Abstract
The production of low-carbon hydrogen based on renewable energy sources is considered a promising direction in the development of the modern world economy. The purpose of the presented research is to develop technologies and study the processes of converting biogases into hydrogen, as well as its use in low-temperature fuel cells. The methodology for organizing a multi-stage laboratory experiment for obtaining biogas, its purification from impurities and, in the future, the production of biohydrogen was developed based on field studies in Peter the Great St. Petersburg Polytechnic University. The results of modeling studies have shown that during biogas reforming, it is possible to obtain a hydrogen mixture with a hydrogen content of 98% vol and methane 2% vol. Based on the results of the research, the possibility of using the significant potential of “weak” biogas containing methane 30–45% vol to produce biohydrogen (more than 93% vol) was proved. A technique for using biohydrogen in low-temperature fuel cells for energy generation has been substantiated and tested.
1. Introduction
The production of low-carbon hydrogen based on renewable energy sources is considered a promising direction in the development of the modern world economy. The use of low-carbon hydrogen as the most important industrial raw material, as well as a high-quality efficient energy carrier, will reduce the consumption of fossil fuels and reduce greenhouse gas emissions, which will contribute to solving the pressing environmental and social problem—global climate change [1].
In 2020, the global demand for hydrogen was about 90 million tons, of which more than 70 million tons were used in the form of pure hydrogen and less than 20 million tons were mixed with carbonaceous gases in the production of methanol and steel. Nearly all of this demand was for oil refining and industrial use. To date, the use of hydrogen in the transport sector is limited to 0.01% of total consumption. In the electric power industry, it is practically not used [2].
Currently, the bulk of hydrogen in the world is obtained from fossil fuels—about 96% of the total, including from natural gas (49%), liquid hydrocarbons (29%), and coal (18%). Steam reforming of natural gas is currently one of the most common, and at the same time the least expensive, processes for producing hydrogen. Its main disadvantage is the emission of greenhouse gases (10 kg CO2/kg H2). Hydrogen production by water electrolysis is 3.9%; an insignificant part (0.1%) is obtained from other sources [3]. The high cost of producing hydrogen limits its wider application.
By 2050, the role of hydrogen in the global energy sector may be comparable to that of fossil fuels. In addition, hydrogen is one of the most effective ways to create long-term energy storage [4].
Biomass is an attractive renewable energy resource for obtaining relatively cheap low-carbon hydrogen due to its large resources, its almost universal availability (wood and wood waste, agricultural crops, aquatic plants, microalgae, waste from the agro-industrial and food sectors, housing and communal services, etc.), the possibility of its effective use in various energy technologies, as well as transport systems [5,6].
Biomass is the largest source of renewable energy used globally today. In 2017, the production of primary energy resources in the world was estimated at 13,972 million toe, including 1992 million toe of renewable energy resources (14.3% of total), of which 1385 million toe (about 70% of renewable) was biomass.
The production of primary energy resources by 2040 in the world is estimated to reach 17,715 million toe. It is assumed that the production of renewable energy resources will reach 3605 million toe (20.3% of the total), of which 1851 million toe (about 51.4%) will be biomass [7]. An analysis of the global energy balance shows that in the foreseeable future, bioenergy will play an important role in the energy supply and decarbonization of transport, housing, agriculture, and other industries due to the possibility of emission-free (without greenhouse gas emissions) efficient conversion into liquid and gaseous fuels, heat and electricity. The special importance of bioenergy in creating a climate-friendly circular economy is noted as a new socially and environmentally acceptable economic model for the development of society [8,9]. Technically realizable resources of primary biomass for the world energy industry, according to a conservative estimate, are a significant value of 160–270 EJ/year (3.82–6.45 billion toe), even considering possible restrictions on use by agriculture and water economy, wood-processing industries, and the social and environmental sphere. This is 3–4.5 times higher than their current use [9].
Russia has significant bioenergy potential. For energy purposes, it is possible to use (annually) [10,11,12,13,14]:
- About 800 million tons of woody biomass;
- About 500 million tons (by dry matter) of organic waste from agricultural production;
- Up to 70 million tons of waste from the forestry and woodworking industries;
- 60 million tons of municipal solid waste;
- Up to 10 million tons of sludge from municipal wastewater.
The main directions of development of bioenergy in the world are [15]:
- The creation of highly efficient technologies for energy waste disposal and obtaining alternative fuel products based on biogas and synthesis gas;
- The production of low-carbon hydrogen for fuel cells.
Obtaining biogas from waste is an effective traditional way of use their energy potential [16]. Biogas can also be used as an available raw material for the subsequent production of low-carbon hydrogen [17], which will allow organizing the local production and use of hydrogen, providing local consumers with a relatively cheap, high-quality, and environmentally friendly energy carrier.
The type of organic waste determines the morphological composition of biogases, the summarized data for which are presented in Table 1 [18]. The methane content of biogas typically ranges from 45% to 75% by volume, with most of the remainder being CO2. This variation means that the energy content of biogas can vary; the lower heating value (LHV) is between 16 megajoules per cubic meter (MJ/m3) and 28 MJ/m3 [19].

Table 1.
Composition of biogases.
The purification of biogas is a complex task and requires the use of a combination of different methods due to its contamination with harmful impurities, the instability of formation, and the variability in time of the content of the methane component [20]. This task is discussed in detail in many publications, e.g., in [21,22].
Solving the problem of biogas purification opens prospects of using biomethane instead of natural gas, which is the main raw material for hydrogen production. Biogas can be obtained almost everywhere since organic waste is constantly generated in the process of human economic activity. Meanwhile, natural gas resources are limited, their use is limited, and they become more expensive over time. The anaerobic decomposition process converts the waste into a valuable fuel, biomethane, which can be steam-reformed to produce low-carbon hydrogen instead of natural gas. At the same time, carbon dioxide contained in biogas, as well as arising from the production of hydrogen, is characterized by an environmentally friendly origin (through biological processes). Carbon dioxide was originally taken from the atmosphere by plants and converted into primary biomass and then through the life cycle into organic waste. The biogas waste disposal process produces a by-product—effluent (fermented residue)—that can be used as a high-quality agricultural fertilizer.
The cost of hydrogen from biogas is comparable to the cost of electrolytic hydrogen produced using electrical energy, hydraulic and wind power plants, and cheaper than hydrogen produced using photovoltaic plants [23,24]. Therefore, the expediency of obtaining hydrogen from biogases with its subsequent use to produce electrical energy is quite obvious.
Biohydrogen can be obtained from the fermentation of various waste products. The article [25] discusses the fermentation of liquid waste from dairy production (whey) to produce biohydrogen. It is shown that the yield of hydrogen and the purity of the hydrogen mixture depend on the pH of the process (optimal pH = 6) and the inoculant. The authors of articles [26,27] showed that industrial wastewater is a cheap substrate for cost-effective production of biohydrogen. Pre-treatment of the inoculant and selective immobilization of hydrogen-producing microorganisms make it possible to obtain the maximum amount of biohydrogen. The processing of organic waste using microbes under dark or photofermentation is an economical and promising way to obtain biohydrogen [28]. In [29,30], the authors showed the possibility of obtaining biohydrogen from the biomass of microalgae and creating a closed biocatalytic system for hydrogen production due to water biophotolysis.
In addition, also of great interest nowadays are the technologies of biological methanation of hydrogen (generated by electrolysis using wind and solar energy) with carbon dioxide in biogas to form methane. This makes it possible to increase the methane yield of the biogas system by 70%. However, due to the complex biochemical processes in bioreactors, these technologies require serious refinement, which is currently being addressed by numerous researchers. [31,32]
It should be noted that for the widespread use of technologies for producing hydrogen from waste, it is necessary to reduce costs, which can be carried out using the existing technological reserve for the key stages of hydrogen production from biogases.
The production of hydrogen for low-temperature PEM (proton exchange membrane) fuel cells is of particular interest because they are most suitable for autonomous consumers in a wide range of loads from hundreds of watts to several tens of kilowatts. Fuel cells can become an effective alternative to burning fuel for primary electricity generation by stationary and mobile sources. The critical question concerns the source of hydrogen, because in many ways, the purity and cost of hydrogen determines the efficiency of fuel cells [33]. Work on the production of hydrogen from biogases and its use in the power supply of small consumers is carried out in EU countries, the USA, and Japan. The following shortcomings of the installations were revealed: the low hydrogen content in the reformate (70–80% vol.) and the presence of harmful impurities in it [34]. This requires the use of expensive and difficult-to-operate systems for its purification, which leads to an increase in the energy intensity of the process and a decrease in efficiency. Therefore, it is extremely important to develop an efficient technology for converting readily available hydrocarbon fuel (biogas) into relatively cheap, “low-carbon” hydrogen as an alternative to electrolysis, as well as methods for its direct use in low-temperature hydrogen fuel cells [35].
The purpose of the presented research is to develop technologies and study the processes of converting biogases into hydrogen, as well as its use in low-temperature fuel cells.
2. Materials and Methods
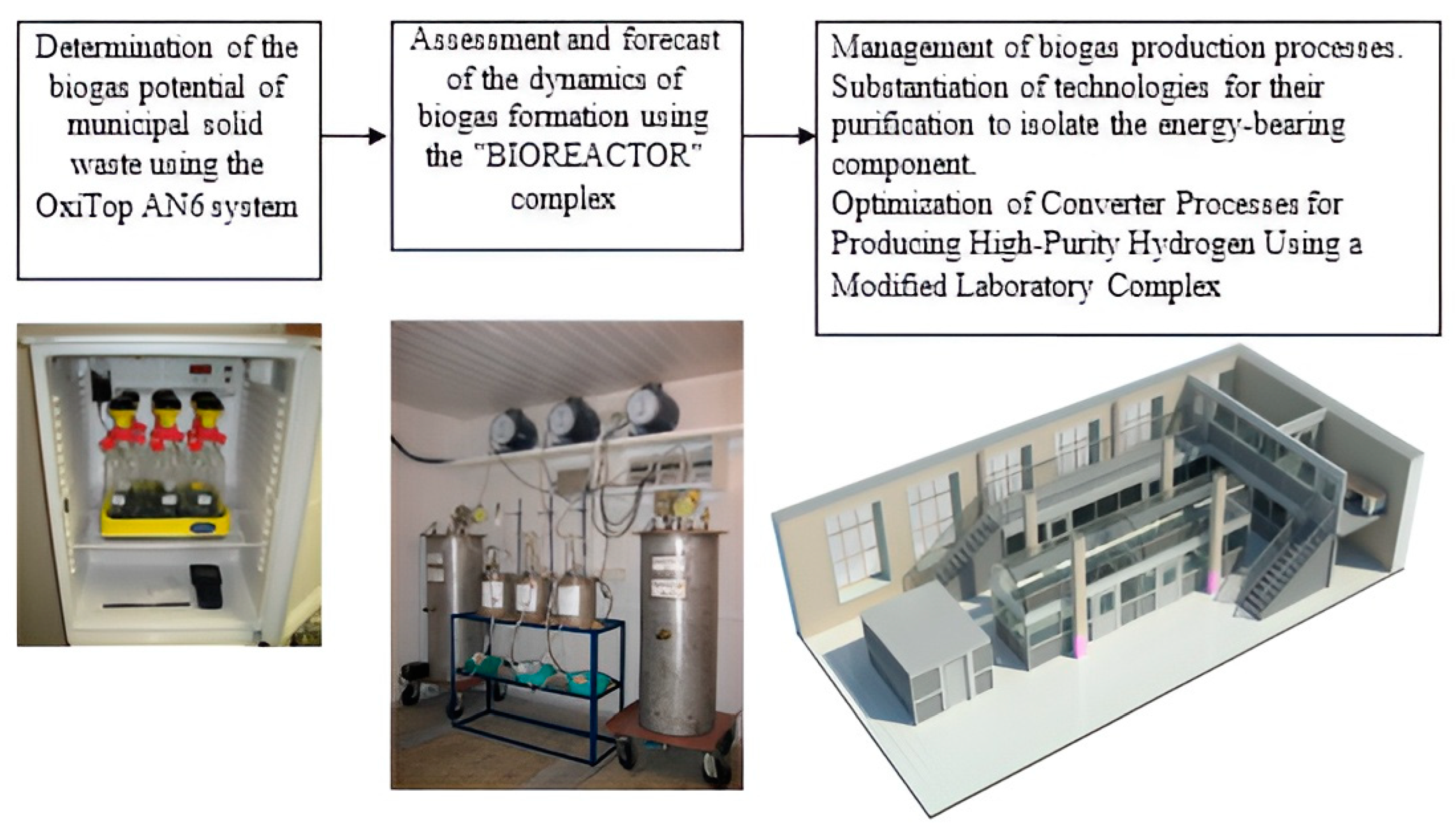
The methodology for organizing a multi-stage laboratory experiment (Figure 1) for obtaining biogas, its purification from impurities and, in the future, the production of biohydrogen was developed based on field studies in Peter the Great St. Petersburg Polytechnic University [36].
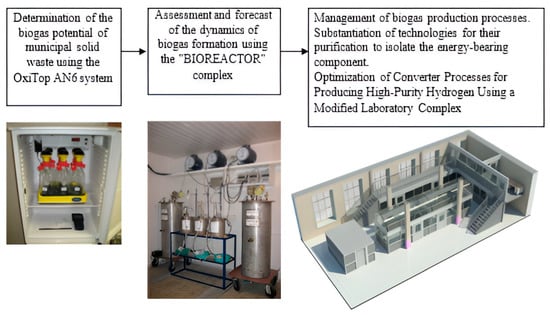
Figure 1.
Scheme of a multi-stage laboratory experiment.
The first stage of the laboratory experiment involved a preliminary assessment of the biogas potential of the studied organic waste using the OxiTop Control AN6|WTW (Germany). In the studied composition, the total and specific volume of biogas, as well as its morphological composition, were determined.
The second stage of the experiment was the assessment and forecast of the dynamics of biogas formation using the automated complex “BIOREACTOR”, certified according to international requirements [37]. The “BIOREACTOR” complex included: a thermobox, fermenters, a system for monitoring and managing waste biodegradation processes, gas meters, etc. [36].
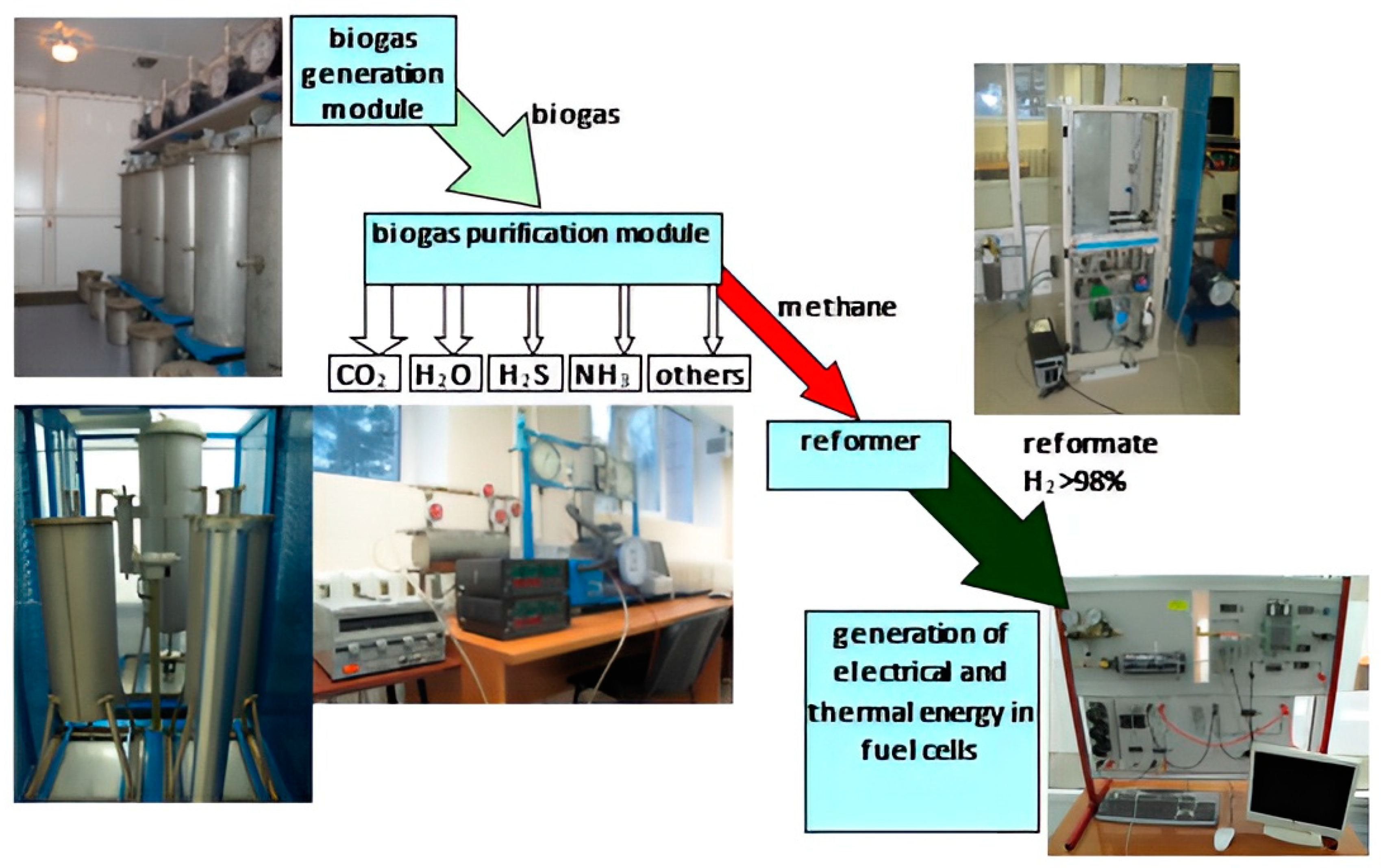
At the third stage of the experiment, studies were carried out on the energy use of biogas based on the created unique laboratory complex [38]. The laboratory complex consisted of the following modules: a biogas generation module (bioreactor module), a biogas storage and purification module, a methane reforming module to produce hydrogen, and a module consisting of low-temperature fuel cells (Figure 2).
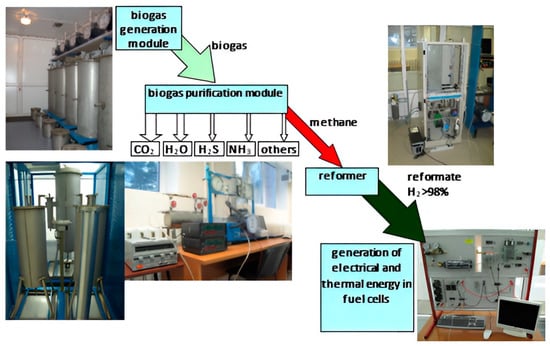
Figure 2.
The composition of the modules of the laboratory complex.
Model samples of the studied wastes, from which biogas was formed in the process of methanogenesis, were placed in the bioreactor module. Within the framework of the first module, the volume and composition of biogas were controlled, and the chemical composition of the filtrate was determined. In the second module, based on adsorption–cryogenic treatment, harmful impurities were removed from biogas and the methane component was isolated. Furthermore, the biomethane purified from impurities was supplied to the reforming module for its steam conversion into biohydrogen. Next, biohydrogen entered the module, consisting of low-temperature fuel cells, where it was converted into electrical energy.
Absorption and catalytic purification (operation in a periodic mode) were used to obtain hydrogen from purified methane in the reforming module [39]. A mixture of methane and water vapor was converted and pre-treated by the adsorption–catalytic method, after which the gas was supplied for additional purification from CO in a methanator—KONIK-TECH. The operating temperature of adsorption–catalytic processes was 600–850 °C, and the methanator was 250 °C. The main technical characteristics of the reformer module are shown in Table 2.

Table 2.
Reformer module specifications.
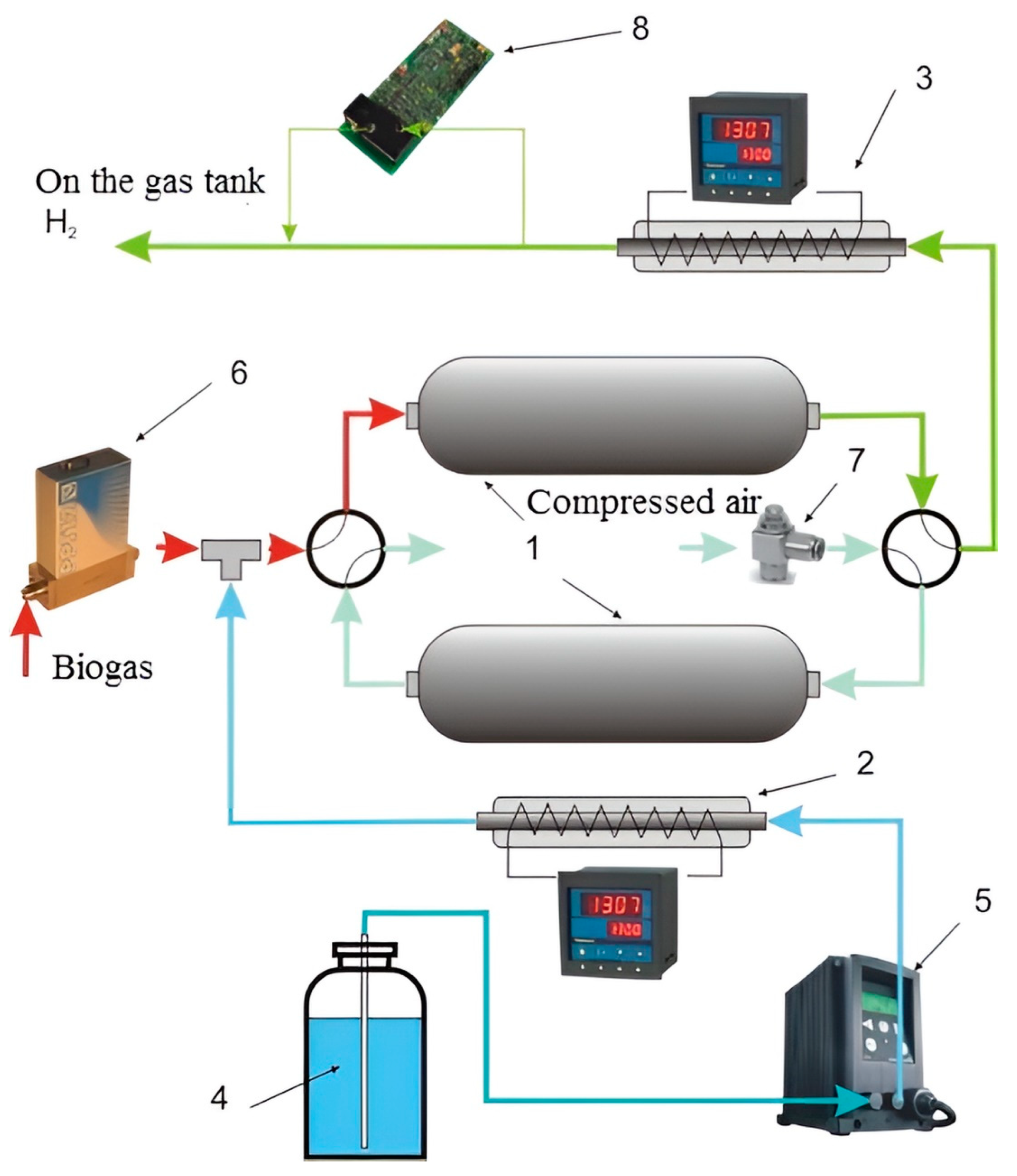
The functional diagram of the reformer module is shown in Figure 3.
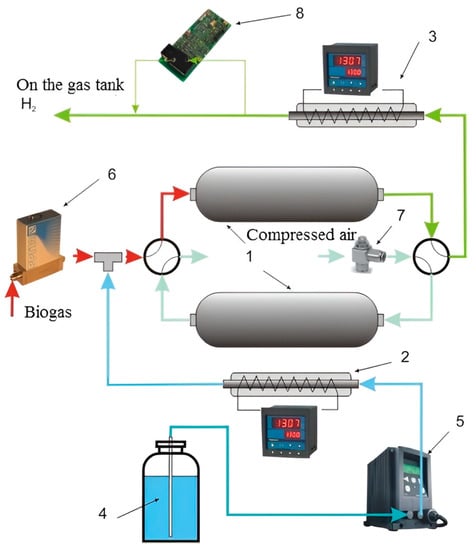
Figure 3.
Scheme of the reformer module: 1—adsorption-catalytic blocks; 2—evaporator with temperature controller; 3—methanator; 4—distillate; 5—liquid pump; 6—gas flow regulator; 7—gas throttle; 8—methane concentration control.
The concentration of hydrogen and impurities in the gas flow was determined using a gas analyzer AVP-02G and an IR Fourier spectrophotometer FSM1202.
It was thermodynamically substantiated that CH4 is not oxidized in a low-temperature fuel cell; however, its presence in a hydrogen mixture can create the effect of shielding the catalytic membrane surface and, therefore, hinder the kinetics of hydrogen oxidation. Traditionally, methods are used to separate hydrogen from CO, CO2, CH4, and other impurities using, for example, expensive membrane technologies [40]. The problem arises of direct use of hydrogen with a residual content of methane in the fuel cell and determination of the effect of non-oxidizing gas on the electrochemical kinetics of hydrogen oxidation.
The possibility of direct use of biohydrogen (obtained from the methane component of biogas) in a fuel cell instead of electrolytic hydrogen was studied on an E-Tek low-temperature fuel cell (P = 5 W with a proton-exchange membrane) [41]. The main characteristics of the E-Tek fuel cell when tested on electrolytic hydrogen and oxygen are shown in Table 3.

Table 3.
E-Tek fuel cell parameters.
The feed rate of the hydrogen-containing gas obtained in the reformer was ~20 mL/min, which provided a sufficient overpressure on the fuel cell membrane (~0.02 atm). The automatic second-by-second registration of realized dependencies was carried out in digital and graphical format, and fuel consumption was also recorded (H2 or H2 + CH4).
3. Results
At the first stage of the laboratory experiment, samples of model waste mixtures were loaded into bioreactors, which correspond to the average morphological composition of municipal solid waste landfilled at Russian solid waste landfills. 6-week-old compost from MSW containing methanogenic bacteria was used as an inoculum. Then, the process of their biodegradation was organized according to the method described in articles [42,43,44]. The volume of biogas generated as a result of waste methanogenesis during the entire duration of the experiment was constantly monitored. The composition of biogas was periodically determined, which made it possible to create model mixtures of biogas with different methane content of 40–51% vol. (the rest is carbon dioxide). The model mixtures were fed into the adsorption–cryogenic module, where impurities were removed. As a result, methane was released with a concentration of 94.1–97.5% vol.
The sorption–catalytic scheme was used to produce hydrogen. This scheme combined the process of steam reforming of hydrocarbons with high-temperature purification from carbon dioxide. The studies were carried out on an experimental setup (Figure 3) with a capacity of up to 60 L H2/hour with thermal regeneration of the absorber. The synthesized granular calcium oxide was used as an absorber [45], as a catalyst for steam reforming—NIAP-03 (11% NiO/Ca2Al2O5) and methanation—NIAP-07 (36 wt.% NiO/Al2O3).
The composition of landfill biogas was modeled with three different gas mixtures: methane, CO2 and nitrogen with a gas content of 94.8; 5.2; 0 vol.% (I mixture—treated biogas), 35.4; 54.9; 9.7 vol.% (II mixture—”weak” biogas) and 44.6; 40; 15.4 vol.% (III mixture), respectively. The temperature at the conversion stage was 680 °C, at the regeneration stage 820 °C, and in the methanator it was 400 °C; the pressure at the conversion stage was 1.6 atm, and the vapor/C ratio = 4.
To assess the stability of the process and the reliability of the laboratory unit, the authors used in addition to the main gas mixture (mixture #1), simulating enriched biogas, also “weak” biogas (mixture #2) and a mixture simulating typical biogas from a landfill in the stage of active methanogenesis (mixture #3). The main results of the experiments on the steam reforming of biogases into hydrogen are presented in Table 4 [46].

Table 4.
Indicators of steam reforming of biogas.
A hydrogen-containing mixture with a hydrogen content of up to 98% vol. and methane up to 2% vol. was obtained experimentally when methane was supplied to the reformer (about 95% vol.) in a wide range of loads (30–100% of the nominal). Furthermore, experiments were carried out on the supply of “weak” biogas containing methane 30–45% vol. to the reformer. At the same time, the hydrogen concentration in the reformate remained high (more than 93% vol). This circumstance expands the possibilities of using the significant potential of “weak” biogases with a relatively low concentration of the methane component in the autonomous energy sector to produce electrical and thermal energy.
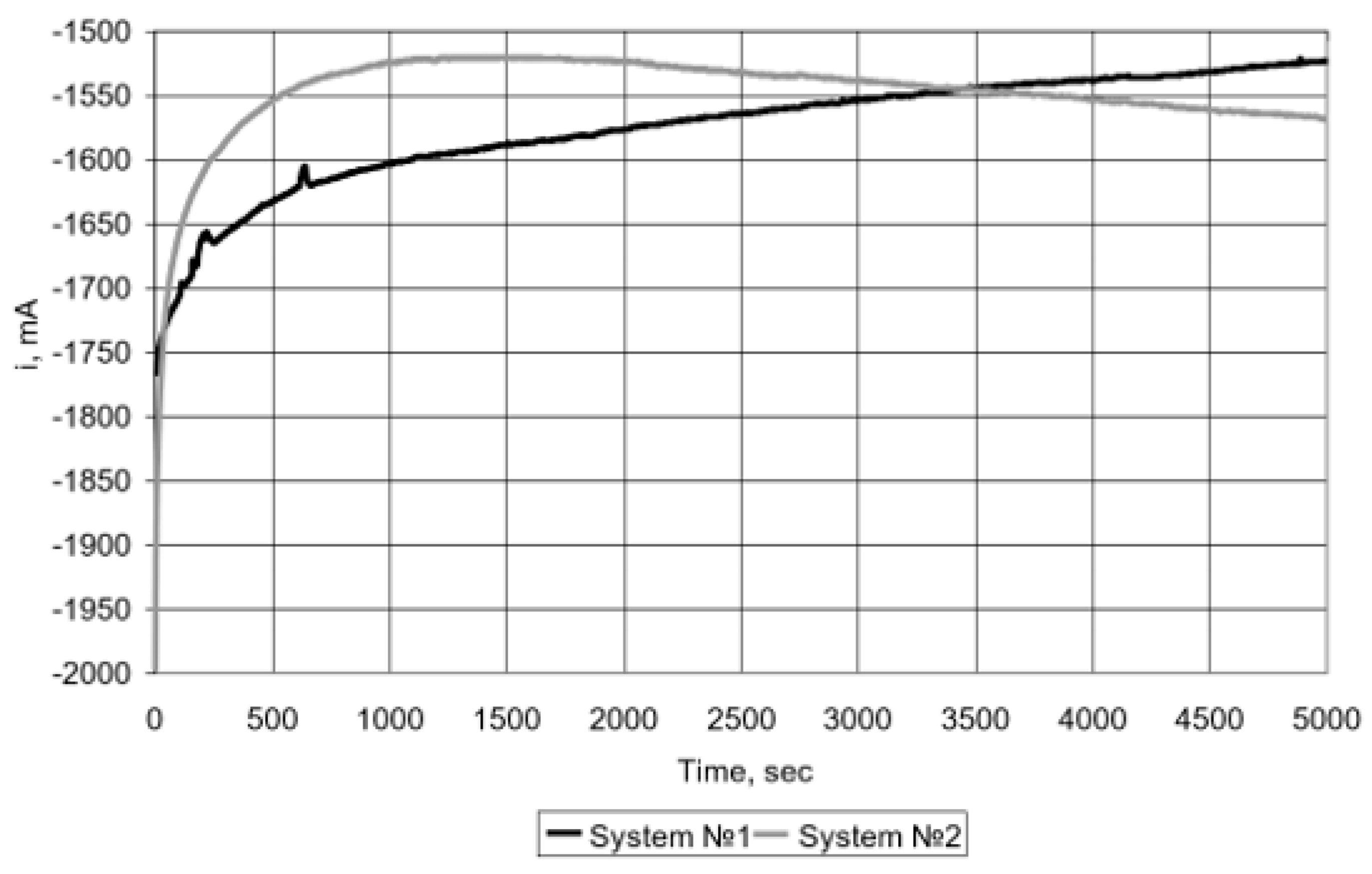
The resulting hydrogen-containing mixture (with a hydrogen content of up to 98% by volume) was fed into a low-temperature fuel cell. Tests have shown that a hydrogen mixture containing up to 2% CH4 (vol.), with direct supply (dead end) to the fuel cell in a potentiostatic mode at a constant potential E = 500 mV, using air oxygen as an oxidizer (Figure 4, curve 1), for a sufficiently long time (~(1.2 ÷ 1.4) 104 s) does not require adjustment for the discharge of unspent fuel from the anode [47,48]. Similar studies were carried out using electrolytic hydrogen (99.999% vol) (Figure 4, curve 2).
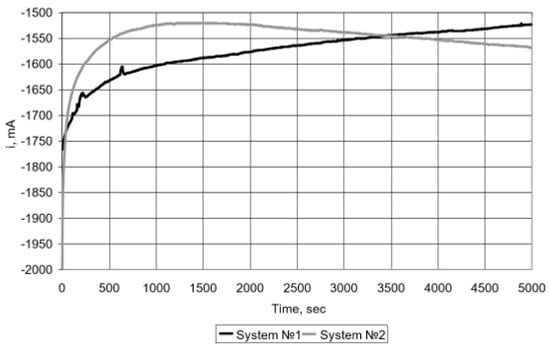
Figure 4.
Dependence of current on time (potentiostatic mode, E = 500 mV) when supplied to the fuel cell: (1) H2–98% + CH4–2% (vol.); (2) H2 (99.999% v/v).
A comparison of the data obtained showed that the fuel cell has similar current characteristics with direct supply of a hydrogen-containing mixture with a residual methane content obtained from biogas, and with the supply of electrolytic hydrogen.
However, it should be noted an increase in the number and frequency of fuel discharges to clean the anode surface of accumulating methane molecules, causing a shielding effect. This leads to some decrease in the fuel utilization factor and increases the loss of the hydrogen-containing mixture. Therefore, it is advisable to clean the hydrogen-containing mixture from residual methane.
The results of the studies performed on a fuel cell confirmed the fundamental possibility of using a hydrogen-containing mixture with a residual methane content obtained from biogas during the fermentation of organic waste (biohydrogen) under conditions of direct supply to a low-temperature fuel cell.
4. Discussion
Based on field studies, a complex was created that allows simulating the process of obtaining biogas from organic waste with its further conversion into hydrogen for supply to a fuel cell to generate electricity.
Now the authors are conducting research in laboratory conditions. The next stage of research may be the creation of a pilot industrial model with a further assessment of the possibility of commercialization of this area of energy use of biogas.
5. Conclusions
The results of modeling studies have shown that during biogas reforming, it is possible to obtain a hydrogen mixture with a hydrogen content of 98% vol and methane 2% vol. Based on the results of the research, the possibility of using the significant potential of “weak” biogas containing methane 30–45% vol to produce biohydrogen (more than 93% vol) was proved. A technique for using biohydrogen in low-temperature fuel cells for energy generation has been substantiated and tested.
Author Contributions
Conceptualization, N.P.; methodology, M.F.; investigation, V.M., V.K. and A.C.; resources, D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation under the strategic academic leadership program “Priority 2030”, agreement 075-15-2021-1333 dated 30 September 2021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ozturka, M.; Dincer, I. A comprehensive review on power-to-gas with hydrogen options for cleaner applications. Int. J. Hydrogen Energy 2021, 46, 31511–31522. [Google Scholar] [CrossRef]
- Global Hydrogen Review. IEA: Paris, France, 2021; 224.
- Baeyens, J.; Zhang, H.; Nie, J.; Appels, L.; Dewil, R.; Ansart, R.; Deng, Y. Reviewing the potential of biohydrogen production by fermentation. In Renewable and Sustainable Energy Reviews; Elsevier: Amsterdam, The Netherlands, 2020; Volume 131, p. 10. [Google Scholar]
- Mitrova, T.; Melnikov, Y.; Chugunov, D. Vodorodnaya E’konomika—put’ k Nizkouglerodnomu Razvitiyu [Hydrogen Economy—The Way to Low-Carbon Development]. In Centr E’nergetiki Moskovskoj Shkoly’ Upravleniya SKOLKOVO [Energy Center of the Moscow School of Management SKOLKOVO]; SKOLKOVO: Moscow, Russia, 2019; p. 62. [Google Scholar]
- World_Energy_Outlook_2018. 2018; p. 661. Available online: https://www.iea.org/reports/world-energy-outlook-2018 (accessed on 24 October 2022).
- Osman Ahmed, A.O.; Deka, T.J.; Baruah, D.C.; Rooney, D. Critical challenges in biohydrogen production processes from the organic feedstocks. Biomass Convers. Biorefin. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Silveira, S. The role of bioenergy in a circular bio-based economy. In Proceedings of the Applied Energy Symposium: MIT A+B, Boston, MA, USA, 22–24 May 2019. [Google Scholar]
- De Schoenmakere, M.; Hoogeveen, Y.; Gillabel, J.; Manshoven, S. The Circular Economy and the Bioeconomy Partners in Sustainability; European Environment Agency: Copenhagen, Denmark, 2018; p. 60.
- Popp, J.; Lakner, Z.; Harangi-Rákos, M.; Fári, M. The effect of bioenergy expansion: Food, energy, and environment. Renew. Sustain. Energy Rev. 2014, 32, 559–578. [Google Scholar] [CrossRef]
- Bezrukix, P.P. Spravochnik po Resursam Vozobnovlyaemy’x Istochnikov R’nergii Rossii i Mestny’m Vidam Topliva (Pokazateli po Territoriyam) [Directory of renewable energy resources in Russia and local fuels (indicators by territories)]. In IACz E’nergiya [Publishing and Analytical Center “Energia”]; Publishing and Analytical Center “Energy” (IAC “Energy”): Moscow, Russia, 2007; p. 272. [Google Scholar]
- Bioe’Nergetika Rossii v 21veke [Bioenergy of Russia in the 21st Century]; Russian Energy Agency of the Energy Ministry of Russia: Moscow, Russia, 2012; p. 37.
- Bioe’nergetika v Rossijskoj Federacii. Dorozhnaya Karta na 2019–2030 [Bioenergy in the Russian Federation. Roadmap for 2019–2030]; TP Bioenergy: Moscow, Russia, 2019; p. 28.
- An, J.; Mikhaylov, A.; Lopatin, E.; Moiseev, N.; Richter, U.H.; Varyash, I.; Dooyum, U.D.; Oganov, A.; Bertelsen, R.G. Bioenergy potential of Russia: Method of evaluating costs. Int. J. Energy Econ. Policy 2019, 9, 244–251. [Google Scholar] [CrossRef]
- Namsaraev, Z.B.; Gotovtsev, P.M.; Komova, A.V.; Vasilov, R.G. Current status and potential of bioenergy in the Russian Federation. Renew. Sustain. Energy Rev. 2018, 81, 625–634. [Google Scholar] [CrossRef]
- D’Adamo, I.; Falcone, P.M.; Huisingh, D.; Morone, P. A circular economy model based on biomethane: What are the opportunities for the municipality of Rome and beyond? Renew. Energy 2021, 163, 166–167. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallem, J.F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Holubar Peter: Steuerung von Biogasanlagen. Protokoll zur Gleichnamigen Open Space Diskussionsrunde bei der 2. österreichischen Biogastagung. 2003. Available online: https://www.nachhaltigwirtschaften.at/resources/pdf/techportraitbiogas.pdf (accessed on 24 October 2022).
- Antonini, C.; Treyer, K.; Streb, A.; van der Spek, M.; Bauer, C.; Mazzotti, M. Hydrogen production from natural gas and biomethane with carbon capture and storage—A techno-environmental analysis. Sustain. Energy Fuels 2020, 4, 2967–2986. [Google Scholar] [CrossRef]
- IEA. Outlook for Biogas and Biomethane. Prospects for Organic Growth; World Energy Outlook Special Report; IEA: Paris, France, 2020; 93p. [Google Scholar]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A Review of Biogas Utilisation, Purification and Upgrading Technologies: Review. Waste and Biomass Valorizatio; Springer: Cham, Switzerland, 2017; Volume 8, pp. 267–283. [Google Scholar]
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for transformation of biogas to biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Kadam, R.; Panwar, N.L. Recent advancement in biogas enrichment and its applications. Renew. Sustain. Energy Rev. 2017, 73, 892–903. [Google Scholar] [CrossRef]
- Rosa, L.; Mazzotti, M. Potential for hydrogen production from sustainable biomass with carbon capture and storage. Renew. Sustain. Energy Rev. 2022, 157, 1–10. [Google Scholar] [CrossRef]
- Sgobbi, A.; Nijs, W.; De Migliob, R.; Chiodi, A.; Gargiulo, M.; Thiel, C. How far away is hydrogen? Its role in the medium and long-term decarbonisation of the European energy system. Int. J. Hydrogen Energy 2016, 41, 19–35. [Google Scholar] [CrossRef]
- De Gioannis, G.; Friargiu, M.; Massi, E.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. Biohydrogen production from dark fermentation of cheese whey: Influence of pH. Int. J. Hydrogen Energy 2014, 39, 20930–20941. [Google Scholar] [CrossRef]
- Usman, T.M.; Banu, J.R.; Gunasekaran, M.; Kumar, G. Biohydrogen production from industrial wastewater: An overview. Bioresour. Technol. Rep. 2019, 7, 100287. [Google Scholar] [CrossRef]
- Zhu, G.; Li, J.; Liu, C.; Huang, X.; Li, L. Simultaneous production of biohydrogen and methane from soybean protein processing wastewater treatment using anaerobic baffled reactor (ABR) Desalin. Water Treat. 2013, 53, 2675–2685. [Google Scholar] [CrossRef]
- Zhu, M.J.; Lin, H.N. Biohydrogen production from waste biomass. Trends Renew. Energy 2016, 2, 54–55. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Nikolskava, A.B.; Lyagin, I.V.; Senko, O.V.; Makhlis, I.A.; Stepanov, N.A.; Maslova, O.V.; Mamedova, F.; Varfolomeev, S.D. Production of biofuels from pretreated microalgae biomass by anaerobic fermentation with immobilized Clostridium acetobutylicum cells. Bioresour. Technol. 2012, 114, 342–348. [Google Scholar] [CrossRef]
- Efremenko, E.; Stepanov, N.; Nikolskava, A.; Senko, O.; Gudkov, D.; Spiricheva, O.; Varfolomeev, S. Immobilized Cells of Various Microorganisms in Production of Liquid Biofuels. In Book 18: European Biomass Conference and Exibition; Spitzer, J., Dallemand, J.F., Baxter, D., Ossenbrink, H., Grassi, A., Helm, P., Eds.; ETA-Florence Renewable Energies: Lyon, France, 2012; pp. 1753–1758. [Google Scholar]
- Bernhard, L.; Lukas, I.; Andreas, L.; Hans, O. Biological hydrogen methanation—A review. Bioresour. Technol. 2017, 245, 1220–1228. [Google Scholar]
- Davis, R.; Richard, O.; David, M.W.; Jerry, D.M. Biological hydrogen methanation systems—An overview of design and efficiency. Bioengineered 2019, 10, 604–634. [Google Scholar]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shahd, N.; Warda, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Kaiwena, L.; Bina, Y.; Taoa, Z. Economic analysis of hydrogen production from steam reforming process: A literature review. Energy Sources B Econ. Plan. Policy 2018, 13, 109–115. [Google Scholar] [CrossRef]
- Liguori, S.; Kian, K.; Buggy, N.; Anzelmo, B.H.; Wilcox, J. Opportunities and challenges of low-carbon hydrogen via metallic membranes. Prog. Energy Combust. Sci. 2020, 80, 23. [Google Scholar] [CrossRef]
- Fyodorov, M.P.; Maslikov, V.I.; Chusov, A.N. Model’ Uchebno-E’Ksperimental’Nogo Kompleksa Dlya Preobrazovaniya E’Nergii Biomassy’ [Model of an Educational and Experimental Complex for the Conversion of Biomass Energy]. Fundamental’ny’e Essledovaniya v Texnicheskix Universitetax. Materialy’ XII Vserossijskoj Konferencii po Problemam Nauki i Vy’sshej Shkoly’. Available online: https://www.elibrary.ru/item.asp?id=21393373 (accessed on 24 October 2022).
- Fedorov, M.P.; Cheremisin, A.V.; Maslikov, V.I. Avtomatizirovanny’j Uchebno-Nauchny’j Laboratorny’j Kompleks “Bioreaktor” Dlya Issledovaniya Processov Biorazlozheniya Tverdy’x By’tovy’x Otxodov [Automated Educational and Scientific Laboratory Complex “Bioreactor” for the Study of the Processes of Biodegradation of Solid Household Waste]. Reg. ‘Naya E’Kologiya. Available online: https://www.elibrary.ru/item.asp?id=21442850 (accessed on 24 October 2022).
- Fedorov, M.P.; Maslikov, V.I.; Chusov, A.N.; Molodczov, D.V. E’ksperimental’ny’j Kompleks Dlya Proizvodstva Vodoroda iz Organosoderzhashhix Otxodov Dlya Primeneniya v Toplivny’x E’lementax [Experimental Complex for the Production of Hydrogen from Organo-Containing waste for Use in Fuel Cells]. Nauchno-Texnicheskie Vedom. SPbGPU. Available online: https://cyberleninka.ru/article/n/eksperimentalnyy-kompleks-po-proizvodstvu-vodoroda-iz-organosoderzhaschih-othodov-dlya-primeneniya-v-toplivnyh-elementah (accessed on 24 October 2022).
- Lysikov, A.I.; Trukhan, S.N.; Okunev, A.G. Sorption enhanced hydrocarbons reforming for fuel cell powered generators. Int. J. Hydrogen Energy 2008, 33, 3061–3066. [Google Scholar] [CrossRef]
- Livshicz, A.I. Sverxproniczaemost’ Metallov po Vodorodu i Membrannoe Vy’delenie Vodoroda iz Gazovy’x Smesej [Super-Permeability of Metals by Hydrogen and Membrane Separation of Hydrogen from Gas Mixtures]. Chetvertaya Rossijskaya Konferenciya «Fizicheskie Problemy’ Vodorodnoj E’nergetiki» [The Fourth Russian Conference “Physical Problems of Hydrogen Energy”]—SPb, St. Petersburg, Russia, 2007; pp. 1–3. Available online: https://engtech.spbstu.ru/userfiles/files/articles/2013/4/8_chusov.pdf (accessed on 24 October 2022).
- Fedorov, M.P.; Zubkova, M.Y.; Thomasov, A.A.; Molodtsov, D.V.; Zelenina, N.K. Investigation of Process Direct Feed Converter Biohydrogen with Residual Methane Content In the Fuel Cell. In Proceedings of the Reports. 7th Russian Conference “Physical Problems of Hydrogen Energy”. St. Petersburg, Russia, 2011. FTI. Joffe. Available online: https://www.scientific.net/AMR.941-944.2107 (accessed on 24 October 2022).
- Maslikov, V.I.; Chusov, A.N.; Negulyaeva, E.Y.; Cheremisin, A.V.; Molodczov, D.V. Laboratorny’e Issledovaniya Razlozheniya Otxodov v Bioreaktorax Dlya Ocenki Biogazovogo Potenciala i Vy’bora Meropri-yatij po Rekul’tivacii Poligonov TBO [Laboratory Studies of Waste Decomposition in Bioreactors to Assess the Biogas Potential and Select Measures for the Reclamation of Landfills]. Nauchno-Texnicheskie Vedom. SPbGPU. Available online: https://www.elibrary.ru/item.asp?id=17937554 (accessed on 24 October 2022).
- Zhazhkov, V.V.; Chusov, A.N.; Politaeva, N.A. Research and assessment of biogas composition at the TKO running and recommendations for its use. Ecol. Ind. Russ. 2021, 25, 4–9. [Google Scholar] [CrossRef]
- Politaeva, N.; Smyatskaya, Y.; Al Afif, R.; Pfeifer, C.; Mukhametova, L. Development of Full-Cycle Utilization of Chlorella sorokiniana Microalgae Biomass for Environmental and Food Purposes. Energies 2020, 13, 2648. [Google Scholar] [CrossRef]
- Lysikov, A.I.; Salanov, A.N.; Okunev, A.G. Change of CO2 carrying capacity of CaO in Isothermal Recarbonation-Decomposition Cycles. Ind. Eng. Chem. Res. 2007, 46, 4633–4638. [Google Scholar] [CrossRef]
- Lysikov, A.I.; Okunev, A.G.; Molodtsov, D.V.; Maslikov, V.I. Novel approach for municipal solid waste biogas reforming into hydrogen for fuel cell powered generators. In Proceedings of the XIX International Conference of Chemical Reactors “Chemreactor-19”, Vienna, Austria, 5–9 September 2010; pp. 192–193. [Google Scholar]
- Chusov, A.N.; Zubkova, M.Y.; Korablev, V.V.; Maslikov, V.I.; Molodczov, D.V. Texnologiya Ispol’zovaniya v Toplivny’x e’lementax Vodorodosoderzhashhej Smesi na Osnove Biogazov Dlya E’nergoobespecheniya Avtonomny’x Potrebitelej [Technology of Using Hydrogen-Containing Mixture Based on Biogas in Fuel Cells for Energy Supply of Autonomous Consumers]. Nauchno-Texnicheskie Vedom. SPbGPU. Available online: https://www.elibrary.ru/item.asp?id=21065414 (accessed on 24 October 2022).
- Zubkova, M.Y.; Maslikov, V.I.; Molodtsov, D.V.; Chusov, A.N. Experimental research of hydrogenous fuel production from biogas for usage in fuel cells of autonomous power supply systems. Adv. Mater. Res. 2014, 941–944, 2107–2111. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).