmol·g−1·h−1 and 4.6 µmol·g−1·h−1, respectively. The ultrathin TiMgAl-LDH and GO created the unsaturated coordination, and introduced Ti3+-Vo and electron-rich carbon defects. The presence of Ti3+-Vo and GO expanded absorption edge to the visible light region, while the electron-rich carbon defects promoted the adsorption/activation of CO2. As shown in Figure 17, electrons in the CB of the ultrathin TiMgAl-LDH would transfer to carbon defects on GO and react with adsorbed CO2 to form superoxide radicals (CO2−). Simultaneously, the photoinduced holes would concentrate in the VB of TiMgAl-LDH, which achieved the spatial separation of electrons and holes.

Figure 16.
Mechanism schematic diagram of improving catalytic efficiency for ZnIn2S4/MgAl-LDH heterojunction [122].

Figure 17.
Schematic diagram of the proposed charge transfer and separation processes in GO/TiMgAl-LDH heterojunction for CO2 photoreduction [101].
In summary, construction of heterojunctions has been widely considered as an efficient method to promote the spatial separation of electrons and holes in LDHs, but MgAl-LDH is rarely selected to construct heterojunctions with other semiconductors due to the wide bandgap. The reduction of bandgap is of significance to improve visible light absorption and construct heterojunctions for MgAl-LDH.
5. Conclusions and Perspectives
LDHs and LDH-based catalysts have been widely applied in the field of CO2 photoreduction due to the excellent structural and chemical advantages. As the most widely used LDH material, MgAl-LDH has the advantages in CO2 adsorption and low cost, but the poor visible light absorption and fast charge recombination of MgAl-LDH limit its application in CO2 photoreduction. Due to the inherent drawbacks of MgAl-LDH, relatively few studies have focused on the improvement of photocatalytic activity for MgAl-LDH compared with other LDHs. More attention should be given to the modification of MgAl-LDH to promote the practical application of LDH materials in CO2 photoreduction. Although some efforts have been devoted to overcome the drawbacks of MgAl-LDH in visible light absorption and charge separation, there are still some scientific issues that need further exploration. The development perspectives of MgAl-LDH in CO2 photoreduction are as follows.
(1) Monolayer MgAl-LDH is beneficial to improve photocatalytic activity in CO2 photoreduction by exposing as many defect sites and metal sites as possible. Bai et al. [92] developed the separate nucleation and aging steps (SNAS) method for scale-up synthesis of monolayer LDHs and more novel methods with the superior adjustment, convenience and feasibility for large-scale production should be further explored. The structure stability of monolayer LDHs in the process of preservation and reaction for practical application should be considered.
(2) A few studies focused on improving the dispersion of active components over MgAl-LDH by utilizing the confinement effect. For example, the anchoring effect of layer structure and the limited space of interlayer can obtain the extremely dispersed active components, such as single-atom catalysts, which may effectively improve the catalytic activity in CO2 photoreduction. More efforts should be devoted to obtaining the highest catalytic efficiency of active components over MgAl-LDH.
(3) The methods to reduce the bandgap of MgAl-LDH are still limited, and basically rely on element doping. More methods should be developed to regulate the bandgap of MgAl-LDH that further improve visible light absorption and make it suitable to construct heterojunction catalysts.
(4) The heterojunction catalysts based on MgAl-LDH for CO2 photoreduction are still scarce and more efforts should be devoted to exploring the suitable semiconductor materials to construct heterojunction with MgAl-LDH. In the process, experiment and theoretical calculation should be combined to explore the properties of different materials and judge the matching degree of band structure. This can provide guidance for the design and development of heterojunction catalysts based on MgAl-LDH.
(5) In the condition without H2 or at low temperature, it is quite difficult to generate C2 product over LDHs in CO2 photoreduction. Developing new methods to regulate the conversion of CO2 to C2 product is important to produce more valuable industrial products.
In summary, MgAl-LDH possesses many advantages in CO2 photoreduction and has the potential to be widely applied. The application of MgAl-LDH in the field of photocatalysis can be further popularized through solving the above problems in the future.
Funding
This work was supported financially by the National Natural Science Foundation of China (No. 52276112), Key R&D Project (Social Development) in Xuzhou (No. KC21290), Joint Funds of the National Natural Science Foundation of China (No. U20A20302) and Innovative group projects in Hebei Province (No. E2021202006).
Data Availability Statement
No data was reported in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral Reefs under Rapid Climate Change and Ocean Acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Albright, R.; Caldeira, L.; Hosfelt, J.; Kwiatkowski, L.; Maclaren, J.K.; Mason, B.M.; Nebuchina, Y.; Ninokawa, A.; Pongratz, J.; Ricke, K.L.; et al. Reversal of ocean acidification enhances net coral reef calcification. Nature 2016, 531, 362–365. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, J.; Hu, Y.; Sun, J.; Yao, S.; Li, Q.; Li, Z.; Zhou, S.; Liu, W. Eutectic doped Li4SiO4 adsorbents using the optimal dopants for highly efficient CO2 removal. J. Mater. Chem. A 2021, 9, 14309–14318. [Google Scholar] [CrossRef]
- Pacala, S.; Socolow, R. Stabilization Wedges: Solving the Climate Problem for the Next 50 Years with Current Technologies. Science 2004, 305, 968–972. [Google Scholar] [CrossRef]
- Din, I.U.; Shaharun, M.S.; Alotaibi, M.A.; Alharthi, A.I.; Naeem, A. Recent developments on heterogeneous catalytic CO2 reduction to methanol. J. CO2 Util. 2019, 34, 20–33. [Google Scholar] [CrossRef]
- Domínguez-Espíndola, R.B.; Arias, D.M.; Rodríguez-González, C.; Sebastian, P.J. A critical review on advances in TiO2-based photocatalytic systems for CO2 reduction. Appl. Therm. Eng. 2022, 216, 119009. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Xu, M.; Cui, Y.; Ren, W.; Zhang, J.; Zhao, H.; Liang, B. Recent intensification strategies of SnO2-based photocatalysts: A review. Chem. Eng. J. 2022, 427, 131564. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Jung, H.S.; Kang, Y.T. A review: Effect of nanostructures on photocatalytic CO2 conversion over metal oxides and compound semiconductors. J. CO2 Util. 2017, 20, 163–177. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, B.; Yu, J. Graphene-Based Photocatalysts for Solar-Fuel Generation. Angew. Chem. Int. Ed. 2015, 54, 11350–11366. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M. Well-designed 2D/2D Ti3C2TA/R MXene coupled g-C3N4 heterojunction with in-situ growth of anatase/rutile TiO2 nucleates to boost photocatalytic dry-reforming of methane (DRM) for syngas production under visible light. Appl. Catal. B Environ. 2021, 285, 119777. [Google Scholar] [CrossRef]
- Tang, J.-Y.; Guo, R.-T.; Zhou, W.-G.; Huang, C.-Y.; Pan, W.-G. Ball-flower like NiO/g-C3N4 heterojunction for efficient visible light photocatalytic CO2 reduction. Appl. Catal. B Environ. 2018, 237, 802–810. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Wang, M.; Shen, M.; Jin, X.; Tian, J.; Shao, Y.; Zhang, L.; Li, Y.; Shi, J. Exploring the enhancement effects of hetero-metal doping in CeO2 on CO2 photocatalytic reduction performance. Chem. Eng. J. 2022, 427, 130987. [Google Scholar] [CrossRef]
- Hailili, R.; Jacobs, D.L.; Zang, L.; Wang, C. Morphology controlled synthesis of CeTiO4 using molten salts and enhanced photocatalytic activity for CO2 reduction. Appl. Surf. Sci. 2018, 456, 360–368. [Google Scholar] [CrossRef]
- Bie, C.; Zhu, B.; Xu, F.; Zhang, L.; Yu, J. In Situ Grown Monolayer N-Doped Graphene on CdS Hollow Spheres with Seamless Contact for Photocatalytic CO2 Reduction. Adv. Mater. 2019, 31, 1902868. [Google Scholar] [CrossRef]
- Xu, M.; Wei, M. Layered Double Hydroxide-Based Catalysts: Recent Advances in Preparation, Structure, and Applications. Adv. Funct. Mater. 2018, 28, 1802943. [Google Scholar] [CrossRef]
- Bi, Z.-X.; Guo, R.-T.; Hu, X.; Wang, J.; Chen, X.; Pan, W.-G. Research progress on photocatalytic reduction of CO2 based on LDH materials. Nanoscale 2022, 14, 3367–3386. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Oh, J.-M.; Hwang, S.-H.; Choy, J.-H. The effect of synthetic conditions on tailoring the size of hydrotalcite particles. Solid State Ion. 2002, 151, 285–291. [Google Scholar] [CrossRef]
- Fang, D.; Huang, L.; Fan, J.; Xiao, H.; Wu, G.; Wang, Y.; Zeng, Z.; Shen, F.; Deng, S.; Ji, F. New insights into the arrangement pattern of layered double hydroxide nanosheets and their ion-exchange behavior with phosphate. Chem. Eng. J. 2022, 441, 136057. [Google Scholar] [CrossRef]
- Szabados, M.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Pálinkó, I. Structural reconstruction of mechanochemically disordered CaFe-layered double hydroxide. Appl. Clay Sci. 2019, 174, 138–145. [Google Scholar] [CrossRef]
- Valeikiene, L.; Paitian, R.; Grigoraviciute-Puroniene, I.; Ishikawa, K.; Kareiva, A. Transition metal substitution effects in sol-gel derived Mg3-xMx/Al1 (M = Mn, Co, Ni, Cu, Zn) layered double hydroxides. Mater. Chem. Phys. 2019, 237, 121863. [Google Scholar] [CrossRef]
- Lv, S.; Kong, X.; Wang, L.; Zhang, F.; Lei, X. Flame-retardant and smoke-suppressing wood obtained by the in situ growth of a hydrotalcite-like compound on the inner surfaces of vessels. New J. Chem. 2019, 43, 16359–16366. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Zhang, S.-H.; Stewart, P.; Zhu, C.-H.; Liu, W.-J.; Hexemer, A.; Schaible, E.; Wang, C. Thermal stability and thermal aging of poly(vinyl chloride)/MgAl layered double hydroxides composites. Chin. J. Polym. Sci. 2016, 34, 542–551. [Google Scholar] [CrossRef]
- Li, T.; Hao, X.; Bai, S.; Zhao, Y.; Song, Y.-F. Controllable synthesis and scale-up production prospect of monolayer layered double hydroxide nanosheets. Acta Phys. Chim. Sin. 2020, 36, 1912005–1912021. [Google Scholar]
- Evans, D.G.; Duan, X. Preparation of layered double hydroxides and their applications as additives in polymers, as precursors to magnetic materials and in biology and medicine. Chem. Commun. 2006, 5, 485–496. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, F.; Zhang, R.; Evans, D.G.; Duan, X. Preparation of Layered Double-Hydroxide Nanomaterials with a Uniform Crystallite Size Using a New Method Involving Separate Nucleation and Aging Steps. Chem. Mater. 2002, 14, 4286–4291. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Wei, J.-J.; Zeng, G.-M.; Zhang, H.-Q.; Tan, X.-F.; Ma, C.; Li, X.-C.; Li, Z.-H.; Zhang, C. A review on strategies to LDH-based materials to improve adsorption capacity and photoreduction efficiency for CO2. Coord. Chem. Rev. 2019, 386, 154–182. [Google Scholar] [CrossRef]
- Jerome, M.P.; Alahmad, F.A.; Salem, M.T.; Tahir, M. Layered double hydroxide (LDH) nanomaterials with engineering aspects for photocatalytic CO2 conversion to energy efficient fuels: Fundamentals, recent advances, and challenges. J. Environ. Chem. Eng. 2022, 10, 108151. [Google Scholar] [CrossRef]
- Bian, X.; Zhang, S.; Zhao, Y.; Shi, R.; Zhang, T. Layered double hydroxide-based photocatalytic materials toward renewable solar fuels production. InfoMat 2021, 3, 719–738. [Google Scholar] [CrossRef]
- Dewangan, N.; Hui, W.M.; Jayaprakash, S.; Bawah, A.-R.; Poerjoto, A.J.; Jie, T.; Jangam, A.; Hidajat, K.; Kawi, S. Recent progress on layered double hydroxide (LDH) derived metal-based catalysts for CO2 conversion to valuable chemicals. Catal. Today 2020, 356, 490–513. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Gong, J. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, Z.; Tong, J.; Yang, M.; Jiang, Z.; Li, C. Direct thermolysis of CO2 into CO and O2. Chem. Commun. 2017, 53, 1188–1191. [Google Scholar] [CrossRef]
- Zhang, K.; Harvey, A.P. CO2 decomposition to CO in the presence of up to 50% O2 using a non-thermal plasma at atmospheric temperature and pressure. Chem. Eng. J. 2021, 405, 126625. [Google Scholar] [CrossRef]
- Kattel, S.; Ramírez, P.J.; Chen, J.G.; Rodriguez, J.A.; Liu, P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 2017, 355, 1296–1299. [Google Scholar] [CrossRef]
- Xu, M.; Yu, D.; Yao, H.; Liu, X.; Qiao, Y. Coal combustion-generated aerosols: Formation and properties. Proc. Combust. Inst. 2011, 33, 1681–1697. [Google Scholar] [CrossRef]
- Yu, D.; Xu, M.; Yao, H.; Sui, J.; Liu, X.; Yu, Y.; Cao, Q. Use of elemental size distributions in identifying particle formation modes. Proc. Combust. Inst. 2007, 31, 1921–1928. [Google Scholar] [CrossRef]
- Guo, Z.; Yu, F.; Yang, Y.; Leung, C.-F.; Ng, S.-M.; Ko, C.-C.; Cometto, C.; Lau, T.-C.; Robert, M. Photocatalytic Conversion of CO2 to CO by a Copper(II) Quaterpyridine Complex. ChemSusChem 2017, 10, 4009–4013. [Google Scholar] [CrossRef]
- Jiang, D.; Zhou, Y.; Zhang, Q.; Song, Q.; Zhou, C.; Shi, X.; Li, D. Synergistic Integration of AuCu Co-Catalyst with Oxygen Vacancies on TiO2 for Efficient Photocatalytic Conversion of CO2 to CH4. ACS Appl. Mater. Interfaces 2021, 13, 46772–46782. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, R.K.; Ram, K.; Aguiar, A.; Koh, J.; Sobral, A.J.F.N. Graphene oxide modified cobalt metallated porphyrin photocatalyst for conversion of formic acid from carbon dioxide. J. CO2 Util. 2018, 27, 107–114. [Google Scholar] [CrossRef]
- Nakata, K.; Ozaki, T.; Terashima, C.; Fujishima, A.; Einaga, Y. High-Yield Electrochemical Production of Formaldehyde from CO2 and Seawater. Angew. Chem. Int. Ed. 2014, 53, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.; Ahmad, M.; Zhao, Z.-P. Rapid and highly selective conversion of CO2 to methanol by heterometallic porous ZIF-8. J. CO2 Util. 2022, 64, 102172. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Hou, W.; Hung, W.H.; Pavaskar, P.; Goeppert, A.; Aykol, M.; Cronin, S.B. Photocatalytic Conversion of CO2 to Hydrocarbon Fuels via Plasmon-Enhanced Absorption and Metallic Interband Transitions. ACS Catal. 2011, 1, 929–936. [Google Scholar] [CrossRef]
- Zhang, K.-L.; Liu, C.-M.; Huang, F.-Q.; Zheng, C.; Wang, W.-D. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. Appl. Catal. B Environ. 2006, 68, 125–129. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic Conversion of CO2 into Renewable Hydrocarbon Fuels: State-of-the-Art Accomplishment, Challenges, and Prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Zhou, Z.; Deng, L.; Liu, L.; Xu, M. Surface defects introduced by metal doping into layered double hydroxide for CO2 photoreduction: The effect of metal species in light absorption, charge transfer and CO2 reduction. Chem. Eng. J. 2022, 442, 136148. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Q.; Shi, X.; Song, Q.; Zhou, C.; Jiang, D. Photocatalytic reduction of CO2 into CH4 over Ru-doped TiO2: Synergy of Ru and oxygen vacancies. J. Colloid Interface Sci. 2022, 608, 2809–2819. [Google Scholar] [CrossRef]
- Han, B.; Ou, X.; Deng, Z.; Song, Y.; Tian, C.; Deng, H.; Xu, Y.-J.; Lin, Z. Nickel Metal–Organic Framework Monolayers for Photoreduction of Diluted CO2: Metal-Node-Dependent Activity and Selectivity. Angew. Chem. Int. Ed. 2018, 57, 16811–16815. [Google Scholar] [CrossRef]
- Izumi, Y. Recent advances in the photocatalytic conversion of carbon dioxide to fuels with water and/or hydrogen using solar energy and beyond. Coord. Chem. Rev. 2013, 257, 171–186. [Google Scholar] [CrossRef]
- Morikawa, M.; Ogura, Y.; Ahmed, N.; Kawamura, S.; Mikami, G.; Okamoto, S.; Izumi, Y. Photocatalytic conversion of carbon dioxide into methanol in reverse fuel cells with tungsten oxide and layered double hydroxide photocatalysts for solar fuel generation. Catal. Sci. Technol. 2014, 4, 1644–1651. [Google Scholar] [CrossRef]
- Miao, Y.-F.; Guo, R.-T.; Gu, J.-W.; Liu, Y.-Z.; Wu, G.-L.; Duan, C.-P.; Pan, W.-G. Z-Scheme Bi/Bi2O2CO3/Layered Double-Hydroxide Nanosheet Heterojunctions for Photocatalytic CO2 Reduction under Visible Light. ACS Appl. Nano Mater. 2021, 4, 4902–4911. [Google Scholar] [CrossRef]
- Khan, A.I.; O’Hare, D. Intercalation chemistry of layered double hydroxides: Recent developments and applications. J. Mater. Chem. 2002, 12, 3191–3198. [Google Scholar] [CrossRef]
- Deng, L.; Liu, X.; Xu, J.; Zhou, Z.; Feng, S.; Wang, Z.; Xu, M. Transfer hydrogenation of CO2 into formaldehyde from aqueous glycerol heterogeneously catalyzed by Ru bound to LDH. Chem. Commun. 2021, 57, 5167–5170. [Google Scholar] [CrossRef]
- Iglesias, A.H.; Ferreira, O.P.; Gouveia, D.X.; Souza Filho, A.G.; de Paiva, J.A.C.; Mendes Filho, J.; Alves, O.L. Structural and thermal properties of Co–Cu–Fe hydrotalcite-like compounds. J. Solid State Chem. 2005, 178, 142–152. [Google Scholar] [CrossRef]
- Xu, S.-M.; Pan, T.; Dou, Y.-B.; Yan, H.; Zhang, S.-T.; Ning, F.-Y.; Shi, W.-Y.; Wei, M. Theoretical and Experimental Study on MIIMIII-Layered Double Hydroxides as Efficient Photocatalysts toward Oxygen Evolution from Water. J. Phys. Chem. C 2015, 119, 18823–18834. [Google Scholar] [CrossRef]
- Miyata, S. Anion-Exchange Properties of Hydrotalcite-Like Compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Weiyang, L.; Ji’an, S.; Yuyuan, Y.; Miao, D.; Qiang, Z. Morphology control of layered double hydroxide and its application in water remediation. Prog. Chem. 2021, 32, 2049. [Google Scholar]
- Boumeriame, H.; Da Silva, E.S.; Cherevan, A.S.; Chafik, T.; Faria, J.L.; Eder, D. Layered double hydroxide (LDH)-based materials: A mini-review on strategies to improve the performance for photocatalytic water splitting. J. Energy Chem. 2022, 64, 406–431. [Google Scholar] [CrossRef]
- Wang, R.; Qiu, Z.; Wan, S.; Wang, Y.; Liu, Q.; Ding, J.; Zhong, Q. Insight into mechanism of divalent metal cations with different d-bands classification in layered double hydroxides for light-driven CO2 reduction. Chem. Eng. J. 2022, 427, 130863. [Google Scholar] [CrossRef]
- Xiong, X.; Zhao, Y.; Shi, R.; Yin, W.; Zhao, Y.; Waterhouse, G.I.N.; Zhang, T. Selective photocatalytic CO2 reduction over Zn-based layered double hydroxides containing tri or tetravalent metals. Sci. Bull. 2020, 65, 987–994. [Google Scholar] [CrossRef]
- Tao, X.; Han, Y.; Sun, C.; Huang, L.; Xu, D. Plasma modification of NiAlCe–LDH as improved photocatalyst for organic dye wastewater degradation. Appl. Clay Sci. 2019, 172, 75–79. [Google Scholar] [CrossRef]
- Mori, K.; Taga, T.; Yamashita, H. Isolated Single-Atomic Ru Catalyst Bound on a Layered Double Hydroxide for Hydrogenation of CO2 to Formic Acid. ACS Catal. 2017, 7, 3147–3151. [Google Scholar] [CrossRef]
- Hirata, N.; Tadanaga, K.; Tatsumisago, M. Photocatalytic O2 evolution from water over Zn–Cr layered double hydroxides intercalated with inorganic anions. Mater. Res. Bull. 2015, 62, 1–4. [Google Scholar] [CrossRef]
- Yan, K.; Liu, Y.; Lu, Y.; Chai, J.; Sun, L. Catalytic application of layered double hydroxide-derived catalysts for the conversion of biomass-derived molecules. Catal. Sci. Technol. 2017, 7, 1622–1645. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Zhou, Z.; Deng, L.; Liu, L.; Xu, M. Platinum Nanoparticles with Low Content and High Dispersion over Exfoliated Layered Double Hydroxide for Photocatalytic CO2 Reduction. Energy Fuels 2021, 35, 10820–10831. [Google Scholar] [CrossRef]
- Flores-Flores, M.; Luévano-Hipólito, E.; Martínez, L.M.T.; Morales-Mendoza, G.; Gómez, R. Photocatalytic CO2 conversion by MgAl layered double hydroxides: Effect of Mg2+ precursor and microwave irradiation time. J. Photochem. Photobiol. A Chem. 2018, 363, 68–73. [Google Scholar] [CrossRef]
- Bravo-Suárez, J.J.; Páez-Mozo, E.A.; Ted Oyama, S. Microtextural properties of layered double hydroxides: A theoretical and structural model. Microporous Mesoporous Mater. 2004, 67, 1–17. [Google Scholar] [CrossRef]
- Iguchi, S.; Teramura, K.; Hosokawa, S.; Tanaka, T. Photocatalytic conversion of CO2 in water using fluorinated layered double hydroxides as photocatalysts. Appl. Catal. A Gen. 2016, 521, 160–167. [Google Scholar] [CrossRef]
- Teramura, K.; Iguchi, S.; Mizuno, Y.; Shishido, T.; Tanaka, T. Photocatalytic Conversion of CO2 in Water over Layered Double Hydroxides. Angew. Chem. Int. Ed. 2012, 51, 8008–8011. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, L.; Su, Y.; Shao, D.; Zeng, S.; Wang, W. Photoreduction of carbon dioxide of atmospheric concentration to methane with water over CoAl-layered double hydroxide nanosheets. J. Mater. Chem. A 2018, 6, 8366–8373. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, W.; Wang, Y.; Zhou, T.; Xu, R. Photocatalytic Reduction of Carbon Dioxide over Self-Assembled Carbon Nitride and Layered Double Hydroxide: The Role of Carbon Dioxide Enrichment. ChemCatChem 2014, 6, 2315–2321. [Google Scholar] [CrossRef]
- Iguchi, S.; Teramura, K.; Hosokawa, S.; Tanaka, T. Photocatalytic conversion of CO2 in an aqueous solution using various kinds of layered double hydroxides. Catal. Today 2015, 251, 140–144. [Google Scholar] [CrossRef]
- Saliba, D.; Ezzeddine, A.; Sougrat, R.; Khashab, N.M.; Hmadeh, M.; Al-Ghoul, M. Cadmium–Aluminum Layered Double Hydroxide Microspheres for Photocatalytic CO2 Reduction. ChemSusChem 2016, 9, 800–805. [Google Scholar] [CrossRef]
- Ahmed, N.; Morikawa, M.; Izumi, Y. Photocatalytic conversion of carbon dioxide into methanol using optimized layered double hydroxide catalysts. Catal. Today 2012, 185, 263–269. [Google Scholar] [CrossRef]
- Ren, J.; Ouyang, S.; Xu, H.; Meng, X.; Wang, T.; Wang, D.; Ye, J. Targeting Activation of CO2 and H2 over Ru-Loaded Ultrathin Layered Double Hydroxides to Achieve Efficient Photothermal CO2 Methanation in Flow-Type System. Adv. Energy Mater. 2017, 7, 1601657. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zang, S.-Q.; Lou, X.W. Hierarchical Hollow Heterostructures for Photocatalytic CO2 Reduction and Water Splitting. Small Methods 2020, 4, 1900586. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, S.; Pan, T.; Xu, S.; Zhou, A.; Pu, M.; Yan, H.; Han, J.; Wei, M.; Evans, D.G.; et al. TiO2@Layered Double Hydroxide Core–Shell Nanospheres with Largely Enhanced Photocatalytic Activity Toward O2 Generation. Adv. Funct. Mater. 2015, 25, 2243–2249. [Google Scholar] [CrossRef]
- Zhao, F.; Zhan, G.; Zhou, S.-F. Intercalation of laminar Cu–Al LDHs with molecular TCPP(M) (M = Zn, Co, Ni, and Fe) towards high-performance CO2 hydrogenation catalysts. Nanoscale 2020, 12, 13145–13156. [Google Scholar] [CrossRef]
- Nayak, S.; Mohapatra, L.; Parida, K. Visible light-driven novel g-C3N4/NiFe-LDH composite photocatalyst with enhanced photocatalytic activity towards water oxidation and reduction reaction. J. Mater. Chem. A 2015, 3, 18622–18635. [Google Scholar] [CrossRef]
- Nayak, S.; Parida, K.M. Nanostructured CeO2/MgAl-LDH composite for visible light induced water reduction reaction. Int. J. Hydrogen Energy 2016, 41, 21166–21180. [Google Scholar] [CrossRef]
- Gao, G.; Zhu, Z.; Zheng, J.; Liu, Z.; Wang, Q.; Yan, Y. Ultrathin magnetic Mg-Al LDH photocatalyst for enhanced CO2 reduction: Fabrication and mechanism. J. Colloid Interface Sci. 2019, 555, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, C.; Zhang, Y.; Guo, Z.; Luo, Y.; Mao, C.-J. Engineering ultrafine NiS cocatalysts as active sites to boost photocatalytic hydrogen production of MgAl layered double hydroxide. Appl. Surf. Sci. 2020, 506, 144999. [Google Scholar] [CrossRef]
- Ning, C.; Wang, Z.; Bai, S.; Tan, L.; Dong, H.; Xu, Y.; Hao, X.; Shen, T.; Zhao, J.; Zhao, P.; et al. 650 nm-driven syngas evolution from photocatalytic CO2 reduction over Co-containing ternary layered double hydroxide nanosheets. Chem. Eng. J. 2021, 412, 128362. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; Zhu, Y.; Zhang, F. Experimental and theoretical investigation into the elimination of organic pollutants from solution by layered double hydroxides. Appl. Catal. B Environ. 2013, 140, 241–248. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Chen, F.; Ma, T.; Zhang, T.; Zhang, Y.; Huang, H. Atomic-Level Charge Separation Strategies in Semiconductor-Based Photocatalysts. Adv. Mater. 2021, 33, 2005256. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Liu, L.; Rao, G.; Zhao, C.; Li, Y. CO2 photoreduction with water vapor by Ti-embedded MgAl layered double hydroxides. J. CO2 Util. 2016, 15, 15–23. [Google Scholar] [CrossRef]
- Bai, S.; Wang, Z.; Tan, L.; Waterhouse, G.I.N.; Zhao, Y.; Song, Y.-F. 600 nm Irradiation-Induced Efficient Photocatalytic CO2 Reduction by Ultrathin Layered Double Hydroxide Nanosheets. Ind. Eng. Chem. Res. 2020, 59, 5848–5857. [Google Scholar] [CrossRef]
- Bai, S.; Li, T.; Wang, H.; Tan, L.; Zhao, Y.; Song, Y.-F. Scale-up synthesis of monolayer layered double hydroxide nanosheets via separate nucleation and aging steps method for efficient CO2 photoreduction. Chem. Eng. J. 2021, 419, 129390. [Google Scholar] [CrossRef]
- Iguchi, S.; Hasegawa, Y.; Teramura, K.; Kidera, S.; Kikkawa, S.; Hosokawa, S.; Asakura, H.; Tanaka, T. Drastic improvement in the photocatalytic activity of Ga2O3 modified with Mg–Al layered double hydroxide for the conversion of CO2 in water. Sustain. Energy Fuels 2017, 1, 1740–1747. [Google Scholar] [CrossRef]
- Tan, L.; Peter, K.; Ren, J.; Du, B.; Hao, X.; Zhao, Y.; Song, Y.-F. Photocatalytic syngas synthesis from CO2 and H2O using ultrafine CeO2-decorated layered double hydroxide nanosheets under visible-light up to 600 nm. Front. Chem. Sci. Eng. 2021, 15, 99–108. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Zhou, Z.; Deng, L.; Liu, L.; Xu, M. Visible Light-Driven CO2 Photocatalytic Reduction by Co-porphyrin-Coupled MgAl Layered Double-Hydroxide Composite. Energy Fuels 2021, 35, 16134–16143. [Google Scholar] [CrossRef]
- Chong, R.; Su, C.; Du, Y.; Fan, Y.; Ling, Z.; Chang, Z.; Li, D. Insights into the role of MgAl layered double oxides interlayer in Pt/TiO2 toward photocatalytic CO2 reduction. J. Catal. 2018, 363, 92–101. [Google Scholar] [CrossRef]
- Song, Q.; Zhou, Y.; Hu, J.; Zhou, C.; Shi, X.; Li, D.; Jiang, D. Synergistic effects of surface Lewis Base/Acid and nitrogen defect in MgAl layered double Oxides/Carbon nitride heterojunction for efficient photoreduction of carbon dioxide. Appl. Surf. Sci. 2021, 563, 150369. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Shi, R.; Waterhouse, G.I.N.; Wen, X.-D.; Zhang, T. Fe-Based Catalysts for the Direct Photohydrogenation of CO2 to Value-Added Hydrocarbons. Adv. Energy Mater. 2021, 11, 2002783. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, C.; Xu, J.; Li, Y. Integrated CO2 capture and photocatalytic conversion by a hybrid adsorbent/photocatalyst material. Appl. Catal. B Environ. 2015, 179, 489–499. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Rao, G.; Zhao, H.; Wang, L.; Xu, J.; Li, Y. Synthesis of novel MgAl layered double oxide grafted TiO2 cuboids and their photocatalytic activity on CO2 reduction with water vapor. Catal. Sci. Technol. 2015, 5, 3288–3295. [Google Scholar] [CrossRef]
- Wang, K.; Miao, C.; Liu, Y.; Cai, L.; Jones, W.; Fan, J.; Li, D.; Feng, J. Vacancy enriched ultrathin TiMgAl-layered double hydroxide/graphene oxides composites as highly efficient visible-light catalysts for CO2 reduction. Appl. Catal. B Environ. 2020, 270, 118878. [Google Scholar] [CrossRef]
- Hao, X.; Tan, L.; Xu, Y.; Wang, Z.; Wang, X.; Bai, S.; Ning, C.; Zhao, J.; Zhao, Y.; Song, Y.-F. Engineering Active Ni Sites in Ternary Layered Double Hydroxide Nanosheets for a Highly Selective Photoreduction of CO2 to CH4 under Irradiation above 500 nm. Ind. Eng. Chem. Res. 2020, 59, 3008–3015. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, X.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; O’Hare, D.; Zhang, T. Layered Double Hydroxide Nanostructured Photocatalysts for Renewable Energy Production. Adv. Energy Mater. 2016, 6, 1501974. [Google Scholar] [CrossRef]
- Tan, L.; Xu, S.-M.; Wang, Z.; Xu, Y.; Wang, X.; Hao, X.; Bai, S.; Ning, C.; Wang, Y.; Zhang, W.; et al. Highly Selective Photoreduction of CO2 with Suppressing H2 Evolution over Monolayer Layered Double Hydroxide under Irradiation above 600 nm. Angew. Chem. Int. Ed. 2019, 58, 11860–11867. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Wu, Y.A.; Jacobs, R.M.J.; Warner, J.H.; Williams, G.R.; O’Hare, D. Reverse Micelle Synthesis of Co−Al LDHs: Control of Particle Size and Magnetic Properties. Chem. Mater. 2011, 23, 171–180. [Google Scholar] [CrossRef]
- Lai, T.; Wang, J.; Xiong, W.; Wang, H.; Yang, M.; Li, T.; Kong, X.; Zou, X.; Zhao, Y.; O’Hare, D.; et al. Photocatalytic CO2 reduction and environmental remediation using mineralization of toxic metal cations products. Chem. Eng. Sci. 2022, 257, 117704. [Google Scholar] [CrossRef]
- Chen, W.; Han, B.; Xie, Y.; Liang, S.; Deng, H.; Lin, Z. Ultrathin Co-Co LDHs nanosheets assembled vertically on MXene: 3D nanoarrays for boosted visible-light-driven CO2 reduction. Chem. Eng. J. 2020, 391, 123519. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, A.; Zhou, Z.; Wang, C.; Deng, L.; Liu, L.; Xia, H.; Xu, M. Elemental Mercury Removal from Flue Gas over Silver-Loaded CuS-Wrapped Fe3O4 Sorbent. Energy Fuels 2021, 35, 13975–13983. [Google Scholar] [CrossRef]
- Miao, C.; Hui, T.; Liu, Y.; Feng, J.; Li, D. Pd/MgAl-LDH nanocatalyst with vacancy-rich sandwich structure: Insight into interfacial effect for selective hydrogenation. J. Catal. 2019, 370, 107–117. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Y.; Zou, J.; Li, L.; Yu, Y.; Wu, L. The cooperation effect in the Au–Pd/LDH for promoting photocatalytic selective oxidation of benzyl alcohol. Catal. Sci. Technol. 2018, 8, 268–275. [Google Scholar] [CrossRef]
- Zhu, P.; Gao, M.; Zhang, J.; Wu, Z.; Wang, R.; Wang, Y.; Waclawik, E.R.; Zheng, Z. Synergistic interaction between Ru and MgAl-LDH support for efficient hydrogen transfer reduction of carbonyl compounds under visible light. Appl. Catal. B Environ. 2021, 283, 119640. [Google Scholar] [CrossRef]
- Kawamura, S.; Puscasu, M.C.; Yoshida, Y.; Izumi, Y.; Carja, G. Tailoring assemblies of plasmonic silver/gold and zinc–gallium layered double hydroxides for photocatalytic conversion of carbon dioxide using UV–visible light. Appl. Catal. A Gen. 2015, 504, 238–247. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, S.-M.; Tan, L.; Liu, G.; Shen, T.; Yu, C.; Wang, H.; Tao, Y.; Cao, X.; Zhao, Y.; et al. 600 nm-driven photoreduction of CO2 through the topological transformation of layered double hydroxides nanosheets. Appl. Catal. B Environ. 2020, 270, 118884. [Google Scholar] [CrossRef]
- Li, P.; Yu, Y.; Huang, P.-P.; Liu, H.; Cao, C.-Y.; Song, W.-G. Core–shell structured MgAl-LDO@Al-MS hexagonal nanocomposite: An all inorganic acid–base bifunctional nanoreactor for one-pot cascade reactions. J. Mater. Chem. A 2014, 2, 339–344. [Google Scholar] [CrossRef]
- He, S.; An, Z.; Wei, M.; Evans, D.G.; Duan, X. Layered double hydroxide-based catalysts: Nanostructure design and catalytic performance. Chem. Commun. 2013, 49, 5912–5920. [Google Scholar] [CrossRef]
- Li, C.; Wei, M.; Evans, D.G.; Duan, X. Layered Double Hydroxide-based Nanomaterials as Highly Efficient Catalysts and Adsorbents. Small 2014, 10, 4469–4486. [Google Scholar] [CrossRef]
- Wu, M.J.; Wu, J.Z.; Zhang, J.; Chen, H.; Zhou, J.Z.; Qian, G.R.; Xu, Z.P.; Du, Z.; Rao, Q.L. A review on fabricating heterostructures from layered double hydroxides for enhanced photocatalytic activities. Catal. Sci. Technol. 2018, 8, 1207–1228. [Google Scholar] [CrossRef]
- Miao, Y.-F.; Guo, R.-T.; Gu, J.-W.; Liu, Y.-Z.; Wu, G.-L.; Duan, C.-P.; Zhang, X.-D.; Pan, W.-G. Fabrication of β-In2S3/NiAl-LDH heterojunction photocatalyst with enhanced separation of charge carriers for efficient CO2 photocatalytic reduction. Appl. Surf. Sci. 2020, 527, 146792. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Xiao, T.; Tang, Z.; Shen, J.; Li, H.; Zhou, Y.; Zou, Z. Urchin-like hierarchical CoZnAl-LDH/RGO/g-C3N4 hybrid as a Z-scheme photocatalyst for efficient and selective CO2 reduction. Appl. Catal. B Environ. 2019, 255, 117771. [Google Scholar] [CrossRef]
- Han, X.; Lu, B.; Huang, X.; Liu, C.; Chen, S.; Chen, J.; Zeng, Z.; Deng, S.; Wang, J. Novel p- and n-type S-scheme heterojunction photocatalyst for boosted CO2 photoreduction activity. Appl. Catal. B Environ. 2022, 316, 121587. [Google Scholar] [CrossRef]
- Yang, Z.; Li, S.; Xia, X.; Liu, Y. Hexagonal MgAl-LDH simultaneously facilitated active facet exposure and holes storage over ZnIn2S4/MgAl-LDH heterojunction for boosting photocatalytic activities and anti-photocorrosion. Sep. Purif. Technol. 2022, 300, 121819. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).