1. Introduction
Mercury (Hg) is a highly toxic element that poses a serious threat to human health. The main source of Hg emissions into the atmosphere is through the combustion of coal for power generation, with the predominant share originating from industrial power plants.
Reported technologies currently applied for Hg separation formed during the coal combustion process may be divided into the following two main groups:
Modifications to the existing flue gas treatment systems (decrease in flue gas temperature, sorbent injection, design modifications);
New technologies, including pre-combustion Hg removal as well as simultaneous removal of multiple gaseous pollutants.
The method for Hg separation from fuel by thermal treatment (low-temperature pyrolysis) before combustion is an interesting alternative to the methods focusing on Hg removal from flue gases. This is due to higher Hg concentrations and much smaller amounts of purified gas compared to flue gas. The possibility of using low-temperature pyrolysis for simultaneous desulfurization and denitrification of fuel is an additional advantage [
1].
Published simulations of systems operating on an industrial scale also indicate that the use of low-temperature pyrolysis is a competitive solution to traditional methods of reducing Hg emissions from the coal combustion process [
2].
The majority of publications examine the effect of temperature and residence time on the degree of Hg removal from coal [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17] and identify the forms of Hg while searching for a relationship between their removal and process temperature [
10,
12,
17,
18,
19,
20]. The published data related to Hg removal by low-temperature pyrolysis implies that, for various sorts of hard coal and different residence times, the average Hg removal was 10–38% at 200 °C, 20–40% at 250 °C, 0–90% at 300 °C, 19–25% at 350 °C, and 20–100% at 400 °C. In most cases, a temperature of 400 °C is sufficient to remove most of the Hg from coal by low-temperature pyrolysis. However, the heterogeneous nature of coal and the lack of research describing the mechanism of Hg removal from coal requires an individual approach for each considered case. The literature concerning Hg removal from coal by low-temperature pyrolysis is listed in
Table 1.
Only a few publications describe the effect of the pyrolysis process, including process temperature, on the properties of the purified fuel, which is critical in terms of practical pyrolysis applications. Similarly, the distribution and characterization of other products generated by the pyrolysis process are of interest for future applications. Merdes et al. [
7] investigated the change of volatile parts in purified fuel and emphasized the importance of maintaining the calorific value of the fuel in addition to achieving high degrees of Hg separation. Wichliński [
15] did not determine the loss of calorific value of fuel when analyzing the loss of carbon (C) element emitted during the pyrolysis process. Skodras [
12] showed the reactivity of the resulting carbonates and co-correlated Hg removal with the removal rate of sulfur, nitrogen, C, and volatile parts. However, their composition or the chemical enthalpy loss of the fuel was not provided. Sotiropoulou [
21] examined the correlations between Hg removal and C conversion expressed as the loss of volatile fuel components. Only the WRI report [
2] on the implementation of pilot studies for the process temperatures of 300 °C contained data related to parameters for specific US coals (550–600 °F).
The effects of temperature and time of the pyrolysis process on the degree of Hg removal and the characteristics of the fuel obtained were studied in this paper. The study was carried out using Polish reference coals used in the power industry. The goal of the study was to determine the pyrolysis process temperatures that allow for efficient Hg removal while reducing the fuel’s degradation rate by measuring the change in the chemical enthalpy flux. Furthermore, we determined the properties of the obtained fuels as a function of process temperature, as well as the distribution of pyrolysis products and the parameters of gaseous products produced by the process.
2. Materials and Methods
2.1. Samples
The presented study was conducted using lignite BC1, BC2 (from the “Bełchatów” coal mine, Bełchatów, Poland), hard coals HC1, HC2 (from the “Janina” coal mine, Libiąż, Poland), and hard coal HC3 (from the “Wieczorek” coal mine, Katowice, Polnad). The fuels’ characteristics are provided in
Table 2. The samples from the pyrolysis tests were prepared in accordance with PN-90/G-04502 (air-dried basis, ground, and sieved < 0.2 mm).
2.2. Pyrolysis
2.2.1. Pyrolysis–Scale 1–19 g
The laboratory studies concerning the impact of coal temperature and residence time on the degree of Hg removal from lignite and hard coals were conducted using a thermogravimetric analyzer TGA 701 (LECO Corporation, St. Joseph, MI, USA). The weight of the sample was approx. 1 g and 19 specimens were analyzed simultaneously in nitrogen. The tests were conducted over a temperature range of 200–500 °C with residence times of 0–10 min for lignite and 0–8 min for hard coals. The process conditions of the performed studies are detailed in
Table 3. For analysis, coal specimens with a grain size below 0.2 mm were employed. A coal specimen was weighed separately into 19 open ceramic crucibles (coal sample per crucible: 1 ± 0.5 g) and introduced into the analyzer. The samples were heated to a specific temperature at the rate of approx. 36 K/min under a nitrogen atmosphere (with nitrogen flow rate of 3.5 L/min). After the pyrolysis process was completed, the analyzer remained closed, and the samples were cooled under a nitrogen atmosphere to room temperature. Then, the charred specimens were collected into one vessel, and Hg content and other relevant physical and chemical properties were measured using procedure Q/LCA/32/A: 2021 of the Institute of Energy and Fuel Processing Technology, based on International Standard ISO 15237:2016 [
22].
2.2.2. Pyrolysis–Scale ~ 100 g
The obtained carried out on lab-scale (~1 g) were verified on the workstation of low-temperature pyrolysis in the fixed-bed (the weight of the sample was approx. ~100 g). The presented research setup was created in cooperation with Joint Research Center, Institute for Energy Petten (The Netherlands). The test setup included an electric furnace, a steel retort (where the tested materials were converted), and a system for collecting the liquid and gaseous fractions. The instrument’s specification required the use of particle size samples in the range of 0.5–3.14 mm. The retort with fuel was introduced into the furnace and heated at a rate of 10 °C/min to 250 °C and 300 °C for lignite, and a temperature range of 350–550 °C for hard coals (temperature was selected based on TGA results and the current lab-scale results). In the case of lignite after reaching the required final temperature of the pyrolysis process, heating of the retort was stopped and the residence time of the coal at the final temperature of the process was 0 min. At this time the retort was immediately removed from the heating zone without opening and cooled down to room temperature under a nitrogen atmosphere. In the case of hard coal, tests were carried out for the following two residence times:
When the test temperature reached the set temperature, heating was immediately stopped by removing the retort from the furnace heating zone and allowed to cool to room temperature under an inert atmosphere by continuously purging it with nitrogen. The residence period of the sample at the given temperature was assumed to be 0 min in the test;
Once the test temperature reached the set temperature, the sample was maintained in the furnace’s heating zone for another 20 min before being removed and cooled to room temperature under a nitrogen atmosphere. The residence period of the sample at the given temperature was supposed to be 20 min in this test. (see
Table 4).
In the process, continuously liquid and gaseous products were collected for detailed qualitative and quantitative analysis. Analysis of the gaseous products (i.e., to determine gaseous products composition, organic compounds content, high-heat value, and density) was carried out using a gas chromatograph with FID (Flame Ionization Detector), TCD (thermal conductivity detector), PFPD (Pulsed Flame Photometric Detector) detector (model CP3800, Varian, Palo Alto, CA, USA). Determination of Hg content in the pyrolytic gas consists of transmission definite volume of research gas by activated carbon sorbents where mercury was adsorbed.
2.3. Mercury Determination
Determination of Hg content was achieved using an MA-2 analyzer (Nippon Instrument Corporation, Osaka, Japan).
The measuring method consisted of the amalgamation of mercury vapors after thermal decomposition of the sample in the analyzer. Detection was performed using a non-dispersive double-beam Cold Vapor-Atomic Absorption (CVAAS) Spectrophotometer. Determination of the total Hg content was performed in accordance with reference [
23] procedure Q/LCA/32/A: 2021 of Institute of Energy and Fuel Processing Technology, based on International Standard ISO 15237:2016. For calibration, NIST Certified Reference Materials were used. The effectiveness of Hg removal from the fuel was established based on the measurements of Hg content in coal and char received in pyrolysis process.
3. Results and Discussion
3.1. Studies of the Impact of Coal Temperature and Residence Time on the Degree of Hg Removal from Fuel
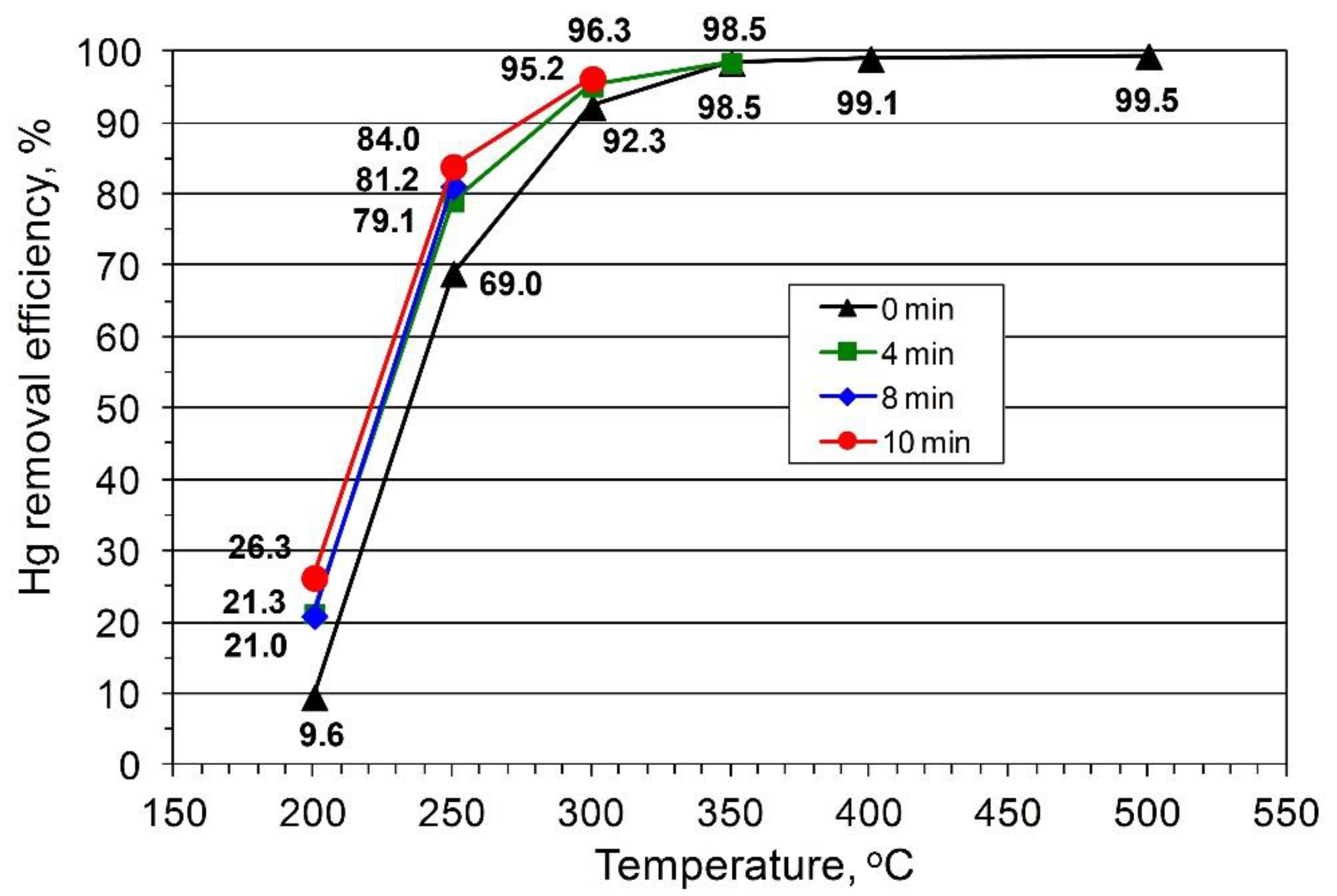
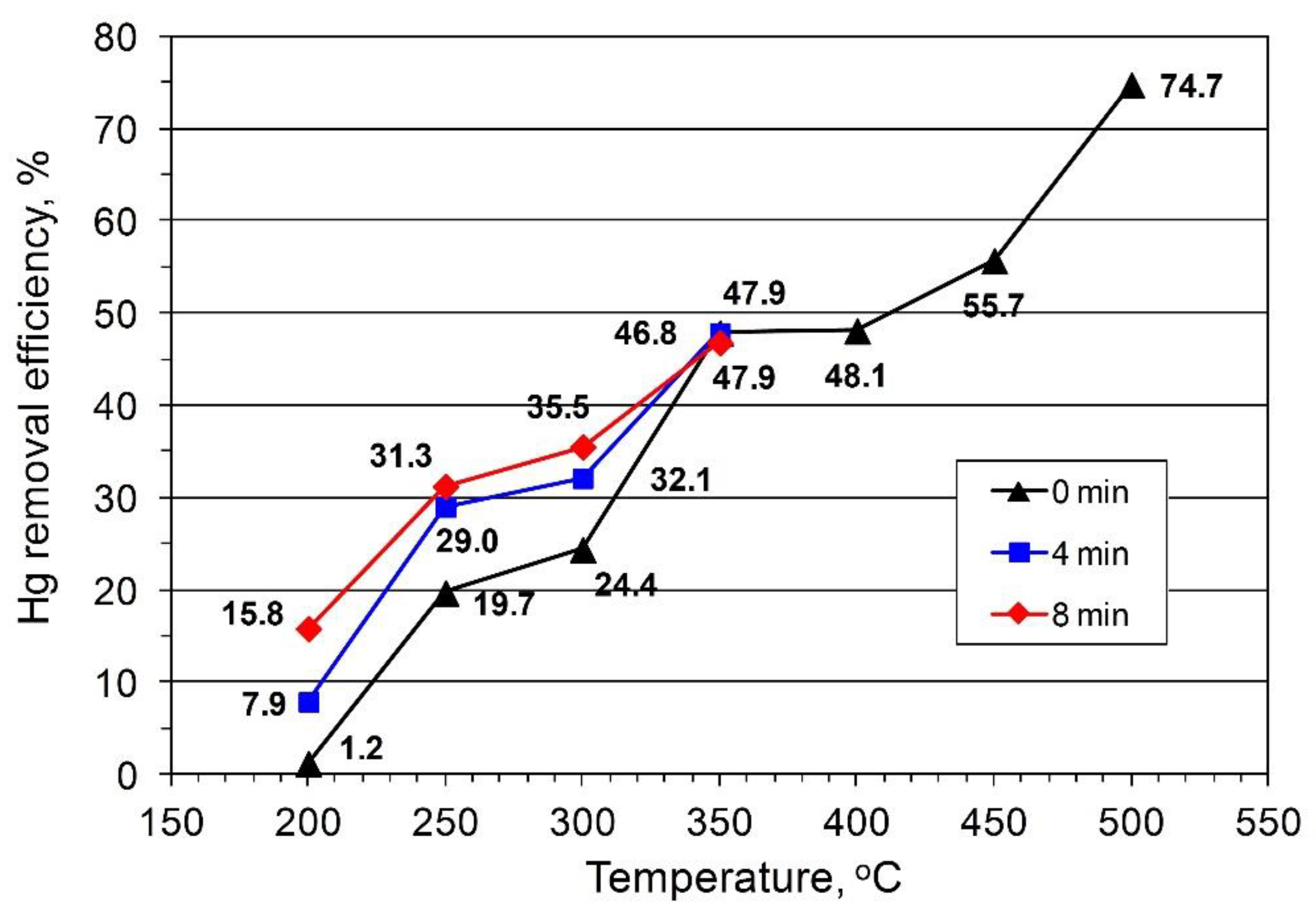
The results of the impact of coal temperature and residence time on the degree of Hg removal from fuel are presented in
Figure 1 and
Figure 2. In the case of lignite (BC1, BC2), the average Hg content decreased during the pyrolysis process at 200 °C (the degree of Hg removal depending on the residence time of the fuel in the reaction zone was 9.6–26%;
Figure 1). When the temperature was increased to 250 °C, the Hg content in the fuel showed a significant decrease and the Hg removal efficiency was 69–84%. A further increase in temperature to 300 °C increased the degree of Hg removal to 90%. The average Hg removal efficiency ratio obtained for hard coals (HC1, HC2, HC3) was far lower. At 200 °C, the decrease in Hg content was insignificant, and, depending on the residence time, the degree of Hg removal varied between 1.2% and 16% (
Figure 2).
Hg content showed a considerable reduction in the fuel and 50% removal efficiency was attained at 350 °C. Compared to the results presented in
Figure 1,
Figure 2 shows that a further increase in the process temperature to 400 °C did not affect Hg removal efficiency, and only at 450–500 °C was an increase up of to 56% and 75% observed, respectively. A temperature increase in the range of 200–500 °C led to an 8–39% loss in the coal weight for lignite (BC1, BC2) and 11–27% for hard coals (HC1, HC2, HC3). The highest decrease occurred within the range of 300–500 °C (27%) and 400–500 °C (12%) for lignite (BC1, BC2) and hard coals (HC1, HC2, HC3), respectively. The residence time of the fuel remaining in the reaction system affected Hg removal efficiency, with the largest impact exerted at low temperatures (200, 250, and 300 °C,
Figure 1 and
Figure 2) and within the residence time range of 0–4 min.
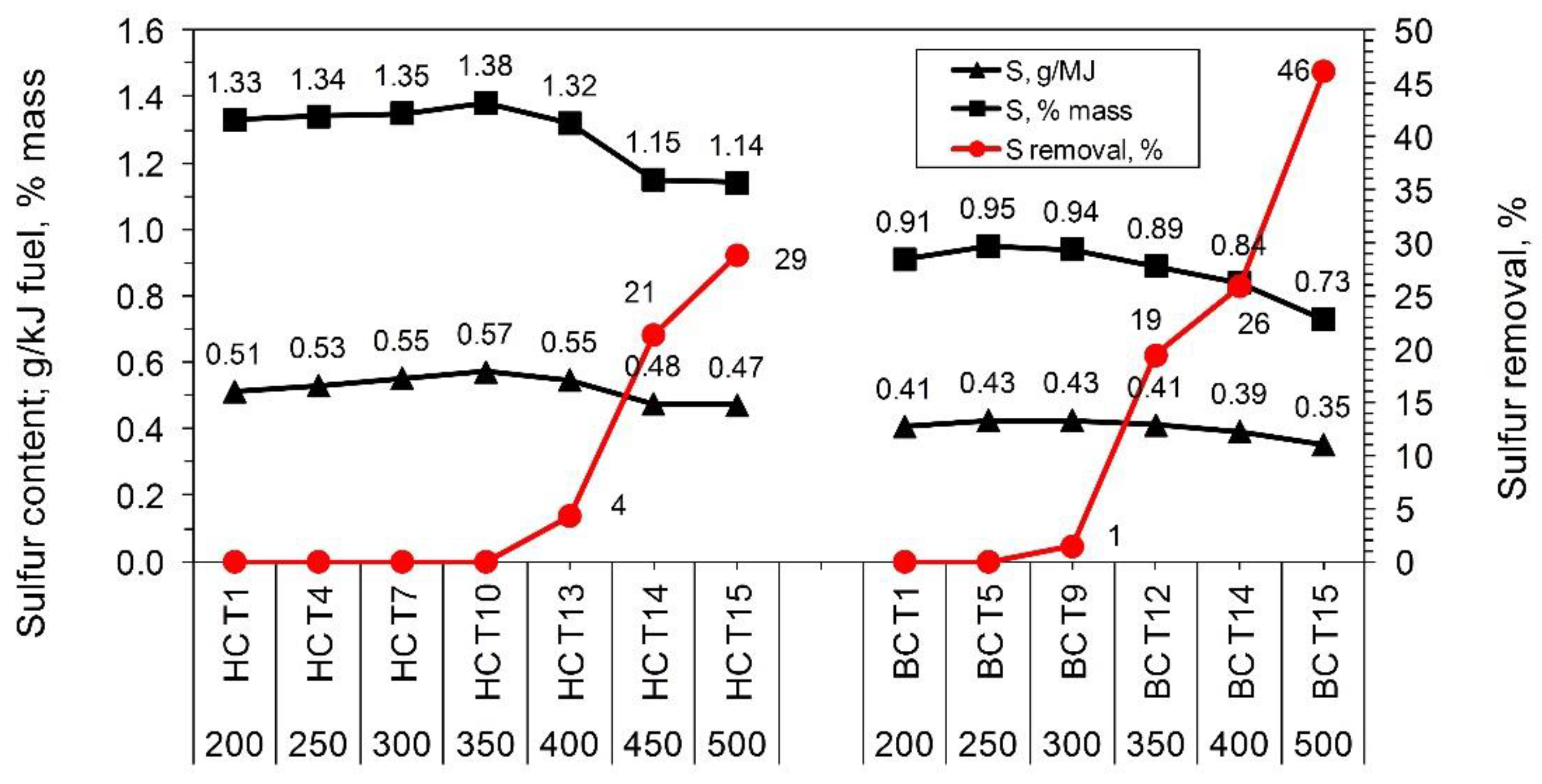
Additionally, thermal coal processing enables sulfur removal from fuel [
1]. The studies conducted showed an increase in the process temperature caused a reduction of sulfur content in solid products. The average removal efficiency obtained at 500 °C was 29% and 46% for hard coals (HC1, HC2, HC3) and lignite (BC1, BC2), respectively (
Figure 3).
For hard coals (HC1, HC2, HC3), within the temperature of 200–250 °C and 350–400 °C, the observed average changes in Hg removal with temperature was negligible and the removal efficiency curve assumed a characteristic shape. The characteristic shape, including the occurrence of a local maximum at a temperature of approx. 400 °C, was explained by the transfer of the coal into its plastic state and a change in its porous structure (porosity loss) [
21,
24]. The temperature at which the maximum removal efficiency occurred depended on the degree of carbonification, heating rate, grain size, and plastic properties of the analyzed fuel. In the case of American bituminous coals with a high content of volatiles, the maximum removal efficiency was 400–500 °C [
21,
24]. The shape of the efficiency curve (
Figure 2) was primarily due to the distribution of Hg forms in the coals because the coals did not reach their plastic state during the temperature range studied. This was evident by analyses conducted according to the PN-G-04565:1994 standard, which could influence the shape of the curve. According to reports, the pyrolysis process, especially the degree of Hg removal, is affected by the type of coal studied. However, the observed discrepancies in the data indicated that the type of Hg contained in the coals tested, rather than the physicochemical features of the coal, affects the efficacy of Hg removal [
9,
10,
18].
Sequential leaching employed (NH
4C
2H
3O
2, HCl, HF, and HNO
3) for testing of Hg distribution in typical American coals indicated that in the case of bituminous coals, more Hg was associated with supplied minerals (up to 65%), while in sub-bituminous coals, Hg was associated mostly with organic matter (up to 100%) [
25].
3.2. Influence of Process Parameters on the Properties of Refined Fuel
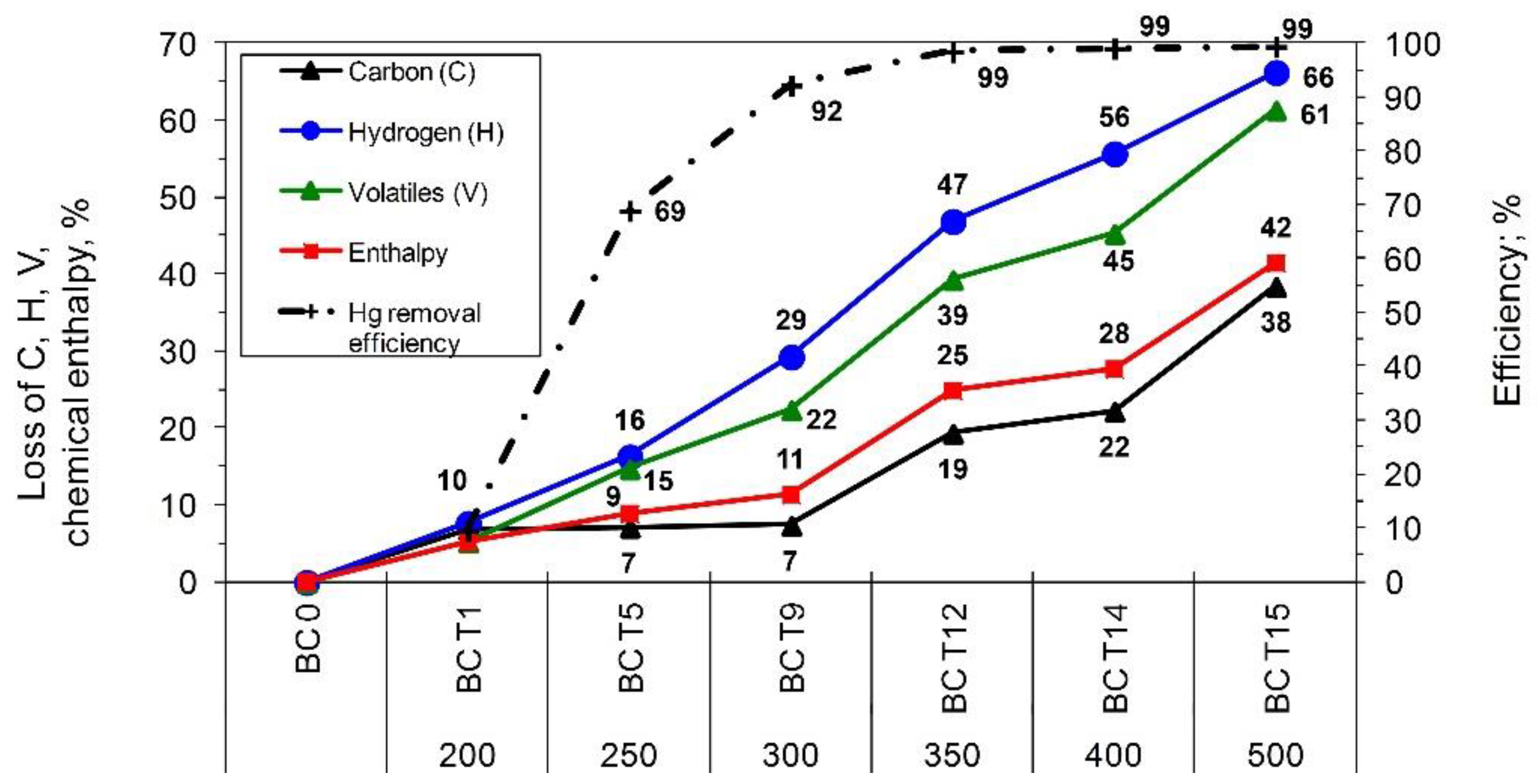
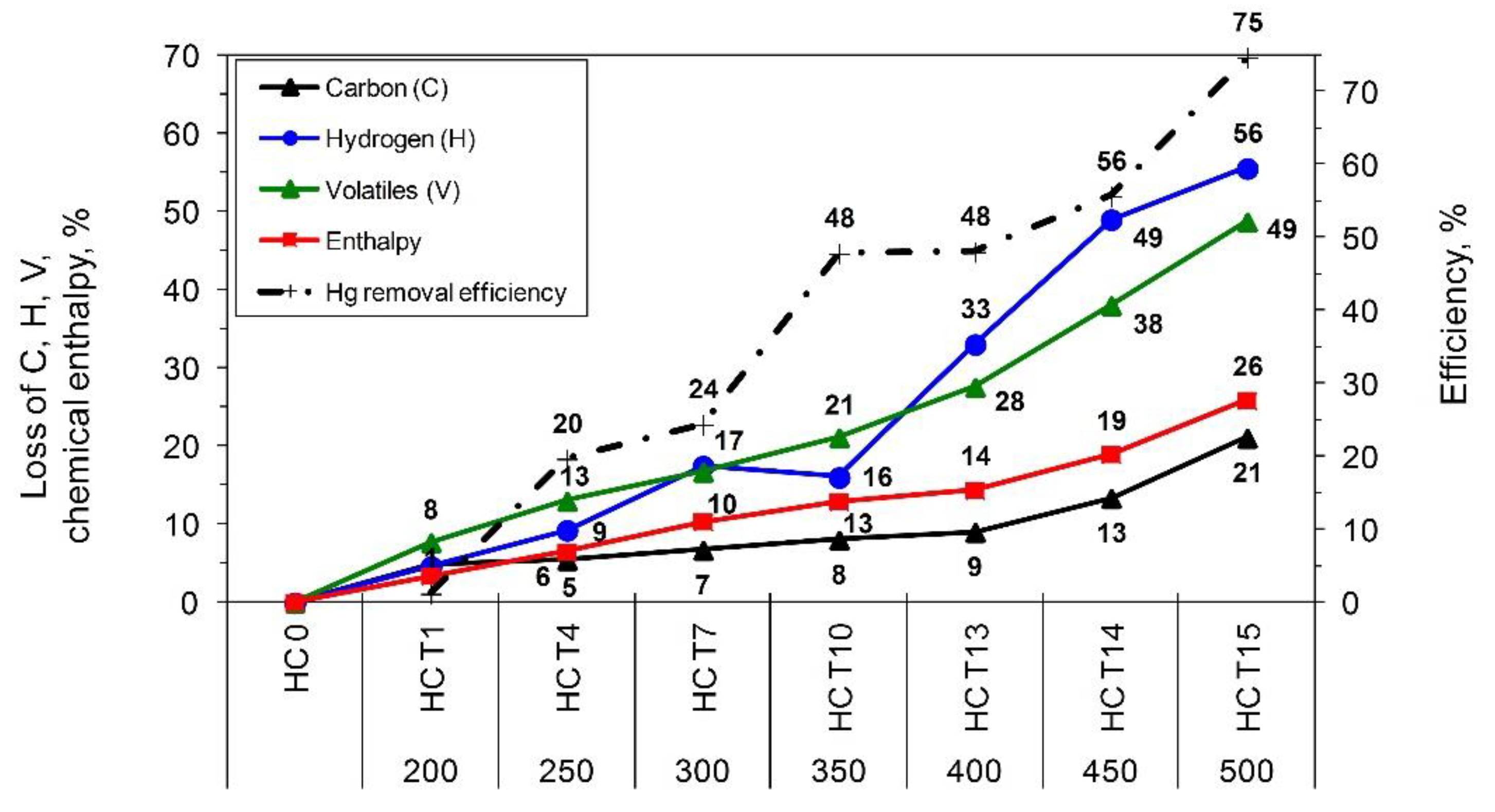
The properties of the coal used and the chars produced are illustrated in
Figure 4 and
Figure 5. Increasing the pyrolysis temperature reduced the moisture and volatile content, which increased the percentage loss of chemical enthalpy of the char. We can speak about refined fuel since the low-temperature pyrolysis method, in this case, is utilized to greatly improve the quality of coal by eliminating mercury from it. Both the lignite and hard coals processes initially led to elevated heating values (due to the removal of moisture from the fuel) and subsequently to its decrease. However, for hard coals (HC1, HC2, HC3) and lignite (BC1, BC2), within the analyzed temperature range, the higher heating value was maintained at a level similar to that of raw coal. For the air-dried state, fuel properties for lignite changed more rapidly with temperature changes, and the changes were observed at 250 °C, whereas further process temperature increases exerted a far larger impact on the char properties than for hard coals. Increasing the pyrolysis process temperature to 500 °C led to an average reduction of C, hydrogen (H), and volatiles (V) content in the char by 38%, 66%, and 61%, respectively, compared to the input fuel. A very sudden drop in chemical enthalpy (by 42%) of the char was also observed (
Figure 4). For hard coals, noticeable changes in the fuel properties were observed within the temperature range of 300–350 °C, and a further increase in temperature to 500 °C caused a considerable reduction in H, C, and V content (56%, 21%, 49%), as well as a reduced fuel enthalpy of 26% (
Figure 5). Comparison of the average changes in the fuel properties and average Hg removal efficiency revealed that the optimum process temperature values for lignite (BC1, BC2) and hard coals (HC1, HC2, HC3) were at 300 °C and 350 °C, respectively (
Figure 4 and
Figure 5).
3.3. Verification of the Results of Bench Scale Experimental Test
The process parameters and refined fuel composition are presented in
Table 5. Additionally,
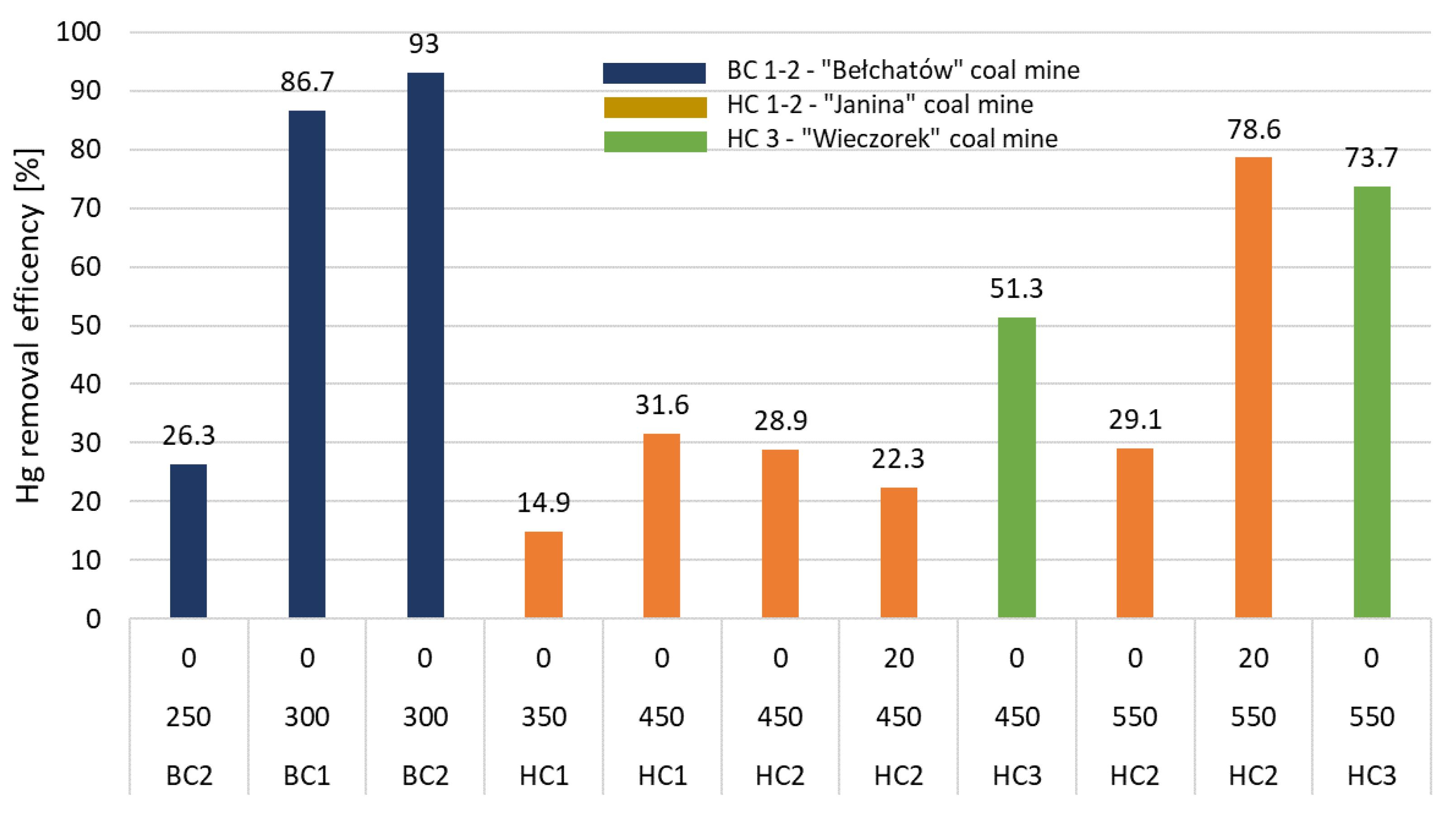
Table 5 shows the Hg removal efficiency from tested coal and the loss of chemical enthalpy of purified fuel compared to the input fuel. The Hg removal efficiency for particular samples of coal is illustrated in
Figure 6.
Results obtained for lignite (BC1, BC2) confirmed high Hg removal efficiency. When the treatment was conducted at 300 °C, an Hg removal efficiency of 86.7% for BC1 and 93.0% for BC2 was achieved. The refined fuel (char) was thought to have a high calorific value and low moisture content. The percentage loss of chem. enthalpy of purified fuel compared to the input fuel reached 10%.
In the case of tests conducted using hard coal HC1 (from the “Janina” coal mine) at 350 °C, the determined Hg removal efficiency was 14.9% (i.e., 24.1% lower than research conducted on a lab-scale-TGA). It was observed that increased temperature to 450 ℃ significantly impacted Hg removal efficiency by approx. 16.7% (the current efficiency of Hg removal was 31.6%). Further tests of low-temperature pyrolysis conducted on a lab scale (~100 g) using a new hard coal sample from the “Janina” coal mine (HC2) confirmed the earlier results. At 450 °C, independent of the residence time of the fuel in the reaction zone, the removal efficiency was determined as 28.9% (
Figure 6,
Table 5). A significant increase in Hg removal efficiency (78.6%,
Figure 6) was obtained at 550 °C with a residence time of 20 min. Unfortunately, this was connected with a significant loss of fuel chem. enthalpy (up to 20%).
The obtained results from hard coal HC3 (from the “Wieczorek” coal mine) showed that a considerable reduction of Hg content in the fuel using low-temperature pyrolysis was possible. Hg removal efficiency at 550 °C reached 73.7% for HC3 (residence time of 0 min). However, as in the case of HC2, a decrease in chem. enthalpy (by 20%) of char was also observed. Yields of the pyrolysis products are provided in
Table 6. Characteristics of the gaseous pyrolysis products are provided in
Table 7.
4. Conclusions
The introduction of Hg emission standards in the coal-fired power industry will, in most cases, result in the necessity of implementing additional technologies to clean flue gases. Apart from the best commercially developed technologies related to sorbent injection, other technological solutions are worth considering, including Hg removal from fuel prior to its combustion by means of thermal treatment.
The results of our research with Polish coals confirmed the possibility of obtaining high Hg removal efficiency from coal before the combustion process using the low-temperature pyrolysis process. The implementation of a moderate process temperature provided removal efficiencies of 50% and 90% for hard coal and lignite, respectively. Moreover, such high Hg removal efficiency was obtained with relatively low losses of chem. enthalpy of the fuel, which did not result in loss of its functional properties according to lab-scale tests and was verified for lignite on a large lab-scale using a rotary kiln system. The results indicated that the optimal process temperature for the tested coal was 300 °C, which gave an Hg removal efficiency of over 90% with a 4% decrease in chem. Enthalpy of the fuel. The obtained results may constitute the development, verification, and demonstration of Hg removal technology using the pyrolysis process at a pilot scale.
Unlike traditional gas purification systems used in power plants, which remove Hg from the waste gas stream, our presented approach is low-cost and easier to implement on a larger scale due to the small gas stream leaving the processing system.
Author Contributions
Conceptualization, E.M. and T.C.; Investigation, E.M.; Methodology, E.M. and T.C.; Supervision, E.M. and T.C.; Writing—original draft, E.M., T.C., I.M., and M.S.; Writing—review and editing, E.M. and T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project “Development of coal gasification technology for high-efficiency prod. of fuels and energy”, Task No. 3 of the Strategic Program for Research and Development: “Advanced energy generation technologies” funded by the Polish National Center for Research and Development and by an internal grand for research in ITPE, Zabrze.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The research results presented were obtained during the course of the project “Development of coal gasification technology for high-efficiency production of fuels and energy”, Task No. 3 of the Strategic Program for Research and Development: “Advanced energy generation technologies” funded by the Polish National Center for Research and Development.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, S.; Pudasainee, D.; Duan, Y.; Gupta, R.; Liu, M.; Lu, J. A review on mercury in coal combustion process: Content and occurrence forms in coal, transformation, sampling methods, emission and control technologies. Progress Energy Combust. Sci. 2019, 73, 26–64. [Google Scholar] [CrossRef]
- Chmielniak, T.M.; Głód, K.; Kopczyński, M. Pyrolysis of Coal to Reduce Mercury Emissions from Combustion into the Atmosphere. In New Technologies for Combustion and Flue Gas Cleaning; Nowak, W., Pronobis, M., Eds.; Silesian Technical University Publishing House: Gliwice, Poland, 2010. [Google Scholar]
- Guffey, F.D.; Bland, A.E. Thermal pretreatment of low-ranked coal for control of mercury emissions. Fuel Process. Technol. 2004, 85, 521–531. [Google Scholar] [CrossRef]
- Bland, A.E.; Newcomer, J.; Sellakumar, K.M. Pilot Testing of WRI’s Novel Mercury Control Technology by Pre-Combustion Thermal Treatment of Coal. Final Tech; Report November 2008 (DOE Award Number: DE-FC26-07NT42785); Western Research Institute: Laramie, WY, USA, 2008. Available online: https://www.osti.gov/bridge/purl.cover.jsp?purl=/948011SCOOSq/948011.pdf (accessed on 8 December 2021).
- Chmielniak, T.M. Reduction of mercury emissions to the atmosphere from coal combustion processes using low temp. pyrolysis-a concept of process implementation on a commercial scale. Rynek Energii 2011, 2, 176–181. [Google Scholar]
- Chmielniak, T.; Słowik, K.; Sajdak, M. Mercury removal by mild thermal treatment of coal. Fuel 2017, 195, 290–298. [Google Scholar] [CrossRef]
- Merdes, A.C.; Keener, T.C.; Khang, S.-J.; Jenkins, R.G. Investigation into the fate of mercury in bituminous coal during mild pyrolysis. Fuel 1998, 77, 1783–1792. [Google Scholar] [CrossRef]
- Wang, M.; Keener, T.C.; Khang, S.-J. The effect of coal volatility on mercury removal from bituminous coal during mild pyrolysis. Fuel Process. Technol. 2000, 67, 147–161. [Google Scholar] [CrossRef]
- Zajusz-Zubek, E.; Konieczyński, J. Dynamics of trace elements release in coal pyrolysis process. Fuel 2003, 82, 1281–1290. [Google Scholar] [CrossRef]
- Iwashita, A.; Tanamachi, S.; Nakajima, T.; Takanashi, H.; Ohki, A. Removal of mercury from coal by mild pyrolysis and leaching behavior of mercury. Fuel 2004, 83, 631–638. [Google Scholar] [CrossRef]
- Skordas, G.E.; Natas, P.; Basinas, P.; Sakellaropoulos, G.P. Effects of pyrolysis temp., residence time on the reactivity of clean coals produced from poor quality coals. Glob. Nest J. 2006, 8, 89–92. [Google Scholar]
- Skordas, G.; Natas, P.; Basinas, P.; Sakellaropoulos, G.P. Removal of pollutants from poor quality coals by pyrolysis. Therm. Sci. 2006, 10, 39–54. [Google Scholar]
- Uruski, L.; Gorecki, J.; Macherzynski, M.; Dziok, T.; Golas, J. The ability of Polish coals to release mercury in the process of thermal treatment. Fuel Process. Technol 2015, 140, 12–20. [Google Scholar] [CrossRef]
- Luo, G.; Ma, J.; Han, J.; Yao, H.; Xu, M.; Zhang, C.; Chen, G.; Gupta, R.; Xu, Z. Hg occurrence in coal and its removal before coal utilization. Fuel 2013, 104, 70–76. [Google Scholar] [CrossRef]
- Wichliński, M.; Kobyłecki, R.; Bis, Z. The release of mercury from polish coals during thermal treatment of fuels in a fluidized bed reactor. Fuel Process. Technol. 2014, 119, 92–97. [Google Scholar] [CrossRef]
- Xu, P.; Luo, G.; Zhang, B.; Zeng, X.; Xu, Y.; Zou, R.; Gan, R.; Yao, H. Influence of low pressure on mercury removal from coals via mild pyrolysis. Appl. Therm. Eng. 2017, 113, 1250–1255. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, L.; Niu, X.; Gao, L.; Cao, Y.; Wei, X.-X.; Li, X. Mercury release characteristics during pyrolysis of eight bituminous coals. Fuel 2018, 222, 250–257. [Google Scholar] [CrossRef]
- Ohki, A.; Sagayama, K.; Tanamachi, S.; Iwashita, A.; Nakajima, T.; Takanashi, H. Release behaviour of mercury during mild pyrolysis of coals and nitric acid-treated coals. Powder Technol. 2008, 180, 30–34. [Google Scholar] [CrossRef]
- Liu, L.; Duan, Y.; Wang, Y.; Wang, H.; Yin, J. Experimental study on mercury release behaviour and speciation during pyrolysis of two different coals. J. Fuel Chem. Technol. 2010, 38, 134–139. [Google Scholar] [CrossRef]
- Luo, G.; Yao, H.; Xu, M.; Grupta, R.; Xu, Z. Identifying modes of occurrence of mercury in coal by temp. programmed pyrolysis. Proc. Combust. Inst. 2011, 33, 2763–2769. [Google Scholar] [CrossRef]
- Sotiropoulou, R.E.P.; Serafidou, M.; Skodras, G. Thermal mercury removal from coals: Effect of pyrolysis conditions and kinetic analysis. Fuel 2019, 238, 44–50. [Google Scholar] [CrossRef]
- Scope of Accreditation of Institute for Chemical Processing of Coal, Laboratory of Analytical Chemistry. Available online: https://www.pca.gov.pl/akredytowane-podmioty/akredytacje-aktywne/laboratoria-badawcze/AB%20081,plik.html (accessed on 8 December 2021).
- ISO 15237:2016; Solid Mineral Fuels—Determination of Total Mercury Content of Coal. International Organization for Standardization: Geneva, Switzerland, 2016.
- Wang, B.; Li, W.; Chen, H.; Li, B.; Wang, G. The removal of mercury from coal via sub-critical water extraction. Fuel Process. Technol 2006, 87, 443–448. [Google Scholar] [CrossRef]
- Ohki, A.; Iwashita, A.; Tanamachi, S.; Nakajima, T.; Takanashi, H. Removal of mercury from coal by mild Pyrolysis and chelate extraction. Fuel Chem. Division Prepr. 2003, 48, 354. [Google Scholar]
Figure 1.
The average percentage efficiency of Hg removal from coal under low-temperature pyrolysis for lignite (BC1, BC2); dependence on process temperature at different residence times (0, 4, 8, 10 min).
Figure 1.
The average percentage efficiency of Hg removal from coal under low-temperature pyrolysis for lignite (BC1, BC2); dependence on process temperature at different residence times (0, 4, 8, 10 min).
Figure 2.
The average percentage efficiency of Hg removal from coal under low-temperature pyrolysis for hard coals (HC1, HC2, HC3); Dependence on process temperature at different residence times (0, 4, 8 min).
Figure 2.
The average percentage efficiency of Hg removal from coal under low-temperature pyrolysis for hard coals (HC1, HC2, HC3); Dependence on process temperature at different residence times (0, 4, 8 min).
Figure 3.
The average sulfur content and degree of sulfur removal vs. process temperature for hard coals (HC1, HC2, HC3) and lignite (BC1, BC2).
Figure 3.
The average sulfur content and degree of sulfur removal vs. process temperature for hard coals (HC1, HC2, HC3) and lignite (BC1, BC2).
Figure 4.
The average change of parameters and composition of refined fuel for lignite; percentage loss of C, H, and V, content, fuel chem. enthalpy vs. process temperature.
Figure 4.
The average change of parameters and composition of refined fuel for lignite; percentage loss of C, H, and V, content, fuel chem. enthalpy vs. process temperature.
Figure 5.
Average change of parameters and composition of refined fuel for hard coals; percentage loss content of C, H, and V, fuel chem. enthalpy vs. process temperature.
Figure 5.
Average change of parameters and composition of refined fuel for hard coals; percentage loss content of C, H, and V, fuel chem. enthalpy vs. process temperature.
Figure 6.
Hg removal efficiency of bench scale experimental test, %.
Figure 6.
Hg removal efficiency of bench scale experimental test, %.
Table 1.
Published data concerning Hg removal from coal by low-temperature pyrolysis [
2,
4,
5,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21].
Table 1.
Published data concerning Hg removal from coal by low-temperature pyrolysis [
2,
4,
5,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21].
| Lp. | Sample Characteristics | Characteristics of the Process | Hg Removal Efficiency | Ref. |
|---|
| Temp. | Residence Time |
|---|
| 1 | Hard coal; m = 0.5 g
Particle size: <115 μm
Dried at 85 °C | 275–600 °C | 10–60 min | LF #6A: 5.3–74% | [7,8] |
| (LF #6A a) |
| 325–450 °C | PT#8: 36–80% |
| (PT#8) |
| 2 | Hard coal, m = 1 g,
Particle size: <200 μm
Dried at 105 °C | 400–1000 °C | 15 min | Hard coal1 b 83–95%
Hard coal2 c 45–85% | [8] |
| 3 | Hard coal; m = 0.5 g | 200–400 °C | 0–60 min | SS coals: 9–90% | [10] |
| Particle size: 106–149 μm | SARM coal d: 0–67% |
| 4 | Hard coal and lignite;
M = 6 g,
Particle size: <74 μm
Dried at 105 °C | 100–300 °C | 0–12 min | PRB e coal: 26–70% | [2] |
| Lignite f: 0–70% |
| 5 | Hard coal and lignite;
m = 0.2 gParticle size: 150–250 μm
Dried at 105 °C | 200–900 °C | 5–120 min | Ptolemais lignite: 29–80%
Bulgarian lignite: 23–95%
Australian hard coal: 32–87% | [10] |
| 6 | Hard coal and lignite; | 200–900 °C | 5–120 min | Ptolemais lignite: 29–80% | [11] |
| m = 0.2 g | Ptolemais lignite (D) g: 23–95% |
| Particle size: 150–250 μm | Megalopolis lignite: 10–70% |
| Dried at 105 °C |
| Australian hard coal: 32–87% |
| 7 | Hard coal, m = 0.5 g | 150–300 °C | 60 min | SS coals h: 24–80% | [12] |
| UF i: ~60% |
| Particle size: <149 μm; | IL #6 j: ~20% |
| PT#8 k: ~60% |
| 8 | Hard coal and lignite;
m = 45 kg/h
(Continuous operation; pilot) | 150–300 °C | 8, 12, 16 min | Gulf Coast Lignite: 63% | [3] |
| Canada Lignite: 90% |
| ND Lignite-C: 55% |
| ND Lignite-A: 44% |
| Southern PRB: 71% |
| Eastern PRB: 80% |
| Northern PRB: 36% |
| Colorado Bituminus: 34% |
| 9 | Hard coal, m = 6 g
Particle size: <150 μm
Dried at 65 °C | 200–800 °C | 0–60 min | Datong coal: 34–95% (800 °C)
Baorixile coal: 29–93% (800 °C) | [18] |
| 10 | Hard coal and brown coal
m = 200 g
Particle size: <200–500 μm
m = 19 g
Particle size: <200 μm
dried at 105 °C | 220–520 °C
200–500 °C | 0, 60 min
10–85 min | Hard coal: 0–85%
Brown coal: 12–99%
Hard coal: 0–85%
Brown coal: 12–99% | [4,5] |
| 11 | Hard coal. m = 100 mg
Particle size: <200 μm
Dried at 105 °C | 20–660 °C | - | Coal C1: 0–55% (600 °C)
Coal C2: 0–70% (650 °C) | [19] |
| 12 | Lignite
Bituminous coals
Particle size: <200 μm
Dried
m = 100 mg | 40–660 °C
Continuous heating process 1 °C/min | | Lignite: 82.4% (400 °C) | [20] |
| Bit. coals: 45.5–59.1% (400 °C) |
The temp. range for
releasing various Hg species was shown to be <150 °C for Hg0, 150–250 °C for HgCl2/org.-bound Hg, 250–400 °C for HgS/silicate-bound Hg and 400–600 °C for pyrite-bound Hg. |
| 13 | Lignite
Hard coals: A and B
Particle size: 500–1000 μm
Dried
m = 500 mg | 170–410 °C | Continuous heating process online measurement | Hard coals: 80% (380 °C)
Lignite: >80% (290 °C) | [14] |
| 14 | Bituminous coals (China)
Size: <200 μm
m = 0.5 g | 100–800 °C | 60 min
0.1, 0.5, 1.0 atm | Coal A, | [15] |
| Maximal efficiencies: |
| 83% at 650 °C (coal A) |
| 89% at 500 °C (coal B) |
| 87% at 400 °C (coal C) |
| 15 | Bituminous coals (China) | 0–1200 °C | Continuous heating process 20 K/min | 150–400 °C; 38.9–80.1% m | [16] |
| Size: 160–270 μm | 500–600 °C; 2.9–29.4% |
| Dried, m = 1 g | t > 750 °C; 8.9–16.2%, |
| 16 | Lignite | 200–900 °C | 5–120 min
0.25, 0.5, 1.0 atm | 200–300 °C; 32–44% l,m
300–600 °C; 36–46%
600–900 °C; 19–31% | [17] |
| Greek: Ptolemais, Megalopolis |
| Bulgarian, Elhovo, |
| Australian, Latrobe Valley. |
| Size: 150–250 μm, dried |
Table 2.
Characteristics of hard coals (HC1, HC2, HC3) and lignite (BC1, BC2).
Table 2.
Characteristics of hard coals (HC1, HC2, HC3) and lignite (BC1, BC2).
Parameter
Unit | W ar | W ad | A d | V d | HHV d | C d | H d | N d | S t,d | Od | Hg d |
|---|
| % | % | % | % | MJ/kg | % | % | % | % | % | ppb |
|---|
| HC1 | 21.3 | 12.4 | 12.10 | 34.86 | 27.3 | 68.9 | 3.95 | 1.07 | 1.39 | 12.53 | 81 |
| HC2 | 8.0 | 5.8 | 15.07 | 31.68 | 25.6 | 65.4 | 4.06 | 0.99 | 2.06 | 12.18 | 116 |
| HC3 | 3.0 | 2.9 | 8.86 | 30.39 | 30.5 | 77.8 | 4.47 | 1.27 | 0.44 | 6.89 | 27 |
| BC1 | 54.2 | 7.8 | 11.06 | 48.07 | 23.5 | 60.7 | 4.39 | 0.74 | 0.90 | 22.17 | 414 |
| BC2 | 4.8 | 4.7 | 9.76 | 50.77 | 23.8 | 60.5 | 4.77 | 0.63 | 0.70 | 23.56 | 392 |
| Uncertainty | 0.4 | 0.2 | 0.2 | 0.19 | 0.16 | 0.5 | 0.1 | 0.03 | 0.05 | - | 0.01 |
Table 3.
Experimental temperature ranges for testing and residence time impact on Hg removal content from lignite (BC1, BC2) and hard coals (HC1, HC2, HC3).
Table 3.
Experimental temperature ranges for testing and residence time impact on Hg removal content from lignite (BC1, BC2) and hard coals (HC1, HC2, HC3).
| Temp. of Pyrolysis Process | BC | HC |
|---|
| Residence Time of Lignites in the Reguired Temp. during the Pyrolysis Process (min) | Residence Time of Hard Coals in the Required Temperature during the Pyrolysis Process (min) |
|---|
| 0 | 4 | 8 | 10 | 0 | 4 | 8 |
|---|
| 200 °C | BCT1 | BCT2 | BCT3 | BCT4 | HCT1 | HCT2 | HCT3 |
| 250 °C | BCT5 | BCT6 | BCT7 | BCT8 | HCT4 | HCT5 | HCT6 |
| 300 °C | BCT9 | BCT10 | | BCT11 | HCT7 | HCT8 | HCT9 |
| 350 °C | BCT12 | BCT13 | | | HCT10 | HCT11 | HCT12 |
| 400 °C | BCT14 | | | | HCT13 | | |
| 450 °C | | | | | HCT14 | | |
| 500 °C | BCT15 | | | | HCT15 | | |
Table 4.
Experimental temperature ranges for testing and residence time impact on Hg content removal from lignite (BC1, BC2) and hard coals (HC1, HC2, HC3). The experiment scale was 100 g.
Table 4.
Experimental temperature ranges for testing and residence time impact on Hg content removal from lignite (BC1, BC2) and hard coals (HC1, HC2, HC3). The experiment scale was 100 g.
| Temp. of Pyrolysis Process | BC1 | BC2 | HC1 | HC2 | HC3 |
|---|
| Residence Time of Lignites in the Reguired Temp. during the Pyrolysis Process (min) | Residence Time of Hard Coals in the Reguired Temp. during the Pyrolysis Process (min) |
|---|
| 0 | 20 | 0 | 20 | 0 | 20 | 0 | 20 | 0 | 20 |
|---|
| 250 °C | | | x | | | | | | | |
| 300 °C | x | | x | | | | | | | |
| 350 °C | | | | | x | | | | | |
| 400 °C | | | | | | | | | | |
| 450 °C | | | | | x | | x | x | x | |
| 500 °C | | | | | | | | | | |
| 550 °C | | | | | | | x | x | x | |
Table 5.
Refined fuel characteristic.
Table 5.
Refined fuel characteristic.
| Coal | Temp. °C;
Residence Time, min | Proximate Analysis, % | HHV d | Ultimate Analysis, % | Hg d | Efficiency Removal Hg | Loss in Entalphy * |
|---|
| W ad | A d | V d | MJ/kg | C t,d | H d | N d | S t,d | Od | ppb | % | % |
|---|
| HC1 | 350; 0 | 2.2 | 12.47 | 29.79 | 27.2 | 69.5 | 4.00 | 1.19 | 1.31 | 11.5 | 66 | 14.9 | - |
| 450; 0 | 0.8 | 13.51 | 30.18 | 27.2 | 68.9 | 4.17 | 1.17 | 1.36 | 11.26 | 51 | 31.6 | - |
| HC2 | 450; 0 | 1.3 | 17.12 | 26.95 | 25.5 | 65.0 | 3.71 | 0.99 | 1.90 | 10.97 | 84 | 28.9 | 2 |
| 450; 20 | 1.8 | 18.43 | 15.17 | 26.4 | 68.7 | 2.89 | 1.12 | 2.08 | 6.47 | 107 | 22.3 | 13.4 |
| 550; 0 | 0.5 | 16.58 | 22.31 | 26.2 | 67.2 | 3.44 | 1.06 | 1.96 | 9.47 | 89 | 29.1 | 5.2 |
| 550; 20 | 1.4 | 19.98 | 8.34 | 27.3 | 72.3 | 2.39 | 1.19 | 1.58 | 2.26 | 33 | 78.6 | 20.0 |
| HC3 | 450; 0 | 0.6 | 8.35 | 15.65 | 30.7 | 80.7 | 3.34 | 1.45 | 0.44 | 5.44 | 15 | 51.3 | 11.8 |
| 550; 0 | 0.6 | 10.06 | 8.51 | 30.9 | 82.3 | 2.63 | 1.49 | 0.41 | 2.83 | 9 | 73.7 | 20.1 |
| BC1 | 300; 0 | 1.7 | 9.97 | 42.74 | 24.9 | 65.0 | 4.10 | 0.76 | 0.80 | 19.36 | 29 | 86.7 | - |
| BC2 | 250; 0 | 1.2 | 10.53 | 48.71 | 24.2 | 61.6 | 4.52 | 0.65 | 0.71 | 21.93 | 306 | 26.3 | 3.8 |
| 300; 0 | 0.8 | 11.49 | 43.99 | 25.7 | 63.4 | 4.61 | 0.60 | 0.69 | 19.14 | 31 | 93.0 | 4.3 |
| Uncertainty | 0.2 | 0.2 | 0.19 | 0.16 | 0.5 | 0.1 | 0.03 | 0.05 | - | 0.01 | 0.05 | 0.05 |
Table 6.
Yields of the pyrolysis products.
Table 6.
Yields of the pyrolysis products.
| Coal | Temp.,
°C | Residence Time, min | Char, % | Liquid Products, % | Gaseous Products, % |
|---|
| HC1 | 350 | 0 | 94.5 | 2.53 | 5.6 |
| 450 | 0 | 91.0 | - | - |
| HC2 | 450 | 0
20 | 91.5 | 6.4 | 2.1 |
| 73.0 | 13.7 | 13.3 |
| 550 | 0
20 | 85.5 | 9.2 | 5.3 |
| 70.0 | 18.1 | 12.0 |
| 550 | 20 | 71.1 | 19.9 | 9.1 |
| HC3 | 450 | 0 | 85.5 | 9.2 | 5.3 |
| 550 | 0 | 77.0 | 14.4 | 8.6 |
| BC1 | 300 | 0 | 93.0 | 3.29 | 7.5 |
| BC2 | 250 | 0 | 85.0 | 8.0 | 7.0 |
| 300 | 0 | 85.0 | 12.8 | 2.2 |
| Uncertainty | 0.02 | 0.02 | 0.05 |
Table 7.
Characteristics of the gaseous pyrolysis products.
Table 7.
Characteristics of the gaseous pyrolysis products.
| Coal | Temp., °C | Residence Time, min | Gaseous Compounds Composition, v/v% | Org. Compounds C2–C5,% v/v | Density, kg/m3 | HHV, MJ/m3 |
|---|
| H2 | N2 | CO | CO2 | CH4 |
|---|
| HC1 | 350 | 0 | 0.13 | 92.95 | 1.10 | 4.84 | 0.10 | 0.12 | 1.28 | - |
| 450 | 0 | 0.07 | 90.06 | 1.23 | 5.44 | 0.13 | 0.14 | 1.29 | 0.50 |
| HC2 | 450 | 0
20 | 0.63 | 81.59 | 2.05 | 11.22 | 0.49 | 0.00 | 0.46 | 0.49 |
| - | - | - | - | - | - | - | - |
| 550 | 0
20 | 2.70 | 57.32 | 5.41 | 24.40 | 4.40 | 0.15 | 0.46 | 2.17 |
| 12.45 | 33.93 | 5.11 | 17.78 | 25.71 | 4.34 | 1.11 | 15.91 |
| 550 | 20 | 8.62 | 39.09 | 8.87 | 17.57 | 16.83 | 6.04 | 1.20 | 14.06 |
| HC3 | 450 | 0 | 3.55 | 62.84 | 2.80 | 9.30 | 16.56 | 4.59 | 1.19 | 10.36 |
| 550 | 0 | 14.38 | 29.76 | 3.23 | 10.37 | 33.31 | 7.25 | 1.02 | 17.49 |
| BC1 | 300 | 0 | 0.10 | 87.84 | 1.43 | 9.16 | 0.10 | 0.05 | 1.32 | 0.30 |
| BC2 | 250 | 0 | 0.01 | 90.95 | 0.11 | 6.34 | 0.03 | 0.01 | 1.29 | 0.02 |
| 300 | 0 | 0.13 | 74.50 | 1.46 | 0.37 | 0.37 | 16.73 | 1.38 | 0.31 |
| Uncertainty | 0.43 | 0.57 | 0.13 | 0.03 | 0.10 | 0.02 | 0.49 | 0.49 |
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).