Abstract
Non-food lignocellulosic biomass is an attractive source owing to its abundance as a renewable resource and cost-effectiveness. Hibiscus cannabinus L., commonly known as kenaf, is a fiber-producing plant with high cellulose yield and non-food biomass. This study aimed to enhance the glucose recovery (GR) of kenaf biomass (KB). The bark and core fibers of KB are rich in glucan content and low in lignin content. Based on its glucan and lignin contents, KB has considerable potential as a feedstock for synthesizing monomer sugars, which can produce biofuel and high-value compounds. Therefore, the bark and core fibers were treated at a moderate temperature with various concentrations of phosphoric acid, followed by enzymatic hydrolysis. After pretreatment, the chemical composition of both feedstocks was changed. Phosphoric acid substantially affected the elimination of partial lignin and hemicellulose, which led to enhanced enzymatic hydrolysis. The maximum hydrolysis efficiency (HE) and GR of bark and core fibers were achieved when both feedstocks were treated with 75% phosphoric acid. Compared with untreated feedstocks, HE increased by approximately 5.6 times for bark and 4.7 times for core fibers. However, GR was enhanced approximately 4.9-fold for bark and 4.3-fold for core fibers.
1. Introduction
The depletion of fossil fuels and global warming are major concerns worldwide owing to the rising energy demand, motivating the exploration of alternative energy sources [1,2]. Consequently, lignocellulosic biomass (LB), a renewable resource, is an attractive feedstock for manufacturing biofuels and biochemical products. These products have emerged as cleaner alternatives to fossil fuels and minimize environmental implications [1,2]. Furthermore, LB can be obtained from various resources, including food and non-food portions of food crops, herbaceous plants, and organic waste generated through farming [3]. LB, particularly non-food feedstock, is highly valuable as it is abundant, globally available, feedstock sustainable, renewable, low in cost, bio-degradable, and environmental-friendly [4,5,6]. Therefore, several lignocellulosic feedstocks (LFs), including rice husks, corn stover, poplar wood chips, sugarcane bagasse, wheat straw, Eucalyptus urograndis chips, and various types of feedstocks, have been characterized and utilized in a sugar platform-based biorefinery to produce bioethanol and other value-added chemicals [7].
Hibiscus cannabinus L., often known as kenaf, is an annual, herbaceous, short-day plant belonging to the Malvaceae family [8] that generates productivity using the C3 photosynthetic pathway [9]. China, India, and Thailand are leading producers of kenaf. Due to its geographical pest resistance and adaptability to various climatic conditions, approximately 0.5 million tons per year of kenaf is produced globally [10]. Kenaf fiber is widely used in manufacturing paper, fabrics, clothing, biomaterials, insulating carpets, absorbent polymers, animal bedding, pharmaceutical preparations, percussion equipment, and plant-based foods with added value [11]. According to previous reports, kenaf has markedly higher photosynthetic activity than other traditional trees and could aid the worldwide drive to attain environmental sustainability [12]. Moreover, kenaf biomass (KB) is an inexpensive, non-food product with a high yield [9]. As mentioned above, KB is recognized as a sustainable lignocellulosic feedstock for sugar platform-based biorefineries that synthesize bioethanol and other chemicals with added value [8,9,12,13,14,15,16,17,18,19].
LB is primarily composed of cellulose, hemicellulose, and lignin, creating a complex polymer architecture that is typically resistant to biological degradation. The recalcitrant structure and rigid persistence features of LB are the most critical barriers to its conversion through biochemical processes [20]. Pretreatment of LB is necessary because it affects the microstructure of most biomass feedstocks, resulting in enhanced enzyme hydrolysis [21]. Various pretreatment strategies have been developed to destroy the complex structures of different types of feedstocks, including physical, chemical, physicochemical, biological, and emerging pretreatments [22]. Chemical pretreatment techniques, including acid and alkali, are widely employed with various types of LFs because they are rapid, efficient, and cost-effective compared with other pretreatments [23]. Acid pretreatment increases the available surface area of cellulose, solubilizes hemicellulose, and alters the structure of lignin, thereby enhancing enzymatic hydrolysis efficiency (HE). However, inhibitor compounds, such as acetic acid, hydroxymethyl furfural, and furfural, are formed during the pretreatment process as a result of the decomposition of sugar when lignocellulosic materials are treated with acid at high pressures and temperatures [23,24]. Phosphoric acid (PA) has been used to pretreat a variety of LBs, including Sesbania grandiflora, Achyranthes aspera, Sida acuta, Alamo switchgrass, Moso bamboo, Miscanthus, poplar, corn stover, switchgrass, hybrid poplar, Douglas fir, hemp stalks, Bermudagrass, reed, rapeseed, and eastern gama [25]. PA is effective at disrupting the resistant structure of lignocellulose feedstocks, which is greatly affected by the reduction of partial lignin and hemicellulose as well as the de-crystallization of cellulose fibers. This results in an improvement in the efficiency of enzymatic hydrolysis of the pretreated biomass. [25,26].
Overall, the purpose of this study was to investigate the effects of the pretreatment of KB with various concentrations of PA. Additionally, the impact of pretreatment on chemical composition, biomass structure, and enzyme hydrolysis were examined.
2. Materials and Methods
2.1. Raw Material
The lignocellulosic material of Hibiscus cannabinus L. (Kenaf KKU 60) employed in this investigation was a non-photosensitive strain purchased from a farmer in the Khon Kaen province of Thailand. The dry bark and core fibers were chopped into approximately 4 × 10 cm sections and pulverized in a rotating mill. The bark and core fibers were then powdered using a 50–100-mesh laboratory test sieve. The two samples were then separated, stored in glass containers with plastic lids, and placed in desiccators for further examination.
2.2. PA Pretreatment
Pretreatment of KB biomass was performed following the protocol of Siripong et al. [27]. In brief, “300 mg of dry sample and 24 mL of 70%, 75%, 80%, or 85% (w/v) PA were combined in a 50 mL centrifuge tube and mixed well with a stirring rod. The tube was subsequently covered and heated in a water bath to 60 °C for 60 min”. “Approximately 20 mL of acetone was added to the treated sample in the tube and vigorously stirred using a stirring rod to complete the process. The liquid mixture in the tube was separated using swing-bucket centrifugation at 2055× g for 10 min, and the supernatant was removed. This step was performed at least three times. In a tube, the solid fraction was rinsed with deionized water until a pH of approximately 7 was achieved”.
The recovery yield (%) and lignin removal (%) were calculated using Equations (1) and (2):
Recovery yields of each content (% DW)* =
[Solid recovery of each content (%) × Treated component of each content (% DW)]
Untreated component of each content (% DW)
[Solid recovery of each content (%) × Treated component of each content (% DW)]
Untreated component of each content (% DW)
Lignin removal (%) = 100 − lignin recovery
- % DW is the percent dry weight.
2.3. Chemical Composition
The specifics of the chemical composition analysis have been described by Siripong et al. [27]. Both treated and untreated samples were analyzed for carbohydrates, lignin, ash, and extractives. Therefore, typical NREL procedures were applied for this analysis [28,29,30].
2.4. Analytical Procedures
In this study, we followed the procedure by Obang et al. [31]. Briefly, “high-performance liquid chromatography (Agilent 1100, Agilent Technologies, Waldbronn, Germany) equipped with Bio-Rad Aminex HPX-87H columns (300 mm × 7.8 mm; Hercules, CA, USA) and a refractive index detector (G1362A; Agilent Technologies, Waldbronn, Germany) were used to analyze all sugars in treated and untreated KB biomasses. The column was maintained at 60 °C, with an injection volume of 20 µL per sample. Filtered 5 mM H2SO4 was used for elution at a flow rate of 0.6 mL/min”.
2.5. Enzymatic Hydrolysis
The treated and untreated KB biomasses were enzymatically hydrolyzed using the method described in a previous study with minor modifications [31,32]. Briefly, “the mixture reaction of enzymatic hydrolysis was performed in a 50 mL Erlenmeyer flask, which was composed of 0.05 M sodium citrate buffer (pH = 4.8), 0.1 mL of 2% sodium azide (w/v), and 0.1 g of biomass (dry basis), with a total volume of 10 mL. Thirty filter paper units (FPU)/g dry biomass of cellulase (Celluclast 1.5 L, Sigma-Aldrich, St. Louis, MO, USA) and 60 U/g dry biomass of β-glucosidase (Oriental Yeast Co. Ltd., Tokyo, Japan) were added to the hydrolysis system. The reaction solution was maintained for 72 h at 50 °C and 150 rpm on a rotary shaker (Innova 4340, New Brunswick Scientific Company, Edison, NJ, USA). Samples of hydrolysates (200 µL) were taken periodically (12, 24, 48, and 72 h) for HPLC measurement of monomer sugars”.
The hydrolysis efficiency (HE) and glucose recovery (GR) were calculated using Equations (3) and (4):
HE (%) = ((Glucose released, g) × 0.9)/(Glucan in initial biomass, g) × 100
GR (%) = (Solid recovery (%DW) × Glucan content (%DW) × 1.11 × HE (%)) × 100
2.6. X-ray Diffraction Analysis
This analysis has been described in a previous report by Obeng et al. 2018 [31]. Briefly, “the X-ray diffraction with a PANalytical X’pert Pro PW 3040/60 diffractometer (Almelo, The Netherlands) was employed to assess the crystallinity of treated and untreated KB biomass”. “The specimen was cleaned three times with acetone and allowed to air-dry at room temperature. The dried sample was pulverized, passed through a sieve with a 150-µm, and scanned between 10°and 30° at a rate of 0.2° per minute”.
The crystallinity index (CrI) was calculated using Equation (5) [33]:
where I002 and Iam represent the estimated intensities at 2θ = 22.5°and 2θ = 15.5°, respectively.
CrI = (I002 − Iam)/I002 × 100%
2.7. Determination of Microstructures of Biomass
“The microstructure of KB was evaluated using scanning electron microscopy (Field Emission Scanning Electron Microscope, FESEM; Thermo Fisher, Apero S), as described in a report by Siripong et al. [27]. Both the treatment and control samples were freeze-dried. The dry sample was then affixed to aluminum stubs and coated with gold”.
2.8. Analytical Statistics
The outcomes were statistically examined using variance and Student’s t-test. All data are shown as mean ± SD (n = 3 and p < 0.5).
3. Results
3.1. Characterization of KB
In this experiment, bark and core fibers from KB were used as raw materials. The chemical compositions of the untreated bark and core fibers were analyzed, as presented in Table 1. The total carbohydrate content (glucan and xylan) was approximately 70% for bark and 65% for core fibers. The ethanol extracts of bark (8.3 ± 0.4%) and core fibers (8.2 ± 0.4%) were equivalent. The contents of xylan (18.4 ± 0.2%) and acid-insoluble lignin (14.2 ± 0.0%) in core fiber were substantially higher than those in bark fiber (13.1 ± 0.1% and 9.5 ± 0.2%, respectively). However, the glucan (56.3 ± 0.2%), acid-soluble lignin (4.3 ± 0.0%), and ash (3.7 ± 0.0%) contents in bark fiber were markedly greater than those in the core fiber (46.3 ± 0.1%, 3.1 ± 0.0%, and 2.7 ± 0.0%, respectively).

Table 1.
Composition of kenaf raw materials.
3.2. Influence of the PA Concentration on the Chemical Composition of Bark and Core
In this study, various concentrations of PA (70%, 75%, 80%, and 85%) were used to treat the bark and core fibers of KB. The effects of PA pretreatment were manifested by changes in the proportions of the three major components (cellulose, hemicellulose, and lignin) in both feedstocks. All the data for bark and core fibers are summarized in Table 2 and Table 3, respectively. In addition, the chemical composition of both samples was remarkably altered when they were pretreated with various concentrations of PA. The amounts of acid-insoluble lignin (AIL), acid-soluble lignin (ASL), and xylan in both feedstocks decreased significantly (p < 0.05) as the PA concentration increased. The xylan content in both feedstocks was significantly reduced (p < 0.05) and ultimately removed because of the PA treatment effect. The solubilization of xylan became more pronounced as the concentration of PA increased. The total xylan content in bark and core fibers was removed entirely by treatment with 80% and 85% PA, respectively (Table 2 and Table 3). A considerable decrease in total lignin was also observed, ranging from 13.8 ± 0.2% (untreated) to 4.4 ± 0.1% for bark fiber and from 17.3 ± 0.0% (untreated) to 4.1 ± 0.2% for core fiber (Table 2 and Table 3). This quantity of total lignin corresponds to roughly 86.8 ± 0.4% and 89.8 ± 0.4% lignin removal for bark and core fibers, respectively (Figure 1). At PA concentrations of 70%, 75%, and 80%, the relative glucose content of bark fiber increased to 78.5 ± 0.2%, 86.6 ± 0.5%, and 89.9 ± 0.4%, whereas that of core fiber improved to 64.0 ± 0.7%, 75.9 ± 0.5%, and 77.4 ± 0.4%, respectively. In contrast, the relative glucose contents of bark fiber (79.4 ± 0.5%) and core fiber (68.9 ± 0.1%) declined upon treatment with 85% PA. Nevertheless, the glucan recovery of bark fiber (59.0 ± 0.3%) and core fiber (64.3 ± 0.1%) was greater than 50%.

Table 2.
Chemical composition of bark fiber in kenaf biomass after pretreatment.

Table 3.
Chemical composition of core fiber in kenaf biomass after pretreatment.
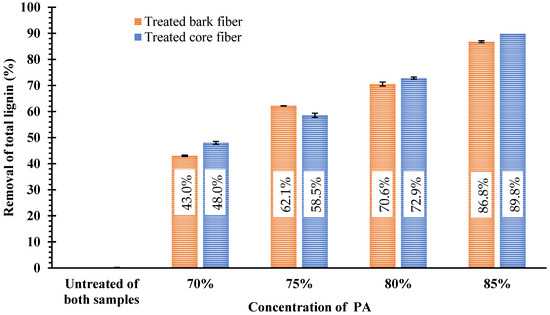
Figure 1.
Lignin removal of both treated bark and core fibers.
3.3. Effect of PA Pretreatment on Enzymatic Hydrolysis
To utilize KB in sugar platform-based biorefineries, a pretreatment process is required to enhance the efficiency of enzymatic hydrolysis to produce monomeric sugars. The impact of PA concentration on HE and GR yields of both feedstocks is presented in Figure 2 for bark fiber and Figure 3 for core fiber. According to the data, the HE and GR yields of untreated samples were 16.3 ± 0.4% and 11.3 ± 0.3, respectively, for bark fiber, and 18.2 ± 0.4% and 10.4 ± 0.2%, respectively, for core fiber (Figure 2 and Figure 3). Incomparable with untreated samples, the HE and GR yields were significantly (p < 0.05) increased after these feedstocks were treated with PA. During enzymatic hydrolysis, the HE and GR yields increased dramatically at 12 h and then progressively at 24, 48, and 72 h. Consequently, the maximum HE and GR yields of both treated and untreated materials were obtained after 72 h incubation. On one hand, at PA concentrations of 70%, 75%, 80%, and 85%, the HE yields were revealed to be 76.9 ± 0.5%, 84.7 ± 0.3%, 80.1 ± 0.3%, and 76.7 ± 0.1%, respectively, for bark fiber, and 80.5 ± 0.9%, 84.6 ± 0.7%, 77.1 ± 0.8%, and 70.8 ± 0.6%, respectively, for core fiber. On the other hand, at PA concentrations of 70%, 75%, 80%, and 85%, the GR yields were determined to be 50.9 ± 0.3%, 55.3 ± 0.2%, 47.4 ± 0.1%, and 31.4 ± 0.0%, respectively, for bark fiber, and 43.4 ± 0.5%, 46.6 ± 0.4%, 36.1 ± 0.4%, and 26.0 ± 0.2%, respectively, for core fiber (Figure 2 and Figure 3). When both feedstocks were processed with 75% PA, the maximum HE and GR yields were observed for bark fiber at 84.7 ± 0.3% and 55.3 ± 0.2% and for core fiber at 84.6 ± 0.7% and 46.6 ± 0.4%, respectively. Nonetheless, as PA increased to 80% and 85%, HE and GR yield decreased, as shown in Figure 2 and Figure 3. Compared with untreated feedstocks, HE yields were increased by approximately 5.6 times for bark and 4.7 times for core fibers. However, GR yields were improved approximately 4.9-fold for bark fiber and 4.3-fold for core fiber.
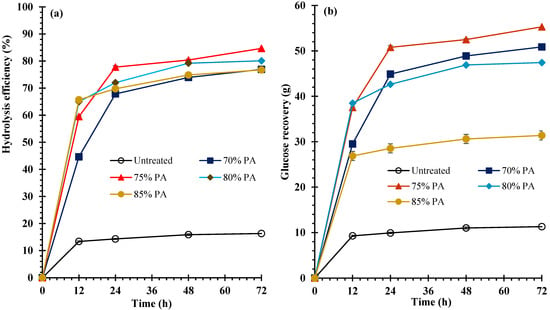
Figure 2.
(a) Hydrolysis efficiency (HE) and (b) Glucose recovery (GR) of bark fiber.
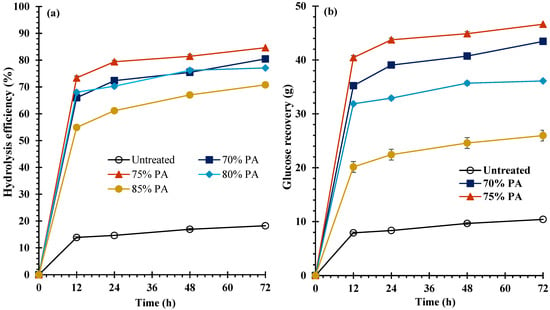
Figure 3.
(a) Hydrolysis efficiency (HE) and (b) Glucose recovery (GR) of core fiber.
3.4. Changes in Surface Morphology
Screening electron microscopy (SEM) was used to assess how pretreatment with PA affected the surface morphology of these materials. The surface morphology of both treated and untreated samples could be observed in the SEM micrographs, as shown in Figure 4a–e for bark fiber and Figure 5a–e for core fiber. Untreated biomass revealed a typical uniform, compacted surface, organized, and stiff fibril structure formed in bundles of intact on the surface of bark and core fibers (Figure 4a and Figure 5a, respectively). After both types of raw biomass were treated with different amounts of PA, the surface structure of each untreated biomass was remarkably altered and subsequently cracked, displaying long rod-shaped vessels. In addition, the fibers inside the intact structures of both samples eventually split, causing them to become highly disordered. (Figure 4b–e and Figure 5b–e).

Figure 4.
Scanning electron micrographs of bark fiber pretreatment with various concentrations of PA; (a) Untreated bark fiber, and that treated with (b) 70% PA, (c) 75% PA, (d) 80% PA, and (e) 85% PA.
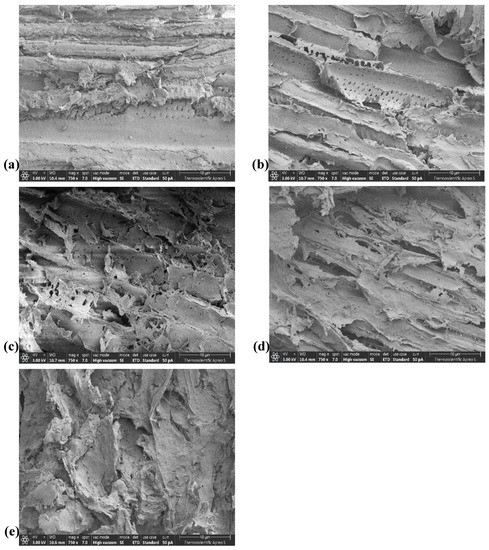
Figure 5.
Scanning electron micrographs of core fiber pretreatment with various concentrations of PA; (a) Untreated core fiber, and that treated with (b) 70% PA, (c) 75% PA, (d) 80% PA, and (e) 85% PA.
3.5. Effect of PA on Cellulose Crystal
The crystallinity index (CrI) values of each sample are listed in Table 4. The X-ray diffractograms of both treated and untreated samples are illustrated in Figure 6 for bark fiber and Figure 7 for core fiber. The X-ray diffractograms of untreated bark and core fibers displayed two significant peaks, characterized as crystalline cellulose I, corresponding to the 101 and 002 lattice planes at 15.5° and 22.5°, respectively. The CrI of untreated bark fibers (70.1%) was higher than that of core fibers (52.0%). When both samples were treated with 70% and 75% PA, the CrI rose to around 74% for bark fiber and approximately 58% for core fiber (Table 4). In addition, the crystallinity of cellulose I was preserved in both feedstocks. When both feedstocks were treated with 80% PA, the two-peak intensity of bark was substantially destroyed (Figure 4d). However, the peak intensity of the core fiber was broad at (2θ)15.5°, and the CrI value increased (61.2%), as shown in Table 4. The crystallite structure of both feedstocks was disrupted and changed from cellulose I to amorphous after treatment with PA 85%. Consequently, the CrI value decreased. Under these conditions, hemicellulose was entirely removed from both samples.

Table 4.
Crystallinity index of the treated and untreated bark and core fibers.
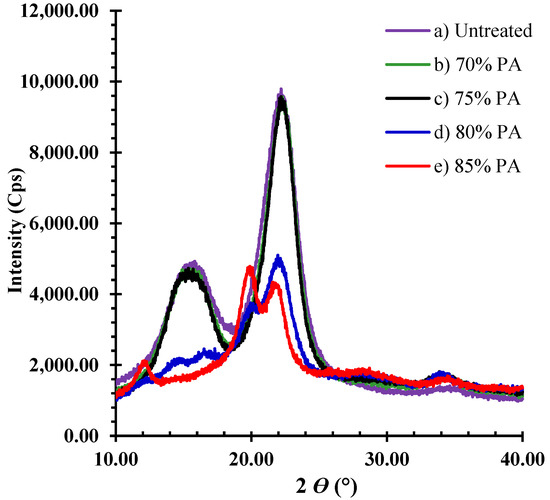
Figure 6.
X-ray diffractogram pattern of treated and untreated bark fibers.
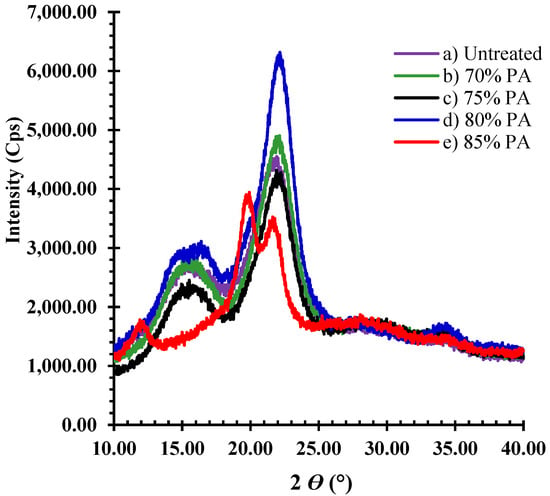
Figure 7.
X-ray diffractogram pattern of treated and untreated core fibers.
4. Discussion
4.1. Characterization of KB
The varying amounts of cellulose, hemicellulose and lignin in lignocellulose depend on the type of lignocellulosic material, plant type, soil quality, harvesting season, and environment [34]. Cellulose is the most important component in synthesizing biofuels derived from biomass and sugar-based biorefineries. The glucan content of bark fiber was greater than that of Japanese cedar (52.7%) [35], reed (49.4%) [36], poplar (49.7%) [37], and aspen (49.0%) [38]. In the case of core fibers, the amount of glucan content was slightly higher than in several lignocellulosic materials such as pine wood (42.0%) [39], oak (41.3) [40], Douglas fir (40.9%) [41], and wheat straw (42.8%) [42]. These results demonstrate the exciting potential of KB for utilization in sugar platform-based biorefineries to produce bioethanol and other value-added compounds.
4.2. Effect of PA Concentration on the Chemical Composition of Bark and Core
The mechanism underlying the main effect of PA on lignocellulosic materials during the pretreatment process involves PA reacting with the biomass to destroy the linkages between cellulose, hemicellulose, and lignin. Consequently, sugars and lignin become partially soluble. Hemicellulose is the component most susceptible to solubilization compared with cellulose and lignin during the pretreatment of feedstock with concentrated PA [43]. Additionally, the crystalline structure of cellulose fiber was transformed into an amorphous structure by this procedure [25,26]. The PA concentration affected both the complete and partial removal of hemicellulose from both samples. Nevertheless, a higher PA concentration could not completely remove lignin from the bark and core fibers, resulting in only a partial decrease. Several authors have observed comparable impacts of PA pretreatment [26,27,31,44,45]. In this study, concentrations of PA at 80% and 85% were found to completely remove xylan from bark and core fibers, respectively. According to reports, hemicellulose is most effectively removed from lignocellulose pretreated with PA concentrations between approximately 75% and 83%. In contrast, cellulose and lignin are only substantially reduced, leading to an increase in relative glucan content [46]. At a higher PA concentration (85%), all linkages between lignin and carbohydrates are destroyed. In addition, the hydrogen bonds connecting the glucan chains are degraded, resulting in a substantial decline in the relative glucan content, glucan recovery, and solid recovery. [25,26,47]. These effects have been reported for several lignocellulosic materials, including weed biomass [45], Thai kenaf [27], and durian peel [31].
4.3. Effect of PA Pretreatment on Enzymatic Hydrolysis
In general, pretreatment of LB is a necessary step for cellulosic biochemical processes, which aim to make cellulose accessible by removing hemicelluloses and lignin from the biomass [48]. The results showed that the lowest HE and GR yields were obtained from untreated feedstocks. This revealed that the recalcitrant structure of both raw materials served as a barrier to restrict cellulase accessibility [20,49,50]. The yields of HE and GR were improved after the pretreatment of each biomass with various PA concentrations. These results suggest that PA pretreatment has a greater impact on the digestibility of cellulose.
Hemicelluloses, a natural barrier surrounding cellulose, can limit enzymatic hydrolysis by blocking enzyme accessibility to cellulose and decreasing the activities of endoglucanase and cellobiohydrolase [25]. According to our findings, approximately 80% of the xylan was removed from both feedstocks when treated with 75% PA. This is the ideal circumstance for maximizing HE and GR yields. However, the levels of HE and GR yields decreased at higher PA concentrations (80% and 85%), owing to the decomposition of glucose during pretreatment.
As a structural barrier, lignin prevents enzymes from penetrating cellulose, restricting carbohydrate availability [20]. Therefore, the removal of lignin is required to enhance enzymatic accessibility to cellulose. In this study, the reduction in total lignin from both materials improved dramatically as the PA concentration increased (Figure 1). Nevertheless, pretreatment with 75% PA generated the maximum HE and GR yields for each treated feedstock. Under these conditions, more than 50% of the total lignin was removed from treated bark fiber (62.1 ± 0.1%) and treated core fiber (58.6 ± 0.5%). These results indicate that the elimination of lignin had an impact on the enhancement of enzyme digestibility. Delignification typically results in disruption of the polymeric network, improvement in porosity, and a decrease in enzyme inhibitory activity [51,52]. The HE yields of both feedstocks were higher than those observed in spruce chips (36.3%), bamboo (39.3%); corn stalks (43.5%); oak chips (50.2%); Jerusalem artichoke stalks (59.6%); wheat straw (68.7%), as reported by Wang et al. 2014 [6]; and common wireweed (82.2%) [45]. Nevertheless, the GR of these feedstocks was larger than that of two varieties of durian peel: 41.7% for monthong peel and 38.5% for chanee peel, which were treated with 75% PA [31].
Thus, the elimination of hemicelluloses and/or lignin by PA pretreatment in this study was associated with an increase in the accessible surface area of cellulose, which is necessary for promoting enzyme digestibility [49].
4.4. Changes in Surface Morphology
In the native structure of lignocellulosic materials, hemicellulose and lignin cover the surface area of cellulose, effectively limiting the accessibility of cellulolytic enzymes [53]. The results indicate that after pretreatment, cellulose had a more accessible surface area when each feedstock was treated with 75% PA; enzymatic hydrolysis generated the highest HE and GR yields. However, as the PA concentration increased, the degradation of the cell wall structure became more severe. At pretreatment degrees of 80% and 85% PA for bark fiber and 85% PA for core fiber, complete disintegration of the structure and generation of more fragments were observed (Figure 4d,e, respectively, and Figure 5e). These results correspond to the decreased HE and GR yields of both the treated feedstocks. In addition, PA pretreatment substantially eliminated hemicellulose and a portion of lignin, consequently enhancing the accessibility of the surface layer of both treated materials. Our results demonstrated that the deletion of barrier coatings on the surface structure was the principal cause of the improvement in HE and GR yields. This confirmed the observations of enzymatic hydrolysis.
4.5. Effect of PA on Cellulose Crystal Structure
The crystallinity of LB is one significant contributor to the susceptibility of cellulose to enzymatic hydrolysis [49,50]. This result is consistent with what other researchers have observed on how pretreatment with approximately 85% concentrated PA makes cellulose less crystalline [47,54,55,56,57]. PA concentrations below 80% were reported to cause cellulose swelling. In contrast, PA concentrations greater than 80% were capable of degrading cellulose and disrupting its crystalline structure [25,55]. These results revealed that the increase in CrI values in both treated samples was unaffected by the improvement in enzymatic hydrolysis. The CrI values of both pretreated samples might improve if the amorphous part of the native cellulose is partially broken down by the acid. Several authors found that increasing the CrI value of LB does not always affect hydrolysis yield [26,31,45]. Even though the percentage of CrIs increased, our research revealed that depleting hemicellulose altered the structure of the cell wall in a strategy that facilitated enzymatic hydrolysis.
The material balance of KB bark and core fibers was transformed into glucose, as illustrated in Figure 8. Both untreated and treated fractions of these feedstocks were exposed to an enzymatic hydrolysis process to convert KB to glucose. Raw material bark and core fibers contained approximately 56.3% and 46.3% glucan, respectively. After loading 1000 g of bark and core fibers into the pretreatment process (75% PA, 60 °C, 60 min), 612 g of solid fraction consisting of 530 g glucan and 36 g xylan for bark fiber, and 588 g of solid fraction composed of 446 g glucan and 39 g xylan for bark fiber were collected. After 72 h of enzymatic hydrolysis, the HE and GR yields for both untreated samples were approximately 16.3% and 113 g, respectively, for bark fiber and 18.2% and 104 g, respectively, for core fiber. The pretreatment of both feedstocks with PA 75% achieved the maximum HE and GR yields, which were approximately 84.7% and 552 g, respectively, for bark fiber and 84.6% and 466 g, respectively, for core fiber. Compared with untreated feedstocks, HE increased by approximately 5.6 times for bark and 4.7 times for core fibers. However, GR was enhanced approximately 4.9-fold for bark fibers and 4.3-fold for core fibers.
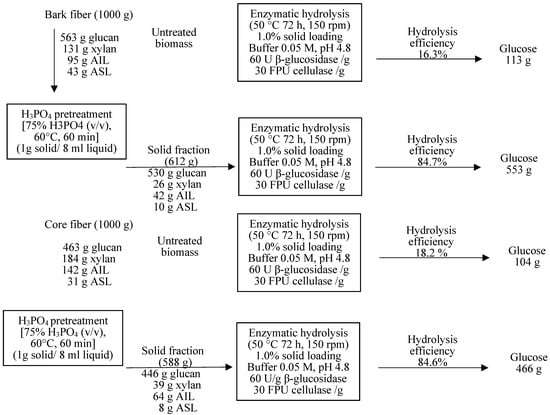
Figure 8.
Material balance of bark and core fibers for glucose production.
5. Conclusions
This research indicates that PA could be utilized for the pretreatment of bark and core fibers inside KB to generate higher enzymatic hydrolysis. Achieving the highest HE and GR yield, 75% PA had the greatest influence on the pretreatment of both feedstocks. The maximum HE and GR yields were observed for bark fiber at 84.7 ± 0.3% and 55.3 ± 0.2% and for core fiber at 84.6 ± 0.7% and 46.6 ± 0.4%, respectively. This condition corresponds to roughly 86.8 ± 0.4% and 89.8 ± 0.4% lignin removal for bark and core fibers, respectively. The pretreatment of these feedstocks with higher concentrations (80% and 85%) led to the degradation of cellulose, resulting in a decrease in HE and GR yield. In addition, the elimination of hemicellulose and partial removal of lignin caused the pretreatment of these feedstocks with PA to dramatically change the surface properties of both bark and core fibers. Thus, PA treatment has a substantial impact on the improvement of HE and GR yield of KB.
Author Contributions
Conceptualization, D.P. and S.P.; methodology, D.P., S.P. and S.W.; software, D.P., S.P. and S.W.; validation, D.P. and S.P.; formal analysis, D.P. and S.P.; investigation, D.P. and S.P.; resources, D.P. and S.P.; data curation, D.P. and S.P.; writing—original draft preparation, D.P. and S.P.; writing—review and editing, D.P. and S.P.; visualization, D.P. and S.P.; supervision, D.P. and S.P.; project administration, S.P.; funding acquisition, D.P. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
The National Research Council of Thailand funded this research (funding number 2556A10702130).
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Faculty of Science for supporting participation in the 4th Asian Federation of Biotechnology Malaysia Chapter International Symposium (AFOBMCIS) 2022 in Malaysia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- An, Y.M.; Zhuang, J.; Li, Y.; Dai, J.Y.; Xiu, Z.L. Pretreatment of Jerusalem Artichoke Stalk Using Hydroxylammonium Ionic Liquids and Their Influences on 2,3-Butanediol Fermentation by Bacillus subtilis. Bioresour. Technol. 2022, 354, 127219. [Google Scholar] [CrossRef] [PubMed]
- Okolie, J.A.; Mukherjee, A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Next-Generation Biofuels and Platform Biochemicals From Lignocellulosic Biomass. Int. J. Energy Res. 2021, 45, 14145–14169. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nizetic, S.; Ong, H.C.; Chong, C.T.; Atabani, A.E.; Pham, V.V. Acid-Based Lignocellulosic Biomass Biorefinery for Bioenergy Production: Advantages, Application Constraints, and Perspectives. J. Environ. Manage. 2021, 296, 113194. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Binod, P.; Pandey, A. Biological Pretreatment of Lignocellulosic Biomass—An Overview. Bioresour. Technol. 2016, 199, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.; Shen, F.; Hu, J.; Sun, F.; Lin, L.; Yang, G.; Zhang, Y.; Deng, S. Pretreating Lignocellulosic Biomass by the Concentrated Phosphoric Acid Plus Hydrogen Peroxide (PHP) for Enzymatic Hydrolysis: Evaluating the Pretreatment Flexibility on Feedstocks and Particle Sizes. Bioresour. Technol. 2014, 166, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; da Silva, S.S. The Path Forward for Lignocellulose Biorefineries: Bottlenecks, Solutions, and Perspective on Commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef]
- Saba, N.; Jawaid, M.; Hakeem, K.R.; Paridah, M.T.; Khalina, A.; Alothman, O.Y. Potential of Bioenergy Production From Industrial Kenaf (Hibiscus cannabinus L.) Based on Malaysian Perspective. Renew. Sustain. Energ. Rev. 2015, 42, 446–459. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Cho, S.K.; Kim, D.S.; Ghodake, G.S.; Kadam, A.; Kumar, G.; Bharagava, R.N.; Banu, R.; Shin, H.S. Pretreatment of Kenaf (Hibiscus cannabinus L.) Biomass Feedstock for Polyhydroxybutyrate (PHB) Production and Characterization. Bioresour. Technol. 2019, 282, 75–80. [Google Scholar] [CrossRef]
- Tye, Y.Y.; Lee, K.T.; Wan Abdullah, W.N.; Leh, C.P. Optimization of Various Pretreatments Condition of Kenaf Core ( Hibiscus cannabinus ) Fibre for Sugar Production: Effect of Chemical Compositions of Pretreated Fibre on Enzymatic Hydrolysability. Renew. Energy 2016, 99, 205–215. [Google Scholar] [CrossRef]
- Kim, J.; Han, G.D.; Muthukathan, G.; Rodrogues, R.; Hyun, D.Y.; Kim, S.H.; Yu, J.K.; Park, J.; Yoo, S.C.; Chung, Y.S. What Traits Should Be Measured for Biomass in Kenaf? Plants 2021, 10, 1394. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.M.; Luthfi, A.A.I.; Low, K.O.; Harun, S.; Manaf, S.F.A.; Illias, R.M.; Jahim, J.M. Preparation of Kenaf Stem Hemicellulosic Hydrolysate and Its Fermentability in Microbial Production of Xylitol by Escherichia coli BL21. Sci. Rep. 2019, 9, 4080. [Google Scholar] [CrossRef]
- Park, H.; Park, S.U.; Jang, B.-K.; Lee, J.J.; Chung, Y.S. Germplasm Evaluation of Kenaf (Hibiscus cannabinus) for Alternative Biomass for Cellulosic Ethanol Production. G.C.B. Bioenergy 2021, 13, 201–210. [Google Scholar] [CrossRef]
- Öztürk, İ.; Irmak, S.; Hesenov, A.; Erbatur, O. Hydrolysis of Kenaf (Hibiscus cannabinus L.) Stems by Catalytical Thermal Treatment in Subcritical Water. Biomass Bioenergy 2010, 34, 1578–1585. [Google Scholar] [CrossRef]
- Ng, S.H.; Tahir, P.M.; Mohamad, R.; Abdullah, L.C.; Choo, A.C.Y.; Liong, Y.Y. Effect of Pretreatment Process on Bioconversion of Kenaf (Hibiscus cannabinus L.) Core to Glucose. BioResources 2013, 8, 2010–2017. [Google Scholar] [CrossRef]
- Jeun, J.-P.; Lee, B.-M.; Lee, J.-Y.; Kang, P.-H.; Park, J.-K. An Irradiation-Alkaline Pretreatment of Kenaf Core for Improving the Sugar Yield. Renew. Energy 2015, 79, 51–55. [Google Scholar] [CrossRef]
- Meryemoğlu, B.; Hasanoğlu, A.; Irmak, S.; Erbatur, O. Biofuel Production by Liquefaction of Kenaf (Hibiscus cannabinus L.) Biomass. Bioresour. Technol. 2014, 151, 278–283. [Google Scholar] [CrossRef]
- Saba, N.; Paridah, M.T.; Jawaid, M.; Abdan, K.; Ibrahim, N.A. Potential Utilization of Kenaf Biomass in Different Applications. In Agricultural Biomass Based Potential Materials; Hakeem, K.R., Jawaid, M., Alothman, Y.O., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–34. [Google Scholar] [CrossRef]
- Saratale, R.G.; Shin, H.S.; Ghodake, G.S.; Kumar, G.; Oh, M.K.; Saratale, G.D. Combined Effect of Inorganic Salts With Calcium Peroxide Pretreatment for Kenaf Core Biomass and Their Utilization for 2,3-Butanediol Production. Bioresour. Technol. 2018, 258, 26–32. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Pandey, A.; Binod, P. Chapter 4. Alkaline Treatment. In Pretreatment of Biomass; Pandey, A., Negi, S., Binod, P., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 51–60. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.F.; Zhang, C.C.; Nan, J.; Ren, N.Q.; Lee, D.J.; Chen, C. Advances in Pretreatment of Lignocellulosic Biomass for Bioenergy Production: Challenges and Perspectives. Bioresour. Technol. 2022, 343, 126123. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, S.; Doherty, W.; Dubal, D.; Atanda, L.; Moghaddam, L.; Sonar, P.; Hessel, V.; Ostrikov, K. Pretreatment and Fermentation of Lignocellulosic Biomass: Reaction Mechanisms and Process Engineering. React. Chem. Eng. 2020, 5, 2017–2047. [Google Scholar] [CrossRef]
- Khir, R.; Pan, Z. Chapter 2. Rice. In Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Zicari, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 21–58. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K.; Kumar, R. Cellulose Solvent-Based Pretreatment for Enhanced Second-Generation Biofuel Production: A Review. Sustain. Energy Fuels 2019, 3, 11–62. [Google Scholar] [CrossRef]
- Sathitsuksanoh, N.; George, A.; Zhang, Y.-H.P. New Lignocellulose Pretreatments Using Cellulose Solvents: A Review. J. Chem. Technol. Biotechnol. 2013, 88, 169–180. [Google Scholar] [CrossRef]
- Premjet, S.; Dana, S.; Obeng, A.K.; Premjet, D. Enzymatic Response to Structural and Chemical Transformations in Hibiscus sabdariffa var. altissima Bark and Core During Phosphoric Acid Pretreatment. BioResources 2018, 13, 6778–6789. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass. In Technical Report NREL/TP-510-42622; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008; pp. 1–8. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. In Technical Report TP-510-42618; National Renewable Energy Laboratory (National Renewable Energy Laboratory, Office of Energy Efficiency and Renewable Energy): Golden, CO, USA, 2012; pp. 1–15. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass In Technical Report NREL/TP-510-42619; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008; pp. 1–12.
- Obeng, A.K.; Premjet, D.; Premjet, S. Fermentable Sugar Production From the Peels of Two Durian (Durio zibethinus Murr.) Cultivars by Phosphoric Acid Pretreatment. Resources 2018, 7, 60. [Google Scholar] [CrossRef]
- Obeng, A.K.; Premjet, D.; Premjet, S. Improved Glucose Recovery From Durian Peel by Alkaline-Catalyzed Steam Pretreatment. PeerJ 2021, 9, e12026. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Premjet, S.; Pumira, B.; Premjet, D. Determining the Potential of Inedible Weed Biomass for Bio-energy and Ethanol Production. BioResources 2013, 8, 701–716. [Google Scholar] [CrossRef]
- Muranaka, Y.; Nakagawa, H.; Hasegawa, I.; Maki, T.; Hosokawa, J.; Ikuta, J.; Mae, K. Lignin-Based Resin Production From Lignocellulosic Biomass Combining Acidic Saccharification and Acetone-Water Treatment. Chem. Eng. J. 2017, 308, 754–759. [Google Scholar] [CrossRef]
- Raud, M.; Tutt, M.; OLT, J.; Kikas, T. Dependence of the Hydrolysis Efficiency on the Lignin Content in Lignocellulosic Material. Int. J. Hydrogr. Energy 2016, 41, 16338–16343. [Google Scholar] [CrossRef]
- Frederick, N.; Zhang, N.; Ge, X.; Xu, J.; Pelkki, M.; Martin, E.; Carrier, D.J. Poplar (Populus deltoides L.): The Effect of Washing Pretreated Biomass on Enzymatic Hydrolysis and Fermentation to Ethanol. A.C.S. Sustain. Chem. Eng. 2014, 2, 1835–1842. [Google Scholar] [CrossRef]
- Goshadrou, A.; Karimi, K.; Lefsrud, M. Characterization of Ionic Liquid Pretreated Aspen Wood Using Semi-quantitative Methods for Ethanol Production. Carbohydr. Polym. 2013, 96, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Lucia, L.A.; Ghiladi, R.A. Development of a Highly Efficient Pretreatment Sequence for the Enzymatic Saccharification of Loblolly Pine Wood. ACS Sustain. Chem. Eng. 2016, 4, 3669–3678. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kim, K.-H.; Ok, Y.S.; Jeon, Y.J.; Kwon, E.E. Pyrolysis of Wastes Generated Through Saccharification of Oak Tree by Using CO2 as Reaction Medium. Appl. Therm. Eng. 2017, 110, 335–345. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Chandra, M.S.; Gu, F.; Gleisner, R.; Reiner, R.; Sessions, J.; Marrs, G.; Gao, J.; Anderson, D. Using Sulfite Chemistry for Robust Bioconversion of Douglas-Fir Forest Residue to Bioethanol at High Titer and Lignosulfonate: A Pilot-Scale Evaluation. Bioresour. Technol. 2015, 179, 390–397. [Google Scholar] [CrossRef]
- Yuan, Z.; Wen, Y.; Li, G. Production of Bioethanol and Value Added Compounds From Wheat Straw Through Combined Alkaline/Alkaline-Peroxide Pretreatment. Bioresour. Technol. 2018, 259, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, W.; Hou, Q.; Wang, S.; Liu, F. Effects of Combined Pretreatment of Dilute Acid Pre-extraction and Chemical-Assisted Mechanical Refining on Enzymatic Hydrolysis of Lignocellulosic Biomass. R.S.C. Adv. 2018, 8, 10207–10214. [Google Scholar] [CrossRef]
- Takata, E.; Tsuruoka, T.; Tsutsumi, K.; Tsutsumi, Y.; Tabata, K. Production of Xylitol and Tetrahydrofurfuryl Alcohol From Xylan in Napier Grass by a Hydrothermal Process With Phosphorus Oxoacids Followed by Aqueous Phase Hydrogenation. Bioresour. Technol. 2014, 167, 74–80. [Google Scholar] [CrossRef]
- Siripong, P.; Duangporn, P.; Takata, E.; Tsutsumi, Y. Phosphoric Acid Pretreatment of Achyranthes aspera and Sida acuta Weed Biomass to Improve Enzymatic Hydrolysis. Bioresour. Technol. 2016, 203, 303–308. [Google Scholar] [CrossRef]
- Kundu, C.; Samudrala, S.P.; Kibria, M.A.; Bhattacharya, S. One-Step Peracetic Acid Pretreatment of Hardwood and Softwood Biomass for Platform Chemicals Production. Sci. Rep. 2021, 11, 11183. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ding, S.Y.; Mielenz, J.R.; Cui, J.B.; Elander, R.T.; Laser, M.; Himmel, M.E.; McMillan, J.R.; Lynd, L.R. Fractionating Recalcitrant Lignocellulose at Modest Reaction Conditions. Biotechnol. Bioeng. 2007, 97, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Gnansounou, E.; Binod, P.; Pandey, A. Bioconversion of Sugarcane Crop Residue for Value Added Products—An Overview. Renew. Energy 2016, 98, 203–215. [Google Scholar] [CrossRef]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of Chemical Pretreatment for Bioconversion of Lignocellulosic Biomass. Renew. Sustain. Energ. Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass Recalcitrance. Part I: The Chemical Compositions and Physical Structures Affecting the Enzymatic Hydrolysis of Lignocellulose. Biofuels Bioprod. Bioref. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Kruyeniski, J.; Ferreira, P.J.T.; Videira Sousa Carvalho, M.d.G.; Vallejos, M.E.; Felissia, F.E.; Area, M.C. Physical and Chemical Characteristics of Pretreated Slash Pine Sawdust Influence Its Enzymatic Hydrolysis. Ind. Crops Prod. 2019, 130, 528–536. [Google Scholar] [CrossRef]
- Pihlajaniemi, V.; Sipponen, M.H.; Liimatainen, H.; Sirviö, J.A.; Nyyssölä, A.; Laakso, S. Weighing the Factors Behind Enzymatic Hydrolyzability of Pretreated Lignocellulose. Green Chem. 2016, 18, 1295–1305. [Google Scholar] [CrossRef]
- Meng, X.; Ragauskas, A.J. Recent Advances in Understanding the Role of Cellulose Accessibility in Enzymatic Hydrolysis of Lignocellulosic Substrates. Curr. Opin. Biotechnol. 2014, 27, 150–158. [Google Scholar] [CrossRef]
- Ishola, M.M.; Isroi; Taherzadeh, M.J. Effect of Fungal and Phosphoric Acid Pretreatment on Ethanol Production From Oil Palm Empty Fruit Bunches (OPEFB). Bioresour. Technol. 2014, 165, 9–12. [Google Scholar] [CrossRef]
- Yoon, S.-Y.; Kim, B.-R.; Han, S.-H.; Shin, S.-J. Different Response Between Woody Core and Bark of Goat Willow (Salix caprea L.) to Concentrated Phosphoric Acid Pretreatment Followed by Enzymatic Saccharification. Energy 2015, 81, 21–26. [Google Scholar] [CrossRef]
- Sathitsuksanoh, N.; Zhu, Z.; Wi, S.; Zhang, Y.H. Cellulose Solvent-Based Biomass Pretreatment Breaks Highly Ordered Hydrogen Bonds in Cellulose Fibers of Switchgrass. Biotechnol. Bioeng. 2011, 108, 521–529. [Google Scholar] [CrossRef]
- Takata, E.; Tsutsumi, K.; Tsutsumi, Y.; Tabata, K. Production of Monosaccharides From Napier Grass by Hydrothermal Process With Phosphoric Acid. Bioresour. Technol. 2013, 143, 53–58. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).