Biochar Acts as an Emerging Soil Amendment and Its Potential Ecological Risks: A Review
Abstract
1. Introduction
2. Basic Properties of Biochar
2.1. Elements
2.2. pH
2.3. Functional Groups
2.4. Electrical Conductance (EC)
2.5. CEC
3. Effects of Biochar on Soil Properties
3.1. pH
3.2. Soil Bulk Density
3.3. Soil Water Retention
3.4. CEC
3.5. Soil Enzyme Activity
4. Effects of Biochar Addition on Soil Nutrients
4.1. C
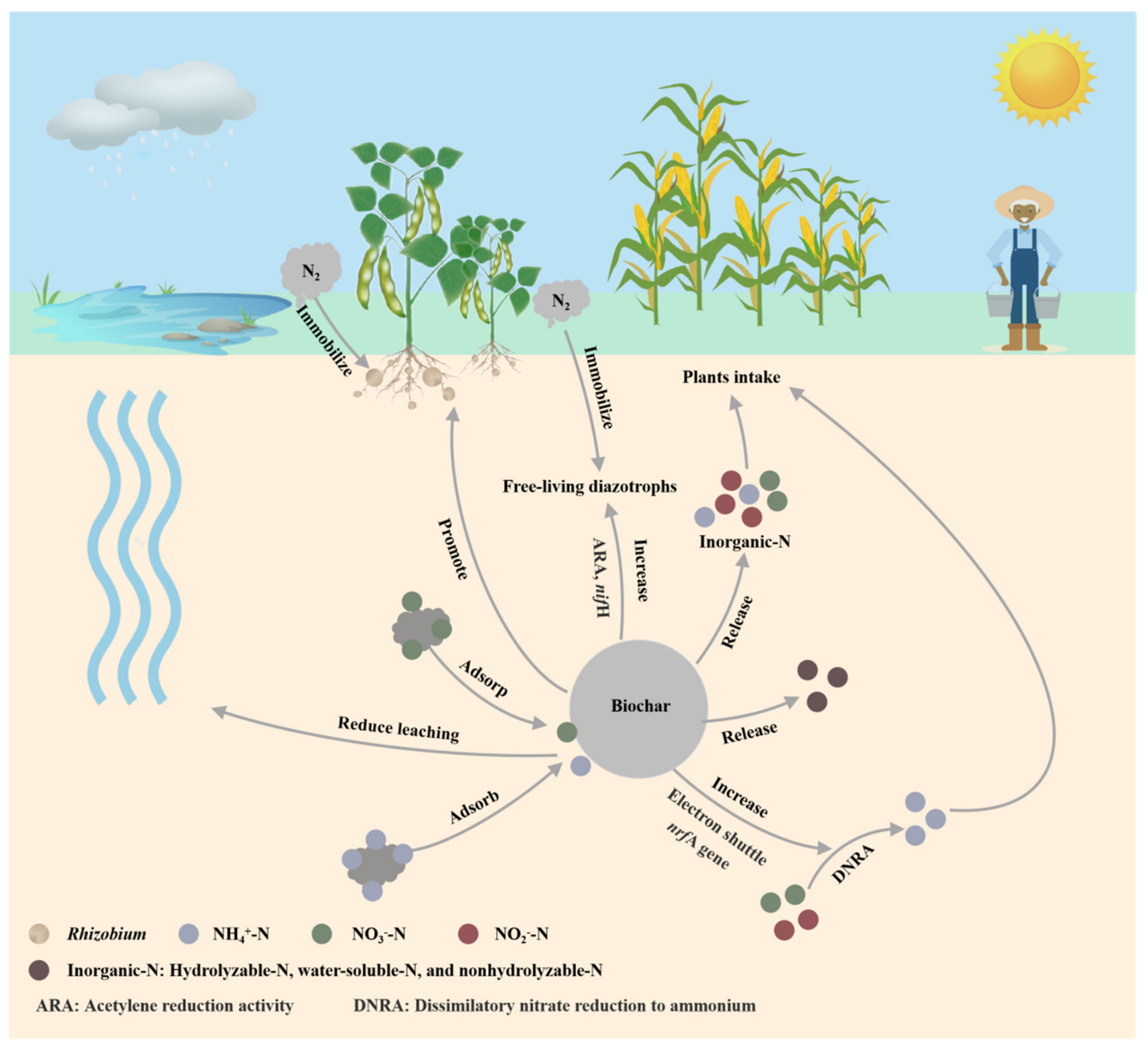
4.2. N
4.3. P
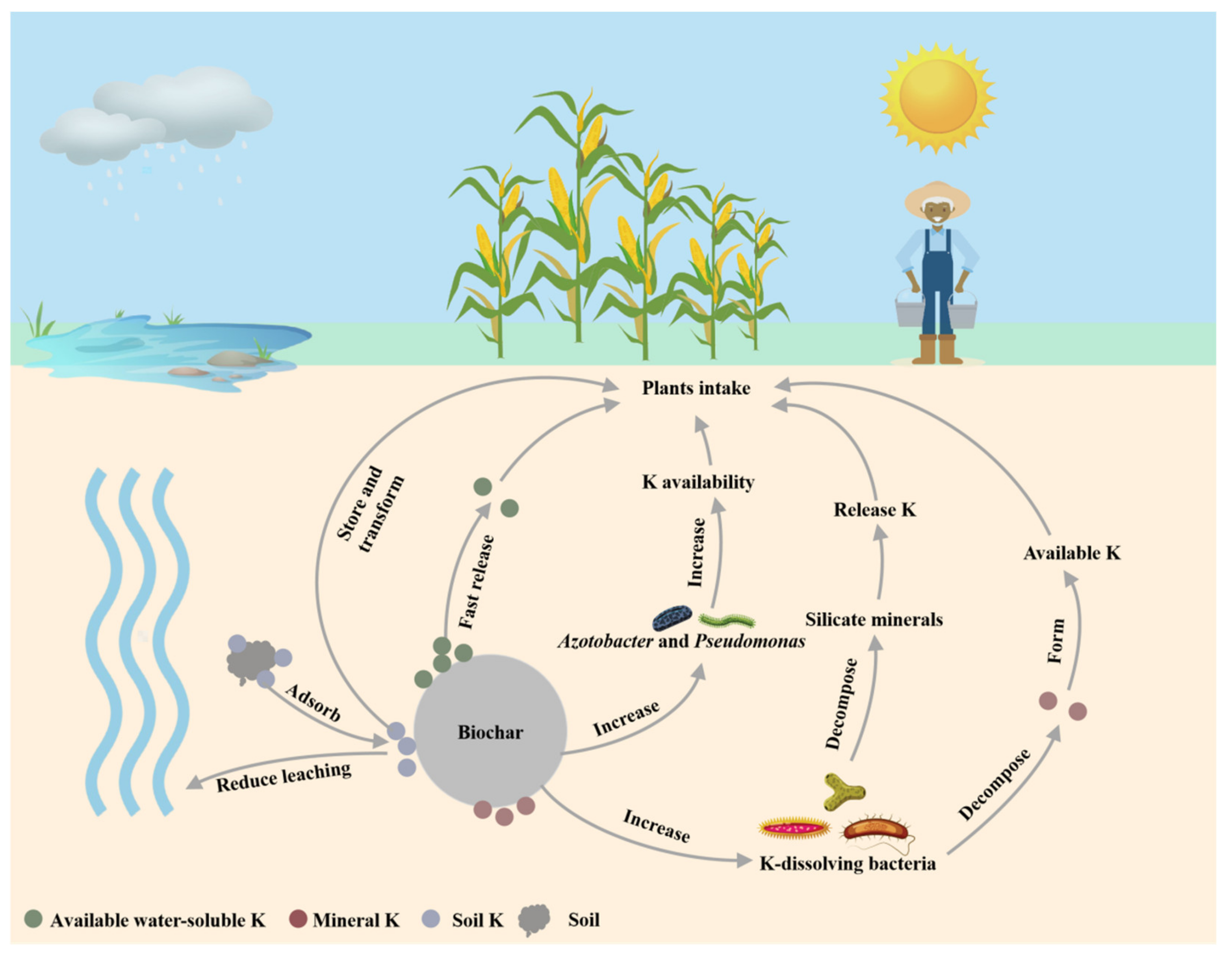
4.4. K
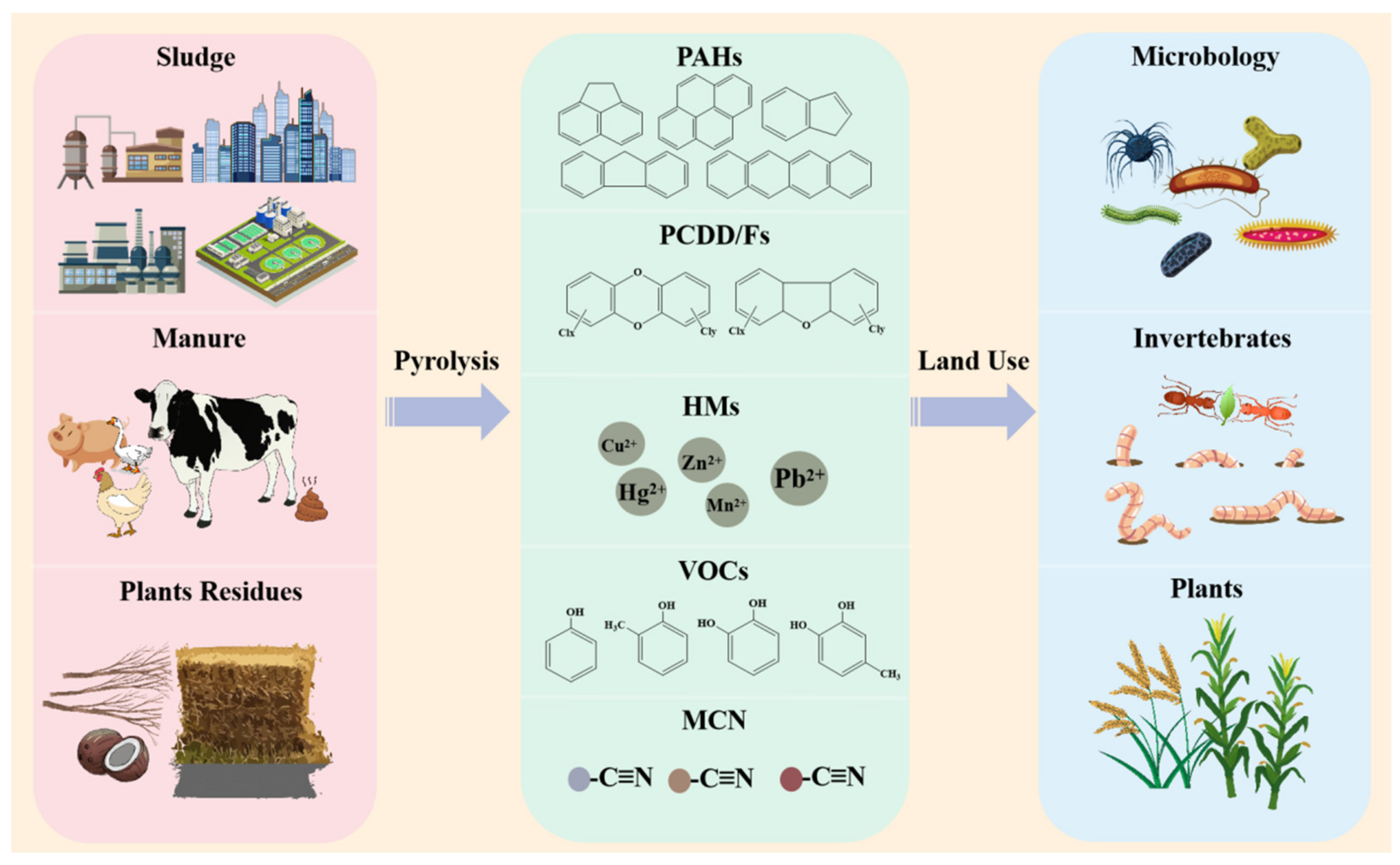
5. The Adverse Effects of Biochar on Soil Organisms
5.1. Toxic Substances of Biochar
5.2. Negative Effects of Biochar on Microbiology
5.3. Negative Effects of Biochar on Invertebrates
5.4. Negative Effects of Biochar on Plants
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Status of the World’s Soil Resources—Main Report; Bulletin of National Institute for Agro-Environmental Sciences; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Borrelli, P.; Robinson, D.A.; Panagos, P.; Lugato, E.; Yang, J.E.; Alewell, C.; Wuepper, D.; Montanarella, L.; Ballabio, C. Land use and climate change impacts on global soil erosion by water (2015–2070). Proc. Natl. Acad. Sci. USA 2020, 117, 21994–22001. [Google Scholar] [CrossRef] [PubMed]
- Miguez-Macho, G.; Fan, Y. Spatiotemporal origin of soil water taken up by vegetation. Nature 2021, 598, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). Recarbonization of Global Soils—A Dynamic Response to Offset Global Emissions; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Ferreira, C.S.S.; Seifollahi-Aghmiuni, S.; Destouni, G.; Ghajarnia, N.; Kalantari, Z. Soil degradation in the European Mediterranean region: Processes, status and consequences. Sci. Total Environ. 2022, 805, 150106. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Wang, C.; Sardans, J.; Vancov, T.; Fang, Y.; Wu, L.; Huang, X.; Gargallo-Garriga, A.; Peñuelas, J.; Wang, W. Effect of soil degradation on the carbon concentration and retention of nitrogen and phosphorus across Chinese rice paddy fields. Catena 2022, 209, 105810. [Google Scholar] [CrossRef]
- Maximillian, J.; Brusseau, M.L.; Glenn, E.P.; Matthias, A.D. Pollution and Environmental Perturbations in the Global System. In Pollution and Environmental Perturbations in the Global System, 3rd ed.; Brusseau, M.L., Pepper, I.L., Gerba, C.P., Eds.; Academic Press: London, UK, 2019; pp. 457–476. [Google Scholar]
- Bashagaluke, J.B.; Logah, V.; Opoku, A.; Sarkodie-Addo, J.; Quansah, C.; Marelli, B. Soil nutrient loss through erosion: Impact of different cropping systems and soil amendments in Ghana. PLoS ONE 2018, 13, e0208250. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Schleuss, P.M.; Kuzyakov, Y. Carbon and Nitrogen Losses from Soil Depend on Degradation of Tibetan Kobresia Pastures. Land Degrad. Dev. 2017, 28, 1253–1262. [Google Scholar] [CrossRef]
- Jin, Q.; Peñuelas, J.; Sardans, J.; Romero, E.; Chen, S.; Liu, X.; Lin, S.; Wang, W. Changes in soil carbon, nitrogen, and phosphorus contents, storages, and stoichiometry during land degradation in jasmine croplands in subtropical China. Exp. Agric. 2021, 57, 113–125. [Google Scholar] [CrossRef]
- Tamene, L.; Sileshi, G.W.; Ndengu, G.; Mponela, P.; Kihara, J.; Sila, A.; Tondoh, J. Soil structural degradation and nutrient limitations across land use categories and climatic zones in Southern Africa. Land Degrad. Dev. 2019, 30, 1288–1299. [Google Scholar] [CrossRef]
- Chen, X.H.; Yu, W.H.; Cai, Y.Y.; Zhang, S.W.; Muneer, M.A.; Zhu, Q.C.; Xu, D.H.; Ma, C.C.; Yan, X.J.; Li, Y.; et al. How to identify and adopt cleaner strategies to improve the continuous acidification in orchard soils? J. Clean. Prod. 2022, 330, 129826. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Cang, L.; Wang, Y.; Zhou, D. Effects of soil properties, nitrogen application, plant phenology, and their interactions on plant uptake of cadmium in wheat. J. Hazard. Mater. 2020, 384, 121452. [Google Scholar] [CrossRef]
- Lu, H.; Wu, Y.; Liang, P.; Song, Q.; Zhang, H.; Wu, J.; Wu, W.; Liu, X.; Dong, C. Alkaline amendments improve the health of soils degraded by metal contamination and acidification: Crop performance and soil bacterial community responses. Chemosphere 2020, 257, 127309. [Google Scholar] [CrossRef] [PubMed]
- Manyà, J.J. Pyrolysis for Biochar Purposes: A Review to Establish Current Knowledge Gaps and Research Needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; DeLuca, T.H.; Cleveland, C.C. Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: A meta-analysis. Sci. Total Environ. 2019, 654, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chen, B. A Direct Observation of the Fine Aromatic Clusters and Molecular Structures of Biochars. Environ. Sci. Technol. 2017, 51, 5473–5482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Feng, Y. The effects of biochar addition on soil physicochemical properties: A review. Catena 2021, 202, 105284. [Google Scholar] [CrossRef]
- Azzi, E.S.; Karltun, E.; Sundberg, C. Prospective Life Cycle Assessment of Large-Scale Biochar Production and Use for Negative Emissions in Stockholm. Environ. Sci. Technol. 2019, 53, 8466–8476. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into Multiple and Multilevel Structures of Biochars and Their Potential Environmental Applications: A Critical Review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef]
- Tang, H.; Chen, M.; Wu, P.; Faheem, M.; Feng, Q.W.; Lee, X.Q.; Wang, S.S.; Wang, B. Engineered biochar effects on soil physicochemical properties and biota communities: A critical review. Chemosphere 2023, 311, 137025. [Google Scholar] [CrossRef]
- Ji, M.Y.; Wang, X.X.; Usman, M.; Liu, F.H.; Dan, Y.T.; Zhou, L.; Campanaro, S.; Luo, G.; Sang, W.J. Effects of different feedstocks-based biochar on soil remediation: A review. Environ. Pollut. 2022, 294, 118655. [Google Scholar] [CrossRef]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Riaz, M.; Roohi, M.; Arif, M.S.; Hussain, Q.; Yasmeen, T.; Shahzad, T.; Shahzad, S.M.; Muhammad, H.F.; Arif, M.; Khalid, M. Corncob-derived biochar decelerates mineralization of native and added organic matter (AOM) in organic matter depleted alkaline soil. Geoderma 2017, 294, 19–28. [Google Scholar] [CrossRef]
- Singh, B.P.; Cowie, A.L.; Smernik, R.J. Biochar carbon stability in a clayey soil as a function of feedstock and pyrolysis temperature. Environ. Sci. Technol. 2012, 46, 11770–11778. [Google Scholar] [CrossRef] [PubMed]
- Nelissen, V.; Rütting, T.; Huygens, D.; Staelens, J.; Ruysschaert, G.; Boeckx, P. Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol. Biochem. 2012, 55, 20–27. [Google Scholar] [CrossRef]
- Karim, A.A.; Kumar, M.; Singh, E.; Kumar, A.; Kumar, S.; Ray, A.; Dhal, N.K. Enrichment of primary macronutrients in biochar for sustainable agriculture: A review. Crit. Rev. Environ. Sci. Technol. 2022, 59, 1449–1490. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Tian, Z.X.; Gong, H.B.; Huang, Q.Y. Migration and transformation mechanisms of nutrient elements (N, P, K) within biochar in straw-biochar-soil-plant systems: A review. ACS Sustain. Chem. Eng. 2019, 7, 22–32. [Google Scholar] [CrossRef]
- Kaal, J.; Schneider, M.P.W.; Schmidt, M.W.I. Rapid molecular screening of black carbon (biochar) thermosequences obtained from chestnut wood and rice straw: A pyrolysis-GC/MS study. Biomass Bioenergy 2012, 45, 115–129. [Google Scholar] [CrossRef]
- Guo, J.; Chen, B. Insights on the Molecular Mechanism for the Recalcitrance of Biochars: Interactive Effects of Carbon and Silicon Components. Environ. Sci. Technol. 2014, 48, 9103–9112. [Google Scholar] [CrossRef]
- Ilyas, M.; Arif, M.; Akhtar, K.; Riaz, M.; Wang, H.Y. Diverse feedstock’s biochars as supplementary K fertilizer improves maize productivity, soil organic C and KUE under semiarid climate. Soil Tillage Res. 2021, 211, 105015. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; Chiu, P.C.; Imhoff, P.T.; Guo, M. Phosphorus release behaviors of poultry litter biochar as a soil amendment. Sci. Total Environ. 2015, 512–513, 454–463. [Google Scholar] [CrossRef]
- Xing, J.; Xu, G.; Li, G. Comparison of pyrolysis process, various fractions and potential soil applications between sewage sludge-based biochars and lignocellulose-based biochars. Ecotoxicol. Environ. Saf. 2021, 208, 111756. [Google Scholar] [CrossRef]
- Kim, H.-B.; Kim, S.-H.; Jeon, E.-K.; Kim, D.-H.; Tsang, D.C.W.; Alessi, D.S.; Kwon, E.E.; Baek, K. Effect of dissolved organic carbon from sludge, Rice straw and spent coffee ground biochar on the mobility of arsenic in soil. Sci. Total Environ. 2018, 636, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, Y.; Lei, H.; Zhang, X.; Wang, L.; Bu, Q.; Wei, Y. Production of hydrocarbons from biomass-derived biochar assisted microwave catalytic pyrolysis. Sustain. Energy Fuels 2018, 2, 1781–1790. [Google Scholar] [CrossRef]
- Jin, Y.; Liang, X.Q.; He, M.M.; Liu, Y.; Tian, G.M.; Shi, J.Y. Manure biochar influence upon soil properties, phosphorus distri-bution and phosphatase activities: A microcosm incubation study. Chemosphere 2016, 142, 128–135. [Google Scholar] [CrossRef]
- Chan, K.Y.; Xu, Z.H. Biochar: Nutrient Properties and Their Enhancement. In Biochar for Environmental Management, 1st ed.; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2009; p. 18. [Google Scholar]
- Shi, R.-Y.; Li, J.-Y.; NI, N.; Xu, R.-K. Understanding the biochar’s role in ameliorating soil acidity. J. Integr. Agric. 2019, 18, 1508–1517. [Google Scholar] [CrossRef]
- Banik, C.; Lawrinenko, M.; Bakshi, S.; Laird, D.A. Impact of pyrolysis temperature and feedstock on surface charge and functional group chemistry of biochars. J. Environ. Qual. 2018, 47, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, Y.; Ma, Q.; Zhou, H.; Luo, X.; Liu, X.; Wang, S. Evolution of the chemical composition, functional group, pore structure and crystallographic structure of biochar from palm kernel shell pyrolysis under different temperatures. J. Anal. Appl. Pyrolysis 2017, 127, 350–359. [Google Scholar] [CrossRef]
- Leng, L.; Xu, S.; Liu, R.; Yu, T.; Zhuo, X.; Leng, S.; Xiong, Q.; Huang, H. Nitrogen containing functional groups of biochar: An overview. Bioresour. Technol. 2020, 298, 122286. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Singh, B.; Dolk, M.M.; Shen, Q.H.; Arbestain, M.C. Biochar pH, Electrical Conductivity and Liming Potential. In A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CSIRO: Victoria, UK, 2017; pp. 23–38. [Google Scholar]
- Li, H.; Meng, J.; Liu, Z.Q.; Lan, Y.; Yang, X.; Huang, Y.W.; He, T.Y.; Chen, W.F. Effects of biochar on N2O emission in denitrification pathway from paddy soil: A drying incubation study. Sci. Total Environ. 2021, 787, 147591. [Google Scholar] [CrossRef]
- Tsadik, Y.K.G.; Hailu, A.M.; Asfaw, S.L.; Mekonnen, Y.S. The effect of brewery sludge biochar on immobilization of bio-available cadmium and growth of Brassica carinata. Heliyon 2020, 6, e05573. [Google Scholar] [CrossRef]
- Ghodszad, L.; Reyhanitabar, A.; Oustan, S.; Alidokht, L. Phosphorus sorption and desorption characteristics of soils as affected by biochar. Soil Tillage Res. 2022, 216, 105251. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H.Y. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A.S. Impact of Biochar Amendment on Fertility of a Southeastern Coastal Plain Soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Rouphael, Y.; Rea, E.; Cardarelli, M.; Bitterlich, M.; Schwarz, D.; Colla, G. Can adverse effects of acidity and aluminum toxicity be alleviated by appropriate rootstock selection in cucumber? Front. Plant Sci. 2016, 7, 1283. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential role of biochars in decreasing soil acidification—A critical review. Sci. Total Environ. 2017, 581–582, 601–611. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Brennan, A.; Jiménez, E.M.; Puschenreiter, M.; Alburquerque, J.A.; Switzer, C. Effects of biochar amendment on root traits and contaminant availability of maize plants in a copper and arsenic impacted soil. Plant Soil 2014, 379, 351–360. [Google Scholar] [CrossRef]
- Brewer, C.E.; Hu, Y.-Y.; Schmidt-Rohr, K.; Loynachan, T.E.; Laird, D.A.; Brown, R.C. Extent of Pyrolysis Impacts on Fast Pyrolysis Biochar Properties. J. Environ. Qual. 2012, 41, 1115–1122. [Google Scholar] [CrossRef]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef]
- Xu, R.-K.; Zhao, A.-Z.; Yuan, J.-H.; Jiang, J. pH buffering capacity of acid soils from tropical and subtropical regions of China as influenced by incorporation of crop straw biochars. J. Soils Sediments 2012, 12, 494–502. [Google Scholar] [CrossRef]
- Palladino, M.; Romano, N.; Pasolli, E.; Nasta, P. Developing pedotransfer functions for predicting soil bulk density in Campania. Geoderma 2022, 412, 115726. [Google Scholar] [CrossRef]
- Rombolà, A.G.; Fabbri, D.; Baronti, S.; Vaccari, F.P.; Genesio, L.; Miglietta, F. Changes in the pattern of polycyclic aromatic hydrocarbons in soil treated with biochar from a multiyear field experiment. Chemosphere 2019, 219, 662–670. [Google Scholar] [CrossRef]
- Khan, I.; Chen, T.; Farooq, M.; Luan, C.; Wu, Q.; Wanning, D.; Xu, S.; Li-Xue, W. The residual impact of straw mulch and biochar amendments on soil physiochemical properties and yield of maize under rainfed system. Agron. J. 2021, 113, 1102–1120. [Google Scholar] [CrossRef]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Oladele, S.; Adeyemo, A.; Awodun, M.; Ajayi, A.; Fasina, A. Effects of biochar and nitrogen fertilizer on soil physicochemical properties, nitrogen use efficiency and upland rice (Oryza sativa) yield grown on an Alfisol in Southwestern Nigeria. Int. J. Recycl. Org. Waste Agric. 2019, 8, 295–308. [Google Scholar] [CrossRef]
- Hussaina, R.K.; Ravia, K.; Ankit, G. Influence of biochar on the soil water retention characteristics (SWRC): Potential application in geotechnical engineering structures. Soil Tillage Res. 2020, 204, 104713. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Sun, J.; Shao, H. Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci. Total Environ. 2016, 568, 910–915. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black Carbon Increases Cation Exchange Capacity in Soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Nelson, P.F. Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere 2010, 78, 1167–1171. [Google Scholar] [CrossRef]
- Chintala, R.; Schumacher, T.E.; Kumar, S.; Malo, D.D.; Rice, J.A.; Bleakley, B.; Chilom, G.; Clay, D.E.; Julson, J.L.; Papiernik, S.K.; et al. Molecular characterization of biochars and their influence on microbiological properties of soil. J. Hazard. Mater. 2014, 279, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Hu, B.; Agathokleous, E.; Wang, L.; Koike, T.; Ma, M.; Rennenberg, H. Biochar application improves karstic lime soil physicochemical properties and enzymes activity and enhances sweet tea seedlings physiological performance. Sci. Total Environ. 2022, 830, 154815. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Patel, A.; Patra, D.D. Biochar ameliorates crop productivity, soil fertility, essential oil yield and aroma profiling in basil (Ocimum basilicum L.). Ecol. Eng. 2016, 90, 361–366. [Google Scholar] [CrossRef]
- Jia, W.; Wang, B.; Wang, C.; Sun, H. Tourmaline and biochar for the remediation of acid soil polluted with heavy metals. J. Environ. Chem. Eng. 2017, 5, 2107–2114. [Google Scholar] [CrossRef]
- Pandey, B.; Suthar, S.; Chand, N. Effect of biochar amendment on metal mobility, phytotoxicity, soil enzymes, and met-al-uptakes by wheat (Triticum aestivum) in contaminated soils. Chemosphere 2022, 307, 135889. [Google Scholar] [CrossRef]
- Song, X.; Li, H.; Song, J.; Chen, W.; Shi, L. Biochar/vermicompost promotes Hybrid Pennisetum plant growth and soil enzyme activity in saline soils. Plant Physiol. Biochem. 2022, 183, 96–110. [Google Scholar] [CrossRef]
- Liao, X.; Kang, H.; Haidar, G.; Wang, W.; Malghani, S. The impact of biochar on the activities of soil nutrients acquisition enzymes is potentially controlled by the pyrolysis temperature: A meta-analysis. Geoderma 2022, 411, 115692. [Google Scholar] [CrossRef]
- Ibrahim, M.; Li, G.; Tang, Y.-T. Biochar effects acidic soil remediation and Brassica oleracea L. toxicity—A case study in subtropical area of China. Environ. Technol. Innov. 2021, 23, 101588. [Google Scholar] [CrossRef]
- Wang, D.Y.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M. Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 2017, 303, 110–117. [Google Scholar] [CrossRef]
- Akolgo, G.A.; Kemausuor, F.; Awafo, E.A.; Amankwah, E.; Atta-Darkwa, T.; Essandoh, E.O.; Bart-Plange, A.; Branco de Freitas Maia, C.M. Biochar as a Soil Amendment Tool: Effects on Soil Properties and Yield of Maize and Cabbage in Brong-Ahafo Region, Ghana. Open J. Soil Sci. 2020, 10, 91–108. [Google Scholar] [CrossRef]
- El-Naggar, A.; El-Naggar, A.H.; Shaheen, S.M.; Sarkar, B.; Chang, S.X.; Tsang, D.C.W.; Rinklebe, J.; Ok, Y.S. Biochar composition-dependent impacts on soil nutrient release, carbon mineralization, and potential environmental risk: A review. J. Environ. Manag. 2019, 241, 458–467. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Yang, Y.; Xia, X.; Li, F.; Yang, Z.; Xing, B. Biochar’s stability and effect on the content, composition and turnover of soil organic carbon. Geoderma 2020, 364, 114184. [Google Scholar] [CrossRef]
- Chagas, J.K.M.; de Figueiredo, C.C.; Ramos, M.L.G. Biochar increases soil carbon pools: Evidence from a global meta-analysis. J. Environ. Manag. 2022, 305, 114403. [Google Scholar] [CrossRef]
- Yin, Y.-F.; He, X.-H.; Gao, R.; Ma, H.-L.; Yang, Y.-S. Effects of Rice Straw and Its Biochar Addition on Soil Labile Carbon and Soil Organic Carbon. J. Integr. Agric. 2014, 13, 491–498. [Google Scholar] [CrossRef]
- Yan, S.; Liu, G. Effect of increasing soil carbon content on tobacco aroma and soil microorganisms. Phytochem. Lett. 2020, 36, 42–48. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Zong, Y.; Hu, Z.; Wu, S.; Zhou, J.; Jin, Y.; Zou, J. Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: A meta-analysis. GCB Bioenergy 2016, 8, 392–406. [Google Scholar] [CrossRef]
- Steiner, C.; Glaser, B.; Geraldes Teixeira, W.; Lehmann, J.; Blum, W.E.H.; Zech, W. Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal. J. Plant Nutr. Soil Sci. 2008, 171, 893–899. [Google Scholar] [CrossRef]
- Buurman, P.; Jongmans, A.G. Podzolisation and soil organic matter dynamics. Geoderma 2005, 125, 71–83. [Google Scholar] [CrossRef]
- Cui, J.L.; Glatzel, S.; Bruckman, V.J.; Wang, B.Z.; Lai, D.Y.F. Long-term effects of biochar application on greenhouse gas production and microbial community in temperate forest soils under increasing temperature. Sci. Total Environ. 2021, 767, 145021. [Google Scholar] [CrossRef]
- Yang, X.Y.; Chang, K.-H.; Kim, Y.J.; Zhang, J.; Yoo, G. Effects of different biochar amendments on carbon loss and leachate characterization from an agricultural soil. Chemosphere 2019, 226, 625–635. [Google Scholar] [CrossRef]
- Hernandez-Soriano, M.C.; Kerré, B.; Kopittke, P.M.; Horemans, B.; Smolders, E. Biochar affects carbon composition and stability in soil: A combined spectroscopy-microscopy study. Sci. Rep. 2016, 6, 25127. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Li, P.; Xian, Q.; Tang, X. Biochar’s impact on dissolved organic matter (DOM) export from a cropland soil during natural rainfalls. Sci. Total Environ. 2019, 650, 1988–1995. [Google Scholar] [CrossRef]
- Lang, T.; Jensen, A.D.; Jensen, P.A. Retention of Organic Elements during Solid Fuel Pyrolysis with Emphasis on the Peculiar Behavior of Nitrogen. Energy Fuels 2005, 19, 1631–1643. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Tian, S.; Tan, Z.; Kasiulienė, A.; Ai, P. Transformation mechanism of nutrient elements in the process of biochar preparation for returning biochar to soil. Chin. J. Chem. Eng. 2017, 25, 477–486. [Google Scholar] [CrossRef]
- Yuan, S.; Tan, Z.; Huang, Q. Migration and transformation mechanism of nitrogen in the biomass–biochar–plant transport process. Renew. Sustain. Energy Rev. 2018, 85, 1–13. [Google Scholar] [CrossRef]
- Azeem, M.; Hayat, R.; Hussain, Q.; Ahmed, M.; Pan, G.; Ibrahim, T.M. Biochar improves soil quality and N2-fixation and reduces net ecosystem CO2 exchange in a dryland legume-cereal cropping system. Soil Tillage Res. 2019, 186, 172–182. [Google Scholar] [CrossRef]
- Thies, J.E.; Rillig, M.C.; Graber, E.R. Biochar Effects on the Abundance Activity and Diversity of the Soil Biota. In Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Lehmann, J., Stephen, J., Eds.; Routledge: London, UK, 2015; pp. 327–389. [Google Scholar]
- Quilliam, R.S.; DeLuca, T.H.; Jones, D.L. Biochar application reduces nodulation but increases nitrogenase activity in clover. Plant Soil 2013, 366, 83–92. [Google Scholar] [CrossRef]
- Xu, C.Y.; Bai, S.H.; Hao, Y.; Rachaputi, R.N.; Wang, H.; Xu, Z.; Wallace, H. Effect of biochar amendment on yield and photo-synthesis of peanut on two types of soils. Environ. Sci. Pollut. Res. 2015, 22, 6112–6125. [Google Scholar] [CrossRef]
- Wu, X.; Sun, Y.; Deng, L.; Meng, Q.; Jiang, X.; Bello, A.; Sheng, S.; Han, Y.; Zhu, H.; Xu, X. Insight to key diazotrophic community during composting of dairy manure with biochar and its role in nitrogen transformation. Waste Manage. 2020, 105, 190–197. [Google Scholar] [CrossRef]
- Zhao, J.W.; Tao, Q.; Li, B.; Luo, J.P.; Zhang, H.Y.; Lu, C.L.; Li, Q.Q.; Xu, Q.; Huang, R.; Li, H.X.; et al. Low-pyrolysis-temperature biochar promoted free-living N2-fixation in calcareous purple soil by affecting diazotrophic composition. Geoderma 2021, 388, 114969. [Google Scholar] [CrossRef]
- Joseph, S.; Pow, D.; Dawson, K.; Mitchell, D.R.G.; Rawal, A.; Hook, J.; Taherymoosavi, S.; Van Zwieten, L.; Rust, J.; Donne, S.; et al. Feeding Biochar to Cows: An Innovative Solution for Improving Soil Fertility and Farm Productivity. Pedosphere 2015, 25, 666–679. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Muller, C.W. A Review of Biochar and Soil Nitrogen Dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, B.; Wang, Q.; Tao, W.; Lin, H. Nano-biochar reduced soil erosion and nitrate loss in sloping fields on the Loess Plateau of China. Catena 2020, 187, 104346. [Google Scholar] [CrossRef]
- Cao, H.; Ning, L.; Xun, M.; Feng, F.; Li, P.; Yue, S.; Song, J.; Zhang, W.; Yang, H. Biochar can increase nitrogen use efficiency of Malus hupehensis by modulating nitrate reduction of soil and root. Appl. Soil Ecol. 2019, 135, 25–32. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Montes-Morán, M.A.; Suárez, D.; Menéndez, J.A.; Fuente, E. On the nature of basic sites on carbon surfaces: An overview. Carbon 2004, 42, 1219–1225. [Google Scholar] [CrossRef]
- Ventura, M.; Sorrenti, G.; Panzacchi, P.; George, E.; Tonon, G. Biochar Reduces Short-Term Nitrate Leaching from A Horizon in an Apple Orchard. J. Environ. Qual. 2013, 42, 76–82. [Google Scholar] [CrossRef]
- Kameyama, K.; Miyamoto, T.; Shiono, T.; Shinogi, Y. Influence of Sugarcane Bagasse-derived Biochar Application on Nitrate Leaching in Calcaric Dark Red Soil. J. Environ. Qual. 2012, 41, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Amonette, J.E.; Joseph, S. Characteristics of Biochar: Microchemical Properties. In Biochar for Environmental Management: Science and Technology, 1st ed.; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 33–52. [Google Scholar]
- Kammann, C.I.; Schmidt, H.-P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.-W.; Conte, P.; Joseph, S. Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 11080. [Google Scholar] [CrossRef] [PubMed]
- Lawrinenko, M. Anion Exchange Capacity of Biochar. Master’s Thesis, Iowa State University, Ames, IA, USA, 2014. [Google Scholar]
- Nguyen, T.T.N.; Xu, C.-Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Santachiara, G.; Salvagiotti, F.; Rotundo, J.L. Nutritional and environmental effects on biological nitrogen fixation in soybean: A meta-analysis. Field Crops Res. 2019, 240, 106–115. [Google Scholar] [CrossRef]
- Reed, S.C.; Cleveland, C.C.; Townsend, A.R. Functional Ecology of Free-Living Nitrogen Fixation: A Contemporary Perspective. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 489–512. [Google Scholar] [CrossRef]
- Ahmad, Z.; Mosa, A.; Zhang, L.; Gao, B. Biochar modulates mineral nitrogen dynamics in soil and terrestrial ecosystems: A critical review. Chemosphere 2021, 278, 130378. [Google Scholar] [CrossRef]
- Ajeng, A.A.; Abdullah, R.; Ling, T.C.; Ismail, S.; Lau, B.F.; Ong, H.C.; Chew, K.W.; Show, P.L.; Chang, J.-S. Bioformulation of biochar as a potential inoculant carrier for sustainable agriculture. Environ. Technol. Innov. 2020, 20, 101168. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Horel, A.; Potyó, I.; Szili-Kovács, T.; Molnár, S. Potential nitrogen fixation changes under different land uses as influenced by seasons and biochar amendments. Arab. J. Geosci. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Rondon, M.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) in-creases with biochar additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Q.; Xiao, K.; Wang, Z.; Wang, K. Divergent responses of biological nitrogen fixation in soil, litter and moss to temperature and moisture in a karst forest, southwest China. Soil Biol. Biochem. 2018, 118, 1–7. [Google Scholar] [CrossRef]
- Yuan, D.; Wang, G.Q.; Hu, C.S.; Zhou, S.G.; Clough, T.J.; Wrage-Mönnig, N.; Luo, J.F.; Qin, S.P. Electron shuttle potential of biochar promotes dissimilatory nitrate reduction to ammonium in paddy soil. Soil Biol. Biochem. 2022, 172, 108760. [Google Scholar] [CrossRef]
- Zhao, Y.; Bu, C.; Yang, H.; Qiao, Z.; Ding, S.; Ni, S.-Q. Survey of dissimilatory nitrate reduction to ammonium microbial community at national wetland of Shanghai, China. Chemosphere 2020, 250, 126195. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, K.; Li, Y.; Sanganyado, E. Electrochemical behavior of biochar and its effects on microbial nitrate reduction: Role of extracellular polymeric substances in extracellular electron transfer. Chem. Eng. J. 2020, 395, 125077. [Google Scholar] [CrossRef]
- Wang, T.; Camps-Arbestain, M.; Hedley, M.; Bishop, P. Predicting phosphorus bioavailability from high-ash biochars. Plant Soil 2012, 357, 173–187. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E.; Yang, H.; Zhang, D. Roles of biochar in improving phosphorus availability in soils: A phosphate adsorbent and a source of available phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Li, F.; Liang, X.; Niyungeko, C.; Sun, T.; Liu, F.; Arai, Y. Effects of biochar amendments on soil phosphorus transformation in agricultural soils. Adv. Agron. 2019, 158, 131–172. [Google Scholar] [CrossRef]
- Qian, T.; Zhang, X.; Hu, J.; Jiang, H. Effects of environmental conditions on the release of phosphorus from biochar. Chemosphere 2013, 93, 2069–2075. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, X.; Zhao, L.; Xu, X.; Harris, W. Phosphorus Release from Dairy Manure, the Manure-Derived Biochar, and Their Amended Soil: Effects of Phosphorus Nature and Soil Property. J. Environ. Qual. 2014, 43, 1504–1509. [Google Scholar] [CrossRef]
- He, H.; Qian, T.-T.; Liu, W.-J.; Jiang, H.; Yu, H.-Q. Biological and chemical phosphorus solubilization from pyrolytical biochar in aqueous solution. Chemosphere 2014, 113, 175–181. [Google Scholar] [CrossRef]
- Qian, T.; Yang, Q.; Jun, D.C.F.; Dong, F.; Zhou, Y. Transformation of phosphorus in sewage sludge biochar mediated by a phosphate-solubilizing microorganism. Chem. Eng. J. 2018, 359, 1573–1580. [Google Scholar] [CrossRef]
- Xu, G.; Sun, J.; Shao, H.; Chang, S.X. Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol. Eng. 2014, 62, 54–60. [Google Scholar] [CrossRef]
- Pastore, G.; Kernchen, S.; Spohn, M. Microbial solubilization of silicon and phosphorus from bedrock in relation to abundance of phosphorus-solubilizing bacteria in temperate forest soils. Soil Biol. Biochem. 2020, 151, 108050. [Google Scholar] [CrossRef]
- Pradhan, A.; Pahari, A.; Mohapatra, S.; Mishra, B.B. Phosphate-Solubilizing Microorganisms in Sustainable Agriculture: Genetic Mechanism and Application. In Advances in Soil Microbiology: Recent Trends and Future Prospects; Adhya, T., Mishra, B., Annapurna, K., Verma, D., Kumar, U., Eds.; Springer Singapore: Singapore, 2017; pp. 81–97. [Google Scholar]
- Jaafar, N.M.; Clode, P.L.; Abbott, L.K. Soil Microbial Responses to Biochars Varying in Particle Size, Surface and Pore Properties. Pedosphere 2015, 25, 770–780. [Google Scholar] [CrossRef]
- Zheng, B.-X.; Ding, K.; Yang, X.-R.; Wadaan, M.A.M.; Hozzein, W.N.; Peñuelas, J.; Zhu, Y.-G. Straw biochar increases the abundance of inorganic phosphate solubilizing bacterial community for better rape (Brassica napus) growth and phosphate uptake. Sci. Total Environ. 2019, 647, 1113–1120. [Google Scholar] [CrossRef]
- Zwetsloot, M.J.; Lehmann, J.; Bauerle, T.; Vanek, S.; Hestrin, R.; Nigussie, A. Phosphorus availability from bone char in a P-fixing soil influenced by root-mycorrhizae-biochar interactions. Plant Soil 2016, 408, 95–105. [Google Scholar] [CrossRef]
- Chen, Q.; Qin, J.; Cheng, Z.; Huang, L.; Sun, P.; Chen, L.; Shen, G. Synthesis of a stable magnesium-impregnated biochar and its reduction of phosphorus leaching from soil. Chemosphere 2018, 199, 402–408. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Wang, Y.; Chen, Y.; Lei, T. Sewage sludge biochar: Nutrient composition and its effect on the leaching of soil nutrients. Geoderma 2016, 267, 17–23. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, Y.; Fan, B.; Zhang, S.; Bolan, N.S.; Chen, Q.; Tsang, D.C. Fe/Al (hydr)oxides engineered biochar for reducing phosphorus leaching from a fertile calcareous soil. J. Clean. Prod. 2021, 279, 123877. [Google Scholar] [CrossRef]
- Sachdeva, V.; Hussain, N.; Husk, B.R.; Whalen, J.K. Biochar-induced soil stability influences phosphorus retention in a temperate agricultural soil. Geoderma 2019, 351, 71–75. [Google Scholar] [CrossRef]
- Ghodszad, L.; Reyhanitabar, A.; Maghsoodi, M.R.; Lajayer, B.A.; Chang, S.X. Biochar affects the fate of phosphorus in soil and water: A critical review. Chemosphere 2021, 283, 131176. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; Wang, Y.; An, W.; Jin, J.; Sun, K.; Wang, X. Effects of biochar addition on the abundance, speciation, availability, and leaching loss of soil phosphorus. Sci. Total Environ. 2021, 758, 143657. [Google Scholar] [CrossRef]
- Rezaeian, M.; Petroudi, E.R.; Mohseni, M.; Haddadi, M.H. Effects of row spacing, nitrogen and potassium fertilizer on yield of silage corn after wheat harvesting. Int. J. Plant Anim. Eviron. Sci. 2014, 4, 358–361. [Google Scholar]
- Li, Z.; Liu, Z.; Zhang, M.; Li, C.; Li, Y.C.; Wan, Y.; Martin, C.G. Long-term effects of controlled-release potassium chloride on soil available potassium, nutrient absorption and yield of maize plants. Soil Tillage Res. 2020, 196, 104438. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Riaz, M.; Xia, H.; Li, Y.X.; Wang, X.L.; Jiang, C.C. Four-year biochar study: Positive response of acidic soil microenvironment and citrus growth to biochar under potassium deficiency conditions. Sci. Total Environ. 2022, 813, 152515. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Liu, B.; Li, Y.; El-Desouki, Z.; Jiang, C. Over two years study: Peanut biochar promoted potassium availability by mediating the relationship between bacterial community and soil properties. Appl. Soil Ecol. 2022, 176, 104485. [Google Scholar] [CrossRef]
- Salo, K.; Mojtahedi, W. Fate of alkali and trace metals in biomass gasification. Biomass Bioenergy 1998, 15, 263–267. [Google Scholar] [CrossRef]
- Orama, N.J.; van de Voorde, T.F.J.; Ouwehand, G.J.; Bezemer, T.M.; Mommer, L.; Jeffery, S.; Groenigena, J.W.V. Soil amendment with biochar increases the competitive ability of legumes via increased potassium availability. Agric. Ecosyst. Environ. 2014, 191, 92–98. [Google Scholar] [CrossRef]
- Amin, A.E.-E.A.Z. Impact of Corn Cob Biochar on Potassium Status and Wheat Growth in a Calcareous Sandy Soil. Commun. Soil Sci. Plant Anal. 2016, 47, 2026–2033. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Biben, B. Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Speir, R.A.; Harris, K.; Das, K.C.; Lee, R.D.; Morris, L.A.; Fisher, D.S. Effect of Peanut Hull and Pine Chip Biochar on Soil Nutrients, Corn Nutrient Status, and Yield. Agron. J. 2010, 102, 623–633. [Google Scholar] [CrossRef]
- Limwikran, T.; Kheoruenromne, I.; Suddhiprakarn, A.; Prakongkep, N.; Gilkes, R.J. Dissolution of K, Ca, and P from biochar grains in tropical soils. Geoderma 2018, 312, 139–150. [Google Scholar] [CrossRef]
- Chi, J.L.; Ge, Y.H. Study on potassium releasing activity of silicate bacteria. J. Microbiol. 1999, 2, 43–51. [Google Scholar]
- Zhang, W.L.; Li, G.H.; Gao, W.D. Effect of biomass charcoal on soil character and crop yield. Chin. Sci. Bull. 2009, 25, 153–157. [Google Scholar]
- Jílková, V.; Angst, G. Biochar and compost amendments to a coarse-textured temperate agricultural soil lead to nutrient leaching. Appl. Soil Ecol. 2022, 173, 104393. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Marsden, K.A.; Gertler, C.; Rousk, J.; DeLuca, T.H.; Jones, D.L. Nutrient dynamics, microbial growth and weed emergence in biochar amended soil are influenced by time since application and reapplication rate. Agric. Ecosyst. Environ. 2012, 158, 192–199. [Google Scholar] [CrossRef]
- Hilber, I.; Bastos, A.C.; Loureiro, S.; Soja, G.; Marsz, A.; Cornelissen, G.; Bucheli, T.D. The different faces of biochar: Contamination risk versus remediation tool. J. Environ. Eng. Landsc. Manag. 2017, 25, 86–104. [Google Scholar] [CrossRef]
- Xing, J.; Li, L.C.; Li, G.B.; Xu, G.R. Feasibility of sludge-based biochar for soil remediation: Characteristics and safety performance of heavy metals influenced by pyrolysis temperatures. Ecotoxicol. Environ. Saf. 2019, 180, 457–465. [Google Scholar] [CrossRef]
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; O’Toole, A.; Sundqvist, K.L.; Arp, H.P.H.; Cornelissen, G. Quantifying the Total and Bioavailable Polycyclic Aromatic Hydrocarbons and Dioxins in Biochars. Environ. Sci. Technol. 2012, 46, 2830–2838. [Google Scholar] [CrossRef]
- Lyu, H.; He, Y.; Tang, J.; Hecker, M.; Liu, Q.; Jones, P.D.; Codling, G.; Giesy, J.P. Effect of pyrolysis temperature on potential toxicity of biochar if applied to the environment. Environ. Pollut. 2016, 218, 1–7. [Google Scholar] [CrossRef]
- Buss, W.; Masek, O.; Graham, M.; Wüstc, D. Inherent organic compounds in biochar-Their content, composition and potential toxic effects. J. Environ. Manag. 2015, 156, 150–157. [Google Scholar] [CrossRef]
- Luo, J.; Lin, L.; Liu, C.; Jia, C.; Chen, T.; Yang, Y.; Shen, M.; Shang, H.; Zhou, S.; Huang, M.; et al. Reveal a hidden highly toxic substance in biochar to support its effective elimination strategy. J. Hazard. Mater. 2020, 399, 123055. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Li, Z.; Yu, G.; Wang, Y. Effect of pyrolysis temperature on characteristics, chemical speciation and risk evaluation of heavy metals in biochar derived from textile dyeing sludge. Ecotoxicol. Environ. Saf. 2019, 168, 45–52. [Google Scholar] [CrossRef]
- Shen, X.; Zeng, J.; Zhang, D.; Wang, F.; Li, Y.; Yi, W. Effect of pyrolysis temperature on characteristics, chemical speciation and environmental risk of Cr, Mn, Cu, and Zn in biochars derived from pig manure. Sci. Total Environ. 2020, 704, 135283. [Google Scholar] [CrossRef]
- Sørmo, E.; Silvani, L.; Thune, G.; Gerber, H.; Schmidt, H.P.; Smebye, A.B.; Cornelissen, G. Waste timber pyrolysis in a medium-scale unit: Emission budgets and biochar quality. Sci. Total Environ. 2020, 718, 137335. [Google Scholar] [CrossRef]
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. The dark side of black gold: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833. [Google Scholar] [CrossRef]
- Kończak, M.; Gao, Y.Z.; Oleszczuk, P. Carbon dioxide as a carrier gas and biomass addition decrease the total and bioavailable polycyclic aromatic hydrocarbons in biochar produced from sewage sludge. Chemosphere 2019, 228, 26–34. [Google Scholar] [CrossRef]
- Tomczyk, B.; Siatecka, A.; Bogusz, A.; Oleszczuk, P. Ecotoxicological assessment of sewage sludge-derived biochars-amended soil. Environ. Pollut. 2021, 275, 116484. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Y.H.; Nan, H.Y.; Yang, F.; Qiu, H.; Xu, X.Y.; Cao, X.D. Suppressed formation of polycyclic aromatic hydro-carbons (PAHs) during pyrolytic production of Fe-enriched composite biochar. J. Hazard. Mater. 2020, 382, 121033. [Google Scholar] [CrossRef]
- Devi, P.; Saroha, A.K. Effect of pyrolysis temperature on polycyclic aromatic hydrocarbons toxicity and sorption behaviour of biochars prepared by pyrolysis of paper mill effluent treatment plant sludge. Bioresour. Technol. 2015, 192, 312–320. [Google Scholar] [CrossRef]
- Chen, X.; Yang, L.; Myneni, S.C.B.; Deng, Y. Leaching of polycyclic aromatic hydrocarbons (PAHs) from sewage sludge-derived biochar. Chem. Eng. J. 2019, 373, 840–845. [Google Scholar] [CrossRef]
- Anjum, R.; Krakat, N.; Reza, M.T.; Klocke, M. Assessment of mutagenic potential of pyrolysis biochars by Ames Salmonella/mammalian-microsomal mutagenicity test. Ecotoxicol. Environ. Saf. 2014, 107, 306–312. [Google Scholar] [CrossRef]
- Meng, J.; Wang, L.; Zhong, L.; Liu, X.; Brookes, P.C.; Xu, J.; Chen, H. Contrasting effects of composting and pyrolysis on bioavailability and speciation of Cu and Zn in pig manure. Chemosphere 2017, 180, 93–99. [Google Scholar] [CrossRef]
- Amoakwah, E.; Arthur, E.; Frimpong, K.A.; Lorenz, F.N.; Rahman, M.A.; Nziguheba, G.; Islam, K.R. Biochar amendment impacts on microbial community structures and biological and enzyme activities in a weathered tropical sandy loam. Appl. Soil Ecol. 2022, 172, 104364. [Google Scholar] [CrossRef]
- Ren, T.; Feng, H.; Xu, C.; Xu, Q.; Fu, B.; Azwar, E.; Wei, Y.; Lam, S.S.; Liu, G. Exogenous application and interaction of biochar with environmental factors for improving functional diversity of rhizosphere’s microbial community and health. Chemosphere 2022, 294, 133710. [Google Scholar] [CrossRef]
- Andrés, P.; Rosell-Melé, A.; Colomer-Ventura, F.; Denef, K.; Cotrufo, M.F.; Riba, M.; Alcañiz, J.M. Belowground biota re-sponses to maize biochar addition to the soil of a Mediterranean vineyard. Sci. Total Environ. 2019, 660, 1522–1532. [Google Scholar] [CrossRef]
- Li, Q.; Lei, Z.; Song, X.; Zhang, Z.; Ying, Y.; Peng, C. Biochar amendment decreases soil microbial biomass and increases bacterial diversity in Moso bamboo (Phyllostachys edulis) plantations under simulated nitrogen deposition. Environ. Res. Lett. 2018, 13, 044029. [Google Scholar] [CrossRef]
- Nan, Q.; Hu, S.; Qin, Y.; Wu, W. Methane oxidation activity inhibition via high amount aged biochar application in paddy soil. Sci. Total Environ. 2021, 796, 149050. [Google Scholar] [CrossRef]
- Han, Y.X.; Douds, D.D., Jr.; Boateng, A.A. Effect of biochar soil-amendments on Allium porrum growth and Arbuscular mycorrhizal fungus colonization. J. Plant Nutr. 2015, 39, 1654–1662. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P.; Bartmiński, P. Chemical and ecotoxicological evaluation of biochar produced from residues of biogas production. J. Hazard. Mater. 2016, 318, 417–424. [Google Scholar] [CrossRef]
- Anyanwu, I.N.; Alo, M.N.; Onyekwere, A.M.; Crosse, J.D.; Nworie, O.; Chamba, E.B. Influence of biochar aged in acidic soil on ecosystem engineers and two tropical agricultural plants. Ecotoxicol. Environ. Saf. 2018, 153, 116–126. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, G.; Liao, X. Negative role of biochars in the dissipation and vegetable uptake of polycyclic aromatic hydrocarbons (PAHs) in an agricultural soil: Cautions for application of biochars to remediate PAHs-contaminated soil. Ecotoxicol. Environ. Saf. 2021, 213, 112075. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleem, M.; Wang, C. Effects of biochar on the earthworm (Eisenia foetida) in soil contaminated with and/or without pesticide mesotrione. Sci. Total Environ. 2019, 671, 52–58. [Google Scholar] [CrossRef]
- Bielská, L.; Škulcová, L.; Neuwirthová, N.; Cornelissen, G.; Hale, S.E. Sorption, bioavailability and ecotoxic effects of hydrophobic organic compounds in biochar amended soils. Sci. Total Environ. 2018, 624, 78–86. [Google Scholar] [CrossRef]
- Baronti, S.; Alberti, G.; Vedove, G.D.; Di Gennaro, F.; Fellet, G.; Genesio, L.; Miglietta, F.; Peressotti, A.; Vaccari, F.P. The Biochar Option to Improve Plant Yields: First Results from Some Field and Pot Experiments in Italy. Ital. J. Agron. 2010, 5, 3–12. [Google Scholar] [CrossRef]
- Hale, S.E.; Jensen, J.; Jakob, L.; Oleszczuk, P.; Hartnik, T.; Henriksen, T.; Okkenhaug, G.; Martinsen, V.; Cornelissen, G. Short-Term Effect of the Soil Amendments Activated Carbon, Biochar, and Ferric Oxyhydroxide on Bacteria and Invertebrates. Environ. Sci. Technol. 2013, 47, 8674–8683. [Google Scholar] [CrossRef]
- Gascó, G.; Cely, P.; Paz-Ferreiro, J.; Plaza, C.; Méndez, A. Relation between biochar properties and effects on seed germination and plant development. Biol. Agric. Hortic. 2016, 32, 237–247. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Ok, Y.S.; Awad, Y.M.; Lee, S.S.; Sung, J.-K.; Koutsospyros, A.; Moon, D.H. Impacts of biochar application on upland agriculture: A review. J. Environ. Manag. 2019, 234, 52–64. [Google Scholar] [CrossRef]
- Gao, X.; Cheng, H.-Y.; Del Valle, I.; Liu, S.; Masiello, C.A.; Silberg, J.J. Charcoal Disrupts Soil Microbial Communication through a Combination of Signal Sorption and Hydrolysis. ACS Omega 2016, 1, 226–233. [Google Scholar] [CrossRef]
- Du, Z.L.; Hu, A.B.; Wang, Q.D.; Ai, J.; Zhang, W.J.; Liang, Y.; Cao, M.X.; Wu, H.J.; Han, D.S. Molecular composition and biotoxicity effects of dissolved organic matters in sludge-based carbon: Effects of pyrolysis temperature. J. Hazard. Mater. 2022, 424, 127326. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, D.; Hong, B.; Wang, H.T.; Li, G.; Ma, Y.B.; Tang, Y.T.; Chen, Q.L. Long-term application of organic fertilization causes the accumulation of antibiotic resistome in earthworm gut microbiota. Environ. Int. 2019, 124, 145–152. [Google Scholar] [CrossRef]
- Xiao, R.; Ali, A.; Xu, Y.; Abdelrahman, H.; Li, R.; Lin, Y.; Bolan, N.; Shaheen, S.M.; Rinklebe, J.; Zhang, Z. Earthworms as candidates for remediation of potentially toxic elements contaminated soils and mitigating the environmental and human health risks: A review. Environ. Int. 2022, 158, 106924. [Google Scholar] [CrossRef]
- Han, J.; Huang, Y.; Meng, J.; Fan, C.; Yang, F.; Tan, H.; Zhang, J. Exposure of earthworm (Eisenia fetida) to rice straw biochar: Ecotoxicity assessments for soil-amended programmes. Sci. Total Environ. 2021, 794, 148802. [Google Scholar] [CrossRef]
- Shi, Z.; Yan, J.; Ren, X.; Wen, M.; Zhao, Y.; Wang, C. Effects of biochar and thermally treated biochar on Eisenia fetida survival, growth, lysosomal membrane stability and oxidative stress. Sci. Total Environ. 2021, 770, 144778. [Google Scholar] [CrossRef]
- Méndez, A.; Gómez, A.; Paz-Ferreiro, J.; Gascó, G. Effects of sewage sludge biochar on plant metal availability after application to a Mediterranean soil. Chemosphere 2012, 89, 1354–1359. [Google Scholar] [CrossRef]
- Visioli, G.; Conti, F.D.; Menta, C.; Bandiera, M.; Malcevschi, A.; Jones, D.L.; Vamerali, T. Assessing biochar ecotoxicology for soil amendment by root phytotoxicity bioassays. Environ. Monit. Assess. 2016, 188, 1–11. [Google Scholar] [CrossRef]
- Ren, N.N.; Tang, Y.Y.; Li, M. Mineral additive enhanced carbon retention and stabilization in sewage sludge-derived biochar. Process Saf. Environ. Prot. 2018, 115, 70–78. [Google Scholar] [CrossRef]
- Alves, B.S.Q.; Zelaya, K.P.S.; Colen, F.; Frazão, L.A.; Napoli, A.; Parikh, S.J.; Fernandes, L.A. Effect of sewage sludge and sugarcane bagasse biochar on soil properties and sugar beet production. Pedosphere 2021, 31, 572–582. [Google Scholar] [CrossRef]
- Chagas, J.K.M.; de Figueiredo, C.C.; da Silva, J.; Paz-Ferreiro, J. The residual effect of sewage sludge biochar on soil availa-bility and bioaccumulation of heavy metals: Evidence from a three-year field experiment. J. Environ. Manag. 2021, 279, 111824. [Google Scholar] [CrossRef]
- Gonzaga, M.I.S.; Mackowiak, C.; de Almeida, A.Q.; Wisniewski, A.; de Souza, D.F.; da Silva Lima, I.; de Jesus, A.N. As-sessing biochar applications and repeated Brassica juncea L. production cycles to remediate Cu contaminated soil. Chemosphere 2018, 201, 278–285. [Google Scholar] [CrossRef]
- Tian, Y.F.; Cui, L.; Lin, Q.M.; Li, G.T.; Zhao, X.R. The sewage sludge biochar at low pyrolysis temperature had better im-provement in urban soil and turf grass. Agronomy 2019, 9, 156. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Nelson, P.F. Comparative assessment of the effect of wastewater sludge biochar on growth, yield and metal bioaccumulation of cherry tomato. Pedosphere 2015, 5, 680–685. [Google Scholar] [CrossRef]
- Yang, C.Y.; Liu, J.J.; Lu, S.G. Pyrolysis temperature affects pore characteristics of rice straw and canola stalk biochars and biochar-amended soils. Geoderma 2021, 397, 115097. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Gu, P.X.; Liu, X.Y.; Huang, X.; Wang, J.Y.; Zhang, S.Y.; Ji, J.H. Effect of crop straw biochars on the remedia-tion of Cd-contaminated farmland soil by hyperaccumulator Bidens pilosa L. Ecotoxicol. Environ. Saf. 2021, 219, 112332. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Sang, M.K.; Igalavithana, A.D.; Zhang, M.; Hou, D.Y.; Oleszczuk, P.; Sung, J.; Ok, Y.S. Biochar alters chemical and microbial properties of microplastic-contaminated soil. Environ. Res. 2022, 209, 112807. [Google Scholar] [CrossRef]
- Qin, J.L.; Qian, S.Y.; Chen, Q.C.; Chen, L.; Yan, L.L.; Shen, G.Q. Cow manure-derived biochar: Its catalytic properties and influential factors. J. Hazard. Mater. 2019, 371, 381–388. [Google Scholar] [CrossRef]
- Xu, Y.G.; Bai, T.X.; Yan, Y.B.; Ma, K.R. Influence of sodium hydroxide addition on characteristics and environmental risk of heavy metals in biochars derived from swine manure. Waste Manag. 2020, 105, 511–519. [Google Scholar] [CrossRef]
- Xu, Y.G.; Qu, W.; Sun, B.Y.; Peng, K.; Zhang, X.Z.; Xu, J.M.; Gao, F.; Yan, Y.B.; Bai, T.X. Effects of added calcium-based additives on swine manure derived biochar characteristics and heavy metals immobilization. Waste Manag. 2021, 123, 69–79. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Wade, P.; Bolan, N. Assessment of the fertilizer potential of biochars produced from slow pyrolysis of biosolid and animal manures. J. Anal. Appl. Pyrolysis 2021, 155, 105043. [Google Scholar] [CrossRef]
- Kiran, Y.K.; Barkat, A.; Cui, X.; Feng, Y.; Pan, F.; Tang, L.; Yang, X.E. Cow manure and cow manure-derived biochar ap-plication as a soil amendment for reducing cadmium availability and accumulation by Brassica chinensis L. in acidic red soil. J. Integr. Agric. 2017, 16, 725–734. [Google Scholar] [CrossRef]
- Wang, F.; Jin, L.T.; Guo, C.N.; Min, L.J.; Zhang, P.; Sun, H.W.; Zhu, H.K.; Zhang, C.P. Enhanced heavy metals sorption by modified biochars derived from pig manure. Sci. Total Environ. 2021, 786, 147595. [Google Scholar] [CrossRef]

| Biochar Type | Pyrolysis Conditions | Highlighted Properties (Biochar) | Soil Type | Highlighted Properties (Soil) | Addition Rate | Duration | pH | CEC | Bulk Density | Water Holding Capacity | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Orchard pruning biomass | 3 h at 500 °C | 71.4% C, 0.7% N, 1.5% H, and 5.9% O (dry weight) | Sandy clay loam | 70% sand, 15% silt and 15% clay. | 0 | 9 months | 5.18 ± 0.30 | 11.8 ± 0.9 meq 100 g−1 | 1.44 ± 0.10 g cm−3 | - | [58] |
| 16.5 t ha−1 in 2009 and further 16.5 t ha−1 in 2010 | 9 months | 6.76 ± 0.18 | 24.3 ± 1.8 meq 100 g−1 | 1.38 ± 0.06 g cm−3 | - | ||||||
| 16.5 t ha−1 | 9 months | 6.54 ± 0.25 | 18.32 ± 1.05 meq 100 g−1 | 1.42 ± 0.07 g cm−3 | - | ||||||
| 0 | 1 year | 5.25 ± 0.15 | 11.5 ± 1.5 meq 100 g−1 | 1.45 ± 0.06 g cm−3 | - | ||||||
| 16.5 t ha−1 in 2009 and further 16.5 t ha−1 in 2010 | 1 year | 6.59 ± 0.20 | 24.1 ± 1.8 meq 100 g−1 | 1.38 ± 0.25 g cm−3 | - | ||||||
| 16.5 t ha−1 | 1 year | 6.32 ± 0.14 | 18.14 ± 0.83 meq 100 g−1 | 1.40 ± 0.03 g cm−3 | - | ||||||
| Peanut shells biochar | 650 °C for 6 h | - | Silt loam acidic soil | 5.58% clay, 49.5% silts, and 44.92% sand | 2% (w/w) | 42 d | 6.11 ±0.15 | - | - | - | [74] |
| 4% (w/w) | 42 d | 6.67 ± 0.16 | - | - | - | ||||||
| 6% (w/w) | 42 d | 6.91 ± 0.18 | - | - | - | ||||||
| Maize straw biochar | 350–550 °C | - | Sandy loam | Bulk density 1.41 g mL−1, water holding capacity 0.38 cm3 cm−3 | 0 tons ha−1 biochar | 1 year | 7.26 | 12.9 cmol kg−1 | 1.45 g cm−3 | - | [59] |
| 4 t ha−1 | 1 year | 7.39 | 15.6 cmol kg−1 | 1.43 g cm−3 | - | ||||||
| 12 t ha−1 | 1 year | 7.54 | 17.4 cmol kg−1 | 1.40 g cm−3 | - | ||||||
| 36 t ha−1 | 1 year | 7.64 | 19.2 cmol kg−1 | 1.36 g cm−3 | - | ||||||
| Grains husks and paper | 500 °C | Total C 531 g kg−1 and total N 14 g kg−1 | Haplic Luvisol | 9.13 g kg−1 soil organic carbon and pH 5.71 | 0 t ha−1 | 1 year | - | 153.93 ± 6.45 Mmol kg−1 | - | - | [75] |
| 10 t ha−1 | 1 year | 160.15 ± 10.40 Mmol kg−1 | - | - | |||||||
| 20 t ha−1 | 1 year | 177.70 ± 13.33 Mmol kg−1 | - | - | |||||||
| Rice husk biochar | 350 °C for 1.25 h | pH 8.50 and 51.13% TOC | Typic Paleustalf Alfisol | 68.8% sand, 25.1% clay and 6.1% silt | 0 | 2 years | 5.27 | 7.18 cmol kg−1 | - | 32.94% | [61] |
| 3 t ha−1 | 2 years | 6.54 | 9.69 cmol kg−1 | - | 32.94% | ||||||
| 6 t ha−1 | 2 years | 6.66 | 9.91 cmol kg−1 | - | 34.87% | ||||||
| 12 t ha−1 | 2 years | 6.73 | 12.30 cmol kg−1 | - | 36.87% | ||||||
| Sawdust | 350 °C for 4 h | - | Loamy sand | 65.3% sand, 25.4% silt, 9.3% clay | 0 | 11 weeks | 6.85 | 13.48 cmol kg−1 | - | - | [76] |
| 10 t ha−1 | 11 weeks | 7.24 | 17.63 cmol kg−1 | - | - | ||||||
| 20 t ha−1 | 11 weeks | 7.30 | 21.57 cmol kg−1 | - | - |
| Pollutants Types | Biochar Types | Pyrolysis Conditions | Concentration | Ref. |
|---|---|---|---|---|
| PAHs | Sewage sludge | 500 °C for 3 h | Total PAHs: 2263 μg kg−1 | [168] |
| 600 °C for 3 h | Total PAHs: 1730 μg kg−1 | |||
| 700 °C for 3 h | Total PAHs: Total PAHs: 1449 μg kg−1 | |||
| Sewage sludge | 500 °C | Total PAHs: 612 to 766 μg kg−1 | [169] | |
| Straw | 350 °C for 2 h | Total PAHs: 3.72 ± 0.422 mg kg−1 | [170] | |
| 500 °C for 2 h | Total PAHs: 5.48 ± 1.02 mg kg−1 | |||
| 650 °C for 2 h | Total PAHs: 4.94 ± 0.315 mg kg−1 | |||
| Paper mill sludge | 200 °C | Nap: 2.5 mg kg−1 Flu: 8.5 mg kg−1 Phe: 32 mg kg−1 Pyr: 15.46 mg kg−1 BaA: 2.01 mg kg−1 CHR: 246.23 mg kg−1 BbF: 1.51 mg kg−1 BkF: 1.07 mg kg−1 | [171] | |
| 300 °C | Nap: 8.5 mg kg−1 Flu: 39.5 mg kg−1 Phe: 55.5 mg kg−1 Pyr: 12.64 mg kg−1 BaA: 24.2 mg kg−1 CHR: 157.42 mg kg−1 BbF: 3.61 mg kg−1 BkF: 2.43 mg kg−1 | |||
| 400 °C | Nap: 860 mg kg−1 Flu: 343.5 mg kg−1 Phe: 913 mg kg−1 Pyr: 492.3 mg kg−1 BaA: 224.7 mg kg−1 CHR: 534.9 mg kg−1 BbF: 195.39 mg kg−1 BkF: 172.3 mg kg−1 | |||
| 500 °C | Nap: 372.5 mg kg−1 Flu: 1198 mg kg−1 Phe: 3700 mg kg−1 Pyr: 1394.7 mg kg−1 BaA: 769.5 mg kg−1 CHR: 859.02 mg kg−1 BbF: 2310 mg kg−1 BkF: 2182 mg kg−1 | |||
| 600 °C | Nap: 4.5 mg kg−1 Flu: 79 mg kg−1 Phe: 197 mg kg−1 Pyr: 165.8 mg kg−1 BaA: 17.5 mg kg−1 CHR: 127.5 mg kg−1 BbF: 134.29 mg kg−1 BkF: 121.8 mg kg−1 | |||
| 700 °C | Flu: 16.5 mg kg−1 Phe: 60 mg kg−1 Pyr: 21 mg kg−1 BaA: 25 mg kg−1 CHR: 12.28 mg kg−1 BbF: 9.01 mg kg−1 BkF: 8.2 mg kg−1 | |||
| Sewage sludge | 300 °C for 3 h | 2 ring PAHs: 5.7 mg kg−1 3 ring PAHs: 4.8 mg kg−1 4 ring PAHs: 1.8 mg kg−1 5 ring PAHs: 0.6 mg kg−1 6 ring PAHs: 0.8 mg kg−1 | [172] | |
| 400 °C for 3 h | 2 ring PAHs: 6.0 mg kg−1 3 ring PAHs: 4.0 mg kg−1 4 ring PAHs: 3.1 mg kg−1 5 ring PAHs: 1.3 mg kg−1 6 ring PAHs: 1.0 mg kg−1 | |||
| 500 °C for 3 h | 2 ring PAHs: 6.9 mg kg−1 3 ring PAHs: 1.4 mg kg−1 4 ring PAHs: 0.3 mg kg−1 5 ring PAHs: 0.6 mg kg−1 | |||
| 600 °C for 3 h | 2 ring PAHs: 1.3 mg kg−1 3 ring PAHs: 2.4 mg kg−1 4 ring PAHs: 2.4 mg kg−1 | |||
| 700 °C for 3 h | 2 ring PAHs: 1.0 mg kg−1 3 ring PAHs: 0.5 mg kg−1 4 ring PAHs: 0.4 mg kg−1 | |||
| Corn stover | 350 °C | Total PAHs: 1609 μg kg−1 | [160] | |
| 450 °C | Total PAHs: 1959 μg kg−1 | |||
| 550 °C | Total PAHs: 1770 μg kg−1 | |||
| Wood pellets | 500 °C for 30 min | Total PAHs: 33,700 μg kg−1 | [173] | |
| PCDD/Fs | Sawdust | 250 °C for 3 h | Total PCDD/Fs: 2.7 × 102 pg g−1 | [161] |
| 300 °C for 3 h | Total PCDD/Fs: 6.1 × 102 pg g−1 | |||
| 400 °C for 3 h | Total PCDD/Fs: 3.6 × 102 pg g−1 | |||
| 500 °C for 3 h | Total PCDD/Fs: 67 pg g−1 | |||
| 700 °C for 3 h | Total PCDD/Fs: 50 pg g−1 | |||
| Food waste | 300 °C | Total 2,3,7,8-substitutes dioxin concentration:13.3 pg g−1 Toxic dioxin concentration: 1.20 pg g−1 | [160] | |
| 400 °C | Total 2,3,7,8-substitutes dioxin concentration: 12.2 pg g−1 Toxic dioxin concentration: 0.15 pg g−1 | |||
| 500 °C | Total 2,3,7,8-substitutes dioxin concentration: 0.39 pg g−1 Toxic dioxin concentration: 0.008 pg g−1 | |||
| 600 °C | Total 2,3,7,8-substitutes dioxin concentration: 7.5 pg g−1 Toxic dioxin concentration: 0.16 pg g−1 | |||
| Digested dairy manure | 600 °C | Total 2,3,7,8-substitutes dioxin concentration: 8.0 pg g−1 Toxic dioxin concentration: 0.13 pg g−1 | ||
| Pine wood | 900 °C | Total 2,3,7,8-substitutes dioxin concentration: 10.7 pg g−1 Toxic dioxin concentration: 0.15 pg g−1 | ||
| Lodgepole pine | - | Total 2,3,7,8-substitutes dioxin concentration: 10.5 pg g−1 Toxic dioxin concentration: 0.18 pg g−1 | ||
| Laurel oak | 650 °C | Total 2,3,7,8-substitutes dioxin concentration: 1.5 pg g−1 Toxic dioxin concentration: 0.02 pg g−1 | ||
| Eastern gamma grass | 650 °C | Total 2,3,7,8-substitutes dioxin concentration: 2.4 pg g−1 Toxic dioxin concentration: 0.02 pg g−1 | ||
| Pine wood | 800 °C | Total 2,3,7,8-substitutes dioxin concentration: 0.5 pg g−1 Toxic dioxin concentration: 0.005 pg g−1 | ||
| Switch grass | 800 °C | Total 2,3,7,8-substitutes dioxin concentration: 0.8 pg g−1 Toxic dioxin concentration: 0.008 pg g−1 | ||
| Switch grass | 900 °C | Total 2,3,7,8-substitutes dioxin concentration: 2.2 pg g−1 Toxic dioxin concentration: 0.22 pg g−1 | ||
| Paper mill waste | 600 °C | Total 2,3,7,8-substitutes dioxin concentration: 0.6 pg g−1 Toxic dioxin concentration: 0.006 pg g−1 | ||
| VOCs | Softwood pellets | At 500 for 20 min (a liquid contaminated biochar) | Phenol: 110 μg g−1 3-methyl-1,2-cyclopentadione: 91 μg g−1 2-methylphenol: 130 μg g−1 3/4-methylphenol: 200 μg g−1 3,4-dimethylphenol: 240 μg g−1 4-ethylphenol: 110 μg g−1 3-ethyl-5-methylphenol: 64 μg g−1 4-ethyl-3-methylphenol: 110 μg g−1 1,2-benzenediol: 66 μg g−1 4-methyl-1,2-benzenediol: 45 μg g−1 | [162] |
| At 500 for 20 min (a gas contaminated biochar) | Phenol: 49 μg g−1 4-methyl-1,2-cyclopentadione: 60 μg g−1 2-methylphenol: 60 μg g−1 3,4-methylphenol: 92 μg g−1 3,4-dimethylphenol: 120 μg g−1 2-methoxy-5-methylphenol: 23 μg g−1 4-ethylphenol: 61 μg g−1 3-ethyl-5-methylphenol: 20 μg g−1 4-ethyl-3-methylphenol: 59 μg g−1 1,2-benzenediol: 49 μg g−1 4-methyl-1,2-benzenediol: 31 μg g−1 | |||
| MCN | Food waste | 800 °C for 1 h | 40,286 mg kg−1 | [163] |
| Soybean residue | 800 °C for 1 h | 17.4 mg kg−1 | ||
| Rapeseed residue | 800 °C for 1 h | 9.6 mg kg−1 | ||
| Phycocyanin | 800 °C for 1 h | 85,870 mg kg−1 | ||
| Algae protein | 800 °C for 1 h | 10.3 mg kg−1 | ||
| Vinasse | 800 °C for 1 h | 6.9 mg kg−1 | ||
| Corn protein | 800 °C for 1 h | 9.3 mg kg−1 | ||
| Wheat straw | 800 °C for 1 h | 1.0 mg kg−1 | ||
| Corn straw | 800 °C for 1 h | 105.4 mg kg−1 | ||
| Cow dung | 800 °C for 1 h | 5.9 mg kg−1 | ||
| Kitchen waste | 800 °C for 1 h | 15.9 mg kg−1 | ||
| Fungi residue | 800 °C for 1 h | 251.1 mg kg−1 | ||
| Biogas residue | 800 °C for 1 h | 50.9 mg kg−1 | ||
| Corn protein with K2CO3 | 800 °C for 1 h | 23,251 mg kg−1 | ||
| Heavy metals | Sewage sludge | 300 °C | Zn: 10,400.17 ± 97.21 mg kg−1 Cu: 316.55 ± 20.08 mg kg−1 Cr: 297.37 ± 9.88 mg kg−1 Ni: 217.32 ± 6.27 mg kg−1 Cd: 0.793 ± 0.103 mg kg−1 Mn: 1859.43 ± 20.75 mg kg−1 | [164] |
| 400 °C | Zn: 11,134.92 ± 69.83 mg kg−1 Cu: 347.53 ± 18.39 mg kg−1 Cr: 314.35 ± 11.37 mg kg−1 Ni: 238.57 ± 8.21 mg kg−1 Cd: 0.852 ± 0.091 mg kg−1 Mn: 2082.52 ± 33.62 mg kg−1 | |||
| 500 °C | Zn: 12,550.41 ± 93.04 mg kg−1 Cu: 374.05 ± 23.01 mg kg−1 Cr: 360.75 ± 8.78 mg kg−1 Ni: 260.14 ± 5.08 mg kg−1 Cd: 0.928 ± 0.055 mg kg−1 Mn: 2256.54 ± 52.01 mg kg−1 | |||
| 600 °C | Zn: 13,080.32 ± 70.55 mg kg−1 Cu: 392.15 ± 10.55 mg kg−1 Cr: 376.82 ± 7.99 mg kg−1 Ni: 272.39 ± 2.09 mg kg−1 Cd: 0.866 ± 0.042 mg kg−1 Mn: 2319.54 ± 41.27 mg kg−1 | |||
| 700 °C | Zn: 14,109.92 ± 91.39 mg kg−1 Cu: 426.92 ± 20.02 mg kg−1 Cr: 411.96 ± 10.11 mg kg−1 Ni: 299.49 ± 7.44 mg kg−1 Cd: 0.139 ± 0.027 mg kg−1 Mn: 2525.01 ± 72.13 mg kg−1 | |||
| Pig manure | 400 °C for 2 h | Water-soluble Cu: 1.21 mg kg−1 Water-soluble Zn: 0.17 mg kg−1 DTPA-Cu: 23.44 mg kg−1 DTPA-Zn: 122.89 mg kg−1 | [174] | |
| 700 °C for 2 h | Water-soluble Cu: 2.38 mg kg−1 Water-soluble Zn: 0.81 mg kg−1 DTPA-Cu: 31.05 mg kg−1 DTPA-Zn: 129.24 mg kg−1 | |||
| Pig manure | 300 °C | Cr: 513.40 ± 3.50 mg kg−1 Mn: 1390.6 ± 5.30 mg kg−1 Cu: 673.40 ± 3.80 mg kg−1 Zn: 4310.30 ± 16.00 mg kg−1 | [165] | |
| 350 °C | Cr: 518.10 ± 7.70 mg kg−1 Mn: 1430.60 ± 6.40 mg kg−1 Cu: 780.90 ± 6.20 mg kg−1 Zn: 4670.90 ± 29.10 mg kg−1 | |||
| 400 °C | Cr: 521.70 ± 4.90 mg kg−1 Mn: 1510.30 ± 1.30 mg kg−1 Cu: 824.60 ± 7.50 mg kg−1 Zn: 4950.80 ± 26.30 mg kg−1 | |||
| 450 °C | Cr: 553.50 ± 10.30 mg kg−1 Mn: 1580.00 ± 8.50 mg kg−1 Cu: 838.70 ± 7.30 mg kg−1 Zn: 5040.70 ± 33.40 mg kg−1 | |||
| 500 °C | Cr: 666.20 ± 9.40 mg kg−1 Mn: 1660.40 ± 6.70 mg kg−1 Cu: 950.70 ± 5.30 mg kg−1 Zn: 5630.00 ± 28.30 mg kg−1 | |||
| 550 °C | Cr: 559.50 ± 5.20 mg kg−1 Mn: 1520.30 ± 4.30 mg kg−1 Cu: 810.20 ± 1.90 mg kg−1 Zn: 4900.80 ± 37.20 mg kg−1 | |||
| 600 °C | Cr: 476.90 ± 2.50 mg kg−1 Mn: 1550.20 ± 8.50 mg kg−1 Cu: 901.50 ± 6.60 mg kg−1 Zn: 5160.80 ± 51.50 mg kg−1 | |||
| 650 °C | Cr: 569.70 ± 11.30 mg kg−1 Mn: 1860.80 ± 9.10 mg kg−1 Cu: 981.80 ± 16.10 mg kg−1 Zn: 6010.80 ± 40.10 mg kg−1 | |||
| 700 °C | Cr: 819.90 ± 10.30 mg kg−1 Mn: 1920.60 ± 2.30 mg kg−1 Cu: 1080.70 ± 9.60 mg kg−1 Zn: 7720.30 ± 46.70 mg kg−1 |
| Negative Impacted Groups | Biochar Feedstock | Pyrolysis Conditions | Soil Type | Addition Rate | Incubation Time | Negative Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Microbiology | Maize corn cob | 450–500 °C | Sandy loam | 5 t·ha−1 | 2, 14 and 24 months | The application of biochar significantly reduced soil microbial biomass (phospholipid fatty acid, PLFA). | [177] |
| Rice straw | At 500 °C for 3 h. | Hydromorphic paddy soil | 10, 15 and 20% (w/w) | 3–4 days | The biochar significantly reduced fungi in soil by 22.2–30.2% compared with control. | [178] | |
| Corn stover, ponderosa pine wood chips, switchgrass | The reactor temperature ramps from 150 °C to 850 °C with residence time of 4 h and 4 min | Sandy, mixed, frigid entic hapludolls | 10 g kg−1, and 50 g kg−1. | 120 days | The short-term incubation study showed that biochar had negative effects on microbial activity (FDA and DHA) and some enzymes including β-glucosidase and protease. | [66] | |
| Rice straw | At 500 °C for 2 h | Clay loam texture | 22.5 t ha−1 | 48 h | The addition of biochar limited the growth of methanotrophs and nitrifiers. | [179] | |
| Sewage sludge | At 500 °C, 600 °C, and 700 °C for 5 h | Loamy sand | 1% (w/w) | 6 months | Biochar derived at 500 °C has a higher toxic effect on Aliivibrio fischeri than that at 600 °C and 700 °C. | [169] | |
| Switchgrass, mixed hardwood, and mixed softwood pellets | 500 °C, 700 °C, 800 °C | - | 10% (w/w) | 6 weeks | Mycorrhizal fungi were inhibited by biochar addition. | [180] | |
| Solid residue from biogas production | 400, 600 and 800 °C | Artificial soil | 0.5–5% (w/w) | - | Depending on the temperature at which it was produced, biochar caused mortality of F. candida at a level from 20 to 30% (0.5% dose) and from 30 to 50% (5.0% dose), and reproduction inhibition occurred from 70 to 100% (0.5% dose) and 100% (5.0% dose) for all temperatures. | [181] | |
| Rice husk | >480 °C | Pristine agricultural soil | 0.5–50% (w/w) | 90 days | Biochar amendment reduced fungal population with shift in community structure and abundance, over time. | [182] | |
| Walnut shells and corn cobs | At 250 °C, 400 °C, and 600 °C for 4 h | - | 2% (w/w) | 75 days | Application of biochars to the soil decreases Proteobacteria richness (23–29%). | [183] | |
| Invertebrates | Rice husk | >480 °C | - | 0.5–50% (w/w) | 90 days | The growth of the exposed earthworms was strongly reduced by biochar, especially for 50% biochar addition. | [182] |
| Sewage sludge | At 500 °C, 600 °C, and 700 °C for 5 h | Loamy sand | 1% (w/w) | 6 months | Biochar exhibited toxic activity towards Folsomia candida after six months incubation, and biochar produced at higher temperatures exhibited lower toxicity than that prepared at lower temperature. | [169] | |
| Wheat straws | At 500 °C for 4 h | Artificial soil | 10% (w/w) | 28 days | About 10% of biochar applications induced DNA damage to earthworms. | [184] | |
| Wood | 700 °C | Sandy loam cambisol | 10% (w/w) | - | Reproduction rates of earthworms reduced by 38% in comparison to unamended soil. | [185] | |
| Rice husk | ~600 °C | Sandy loam cambisol | 10% (w/w) | - | Reproduction rates of earthworms reduced by27% compared to unamended soil. | ||
| Plants | Walnut shells and corn cobs | 250 °C, 400 °C, and 600 °C for 4 h | - | 2% (w/w) | 75 days | Most of six biochars significantly increased the concentrations of Σ16PAHs in Chinese cabbage by 30.10–74.22%. | [183] |
| Beech, hazel, oak, birch | 500 °C | - | 100 and 120 t ha−1 | - | A general reduction of wheat biomass was observed. | [186] | |
| Rice husk | >480 °C | - | 0.5–50% (w/w) | 90 days | Biochar led to the direct toxic influence on the roots of Oryza sativa and Solanum lycopersicum | [182] | |
| Sewage sludge | At 500 °C, 600 °C, and 700 °C for 5 h | Loamy sand | 1% (w/w) | 6 months | The biochar exhibited toxic activity towards Lepidium sativum after six months incubation, and biochar produced at higher temperatures exhibited lower toxicity than that prepared at lower temperature. | [169] | |
| Switchgrass, mixed hardwood, and mixed softwood pellets | 500 °C, 700 °C, 800 °C | - | 10% (w/w) | 6 weeks | Biochar amendment tended to reduce the aboveground biomass of Allium porrum ‘ Musselburgh’. | [180] | |
| Corn stover | At 600 °C for 20 min | - | 2, and 5% (w/w) | 28 days | The addition of biochar contributed to weight loss in Aporrectodea caliginosa. | [187] | |
| Mixed wood sievings | At 500 °C for 20 min | Haplic Cambisols | 8% (w/w) | 1 month | The biochar led to a decline of Lactuca sativa biomass. | [188] | |
| Paper sludge + wheat husks | At 500 °C for 20 min | Haplic Cambisols | 8% (w/w) | 1 month | About 33% lower stem length of Lactuca Sativa was found after biochar amendment. | ||
| Sewage sludge | At 500 °C for 20 min | Haplic Cambisols | 8% (w/w) | 1 month | The biochar reduced 15–38% of the stem length and root (dry weight). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Li, X.; Li, Y.; Bao, H.; Xing, J.; Zhu, Y.; Nan, J.; Xu, G. Biochar Acts as an Emerging Soil Amendment and Its Potential Ecological Risks: A Review. Energies 2023, 16, 410. https://doi.org/10.3390/en16010410
Zhao Y, Li X, Li Y, Bao H, Xing J, Zhu Y, Nan J, Xu G. Biochar Acts as an Emerging Soil Amendment and Its Potential Ecological Risks: A Review. Energies. 2023; 16(1):410. https://doi.org/10.3390/en16010410
Chicago/Turabian StyleZhao, Yue, Xin Li, Yunyang Li, Huanyu Bao, Jia Xing, Yongzhao Zhu, Jun Nan, and Guoren Xu. 2023. "Biochar Acts as an Emerging Soil Amendment and Its Potential Ecological Risks: A Review" Energies 16, no. 1: 410. https://doi.org/10.3390/en16010410
APA StyleZhao, Y., Li, X., Li, Y., Bao, H., Xing, J., Zhu, Y., Nan, J., & Xu, G. (2023). Biochar Acts as an Emerging Soil Amendment and Its Potential Ecological Risks: A Review. Energies, 16(1), 410. https://doi.org/10.3390/en16010410