A Novel Mesoporous Activated Carbon Derived from Calliandra calothyrsus via Physical Activation: Saturation and Superheated
Abstract
1. Introduction
2. Experimental
2.1. Materials and Preparation
2.2. Experimental Procedure
2.3. Product Characterization
3. Results and Discussion
3.1. Chemical Properties of Raw Material, Char and Activated Carbon
3.2. Elementary Contecture
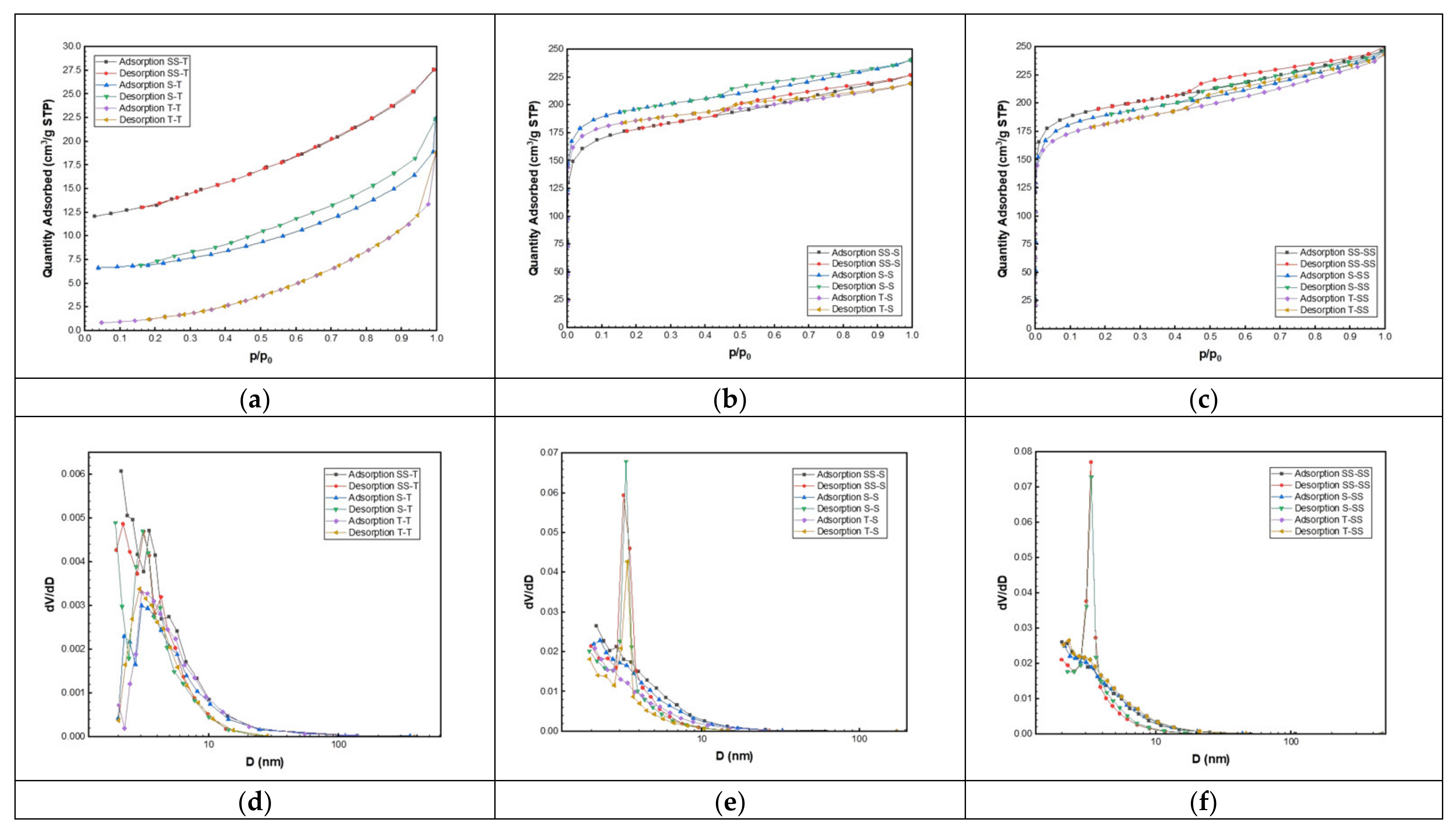
3.3. Surface Texture Property
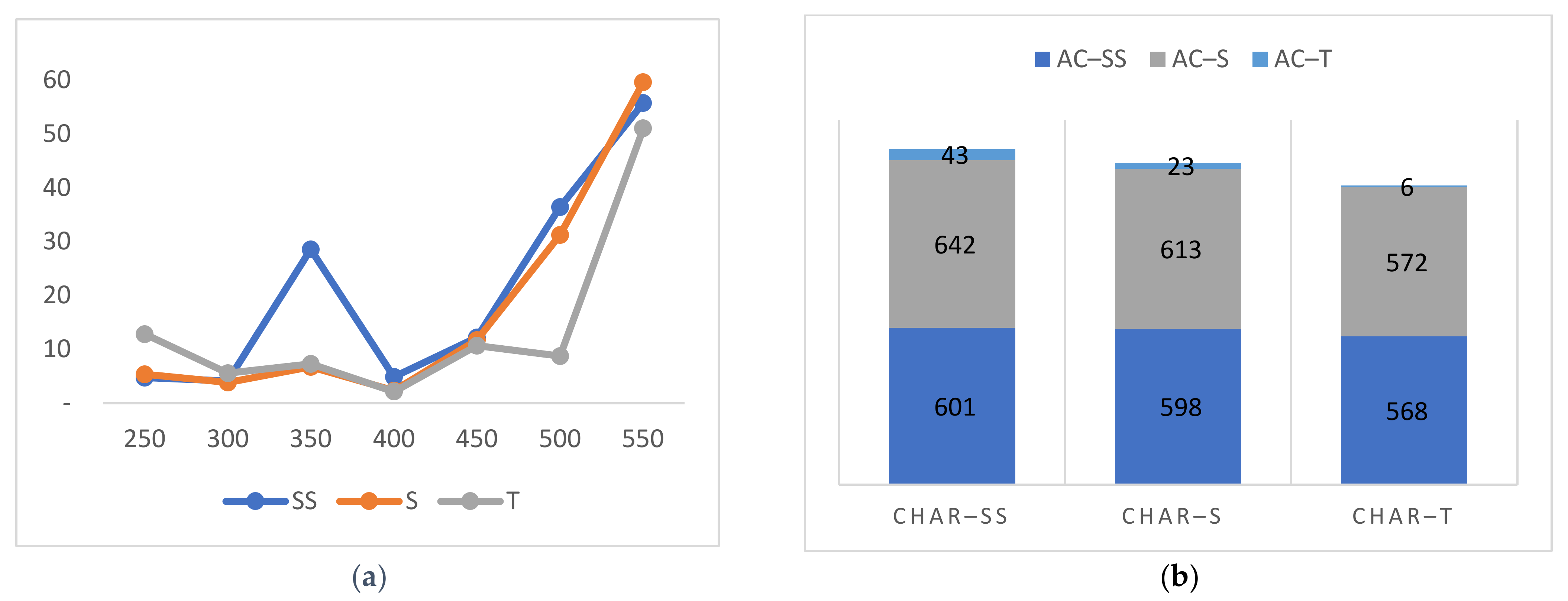
3.4. Iodine Adsorption Performance
3.5. Activated Carbon Structure
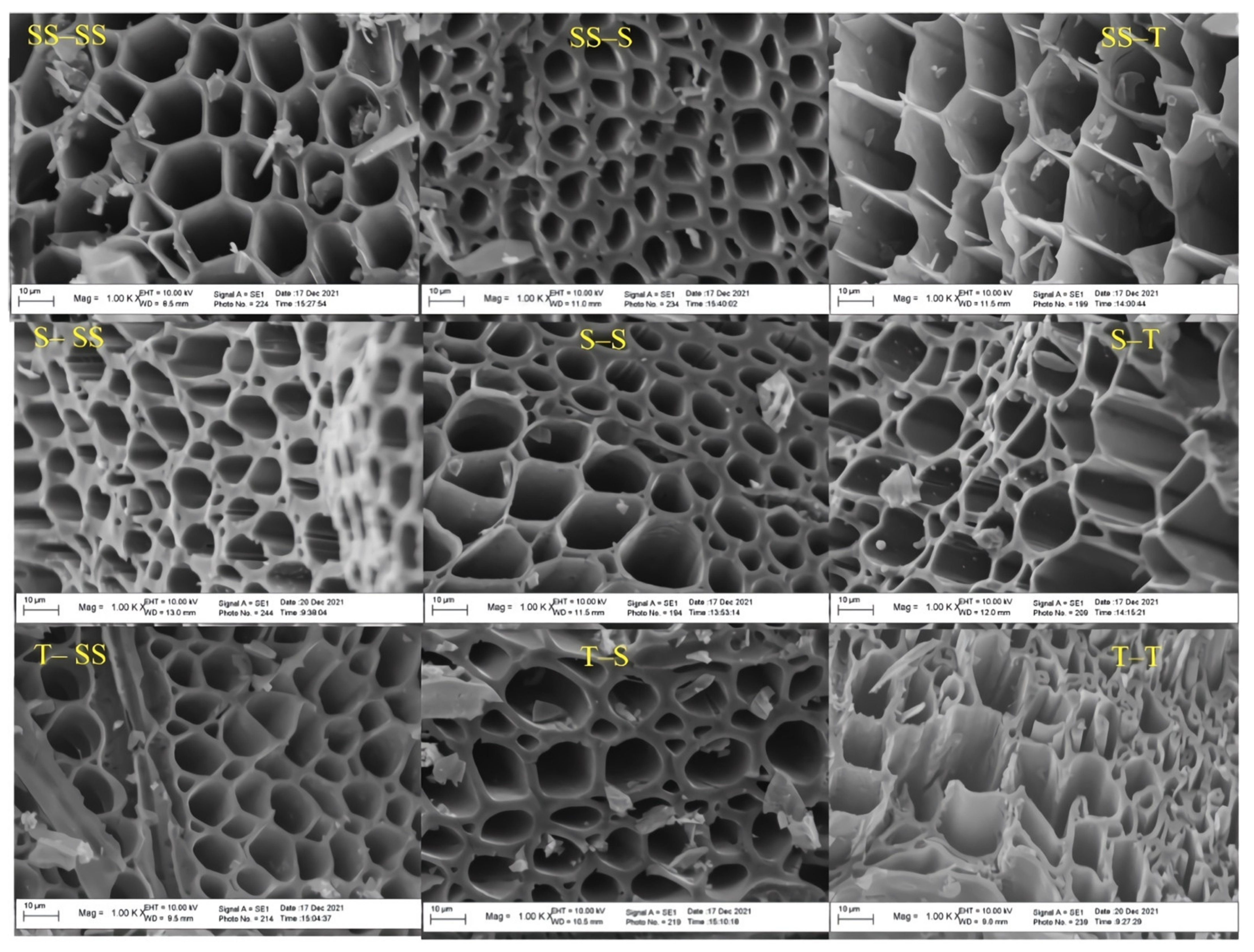
3.6. Surface Topography
3.7. Thermogravimetric (TGA)
3.8. Functional Group of Activated Carbon
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, A.; Balasubramanian, R.; Srinivasan, M. Production of high surface area mesoporous activated carbons from waste biomass using hydrogen peroxide-mediated hydrothermal treatment for adsorption applications. Chem. Eng. J. 2015, 273, 622–629. [Google Scholar] [CrossRef]
- Yuan, J.; Amano, Y.; Machida, M. Surface characterization of mesoporous biomass activated carbon modified by thermal chemical vapor deposition and adsorptive mechanism of nitrate ions in aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 616, 126213. [Google Scholar] [CrossRef]
- Ekanayake, U.M.; Rahmati, S.; Zhou, R.; Zhou, R.; Cullen, P.J.; O’Mullane, A.P.; MacLeod, J.; Ostrikov, K.K. Power-to-decarbonization: Mesoporous carbon-MgO nanohybrid derived from plasma-activated seawater salt-loaded biomass for efficient CO2 capture. J. CO2 Util. 2021, 53, 101711. [Google Scholar] [CrossRef]
- Khalil, K.M.; Elhamdy, W.A.; Mohammed, K.M.; Said, A.E.-A.A. Nanostructured P-doped activated carbon with improved mesoporous texture derived from biomass for enhanced adsorption of industrial cationic dye contaminants. Mater. Chem. Phys. 2022, 282, 125881. [Google Scholar]
- Cheng, H.; Ye, G.; Wang, X.; Su, C.; Zhang, W.; Yao, F.; Wang, Y.; Jiao, Y.; Huang, H.; Ye, D. Micro-mesoporous carbon fabricated by Phanerochaete chrysosporium pretreatment coupling with chemical activation: Promoting effect and toluene adsorption performance. J. Environ. Chem. Eng. 2021, 9, 105054. [Google Scholar] [CrossRef]
- Zhang, G.; Lei, B.; Chen, S.; Xie, H.; Zhou, G. Activated carbon adsorbents with micro-mesoporous structure derived from waste biomass by stepwise activation for toluene removal from air. J. Environ. Chem. Eng. 2021, 9, 105387. [Google Scholar] [CrossRef]
- Khalil, K.M.; Elhamdy, W.A.; Elsamahy, A.A. Biomass derived P−doped activated carbon as nanostructured mesoporous adsorbent for chromium(VI) pollutants with pronounced functional efficiency and recyclability. Colloids Surfaces A: Physicochem. Eng. Asp. 2022, 641, 128553. [Google Scholar] [CrossRef]
- Neolaka, Y.A.; Lawa, Y.; Naat, J.; Riwu, A.A.; Darmokoesoemo, H.; Widyaningrum, B.A.; Iqbal, M.; Kusuma, H.S. Indonesian Kesambi wood (Schleichera oleosa) activated with pyrolysis and H2SO4 combination methods to produce mesoporous activated carbon for Pb(II) adsorption from aqueous solution. Environ. Technol. Innov. 2021, 24, 101997. [Google Scholar]
- Liou, T.-H. Development of mesoporous structure and high adsorption capacity of biomass-based activated carbon by phosphoric acid and zinc chloride activation. Chem. Eng. J. 2010, 158, 129–142. [Google Scholar] [CrossRef]
- Saputra, N.A.; Saputra, I.S.; Yuniarti, K. Andianto Preparation and characterization of Gigantochloa robusta activated carbon to reduce COD levels of pharmaceutical waste. IOP Conf. Ser. Mater. Sci. Eng. 2020, 935. [Google Scholar] [CrossRef]
- Rutherford, D.W.; Wershaw, R.L.; Rostad, C.E.; Kelly, C.N. Effect of formation conditions on biochars: Compositional and structural properties of cellulose, lignin, and pine biochars. Biomass Bioenergy 2012, 46, 693–701. [Google Scholar] [CrossRef]
- Lewoyehu, M. Comprehensive review on synthesis and application of activated carbon from agricultural residues for the remediation of venomous pollutants in wastewater. J. Anal. Appl. Pyrolysis 2021, 159, 105279. [Google Scholar]
- Joshi, S.; Shrestha, R.G.; Pradhananga, R.R.; Ariga, K.; Shrestha, K.L. High surface area nanoporous activated carbons materials. J. Carbon Res. 2022, 8, 2. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, X.; Zhu, B.; Liu, P.-F.; Lu, H.-T. Micro-mesoporous carbon spheres derived from carrageenan as electrode material for supercapacitors. J. Power Sources 2014, 268, 584–590. [Google Scholar] [CrossRef]
- Rustamaji, H.; Prakoso, T.; Devianto, H.; Widiatmoko, P.; Saputera, W.H. Urea nitrogenated mesoporous activated carbon derived from oil palm empty fruit bunch for high-performance supercapacitor. J. Energy Storage 2022, 52, 104724. [Google Scholar]
- Ma, X.; Yang, H.; Yu, L.; Chen, Y.; Li, Y. Preparation, Surface and Pore Structure of High Surface Area Activated Carbon Fibers from Bamboo by Steam Activation. Materials 2014, 7, 4431–4441. [Google Scholar] [CrossRef]
- Pallarés, J.; González-Cencerrado, A.; Arauzo, I. Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenergy 2018, 115, 64–73. [Google Scholar] [CrossRef]
- Chen, D.; Chen, X.; Sun, J.; Zheng, Z.; Fu, K. Pyrolysis polygeneration of pine nut shell: Quality of pyrolysis products and study on the preparation of activated carbon from biochar. Bioresour. Technol. 2016, 216, 629–636. [Google Scholar] [CrossRef]
- Bardestani, R.; Kaliaguine, S. Steam activation and mild air oxidation of vacuum pyrolysis biochar. Biomass-Bioenergy 2017, 108, 101–112. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Upgrading the rice husk char obtained by flash pyrolysis for the production of amorphous silica and high quality activated carbon. Bioresour. Technol. 2014, 170, 132–137. [Google Scholar] [CrossRef]
- Yi, H.; Nakabayashi, K.; Yoon, S.-H.; Miyawaki, J. Pressurized physical activation: A simple production method for activated carbon with a highly developed pore structure. Carbon 2021, 183, 735–742. [Google Scholar] [CrossRef]
- Zaini, M.A.A.; Zhi, L.L.; Hui, T.S.; Amano, Y.; Machida, M. Effects of physical activation on pore textures and heavy metals removal of fiber-based activated carbons. Mater. Today Proc. 2020, 39, 917–921. [Google Scholar] [CrossRef]
- Williams, P.; Reed, A. Development of activated carbon pore structure via physical and chemical activation of biomass fibre waste. Biomass-Bioenergy 2006, 30, 144–152. [Google Scholar] [CrossRef]
- Powell, M.H.; Roshetko, J.M. International workshop on the Genus Calliandra. In International Workshop on the Genus Calliandra; Winrock International: Bogor, Indonesia, 1996. [Google Scholar]
- Chamberlain, J.R. Calliandra Calothyrsus: An Agroforestry Tree for the Humid Tropics 40; Oxford Forestry Institute, University of Oxford: Oxford, UK, 2001. [Google Scholar]
- Adaganti, S.Y.; Kulkarni, B.M.; Desai, G.P.; Shanmukhappa, S. Effect of hydrothermal explosion pretreatment on the composition and structure of Calliandra calothyrsus shrub—A lignocellulosic biomass. Int. J. Renew. Sustain. Energy 2014, 3, 1–5. [Google Scholar] [CrossRef][Green Version]
- Hendrati, R.L.; Hidayati, N. Budidaya kaliandra (Calliandra calothyrsus) Untuk Bahan Baku Sumber Energi. Cetakan 1. IPB Press: Bogor, Indonesia, 2014. [Google Scholar]
- Haqiqi, M.T.; Yuliansyah; Suwinarti, W.; Amirta, R. Response surface methodology to simplify calculation of wood energy potency from tropical short rotation coppice species. IOP Conf. Ser. Earth Environ. Sci. 2018, 144, 012041. [Google Scholar] [CrossRef]
- Cagnon, B.; Py, X.; Guillot, A.; Stoeckli, F.; Chambat, G. Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors. Bioresour. Technol. 2009, 100, 292–298. [Google Scholar] [CrossRef]
- Rouatbi, M.; Duquenoy, A.; Giampaoli, P. Extraction of the essential oil of thyme and black pepper by superheated steam. J. Food Eng. 2007, 78, 708–714. [Google Scholar] [CrossRef]
- Sagehashi, M.; Miyasaka, N.; Shishido, H.; Sakoda, A. Superheated steam pyrolysis of biomass elemental components and Sugi (Japanese cedar) for fuels and chemicals. Bioresour. Technol. 2006, 97, 1272–1283. [Google Scholar] [CrossRef]
- Berghel, J.; Renström, R. Superheated steam drying of sawdust in continuous feed spouted beds—A design perspective. Biomass-Bioenergy 2014, 71, 228–234. [Google Scholar] [CrossRef]
- Xu, K.; Tu, D.; Chen, T.; Zhong, T.; Lu, J. Effects of environmental-friendly modified rubber seed shell on the comprehensive properties of high density polyethylene/rubber seed shell composites. Ind. Crop. Prod. 2016, 91, 132–141. [Google Scholar] [CrossRef]
- Behera, G.; Sutar, P. A comprehensive review of mathematical modeling of paddy parboiling and drying: Effects of modern techniques on process kinetics and rice quality. Trends Food Sci. Technol. 2018, 75, 206–230. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, X.; Qi, Z.; Wang, H.; Yang, R.; Lin, W.; Li, J.; Zhou, W.; Ronsse, F. Superheated steam as carrier gas and the sole heat source to enhance biomass torrefaction. Bioresour. Technol. 2021, 331, 124955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Han, P.; Yang, R.; Wang, H.; Lin, W.; Zhou, W.; Yan, Z.; Qi, Z. Fuel properties and combustion behaviors of fast torrefied pinewood in a heavily loaded fixed-bed reactor by superheated steam. Bioresour. Technol. 2021, 342, 125929. [Google Scholar] [CrossRef] [PubMed]
- Dence, C.W. The Determination of Lignin. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 33–61. [Google Scholar]
- Yoshihara, K.; Kobayashi, T.; Fujii, T.; Akamatsu, I. A novel modification of Klason lignin quantitaitve method. Jpn. Tappi J. 1984, 38, 466–475. [Google Scholar] [CrossRef]
- Niksiar, A.; Nasernejad, B. Activated carbon preparation from pistachio shell pyrolysis and gasification in a spouted bed reactor. Biomass-Bioenergy 2017, 106, 43–50. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Xing, Z.-J.; Duan, Z.-K.; Li, M.; Wang, Y. Effects of steam activation on the pore structure and surface chemistry of activated carbon derived from bamboo waste. Appl. Surf. Sci. 2014, 315, 279–286. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Su, Y.; Liu, L.; Zhang, S.; Xu, D.; Du, H.; Cheng, Y.; Wang, Z.; Xiong, Y. A green route for pyrolysis poly-generation of typical high ash biomass, rice husk: Effects on simultaneous production of carbonic oxide-rich syngas, phenol-abundant bio-oil, high-adsorption porous carbon and amorphous silicon dioxide. Bioresour. Technol. 2019, 295, 122243. [Google Scholar] [CrossRef]
- Angın, D. Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour. Technol. 2013, 128, 593–597. [Google Scholar] [CrossRef]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Xu, C.; Chen, Z.; Zhang, S. Properties of biomass-derived biochars: Combined effects of operating conditions and biomass types. Bioresour. Technol. 2015, 192, 83–89. [Google Scholar] [CrossRef]
- Ghani, W.A.W.A.K.; Mohd, A.; da Silva, G.; Bachmann, R.T.; Taufiq-Yap, Y.H.; Rashid, U.; Al-Muhtaseb, A.H. Biochar production from waste rubber-wood-sawdust and its potential use in C sequestration: Chemical and physical characterization. Ind. Crop. Prod. 2013, 44, 18–24. [Google Scholar] [CrossRef]
- Yakaboylu, O.; Albrecht, I.; Harinck, J.; Smit, K.; Tsalidis, G.-A.; Di Marcello, M.; Anastasakis, K.; de Jong, W. Supercritical water gasification of biomass in fluidized bed: First results and experiences obtained from TU Delft/Gensos semi-pilot scale setup. Biomass-Bioenergy 2018, 111, 330–342. [Google Scholar] [CrossRef]
- Sumathi, S.; Bhatia, S.; Lee, K.T.; Mohamed, A.R. Optimization of microporous palm shell activated carbon production for flue gas desulphurization: Experimental and statistical studies. Bioresour. Technol. 2009, 100, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Manyà, J.J.; Azuara, M.; Manso, J.A. Biochar production through slow pyrolysis of different biomass materials: Seeking the best operating conditions. Biomass-Bioenergy 2018, 117, 115–123. [Google Scholar] [CrossRef]
- Chen, F.-X.; Gong, P.; Zhang, H.-K.; Bai, X.-H.; Gao, Y.-F.; Zhou, A.-N. Biomass Pyrolysis of Helianthus annuus Stems: Qualitative and Quantitative Study Based on Py-GC/MS. BioResources 2016, 11, 8589–8614. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Yang, H.; Wang, X.; Zhang, S.; Chen, H. Biomass-based pyrolytic polygeneration system on cotton stalk pyrolysis: Influence of temperature. Bioresour. Technol. 2011, 107, 411–418. [Google Scholar] [CrossRef]
- Li, W.; Yang, K.; Peng, J.; Zhang, L.; Guo, S.; Xia, H. Effects of carbonization temperatures on characteristics of porosity in coconut shell chars and activated carbons derived from carbonized coconut shell chars. Ind. Crop. Prod. 2008, 28, 190–198. [Google Scholar] [CrossRef]
- Zhu, X.; Li, C.; Li, J.; Xie, B.; Lü, J.; Li, Y. Thermal treatment of biochar in the air/nitrogen atmosphere for developed mesoporosity and enhanced adsorption to tetracycline. Bioresour. Technol. 2018, 263, 475–482. [Google Scholar]
- Sahin, Ö.; Saka, C. Preparation and characterization of activated carbon from acorn shell by physical activation with H2O–CO2 in two-step pretreatment. Bioresour. Technol. 2013, 136, 163–168. [Google Scholar] [CrossRef]
- Maneerung, T.; Liew, J.; Dai, Y.; Kawi, S.; Chong, C.; Wang, C.H. Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: Kinetics, isotherms and thermodynamic studies. Bioresour. Technol. 2016, 200, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Liu, W.; Jiang, R.; Wang, Z. Physicochemical and porosity characteristics of thermally regenerated activated carbon polluted with biological activated carbon process. Bioresour. Technol. 2014, 171, 260–264. [Google Scholar] [CrossRef]
- An, D.; Guo, Y.; Zou, B.; Zhu, Y.; Wang, Z. A study on the consecutive preparation of silica powders and active carbon from rice husk ash. Biomass-Bioenergy 2011, 35, 1227–1234. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Lin, P.; Gao, Y.; Wen, Y.; Li, K.; Li, L. Oxygen-rich microporous carbons with exceptionally high adsorption of iodine. Mater. Chem. Phys. 2022, 285, 126193. [Google Scholar] [CrossRef]
- Kanjana, K.; Harding, P.; Kwamman, T.; Kingkam, W.; Chutimasakul, T. Biomass-derived activated carbons with extremely narrow pore size distribution via eco-friendly synthesis for supercapacitor application. Biomass-Bioenergy 2021, 153, 106206. [Google Scholar] [CrossRef]
- Subramanian, S.; Pande, G.; De Weireld, G.; Giraudon, J.-M.; Lamonier, J.-F.; Batra, V.S. Sugarcane bagasse fly ash as an attractive agro-industry source for VOC removal on porous carbon. Ind. Crop. Prod. 2013, 49, 108–116. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Luo, H.; Zhang, Y.; Xu, Q.; Zhang, Z.; Xu, H.; Wang, Z. Biomass activated carbon supported with high crystallinity and dispersion Fe 3 O 4 nanoparticle for preconcentration and effective degradation of methylene blue. J. Taiwan Inst. Chem. Eng. 2017, 81, 265–274. [Google Scholar] [CrossRef]
- Pari, G.; Sofyan, K.; Syafii, W.; Buchari, B.; Yamamoto, H. Kajian Struktur Arang Dari Lignin. J. Penelit. Has. Hutan 2006, 24, 9–20. [Google Scholar] [CrossRef]
- Shvalagin, V.; Kuchmiy, S.; Skoryk, M.; Bondarenko, M.; Khyzhun, O. Acid treated crystalline graphitic carbon nitride with improved efficiency in photocatalytic ethanol oxidation under visible light. Mater. Sci. Eng. B 2021, 271, 115304. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S.; Arockiam, L. Study on kinetic parameters of different biomass samples using thermo-gravimetric analysis. Biomass-Bioenergy 2013, 58, 58–66. [Google Scholar] [CrossRef]
- Mohammad-Khah, A.; Ansari, R. Activated charcoal: Preparation, characterization and applications: A review article. Int. J. Chem. Tech. Res. 2009, 1, 859–864. [Google Scholar]
- Allwar, A.; Winarsi, R.; Fitriyani, N.; Merdekawati, K. Characterization and Application of Activated Carbon from Oil Palm Shell Prepared By Physical Activation and Nitric Acid for the Removal of Phenol and 2-Chlorophenol. Int. J. Sci. Res. 2017, 1528–1534. [Google Scholar]
- Maulina, S.; Mentari, V.A. Comparison of Functional Group and Morphological Surface of Activated Carbon from Oil Palm Fronds Using Phosphoric Acid (H3PO4) and Nitric Acid (HNO3) as an Activator. IOP Conf. Ser. Mater. Sci. Eng. 2019, 505, 012023. [Google Scholar] [CrossRef]
- Shu, J.; Cheng, S.; Xia, H.; Zhang, L.; Peng, J.; Li, C.; Zhang, S. Copper loaded on activated carbon as an efficient adsorbent for removal of methylene blue. RSC Adv. 2017, 7, 14395–14405. [Google Scholar] [CrossRef]
- García, J.R.; Sedran, U.; Zaini, M.A.A.; Zakaria, Z.A. Preparation, characterization, and dye removal study of activated carbon prepared from palm kernel shell. Environ. Sci. Pollut. Res. 2017, 25, 5076–5085. [Google Scholar] [CrossRef] [PubMed]



| Proximate | Ultimate | Wood Chemical | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Volatile | Ash | FC | C | H | N | O | Hemicellulose | Cellulose | Lignin |
| 77 | 1.2 | 18 | 45.23 | 6.32 | 0.63 | 47.82 | 29.9 | 58.36 | 28.8 |
| Samples | 2θ Lc(002) | d(002) (nm) | 2θ La(100) | d(100) (nm) | Lc (nm) | N | La (nm) | X (%) |
|---|---|---|---|---|---|---|---|---|
| T–T | 23.30 | 0.3814 | 44.00 | 0.2056 | 1.2414 | 3.26 | 2.8500 | 21.29 |
| T–S | 23.80 | 0.3735 | 44.72 | 0.2024 | 1.6086 | 4.31 | 3.4210 | 21.59 |
| T–SS | 23.98 | 0.3707 | 44.75 | 0.2023 | 1.9851 | 5.35 | 3.8764 | 23.82 |
| S–T | 23.98 | 0.3707 | 43.36 | 0.2085 | 1.5065 | 4.06 | 5.2317 | 22.26 |
| S–S | 24.16 | 0.3680 | 43.86 | 0.2062 | 1.5070 | 4.10 | 4.6992 | 24.71 |
| S–SS | 24.94 | 0.3567 | 43.92 | 0.2059 | 1.6706 | 4.68 | 5.0529 | 23.08 |
| SS–T | 24.26 | 0.3665 | 43.16 | 0.2094 | 1.2303 | 3.36 | 6.3730 | 24.66 |
| SS–S | 26.72 | 0.3333 | 44.79 | 0.2021 | 1.3191 | 3.96 | 5.5577 | 23.56 |
| SS–SS | 25.58 | 0.3479 | 43.96 | 0.2058 | 1.2928 | 3.72 | 7.5968 | 32.76 |
| Sample Code | Mass mg | % Mass Loss at ±105 °C | Tig | Tbo | Mass Loss at Tbo | Rmax | Onset Point | Offset Point | Point of Reaction | Tmax | Enthalpy (J/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | % | °C | % | °C | mg/s | % | °C | min | °C | min | °C | min | °C | min | ||||
| Crude | 7.92 | 7.2 | 285.1 | 12.9 | 562.3 | 87.6 | 323.2 | 0.0176 | 35.2 | 234.9 | 11.0 | 508.9 | 28.0 | 284.1 | 13.0 | 345.6 | 17.0 | 20,121 |
| SS-SS | 12.95 | 15.3 | 340.4 | 21.9 | 217.1 | 90.8 | 365.5 | 0.0213 | 33.8 | 320.8 | 19.0 | 472.4 | 30.0 | 345.9 | 20.0 | 368.5 | 22.0 | 28,013 |
| S-SS | 7.73 | 17.4 | 348.2 | 25.7 | 597.4 | 91.6 | 375.0 | 0.0197 | 45.4 | 328.6 | 19.0 | 434.8 | 27.0 | 354.8 | 21.0 | 382.0 | 23.0 | 37,004 |
| T-SS | 12.2 | 16.3 | 344.2 | 22.7 | 492.2 | 90.5 | 369.1 | 0.0236 | 36.7 | 325.0 | 19.0 | 456.1 | 29.0 | 345.7 | 20.0 | 374.5 | 22.0 | 23,614 |
| SS-S | 8.96 | 18.1 | 351.2 | 27.0 | 517.4 | 78.3 | 379.7 | 0.0160 | 46.9 | 333.0 | 20.0 | 479.4 | 30.0 | 358.4 | 21.0 | 384.3 | 23.0 | 30,485 |
| S-S | 25.28 | 15.0 | 321.7 | 17.1 | 510.9 | 75.1 | 348.8 | 0.0305 | 26.1 | 298.5 | 17.0 | 487.3 | 30.0 | 325.5 | 18.0 | 355.6 | 20.0 | 18,620 |
| T-S | 9.26 | 17.6 | 335.0 | 24.7 | 496.5 | 82.3 | 364.8 | 0.0175 | 40.5 | 315.6 | 18.0 | 471.1 | 29.0 | 344.5 | 20.0 | 370.4 | 22.0 | 34,224 |
| SS-T | 7.67 | 15.2 | 450.4 | 31.1 | 712.3 | 50.7 | 549.7 | 0.0114 | 74.1 | 511.9 | 32.0 | 652.3 | 42.0 | 548.4 | 34.0 | 568.9 | 36.0 | 48,543 |
| S-T | 15.61 | 10.5 | 434.5 | 18.3 | 568.1 | 44.8 | 571.6 | 0.0103 | 45.6 | 387.7 | 23.0 | 552.7 | 35.0 | 398.1 | 24.0 | 528.3 | 33.0 | 5,622 |
| T-T | 11.50 | 10.7 | 440.9 | 19.1 | 600.6 | 87.6 | 517.9 | 0.0192 | 61.7 | 406.9 | 25.0 | 568.6 | 36.0 | 459.3 | 28.0 | 531.2 | 33.0 | 31,384 |
| Sample | O–H | C≡C | C=O | Alkanes | NO2 | C–N | C–O | Alkenes |
|---|---|---|---|---|---|---|---|---|
| T–SS | 58.5 | 38.6 | 45.5 | 54.6 | 56.8 | 45.1 | 52.6 | 52.4 |
| S–SS | 70.1 | 46.5 | 54.3 | 62.3 | 65.4 | 53.1 | 59.9 | 59.2 |
| SS–SS | 58.5 | 38.6 | 45.5 | 54.6 | 56.4 | 45.1 | 57.7 | 52.3 |
| T–S | 63.2 | 40.0 | 45.8 | 53.2 | 57.8 | 46.2 | 52.7 | 52.3 |
| S–S | 137.5 | 65.9 | 77.5 | 68.4 | 90.8 | 68.4 | 68.4 | 81.3 |
| SS–S | 155.5 | 63.7 | 89.1 | 93.7 | 103.7 | 79.1 | 93.3 | 92.9 |
| T–T | 65.9 | 41.0 | 47.9 | 54.1 | 58.5 | 46.1 | 51.9 | 50.1 |
| S–T | 75.6 | 47.8 | 54.7 | 61.6 | 67.0 | 53.7 | 60.8 | 58.7 |
| SS–T | 97.2 | 59.0 | 66.8 | 74.2 | 80.1 | 64.7 | 72.0 | 68.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saputra, N.A.; Darmawan, S.; Efiyanti, L.; Hendra, D.; Wibowo, S.; Santoso, A.; Djarwanto; Gusmailina; Komarayati, S.; Indrawan, D.A.; et al. A Novel Mesoporous Activated Carbon Derived from Calliandra calothyrsus via Physical Activation: Saturation and Superheated. Energies 2022, 15, 6675. https://doi.org/10.3390/en15186675
Saputra NA, Darmawan S, Efiyanti L, Hendra D, Wibowo S, Santoso A, Djarwanto, Gusmailina, Komarayati S, Indrawan DA, et al. A Novel Mesoporous Activated Carbon Derived from Calliandra calothyrsus via Physical Activation: Saturation and Superheated. Energies. 2022; 15(18):6675. https://doi.org/10.3390/en15186675
Chicago/Turabian StyleSaputra, Nur Adi, Saptadi Darmawan, Lisna Efiyanti, Djeni Hendra, Santiyo Wibowo, Adi Santoso, Djarwanto, Gusmailina, Sri Komarayati, Dian Anggraini Indrawan, and et al. 2022. "A Novel Mesoporous Activated Carbon Derived from Calliandra calothyrsus via Physical Activation: Saturation and Superheated" Energies 15, no. 18: 6675. https://doi.org/10.3390/en15186675
APA StyleSaputra, N. A., Darmawan, S., Efiyanti, L., Hendra, D., Wibowo, S., Santoso, A., Djarwanto, Gusmailina, Komarayati, S., Indrawan, D. A., Yuniawati, Nawawi, D. S., Maddu, A., Pari, G., & Syafii, W. (2022). A Novel Mesoporous Activated Carbon Derived from Calliandra calothyrsus via Physical Activation: Saturation and Superheated. Energies, 15(18), 6675. https://doi.org/10.3390/en15186675








