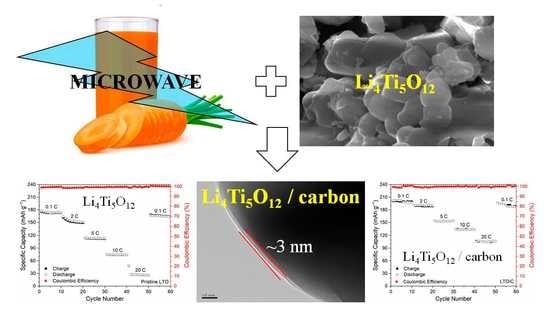
Li4Ti5O12 Coated by Biomass-Derived Carbon Quantum Dots as Anode Material with Enhanced Electrochemical Performance for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Biomass-Derived Carbon Precursor
2.2. Preparation of Pristine and Carbon-Coated LTO Materials
2.3. Characterization of Materials
2.4. Characterization of Materials
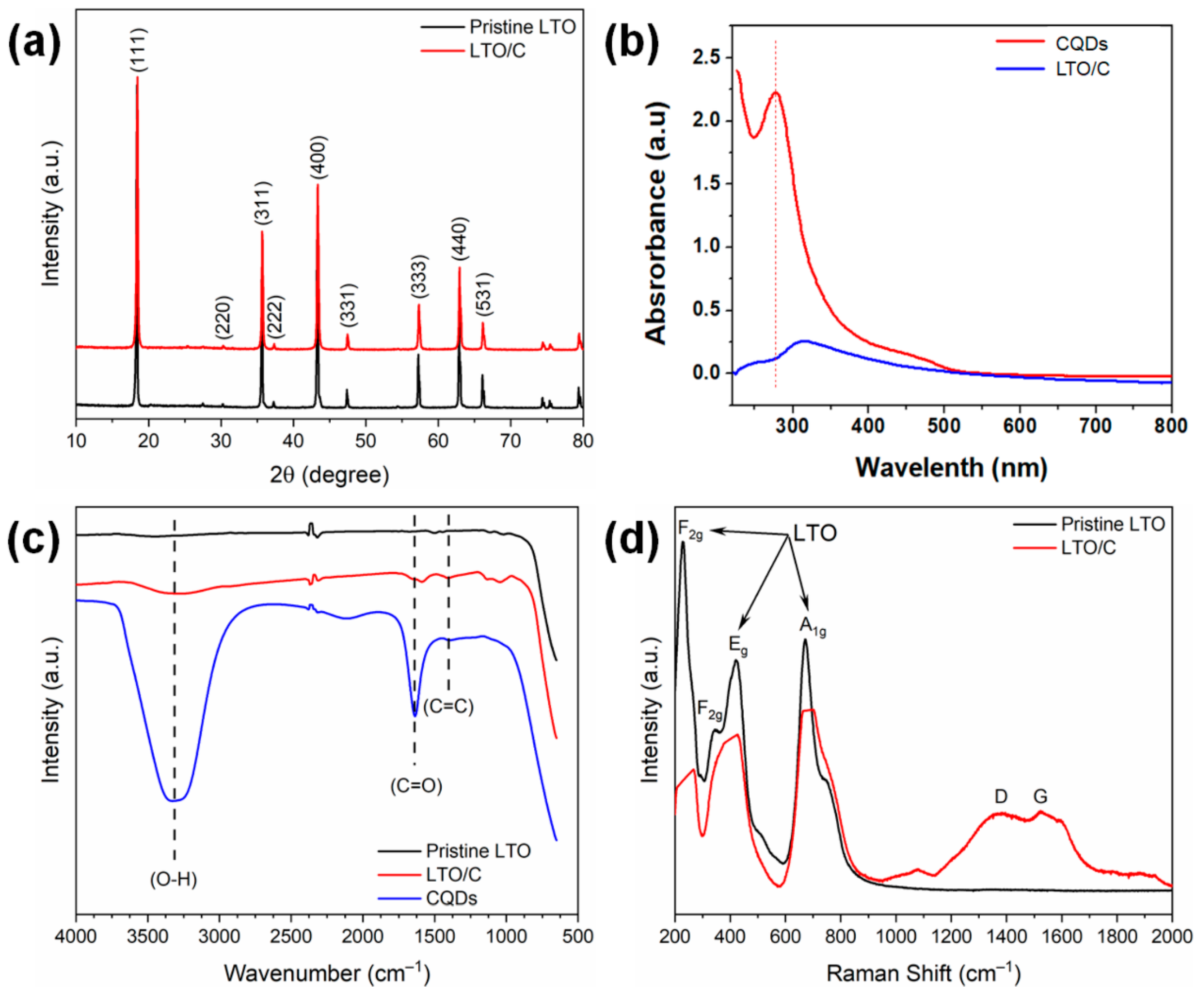
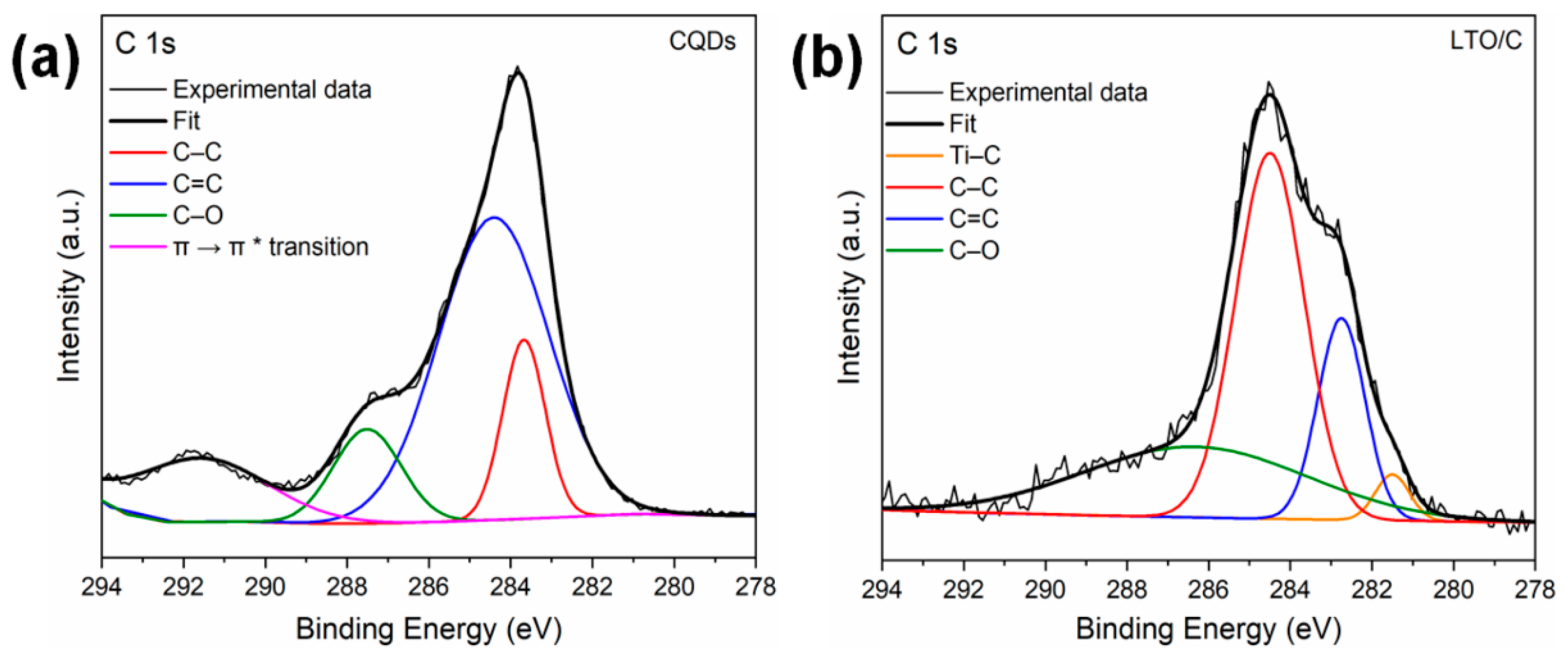
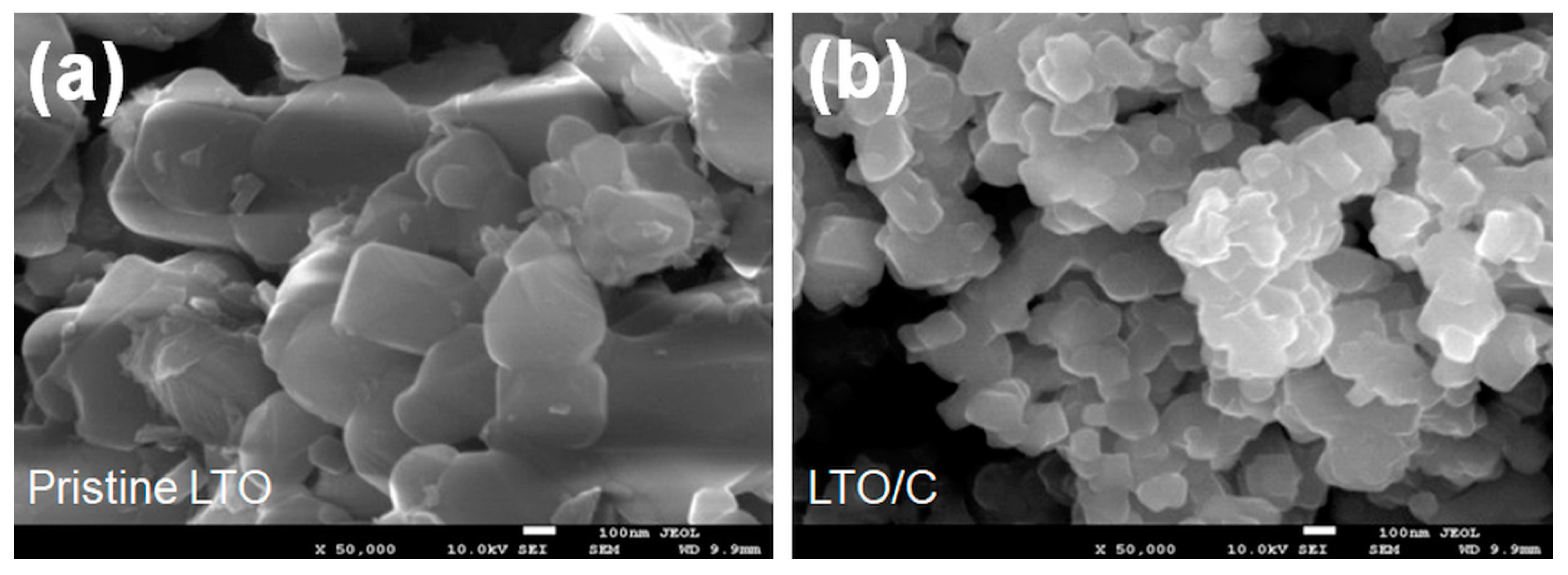
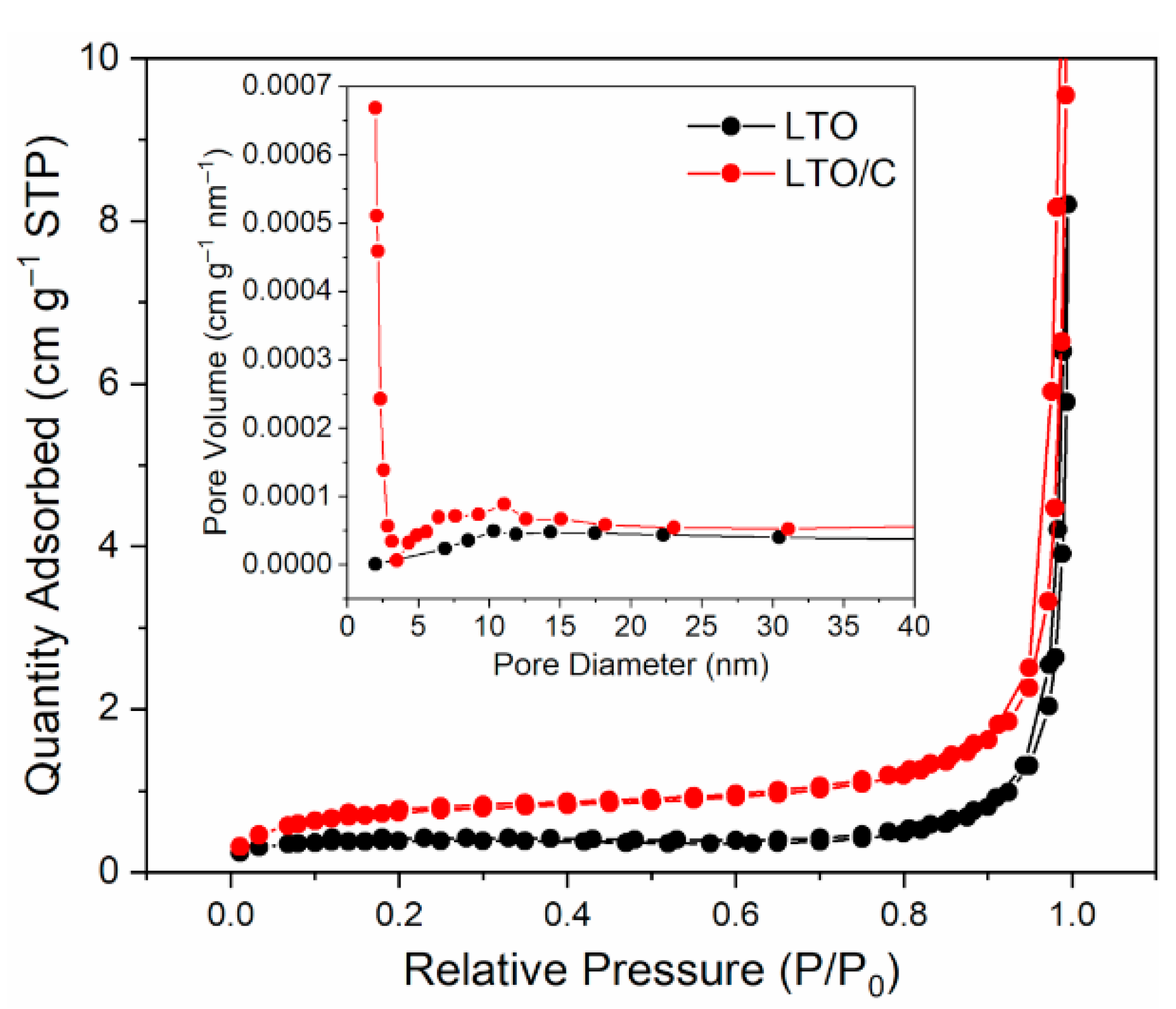
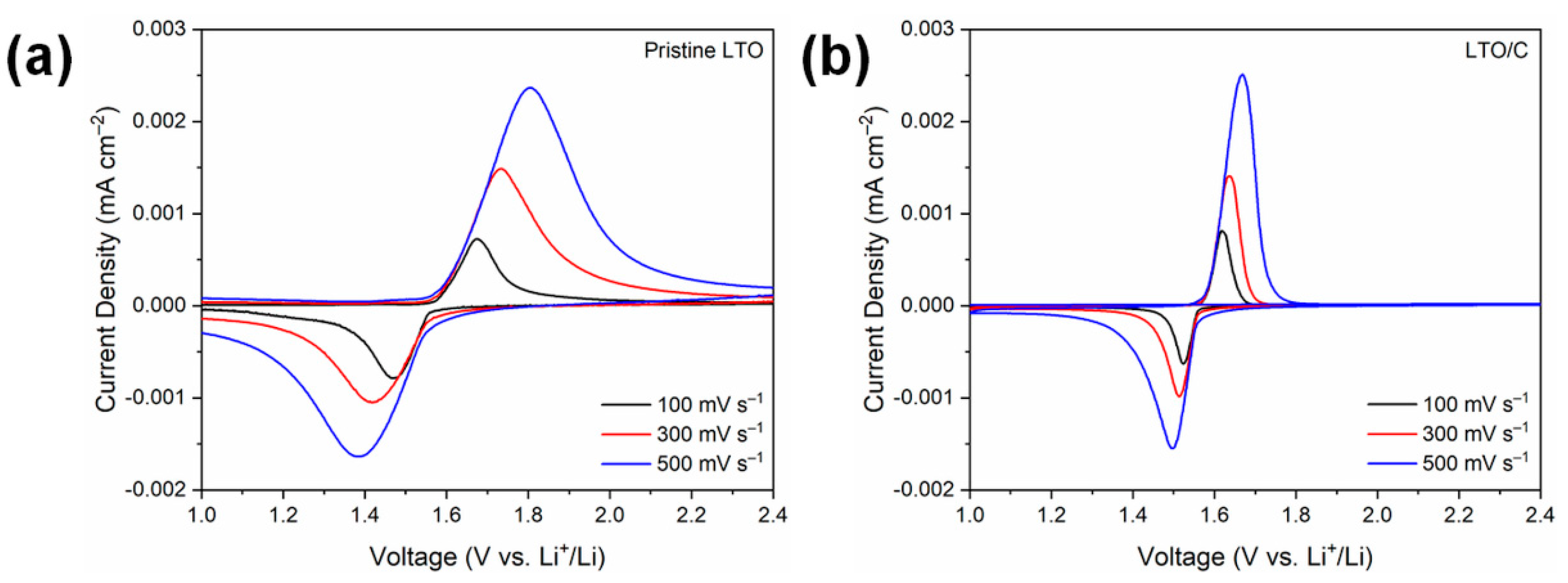
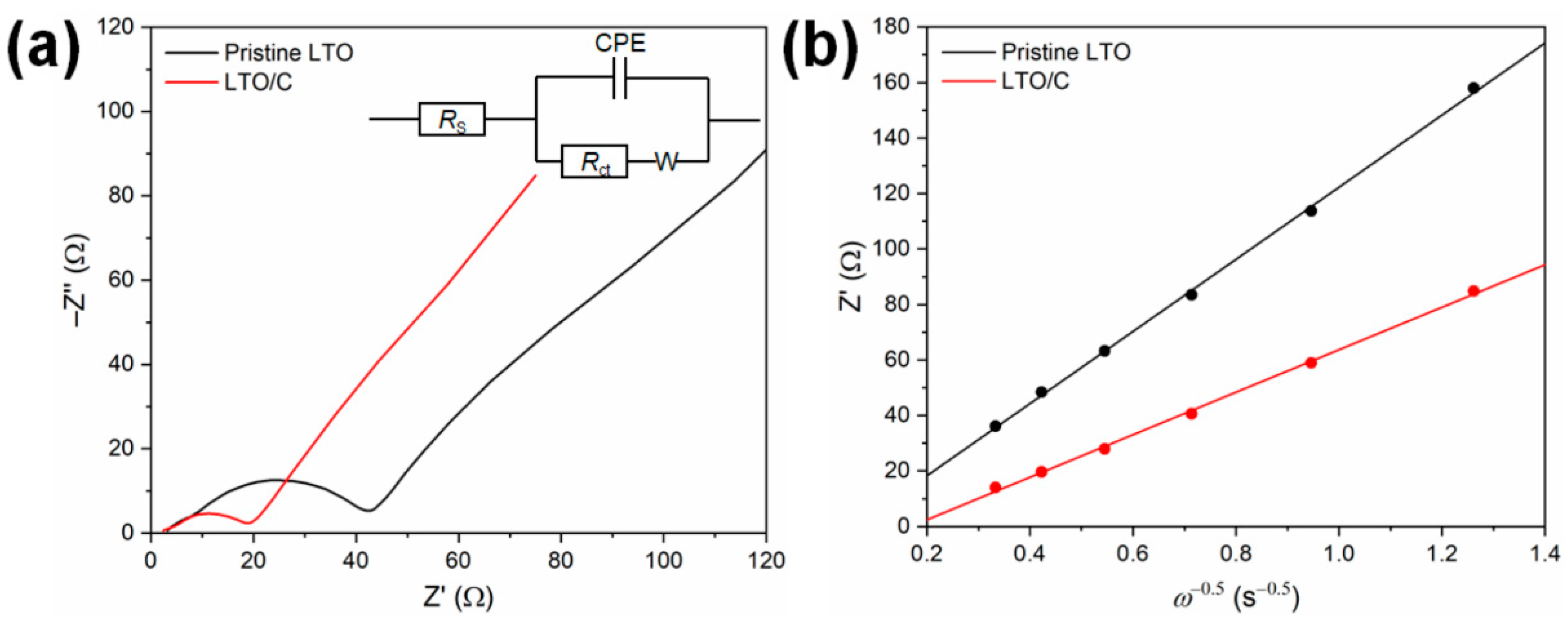
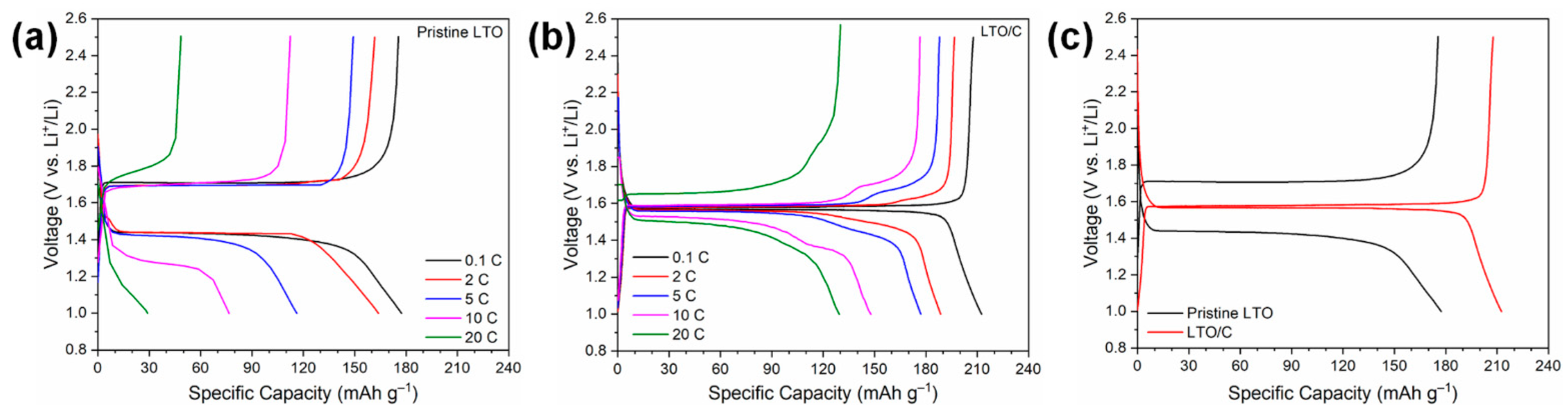
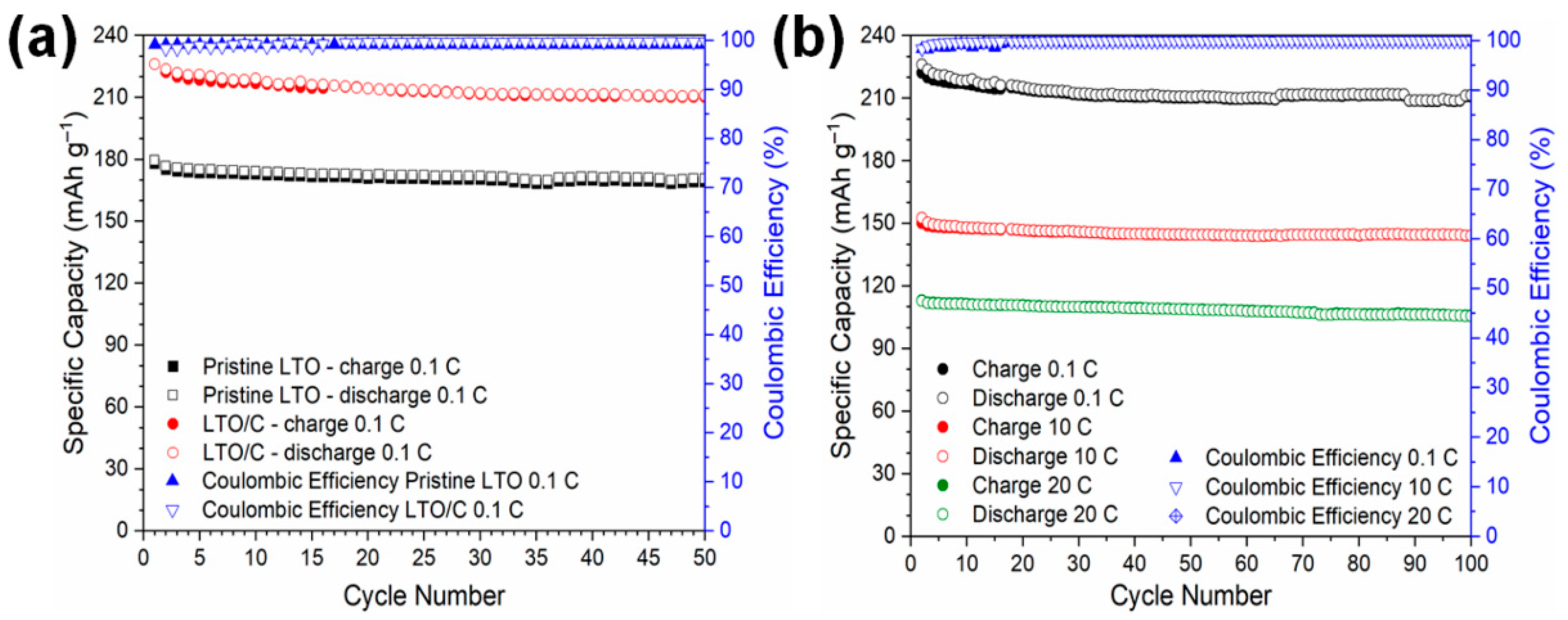
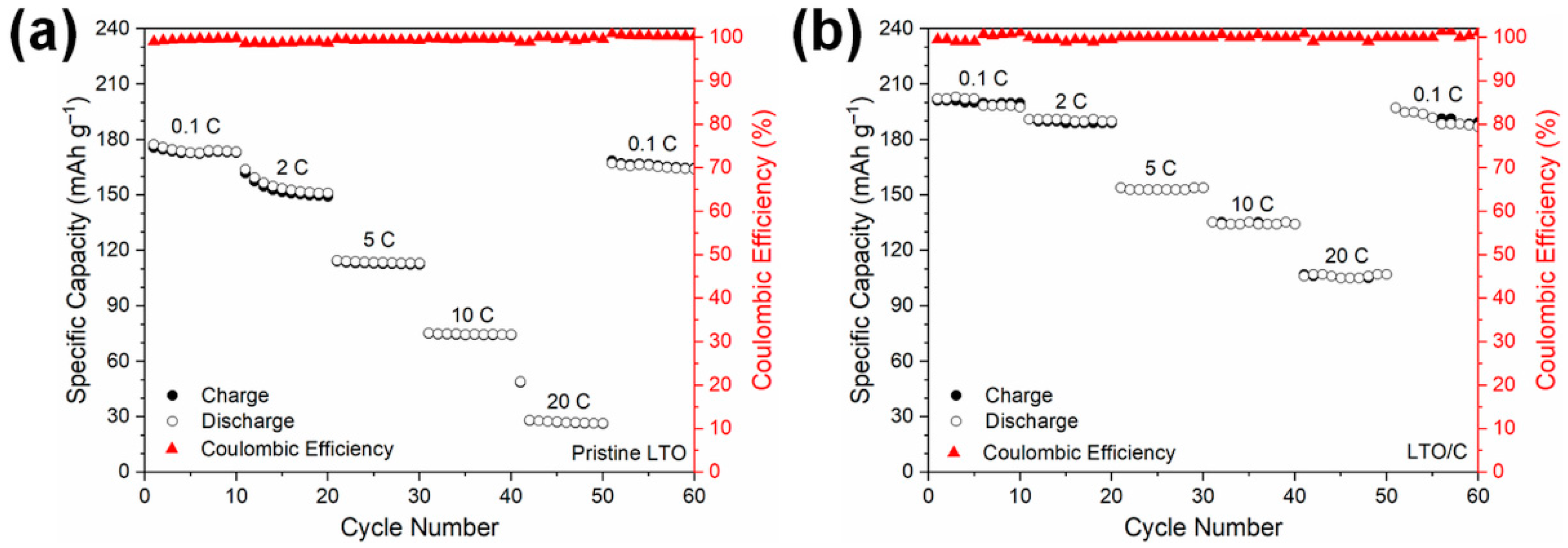
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.Y.; Zhou, G.M.; Liu, K.; Cui, Y. Design of complex nanomaterials for energy storage: Past success and future opportunity. Acc. Chem. Res. 2017, 50, 2895–2905. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Wu, J.X.; Cao, Y.L.; Zhao, H.M.; Mao, J.F.; Guo, Z.P. The critical role of carbon in marrying silicon and graphite anodes for high-energy lithium-ion batteries. Carbon Energy 2019, 1, 57–76. [Google Scholar] [CrossRef]
- Seo, J.H.; Verlinde, K.; Rajagopalan, R.; Gomez, E.D.; Mallouk, T.E.; Randall, C.A. Cold sintering process for fabrication of a high volumetric capacity Li4Ti5O12 anode. Mater. Sci. Eng. B 2019, 250, 114435. [Google Scholar] [CrossRef]
- Iniguez, F.B.; Jeong, H.; Mohamed, A.Y.; Nogales, P.M.; Choi, H.; Jeong, S.K.; Park, J.B.; Kim, Y.S.; Cho, D.Y. Structure and electrochemical properties of CNT-supported Li-Ti-O anode material for Li-ion battery. J. Ind. Eng. Chem. 2022, 112, 125–133. [Google Scholar] [CrossRef]
- Yi, T.F.; Yang, S.Y.; Xie, Y. Recent advances of Li4Ti5O12 as a promising next generation anode material for high power lithium-ion batteries. J. Mater. Chem. A 2015, 3, 5750–5777. [Google Scholar] [CrossRef]
- Pang, S.P.; Zhao, Y.Y.; Zhang, C.J.; Zhang, Q.H.; Gu, L.; Zhou, X.H.; Li, G.C.; Cui, G.L. Electrostatic assembly of mesoporous Li4Ti5O12/graphene hybrid as high-rate anode materials. Scr. Mater. 2013, 69, 171–174. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.M.; Yang, H.; Shen, X.D. Characterization and electrochemical properties of carbon-coated Li4Ti5O12 prepared by a citric acid sol–gel method. J. Alloys Compd. 2011, 509, 712–718. [Google Scholar] [CrossRef]
- Li, J.R.; Tang, Z.L.; Zhang, Z.T. Controllable formation and electrochemical properties of one-dimensional nanostructured spinel Li4Ti5O12. Electrochem. Commun. 2005, 7, 894–899. [Google Scholar] [CrossRef]
- Prakash, A.S.; Manikandan, P.; Ramesha, K.; Sathiya, M.; Tarascon, J.M.; Shukla, A.K. Solution-combustion synthesized nanocrystalline Li4Ti5O12 as high-rate performance Li-ion battery anode. Chem. Mat. 2010, 22, 2857–2863. [Google Scholar] [CrossRef]
- Li, K.M.; Dai, X.D.; Manawan, M.; Wang, Q.; Pan, J.H. Unveiling the formation mechanism and phase purity control of nanostructured Li4Ti5O12 via a hydrothermal process. Cryst. Growth Des. 2021, 21, 5440–5450. [Google Scholar] [CrossRef]
- Du, G.D.; Sharma, N.; Peterson, V.K.; Kimpton, J.A.; Jia, D.Z.; Guo, Z.P. Br-doped Li4Ti5O12 and composite TiO2 anodes for Li-ion batteries: Synchrotron X-ray and in situ neutron diffraction studies. Adv. Funct. Mater. 2011, 21, 3990–3997. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, B.; Ji, G.; Lee, J.Y. Carbon-encapsulated F-doped Li4Ti5O12 as a high rate anode material for Li+ batteries. ACS Nano 2013, 7, 10870–10878. [Google Scholar] [CrossRef]
- Lin, C.F.; Lai, M.O.; Lu, L.; Zhou, H.H.; Xin, Y.L. Structure and high rate performance of Ni2+ doped Li4Ti5O12 for lithium ion battery. J. Power Sources 2013, 244, 272–279. [Google Scholar] [CrossRef]
- Xue, X.X.; Yan, H.Y.; Fu, Y.Q. Preparation of pure and metal-doped Li4Ti5O12 composites and their lithium-storage performances for lithium-ion batteries. Solid State Ion. 2019, 335, 1–6. [Google Scholar] [CrossRef]
- Liu, Z.F.; Cao, L.; He, F.L.; Zhang, Z.W.; Li, D.; Zhao, W.; Qi, T. Study on the possibility of diagonal line rule in elemental doping effects in Li4Ti5O12 by mechanochemical method. Electrochim. Acta 2022, 422, 140485. [Google Scholar] [CrossRef]
- Kang, C.Y.; Krajewski, M.; Lin, J.Y. Impact of titanium precursors on formation and electrochemical properties of Li4Ti5O12 anode materials for lithium-ion batteries. J. Solid State Electrochem. 2021, 25, 575–582. [Google Scholar] [CrossRef]
- Wang, Y.G.; Liu, H.M.; Wang, K.X.; Eiji, H.; Wang, Y.R.; Zhou, H.S. Synthesis and electrochemical performance of nano-sized Li4Ti5O12 with double surface modification of Ti(III) and carbon. J. Mater. Chem. 2009, 19, 6789–6795. [Google Scholar] [CrossRef]
- Jung, H.G.; Myung, S.T.; Yoon, C.S.; Son, S.B.; Oh, K.H.; Amine, K.; Scrosati, B.; Sun, Y.K. Microscale spherical carbon-coated Li4Ti5O12 as ultra high power anode material for lithium batteries. Energy Environ. Sci. 2011, 4, 1345–1351. [Google Scholar] [CrossRef]
- Zhu, Z.Q.; Cheng, F.Y.; Chen, J. Investigation of effects of carbon coating on the electrochemical performance of Li4Ti5O12/C nanocomposites. J. Mater. Chem. A 2013, 1, 9484–9490. [Google Scholar] [CrossRef]
- Li, Z.Y.; Ding, F.X.; Zhao, Y.G.; Wang, Y.D.; Li, J.L.; Yang, K.; Gao, F. Synthesis and electrochemical performance of Li4Ti5O12 submicrospheres coated with TiN as anode materials for lithium-ion battery. Ceram. Int. 2016, 42, 15464–15470. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, H.; Gao, Y.; He, X.Q.; White, T.A.; Liang, X.H. Li4Ti5O12 coated with ultrathin aluminum-doped zinc oxide films as an anode material for lithium-ion batteries. J. Power Sources 2019, 436, 226859. [Google Scholar] [CrossRef]
- Lv, S.X.; Chen, Q.L.; Song, F.X.; Li, Y.N. One-step synthesis of a double conductive layer C-SiOx-TiO2 co-coated Li4Ti5O12 anode material toward a high-rate and large-capacity lithium-ion battery. Appl. Surf. Sci. 2021, 555, 149637. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Pang, S.P.; Zhang, C.J.; Zhang, Q.H.; Gu, L.; Zhou, X.H.; Li, G.C.; Cui, G.L. Nitridated mesoporous Li4Ti5O12 spheres for high-rate lithium-ion batteries anode material. J. Solid State Electrochem. 2013, 17, 1479–1485. [Google Scholar] [CrossRef]
- Dhaiveegan, P.; Peng, H.T.; Michalska, M.; Xiao, Y.M.; Lin, J.Y.; Hsieh, C.K. Investigation of carbon coating approach on electrochemical performance of Li4Ti5O12/C composite anodes for high-rate lithium-ion batteries. J. Solid State Electrochem. 2018, 22, 1851–1861. [Google Scholar] [CrossRef]
- Hsu, S.C.; Huang, T.T.; Wu, Y.J.; Lu, C.Z.; Weng, H.C.; Huang, J.H.; Chang-Jian, C.W.; Liu, T.Y. Polyimide-derived carbon-coated Li4Ti5O12 as high-rate anode materials for lithium ion batteries. Polymers 2021, 13, 1672. [Google Scholar] [CrossRef]
- Li, K.Q.; Zhang, Y.; Sun, Y.N.; Xu, Y.L.; Zhang, H.; Ye, P.; Zheng, M.D.; Zhou, N.; Wang, D. Template-free synthesis of biomass-derived carbon coated Li4Ti5O12 microspheres as high performance anodes for lithium-ion batteries. Appl. Surf. Sci. 2018, 459, 572–582. [Google Scholar] [CrossRef]
- Dager, A.; Uchida, T.; Maekawa, T.; Tachibana, M. Synthesis and characterization of monodisperse carbon quantum dots from fennel seeds: Photoluminescence analysis using machine learning. Sci. Rep. 2019, 9, 14004. [Google Scholar] [CrossRef]
- Ghosh, S.; Satapathy, S.S.; Ghosh, K.; Jauhari, S.; Panda, S.K.; Si, S. Carbon dots assisted synthesis of gold nanoparticles and their catalytic activity in 4-nitrophenol reduction. ChemistrySelect 2019, 4, 3416–3422. [Google Scholar] [CrossRef]
- Meng, X.D.; Wang, X.C.; Zhou, Y.X.; Tong, L.; Zeng, X.H.; Chen, X.B. Spinel lithium titanate from brookite nanocrystallites. Ceram. Int. 2014, 40, 4989–4993. [Google Scholar] [CrossRef]
- Jhan, Y.R.; Duh, J.G. Electrochemical performance and low discharge cut-off voltage behavior of ruthenium doped Li4Ti5O12 with improved energy density. Electrochim. Acta 2012, 63, 9–15. [Google Scholar] [CrossRef]
- Li, Y.A.; Chen, Q.L.; Meng, Q.Q.; Lei, S.L.; Li, C.Q.; Li, X.Y.; Ma, J.B. One-step synthesis of a nanosized cubic Li2TiO3-Coated Br, C, and N Co-Doped Li4Ti5O12 anode material for stable high-rate lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 25804–25816. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, H.C.; Cui, W.J.; Xiao, Q.; Zhao, J.B. Fast solution-combustion synthesis of nitrogen-modified Li4Ti5O12 nanomaterials with improved electrochemical performance. ACS Appl. Mater. Interfaces 2014, 6, 7895–7901. [Google Scholar] [CrossRef]
- Wu, C.Y.; Wang, Y.X.; Xie, J.; Cao, G.S.; Zhu, T.J.; Zhao, X.B. Electrochemical performance of Li4Ti5O12/carbon nanofibers composite prepared by an in situ route for Li-ion batteries. J. Solid State Electrochem. 2012, 16, 3915–3921. [Google Scholar] [CrossRef]
- Lan, C.K.; Chang, C.C.; Wu, C.Y.; Chen, B.H.; Duh, J.G. Improvement of the Ar/N2 binary plasma-treated carbon passivation layer deposited on Li4Ti5O12 electrodes for stable high-rate lithium ion batteries. RSC Adv. 2015, 5, 92554–92563. [Google Scholar] [CrossRef]
- Paillard, V. On the origin of the 1100 cm−1 Raman band in amorphous and nanocrystalline sp3 carbon. Europhys. Lett. 2001, 54, 194–198. [Google Scholar] [CrossRef]
- Korepanov, V.I.; Hamaguchi, H.O.; Osawa, E.; Ermolenkov, V.; Lednev, I.K.; Etzold, B.J.M.; Levinson, O.; Zousman, B.; Epperla, C.P.; Chang, H.C. Carbon structure in nanodiamonds elucidated from Raman spectroscopy. Carbon 2017, 121, 322–329. [Google Scholar] [CrossRef]
- Zhang, L.H.; Koka, R.V. A study on the oxidation and carbon diffusion of TiC in alumina titanium carbide ceramics using XPS and Raman spectroscopy. Mater. Chem. Phys. 1998, 57, 23–32. [Google Scholar] [CrossRef]
- Friederici, L.; Mesceriakove, S.M.; Neumann, A.; Sermyagina, E.; Mescerlakovas, A.; Lahde, A.; Grimmer, C.; Streibel, T.; Ruger, C.P.; Zimmermann, R. Effect of hydrothermal carbonization and eutectic salt mixture (KCl/LiCl) on the pyrolysis of Kraft lignin as revealed by thermal analysis coupled to advanced high-resolution mass spectrometry. J. Anal. Appl. Pyrolysis 2022, 166, 105604. [Google Scholar] [CrossRef]
- Cong, Y.; Li, X.K.; Qin, Y.; Dong, Z.J.; Yuan, G.M.; Cui, Z.W.; Lai, X.J. Carbon-doped TiO2 coating on multiwalled carbon nanotubes with higher visible light photocatalytic activity. Appl. Catal. B Environ. 2011, 107, 128–134. [Google Scholar] [CrossRef]
- Yun, B.N.; Du, H.L.; Hwang, J.Y.; Jung, H.G.; Sun, Y.K. Improved electrochemical performance of boron-doped carbon-coated lithium titanate as an anode material for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 2802–2810. [Google Scholar] [CrossRef]
- Chaisit, S.; Chanlek, N.; Khajonrit, J.; Sichumsaeng, T.; Maensiri, S. Preparation, characterization, and electrochemical properties of KOH-activated carbon from cassava root. Mater. Res. Express 2020, 7, 105605. [Google Scholar] [CrossRef]
- Park, J.; Back, T.; Mitchel, W.C.; Kim, S.S.; Elhamri, S.; Boeckl, J.; Fairchild, S.B.; Naik, R.; Voevodin, A.A. Approach to multifunctional device platform with epitaxial graphene on transition metal oxide. Sci. Rep. 2015, 5, 14374. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, X.Q.; Chen, X.G.; Soutis, C. π-π interaction between carbon fibre and epoxy resin for interface improvement in composites. Compos. Part B Eng. 2021, 220, 108983. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Ding, Y.; Li, G.R.; Xiao, C.W.; Gao, X.P. Insight into effects of graphene in Li4Ti5O12/carbon composite with high rate capability as anode materials for lithium ion batteries. Electrochim. Acta 2013, 102, 282–289. [Google Scholar] [CrossRef]
- Lai, C.; Dou, Y.Y.; Li, X.; Gao, X.P. Improvement of the high rate capability of hierarchical structured Li4Ti5O12 induced by the pseudocapacitive effect. J. Power Sources 2010, 195, 3676–3679. [Google Scholar] [CrossRef]
- Li, X.; Lai, C.; Xiao, C.W.; Gao, X.P. Enhanced high rate capability of dual-phase Li4Ti5O12-TiO2 induced by pseudocapacitive effect. Electrochim. Acta 2011, 56, 9152–9158. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.Q.; Zhou, X.Y.; You, M.; Zhang, C.; Yue, W.B. Two-dimensional graphene-based Li4Ti5O12 with hierarchical pore structure and large pseudocapacitive effect as high-rate and long-cycle anode material for lithium-ion batteries. Electrochim. Acta 2022, 405, 139814. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajewski, M.; Chen, C.-H.; Huang, Z.-T.; Lin, J.-Y. Li4Ti5O12 Coated by Biomass-Derived Carbon Quantum Dots as Anode Material with Enhanced Electrochemical Performance for Lithium-Ion Batteries. Energies 2022, 15, 7715. https://doi.org/10.3390/en15207715
Krajewski M, Chen C-H, Huang Z-T, Lin J-Y. Li4Ti5O12 Coated by Biomass-Derived Carbon Quantum Dots as Anode Material with Enhanced Electrochemical Performance for Lithium-Ion Batteries. Energies. 2022; 15(20):7715. https://doi.org/10.3390/en15207715
Chicago/Turabian StyleKrajewski, Marcin, Chun-Hao Chen, Zhi-Ting Huang, and Jeng-Yu Lin. 2022. "Li4Ti5O12 Coated by Biomass-Derived Carbon Quantum Dots as Anode Material with Enhanced Electrochemical Performance for Lithium-Ion Batteries" Energies 15, no. 20: 7715. https://doi.org/10.3390/en15207715
APA StyleKrajewski, M., Chen, C.-H., Huang, Z.-T., & Lin, J.-Y. (2022). Li4Ti5O12 Coated by Biomass-Derived Carbon Quantum Dots as Anode Material with Enhanced Electrochemical Performance for Lithium-Ion Batteries. Energies, 15(20), 7715. https://doi.org/10.3390/en15207715