A Recent and Systematic Review on Water Extraction from the Atmosphere for Arid Zones
Abstract
1. Introduction
2. Water Extraction Methods
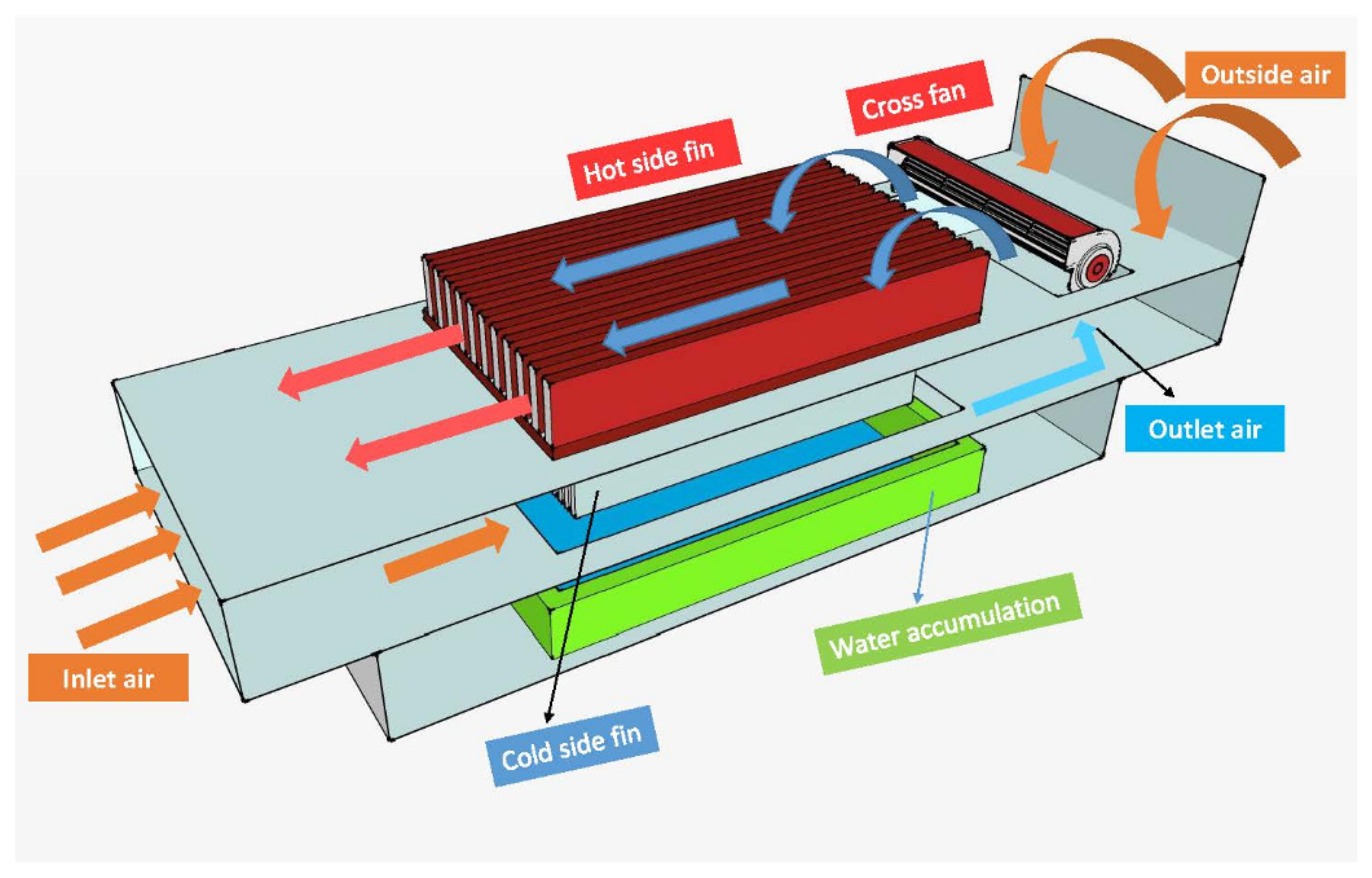
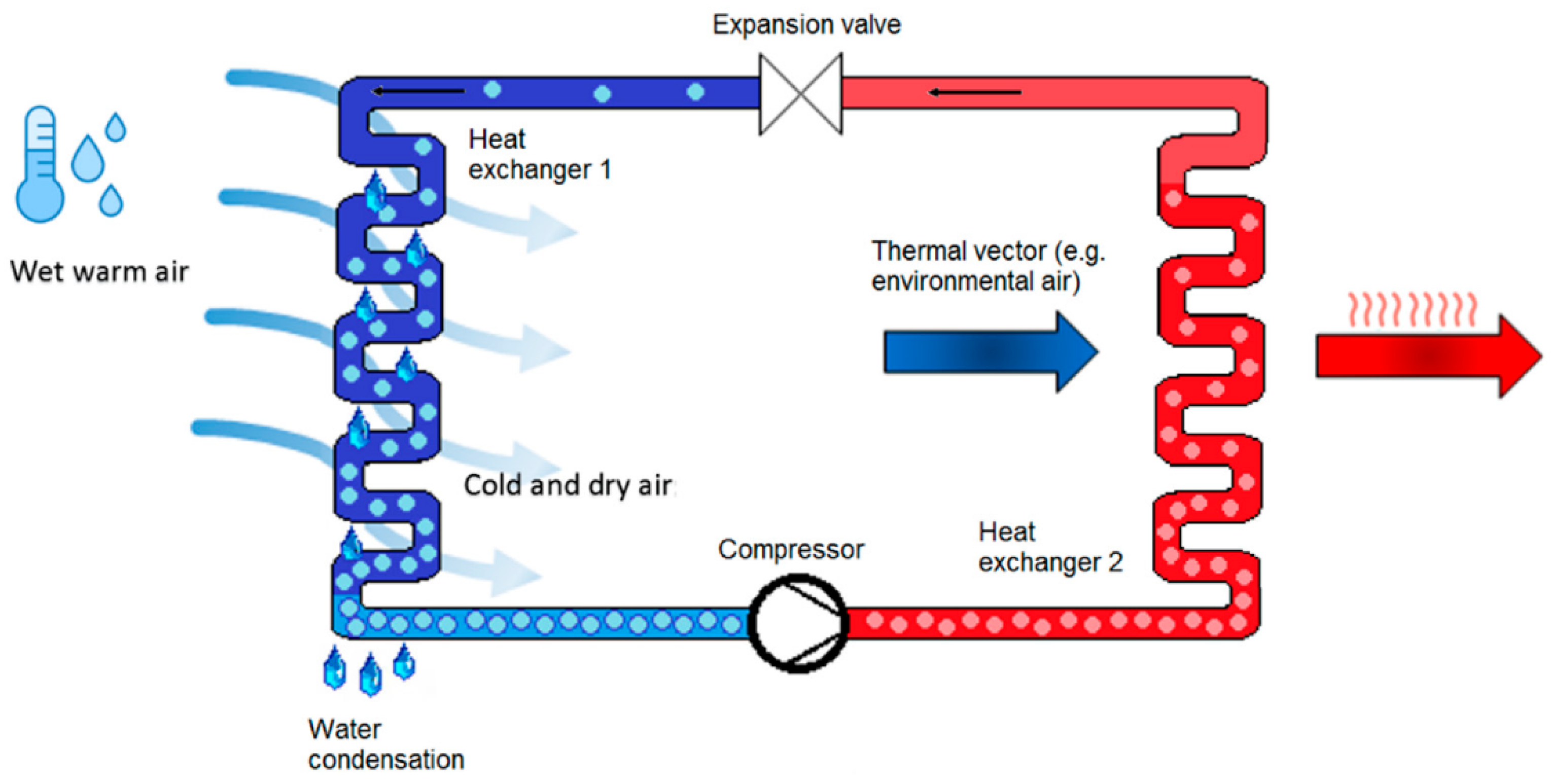
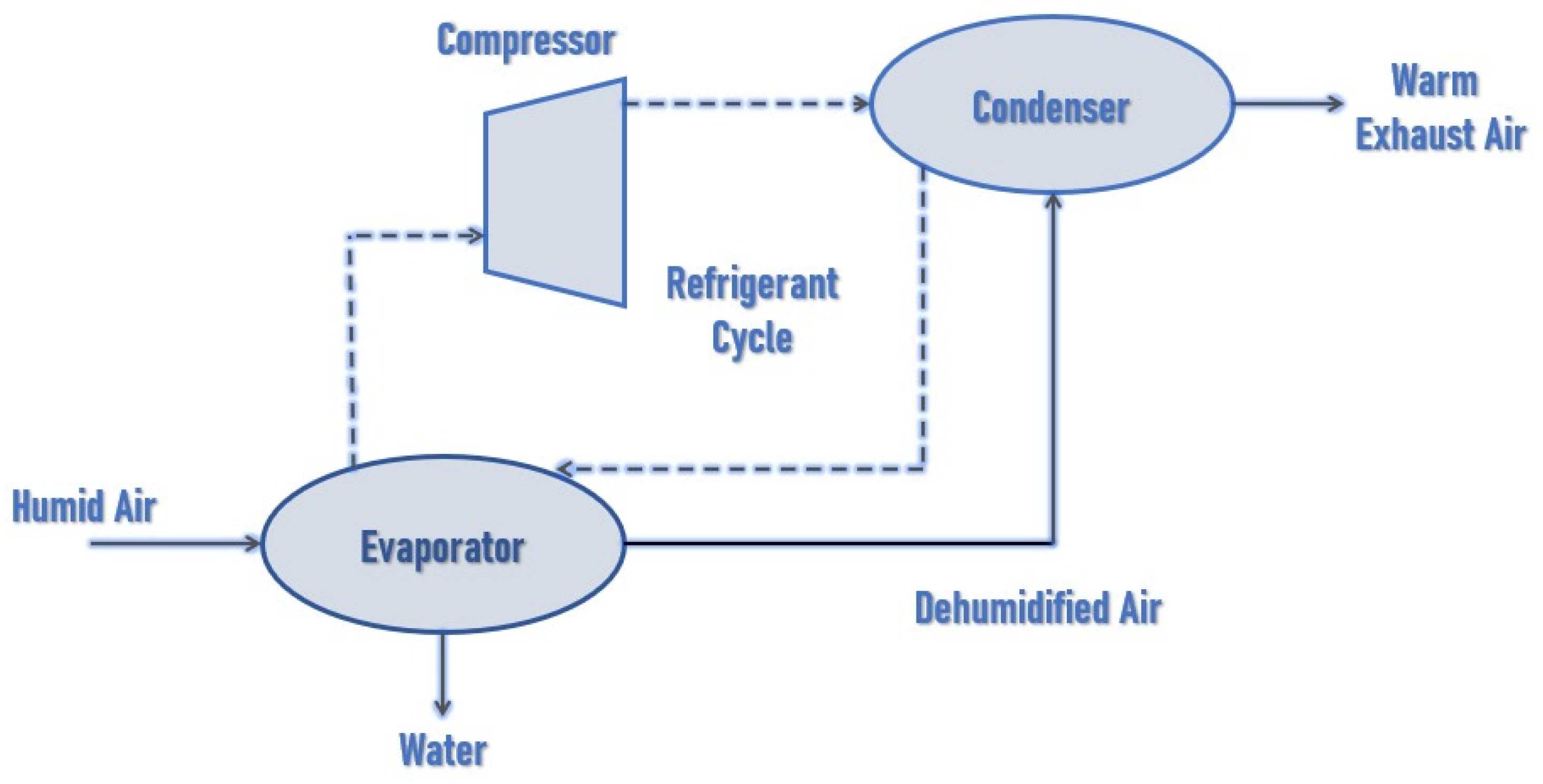
2.1. Air-to-Water Generators (AWGs)
2.2. Earth-Water Collector
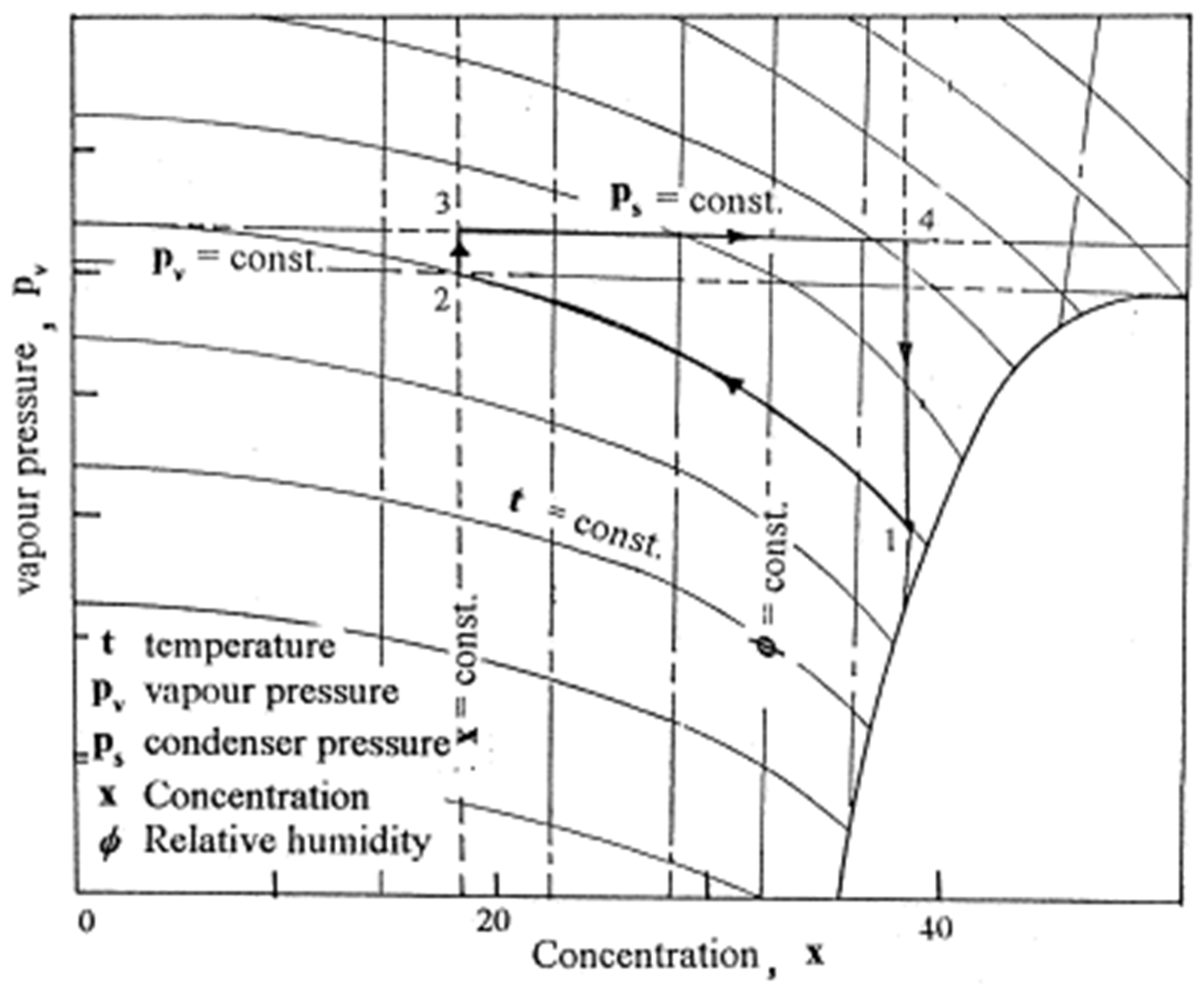
2.3. Absorption–Regeneration Cycle
- (a)
- Process 1_2: isothermal water vapor absorption from the air;
- (b)
- Process 2_3: heating the absorbent at a steady concentration;
- (c)
- Process 3_4: constant pressure absorbent regeneration;
- (d)
- Process 4_1: cooling the absorbent at a steady concentration.
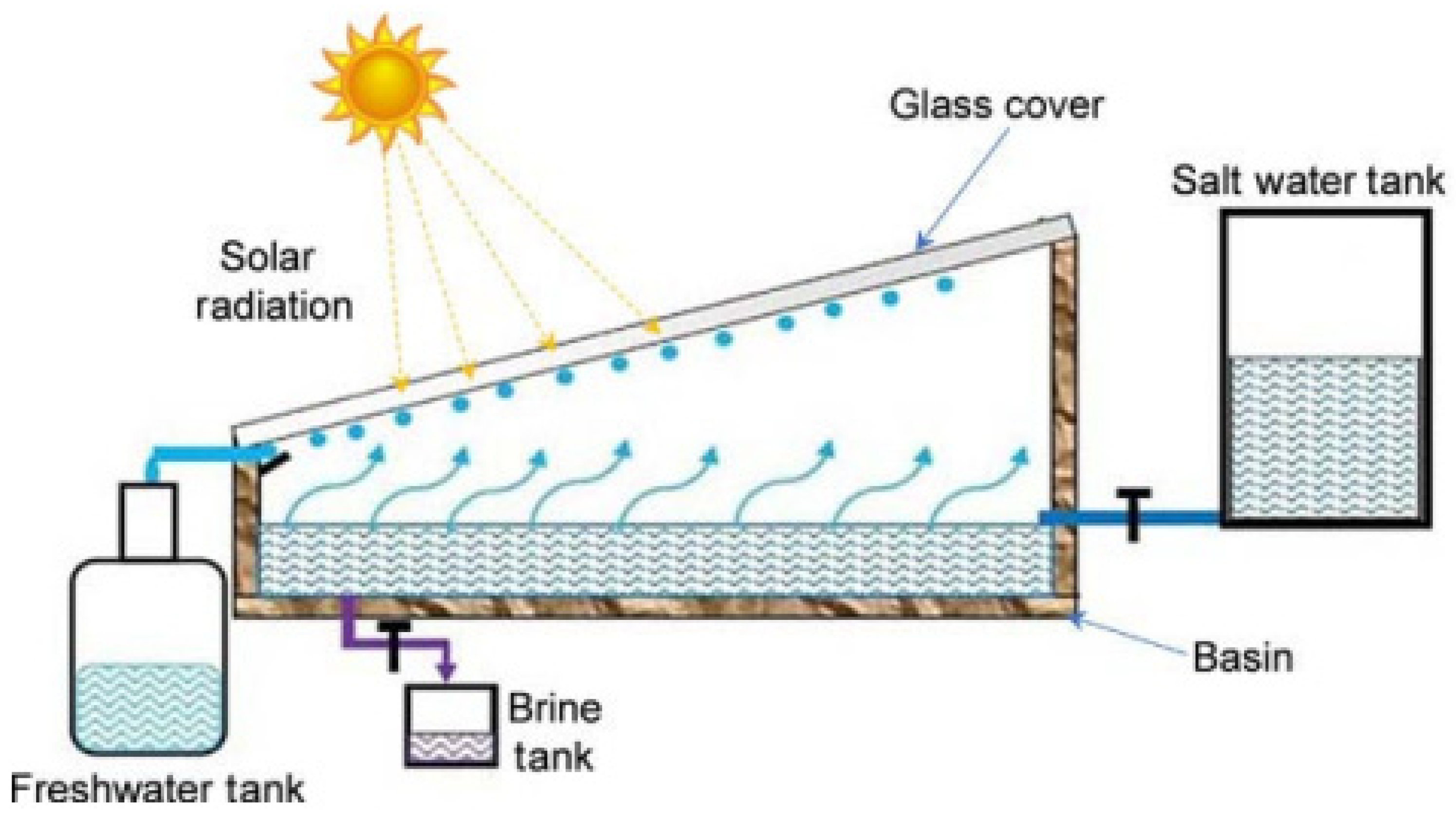
2.4. Dew Collection
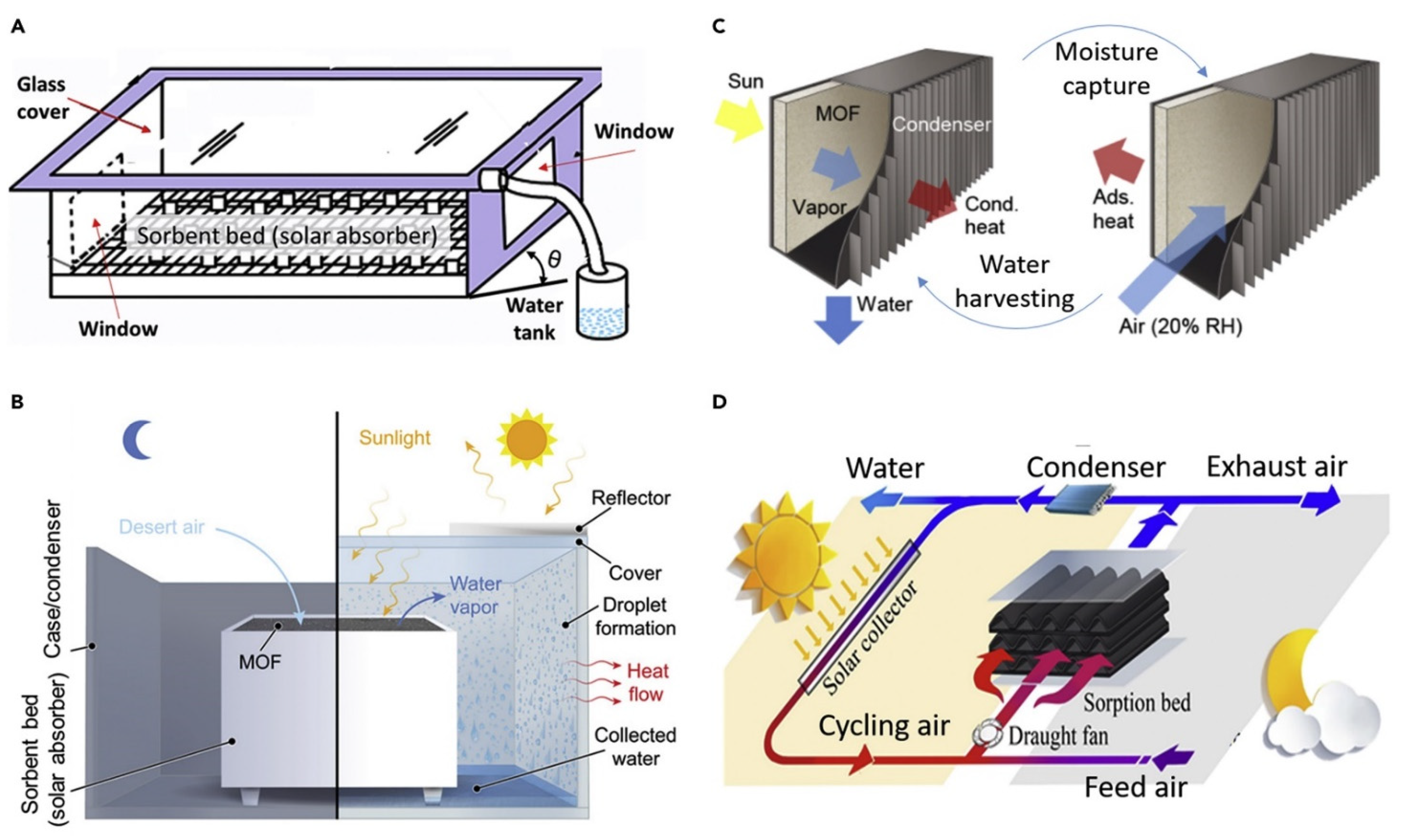
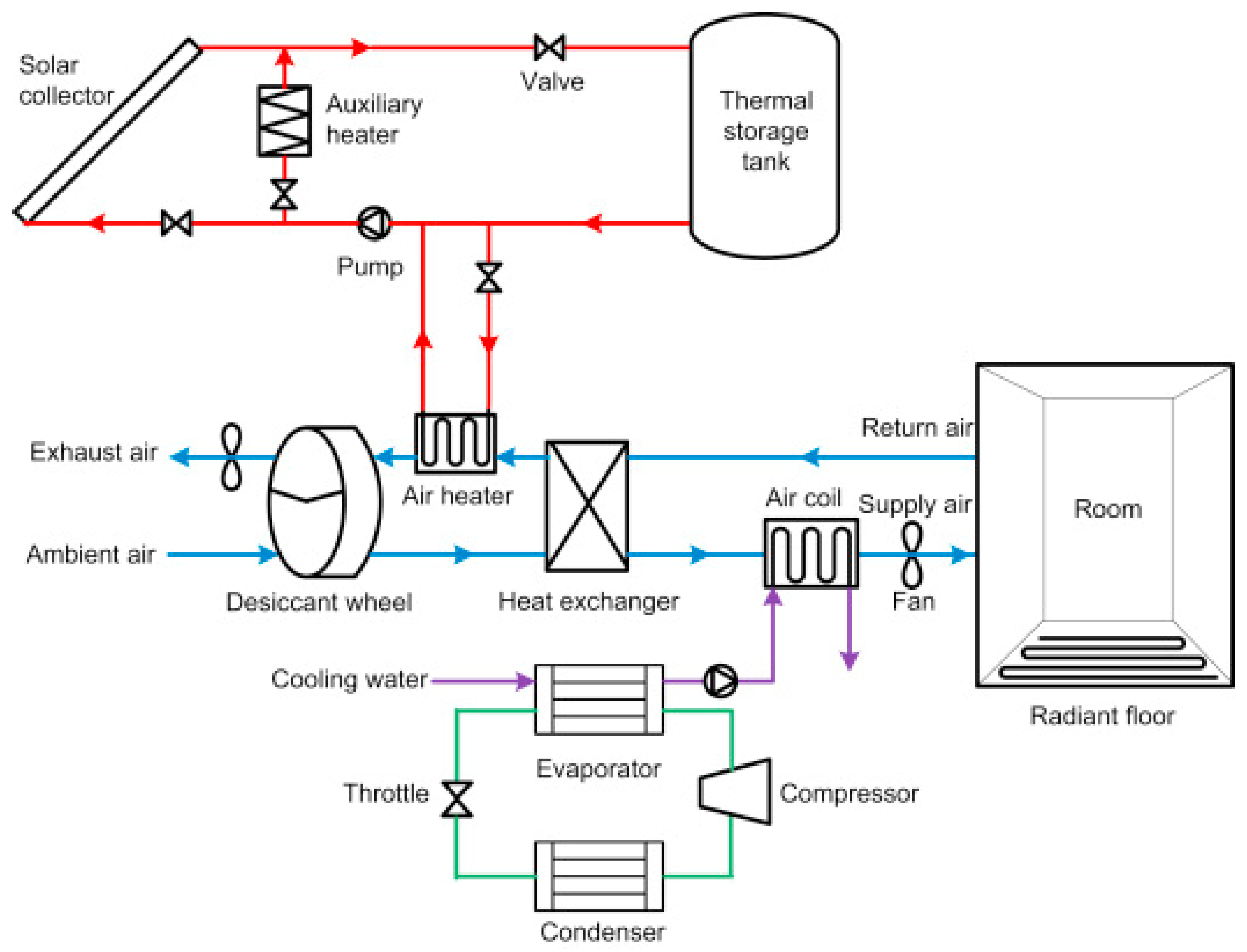
2.5. Desiccant Systems
3. Policy Perspective
4. Conclusions
5. Future Work and Limitations
Author Contributions
Funding
Conflicts of Interest
References
- Singh, L.K.; Jha, M.K.; Chowdary, V. Multi-criteria analysis and GIS modeling for identifying prospective water harvesting and artificial recharge sites for sustainable water supply. J. Clean. Prod. 2017, 142, 1436–1456. [Google Scholar] [CrossRef]
- Gao, M.; Peh, C.K.; Meng, F.L.; Ho, G.W. Photothermal Membrane Distillation toward Solar Water Production. Small Methods 2021, 5, 2001200. [Google Scholar] [CrossRef]
- Morciano, M.; Fasano, M.; Bergamasco, L.; Albiero, A.; Curzio, M.L.; Asinari, P.; Chiavazzo, E. Sustainable freshwater production using passive membrane distillation and waste heat recovery from portable generator sets. Appl. Energy 2020, 258, 114086. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, A. Experimental investigation of solar powered water production from atmospheric air by using composite desiccant material “CaCl2/saw wood”. Desalination 2015, 367, 216–222. [Google Scholar] [CrossRef]
- Kalidasan, B.; Divyabharathi, R.; Pandey, A.; Subramaniyan, C.; Mohankumar, S. Technological Advancement of Solar Thermal System Desalination Process—A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1059, 012061. [Google Scholar] [CrossRef]
- Meran, G.; Siehlow, M.; Von Hirschhausen, C. Water Availability: A Hydrological View. In Agriculture Productivity in Tunisia Under Stressed Environment; Springer: New York, NY, USA, 2020; pp. 9–21. [Google Scholar]
- Hamed, A.M.; Aly, A.A.; Zeidan, E.-S.B. Application of Solar Energy for Recovery of Water from Atmospheric Air in Climatic Zones of Saudi Arabia. Nat. Resour. 2011, 2, 8–17. [Google Scholar] [CrossRef]
- Kabeel, A. Application of sandy bed solar collector system for extraction of water from the air. In Proceedings of the 8th International Water Technology Conference, Alexandria, Egypt, 26–28 March 2004; pp. 231–249. [Google Scholar]
- Hamed, A.M.; Kabeel, A.; Zeidan, E.-S.B.; Aly, A.A. A technical review on the extraction of water from atmospheric air in arid zones. JP J. Heat Mass Transf. 2010, 4, 213–228. [Google Scholar]
- Ingold, T.; Mätzler, C.; Kampfer, N.; Schmid, B.; Démoulin, P. Modeled and empirical approaches for retrieving columnar water vapor from solar transmittance measurements in the 0.72, 0.82, and 0.94 μm absorption bands. J. Geophys. Res. Space Phys. 2000, 105, 24327–24343. [Google Scholar] [CrossRef]
- Bar, E. Extraction of water from air—An alternative solution for water supply. Desalination 2004, 165, 335. [Google Scholar] [CrossRef]
- Cattani, L.; Magrini, A.; Cattani, P. Water Extraction from Air: A Proposal for a New Indicator to Compare Air Water Generators Efficiency. Energies 2021, 14, 224. [Google Scholar] [CrossRef]
- He, W.; Yu, P.; Hu, Z.; Lv, S.; Qin, M.; Yu, C. Experimental Study and Performance Analysis of a Portable Atmospheric Water Generator. Energies 2019, 13, 73. [Google Scholar] [CrossRef]
- Khalil, B.; Adamowski, J.; Shabbir, A.; Jang, C.; Rojas, M.; Reilly, K.; Ozga-Zielinski, B. A review: Dew water collection from radiative passive collectors to recent developments of active collectors. Sustain. Water Resour. Manag. 2016, 2, 71–86. [Google Scholar] [CrossRef]
- Tripathi, A.; Tushar, S.; Pal, S.; Lodh, S.; Tiwari, S.; Desai, R.S. Atmospheric water generator. Int. J. Enhanc. Res. Sci. 2016, 5, 69–72. [Google Scholar]
- Khalil, A. Dehumidification of atmospheric air as a potential source of fresh water in the UAE. Desalination 1993, 93, 587–596. [Google Scholar] [CrossRef]
- Habeebullah, B.A. Potential use of evaporator coils for water extraction in hot and humid areas. Desalination 2009, 237, 330–345. [Google Scholar] [CrossRef]
- Li, Y.; Lu, L.; Yang, H. Energy and economic performance analysis of an open cycle solar desiccant dehumidification air-conditioning system for application in Hong Kong. Sol. Energy 2010, 84, 2085–2095. [Google Scholar] [CrossRef]
- Alahmer, A.; Al-Dabbas, M.; AlSaqoor, S.; Al-Sarayreh, A. Utilizing of Solar Energy for Extracting Freshwater from Atmospheric Air. Appl. Sol. Energy 2018, 54, 110–118. [Google Scholar] [CrossRef]
- Uglanov, D.; Zheleznyak, K.; Chertykovsev, P. The Development and Calculation of an Energy-saving Plant for Obtaining Water from Atmospheric Air. IOP Conf. Ser. Mater. Sci. Eng. 2018, 302, 012053. [Google Scholar] [CrossRef]
- Vinay, M.V.; Suman, A.; Shadakshari, R. Dehumidification of Atmospheric Air for Water Production. Int. J. Innov. Res. Sci. Eng. Technol. 2018, 7, 3808–3813. [Google Scholar]
- Thippeswamy, J.B.; Praveen Kumar, B.S.; Sandeep, G.P.; Rudresh, M.P. Extraction of Water from Atmospheric Moisture; Kalpataru Institute of Technology: Tiptur, India, 2015. [Google Scholar]
- Dash, A.; Mohapatra, A. Atmospheric Water Generator: To Meet the Drinking Water Requirements of a Household in Coastal Regions of India. Ph.D. Thesis, Department of Mechanical Engineering, National Institute of Technology, Rourkela, India, 2015. [Google Scholar]
- North, G.R.; Pyle, J.A.; Zhang, F. (Eds.) Encyclopedia of Atmospheric Sciences; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1. [Google Scholar]
- Dickinson, R.E. Land-atmosphere interaction. Rev. Geophys. 1995, 33, 917–922. [Google Scholar] [CrossRef]
- Kobayashi, M. A method of obtaining water in arid lands. Sol. Energy 1963, 7, 93–99. [Google Scholar] [CrossRef]
- Roderick, M.L.; Sun, F.; Farquhar, G.D. Water Cycle Varies over Land and Sea. Science 2012, 336, 1230–1231. [Google Scholar] [CrossRef]
- Sharshir, S.; Yang, N.; Peng, G.; Kabeel, A. Factors affecting solar stills productivity and improvement techniques: A detailed review. Appl. Therm. Eng. 2016, 100, 267–284. [Google Scholar] [CrossRef]
- Hamed, A.M. Absorption-regeneration cycle for water production from air-theoretical approach. Renew. Energy 2000, 19, 625–635. [Google Scholar] [CrossRef]
- Sultan, A. Absorption/regeneration non-conventional system for water extraction from atmospheric air. Renew. Energy 2004, 29, 1515–1535. [Google Scholar] [CrossRef]
- Kim, H.; Rao, S.R.; Kapustin, E.A.; Zhao, L.; Yang, S.; Yaghi, O.M.; Wang, E.N. Adsorption-based atmospheric water harvesting device for arid climates. Nat. Commun. 2018, 9, 1191. [Google Scholar] [CrossRef]
- Rajvanshi, A.K. Large scale dew collection as a source of fresh water supply. Desalination 1981, 36, 299–306. [Google Scholar] [CrossRef]
- Nilsson, T. Initial experiments on dew collection in Sweden and Tanzania. Sol. Energy Mater. Sol. Cells 1996, 40, 23–32. [Google Scholar] [CrossRef]
- Gandhidasan, P.; Abualhamayel, H. Modeling and testing of a dew collection system. Desalination 2005, 180, 47–51. [Google Scholar] [CrossRef]
- Ziatdinov, R.; Nabiyev, R.; Kim, H.-S.; Lim, S.H. The Concept of a Dew Collection Device Based on the Mathematical Model of Sliding Liquid Drops on an Inclined Solid Surface. IOP Conf. Ser. Earth Environ. Sci. 2019, 272, 022091. [Google Scholar] [CrossRef]
- Beysens, D.; Clus, O.; Mileta, M.; Milimouk, I.; Muselli, M.; Nikolayev, V. Collecting dew as a water source on small islands: The dew equipment for water project in Bisevo (Croatia). Energy 2007, 32, 1032–1037. [Google Scholar] [CrossRef]
- Mousavi-baygi, M. The implementation of fog water collection systems in Northeast Iran. Int. J. Pure Appl. Phys. 2008, 4, 13–21. [Google Scholar]
- Jacobs, A.; Heusinkveld, B.; Berkowicz, S. Passive dew collection in a grassland area, The Netherlands. Atmos. Res. 2008, 87, 377–385. [Google Scholar] [CrossRef]
- Tariq, N.; Asim, M.; Al-Obeidat, F.; Farooqi, M.Z.; Baker, T.; Hammoudeh, M.; Ghafir, I. The Security of Big Data in Fog-Enabled IoT Applications Including Blockchain: A Survey. Sensors 2019, 19, 1788. [Google Scholar] [CrossRef]
- Al-Hassan, G.A. Fog Water Collection Evaluation in Asir Region–Saudi Arabia. Water Resour. Manag. 2009, 23, 2805–2813. [Google Scholar] [CrossRef]
- Katejanekarn, T.; Chirarattananon, S.; Kumar, S. An experimental study of a solar-regenerated liquid desiccant ventilation pre-conditioning system. Sol. Energy 2009, 83, 920–933. [Google Scholar] [CrossRef]
- William, G.E.; Hassan, M.; Fatouh, M. Water Production by Using Solar Energy: Water Recovery from Atmospheric Air; LAP Lambert Academic Publishing: Sunnyvale, CA, USA, 2014. [Google Scholar]
- Hall, R.C. Theoretical calculations on the production of water from the atmosphere by absorption with subsequent recovery in a solar still. Sol. Energy 1966, 10, 41–45. [Google Scholar] [CrossRef]
- Gad, H.; Hamed, A.; El-Sharkawy, I.I. Application of a solar desiccant/collector system for water recovery from atmospheric air. Renew. Energy 2001, 22, 541–556. [Google Scholar] [CrossRef]
- Clarke, N.P. Atmospheric Moisture Collection Device. U.S. Patent 5,233,843A, 10 August 1993. [Google Scholar]
- Alayli, Y.; Hadji, N.; Leblond, J. A new process for the extraction of water from air. Desalination 1987, 67, 227–229. [Google Scholar] [CrossRef]
- Gandhidasan, P.; Abualhamayel, H. Water recovery from the atmosphere. Renew. Energy 1996, 9, 745–748. [Google Scholar] [CrossRef]
- Abualhamayel, H.; Gandhidasan, P. A method of obtaining fresh water from the humid atmosphere. Desalination 1997, 113, 51–63. [Google Scholar] [CrossRef]
- Aristov, Y.; Tokarev, M.; Gordeeva, L.; Snytnikov, V.; Parmon, V. New Composite Sorbents for Solar-Driven Technology of Fresh Water Production from the Atmosphere. Sol. Energy 1999, 66, 165–168. [Google Scholar] [CrossRef]
- Hamed, A. Experimental investigation on the natural absorption on the surface of sandy layer impregnated with liquid desiccant. Renew. Energy 2003, 28, 1587–1596. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, R.; Zhang, Y.; Wang, J. Progress and Expectation of Atmospheric Water Harvesting. Joule 2018, 2, 1452–1475. [Google Scholar] [CrossRef]
- Scrivani, A.; el Asmar, T.; Bardi, U. Solar trough concentration for freshwater production and wastewater treatment. Desalination 2007, 206, 485–493. [Google Scholar] [CrossRef]
- Ge, T.; Xu, J. Review of solar-powered desiccant cooling systems. Adv. Sol. Heat. Cool. 2016, 329–379. [Google Scholar] [CrossRef]
- Kabeel, A. Water production from air using multi-shelves solar glass pyramid system. Renew. Energy 2007, 32, 157–172. [Google Scholar] [CrossRef]
- Abdelal, N.; Taamneh, Y. Enhancement of pyramid solar still productivity using absorber plates made of carbon fiber/CNT-modified epoxy composites. Desalination 2017, 419, 117–124. [Google Scholar] [CrossRef]
- Ji, J.; Wang, R.; Li, L. New composite adsorbent for solar-driven freshwater production from the atmosphere. Desalination 2007, 212, 176–182. [Google Scholar] [CrossRef]
- Bardi, U. Freshwater production using solar concentration: The AQUASOLIS project. Desalination 2008, 220, 588–591. [Google Scholar] [CrossRef]
- Gandhidasan, P.; Abualhamayel, H.I. Investigation of humidity harvest as an alternative water source in the Kingdom of Saudi Arabia. Water Environ. J. 2009, 24, 282–292. [Google Scholar] [CrossRef]
- Kabeel, A.; Abdulaziz, M.; El-Said, E.M. Solar-based atmospheric water generator utilization of a freshwater recovery: A numerical study. Int. J. Ambient. Energy 2016, 37, 68–75. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, A. Solar-driven technology for freshwater production from atmospheric air by using the composite desiccant material “CaCl2/floral foam”. Environ. Dev. Sustain. 2015, 18, 1151–1165. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, A. Experimental investigation of design parameters of solar glass desiccant box type system for water production from atmospheric air. J. Renew. Sustain. Energy 2015, 7, 033122. [Google Scholar] [CrossRef]
- William, G.; Mohamed, M.; Fatouh, M. Desiccant system for water production from humid air using solar energy. Energy 2015, 90, 1707–1720. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, A. Composite desiccant material “CaCl2/Vermiculite/Saw wood”: A new material for freshwater production from atmospheric air. Appl. Water Sci. 2017, 7, 2103–2111. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Wang, R.; Wang, L.; Liu, J. A high efficient semi-open system for freshwater production from the atmosphere. Energy 2017, 138, 542–551. [Google Scholar] [CrossRef]
- Talaat, M.; Awad, M.; Zeidan, E.; Hamed, A. Solar-powered portable apparatus for extracting water from air using desiccant solution. Renew. Energy 2018, 119, 662–674. [Google Scholar] [CrossRef]
- Srivastava, S.; Yadav, A. Water generation from atmospheric air by using composite desiccant material through fixed focus concentrating solar thermal power. Sol. Energy 2018, 169, 302–315. [Google Scholar] [CrossRef]
- Estahbanati, M.R.K.; Ahsan, A.; Feilizadeh, M.; Jafarpur, K.; Ashrafmansouri, S.-S.; Feilizadeh, M. Theoretical and experimental investigation on internal reflectors in a single-slope solar still. Appl. Energy 2016, 165, 537–547. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, C.; Xian, H.; Du, X. Thermal Properties of Solar Collector Comprising Oscillating Heat Pipe in a Flat-Plate Structure and Water Heating System in Low-Temperature Conditions. Energies 2018, 11, 2553. [Google Scholar] [CrossRef]
- Riahi, A.; Zakaria, N.A.; Noh, N.M.; Mat Amin, M.Z.; Mat Jusoh, A.; Mohamad Ideris, M.; Muhammad, M.Z.; Ramli, M.A.; Mohd Arif Zainol, M.R.R.; Shaharuddin, S.; et al. Performance Investigation of 18 Thermoelectric Cooler (TEC) Units to Supply Continuous Daily Fresh Water from Malaysia’s Atmosphere. Sustainability 2021, 13, 1399. [Google Scholar] [CrossRef]
- Moghimi, F.; Ghoddusi, H.; Asiabanpour, B.; Behroozikhah, M. Is atmospheric water generation an economically viable solution? Clean Technol. Environ. Policy 2021, 23, 1045–1062. [Google Scholar] [CrossRef]

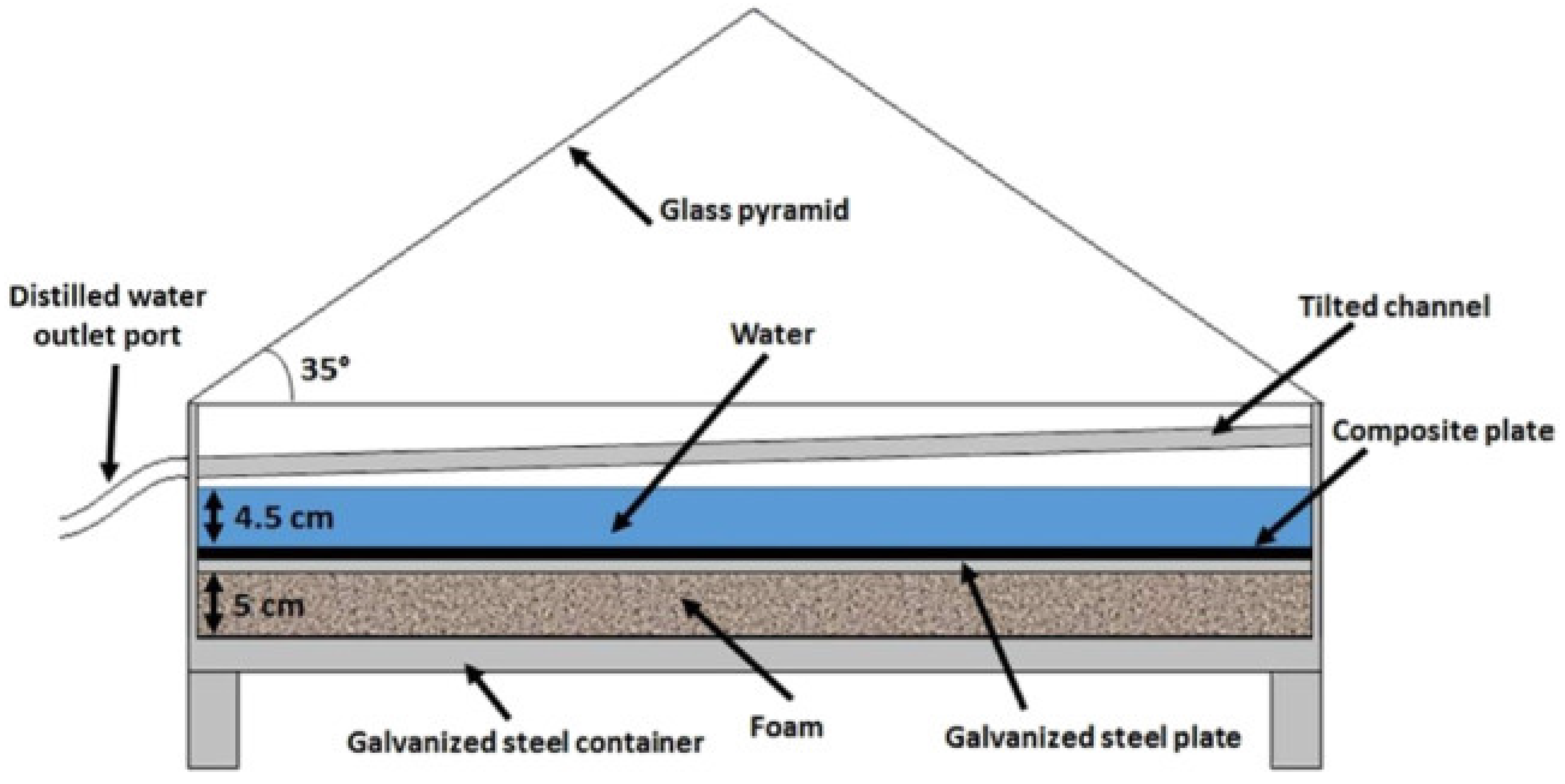


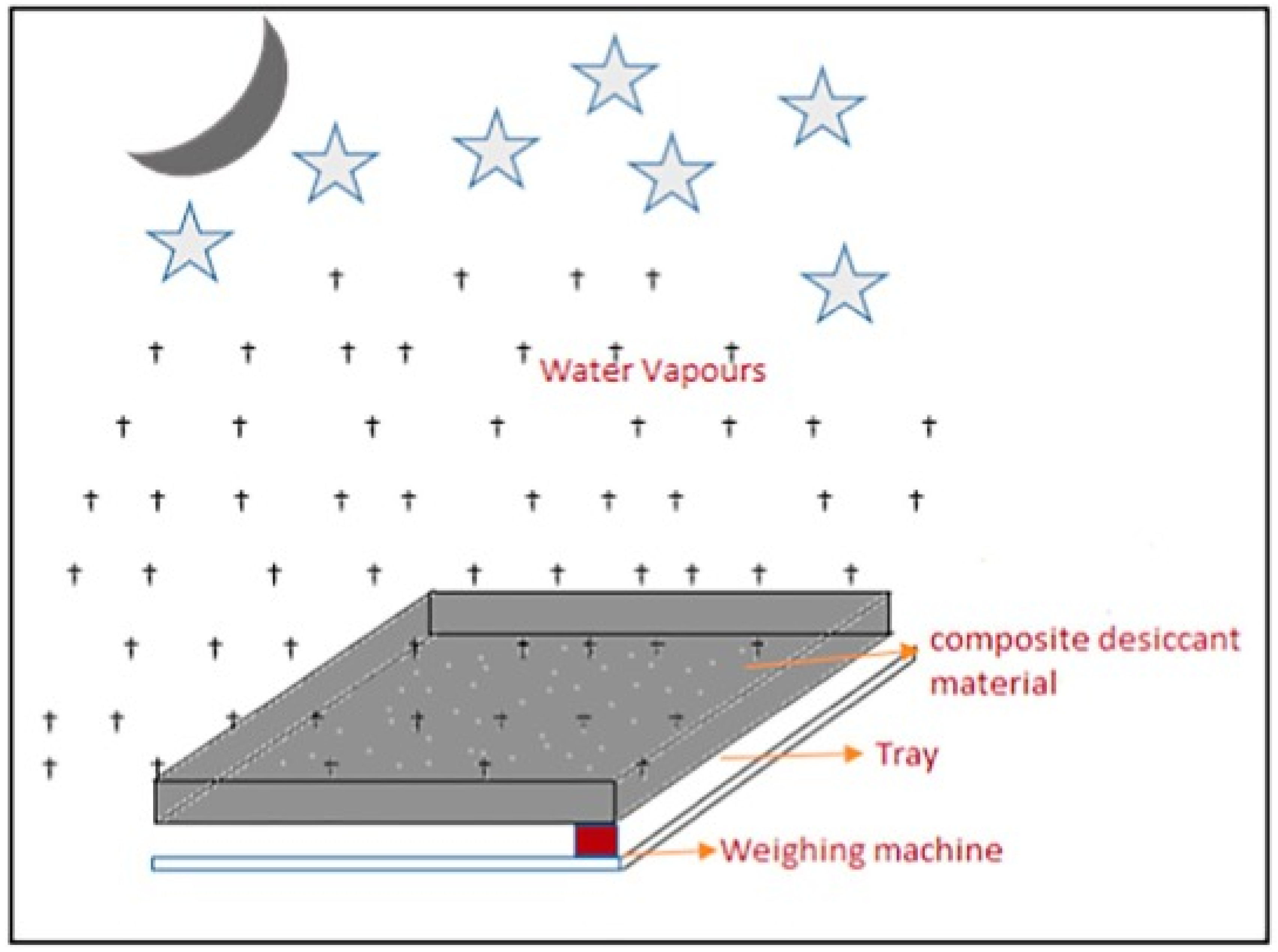
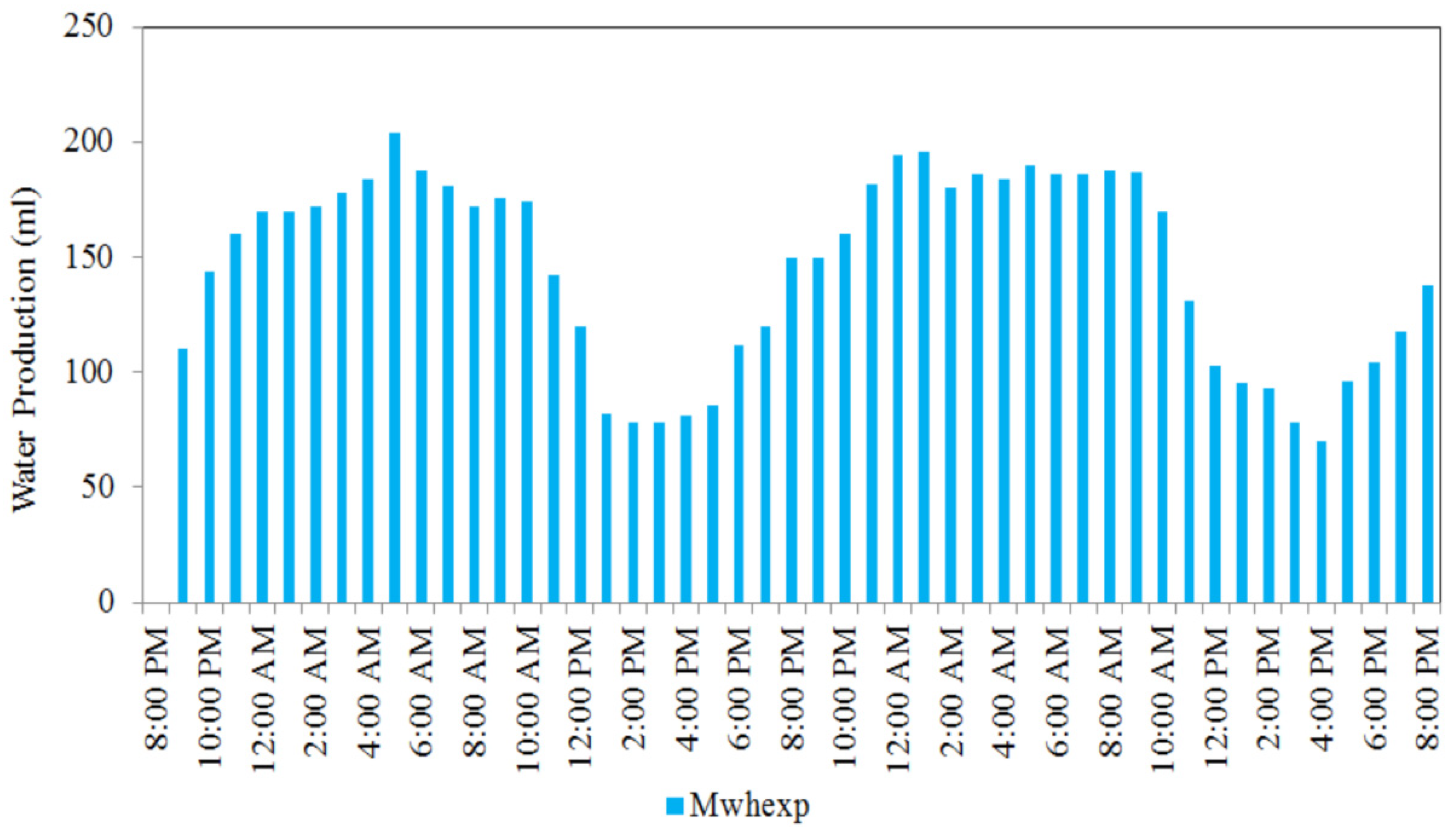
| Reference No. | Bed Type | Desiccant Type | Production Amount | Test Location | Test Date |
|---|---|---|---|---|---|
| [43] | Sheet of plywood | Ethylene glycol | - | Manhattan, USA | 1966 |
| [46] | S-shaped composite material | Physicochemical adsorption | 1 L/m2 | Holland | 1987 |
| [45] | - | Triethylene glycol | - | USA | 1993 |
| [47] | - | CaCl2 | - | Dhahran, KSA | 1996 |
| [48] | - | - | 1.92 L/m2 | Dhahran, KSA | 1997 |
| [49] | SiO2 or Al2O3 or C | CaCl2 or LiBr | 3–5 ton/ton of dry SWSs | Novosibirsk, Russia | 1998 |
| [44] | Thick corrugated layer of cloth | CaCl2 | 1.5 L/m2 | Egypt | 2000 |
| [50] | Sand | CaCl2 | - | Egypt | 2002 |
| [8] | Sand | CaCl2 | 1.2 L/m2 | Egypt | 2004 |
| [54] | Saw wood and corrugated cloth layer | CaCl2 | 2.5 L/m2 | Egypt | 2007 |
| [32] | MCM-41 material | CaCl2 | 1.2 L/m2 | Shanghai, China | 2007 |
| [56] | - | CaCl2 | Absorption: 2.11 L/m2 Desorption: 1.15 L/m2 | Dhahran, KSA | 2010 |
| [24] | Sand | CaCl2 | 1 L/m2 | Taif city, KSA | 2011 |
| [59] | - | CaCl2 | 3.9 L/h/m2 | Tanta, Egypt | 2014 |
| [62] | Sand and cloth layer | - | 1.23 slit/m2/d 2.32 slit/m2/d | Cairo, Egypt | 2015 |
| [4] | Saw wood | CaCl2 | 180 mL/kg/d | Kurukshetra, India | 2015 |
| [60] | Floral foam | CaCl2 | 0.35 mL/cm3/d | Kurukshetra, India | 2015 |
| [63] | Vermiculite + Saw wood | CaCl2 | 195 mL/kg/d | Kurukshetra, India | 2015 |
| [64] | Active carbon felt + Nano silica grains | LiCl | 14.7 kg/kg of water | Shanghai, China | 2017 |
| [65] | Cloth layer | CaCl2 | 0.3295–0.6310 kg/m2/d | Mansoura, Egypt | 2018 |
| [66] | Sand | CM1: LiCl CM2: CaCl2 CM3: LiBr | CM1: 90 mL/d CM2: 115 mL/d CM3: 73 mL/d | Kurukshetra, India | 2018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Danook, S.H.; AL-bonsrulah, H.A.Z.; Veeman, D.; Wang, F. A Recent and Systematic Review on Water Extraction from the Atmosphere for Arid Zones. Energies 2022, 15, 421. https://doi.org/10.3390/en15020421
Wang Y, Danook SH, AL-bonsrulah HAZ, Veeman D, Wang F. A Recent and Systematic Review on Water Extraction from the Atmosphere for Arid Zones. Energies. 2022; 15(2):421. https://doi.org/10.3390/en15020421
Chicago/Turabian StyleWang, Yinyin, Suad Hassan Danook, Hussein A.Z. AL-bonsrulah, Dhinakaran Veeman, and Fuzhang Wang. 2022. "A Recent and Systematic Review on Water Extraction from the Atmosphere for Arid Zones" Energies 15, no. 2: 421. https://doi.org/10.3390/en15020421
APA StyleWang, Y., Danook, S. H., AL-bonsrulah, H. A. Z., Veeman, D., & Wang, F. (2022). A Recent and Systematic Review on Water Extraction from the Atmosphere for Arid Zones. Energies, 15(2), 421. https://doi.org/10.3390/en15020421









