Abstract
In this study, a new method for biomass thermal treatment was introduced. The volatile organic compounds (VOCs) of Ficus hispida biomass were obtained via hydrodistillation. The qualitative analysis of VOCs performed by GC–MS and GC–FID techniques identified pentadecanal (14.65%), 2-(E)-hexenal (11.15%), and 2-butyl-5-methyl-2-hexenoic acid ethyl ester (8.53%) as the major compounds. The chemical components varied significantly from the previous study. The results of the DPPH, ABTS, and FRAP methods gave IC50 and antioxidant capacity values of 3.08 ± 0.024 mg/mL, 0.44 ± 0.009 mg/mL, and 135.64 ± 25.49 mM/g, respectively. From the results, the VOCs distilled from F. hispida leaves have an antioxidant property that can be utilized as a natural botanical supplement as an antioxidant and preservative. In addition, the present research offers additional scientific support and a chemical basis for future natural drug discovery.
1. Introduction
Biomass is utilized in the annual energy level, agricultural residue, forestry, plantation, and urban solid waste. Biomass conversion can be carried out by several processes. Widely used processes are carbonization, densification, gasification, pyrolysis, and anaerobic digestion [1]. However, one method of thermal treatment, hydrodistillation, has only been reported relatively little.
Plants produce a large, diverse, multifarious array of organic compounds that appear to have no direct function in growth and development. These substances are known as secondary products or secondary metabolites [2]. Volatile organic compounds (VOCs), or essential oils (EOs), are liquid mixtures of small-polar or non-polar compounds derived from aromatic plant biomass, commonly by hydrodistillation through thermal treatment. The mixtures of volatile organic compounds (VOCs) usually have pleasantly scented fragrances and constitute what is called the “essence” of the plants. Despite their complex and rich composition, the use of VOCs remains widespread, including in aroma-therapeutic, medicinal, culinary, and other anthropogenic applications [3]. Moreover, essential oils or “essences” owe their name to their flammability. A previous study has investigated the influence of essential oil on engine performance and the combustion characteristics of a multi-cylinder compression ignition engine [4].
Ficus (Moraceae) is one of the largest genera of angiosperms, including trees, shrubs, creepers, hemiepiphytes, and climbers located in the tropics and subtropics worldwide, totaling about 900 species, whose pharmacological uses have been supported by several studies [5]. This large genus, with a high economic and nutritional value, plays a vital role as a genetic resource, making up a significant part of biodiversity in the rainforest ecosystem. Animals and human beings in tropical and subtropical areas see figs as a good and important source of nourishment. F. hispida L.f., commonly known as “hairy fig” (English), “peyatti” (Tamil), “gobla” (Hindi), “dumoor” (Bengali), and “Niunai shu” (China), is an evergreen tree and the species name, hispida, is Latin for “wirehaired”, indicating the densely hispid morphological feature of the plant. F. hispida is an herbal medicine that is traditionally used as a remedy for various ailments, including ulcers, anemia, piles, jaundice, hemorrhage, diabetes, convulsion, dysentery, diarrhea, and biliousness [6,7]. The latex of the plant is also documented for treating a ringworm infection, and the paste of its ripe fruits is used for the treatment of goiter [8]. There is growing evidence that free radicals produce molecules associated with various degenerative human diseases such as arteriosclerosis and diabetes [9]. Plant extracts and VOCs contain antioxidant compounds as reducing agents, free radical scavengers, and quenchers of singlet oxygen formation [10]. At present, millions of people cover a significant proportion of their subsistence needs and earn an income through the harvesting of non-timber forest products. Some of these resources include using aromatic plants, especially leaves, as an alternative to generate economic benefits without compromising forest conservation [11,12]. Because of its wide range of habitats and multiple medicinal properties, the treatment and utilization of this species is of paramount importance and should be investigated.
However, through careful bibliographic retrieval, only one research work has briefly reported the chemical constituents of F. hispida VOCs [13]. To the best of our knowledge, the antioxidant activity of the essential oils from F. hispida has not yet been reported. To fill the gap, and offer scientific support and an updated phytochemical basis, jointly with introducing a thermal treatment method of the plant biomass, the present work was undertaken with the main objective to investigate the phytochemical composition of the VOCs distilled from F. hispida leaves biomass, along with their antioxidant activity.
2. Materials and Methods
2.1. Chemicals Used and Plant Biomass
Trolox (6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid), BHT (butylated hydroxytoluene), DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,20-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid), and TPTZ (2,4,6-tri-(2-pyridyl)-s-triazin) were used. All chemicals were acquired from Sigma-Aldrich, Shanghai, China.
The fresh leaves of F. hispida were collected from Lingshan County, Qinzhou City, Guangxi Province, China (22°43′ N, 109°30′ E), in May 2022. Initially, the plant biomass of F. hispida leaves was identified by morphological features by Prof. Hong Zhao. The voucher specimen was deposited at Marine College, Shandong University, Weihai, China.
2.2. Thermal Treatment and Essential Oil Extraction
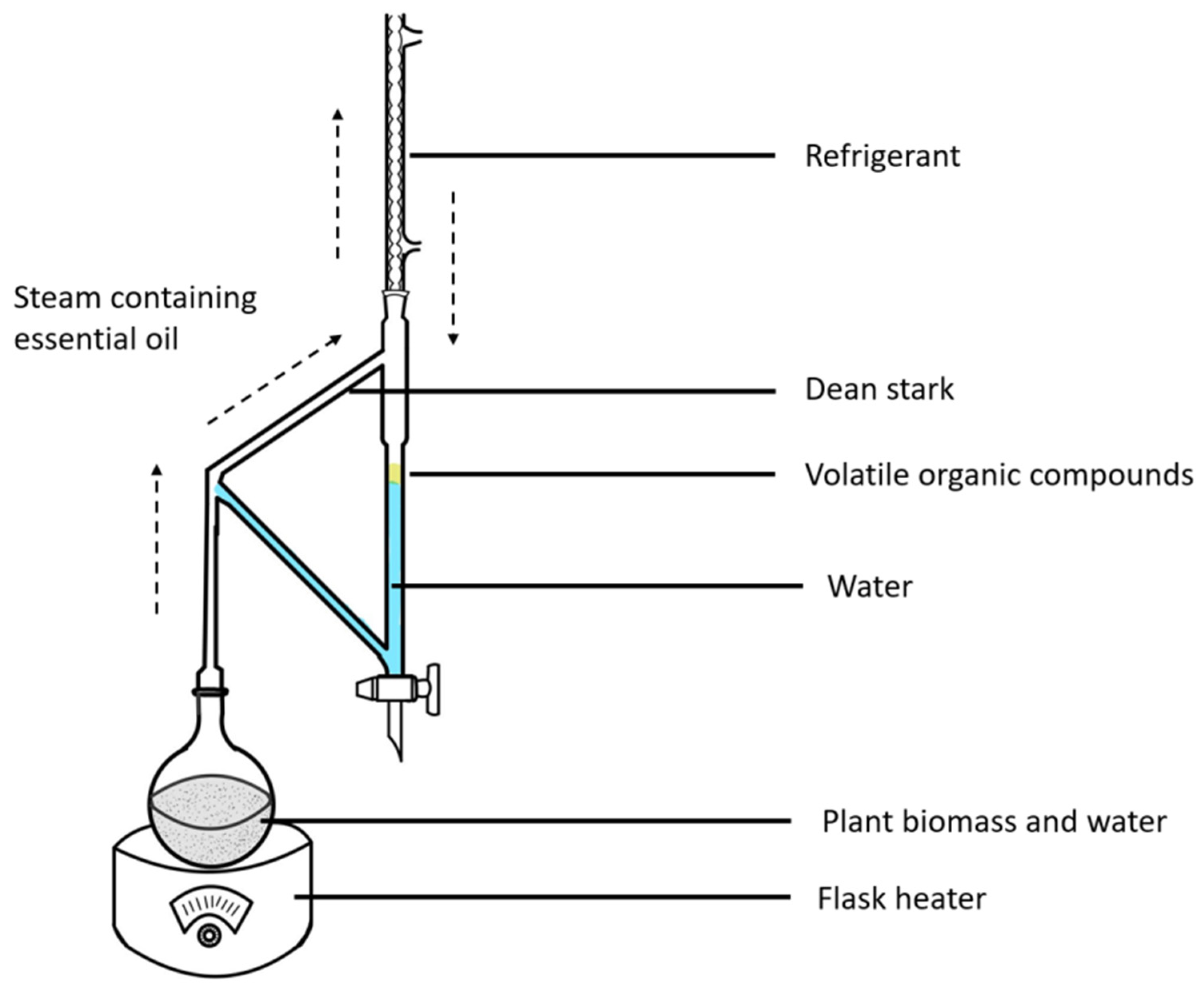
The fresh plant biomass (3 kg) of F. hispida slated for further analysis was milled into a powder and then subjected to hydrodistillation for 5 h using a Clevenger-type apparatus [14] with a sufficient amount of water. In order to obtain the greatest yield, the present experiment used a sample size to solvent amount ratio of approximately 1:3 under the consideration of previous studies [15]. The schematic is shown in Figure 1. The obtained VOCs were treated with anhydrous sodium sulfate and further dried using a Termovap Sample Concentrator. The dried oil was stored in sealed vials at 4 °C for further analysis.
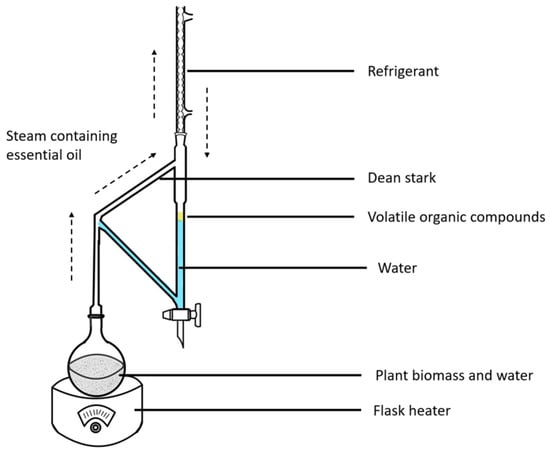
Figure 1.
The Clevenger apparatus is a tool used for essential oil extraction using steam. This technique uses temperature to separate the volatile compounds from the plant biomass, and the organic compounds are separated using steam so as not to degrade the VOCs. The Clevenger-type apparatus conducts the distillation process by boiling, condensing, and decantation to separate the oil. This is the official standard method for extracting essential oils for quality control.
2.3. Essential Oil Chemical Component Acquisition
The confirmation of the phytochemical constituents of F. hispida EOs was performed using an Agilent gas chromatograph–mass spectrometer (GC–MS) (7890-5975C). Briefly, the chromatography conditions were as follows: chromatographic column, HP-5MS (30 m × 0.25 mm × 0.25 μm); injector temperature, 270 °C; carrier gas, helium at a flow rate of 1.10 mL/min; temperature-rising program, initial oven temperature, 60 °C, increasing by 7 °C/min to 220 °C and held stable for 6 min, and then increasing by 10 °C/min to 280 °C and held stable for 6 min. The mass spectrometer conditions were as follows: EI: 70 eV, 230 °C, the mass scan range of 20–450 Da, and an acquisition frequency of 2. The quadrupole temperature was 150 °C, and 0.5 μL samples were injected.
The EOs and the n-alkane (C7–C30) were analyzed under the same GC conditions. The data processing was carried out by Agilent MassHunter Qualitative Analysis 10.0 program, and the relative abundance of each compound in the EOs was determined by the peak area normalization. The identification of the essential oil components was carried out by calculating their retention indices (RI) in a temperature-dependent programmed condition and was compared with the spectral library (NIST/EPA/NIH 2020).
2.4. Antioxidant Activity
The antioxidant capacity was evaluated to assess the radical-scavenging activity (DPPH and ABTS methods) and ferric-reducing antioxidant power from EOs distilled from the F. hispida biomass. The EOs were dissolved in ethanol at 20, 4, 2, 1, 0.5, 0.25, and 0.1 mg/mL. Hydrophobic (lipid-soluble) antioxidant compound BHT and hydrophilic (water-soluble) antioxidant Trolox were used as the reference compounds for ABTS, DPPH, and FRAP (ferric reducing antioxidant power) assays. All measurements were done in triplicate, and the mean values were calculated.
2.4.1. DPPH Assay
The experimental procedure was adapted from Nenadis et al., (2002) and Munteanu et al., (2021) [16,17], with some modifications. Briefly, a DPPH 0.1 mg/mL solution was prepared in ethanol. The mixed solution was shaken vigorously before incubating in the dark at room temperature for 1 h. Then, 100 μL of ethanol and 150 μL of prepared DPPH were added to the microplate as a control. Aliquots of 50 μL BHT solutions or EOs at different concentrations, as mentioned above, were added to 200 μL ethanol without DPPH in 96-well microplates, serving as a sample blank. Aliquots of 50 μL of the BHT mentioned above or EOs solutions were pipetted to prepare 100 μL ethanolic DPPH in the 96-well microplate.
The absorbance was measured at 516 nm using an Epoch microplate absorbance spectro-photometer after the incubation of compounds to be tested for 30 min under dark conditions. The readings for each sample were recorded using the software Microplate Manager.
Tests were carried out in triplicate. The radical scavenging activity (RSA%) was calculated according to the following equation:
where ASample is the absorbance of the tested sample at different concentrations, AControl is the absorbance of the control (ethanolic DPPH solution), and ASample Blank is the absorbance of the ethanolic sample without DPPH. The IC50 was then calculated.
2.4.2. ABTS Assay
In the ABTS assay, the method of Li et al. was adopted with minor changes [18]. The pre-formed radical mono cation of ABTS was generated by oxidation of the ABTS solution (7.4 mmol/L) with potassium persulfate (K2S2O8) solution (2.6 mmol/L) in equal amounts. The mixture was kept in the dark at 25 °C for 12 h to allow for the completion of the radical generation. To determine the radical scavenging activity of the EOs, 200 μL aliquot of ABTS•+ reagent was mixed with 50 μL of sample ethanolic solutions (25–2000 μg/mL) in 96-well microplates. After incubation for 7 min, the absorbance at 734 nm was read on an Epoch microplate absorbance spectrophotometer. The percentage inhibition (scavenging activity) of the samples was calculated as follows:
where A0 is the absorbance of mixtures without samples at 734 nm, while A is the absorbance at 734 nm with samples.
2.4.3. FRAP Assay
The FRAP experiment was conducted as described in previous reports [19,20], with some modifications. The sample was ethanolic EOs. A standard solution of Trolox represents the positive control. The working agent was prepared as follows, A: PH 3.6 acetate buffer solution, B: 10 mmol/L TPTZ solution, C: 20 mmol/L Fe3+ solutions, the working agent was mixed at a proportion of 10:1:1, respectively. Solutions of 1M HCl and 40 mM HCl were used to acidify the working agent. Then, 50 μL of different dilutions of EOs (1000, 500, 250, 100, and 25 μg/mL) and Trolox (2, 5, 10, 15, and 20 μL) were mixed with 200 μL of the FRAP working reagent in a 96-well microplate. The blank solution was prepared similarly by replacing EOs with distilled water. All of the tests were run in triplicates and averaged.
The yellow color of the solution to be tested was transformed into green and blue. After 30 min of reaction, the absorbance of the resulting solution was measured at 593 nm by an Epoch microplate absorbance spectrophotometer. The concentration of Fe2+-TPTZ (antioxidant capacity) was calculated by comparing the absorbance at 593 nm with the standard curve of the Trolox standard solutions.
2.5. Statistical Analysis
The retention indexes (RI) of the identified compounds were determined by Kovat’s method [21] and the formula used was
where RT is the retention time of the respective compound, X is the compound to be determined, and Z is the number of carbon atoms in the smaller alkane.
RI = 100 Z + 100 [Log RT(X) − (log RT(Z)/(log RT (Z + 1) − (log RT (Z)]
All of the experiments were performed thrice. Data are expressed as mean ± SD of three independent experiments.
3. Results and Discussion
3.1. Essential Oil Yield and Chemical Composition
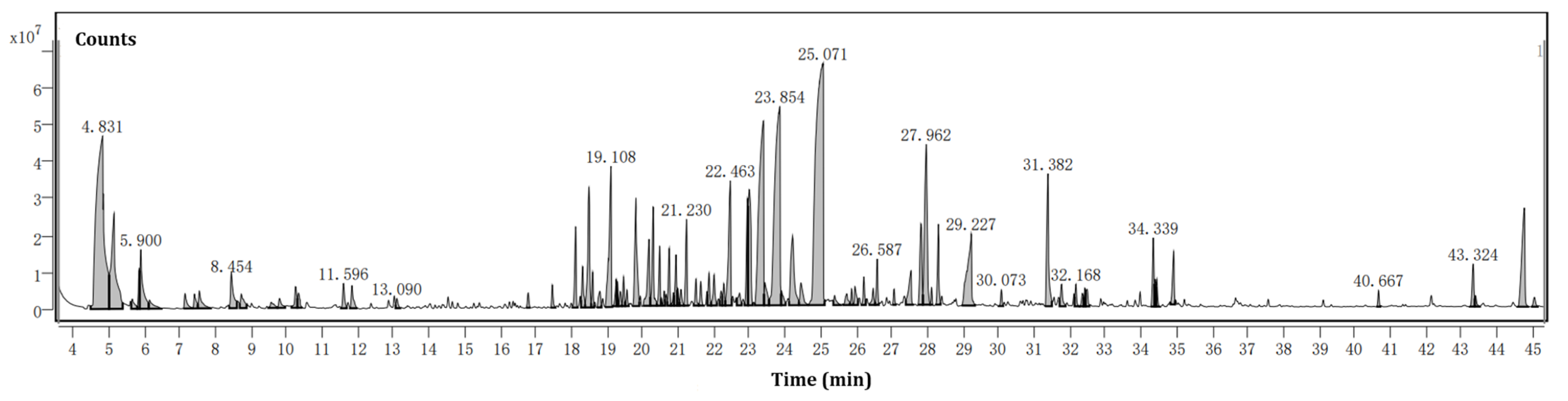
The hydrodistillation of the biomass of 3 kg of F. hispida leaves produced VOCs with a yield of 0.02% (w/w), and the VOCs produced a light yellow color with a strong odor. In this respect, we compared our yield with the yields from other authors. Similar VOC extraction efficiencies were presented in the Piperaceae plant Peperomia pellucida (0.03%) [22]. However, higher yields of VOCs were also reported by previous publications. The hydro-distilled VOCs of the Anacardiaceae plant Pistacia atlantica using a Clevenger permitted researchers to obtain an essential oil with a yield of 0.32% [23]. The chromatogram of the VOCs distilled from the biomass of F. hispida is shown in Figure 2.
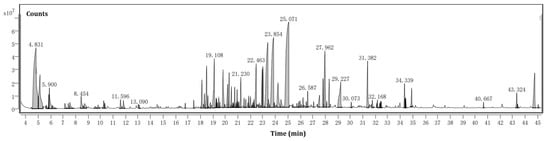
Figure 2.
Chromatogram of the VOCs distilled from the biomass of F. hispida.
The phytoconstituents present in the F. hispida biomass VOCs were identified and are presented in Table 1 according to the order of elution on the HP-5MS column in GC–MS. Seventy-seven compounds were identified and quantified, corresponding to 88.48% of the total VOCs.

Table 1.
The chemical composition of the VOCs distilled from F. hispida.
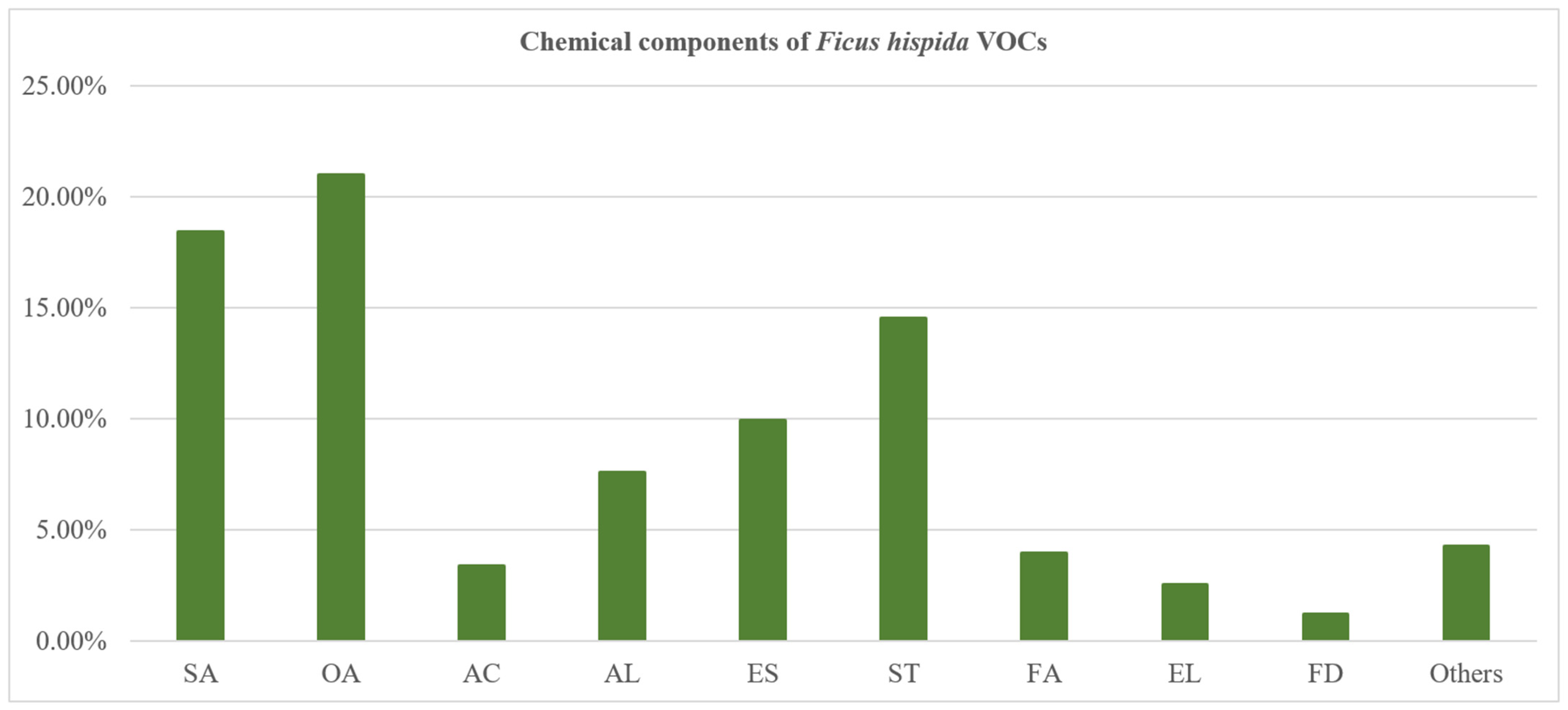
The GC–MS and GC–FID (gas chromatograph–flame ionization detector) analysis showed that F. hispida VOCs taking a large proportion were pentadecanal (14.65%), 2-(E)-hexenal (11.15%), and 2-butyl-5-methyl-2-hexenoic acid ethyl ester (8.53%). The compounds that were identified were separated into several compound classes: olefine aldehydes (20.96%), saturated aldehydes (18.41%), sesquiterpenoids (14.51%), esters (9.92%), alcohols (7.55%), fatty acids (3.94%), aromatic compounds (3.36%), enol (2.51%), furan derivatives (1.19%), and other components (Figure 3). The olefine aldehydes were the most abundant phyto-compounds in F. hispida VOCs, dominated by 2-(E)-hexenal (11.15%) and (Z)-7-tetradecenal (3.39%), while the dominating saturated aldehyde was pentadecanal (14.65%).
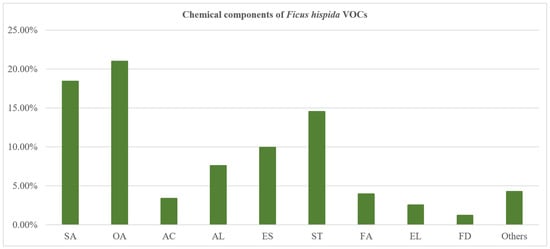
Figure 3.
The percentage composition of chemical composition classes distilled from F. hispida L.f. SA: saturated aldehyde; OA: olefine aldehyde; AC: aromatic compounds; AL: alcohol; ES: ester; ST: sesquiterpenoids; FA: fatty acids; EL: enol; FD: furan derivatives.
The distilled VOCs of 3 kg F. hispida leaves using a Clevenger-type apparatus permitted us to obtain EOs with a yield of 0.6 mL. In the previous study, volatiles of F. hispida from steam distillation was present in small quantities, about 20 µL/kg fresh wt of F. hispida plant material, which is much less than the yield in the present study [13]. The differences between the volatile oils yield in this study and other studies reported in the literature are probably due to the thermal treatment method (hydro-distillation and steam distillation) [24]. In addition, studies conducted by Martinez-Nataren et al. showed that climatic and edaphic conditions have a significant effect on the VOCs yield, which suggests that each particular population presents specific microclimatic and edaphic conditions that influence the secondary metabolism of individual plants.
In the present study, the chemical composition of F. hispida VOCs was rich in olefine aldehydes (20.96%) and saturated aldehydes (18.41%). However, the F. hispida composition results of this study are not consistent with the previous study carried out in 2001 [13], which found that F. hispida VOCs were mainly composed of palmitic acid (30.74% to 27.80%) as the major compounds, followed by 9,12-octadecadienoic acid (9.31% to 5.43%). The chemical composition variations of F. hispida VOCs could also be attributed to several factors, such as the physiological and anatomical characteristics of the plant, the period of collection, the storage conditions, and the oil extraction techniques [25,26,27]. The plant biomass in the previous study was collected in the Xishuangbanna Tropical Botanical Garden, Yunnan, China, in 2001. However, in our research, the plant sample was collected in Lingshan County, Qinzhou city, Guangxi Province, China, in May 2022. Hence, considerable geographic variation in the VOCs composition was observed across bioclimatic regions, as well as among populations and individuals.
3.2. Antioxidant Activity
The method for evaluating antioxidant activity is still evolving. In the present study, the antioxidant activities of F. hispida VOCs obtained after thermal treatment were determined using three different methods: DPPH, ABTS, and FRAP. Table 2 shows the antioxidant tests of the VOCs obtained from the F. hispida biomass.

Table 2.
Antioxidant activities of F. hispida volatile organic compounds expressed as IC50 values (μg/mL) for DPPH and ABTS, and antioxidant capacity for the FRAP assays.
3.2.1. DPPH Assay
The rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH•) test is one of the most acceptable, simple, and widely used antioxidant methods often used to evaluate the scavenging ability of the phenolic compounds. It is typically applied in essential oils, plant extracts, and other isolated organic compounds [28]. The deep purple DPPH radical transforms into a stable pale-yellow molecule when the odd electron of the DPPH free radical is paired with hydrogen from a free radical scavenging antioxidant via the HAT (hydrogen atom transfer) mechanism. Accordingly, the degree of DPPH discoloration could indicate the scavenging activity of the antioxidant to be tested [17].
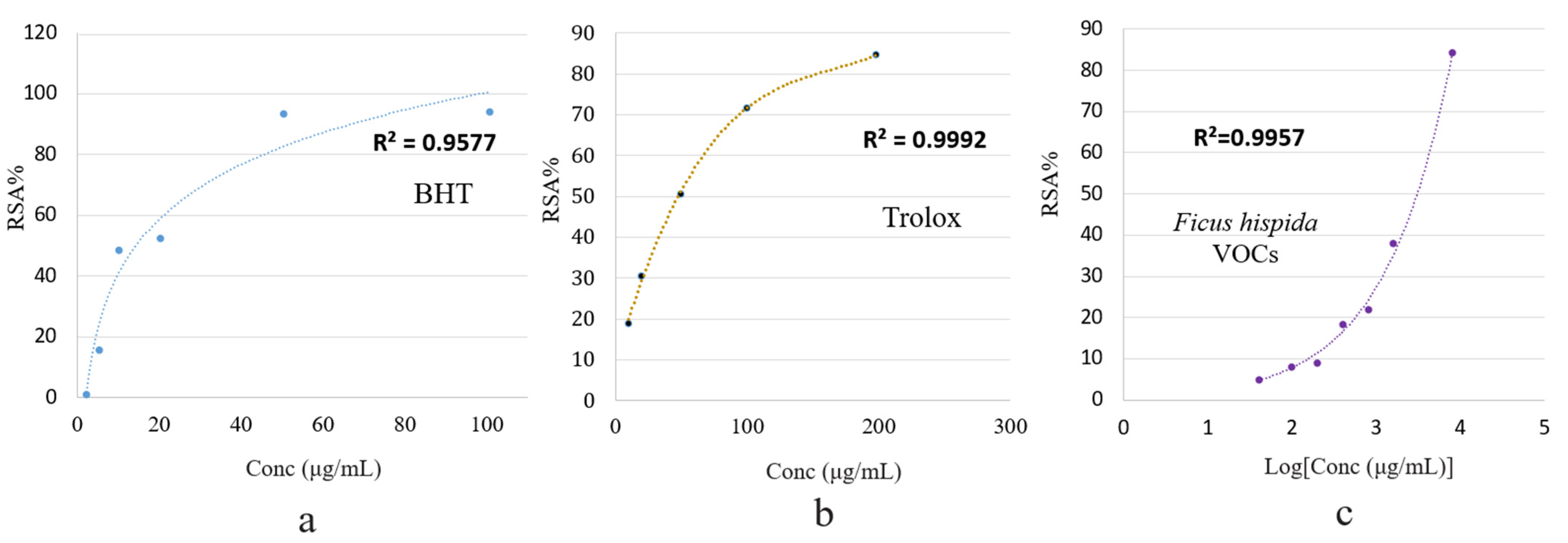
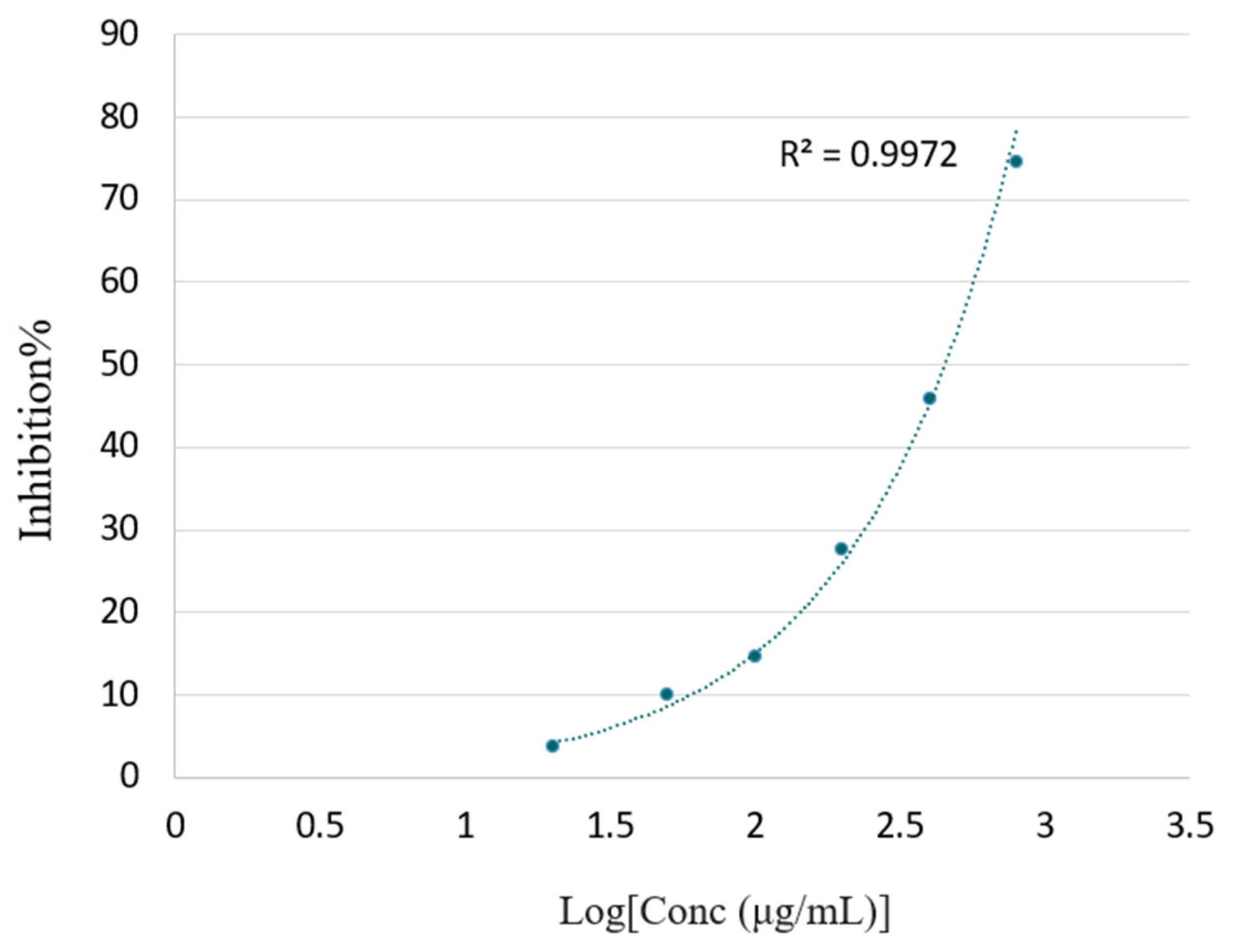
Figure 4 shows the effective concentrations of the F. hispida VOCs required to scavenge DPPH free radicals and the scavenging values (RSA%) as inhibition percentages. The inhibition percentage (RSA%) increased with the VOCs concentration in a dose-dependent manner.
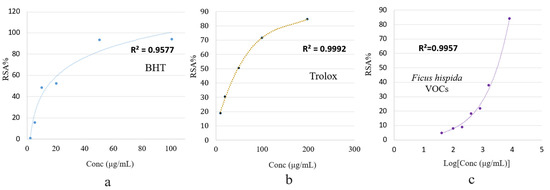
Figure 4.
Percentage of radical scavenging activity (RSA%) of BHT (a), trolox (b), and F. hispida volatile organic compounds (c) using the DPPH method.
At the highest tested concentration (4000 μg/mL), the F. hispida VOCs ethanolic solution showed an 84.52% antioxidant activity. The antioxidant standards, BHT and Trolox, showed 84.70% (200 μg/μL) and 94.24% (100 μg/μL) antioxidant activity, respectively. Compared with the antioxidant capacity of BHT and Trolox, expressed as RSA%, the F. hispida VOCs showed a relatively low antioxidant effect, as the activities of the two antioxidant standards at a low concentration were higher than the VOCs solutions at a highest concentration. This low DPPH free radical scavenging activity may result from the relatively low concentration of phenolic compounds (3.36%), as shown in Figure 3 [16]. A plethora of literature revealed a positive relationship between specific phytochemical compounds and antioxidant activity. Hussain et al., (2008) recorded the excellent antioxidant activity of basil VOCs in a DPPH assay (IC50 4.8–6.7 µg/mL) in accordance with the standard BHT, wherein the linalool contents were calculated as 56.7% [29]. In an earlier study, the antioxidant activity of Ocimum basilicum essential oil was reported as being IC50 5.92 ± 0.15 µg/mL in the DPPH assay and was ascribed to the presence of estragole [30].
3.2.2. ABTS Assay
The free radical scavenging capacities of the VOCs were also measured using the ABTS assay, and the results are given in Figure 5. In the ABTS assay, the degree of discoloration of the blue-green color, quantified as a drop in absorbance at 734 nm, depends on the sample concentration and intrinsic antioxidant activity. As shown in Figure 5, F. hispida VOCs exhibited a 92.43% inhibition rate against the ABTS free radical at its highest concentration (4000 μg/mL) and a 74.62% inhibition rate at a concentration of 800 μg/mL. Hence, F. hispida VOCs at a high concentration exhibited a relatively good antioxidant capacity. Valeriana pilosa VOCs containing patchoulol (20.8%) also displayed a high ABTS antioxidant activity [31].
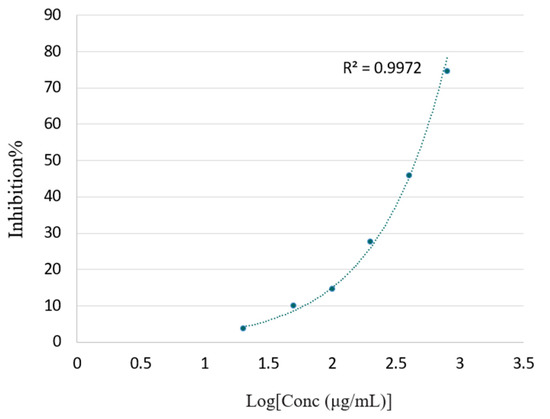
Figure 5.
Percentage of the inhibitory effect of the F. hispida volatile organic compounds in the ABTS free radical scavenging assay.
However, the ABTS assay has also been challenged for the lack of biological relevance owing to the use of the artificial ABTS radical cation, which was not found in biological systems or food [32]. In addition, the ABTS reaction was found to vary between slow reactions, and it could take a long time to reach the endpoint. In that case, using an endpoint of short duration (5 or 7 min) may lead to an underestimation of the antioxidant activity owing to reading before the reaction was completely finished [33]. Similar cases were also observed in this study.
3.2.3. FRAP Assay
The FRAP test is a typical SET (single electron transfer) method measuring the reduction of the complex of colorless Fe3+ ligand to the intensely blue Fe2+ by means of antioxidant compounds in acid environments [17]. Moreover, Cao and Prior suggested no correlation between the FRAP and ABTS methods [34]. Hence, the antioxidant evaluation of the FRAP assay would be beneficial for generating a more comprehensive “antioxidant profile” [35]. The antioxidant activity of F. hispida VOCs was 135.64 ± 25.49 mM/g, which indicates that the reducing power of the essential oil in the metal ion may play an important role in its antioxidant activity.
Overall, relatively low antioxidant capacities were observed herein for F. hispida VOCs. However, the chemical composition of F. hispida VOCs differed within species due to the different thermal treatment methods and growing status, reflecting the changes in the biological activities. This case was observed in previous studies of the Asteraceae genus. Essential oils of some species in the genus Artemisia L. showed weak antioxidant activity [36,37,38], but some species in this genus presented strong antioxidant activity [39]. Hence, despite the relatively low antioxidant properties of F. hispida VOCs, other species such as F. carica, F. pumila, and F. indica of the Ficus genus should also be investigated via various thermal treatment methods and bioactive determining assays.
4. Conclusions
Hydrodistillation is one of the methods of thermal treatment when it comes to biomass utilization. The data in this study confirmed the chemical composition and antioxidant activity of F. hispida VOCs. The qualitative analysis of VOCs performed by the GC–MS and GC–FID technique identified pentadecanal (14.65%), 2-(E)-hexenal (11.15%), and 2-butyl-5-methyl-2-hexenoic acid ethyl ester (8.53%) as the major compounds. The research showed that the VOCs were rich in olefine aldehydes. In addition, the antioxidant capacity was investigated through methods of trapping free radical DPPH, ABTS, and iron reduction (FRAP). The samples possessed a considerable FRAP antioxidant activity, suggesting it would serve as an alternative to undesirable synthetic additives. To the best of our knowledge, this is the first report dealing with the antioxidant activity of volatile organic components from F. hispida, and this report demonstrated a method of biomass thermal treatment.
Author Contributions
Conceptualization, Z.X., X.R. and X.L.; methodology, Z.X., P.G. and X.L.; software, Z.X., P.G. and X.L.; validation, X.L.; formal analysis, Z.X.; investigation, Z.X.; resources, X.L.; data curation, Z.X., P.G. and X.L.; writing—original draft preparation, Z.X.; writing—review and editing, Z.X.; visualization, Z.X.; supervision, X.R. and X.L.; project administration, X.L.; funding acquisition, X.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author.
Acknowledgments
We express our gratitude to Hong Zhao for his kind plant identification and instruction of Latin botanical nomenclature.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| VOC | Volatile Organic Compound |
| F. hispida | Ficus hispida L. |
| GC–MS | Gas chromatography-mass spectrometry |
| GC–FID | Gas chromatography–flame-ionization detection |
| RI | Retention index |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,20-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid |
| FRAP | Ferric reducing antioxidant power |
| TPTZ | 2,4,6-tri-(2-pyridyl)-s-triazin |
| BHT | butylated hydroxytoluene |
| Trolox | 6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid |
References
- Isnugroho, K.; Birawidha, D.C.; Hendronursito, Y. The Biomass Waste Use as a Secondary Energy Source for Metal Foundry Process. CONFAST 2016, 1746, 1–6. [Google Scholar]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.A.; Nabi, M.N.; Van, T.C.; Suara, K.; Jafari, M.; Dowell, A.; Islam, M.A.; Marchese, A.J.; Tryner, J.; Hossain, M.F.; et al. Performance and Combustion Characteristics Analysis of Multi-Cylinder CI Engine Using Essential Oil Blends. Energies 2018, 11, 738. [Google Scholar] [CrossRef]
- Ali, M.; Chaudhary, N. Ficus hispida Linn.: A review of its pharmacognostic and ethnomedicinal properties. Pharmacogn. Rev. 2011, 5, 96–102. [Google Scholar] [PubMed]
- Shahriar, M.; Islam, S.; Parvin, S.; Hoque, S. Thrombolytic activity and antimicrobial properties of Ficus hispida. J. Sci. Res. 2013, 5, 393–397. [Google Scholar] [CrossRef]
- Mandal, S.C.; Kumar, C.K.A. Studies on the anti-diarrhoeal activity of Ficus hispida leaf extract in rats. Fitoterapia 2002, 73, 663–667. [Google Scholar] [CrossRef]
- Salvi, V.; Joshi, Y.; Dhande, S.; Kadam, V. A review on Ficus hispida. Res. J. Pharmacogn. Phytochem. 2013, 5, 149–154. [Google Scholar]
- Okoh, S.O.; Asekun, O.T.; Familoni, O.B.; Afolayan, A.J. Antioxidant and Free Radical Scavenging Capacity of Seed and Shell Essential Oils Extracted from Abrus precatorius (L). Antioxidants 2014, 3, 278–287. [Google Scholar] [CrossRef]
- Millogo-Kone, M.; Lompo, F.; Nacoulma, O. Evaluation of flavonoids, total phenolic contents of P. biglobosa and free radical scavenging and antimicrobial activities. Res. J. Med. Sci. 2009, 3, 70–74. [Google Scholar]
- Ticktin, T. The ecological implications of harvesting non-timber forest products. J. Appl. Ecol. 2004, 41, 11–21. [Google Scholar] [CrossRef]
- Paumgarten, F. The role of non-timber forest products as safety-nets: A review of evidence with a focus on South Africa. GeoJournal 2005, 64, 189–197. [Google Scholar] [CrossRef]
- Song, Q.S.; Yang, D.R.; Zhang, G.M.; Yang, C.R. Volatiles from Ficus hispida and their attractiveness to fig wasps. J. Chem. Eco. 2001, 27, 1929–1942. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Wu, J.J.; Xu, Y.J.; Fu, M.Q.; Xiao, G.S. Effect of Second Cooling on the Chemical Components of Essential Oils from Orange Peel (Citrus sinensis). J. Agric. Food Chem. 2014, 62, 8786–8790. [Google Scholar] [CrossRef]
- Katekar, V.P.; Rao, A.B.; Sardeshpande, V.R. Review of the rose essential oil extraction by hydrodistillation: An investigation for the optimum operating condition for maximum yield. Sustain. Chem. Pharm. 2022, 29, 100783. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH center dot) tests. J. Am. Oil Chem. Soc. 2002, 79, 1191–1195. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Li, X.C.; Lin, J.; Gao, Y.X.; Han, W.J.; Chen, D.F. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem. Cent. J. 2012, 6, 140. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Szafranska, K.; Szewczyk, R.; Janas, K.M. Involvement of melatonin applied to Vigna radiata, L. seeds in plant response to chilling stress. Cent. Eur. J. Biol. 2014, 9, 1117–1126. [Google Scholar] [CrossRef]
- Paw, M.; Begum, T.; Gogoi, R.; Pandey, S.K.; Lal, M. Chemical Composition of Citrus limon L. Burmf Peel Essential Oil from North East India. J. Essent. Oil Bear. Plants 2020, 23, 337–344. [Google Scholar] [CrossRef]
- Usman, L.A.; Ismaeel, R.O. Chemical Composition of Root Essential oil of Peperomia pellucida (L.) Kunth. Grown in Nigeria. J. Essent. Oil Bear. Plants 2020, 23, 628–632. [Google Scholar] [CrossRef]
- Khiya, Z.; Oualcadi, Y.; Zerkani, H.; Gamar, A.; Amine, S.; EL Hamzaoui, N.; Berrekhis, F.; Zair, T.; EL Hilali, F. Chemical Composition and Biological Activities of Pistacia atlantica Desf. Essential Oil from Morocco. J. Essent. Oil Bear. Plants 2021, 24, 254–265. [Google Scholar] [CrossRef]
- Lim, A.C.; Tang, S.G.H.; Zin, N.M.; Maisarah, A.M.; Ariffin, I.A.; Ker, P.J.; Mahlia, T.M.I. Chemical Composition, Antioxidant, Antibacterial, and Antibiofilm Activities of Backhousia citriodora Essential Oil. Molecules 2022, 27, 4895. [Google Scholar] [CrossRef] [PubMed]
- Heikal, A.A.-E.M. Variation in the Essential Oil Content and its Composition in Eucalyptus cinerea Leaves and its Relation to Some Environmental Factors. J. Essent. Oil Bear. Plants 2017, 20, 995–1005. [Google Scholar] [CrossRef]
- Jardak, M.; Elloumi-Mseddi, J.; Aifa, S.; Mnif, S. Chemical composition, anti-biofilm activity and potential cytotoxic effect on cancer cells of Rosmarinus officinalis L. essential oil from Tunisia. Lipids Health Dis. 2017, 16, 190. [Google Scholar] [CrossRef]
- Barra, A. Factors Affecting Chemical Variability of Essential Oils: A Review of Recent Developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; De Moraes, A.A.B.; Da Costa, K.S.; Galúcio, J.M.P.; Taube, P.S.; Costa, C.M.L.; Cruz, J.N.; Andrade, E.H.D.A.; De Faria, L.J.G. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Nadeem, H.R.; Akhtar, S.; Ismail, T.; Qamar, M.; Sestili, P.; Saeed, W.; Azeem, M.; Esatbeyoglu, T. Antioxidant Effect of Ocimum basilicum Essential Oil and Its Effect on Cooking Qualities of Supplemented Chicken Nuggets. Antioxidants 2022, 11, 1882. [Google Scholar] [CrossRef]
- Minchán-Herrera, P.; Ybañez-Julca, R.O.; Quispe-Díaz, I.M.; Venegas-Casanova, E.A.; Jara-Aguilar, R.; Salas, F.; Zevallos-Escobar, L.; Yáñez, O.; Pino-Rios, R.; Calderon, P.B.; et al. Valeriana pilosa Roots Essential Oil: Chemical Composition, Antioxidant Activities, and Molecular Docking Studies on Enzymes Involved in Redox Biological Processes. Antioxidants 2022, 11, 1337. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Tian, X.; Schaich, K.M. Effects of molecular structure on kinetics and dynamics of the Trolox Equivalent Antioxidant Capacity assay with ABTS+•. J. Agric. Food Chem. 2013, 61, 5511–5519. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Cao, R.L. In vivo total antioxidant capacity: Comparison of different analytical methods. Free Radical Biol. Med. 1999, 27, 1173–1181. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Kotan, R.; Mavi, A.; Cakir, A.; Ala, A.; Yildirim, A. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum, and Artemisia spicigera essential oils. J. Agric. Food Chem. 2005, 53, 9452–9458. [Google Scholar]
- Mighri, H.; Hajlaoui, H.; Akrout, A.; Najjaa, H.; Neffati, M. Antimicrobial and antioxidant activities of Artemisia herba-alba essential oil cultivated in Tunisian arid zone. CR Chim. 2010, 13, 380–386. [Google Scholar] [CrossRef]
- Lopes-Lutz, D.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 2008, 69, 1732–1738. [Google Scholar] [CrossRef]
- Rashid, S.; Ahmad, M.; Amin, W.; Ahmad, B. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013, 138, 693–700. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).