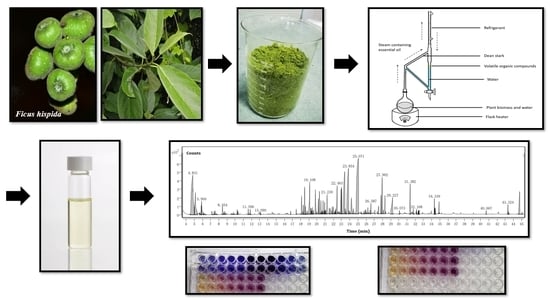
Thermal Treatment (Hydrodistillation) on The Biomass of Ficus hispida L. f.: Volatile Organic Compounds Yield, Phytochemical Composition, and Antioxidant Activity Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals Used and Plant Biomass
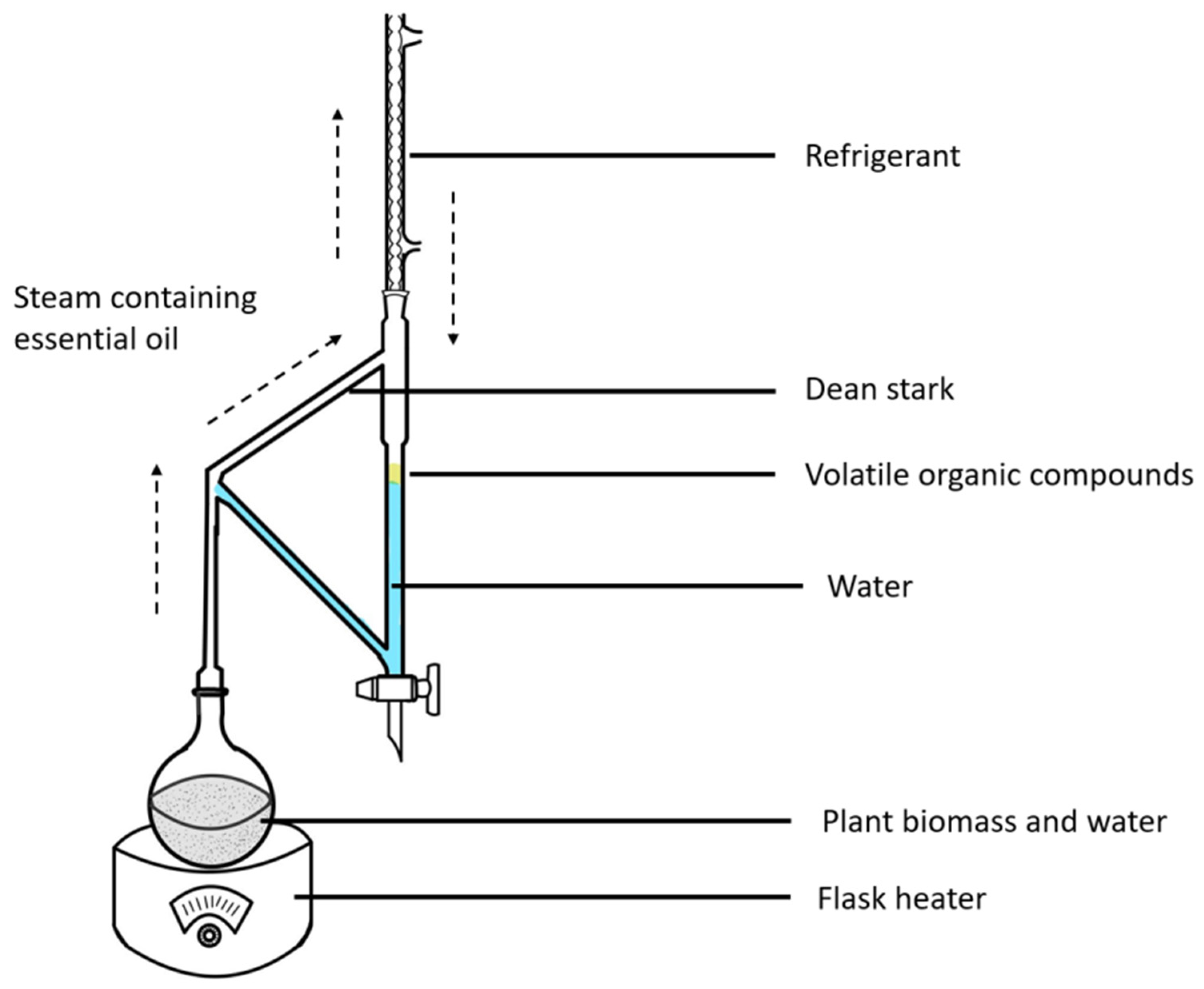
2.2. Thermal Treatment and Essential Oil Extraction
2.3. Essential Oil Chemical Component Acquisition
2.4. Antioxidant Activity
2.4.1. DPPH Assay
2.4.2. ABTS Assay
2.4.3. FRAP Assay
2.5. Statistical Analysis
3. Results and Discussion
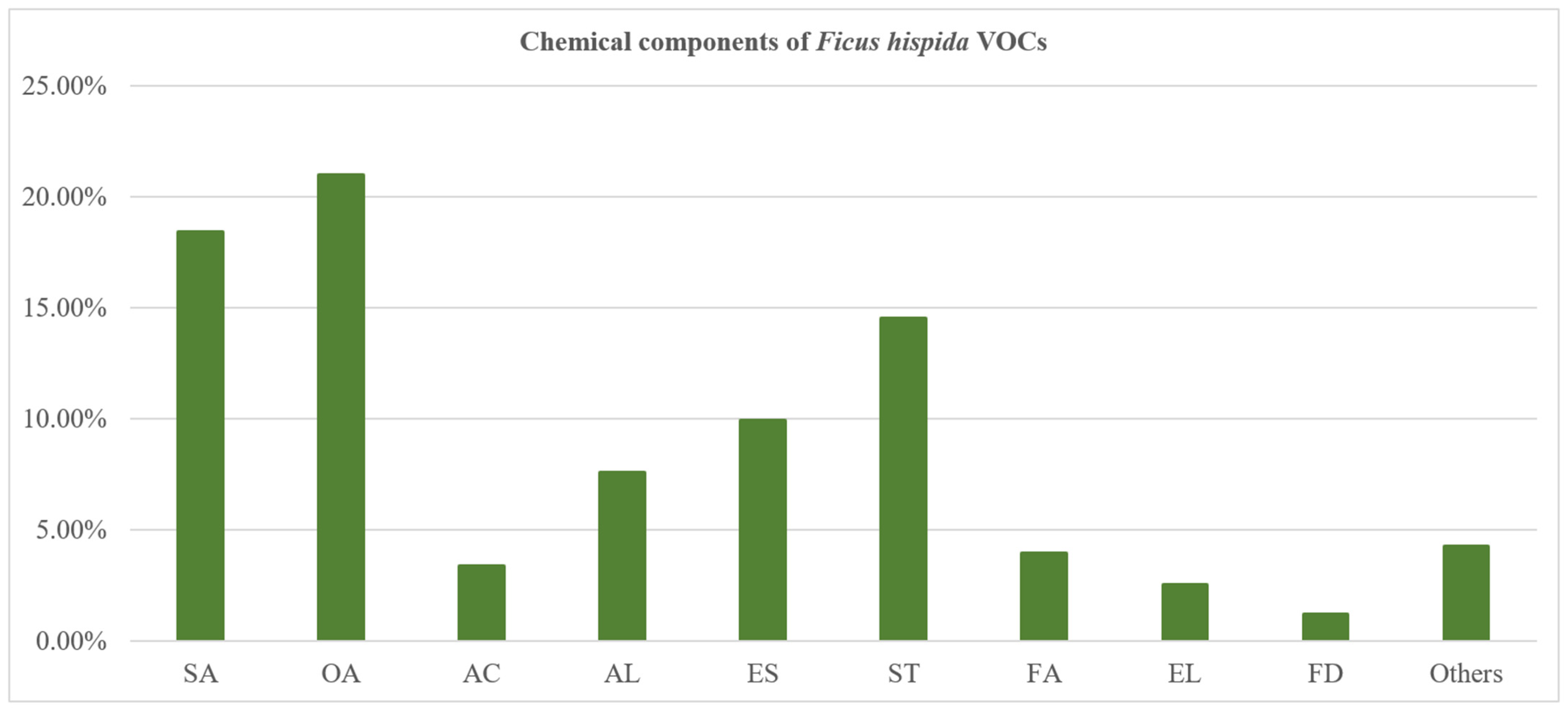
3.1. Essential Oil Yield and Chemical Composition
3.2. Antioxidant Activity
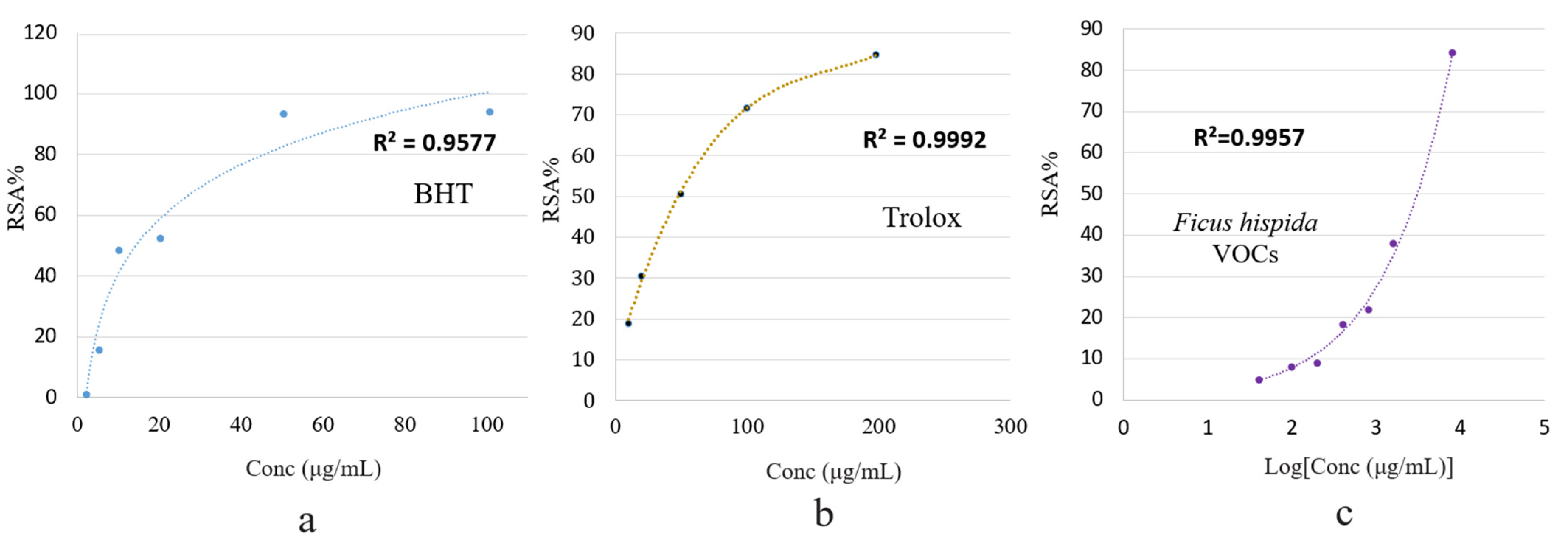
3.2.1. DPPH Assay
3.2.2. ABTS Assay
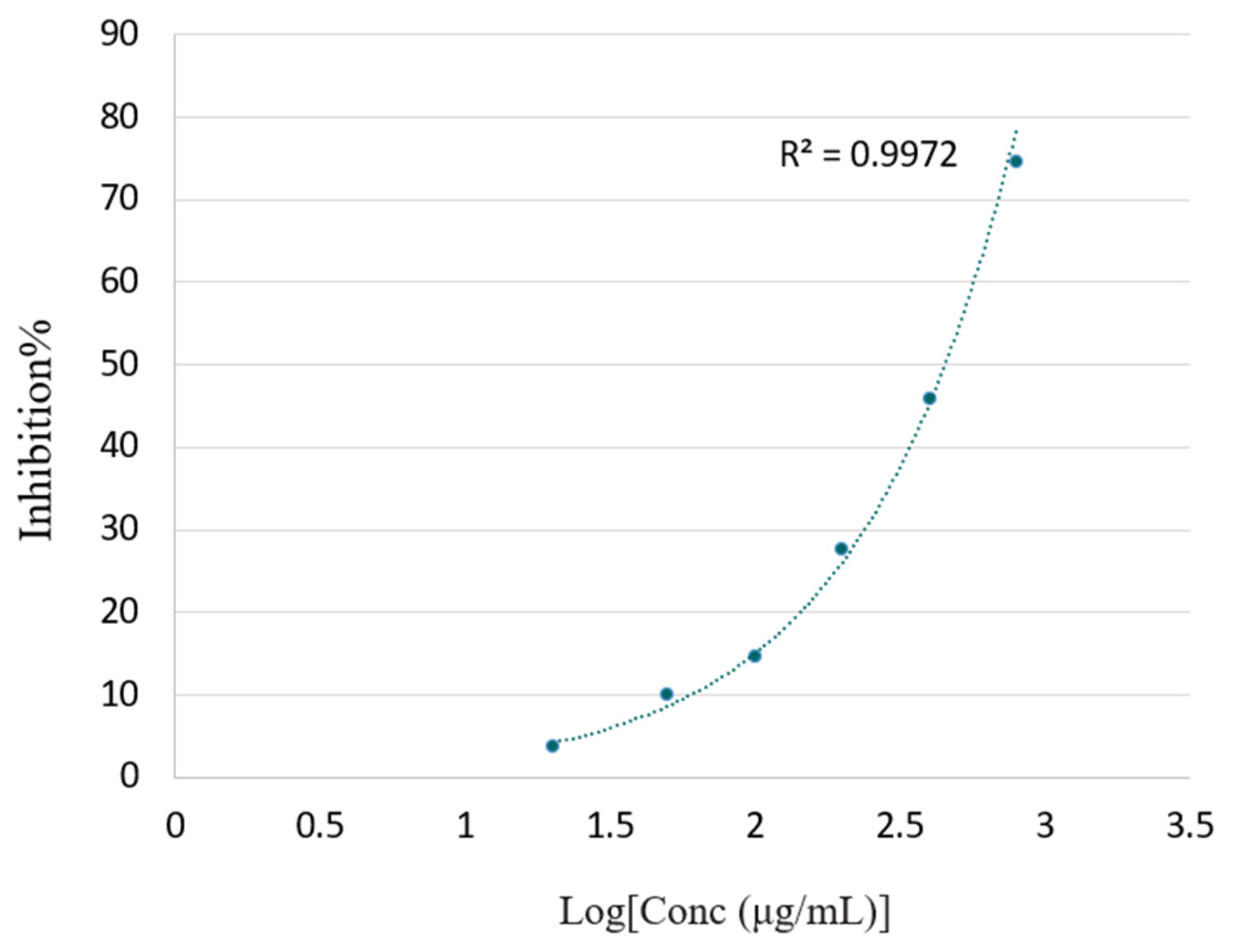
3.2.3. FRAP Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VOC | Volatile Organic Compound |
| F. hispida | Ficus hispida L. |
| GC–MS | Gas chromatography-mass spectrometry |
| GC–FID | Gas chromatography–flame-ionization detection |
| RI | Retention index |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,20-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid |
| FRAP | Ferric reducing antioxidant power |
| TPTZ | 2,4,6-tri-(2-pyridyl)-s-triazin |
| BHT | butylated hydroxytoluene |
| Trolox | 6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid |
References
- Isnugroho, K.; Birawidha, D.C.; Hendronursito, Y. The Biomass Waste Use as a Secondary Energy Source for Metal Foundry Process. CONFAST 2016, 1746, 1–6. [Google Scholar]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.A.; Nabi, M.N.; Van, T.C.; Suara, K.; Jafari, M.; Dowell, A.; Islam, M.A.; Marchese, A.J.; Tryner, J.; Hossain, M.F.; et al. Performance and Combustion Characteristics Analysis of Multi-Cylinder CI Engine Using Essential Oil Blends. Energies 2018, 11, 738. [Google Scholar] [CrossRef]
- Ali, M.; Chaudhary, N. Ficus hispida Linn.: A review of its pharmacognostic and ethnomedicinal properties. Pharmacogn. Rev. 2011, 5, 96–102. [Google Scholar] [PubMed]
- Shahriar, M.; Islam, S.; Parvin, S.; Hoque, S. Thrombolytic activity and antimicrobial properties of Ficus hispida. J. Sci. Res. 2013, 5, 393–397. [Google Scholar] [CrossRef]
- Mandal, S.C.; Kumar, C.K.A. Studies on the anti-diarrhoeal activity of Ficus hispida leaf extract in rats. Fitoterapia 2002, 73, 663–667. [Google Scholar] [CrossRef]
- Salvi, V.; Joshi, Y.; Dhande, S.; Kadam, V. A review on Ficus hispida. Res. J. Pharmacogn. Phytochem. 2013, 5, 149–154. [Google Scholar]
- Okoh, S.O.; Asekun, O.T.; Familoni, O.B.; Afolayan, A.J. Antioxidant and Free Radical Scavenging Capacity of Seed and Shell Essential Oils Extracted from Abrus precatorius (L). Antioxidants 2014, 3, 278–287. [Google Scholar] [CrossRef]
- Millogo-Kone, M.; Lompo, F.; Nacoulma, O. Evaluation of flavonoids, total phenolic contents of P. biglobosa and free radical scavenging and antimicrobial activities. Res. J. Med. Sci. 2009, 3, 70–74. [Google Scholar]
- Ticktin, T. The ecological implications of harvesting non-timber forest products. J. Appl. Ecol. 2004, 41, 11–21. [Google Scholar] [CrossRef]
- Paumgarten, F. The role of non-timber forest products as safety-nets: A review of evidence with a focus on South Africa. GeoJournal 2005, 64, 189–197. [Google Scholar] [CrossRef]
- Song, Q.S.; Yang, D.R.; Zhang, G.M.; Yang, C.R. Volatiles from Ficus hispida and their attractiveness to fig wasps. J. Chem. Eco. 2001, 27, 1929–1942. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Wu, J.J.; Xu, Y.J.; Fu, M.Q.; Xiao, G.S. Effect of Second Cooling on the Chemical Components of Essential Oils from Orange Peel (Citrus sinensis). J. Agric. Food Chem. 2014, 62, 8786–8790. [Google Scholar] [CrossRef]
- Katekar, V.P.; Rao, A.B.; Sardeshpande, V.R. Review of the rose essential oil extraction by hydrodistillation: An investigation for the optimum operating condition for maximum yield. Sustain. Chem. Pharm. 2022, 29, 100783. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH center dot) tests. J. Am. Oil Chem. Soc. 2002, 79, 1191–1195. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Li, X.C.; Lin, J.; Gao, Y.X.; Han, W.J.; Chen, D.F. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem. Cent. J. 2012, 6, 140. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Szafranska, K.; Szewczyk, R.; Janas, K.M. Involvement of melatonin applied to Vigna radiata, L. seeds in plant response to chilling stress. Cent. Eur. J. Biol. 2014, 9, 1117–1126. [Google Scholar] [CrossRef]
- Paw, M.; Begum, T.; Gogoi, R.; Pandey, S.K.; Lal, M. Chemical Composition of Citrus limon L. Burmf Peel Essential Oil from North East India. J. Essent. Oil Bear. Plants 2020, 23, 337–344. [Google Scholar] [CrossRef]
- Usman, L.A.; Ismaeel, R.O. Chemical Composition of Root Essential oil of Peperomia pellucida (L.) Kunth. Grown in Nigeria. J. Essent. Oil Bear. Plants 2020, 23, 628–632. [Google Scholar] [CrossRef]
- Khiya, Z.; Oualcadi, Y.; Zerkani, H.; Gamar, A.; Amine, S.; EL Hamzaoui, N.; Berrekhis, F.; Zair, T.; EL Hilali, F. Chemical Composition and Biological Activities of Pistacia atlantica Desf. Essential Oil from Morocco. J. Essent. Oil Bear. Plants 2021, 24, 254–265. [Google Scholar] [CrossRef]
- Lim, A.C.; Tang, S.G.H.; Zin, N.M.; Maisarah, A.M.; Ariffin, I.A.; Ker, P.J.; Mahlia, T.M.I. Chemical Composition, Antioxidant, Antibacterial, and Antibiofilm Activities of Backhousia citriodora Essential Oil. Molecules 2022, 27, 4895. [Google Scholar] [CrossRef] [PubMed]
- Heikal, A.A.-E.M. Variation in the Essential Oil Content and its Composition in Eucalyptus cinerea Leaves and its Relation to Some Environmental Factors. J. Essent. Oil Bear. Plants 2017, 20, 995–1005. [Google Scholar] [CrossRef]
- Jardak, M.; Elloumi-Mseddi, J.; Aifa, S.; Mnif, S. Chemical composition, anti-biofilm activity and potential cytotoxic effect on cancer cells of Rosmarinus officinalis L. essential oil from Tunisia. Lipids Health Dis. 2017, 16, 190. [Google Scholar] [CrossRef]
- Barra, A. Factors Affecting Chemical Variability of Essential Oils: A Review of Recent Developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; De Moraes, A.A.B.; Da Costa, K.S.; Galúcio, J.M.P.; Taube, P.S.; Costa, C.M.L.; Cruz, J.N.; Andrade, E.H.D.A.; De Faria, L.J.G. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Nadeem, H.R.; Akhtar, S.; Ismail, T.; Qamar, M.; Sestili, P.; Saeed, W.; Azeem, M.; Esatbeyoglu, T. Antioxidant Effect of Ocimum basilicum Essential Oil and Its Effect on Cooking Qualities of Supplemented Chicken Nuggets. Antioxidants 2022, 11, 1882. [Google Scholar] [CrossRef]
- Minchán-Herrera, P.; Ybañez-Julca, R.O.; Quispe-Díaz, I.M.; Venegas-Casanova, E.A.; Jara-Aguilar, R.; Salas, F.; Zevallos-Escobar, L.; Yáñez, O.; Pino-Rios, R.; Calderon, P.B.; et al. Valeriana pilosa Roots Essential Oil: Chemical Composition, Antioxidant Activities, and Molecular Docking Studies on Enzymes Involved in Redox Biological Processes. Antioxidants 2022, 11, 1337. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Tian, X.; Schaich, K.M. Effects of molecular structure on kinetics and dynamics of the Trolox Equivalent Antioxidant Capacity assay with ABTS+•. J. Agric. Food Chem. 2013, 61, 5511–5519. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Cao, R.L. In vivo total antioxidant capacity: Comparison of different analytical methods. Free Radical Biol. Med. 1999, 27, 1173–1181. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Kotan, R.; Mavi, A.; Cakir, A.; Ala, A.; Yildirim, A. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum, and Artemisia spicigera essential oils. J. Agric. Food Chem. 2005, 53, 9452–9458. [Google Scholar]
- Mighri, H.; Hajlaoui, H.; Akrout, A.; Najjaa, H.; Neffati, M. Antimicrobial and antioxidant activities of Artemisia herba-alba essential oil cultivated in Tunisian arid zone. CR Chim. 2010, 13, 380–386. [Google Scholar] [CrossRef]
- Lopes-Lutz, D.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 2008, 69, 1732–1738. [Google Scholar] [CrossRef]
- Rashid, S.; Ahmad, M.; Amin, W.; Ahmad, B. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013, 138, 693–700. [Google Scholar] [CrossRef]
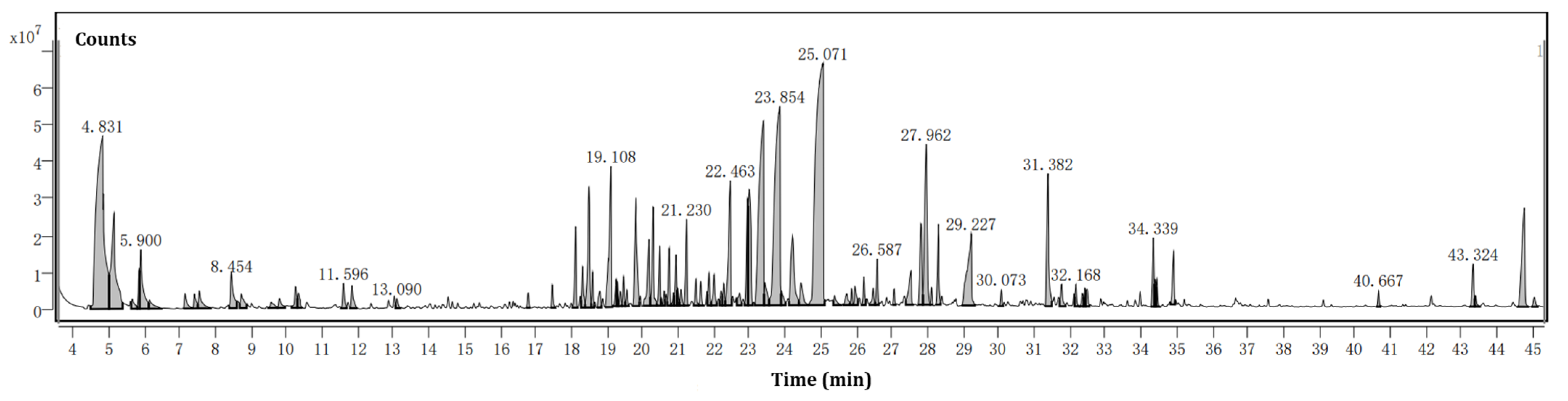
| No. | Molecular Formula | RT a | Components b | CAS ID | Percentage (%) c |
|---|---|---|---|---|---|
| 1 | C6H10O | 4.831 | (E)-2-Hexenal | 6728-26-3 | 11.15% |
| 2 | C6H14O | 5.153 | 1-Hexanol | 111-27-3 | 3.61% |
| 3 | C7H10Cl2O3 | 5.660 | (Tetrahydro-2-furanyl)methyl 2,2-dichloroacetate | 4697-00-1 | 0.23% |
| 4 | C7H12O | 5.857 | (Z)-4-Heptenal | 6728-31-0 | 0.42% |
| 5 | C7H14O | 5.900 | Heptanal | 111-71-7 | 1.35% |
| 6 | C6H8O | 6.146 | (E,E)-2,4-Hexadienal | 142-83-6 | 0.31% |
| 7 | C8H14O2 | 7.144 | Acrylic acid isoamyl ester | 4245-35-6 | 0.31% |
| 8 | C8H18O | 7.411 | 4-Methyl-3-heptanol | 14979-39-6 | 0.26% |
| 9 | C7H6O | 7.548 | Benzaldehyde | 100-52-7 | 0.45% |
| 10 | C9H14O | 8.454 | 2-Pentyl-furan | 3777-69-3 | 0.80% |
| 11 | C9H12O | 8.726 | cis-2-(2-Pentenyl)furan | 70424-13-4 | 0.39% |
| 12 | NA d | 9.566 | Unidentified | NA | 0.18% |
| 13 | C6H10O2 | 9.795 | (E)-Hexenoic acid | 13419-69-7 | 0.22% |
| 14 | C10H18 | 10.248 | Isocamphane | 473-19-8 | 0.35% |
| 15 | C8H14O | 10.330 | (E)-2-octenal | 2548-87-0 | 0.26% |
| 16 | C9H18O | 11.596 | Nonanal | 124-19-6 | 0.38% |
| 17 | C8H12O | 11.836 | 2-Methyl-3-methylene-cyclopentanecarboxaldehyde | 826337-64-8 | 0.41% |
| 18 | C9H10O | 13.090 | 3-Ethyl-benzaldehyde | 34246-54-3 | 0.15% |
| 19 | C10H16O | 16.784 | (E,E)-2,4-Decadienal | 25152-84-5 | 0.18% |
| 20 | C13H22 | 17.46 | (E)-6-Tridecen-4-yne | 74744-46-0 | 0.25% |
| 21 | C15H24 | 18.115 | Copaene | 3856-25-5 | 0.93% |
| 22 | C11H20O3 | 18.311 | 4-Methylpentyl 3-hydroxy-2-methylenebutanoate | NA | 0.56% |
| 23 | C15H24 | 18.488 | β-Elemene | 515-13-9 | 1.97% |
| 24 | C14H30 | 18.595 | Tetradecane | 629-59-4 | 0.37% |
| 25 | C15H24 | 18.791 | (−)-Cedrene | 469-61-4 | 0.30% |
| 26 | C15H24 | 19.108 | Caryophyllene | 87-44-5 | 2.63% |
| 27 | C13H20O | 19.255 | α-Ionone | 127-41-3 | 0.31% |
| 28 | C10H12O3 | 19.293 | 2,3-Dihydro-2,2-dimethyl-3,7-benzofurandiol | 17781-15-6 | 0.22% |
| 29 | C15H24 | 19.37 | α-Bergamotene | 17699-05-7 | 0.19% |
| 30 | C15H24 | 19.462 | α-Guaiene | 3691-12-1 | 0.31% |
| 31 | C15H24 | 19.555 | cis-β-Farnesene | 28973-97-9 | 0.19% |
| 32 | C15H24 | 19.812 | Humulene | 6753-98-6 | 1.93% |
| 33 | C10H14O3 | 20.177 | 2,4,6-Trimethoxytoluene | 14107-97-2 | 1.16% |
| 34 | C15H24 | 20.292 | γ-Muurolene | 30021-74-0 | 1.35% |
| 35 | C13H20O | 20.477 | trans-β-Ionone | 79-77-6 | 0.68% |
| 36 | C10H15I | 20.603 | 1-Iodoadamantane | 768-93-4 | 0.15% |
| 37 | C15H24 | 20.744 | α-Bulnesene | 3691-11-0 | 0.67% |
| 38 | C15H24 | 20.859 | Valencene | 24741-64-8 | 0.15% |
| 39 | C13H26O | 20.935 | Tridecanal | 10486-19-8 | 0.62% |
| 40 | C15H24O | 20.979 | Butylated Hydroxytoluene | 128-37-0 | 0.19% |
| 41 | C15H26O | 21.077 | Cubebol | 23445-02-5 | 0.23% |
| 42 | C15H24 | 21.230 | δ-Cadinene | 483-76-1 | 0.98% |
| 43 | C12H22S | 21.497 | 3-n-Propyl-2-thiabicyclo[4.4.0]decane | 123192-50-7 | 0.32% |
| 44 | NA | 21.628 | Unidentified | NA | 0.30% |
| 45 | NA | 21.863 | Unidentified | NA | 0.42% |
| 46 | NA | 22.005 | Unidentified | NA | 0.47% |
| 47 | C15H24O | 22.201 | Caryophyllene oxide | 1139-30-6 | 0.20% |
| 48 | C16H26 | 22.277 | (3E,7E)-4,8,12-Trimethyltrideca-1,3,7,11-tetraene | 62235-06-7 | 0.28% |
| 49 | C15H24O | 22.463 | Humulene epoxide I | 19888-33-6 | 2.29% |
| 50 | NA | 22.719 | Unidentified | NA | 0.27% |
| 51 | C12H24O | 22.943 | Cyclododecanol | 1724-39-6 | 1.34% |
| 52 | NA | 22.992 | Unidentified | NA | 2.44% |
| 53 | NA | 23.390 | Unidentified | NA | 6.76% |
| 54 | C9H14N4O2 | 23.434 | 2-Diethylamino-6,7-dihydro-4H-oxazolo[3,2-a]-1,3,5-triazin-4-one | 62627-00-3 | 0.51% |
| 55 | C13H24O2 | 23.854 | 2-butyl-5-methyl-2-hexenoic acid, ethyl ester | 2242694-57-9 | 8.53% |
| 56 | C15H22O | 23.903 | cis-10-Hydroxycalamene | 123932-45-6 | 0.26% |
| 57 | C14H30O | 24.209 | 1-Tetradecanol | 112-72-1 | 2.03% |
| 58 | C14H26O | 24.443 | (Z)-7-Tetradecenal | 65128-96-3 | 0.54% |
| 59 | C15H30O | 25.070 | Pentadecanal | 2765-11-9 | 14.65% |
| 60 | NA | 25.387 | Unidentified | NA | 0.19% |
| 61 | C14H28O2 | 25.731 | Tetradecanoic acid | 544-63-8 | 0.25% |
| 62 | C16H30O | 25.867 | (E)-Hexadec-2-enal | 22644-96-8 | 0.16% |
| 63 | C15H32O | 25.960 | n-Pentadecanol | 629-76-5 | 0.31% |
| 64 | C16H30O | 26.211 | cis-9-Hexadecenal | 56219-04-6 | 0.33% |
| 65 | C16H32O | 26.587 | Hexadecanal | 629-80-1 | 0.49% |
| 66 | C18H36O | 27.062 | 2-Pentadecanone, 6,10,14-trimethyl- | 502-69-2 | 0.16% |
| 67 | C15H30O2 | 27.536 | Pentadecanoic acid | 1002-84-2 | 0.76% |
| 68 | C16H28O | 27.815 | (Z,Z)-7,10,-Hexadecadienal | 56829-23-3 | 1.38% |
| 69 | C14H26O | 27.962 | (Z)-7-Tetradecenal | 65128-96-3 | 3.39% |
| 70 | C18H32O | 28.109 | (Z,Z,Z)-9,12,15-Octadecatrien-1-ol | 506-44-5 | 0.15% |
| 71 | C17H34O | 28.306 | Heptadecanal | 629-90-3 | 0.92% |
| 72 | C16H32O2 | 29.227 | n-Hexadecanoic acid | 57-10-3 | 2.49% |
| 73 | C20H34O | 30.073 | Geranyl linalool | 1113-21-9 | 0.18% |
| 74 | C20H40O | 31.382 | Phytol | 150-86-7 | 2.17% |
| 75 | C18H34O2 | 31.764 | cis-Vaccenic acid | 506-17-2 | 0.37% |
| 76 | C9H16O | 32.168 | Cyclononanone | 3350-30-9 | 0.28% |
| 77 | C16H33NO | 32.348 | Hexadecanamide | 629-54-9 | 0.18% |
| 78 | C13H22O2 | 32.419 | 10-Methyldodec-2-en-4-olide | 1000370-41-0 | 0.21% |
| 79 | C13H17NO4 | 32.484 | Heptanoic acid, 3-nitrophenyl ester | 56052-18-7 | 0.28% |
| 80 | C17H29N | 34.339 | 2-Methyl-5-undecylpyridine | 52535-38-3 | 0.82% |
| 81 | C18H12O2S | 34.437 | 4-oxo-2,6-diphenyl-4H-thiopyran-3-carboxaldehyde | 70940-96-4 | 0.29% |
| 82 | C18H35NO | 34.912 | (Z)-9-Octadecenamide | 301-02-0 | 0.72% |
| 83 | C30H50 | 40.667 | Squalene | 111-02-4 | 0.17% |
| 84 | C17H32O | 43.324 | (Z)-14-methyl-8-Hexadecenal, | 60609-53-2 | 0.80% |
| 85 | NA | 43.390 | Unidentified | NA | 0.16% |
| 86 | C18H34O | 44.764 | (Z)-9-Octadecenal | 2423-10-1 | 2.48% |
| Samples | DPPH 50% Effective Concentration (mg/mL) | ABTS 50% Effective Concentration (mg/mL) | FRAP Antioxidant Capacity (mM/g) |
|---|---|---|---|
| F. hispida VOCs | 3.08 ± 0.024 | 0.44 ± 0.009 | 135.64 ± 25.49 |
| BHT | 0.042 ± 0.002 | 0.006 ± 0.001 | - |
| Trolox | 0.015 ± 0.001 | 0.014 ± 0.001 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Gao, P.; Ren, X.; Liu, X. Thermal Treatment (Hydrodistillation) on The Biomass of Ficus hispida L. f.: Volatile Organic Compounds Yield, Phytochemical Composition, and Antioxidant Activity Evaluation. Energies 2022, 15, 8092. https://doi.org/10.3390/en15218092
Xu Z, Gao P, Ren X, Liu X. Thermal Treatment (Hydrodistillation) on The Biomass of Ficus hispida L. f.: Volatile Organic Compounds Yield, Phytochemical Composition, and Antioxidant Activity Evaluation. Energies. 2022; 15(21):8092. https://doi.org/10.3390/en15218092
Chicago/Turabian StyleXu, Ziyue, Peizhong Gao, Xiaohan Ren, and Xu Liu. 2022. "Thermal Treatment (Hydrodistillation) on The Biomass of Ficus hispida L. f.: Volatile Organic Compounds Yield, Phytochemical Composition, and Antioxidant Activity Evaluation" Energies 15, no. 21: 8092. https://doi.org/10.3390/en15218092
APA StyleXu, Z., Gao, P., Ren, X., & Liu, X. (2022). Thermal Treatment (Hydrodistillation) on The Biomass of Ficus hispida L. f.: Volatile Organic Compounds Yield, Phytochemical Composition, and Antioxidant Activity Evaluation. Energies, 15(21), 8092. https://doi.org/10.3390/en15218092