1. Introduction
In modern society, semiconductors are essential parts that are widely used not only in PCs and smartphones, but also in various home appliances and automobiles. Semiconductors, which are essential parts indispensable to our lives as such, are sensitive to changes in the market, and technological developments are constantly made to respond to changes. Recently, the semiconductor industry has been promoting the development of ultra-miniaturized processes for semiconductors smaller than 5 nm, and accordingly, the modernization of manufacturing facilities and the use of special gases have been increasing [
1].
Methods of supplying process gases to semiconductor equipment include trailer gas supply equipment that supplies gases from a trailer, bulk specialty gas supply (BSGS) systems that receive bulk specialty gases from the Y cylinder and distribute the process gases with a distribution device, and gas cabinet supply systems that supply cylinders filled with highly dangerous specialty gases by putting them into the gas cabinet [
1].
In general, various hazardous materials such as flammable, toxic, and corrosive materials are used in manufacturing semiconductors. In particular, semiconductors are manufactured using special gases in processes such as chemical vapor deposition (CVD) and etching. Special gas is filled in a container as compressed or liquefied gas, and a gas cylinder is used as a facility to supply the special gas to the semiconductor manufacturing process. When an abnormal situation (i.e., high pressure) for the cylinder occurs in the gas supply system, the gas can be released through the pressure relief device installed in the gas cylinder to ensure the safety of the supply system such as piping and measuring instruments. In the case protection devices are not working, it would be problematic.
Among them, the gas cylinder cabinet supply system can be easily managed with the connection between the cylinder and line pipes, the management of the flow rate of the gas going out of the cylinder, detection of leaks, and monitoring of the condition of the cylinder and can be optimized for the characteristics of manufacturing sites such as playing the role of connecting lines pipes and the cylinder instead of using different sizes of valves by chemical. Therefore, it is widely used in the fields of the semiconductor industry. However, due to the nature of specialty gases, if the gas is leaked, this system will be very dangerous due to diffusion, explosion, etc., of the gas and can have fatal effects on the workers.
In Japan, where the development of the semiconductor industry has preceded South Korea, many accident cases have already been analyzed. In 1984, in Japan, mono-germane, which is used in the semiconductor single crystal growth process (Epitaxy), exploded while being unloaded from a truck, causing serious injuries to two people. In addition, in 1989, there was an accident where mono-silane in the cylinder cabinet exploded due to damage to the gasket sealing in the semiconductor CVD process. In February 2000, an explosion accident occurred at a mixture of tri-fluoro-nitrogen and a lubricant in the compressor intake pipeline during the CVD process [
2]. In South Korea, too, in 2012, a hydrogen fluoride leakage accident occurred during the unloading process of hydrogen fluoride in Gumi, leading to the death of five persons and the discarding of all contaminated livestock products, including 3654 livestock in the affected area. In 2013, a hydrofluoric acid leakage accident occurred in the central chemical supply system of A Electronics Company leading to the death of one person and burns all over the bodies of four persons. As such, large and small accidents due to specialty gases used in semiconductors have been occurring constantly.
To reduce such accidents, existing accident cases should be investigated and analyzed, and measures to prevent the recurrence of accidents and prevent accidents in advance should be studied based on the results of the investigation and analysis. Yanagida and Matsumoto (2000) classified accidents that occurred in the Japanese semiconductor processes into Fatality, H. Injury, and L. Injury accidents and investigated and analyzed the parts where accidents occurred and the causes of accidents with a view to preventing accidents that may occur later. Ngai (2007) conducted a field test on leakage from the silane cylinder valve used in the semiconductor process and applied the leakage to an actual cylinder cabinet to study the effects of leakage and explosion in the semiconductor cylinder cabinet [
3]. In the case of diffusion instead of explosion accidents, when a gas is leaked into the semiconductor, the analysis of the diffusion air current inside the fab is also important. I et al. (2009) used CFD simulations to identify the air current pattern through ventilation when process material is leaked and applied the leakage locations and leakage rates of various scenarios to identify the pattern of leakage, thermal radiation [
4,
5], etc., inside a clean room. In addition, Min et al. (2021) studied the diffusion patterns according to whether there are buildings or not and the arrangement of buildings when chlorine gas leaked in a downtown area, placed physical barriers of 3, 6, and 9 m as a way to reduce the diffusion distance, and studied the effects.
As such, it is important to prevent and manage specialty gases used in semiconductor fabs (fabrication facilities) before accidents occur, and accordingly, the High-Pressure Gas Safety Control Act of South Korea stipulated safety regulations for gas cylinder cabinets of semiconductor fabs indicating that the gas cylinder cabinet should play the roles of blocking fire and direct sunlight, maintaining appropriate temperatures, storing gases, preventing movements, and ventilating and also indicating that additional protective walls must be installed to protect the gas cylinder [
6,
7,
8,
9].
According to domestic law, the protective walls should be installed with a buried depth of at least 450 mm from the floor. However, since the slab of the existing semiconductor fabs has been constructed to be 250 mm thick, difficulties are experienced in changing the layout of semiconductor fabs because the legal standard cannot be met when the gas facility rooms are newly constructed or expanded. This can be an element that hinders the development of the semiconductor industry in which the layout is frequently changed.
Therefore, this study investigated the protective performance of the gas cylinder cabinet to see whether the cabinet alone can replace the protective wall when the gas explodes in the gas cylinder cabinet used in semiconductor facilities in order to provide a basis for safe and simplified fab layout changes. To that end, the study was conducted in two stages. First, in the first stage of the study, the risk due to cabinet deformation when hydrogen gas exploded in the cabinet was evaluated, and the explosion pressure value was calculated. In the second stage, the protection performance was studied by simulating the AUTODYN using the resultant value. In particular, in the first stage, a gas cylinder cabinet with the same specifications as those used in the actual field was fabricated, an experimental demonstration was carried out thereafter, and the blast pressure propagation profile was calculated using the TNT equivalence method.
The above study is intended to evaluate the safety of the semiconductor gas cylinder cabinet and prepare a basis for simplification of the installation of protective walls when the semiconductor fab layout is changed in order to help strengthen the competitiveness of the semiconductor industry.
2. Verification Experiment
2.1. Experimental Gas Cylinder Cabinet Specifications and Target Gas Selection
In order to set the specifications of the cabinet to be used for the protection performance verification experiment, the Korean Industrial Standard KSB 6210, which stipulates domestic and foreign gas cylinder cabinet makers and gas cylinder capacities, was investigated, and domestic and foreign gas cylinder cabinet specifications were classified according to the gas cylinder storage capacities. The results are shown in
Table 1 and
Table 2.
Based on the contents shown above, a gas cylinder cabinet with the following specifications (
Table 3) was fabricated and used in the protective performance verification experiment; provided that, in order to identify the external deformation caused by the explosion inside the cabinet, the cylinder inside the cabinet was removed, and a situation where gas leaked inside the cabinet was assumed. The real pictures of the gas cabinet is shown in
Figure 1.
When a gas leaks from a gas cabinet cylinder, the pattern of explosion shows big differences depending on the type of the gas, whether the container explodes, whether there is an explosion due to the leakage, whether there is a physical explosion, whether there is a chemical explosion, and the amount of the stored and leaked gas. In the semiconductor manufacturing site, hydrogen, oxygen, methane, acetylene, nitrogen, carbon dioxide, etc., are generally used. Among them, combustible gases form standardized explosion ranges according to the types of the gases. In this study in order to apply an extreme state to the gas cylinder cabinet, hydrogen gas with a difference between UFL and LFL exceeding 20% was selected.
Hydrogen gas has an explosion range of 4–75%, and according to a previous study by Kim et al. [
10], the maximum explosion pressure occurs at 9%, which is the median value between the lower and upper explosion limits. Therefore, in this study, it was assumed that about 39%, the median value between the lower and upper explosion limits of hydrogen would leak inside the gas cylinder cabinet leading to the explosion.
2.2. Gas Cylinder Cabinet Experimental Condition Setting
The gas cylinder cabinet experiments were carried out in two states: an open state and a closed state according to the sealing state of the upper pipe.
- -
Open state: The hole in the upper part of the gas cylinder cabinet was sealed using thin vinyl.
- -
Closed state: The hole at the top of the gas cylinder cabinet was sealed using a 2.4 t steel sheet.
Thereafter, the hole for injecting hydrogen gas into each gas cylinder cabinet was sealed using a rubber sealing, and the gas cylinder cabinet was fixed to the concrete floor using anchor bolts.
To look at the experimental condition in a little more detail, in the case of the open gas cylinder cabinet, the inside was sealed using tapes on the hole perforated for internal piping connection and ventilation, and in the case of the closed gas cylinder cabinet, the inside was sealed using a 2.4 t steel plate on the perforated hole and two 0.3 t steel sheets (0.6 t) on the air intake hole under the door, and additional using tapes on the outside. Thereafter, no separate additional sealing of the sealed inner hole was carried out in the cabinets in both states. In addition, an environment in which the injected hydrogen gas can leak through the gas cylinder cabinet door was created to satisfy the actual usage conditions. The door was fixed using the same locking device as that in actual use, and the gas cylinder cabinet was buried in concrete to a 100 mm depth using a 12 mm chemical anchor.
Figure 2. shows the schematic diagram of the test set-up for gas cabinet explosion verifiation test equipment.
2.3. Hydrogen Gas Injection and Ignition
The hydrogen gas concentration inside the gas cylinder cabinet is set to 39 ± 5 vol%, and hydrogen is injected while controlling the pressure and flow rate using a commercially available hydrogen container.
Figure 3 shows the P&ID of hydrogen gas supply monitoring system. The method is as follows. First, open the hydrogen container and adjust it so that hydrogen gas can flow into the gas cylinder cabinet through the pipe, and set the pressure so that it can be maintained at 400 kPa by operating the pressure regulator. Thereafter, adjust the hydrogen gas injection so that the flow rate can be maintained at 12 m
3/h.
The hydrogen gas supply was controlled remotely through a computer program. The gas supply and shutoff were controlled, and the flow rate of the injected hydrogen gas was controlled using a monitoring system. A monitoring system was also used for the ignition device for the explosion, and in this case, the ignition time was set to 0.1 s.
2.4. Measuring Equipment Installation and Configuration
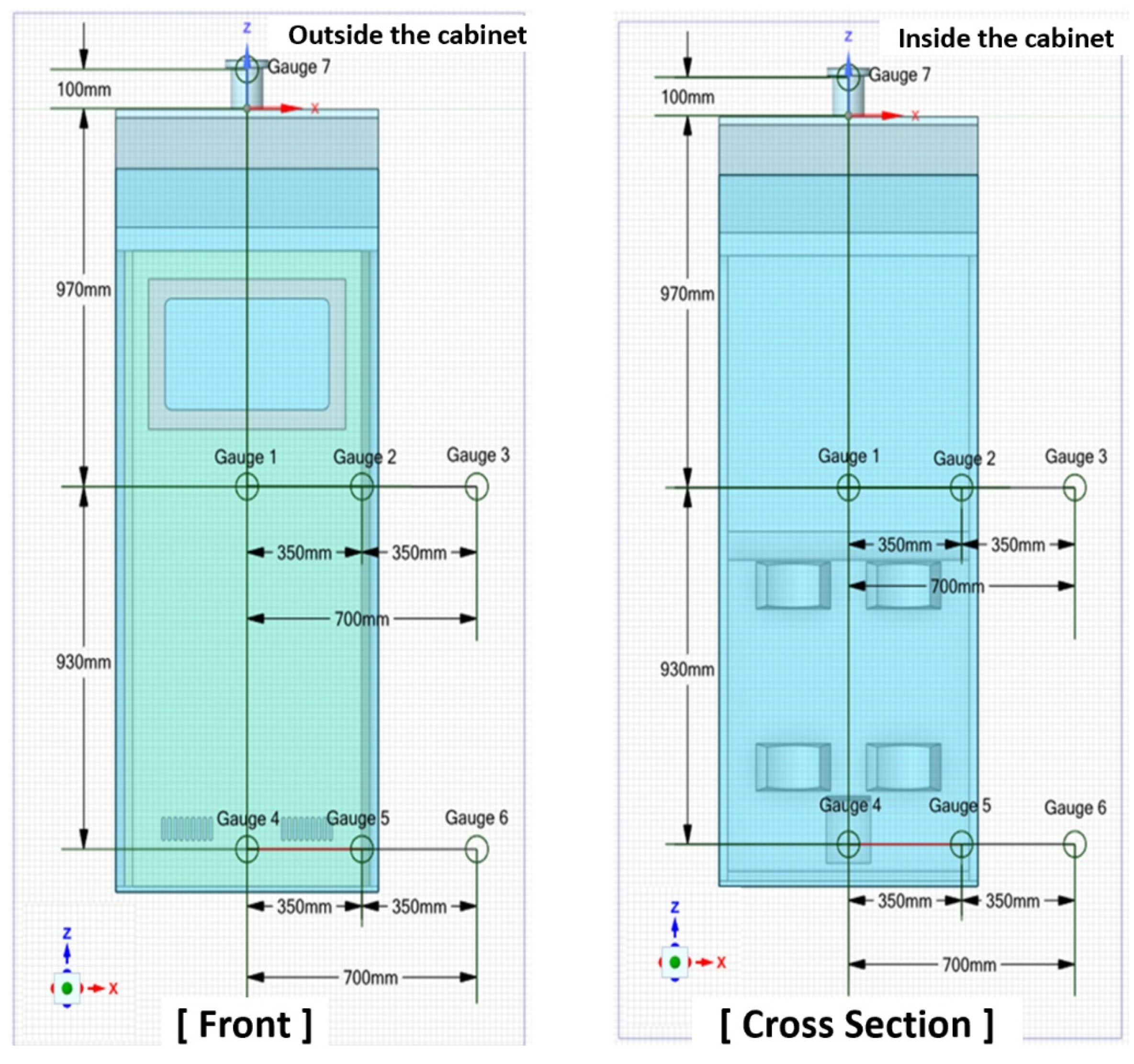
In each experiment, the pressure was measured by measuring internal pressure at 6 points, external pressure at 2 points, and upper pipe pressure at 1 point. The strain rate was measured at 4 points, and a 3D scan was performed.
Figure 4 shows the diagram of internal sensor positions.
Pressure gauge sensors were installed at the center, at the rear center, at the side center of the inside of the gas cylinder cabinet at the center height (P1, P2, P3), at the center, at the rear center, at the side center of the inside of the gas cylinder at the lower height (P4, P5, P6), at the center of the side wall (P7), at the upper pipe part (P8), and incident pressure gauges were installed at the center (P9) and lower part (P10) of the height of the gas cylinder cabinet at a position 350 mm away from the door in the diagonal direction in order to measure the incident pressure. In addition, in order to measure the strain rate of the gas cylinder cabinet according to the explosion load, two-axis strain gauges (S1-X, S1-Y, S2-X, S2-Y) were installed on the left and right sides of the center of the outer side walls of the gas cylinder cabinet.
2.5. Experimental Results
2.5.1. Scenario 1: State where the Pipe above the Gas Cylinder Cabinet Is Opened
Hydrogen was injected at a concentration of 39 ± 5 vol% in consideration of actual usage conditions to conduct internal explosion pressure and hydrogen gas injection tests. The hydrogen concentration was measured at the top, center, and bottom, respectively, and the concentration at the center where the ignition device was located was set as the reference concentration. A value of 40.382 vol%, which is the maximum concentration value, was shown when 317.6 s passed after the supply of hydrogen began, and the concentration gradually decreased. The ignition was carried out at a hydrogen concentration of 38.357 vol%. The changes in the hydrogen supply and hydrogen concentration are shown in
Figure 5.
As can be seen from
Figure 5, the hydrogen gas concentration dropped sharply after ignition, and in this case, the maximum explosion pressure and explosive velocity in the gas cylinder cabinet were measured. The maximum explosion pressure was −0.21 and 0.36 bar at point P1. In addition, the central explosion pressure shown on the P2 explosion pressure gauge, which is at the closest point to the electric ignition system, is −0.15, 0.22 bar. Thereafter, based on the P2 pressure gauge, each explosive velocity was calculated with the time to reach the explosion pressure at each point, and the results are in
Table 4. The calculation method is as follows.
Table 5 shows the maximum explosion pressure and the maximum negative pressure (−) of each sensor according to Scenario 1. As can be seen from
Table 5, it could be seen that P4 to P6 were not affected by the explosion as all pressures escaped through the open upper pipe. In addition, the pressure gauges installed on the left and right sides were hardly affected in terms of stress, so it was assumed that the gas cylinder cabinet per se was not deformed and no separate flame due to the explosion was observed by the naked eyes. Two experiments were performed for Scenario 1, and the higher values in terms of overpressure were chosen for this analysis. We have not shown the repeated experiment results.
2.5.2. Scenario 2: State where the Pipe above the Gas Cylinder Cabinet Is Closed
In the state where the pipe above the gas cylinder cabinet was closed too, the hydrogen concentration was measured at the top, center, and bottom, respectively, identically to the state where the pipe was opened, and the concentration at the center where the ignition device is located was set as the reference concentration. A value of 44.868 vol%, which is the maximum concentration value, was shown when 145.9 s passed after the supply of hydrogen began, and the concentration gradually decreased. The ignition was carried out at 258.4 s, when a hydrogen concentration of 39.07 vol% was reached. The changes in the hydrogen supply and hydrogen concentration are shown in
Figure 6.
In Scenario 2, a rapid drop of the hydrogen gas concentration inside the cabinet dropped was observed after ignition identically to Scenario 1. In this case, the maximum explosion pressure and explosive velocity inside the gas cylinder cabinet were measured. In some MFCs, hunting occurred in the process of adjusting the flow due to a decrease in the H
2 injection volume. In Scenario 2, the maximum explosion pressure occurred at point P8, and the pressure value at this time was shown to be −0.40 and 0.36 bar. The central explosion pressure shown on the P2 explosion pressure gauge, which is at the closest point to the electric ignition system, is −0.12 and 0.12 bar. In Scenario 2, based on the pressure gauge P2, each explosion velocity was calculated as the time to reach the explosion pressure at each point, and the results are shown in
Table 6. Two experiments were performed for Scenario 2 and the higher values in terms of overpressure were chosen for this analysis. We have not shown the repeated experiment results.
Table 7 shows the maximum explosion pressure and the maximum negative pressure (−) of each sensor according to Scenario 2.
The explosion velocity from P2 was measured to be 557.27–994.00 mm/ms, but the probabilities by location were not identified, and the first pressure was generated from the upper part (P8). This is considered to be a phenomenon occurring because the shape of the explosion pressure does not have isotropy. In addition, the strain gauges installed on the left and right sides showed a maximum stress of −2.87 MPa (compression), indicating that the effect is very small in terms of stress, Therefore, it was identified that no large deformation occurred in the gas cylinder cabinet per se.
3. Review of Protection Performance
Next, the protective performance of the protective wall was analyzed by applying a protective wall that meets the domestic legal standards based on the explosion of about 0.3 bar that was generated at the center point in the gas cylinder cabinet during the verification experiment. To that end, boundary conditions such as explosion location, explosion pressure, and explosion pressure propagation profile calculation are required before performing the explosion analysis. The center of the cabinet model was applied as the explosion location, and the explosion pressure propagation profile using the TNT equivalent method was applied to the explosion pressure.
3.1. Explosion Analysis Stage 1, TNT Equivalent Method
In the case of combustible gases, the explosion pressure and explosive velocity vary greatly depending on the gas composition ratio, mixing characteristics, and compression state, and it is difficult to apply directly to AUTODYN due to its atypical shape. Therefore, in AUTODYNU, the TNT equivalent method is used to define the characteristics of gas explosion.
The TNT equivalent method refers to expressing the energy generated when a material explodes as the weight of TNT that generates the same energy, and is mainly used for conversion of explosion diffusion distances and prediction of explosion damage. TNT is highly reliable because its explosion characteristics such as explosion pressure and explosion energy in the event of explosion have been measured in detail in previous studies, and has characteristics of enabling the calculation of explosion pressure characteristics according to the composition ratio and the amount of leakage of combustible gases.
The formula for calculating TNT equivalent is as shown by Equation (1), and the variables for calculating TNT equivalent are shown in
Table 8.
: TNT equivalent (kg);
: Explosion yield coefficient (0.5);
: Quantity of leaked combustible gas (kg);
: Heat of combustion of the material that caused the explosion (Kcal/kg);
: Heat of combustion of TNT (1110 Kcal/kg).
A two-dimensional analysis was performed to calculate the gas explosion pressure data using the TNT equivalent calculated in
Table 8, and the two-dimensional analysis model is shown in
Figure 7. Thereafter, to simulate the pressure propagation shape (spherical), an air region was constructed using a 2D wedge shape, and the size of the TNT region was determined considering the volume corresponding to the TNT equivalent.
Figure 8 shows the results of explosion profile in 2D.
3.2. Protection Performance Analysis, Stage 2
In Stage 2, analysis was performed applying the explosion pressure propagation profile calculated using the TNT equivalence method in Stage 1. The legal standard protective wall bar arrangement drawing and physical properties used in the analysis are as follows in
Figure 9.
In addition, the physical properties of the protective wall model used in the analysis are shown in
Table 9.
The boundary conditions to conduct structural analysis for the evaluation of the protection performance of the protective wall were applied based on the explosion pressure of about 0.3 bar generated at the center point in the gas cylinder cabinet during the verification experiment, and the state where the boundary conditions were applied was as follows in
Figure 10.
The structural analysis of the protective wall was conducted, and as a result, the maximum stress of the concrete model was measured to be 30.211 MPa, and the maximum stress of the reinforcing bar model was measured to be 112.88 MPa as in
Figure 11. These values do not exceed 45 MPa, the tensile strength of concrete, and 420 MPa, the yield strength of reinforcing bars, respectively, indicating that the effect of hydrogen explosion in the cylinder cabinet on damage to the protective wall is very small.
4. Conclusions
The purpose of this study was to propose a new alternative to the domestic protective wall installation standard, which is problematic in the frequent semiconductor fab layout changes following the development of semiconductor technologies. To that end, hydrogen gas leakage from the cylinder cabinet being used was assumed, the explosion pressure was experimented, and the effect of the explosion pressure on the protective wall was examined. To this end, first, in the first stage, a cabinet cylinder with the same specifications as those used in the actual semiconductor field was fabricated, hydrogen gas was leaked, and the explosion pressure was measured. Thereafter, in the second stage, the cabinet cylinder center point explosion pressure value of 0.3 bar, which is the result of the experiment conducted earlier, was applied to the protective wall to find out the protection performance. As a result, the maximum stress in the concrete model was measured to be 30.211 MPa, and the maximum stress in the reinforcing bar model was measured to be 112.88 MPa. These values do not exceed 45 MPa, the tensile strength of concrete, and 420 MPa, the yield strength of rebar, respectively. These resultant values mean that the effect of the explosion pressure due to the explosion of the hydrogen gas in the gas cylinder cabinet on damage to the protective wall is very small. Therefore, this study prepared and presented a basis for the construction of simplified protective walls that fit the situation of the site when the semiconductor fab layout is changed and is expected to be very helpful for the development of the semiconductor industry through the foregoing.
However, the present test simply experimented the situation of explosion of the leaked gas inside the gas cylinder cabinet without considering external factors. Additional reviews of external factors such as external flames, temperature changes, or impact are judged to be necessary. In addition, in the case of the present test, matters regarding the installation and operation of the gas cylinder cabinet were not considered, and the tests were conducted under different sealing conditions for the upper and lower exhaust holes and sides of the gas cylinder cabinet.
Therefore, in the gas cylinder cabinet operating situation cabinet where the gas leak alarm device and the exhaust device are installed, the gas is exhausted before it is retained. Therefore, it is assumed that no explosive atmosphere will be formed or lower explosion pressures than the result of the present experiment in which 39% of hydrogen was injected will be shown.